Abstract
In yeast, sphingoid base synthesis is required for the internalization step of endocytosis and organization of the actin cytoskeleton. We show that overexpression of either one of the two kinases Pkh1p or Pkh2p, that are homologous to mammalian 3-phosphoinositide-dependent kinase-1 (PDK1), can specifically suppress the sphingoid base synthesis requirement for endocytosis. Pkh1p and Pkh2p have an overlapping function because only a mutant with impaired function of both kinases is defective for endocytosis. Pkh1/2p kinases are activated in vitro by nanomolar concentrations of sphingoid base. These results suggest that Pkh1/2p kinases are part of a sphingoid base-mediated signaling pathway that is required for the internalization step of endocytosis. The Pkc1p kinase that is phosphorylated by Pkh1/2p kinases and plays a role in endocytosis was identified as one of the downstream effectors of this signaling cascade.
Keywords: endocytosis/PKH, PKC1 and PDK1 kinases/signaling pathway/sphingoid base
Introduction
Endocytosis is the process whereby eukaryotic cells internalize extracellular material and part of their own plasma membrane. This pathway is used commonly for uptake of nutrients, down-regulation of receptors and removal of other proteins from the cell surface. The development of several reporter systems to study endocytosis, as well as the use of genetic studies in the yeast Saccharomyces cerevisiae, has permitted the identification of many components required for this pathway. The internalization step of endocytosis requires actin, proteins that mediate actin cytoskeleton organization, such as fimbrin, calmodulin, type I myosin, the amphiphysin homologs Rvs161p and Rvs167p and clathrin, and a large set of proteins associated with these components (Geli and Riezman, 1998; D’Hondt et al., 2000; Lombardi et al., 2001).
Recently, not only proteins but also lipids have been implicated in several stages of membrane trafficking, but the role of lipids in vesicle budding and fusion in living cells is poorly understood. It is known that the sphingolipid synthesis pathway is necessary for trafficking of glycosylphosphatidylinositol (GPI)-anchored proteins from the endoplasmic reticulum to the Golgi apparatus (Horvath et al., 1994; Sütterlin et al., 1997). Phospho lipids, in particular phosphorylated derivatives of phosphatidylinositol (PI), also appear to play a critical role in regulating transport events (De Camilli et al., 1996). Sterols have been implicated in control of the internalization step of endocytosis in both yeast and mammalian cells (Munn et al., 1999; Rodal et al., 1999; Subtil et al., 1999). Sphingoid bases have also been shown to play a role in regulation of cell surface expression of amino acid permeases (Skrzypek et al., 1998).
Using the S.cerevisiae lcb1-100 mutant, a requirement for sphingoid base synthesis for the internalization step of endocytosis and for actin cytoskeleton organization was revealed (Zanolari et al., 2000). The LCB1 gene encodes a subunit of serine palmitoyltransferase that catalyzes the first step in sphingolipid synthesis, the condensation of serine and palmitoyl-CoA to yield the 3-ketosphinganine (Nagiec et al., 1994). The sphingoid base requirement for endocytosis can be suppressed by loss of protein phosphatase 2A activity or by overexpression of two kinases, Pkc1p or Yck2p, suggesting a signaling function for sphingosine in activation of a protein kinase and a protein phosphatase acting sequentially in endocytosis (Friant et al., 2000).
These results imply that the function of sphingoid bases in endocytosis is to control protein phosphorylation. In mammalian cells, sphingosine induces in vitro phosphorylation of endogenous proteins through the activation of protein kinases (Pushkareva et al., 1992), and two unidentified sphingosine-activated protein kinases were characterized by their substrate specificity and their sphingosine requirement (Pushkareva et al., 1993). Sphingosine also acts by inhibiting some kinases such as protein kinase C (PKC) (Hannun et al., 1986) or by activating others, such as the casein kinase II (McDonald et al., 1991), the atypical PKC isoform ζ (Muller et al., 1995), the p21-activated kinase-1 (PAK1) (Bokoch et al., 1998) and the 3-phosphoinositide-dependent kinase-1 (PDK1) (King et al., 2000). Thus sphingoid bases play a dual role in the regulation of protein phosphorylation.
It was shown recently that sphingosine stimulates PDK1 kinase autophosphorylation and increases the phosphorylation of known PDK1 substrates such as PAK1, Akt and PKC β kinases in vitro, suggesting that sphingosine is a novel activator of PDK1 (King et al., 2000). PDK1 phosphorylates several protein kinases in vitro and is responsible for activating these enzymes in vivo (Alessi et al., 1998; Le Good et al., 1998; Pullen et al., 1998). Saccharomyces cerevisiae has two PDK1 homologs encoded by the PKH1 and PKH2 genes. Similarly, Pkh1/2p kinases were shown to phosphorylate and activate several protein kinases including Ypk1/2p and Pkc1p both in vitro and in vivo (Casamayor et al., 1999; Inagaki et al., 1999). Pkh1p and Pkh2p kinases share an essential role for cell growth since the double knockout pkh1Δ pkh2Δ yeast strain is not viable. Synthetic lethality of the pkh1Δ pkh2Δ double mutations is complemented by full-length human PDK1, or human PDK1 lacking the C-terminal pleckstrin homology (PH) domain that binds phosphoinositides (Casamayor et al., 1999). In contrast to human and Drosophila PDK1, neither Pkh1p nor Pkh2p kinase contain an obvious PH domain, suggesting that in yeast the activation of the Pkh1/2p kinases may not be dependent on phosphoinositides.
A recent study revealed that yeast cells overproducing PKH1 display an increase in resistance to treatment with myriocin, a serine palmitoyl transferase (Lcb1p) inhibitor (Sun et al., 2000). Pkh2p was shown to phosphorylate Pkc1p kinase in vitro and the temperature sensitivity of a pkh-ts (pkh1-ts pkh2Δ) mutant is partially suppressed by a PKC1-R398P dominant mutation (Inagaki et al., 1999). In a previous study, we showed that PKC1 overexpression or PKC1-R398P expression can suppress the sphingoid base requirement for endocytosis, suggesting a link between sphingoid base synthesis and Pkc1p activation (Friant et al., 2000). These results suggest that the Pkh1/2p kinases may be activated by sphingoid bases and play a role in endocytosis via Pkc1p activation. To test this hypothesis, an involvement of the Pkh1/2p kinases in endocytosis was examined.
Here, we show that overexpression of either one of the two protein kinases Pkh1p or Pkh2p can abrogate the sphingoid base synthesis requirement for endocytosis and restore organization of the actin cytoskeleton in lcb1-100 mutant cells. The two kinases have an overlapping function in endocytosis because only a mutant with impaired function of both kinases is defective in the internalization step of endocytosis. Phosphorylation of Pkc1p by Pkh1p or Pkh2p kinase is increased in the presence of nanomolar concentrations of sphingoid base. These results suggest that the function of sphingoid base in endocytosis is to activate a signaling pathway containing the Pkh1/2p kinases and its downstream effector Pkc1p.
