Abstract
Decreased albumin expression is a frequent feature of cachexia patients afflicted with chronic diseases, including cancer, and a major contributor to their morbidity. Here we show that tumor necrosis-α (TNF-α) treatment of primary mouse hepatocytes or TNF-α overexpression in a mouse model of cachexia induces oxidative stress, nitric oxide synthase (NOS) expression and phosphorylation of C/EBPβ on Ser239, within the nuclear localization signal, thus inducing its nuclear export, which inhibits transcription from the albumin gene. SIN-1, a NO donor, duplicated the TNF-α effects on hepatocytes. We found similar molecular abnormalities in the liver of patients with cancer-cachexia. The cytoplasmic localization and association of C/EBPβ-PSer239 with CRM1 (exportin-1) in TNF-α-treated hepatocytes was inhibited by leptomycin B, a blocker of CRM1 activity. Hepatic cells expressing the non-phosphorylatable C/EBPβ alanine mutant were refractory to the inhibitory effects of TNF-α on albumin transcription since the mutant remained localized to the nucleus. Treatment of TNF-α mice with antioxidants or NOS inhibitors prevented phosphorylation of C/EBPβ on Ser239 and its nuclear export, and rescued the abnormal albumin gene expression.
Keywords: AIDS/albumin/cancer/NOS/oxidative stress
Introduction
Albumin is the most abundant protein in plasma, and the colloid pressure of plasma is maintained principally by the levels of circulating albumin (West, 1990). Albumin also performs important metabolic functions in the transport of free fatty acids, bilirubin and many drugs (West, 1990; Chojkier, 1995). In a normal individual, ∼15 g of albumin are synthesized daily to maintain the albumin plasma steady-state concentration (∼4 g/100 ml) (West, 1990). Therefore, decreased albumin synthesis results in hypoalbuminemia, which facilitates excessive transudation of fluids into extravascular spaces (edema and ascites) (Braunwald, 1994). Hypoalbuminemia is a frequent feature of cachectic patients afflicted with chronic diseases (Tracey and Cerami, 1993), including cancer, AIDS and inflammatory disorders, and a major contributor to their morbidity (Voth et al., 1990; Beutler, 1992; Grunfeld and Feingold, 1992; Roubenoff et al., 1994). There is strong evidence to suggest that tumor necrosis factor-α (TNF-α) is a critical mediator (Fong et al., 1989; Yoneda et al., 1991; Cheng et al., 1992), in concert with other cytokines (Grunfeld and Feingold, 1992; Strassman et al., 1992; Spiegelman and Hotamisligil, 1993; Todorov et al., 1996), of cachexia. Therefore, we evaluated the mechanisms leading to decreased albumin transcription (Brenner et al., 1990) in a murine model of cachexia (TNF-α mice), induced by chronically elevated serum TNF-α (Oliff et al., 1987; Brenner et al., 1990; Buck and Chojkier, 1996) in hepatocytes and hepatoma cells treated with TNF-α and in patients with cancer-cachexia.
Here we show that TNF-α treatment of primary mouse hepatocytes or TNF-α overexpression in a mouse model of cachexia induces oxidative stress, nitric oxide synthase (NOS) expression and phosphorylation of C/EBPβ on Ser239, within the nuclear localization signal (NLS), thus inducing its nuclear export, which inhibits transcription from the albumin gene. We found similar molecular abnormalities in the liver of patients with cancer-cachexia. The cytoplasmic localization and association of C/EBPβ-PSer239 with CRM1 (exportin-1) in TNF-α-treated hepatocytes was inhibited by leptomycin B, a blocker of CRM1 activity. Hepatic cells expressing the non-phosphorylatable C/EBPβ alanine mutant were refractory to the inhibitory effects of TNF-α on albumin transcription since the mutant remained localized to the nucleus. Treatment of TNF-α mice with antioxidants or NOS inhibitors prevented phosphorylation of C/EBPβ on Ser239 and its nuclear export, and rescued the abnormal albumin gene expression.
Results
Oxidative stress inhibits albumin expression in the liver of TNF-α mice
Chinese hamster ovary (CHO) cells stably transfected with either a cytomegalovirus (CMV) vector expressing human TNF-α/neo (TNF-α cells) or neo alone (CHO cells, control) (Oliff et al., 1987; Brenner et al., 1990; Buck and Chojkier, 1996) were used in our experiments. The TNF-α cells, but not the CHO cells, secreted human TNF-α. Athymic nude mice were injected intramuscularly with either CHO cells or TNF-α cells. The TNF-α and CHO mice maintained essentially the same weight for the first 2–3 weeks after inoculation. We have reported that albumin mRNA and albumin transcription are markedly decreased in TNF-α mice before the onset of weight loss (Brenner et al., 1990). After this period, TNF-α animals began to develop symptoms of cachexia, including decreased weight, muscle wasting, anemia and abnormal wound healing (Buck and Chojkier, 1996; Buck et al., 1996).
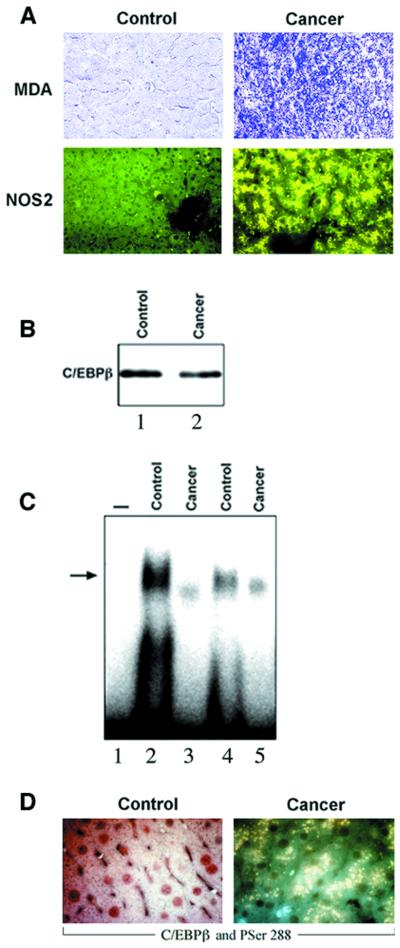
Because TNF-α may initiate a cascade leading to oxidative stress (Wong et al., 1989; Henkel et al., 1993; Schulze-Osthoff et al., 1993), we assessed whether aldehyde products of lipid peroxidation, such as malondialdehyde (MDA) (Chaudhary et al., 1994), were present in the liver of TNF-α mice. Using specific antibodies against MDA–lysine adducts for immunostaining (Houglum et al., 1990; Buck and Chojkier, 1996), we found a high level of MDA–protein adducts in the liver of TNF-α mice, while the liver of control mice had negligible levels (Figure 1).
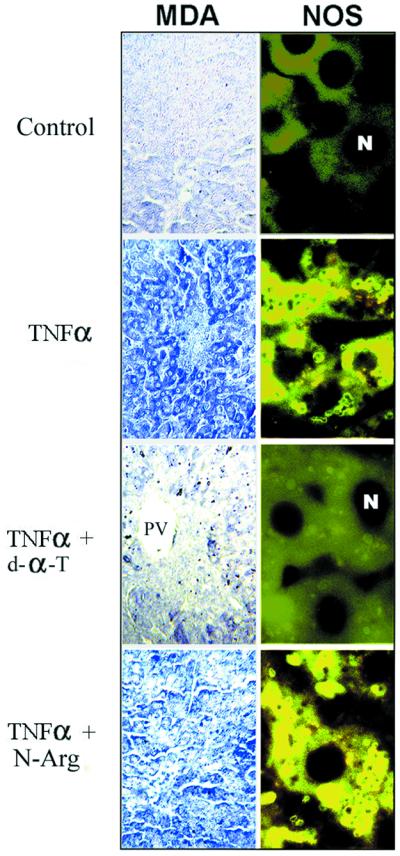
Fig. 1. Increased oxidative stress and NOS2 in the liver of cachectic mice. The experimental groups are as described in Materials and methods. Representative examples (n = 8 in each group) of the immunohistochemistry for malondialdehyde (MDA)–protein adducts and NOS2, using antibodies specific for MDA–lysine adducts and NOS2. PV indicates portal venules. Negligible staining was observed in all immunohistochemistries when the first antibody was omitted.
Because of this evidence, supporting an oxidative stress pathway in the liver of TNF-α mice, and because aldehydes may form adducts with proteins critical for differentiated function, we treated these animals with antioxidants in an attempt to elucidate the mechanisms responsible for the decrease in albumin gene expression of TNF-α mice (Brenner et al., 1990). When TNF-α mice received a diet supplemented with d-α-tocopherol (Buck and Chojkier, 1996), a lipophilic antioxidant, the increased oxidative stress in the liver was blocked, as determined by the absence of MDA–protein adducts (Figure 1), a sensitive indicator of lipid peroxidation (Houglum et al., 1990). More importantly, the characteristic decrease in albumin mRNA of TNF-α mice was prevented, to a significant extent, by a 30-day treatment with d-α-toco pherol (Brenner et al., 1990) (Figure 2A). We also assessed whether the inhibition of albumin mRNA induced by TNF-α could be rescued in short-term experiments with antioxidants. In normal mice that had received eight high-dose TNF-α injections (Buck et al., 1996) to induce a rapid decrease in liver albumin mRNA (Figure 2B), three high-dose d-α-tocopherol injections (Chojkier et al., 1998) were sufficient to rescue the impaired albumin gene expression observed in this animal model (Figure 2B). As expected, the non-lipophilic antioxidant, 3-amino-1-(3-trifluoromethylphenyl)-2-pyrazoline hydrochloride (BW755c) (Buck and Chojkier, 1996), did not affect the generation of MDA–protein adducts (not shown) since it does not alter the peroxidation of polyunsaturated fatty acids in membranes. However, BW755c treatment normalized the albumin mRNA in TNF-α mice (not shown), indicating that the oxidative stress cascade initiated by TNF-α can be blocked effectively at different levels.
