Abstract
Calreticulin and calnexin are Ca2+-binding proteins with chaperone activity in the endoplasmic reticulum. These proteins have been eliminated by gene replacement in Dictyostelium, the only microorganism known to harbor both proteins; family members in Dictyostelium are located at the base of phylogenetic trees. A dramatic decline in the rate of phagocytosis was observed in double mutants lacking calreticulin and calnexin, whereas only mild changes occurred in single mutants. Dictyostelium cells are professional phagocytes, capable of internalizing particles by a sequence of activities: adhesion of the particle to the cell surface, actin-dependent outgrowth of a phagocytic cup, and separation of the phagosome from the plasma membrane. In the double-null mutants, particles still adhered to the cell surface, but the outgrowth of phagocytic cups was compromised. Green fluorescent protein-tagged calreticulin and calnexin, expressed in wild-type cells, revealed a direct link of the endoplasmic reticulum to the phagocytic cup enclosing a particle, such that the Ca2+ storage capacity of calreticulin and calnexin might directly modulate activities of the actin system during particle uptake.
Keywords: calnexin/calreticulin/Dictyostelium/endoplasmic reticulum/phagocytosis
Introduction
Calreticulin and calnexin are ubiquitous Ca2+ storage proteins in the endoplasmic reticulum (ER). In addition, these proteins act as chaperones preferentially directed toward glycans N-glycosidically linked to a protein close to its N-terminus (Molinari and Helenius, 2000; for reviews see Helenius et al., 1997; Trombetta and Helenius, 1998; High et al., 2000; Ellgaard and Helenius, 2001). Calreticulin is an ER luminal protein supplied with a K(H)DEL recognition signal for retrieval. Calnexin is an ER-specific type I transmembrane protein. Calreticulin has also been implicated in a variety of functions outside of the ER, ranging from cell adhesion, integrin-dependent Ca2+ signaling and steroid-sensitive gene expression (Michalak, 1996; Michalak et al., 1999; Corbett and Michalak, 2000; Johnson et al., 2001) to nuclear export (Holaska et al., 2001).
In yeasts and mice, the roles of calreticulin and calnexin in vivo have been analyzed using gene disruption. Inactiv ation of the single calnexin gene CNE1 in Saccharomyces cerevisiae causes no gross defects in growth, but leads to enhanced secretion of immature glycoproteins, indicating a function of CNE1 in the ER quality control apparatus (Parlati et al., 1995a; Arima et al., 1998). The Schizo saccharomyces pombe homolog of calnexin is essential for viability (Jannatipour and Rokeach, 1995; Parlati et al., 1995b). Calreticulin-deficient mice are embryonic lethal due to changes in cell adhesiveness and impairment of cardiac development and function (Coppolino et al., 1997; Mesaeli et al., 1999; Rauch et al., 2000). In the present paper we investigate calreticulin and calnexin in Dictyo stelium discoideum, the only microorganism known to contain both these proteins. The Dictyostelium calreticulin and calnexin sequences indicate basal positions of these proteins in the eukaryote phylogenetic tree. This is of interest since the Dictyostelium proteins may have maintained primary functions not modified to meet the special requirements of organ and tissue organization in higher eukaryotes (Johnson et al., 2001).
Here we focus on the role of calreticulin and calnexin in phagocytosis. The sequence of events involved in particle uptake by Dictyostelium cells resembles that of professional phagocytes in vertebrates. Phagocytosis begins with adhesion of a particle to the phagocyte surface, followed by transmembrane signal transduction and restructuring of the cortical actin network in line with phagocytic cup formation (see the review by May and Machesky, 2001). In Dictyostelium, two types of receptors mediate the adhesion of particles: a pair of integral membrane proteins, also represented in humans (Cornillon et al., 2000), and lectins with a preference for d-glucose as the terminal sugar of bacterial liposaccharides (Vogel et al., 1980). Signal transduction to the actin system requires a heterotrimeric G-protein (Peracino et al., 1998). As in mammalian cells, the IP3 kinase pathway is involved in Dictyostelium phagocytosis (Zhou et al., 1998; Cardelli, 2001), although its major role is in phagosome fusion rather than particle uptake (Rupper et al., 2001). An immediate response to particle attachment is the recruitment of the PH domain of CRAC, a protein also implicated in chemotactic signal transduction and receptor-mediated adenylate cyclase activation (Parent et al., 1998).
Both chemotactic stimulation and particle attachment induce the polymerization of actin at the site of stimulation, together with the recruitment of a number of actin-binding proteins from the cytoplasm. These proteins include coronin (Maniak et al., 1995), cofilin (Aizawa et al., 1995), Aip1 (Konzok et al., 1999) and the Arp2/3 complex (Insall et al., 2001), all of which are common to Dictyostelium and mammalian cells. A special function has been attributed to myosin VII, a motor protein with two FERM domains per subunit which accumulates at the border of phagocytic cups. Myosin VII promotes phagocytosis by enhancing the adhesiveness of the Dicyto stelium cell surface (Tuxworth et al., 2001). In the present paper we show that calreticulin and calnexin are not essential for the adhesion of particles, but are important for the subsequent step of particle uptake: the outgrowth of a phagocytic cup enveloping the particle surface.
Results
Positions of Dictyostelium calnexin and calreticulin in the phylogenetic tree
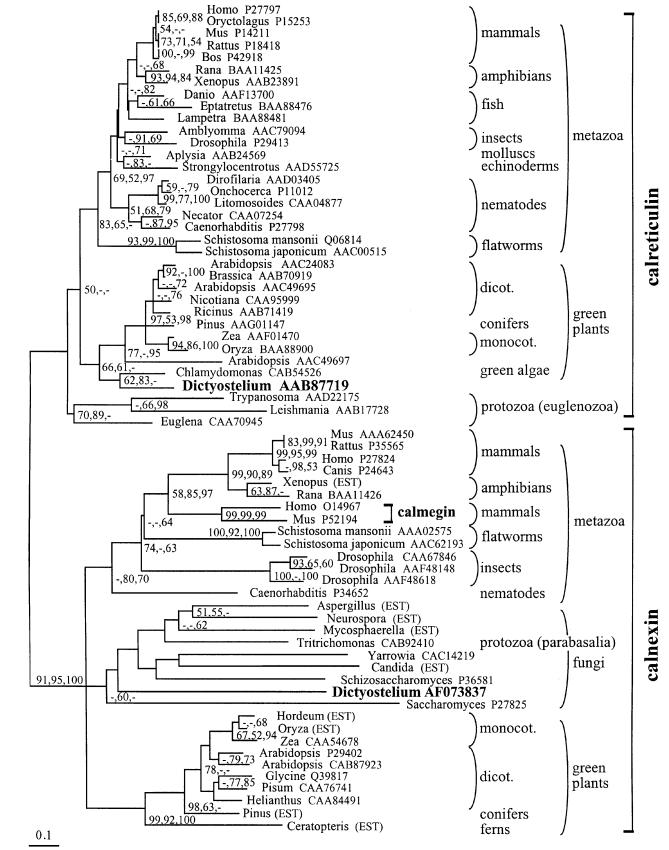
Although similar, calnexin and calreticulin sequences can be distinguished readily by their unique C-terminal ends. Calnexins are type I transmembrane proteins with a cytoplasmic C-terminal tail, whereas calreticulins are soluble proteins supplied with a C-terminal K(H)DEL signal for retention in the lumen of the ER. Phylogenetic calculations using distance, maximum parsimony and maximum likelihood methods uniformly yield distinct monophyletic clades for calnexin and calreticulin with high bootstrap support (Figure 1). Even partial sequences that do not include the C-terminal regions or the two repeated motifs A and B in the central region of the sequences can be assigned unambiguously to one of the two families. This clear separation between the two families, even in the most deeply branching eukaryotes, suggests that calnexins and calreticulins already existed as different proteins in the last common ancestor of present-day eukaryotes. Calmegin, a paralog of calnexin, is found at the root of vertebrate calnexins, suggesting that it evolved by duplication and divergence in early vertebrates.