Results
Overexpression of Pkh1/2p kinases restores endocytosis in the lcb1-100 mutant
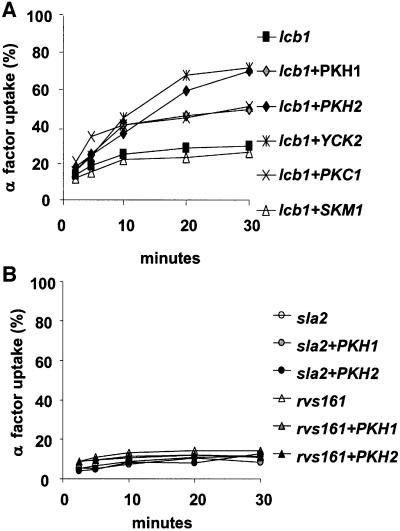
To test if the yeast PDK1 homologs Pkh1p and Pkh2p could be part of the sphingoid base requirement for endocytosis, we analyzed if PKH1 or PKH2 overexpression could suppress the endocytic defect of the lcb1-100 mutant. The lcb1-100 strain was transformed with high copy (2µ) plasmids bearing the PKH1 or PKH2 gene (Table I). Neither of the two protein kinases tested was able to suppress the temperature-sensitive growth phenotype displayed by the lcb1-100 mutant (data not shown). The strains overexpressing the Pkh1p or Pkh2p kinases were tested for internalization of [35S]α-factor at 37°C. Overexpression of PKH2 and, to a lesser extent, PKH1 restored the defect in α-factor internalization of the lcb1-100 mutant cells at 37°C (Figure 1A). To compare the suppressor effect of the Pkh1/2p kinases, we tested in parallel three other kinases, Skm1p, Yck2p and Pkc1p (Table I). Skm1p is a yeast protein kinase that shares homology (40% identity and 57% similarity) with the mammalian PAK1, a kinase reported to be activated by sphingosine (Bokoch et al., 1998). Yck2p and Pkc1p kinases were already shown to suppress the lcb1-100 defect in endocytosis (Friant et al., 2000). SKM1 overexpression did not restore the internalization defect observed in the lcb1-100 mutant cells. The α-factor uptake rate displayed by the lcb1-100 cells overexpressing PKH2 is similar to that of cells overexpressing YCK2, whereas the PKH1 suppressor effect is more similar to that of PKC1 (Figure 1A).
Table I. Plasmids.
| Plasmid | Yeast Ori | Insert | Source |
|---|---|---|---|
| YEp195-PKH1 | 2µ | PKH1 | Inagaki et al. (1999) |
| YEp195-PKH2 | 2µ | PKH2 | Inagaki et al. (1999) |
| PSEY18-SKM1 | 2µ | SKM1 | T.Beck |
| YEp-CKA2 | 2µ | CKA2 | C.Schaerer-Brodbeck |
| YEp-CKB1 | 2µ | CKB1 | C.Schaerer-Brodbeck |
| YEp-CKB2 | 2µ | CKB2 | C. Schaerer-Brodbeck |
| pTS80 | 2µ | PKH1-HA3 | Schmelzle and Hall |
| pTS81 | 2µ | PKH2-HA3 | Schmelzle and Hall |
| pSH24 | 2µ | PKC1 | Helliwell et al. (1998) |
| pTS101 | 2µ | PKC1-HA3 | Schmelzle and Hall |
| pL2.3 | 2µ | YCK2 | Robinson et al. (1992) |
| pKT10 | 2µ | GAL-HA2-pkh2-K208R | Inagaki et al. (1999) |
Fig. 1. Overexpression of PKH1 and PKH2 specifically suppresses the lcb1-100 endocytic defect. (A) Strain RH3809 (lcb1) was transformed with high copy plasmids carrying PKH1, PKH2, SKM1, PKC1 or YCK2 kinases genes (Table I), and the corresponding transformants were assayed for α-factor internalization at 37°C and compared with lcb1-100 cells. (B) The sla2-41 (end4-1) and rvs161 (end6-1) temperature-sensitive mutants (RH1597 and RH2082) were transformed by a high-copy number plasmid bearing PKH1 or PKH2 and assayed for α-factor uptake at 37°C.
To determine if other endocytosis mutants could also be suppressed by PKH1 or PKH2 overexpression, the sla2-41 (end4-1) and rvs161 (end6-1) strains were transformed by the 2µ plasmid bearing the PKH1/2 genes and assayed for α-factor uptake at 37°C (Figure 1B). These two mutants are defective for α-factor internalization at 37°C (Raths et al., 1993; Munn et al., 1995). The overexpression of PKH1 or PKH2 did not restore endocytosis in these strains (Figure 1B), suggesting that Pkh1/2p overexpression specifically suppresses the lcb1-100 endocytic defect.
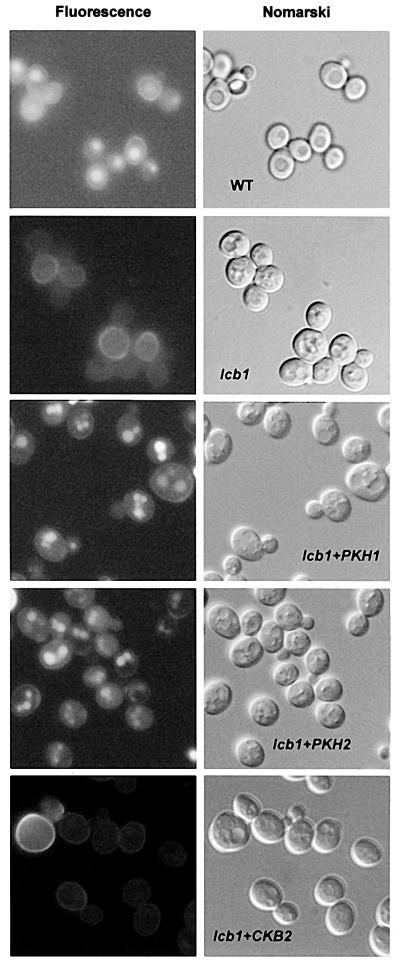
The ability of overexpressed PKH1 or PKH2 to restore fluid-phase endocytosis in lcb1-100 cells at 37°C was tested and compared with wild-type, lcb1-100 and lcb1-100 cells transformed by episomal plasmids bearing different subunits of the yeast casein kinase II (CKA2, CKB1 and CKB2) (Table I and Figure 2). Yeast casein kinase II overexpression was used as a control, because sphingosine was shown to be a potent activator of mammalian casein kinase II (McDonald et al., 1991). The lcb1-100 cells and the lcb1-100 mutants overexpressing CKA2, CKB1 or CKB2 were defective for uptake and accumulation of the fluorescent dye lucifer yellow (LY) in the vacuole at 37°C (Figure 2 and data not shown). Consistent with the α-factor uptake results, overexpression of PKH1 or PKH2 allowed the lcb1-100 mutant to accumulate LY in the vacuole (Figure 2).
Fig. 2. PKH1/2 overexpression suppresses the fluid-phase endocytic defect of the lcb1-100 mutant. Wild-type cells (wt, RH448) and lcb1-100 (lcb1, RH3809) cells carrying a PKH1, PKH2 or CKB2 high-copy number plasmid (lcb1+PKH1, lcb1+PKH2 or lcb1+CKB2) were assayed for lucifer yellow (LY) accumulation in the vacuole at 37°C. The same field of cells viewed by fluorescence (left panel) and by Nomarski optics (right panel) is shown.
Taken together, the above results show that overexpression of Pkh1/2p kinases specifically suppresses both receptor-mediated and fluid-phase endocytosis defects of the lcb1-100 mutant. These results suggest that sphingoid base may activate a protein phosphorylation pathway that is required for the internalization step of endocytosis. The Pkh1/2p kinases may be part of this cascade.