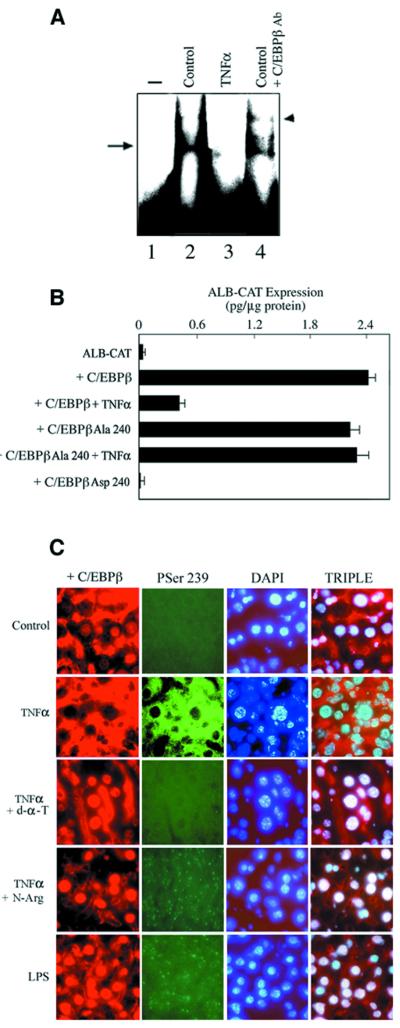
Fig. 2. Oxidative stress and NO inhibit albumin expression in the liver of cachectic mice. (A) The experimental groups are as described in Materials and methods. Representative examples (n = 6 in each group) of albumin mRNA detected by an RNase protection assay with a specific riboprobe, using equal amounts of total RNA. The 18S RNA was utilized as a correction factor for loading. Albumin mRNA in livers from CHO (lane 1), TNF-α (lane 2), TNF-α/d-α-tocopherol (lane 3) and TNF-α/nitro-l-arginine (lane 4). P <0.05 for TNF-α. (B) Mice received TNF-α alone from 8 to 92 h (closed circles) or with d-α-tocopherol from 24 to 72 h (open circles) as described in Materials and methods. Animals were sacrificed as indicated and liver albumin mRNA was determined as in (A). The results are averages of at least triplicate samples. P <0.05 for d-α-tocopherol at 72 and 96 h. (C) Mobility shift analysis of liver nuclear extracts was performed using equal amounts of nuclear protein (5 µg) and following incubation with a 32P-labeled oligonucleotide (1 ng) spanning the D-site or the B-site of the albumin enhancer/promoter or the control Sp1 site. The position of the bound DNA is indicated by arrows. Representative samples (n = 5 in each group) are shown: CHO (2); TNF-α (3); TNF-α/d-α-tocopherol (4); TNF-α/BW755c (5); and TNF-α/nitro-l-arginine (6). On lane 1, the probe was processed without nuclear extracts. The D-site binding activities were CHO (100%), TNF-α (22%), TNF-α/d-α-tocopherol (84%), TNF-α/BW755c (67%) and TNF-α/nitro-l-arginine (76%). (D) HepG2 human hepatoma cells were transfected with ALB-CAT (1 µg) alone or with CMV-C/EBPβ (1 µg) and treated every 24 h with TNF-α (10 ng/ml); TNF-α + d-α-tocopherol (50 µM); TNF-α + nitro-l-arginine (500 µM); and SIN-1 (0.6 µM) as indicated. After 48 h, the cells were collected and CAT expression was determined. The results are averages (± SEM) of triplicate samples, and representative of four independent experiments. P <0.05 for C/EBPβ + TNF-α and C/EBPβ + SIN-1 compared with C/EBPβ.
The D-site, which is present in both the enhancer and promoter of the albumin gene (Zaret, 1994; Chojkier, 1995), is critical for the expression of liver-specific genes (Maire et al., 1989; Descombes et al., 1990; Zaret, 1994; Chojkier, 1995). Therefore, its binding and transcriptional activities, which are contributed mainly by C/EBPβ and C/EBPα, have been used as indicators of liver-specific differentiation (Descombes et al., 1990). We analyzed the D-site binding activities of liver nuclear proteins from the various experimental groups. There was substantially less D-site binding activity in liver nuclear extracts from TNF-α mice than in those from control animals (Figure 2C), and this deficiency was reversed, albeit incompletely, in TNF-α mice treated with either d-α-tocopherol or BW775c (Figure 2C). The D-site nuclear protein complex was supershifted by specific anti-C/EBPβ antibodies as described previously (Descombes et al., 1990) (data not shown). In contrast, the level of protein binding to the B-site, another important cis-element for the efficient transcription of the albumin gene (Maire et al., 1989; Zaret, 1994; Chojkier, 1995), was only slightly decreased in liver nuclear extracts from TNF-α mice (Figure 2C). Liver nuclear extracts from TNF-α mice also showed normal levels of protein binding to the Sp1 site, a site commonly used in housekeeping genes (Figure 2C), indicating a selective defect in the binding of C/EBPβ to the albumin D-site. The binding of liver nuclear extracts to these labeled cognate DNAs was suppressed by competition with excess unlabeled homologous, but not heterologous, oligonucleotide (data not shown).
Next, we assessed the effects of TNF-α on albumin transcription in HepG2 human hepatoma cells. Because HepG2 cells express negligible amounts of C/EBPβ (Descombes et al., 1990; Trautwein et al., 1993; Buck et al., 1994), in these experiments, cells were transfected with a CMV vector expressing C/EBPβ (Buck et al., 1994, 1999) and with an albumin–enhancer (–9 to –12 kb)/promoter (–282/+28bp) chimeric reporter gene (ALB-CAT) (Kioussis et al., 1979). C/EBPβ binds to two critical cis-elements within the promoter (D-site) and the enhancer of the albumin gene (Descombes et al., 1990; Zaret, 1994; Chojkier, 1995). As expected, C/EBPβ stimulated transcription from the ALB-CAT reporter gene (Figure 2D). TNF-α treatment of HepG2 cells markedly inhibited ALB-CAT expression induced by C/EBPβ, and this effect was blocked with the antioxidants d-α-tocopherol (Figure 2D) or BW755c (data not shown), suggesting that an oxidative stress pathway mediates the inhibitory effects of TNFα on albumin transcription.
NO inhibits albumin gene expression
Because oxidative stress pathways interact with NO to modulate cytoprotective or cytotoxic effects (Lipton et al., 1993; Stamler, 1994; Buck and Chojkier, 1996), and NO itself plays a role in liver function (Geller, 1993), we analyzed whether this pathway affects albumin transcription. N5-[nitroamidino]-l-2,5-diaminopentanoic acid (nitro- l-arginine), a potent inhibitor of NOS (Kobzik et al., 1994; Buck and Chojkier, 1996), blocked the inhibition of ALB-CAT transcription initiated by TNF-α (Figure 2D). Additional support for a role for NO was obtained by treating the hepatoma cells with 3-morpholinosydnonomine (SIN-1), an NO donor (Lipton et al., 1993; Buck and Chojkier, 1996). SIN-1 treatment (Lipton et al., 1993; Buck and Chojkier, 1996) markedly decreased ALB-CAT transcription in HepG2 cells in the absence of TNF-α (Figure 2D).
Because the data derived from HepG2 cells suggested that the NO pathway may mediate (or act synergistically with) the effects of oxidative stress on liver cells, we studied these interactions in TNF-α mice. As shown in Figure 1, the expression of NOS was increased substantially in the livers of TNF-α mice compared with control mice. The increased expression of NOS in TNF-α mice was prevented by treatment with the antioxidants d-α-tocopherol (Figure 1) or BW755c. These results support the hypothesis that oxidative stress induces NOS expression in the liver of TNF-α mice.
Treatment of TNF-α mice with the NOS inhibitor, nitro-l-arginine (Buck and Chojkier, 1996), ameliorated the decrease in both albumin mRNA (Figure 2A) and binding of liver nuclear extracts to the albumin D-site (Figure 2C). Nitro-l-arginine did not affect the induction of either MDA–protein adducts or NOS (Figure 1), upstream components of the cascade leading to the synthesis of NO, in the liver of TNF-α mice. Treatment of TNF-α mice with either antioxidants or nitro-l-arginine did not affect the production of TNF-α, or some of the other end-organ biological effects of TNF-α, such as anemia or inhibition of collagen α1(I) expression (Buck and Chojkier, 1996; Buck et al., 1996), indicating a selective effect on specific TNF-α pathways.
Phosphorylation of C/EBPβ on its DNA-binding domain mediates the inhibition of albumin transcription by TNF-α
C/EBPβ contributes, as homo- and heterodimers, ∼70% of the binding activity of liver nuclear proteins to the D-site (Descombes et al., 1990). However, the expression of C/EBPβ (LAP, NF-IL6, IL-6DBP) protein (Akira et al., 1990a; Descombes et al., 1990; Poli et al., 1990) was not decreased in whole liver lysates from TNF-α mice (Figure 3A). This observation could be reconciled with the decreased D-site binding activities of liver nuclear extracts from TNF-α mice, if TNF-α stimuli were to induce a modification of C/EBPβ, such as phosphorylation, that impairs its function. Therefore, we investigated the role of C/EBPβ phosphorylation in the inhibition of albumin transcription induced by TNF-α in primary mouse hepatocytes. As in normal adult liver, these quiescent hepatocytes expressed nuclear C/EBPβ (Buck et al., 1999).
