ADP-ribosyl cyclases are a family of multifunctional enzymes that appear to be expressed ubiquitously in eukaryotic systems (reviewed in refs. 1 and 2). These enzymes are known to metabolize NAD+ and produce at least three molecules that have been demonstrated to be involved in calcium signaling. These enzymes are named for their ability to convert NAD+ to cyclic ADP-ribose (cADPR), a signaling molecule that has been shown to mobilize calcium from intracellular stores by means of a ryanodine receptor/calcium channeldependent system (reviewed in refs. 3 and 4). In addition to cADPR, ADP-ribosyl cyclases have been shown to produce ADP-ribose (ADPR) and nicotinic acid adenine dinucleotide 2′-phosphate (NAADP) (1, 2, 5). These molecules are also involved in regulating calcium signaling: ADPR has been shown to regulate plasma membrane LTRPC2 calcium channels in mammalian cells (6), and NAADP induces calcium mobilization from intracellular stores in several systems (7). In mammalian systems, the ectoenzymes CD38 and CD157 appear to be the major enzymes with ADP-ribosyl cyclase activity (2), although a non-CD38/CD157 ADP-ribosyl cyclase has been detected recently in brains from CD38 knockout mice (8). The work of Basile et al. (9) in a recent issue of PNAS demonstrates that, in addition to producing cADPR, ADPR, and NAADP, ADP-ribosyl cyclases can also generate three adenine dinucleotides (9). Interestingly, these adenine dinucleotides are shown to exist naturally and to have biological activity (9). These findings have several important implications, including expanding the signaling capabilities of the ADP-ribosyl cyclase family and providing a potential mechanism for the synthesis of adenine dinucleotides.
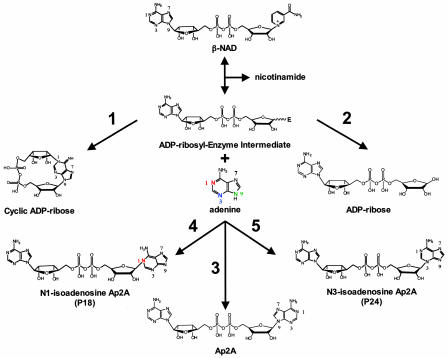
Adenine homodinucleotides with the general structure of adenosine 5′-oligophospho-5″-adenosine have been known to exist naturally for >30 years (see refs. 10 and 11 for reviews). Adenine dinucleotides with a backbone of two to seven phosphate moieties have been described, and these compounds display a wide variety of biological activity (10, 11). All of the adenine dinucleotides described to date contain N-glycosidic linkages between the anomeric carbon of ribose and N9 of adenine. Basile et al. (9) describe the synthesis of adenosine 5′-pyrophosphate-5′-adenosine (Ap2A) with the normal C1′–N9 linkage as well as two previously unidentified Ap2A isomers containing C1′–N1 (named P18) and C1′–N3 (named P24) linkages (see Fig. 1 for the structures of these products) by ADP-ribosyl cyclases, including CD38, in the presence of adenine. Ap2A is present in platelet secretory granules (12) and cardiac tissue (13); however, the system responsible for generating this molecule is unknown. Fig. 1 depicts a plausible mechanism for the production of Ap2A and the N1 (P18) and N3 (P24) isomers of Ap2A by ADP-ribosyl cyclases. There is evidence that the ADP-ribosyl cyclase reaction proceeds through a reactive enzymeADP-ribosyl intermediate (2). This intermediate can react with various nucleophiles to form different products. In pathway 1, an intramolecular reaction between the N1 nitrogen of adenine and C1′ of the ADP-ribosyl-enzyme intermediate produces cADPR. Attack of the intermediate by water yields ADPR in pathway 2. Similar to the base exchange reaction for the synthesis of NAADP (14), the production of Ap2A and the N1 and N3 isomers most likely occurs through the attack of the N9, N1, and N3 nitrogen atoms of adenine (pathways 3, 4, and 5 in Fig. 1), respectively.
Fig. 1.
