Abstract
IκB kinase (IKK) is a key mediator of NF-κB activation induced by various immunological signals. In T cells and most other cell types, the primary target of IKK is a labile inhibitor of NF-κB, IκBα, which is responsible for the canonical NF-κB activation. Here, we show that in T cells infected with the human T-cell leukemia virus (HTLV), IKKα is targeted to a novel signaling pathway that mediates processing of the nfκb2 precursor protein p100, resulting in active production of the NF-κB subunit, p52. This pathogenic action is mediated by the HTLV-encoded oncoprotein Tax, which appears to act by physically recruiting IKKα to p100, triggering phosphorylation-dependent ubiquitylation and processing of p100. These findings suggest a novel mechanism by which Tax modulates the NF-κB signaling pathway.
Keywords: IKK/NF-κB2 p100–Tax/proteasome/ubiquitylation/virus–host interaction
Introduction
The NF-κB family of transcription factors participates in regulation of diverse biological processes, including immune responses, cell growth and apoptosis (Gilmore et al., 1996; Ghosh et al., 1998; Sha, 1998; Barkett and Gilmore, 1999). Mammalian cells express five NF-κB members, RelA, RelB, c-Rel, p50 and p52, which function as various homo- and heterodimers (Siebenlist et al., 1994). The NF-κB factors are normally sequestered in the cytoplasm through physical interaction with ankyrin repeat-containing inhibitors, including IκBα and related proteins (Baldwin, 1996). A well-characterized pathway leading to NF-κB activation is through phosphorylation and subsequent degradation of IκBα (Brockman et al., 1995; Brown et al., 1995). This canonical NF-κB signaling pathway depends on a multisubunit IκB kinase (IKK), which responds to various stimuli, such as the inflammatory cytokine tumor necrosis factor α (TNF-α), the mitogen phorbol 12-myristate 13-acetate (PMA) and certain viral proteins (Sun and Ballard, 1999; Karin and Ben-Neriah, 2000; Hiscott et al., 2001). The IKK is composed of two catalytic subunits, IKKα and IKKβ, and a regulatory subunit, IKKγ (also named NEMO, IKKAP1 or FIP-3) (Karin and Ben-Neriah, 2000). Recent gene knockout studies suggest that the two catalytic subunits of IKK have distinct physiological functions. While IKKβ is essential for signal-induced IκBα phosphorylation, IKKα is largely dispensable for this function (Hu et al., 1999; Li et al., 1999a,b,d; Tanaka et al., 1999). IKKα appears to regulate keratinocyte differentiation in a NF-κB- independent manner (Hu et al., 2001).
Another level of NF-κB regulation is via processing of the NF-κB1 and NF-κB2 precursor proteins p105 and p100, a proteasome-catalyzed event required to generate p50 and p52, respectively (Fan and Maniatis, 1991; Siebenlist et al., 1994). These precursor proteins contain ankyrin repeats at their C-terminal portions and function as IκB-like NF-κB inhibitors (Rice et al., 1992; Mercurio et al., 1993). The processing of p105 and p100 not only generates mature p50 and p52 but also results in liberation of the sequestered NF-κB members. By and large, the processing of p105 occurs constitutively and seems to be mediated primarily by a cotranslational mechanism (Lin et al., 1998a, 2000). Although p105 serves as a target of IKK, the IKK-mediated p105 phosphorylation may regulate signal-induced degradation, rather than limited processing, of p105 (Belich et al., 1999; Heissmeyer et al., 1999). However, inducible processing of p105 may occur under certain conditions (Orian et al., 2000). In contrast to p105, the cotranslational processing of p100 is extremely poor (Heusch et al., 1999), and p100 does not undergo inducible processing or degradation in response to various cellular stimuli (Sun et al., 1994). The poor basal processing of p100 seems to be due to the presence of a C-terminal processing-inhibitory domain (PID) (Xiao et al., 2001). Thus, in most cell types, p100 is expressed largely as its unprocessed form, while a large proportion of p105 is processed to p50. Consistently, p50 forms prototypical NF-κB heterodimers, but p52 is only involved in specific functions of NF-κB such as B-cell growth and formation of germinal centers in peripheral lymphoid organs (Caamano et al., 1998; Franzoso et al., 1998).
Although the cellular signals stimulating p100 processing remain unknown, the NF-κB-inducing kinase (NIK) has been shown to play a key regulatory role in this proteolysis event (Xiao et al., 2001). Expression of NIK, but not of other MAP kinase kinase kinases (MAP3Ks), in mammalian cells induces p100 processing (Xiao et al., 2001). Consistently, nik gene mutation in alymphoplasia (aly) mice leads to a block of p100 processing in vivo (Xiao et al., 2001). The NIK-induced p100 processing involves site-specific phosphorylation and subsequent ubiquitylation of p100, although it is unclear whether NIK or a NIK-associated kinase catalyzes the p100 phosphorylation (Xiao et al., 2001). A recent study indicates that IKKα may be involved in NIK-induced p100 processing (Senftleben et al., 2001). NIK does not induce p100 processing in mouse embryonic fibroblasts (MEF) lacking IKKα, and this defect can be rescued by transfection of IKKα. However, since many cellular stimuli capable of activation of both IKKα and IKKβ fail to induce p100 processing, it is clear that a novel mechanism is involved in the NIK/p100 pathway. It is likely that NIK-induced p100 processing may involve additional mechanisms other than activation of IKKα.
The tight control of p52 generation may be important for proper regulation of NF-κB function in cell growth and survival. Indeed, emerging evidence suggests that deregulated production of p52 may cause abnormal lymphocyte proliferation and transformation. Mice overexpressing p52, in the absence of its precursor p100, develop gastric and lymphoid hyperplasia (Ishikawa et al., 1997). In humans, the nfκb2 gene is frequently involved in chromosomal translocations associated with various lymphomas (Rayet and Gelinas, 1999). In all cases studied, the rearranged nfκb2 genes encode p100 mutants lacking their C-terminal region (Rayet and Gelinas, 1999), which contains the PID, thus rendering them capable of constitutive processing (Xiao et al., 2001). Interestingly, overproduction of p52 is also associated with T-cell transformation induced by the human T-cell leukemia virus type 1 (HTLV-I) (Lanoix et al., 1994).
HTLV-I is an oncogenic retrovirus etiologically associated with the development of an acute T-cell malignancy, adult T-cell leukemia (ATL) (Poiesz et al., 1980; Yoshida et al., 1982). HTLV-I transforms T cells via its regulatory protein Tax, which acts by inducing aberrant expression of a large array of cellular genes involved in T-cell growth and survival (Ressler et al., 1996). Tax induces many of these genes through activation of the transcription factor NF-κB (Sun and Ballard, 1999). Recent studies suggest that Tax physically associates with IKK (Chu et al., 1998) and stimulates the catalytic activity of this cellular kinase (Geleziunas et al., 1998; Uhlik et al., 1998; Yin et al., 1998; reviewed by Sun and Ballard, 1999). This virus-specific effect is dependent on IKKγ (Yamaoka et al., 1998; Harhaj et al., 2000), which serves as an adaptor for recruiting Tax to the IKK catalytic subunits (Chu et al., 1999; Harhaj and Sun, 1999; Jin et al., 1999; Xiao et al., 2000). Tax-induced IKK activation is responsible for the persistent phosphorylation of IκBα and nuclear expression of NF-κB in HTLV-infected T cells (Sun and Ballard, 1999).
Since IKK activation by cellular stimuli does not enhance p100 processing, it has remained unclear how HTLV-I induces active production of p52. In this study, we have demonstrated that the Tax protein functions as a potent inducer of p100 processing. Tax interacts specifically with p100 via two short helices, and this molecular interaction is essential for Tax induction of p100 processing. Interestingly, the Tax-induced p100 processing does not require NIK but involves the non-canonical IKK component IKKα, which phosphorylates specific serines at the C-terminal region of p100. An important mechanism of Tax action in this virus-specific pathway is to recruit IKKα to p100. These studies provide an example of how a retroviral oncoprotein modifies the function of a cellular protein kinase and targets it to a pathogenic pathway.