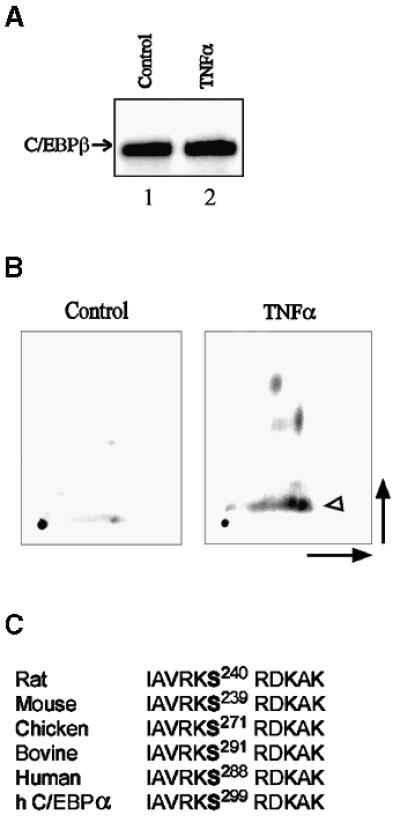
Fig. 3. TNF-α stimulates phosphorylation of C/EBPβ in its DNA-binding domain. (A) Representative examples (n = 5 in each group) of C/EBPβ protein immunoblots in liver lysates from CHO (lane 1) and TNF-α (lane 2) groups. The antigen–antibody complexes were visualized using the Renaissance detection system (DuPont). (B) Tryptic phosphopeptide maps of C/EBPβ immunopurified from day-5 primary mouse hepatocytes cultured on a collagen type I matrix. Cells were labeled with [32P]orthophosphate (2 mCi/ml; total 30 mCi) for 18 h and either treated with TNF-α (10 ng/ml) for 30 min before harvest or not treated (control). C/EBPβ tryptic peptides were separated by high-voltage electrophoresis (horizontal dimension) followed by ascending thin-layer chromatography (vertical dimension). The level of the phosphopeptide containing Ser239 (arrowhead), identified as described previously (Trautwein et al., 1994), was increased after treatment with TNF-α. (C) The basic domain serine phosphoacceptor is conserved through evolution. Rat (S240), mouse (S239), chicken (S271), bovine (S291) and human (S288) phosphoacceptors, within the basic domain of C/EBPβ, are shown. An identical phosphoacceptor is present in human (h) C/EBPα (S299).
To test whether TNF-α induces C/EBPβ phosphorylation, highly differentiated, quiescent mouse hepatocytes cultured on a collagen type I matrix (Buck et al., 1999) were incubated with 30 mCi of [32P]orthophosphate before treatment for 30 min with TNF-α. Phosphopeptide mapping of immunoprecipitated endogenous C/EBPβ showed that TNF-α induced a major site-specific phosphorylation of C/EBPβ on Ser239, as well as other, as yet unidentified, sites (Figure 3B). Phosphorylation of Ser240 in rat C/EBPβ (homologous to mouse PSer239), which was identified previously using recombinant wild-type and mutant C/EBPβ-Ala240 and activated protein kinase C (Trautwein et al., 1994), was shown to inhibit in vitro binding of C/EBPβ to cognate DNA (Trautwein et al., 1994). This serine phosphoacceptor, within the DNA-binding domain and NLS of C/EBPβ (Williams et al., 1997), is identical in rat, mouse, chicken, bovine and human, as well as in C/EBPα (Akira et al., 1990a; Descombes et al., 1990; Cao et al., 1991; Katz et al., 1993; Yamaoka et al., 1997) (Figure 3C). Endogenous C/EBPβ was not phosphorylated on Ser239 in control hepatocytes (Figure 3B). Furthermore, nuclear extracts from these mouse hepatocytes treated for 30 min with TNF-α displayed decreased binding activity for the albumin D-site (Figure 4A), which was restored (data not shown) by phosphatase treatment of the nuclear extracts as described previously (Trautwein et al., 1994).
Fig. 4. Phosphorylation of C/EBPβ in its basic domain induced by TNF-α inhibits albumin expression. (A) Mobility shift analysis of nuclear extracts from day 5 primary mouse hepatocytes was performed using equal amounts of nuclear protein (5 µg) and following incubation with a 32P-labeled oligonucleotide (1 ng) spanning the D-site of the albumin enhancer/promoter. The position of the bound DNA is indicated by the arrow and the supershifted DNA is indicated by the arrowhead. Representative samples are shown: control (2); TNF-α (10 ng/ml for 30 min) (3); and control + C/EBPβ antibody (4). On lane 1, the probe was processed without nuclear extracts. (B) HepG2 cells were transfected with ALB-CAT (1 µg) alone or with 1 µg of CMV-C/EBPβ, CMV-C/EBPβ-Ala240 or CMV-C/EBPβ-Asp240, and treated with TNF-α (10 ng/ml) every 24 h as indicated. After 48 h, the cells were collected and CAT expression was determined. The results are averages (± SEM) of triplicate samples, and representative of five independent experiments. P <0.05 for C/EBPβ + TNF-α and C/EBPβ-Asp240 compared with C/EBPβ. (C) Representative examples (n = 8 in each group) of the triple channel immunofluorescence microscopy using antibodies for C/EBPβ and C/EBPβ-PSer239 simultaneously. Hepatocytes from control, TNFα/d-α-tocopherol-, TNF-α/nitro-l-arginine- and LPS-treated mice displayed nuclear C/EBPβ (in red) but not C/EBPβ-PSer-239 (in green). In hepatocytes from TNF-α livers, the cytoplasmic co-localization of antibodies against C/EBPβ (red) and C/EBPβ-PSer-239 (green) is shown in yellow. Nuclei are stained with DAPI (blue); co-localization of C/EBPβ (red) and DAPI (blue) is shown in white.
Because phosphorylation of C/EBPβ within its basic domain impaired binding of this transcription factor to cognate DNA (Figure 4A), we analyzed the role of this phosphoacceptor in albumin gene expression. In HepG2 cells, the rat C/EBPβ-Ala240 mutant protein, which lacks the Ser240 phosphoacceptor (Trautwein et al., 1994), stimulated albumin transcription as much as wild-type C/EBPβ but was refractory to the inhibitory effects of TNF-α (Figure 4B). These findings suggest that phosphorylation of rat C/EBPβ on Ser240 is critical for the TNF-α signal transduction pathway to cause inhibition of albumin transcription. The effects of Ser240 phosphorylation of rat C/EBPβ on its DNA binding affinity can be mimicked by introduction of a negatively charged residue (Trautwein et al., 1994). As expected, cells expressing the mutant C/EBPβ-Asp240 protein exhibited minimal transcription from the ALB-CAT reporter gene (Figure 4B).
Next, we developed antibodies against a peptide containing the phosphorylated Ser239 domain, that recognized C/EBPβ-PSer239 but not unphosphorylated C/EBPβ. These antibodies were purified by affinity chromatography on the PSer239 phosphoacceptor peptide (CIAVRK-PSer239-RDKAK). Immunohistochemical staining with purified anti-C/EBPβ-PSer239 antibodies was positive for this epitope in liver sections from TNF-α mice, but negative in liver sections from control and d-α-tocopherol- or nitro-l-arginine-treated TNF-α mice (Figure 4C). Unexpectedly, the localization of C/EBPβ was almost exclusively cytoplasmic, correlating with Ser239 phosphorylation in TNF-α mice, whereas C/EBPβ was mainly nuclear in control and d-α-tocopherol- or nitro-l-arginine-treated TNF-α mice, correlating with lack of Ser239 phosphorylation. In contrast to the findings in TNF-α mice, following the administration of lipopolysaccharide (LPS), another inducer of inflammation (Akira et al., 1990a; Alonzi et al., 2001), to normal mice, C/EBPβ expression, although increased, was predominantly nuclear and not phosphorylated on Ser239 (Figure 4C).
To delineate the mechanisms responsible for the nuclear exclusion of C/EBPβ-PSer239, we transfected primary mouse hepatocytes isolated from C/EBPβ–/– mice (Screpanti et al., 1995; Buck et al., 1999) with CMV vectors expressing mouse C/EBPβ-hemagglutinin (HA), C/EBPβ-Ala239-HA, or C/EBPβ-Asp239-HA. Using confocal microscopy, we detected C/EBPβ mainly in the nucleus, and CRM1 (exportin-1), a nuclear export receptor (Ossareh-Nazari et al., 1997; Kudo et al., 1999; Nachury and Weis, 1999), in a nuclear and perinuclear localization in hepatocytes (Figure 5A). Following treatment for 30 min with TNF-α, C/EBPβ was mainly cytoplasmic and co-localized in the perinuclear region with CRM1. Treatment of hepatocytes with leptomycin B, a blocker of protein nuclear export through its interactions with CRM1 (Kudo et al., 1999), prevented the effects of TNF-α on C/EBPβ nuclear export (Figure 5A), but nuclear C/EBPβ was still phosphorylated on Ser239 judging by the positive staining with anti-PSer239 antibodies (data not shown; see below). Hepatocytes treated only with leptomycin B displayed unphosphorylated, nuclear C/EBPβ (data not shown). These experiments indicate that the cytoplasmic localization of C/EBPβ-PSer239 induced by TNF-α is mediated by CRM1. Like wild-type C/EBPβ, the non-phosphorylatable C/EBPβ-Ala239 mutant displayed a predominantly nuclear localization but it was refractory to the induction of nuclear export by TNF-α (Figure 5B). Furthermore, the phosphorylation-mimic C/EBPβ-Asp239 mutant was excluded from the nucleus and co-localized in the perinuclear region with CRM1 (Figure 5B), closely resembling the pattern of C/EBPβ-PSer239 induced by TNF-α treatment in both mice and cells. These data strongly suggest that phosphorylation of Ser239 is required for the nuclear export of C/EBPβ following TNF-α treatment.
