Abstract
Objective
Gestational diabetes mellitus (GDM) is a common pregnancy complication associated with adverse maternal and neonatal outcomes. The gut microbiota has been implicated in the pathogenesis of metabolic disorders, including GDM. To characterize the gut microbiota in GDM patients with different fetal birth weight outcomes and to compare these profiles with those of their newborns and non-GDM controls.
Methods
This study included 54 patients with normal blood and 70 patients with GDM, which were categorized based on fetal birth weight outcomes including GDM with fetal growth restriction (FGR), normal birth weight, and macrosomia. Maternal fecal samples were collected before delivery. Neonatal meconium samples were obtained post-delivery. 16S rRNA gene sequencing was performed to analyze the gut microbiota composition.
Results
GDM patients exhibited a decrease in Firmicutes and an increase in Bacteroidetes compared to non-GDM controls. Mothers with GDM with FGR had higher levels of Firmicutes and lower levels of Bacteroidetes and Actinobacteria compared to those with normal birth weight or macrosomia. Neonates of mothers with GDM with macrosomia showed a decrease in Firmicutes and an increase in Proteobacteria compared to the other groups. Neonates of mothers with GDM with FGR had increased Firmicutes and decreased Proteobacteria and Actinobacteria. By α-diversity analysis, the Shannon index and OTUs of mothers in GDM with FGR were higher than others. The neonatal Shannon index and OTUs in the GDM with normal macrosomia group were lower than those in the other two groups, but the differences were not statistically significant. Correlation analysis revealed significant associations between specific microbial taxa and maternal biochemical indicators, as well as neonatal Apgar scores. No environmental factors were found to be related to maternal α-diversity among them. Neonatal α-diversity were correlated with c-glutamyltranspeptidase (GGT), high-density lipoprotein (HDL), aspartate transaminase (AST) and maternal age.
Conclusions
GDM is associated with distinct gut microbiota profiles that vary with different fetal birth weight outcomes. These findings suggest that the gut microbiota may play a role in the pathogenesis of GDM and its associated adverse pregnancy outcomes. Further research is warranted to explore the potential of gut microbiota-based interventions for improving maternal and neonatal health in GDM.
Keywords: Gestational diabetes mellitus, Gut microbiota, Macrosomia, Fetal growth restriction, 16S rRNA gene sequencing
Introduction
Gestational diabetes mellitus (GDM) is defined as glucose intolerance first detected during pregnancy [1]. According to the latest estimates from the International Diabetes Federation (IDF), GDM affects about 10% of pregnant women worldwide. This prevalence varies significantly across regions, with higher prevalence observed in certain populations [2]. GDM significantly increases maternal adverse perinatal and postpartum outcomes, including miscarriage, fetal malformation, preeclampsia, fetal growth restriction, fetal death, macrosomia, neonatal hypoglycemia, neonatal hyperbilirubinemia, and neonatal respiratory distress syndrome [3]. The impact on offspring is to increase the risk of obesity and metabolic syndrome in the later period.
Macrosomia is a common bad outcome of GDM. For mothers, macrosomia increases the risk of cesarean section, postpartum hemorrhage and vaginal avulsion. At the same time, macrosomia increases the risk of shoulder dystocia, clavicle fracture and brachial plexus injury, and increases the incidence of neonatal hypoglycemia. In the long term, the offspring of macrosomia at birth had an increased risk of obesity, metabolic syndrome, type 2 diabetes mellitus, and impaired islet insulin secretion and insulin sensitivity [4]. On the other hand, unsatisfactory blood glucose control during pregnancy and complicated with hypertensive disorders of pregnancy are risk factors for gestational diabetes mellitus complicated with fetal growth restriction (FGR), which affects fetal development [5]. As a result, the risk of perinatal complications such as fetal distress, fetal malformation, neonatal asphyxia and hyperbilirubinemia is increased, and there is a certain correlation with physical and intellectual development in children and adolescents. Studies have found that even newborns born to mothers with GDM have normal weight, compared with non-GDM neonates, there are some problems in future metabolism [6]. Therefore, understanding the differences in different pregnancy outcomes of GDM will be the key to how to effectively prevent the development of GDM in the early stage.
Previous studies have found that GDM can induce gut microbiota dysregulation and changes in gut function in mothers with GDM, and the gut microbiota of neonates born to mothers with GDM is different from that of healthy controls [7]. Gut microbiota plays a key role in regulating insulin resistance and inflammatory response in GDM patients, which may become a new idea for the prevention or treatment of GDM plays a key role in regulating insulin resistance and inflammatory response in GDM patients, which may become a new idea for the prevention or treatment of GDM [8]. Most of the colonization of the human gut microbiota begins at neonatal birth, and during the first few days of life, the gut is colonized by a heterogeneous microbial population unrelated to nutrient sources that becomes more stable during the first week of life. Studies have shown that early bacterial colonization is essential for gut development and immune system maturation, reflecting the influence of maternal gut microbiota on the colonization process of children’s gut microbiota. In recent years, with the wide application of 16S rRNA high-throughput sequencing technology and the development of proteomics and metabolomics, numerous studies have found that changes in the abundance and diversity of gut microbiota play a crucial role in the development of GDM [9].
Metagenomic studies have shown that the imbalance of gut microbiota in pregnant women with GDM is related to metabolic pathways of carbohydrate metabolism and insulin signaling, suggesting that pregnant women with GDM may have “characteristic gut microbiota”. The correlation between gut microbiota imbalance in pregnant women with GDM and host metabolism has gradually become a new direction for the prevention and control of GDM [10]. This provides us with a new direction for early prevention and treatment of GDM and reducing the occurrence of adverse pregnancy outcomes of GDM.
Previous studies mainly focused on the differences in gut microbiome changes between mothers with GDM pregnancy and their newborns after delivery, and there were few reports on the differences in gut microbiota between GDM mothers and newborns with different pregnancy outcomes. Therefore, we aimed to investigate potential differences in the abundance and composition of gut microbiota between mothers with GDM combined with different fetal birth weight and newborns after delivery, and their association with clinical measures related to delivery. In this study, 70 GDM patients were divided into three groups according to weight at birth: fetal growth restriction group, normal birth weight group and macrosomia group. 16SrRNA gene sequencing was performed on fecal samples of mothers and newborns in the third trimester to analyze the composition of gut microbiota, and to explore the changes of gut microbiota composition in GDM mothers and neonates with different fetal birth weight. In addition, the results may provide guidance for the different prognosis of GDM through early gut microbiome intervention.