Pkh1/2p kinases are required for endocytosis
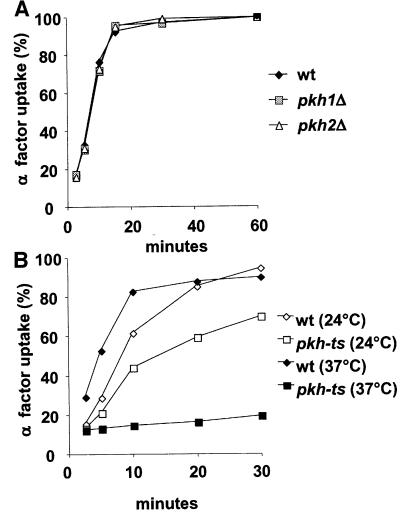
To determine whether Pkh1/2p kinase activity is required for the internalization step of endocytosis, α-factor uptake and LY accumulation of pkh1Δ, pkh2Δ and pkh-ts strains were assayed (Figures 3 and 4). PKH1 and PKH2 have overlapping function for cell growth (Casamayor et al., 1999; Inagaki et al., 1999). The pkh-ts strain harbors a chromosomal deletion of the PKH2 gene (pkh2::LEU2) and a temperature-sensitive pkh1-ts mutant allele, pkh1D398G (Inagaki et al., 1999). The single disruptants and temperature-sensitive mutant strains were assayed for α-factor uptake and compared with wild-type cells (Figure 3A and B). The single mutant cells pkh1Δ and pkh2Δ internalized α-factor at the same rate as wild-type cells at 37°C (Figure 3A). At 24°C, the pkh-ts strain showed a partial α-factor internalization defect when compared with wild-type cells, and at 37°C the mutant strain was unable to internalize α-factor (Figure 3B). We conclude that Pkh1p and Pkh2p kinases have an overlapping function for endocytosis and that the loss of Pkh1/2p kinase activity affects the internalization step of receptor-mediated endocytosis.
Fig. 3. (A) Pkh1p and Pkh2p have redundant function in endocytosis. The single mutant pkh1Δ (RH5413) and pkh2Δ (RH5388) cells were assayed for α-factor uptake at 37°C and compared with the wild-type cells (wt, RH2964). (B) Pkh1/2p kinases are required for endocytosis. Radiolabeled α-factor uptake assays were performed at 24°C (open symbols) or 37°C (closed symbols) on wild-type (RH5411) and pkh-ts (RH5412) strains.
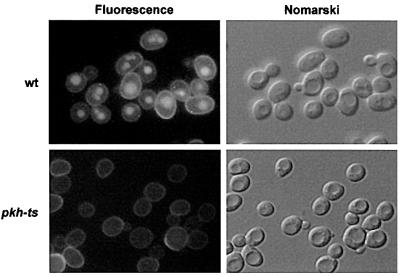
Fig. 4. Pkh1/2p kinases are required for fluid-phase endocytosis. Wild-type (wt, RH5411) and pkh-ts (RH5412) cells were incubated with LY for 1 h at 37°C. To visualize LY uptake, cells were viewed by FITC fluorescence optics (left panel). The same fields of cells were viewed by Nomarski optics (right panel) to visualize the vacuoles.
As the pkh-ts mutant is defective for receptor-mediated internalization at 37°C, it was important to examine whether this mutant is also defective for fluid-phase endocytosis. LY accumulation in the wild-type and pkh-ts mutant cells was assayed at 37°C (Figure 4). The pkh-ts cells were completely defective in LY accumulation at 37°C (Figure 4). These results show that Pkh1p and Pkh2p have an overlapping function in endocytosis, because only the cells with impaired activity for both kinases are defective for the internalization step of endocytosis.
Overexpression of Pkh1/2p specifically corrects the actin defect of the lcb1-100 mutant
The lcb1-100 mutant is defective in the organization of the actin cytoskeleton at 37°C. This defect, like the endocytic defect, can be suppressed by addition of phytosphingosine (PHS) or overexpression of the kinases Yck2p or Pkc1p (Friant et al., 2000; Zanolari et al., 2000). Therefore, it is conceivable that overexpression of the Pkh1/2p kinases, which restored endocytosis in the lcb1-100 mutant, may also correct the actin organization defect of this mutant. To test this, we examined whether Pkh1p or Pkh2p overexpression in the lcb1-100 mutant could restore the polarized distribution of actin at 37°C. Wild-type, lcb1-100 and lcb1-100 cells overexpressing PKH1 or PKH2 were grown at 24°C, shifted to 37°C for 2 h and the cells were fixed and stained with tetramethylrhodamine isothiocyanate (TRITC)–phalloidin to visualize F-actin (Figure 5). A shift from 24 to 37°C causes a heat-induced depolarization of the actin cytoskeleton in wild-type cells. Normal polarized actin localization is restored after 1.5–2 h at 37°C in wild-type cells (Figure 5, panel wt). In contrast, this perturbation was irreversible in the lcb1-100 mutant cells, as seen by the accumulation of actin patches in the mother cell of the budded cells (Figure 5, panel lcb1) (Zanolari et al., 2000). However, lcb1-100 mutant cells overexpressing either Pkh1p or Pkh2p kinases displayed polarized distribution of actin that was more similar to wild-type cells (Figure 5, panels lcb1+PKH1 and lcb1+PKH2).
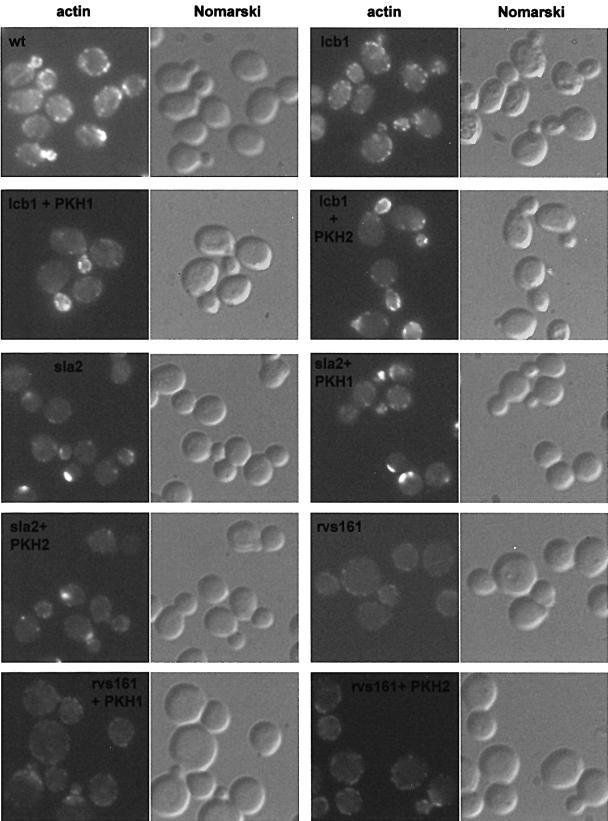
Fig. 5. Pkh1/2p kinase overexpression specifically suppresses the lcb1-100 actin organization defect. Logarithmic cultures of wild-type cells (wt, RH448), lcb1-100, sla2-41 (end4-1) and rvs161 (end6-1) mutant cells (RH3802, RH1597 and RH2082), with or without the PKH1 and PKH2 plasmids, were grown at 24°C, shifted to 37°C for 2 h, fixed, stained with TRITC–phalloidin and observed by fluorescence (actin) and Nomarski microscopy.
