Abstract
Heparan sulfate (HS) side chains of HS proteoglycans bind to and assemble extracellular matrix proteins and play important roles in cell–cell and cell–extracellular matrix interactions. HS chains bind a multitude of bioactive molecules and thereby function in the control of multiple normal and pathological processes. Enzymatic degradation of HS by heparanase, a mammalian endoglycosidase, affects the integrity and functional state of tissues and is involved in, among other processes, inflammation, angiogenesis, and cancer metastasis. Here, we report the cloning of heparanase from four Israeli species of the blind subterranean mole rat (Spalax ehrenbergi superspecies), 85% homologous to the human enzyme. Unlike its limited expression in human tissues, heparanase is highly expressed in diverse Spalax tissues. Moreover, we have identified a unique splice variant of the Spalax enzyme lacking 16 aa encoded by exon 7. This deletion resulted in a major defect in trafficking and processing of the heparanase protein, leading to a loss of its enzymatic activity. Interspecies variation was noted in the sequence and in the expression of the splice variant of the heparanase gene in blind mole rats living under different ecogeographical stresses, indicating a possible role in adaptation to stress in Spalax evolution.
Keywords: alternative splicing, heparan sulfate, blind mole rat, angiogenesis, cancer
Heparan sulfate (HS) proteoglycans are macromolecules associated with the cell surface and the extracellular matrix (ECM) of a wide range of cells of vertebrate and invertebrate tissues (1–3). HS binds to and assembles ECM proteins and plays important roles in the structural integrity of the ECM and in cell–cell and cell–ECM interactions. HS chains sequester a multitude of proteins and bioactive molecules and thereby function in the control of a large number of normal and pathological processes (1–4). Apart from sequestration of bioactive molecules, HS proteoglycans have a coreceptor role in which the proteoglycan, in concert with the other cell surface molecule, comprises a functional receptor complex that binds the ligand and mediates its action (3–5).
Enzymatic degradation of HS by heparanase, a mammalian endoglucuronidase, affects the integrity and functional state of tissues and is involved in fundamental biological phenomena, ranging from pregnancy, morphogenesis, and development to inflammation, angiogenesis, and cancer metastasis (6–10). Heparanase elicits an indirect angiogenic response by releasing HS-bound angiogenic growth factors [e.g., basic fibroblast growth factor and vascular endothelial growth factor (VEGF)] from the ECM and by generating HS fragments that potentiate basic fibroblast growth factor receptor binding, dimerization, and signaling (5, 8). Despite earlier reports on the existence of several distinct mammalian HS-degrading endoglycosidases (heparanases), the cloning of the same single gene by several groups (6, 7, 11, 12) suggests that mammalian cells express primarily a single dominant functional heparanase enzyme. Human heparanase is synthesized as a latent 65-kDa precursor whose processing involves proteolytic cleavage and formation of an active enzyme composed of 50- and 8-kDa subunits (13–15). Because heparanase promotes angiogenesis and cancer progression, we decided to investigate the evolution of this unique enzyme in a wild mammal that was exposed to underground hypoxic stress throughout the family Spalacidae evolutionary history (16).
The subterranean blind mole rat of the genus Spalax in Israel belongs to the superspecies Spalax ehrenbergi, consisting of at least 12 allospecies in the Near East. The four Israeli species have been the subject of intensive and extensive interdisciplinary evolutionary studies (16, 17). They represent four species with different diploid chromosome numbers (2n) associated with four climatic regimes in Israel. These include: Spalax galili (2n = 52), which lives in the humid, cool upper Galilee mountains; Spalax golani (2n = 54), which lives in the semidry, cool Golan heights; Spalax carmeli (2n = 58), which ranges in humid, warm central Israel; and Spalax judaei (2n = 60), which lives in the dry and warm Samaria, Judea, and the northern Negev (16–18). Spalax lives all of its life, averaging 3 years, in sealed underground tunnels (19), evolving a unique adaptive complex to cope with hypoxia and hypercapnia (20, 17).
Among the strategies used by Spalax to tolerate hypoxia are higher myocardial maximal oxygen consumption (21), structural adaptations in tissues that result in a decreased diffusion distance of oxygen to the mitochondria (22), increase in the lung diffusion capacity (22), specific differences in myoglobin that augment oxygen delivery at low oxygen tensions (23), and increased density of blood vessels correlated with a unique VEGF expression pattern (19, 24, 25). Hemoglobin and hematocrit are higher in the northern species, which survive higher hypoxia than the southern species (17).