Results
Active processing of p100 in HTLV-I infected human T cells
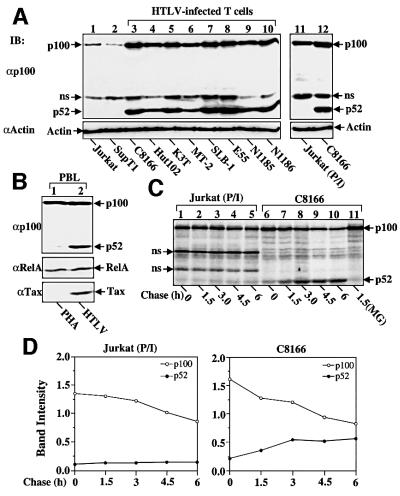
To investigate the mechanism underlying the aberrant expression of p52 in HTLV-infected T cells, we examined whether HTLV infection induces processing of p100. The level of p100 and p52 was analyzed in a large panel of HTLV-transformed T-cell lines, established either from ATL patients or by in vitro HTLV infection of human primary T cells (Uhlik et al., 1998). In two HTLV-negative control T-cell lines (Jurkat and Sup T1), little p52 could be detected (Figure 1A); in contrast, a remark ably high level of p52 was detected in each of the HTLV-transformed T-cell lines (Figure 1A and see Supplementary figure 1 available at The EMBO Journal Online). The amount of p100 was relatively high in the HTLV-positive cells; this was likely due to nfκb2 gene induction by the activated NF-κB in these cells (Liptay et al., 1994; Sun et al., 1994). Indeed, activation of NF-κB in the control Jurkat cells by mitogen treatment also led to heightened expression of p100 (lane 11). However, consistent with the inability of p100 to respond to cellular activation signals (Sun et al., 1994), the level of p52 in the mitogen-stimulated cells was still extremely low (lane 11); a faint p52 band could be detected only after prolonged exposure of the immunoblotting films (data not shown). A similar result was obtained with TNF-α-stimulated T cells (data not shown). Thus, the induction of p100 processing in HTLV-transformed T cells appeared to be mediated specifically by HTLV. This idea was confirmed in freshly isolated human T cells. When these cells were activated by the polyclonal T-cell activator phytohemagglutinin (PHA), only a little p52 could be detected, although these cells expressed an abundant level of p100 (Figure 1B, lane 1). In contrast, infection of the cells with HTLV led to potent p52 production (lane 2). A parallel control immunoblotting assay showed that the level of the RelA subunit of NF-κB was comparable in both the normal and HTLV-infected T cells. These results clearly demonstrate that HTLV infection induces abnormal processing of p100.
Fig. 1. Active processing of p100 associated with HTLV-I infection. (A) Constitutive processing of p100 in HTLV-transformed T cell lines. Whole-cell extracts (20 µg), isolated from the control or HTLV-infected T-cell lines, were subjected to IB using anti-p100 (upper panel) or anti-actin (lower panel) antibodies. In lane 11, the cells were stimulated with the mitogen PMA (10 ng/ml) and ionomycin (1 µM) for 8 h before extract preparation. (B) Processing of p100 induced by HTLV-I infection of human primary T cells. Cell lysates were isolated from PHA-stimulated or HTLV-infected human primary T cells and subjected to IB using the antibodies indicated. (C) Pulse–chase labeling. Jurkat cells (stimulated for 2 h with PMA and ionomycin, P/I) or HTLV-infected C8166 cells were pulse-labeled for 45 min followed by chase for the time periods indicated. In lane 11, the cells were chased in the presence of a proteasome inhibitor, MG132 (50 µM). p100 and its processing product p52 are indicated. Two non-specific bands are indicated by ns. (D) Densitometry quantitation of the radiolabeled p100 and p52 bands presented in (C).
We then performed a pulse–chase labeling study to examine whether HTLV-induced p100 processing occurs at the post-translational or cotranslational levels. As shown in Figure 1C and D, in HTLV-infected T cells, p52 was actively generated from the pulse-labeled precursor protein p100, which was sensitive to a proteasome inhibitor, MG132 (lane 11). The precursor–product relationship was most evident within the first 3 h of chase (Figure 1D, right panel), but the processing rate was gradually retarded after longer periods of chase. Although the mechanism mediating such a pattern of processing remains unclear, it may involve feedback inhibition by the processed product (p52), a mechanism proposed for the regulation of p105 processing (Harhaj et al., 1996). During the late time points of chase, the level of p100 in Jurkat cells was also decreasing, but it was not associated with significant generation of p52 (Figure 1D, left panel). This slow loss of p100 was likely due to protein decay, although it might also result from mitogen-stimulated degradation (the Jurkat cells were stimulated with mitogens). Never theless, generation of p52 was clearly a specific event for the HTLV-infected cells. These results suggest the involvement of a post-translational mechanism in HTLV-induced p100 processing. It remains to be determined whether Tax also enhances the cotranslational processing of p100, a mechanism known to mediate the basal processing of p100 (Heusch et al., 1999).
Induction of p100 processing is mediated by the Tax oncoprotein
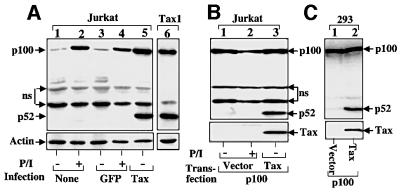
Since Tax serves as the key mediator of HTLV-induced T-cell transformation (Ressler et al., 1996), we investigated whether this viral protein was responsible for the induction of p100 processing. Jurkat T cells were infected with retroviral expression vectors encoding either Tax or the green fluorescent protein (GFP), followed by analysis of the processing of endogenous p100. Expression of Tax led to active production of p52 (Figure 2A, lane 5), a result reminiscent of that obtained with HTLV-infected T cells (see Figure 1). The aberrant p100 processing was also detected in a Tax-transformed T-cell line, Tax1 (lane 6), which was generated by delivering the Tax cDNA into human cord blood T cells (Grassmann et al., 1992). In contrast, GFP expression in Jurkat cells did not alter the fate of p100 (lane 3), as compared with uninfected cells (lane 1). Moreover, mitogen stimulation of both uninfected and GFP-infected cells failed to induce p52 production (lanes 2 and 4). Thus, Tax appeared to be an inducer of p100 processing. We further examined this possibility by transient transfection studies. When expressed in Jurkat cells, the exogenous p100 remained largely unprocessed (Figure 2B, upper panel, lane 1), and this state was not altered by mitogen stimulation (lane 2). However, the p100 was processed efficiently when it was coexpressed with Tax (lane 3). A similar result was obtained with non-lymphoid 293 cells (Figure 2C). These results further demonstrate that the productive processing of p100 associated with HTLV infection results from a specific action of Tax.
Fig. 2. Induction of p100 processing by Tax. (A) Jurkat cells were either not infected or infected with retroviral vectors encoding GFP or Tax. The cells were either not treated (–) or stimulated for 8 h with PMA plus ionomycin (+) followed by extract preparation and IB analysis using anti-p100 (upper panel) or anti-actin (lower panel). In lane 6, IB was performed using an extract isolated from a Tax-transformed T-cell line, Tax1. Non-specific bands are indicated by ns. (B) Jurkat cells (5 × 106) were transfected with p100 (1 µg) together with either an empty vector or Tax (1 µg) followed by mitogen treatment as indicated. Exogenous p100/p52 and Tax were analyzed by IB using anti-p100 (upper panel) and anti-Tax (lower panel). (C) Induction of p100 processing by Tax in 293 cells. 293 cells were transfected with p100 (0.5 µg) together with either an empty vector or Tax (1 µg) followed by IB assays.
IKKγ, but not NIK, is essential for Tax-induced p100 processing
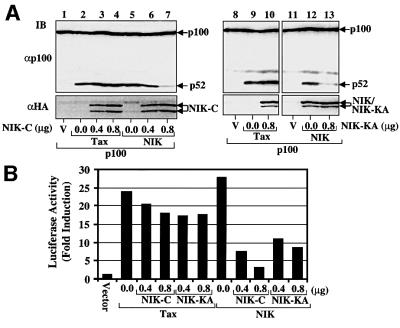
We have recently shown that NIK is a kinase that specifically induces p100 processing, although the cellular signals triggering this cellular pathway remain unknown (Xiao et al., 2001). We investigated the role of NIK in Tax-induced p100 processing by dominant-negative inhibition assays. The C-terminal portion of NIK (NIK-C) is known to serve as a potent inhibitor of the wild-type NIK (Lin et al., 1998b). Consistently, we found that overexpression of NIK-C efficiently blocked NIK-induced p100 processing (Figure 3A, lanes 5–7). However, NIK-C had no effect on Tax-induced p100 processing (lanes 2–4). A similar result was obtained with a catalytically inactive NIK (lanes 8–13). This result indicates strongly that NIK is not involved in Tax induction of p100 processing. Parallel reporter gene assays showed that the NIK mutants partially inhibited Tax-induced κB enhancer activity and more efficiently inhibited the NIK-induced κB activation (Figure 3B). Since the Tax-induced p100 processing was even slightly enhanced by the NIK mutants, the inhibitory effect of NIK mutants on Tax activation of κB may not be linked to the p100 processing.
Fig. 3. Effect of dominant-negative NIK mutants on Tax- and NIK-induced p100 processing. (A) 293 cells were transfected with Tax (1 µg) or HA-tagged NIK (0.8 µg) together with the indicated amounts of HA-tagged NIK(650–947) (NIK-C) or NIK(KK429–430AA) (NIK-KA). All the cells were also transfected with p100 (0.5 µg). The processing of p100 and expression of HA-tagged NIK proteins were analyzed by IB using anti-p100 (upper panel) and anti-HA (lower panel), respectively. Wild-type NIK and NIK-KA comigrate in the gel. (B) 293 cells were transfected as in (A) except that p100 was replaced with κB–TATA–luciferase and a control Rennila luciferase reporter driven by the constitutive thymidine kinase promoter. Dual luciferase assays were performed as described in Materials and methods. The κB-specific luciferase activity is presented as fold induction relative to the basal level measured in cells transfected with the pcDNA empty vector. The values shown are representative of three independent experiments.