Methods
Study design and participants
Patients with GDM according to the diagnostic criteria for GDM [11] who were diagnosed and successfully delivered in Changzhou Maternal and Child Health Care Center from 2023 to 2024 were selected, and patients with normal blood glucose during the same period were selected as controls. All participants were diagnosed using a standardized 75-g oral glucose tolerance test (OGTT) performed at 24–28 weeks of gestation, in accordance with the criteria of the International Association of Diabetes and Pregnancy Study Groups (IADPSG), in which a fasting blood glucose (FBG) value greater than or equal to 5.1 mmol/L, a 1-h OGTT glucose value greater than or equal to 10.0 mmol/L, or a 2-h OGTT glucose value greater than or equal to 8.5 mmol/L [12]. We grouped patients with GDM according to fetal birth weight: GDM combined with FGR, with normal birth weight, and with macrosomia. FGR: A birth weight of less than 2500 g after 37 weeks of gestation, or two standard deviations below the average weight for the gestational age or below the 10th percentile of normal weight for the gestational age. Macrosomia: birth weight of 4000 g or more. Demographic information and clinical records of the study population were extracted from a structured questionnaire and the Hospital Information Systems, respectively. This study was conducted in accordance with the Declaration of Helsinki and approved by the Medicine Ethics Committee of Changzhou Maternal and Child Health Care Hospital (reference number: [2023] 367), and all participants provided informed consent.
Sample collection, DNA extraction, and 16S rRNA sequencing
We obtained maternal fecal samples before delivery and collected the first-pass meconium samples (approximately 200 mg) from infants on sterilized diapers within a few hours after birth to capture initial microbial colonization. All samples were collected in sterile containers. Samples were transported from the collection site to the laboratory within 2 h in insulated containers with ice packs to maintain the temperature at approximately 4 °C. Upon arrival at the laboratory, samples were processed for DNA extraction immediately. Any remaining sample material was stored at −80 °C until further analysis.16S rRNA amplicon sequencing was performed by Genesky Biotechnologies Inc., Shanghai, 201,315 (China). Briefly, total genomic DNA was extracted using the FastDNA SPIN Kit for Soil (MP Biomedicals, Santa Ana, CA). The integrity of genomic DNA was detected through agarose gel electrophoresis, and the concentration and purity of genomic DNA were detected through the Nanodrop 2000 and Qubit3.0 Spectrophotometer. The V3-V4 hypervariable regions of the 16S rRNA gene were amplified with the primers 341 F (5’- CCTACGGGNGGCWGCAG − 3’) and 805R (5’- GACTACHVGGGTATCTAATCC − 3’) and then sequenced using Illumina NovaSeq 6000 sequencer. To ensure optimal amplification of the V3-V4 region of the 16S rRNA gene, the PCR was performed under the following conditions: Initial denaturation at 95 °C for 3 min, followed by 35 cycles of denaturation at 95 °C for 30 s, annealing at 55 °C for 30 s, and extension at 72 °C for 30 s. A final extension step was performed at 72 °C for 5 min, followed by cooling to 4 °C. These conditions were optimized to ensure specific binding and efficient amplification of the target region.
Illumina read data processing and analysis
Amplicons were quantified using the Qubit 3.0 fluorometer and pooled in equimolar amounts. The pooled library was then purified using the AMPure XP beads (Beckman Coulter, Brea, CA, USA) to remove any remaining contaminants. The final library concentration was measured using the KAPA Library Quantification Kit (KAPA Biosystems) on a Roche LightCycler 480 real-time PCR system (Roche, Basel, Switzerland). Sequencing was performed on an Illumina MiSeq platform (Illumina, San Diego, CA, USA) using the MiSeq Reagent Kit v3 (600-cycle) according to the manufacturer’s protocol. Paired-end reads (2 × 300 bp) were generated.
The raw read sequences were processed in QIIME2. The adaptor and primer sequences were trimmed using the cutadapt plugin. DADA2 plugin was used for quality control and to identify amplicon sequence variants (ASVs). Taxonomic assignments of ASV representative sequences were performed with confidence threshold 0.8 by a pre-trained Naive Bayes classifier which was trained on the RDP (version 11.5).
Statistical analysis
The general data of different GDM groups were collected, and the continuous variables were expressed as mean ± SD, and the categorical variables were expressed as numbers (percentages). To ensure the robustness of our analyses, we used multiple strategies to handle missing data. For continuous variables with missing values, we used mean imputation, in which missing values were replaced by the mean of the observed data for the variable. For categorical variables, we used pattern imputation, in which missing values were replaced with the most common category observed in the data set. In addition, we performed sensitivity analyses to assess the effect of missing data on the results and to compare results with and without imputation. Analysis of variance and Kruskal-Wallis H test were used to compare the statistical differences. The difference of α-diversity index between groups was analyzed by ANOVA. P-value < 0.05 was used as the significance screening threshold.
Results
Maternal and infant data after GDM was grouped according to fetal delivery outcome
Compared with the control group, the pre-pregnancy BMI and the levels of fasting blood glucose, 1-hour and 2-hour blood glucose after OGTT at 24–28 weeks of pregnancy in the GDM group were significantly higher (P < 0.01), but the gestational age at delivery was earlier and the rate of cesarean section was higher in the GDM group (P < 0.05) (Table 1). The characteristics of 43 GDM patients with normal fetal weight, 15 GDM patients with macrosomia, and 12 GDM patients with FGR were summarized in Table 2. There was no significant difference in maternal age among the three groups (P = 0.541). The pre-pregnancy body mass index, weight gain during pregnancy, gestational age at delivery and parity were the highest in the GDM with macrosomia group (P = 0.000, 0.021, 0.000, respectively). The 1-hour postprandial blood glucose in the GDM with macrosomia group was higher than that in the GDM with normal weight group (P = 0.032), and the HbA1c in the GDM with macrosomia group was higher than that in the other two groups (P = 0.000). The level of pre-pregnancy albumin was the highest in the GDM with FGR group (P = 0.013), the level of c-glutamyltranspeptidase was the highest in the GDM with macrosomia group (P = 0.007), and the level of alkaline phosphatase was the highest in the GDM with normal weight group (P = 0.016). However, there were no significant differences in TGs, TC, HDL, LDL, VLDL, ALT, AST, GR, ADA, cystatin C, RBP, TRF, Apolipoprotein A1, Apolipoprotein B and lipoprotein a among the three groups. In terms of neonatal delivery outcomes, the cesarean section rate in the GDM with macrosomia and FGR groups was higher than that in the GDM with normal weight group (P = 0.015), but there was no difference between the two groups. No neonatal asphyxia or NICU admission occurred in the GDM with normal weight and macrosomia group, but 3 cases of neonatal hypoglycemia occurred in the GDM with macrosomia group. Two cases in the GDM combined with FGR group had neonatal asphyxia and were admitted to NICU and discharged after treatment (As shown in Table 2).
Table 1.