Our group has recently cloned and elucidated the expression of p53 (26, 27) and VEGF (24, 25) in Spalax. p53 gene in healthy Spalax individuals possesses two amino acid substitutions in its DNA-binding domain identical to mutations found in human tumors. These adaptive substitutions endow Spalax p53 with severalfold higher activation of cell arrest and DNA repair genes compared to human p53, and they also favor activation of DNA repair genes over apoptotic genes. Expression of VEGF was constitutively high in Spalax muscles, regardless of the oxygen levels, similar to its expression in highly metastatic tumor cells (28) and unlike its levels in rat muscle (25).
Heparanase plays important roles in cancer metastasis and angiogenesis (6–10). Those roles and the cancer-like expression pattern of VEGF and p53 in Spalax, as well as the higher blood vessel density in some tissues of Spalax compared to other rodents (19, 23–25), led us to clone the Spalax heparanase and investigate its putative contribution to Spalax adaptation to life underground. In the course of this research, we identified a unique splice variant of the enzyme that lacks exon 7 and constitutes, to the best of our knowledge, the first naturally occurring splice variant of the heparanase-coding region described to date.
Materials and Methods
Animals. The animals used for cloning of the Spalax heparanase belong to the four species of the S. ehrenbergi superspecies in Israel. All of the animals were captured in the field and kept in our animal facility for at least 3 months before use. Animals were housed in individual cages, and each species was housed in a separate room. They were kept under controlled conditions at 22–24°C and fed with carrots and apples. Animals used in this study were adults and ranged in weight from 100 to 150 g.
Tissues. Animals were killed by injection of Ketaset CIII (Fort Dodge Animal Health, Fort Dodge, IA) at 5 mg/kg of body weight. Whole organs were taken out and immediately frozen in liquid nitrogen. The ethics committee of the University of Haifa approved all experiments.
RNA and cDNA Preparation. Total RNA was extracted from tissues by using TRI Reagent (Molecular Research Center, Cincinnati) according to the manufacturer's instructions. cDNA was prepared by reverse transcription (Moloney murine leukemia virus reverse transcriptase, Promega) of 1 μg of total RNA, by using oligo(dT)15 and random primers (6).
Gene Cloning. For cloning of Spalax heparanase, kidney cDNAs from four Spalax species were prepared. The ORF of heparanase was isolated by PCR using Taq DNA polymerase (Qbiogene, Illkirch, France). The oligonucleotides (Sigma Genosys, Rehovot, Israel) used for cloning were designed according to published sequences of the mouse, rat, and human heparanases (6, 7, 11, 12). Spalax heparanase cDNAs were subcloned into the eukaryotic expression plasmid pcDNA3 (Invitrogen, Leek, The Netherlands) at the EcoRI site. For cloning the 3′ end and the 3′ UTR, 3′ RACE (RLM-RACE, Ambion, Austin, TX) was performed, by using the Spalax-specific sense primer 5′-AGTGTACCTCCACTGCACAAA-3′, according to the manufacturer's instructions.
Tissue Distribution of the Wild-Type Spalax Heparanase and Its Splice Variant, No. 7. Screening of cDNAs from a variety of tissues for expression of wild-type heparanase, or its splice variant, no. 7, was performed by means of PCR. The primers used were located around the Spalax heparanase cDNA region encoded by exon 7 (sense, 5′-GGTCAACCTCGAGGAAAGACAGTTAA-3′; antisense, 5′-CATAAAGCCAGCTGCAAAGGTG-3′).
DNA Sequencing. DNA sequencing was performed by using vector-specific and gene-specific primers, with an automated DNA sequencer (Applied Biosystems Prism model 310 Genetic Analyzer, PerkinElmer, Foster City, CA).
Similarity Tree. A protein (amino acids)-based tree was established by using Kimura's protein distance (29) and the neighbor-joining method. The tree is derived from the Wisconsin Package version 103 (Genetics Computer Group, Madison, WI).
Cells and Transfections. Human embryonic kidney cells (HEK293) were cultured in Dulbecco's modified Eagle's medium (DMEM, 4.5 g of glucose per liter) containing 10% FCS and antibiotics, as described (30, 31). Cells were grown in 60-mm tissue culture dishes and transfected with a total of 1–2 μg of plasmid DNA mixed with 6 μl of FuGene transfection reagent (Roche Applied Science, Mannheim, Germany) and 94 μl of DMEM. Transiently transfected cells were obtained after 24–48 h of incubation at 37°C. Stable populations of transfected cells were selected with G418 (6, 30, 31).
Heparanase Activity. Cell lysates prepared from 1 × 106 cells by three cycles of freezing and thawing in heparanase reaction buffer (20 mM phosphate–citrate buffer, pH 6.0/1 mM DTT/1 mM CaCl2/50 mM NaCl) were incubated (4 h, 37°C, pH 6.0) with 35S-labeled ECM, prepared as described (6). The incubation medium containing 35S-labeled HS degradation fragments was analyzed by gel filtration on a Sepharose CL-6B column (6, 31). Fractions (0.2 ml) were eluted with PBS, and their radioactivity was counted in a β-scintillation counter. Degradation fragments of HS side chains were eluted from Sepharose 6B at 0.5 < Kav < 0.8 (peak II, fractions 20–30) (6, 31, 32). Each experiment was performed at least three times, and the variation in elution positions (Kav values) did not exceed ±15% of the mean.