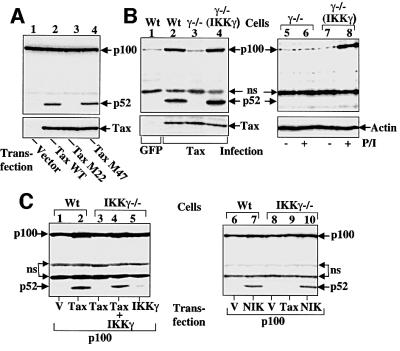
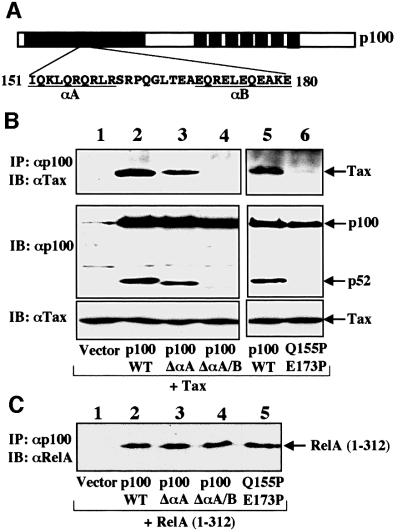
To further assess the mechanism by which Tax induces p100 processing, we analyzed the effect of two well-studied Tax mutants, M22 and M47 (Smith and Greene, 1990), on this specific function. M22 is defective in IKK activation due to its inability to bind IKKγ, while M47 retains this specific activity (Chu et al., 1999; Harhaj and Sun, 1999; Jin et al., 1999). Interestingly, M47, but not M22, induced the processing of p100 (Figure 4A, lanes 3 and 4). This finding indicated the involvement of the IKK signaling pathway in Tax-induced p100 processing. We tested this idea further using an IKKγ-deficient Jurkat T-cell mutant, JM4.5.2, which is completely defective in NF-κB activation by both cellular stimuli and Tax (Harhaj et al., 2000). Tax was delivered to the wild-type or mutant Jurkat cells via retroviral infection. As expected, Tax induced p100 processing in the parental Jurkat cells (Figure 4B, lane 2). However, this proteolytic event did not occur in the IKKγ–/– cells (lane 3). This defect was completely rescued when the JM4.5.2 cells were reconstituted with exogenous IKKγ (lane 4). Expression of exogenous IKKγ alone or together with mitogens did not induce the processing of endogenous p100 (lanes 7 and 8), although IKKγ reconstitution rescued the induction of p100 de novo synthesis by the mitogens (lane 8).
Fig. 4. Requirement of IKK in Tax-induced p100 processing. (A) Induction of p100 processing by Tax and its mutants. 293 cells were transfected with an empty vector or the indicated Tax constructs followed by analyzing the p100 processing and expression of Tax by IB. (B) Parental Jurkat (Wt), an IKKγ-deficient (γ–/–) Jurkat derivative JM4.5.2 or an IKKγ-reconstituted JM4.5.2 [γ–/–(IKKγ)] (Rivera-Walsh et al., 2000) was infected with retroviral vectors encoding GFP or Tax, followed by IB analysis of endogenous p100/p52 (upper panel) and infected Tax (lower panel) (lanes 1–4). In lanes 5–8, the uninfected cells were either not treated (–) or stimulated for 8 h with PMA plus ionomycin (+), followed by IB analysis of endogenous p100/p52 (upper panel) and actin (lower panel). (C) Transient transfection was performed with the parental and IKKγ-deficient Jurkat cells and the expression vectors indicated (1 µg for p100 and Tax, 0.2 µg for IKKγ, 0.8 µg for NIK; V stands for pcDNA vector). Processing of exogenous p100 was analyzed by IB.
To confirm that the lack of p52 production in the IKKγ-deficient T cells was not due to the low level of p100, exogenous p100 was transfected to the parental and IKKγ-deficient Jurkat T cells either alone or together with Tax (Figure 4C). Tax-induced processing of the exogenous p100 occurred in wild-type (lane 2) but not IKKγ-deficient cells (lane 3), unless IKKγ was transfected (lane 4). Thus, IKKγ is an essential factor involved in Tax-induced p100 processing. Interestingly, in a parallel experiment, we found that NIK induced p100 processing in both the wild-type and IKKγ-deficient T cells (Figure 4C, lanes 7 and 10). This finding provides another piece of evidence indicating that Tax-induced p100 processing is not mediated through NIK.
Tax-induced p100 processing requires Tax–p100 physical interaction
An important question to consider is why other IKK stimulators could not induce p100 processing. One potential answer is that Tax not only activates IKK but may also functionally modify this cellular kinase in the induction of p100 processing. In this regard, Tax is known to interact physically with p100 (Béraud et al., 1994). Although the functional significance of this molecular interaction remains unclear, it is interesting to note that the M22 mutant of Tax, incapable of inducing p100 processing (Figure 4A), is inactive in p100 binding (Béraud et al., 1994). To assess the role of the Tax–p100 interaction in regulating p100 processing, we mapped the domain within p100 involved in Tax interaction. Interestingly, a region of p100 containing two short helices was found to be important for Tax binding (Figure 5A and B). Prior structural studies demonstrated that these two helices (αA and αB) (Figure 5A) are exposed on the surface of the protein but are not involved in DNA binding, dimerization or general folding of p52 (Cramer et al., 1997). It has been proposed that this region of p100 may interact with certain regulatory proteins (Cramer et al., 1997). Both αA and αB appeared to be involved in the binding of p100 to Tax. Deletion of αA partially inhibited the p100–Tax interaction (Figure 5B, upper panel, lane 3), while deletion of both helices largely abolished the binding (lane 4). Similarly, disruption of the α helices by proline substitutions also prevented the binding of p100 to Tax (lane 6). On the other hand, none of these structural alterations affected the dimerization function of p100 (Figure 5C), which was in agreement with the previous structural studies. More importantly, the p100 mutants defective in Tax binding failed to respond to Tax-induced processing (Figure 5B, middle panel, lanes 4 and 6). These results indicate that Tax associates physically with p100 via two α helices, and this interaction is essential for Tax induction of p100 processing.
Fig. 5. Interaction of Tax with p100 via two α helices, which is correlated with Tax-induced p100 processing. (A) Scheme of p100 indicating the two α helices, αA and αB, previously identified by X-ray crystallography (Cramer et al., 1997). (B) 293 cells were transfected with Tax together with the indicated p100 constructs. The p100 proteins were isolated from cell lysates by IP followed by detection of the coprecipitated Tax by IB (upper panel). The cell lysates were also subjected to IB to monitor the p100 processing (middle panel) and Tax expression (lower panel). (C) Dimerization of p100 mutants with the Rel homology domain of RelA, RelA(1–312). 293 cells were transfected with RelA(1–312) together with the p100 constructs, followed by coIP assays.
Tax recruits IKKα to p100
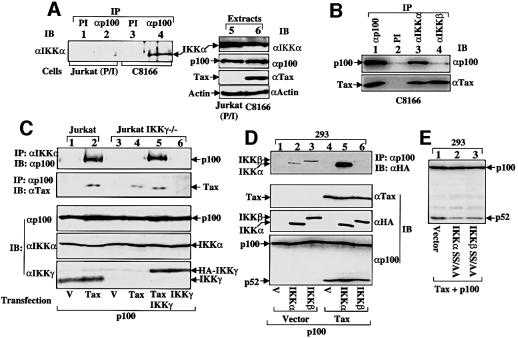
Since Tax interacts with both IKK (via IKKγ) (Chu et al., 1999; Harhaj and Sun, 1999; Jin et al., 1999) and p100, this raised the intriguing possibility that Tax specifically targets the IKK complex to p100, triggering phosphorylation-dependent p100 processing. We examined whether IKK is in the same complex with p100 in control Jurkat cells and HTLV-infected cells by coimmunoprecipitation (coIP). No significant binding between p100 and IKKα was detected in either untreated (data not shown) or mitogen-stimulated Jurkat T cells (Figure 6A, lane 2). Interestingly, a stable p100–IKKα association was readily demonstrated in the HTLV-infected C8166 cells (lane 4) as well as a number of other HTLV-infected T-cell lines (data not shown). The levels of IKKα and p100 proteins were similar in the mitogen-treated Jurkat cells and the C8166 cells (lanes 5 and 6). Interestingly, a parallel coIP assay revealed that IKKβ was not associated with the p100 complex (Figure 6B, upper panel, lane 4). This biochemical defect was not due to the inefficiency of the IKKβ antibody in IP, since it efficiently pulled down Tax (lower panel, lane 4). This finding suggests that although both IKKα and IKKβ form complexes with Tax (via IKKγ), only IKKα is present in the p100–Tax complex.