Fig. 1. Distance-based phylogenetic tree of calnexins and calreticulins, with highlighted positions of the Dictyostelium proteins. The tree was computed with the Protdist, Seqboot and Neighbor modules of Phylip, and the alignment underlying the phylogenetic calculations was obtained as described in Materials and methods. Bootstrap support exceeding 50% of replicates by distance (Clustal; 1000 replicates), maximum likelihood (Puzzle; 1000 replicates) and maximum parsimony (PAUP; 100 replicates) methods is shown in that order, next to the corresponding node. The tree shown here was selected from 100 Seqboot replicates for the largest number of nodes supported by bootstrap analysis in Clustal, Puzzle and PAUP. Database accession codes for the non-redundant protein database at NCBI (http://www.ncbi.nlm.nih.gov/Entrez/) are shown next to each sequence, except where the sequence was reconstructed from ESTs.
So far, only Metazoa and Viridiplantae consistently contain both calreticulin and calnexin. Dictyostelium is the first microorganism known to contain both proteins. Dictyostelium calreticulin was found, with moderate bootstrap support, to be phylogenetically close to plant sequences (Figure 1). Dictyostelium calnexin, on the other hand, was excluded from the plant calnexin clade with high bootstrap support, and instead was included with marginal support in the fungal clade. The fungal clade is poorly resolved and contains divergent, deeply branching sequences and members that may not be closely related to other ones (such as the parabasalian protozoan Tritricho monas). The significance of this assignment is therefore questionable.
Cellular localization of endogenous and green fluorescent protein (GFP)-tagged calnexin and calreticulin
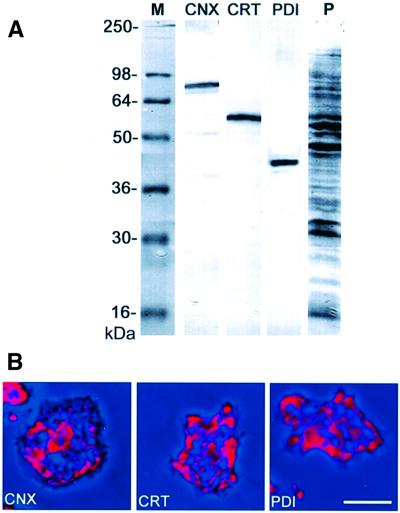
Antibodies raised against calreticulin or the cytoplasmic C-terminal region of calnexin, which is missing in calreticulin, were checked in western blots for monospecificity (Figure 2A). When these antibodies were used for immunofluorescence labeling, they recognized exclusively the reticulate structure of the ER, similar to an antibody against an inherent ER marker protein, disulfide isomerase (PDI), which was used as a reference (Figure 2B).
Fig. 2. Immunolabeling of endogenous calreticulin and calnexin in Dictyostelium cells. (A) Specificity of mAbs used as ER markers, assayed in western blots of total cellular proteins. CNX, calnexin labeled with mAb 270-390-2; CRT, calreticulin labeled with mAb 252-234-2; PDI, protein disulfide isomerase labeled with mAb 221-135-1. The last lane (P) shows a parallel lane stained for proteins with Ponceau S. (B) Immunofluorescence labeling of fixed cells using the antibodies shown in (A). Immunofluorescence in red is superimposed on phase-contrast images in dark blue. All three antibodies label the reticulate structure of the ER. The perinuclear layer in the center of the cell is most prominently labeled with the calnexin antibody. Bar in (B), 10 µm.
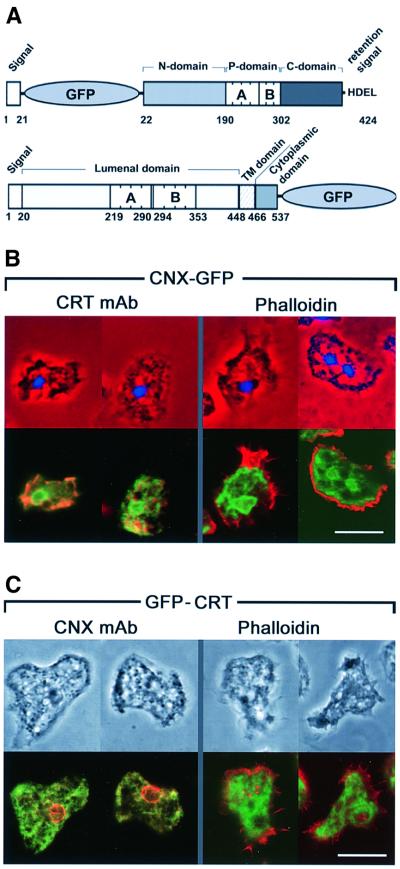
For visualizing the proteins in live cells, GFP fusions were constructed. In calreticulin, the GFP tag was placed behind the N-terminal leader sequence to keep the C-terminal HDEL retention signal unaffected. In calnexin, the GFP was fused to the C-terminal end of the cytoplasmic tail (Figure 3A). Both GFP-tagged proteins localized to the ER (Figure 3B and C), in accord with antibody labeling of the endogenous calreticulin or calnexin. Calnexin–GFP or calnexin antibody provided the most distinct label of the entire ER, in particular accentuating the outer nuclear membrane (Figures 2B, 3B and C). Perinuclear fluorescence was less prominent when calreticulin was labeled either by a GFP tag or with antibody. All the markers used visualized the peripheral portions of the ER, which extended into lobes immediately beneath the cortical layer of actin filaments (Figure 3B and C).
Fig. 3. GFP fusion constructs of calreticulin and calnexin. (A) Dictyostelium calreticulin (top) shows the typical tripartite structure comprising an N-domain, a P-domain consisting of A and B repeats, and a C-domain ending with a HDEL retention signal. The GFP moiety has been placed behind the signal sequence of calreticulin. In calnexin (bottom), a signal sequence is followed by the luminal domain including a cluster of A and B repeats, which is separated by a transmembrane (TM) domain from the cytoplasmic domain. GFP is joined to the C-terminal end of this domain. (B) Cells expressing calnexin–GFP labeled with calreticulin-specific antibody or with phalloidin for F-actin. Upper panels show nuclei stained with 4′,6-diamidino-2-phenylindole in blue, superimposed on phase-contrast images in red. Lower panels depict the same cells with the fluorescence of calnexin–GFP in green, and antibody or phalloidin label in red. Areas of merging labels appear in yellow to brownish color. (C) Cells expressing GFP–calreticulin labeled with calnexin-specific antibody or with phalloidin. Top panels show phase-contrast images; lower panels show calnexin–GFP fluorescence in green, and the antibody or phalloidin label in red. Bars in (B) and (C), 10 µm.
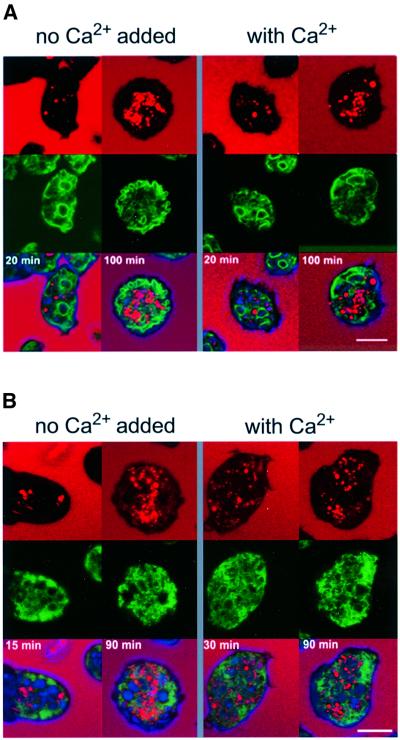
To establish that the GFP-tagged proteins are efficiently retrieved to the ER, cells expressing either calnexin–GFP or GFP–calreticulin were co-labeled with tetramethylrhodamine isothiocyanate (TRITC)–dextran, a fluid-phase marker for endosomes that is internalized by macropinocytosis (Hacker et al., 1997). Careful analysis of the original images on computer screens revealed that none of the GFP-tagged proteins co-localized with this marker at early or late stages of the endosomal pathway, as illustrated in Figure 4. This exclusion from the endosomal pathway remained unchanged in cells that were stressed by exposure to 10 mM Ca2+. Within the limits of detection we did not observe any fluorescence of GFP–calreticulin in the cytoplasm or on the cell surface, which is consistent with the undetectability of endogenous calreticulin at these locations by immunofluorescence labeling (Figure 2B).