Characteristics of the study participants
| Group | Control(n = 54) | GDM(n = 70) | P |
|---|---|---|---|
| Age, years (mean ± SD) | 32.91 ± 3.02 | 31.01 ± 4.41 | 0.008** |
| Pre-pregnancy BMI, kg/m2 (mean ± SD) | 22.29 ± 1.76 | 23.60 ± 3.22 | 0.005** |
| Weight Gain during Pregnancy(Kg) | 10.70 ± 2.20 | 11.83 ± 5.03 | 0.097 |
| Gestational age (mean ± SD, wk) | 39.15 ± 0.94 | 38.74 ± 1.06 | 0.028* |
| OGTT_FBG (mean ± SD, mmol/L) | 4.40 ± 0.35 | 5.01 ± 0.62 | 0.000*** |
| OGTT_1 h (mean ± SD, mmol/L) | 8.04 ± 1.72 | 10.14 ± 1.74 | 0.000*** |
| OGTT_2 h (mean ± SD, mmol/L) | 6.86 ± 1.15 | 8.85 ± 1.72 | 0.000*** |
| HbA1c(%) | 4.74 ± 0.16 | 4.58 ± 0.49 | 0.019* |
| Parity [no. (%)] | 0.185 | ||
| Nulliparae | 39 | 42 | |
| Multiparae | 15 | 28 | |
| Delivery mode [no. (%)] | 0.004** | ||
| Cesarean | 21 | 46 | |
| Vaginal | 33 | 24 | |
| Gender of newborn | 0.590 | ||
| Boy | 22 | 32 | |
| Girl | 32 | 38 | |
| Birth wt (mean ± SD, g) | 3317.41 ± 235.54 | 3393.71 ± 614.96 | 0.389 |
BMI Body Mass Index, kg/m2, HbA1C glycosylated hemoglobin, OGTT_FBG OGTT fasting blood glucose value, OGTT_1 h 1-h OGTT value, OGTT_2 h 2-h OGTT value
*P<0.05
**P<0.01
***P<0.001
Table 2.
General data of gestational diabetes mellitus patients with different delivery outcomes
| Group | GN(n = 43) | GM(n = 15) | GF(n = 12) | P |
|---|---|---|---|---|
| Maternal | ||||
| Age, years (mean ± SD) | 31.47 ± 0.71 | 30.07 ± 1.28 | 30.58 ± 0.65 | 0.541 |
| Height, cm(mean ± SD) | 160.51 ± 096 | 163.33 ± 1.08 | 156.92 ± 1.55#,& | 0.020 |
| Pre-pregnancy BMI, kg/m2 (mean ± SD) | 22.71 ± 0.32 | 28.32 ± 0.49* | 20.89 ± 0.45#,& | 0.000 |
| Weight Gain during Pregnancy(Kg) | 11.59 ± 0.71 | 14.53 ± 1.38* | 9.29 ± 1.33# | 0.021 |
| Gestational age (mean ± SD, wk) | 39.12 ± 0.15 | 38.53 ± 0.19* | 37.67 ± 0.23#,& | 0.000 |
| OGTT_FBG (mean ± SD, mmol/L) | 4.98 ± 0.10 | 5.16 ± 0.11 | 4.92 ± 0.17 | 0.537 |
| OGTT_1 h (mean ± SD, mmol/L) | 9.74 ± 0.27 | 10.85 ± 0.24* | 10.14 ± 0.58 | 0.046 |
| OGTT_2 h (mean ± SD, mmol/L) | 8.67 ± 0.28 | 8.96 ± 0.32 | 9.36 ± 0.56 | 0.459 |
| HbA1c(%) | 4.25 ± 0.03 | 5.40 ± 0.05* | 4.76 ± 0.04#,& | 0.000 |
| Parity [no. (%)] | 0.000 | |||
| Nulliparae | 28(65.1%) | 3(20%) | 11(91.7%) | |
| Multiparae | 15(34.9) | 12(80%)* | 1(8.3%)# | |
| TGs (mmol/L) | 5.98 ± 0.18 | 5.85 ± 0.38 | 6.15 ± 0.40 | 0.847 |
| TC (mmol/L) | 3.95 ± 0.23 | 4.89 ± 0.75 | 3.69 ± 0.55 | 0.205 |
| ALB (g/L) | 33.93 ± 0.32 | 34.84 ± 0.64 | 36.14 ± 0.78& | 0.013 |
| HDL(mmol/L) | 1.87 ± 0.05 | 1.76 ± 0.09 | 2.09 ± 0.17 | 0.086 |
| LDL(mmol/L) | 3.58 ± 0.13 | 3.44 ± 0.23 | 3.74 ± 0.29 | 0.681 |
| VLDL | 473.26 ± 19.57 | 462.93 ± 34.14 | 529.03 ± 45.16 | 0.382 |
| GGT | 10.77 ± 0.84 | 23.74 ± 5.64* | 18.87 ± 9.16 | 0.007 |
| ALT | 12.83 ± 1.26 | 26.65 ± 9.62 | 23.42 ± 6.53 | 0.050 |
| AST | 21.98 ± 4.66 | 22.27 ± 4.58 | 21.09 ± 2.92 | 0.992 |
| AKP | 163.42 ± 8.25 | 127.33 ± 11.02* | 123.29 ± 15.85& | 0.016 |
| GR | 41.83 ± 1.10 | 42.39 ± 3.14 | 41.09 ± 3.41 | 0.937 |
| ADA | 10.50 ± 0.32 | 11.81 ± 0.43 | 10.49 ± 0.87 | 0.127 |
| cystatin C | 8.32 ± 5.05 | 9.79 ± 8.91 | 10.07 ± 8.93 | 0.972 |
| RBP | 38.99 ± 1.44 | 41.49 ± 1.41 | 45.00 ± 2.43 | 0.097 |
| TRF | 3.15 ± 0.08 | 3.25 ± 0.24 | 3.17 ± 0.14 | 0.862 |
| Apolipoprotein A1 | 2.08 ± 0.05 | 2.09 ± 0.07 | 2.11 ± 0.04 | 0.162 |
| Apolipoprotein B | 2.44 ± 1.27 | 1.17 ± 0.08 | 1. 21 ± 0.09 | 0.742 |
| Lipoprotein a | 189.69 ± 25.71 | 105.88 ± 14.83 | 136.69 ± 16.83 | 0.109 |
| Neonatal | ||||
| Delivery mode [no. (%)] | 0.015 | |||
| Cesarean | 23(53.5%) | 14(93.3%)* | 9(75%)# | |
| Vaginal | 20(46.5%) | 1(6.7%) | 3(25%) | |
| Gender [no. (%)] | 0.365 | |||
| Boy | 19(44.2%) | 9(60%) | 4(33.3%) | |
| Girl | 24(55.8%) | 6(40%) | 8(66.7%) | |
| Birth wt (mean ± SD, g) | 3323.95 ± 35.98 | 4270.67 ± 119.79* | 2547.50 ± 55.42#,& | 0.000 |
| neonatal asphyxia[no. (%)] | 0(0%) | 0(0%) | 2(16.7%)#,& | 0.007 |
| NICU[no. (%)] | 0(0%) | 0(0%) | 2(16.7%)#,& | 0.007 |
| neonatal hypoglycemia[no. (%)] | 0(0%) | 3(20%)* | 0(0%)# | 0.003 |
GN GDM with normal birth weight, GM GDM with macronesia, GF GDM with fetal growth restriction, TGs triglycerides, TC triglyceride, ALB albumin, HDL high-density lipoprotein, LDL low-density lipoprotein, VLDL very low density lipoprotein, GGT c-glutamyltranspeptidase, ALT alanine aminotransferase, AST aspartate transaminase, AKP alkaline phosphatase, GR glutathione reductase, ADA adenosine deaminase, RBP retinol conjugated protein, TRF transferrin
*GM vs. GN, #GF vs. GM, &GF vs. GN
*P<0.05; # P<0.05; &P<0.05
Differences in maternal and neonatal gut microbiota between euglycemic and GDM groups
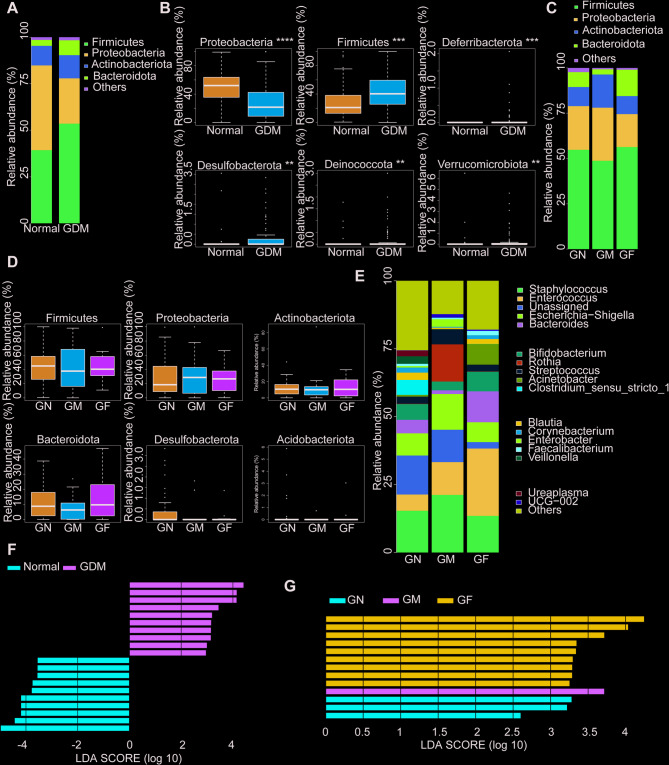