The Pkh1/2p kinases have been shown to be required for actin cytoskeleton organization in yeast (Inagaki et al., 1999). To determine if the suppression by PKH1/2 was specific for the lcb1-100 mutant cells, two other endocytic mutants that display actin cytoskeleton defects, sla2-41 (end4-1) and rvs161 (end6-1), were tested (Figure 5, panels sla2 and rvs161) (Raths et al., 1993; Munn et al., 1995). The sla2-41 and rvs161 mutants were transformed by high copy number plasmids bearing the PKH1 or PKH2 gene and assayed for actin localization at 37°C (Figure 5, panels sla2+PKH1, sla2+PKH2, rvs161+PKH1 and rvs161+PKH2). Proper actin localization was not restored by either PKH1 or PKH2 overexpression in these mutants, showing that suppression is specific for the lcb1-100 mutant. The above results suggest that one target of the sphingoid base synthesis requirement is likely to be the actin cytoskeleton because overexpression of the Pkh1/2p kinases corrected the actin defect specifically in the lcb1-100 mutant.
Sphingoid base activates Pkh1p and Pkh2p kinases activity in vitro
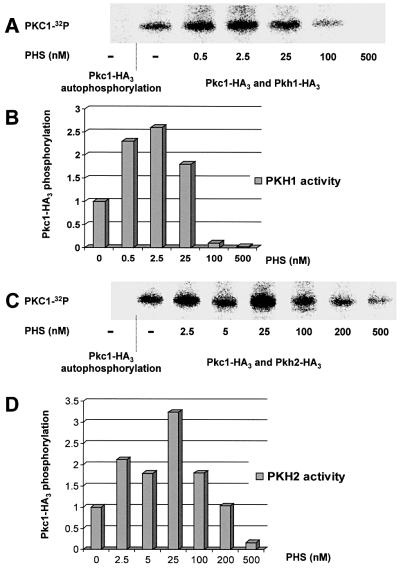
The above results suggest that, like their mammalian homolog PDK1, Pkh1p and Pkh2p could be activated directly by sphingoid base as part of a signaling cascade required for the internalization step of endocytosis. To test this hypothesis, we developed an in vitro phosphorylation assay by using immunoprecipitated Pkh1p or Pkh2p incubated in the presence of increasing concentration of PHS. Wild-type cells were transformed by plasmids bearing the PKH1 and PKH2 genes tagged at their C-terminus with a triple hemagglutinin (HA) epitope or the pkh2-KR kinase-dead mutant tagged at the N-terminus with a double HA (Table I). The Pkh1-HA3 and Pkh2-HA3 constructs are functional because they can suppress the temperature-sensitive growth phenotype displayed by the pkh-ts strain (data not shown). It was shown recently that Pkh1/2p kinases directly phosphorylate and activate Pkc1p (Inagaki et al., 1999). Therefore, we used immunoprecipitated Pkc1p as the natural substrate for the Pkh1p and Pkh2p kinases in our in vitro assay. A wild-type strain was transformed with a multicopy plasmid encoding Pkc1p triple HA tagged at its C-terminus (pTS94). This construct is functional since a pkc1-2-ts strain transformed by this plasmid shows no growth defect at 37°C. Pkh1-HA3, Pkh2-HA3, pkh2-KR-HA2 or Pkc1-HA3 were immunoprecipitated with an anti-HA antibody. Immuno precipitated Pkc1-HA3 was folded properly because the immunoprecipitated Pkc1-HA3 phosphorylated its substrate (myelin basic protein) correctly (Antonsson et al., 1994; data not shown). Immunoprecipitated Pkh1-HA3 or Pkh2-HA3 was incubated in the presence of its substrate Pkc1-HA3, and aliquots of this kinase–substrate mix were incubated with [γ-32P]ATP in the presence of increasing concentrations of PHS from 0.5 up to 500 nM (Figure 6). A control without Pkh1/2p kinase shows no Pkc1-HA3 autophosphorylation, meaning that Pkc1p phosphorylation requires addition of the Pkh1p or Pkh2p immunoprecipitate. Phosphorylated [32P]Pkc1-HA3 was revealed by using a Cyclone Storage Phosphor Imager (Packard) after electrophoresis on a 7.5% SDS–polyacrylamide gel (Figures 6A and C, and 7A and C) and the amount of radiolabeled Pkc1-HA3 was quantified (Figures 6B and D, and 7B). All phosphorylation assays were performed at least twice; the results shown are from one of the independent experiments that gave nearly identical results. PHS was found to be an activator of both Pkh1p and Pkh2p kinases, since at concentrations as low as 0.5 nM there is an increase in Pkc1-HA3 phosphorylation (Figure 6A and C), and therefore an activation of the kinase activity. A 3-fold activation of both Pkh1p and Pkh2p is observed in the presence of 2.5–25 nM PHS (Figure 6B and D). At higher concentrations, however, PHS became less effective and at 500 nM PHS, it inhibited both Pkh1p and Pkh2p phosphorylation of Pkc1p (Figure 6). PHS has a bifunctional effect on Pkh1/2p activity in vitro, stimulating the enzyme activity at low concentrations and inhibiting it at high concentrations.
Fig. 6. Phytosphingosine activates the Pkh1/2p kinases. (A) Immunoprecipitated Pkh1-HA3 was incubated with its substrate Pkc1-HA3 in the presence of [γ-32P]ATP and increasing amounts of PHS. Phosphorylated Pkc1-HA3 was revealed by a Cyclone Storage Phosphor Imager (Packard) after separation on a 7.5% SDS–polyacrylamide gel. (B) The amount of radiolabeled [32P]Pkc1-HA3 was quantified in each lane and basal phosphorylation of Pkc1p by Pkh1p without PHS was set as 1. (C and D) The same assay was done using Pkh2-HA3 as the kinase.
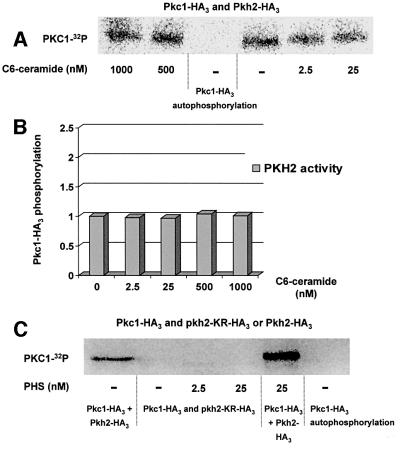
Fig. 7. Pkh2p kinase specifically phosphorylates Pkc1p and is not activated by C6-ceramide. (A) Immunoprecipitated Pkh2-HA3 was incubated with Pkc1-HA3 in the presence of [γ-32P]ATP and increasing amounts of C6-ceramide. Phosphorylated Pkc1-HA3 was revealed by a Cyclone Storage Phosphor Imager (Packard) after separation on a 7.5% SDS–polyacrylamide gel. (B) The amount of radiolabeled [32P]Pkc1-HA3 was quantified in each lane and basal phosphorylation of Pkc1p by Pkh2p without C6-ceramide was set to 1. (C) Immunoprecipitated Pkh2-HA3 or the kinase-dead mutant pkh2-KR-HA2 was incubated with Pkc1-HA3 in the presence of [γ-32P]ATP and increasing amounts of PHS. Phosphorylated Pkc1-HA3 was revealed by a Cyclone Storage Phosphor Imager (Packard) after separation on a 7.5% SDS–polyacrylamide gel.