Western Blot Analysis. Cells (2 × 106) transfected with either insert-free pcDNA3 vector alone or pcDNA3, containing the Spalax heparanase, were lysed in 1 ml of lysis buffer containing 50 mM Tris·HCl (pH 7.4), 150 mM NaCl, 0.5% Triton X-100, and a mixture of protease inhibitors (Roche Applied Science, Mannheim, Germany). Heparanase was concentrated by incubating (4°C, 1 h) the cell lysate with Con A beads (Amersham Pharmacia Biosciences, Uppsala) or Fractogel (Merck, Darmstadt, Germany) and washing twice with PBS (33–35). The beads were boiled (3 min) in sample buffer and centrifuged, and the supernatant was subjected to SDS/PAGE and immunoblot analysis with polyclonal anti-heparanase antibodies 1453 or 810 (1:2,500) as described (33–35). Antibody 1453 was raised in rabbit against the entire 65-kDa heparanase precursor (35). Antibody 810 was raised in rabbit against the C terminus of the 8-kDa human heparanase subunit (14, 35). Immunoreactive bands were detected by the enhanced chemiluminescence reagent as described (6, 33–35).
Results
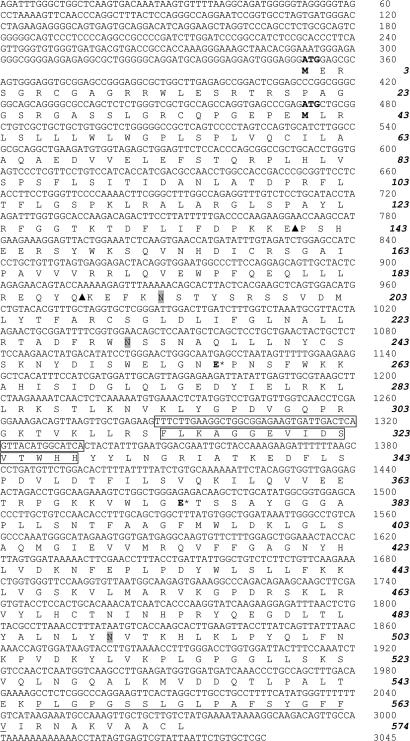
Cloning of the Spalax Heparanase cDNA. The full-length Spalax heparanase cDNAs (including 351 nucleotides in the 5′ UTR and 74 nucleotides in the 3′ UTR) from the four Israeli species were obtained and sequenced (Fig. 1). The presented sequences (nucleic and amino acids) are those of S. carmeli (2n = 58), AM085492, Muhraga. The amino acid sequences of the other species vary in a few amino acids (S. judaei, AM085493, Anza, Arg-285 is changed to Lys, Cys-483 to Val, and Gly-548 to Arg; S. galili, AM085490, Dalton, Glu-190 to Asp and Gly-548 to Arg; S. golani, AM085491, Quneitra, Ala-404 to Ser and Gly-548 to Arg) and are otherwise identical.
Fig. 1.
Nucleotide and predicted amino acid sequences of Spalax heparanase. Nucleotide sequences are shown above the predicted amino acid sequences. Numbers on the right correspond to nucleotides (Roman) and amino acid residues (bold italic). The two initiation codons (ATG) and their corresponding methionine residues (M) are in bold. The three potential N-glycosylation sites are shaded. Arrowheads mark the two cleavage sites generating the two subunits and releasing the linker peptide residing in between. The nucleotide and amino acid sequences lacking in splice 7 are boxed. The hydrophobic potential membrane-spanning domain of 19 aa is underlined.
The cloned Spalax heparanase (Fig. 1) contains two initiation codons (ATG1 and ATG2) of which ATG2 corresponds to that of heparanase cloned from other species. The ORF starting from ATG2 consists of 1,602 bp that encode for a polypeptide of 534 aa (compared with 543 aa of the human protein). Alignment of the amino acid sequences shows that the signal peptide in the N terminus contains 26 aa, compared with 35 residues in the human enzyme (Fig. 2). A hydrophobic, possibly transmembrane region was identified at the C terminus (Pro-546–Val-564) (Fig. 1). Similar to other glycosylhydrolases and to the human enzyme (7, 9), Spalax heparanase has a catalytic mechanism that involves two conserved acidic residues, a putative proton donor (Glu-256), and a nucleophile (Glu-374) (Fig. 1).