Plausible mechanisms for the generation of cADPR, ADPR, Ap2A, the N1 isomer of Ap2A, and the N3 isomer of Ap2A by ADP-ribosyl cyclases. The mechanisms are labeled 1–5, respectively. See the text for details. The nitrogen atoms in adenine and the various products are numbered.
ADP-ribosyl cyclases can generate adenine dinucleotides.
The data presented by Basile et al. (9) indicate that Ap2A and the N1 and N3 isomers of Ap2A (P18 and P24) can be detected in acid extracts from ADP-ribosyl cyclase-positive cells in both sponge tissue and mammalian cell lines (9). Only CD38-positive mammalian cells had the capacity to metabolize Ap2A, P18, and P24 (9). This finding is interesting because the initial metabolic pathway was found to include a nucleotide pyrophosphatase (9), which CD38 is not known to have. It is possible that the expression of CD38 and the production of Ap2A, P18, and/or P24 provides a signal (or signals) for the expression of enzymes that are capable of metabolizing adenine dinucleotides. Ap2A, P18, and P24 also accumulated in mammalian cells when added exogenously, indicating that an uptake system for these dinucleotides exists (9). These adenine dinucleotides were shown to have interesting effects on mammalian cells (9). Both long- and short-term incubation of CD38-positive or CD38-negative HeLa cells with Ap2A, P18, and P24 had effects on basal calcium and endoplasmic reticulum calcium stores (9). These effects ranged from decreasing (P18) or increasing (Ap2A and P24) basal calcium levels in the long term to stimulating calcium influx acutely (P24). Some of these effects were more pronounced in CD38-positive cells, suggesting a possible synergism with cADPR. It is known that Ap2A acts synergistically with cADPR by increasing the sensitivity of the ryanodine receptor to cADPR (15). cADPR is known to stimulate the proliferation of hemopoietic progenitors by means of a calcium-mediated process (16). P18 and P24 were shown to inhibit hemopoietic progenitor cell growth, whereas Ap2A increased progenitor proliferation and displayed a synergistic effect with cADPR (9). Thus, ADP-ribosyl cyclases generate products that stimulate (cADPR and Ap2A) and inhibit (P18 and P24) progenitor proliferation. Understanding the complex interplay among these signaling molecules may have potential therapeutic benefits, especially in procedures such as ex vivo expansion of hemopoietic progenitor cells for clinical purposes.
cADPR, ADPR, NAADP, Ap2A, P18, and P24 could represent a small fraction of the potential products of ADP-ribosyl cyclases that are possible, given the mechanism shown in Fig. 1. In this mechanism, any molecule containing a nucleophile could attack the ADP-ribosyl-enzyme intermediate to generate a product. The generation of these products could conceivably produce signaling molecules in their own right or have deleterious effects on cell signaling by interfering with the synthesis of the natural products of ADP-ribosyl cyclase (cADPR, ADPR, NAADP, etc.). Several examples of this phenomenon are known and may be biologically relevant. Heterodinucleotides containing guanine and adenine (Ap2G) have been detected in platelet secretory granules (12). This nucleotide could be synthesized if guanine were to attack the ADP-ribosyl-enzyme intermediate. Ap2G is known to have proliferative activity on vascular smooth muscle cells (12). Examples of ADP-ribosyl cyclase reactions that could produce potentially harmful effects on cells include the synthesis of a methanol-ADPR product by CD38 in the presence of methanol (2) and the presence of nicotine adenine dinucleotides in tissues of rabbits that have been treated with nicotine (17). Not only could ADP-ribosyl cyclases such as CD38 be involved in producing these methanol or nicotine metabolites, but their production could potentially have profound effects on cell function by altering calcium signaling and/or pyridine nucleotide levels.
Adenine dinucleotides had effects on basal calcium and endoplasmic reticulum calcium stores.
The results presented by Basile et al. (9) suggest a potential mechanism for the synthesis of adenine dinucleotides (Ap2A and isomers) and indicate that the ADP-ribosyl cyclases are more versatile than originally thought. The data should represent the framework for further investigation into the metabolism and action of Ap2A, P18, and P24 and the interaction of these adenine dinucleotides with other products of ADP-ribosyl cyclase (cADPR, ADPR, and NAADP).