To test if this activator effect is specific for sphingoid bases, the same assay was done in the presence of increasing concentrations of C6-ceramide (Figure 7A and B). C6-ceramide is a soluble sphingolipid containing a short fatty acid chain (C6) bound to the sphingoid base moiety. C6-ceramide can be used as a negative control in our phosphorylation assay, because addition of C6-ceramide is not able to suppress the internalization defect observed in the lcb1-100 mutant cells, in contrast to PHS or dihydrosphingosine addition (B.Zanolari and H.Riezman, unpublished data) (Zanolari et al., 2000). Addition of C6-ceramide to Pkh2p kinase did not result in a change in the basal level of Pkc1p phosphorylation (Figure 7A and B), meaning that C6-ceramide has no effect on Pkh2p kinase activity at either low (2.5 nM) or high (1 µM) concentrations.
To rule out the possibility that Pkc1p phosphorylation could be due to another kinase co-immunoprecipitated with Pkh1p or Pkh2p kinases, we also tested an HA-tagged kinase-dead mutant (pkh2-KR-HA2) bearing a lysine to arginine change in the kinase domain (Inagaki et al., 1999). The in vitro phosphorylation experiments were done under the same conditions as for the wild-type Pkh2-HA3 kinase. There was no Pkc1p phosphorylation observed in the presence of the pkh2-KR-HA2 kinase-dead mutant, even in the presence of PHS concentrations that were shown to activate Pkh2p activity (Figure 7C). This result shows that Pkc1p is phosphorylated specifically by Pkh2p kinase in our in vitro assay. The above results show that Pkh1/2p kinases are activated specifically by PHS in vitro and identify them as yeast sphingoid base-activated kinases.
Discussion
The major finding of this study is that Pkh1/2p kinases are part of a sphingoid base-mediated signaling pathway that is required for the internalization step of endocytosis and for actin cytoskeleton organization in yeast. As shown previously, the actin cytoskeleton plays an essential role in the internalization step of endocytosis in yeast; yeast mutants altered in actin and actin-binding proteins are defective in endocytosis (Kübler and Riezman, 1993; Munn et al., 1995; Riezman et al., 1996). Here we showed that overexpression of PKH1/2 suppresses the endocytic and actin cytoskeleton organization defect of the lcb1-100 mutant. It is noteworthy that overexpression of neither PKH1 nor PKH2 suppressed the endocytic or actin defect of other mutants that are defective in the internalization step of endocytosis and in actin cytoskeleton organization, suggesting that this suppressor effect is specific for the lcb1-100 mutation. The Pkh1p and Pkh2p kinases are functionally redundant for both fluid-phase and receptor-mediated endocytosis. A previous study has shown that these two kinases are also required for actin cytoskeleton organization, since the pkh-ts strain displays a defect in actin polarization upon shift to 37°C (,Inagaki et al., 1999). These results suggest that Pkh1/2p kinases play a direct role in endocytosis perhaps by regulating the endocytic machinery and/or the actin cytoskeleton. Here, we showed that sphingoid base activates both Pkh1p and Pkh2p protein kinases in vitro. All these results together suggest that there is a sphingoid base-mediated signaling pathway required for endocytosis and that Pkh1/2p kinase is the sphingoid base-activated kinase that probably initiates this cascade.
Pkh1p and Pkh2p are yeast homologs of mammalian PDK1 kinase. Human, Drosophila and Caenorhabditis elegans PDK1 kinases contain a PH which binds PI(3,4,5)P3 and PI(3,4)P2 (Stephens et al., 1998; Currie et al., 1999; Fruman et al., 1999). In contrast, the two yeast members of this kinase family, Pkh1p and Pkh2p, lack the PH domain (Casamayor et al., 1999). It was shown previously that mammalian PDK1 lacking the PH domain is sufficient to rescue pkh1Δ pkh2Δ mutant cells from lethality (Casamayor et al., 1999). Furthermore, while S.cerevisiae contain PI(3)P, they do not have PI(3,4)P2 nor PI(3,4,5)P3 (Hawkins et al., 1993; De Camilli et al., 1996). All these results suggest that Pkh1/2p kinases are not regulated by phosphoinositides like their mammalian counterpart. The best candidate for regulating Pkh1/2p kinases activity is the sphingoid bases, because a previous study showed that mammalian PDK1 kinase is activated in vitro in the presence of sphingosine and that an elevated intracellular sphingosine level increases PDK1 activity in vivo (King et al., 2000). Here, we showed that overexpression of PKH1 or PKH2 suppresses the endocytic defect of the lcb1-100 mutant strain that is impaired in sphingoid base synthesis. This suppression is specific for Pkh1/2p because overexpression of other protein kinases such as Skm1p or subunits of casein kinase II are not able to restore endocytosis of the lcb1-100 mutant cells. Skm1p is a yeast homolog of mammalian PAK1. PAK1 and mammalian casein kinase II were shown to be activated by sphingosine (McDonald et al., 1991; Bokoch et al., 1998). This result further demonstrates the specificity of Pkh1/2p in this signaling cascade, because overexpression of other protein kinases which have been reported to be activated by sphingosine bases had no suppressor effect on lcb1-100 mutant cells. Moreover, by using an in vitro kinase assay, we could show that both Pkh1p and Pkh2p are activated by nanomolar concentrations of PHS. There is a 3-fold increase in Pkh1/2p kinase activity in the presence of very low PHS concentration (0.5–2.5 nM). This concentration mimics the cellular concentrations of sphingoid bases (sphinganine and PHS) that are expected in exponential phase yeast cells (Wu et al., 1995). Using the same in vitro kinase assay, we also tested the ability of C6-ceramide, a sphingolipid that is unable to suppress the lcb1-100 endocytic defect (B.Zanolari and H.Riezman, unpublished data), to activate Pkh2p kinase. We did not observe any activation or inhibition of Pkh2p in the presence of C6-ceramide, meaning that activation of Pkh2p by PHS is specific. All these results together suggest that both PDK1 and its yeast counterparts Pkh1/2p kinases are sphingoid base-activated kinases. The results obtained here and previous results showing that neither phosphorylated sphingoid bases nor ceramides or sphingolipids are required for endocytosis (Zanolari et al., 2000) suggest that sphingoid bases (DHS and/or PHS) are required for Pkh1/2p kinase activation. However, we cannot rule out the possibility that another as yet unidentified derivative of PHS/DHS could be the true Pkh1/2p kinase activator, but this derivative cannot be a phosphorylated sphingoid base because yeast incapable of generating phosphorylated sphingoid bases are capable of endocytosis (Zanolari et al., 2000).