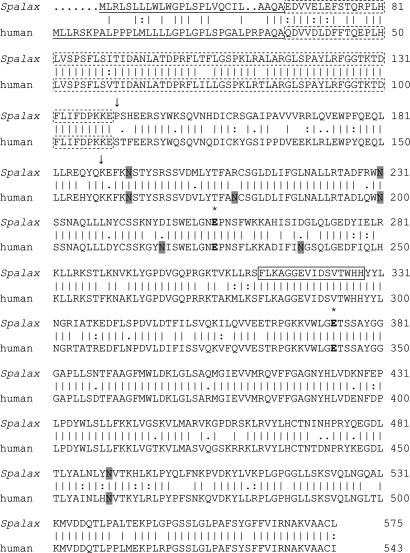
Fig. 2.
Comparison of Spalax and human heparanase amino acid sequences. Vertical lines denote conserved amino acids, and double or single dots mark similar amino acids (Wisconsin Package, Version 103). The putative two catalytic Glu residues, the proton donor and the nucleophile, are marked in bold with * above. The potential N-glycosylation sites are shaded. The 8-kDa subunit is marked with a dotted box. The cleavage sites generating the mature enzyme are marked by arrows, and amino acids between the two arrows denote the linker sequence. The sequence boxed with a continuous line denotes the amino acids lacking in splice 7 of the Spalax heparanase.
Human heparanase is synthesized as a latent 65-kDa precursor whose activation involves proteolytic cleavage at two potential sites located at the N-terminal region of the molecule (Glu-109–Ser-110 and Gln-157–Lys-158), resulting in the formation of two subunits that heterodimerize to form the active heparanase enzyme (13–15). Homologous cleavage sites were identified in the Spalax heparanase at Glu-140–Pro-141 and Gln-188–Lys-189. Alignment of the Spalax amino acid sequence with that of the rat, mouse, human, bovine, and chicken showed 86.7%, 88.6%, 85%, 83.7%, and 67.2% identity, respectively. The predicted amino acid sequence of the Spalax heparanase has three potential N-glycosylation sites (Fig. 1), compared with six in the human (Fig. 2) and four in the mouse and rat heparanases (6–8, 36). All three N-glycosylation sites of the Spalax enzyme are conserved in the human, mouse, and rat heparanases.
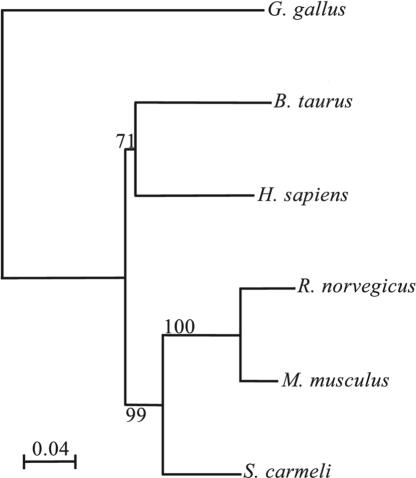
Heparanase Similarity Tree. We have used the Kimura distances to generate a tree based on amino acid distances (Fig. 3). The similarity tree shows that Spalax is situated on a branch separate from the mouse and rat heparanases, and rodents are situated in a cluster separate from the other mammals (human and bovine) and markedly different from the chicken heparanase. The highest similarity in amino acids is between Spalax and mouse (88.6%). Alignment of human heparanase with Spalax, mouse, or rat heparanases revealed that the Spalax enzyme possesses the highest similarity to human (85% vs. 81% and 80.5% similarity for the mouse and rat enzymes, respectively).
Fig. 3.
Heparanase similarity tree: an amino acid-based tree using the Kimura distances. The bar represents substitutions per amino acid. The numbers in the junctions are bootstrapping (in percentage) based on 1,000 replications. Alignment of the Spalax amino acids sequence with that of the rat, mouse, human, bovine, and chicken shows 86.7%, 88.6%, 85%, 83.7%, and 67.2% identity, respectively.
Identification and Cloning of a Splice Variant of Spalax Heparanase Lacking Exon 7. A splice variant of Spalax heparanase was cloned from S. judaei kidney, accession no. AM085494. Sequence analysis revealed that it originates from splicing out of exon 7 (37) (nucleotides 1287–1334), resulting in shortening of the wild-type cDNA by 48 base pairs with no frameshift (Fig. 1). Gel electrophoresis of PCR products amplified by using primers designed around this deletion segment and kidney cDNA as a template revealed both the wild-type and spliced forms. Plasmids containing the coding region of either form were subjected to PCR and used as positive controls (Fig. 4A). The amino acid sequence of the splice variant lacks 16 aa in comparison with the wild-type protein, the deletion (Phe-313–His-328) located in a region between the nucleophile and proton donor residues (Figs. 1 and 2).