Fig. 4. Retention of calnexin–GFP and GFP–calreticulin in the ER, shown in live Dictyostelium cells labeled with the endosome marker TRITC–dextran, which is taken up by macropinocytosis. (A) Cells expressing calnexin–GFP. (B) Cells expressing GFP–calreticulin. Upper panels show the fluorescence of TRITC–dextran in red. In the confocal images, cell bodies appear black with the exception of endosomes loaded with the marker. In the middle panels, GFP fluorescences are shown in green. In the lower panels, both images are superimposed on each other. Cells were incubated for the times indicated with TRITC–dextran. Until 30 min after internalization, endosomes are in the acidic phase (Aubry et al., 1997). After ≥90 min, endosomes of all stages until exocytosis should be loaded with the marker. Cells were incubated either in 10 mM TRICIN–HCl buffer pH 7.0 without Ca2+ or with 10 mM Ca2+. Bars, 10 µm.
Calnexin and calreticulin single and double knock-out mutants
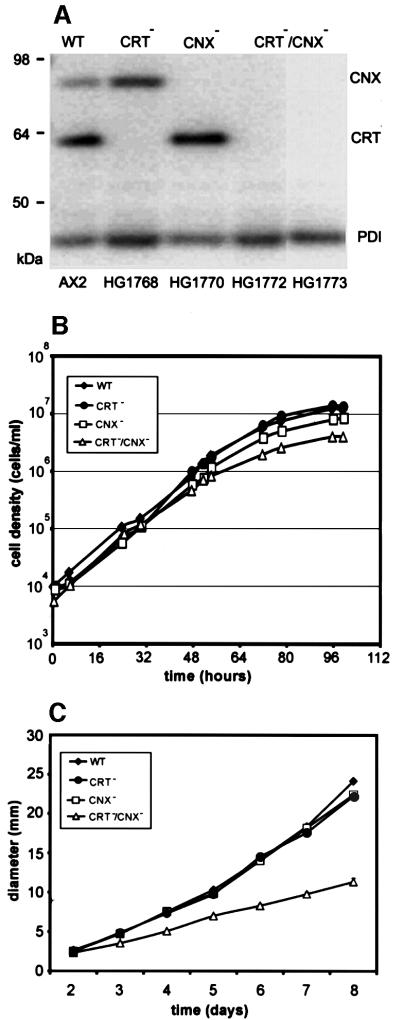
To assess the function of calreticulin and calnexin in Dictyostelium cells, we eliminated these proteins individually or together by interruption of the corresponding genes. Correct integration of the gene replacement vectors was established by Southern blotting of genomic DNA. (Details of the gene targeting strategies are outlined in Materials and methods.) Western analysis confirmed the absence of calreticulin or calnexin in the single knock-out mutants, and of both proteins in the double mutants CRT–/CNX– (Figure 5A). The expression of PDI, another luminal ER-resident chaperone, was essentially unchanged in single as well as double knock-out strains.
Fig. 5. Wild-type AX2, single and double mutants, and their growth in liquid medium or on agar plates with bacteria. (A) Western blot of wild-type (WT), calreticulin-null (CRT–), calnexin-null (CNX–) and two independent CRT/CNX double-null mutants (CRT–/CNX–). Strain designations are given at the bottom. Equal amounts of protein were applied per lane, and the blot was probed for the presence of calreticulin, calnexin and PDI using a combination of calreticulin-, calnexin- and PDI-specific mAbs. (B) Semi-logarithmic plot of axenic growth with liquid nutrient medium in shaken suspension. Data are from one experiment. (C) Growth on bacterial lawns on agar plates measured as the increase in plaque diameter over time.
In liquid nutrient medium, the double mutants had a moderately reduced growth rate, as shown in Figure 5B. On average over four experiments, the mean generation times during exponential growth were 8.3 h for wild type, 9.2 h for calreticulin-null cells, 10.7 h for calnexin-null cells and 12.9 h for double-null cells. When cultivated with bacteria on agar plates, the CRT/CNX double-null mutant grew more slowly than wild type (Figure 5C). In contrast, the single knock-out mutants did not differ significantly from wild type, indicating that calreticulin and calnexin overlap in their capability for supporting growth under the conditions used.
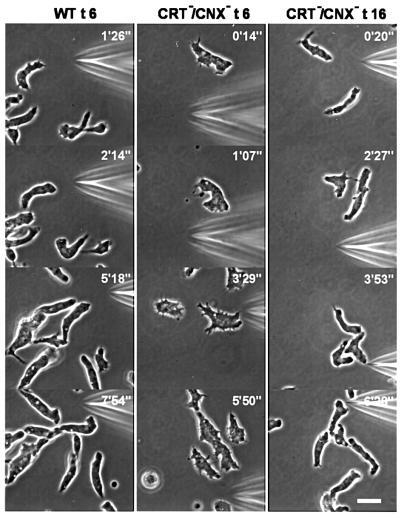
The double knock-out mutants were not only viable, but also capable of undergoing development. Starving cells aggregated into streams radially oriented towards aggregation centers, indicating that cAMP signals were produced and chemotactic responses were elicited. This was confirmed by stimulating double-mutant cells through a micropipette with cyclic AMP (Figure 6). The mutant cells were responsive to the chemoattractant after 6 h of development, like wild-type cells, but needed longer before they were maximally elongated. In the elongated cells, the front region often appeared broader than in wild-type cells, but apart from this slight alteration, the mutant cells responded normally to the attractant. Like wild-type cells, they often accommodated to changing directions of a gradient by protruding new leading edges from the side of the cell body or by converting a tail into a front (Figure 6). These results show that neither the activity of cAMP receptors on the cell surface nor the translation of an external attractant gradient into a motility response depended on the presence of the two Ca2+-binding chaperones in the ER.
Fig. 6. Chemotaxis of CRT/CNX double-null mutant cells in comparison to wild type (WT). Cells were starved either for 6 h (t6) or 16 h (t16) and subsequently stimulated with cAMP through a micropipette. Orientation of cells in gradients of the chemoattractant is seen in all examples of wild-type and mutant cells. In the mutants, the typical elongation of aggregating cells needed a longer period of starvation than 6 h, which is sufficient for wild type. Numbers indicate times after positioning of the micropipette. Bar, 10 µm.
A severe defect of phagocytosis in CRT/CNX double-null mutants
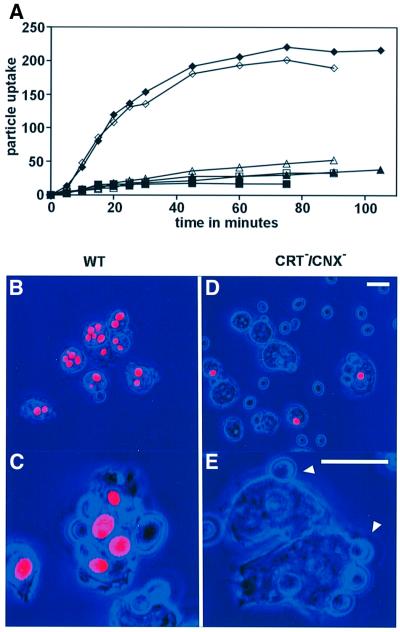
The slower growth rate of double-mutant cells on agar plates, as shown in Figure 5C, might be due to a role of calreticulin and calnexin in phagocytosis, endosome processing or cell motility. In order to single out an alteration in phagocytosis, we measured the initial uptake rate of yeast particles. The internalization of fluorescent yeast particles by wild-type and double-null mutant cells was compared in shaken suspension by quenching the fluorescence of free or cell surface-attached particles with Trypan blue (Hed, 1986; Maniak et al., 1995). The uptake of particles into wild-type cells was almost linear for the first 20 min, and reached a steady-state value after ∼1 h due to equal endo- and exocytosis rates of yeast particles.
As shown in Figure 7A, the rate of particle uptake was dramatically reduced in the double-null cells to <10% of the wild-type rate. This impairment of particle uptake was confirmed by microscopic observation. After 45 min of incubation, most of the wild-type cells contained several ingested yeast particles (Figure 7B and C), while in the mutant cells internalized particles were only found occasionally (Figure 7D and E). Excess yeast particles were left in the medium, and many were attached to the cell surfaces. Often, shallow rudiments of cups were observed at sites of adherent yeast particles (arrowheads in Figure 7E), suggesting an arrest of phagocytosis at a stage preceding cup extension.
