Abstract
Terminal differentiation of muscle cells follows a precisely orchestrated program of transcriptional regulatory events at the promoters of both muscle-specific and ubiquitous genes. Two distinct families of transcriptional co-activators, GCN5/PCAF and CREB-binding protein (CBP)/p300, are crucial to this process. While both possess histone acetyl-transferase (HAT) activity, previous studies have failed to identify a requirement for CBP/p300 HAT function in myogenic differentiation. We have addressed this issue directly using a chemical inhibitor of CBP/p300 in addition to a negative transdominant mutant. Our results clearly demonstrate that CBP/p300 HAT activity is critical for myogenic terminal differentiation. Furthermore, this requirement is restricted to a subset of events in the differentiation program: cell fusion and specific gene expression. These data help to define the requirements for enzymatic function of distinct coactivators at different stages of the muscle cell differentiation program.
Keywords: CREB-binding protein (CBP)/histone acetyl-transferase/MyoD/myogenesis/p300
Introduction
Muscle cell terminal differentiation represents a paradigm for multi-step genetic programs regulated at the level of transcription. It is orchestrated by two families of transcription factors, MEF-1 (or myogenic bHLHs) and MEF-2 (Buckingham, 1994; Weintraub, 1994), which act at specific stages during the differentiation process (Rudnicki and Jaenisch, 1995; Buckingham, 1996). Myogenic bHLHs (MyoD, Myf-5, myogenin and MRF-4) are necessary and sufficient to induce a muscle-specific genetic program in non-muscle cells. MyoD and Myf-5 are involved in the initial step of myoblast (the muscle precursor cell) determination (Rudnicki et al., 1993). Myogenin is required later for the terminal differentiation step (Hasty et al., 1993), and MRF-4 during muscle fiber maturation.
Muscle cell differentiation involves the fusion of muscle precursors into multi-nucleated myotubes, a process regulated by the activation of the muscle genetic program. This program requires the timely ordered expression of both ubiquitous and muscle-specific proteins. On terminal differentiation, myoblastic precursor cells exit from the proliferative cycle in an irreversible manner. MyoD triggers muscle terminal differentiation by directly or indirectly activating the muscle genetic program. This results in the activation of two categories of genes, i.e. genes whose products are involved in growth arrest and genes whose products determine the muscle phenotype. Growth arrest is accomplished via the action of pocket proteins (Harbour and Dean, 2000) and through the repression of cyclin-dependent kinases by expression of inhibitors such as p21 (Mal et al., 2000). Muscle cell phenotype is determined by muscle structural proteins—such as myosin heavy chain (MHC), actin and others—and by proteins integral to muscle function, such as muscle creatine kinase (MCK). Early stages of this program involve the activation of the muscle-specific transcription factor myogenin (Venuti et al., 1995), as well as the ubiquitous p21 gene (Halevy et al., 1995; Parker et al., 1995), followed by the activation of other muscle-specific genes, such as MHC or MCK (Lassar et al., 1989).
MyoD and myogenic bHLHs require co-regulator proteins that do not bind directly to DNA but are recruited to promoters through interactions with sequence-specific DNA binding proteins. These co-regulators include pocket proteins (Novitch et al., 1996; Sellers et al., 1998), histone deacetylases (Lu et al., 2000; McKinsey et al., 2000; Steinbac et al., 2000) and histone acetyl-transferases (HATs) (Missero et al., 1995; Eckner et al., 1996b; Yuan et al., 1996; Puri et al., 1997a). HATs transfer an acetyl group to lysines of histone and non-histone proteins. Histone acetylation is often associated with active transcription (Brownell and Allis, 1996); and a number of HATs are transcriptional co-activators (Mizzen and Allis, 1998; Utley et al., 1998).
In mammals, several HATs have been characterized, including CREB-binding protein (CBP) and p300 (Bannister and Kouzarides, 1996; Ogryzko et al., 1996), GCN5 and PCAF (Yang et al., 1996; Dyda et al., 2000), TAF II 250 (Mizzen et al., 1996), SRC-1 (Spencer et al., 1997) and others. These enzymes are found in multi-molecular complexes that include both HAT and non-HAT proteins. Among the HATs, two families have been linked to the muscle differentiation program: CBP/p300 (Missero et al., 1995; Eckner et al., 1996b; Yuan et al., 1996; Puri et al., 1997a) and GCN5/PCAF (Puri et al., 1997b; Xu et al., 2000). CBP/p300 and PCAF are found in the same complexes in cells, but they display distinct patterns of substrate specificity and/or modification in vitro. PCAF preferentially acetylates histone H3 on lysine 14, while CBP and p300 acetylate all lysines on all four histones (Schiltz et al., 1999; Trievel et al., 2000).
Both families have essential functions in a number of systems (Goodman and Smolik, 2000; Schiltz and Nakatani, 2000). PCAF behaves as a proliferation repressor in cultured cells (Yang et al., 1996) and is not essential in mice—perhaps due to functional redundancy with GCN5 (Yamauchi et al., 2000). In contrast, mice in which GCN5 is inactivated die at early embryonic stages with defects in muscle precursor tissues (Xu et al., 2000; Yamauchi et al., 2000). PCAF and GCN5 have also been shown to be required for myogenic differentiation in culture (Puri et al., 1997b).
The CBP/p300 family is essential for the balance between cell proliferation and differentiation. In vivo, both CBP and p300 are required for embryonic development in mice (Yao et al., 1998; Oike et al., 1999b), and their importance is further documented by gene dosage-associated phenotypes in man (Petrij et al., 1995) and in mice (Oike et al., 1999a). In cultured cells, however, these two highly homologous proteins (Arany et al., 1994) are generally, with few exceptions, functionally interchangeable (Kawasaki et al., 1998), and thus are referred to as CBP/p300. CBP and/or p300 function as co-activators for a variety of transcription factors involved in proliferation or differentiation, such as nuclear receptors and others (Chakravarti et al., 1996; Goodman and Smolik, 2000). They are essential targets for transforming viral proteins such as E1A or SV40 T (Eckner et al., 1996a), and are required for the G1/S transition in cycling cells (Ait-Si-Ali et al., 2000). On the other hand, they are critical for muscle cell terminal differentiation ex vivo, and both p300 and CBP interact physically with MyoD (Sartorelli et al., 1997; Riou et al., 2000).
The specific functions of the various HAT families—well established by genetic studies in yeast (Mizzen and Allis, 1998)—remain to be clarified in mammals. In particular, the functions of CBP/p300 and GCN5/PCAF in the muscle differentiation program could not be determined at the molecular level by gene inactivation experiments in mice. In vitro, both GCN5/PCAF and CBP/p300 acetylate MyoD (Sartorelli et al., 1999; Polesskaya et al., 2000). In cultured myoblastic cells, multiple lines of evidence implicate the PCAF protein (as well as its intrinsic HAT activity) in myotube formation, and in both muscle-specific and ubiquitous promoter activation (Puri et al., 1997b). Similarly, CBP and/or p300 have also been implicated in the execution of the muscle-specific genetic program (Eckner et al., 1996b; Puri et al., 1997a). However, transient transfection experiments suggested that CBP/p300 HAT enzymatic activity was dispensable for the activation of at least some promoters in myoblastic cells (Puri et al., 1997b). From these experiments, it was concluded that CBP HAT activity is not involved in MyoD-dependent transcriptional activation or in muscle cell differentiation (Puri et al., 1997b). However, only one promoter was investigated in that study.