The analysis of maternal gut microbiota showed that Firmicutes and Bacteroidetes represented the dominant taxa in all samples at the phylum level. Under the same delivery outcome, the GDM group showed a decrease in Firmicutes and an increase in Bacteroidetes (Fig. 1A). There were significant increases in Bacteroidota (P < 0.01), Desulfobacterota (P <0.01), and Fusobacterium (P < 0.05) in the GDM group compared with the normal group (Fig. 1B). According to the grouping of fetal birth weight, compared with the GDM with normal weight group, the GDM with macrosomia group showed a decrease in Firmicutes and Actinobacteria, an increase in Proteobacteria and Bacteroidetes, and a significant increase in Bacteroidetes. The GDM with fetal growth restriction group showed a decrease in Bacteroidetes, Actinobacteria and Bacteroidetes, and a significant increase in Firmicutes (Fig. 1C, D). At the genus level, Bacteroides, Lachnospira, Faecalibacterium, Bifidobacterium and Bacteroides Blautia were the dominant genera in the three groups. Compared with the normal weight group, the GDM complicated with macrosomia group had an increase in Bacteroides and a decrease in Bifidobacterium, while the GDM complicated with FGR group had a decrease in Bacteroides and Bifidobacterium and an increase in Faecalibacterium (Fig. 1E). The LEfSe analysis revealed distinct differences in the gut microbiota composition between groups, suggesting that targeted microbiota interventions could be beneficial in managing these conditions. Key findings included increased levels of Streptococcus and Prevotella colorans in the GDM group, potentially linked to metabolic disturbances and inflammation. But, there was a notable decrease in Lachnoclostridium, a genus associated with a healthy gut microbiome (Fig. 1F). In the GM group, Subdoligranulum and Acidaminococcus were significantly enriched. Conversely, the GF group exhibited higher levels of Corynebacterium durum and Slackia piriformis, suggesting that targeted microbiota interventions could be beneficial in managing these conditions (Fig. 1G).
Fig. 1.
Microbiome characteristics of mothers in the study population. A Relative abundance of dominant gut microbiota taxa in women with gestational diabetes mellitus (GDM) and healthy controls. B The relative abundance of differential flora phyla between GDM patients and healthy controls. C Relative abundance of dominant gut microbiota groups among the three GDM groups grouped by fetal birth weight. D The relative abundance of differentially expressed phyla among the three GDM groups according to fetal birth weight. E The relative abundance of dominant genera of gut microbiota among the three GDM groups. F The LEfSe analysis between GDM and normal patients. G The LEfSe analysis among the GM and GF groups.*P < 0.05, **P < 0.01.GN, GDM with normal birth weight; GM, GDM with macrosomia; GF, GDM with fetal growth restriction
In the neonatal group, Proteobacteria, Firmicutes, Actinobacteria, and Bacteroidetes were the dominant taxa in all samples at the phylum level (Fig. 2A). Under the same delivery outcome, the GDM neonatal group showed a decrease in Proteobacteria and Actinobacteria, and an increase in Firmicutes and Bacteroidetes. Proteobacteria was the phylum with a decreased difference, while Firmicutes and Desulfobacterota were the phylum with an increased difference (Fig. 2B). Grouped analysis showed that the proportion of Firmicutes and Bacteroidetes decreased and the proportion of Proteobacteria, and Actinobacteria increased in the GDM complicated with fetal growth restriction group. The proportion of Firmicutes and Bacteroidetes increased and the proportion of Proteobacteria, and Actinobacteria decreased in the GDM complicated with fetal growth restriction group (Fig. 2C, D). However, the dominant genera in the three GDM groups were Staphylococcus, Enterococcus and Escherichia - shigella. Compared with normal weight neonates, the dominant bacteria in macrosomia caused by GDM increased. Neonates with fetal growth restriction caused by GDM showed a higher reduction in Enterococcus and Staphylococcus (Fig. 2E). The neonatal GDM group had higher levels of Proteobacteria, Sphingomonas and Megamonas, and significantly lower numbers of Coprococcus and Bacteroides than the euglycemic group (Fig. 2F). In the GM group, there was a notable increase in Lactobacillus and Prevotella pallens. The GF group exhibited higher levels of Intestinimonas and Prevotella_9 (Fig. 2G).
Fig. 2.
Microbiome characteristics of newborns in the study population. A The relative abundance of dominant gut microbiota groups in neonates with GDM and healthy controls. B The relative abundance of differential phyla in gut microbiota between GDM and healthy controls. C The relative abundance of dominant gut microbiota groups among the three groups of GDM neonates grouped according to fetal birth weight. D The relative abundance of different flora phyla among the three GDM groups according to fetal birth weight. E The relative abundance of dominant genera of gut microbiota among the three GDM groups. F The LEfSe analysis between GDM and euglycemic neonates. G The LEfSe analysis of neonates among the three groups of GDM
In summary, compared with the GDM with normal weight group, the GDM with macrosomia group showed a decrease in Firmicutes and an increase in Proteobacteria in both mothers and neonates. The increase of Firmicutes and the decrease of Proteobacteria and Actinobacteria were observed in both mothers and neonates of GDM with FGR group. Bacteroides, Lachnospira, Faecalibacterium and Bifidobacterium were significantly different among the three groups, while Staphylococcus, Enterococcus, Escherichia coli and Shigella were significantly different among the newborns.