Fig. 5. Nuclear export of C/EBPβ-PSer-239 is mediated by CRM1 in hepatocytes treated with TNFα. Day 5 primary hepatocytes were isolated form C/EBPβ–/– mice. Representative confocal microscopy (n = 5 in each group). (A) Mouse hepatocytes were transfected with C/EBPβ, treated as described in Materials and methods and stained with antibodies against HA and CRM1. In C/EBPβ and TNF-α + leptomycin B cells, C/EBPβ (red) is nuclear and CRM1 (green) is nuclear and perinuclear. In TNF-α cells, C/EBPβ and CRM1 are co-localized in the cytoplasm (in yellow). Nuclei are stained with TOTO-3 (blue). (B) Mouse hepatocytes were transfected with CMV vectors expressing C/EBPβ-Ala239 or C/EBPβ-Asp239 for 24 h, treated as described in Materials and methods and stained with antibodies against HA and CRM1. C/EBPβ-Ala239 localized to the nucleus (in red) in untreated and TNF-α-treated hepatocytes. C/EBPβ-Asp239 co-localized with CRM1 in the perinuclear region (in yellow). Nuclei are stained with TOTO-3 (blue). (C) Cells were stained with antibodies against C/EBPα and CRM1. Control hepatocytes expressed nuclear C/EBPα. TNF-α treatment induced the nuclear export of C/EBPα, which co-localized with CRM1 in the perinuclear region (in yellow). Nuclei are stained with TOTO-3 (blue). (D) Mouse hepatocytes were treated with TNF-α or SIN-1 as described in Materials and methods, and stained with antibodies against HA and PSer239. TNFα and SIN-1 treatments induced the phosphorylation of C/EBPβ on Ser239 and its nuclear export. The cytoplasmic co-localization of C/EBPβ-PSer239 (green) and C/EBPβ (red) antibodies is shown in yellow. Control hepatocytes displayed mainly nuclear C/EBPβ (red) but not C/EBPβ-PSer239. Nuclei are stained with TOTO-3 (blue).
Because mouse C/EBPα also has the same phosphoacceptor (Ser300) (Christy et al., 1991) and because C/EBPα also plays an important role in liver-specific gene transcription (Landschulz et al., 1988), we analyzed the effects of TNF-α on C/EBPα, in primary hepatocytes isolated from C/EBPβ–/– mice as described previously (Buck et al., 1999). C/EBPα was found mainly in the nucleus of control C/EBPβ–/– hepatocytes (Figure 5C). However, following TNF-α treatment, C/EBPα was excluded from the nucleus and co-localized predominantly in the perinuclear region with CRM1 (Figure 5C). These results indicate that C/EBPα is also susceptible to the TNF-α induction of both phosphorylation within its NLS (Williams et al., 1997) and nuclear export.
The mechanisms affecting C/EBPβ following TNF-α treatment of hepatocytes appear to involve CRM1. Therefore, we analyzed whether CRM1 was associated with C/EBPβ in primary mouse C/EBPβ–/– hepatocytes by immunoprecipitation of C/EBPβ followed by immunoblotting with anti-CRM1 antibodies. Consistent with the confocal microscopy studies, immunoprecipitation of C/EBPβ-HA from TNF-α-treated hepatocytes immunoprecipitated with antibodies against HA was associated with CRM1 (Figure 6). This association was negligible in control and in TNF-α/leptomycin B-treated hepatocytes (Figure 6). Moreover, phosphorylation of C/EBPβ on Ser239 was induced by TNF-α (Figure 6), as we determined by phosphopeptide mapping (Figure 3B) and by immunohistochemistry (Figure 5D). Also, TNF-α treatment of C/EBPβ–/– hepatocytes induced the phosphorylation of endogenous C/EBPα on Ser300 (Christy et al., 1991) (homologous to human C/EBPα Ser299), as detected with anti-PSer239 antibodies, as well as the association of C/EBPα with CRM1 (Figure 6). In addition, leptomycin B decreased the phosphorylation of C/EBPβ on Ser239 induced by TNF-α (Figure 6).
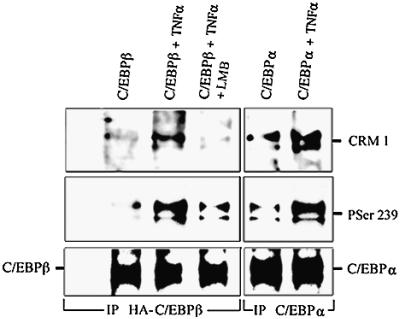
Fig. 6. C/EBPβ-PSer239 is associated with CRM1 in hepatocytes treated with TNF-α. Day 5 primary mouse C/EBPβ–/– hepatocytes were transfected with C/EBPβ-HA or not. After 24 h, cells were treated for 30 min as indicated. Cell lysates (500 µg) were precipitated with either HA or C/EBPα antibodies. Protein blotting of immunoprecipitates was performed as described in Materials and methods, using specific antibodies against CRM1, C/EBPβ-PSer239, C/EBPβ or C/EBPα.
Because inducible NOS (NOS2) expression is increased in the livers of TNF-α mice, and nitro-l-arginine, a blocker of NOS, prevents the phosphorylation of C/EBPβ and its nuclear export (Figure 4C), as well as the inhibition of albumin gene expression (Figure 2) induced by TNF-α, we treated primary mouse hepatocytes with SIN-1, a NO donor, and assessed the cellular localization of C/EBPβ using confocal microscopy. SIN-1, like TNF-α, was sufficient to induce the phosphorylation of C/EBPβ on Ser239 and its cytoplasmic localization. The cytoplasmic co-localization of C/EBPβ-PSer239 and C/EBPβ in TNF-α- and SIN-1-treated cells is depicted in yellow by an overlay of both channels (Figure 5D). These results suggest that NO, following its induction by TNF-α, is sufficient to stimulate the phosphorylation of C/EBPβ on Ser239 and its cytoplasmic localization.
Given that NO may activate guanylyl cyclase, which in turn increases cGMP levels and the cGMP-dependent protein kinase II (PKG) (El-Husseini et al., 1998; Idriss et al., 1999), we analyzed whether this signal transduction pathway mediates phosphorylation of C/EBPβ on Ser239. We found no association of C/EBPβ with PKG in livers from TNF-α mice or in TNF-α-treated hepatocytes (data not shown), suggesting that PKG is not the protein kinase that phosphorylates C/EBPβ following TNF-α signaling.
Enhanced oxidative stress and NOS expression in the liver of patients with cancer-cachexia
To assess the relevance to human cachexia of the cellular and animal models of cachexia, we analyzed, in preliminary studies, the mechanisms leading to decreased albumin gene expression in six patients with cancer-cachexia (see Materials and methods). These patients (62 ± 5 years) had low serum albumin (2.5 ± 0.4 g/dl; P <0.05). Liver biopsies from cachectic patients afflicted with cancer also displayed a high level of both MDA–protein adducts and NOS2 expression (Figure 7A). Although whole lysates of liver biopsies from cachectic patients had normal amounts of C/EBPβ protein (Figure 7B), there was a substantial decrease in the DNA-binding activity of nuclear extracts to the D-site (Figure 7C), congruent with the abnormal cytoplasmic localization of C/EBPβ (Figure 7D) compared with samples from four control patients (67 ± 4 years; serum albumin 3.7 ± 0.2 g/dl). We detected cytoplasmic C/EBPβ-PSer288 (homologous to mouse C/EBPβ-PSer239) in the cytoplasm of hepatocytes from patients with cancer-cachexia, while in control subjects C/EBPβ was mainly nuclear and not phosphorylated on Ser288 (Figure 7D). The impaired D-site binding activities of nuclear extracts from cachectic patients were normalized by the addition of recombinant C/EBPβ or nuclear extracts from control individuals (data not shown). The binding of liver nuclear extracts to the cognate DNA was suppressed by competition with excess unlabeled homologous, but not heterologous, oligonucleotide.
Fig. 7. Nuclear export of C/EBPβ-Ser288 in the liver of patients with cancer-cachexia. (A) Representative immunohistochemistry for MDA–protein adducts, NOS2, C/EBPβ and C/EBPβ-PSer288 of control individuals (control) and cancer-cachexia patients (cachexia) was performed as described in Figures 1 and 4. (B) A representative C/EBPβ protein immunoblot in C/EBPβ immunoprecipitates from liver protein lysates (250 µg) of control (lane 1) and cancer-cachexia (lane 2) subjects was performed as described in Materials and methods. (C) Mobility shift analysis of liver nuclear extracts (5 µg of protein) and the 32P-labeled D-site of the albumin enhancer/promoter (1 ng) was performed as described in Figure 2. The position of the bound DNA is indicated by an arrow. Samples shown are: control (lanes 2 and 4); cancer-cachexia (lanes 3 and 5). On lane 1, the probe was processed without nuclear extracts. (D) Representative immunohistochemistry using antibodies for C/EBPβ (in red) and C/EBPβ-PSer288 (in green), simultaneously. In control liver, C/EBPβ was localized in the nucleus and C/EBPβ-PSer288 was undetectable. In cancer-cachexia liver, C/EBPβ-PSer288 was detected in the cytoplasm in yellow due to the superimposition of C/EBPβ (red) and C/EBPβ-PSer288 (green).