Fig. 7. Phagocytosis of yeast particles in wild-type and CRT/CNX double-null mutants. (A) Internalization of particles over time in two experiments (open and closed symbols) by wild-type AX2 cells (diamonds) and cells of two independent double mutants HG1772 (squares) and HG1773 (triangles). Particle uptake is plotted in arbitrary fluorescence units reflecting the number of internalized yeast particles. (B–E) Internalized particles distinguished by red fluorescence from adherent particles whose fluorescence is quenched. Phase-contrast images are colored in blue. Wild-type cells (B and C) show numerous ingested particles. In double-null cells (D and E), only few internalized particles are found. Arrowheads in (E) point to adherent particles not enveloped by a phagocytic cup. Bars for the upper and lower panels, 10 µm.
Outgrowth of phagocytic cups is rate limiting in CRT/CNX double mutants
A defect in phagocytosis can be due to impairment of particle adhesion, of phagocytic cup extension, or of the final closure and separation of the phagosome from the plasma membrane. The slightly concave shape of the contact region between the double-mutant cells and yeast particles indicated that uptake is blocked at an early stage; therefore, adhesion or outgrowth might be impaired in the mutants. To distinguish between these possibilities, we inhibited particle uptake either by latrunculin A to inactivate the actin system or by cold treatment, and determined the number of adherent yeast particles per Dictyostelium cell.
Table I shows the number of yeast particles sticking to wild-type and mutant Dictyostelium cells in a shaken suspension with 10 µM latrunculin A. Conditions were the same as for the measurement of uptake as shown in Figure 7A, except that the excess of yeast particles over Dictyostelium cells was reduced from six to three. The data reveal a moderate decline in adherence; less extreme however, than is necessary to explain the massive reduction in the rate of phagocytosis.
Table I. Adherence of yeast particles to Dictyostelium cells in the presence of 10 µM latrunculin to inactivate the actin system.
| Wild-type and mutant strains | Percentage of Dictyostelium cells decorated with these numbers of yeast particles |
Average no. of yeast particles per | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | Dictyostelium cell | |
| Wild type AX2 | 63.3 | 24.2 | 7.0 | 3.1 | 1.6 | 0.8 | 0.0 | 0.0 | 0.58 |
| CRT–/CNX– HG1772 | 69.8 | 22.1 | 3.5 | 4.7 | 0.0 | 0.0 | 0.0 | 0.0 | 0.43 |
| CRT–/CNX– HG1773 | 71.0 | 23.4 | 5.0 | 0.7 | 0.0 | 0.0 | 0.0 | 0.0 | 0.35 |
| Mean of CRT–/CNX– | 70.4 | 22.8 | 4.3 | 2.7 | 0.0 | 0.0 | 0.0 | 0.0 | 0.39 |
Inhibition of particle uptake by cold treatment was performed as described by Tuxworth et al. (2001). Again, particle adhesion was only moderately diminished in CRT/CNX-deficient mutants (Table II). This result contrasts with the strong suppression of particle adherence observed by Tuxworth et al. (2001) in myosin VII-null mutants, confirming that a step after particle adhesion is rate limiting in phagocytosis of CRT/CNX double-null mutants.
Table II. Adherence of yeast particles to Dictyostelium cells under conditions of suppressed uptake in the colda.
| Wild-type and mutant strains | Percentage of Dictyostelium cells decorated with these numbers of yeast particles | Average no. of yeast particles per | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | Dictyostelium cell | |
| Wild type AX2 | 62.4 | 24.8 | 8.0 | 3.2 | 0.8 | 0.5 | 0.2 | 0.2 | 0.56 |
| CRT–/CNX– HG1772 | 77.0 | 18.0 | 4.1 | 0.8 | 0.1 | 0.1 | 0.0 | 0.0 | 0.29 |
| CRT–/CNX– HG1773 | 64.8 | 25.5 | 7.3 | 1.7 | 0.3 | 0.5 | 0.0 | 0.0 | 0.48 |
| Mean of CRT–/CNX– | 71.0 | 22.0 | 5.7 | 1.3 | 0.2 | 0.3 | 0.0 | 0.0 | 0.39 |
aFor the method, see Tuxworth et al. (2001).
Connection of the ER to phagocytic cups
The impairment of phagocytosis in calreticulin- and calnexin-deficient mutants raises the question of the mechanism of action of the two ER proteins in particle uptake. We addressed this question by exploring whether the ER in Dictyostelium cells associates closely enough with phagocytic cups to exert a direct, local influence on cup extension.
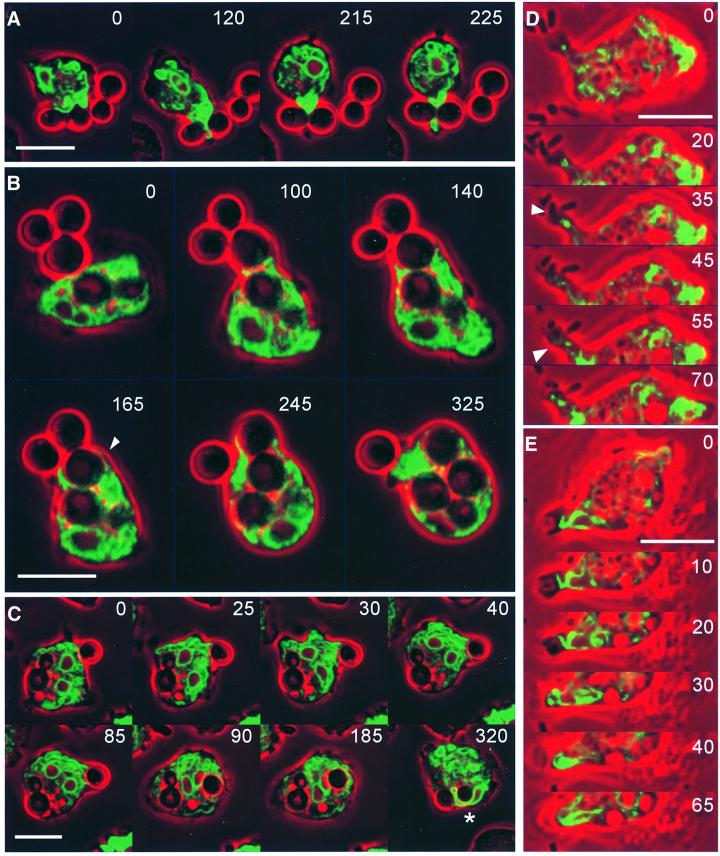
The phagocytic cup is a thin circular lamella containing actin filaments and actin-binding proteins sandwiched between two membranes, one surrounding the particle and the other forming the outer cup surface (Maniak et al., 1995). To visualize ER dynamics in connection with cup formation, we have recorded phagocytosis in a confocal time series of wild-type cells using either GFP–calreticulin or calnexin–GFP as fluorescent labels. With both markers, the ER was found to extend into outgrowing phagocytic cups.
Figure 8A–C illustrates ER association with phagocytic cups formed around yeast particles. As Dictyostelium cells prefer to embrace particles at sites of high curvature, they tend to protrude extensions into the cleft between two adjacent particles attached to the phagocyte (Insall et al., 2001). Figure 8A shows an ER tube invading this space. Evidence for extension of the luminal space of the ER into phagocytic cups is provided by Figure 8B, depicting a cell labeled with GFP–calreticulin. This cell takes up two yeast particles in tandem, enveloping each particle in a layer of the ER. The sequence of Figure 8C shows accumulation of ER membranes at the site of particle adhesion (0–25 s frames) followed by an overall orientation of cisternae towards the particle being engulfed (30–85 s frames). The phagosome remained enveloped by ER membranes for no longer than 30 s after internalization of the particle. Afterwards, contacts of the ER to the phagosome became transitory. Phagosomes that had been formed before recording of the cell commenced were not consistently associated with the ER, emphasizing that firm association occurs primarily at the onset of, and during, the uptake process.