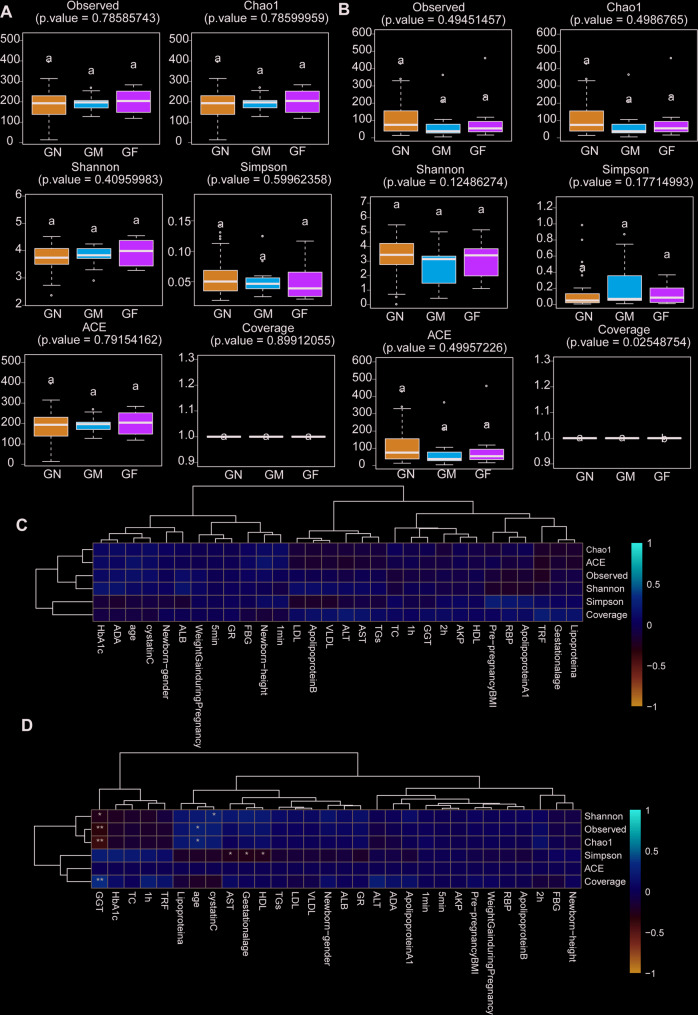
Results of α-diversity analysis
By α-diversity analysis, the Shannon index (P = 0.4096) and OTUs (P = 0.7859) of mothers in GDM with fetal growth restriction group were higher than those in the other two groups (Fig. 3A). However, the neonatal Shannon index (P = 0.1249) and OTUs (P = 0.4945) in the GDM with normal macrosomia group were lower than those in the other two groups, but the differences were not statistically significant, which may be related to the small sample size (Fig. 3B).
Fig. 3.
α-Diversity indexes of the fecal microbiome. A α-Diversity indexes of the fecal microbiome between the three groups of GDM patients. B α-Diversity indexes of the fecal microbiome between the three groups of GDM newborns. C The correlation between α-diversity indicators and environmental factors among the three groups of GDM patients was analyzed by regression analysis. D The correlation between α-diversity indicators and environmental factors among the three groups of GDM newborns was analyzed by regression analysis. FBG, glucose at time 0 min; 1 h, glucose at time 60 min; 2 h, glucose at time 120 min; HbAlc, glycated hemoglobin; TGs, triglycerides; TC, triglyceride; ALB, albumin; HDL, high-density lipoprotein; LDL, low-density lipoprotein; VLDL, very low density lipoprotein; GGT, c-glutamyltranspeptidase; ALT, alanine aminotransferase; AST, aspartate transaminase; AKP, alkaline phosphatase; GR, glutathione reductase; ADA, adenosine deaminase; RBP, retinol conjugated protein; TRF, transferrin; 1 min, Neonatal 1-minute Apgar score; 5 min, Neonatal 5-minute Apgar score
Correlation analysis of gut microbiota species and α-diversity with environmental factors
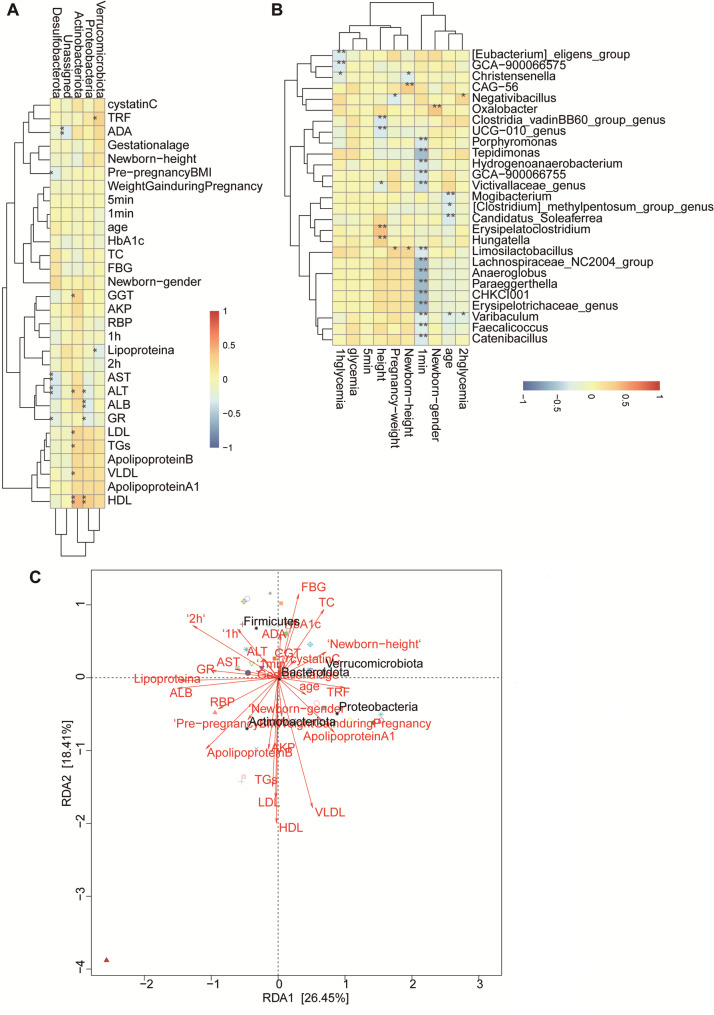
Regression analysis showed that Actinobacteriota, Proteobacteria, Verrucomicrobiota and Firmicutes were closely related to maternal gut microbiota and maternal biochemical indicators. Actinobacteriota was positively correlated with maternal HDL, LDL, TGs, VLDL, AST, GGT. Proteobacteria were closely and positively correlated with GR, ALB and ALT, and Verrucomicrobiota and Firmicutes were correlated with OGTT results (Fig. 4A). The score of newborns at 1 min after birth was closely negatively correlated with the genus level of maternal gut microbiota Tepidimonas, Anaeroglobus, and Erysipelotrichaceae-genus, which mostly belonged to Proteobacteria and Firmicutes, and were mostly Gram-negative anaerobic bacteria (|cor|>0.3, P < 0.05), further suggesting that maternal gut microbiota Firmicutes, Defensiobacteria and Proteobacteria play an important role in the differences of blood glucose and biochemical metabolic indicators in GDM (Fig. 4B, C).Among the three groups of GDM neonates, the level of Fusobacteriota phylum in neonatal gut microbiota was correlated with maternal HbA1c. The newborn-gender were correlated with neonatal level of Proteobacteria, Deinococcota and Planctomycetota (Fig. 5A). The levels of Fusobacteriota, Patescibacteria, Deferribacterota, and Deinococcota in the three groups were correlated with maternal biochemical indicators, and the levels of Actinobacteria and Firmicutes in the three groups were correlated with maternal OGTT results. Neonatal score at 1 min after birth was positively correlated with neonatal Proteobacteria phylum level (Fig. 5B, C). No environmental factors were found to be related to maternal α-diversity among the three GDM groups (Fig. 3C). Neonatal α-diversity were correlated with GGT, HDL, AST and maternal age (Fig. 3D).