Discussion
In this study, we have shown that in normal hepatocytes, TNF-α induces both a site-specific phosphorylation of C/EBPβ within the NLS and its cytoplasmic localization. This phosphorylation is required for C/EBPβ’s cytoplasmic localization, which is mediated by CRM1 and results in decreased transcription from the albumin enhancer/promoter. Our results provide a relevant physiological example of mammalian transcriptional regulation exerted through phosphorylation-dependent nucleocytoplasmic protein transport (Hogan and Rao, 1999). Inhibition of albumin synthesis is a common finding in patients with cachexia of cancer, AIDS and diseases characterized by chronic inflammation, and a major contributor to the morbidity of these diseases (Tracey and Cerami, 1993). Because the TNF-α mouse model of cachexia closely resembles human cachexia (Oliff et al., 1987; Brenner et al., 1990; Tracey et al., 1990), it provides a valuable system to analyze the molecular mechanisms responsible for inhibition of albumin synthesis. The TNF-α serum levels in TNF-α mice are only moderately increased (100–300 pg/ml) at the onset of weight loss (Buck and Chojkier, 1996) but, at the time of sacrifice, values are similar to those found in patients with trauma or infectious, parasitic and neoplastic diseases (Scuderi et al., 1986; Waage et al., 1987; Grau et al., 1989; Goodman et al., 1990).
We demonstrated the presence of MDA–protein adducts in the livers of TNF-α mice, indicating activation of an oxidative pathway (Houglum et al., 1990; Chaudhary et al., 1994). These findings are in agreement with evidence that TNF-α can stimulate oxidative stress in many cells and tissues (Wong et al., 1989; Schulze-Osthoff et al., 1993). In addition, NOS2 expression was induced markedly in the livers of TNF-α mice. This effect was rescued by treating these animals with the antioxidants d-α-tocopherol or BW755c, indicating that the liver induction of NOS2 in TNF-α mice is mediated by an enhanced oxidative stress. In addition, in primary mouse hepatocytes, SIN-1, a NO donor, was sufficient to induce the phosphorylation of C/EBPβ on Ser239 within the NLS, and its nuclear export. NO plays an important role in redox signaling by interacting with superoxide to generate peroxynitrite (Lipton et al., 1993; Stamler, 1994) and by nitrosylation of mitochondrial complex I (Clementi et al., 1998). NOS expression is increased markedly in both the liver of patients with chronic viral hepatitis and hepatoma cells transfected with the hepatitis B virus cDNA (Majano et al., 1998). Therefore, NO may also mediate the inhibition of albumin expression in chronic viral hepatitis. In addition, wild-type p53 and tumor-derived p53 mutants can repress C/EBPβ-mediated transactivation of the albumin promoter (Kubicka et al., 1999).
The phosphorylation of C/EBPβ on Ser239 and its cytoplasmic localization, as well as the decreased albumin gene expression in TNF-α mice, were reversed by treating these animals with antioxidants (d-α-tocopherol or BW755c) or a NOS inhibitor (nitro-l-arginine), indicating that oxidative pathways and activation of NOS are critical for the phosphorylation of C/EBPβ on Ser239 and the inhibition of albumin transcription in TNF-α mice (Brenner et al., 1990). Neither the supplemental dose of d-α-tocopherol nor the nitro-l-arginine treatment utilized in our study were toxic to control or TNF-α mice (Buck and Chojkier, 1996; Chojkier et al., 1998) for up to 8 weeks. The effects of antioxidants and nitro-l-arginine were not the spurious result of decreased synthesis of TNF-α, since they affected neither the secretion of biologically active TNF-α by these cells, nor the serum levels of TNF-α in TNF-α animals (Buck and Chojkier, 1996). This pathway may include activation of other cytokines such as interleukin (IL)-1β and IL-6 (Dinarello et al., 1986; Akira et al., 1990b; Schulze-Osthoff et al., 1993), which in turn could contribute to the inhibition of albumin gene expression in cachexia (Flores et al., 1989; Fong et al., 1989; Strassman et al., 1992; Spiegelman and Hotamisligil, 1993). However, LPS administration to mice, another inducer of inflammation, resulted in the increased nuclear expression of C/EBPβ, which remained unphosphorylated on Ser239. The induction of C/EBPβ mRNA, C/EBPβ protein in the nucleus and C/EBPβ binding activities by LPS has been reported previously (Akira et al., 1990a; Alonzi et al., 2001).
Although binding of the albumin enhancer/promoter D-site (Zaret, 1994; Chojkier, 1995) by liver nuclear proteins from TNF-α mice and cachectic patients was substantially decreased, the total hepatocyte expression of C/EBPβ, a major D-site activator protein (Akira et al., 1990a; Poli et al., 1990; Trautwein et al., 1993; Chojkier, 1995), remained unchanged. We demonstrated that in highly differentiated, quiescent primary mouse hepatocytes, TNF-α was able to stimulate phosphorylation of endogenous mouse C/EBPβ on Ser239 within its NLS (Williams et al., 1997), which inhibits C/EBPβ’s characteristic nuclear localization in normal hepatocytes (Descombes et al., 1990; Buck et al., 1994), and its binding to cognate DNA sequences necessary for high level transcription from the albumin gene (Maire et al., 1989; Descombes et al., 1990; Trautwein et al., 1993; Chojkier, 1995). Serine phosphorylation of the Saccharo myces cerevisiae transcription factor SWI5 within the NLS prevents its nuclear import (Moll et al., 1991). In addition to the neutralization by phosphorylation on Ser239 of the mouse C/EBPβ NLS, C/EBPβ also has a sequence within the leucine zipper domain (L271-SRE-L-ST-L-RN-L) that closely conforms to the consensus leucine-rich nuclear export signal (NES) (Mattaj and Englmeir, 1998). Our results suggest that this putative NES becomes available for interaction with CREM1 following the phosphorylation of C/EBPβ’s NLS induced by TNF-α or NO. Cells expressing the non-phosphorylatable rat C/EBPβ-Ala240 mutant (homologous to mouse C/EBPβ Ala239) were refractory to the inhibitory effects of TNF-α on albumin transcription. As expected, TNF-α did not induce the cytoplasmic localization of the non-phosphorylatable mutants of rat C/EBPβ-Ala240 or mouse C/EBPβ-Ala239. Nuclear proteins from cells expressing the phosphorylation-mimic rat C/EBPβ-Asp240 mutant, which, like phosphorylated C/EBPβ did not localize to the nucleus, displayed negligible binding to the D-site and these cells did not transcribe albumin reporter chimeric genes. There are other examples of how modification of nucleocytoplasmic transport can regulate gene expression (for reviews see Mattaj and Englmeir, 1998; Hogan and Rao, 1999).
In our experiments, C/EBPβ’s normal nuclear localization in differentiated hepatocytes was disrupted by TNF-α in both mice and primary cell cultures. When C/EBPβ is phosphorylated on Ser239 by TNF-α (or by its mediator, NO), it is found in the cytoplasm. This could be the result of either impaired nuclear import or enhanced nuclear export of C/EBPβ. However, our studies suggest that the latter is a probable explanation for the following reasons: (i) in hepatocytes, a 30 min TNF-α or SIN-1 treatment was sufficient to redistribute C/EBPβ from the nucleus to the cytoplasm, before protein synthesis and impaired nuclear import could contribute significantly to the cytoplasmic localization; (ii) in TNF-α mice, although C/EBPβ was localized predominantly in the cytoplasm of hepatocytes, the total hepatic C/EBPβ was unchanged, ruling out increased nuclear degradation of C/EBPβ as an explanation; (iii) C/EBPβ’s nuclear localization is conserved in TNF-α-treated hepatocytes, by blocking with leptomycin B the activity of CRM1, which is critical for nuclear protein export (Ossareh-Nazari et al., 1997; Kudo et al., 1999); and (iv) in TNF-α-treated hepatocytes, C/EBPβ was co-localized in the cytoplasm and associated with CRM1. Nonetheless, the inhibition of CRM1 by leptomycin B was associated with a more moderate phosphorylation of C/EBPβ on Ser239 compared with TNF-α-treated cells, suggesting that the impaired nuclear export of C/EBPβ-PSer239 may allow dephosphorylation of C/EBPβ-PSer239 by a protein phosphatase within the nucleus. Alternatively, the appropriate localization and/or activity of the as yet unidentified TNF-α-induced protein kinase for C/EBPβ-Ser239 could be decreased by leptomycin B.
It is of interest that, in transformed rat hepatocyte and human colorectal cancer cell lines, which exhibit the abnormal absence of nuclear C/EBPβ, TNF-α and antioxidants, respectively, promote the nuclear import of C/EBPβ (Yin et al., 1996; Chinery et al., 1997), indicating a reversal of the normal nucleocytoplasmic transport in transformed and cancer cell lines through as yet unidentified mechanisms. Furthermore, in the human colorectal cancer cell line, activation of PKA leads to phosphorylation of C/EBPβ on Ser299, which is required for C/EBPβ’s nuclear translocation (Chinery et al., 1997). Nonetheless, following the administration of LPS or partial hepatectomy, there is an increase in the nuclear expression of C/EBPβ and of the transcriptional repressor C/EBPβ 21 kDa (LIP) (Akira et al., 1990a; Diehl and Yang, 1994; Alonzi et al., 2001), which may be physiologically relevant for liver regeneration induced by hepatectomy and acute phase reaction induced by LPS. In contrast to the partial hepatectomy model, CCl4-induced hepatic regeneration following hepatocellular necrosis and inflammation (a common feature of human liver disease) (Houglum et al., 1995; Buck et al., 1999; Rudolph et al., 2000) is associated with a marked decrease in D-site binding affinity of liver nuclear extracts (Mueller et al., 1990).