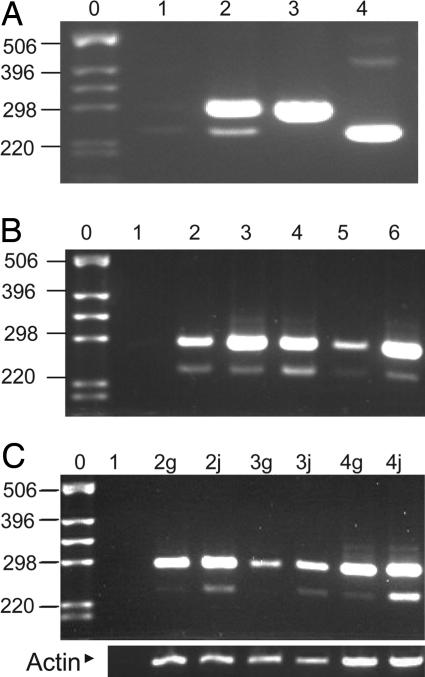
Fig. 4.
Expression of heparanase in different Spalax tissues. Semiquantitive RT-PCR using Spalax-specific primers located around the heparanase cDNA region encoded by exon 7. Bands of 288 bp represent the wild-type enzyme, and those of 240 bp represent its splice 7 form. (A) Lane 1, reaction mixture alone; lane 2, cDNA of kidney; lanes 3 and 4, plasmids containing the cDNA sequence of the wild-type Spalax heparanase and the splice 7 variant, respectively. (B) Lane 1, reaction mixture alone; lanes 2–6, cDNAs of S. judaei kidney, liver, heart, brain, and eye, respectively. (C) Comparison of heparanase expression of S. galili and S. judaei (g or j added to the lane number, respectively). Lane 1, reaction mixture alone; lanes 2–4, cDNAs from kidney, brain, and liver, respectively. The same cDNA preparations were subjected to RT-PCR using primers specific for Spalax β-actin to control for equal loading. Note the higher expression of splice 7 in S. judaei. DNA ladder lanes are marked by 0. Shown to the left of the DNA ladder are the corresponding number of base pairs.
To evaluate the tissue distribution of heparanase and its splice variant in Spalax, cDNAs from different tissues were prepared and subjected to PCR using specific primers designed around exon 7. Both the splice variant and wild-type heparanases were detected in cDNAs from kidney, liver, heart, brain, and eye (Fig. 4B). The wild-type cDNA constituted the principal form of heparanase, whereas splice 7 ranged from 0% to 25% of the total heparanase in different tissues and animals. A marked variation in tissue expression of Spalax heparanase splice 7 was noted between individual animals from different ecogeographical locations. Splice 7 was markedly higher in S. judaei, which lives in a dry, normoxic environment, than in S. galili, which resides in humid, cool, and frequently hypoxic conditions during the winter (Fig. 4C).
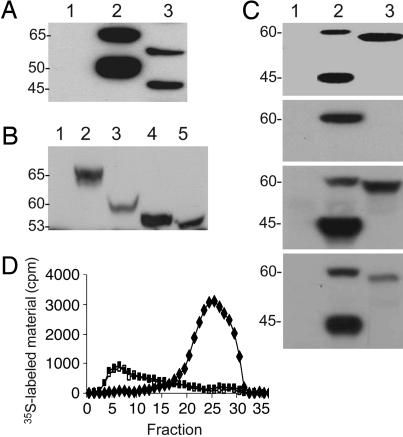
Functional Expression of Wild-Type and Splice 7 Spalax Heparanases in Mammalian Cells. The full-length human and Spalax heparanase cDNAs, as well as Spalax splice 7 cDNA, were subcloned into the expression vector pcDNA3 and transfected into HEK293 cells. Stable transfected cells were obtained after selection with G418. Western blot analysis of wild-type Spalax heparanase partially purified from cell lysates (using anti-heparanase antibody 1453, which recognizes both the unprocessed and processed enzyme) revealed 60- and 45-kDa protein bands (Fig. 5A, lane 3) compared with the 65- and 50-kDa latent and active forms of the human enzyme (Fig. 5A, lane 2). To evaluate the contribution of glycosylation to the molecular mass difference between the human and Spalax heparanases, cells stably transfected with each heparanase species were incubated (48 h, 37°C) with or without 10 μg/ml tunicamycin (N-glycosylation inhibitor). Western blotting of cell lysates by using anti-heparanase antibody 810, which recognizes the unprocessed protein, revealed a single band in both species that corresponds to the unprocessed heparanase. In cells that were not treated with tunicamycin, the human heparanase appeared as a 65-kDa band (Fig. 5B, lane 2), whereas that of Spalax corresponded to a 60-kDa protein (Fig. 5B, lane 3). After treatment with tunicamycin, both the human and Spalax heparanases appeared as 53-kDa proteins (Fig. 5B, lanes 4 and 5), most likely because of their complete deglycosylation.