Fig. 4.
Correlation between gut microbiota and environmental factors among the three groups of GDM patients. A Correlation between the phylum level of gut microbiota and environmental factors among the three groups of GDM patients. B Correlation between the genus level of gut microbiota and environmental factors among the three groups of GDM patients. C RDA/CCA plots (species, sample, and environmental factors) among the three groups of GDM patients
Fig. 5.
Correlation between gut microbiota and environmental factors among the three groups of GDM newborns. A Correlation between the phylum level of gut microbiota and environmental factors among the three groups of GDM newborns. B Correlation between the genus level of gut microbiota and environmental factors among the three groups of GDM newborns. C RDA/CCA plots (species, sample, and environmental factors) among the three groups of GDM newborns
Discussion
The findings of our study offer a nuanced perspective on the intricate relationship between GDM and alterations in the gut microbiota. Our results indicate that GDM is associated with a distinct gut microbiota signature, which varies based on fetal birth weight outcomes. This observation is pivotal as it suggests that the gut microbiota may not only be a biomarker of GDM but could also be implicated in the pathophysiology of adverse pregnancy outcomes.
The decrease in Firmicutes and increase in Bacteroidetes observed in GDM patients is consistent with previous studies that have reported similar shifts in gut microbiota composition in obesity and type 2 diabetes mellitus [13]. This pattern of dysbiosis has been previously implicated in the development of insulin resistance and the metabolic syndrome, both of which are hallmarks of GDM. These phyla are known to influence the production of short-chain fatty acids (SCFAs), which play a crucial role in glucose homeostasis and energy regulation [14]. The reduced abundance of Firmicutes, particularly those genera that are efficient SCFA producers, may contribute to the insulin resistance characteristic of GDM. Conversely, the increased Bacteroidetes could reflect a pro-inflammatory state, which is often associated with metabolic disorders and may exacerbate the inflammatory response seen in GDM [15]. Recent studies have highlighted the role of gut microbiota-derived metabolites in the development of insulin resistance and inflammation in GDM patients. Studies integrating metagenomics and metabolomics analyses have shown significant changes in gut microbiota composition and related metabolite profiles in GDM patients compared with controls. Specifically, a decrease in SCFAs such as acetate, propionate, and butyrate, which are known to enhance insulin sensitivity and regulate glucose metabolism [16]. The reduced abundance of SCFA-producing bacteria such as Faecalibacterium, Prevotella and strecoccus may be involved in insulin resistance in GDM patients. In addition, there was an increase in proinflammatory bacteria, such as shahii Alistipes and Ruminococcus gnavus, which were associated with elevated levels of circulating inflammatory markers. These bacteria can produce metabolites that trigger low-grade inflammation, which is a hallmark of metabolic disorders such as GDM [17]. Dopamine levels are significantly reduced in GDM patients, which may exacerbate the inflammatory process and further promote insulin resistance [18]. These findings suggest that gut microbiota-derived metabolites can affect insulin resistance and inflammation in GDM patients through multiple pathways, including imbalance of SCFA production, increased production of proinflammatory metabolites, and dysregulation of neuroendocrine pathways such as dopamine metabolism. Future studies should focus on elucidating the specific molecular mechanisms by which these metabolites interact with host metabolic and immune pathways.
The variation in gut microbiota composition among GDM patients with different fetal birth weight outcomes is particularly noteworthy [19]. The macrosomia group exhibited a decrease in Firmicutes and an increase in Proteobacteria, which includes potentially pathogenic bacteria. This shift could have significant implications for neonatal health, as early-life exposure to such microbes may predispose offspring to metabolic disorders later in life. In contrast, the FGR group showed an increase in Firmicutes, suggesting a different metabolic perturbation that could impact fetal growth and development. The increased abundance of Firmicutes in the FGR group may be a potential factor for its adverse pregnancy outcome. Overexpression of Firmicutes, especially metabolically active Firmicutes, not only leads to disruption of SCFAs production, which leads to dysregulation of glucose homeostasis and energy regulation and exacerbates insulin resistance. In addition, a shift to higher firmicutes levels may trigger a proinflammatory state that is often associated with metabolic disorders and can negatively affect fetal growth by compromising placental function and nutrient transport [20]. The increase in Firmicutes may trigger a low-grade inflammatory response by releasing endotoxins such as lipopolysaccharide (LPS). This inflammatory response may affect placental function and reduce the nutrient supply to the fetus, thereby causing FGR [21]. In addition, alterations in the composition of the microbiota may affect the maternal metabolic environment and the angiogenesis and nutrient transport function of placenta, potentially reducing the availability of nutrients required for fetal development [22, 23]. Taken together, these mechanisms highlight the multifaceted role of Firmictes in GDM with FGR, underlining the need for further studies to elucidate the exact pathways and potential therapeutic targets [24].
LEfSe analysis can show the composition of gut microbiota with significant differences in different groups, which can be used to guide targeted microbiota interventions. Streptococcus and Prevotella coloratum were significantly increased in the group of GDM mothers, often associated with metabolic disorders and inflammation. These taxa may be involved in the insulin resistance and overall metabolic disorders characteristic of GDM. In contrast, Lachnoclostridium, a genus typically associated with a healthy gut microbiome, was significantly reduced. This reduction may indicate a disturbance of the microbiota that may exacerbate the metabolic complications of GDM. Subdoligranulum and Acidaminococcus were significantly enriched in mothers with GDM complicated with macrosomia, and those with GDM complicated with FGR showed higher levels of Corynebacterium durum and Slackia piriformis. It may affect nutrient absorption and immune response in the neonatal period [25]. The neonatal GDM group had higher levels of Proteobacteria, Sphingomonas, and Meganomonas and significantly lower numbers of Coprococcus and Bacteroides than the euglycemic group. These microbial changes may reflect metabolic and immune adaptation to maternal GDM in early life. The GM group had a significant increase in Lactobacillus and Prevotella, which may affect neonatal intestinal health and development. GDM with fetal growth restriction had higher levels of Enteromonas and Prevotella a_9, and elevated levels of these proinflammatory flora may contribute to insulin resistance and metabolic inflammation [17]. Similarly, the enrichment of Enteromonas in growth factors may reflect altered nutrient absorption and immune responses that affect fetal growth [26]. Future research should focus on elucidating the mechanisms by which these microbial changes affect maternal and newborn health.