Why do TNF-α mice, but not C/EBPβ–/– mice (Screpanti et al., 1995; M.Buck, unpublished data), have a decreased expression of albumin? Based on our results, there are two mechanisms that can explain the apparent discrepancy. One would expect other C/EBPs expressed in hepatocytes to substitute, but because C/EBPβ-PSer239 has a normal leucine zipper domain, it could act in hepatocytes of TNF-α mice as a dominant-negative by forming homo- and heterodimers with leucine zipper proteins, including other C/EBPs (Descombes et al., 1990; Cao et al., 1991), and inducing their nuclear export. Moreover, C/EBPα, which is expressed (Greenbaum et al., 1998) and functions as a transcription factor (Wang et al., 1995; Greenbaum et al., 1998) normally, under basal conditions, in C/EBPβ–/– mice (Screpanti et al., 1995) is also a target of the TNF-α signal transduction pathway, given the presence of the conserved serine phosphoacceptor in its DNA-binding domain (Cao et al., 1991). We found that TNF-α also stimulates the phosphorylation on Ser300 and the nuclear export of C/EBPα in C/EBPβ–/– hepatocytes.
Our preliminary findings in patients with cancer-cachexia indicate that the cascade leading to decreased albumin gene expression also involves oxidative stress, NOS2 expression, phosphorylation of C/EBPβ on Ser288 (the human homolog to mouse Ser239), impaired nuclear localization of C/EBPβ and albumin-binding activities. Therefore, this study provides insights into the mechanisms responsible for the decreased albumin expression in cachexia, which may in turn lead to novel therapeutic approaches for patients with cancer, AIDS and chronic inflammatory diseases.
Materials and methods
Mouse model of cachexia and LPS injection
CHO cells stably transfected with either the human TNF-α gene cloned into a CMV mammalian expression vector (TNF-α cells) or with the mammalian expression vector alone (CHO cells, control) were grown in Dulbecco’s modified Eagle’s essential medium (DMEM) supplemented with 10% fetal calf serum. The TNF-α cells, but not the CHO cells, produced TNF-α. Four-week-old male homozygous nude mice were injected intramuscularly with either 3.5 × 106 CHO cells or 3.5 × 106 TNF-α cells as described (Brenner et al., 1990; Buck and Chojkier, 1996). Animals in the treatment groups received d-α-tocopherol (8 IU/g of foodstuff), BW755c (10 mg/kg in 100 µl of 1% sucrose, twice a day orally) or nitro-l-arginine (50 µg/ml of drinking water) (Buck and Chojkier, 1996). Animals had free access to food and water and they were sacrificed at 30 days post-inoculation. Also, 4-week-old male heterozygous nude mice received hTNF-α (R&D) (500 ng, intramuscularly every 12 h from 8 to 92 h) alone or with D-α-tocopherol acetate (150 IU, intraperitoneally every 24 h from 24 to 72 h). Animals were sacrificed throughout a 96 h period. TNF-α levels in cell culture media and mouse sera were measured by a biological cytolytic assay and by an enzyme-linked immunosorbent assay (ELISA), using monoclonal antibodies against human TNF-α (Brenner et al., 1990; Buck and Chojkier, 1996). LPS (Escherichia coli serotype 026:B6; Sigma, St Louis, MO) (1 mg/kg body weight) was administered intraperitoneally and mice were sacrificed after 9 h.
Cell culture and transfection
Primary hepatocytes isolated from C/EBPβ+/+ and C/EBPβ–/– mice and cultured in a serum-free medium on a collagen type I matrix remained quiescent and highly differentiated until day 12 (Buck et al., 1999). Hepatocytes were either treated with TNF-α for 30 min (10 ng/ml) or not treated (control). In some experiments, hepatocytes were treated for 30 min with SIN-1 (0.6 µM) or for 45 min with leptomycin B (10 ng/ml). HepG2 cells (ATTC) were cultured in DMEM supplemented with 10% fetal bovine serum (Gibco). Treatments were started after transfection and were provided every 24 h. DNA transfection was carried out with Lipofectin as recommended by the manufacturer (BRL). The ALB-CAT plasmid (1 µg) (Kioussis et al., 1979) was transfected with CMV- C/EBPβ, CMV-C/EBPβ-Ala240, CMV-C/EBPβ- Asp240 and/or control pcDNA (1 µg each). Cells were harvested 48 h after transfection and CAT content determined by ELISA following the manufacturer’s (5 Prime→3 Prime) recommendations (Buck and Chojkier, 1996).
Nuclear extracts preparation and gel retardation assay
Tissue or cells were homogenized in the presence of protease and phosphatase inhibitors as described (Descombes et al., 1990; Buck et al., 1994). The nuclei were sedimented in a 30% sucrose cushion by a 4000 g centrifugation at 4°C for 20 min, and lysed (Descombes et al., 1990; Buck et al., 1994; Buck and Chojkier, 1996). Gel retardation analyses of protein–DNA complexes were performed with equal amounts of nuclear extracts (5 µg protein) as described (Descombes et al., 1990; Trautwein et al., 1993; Buck et al., 1994). The sense oligonucleotides were D-site (5′-TGGTATGATTTTGTAATGGGG-3′), B-site (5′-AGTATGGTTAATGATCTA-3′) and Sp1 (5′-GGGGCGGGGC-3′). Expression of recombinant C/EBPβ protein was induced with isopropyl-β-d-thiogalactopyranoside (IPTG), from the coding region of C/EBPβ cDNA (Descombes et al., 1990) cloned into a pRSET vector, and purified with Probond Resin (Invitrogen) (Buck and Chojkier, 1996).
Phosphorylation of C/EBPβ in vivo
Day 5 hepatocytes (60 × 106 in 30 ml of serum-free, phosphate-free hepatocyte medium) were labeled with 2 mCi/ml (total 30 mCi) of [32P]orthophosphate (ICN, Irvine, CA) for 18 h (Buck et al., 1999). Cells were either treated with TNF-α (10 ng/ml) for 30 min before harvest or not treated (control). Phosphopeptide mapping of endogenous C/EBPβ was performed as described (Boyle et al., 1991).
Immunohistochemistry, RNA determination and immunoblotting
Immunohistochemical detection of MDA–protein adducts and NOS2 was performed in liver sections (Houglum et al., 1990; Buck et al., 1994), using the avidin–biotin–alkaline phosphatase system (Vector Labora tories, Inc.) and antibodies against MDA-lysine epitopes (Houglum et al., 1990) and NOS2 (Transduction Laboratories). Immunofluorescence staining was observed in livers using a triple-channel fluorescence microscope. Fluorochromes utilized were fluorescein isothiocyanate (FITC), Texas red, 4′,6-diamidino-2-phenylindole (DAPI) and TOTO-3 (Molecular Probes). Fluorescent labels were also analyzed by confocal microscopy in hepatocytes using purified antibodies reactive to both human and mouse C/EBPβ, C/EBPα, CRM1 (Santa Cruz Biotechnologies) and C/EBPβ-PSer239 (induced in rabbits with the epitope CIAVRK-PSer239-RDKAK linked to keyhole limpet hemocyanin). RNA was purified from liver with Trizol following the manufacturer’s protocol (BRL). RNase protection assays were performed with specific riboprobes for albumin or 18S RNA (Ambion) as described previously (Buck and Chojkier, 1996).
Immunoprecipitation and immunoblotting
C/EBPβ, C/EBPβ-Ala239, C/EBPβ-Asp239, C/EBPα and CRM1 were detected by immunoblotting immunoprecipitates from hepatocyte lysates (Buck et al., 1994) following the chemiluminescence protocol (DuPont) and using purified IgG antibodies as described (Trautwein et al., 1993).
Human subjects
Preliminary studies were conducted in six male patients (62 ± 5 years; serum albumin 2.5 ± 0.4 g/dl) with cachexia (30% decrease in ideal body weight over the preceding 6 months) due to cancer (anal, esophageal, lung and kidney carcinomas, multiple myeloma and malignant mesothelioma) but without liver metastasis, and in four male control subjects (67 ± 4 years; serum albumin 3.7 ± 0.2 g/dl) with normal body weight and without cancer (University of California, San Diego, CA IRB #991275). Liver biopsies were obtained during elective intra-abdominal surgery and processed as described above.
Statistical analysis
Results are expressed as the mean (± SEM) of at least triplicate experiments unless stated otherwise. Either Student’s t- or Fisher’s exact test was used to evaluate the differences of the means between groups, with a P-value of <0.05 as significant.
Acknowledgments
Acknowledgements
We are grateful to W.G.Hardison (University of California, San Diego) for his valuable comments, S.Moncada (University College London, UK) for providing BW755c, V.Poli (University of Dundee, UK) for the C/EBPβ–/– KO founder mice, and S.Tilghman (Princeton University) for the [palb(9–12) CAT] reporter plasmid. We thank J.Meisenhelder for phosphopeptide synthesis, Dean Kirby for phosphopeptide mass spectroscopy, Chon Garcia for rabbit immunization, Tao Li for her technical assistance, and Jennifer Cordeau and Amy King for the preparation of this manuscript. This study was supported in part by grants from the US Public Health Service, Department of Veterans Affairs and the American Liver Foundation. M.B. was supported by the NIH Program Project NCI-CA54418. T.H. is a Frank and Else Schilling American Cancer Society Research Professor.
References
- Akira S., Isshiki,H., Sugita,T., Tanabe,O., Kinoshita,S., Nishio,Y., Nakajima,T., Hirano,T. and Kishimoto,T. (1990a) A nuclear factor for IL-6 expression (NF-IL6) is a member of a C/EBP family. EMBO J., 9, 1897–1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akira S., Hirano,T., Taga,T. and Kishimoto,T. (1990b) Biology of multifunctional cytokines; IL 6 and related molecules (IL and TNF). FASEB J., 4, 2860–2867. [PubMed] [Google Scholar]
- Alonzi T., Maritano,D., Gorgoni,B., Rizzuto,G., Libert,C. and Poli,V. (2001) Essential role of STAT3 in the control of the acute-phase response as revealed by inducible gene activation in the liver. Mol. Cell. Biol., 21, 1621–1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutler B. (1992) Tumor Necrosis Factors: The Molecules and Their Emerging Role in Medicine. Raven Press, New York.