Fig. 6. Induction of p100–IKKα binding by Tax. (A) Stable association of p100 with IKKα in HTLV-infected T cells. Cell extracts were prepared from mitogen-stimulated Jurkat cells or the HTLV-infected C8166 cells and subjected to IP using either a preimmune serum (PI) or anti-p100 antibody, and the coprecipitated IKKα was detected by IB (lanes 1–4). The extracts were also analyzed directly by IB to detect the expression levels of IKKα, p100, Tax and actin (lanes 5 and 6). (B) IKKα, but not IKKβ, is in the p100 complex in HTLV-infected T cells. The C8166 cell lysates were subjected to IP using the indicated PI and immune sera. The p100 (upper panel) and Tax (lower panel) proteins in the immune complexes were analyzed by IB. (C) Binding of transfected p100 to endogenous IKKα in Tax-expressing cells. Parental Jurkat or its IKKγ-deficient variant was transfected with the pcDNA vector (V) or the expression vectors indicated below the figure (1 µg for Tax, 0.2 µg for HA-tagged IKKγ and 1 µg for p100). The p100–IKKα (top panel) and p100–Tax (second panel) interactions were analyzed by coIP. The lower three panels show IB analyses of the expression levels of p100, IKKα and IKKγ in the cell lysates. The larger molecular size of the exogenously transfected IKKγ (bottom panel, lanes 5 and 6) was due to its HA epitope. (D) Induction of p100 association with IKKα but not IKKβ by Tax. 293 cells were transfected with the expression vectors indicated (0.25 µg for IKKα and IKKβ, 1 µg for Tax, 0.5 µg for p100). All the cells also received the IKKγ expression vector (0.2 µg), since endogenous IKKγ is low in these non-lymphoid cells (Xiao et al., 2000). The p100–IKKα and p100–IKKβ interactions were analyzed by coIP (top panel), and the protein expression was analyzed by IB (lower panels). (E) Kinase-dead IKKα and IKKβ do not support Tax-induced p100 processing. The transfection was performed as in (D), followed by IB analysis of p100/p52 (upper), HA-tagged IKK mutants and Tax.
We then investigated whether Tax induces the binding of p100 to IKK in transiently transfected Jurkat T cells. Exogenous p100 was expressed in Jurkat T cells along with either an empty vector (Figure 6C, lane 1) or Tax (lane 2) followed by analysis of the binding of p100 to endogenous IKKα. Consistent with the results obtained with HTLV-infected T cells, Tax strongly induced the binding of exogenous p100 to IKKα (Figure 6C, top panel, lane 2). One potential mechanism by which Tax induces IKKα–p100 binding is through the physical interactions of Tax with IKK and p100. Since the Tax–IKK interaction is mediated through IKKγ, we examined whether this IKK regulatory subunit was essential for Tax-induced p100– IKKα complex formation. Transient transfection assays were performed using IKKγ-deficient Jurkat T cells. Interestingly, Tax failed to induce the binding of IKKα to p100 in these mutant cells (Figure 6C, top panel, lane 4). This functional defect was not due to problems in p100 transfection or expression of endogenous IKKα, since the level of these proteins was similar in the wild-type and IKKγ-deficient cells (panels 3 and 4). Furthermore, the binding of p100 to Tax was not affected in the IKKγ–/– cells (panel 2, lane 4). Finally, the defect in Tax-induced IKKα–p100 interaction was completely rescued when the cells were reconstituted with exogenous IKKγ (top panel, lane 5). Thus, IKKγ is required for Tax-mediated recruitment of IKKα to p100. This result is in full agreement with the finding that IKKγ is required for Tax induction of p100 processing (see Figure 4).
To further confirm that Tax induces the formation of a stable complex between p100 and IKKα, we performed coIP assays using 293 cells, which contain only low levels of endogenous IKK proteins. When overexpressed with p100, both IKKα and IKKβ could weakly interact with p100 (Figure 6D, top panel, lanes 2 and 3). However, only the p100–IKKα interaction was markedly enhanced in cells cotransfected with Tax (lane 5). In fact, the interaction of p100 with IKKβ diminished with Tax expression (lane 6), a result observed in multiple experiments (data not shown). Consistently, IKKα, but not IKKβ, potentiated Tax-induced p100 processing (bottom panel, lanes 5 and 6). Furthermore, kinase-dead mutants of IKKα or IKKβ did not support Tax-induced p100 processing (Figure 6E). Together, these studies indicate that Tax induction of p100 processing involves selective targeting of IKKα to p100.
Tax-induced p100 processing involves p100 phosphorylation and ubiquitylation
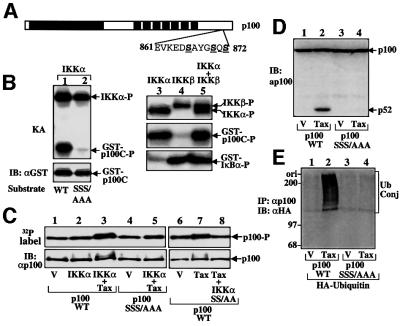
We have recently shown that p100 contains a serine cluster (S866, S870, S872, Figure 7A) phosphorylated by NIK or a NIK-associated kinase (Xiao et al., 2001). We examined whether these serines are targets of IKKα by in vitro kinase assays using purified IKKα. Interestingly, a glutathione S-transferase (GST) fusion protein containing the C-terminal region of p100 [GST–p100(860–898) or GST–p100C] was efficiently phosphorylated by IKKα (Figure 7B, upper panel, lane 1); this phosphorylation was completely abolished when the three C-terminal serines of p100 were substituted with alanines (lane 2). In a comparison kinase assay, we found that IKKα was much more efficient than IKKβ in phosphorylating p100C (Figure 7B, lanes 3–5, middle panel), although IKKβ was a better kinase for IκBα (lower panel). A similar result has been obtained using a longer p100C substrate (Senftleben et al., 2001).
Fig. 7. Involvement of phosphorylation and ubiquitylation in Tax-induced p100 processing. (A) Schematic diagram of p100 showing the phosphorylation site. (B) IKKα, but not IKKβ, is a strong p100 kinase in vitro. In vitro kinase assays were performed using recombinant IKKα, IKKβ or both (as indicated at the top of the figure) and different substrates: lane 1, GST–p100(860–898) (or GST–p100C) wild type; lane 2, GST–p100(860–898)SSS/AAA; lanes 3–5 middle panel, GST–p100(860–898); lanes 3–5 bottom panel, GST–IκBα(1–54). The phosphorylated p100 and IκBα substrates and autophosphorylated IKKs are indicated, and the levels of the wild-type and mutant forms of p100 substrates were monitored by IB analysis of the kinase assay membrane (lanes 1 and 2, lower panel). (C) Phosphorylation of p100 in vivo. 293 cells were transfected with the vectors indicated and metabolically labeled with [32P]orthophosphate. The radiolabeled p100 was isolated by IP, fractionated by SDS–PAGE, transferred onto nitrocellulose membrane and visualized by autoradiography (upper panel). The membrane was subsequently subjected to IB (using anti-p100) to monitor the total protein level of p100. (D) Processing analyses of the wild-type p100 and p100SSS/AAA in 293 cells. Tax induces the processing of p100WT but not the mutant. (E) Tax-induced p100 processing is associated with its ubiquitylation. 293 cells were transfected with the expression vectors indicated (1 µg for Tax, 0.5 µg for p100 and HA-ubiquitin). The p100 wild type and its phosphorylation-defective mutant were isolated by IP with anti-p100 followed by IB using anti-HA to detect the ubiquitin-conjugated p100 (Ub Conj).
To examine whether IKKα also phosphorylates p100 in vivo, wild-type p100 or p100 harboring the serine to alanine mutations (p100SSS/AAA) was transfected into 293 cells together with IKKα, either in the absence or presence of Tax. Consistent with a previous study (Betts and Nabel, 1996), p100 appeared to have constitutive phosphorylation sites, since it was labeled with 32P when expressed alone in 293 cells (Figure 7C, upper panel, lanes 1 and 6). Expression of IKKα alone did not lead to significant enhancement of p100 phosphorylation (lane 2). However, in the presence of Tax, IKKα induced potent phosphorylation of p100, as demonstrated by both enhanced 32P incorporation (Figure 7C, upper panel, lane 3) and generation of a band shift (lower panel, lane 3). Under these conditions, Tax alone only moderately induced p100 phosphorylation (lane 7), which was not enhanced by a mutant form of IKKα (IKKαSS/AA) (lane 8). These results are in agreement with the p100 processing data presented in Figure 6D, lower panel, and E. The generation of a band shift along with IKKα-induced p100 phosphorylation suggests that this inducible phosphorylation occurs at sites different from those for constitutive phosphorylation. More importantly, the inducible phosphorylation of p100 in vivo appears to require the same serines phosphorylated by IKKα in vitro, since the p100SSS/AAA mutant was not super-phosphorylated in cells expressing IKKα and Tax (lane 5).
Processing assays revealed that the p100SSS/AAA mutant was defective in Tax-induced processing (Figure 7D, lane 4), although it remained competent in Tax binding (Supplementary figure 3). This result suggests that phosphorylation of p100 at the specific C-terminal serines is required for its processing by Tax. Additionally, Tax-induced p100 processing was associated with ubiquitylation of this NF-κB precursor protein (Figure 7E, lane 2), and this modification was not detected with the phosphorylation-defective p100 mutant (lane 4). These results indicate that Tax induction of p100 processing involves IKKα-mediated phosphorylation and subsequent ubiquitylation of p100.