Fig. 8. ER dynamics in phagocytosis. (A–C) Uptake of yeast particles by cells labeled with either calnexin–GFP (A and C) or GFP–calreticulin (B). The sequence in (A) shows the first engagement of a cell with a bipartite yeast particle. Initially, the ER forms a layer adjacent to the particle surface (0 s), and subsequently a hook around the cleft between the two halves of the particle (120 s). Strong accumulation of ER membranes at this cleft (215 s) is followed by the appearance in cross-section of an ER tube at the distal side of the particle (225 s). (B) Uptake of two yeast particles in tandem showing in optical section the extension of the ER along the particle surfaces. The arrowhead at 165 s points to a thin ER layer surrounding the particle that is taken up first. GFP fluorescence shown in green is superimposed on phase-contrast images in red. (C) Accumulation of the ER at a site of ongoing phagocytosis at the right edge of the cell. A previously internalized yeast cell with a bud located in the left half of the phagocyte shows no consistent association with ER membranes. The two nuclei of this cell are recognizable by their calnexin-enriched outer membrane; one of them is seen to be strongly deformed when squeezed between the yeast particles (asterisk in the last frame). (D and E) Phagocytosis of bacteria by cells labeled with either calnexin–GFP (D) or GFP–calreticulin (E). Both cells engulf bacteria at the tip of extensions that contain an ER core. In (D), a strand of the ER connecting the site of bacteria attachment with the cell body (arrowhead at 35 s) widens into a delicate bowl-shaped layer at the time of uptake (arrowhead at 55 s). In (E), the ER forms a layer on the bottom of the phagocytic cup (10 s) and turns into loops flanking the bacterium during its internalization (20–40 s). GFP fluorescence is shown in green, phase-contrast images are in red. Bars, 10 µm.
Association of the ER with sites of particle uptake was also observed when yeast particles were replaced with smaller bacteria. Typically, the bacteria attached to protrusions of Dictyostelium cells before being engulfed. Figure 8D and E shows two sequences of bacteria uptake: one for a cell labeled with calnexin–GFP, the other with GFP–calreticulin. In both sequences, an ER strand is seen to extend towards the phagocytic cup, with the ER forming a loop or a bowl-shaped terminus below the nascent phagosome.
Discussion
In this paper we present two findings: (i) that elimination of both calreticulin and calnexin by gene replacement causes a severe impairment of particle uptake; and (ii) that cisternae of the ER are drawn into the cup-shaped cell surface extensions that envelop a particle during its internalization. The first finding demonstrates that the two Ca2+-binding proteins are either directly or indirectly involved in phagocytosis. The second set of data points to a close association of the ER with the membrane of the incipient phagosome throughout the uptake process. This connection raises the possibility that the Ca2+-buffering capacity of calreticulin and calnexin in the ER directly influences the Ca2+ concentration in the slim cytoplasmic space of the phagocytic cup. Since a strong reduction in the rate of growth on bacteria was observed in double-null mutants and only a marginal effect was seen in single calreticulin- or calnexin-deficient mutants, the two proteins are thought, in this particular aspect, to mutually complement each other.
An effect of calreticulin and calnexin from within the ER, extending into the microenvironment of the phagocytic cup, appears more likely than an exit of either protein from the ER. We have found neither calreticulin nor calnexin in the endosomes, in the cytoplasm, or on the surface of Dictyostelium cells. For localization, the use of GFP-tagged calreticulin and calnexin in live cells proved to be superior to immunofluorescence detection of the proteins in fixed cells. As reported for other cells (Prinz et al., 2000), the ER tends to become fragmented in Dictyostelium under the mild fixation conditions that preserve immunoreactivity.
The correct localization of GFP–calreticulin was favored by placement of the GFP moiety at the N-terminus of the protein, so as not to disturb the HDEL retention signal at the C-terminus. The correct localization of calnexin blocked at its cytoplasmic tail by GFP was not a certainty, but fortunately this fusion protein proved to be a superb means of visualizing the dynamics of the highly convoluted ER membrane system in vivo.
The demonstration of calreticulin and ER membranes in phagocytic cups is in accord with previous results for mammalian phagocytes. In neutrophils, SERCA Ca2+-ATPase and calreticulin accumulate at the phagosome, the Ca2+ store giving rise to a cytosolic Ca2+ increase localized to the phagocytic cup (Stendahl et al., 1994). Proteome analysis established that calreticulin and calnexin are constituents of phagosomes in a mouse macrophage-like cell line (Garin et al., 2001). Our studies using GFP fusion proteins in Dictyostelium indicate that association of the ER with the phagocytic cup is transient and readily dissolved after uptake of a particle. We have no evidence for the fusion of ER and phagosome membranes, since neither calreticulin nor calnexin could be detected at the phagosome membrane after the ER became detached.
In neutrophil granulocytes, an increase in the cytosolic Ca2+ concentration is crucial for phagocytosis (Lundquist-Gustafsson et al., 2000), and Ca2+ is also required in Dictyostelium (Yuan et al., 2001). In the light of this general requirement of Ca2+ for phagocytosis, Ca2+ storage appears to be the relevant activity of calreticulin and calnexin in phagocytic cup extension. However, since calreticulin and calnexin also act as chaperones, we cannot rule out that impairment of protein folding contributes to the deficiency in phagocytosis. On the other hand, cellular activities that strictly depend on the folding of membrane proteins persisted in the CRT/CNX double-null mutants. Particle adhesion mediated by transmembrane proteins (Cornillon et al., 2000) was only moderately affected, and was definitely not the rate-limiting step of phagocytosis in these mutants (Tables I and II as compared with Figure 7A). Overlapping activities of Bip and other ER-resident chaperones might partially compensate for the loss of chaperone activity in the double mutants. Furthermore, neither calreticulin nor calnexin proved to be essential for chemotaxis, which depends on cell surface receptors that must be folded within the ER or Golgi apparatus. The maintenance of chemotactic responsiveness in cells lacking calreticulin and calnexin is consistent with the persistence of chemotaxis in mutant cells where cytoplasmic Ca2+ changes in response to chemoattractant had been suppressed by eliminating a member of the InsP3/RyR receptor family of Ca2+ channels (Traynor et al., 2000). Together, these data show that phagocytosis is unusual in its critical dependence on calreticulin and calnexin.
The finding that ER is rapidly drawn into the outgrowing cup raises the question as to the mechanism of this organelle movement. Candidate proteins for anchoring ER cisternae are coronin and Aip1, both recruited to the border of phagocytic cups and both shown by gene disruption to be important for the efficiency of phagocytosis (Maniak et al., 1995; Konzok et al., 1999). As members of the WD40 repeat family, coronin and Aip1 are capable of undergoing multiple protein–protein interactions, and their conservation in metazoans is consistent with a general function in phagocytosis (Ferrari et al., 1999). However, the reduction by 60–70% in the rate of particle uptake observed in cells lacking coronin or Aip1 (Maniak et al., 1995; Konzok et al., 1999) is less severe than the impairment of phagocytosis in CRT/CNX double-null mutants, suggesting the presence of additional players in this process.
Deficiencies in phagocytosis as severe as those in CRT/CNX double-null mutants have previously been found in Dictyostelium mutants lacking talin (Niewöhner et al., 1997) or myosin VII (Tuxworth et al., 2001). In both cases, the defect was traced to a loss of adhesiveness of the Dictyostelium cell surface, which strongly reduced the probability of a particle persisting on the phagocyte surface long enough to be engulfed. Accordingly, it has been shown for myosin VII-null mutants that in the few examples where uptake progressed, the phagocytic cup protruded around the particle at a normal rate.
The CRT/CNX double-null mutants differed from myosin VII-null mutants by the failure of phagocytic cup extension around adherent particles. The difference between myosin VII-null and CRT/CNX double-null mutants is obvious if one compares the number of adherent yeast particles. Under comparable conditions, the average number of yeast particles per Dictyostelium cell declined from 0.7 in wild-type to 0.05 in myosin VII- null cells (Tuxworth et al., 2001), but only from 0.6 to 0.4 in CRT/CNX double-null cells (Table II). The effect of eliminating calreticulin and calnexin in Dictyostelium seems to be related to calreticulin-dependent changes in retinal pigment epithelial cells. If these cells underexpress calreticulin, they ‘stick’ to a substratum by close contacts, but only if they overexpress calreticulin do they ‘grip’ the substratum by organizing the actin system into focal contacts (Fadel et al., 1999).
In macrophages (Swanson and Baer, 1995) as well as in Dictyostelium cells (Maniak et al., 1995), a zipper rather than a trigger mechanism for phagocytic cup extension has been proposed. This implies that extension of the cup is continuously controlled and can be reversed at any step prior to terminal membrane fusion. Our data implicating calreticulin and calnexin in phagocytic cup extension, and showing that the ER is tightly associated with the cup, suggest that these proteins might be part of the system that controls the opening and closing of the zipper.