α-Diversity, a measure of the richness and evenness of microbial species within a sample, is pivotal for understanding the complexity of the gut ecosystem and its metabolic functions [27]. The variation in α-diversity among GDM patients with different fetal birth weight outcomes is particularly noteworthy [28]. Studies suggest that pre-pregnancy obesity may affect the neonatal meconium microbiota by changing the composition of maternal gut microbiota, thereby affecting the changes of fetal growth indicators. For example, birth weight and weight to height ratio are negatively correlated with α-diversity index [29]. The FGR group showed higher α-diversity in our studies, which may suggest a different metabolic perturbation that could impact fetal growth and development. In contrast, the macrosomia group exhibited a decrease in α-diversity, which could be linked to the overgrowth of certain bacterial taxa that promote energy harvest and potentially contribute to fetal overgrowth. Our study highlights the importance of considering the gut microbiota α-diversity as a potential therapeutic target for managing GDM and its associated risks.
Our study also revealed correlations between specific gut microbial taxa and clinical parameters, such as maternal biochemical indicators and neonatal Apgar scores. These correlations suggest that the gut microbiota may influence metabolic health and pregnancy outcomes through modulation of maternal glucose metabolism and inflammation [30]. For instance, the positive correlation between Actinobacteriota and maternal HDL levels implies a potential role for this microbial group in lipid metabolism, which is often disrupted in GDM. Similarly, the negative correlation between Proteobacteria and neonatal Apgar scores at one minute post-partum indicates that maternal gut microbiota may influence neonatal health outcomes, potentially through its impact on maternal glucose metabolism. Previous studies have suggested that the differential abundance of taxa at the genus and species levels between GDM group and control group is related to blood glucose levels, which may be used as biomarkers and therapeutic targets for the diagnosis of probiotics or synbiotics [31].
The implications of these findings are profound, as they suggest that interventions targeting the gut microbiota could potentially improve maternal metabolic health and reduce the risk of adverse pregnancy outcomes [32]. Probiotics or prebiotics that modulate the gut microbiota composition towards a more beneficial profile could potentially improve maternal metabolic health and reduce the risk of adverse pregnancy outcomes [20]. However, the potential side effects and safety concerns associated with the use of these agents must be considered, and the potential risk of infection must be carefully evaluated, especially in immunocompromised individuals. Furthermore, given the potential for probiotics and prebiotics to influence the maternal gut microbiota and subsequently the fetal environment, the maternal and fetal effects of these interventions should be closely monitored. Furthermore, fecal microbiota transplantation, which has shown promise in treating Clostridioides difficile infections, could be explored as a potential therapeutic approach for GDM.
Of course, there are limitations to our study. First, the cross-sectional design limited our ability to establish a causal relationship between the gut microbiota and GDM. Because the data were collected at a single time point, we were unable to determine whether the observed differences in gut microbiota were the result before or after the onset of GDM. In addition, our cross-sectional design may have introduced confounding factors that we cannot fully account for, such as dietary habits, physical activity level, and other lifestyle factors that influence the gut microbiota. The small sample size (70 women with GDM) also limited our ability to detect significant differences in some analyses. For example, the lack of statistical significance for neonatal Shannon and otu indices may be due to underpower rather than a true absence of difference. A larger sample size would provide more robust effect size estimates and increase the likelihood of detecting a significant association. In addition, the small sample size limits the generalizability of our findings. Future studies should employ a longitudinal approach and include larger, diverse cohorts to elucidate the causal relationship between gut microbiota and GDM. Although our study focused primarily on the relationship between gut microbiota and GDM, it must be acknowledged that other factors such as diet, lifestyle, and genetics can significantly influence this relationship. Diet is a major determinant of gut microbiota composition, and studies have shown that dietary interventions can significantly alter gut microbiota in overweight and obese GDM women [20]. Lifestyle factors, including physical activity and sleep patterns, can also influence gut microbiota composition. Physical activity has been shown to increase microbial diversity and increase the abundance of beneficial taxa [33]. Similarly, sleep quality can affect gut microbiota through circadian regulation. Future studies should explore how lifestyle changes can be leveraged to improve gut microbiota health and reduce GDM risk. Studies have identified specific genetic variants associated with altered gut microbiota composition and metabolic phenotypes [34]. These genetic factors may interact with environmental factors to modulate the relationship between gut microbiota and GDM. Future studies should investigate the genetic determinants of gut microbiota composition and their interactions with environmental factors in the context of GDM. Integrating data on diet, physical activity, and genetic factors would provide a more complete picture of the dynamic effects of multiple factors.
Conclusions
In conclusion, GDM was associated with significant changes in gut microbiota composition in affected mothers and their newborns, and different patterns were observed across fetal birth-weight outcomes. The results of this study suggest a potential association between changes in gut microbiota and GDM pathogenesis and adverse pregnancy outcomes, but further studies with larger sample sizes and longitudinal designs are needed to confirm this relationship. It is of interest to consider the gut microbiota as a potential therapeutic target for the management of GDM and its associated risks. By deepening the understanding of the role of the gut microbiota in GDM, the way could be paved for innovative preventive and therapeutic strategies for the health of mothers and their offspring.
Acknowledgements
We would like to acknowledge the whole team members involved in the management.
Abbreviations
- GDM
Gestational Diabetes Mellitus
- FGR
Fetal growth restriction
- GN
GDM with normal birth weight
- GM
GDM with macronesia
- GF
GDM with fetal growth restriction
- TC
Triglyceride
- ALB
Albumin
- HDL
High-density lipoprotein
- LDL
Low-density lipoprotein
- VLDL
Very low density lipoprotein
- GGT
c-glutamyltranspeptidase
- ALT
Alanine aminotransferase
- AST
Aspartate transaminase
- AKP
Alkaline phosphatase
- GR
Glutathione reductase
- ADA
Adenosine deaminase
- RBP
Retinol conjugated protein
- TRF
Transferrin
Authors’ contributions
DX contributed to data collection, analysis, and manuscript writing. HW analyzed the data and revised the manuscript. WW designed the research, revised the manuscript. All authors read and approved the final manuscript.
Funding
This study was supported by Nanjing Medical University - Changzhou Medical Center - Clinical Project under Grant No. CMCC202205,Changzhou Municipal Health Commission Youth Project under Grant No. QN202374.