Discussion
Processing of the nfκb2 gene product, p100, to generate p52 is a tightly controlled process. Unlike IκBα degradation, p100 processing can not be induced by various cellular signals that stimulate the activity of IKK. Under physiological conditions, active processing of p100 appears to be a B-cell-specific event associated with B-cell differentiation (Liou et al., 1994). While the physiological signal triggering p100 processing remains unknown, the data presented here demonstrate that the HTLV-encoded Tax oncoprotein serves as a pathological stimulator of p100 processing in T cells. In both HTLV-infected and Tax-expressing T cells, p100 is actively converted to its product p52, an event that is associated with abundant nuclear expression of p52–cRel–NF-κB complexes (Supplementary figure 4). Since overproduction of p52 or loss of intact p100 is associated with lymphoid hyperplasia and transformation (Ishikawa et al., 1997; Rayet and Gelinas, 1999), induction of p100 processing may be a part of the oncogenic mechanism of HTLV.
Previous studies suggest that the nfκb2 gene is positively regulated by nuclear NF-κB (Liptay et al., 1994; Sun et al., 1994). Indeed, activation of NF-κB in both human primary T cells and the Jurkat leukemia T cells leads to enhanced expression of p100 (Figure 1). However, the induction of p100 protein synthesis by cellular signals is not associated with its active processing or generation of p52. Since p100 functions as an IκB-like molecule (Mercurio et al., 1993; Sun et al., 1994), the NF-κB- mediated nfκb2 gene induction appears to serve as an autoregulatory mechanism to prevent uncontrolled NF-κB activation during T-cell activation (Sun et al., 1994). Thus, the active processing of p100 in HTLV-infected T cells may not only generate large amounts of p52 but also disrupt the autoregulatory function of p100.
The mechanisms mediating NF-κB precursor processing have been extensively studied using the nfκb1 gene product p105, the precursor of p50. Recent evidence suggests that production of p50 is largely mediated through a cotranslational mechanism (Lin et al., 1998a). This process appears to be constitutive, since the ratio of p50 to p105 is close to 1:1 in most cell types. Such a pattern of p50 biogenesis is consistent with its involvement in the formation of the prototypical form of NF-κB, the p50–RelA heterodimer (Siebenlist et al., 1994). Recent studies suggest that p105 serves as a target for IKK. The C-terminus of p105 contains a sequence that is homologous to the IKK phosphorylation site in IκBα and can be phosphorylated by both IKKα and IKKβ (Heissmeyer et al., 1999; Orian et al., 2000; Salmerón et al., 2001). The IKK-mediated p105 phosphorylation may regulate the inducible degradation of this IκB-like molecule (Heissmeyer et al., 1999, 2001; Salmerón et al., 2001), a process that can be triggered by various extracellular stimuli (Harhaj et al., 1996; Salmerón et al., 2001; Syrovets et al., 2001). IKK has also been shown to promote p105 processing(Orian et al., 2000), although under what physiological conditions this regulation may play a role remains unknown.
As seen with p50, the basal production of p52 from the nfκb2 gene also occurs cotranslationally (Heusch et al., 1999). However, the efficiency of the cotranslational production of p52 is extremely poor (Heusch et al., 1999). Thus, in most cell types, the predominant nfκb2 gene product is p100. Since active production of p52 is essential for some biological processes, such as B-cell proliferation and the formation of germinal centers (Caamano et al., 1998; Franzoso et al., 1998), mechanisms regulating inducible p100 processing must exist. Indeed, we have recently shown that the NIK kinase plays a central role in regulating the productive processing of p100 (Xiao et al., 2001). When expressed in mammalian cells, NIK, but not various other MAP3Ks, induces productive processing of p100. Consistently, nik gene mutation in the aly mice is associated with a severe defect in p100 processing (Xiao et al., 2001), and they exhibit certain lymphoid deficiencies observed in nfκb2 knockout mice (Miyawaki et al., 1994). The mechanism by which NIK induces p100 processing has not been completely defined. When isolated from mammalian cells by IP, the NIK immune complex induces p100 phosphorylation at specific C-terminal serines, a modification that appears to trigger p100 ubiquitylation and processing (Xiao et al., 2001). This finding suggests that NIK or a NIK-associated protein kinase catalyzes the p100 phosphorylation. A more recent study suggests that IKKα may be involved in NIK-induced p100 processing. As seen with aly mice, IKKα–/– radiation chimera mice have a defect in p100 processing and exhibit deficiencies in germinal center formation (Senftleben et al., 2001). At least in MEF, IKKα is required for NIK-induced p100 processing. However, it seems clear that p100 processing is not simply the result of activation of IKKα by NIK, since many NF-κB inducers capable of activating both IKKα and IKKβ fail to induce p100 processing. Similarly, expression of IKK subunits in mammalian cells does not lead to effective p100 processing (Xiao et al., 2001). It is likely that NIK both activates IKKα and functionally cooperates with this downstream kinase in the induction of p100 processing. Clearly, more studies are warranted for a complete understanding of how the inducible processing of p100 is regulated by this cellular regulatory pathway.
The current study not only demonstrates a novel function of the retroviral protein Tax but also provides some insights into the general mechanism of p100 processing. Our data indicate that the engagement of p100 by IKKα requires Tax; this finding suggests that physical recruitment of IKKα to p100 may be a part of the mechanism mediating Tax-induced p100 processing. In this regard, Tax is known to interact with both IKK (via IKKγ) (Sun and Ballard, 1999) and p100 (Béraud et al., 1994). Although one consequence of the Tax–p100 interaction is the relocation of Tax from nucleus to the cytoplasm, a positive effect on NF-κB activation is also indicated, as the Tax M22 mutant (inactive in NF-κB activation) is defective in p100 binding (Béraud et al., 1994). Our current study shows that Tax binds to p100 via two α helices (Figure 5), previously revealed by three-dimensional structural analysis of p52 (Cramer et al., 1997). Results from mutagenesis analysis indicate that the Tax–p100 physical interaction is required for Tax induction of p100 processing. Disruption of the two short helices by deletion or proline substitutions abolishes Tax–p100 interaction and Tax-induced p100 processing (Figure 5B). The essential role of Tax–IKK interaction in inducing p100 processing is suggested by the requirement of IKKγ (Figure 4), an essential adaptor for Tax binding to both IKKα and IKKβ (Chu et al., 1999; Harhaj and Sun, 1999; Jin et al., 1999). Notably, IKKγ is not required for NIK-induced p100 processing (Figure 4C), suggesting the involvement of different mechanisms in the cellular and viral pathways of p100 processing. Dominant inhibition assays suggest that Tax-mediated p100 processing does not go through NIK (Figure 3). Furthermore, our preliminary studies suggest that NIK, but not Tax, is effective in the induction of p100 processing in mouse fibroblasts (Supplementary figure 2).
IKKα and IKKβ have been isolated as subunits of the same holoenzyme (Karin, 1999). However, increasing evidence indicates that these two kinases can mediate different functions (Hu et al., 1999, 2001; Li et al., 1999a,b,d; Tanaka et al., 1999). While IKKβ is an essential component of the canonical IKK complex, it does not seem to participate in regulation of p100 processing. This kinase is not effective in phosphorylating p100 in vitro (Figure 7; Senftleben et al., 2001) and does not promote Tax-induced p100 processing in transfected cells (Figure 6D). More interestingly, IKKα, but not IKKβ, forms a stable complex with p100 in Tax-expressing cells (Figure 6B and D). This finding indicates that Tax selectively targets IKKα to p100. Biochemical differences between IKKα and IKKβ have also been observed in a previous study (Li et al., 1999c). In HTLV-infected T cells, IKKβ is mostly present in a 300 kDa complex, while IKKα is present in a 700 kDa complex (Li et al., 1999c).
In summary, the results presented here demonstrate a novel mechanism by which retroviruses alter the cellular signaling machinery. We show that HTLV infection of human T cells causes productive processing of p100, an event that does not occur in mitogen-activated T cells. This pathological action of HTLV is mediated by the Tax oncoprotein, a finding that makes Tax the first agent known to stimulate the p100 processing pathway. Our data indicate that Tax not only stimulates the catalytic activity of IKK but also functionally cooperates with this kinase. Tax appears to specifically target IKKα to p100, triggering phosphorylation- and ubiquitylation-dependent p100 processing.