Materials and methods
Dictyostelium discoideum sequences of calreticulin and calnexin were obtained by strains AX2 or AX3 cDNA and genomic cloning. Calnexin and calreticulin sequences of other species were identified in the non-redundant database at NCBI using PSI-Blast (http://www.ncbi.nlm.nih.gov/blast/psiblast.cgi) and retrieved using Entrez (http://www.ncbi.nlm.nih.gov/Entrez/). In several cases, such as the calreticulins from Trypanosoma cruzi (AAD22175) and Leishmania donovani (AAB17728), the sequences contained internal frame-shifts, which were judged to be sequencing errors. The frame-shifted regions were corrected and the junctions were encoded as ‘missing data’. Where possible, expressed sequence tag (EST) sequence data from the ‘EST other’ database at NCBI were used to complete the phylogenetic spectrum, particularly among plant and fungal calnexins.
Calnexins (including calmegin) and calreticulins were aligned separately in MACAW (Schuler et al., 1991), and gapped or unalignable regions were eliminated. The two alignments were then merged in Clustal (Thompson et al., 1994), and gapped or unalignable regions were again eliminated. The final alignment included regions corresponding to residues 21–29, 34–40, 45–53, 62–81, 83–87, 89–118, 122–138, 140–159, 162–237, 239–308 and 312–348 in Dictyostelium calreticulin, and residues 26–34, 41–47, 49–57, 65–88, 92–123, 129–145, 147–159, 166–172, 174–212, 215–252, 274–304, 323–360 and 364–400 in Dictyostelium calnexin. The alignment was used as an input file to the phylogeny programs Phylip 3.572 (Protdist, Seqboot) (Felsenstein, 1993), Puzzle 3.1 (Strimmer and von Haeseler, 1996) and PAUP 3.1.1 (Swofford, 1993), which were used in default settings.
Cell strains, growth and transformation
Dictyostelium discoideum AX2 wild-type or mutant cells were cultivated in nutrient medium (Watts and Ashworth, 1970) either in shaken suspension or submerged on Petri dishes at 23°C. To induce starvation, cells were washed twice in 17 mM K-Na-phosphate buffer (PB) pH 6.0, and shaken at a density of 107 cells/ml in the buffer. For cells grown on a bacterial food source, Klebsiella aerogenes was spread on SM agar plates, and cells of D.discoideum were inoculated onto the surface with a toothpick.
Transformation of 5 × 107 cells/ml was performed with 20–25 µg of DNA either by the calcium phosphate method or by electroporation using a Bio-Rad gene pulser at 0.8–0.9 V, 3 µF with 4 mm cuvettes. After 24 h, 10 µg/ml blasticidin-S (ICN) or 20 µg/ml G418 (Sigma) was added. After 10–12 days of antibiotic selection, transformants were cloned on lawns of Klebsiella aerogenes growing on SM agar and identified by the colony blot technique (Wallraff and Gerisch, 1991) using mouse monoclonal antibodies (mAbs) specific for calreticulin (mAb 252-234-3) or calnexin (a mixture of mAbs 270-317-1, 270-390-2 and 270-463-2).
In this study, the following independent knock-out strains were used: HG1768 and HG1769 (crtA–); HG1770 and HG1771 (cnxA–); HG1772 and HG1773 (crtA–/cnxA–). To generate strain HG1767 expressing GFP–calreticulin, AX2 cells were transformed with a vector encoding act15/crtA(1-21)-gfp(S65T)-crtA(22-424). Strain HG1738 expressing calnexin–GFP was generated in AX2 using a vector encoding act15/cnxA-RSSSKLK–gfp(S65T).
Vector construction and Southern analysis
For single knock-outs, the blasticidin resistance cassette was introduced into either the calreticulin or the calnexin gene by homologous recombination. For construction of the calreticulin targeting vector, 5′ and 3′ fragments encompassing nucleotides 162–441 and 467–1269 of the calreticulin coding sequence were generated by PCR. The 3′ fragment was cleaved by BamHI and EcoRI, and the 5′ fragment by HindIII, and both fragments flanking the blasticidin cassette were cloned into pBsr2 (Sutoh, 1993). The construct was excised from the vector using PstI and EcoRI, dephosphorylated and used for transformation of AX2 wild-type cells. The same strategy was employed for construction of a calnexin gene replacement vector using nucleotides 54–430 and 475–1611 of the calnexin coding sequence separated by the blasticidin resistance cassette. The CRT/CNX double mutant was obtained by transforming the calreticulin-null mutant HG1768 with a gene replacement construct carrying the G418 resistance cassette with fragments encompassing nucleotides 134–438 and 475–1611 of the calnexin coding region.
Southern blotting was performed as described (Kreitmeier et al., 1995). Blots were hybridized under high stringency conditions and probed with PCR-generated sequences to confirm the integration of the resistance cassette into the respective gene.
Immunofluorescence labeling
mAbs against calreticulin and calnexin were obtained by immunizing BALB/c mice with recombinant calreticulin or a calnexin fragment, together with Freund’s adjuvant or Alugel S (Serva) and pertussis toxin (Sigma). Spleen cells were fused with PAIB3Ag8I myeloma cells. Specificity of the antibodies was determined by western blotting of proteins resolved by 10% SDS–PAGE. PDI was labeled with mAb 221-135-1 (Monnat et al., 1997). Primary antibodies were detected with phosphatase-coupled anti-mouse IgG (Dianova).
For immunofluorescence labeling, AX2 wild-type or mutant cells allowed to settle onto glass coverslips for 30 min were fixed with picric acid/formaldehyde for 20 min and post-fixed with 70% ethanol. Subsequently, the cells were processed for immunolabeling according to Humbel and Biegelmann (1992). Calreticulin was detected with mAb 251-67-4, calnexin was labeled with mAb 270-390-2, and Cy2-conjugated (BioTrend Chemicals) or TRITC-conjugated (Jackson ImmunoResearch) goat anti-mouse IgG was employed to detect primary antibodies. F-actin was labeled with TRITC-conjugated phalloidin (Sigma Chemical Co.). Confocal immunofluorescence microscopy was performed with a Zeiss LSM 410 equipped with a 100×/1.3 Plan-Neofluar objective.
GFP fusion proteins and live imaging
The GFP–calreticulin vector was constructed by cloning the sequence encoding the red-shifted GFP S65T (Heim and Tsien, 1996) in-frame between the sequences encoding the leader and the processed calreticulin. The construct was expressed under the control of an actin-15 promoter in pDEXRH (Faix et al., 1992). For expression of calnexin–GFP, S65T GFP was fused to the C-terminus of calnexin with a spacer (RSSSKLK) in between. Clones HG1772 expressing GFP–calreticulin and HG1738 expressing calnexin–GFP were obtained by transforming AX2 wild-type cells.
For studying the localization of GFP fusion proteins, cells were washed twice in PB or 10 mM TRICIN–HCl pH 7.0 and transferred to a glass coverslip in an open chamber to record GFP fluorescence in parallel with phase-contrast images, using an inverted Zeiss LSM 410 confocal microscope equipped with a 488 nm argon ion laser and a 100×/1.3 Plan-Neofluar objective. For recording ER association with phagocytic cups, cells were fed living S.cerevisiae or K.aerogenes. Uptake of TRITC–dextran MW 70 000 (Molecular Probes) into endosomes was recorded simultaneously with GFP fluorescence using the He/Ne laser line 543 nm as described (Schneider et al., 2000).
Phagocytosis and chemotaxis assays
Phagocytosis assays using TRITC-labeled, heat-killed yeast particles in shaken suspension were carried out essentially as described by Maniak et al. (1995). Before use in uptake and adhesion experiments, the yeast suspension was sonicated and filtered through gauze of 8 µm mesh width, so that only single particles remained present. To quantify uptake, we added 1.2 × 108 TRITC-labeled, heat-killed yeast particles to 2 × 107 Dictyostelium cells resuspended in 10 ml of nutrient medium. The suspension was shaken in siliconized 25 ml Erlenmeyer flasks at 150 r.p.m. Samples of 1 ml were withdrawn at intervals, pipetted into 100 µl of 2 mM Trypan blue to quench the fluorescence of non-internalized yeast particles (Hed, 1986), shaken for 3 min, and centrifuged for 3 min at 500 g. The pellet was resuspended in 1 ml of PB and immediately measured in a Perkin Elmer LS50B spectrofluorimeter at 544 nm for excitation and 574 nm for emission.