Fig. 5.
Expression, glycosylation, secretion, and enzymatic activity of splice 7 vs. Spalax heparanases. (A–C) Western blots using anti-heparanase antibodies 1453 in A and C and 810 in B. (A) Lysates of 293HEK cells transfected with mock (lane 1), human (lane 2), or Spalax (lane 3) heparanases. (B) 293HEK cells transfected with mock (lane 1), human (lanes 2 and 4), or Spalax (lanes 3 and 5) heparanases were preincubated without (lanes 2 and 3) or with (lanes 4 and 5) tunicamycin. Cell lysates were subjected to SDS/PAGE and Western blotting, as described in Materials and Methods. Note that the molecular weight difference between the human (lane 2) and Spalax (lane 3) heparanases is abolished after treatment with tunicamycin (lanes 4 and 5). (C) Comparison of Spalax wild-type and splice 7 heparanase processing, secretion, and heparin binding. The first blot shows lysates, and the second blot shows conditioned medium of cells transfected with mock (lane 1), Spalax wild-type (lane 2), and splice 7 (lane 3) heparanases. Note the lack of processing (first blot) and secretion (second blot) of splice 7. The third and fourth blots show heparin-binding capacity. Lysates of 293HEK cells transfected with mock (lanes 1), Spalax wild-type (lane 2), or splice 7 (lane 3) heparanases were incubated with Fractogel (third blot), as a positive control, or with heparin beads (fourth blot). Proteins remaining bound to the resin and beads after washing were subjected to Western blot analysis using anti-heparanase antibodies, as described in Materials and Methods. Both wild-type and splice 7 Spalax heparanases bind to the heparin beads. (D) Heparanase enzymatic activity. Lysates of cell stably transfected with pcDNA3 vectors containing Spalax wild-type (♦) or splice 7 (□) heparanases vs. mock, insert-free plasmid alone (▪) were incubated (4 h, 37°C, pH 6.0) with 35S-labeled ECM. Labeled degradation fragments released into the incubation medium were analyzed by gel filtration on Sepharose 6B. Peak I (fractions 1–10), representing nearly intact HS proteoglycans, was noticed in the mock (▪) and splice 7 (□) transfected cells. Peak II (fractions 20–30), representing HS degradation products, was obtained in cells transfected with the wild-type Spalax heparanase (♦).
Next, we compared the expression pattern of splice 7 and wild-type Spalax heparanases, applying HEK293 cells transfected with each form. As shown in Fig. 5B (lane 1, mock; lane 2, wild type; lane 3, splice 7), splice 7 appeared as an ≈59-kDa band, as compared with the 60- and 45-kDa proteins of the wild-type latent and active Spalax enzymes, respectively (Fig. 5C, first blot). To evaluate secretion of the Spalax heparanase and its splice 7, we cultured (24 h, 37°C) HEK293 cells stably transfected with Spalax heparanase, splice 7, or insert-free mock plasmid in the absence or presence of 20 μg/ml heparin. We have previously demonstrated accumulation of secreted heparanase in the presence of heparin (38). Western blot analysis of the incubation medium by using anti-heparanase antibodies revealed secretion and accumulation of the wild-type latent enzyme in the culture medium (Fig. 5C, second blot). In contrast, splice 7 was not detected in the incubation medium (Fig. 5C, second blot) regardless of the presence of heparin, indicating its inability to be secreted and to accumulate in the culture medium (38). To assess the binding of Spalax heparanase and splice 7 to heparin, lysates of cells transfected with each variant or with a mock control plasmid were incubated with heparin-Sepharose beads or with Fractogel (cation exchange resin) as a positive control. The beads were washed with PBS, and the bound proteins were subjected to immunoblotting. Both the wild-type and splice 7 Spalax heparanases bound to heparin beads and were readily detected after SDS/PAGE of the bound proteins and Western blotting (Fig. 5C, fourth blot).
Heparanase Enzymatic Activity. We assessed the ability of Spalax heparanase and its splice variant to degrade HS in intact ECM. For this purpose, lysates of HEK293 cells stably transfected with the full-length Spalax heparanase, splice 7, or a mock control were incubated (4 h, 37°C, pH 6.0) with intact, naturally produced, sulfate-labeled ECM. Labeled degradation fragments released into the incubation medium were then analyzed by gel filtration on Sepharose 6B. Sulfate-labeled material released by the mock-transfected cells eluted just after the void volume (V0) (peak I, fractions 1–10, Kav < 0.2) and consisted almost entirely of intact, high-molecular-weight HS proteoglycans released from the ECM by proteolytic enzymes present in the cell lysate and/or residing in the ECM itself (39). Similar results were obtained with splice 7 transfected cells. In contrast, incubation of the ECM with lysates of cells transfected with the wild-type Spalax heparanase resulted in release of low-molecular-weight-labeled degradation fragments eluted toward the Vt of the column (peak II, fractions 20–30, 0.5 < Kav < 0.8) (Fig. 5D). These fragments were shown to be degradation products of HS because they were (i) 5- to 6-fold smaller than intact HS side chains, (ii) resistant to further digestion with papain and chondroitinase ABC, and (iii) susceptible to deamination by nitrous acid (39).