In order to clarify this issue, we have employed two complementary approaches. First, we used a chemical HAT inhibitor, Lys CoA (Lau et al., 2000), which inhibits CBP/p300 but does not affect PCAF. Secondly, we challenged the muscle genetic program with a negative transdominant mutant of CBP. Surprisingly, cell fusion into myotubes was dramatically inhibited in cells devoid of CBP or p300 HAT activity, suggesting that CBP/p300 or a related HAT enzymatic activity is critical for this process. Furthermore, CBP/p300 HAT activity is dispensable for the activation of some early genes (p21 or myogenin), in agreement with the results of Puri et al. (1997b), but is critical for expression, promoter activation and local histone acetylation of some late markers.
Results
CBP/p300 HAT enzymatic activity increases at late stages of differentiation
CBP/p300 HAT activity was monitored during the course of myoblastic cell differentiation. C2C12 cells were placed in differentiation medium and CBP or p300 was immunoprecipitated and assayed for HAT activity after various periods of time. Results indicated that the enzymatic activities of CBP and p300 varied during the terminal differentiation process (Figure 1). During the first hour, the activity was stable, or even decreased depending on the status of the cells with regard to synchronization (data not shown), but increased at 24 h of differentiation. This enzymatic activation is coherent with a role for CBP/p300 HAT activity during the differentiation process. To test this hypothesis, we next used Lys-CoA, a synthetic inhibitor of the HATs CBP and p300.
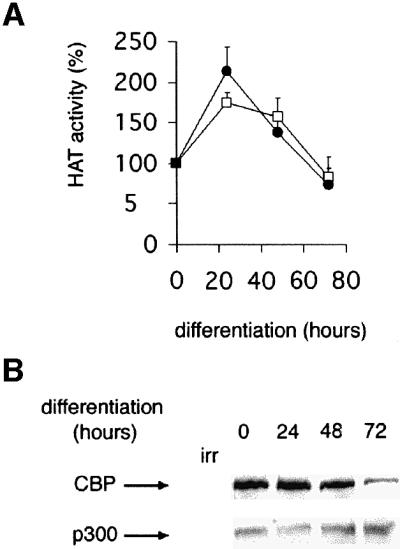
Fig. 1. Time course of CBP and p300 HAT activities during myogenic terminal differentiation. C2C12 cells were placed in differentiation medium, and CBP or p300 was immunoprecipitated at the times indicated and assayed for HAT activity (A) or analyzed by western blotting (B). Closed circle, CBP; open square, p300. The mean of three independent experiments is shown in (A). A value of 100% corresponds to 7470 c.p.m. for CBP and 8180 c.p.m. for p300.
Lys-CoA inhibits CBP/p300 HAT but not PCAF
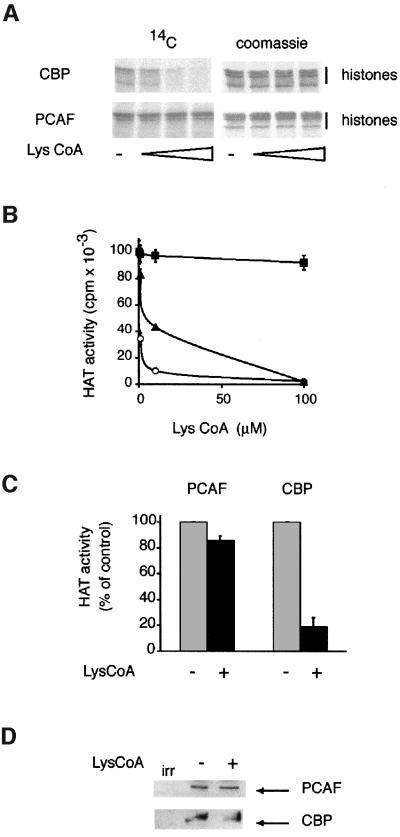
Lys-CoA was previously shown to inhibit selectively the CBP/p300 family of HATs but not PCAF (Lau et al., 2000). As these data were obtained largely in vitro under conditions in which the acetyl-transferase reaction time was short, the specificity of inhibition was verified under conditions that may be closer to those anticipated in cells, in particular using extended reaction times (see the legend to Figure 2). Recombinant CBP or PCAF was incubated with purified nucleosomes, 14C-labeled acetyl-CoA and increasing doses of Lys-CoA. Histones were analyzed after 1 h (Figure 2A). Lys-CoA inhibited histone acetylation by CBP, but not by PCAF. The effect of Lys-CoA on CBP and PCAF enzymatic activities was also monitored using a more quantitative assay and a synthetic peptide substrate (corresponding to the first 24 amino acids of histone H3) (Ait-Si-Ali et al., 1998). Lys-CoA potently repressed the HAT activities of CBP and p300 (Figure 2B) but not that of PCAF—at least in the concentration range tested (up to 100-fold greater than the 50% inhibitory dose for CBP).
Fig. 2. Lys-CoA specifically inhibits CBP/p300. (A) Bacterially produced recombinant CBP or PCAF (as indicated) was incubated with nucleosomes purified from HeLa cells and 14C-labeled acetyl-CoA; histones were analyzed by SDS–PAGE followed by autoradiography. (B) Recombinant CBP (closed triangles), p300 (open circles) or PCAF (closed squares) was incubated with a synthetic peptide corresponding to the first 24 amino acids of histone H3, together with [14C]AcCoA and the doses of Lys-CoA indicated. The radioactivity incorporated in the peptide was measured after 1 h; the mean of three independent experiments is shown. (C and D) C2C12 cells were permeabilized in the presence (+) or the absence (–) of 1 mM Lys-CoA. Extracts were prepared after 1 h. CBP/p300 and PCAF were immunoprecipitated and assayed for HAT activity (C) or analyzed by western blotting (D). The mean of three independent experiments is shown. A value of 100% corresponds to 15 053 c.p.m. for CBP and 2109 c.p.m. for PCAF.
The effect of Lys-CoA on the two enzymes was next assessed in live cells (C2C12, a mouse myoblastic cell line). Experiments using recombinant CBP adsorbed onto beads demonstrated that at 4°C the inhibition was resistant to stringent washes (A.Polesskaya, unpublished observations). Thus, the effect of Lys-CoA could be monitored on endogenous HATs immunoprecipitated from cells. As the inhibitor does not penetrate the cells, they were first permeabilized using TransPort™ (Gibco), under conditions such that ∼80–90% were permeabilized, as assessed by Trypan Blue penetration (data not shown). CBP or PCAF was immunoprecipitated and assayed for HAT activity 1 h later. Consistent with the in vitro results, Lys-CoA inhibited CBP and had no effect on PCAF (Figure 2C). This result indicates that the complex between the inhibitor and the enzyme is stable enough to resist the stringent washing procedures used in immunoprecipitation. These results confirmed that Lys-CoA can be used effectively to discriminate between CBP/p300 and PCAF HAT activities in live cells.