Adhesion of yeast particles in the presence of latrunculin A was determined by suspending 2 × 107 Dictyostelium cells in 10 ml of nutrient medium. The suspension was shaken as described above. After 30 min of equilibration, 10 µM latrunculin A (Molecular Probes) was added, and 5 min later 6 × 107 fluorescent yeast particles. After 5 min, aliquots were fixed for 30 min with an equal volume of 8% glutaraldehyde. After the cells had settled on a glass surface, 10 arbitrarily chosen microscope fields were photographed, and the numbers of yeast particles adhering to each Dictyostelium cell were counted.
Adhesion of yeast particles in the cold was assayed essentially according to Tuxworth et al. (2001). Cells attached to coverslips were incubated on ice for 15 min in SB, consisting of PB supplemented with 1 mM MgCl2. Subsequently, the cells were overlayed with a suspension of 6 × 106 fluorescent yeast particles per milliliter. After 15 min on ice, the specimens were fixed with 4% glutaraldehyde for 30 min, and non-adherent particles were removed by washing. Four areas each on four coverslips were counted per data point.
Chemotactic responses were recorded from cells developed in PB and stimulated with a micropipette (Gerisch and Keller, 1981) filled with a solution of 10–4 M cAMP.
Acknowledgments
Acknowledgements
We thank Barbara Knoblach for assistance in calreticulin antibody production. We thank the Deutsche Forschungsgemeinschaft for grants to G.G. and A.M.-T., and the staff of the institute for the support that enabled us to finish this study.
References
- Aizawa H., Sutoh,K., Tsubuki,S., Kawashima,S., Ishii,A. and Yahara,I. (1995) Identification, characterization and intracellular distribution of cofilin in Dictyostelium discoideum. J. Biol. Chem., 270, 10923–10932. [DOI] [PubMed] [Google Scholar]
- Arima H., Kinoshita,T., Ibrahim,H.R., Azakami,H. and Kato,A. (1998) Enhanced secretion of hydrophobic peptide fused lysozyme by the introduction of N-glycosylation signal and the disruption of calnexin gene in Saccharomyces cerevisiae. FEBS Lett., 440, 89–92. [DOI] [PubMed] [Google Scholar]
- Aubry L., Klein,G., Martiel,J.-L. and Satre,M. (1997) Fluid-phase endocytosis in the amoebae of the cellular slime mould Dictyostelium discoideum: mathematical modelling of kinetics and pH evolution. J. Theor. Biol., 184, 89–98. [Google Scholar]
- Cardelli J. (2001) Phagocytosis and macropinocytosis in Dictyostelium: phosphoinositide-based processes, biochemically distinct. Traffic, 2, 311–320. [DOI] [PubMed] [Google Scholar]
- Coppolino M.G., Woodside,M.J., Demaurex,N., Grinstein,S., St-Arnaud,R. and Dedhar,S. (1997) Calreticulin is essential for integrin-mediated calcium signalling and cell adhesion. Nature, 386, 843–847. [DOI] [PubMed] [Google Scholar]
- Corbett E.F. and Michalak,M. (2000) Calcium, a signaling molecule in the endoplasmic reticulum? TIBS, 25, 307–311. [DOI] [PubMed] [Google Scholar]
- Cornillon S., Pech,E., Benghezal,M., Ravanel,K., Gaynor,E., Letourneur,F., Brückert,F. and Cosson,P. (2000) Phg1p is a nine-transmembrane protein superfamily member involved in Dictyostelium adhesion and phagocytosis. J. Biol. Chem., 275, 34287–34292. [DOI] [PubMed] [Google Scholar]
- Ellgaard L. and Helenius,A. (2001) ER quality control: towards an understanding at the molecular level. Curr. Opin. Cell Biol., 13, 431–437. [DOI] [PubMed] [Google Scholar]
- Fadel M.P., Dziak,E., Lo,C.-M., Ferrier,J., Mesaeli,N., Michalak,M. and Opas,M. (1999) Calreticulin affects focal contact-dependent but not close contact-dependent cell–substratum adhesion. J. Biol. Chem., 274, 15085–15094. [DOI] [PubMed] [Google Scholar]
- Faix J., Gerisch,G. and Noegel,A.A. (1992) Overexpression of the csA cell adhesion molecule under its own cAMP-regulated promoter impairs morphogenesis in Dictyostelium. J. Cell Sci., 102, 203–214. [DOI] [PubMed] [Google Scholar]
- Felsenstein J. (1993) PHYLIP (Phylogeny Inference Package) version 3.57c. Distributed by the author. Department of Genetics, University of Washington, Seattle, WA. http://evolution.genetics.washington. edu/phylip.html
- Ferrari G., Langen,H., Naito,M. and Pieters,J. (1999) A coat protein on phagosomes involved in the intracellular survival of mycobacteria. Cell, 97, 435–447. [DOI] [PubMed] [Google Scholar]
- Garin J., Diez,R., Kieffer,S., Dermine,J.-F., Duclos,S., Gagnon,E., Sadoul,R., Rondeau,C. and Dejardins,M. (2001) The phagosome proteome: insights into phagosome function. J. Cell Biol., 152, 165–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerisch G. and Keller,H.U. (1981) Chemotactic reorientation of granulocytes stimulated with micropipettes containing fMet-Leu-Phe. J. Cell Sci., 52, 1–10. [DOI] [PubMed] [Google Scholar]
- Hacker U., Albrecht,R. and Maniak,M. (1997) Fluid-phase uptake by macropinocytosis in Dictyostelium. J. Cell Sci., 110, 105–112. [DOI] [PubMed] [Google Scholar]
- Hed J. (1986) Methods for distinguishing ingested from adhering particles. Methods Enzymol., 132, 198–204. [DOI] [PubMed] [Google Scholar]
- Heim R. and Tsien,R.Y. (1996) Engineering green fluorescent protein for improved brightness, longer wavelengths and fluoresence energy transfer. Curr. Biol., 6, 178–182. [DOI] [PubMed] [Google Scholar]
- Helenius A., Trombetta,E.S., Hebert,D.N. and Simons,J.F. (1997) Calnexin, calreticulin and the folding of glycoproteins. Trends Cell Biol., 7, 193–200. [DOI] [PubMed] [Google Scholar]
- High S., Lecomte,F.J.L., Russell,S.J., Abell,B.M. and Oliver,J.D. (2000) Glycoprotein folding in the endoplasmic reticulum: a tale of three chaperons? FEBS Lett., 476, 38–41. [DOI] [PubMed] [Google Scholar]
- Holaska J.M., Black,B.E., Love,D.C., Hanover,J.A., Leszyk,J. and Paschal,B.M. (2001) Calreticulin is a receptor for nuclear export. J. Cell Biol., 152, 127–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humbel B.M. and Biegelmann,E. (1992) A preparation protocol for postembedding immunoelectron microscopy of Dictyostelium discoideum with monoclonal antibodies. Scanning Microsc., 6, 817–825. [Google Scholar]
- Insall R., Müller-Taubenberger,A., Machesky,L., Köhler,J., Simmeth,E., Atkinson,S.J., Weber,I. and Gerisch,G. (2001) Dynamics of the Dictyostelium Arp2/3 complex in endocytosis, cytokinesis and chemotaxis. Cell Motil. Cytoskel., in press. [DOI] [PubMed] [Google Scholar]
- Jannatipour M. and Rokeach,L.A. (1995) The Schizosaccharomyces pombe homologue of the chaperone calnexin is essential for viability. J. Biol. Chem., 270, 4845–4853. [DOI] [PubMed] [Google Scholar]
- Johnson S., Michalak,M., Opas,M. and Eggleton,P. (2001) The ins and outs of calreticulin: from the ER lumen to the extracellular space. Trends Cell Biol., 11, 122–129. [DOI] [PubMed] [Google Scholar]
- Konzok A., Weber,I., Simmeth,E., Hacker,U., Maniak,M. and Müller-Taubenberger,A. (1999) DAip1, a Dictyostelium homologue of the yeast actin-interacting protein 1, is involved in endocytosis, cytokinesis and motility. J. Cell Biol., 146, 453–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitmeier M., Gerisch,G., Heizer,C. and Müller-Taubenberger,A. (1995) A talin homologue of Dictyostelium rapidly assembles at the leading edge of cells in response to chemoattractant. J. Cell Biol., 129, 179–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundquist-Gustafsson H., Gustafsson,M. and Dahlgren,C. (2000) Dynamic Ca2+ changes in neutrophil phagosomes. A source for intracellular Ca2+ during phagolysosome formation? Cell Calcium, 27, 353–362. [DOI] [PubMed] [Google Scholar]
- Maniak M., Rauchenberger,R., Albrecht,R., Murphy,J. and Gerisch,G. (1995) Coronin involved in phagocytosis: dynamics of particle-induced relocalization visualized by a green fluorescent protein tag. Cell, 83, 915–924. [DOI] [PubMed] [Google Scholar]
- May R.C. and Machesky,L.M. (2001) Phagocytosis and the actin cytoskeleton. J. Cell Sci., 114, 1061–1077. [DOI] [PubMed] [Google Scholar]
- Mesaeli N., Nakamura,K., Zvaritch,E., Dickie,P., Dziak,E., Krause,K.-H., Opas,M., MacLennan,D.H. and Michalak,M. (1999) Calreticulin is essential for cardiac development. J. Cell Biol., 144, 857–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalak M. (1996) Calreticulin. Springer-Verlag, Heidelberg, Germany.