Materials and methods
Plasmid constructs and antibodies
Expression vectors encoding p100, Tax and its mutants, HA-tagged IKKα, IKKβ, IKKγ, NIK, NIK(KK429–430AA) and NIK(650–947) have been described (Sun et al., 1994; Uhlik et al., 1998; Xiao and Sun, 2000). HA-tagged IKKαSS/AA and IKKβSS/AA were created by site-directed mutagenesis; these mutants harbor alanine substitutions at Ser176 and Ser180 located in their activation loops and are catalytically inactive. RelA(1–312) encodes the N-terminal Rel homology domain of RelA (also named p65) and was provided by Dr W.C.Greene (Ganchi et al., 1992). The p100 mutants were generated by site-directed mutagenesis using pCMV4-p100 (Sun et al., 1994) as template. p100SSS/AAA harbors serine to alanine substitutions at residues 866, 870 and 872. p100ΔαA and p100ΔαB carry deletions of amino acids 151–160 and 170–181, respectively, and p100ΔαA/B carries both deletions. p100Q155P/E173P harbors proline substitutions at Gln155 and Glu173. GST–p100(860–898) (labeled GST–p100C in the figures) was generated by cloning the C-terminal fragment (amino acids 860–898) of p100 into the pGEX4T-3 vector (Pharmacia Biotech), and GST–p100(860–898)SSS/AAA was created by site-directed mutagenesis to substitute serines 866, 870 and 872 with alanines. The retroviral expression vector encoding Tax was constructed by inserting Tax cDNA (gift from Dr W.C.Greene) into the pCLXSN vector (Naviaux et al., 1996). The same vector was used to construct the GFP retroviral expression vector. The κB–TATA–luciferase reporter plasmid has been described (Sun et al., 1996). The HA-ubiquitin was generated by cloning human ubiquitin precursor cDNA (Lund et al., 1985) into the pcDNA vector downstream of an HA epitope tag. The anti-HA monoclonal antibody [horseradish peroxidase (HRP)-conjugated, 3F10] was from Roche Molecular Biochemicals. The anti-Tax monoclonal antibody was prepared from a hybridoma (168B17-46-34) provided by the AIDS Research and Reference Program, NIAID, NIH. The antibodies recognizing the N-terminus of p100 (anti-p100) and the N-terminus of RelA (anti-RelA or anti-p65) were kindly provided by Dr W.C.Greene (Sun et al., 1994). The antibodies for actin (C-2), IKKα (H744), IKKβ (H-4) and IKKγ (FL-419) were purchased from Santa Cruz Biotechnology, Inc.
Cell culture, transfection and retroviral infection
Human Jurkat (SVT35) (Harhaj et al., 2000), Sup T1 and HTLV-I-transformed T-cell lines (Uhlik et al., 1998) were cultured in RPMI medium supplemented with 10% fetal bovine serum, 2 mM l-glutamine and antibiotics. The kidney carcinoma cell line 293 was cultured in Dulbecco’s medium with the same supplements. The IKKγ-deficient Jurkat derivatives were generated by somatic mutagenesis of the parental SVT35 Jurkat cells (Harhaj et al., 2000; Rivera-Walsh et al., 2000). Human peripheral blood T cells were prepared from T-cell-enriched human blood (Biological Specialty Corporation, Colmar), activated with PHA (2 µg/ml) and infected with HTLV-I in vitro as described (Harhaj et al., 1999). Jurkat cells (5 × 106) and 293 cells (1 × 105, seeded in six-well plates) were transfected with DEAE–dextran (Holbrook et al., 1987). Retroviral transduction was performed using the pCLXSN system provided by Dr I.Verma (Naviaux et al., 1996). The cDNAs for GFP and Tax were cloned into the pCLXSN retroviral vector by standard methods, and the procedure for retrovirus production and infection was as described previously (Rivera-Walsh et al., 2000), except the inclusion of vesicular stomatitis virus glycoprotein (provided by Dr T.Friedmann, see Sharma et al., 1996) in the packaging.
Immunoblotting and coIP
Jurkat cells and the various HTLV-infected T-cell lines were either not treated or stimulated with PMA (10 ng/ml) and ionomycin (1 µM) for 8 h and then lysed in RIPA buffer [50 mM Tris–HCl pH 7.4, 150 mM NaCl, 1 mM EDTA, 0.25% Na-deoxycholate, 1% NP-40, 1 mM dithiothreitol (DTT), 0.1 mM phenylmethylsulfonyl fluoride (PMSF)] supplemented with 1/100 vol of a protease inhibitor cocktail (Ballard et al., 1990). For transfected and retrovirally infected cells, the cells were harvested 36–48 h post-transfection or 72 h after retroviral infection and lysed in RIPA buffer. The cell lysates (7 µg for 293 cells, 20 µg for the various T-cell lines and primary T cells) were subjected to SDS–PAGE and immunoblotting (IB) (Uhlik et al., 1998). CoIP assays were performed as described previously (Xiao et al., 2001). The amounts of cell lysate used in coIP were 250 µg for transfected 293 cells and 500 µg for the Jurkat and HTLV-infected T cells.
Cell labeling and pulse–chase assays
C8166 cells and mitogen-stimulated (2 h with PMA and ionomycin) Jurkat cells were starved for 1 h in Dulbecco’s modified Eagle’s medium (DMEM) lacking methionine and cysteine, and then metabolically labeled for 45 min with 350 µCi/ml [35S]methionine/[35S]cysteine. The pulse-labeled cells were chased for different time periods in regular DMEM supplemented with 10 mM cold methionine and cysteine, and then lysed in RIPA buffer supplemented with protease inhibitors. The radiolabeled p100 and p52 were isolated by IP using anti-p100, fractionated by SDS–PAGE and visualized by autoradiography. The radiolabeled protein bands were also quantitated by densitometry.
Luciferase reporter gene assays
Luciferase reporter gene assays were performed using the Dual-Luciferase Reporter Assay System (Promega). Briefly, 293 cells (1 × 105) were transfected in six-well plates with κB–TATA– luciferase (100 ng) and a control Rennila luciferase driven by the constitutively active thymidine kinase promoter (pRL-TK, 40 ng). At ∼40 h post-transfection, the cells were lysed in a reporter lysis buffer (Passive Lysis Buffer; Promega) at 500 µl/well. Luciferase activity was detected by mixing 5 µl of extract with 20 µl of luciferase substrates and measured in an FB12 luminometer (Zylux, Maryville, TN). The κB-specific luciferase activity was normalized based on the Rennila luciferase activity.
In vitro kinase assay and in vivo protein phosphorylation assay
In vitro kinase assays were performed essentially as described (Delhase et al., 1999). Purified recombinant IKKα and IKKβ (15 ng, provided by Dr M.Karin) were incubated for 20 min in a kinase assay buffer containing 1.5 µg of substrates [GST–p100(860–898), GST–p100(860– 898)SSS/AAA or GST–IκBα(1–54)]. The phosphorylated proteins were fractionated by SDS–PAGE, transferred onto nitrocellulose membranes and visualized by autoradiography.
For in vivo p100 phosphorylation assays, p100 was transfected into 293 cells together with the indicated expression vectors. About 40 h post-transfection, the cells were starved for 1 h in a phosphate-free medium and then metabolically labeled for 1.5 h with [32P]orthophosphate (0.5 mCi/ml). The cells were lysed in RIPA buffer supplemented with protease inhibitors and various phosphatase inhibitors (Sun et al., 1994). The radiolabeled p100 protein was isolated by IP using anti-p100, fractionated by SDS–PAGE, transferred onto nitrocellulose membrane and then visualized by autoradiography. The membrane was subsequently used for immunoblotting to analyze the protein level of p100.
In vivo ubiquitin conjugation assays
293 cells were transfected in six-well plates with HA-ubiquitin and p100 together with the indicated expression vectors. About 36–48 h post-transfection, the cells were incubated with a proteasome inhibitor, MG132 (50 µM), for 1.5 h and then lysed in RIPA buffer. The cell lysates were immediately subjected to IP using anti-p100. The agarose beads were washed three times with RIPA buffer followed by two additional washes with RIPA supplemented with 1 M urea. The eluted ubiquitin-conjugated p100 was analyzed by IB using HRP-conjugated anti-HA.
Supplementary data
Supplementary data for this paper are available at The EMBO Journal Online.
Acknowledgments
Acknowledgements
We thank W.C.Greene, T.Friedmann, M.Karin, I.Verma and the AIDS Research and Reference Program of NIAID for reagents. This work was supported by Public Health Service grant 2R01 CA68471 to S.-C.S. M.E.C. is supported by NIH postdoctoral fellowship 1F32CA83280.