CBP/p300 HAT enzymatic activity is required for myotube formation
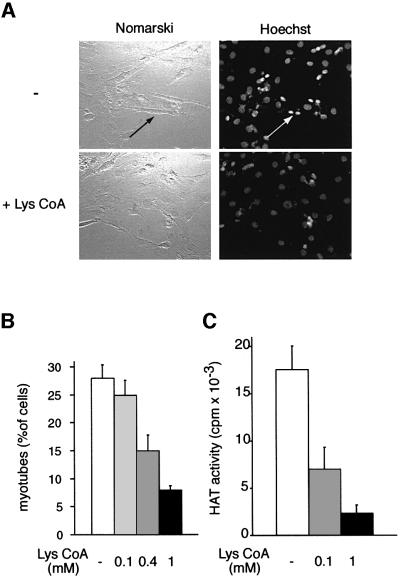
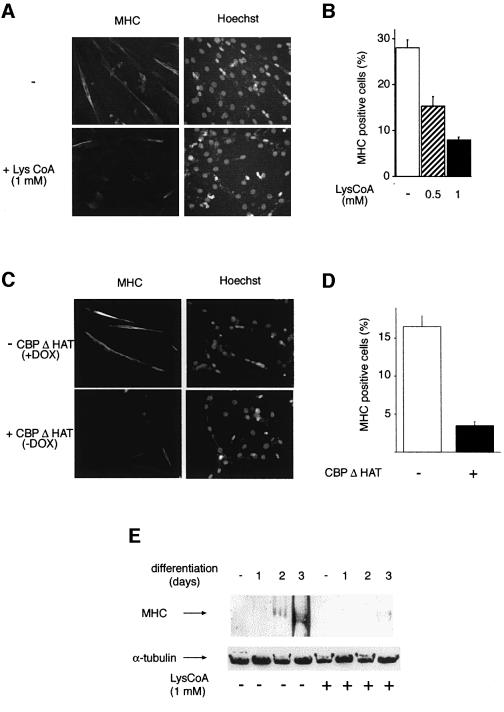
To assess the involvement of CBP/p300 HAT enzymatic activity in myogenic terminal differentiation, myoblastic cells (C2C12) were permeabilized in the presence or absence of Lys-CoA and placed in differentiation medium; myotube formation was monitored 72 h later. In the absence of inhibitor, ∼30% of the cells had fused into bona fide multi-nucleated myotubes (Figure 3A and B). This proportion decreased in the presence of the inhibitor, in a dose-dependent manner, to ∼5% at the maximal dose of inhibitor tested. This inhibition correlated well with the reduction in endogenous CBP HAT activity, assayed in parallel samples (Figure 3C). In addition, it is noteworthy that the nuclei in Lys-CoA-treated cells did not have the condensed appearance of those associated with myotubes in mock-treated cells (arrows in Figure 3A). Residual myotube formation and HAT activity most likely correspond to cells that had not been permeabilized (∼15–20%). Taken together, these results indicate that the formation of myotubes is strongly diminished by the inhibition of CBP/p300 by Lys-CoA. Therefore, GCN5/PCAF HAT activity, which is not sensitive to Lys-CoA, is not sufficient to sustain the muscle differentiation program, and CBP/p300 HAT activity seems to be required for at least some step(s) of this program.
Fig. 3. Lys-CoA inhibits myotube formation. C2C12 cells were permeabilized in the presence or absence of Lys-CoA, and placed in differentiation medium; after 3 days, cells were fixed and labeled with Hoechst 33258. (A) Typical fields: the arrows point to a myotube and its condensed nuclei. (B) Statistical analysis of the results: shown is the mean ± SE of three independent experiments; a minimum of 300 cells were counted in each experiment. (C) CBP/p300 HAT activity was measured as in Figure 2C. Shown is the mean ± SE of three independent experiments.
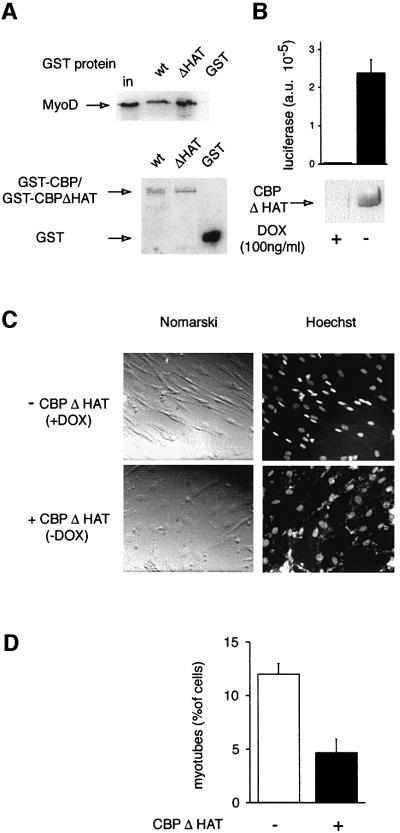
Lys-CoA is likely to inhibit other HATs in addition to CBP. Consequently, in order to confirm the involvement of CBP/p300 in muscle differentiation, we used a more selective approach involving a negative transdominant mutant, CBPΔHAT, in which a deletion in the HAT domain abolishes enzymatic activity (Ait-Si-Ali et al., 2000). This mutant protein retains other functions of CBP such as protein–protein interactions (Ait-Si-Ali et al., 2000) and, in particular, fully retains the ability to interact with MyoD, as assessed using a glutathione S-transferase (GST) pull-down assay (Figure 4A). CBPΔHAT was expressed in an inducible manner (100- to 200-fold, as estimated from a co-induced luciferase gene; Figure 4B), using the ‘tet-off’ system (Gossen and Bujard, 1992) in a clone derived from the C2C12 myogenic cell line. Expression of the mutant strongly impaired the ability of the cells to fuse into myotubes, in a manner indistinguishable from Lys-CoA treatment (Figure 4C and D). A control cell line in which the backbone expression vector was transfected differentiated normally both in the presence and absence of doxycycline (data not shown). Furthermore, expression of wild-type (WT) CBP did not affect the differentiation of stably transfected C2C12 cells (data not shown). This result independently confirms the HAT inhibitor data and clearly demonstrates that CBP or p300 HAT activity is required for myogenic terminal differentiation.
Fig. 4. CBPΔHAT, a negative transdominant mutant of CBP, inhibits myotube formation. (A) CBPΔHAT interacts with MyoD: GST pull-down analysis of CBP and CBPΔHAT. Upper panel: MyoD was incubated with beads coated with the GST proteins indicated, and bound MyoD was analyzed by western blotting; in, 10% of the input. Lower panel: the protein content of the beads used above was monitored by western blotting using an anti-GST antibody. (B) Induction of the mutant by doxycycline (DOX) removal: extracts of C2C12-CBPΔHAT cells were assayed for luciferase activity or immunoprecipitated with anti-CBP antibodies followed by western blot analysis with anti-HA. (C and D) C2C12-CBPΔHAT cells cultured in the presence of doxycycline or in its absence (as indicated) were fixed and labeled with Hoechst after 72 h in differentiation medium. (C) Typical fields. (D) Statistical analysis (mean of three independent experiments with 300 cells analyzed in each).