- Michalak M., Corbett,E.F., Mesaeli,N., Nakamura,K. and Opas,M. (1999) Calreticulin: one protein, one gene, many functions. Biochem. J., 344, 281–292. [PMC free article] [PubMed] [Google Scholar]
- Molinari M. and Helenius,A. (2000) Chaperone selection during glycoprotein translocation into the endoplasmic reticulum. Science, 288, 331–333. [DOI] [PubMed] [Google Scholar]
- Monnat J., Hacker,U., Geissler,H., Rauchenberger,R., Neuhaus,E.M., Maniak,M. and Soldati,T. (1997) Dictyostelium discoideum protein disulfide isomerase, an endoplasmic reticulum resident enzyme lacking a KDEL-type retrieval signal. FEBS Lett., 418, 357–362. [DOI] [PubMed] [Google Scholar]
- Niewöhner J., Weber,I., Maniak,M., Müller-Taubenberger,A. and Gerisch,G. (1997) Talin-null cells of Dictyostelium are strongly defective in adhesion to particle and substrate surfaces and slightly impaired in cytokinesis. J. Cell Biol., 138, 349–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent C.A., Blacklock,B.J., Froehlich,W.M., Murphy,D.B. and Devreotes,P.N. (1998) G protein signaling events are activated at the leading edge of chemotactic cells. Cell, 95, 81–91. [DOI] [PubMed] [Google Scholar]
- Parlati F., Dominguez,M., Bergeron,J.J.M. and Thomas,D.Y. (1995a) Saccharomyces cerevisiae CNE1 encodes an endoplasmic reticulum (ER) membrane protein with sequence similarity to calnexin and calreticulin and functions as a constituent of the ER quality control apparatus. J. Biol. Chem., 270, 244–253. [DOI] [PubMed] [Google Scholar]
- Parlati F., Dignard,D., Bergeron,J.J.M. and Thomas,D.Y. (1995b) The calnexin homologue CNX1+ in Schizosaccharomyces pombe is an essential gene which can be complemented by its soluble ER domain. EMBO J., 14, 3064–3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peracino B. et al. (1998) G protein β subunit-null mutants are impaired in phagocytosis and chemotaxis due to inappropriate regulation of the actin cytoskeleton. J. Cell Biol., 141, 1529–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinz W.A., Grzyb,L., Veenhuis,M., Kahana,J.A., Silver,P.A. and Rapoport,T.A. (2000) Mutants affecting the structure of the cortical endoplasmic reticulum in Saccharomyces cerevisiae. J. Cell Biol., 150, 461–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch F., Prud’homme,J., Arabian,A., Dedhar,S. and St-Arnaud,R. (2000) Heart, brain and body wall defects in mice lacking calreticulin. Exp. Cell Res., 256, 105–111. [DOI] [PubMed] [Google Scholar]
- Rupper A.C., Rodriguez-Paris,J.M., Grove,B.D. and Cardelli,J.A. (2001) p110-related PI 3-kinases regulate phagosome–phagosome fusion and phagosomal pH through a PKB/Akt dependent pathway in Dictyostelium. J. Cell Sci., 114, 1283–1295. [DOI] [PubMed] [Google Scholar]
- Schneider N., Schwartz,J.-M., Köhler,J., Becker,M., Schwarz,H. and Gerisch,G. (2000) Golvesin-GFP fusions as distinct markers for Golgi and post-Golgi vesicles in Dictyostelium cells. Biol. Cell, 92, 495–511. [DOI] [PubMed] [Google Scholar]
- Schuler G.D., Altschul,S.F. and Lipman,D.J. (1991) A workbench for multiple alignment construction and analysis. Proteins, 9, 180–190. [DOI] [PubMed] [Google Scholar]
- Stendahl O., Krause,K.-H., Krischer,J., Jerström,P., Theler,J.-M., Clark,R.A., Carpentier,J.-L. and Lew,D.P. (1994) Redistribution of intracellular Ca2+ stores during phagocytosis in human neutrophils. Science, 265, 1439–1441. [DOI] [PubMed] [Google Scholar]
- Strimmer K. and von Haeseler,A. (1996) Quartet puzzling: a quartet maximum-likelihood method for reconstructing tree topologies. Mol. Biol. Evol., 13, 964–969. [Google Scholar]
- Sutoh K. (1993) A transformation vector for Dictyostelium discoideum with a new selectable marker, bsr. Plasmid, 30, 150–154. [DOI] [PubMed] [Google Scholar]
- Swanson J.A. and Baer,S.C. (1995) Phagocytosis by zippers and triggers. Trends Cell Biol., 5, 89–93. [DOI] [PubMed] [Google Scholar]
- Swofford D.L. (1993) PAUP: Phylogenetic Analysis Using Parsimony, Version 3.1.1. Illinois Natural History Survey, Champaign, IL.
- Thompson J.D., Higgins,D.G. and Gibson,T.J. (1994) ClustalW: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res., 22, 4673–4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traynor D., Milne,J.L.S., Insall,R.H. and Kay,R.R. (2000) Ca2+ signalling is not required for chemotaxis in Dictyostelium. EMBO J., 19, 4846–4854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trombetta E.S. and Helenius,A. (1998) Lectins as chaperones in glycoprotein folding. Curr. Opin. Struct. Biol., 8, 587–592. [DOI] [PubMed] [Google Scholar]
- Tuxworth R.I., Weber,I., Wessels,D., Addicks,G.C., Soll,D.R., Gerisch,G. and Titus,M.A. (2001) A role for myosin VII in dynamic cell adhesion. Curr. Biol., 11, 318–329. [DOI] [PubMed] [Google Scholar]
- Vogel G., Thilo,L., Schwarz,H. and Steinhart,R. (1980) Mechanism of phagocytosis in Dictyostelium discoideum: phagocytosis is mediated by different recognition sites as disclosed by mutants with altered phagocytic properties. J. Cell Biol., 86, 456–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallraff E. and Gerisch,G. (1991) Screening of Dictyostelium mutants defective in cytoskeletal proteins by colony immunoblotting. Methods Enzymol., 196, 334–348. [DOI] [PubMed] [Google Scholar]
- Watts D.J. and Ashworth,J.M. (1970) Growth of myxamoebae of the cellular slime mould Dictyostelium discoideum in axenic culture. Biochem. J., 119, 171–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan A., Siu,C.-H. and Chia,C.P. (2001) Calcium requirement for efficient phagocytosis by Dictyostelium discoideum. Cell Calcium, 29, 229–238. [DOI] [PubMed] [Google Scholar]
- Zhou K., Pandol,S., Bokoch,G. and Traynor-Kaplan,A.E. (1998) Disrup tion of Dictyostelium PI3K genes reduces [32P]phosphatidylinositol 3,4 biphosphate and [32P]phosphatidylinositol triphosphate levels, alters F-actin distribution and impairs pinocytosis. J. Cell Sci., 111, 283–294. [DOI] [PubMed] [Google Scholar]