References
- Baldwin A.S. Jr, (1996) The NF-κB and IκB proteins: new discoveries and insights. Annu. Rev. Immunol., 14, 649–683. [DOI] [PubMed] [Google Scholar]
- Ballard D.W., Walker,W.H., Doerre,S., Sista,P., Molitor,J.A., Dixon,E.P., Peffer,N.J., Hannink,M. and Greene,W.C. (1990) The v-rel oncogene encodes a κB enhancer binding protein that inhibits NF-κB function. Cell, 63, 803–814. [DOI] [PubMed] [Google Scholar]
- Barkett M. and Gilmore,T.D. (1999) Control of apoptosis by Rel/NF-κB transcription factors. Oncogene, 18, 6910–6924. [DOI] [PubMed] [Google Scholar]
- Belich M.P., Salmerón,A., Johnston,L.H. and Ley,S.C. (1999) TPL-2 kinase regulates the proteolysis of the NF-κB-inhibitory protein NF-κB1 p105. Nature, 397, 363–368. [DOI] [PubMed] [Google Scholar]
- Béraud C., Sun,S.-C., Ganchi,P.A., Ballard,D.W. and Greene,W.C. (1994) Human T-cell leukemia virus type I Tax associates with and is negatively regulated by the NF-κB2 p100 gene product: implications for viral latency. Mol. Cell. Biol., 14, 1374–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betts J.C. and Nabel,G.J. (1996) Differential regulation of NF-κB2(p100) processing and control by amino-terminal sequences. Mol. Cell. Biol., 16, 6363–6371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockman J.A., Scherer,D.C., McKinsey,T.A., Hall,S.M., Qi,X., Lee,W.Y. and Ballard,D.A. (1995) Coupling of a signal response domain in IκBα to multiple pathways for NF-κB activation. Mol. Cell. Biol., 15, 2809–2818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown K., Gerstberger,S., Carlson,L., Franzoso,G. and Siebenlist,U. (1995) Control of IκBα proteolysis by site-specific, signal-induced phosphorylation. Science, 267, 1485–1488. [DOI] [PubMed] [Google Scholar]
- Caamano J.H., Rizzo,C.A., Durham,S.K., Barton,D.S., Raventos-Suarez,C., Snapper,C.M. and Bravo,R. (1998) Nuclear factor (NF)-κB2 (p100/p52) is required for normal splenic microarchitecture and B cell-mediated immune responses. J. Exp. Med., 187, 185–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu Z.-L., DiDonato,J.A., Hawiger,J. and Ballard,D.W. (1998) The Tax oncoprotein of human T-cell leukemia virus type 1 associates with and persistently activates IκB kinases containing IKKα and IKKβ. J. Biol. Chem., 273, 15891–15894. [DOI] [PubMed] [Google Scholar]
- Chu Z.-L., Shin,Y.-A., Yang,J.-M., DiDonato,J.A. and Ballard,D.W. (1999) IKKγ mediates the interaction of cellular IκB kinases with the Tax transforming protein of human T cell leukemia virus type 1. J. Biol. Chem., 274, 15297–15300. [DOI] [PubMed] [Google Scholar]
- Cramer P., Larson,C.J., Verdine,G.L. and Muller,C.W. (1997) Structure of the human NF-κB p52 homodimer–DNA complex at 2.1 Å resolution. EMBO J., 16, 7078–7090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delhase M., Hayakawa,M., Chen,Y. and Karin,M. (1999) Positive and negative regulation of IκB kinase activity through IKKβ subunit phosphorylation. Science, 284, 309–313. [DOI] [PubMed] [Google Scholar]
- Fan C.M. and Maniatis,T. (1991) Generation of p50 subunit of NF-κB by processing of p105 through an ATP-dependent pathway. Nature, 354, 395–398. [DOI] [PubMed] [Google Scholar]
- Franzoso G. et al. (1998) Mice deficient in nuclear factor (NF)-κB/p52 present with defects in humoral responses, germinal center reactions and splenic microarchitecture. J. Exp. Med., 187, 147–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganchi P.A., Sun,S.-C., Greene,W.C. and Ballard,D.W. (1992) IκB/MAD-3 masks the nuclear localization signal of NF-κB p65 and acts with the C-terminal activation domain to inhibit NF-κB p65 DNA binding. Mol. Biol. Cell, 3, 1339–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geleziunas R. et al. (1998) Human T-cell leukemia virus type 1 Tax induction of NF-κB involves activation of the IκB kinase α (IKKα) and IKKβ cellular kinases. Mol. Cell. Biol., 18, 5157–5165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S., May,M.J. and Kopp,E.B. (1998) NF-κB and Rel proteins: evolutionarily conserved mediators of immune responses. Annu. Rev. Immunol., 16, 225–260. [DOI] [PubMed] [Google Scholar]
- Gilmore T.D., Koedood,M., Piffat,K.A. and White,D.W. (1996) Rel/NF-κB/IκB proteins and cancer. Oncogene, 13, 1367–1378. [PubMed] [Google Scholar]
- Grassmann R., Berchtold,S., Radant,I., Alt,M., Fleckenstein,B., Sodroski,J.G., Haseltine,W.A. and Ramstedt,U. (1992) Role of human T-cell leukemia virus type I x region proteins in immortalization of primary human lymphocytes in culture. J. Virol., 66, 4570–4575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harhaj E.W. and Sun,S.-C. (1999) IKKγ serves as a docking subunit of the IκB kinase and mediates interaction of IKK with the human T-cell leukemia virus Tax protein. J. Biol. Chem., 274, 22911–22914. [DOI] [PubMed] [Google Scholar]
- Harhaj E.W., Maggirwar,S.B. and Sun,S.-C. (1996) Inhibition of p105 processing by NF-κB proteins in transiently transfected cells. Oncogene, 12, 2385–2392. [PubMed] [Google Scholar]
- Harhaj E.W., Good,L., Xiao,G. and Sun,S.-C. (1999) Gene expression profiles in HTLV-I-immortalized T cells: deregulated expression of genes involved in apoptosis regulation. Oncogene, 18, 1341–1349. [DOI] [PubMed] [Google Scholar]
- Harhaj E.W., Good,L., Xiao,G.-T., Uhlik,M., Cvijic,M.E., Rivera,I. and Sun,S.-C. (2000) Somatic mutagenesis studies of NF-κB signaling in human T cells: evidence for an essential role of IKK gamma in NF-κB activation by T-cell costimulatory signals and HTLV-I Tax protein. Oncogene, 19, 1386–1391. [DOI] [PubMed] [Google Scholar]
- Heissmeyer V., Krappmann,D., Wulczyn,F.G. and Scheidereit,C. (1999) NF-κB p105 is a target of IκB kinases and controls signal induction of Bcl-3–p50 complexes. EMBO J., 18, 4766–4778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heissmeyer V., Krappmann,D., Hatada,E.N. and Scheidereit,C. (2001) Shared pathways of IκB kinase-induced SCFbTrCP-mediated ubiquitination and degradation for the NF-κB precursor p105 and IκBα. Mol. Cell. Biol., 21, 1024–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heusch M., Lin,L., Geleziunas,R. and Greene,W.C. (1999) The generation of nfkb2 p52: mechanism and efficiency. Oncogene, 18, 6201–6208. [DOI] [PubMed] [Google Scholar]
- Hiscott J., Kwon,H. and Genin,P. (2001) Hostile takeovers: viral appropriation of the NF-κB pathway. J. Clin. Invest., 107, 143–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holbrook N., Gulino,A. and Ruscetti,F. (1987) Cis-acting transcriptional regulatory sequences in the gibbon ape leukemia virus (GALV) long terminal repeat. Virology, 157, 211–219. [DOI] [PubMed] [Google Scholar]
- Hu Y., Baud,V., Delhase,M., Zhang,P., Deerinck,T., Ellisman,M., Johnson,R. and Karin,M. (1999) Abnormal morphogenesis but intact IKK activation in mice lacking the IKKα subunit of IκB kinase. Science, 284, 316–320. [DOI] [PubMed] [Google Scholar]
- Hu Y., Baud,V., Oga,T., Kim,K.I., Yoshida,K. and Karin,M. (2001) IKKα controls formation of the epidermis independently of NF-κB. Nature, 410, 710–714. [DOI] [PubMed] [Google Scholar]
- Ishikawa H., Carrasco,D., Claudio,E., Ryseck,R.P. and Bravo,R. (1997) Gastric hyperplasia and increased proliferative responses of lymphocytes in mice lacking the COOH-terminal ankyrin domain of NF-κB2. J. Exp. Med., 186, 999–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin D.-Y., Giordano,V., Kibler,K.V., Nakano,H. and Jeang,K.-T. (1999) Role of adaptor function in oncoprotein-mediated activation of NF-κB: HTLV-I Tax interacts directly with IκB kinase γ. J. Biol. Chem., 274, 17402–17405. [DOI] [PubMed] [Google Scholar]
- Karin M. (1999) How NF-κB is activated: the role of the IκB kinase (IKK) complex. Oncogene, 18, 6867–6874. [DOI] [PubMed] [Google Scholar]
- Karin M. and Ben-Neriah,Y. (2000) Phosphorylation meets ubiquitination: the control of NF-κB activity. Annu. Rev. Immunol., 18, 621–663. [DOI] [PubMed] [Google Scholar]
- Lanoix J., Lacoste,J., Pepin,N., Rice,N. and Hiscott,J. (1994) Overproduction of NF-κB2 (lyt-10) and c-Rel: a mechanism for HTLV-I Tax-mediated trans-activation via the NF-κB signalling pathway. Oncogene, 9, 841–852. [PubMed] [Google Scholar]