p21 and myogenin gene expression is not affected by the absence of CBP/p300 HAT enzymatic activity
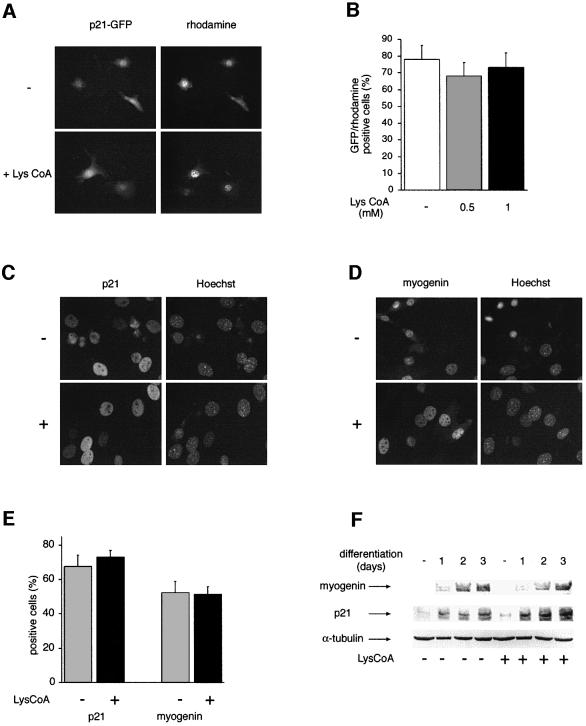
In order to define the step of the myogenic differentiation program blocked by CBP/p300 inhibition, we next analyzed the expression of differentiation markers. p21 is a ubiquitous protein involved in myoblast growth arrest (Halevy et al., 1995; Missero et al., 1995; Parker et al., 1995). This protein begins to accumulate early in the differentiation program, following the transcriptional activation of its promoter by MyoD (Missero et al., 1995; Eckner et al., 1996b). First, we assessed the effect of Lys-CoA on the activity of p21 promoter. C3H 10T1/2 cells were microinjected with a p21–green fluorescent protein (GFP) reporter, together with recombinant MyoD and an injection marker, in the presence or absence of Lys-CoA (4 mM in the injection mix, corresponding to 0.1–0.5 mM final concentration in the injected nuclei). p21 promoter activity was not affected by the inhibitor (Figure 5A and B), similar to results published previously (Puri et al., 1997b). Expression of the endogenous p21 protein was next analyzed by immunofluorescence in C2C12 myoblastic cells permeabilized in the presence or absence of Lys-CoA and subsequently placed in differentiation medium. As observed on the exogenous promoter, the proportion of cells expressing p21 was identical in the presence or absence of the inhibitor (Figure 5C and E). Similar results (Figure 5D and E) were obtained with another marker, myogenin, which represents a muscle-specific gene instrumental to the differentiation program (Rudnicki and Jaenisch, 1995), and which also accumulates early (Edmondson and Olson, 1989). A statistical analysis of the results indicated that neither of these proteins was affected by Lys-CoA (Figure 5E). This result was confirmed by western blotting (Figure 5F), which showed similar levels of both myogenin and p21 proteins in treated versus untreated cells. Identical results were obtained using the CBP negative transdominant CBPΔHAT, which had no effect on p21 or myogenin proteins (data not shown). Taken together, these results indicate that CBP/p300 HAT activity is not required for activation of p21 and myogenin genes.
Fig. 5. Myogenin and p21 expression is not affected by Lys-CoA. (A and B) Lys-CoA has no effect on the activity of the p21 promoter. C3H 10T1/2 cells were microinjected with a p21–GFP construct, together with Lys-CoA where indicated (4 mM in the injection mix), recombinant MyoD and an injection marker coupled to rhodamine. Cells were analyzed 24 h later. (A) Typical fields; note that each field contains few microinjected cells, but significant numbers of cells were analyzed by cumulating numerous fields for each measure. (B) Statistical analysis: shown is the mean ± SE of three independent experiments, with 300 cells counted per experiment. (C–F) Endogenous p21 and myogenin proteins are not affected by Lys-CoA. C2C12 cells were permeabilized in the presence or absence of Lys-CoA (1 mM) as indicated, placed in differentiation medium for 24 h (C–E) for the periods of time indicated (F), fixed and labeled with Hoechst and either anti-p21 (C) or anti-myogenin (D) antibodies. (E) Statistical analysis of the results (mean ± SE of three independent experiments, with 300 cells analyzed per experiment). (F) Extracts of permeabilized cells were prepared at the times indicated and analyzed by western blotting using anti-myogenin, anti-p21 or anti-α-tubulin antibodies as indicated.
MHC synthesis requires CBP/p300 HAT activity
We next analyzed the effect of CBP/p300 inhibition on the expression of some late muscle-specific markers by immunofluorescence. C2C12 cells treated with inhibitor showed a dramatic reduction in the expression of MHC compared with mock-treated controls (Figure 6A and B). The residual positive cells had most likely failed to incorporate the inhibitor. Similar results were obtained when CBP/p300 activity was inhibited by induction of CBPΔHAT expression (Figure 6C and D). Western blot analysis of cells treated with Lys-CoA confirmed that MHC protein expression was strongly repressed following CBP/p300 inhibition (Figure 6E).
Fig. 6. Lys-CoA and CBPΔHAT both inhibit MHC protein expression. (A and B) C2C12 cells were permeabilized in the presence of Lys-CoA or in its absence (as indicated) and placed in differentiation medium for 72 h. Cells were fixed and analyzed by immunofluorescence using anti-MHC antibodies. (A) Typical fields. (B) Statistical analysis of the results (mean ± SE of three independent experiments, with 300 cells counted per experiment). (C and D) CBPΔHAT inhibits MHC expression. C2C12-CBPΔHAT were maintained in doxycycline (–) or cultured in the absence of doxycycline for 3 days (CBPΔHAT), then placed in differentiation medium for 72 h, fixed and analyzed by immunofluorescence using anti-MHC antibodies. (C) Typical fields; (D) statistical analysis of the results (mean ± SE of three independent experiments with 300 cells counted per experiment). (E) Western blot analysis; cells were treated with Lys-CoA as in (A) and placed in differentiation medium for the periods of time indicated; extracts were analyzed by western blotting using anti MHC or anti-α-tubulin antibodies.
MCK expression and promoter activity requires CBP/p300 HAT function
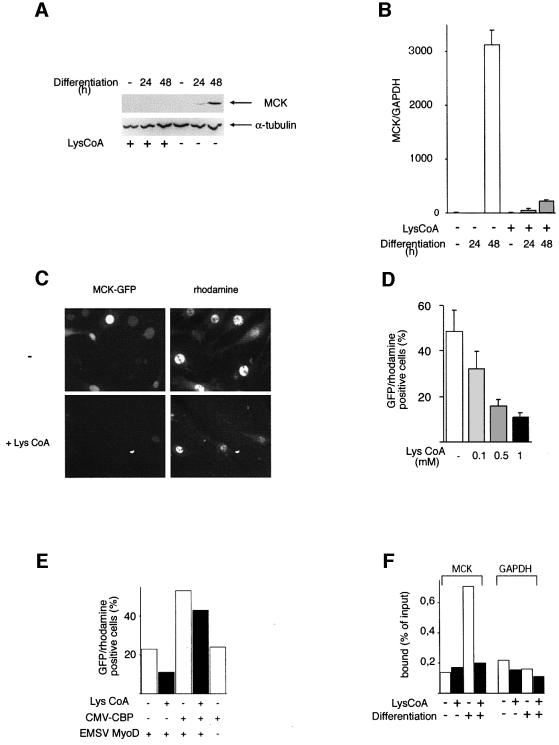
Identical results were observed with another late muscle gene, MCK, which showed a strongly impaired expression at the protein level (Figure 7A), as well as at the level of mRNA (Figure 7B). Furthermore, when the activity of the MCK promoter was assessed by microinjection following the protocol described above for p21, MCK promoter-driven GFP expression was dramatically decreased in the presence of the inhibitor (Figure 7C and D). MCK promoter activity could be rescued by CBP expression (Figure 7E, inhibition was three times better in the absence of CBP than in its presence), consistent with CBP or p300 HAT activity being a target of inhibition by Lys-CoA.