Author contributions: T.F.W. wrote the paper.
Conflict of interest statement: No conflicts declared.
See companion article on page 14509 in issue 41 of volume 102.
References
- 1.Lee, H. C. (2000) Chem. Immunol. 75, 39–59. [DOI] [PubMed] [Google Scholar]
- 2.Schuber, F. & Lund, F. E. (2004) Curr. Mol. Med. 4, 249–261. [DOI] [PubMed] [Google Scholar]
- 3.Lee, H. C. (2001) Annu. Rev. Pharmacol. Toxicol. 41, 317–345. [DOI] [PubMed] [Google Scholar]
- 4.Lee, H. C. (2004) Curr. Mol. Med. 4, 227–237. [DOI] [PubMed] [Google Scholar]
- 5.Lee, H. C., Graeff, R. M. & Walseth, T. F. (1997) Adv. Exp. Med. Biol. 419, 411–419. [PubMed] [Google Scholar]
- 6.Perraud, A. L., Fleig, A., Dunn, C. A., Bagley, L. A., Launay, P., Schmitz, C., Stokes, A. J., Zhu, Q., Bessman, M. J., Penner, R., et al. (2001) Nature 411, 595–599. [DOI] [PubMed] [Google Scholar]
- 7.Lee, H. C. (2000) J. Membr. Biol. 173, 1–8. [DOI] [PubMed] [Google Scholar]
- 8.Ceni, C., Muller-Steffner, H., Lund, F., Pochon, N., Schweitzer, A., De Waard, M., Schuber, F., Villaz, M. & Moutin, M. J. (2003) J. Biol. Chem. 278, 40670–40678. [DOI] [PubMed] [Google Scholar]
- 9.Basile, G., Taglialatela-Scafati, O., Damonte, G., Armirotti, A., Bruzzone, S., Guida, L., Franco, L., Usai, C., Fattorusso, E., De Flora, A. & Zocchi, E. (2005) Proc. Natl. Acad. Sci. USA 102, 14509–14514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoyle, C. H. V., Hilderman, R. H., Pintor, J. J., Schluter, H. & King, B. F. (2001) Drug Dev. Res. 52, 260–273. [Google Scholar]
- 11.McLennan, A. G. (2000) Pharmacol. Ther. 87, 73–89. [DOI] [PubMed] [Google Scholar]
- 12.Jankowski, J., Hagemann, J., Tepel, M., van Der Giet, M., Stephan, N., Henning, L., GouniBerthold, I., Sachinidis, A., Zidek, W. & Schluter, H. (2001) J. Biol. Chem. 276, 8904–8909. [DOI] [PubMed] [Google Scholar]
- 13.Luo, J., Jankowski, J., Knobloch, M., Van der Giet, M., Gardanis, K., Russ, T., Vahlensieck, U., Neumann, J., Schmitz, W., Tepel, M., et al. (1999) FASEB J. 13, 695–705. [DOI] [PubMed] [Google Scholar]
- 14.Aarhus, R., Graeff, R. M., Dickey, D. M., Walseth, T. F. & Lee, H. C. (1995) J. Biol. Chem. 270, 30327–30333. [DOI] [PubMed] [Google Scholar]
- 15.Holden, P., Padua, R. A. & Geiger, J. D. (1996) J. Neurochem. 67, 574–580. [DOI] [PubMed] [Google Scholar]
- 16.Podesta, M., Zocchi, E., Pitto, A., Usai, C., Franco, L., Bruzzone, S., Guida, L., Bacigalupo, A., Scadden, D. T., Walseth, T. F., et al. (2000) FASEB J. 14, 680–690. [DOI] [PubMed] [Google Scholar]
- 17.Shen, W. C., Greene, K. M. & Van Vunakis, H. (1977) Biochem. Pharmacol. 26, 1841–1846. [DOI] [PubMed] [Google Scholar]