- Boyle W.J., van der Geer,P. and Hunter,T. (1991) Phosphopeptide mapping and phosphoamino acid analysis by two-dimensional separation on thin-layer cellulose plates. Methods Enzymol., 201, 110–149. [DOI] [PubMed] [Google Scholar]
- Braunwald E. (1994) Edema. In Isselbacher,K.J., Braunwald,E., Wilson,J.D., Martin,J.B. and Fauci,A.S. (eds), Harrison’s Principles of Internal Medicine. McGraw-Hill, New York, pp. 210–214.
- Brenner D.A., Buck,M., Feitelberg,S.P. and Chojkier,M. (1990) Tumor necrosis factor α inhibits albumin gene expression in a murine model of cachexia. J. Clin. Invest., 85, 248–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck M. and Chojkier,M. (1996) Muscle wasting and dedifferentiation induced by oxidative stress in a murine model of cachexia is prevented by inhibitors of nitric oxide synthesis and antioxidants. EMBO J., 15, 1753–1765. [PMC free article] [PubMed] [Google Scholar]
- Buck M., Turler,H. and Chojkier,M. (1994) LAP (NF-IL6), a tissue-specific transcriptional activator, is an inhibitor of hepatoma cell proliferation. EMBO J., 13, 851–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck M., Houglum,K. and Chojkier,M. (1996) Tumor necrosis factor α inhibits collagen α1(I) gene expression and wound healing in a murine model of cachexia. Am. J. Pathol., 149, 195–204. [PMC free article] [PubMed] [Google Scholar]
- Buck M., Poli,V., van der Geer,P., Chojkier,M. and Hunter,T. (1999) Phosphorylation of rat serine 105 or mouse threonine 217 in C/EBPβ is required for hepatocyte proliferation induced by TGFα. Mol. Cell, 4, 1087–1092. [DOI] [PubMed] [Google Scholar]
- Cao Z., Umek,R.M. and McKnight,S.L. (1991) Regulated expression of three C/EBP isoforms during adipose conversion of 3T3-L1 cells. Genes Dev., 5, 1538–1552. [DOI] [PubMed] [Google Scholar]
- Chaudhary A.K., Nokubo,M., Reddy,G.R., Yeola,S.N., Morrow,J.D., Blair,I.A. and Marnett,L.J. (1994) Detection of endogenous malondialdehyde–deoxyguanosine adducts in human liver. Science, 265, 1580–1582. [DOI] [PubMed] [Google Scholar]
- Cheng J., Turksen,K., Yu,Q.C., Schreiber,H., Teng,M. and Fuchs,E. (1992) Cachexia and graft-vs.-host-disease-type skin changes in keratin promoter-driven TNFα transgenic mice. Genes Dev., 6, 1444–1456. [DOI] [PubMed] [Google Scholar]
- Chinery R., Brockman,J.A., Dransfield,D.T. and Coffey,R.J. (1997) Antioxidant-induced nuclear translocation of CCAAT/enhancer-binding protein β. A critical role for protein kinase A-mediated phosphorylation or Ser299. J. Biol. Chem., 272, 30356–30361. [DOI] [PubMed] [Google Scholar]
- Chojkier M. (1995) Regulation of liver-specific gene expression. In Boyer,J. and Ockner,R. (eds), Progress in Liver Diseases. W.B. Saunders, Orlando, pp. 37–61. [PubMed]
- Chojkier M., Houglum,K., Lee,K.S. and Buck,M. (1998) Long- and short-term d-α-tocopherol supplementation inhibits liver collagen α1(I) gene expression. Am. J. Physiol., 275, G1480–G1485. [DOI] [PubMed] [Google Scholar]
- Christy R.J., Kaestner,K.H., Geiman,D.E. and Lane,M.D. (1991) CCAAT/enhancer binding protein gene promoter: binding of nuclear factors during differentation of 3T3-L1 preadipocytes. Proc. Natl Acad. Sci. USA, 88, 2593–2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clementi E., Brown,G., Feelisch,M. and Moncada,S. (1998) Persistent inhibition of cell respiration by nitric oxide: crucial role of S-nitrosylation of mitochondrial complex I and protective action of glutathione. Proc. Natl Acad. Sci. USA, 95, 7631–7636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Descombes P., Chojkier,M., Lichtsteiner,S., Falvey,E. and Schibler,U. (1990) LAP, a novel member of the C/EBP gene family, encodes a liver-enriched transcriptional activator protein. Genes Dev., 4, 1541–1551. [DOI] [PubMed] [Google Scholar]
- Diehl A.M. and Yang,S.Q. (1994) Regenerative changes in C/EBPα and C/EBPβ modulate expression binding to the C/EBP site in the c-fos promoter. Hepatology, 19, 447–456. [PubMed] [Google Scholar]
- Dinarello C.A., Cannon,J.G., Wolff,S.M., Bernheim,H.A., Beutler,B., Cerami,A., Figari,I.S., Palladino,M.A.,Jr and O’Connor,J.V. (1986) Tumor necrosis factor (cachectin) is an endogenous pyrogen and induces production of IL-1. J. Exp. Med., 163, 1433–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Husseini A.E., Bladen,C., Williams,J.A., Reiner,P.B. and Vincent,S.R. (1998) Nitric oxide regulates cyclic GMP-dependent protein kinase phosphorylation in rat brain. J. Neurochem., 71, 676–683. [DOI] [PubMed] [Google Scholar]
- Flores E.A., Bistran,B.R., Pompselli,J.J., Dinarello,C.A., Blackburn,G.L. and Istfan,N.W. (1989) Infusion of tumor necrosis/cachectin promotes muscle catabolism in the rat: a synergistic effect with interleukin 1. J. Clin. Invest., 83, 1614–1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong Y. et al. (1989) Cachectin/TNF or IL-1 induces cachexia with redistribution of body proteins. Am. J. Physiol., 256, R659–R665. [DOI] [PubMed] [Google Scholar]
- Geller D.A. (1993) Cytokines, endotoxin and glucocorticoids regulate the expression of inducible nitric oxide synthase in hepatocytes. Proc. Natl Acad. Sci. USA, 90, 522–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman J.C., Robertson,C.S., Grossman,R.G. and Narayan,R.K. (1990) Elevation of tumor necrosis factor in head injury. J. Neuroimmunol., 30, 213–217. [DOI] [PubMed] [Google Scholar]
- Grau G.E., Taylor,T.E., Molyneux,M.E., Wirima,J.J., Vassalli,P., Hommel,M. and Lambert,P. (1989) Tumor necrosis factor and disease severity in children with Falciparum malaria. N. Engl. J. Med., 320, 1586–1591. [DOI] [PubMed] [Google Scholar]
- Greenbaum L., Li,W., Cressman,D., Peng,Y., Ciliberto,G., Poli,V. and Taub,R. (1998) CCAAT enhancer-binding protein β is required for normal hepatocyte proliferation in mice after partial hepatectomy. J. Clin. Invest., 102, 996–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunfeld C. and Feingold,K.R. (1992) Metabolic disturbances and wasting in the acquired immunodeficiency syndrome. N. Engl. J. Med., 327, 329–337. [DOI] [PubMed] [Google Scholar]
- Henkel T., Machleidt,T., Alkalay,I., Kronke,M., Ben-Neriah,Y. and Baeuerle,P. (1993) Rapid proteolysis of IκB-α is necessary for activation of transcription factor NF-κB. Nature, 365, 182–185. [DOI] [PubMed] [Google Scholar]
- Hogan P.G. and Rao,A. (1999) Transcriptional regulation. Modification of nuclear export? Nature, 398, 200–201. [DOI] [PubMed] [Google Scholar]
- Houglum K., Filip,M., Witztum,J.L. and Chojkier,M. (1990) Malondialdehyde and 4-hydroxynonenal protein adducts in plasma and liver of rats with iron overload. J. Clin. Invest., 86, 1991–1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houglum K., Buck,M., Alcorn,J., Contreras,S., Bornstein,P. and Chojkier,M. (1995) Two different cis-acting regulatory regions direct cell-specific transcription of the collagen α1(I) gene in hepatic stellate cells and in skin and tendon fibroblasts. J. Clin. Invest., 96, 2269–2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idriss S., Gudi,T., Casteel,D., Kharitonov,V., Pilz,R.B. and Boss,G. (1999) Nitric oxide regulation of gene transcription via soluble guanylate cyclase and type I cGMP-dependent protein kinase. J. Biol. Chem., 274, 9489–9493. [DOI] [PubMed] [Google Scholar]
- Katz S., Kowenz-Leutz,E., Muller,C., Meese,K., Ness,S.A. and Leutz,A. (1993) The NF-M transcription factor is related to C/EBPβ and plays a role in signal transduction, differentiation and leukemogenesis of avian myelomonocytic cells. EMBO J., 12, 1321–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kioussis D., Hamilton,R., Hanson,R.W., Tilghman,S.M. and Taylor,J.M. (1979) Construction and cloning of rat albumin structural gene sequences. Proc. Natl Acad. Sci. USA, 76, 4370–4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobzik L., Reid,M.B., Bredt,D.S. and Stamler,J.S. (1994) Nitric oxide in skeletal muscle. Nature, 372, 546–548. [DOI] [PubMed] [Google Scholar]