Fig. 7. Lys-CoA inhibits MCK expression. (A and B) C2C12 cells were permeabilized in the presence of Lys-CoA (1 mM) or in its absence (as indicated) and placed in differentiation medium for the periods of time indicated. (A) MCK protein expression was analyzed by western blotting, using α-tubulin as an internal standard. (B) The MCK RNA steady-state level was analyzed by RT–PCR, using GAPDH as an internal standard; the ratio between MCK and GAPDH RNA is shown. (C–F) C3H 10T1/2 cells were microinjected with a mixture containing an MCK–GFP reporter construct, an expression vector for MyoD, an injection marker coupled to rhodamine, and Lys-CoA (as indicated). (C) Typical fields. (D) Statistical analysis of the results; shown is the mean ± SE of three independent experiments. (E) Cells were microinjected as in (A), but an expression vector for CBP was added where indicated (results of a typical experiment performed twice). (F) Lys-CoA inhibits histone H4 acetylation on the MCK promoter. Chromatin was prepared from C2C12 cells treated with Lys-CoA and induced to differentiate as indicated, immunoprecipitated using anti-acetylated H4 antibodies and analyzed by real-time PCR (on a Light Cycler) with primers amplifying the MCK promoter, or a GAPDH sequence used as a control. Shown are the numbers of copies as a percentage of the inputs (results of a typical experiment performed twice).
We have previously shown that activation of the MCK promoter is accompanied by a local increase in H4 acetylation (Polesskaya et al., 2001). In order to explore whether CBP/p300 might be involved, cells were permeabilized in the presence or absence of Lys-CoA and induced to differentiate. Chromatin was prepared from these cells and immunoprecipitated using an anti-acetylated H4 antibody. MCK promoter DNA was detected in immunoprecipitates using real-time PCR. Glyceralde(GAPDH) was used as a constitutively expressed control. When C2C12 cells were induced to differentiate in the absence of inhibitors, histone H4 acetylation increased significantly on the MCK promoter (Figure 7F); Lys-CoA treatment dramatically inhibited this acetylation. A global analysis suggested that the steady-state level of histone H3 and H4 acetylation did not vary during differentiation, nor was it affected by Lys-CoA (data not shown). Furthermore, histone acetylation on GAPDH was not affected either by differentiation or by Lys-CoA. This result implies that CBP or p300 is involved in H4 acetylation on the MCK promoter.
Discussion
The CBP/p300 family of proteins is involved in the muscle-cell terminal differentiation program (Missero et al., 1995; Eckner et al., 1996b; Yuan et al., 1996; Puri et al., 1997a), although it was proposed that their HAT enzymatic activity is dispensable in this process (Puri et al., 1997b). Here, we show that treatment with Lys-CoA, a CBP/p300 inhibitor, dramatically reduced the proportion of cells fusing into myotubes under differentiation conditions. The effect of Lys-CoA could be mimicked by use of a negative transdominant mutant of CBP (CBPΔHAT), whose targets are most likely CBP and p300. This confirmed the involvement of CBP/p300 and not that of a distinct HAT. These results indicate an essential function for CBP/p300 HAT in the cell fusion process, perhaps through the activation of gene(s) such as meltrin-α (Kurisaki et al., 1998), whose products participate in this process.
In order to elucidate the role(s) for this family of HATs in the differentiation process, the effect of CBP/p300 HAT inhibition was also analyzed at the molecular level. Expression of two early markers (p21 and myogenin) was not affected by inhibition of CBP/p300 HAT activity by Lys-CoA nor by CBPΔHAT. In contrast, both Lys-CoA and the CBPΔHAT transdominant mutant inhibited two late markers: MHC and MCK. This inhibition was documented at several levels, including protein expression, promoter activity and promoter histone acetylation status. These data suggest that CBP/p300 HAT activity is dispensable for at least some early steps of the differentiation program, but is critically required for at least some late steps. Alternatively, the observed differences between early and late promoters could result from a delayed effect of the inhibitors, with inhibition in permeabilized cells evident only beyond 24 h after permeabilization. How ever, a direct analysis of CBP HAT enzymatic activity from permeabilized cells demonstrated inhibition as early as 1 h after permeabilization (Figure 2C). Further, a comparison of the two categories of promoters in the same assay (after microinjection) confirmed that Lys-CoA inhibited only the MCK promoter, and not the p21 promoter. In addition, similar results were observed using the negative transdominant mutant to inhibit CBP/p300; in these experiments, the maximal level of mutant expression was reached prior to the induction of cell differentiation.
Inhibition of late—and not early—promoters by Lys- CoA could also result from toxicity at late stages. Toxicity as a cause for late marker inhibition was ruled out because Lys-CoA-treated cells expressed normal levels of myogenin, p21 and α-tubulin at late stages, as assessed by western blotting (Figure 5F) or by immunofluorescence (data not shown), even though some of these proteins have very short half-lives (Edmondson et al., 1991). In addition, dose–response analysis revealed a good correlation between HAT inhibition and cell differentiation inhibition. Also, similar results were observed with the CBP/p300 negative transdominant mutant. Furthermore, when promoters were directly compared in the same reporter assay (analysis of microinjected reporter constructs, Figures 5A and 6C), only the MCK promoter, and not the p21 promoter, was inhibited under these identical conditions. Finally, a direct analysis of viability using Trypan Blue exclusion indicated that cell death was not increased in cells where CBP/p300 was inhibited prior to differentiation (data not shown). Thus, our results suggest that CBP/p300 HAT is required for the activation of late, and not early, genes in muscle. We cannot rule out a role for CBP/p300 in the activation of early genes that we have not analyzed. Although it thus remains possible that both CBP/p300 and PCAF are involved in early stages of differentiation, it is of note that CBP/p300 enzymatic activity is not maximal during this step (Figure 1). We cannot currently distinguish direct from indirect effects of CBP/p300 on late gene expression. It is possible that CBP/p300 HAT acts at early stages on unknown gene(s) which, in turn, are indispensable for late gene activation. A more global analysis of genes expressed in treated cells will allow further clarification of this point. In any case, our results clearly demonstrate that distinct mechanisms are used for some of the early versus later events of this program. The mechanisms through which the early promoters that do not require CBP HAT activity are induced might involve other HATs, in particular GCN5/PCAF as suggested previously (Puri et al., 1997b). Indeed, at 24 h of differentiation, histone acetylation increased on the myogenin promoter, and Lys-CoA had little or no effect on this increase (data not shown).