- Li Q., Lu,Q., Hwang,J.Y., Buscher,D., Lee,K.F., Izpisua-Belmonte,J.C. and Verma,I.M. (1999a) IKK1-deficient mice exhibit abnormal development of skin and skeleton. Genes Dev., 13, 1322–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Van Antwerp,D., Mercurio,F., Lee,K.F. and Verma,I.M. (1999b) Severe liver degeneration in mice lacking the IκB kinase 2 gene. Science, 284, 321–325. [DOI] [PubMed] [Google Scholar]
- Li X.H., Murphy,K.M., Palka,K.T., Surabhi,R.M. and Gaynor,R.B. (1999c) The human T-cell leukemia virus type-1 Tax protein regulates the activity of the IκB kinase complex. J. Biol. Chem., 274, 34417–34424. [DOI] [PubMed] [Google Scholar]
- Li Z.W., Chu,W., Hu,Y., Delhase,M., Deerinck,T., Ellisman,M., Johnson,R. and Karin,M. (1999d) The IKKβ subunit of IκB kinase (IKK) is essential for nuclear factor κB activation and prevention of apoptosis. J. Exp. Med., 189, 1839–1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L., DeMartino,G.N. and Greene,W.C. (1998a) Cotranslational biogenesis of NF-κB p50 by the 26S proteasome. Cell, 92, 819–828. [DOI] [PubMed] [Google Scholar]
- Lin X., Mu,Y., Cunningham,E.T.J., Marcu,K.B., Geleziunas,R. and Greene,W.C. (1998b) Molecular determinants of NF-κB-inducing kinase action. Mol. Cell. Biol., 18, 5899–5907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L., DeMartino,G.N. and Greene,W.C. (2000) Cotranslational dimerization of the rel homology domain of NF-κB1 generates p50-p105 heterodimers and is required for effective p50 production. EMBO J., 19, 4712–4722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liou H.C., Sha,W.C., Scott,M.L. and Baltimore,D. (1994) Sequential induction of NF-κB/Rel family proteins during B-cell terminal differentiation. Mol. Cell. Biol., 14, 5349–5359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liptay S., Schmid,R.M., Nabel,E.G. and Nabel,G.J. (1994) Tran scriptional regulation of NF-κB2: evidence for κB-mediated positive and negative autoregulation. Mol. Cell. Biol., 14, 7695–7703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund P.K., Moats-Staats,B.M., Simmons,J.G., Hoyt,E., D’Ercole,A.J., Martin,F. and Van Wyk,J.J. (1985) Nucleotide sequence analysis of a cDNA encoding human ubiquitin reveals that ubiquitin is synthesized as a precursor. J. Biol. Chem., 260, 7609–7613. [PubMed] [Google Scholar]
- Mercurio F., DiDonato,J.A., Rosette,C. and Karin,M. (1993) p105 and p98 precursor proteins play an active role in NF-κB-mediated signal transduction. Genes Dev., 7, 705–718. [DOI] [PubMed] [Google Scholar]
- Miyawaki S., Nakamura,Y., Suzuka,H., Koba,M., Yasumizu,R., Ikehara,S. and Shibata,Y. (1994) A new mutation, aly, that induces a generalized lack of lymph nodes accompanied by immunodeficiency in mice. Eur. J. Immunol., 24, 429–434. [DOI] [PubMed] [Google Scholar]
- Naviaux R.N., Costanzi,E., Haas,M. and Verma,I.M. (1996) The pCL vector system: rapid production of helper-free high-titer, recombinant retroviruses. J. Virol., 70, 5701–5705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orian A. et al. (2000) SCFβ(-TrCP) ubiquitin ligase-mediated processing of NF-κB p105 requires phosphorylation of its C-terminus by IκB kinase. EMBO J., 19, 2580–2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poiesz B.F., Ruscetti,F.W., Gazdar,A.F., Bunn,P.A., Minna,J.D. and Gallo,R.C. (1980) Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc. Natl Acad. Sci. USA, 77, 7415–7419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayet B. and Gelinas,C. (1999) Aberrant rel/nfkb genes and activity in human cancer. Oncogene, 18, 6938–6947. [DOI] [PubMed] [Google Scholar]
- Ressler S., Connor,L.M. and Marriott,S.J. (1996) Cellular transformation by human T-cell leukemia virus type I. FEMS Microbiol. Lett., 140, 99–109. [DOI] [PubMed] [Google Scholar]
- Rice N.R., MacKichan,M.L. and Israel,A. (1992) The precursor of NF-κB p50 has IκB-like functions. Cell, 71, 243–253. [DOI] [PubMed] [Google Scholar]
- Rivera-Walsh I., Cvijic,M.E., Xiao,G. and Sun,S.C. (2000) The NF-κB signaling pathway is not required for Fas ligand gene induction but mediates protection from activation-induced cell death. J. Biol. Chem., 275, 25222–25230. [DOI] [PubMed] [Google Scholar]
- Salmerón A., Janzen,J., Soneji,Y., Bump,N., Kamens,J., Allen,H. and Ley,S.C. (2001) Direct phosphorylation of NF-κB1 p105 by the IκB kinase complex on serine 927 is essential for signal-induced p105 proteolysis. J. Biol. Chem., 276, 22215–22222. [DOI] [PubMed] [Google Scholar]
- Senftleben U. et al. (2001) Activation by IKKα of a second, evolutionary conserved, NF-κB signaling pathway. Science, 293, 1495–1499. [DOI] [PubMed] [Google Scholar]
- Sha W.C. (1998) Regulation of immune responses by NF-κB/Rel transcription factors. J. Exp. Med., 187, 143–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S., Cantwell,M., Kipps,T.J. and Friedmann,T. (1996) Efficient infection of a human T-cell line and of human primary peripheral blood leukocytes with a pseudotyped retrovirus vector. Proc. Natl Acad. Sci. USA, 93, 11842–11847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebenlist U., Franzoso,G. and Brown,K. (1994) Structure, regulation and function of NF-κB. Annu. Rev. Cell Biol., 10, 405–455. [DOI] [PubMed] [Google Scholar]
- Smith M.R. and Greene,W.C. (1990) Identification of HTLV-1 tax transactivator mutants exhibiting novel transcriptional phenotypes. Genes Dev., 4, 1875–1885. [DOI] [PubMed] [Google Scholar]
- Sun S.-C. and Ballard,D.W. (1999) Persistent activation of NF-κB by the Tax transforming protein of HTLV-1: hijacking cellular IκB kinases. Oncogene, 18, 6948–6958. [DOI] [PubMed] [Google Scholar]
- Sun S.-C., Ganchi,P.A., Beraud,C., Ballard,D.W. and Greene,W.C. (1994) Autoregulation of the NF-κB transactivator Rel A (p65) by multiple cytoplasmic inhibitors containing ankyrin motifs. Proc. Natl Acad. Sci. USA, 91, 1346–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun S.-C., Elwood,J. and Greene,W.C. (1996) Both amino- and carboxyl-terminal sequences within IκBα regulate its inducible degradation. Mol. Cell. Biol., 16, 1058–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syrovets T., Jendrach,M., Rohwedder,A., Schule,A. and Simmet,T. (2001) Plasmin-induced expression of cytokines and tissue factor in human monocytes involves AP-1 and IKKβ-mediated NF-κB activation. Blood, 97, 3941–3950. [DOI] [PubMed] [Google Scholar]
- Tanaka M., Fuentes,M.E., Yamaguchi,K., Durnin,M.H., Dalrymple,S.A., Hardy,K.L. and Goeddel,D.V. (1999) Embryonic lethality, liver degeneration and impaired NF-κB activation in IKKβ-deficient mice. Immunity, 10, 421–429. [DOI] [PubMed] [Google Scholar]
- Uhlik M., Good,L., Xiao,G., Harhaj,E.W., Zandi,E., Karin,M. and Sun,S.-C. (1998) NF-κB-inducing kinase and IκB kinase participate in human T-cell leukemia virus I Tax-mediated NF-κB activation. J. Biol. Chem., 273, 21132–21136. [DOI] [PubMed] [Google Scholar]
- Xiao G. and Sun,S.C. (2000) Negative regulation of the nuclear factor κB-inducing kinase by a cis-acting domain. J. Biol. Chem., 275, 21081–21085. [DOI] [PubMed] [Google Scholar]
- Xiao G., Harhaj,E.W. and Sun,S.-C. (2000) Domain-specific interaction with IKKγ is an essential step in Tax-mediated activation of IKK. J. Biol. Chem., 275, 34060–34067. [DOI] [PubMed] [Google Scholar]
- Xiao G., Harhaj,E.W. and Sun,S.C. (2001) NF-κB-inducing kinase regulates the processing of NF-κB2 p100. Mol. Cell, 7, 401–409. [DOI] [PubMed] [Google Scholar]
- Yamaoka S., Courtois,G., Bessia,C., Whiteside,S.T., Weil,R., Agou,F., Kirk,H.E., Kay,R.J. and Israel,A. (1998) Complementation cloning of NEMO, a component of the IκB kinase complex essential for NF-κB activation. Cell, 93, 1231–1240. [DOI] [PubMed] [Google Scholar]
- Yin M.-J., Christerson,L.B., Yamamoto,Y., Kwak,Y.-T., Xu,S., Mercurio,F., Barbose,M., Cobb,M.H. and Gaynor,R.B. (1998) HTLV-I Tax protein binds to MEKK1 to stimulate IκB kinase activity and NF-κB activation. Cell, 93, 875–884. [DOI] [PubMed] [Google Scholar]
- Yoshida M., Miyoshi,I. and Hinuma,Y. (1982) Isolation and characterization of retrovirus from cell lines of human adult T-cell leukemia and its implication in the disease. Proc. Natl Acad. Sci. USA, 79, 2031–2035. [DOI] [PMC free article] [PubMed] [Google Scholar]