- Kubicka S., Kuhnel,F., Zender,L., Lenhard Rudolph,K., Plümpe,J., Manns,M. and Trautwein,C. (1999) p53 represses CAAT enhancer-binding protein (C/EBP)-dependent transcription of the albumin gene. J. Biol. Chem., 274, 32137–32144. [DOI] [PubMed] [Google Scholar]
- Kudo N., Matsumori,N., Taoka,H., Fujiwara,D., Schreiner,E.P., Wolff,B., Yoshida,M. and Horinouchi,S. (1999) Leptomycin B inactivates CRM1/exportin 1 by covalent modification at a cysteine residue in the central conserved region. Proc. Natl Acad. Sci. USA, 96, 9112–9117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landschulz W.H., Johnson,P.F., Adashi,E.Y., Graves,B.J. and McKnight,S.L. (1988) Isolation of a recombinant copy of the gene encoding C/EBP. Genes Dev., 2, 786–800. [DOI] [PubMed] [Google Scholar]
- Lipton S.A., Choi,Y.-B., Pan,Z.-H., Lei,S.Z., Chen,H.-S.V., Sucher,N.J., Loscalzo,J., Singel,D.J. and Stamler,J.S. (1993) A redox-based mechanism for the neuroprotective and neurodestructive effects of nitric oxide and related nitroso-compounds. Nature, 364, 626–632. [DOI] [PubMed] [Google Scholar]
- Maire P., Wuarin,J. and Schibler,U. (1989) The role of cis-acting promoter elements in tissue-specific albumin gene expression. Science, 244, 343–346. [DOI] [PubMed] [Google Scholar]
- Majano P.L., Garciá-Monzón,C., López-Cabrera,M., Lara-Pezzi,E., Fernández-Ruiz,E., Garcia-Iglesias,C., Borque,M.J. and Moreno-Otero,R. (1998) Inducible nitric oxide synthase expression in chronic viral hepatitis. J. Clin. Invest., 101, 1343–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattaj I. and Englmeir,L. (1998) Nucleocytoplasmic transport: the soluble phase. Annu. Rev. Biochem., 67, 265–306. [DOI] [PubMed] [Google Scholar]
- Moll T., Tebb,G., Surana,U., Robitsch,H. and Nasmyth,K. (1991) The role of phosphorylation and the CDC28 protein kinase in cell cycle-regulated nuclear import of the S.cerevisiae transcription factor SWI5. Cell, 66, 743–758. [DOI] [PubMed] [Google Scholar]
- Mueller C.R., Maire,P. and Schibler,U. (1990) DBP, a liver-enriched transcriptional activator, is expressed late in ontogeny and its tissue specificity is determined posttranscriptionally. Cell, 61, 279–291. [DOI] [PubMed] [Google Scholar]
- Nachury M.V. and Weis,K. (1999) The direction of transport through the nuclear pore can be inverted. Proc. Natl Acad. Sci. USA, 96, 9622–9627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliff A., Defeo-Jones,D., Boyer,M., Martinez,D., Kiefer,D., Vuocolo,G., Wolfe,A. and Socher,S.H. (1987) Tumors secreting human TNFα/cachectin induce cachexia in mice. Cell, 50, 555–563. [DOI] [PubMed] [Google Scholar]
- Ossareh-Nazari B., Bachelerie,F. and Dargemont,C. (1997) Evidence for a role of CRM1 in signal-mediated nuclear protein export. Science, 278, 141–144. [DOI] [PubMed] [Google Scholar]
- Poli V., Mancini,F.P. and Cortese,R. (1990) IL6-DBP, a nuclear protein involved in interleukin-6 signal transduction, defines a new family of leucine zipper proteins related to C/EBP. Cell, 63, 643–653. [DOI] [PubMed] [Google Scholar]
- Roubenoff R., Roubenoff,R.A., Cannon,J.G., Kehayias,J.J., Zhuang,H., Dawson-Hughes,B., Dinarello,C.A. and Rosenberg,I.H. (1994) Rheumatoid cachexia: cytokine-driven hypermetabolism accompany ing reduced body cell mass in chronic inflammation. J. Clin. Invest., 93, 2379–2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph K., Chang,S., Millard,M., Schreiber-Agus,N. and DePinho,R. (2000) Inhibition of experimental liver cirrhosis in mice by telomerase gene delivery. Science, 287, 1253–1258. [DOI] [PubMed] [Google Scholar]
- Schulze-Osthoff K., Beyaert,R., Vandevoorde,V., Haegeman,G. and Fiers,W. (1993) Depletion of the mitochondrial electron transport abrogates the cytotoxic and gene-inductive effects of TNF. EMBO J., 12, 3095–3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Screpanti I. et al. (1995) Lymphoproliferative disorder and imbalanced T-helper response in C/EBPβ-deficient mice. EMBO J., 14, 1932–1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scuderi P., Lam,K.S., Ryan,K.J., Peterson,E., Sterling,K.E., Finley,P.R., Ray,C.G., Slymen,D.J. and Salmon,S.E. (1986) Raised serum levels of tumor necrosis factor in parasitic infections. Lancet, ii, 1364–1365. [DOI] [PubMed] [Google Scholar]
- Spiegelman B.M. and Hotamisligil,G.S. (1993) Through thick and thin: wasting, obesity and TNFα. Cell, 73, 625–627. [DOI] [PubMed] [Google Scholar]
- Stamler J.S. (1994) Redox signaling: nitrosylation and related interactions of nitric oxide. Cell, 78, 931–936. [DOI] [PubMed] [Google Scholar]
- Strassman G., Fong,M., Kenney,J.S. and Jacob,C.O. (1992) Evidence for the involvement of interleukin 6 in experimental cancer cachexia. J. Clin. Invest., 89, 1681–1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todorov P., Carluk,P., McDevitt,T., Coles,B., Fearon,K. and Tisdale,M. (1996) Characterization of a cancer cachectic factor. Nature, 379, 739–742. [DOI] [PubMed] [Google Scholar]
- Tracey K.J. and Cerami,A. (1993) Tumor necrosis factor, other cytokines and disease. Annu. Rev. Cell. Biol., 9, 317–343. [DOI] [PubMed] [Google Scholar]
- Tracey K.J., Morgello,S., Koplin,B., Fahey,T.J.I., Fox,J., Aledo,A., Manogue,K.R. and Cerami,A. (1990) Metabolic effects of cachectin/tumor necrosis factor are modified by site of production. J. Clin. Invest., 86, 2014–2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trautwein C., Caelles,C., van der Geer,P., Hunter,T., Karin,M. and Chojkier,M. (1993) Transactivation by NF-IL6/LAP is enhanced by phosphorylation of its activation domain. Nature, 364, 544–547. [DOI] [PubMed] [Google Scholar]
- Trautwein C., van der Geer,P., Karin,M., Hunter,T. and Chojkier,M. (1994) Protein kinase A and C site-specific phosphorylations of LAP (NF-IL6) modulate its binding affinity to DNA-recognition elements. J. Clin. Invest., 93, 2554–2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voth R., Rossol,S., Klein,K., Hess,G., Schutt,K.H., Schroder,H.C., Büschenfelde,K.-H.M.Z. and Müller,W.E.G. (1990) Differential gene expression of IFN-α and tumor necrosis factor-α in peripheral blood mononuclear cells from patients with AIDS related complex and AIDS. J. Immunol., 144, 970–975. [PubMed] [Google Scholar]
- Waage A., Halstensen,A. and Espevik,T. (1987) Association between tumor necrosis factor in serum and fatal outcome in patients with meningococcal disease. Lancet, i, 355–357. [DOI] [PubMed] [Google Scholar]
- Wang N.D., Finegold,M.J., Bradley,A., Ou,C.N., Abdelsayed,S.V., Wilde,M.D., Taylor,L.R., Wilson,D.R. and Darlington,G.J. (1995) Impaired energy homeostasis in C/EBPα knockout mice. Science, 269, 1108–1112. [DOI] [PubMed] [Google Scholar]
- West J.B. (1990) Physiological Basis of Medical Practice. Williams & Wilkins, Baltimore, MD.
- Williams S.C., Angerer,N. and Johnson,P.F. (1997) C/EBP proteins contain nuclear localization signals imbedded in their basic regions. Gene Expression, 6, 371–385. [PMC free article] [PubMed] [Google Scholar]
- Wong G.H.W., Elwell,J.H., Oberley,L.W. and Goeddel,D.V. (1989) Manganous superoxide dismutase is essential for cellular resistance to cytotoxicity of tumor necrosis factor. Cell, 58, 923–931. [DOI] [PubMed] [Google Scholar]
- Yamaoka I., Taniguchi,Y. and Sasaki,Y. (1997) Rapid communication: nucleotide sequence of bovine C/EBPβ gene. J. Anim. Sci., 75, 587–587. [DOI] [PubMed] [Google Scholar]
- Yin M., Yang,S.Q., Lin,H.Z., Lane,M.D., Chatterjee,S. and Diehl,A. (1996) Tumor necrosis factor α promotes nuclear localization of cytokine-inducible CCAAT/enhancer binding protein isoforms in hepatocytes. J. Biol. Chem., 271, 17974–17978. [DOI] [PubMed] [Google Scholar]
- Yoneda T., Alsina,M.A., Chavez,J.B., Bonewald,L., Nishimura,R. and Mundy,G.R. (1991) Evidence that tumor necrosis factor plays a pathogenetic role in the paraneoplastic syndromes of cachexia, hypercalcemia and leukocytosis in a human tumor in nude mice. J. Clin. Invest., 87, 977–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaret K. (1994) Genetic control of hepatocyte differentiation. In Arias,I.M., Boyer,J.L., Fausto,N., Jakoby,W., Schachter,D. and Shafritz,D.A. (eds), The Liver: Biology and Pathobiology. Raven Press, New York, NY, pp. 53–68.