Pkc1p and Ypk1/2p kinases were identified as downstream effectors of the Pkh1/2p protein kinase cascade. These two kinases are phosphorylated directly by Pkh kinase in vitro (Casamayor et al., 1999; Inagaki et al., 1999). Reduced Pkc1p activity was observed in a pkh-ts mutant strain, indicating that Pkh1/2p kinases are required for Pkc1p function in vivo (Inagaki et al., 1999). In a previous study, we could show that an increased gene dosage of PKC1 or expression of a dominant, activated allele of PKC1 suppresses the endocytic defect of the lcb1-100 mutant (Friant et al., 2000). The above results show that in vitro Pkc1p phosphorylation by Pkh1/2p kinases is activated by sphingoid base. These results suggest that Pkc1p may be activated by Pkh1/2p kinases in response to sphingoid base and that this signaling cascade is required for the internalization step of endocytosis. The essential role for Pkh1/2p kinases in endocytosis is confirmed further by the strong endocytic defect that is observed in a pkh-ts mutant strain. The lcb1-100 mutant strain blocked in sphingoid base synthesis shows a similar endocytosis defect (Zanolari et al., 2000), whereas the pkc1-ts mutant cells showed wild-type endocytosis (Friant et al., 2000). Therefore, the Pkc1p kinase is one of the downstream effectors of the Pkh1/2p signaling cascade, but it is not the only one required for the internalization step of endocytosis. Yck2p kinase could be another downstream effector, because its overexpression also suppresses the lcb1-100 endocytosis defect and only the double mutant pkc1-ts yck-ts strain is completely defective for endocytosis (Friant et al., 2000). We did not find a consensus PDK1/2-Pkh1/2 phosphorylation site in the Yck2p kinase, whereas both Pkc1p and Ypk1/2p kinases have these sites (Casamayor et al., 1999), meaning that Yck2p is probably not activated directly by Pkh1/2p. There could be another kinase upstream of Yck2p in the signaling cascade. The Ypk1/2p kinases would be good candidates.
The Ypk1p kinase recently was identified as a multicopy suppressor gene that restores growth in the presence of a serine palmitoyl transferase (Lcb1p) inhibitor. Furthermore, phosphorylation of the Ypk1p kinase is increased in the presence of PHS (Sun et al., 2000). This phosphorylation could be due to activation of Pkh1/2p kinases by PHS, because Ypk1p is phosphorylated directly by Pkh2p in vitro (Casamayor et al., 1999). In agreement with this hypothesis, it was shown that Pkh kinase activity was required for maximal Ypk1p phosphorylation in vivo (Casamayor et al., 1999). This suggests that Pkh1/2p kinases are tightly regulated by sphingoid bases, not only for their function in endocytosis, but also for their overall function in regulating several yeast processes, such as cell growth, control of the mitogen-activated protein kinases and organization of the actin cytoskeleton.
In a previous study, we could show that YPK1 or YPK2 overexpression did not suppress the lcb1-100 endocytosis defect (Friant et al., 2000), suggesting that these kinases are not part of the signaling cascade required for the internalization step of endocytosis. However, we cannot exclude that YPK1/2 overexpression would be insufficient to mimic the Pkh1/2p activation effect on these kinases, because they may not be active in their dephosphorylated state, even if overexpressed.
In summary, our results are the first example of a sphingoid base-activated signaling pathway being used to regulate a step of membrane trafficking. Many details remain to be discovered, including the identification of the downstream effectors of the Pkh1/2p sphingoid base-activated protein kinase and the mechanism whereby sphingoid base controls the relative activities of protein kinases. The ultimate target of the sphingoid base-mediated signaling pathway may be the endocytic machinery and/or the actin cytoskeleton via the Pkc1p kinase. The ease of genetic and molecular studies in yeast should help to unravel the mechanism.
Materials and methods
Plasmids, strains, media and genetic manipulations
Plasmids and yeast strains used in this study are listed in Tables I and II, respectively. Disruption mutants were created by integrative transformation using standard techniques. Yeast cell cultures and genetic manipulations were carried out essentially as described by Sherman et al. (1983). Yeast cells were transformed by the LiAc method using single-stranded carrier DNA and dimethylsulfoxide (DMSO) (Schiestl and Gietz, 1989; Hill et al., 1991). Rich YPUAD medium and synthetic minimal media (SD) complemented with the appropriate nutrients for plasmid maintenance were prepared as described (Munn et al., 1995).
Table II. Yeast strains used in this study.
| Strains | Genotype | Source |
|---|---|---|
| RH448 | leu2 ura3 his4 lys2 bar1 | laboratory strain |
| RH1597 | leu2 ura3 his4 bar1 sla2-41 | Raths et al. (1993) |
| RH2082 | leu2 ura3 his4 bar1 rvs161 | Munn et al. (1995) |
| RH2964 | leu2 ura3 his3 ade2 trp1 lys2 bar1 | laboratory strain |
| RH3802 | leu2 ura3 his4 his3 ade2 lys2 bar1 lcb1-100 | Friant et al. (2000) |
| RH3809 | leu2 ura3 his4 bar1 lcb1-100 | Friant et al. (2000) |
| RH5388 | leu2 ura3 ade2 trp1 bar1::URA3 pkh2::LEU2 | this study |
| RH5411 | leu2 ura3 his2 ade1 trp1 bar1::URA3 | this study |
| RH5412 | leu2 ura3 his2 ade1 trp1 bar1::URA3 pkh1-ts pkh2::LEU2 | this study |
| RH5413 | leu2 ura3 his3 ade2 trp1 lys2 bar1 pkh1::TRP1 | this study |
All strains listed in this table are MATa.
Endocytosis assays
Lucifer yellow-carbohydrazide (LY) (Fluka, Buchs, Switzerland) assays were performed as described (Dulic et al., 1991; Munn and Riezman, 1994). Yeast pre-cultures were grown at 24°C in YPUAD or in SD selective media in order to maintain the plasmids. Cells taken from the pre-culture were then grown at 24°C in YPUAD to mid-log phase, shifted to 37°C for 15 min and incubated for 1 h at 37°C with LY. [35S]α-factor uptake assays were performed on mid-log phase cells using the continuous presence protocol as described (Dulic et al., 1991). Pre-cultures were grown at 24°C in YPUAD or in SD selective media in order to maintain the plasmids. Cells taken from the pre-culture were then grown at 24°C in YPUAD medium; all α-factor uptake assays were carried out at 24 or 37°C after 15 min pre-incubation at the respective temperature. All uptake assays were performed at least twice; the results shown are from one of the independent experiments, which gave nearly identical results.
Rhodamine–phalloidin staining of actin
Yeast cell pre-cultures were grown at 24°C in SD selective media in order to maintain the plasmids. Cells taken from the pre-culture were grown at 24°C in YPUAD medium to early log phase. Cells (at 1 × 107 cells/ml) were then incubated for 2 h at 37°C, fixed in formaldehyde and stained with TRITC–phalloidin (Sigma, St Louis, MO) to visualize F-actin essentially as described previously (Bénédetti et al., 1994).