Taken together, our results show that CBP/p300 HAT activity is required for muscle cell terminal differentiation, contrary to the suggestion by Puri et al. (1997b), who found CBP/p300 HAT dispensable. Our data refine their observation, showing that CBP/p300 HAT activity is indeed dispensable for activation of some of the genes in the myogenic program, but is critical for others and for cell fusion. These data shed new light on CBP/p300 function and reveal the existence of both CBP/p300 HAT-dependent and -independent pathways during muscle cell differentiation. Finally, they raise the possibility of using inhibitors of specific chromatin modification enzymes to manipulate gene transcription in vivo.
Materials and methods
Cell permeabilization
C2C12 cells were incubated in suspension in warm buffer (ICB, supplied with the TransPort kit) supplemented with Trypan Blue, Lys-CoA where appropriate, and TransPort Reagent (Gibco-BRL) at 37°C for 10 min. Stop Reagent was added and the cells were seeded onto glass slides in 24-well culture plates. Differentiation was triggered 1 h later by changing the medium to Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 0.5% fetal calf serum (FCS).
HAT assays
GST–CBP and GST–PCAF were prepared as described previously (Polesskaya et al., 2000). Flag-tagged p300 was produced in insect cells using a baculovirus-driven expression system. Endogenous CBP/p300 and stably transfected flag-PCAF (ectopically expressed PCAF had to be used due to the lack of antibodies available to immunoprecipitate endogenous PCAF without co-immunoprecipitating CBP) were immunoprecipitated from C2C12 cells, permeabilized in the presence or absence of Lys-CoA, using the NM11 antibody (PharMingen) and the M-2 antibody (Sigma), respectively. HAT activity was measured as described previously (Ait-Si-Ali et al., 1998) using a peptide corresponding to the first 24 amino acids of histone H3.
Global histone acetylation was monitored according to Kruhlak et al. (2001).
Microinjection assays
The injection mix included 10 µg of reporter construct (MCK-EGFP or p21-EGFP), 2 µg of recombinant non-tagged MyoD, increasing amounts of Lys-CoA, increasing amounts of pCMV-CBP expression plasmid (when appropriate) and dextran–rhodamine as described previously (Polesskaya et al., 2000). Cells were placed in differentiation medium 6 h after injection and analyzed 48 h later.
Stable transfections
C2C12-TtA cells were derived by transfection of C2C12 cells with a plasmid driving the expression of the chimeric tTA protein (Gossen and Bujard, 1992) under the control of the promoter of elongation factor 1 (a generous gift of Dr M.Lacasa). CBPΔHAT coding sequence (HA tagged) was cloned into pBIL (Clontech), a bidirectional vector which in the absence of doxycycline directs the expression of both the cloned protein of interest and luciferase. C2C12-CBPΔHAT was derived by transfection of C2C12-TtA with the pBIL-CBPΔHAT construct. A clone in which CBPΔHAT expression was induced >200-fold, as calculated from luciferase activity, was selected for further studies. Doxycycline had no influence on C2C12 differentiation.
Immunoprecipitation, western blotting and immunohistochemistry
Immunoprecipitation and western blotting were performed using standard procedures in RIPA buffer [50 mM Tris pH 7.5, 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate (SDS), 1 mM EDTA] supplemented with a cocktail of protease inhibitors.
CBP was precipitated from C2C12 myoblasts with anti-CBP antibody (A-22; Santa Cruz) and analyzed by western blotting with anti-p300/CBP (NM11; PharMingen). Monitoring of p21, myogenin and MHC expression was performed by western blotting on total extracts of C2C12 cells using anti-p21 (65951A; PharMingen), anti-myogenin (F.5D; a kind gift of Dr W.E.Wright), anti-MHC (MY-32; Sigma) or anti-MCK (Ito et al., 2001). Protein in total extracts was standardized by monitoring α-tubulin expression (DM1A; Sigma). For HAT assays, beads were washed twice in HAT buffer (50 mM Tris pH 7.5, 1 mM EDTA, protease inhibitors).
Expression of early and late differentiation markers was monitored by immunohistochemical analysis, using standard procedures.
RNA analysis
RNA was analyzed by RT–PCR using real-time PCR performed on a Light Cycler (Roche Diagnostics) using an RNA amplification kit from Roche and according to the manufacturer’s instructions. The primers used were 5′-CACCATGCCGTTCGGCAACA-3′ and 5′-GGTTGTCCACCCCAGTCT-3′ for MCK-3′, 5′-CCAATGTGTCCGTCGTGGATCT-3′ and 5′-GTTGAAGTCGCAGGAGACAACC-3′ for GAPDH. Negative controls included samples without reverse transcriptase. Three determinations were performed for each sample.
Chromatin immunoprecipitation
Chromatin immunoprecipitation was performed as described previously (Ferreira et al., 2001). Immunoprecipitated DNA was analyzed by quantitative PCR (Light Cycler as above) using SYBR green dye. The primers used were CACACAGCACAGACAGACACT-fw and GGTGGCTATTGAGACCTA-rev for MCK, yielding a 178 bp fragment (18 bp downstream from the transcription start), and CCAATGTGTCCGTCGTGGATCT-fw and GTTGAAGTCGCAGGAGACAACC-rev for the GAPDH gene (used as internal control), yielding a 190 bp fragment. Copy numbers were calculated by reference to a standard curve, generated using a plasmid (data not shown). Two to three dilutions of each sample were analyzed.
Acknowledgments
Acknowledgements
We thank Vasia Ogryzko and Paul Wade for critical reading of the manuscript, Michel Lacasa for the kind gift of pEf1-TtA, Kanefusa Kato for the kind gift of anti-MCK antibodies and M.Cabanis for the preparation of recombinant proteins. This work was supported by grants from the Association Française contre les Myopathies and from the European 5th PCRDT (grant QLG1-CT-1999-00866). A.P. and I.N. were supported by the Association pour la Recherche sur le Cancer.