Discussion
The Spalax Evolutionary Model. The subterranean blind mole rat of the genus Spalax in Israel is an excellent model of the twin evolutionary processes of adaptation and speciation (16, 17). The hypoxic, dark, low productive, and energetically stressful environment in which Spalax lives resulted in a variety of adaptations at the structural, functional, organismal, and molecular levels. Structural adaptations include regression of less important organs (e.g., the eyes, which are subcutaneous and atrophic but still have an active retina used in photoperiodic perception) and progression of others (e.g., big teeth and strong neck muscles needed for underground digging). The hypoxic environment that Spalax tissues survive (20) is probably similar to the hypoxic conditions in tumor cores. This similarity may explain the evolution in Spalax of physiological variants of oncogenes and angiogenic proteins with similarities to mutations found in human cancer cells (26, 27). For example, our group has recently shown that amino acid substitutions in the Spalax p53 gene are identical to known tumor-associated mutations (26, 27). VEGF expression in Spalax muscle was constitutively high, a pattern similar to its expression in highly metastatic tumor cells (24, 25, 28). Also, erythropoietin expression levels in Spalax exhibit a higher increment under hypoxia, relative to other rodents (20). The four allospecies of Spalax developed in Israel and share similar morphology, but differ in their unique adaptive complex to the different climatic stresses. Major changes in genomic DNA structure resulted in different chromosome number and structure (16, 17). Here, we report that heparanase, which in humans is expressed mainly in malignant cancer cells, is highly expressed in diverse Spalax tissues (Fig. 4B).
Spalax Heparanase: Structure and Function. We have demonstrated that, despite some differences in sequence, Spalax heparanase is as active as the human enzyme in degrading HS in the ECM. Spalax expresses heparanase in a multitude of tissues and, hence, may contribute to the increased density of blood vessels observed in some of these tissues, relative to mammals residing aboveground. Of special interest is the high expression of heparanase in the Spalax eye (Fig. 4B, lane 6), which is atrophic and visually blind (16, 17), but is assessing photoperiodicity by responding to signals that penetrate the soil and is also involved in the circadian rhythm control (40–42).
Spalax heparanase posesses fewer N-glycosylation sites than any other described mammalian heparanase. Our results suggest that differences in molecular weights between Spalax and human heparanases are primarily due to a lower glycosylation of the Spalax protein, which lacks three of the six N-glycosylation sites of the human heparanase. Glycosylation of heparanase is not required for its enzymatic activity but may function in heparanase trafficking and secretion (43). Secretion of heparanase appears to be a cardinal step in its processing and activation (34). In fact, transient transfection of cells with the Spalax vs. the human heparanase revealed a slower processing of the Spalax protein (our unpublished results).
Alternative Splicing of Spalax Heparanase. Apart from cloning the Spalax heparanase gene, we describe a unique splice variant of heparanase with interspecies variability of its expression. This splice variant lacks 48 base pairs that encode for 16 aa residing between the proton donor (Glu-256) and nucleophile (Glu-374) sites. This deletion did not prevent heparanase's binding to heparin (Fig. 5C). Unlike the wild-type heparanase, splice 7 was not detected in the medium of transfected cells, suggesting a defect in its secretion (Fig. 5C). Likewise, processing of splice 7 (i.e., conversion of the latent enzyme into its active form) could not be detected (Fig. 5C), and hence this splice variant showed no heparanase enzymatic activity (Fig. 5D). A recently constructed human homolog of splice 7 exhibited characteristics similar to the Spalax splice variant (unpublished results).
The lesser expression of splice 7 in S. galili compared to S. judaei (zFig. 4C) may be due to evolutionary adaptations to the burrow atmosphere differences experienced by these species. S. galili survives more severe hypoxic conditions than does S. judaei and thus may need all its heparanase molecules in the nonspliced, enzymatically active form to generate enough blood vessels. Preferential production of splice 7 in S. judaei may be a regulatory process by which heparanase enzymatic activity is down-regulated at the transcriptional level. Of interest is the high expression of splice 7 in the Spalax heart and eye, although the functional significance of this observation is not known. Recently, heparanase was implicated in a variety of nonenzymatic functions (e.g., cell adhesion and survival) (30, 35) that may still be conserved in splice 7. These functions are currently being investigated. Several other splice variants of Spalax heparanase were identified, resulting in expression of truncated forms compared with the wild-type protein (unpublished results). Altogether, these results indicate that alternative splicing (44, 45) in Spalax plays a key role in modulating gene functions in response to hypoxic stresses and to the unique evolution of this mammal under diverse fluctuating burrow oxygen levels. The high rate of alternative splicing enabled the identification of heparanase splice variants that, until now, could not be detected in other species. The present results will, in all likelihood, enable the identification of the human counterpart of the Spalax splice variant and elucidate its function and physiological significance. Moreover, the Spalax splice variants could be important in human cancer research and the design of heparanase-based therapeutic approaches.