In vitro phosphorylation assay
Wild-type yeast cells (RH448) transformed by plasmids bearing PKC1-HA3, PKH1-HA3, PKH2-HA3 or pkh2-KR-HA2 were grown at 24°C in SD selective media in order to maintain the plasmids. Cells (at ∼1.5 × 107 cells/ml) were harvested and total yeast proteins were extracted by glass bead lysis in a buffer containing, 50 mM HEPES pH 7.5, 150 mM KCl, 1 mM EDTA, 1 mM EGTA, 10% glycerol and 1 µg/ml each of the protease inhibitors aprotinin, leupeptin, pepstatin A, chymostatin and antipain. The protein concentration of the lysates was measured using the Bio-Rad protein assay. Equal amounts of total protein for the different kinases were taken and triple HA-tagged kinases were immunoprecipitated overnight at 4°C from these extracts using 5 µl of anti-HA rat monoclonal antibody (Clone 3F10, Roche Molecular Biochemicals), 50 µl of 30% protein G–Sepharose beads (v/v) in a final volume of 750 µl of immunoprecipitation buffer (50 mM Tris–HCl pH 7.5, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% NP-40 and 1 µg/ml each of the protease inhibitors aprotinin, leupeptin, pepstatin A, chymostatin and antipain). Beads were then washed three times in immunoprecipitation buffer and twice in 40 mM MOPS pH 7.5 buffer and resuspended in phosphorylation buffer [40 mM MOPS pH 7.5, 1 mM dithiothreitol (DTT) and 10 mM MgCl2]. In order to control that there is a comparable level of protein expression and immunoprecipitation, the total lysate and the immune complexes were subjected to western blot analysis using a mouse anti-HA antibody. Equal amounts of kinase beads (Pkh1p or Pkh2p beads) were mixed with Pkc1p beads (substrate). In order to determine the amount of autophosphorylation, a control containing the same amount of Pkc1p beads mixed with phosphorylation buffer was treated in parallel. The kinase–substrate mix was aliquoted and 1 µl of d-erythro-phytosphingosine (PHS) or C6-ceramide at the proper dilution in ethanol was added to the mix in order to give a final concentration of 0.5, 2.5, 25, 100, 200, 500 or 1000 nM of PHS or C6-ceramide in each reaction assay. The control reaction without lipid was done by adding 1 µl of ethanol to the reaction. After 10 min of pre-incubation at room temperature, 2 µl of [32P]ATP mix (1 mM ATP and 4 µCi of [γ-32P]ATP) was added and the phosphorylation reaction was run for 30 min at room temperature. The reaction was stopped by addition of cold ATP at a final concentration of 50 mM and Laemmli protein sample buffer. Samples were boiled for 5 min and the total reaction was loaded on a 7.5% SDS–polyacrylamide gel. After electrophoresis, the gel was washed once in trichloroacetic acid (TCA) (12.5%) and twice in Coomassie blue destaining solution. Phosphorylated Pkc1-HA3 was revealed and quantified by using a Cyclone Storage Phosphor Imager (Packard). All phosphorylation assays were performed at least twice. The results shown are from one of the independent experiments, which gave nearly identical results.
Acknowledgments
Acknowledgements
We thank K.Matsumoto and C.Schaerer-Brodbeck for strains and plasmids, J.Holenstein and T.Aust for technical assistance, B.Zanolari for communicating unpublished results, and A.Imbo and members of the Riezman laboratory for helpful comments on the manuscript. This work was supported by the Canton of Basel-Stadt, a Human Frontier Science Program Organization (HFSP) long-term fellowship (to S.F.), Boehringer Ingelheim Fonds (to T.S.) and grants from the Swiss National Science Foundation (to H.R. and M.N.H.) and from the HSFP (to H.R., grant RG 0288).
References
- Alessi D.R., Kozlowski,M.T., Weng,Q.P., Morrice,N. and Avruch,J. (1998) 3-Phosphoinositide-dependent protein kinase 1 (PDK1) phosphorylates and activates the p70 S6 kinase in vivo and in vitro. Curr. Biol., 8, 69–81. [DOI] [PubMed] [Google Scholar]
- Antonsson B., Montessuit,S., Friedli,L., Payton,M.A. and Paravicini,G. (1994) Protein kinase C in yeast. Characteristics of the Saccharomyces cerevisiae PKC1 gene product. J. Biol. Chem., 269, 16821–16828. [PubMed] [Google Scholar]
- Bénédetti H., Raths,S., Crausaz,F. and Riezman,H. (1994) The END3 gene encodes a protein that is required for the internalization step of endocytosis and for actin cytoskeleton organization in yeast. Mol. Biol. Cell, 5, 1023–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokoch G.M., Reilly,A.M., Daniels,R.H., King,C.C., Olivera,A., Spiegel,S. and Knaus,U.G. (1998) A GTPase-independent mechanism of p21-activated kinase activation. Regulation by sphingosine and other biologically active lipids. J. Biol. Chem., 273, 8137–8144. [DOI] [PubMed] [Google Scholar]
- Casamayor A., Torrance,P.D., Kobayashi,T., Thorner,J. and Alessi,D.R. (1999) Functional counterparts of mammalian protein kinases PDK1 and SGK in budding yeast. Curr. Biol., 9, 186–197. [DOI] [PubMed] [Google Scholar]
- Currie R.A., Walker,K.S., Gray,A., Deak,M., Casamayor,A., Downes,C.P., Cohen,P., Alessi,D.R. and Lucocq,J. (1999) Role of phosphatidylinositol 3,4,5-trisphosphate in regulating the activity and localization of 3-phosphoinositide-dependent protein kinase-1. Biochem. J., 337, 575–583. [PMC free article] [PubMed] [Google Scholar]
- De Camilli P., Emr,S.D., McPherson,P.S. and Novick,P. (1996) Phosphoinositides as regulators in membrane traffic. Science, 271, 1533–1539. [DOI] [PubMed] [Google Scholar]
- D’Hondt K., Heese-Peck,A. and Riezman,H. (2000) Protein and lipid requirements for endocytosis. Annu. Rev. Genet., 34, 255–295. [DOI] [PubMed] [Google Scholar]
- Dulic V., Egerton,M., Elguindi,I., Raths,S., Singer,B. and Riezman,H. (1991) Yeast endocytosis assays. Methods Enzymol., 194, 697–710. [DOI] [PubMed] [Google Scholar]
- Friant S., Zanolari,B. and Riezman,H. (2000) Increased protein kinase or decreased PP2A activity bypasses sphingoid base requirement in endocytosis. EMBO J., 19, 2834–2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fruman D.A., Rameh,L.E. and Cantley,L.C. (1999) Phosphoinositide binding domains: embracing 3-phosphate. Cell, 97, 817–820. [DOI] [PubMed] [Google Scholar]
- Geli M.I. and Riezman,H. (1998) Endocytic internalization in yeast and animal cells: similar and different. J. Cell Sci., 111, 1031–1037. [DOI] [PubMed] [Google Scholar]
- Hannun Y.A., Loomis,C.R., Merrill,A.H.,Jr and Bell,R.M. (1986) Sphingosine inhibition of protein kinase C activity and of phorbol dibutyrate binding in vitro and in human platelets. J. Biol. Chem., 261, 12604–12609. [PubMed] [Google Scholar]
- Hawkins P.T., Stephens,L.R. and Piggott,J.R. (1993) Analysis of inositol metabolites produced by Saccharomyces cerevisiae in response to glucose stimulation. J. Biol. Chem., 268, 3374–3383. [PubMed] [Google Scholar]
- Helliwell S.B., Schmidt,A., Ohya,Y. and Hall,M.N. (1998) The Rho1 effector Pkc1, but not Bni1, mediates signalling from Tor2 to the actin cytoskeleton. Curr. Biol., 8, 1211–1214. [DOI] [PubMed] [Google Scholar]