References
- Ait-Si-Ali S., Ramirez,S., Robin,P., Trouche,D. and Harel-Bellan,A. (1998) A rapid and sensitive assay for histone acetyl-transferase activity. Nucleic Acids Res., 26, 3869–3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ait-Si-Ali S. et al. (2000) CBP/p300 histone acetyl-transferase activity is important for the G1/S transition. Oncogene, 19, 2430–2437. [DOI] [PubMed] [Google Scholar]
- Arany Z., Sellers,W.R., Livingston,D.M. and Eckner,R. (1994) E1A-associated p300 and CREB-associated CBP belong to a conserved family of coactivators. Cell, 77, 799–800. [DOI] [PubMed] [Google Scholar]
- Bannister A.J. and Kouzarides,T. (1996) The CBP co-activator is a histone acetyltransferase. Nature, 384, 641–643. [DOI] [PubMed] [Google Scholar]
- Brownell J.E. and Allis,C.D. (1996) Special HATs for special occasions: linking histone acetylation to chromatin assembly and gene activation. Curr. Opin. Genet. Dev., 6, 176–184. [DOI] [PubMed] [Google Scholar]
- Buckingham M. (1994) Muscle differentiation. Which myogenic factors make muscle? Curr. Biol., 4, 61–63. [DOI] [PubMed] [Google Scholar]
- Buckingham M. (1996) Skeletal muscle development and the role of the myogenic regulatory factors. Biochem. Soc. Trans., 24, 506–509. [DOI] [PubMed] [Google Scholar]
- Chakravarti D., LaMorte,V.J., Nelson,M.C., Nakajima,T., Schulman,I.G., Juguilon,H., Montminy,M. and Evans,R.M. (1996) Role of CBP/P300 in nuclear receptor signaling. Nature, 383, 99–103. [DOI] [PubMed] [Google Scholar]
- Dyda F., Klein,D.C. and Hickman,A.B. (2000) GCN5-related N-acetyltransferases: a structural overview. Annu. Rev. Biophys. Biomol. Struct., 29, 81–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckner R., Ludlow,J.W., Lill,N.L., Oldread,E., Arany,Z., Modjtahedi,N., DeCaprio,J.A., Livingston,D.M. and Morgan,J.A. (1996a) Association of p300 and CBP with simian virus 40 large T antigen. Mol. Cell. Biol., 16, 3454–3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckner R., Yao,T.P., Oldread,E. and Livingston,D.M. (1996b) Inter action and functional collaboration of p300/CBP and bHLH proteins in muscle and B-cell differentiation. Genes Dev., 10, 2478–2490. [DOI] [PubMed] [Google Scholar]
- Edmondson D.G. and Olson,E.N. (1989) A gene with homology to the myc similarity region of MyoD1 is expressed during myogenesis and is sufficient to activate the muscle differentiation programme. Genes Dev., 3, 628–640. [DOI] [PubMed] [Google Scholar]
- Edmondson D.G., Brennan,T.J. and Olson,E.N. (1991) Mitogenic repression of myogenin autoregulation. J. Biol. Chem., 266, 21343–21346. [PubMed] [Google Scholar]
- Ferreira R., Naguibneva,I., Ait-Si-Ali,S., Mathieu,M., Pritchard,L.L., Robin,P. and Harel-Bellan,A. (2001) Cell cycle dependent stable recruitment of a histone deacetylase correlates with histone H4 lysine 5 and 12 deacetylation on an E2F target promoter. EMBO Rep., 2, 794–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman R.H. and Smolik,S. (2000) CBP/p300 in cell growth, transformation and development. Genes Dev., 14, 1553–1577. [PubMed] [Google Scholar]
- Gossen M. and Bujard,H. (1992) Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc. Natl Acad. Sci. USA, 89, 5547–5551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halevy O., Novitch,B.G., Spicer,D.B., Skapek,S.X., Rhee,J., Hannon,G.J., Beach,D. and Lassar,A.B. (1995) Correlation of terminal cell cycle arrest of skeletal muscle with induction of p21 by MyoD. Science, 267, 1018–1021. [DOI] [PubMed] [Google Scholar]
- Harbour J.W. and Dean,D.C. (2000) Rb function in cell-cycle regulation and apoptosis. Nature Cell Biol., 2, E65–E67. [DOI] [PubMed] [Google Scholar]
- Hasty P., Bradley,A., Morris,J.H., Edmondson,D.G., Venuti,J.M., Olson,E.N. and Klein,W.H. (1993) Muscle deficiency and neonatal death in mice with a targeted mutation in the myogenin gene. Nature, 364, 501–506. [DOI] [PubMed] [Google Scholar]
- Ito H., Kamei,K., Iwamoto,I., Inaguma,Y. and Kato,K. (2001) Regulation of the levels of small heat-shock proteins during differentiation of C2C12 cells. Exp. Cell Res., 266, 213–221. [DOI] [PubMed] [Google Scholar]
- Kawasaki H., Eckner,R., Yao,T.P., Taira,K., Chiu,R., Livingston,D.M. and Yokoyama,K.K. (1998) Distinct roles of the co-activators p300 and CBP in retinoic-acid-induced F9-cell differentiation. Nature, 393, 284–289. [DOI] [PubMed] [Google Scholar]
- Kruhlak M.J., Hendzel,M.J., Fischle,W., Bertos,N.R., Hameed,S., Yang,X.J., Verdin,E. and Bazett-Jones,D.P. (2001) Regulation of global acetylation in mitosis through loss of histone acetyltransferases and deacetylases from chromatin. J. Biol. Chem., 276, 38307–38319. [DOI] [PubMed] [Google Scholar]
- Kurisaki T., Masuda,A., Osumi,N., Nabeshima,Y. and Fujisawa-Sehara,A. (1998) Spatially- and temporally-restricted expression of meltrin α (ADAM12) and β (ADAM19) in mouse embryo. Mech. Dev., 73, 211–215. [DOI] [PubMed] [Google Scholar]
- Lassar A.B., Buskin,J.N., Lockshon,D., Davis,R.L., Apone,S., Hauschka,S.D. and Weintraub,H. (1989) MyoD is a sequence-specific DNA binding protein requiring a region of myc homology to bind to the muscle creatine kinase enhancer. Cell, 58, 823–831. [DOI] [PubMed] [Google Scholar]
- Lau O.D. et al. (2000) HATs off: selective synthetic inhibitors of the histone acetyltransferases p300 and PCAF. Mol. Cell, 5, 589–595. [DOI] [PubMed] [Google Scholar]
- Lu J., McKinsey,T.A., Zhang,C.L. and Olson,E.N. (2000) Regulation of skeletal myogenesis by association of the MEF2 transcription factor with class II histone deacetylases. Mol. Cell, 6, 233–244. [DOI] [PubMed] [Google Scholar]
- Mal A., Chattopadhyay,D., Ghosh,M.K., Poon,R.Y., Hunter,T. and Harter,M.L. (2000) p21 and retinoblastoma protein control the absence of DNA replication in terminally differentiated muscle cells. J. Cell Biol., 149, 281–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinsey T.A., Zhang,C.L., Lu,J. and Olson,E.N. (2000) Signal-dependent nuclear export of a histone deacetylase regulates muscle differentiation. Nature, 408, 106–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missero C., Calautti,E., Eckner,R., Chin,J., Tsai,L.H., Livingston,D.M. and Dotto,G.P. (1995) Involvement of the cell-cycle inhibitor Cip1/WAF1 and the E1A-associated p300 protein in terminal differentiation. Proc. Natl Acad. Sci. USA, 92, 5451–5455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizzen C.A. and Allis,C.D. (1998) Linking histone acetylation to transcriptional regulation. Cell. Mol. Life Sci., 54, 6–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizzen C.A. et al. (1996) The TAF(II)250 subunit of TFIID has histone acetyltransferase activity. Cell, 87, 1261–1270. [DOI] [PubMed] [Google Scholar]