Data availability
The datasets generated and/or analysed during the current study are not publicly available due to reasons for non-disclosure of data but are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This study was conducted in accordance with the Declaration of Helsinki and approved by the Medicine Ethics Committee of Changzhou Maternal and Child Health Care Hospital (reference number: [2023] 367), and all participants provided informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sweeting A, Wong J, Murphy HR, Ross GP. A clinical update on gestational diabetes mellitus. Endocr Rev. 2022;43(5):763–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sun H, Saeedi P, Karuranga S, et al. IDF diabetes atlas: global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. 2022;183:109119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ye W, Luo C, Huang J, Li C, Liu Z, Liu F. Gestational diabetes mellitus and adverse pregnancy outcomes: systematic review and meta-analysis. BMJ. 2022;377:e067946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Song X, Chen L, Zhang S, et al. Gestational diabetes mellitus and high triglyceride levels mediate the association between pre-pregnancy overweight/obesity and macrosomia: a prospective cohort study in central China. Nutrients. 2022;14(16):3347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xiong Y, Wang Y, Wu M, et al. Aberrant NK cell profile in gestational diabetes mellitus with fetal growth restriction. Front Immunol. 2024;15:1346231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mor L, Tamayev L, Laxer B, et al. Improved neonatal outcomes in pregnancies with coexisting gestational diabetes and preeclampsia in normal birthweight neonates- insights from a retrospective cohort study. Placenta. 2024;149:1–6. [DOI] [PubMed] [Google Scholar]
- 7.Hu R, Liu Z, Geng Y, et al. Gut microbiota and critical metabolites: potential target in preventing gestational diabetes mellitus. Microorganisms. 2023;11(7):1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cruz MC, Azinheiro S, Pereira SG. Modulation of gut microbiota by diet and probiotics: potential approaches to prevent gestational diabetes mellitus. Gut Microbiome (Camb). 2023;4:e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wei J, Qing Y, Zhou H, Liu J, Qi C, Gao J. 16S rRNA gene amplicon sequencing of gut microbiota in gestational diabetes mellitus and their correlation with disease risk factors. J Endocrinol Invest. 2022;45(2):279–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pheiffer C, Riedel S, Dias S, Adam S. Gestational diabetes and the gut microbiota: fibre and polyphenol supplementation as a therapeutic strategy. Microorganisms. 2024;12(4):633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.ElSayed NA, Aleppo G, Aroda VR, et al. 15. Management of diabetes in pregnancy: standards of care in diabetes-2023. Diabetes Care. 2023;46(Suppl 1):S254–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.International Association of Diabetes and Pregnancy Study Groups Consensus Panel, Metzger BE, et al. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care. 2010;33(3):676–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martínez-López YE, Esquivel-Hernández DA, Sánchez-Castañeda JP, Neri-Rosario D, Guardado-Mendoza R, Resendis-Antonio O. Type 2 diabetes, gut microbiome, and systems biology: a novel perspective for a new era. Gut Microbes. 2022;14(1):2111952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pinto Y, Frishman S, Turjeman S, et al. Gestational diabetes is driven by microbiota-induced inflammation months before diagnosis. Gut. 2023;72(5):918–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ye D, Huang J, Wu J, et al. Integrative metagenomic and metabolomic analyses reveal gut microbiota-derived multiple hits connected to development of gestational diabetes mellitus in humans. Gut Microbes. 2023;15(1):2154552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Portincasa P, Bonfrate L, Vacca M, et al. Gut microbiota and short chain fatty acids: implications in glucose homeostasis. Int J Mol Sci. 2022;23(3):1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tilg H, Zmora N, Adolph TE, Elinav E. The intestinal microbiota fuelling metabolic inflammation. Nat Rev Immunol. 2020;20(1):40–54. [DOI] [PubMed] [Google Scholar]
- 18.Ter Horst KW, Lammers NM, Trinko R, et al. Striatal dopamine regulates systemic glucose metabolism in humans and mice. Sci Transl Med. 2018;10(442):eaar3752. [pii]. [DOI] [PubMed] [Google Scholar]
- 19.Chen T, Qin Y, Chen M, et al. Gestational diabetes mellitus is associated with the neonatal gut microbiota and metabolome. BMC Med. 2021;19(1):120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mokkala K, Paulin N, Houttu N, et al. Metagenomics analysis of gut microbiota in response to diet intervention and gestational diabetes in overweight and obese women: a randomised, double-blind, placebo-controlled clinical trial. Gut. 2021;70(2):309–18. [DOI] [PubMed] [Google Scholar]
- 21.Canfora EE, Meex R, Venema K, Blaak EE. Gut microbial metabolites in obesity, NAFLD and T2DM. Nat Rev Endocrinol. 2019;15(5):261–73. [DOI] [PubMed] [Google Scholar]
- 22.Kovatcheva-Datchary P, Nilsson A, Akrami R, et al. Dietary fiber-induced improvement in glucose metabolism is associated with increased abundance of prevotella. Cell Metab. 2015;22(6):971–82. [DOI] [PubMed] [Google Scholar]
- 23.Louis P, Flint HJ. Formation of propionate and butyrate by the human colonic microbiota. Environ Microbiol. 2017;19(1):29–41. [DOI] [PubMed] [Google Scholar]
- 24.Ziętek M, Celewicz Z, Szczuko M. Short-chain fatty acids, maternal microbiota and metabolism in pregnancy. Nutrients. 2021;13(4):1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang J, Zheng J, Shi W, et al. Dysbiosis of maternal and neonatal microbiota associated with gestational diabetes mellitus. Gut. 2018;67(9):1614–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arumugam R, Horowitz E, Lu D, et al. The interplay of prolactin and the glucocorticoids in the regulation of beta-cell gene expression, fatty acid oxidation, and glucose-stimulated insulin secretion: implications for carbohydrate metabolism in pregnancy. Endocrinology. 2008;149(11):5401–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Su Y, Wang HK, Gan XP, et al. Alterations of gut microbiota in gestational diabetes patients during the second trimester of pregnancy in the Shanghai Han population. J Transl Med. 2021;19(1):366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grech A, Collins CE, Holmes A, et al. Maternal exposures and the infant gut microbiome: a systematic review with meta-analysis. Gut Microbes. 2021;13(1):1–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cömert TK, Akpinar F, Erkaya S, Durmaz B, Durmaz R. The effect of pre-pregnancy obesity on gut and meconium microbiome and relationship with fetal growth. J Matern Fetal Neonatal Med. 2022;35(26):10629–37. [DOI] [PubMed] [Google Scholar]
- 30.Wang S, Liu Y, Tam WH, et al. Maternal gestational diabetes mellitus associates with altered gut Microbiome composition and head circumference abnormalities in male offspring. Cell Host Microbe. 2024;32(7):1192–e12065. [DOI] [PubMed] [Google Scholar]
- 31.Chen F, Gan Y, Li Y, et al. Association of gestational diabetes mellitus with changes in gut microbiota composition at the species level. BMC Microbiol. 2021;21(1):147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Davidson SJ, Barrett HL, Price SA, Callaway LK, Dekker Nitert M. Probiotics for preventing gestational diabetes. Cochrane Database Syst Rev. 2021;4(4):CD009951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zmora N, Suez J, Elinav E. You are what you eat: diet, health and the gut microbiota. Nat Rev Gastroenterol Hepatol. 2019;16(1):35–56. [DOI] [PubMed] [Google Scholar]
- 34.Goodrich JK, Waters JL, Poole AC, et al. Human genetics shape the gut microbiome. Cell. 2014;159(4):789–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analysed during the current study are not publicly available due to reasons for non-disclosure of data but are available from the corresponding author on reasonable request.