- Hill J., Donald,K.A., Griffiths,D.E. and Donald,G. (1991) DMSO-enhanced whole cell yeast transformation. Nucleic Acids Res., 19, 5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath A., Sütterlin,C., Manning-Krieg,U., Movva,N.R. and Riezman,H. (1994) Ceramide synthesis enhances transport of GPI-anchored proteins to the Golgi apparatus in yeast. EMBO J., 13, 3687–3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki M., Schmelzle,T., Yamaguchi,K., Irie,K., Hall,M.N. and Matsumoto,K. (1999) PDK1 homologs activate the Pkc1-mitogen-activated protein kinase pathway in yeast. Mol. Cell. Biol., 19, 8344–8352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King C.C., Zenke,F.T., Dawson,P.E., Dutil,E.M., Newton,A.C., Hemmings,B.A. and Bokoch,G.M. (2000) Sphingosine is a novel activator of 3-phosphoinositide- dependent kinase-1. J. Biol. Chem., 29, 29. [DOI] [PubMed] [Google Scholar]
- Kübler E. and Riezman,H. (1993) Actin and fimbrin are required for the internalization step of endocytosis in yeast. EMBO J., 12, 2855–2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeGood J.A., Ziegler,W.H., Parekh,D.B., Alessi,D.R., Cohen,P. and Parker,P.J. (1998) Protein kinase C isotypes controlled by phosphoinositide 3-kinase through the protein kinase PDK1. Science, 281, 2042–2045. [DOI] [PubMed] [Google Scholar]
- Lombardi R., Friant,S. and Riezman,H. (2001) Endocytosis in Saccharomyces cerevisiae: involvement of actin, actin-associated protein complexes and lipids in the internalization step. Frontiers Mol. Biol., 36, 218–246. [Google Scholar]
- McDonald O.B., Hannun,Y.A., Reynolds,C.H. and Sahyoun,N. (1991) Activation of casein kinase II by sphingosine. J. Biol. Chem., 266, 21773–21776. [PubMed] [Google Scholar]
- Muller G., Ayoub,M., Storz,P., Rennecke,J., Fabbro,D. and Pfizenmaier,K. (1995) PKC ζ is a molecular switch in signal transduction of TNF-α, bifunctionally regulated by ceramide and arachidonic acid. EMBO J., 14, 1961–1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munn A.L. and Riezman,H. (1994) Endocytosis is required for the growth of vacuolar H(+)-ATPase-defective yeast: identification of six new END genes. J. Cell Biol., 127, 373–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munn A.L., Stevenson,B.J., Geli,M.I. and Riezman,H. (1995) end5, end6 and end7: mutations that cause actin delocalization and block the internalization step of endocytosis in Saccharomyces cerevisiae. Mol. Biol. Cell., 6, 1721–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munn A.L., Heese-Peck,A., Stevenson,B.J., Pichler,H. and Riezman,H. (1999) Specific sterols required for the internalization step of endocytosis in yeast. Mol. Biol. Cell, 10, 3943–3957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagiec M.M., Baltisberger,J.A., Wells,G.B., Lester,R.L. and Dickson,R.C. (1994) The LCB2 gene of Saccharomyces and the related LCB1 gene encode subunits of serine palmitoyltransferase, the initial enzyme in sphingolipid synthesis. Proc. Natl Acad. Sci. USA, 91, 7899–7902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pullen N., Dennis,P.B., Andjelkovic,M., Dufner,A., Kozma,S.C., Hemmings,B.A. and Thomas,G. (1998) Phosphorylation and activation of p70s6k by PDK1. Science, 279, 707–710. [DOI] [PubMed] [Google Scholar]
- Pushkareva M., Khan,W.A., Alessenko,A.V., Sahyoun,N. and Hannun,Y.A. (1992) Sphingosine activation of protein kinases in Jurkat T cells. In vitro phosphorylation of endogenous protein substrates and specificity of action. J. Biol. Chem., 267, 15246–15251. [PubMed] [Google Scholar]
- Pushkareva M., Bielawska,A., Menaldiv,D., Liotta,D. and Hannun,Y.A. (1993) Regulation of sphingosine-activated protein kinases: selectivity of activation by sphingoid bases and inhibition by non-esterified fatty acids. Biochem. J., 294, 699–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raths S., Rohrer,J., Crausaz,F. and Riezman,H. (1993) end3 and end4: two mutants defective in receptor-mediated and fluid-phase endocytosis in Saccharomyces cerevisiae. J. Cell Biol., 120, 55–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riezman H., Munn,A., Geli,M.I. and Hicke,L. (1996) Actin-, myosin- and ubiquitin-dependent endocytosis. Experientia, 52, 1033–1041. [DOI] [PubMed] [Google Scholar]
- Robinson L.C. et al. (1992) Yeast casein kinase I homologues: an essential gene pair. Proc. Natl Acad. Sci. USA, 89, 28–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodal S.K., Skretting,G., Garred,O., Vilhardt,F., van Deurs,B. and Sandvig,K. (1999) Extraction of cholesterol with methyl-β-cyclodextrin perturbs formation of clathrin-coated endocytic vesicles. Mol. Biol. Cell, 10, 961–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiestl R.H. and Gietz,R.D. (1989) High efficiency transformation of intact yeast cells using single stranded nucleic acids as a carrier. Curr. Genet., 16, 339–346. [DOI] [PubMed] [Google Scholar]
- Sherman F., Fink,G. and Hicks,J.B. (1983) Methods in Yeast Genetics: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Skrzypek M.S., Nagiec,M.M., Lester,R.L. and Dickson,R.C. (1998) Inhibition of amino acid transport by sphingoid long chain bases in Saccharomyces cerevisiae. J. Biol. Chem., 273, 2829–2834. [DOI] [PubMed] [Google Scholar]
- Stephens L. et al. (1998) Protein kinase B kinases that mediate phosphatidylinositol 3,4,5-trisphosphate-dependent activation of protein kinase B. Science, 279, 710–714. [DOI] [PubMed] [Google Scholar]
- Subtil A., Gaidarov,I., Kobylarz,K., Lampson,M.A., Keen,J.H. and McGraw,T.E. (1999) Acute cholesterol depletion inhibits clathrin-coated pit budding. Proc. Natl Acad. Sci. USA, 96, 6775–6780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y., Taniguchi,R., Tanoue,D., Yamaji,T., Takematsu,H., Mori,K., Fujita,T., Kawasaki,T. and Kozutsumi,Y. (2000) Sli2 (Ypk1), a homologue of mammalian protein kinase SGK, is a downstream kinase in the sphingolipid-mediated signaling pathway of yeast. Mol. Cell. Biol., 20, 4411–4419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sütterlin C., Doering,T.L., Schimmoller,F., Schröder,S. and Riezman,H. (1997) Specific requirements for the ER to Golgi transport of GPI-anchored proteins in yeast. J. Cell Sci., 110, 2703–2714. [DOI] [PubMed] [Google Scholar]
- Wu W.-I., McDonough,V.M., Nickels,J.T.,Jr, Ko,J., Fischl,A.S., Vales,T.R., Merrill,A.H.,Jr. and Carman,G.M. (1995) Regulation of lipid biosynthesis in Saccharomyces cerevisiae by fumonisin B1. J. Biol. Chem., 270, 13171–13178. [DOI] [PubMed] [Google Scholar]
- Zanolari B., Friant,S., Funato,K., Sütterlin,C., Stevenson,B.J. and Riezman,H. (2000) Sphingoid base synthesis requirement for endocytosis in Saccharomyces cerevisiae. EMBO J., 19, 2824–2833. [DOI] [PMC free article] [PubMed] [Google Scholar]