- Novitch B.G., Mulligan,G.J., Jacks,T. and Lassar,A.B. (1996) Skeletal muscle cells lacking the retinoblastoma protein display defects in muscle gene expression and accumulate in S and G2 phases of the cell cycle. J. Cell Biol., 135, 441–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogryzko V.V., Schiltz,R.L., Russanova,V., Howard,B.H. and Nakatani,Y. (1996) The transcriptional coactivators p300 and CBP are histone acetyltransferases. Cell, 87, 953–959. [DOI] [PubMed] [Google Scholar]
- Oike Y. et al. (1999a) Truncated CBP protein leads to classical Rubinstein–Taybi syndrome phenotypes in mice: implications for a dominant-negative mechanism. Hum. Mol. Genet., 8, 387–396. [DOI] [PubMed] [Google Scholar]
- Oike Y. et al. (1999b) Mice homozygous for a truncated form of CREB binding protein exhibit defects in hematopoiesis and vasculo-angiogenesis. Blood, 93, 2771–2779. [PubMed] [Google Scholar]
- Parker S.B., Eichele,G., Zhang,P., Rawls,A., Sands,A.T., Bradley,A., Olson,E.N., Harper,J.W. and Elledge,S.J. (1995) p53-independent expression of p21Cip1 in muscle and other terminally differentiating cells. Science, 267, 1024–1027. [DOI] [PubMed] [Google Scholar]
- Petrij F. et al. (1995) Rubinstein–Taybi syndrome caused by mutations in the transcriptional co-activator CBP. Nature, 376, 348–351. [DOI] [PubMed] [Google Scholar]
- Polesskaya A., Duquet,A., Naguibneva,I., Weise,C., Vervisch,A., Bengal,E., Hucho,F., Robin,P. and Harel-Bellan,A. (2000) CREB-binding protein/p300 activates MyoD by acetylation. J. Biol. Chem., 275, 34359–34364. [DOI] [PubMed] [Google Scholar]
- Polesskaya A., Naguibneva,I., Duquet,A., Bengal,E., Robin,P. and Harel-Bellan,A. (2001) Interaction between acetylated MyoD and the bromodomain of CBP and/or p300. Mol. Cell. Biol., 21, 5312–5320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puri P.L., Avantaggiati,M.L., Balsano,C., Sang,N., Graessmann,A., Giordano,A. and Levrero,M. (1997a) p300 is required for MyoD-dependent cell cycle arrest and muscle-specific gene transcription. EMBO J., 16, 369–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puri P.L. et al. (1997b) Differential roles of p300 and PCAF acetyltransferases in muscle differentiation. Mol. Cell, 1, 35–45. [DOI] [PubMed] [Google Scholar]
- Riou P., Bex,F. and Gazzolo,L. (2000) The human T cell leukemia/lymphotropic virus type 1 Tax protein represses MyoD-dependent transcription by inhibiting MyoD-binding to the KIX domain of p300. A potential mechanism for Tax-mediated repression of the transcriptional activity of basic helix–loop–helix factors. J. Biol. Chem., 275, 10551–10560. [DOI] [PubMed] [Google Scholar]
- Rudnicki M.A. and Jaenisch,R. (1995) The MyoD family of transcription factors and skeletal myogenesis. BioEssays, 17, 203–209. [DOI] [PubMed] [Google Scholar]
- Rudnicki M.A., Schnegelsberg,P.N., Stead,R.H., Braun,T., Arnold,H.H. and Jaenisch,R. (1993) MyoD or Myf-5 is required for the formation of skeletal muscle. Cell, 75, 1351–1359. [DOI] [PubMed] [Google Scholar]
- Sartorelli V., Huang,J., Hamamori,Y. and Kedes,L. (1997) Molecular mechanisms of myogenic coactivation by p300: direct interaction with the activation domain of MyoD and with the MADS box of MEF2C. Mol. Cell. Biol., 17, 1010–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartorelli V., Puri,P.L., Hamamori,Y., Ogryzko,V., Chung,G., Nakatani,Y., Wang,J.Y. and Kedes,L. (1999) Acetylation of MyoD directed by PCAF is necessary for the execution of the muscle programme. Mol. Cell, 4, 725–734. [DOI] [PubMed] [Google Scholar]
- Schiltz R.L. and Nakatani,Y. (2000) The PCAF acetylase complex as a potential tumor suppressor. Biochim. Biophys. Acta, 1470, M37–M53. [DOI] [PubMed] [Google Scholar]
- Schiltz R.L., Mizzen,C.A., Vassilev,A., Cook,R.G., Allis,C.D. and Nakatani,Y. (1999) Overlapping but distinct patterns of histone acetylation by the human coactivators p300 and PCAF within nucleosomal substrates. J. Biol. Chem., 274, 1189–1192. [DOI] [PubMed] [Google Scholar]
- Sellers W.R., Novitch,B.G., Miyake,S., Heith,A., Otterson,G.A., Kaye,F.J., Lassar,A.B. and Kaelin,W.G. (1998) Stable binding to E2F is not required for the retinoblastoma protein to activate transcription, promote differentiation and suppress tumor cell growth. Genes Dev., 12, 95–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer T.E. et al. (1997) Steroid receptor coactivator-1 is a histone acetyltransferase. Nature, 389, 194–198. [DOI] [PubMed] [Google Scholar]
- Steinbac O.C., Wolffe,A.P. and Rupp,R.A. (2000) Histone deacetylase activity is required for the induction of the MyoD muscle cell lineage in Xenopus. Biol. Chem., 381, 1013–1016. [DOI] [PubMed] [Google Scholar]
- Trievel R.C., Li,F.Y. and Marmorstein,R. (2000) Application of a fluorescent histone acetyltransferase assay to probe the substrate specificity of the human p300/CBP-associated factor. Anal. Biochem., 287, 319–328. [DOI] [PubMed] [Google Scholar]
- Utley R.T., Ikeda,K., Grant,P.A., Cote,J., Steger,D.J., Eberharter,A., John,S. and Workman,J.L. (1998) Transcriptional activators direct histone acetyltransferase complexes to nucleosomes. Nature, 394, 498–502. [DOI] [PubMed] [Google Scholar]
- Venuti J.M., Morris,J.H., Vivian,J.L., Olson,E.N. and Klein,W.H. (1995) Myogenin is required for late but not early aspects of myogenesis during mouse development. J. Cell Biol., 128, 563–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub H. (1994) The MyoD family and myogenesis: redundancy, networks and thresholds. Cell, 75, 1241–1244. [DOI] [PubMed] [Google Scholar]
- Xu W., Edmondson,D.G., Evrard,Y.A., Wakamiya,M., Behringer,R.R. and Roth,S.Y. (2000) Loss of gcn5l2 leads to increased apoptosis and mesodermal defects during mouse development. Nature Genet., 26, 229–232. [DOI] [PubMed] [Google Scholar]
- Yamauchi T., Yamauchi,J., Kuwata,T., Tamura,T., Yamashita,T., Bae,N., Westphal,H., Ozato,K. and Nakatani,Y. (2000) Distinct but overlapping roles of histone acetylase PCAF and of the closely related PCAF-B/GCN5 in mouse embryogenesis. Proc. Natl Acad. Sci. USA, 97, 11303–11306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X.J., Ogryzko,V.V., Nishikawa,J., Howard,B.H. and Nakatani,Y. (1996) A p300/CBP-associated factor that competes with the adenoviral oncoprotein E1A. Nature, 382, 319–324. [DOI] [PubMed] [Google Scholar]
- Yao T.P. et al. (1998) Gene dosage-dependent embryonic development and proliferation defects in mice lacking the transcriptional integrator p300. Cell, 93, 361–372. [DOI] [PubMed] [Google Scholar]
- Yuan W., Condorelli,G., Caruso,M., Felsani,A. and Giordano,A. (1996) Human p300 protein is a coactivator for the transcription factor MyoD. J. Biol. Chem., 271, 9009–9013. [DOI] [PubMed] [Google Scholar]