Acknowledgments
We thank Alma Joel and Michael Margulis (Institute of Evolution, University of Haifa) for technical assistance, and Robin Permut (Institute of Evolution) for reviewing the manuscript. This work was supported by a scholarship awarded by the Israeli Ministry of Science and Technology to Israeli Arab, Druze, and Charkas students (to N.J.N.); by Grant 532/02 from the Israel Science Foundation; by United States Public Health Service Grant R01 CA106456-01 from National Cancer Institute, National Institutes of Health (to I.V.); and by the Ancell-Teicher Foundation for Genetics and Molecular Evolution (E.N.). This work is a partial fulfillment of requirements for Ph.D. (N.J.N.).
Author contributions: N.J.N., E.N., N.I., I.V., and A.A. designed research; N.J.N., I.S., and A.A. performed research; N.J.N., E.N., I.V., and A.A. analyzed data; and N.J.N., E.N., I.V., and A.A. wrote the paper.
Abbreviations: HS, heparan sulfate; ECM, extracellular matrix.
Data deposition: The sequences reported in this paper have been deposited in the GenBank database (accession nos. AM085490–AM085494).
References
- 1.Kjellen, L. & Lindahl, U. (1991) Annu. Rev. Biochem. 60, 443-475. [DOI] [PubMed] [Google Scholar]
- 2.Bernfield, M., Gotte, M., Park, P. W., Reizes, O., Fitzgerald, M. L., Lincecum, J. & Zako, M. (1999) Annu. Rev. Biochem. 68, 729-777. [DOI] [PubMed] [Google Scholar]
- 3.Iozzo, R. V. (1998) Annu. Rev. Biochem. 67, 609-652. [DOI] [PubMed] [Google Scholar]
- 4.Vlodavsky, I., Bar-Shavit, R., Korner, G. & Fuks, Z. (1993) in Basement Membranes: Cellular and Molecular Aspects, eds. Rohrbach, D. H. & Timpl, R. (Academic, Orlando, FL), pp. 327-343.
- 5.Vlodavsky, I., Miao, H.-Q., Medalion, B., Danagher, P. & Ron, D. (1996) Cancer Metastasis Rev. 15, 177-186. [DOI] [PubMed] [Google Scholar]
- 6.Vlodavsky, I., Friedmann, Y., Elkin, M., Aingorn, H., Atzmon, R., Ishai-Michaeli, R., Bitan, M., Pappo, O., Peretz, T., Michal, I., et al. (1999) Nat. Med. 5, 793-802. [DOI] [PubMed] [Google Scholar]
- 7.Hulett, M. D., Freeman, C., Hamdorf, B. J., Baker, R. T., Harris, M. J. & Parish, C. R. (1999) Nat. Med. 5, 803-809. [DOI] [PubMed] [Google Scholar]
- 8.Vlodavsky, I. & Friedmann, Y. (2001) J. Clin. Invest. 108, 341-347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parish, C. R., Freeman, C. & Hulett, M. D. (2001) Biochim. Biophys. Acta 1471, M99-M108. [DOI] [PubMed] [Google Scholar]
- 10.Edovitsky, E., Elkin, M., Zcharia, E., Peretz, T. & Vlodavsky, I. (2004) J. Natl. Cancer Inst. 96, 1219-1230. [DOI] [PubMed] [Google Scholar]
- 11.Kussie, P. H., Hulmes, J. D., Ludwig, D. L., Patel, S., Navarro, E. C., Seddon, A. P., Giorgio, N. A. & Bohlen, P. (1999) Biochem. Biophys. Res. Commun. 261, 183-187. [DOI] [PubMed] [Google Scholar]
- 12.Toyoshima, M. & Nakajima, M. (1999) J. Biol. Chem. 274, 24153-24160. [DOI] [PubMed] [Google Scholar]
- 13.Abboud-Jarrous, G., Aingorn, H., Rangini-Guetta, Z., Atzmon, R., Elgavish, S., Peretz, T. & Vlodavsky, I. (2005) J. Biol. Chem. 280, 13568-13575. [DOI] [PubMed] [Google Scholar]
- 14.Levy-Adam, F., Miao, H.-Q., Heinrikson, R. L., Vlodavsky, I. & Ilan, N. (2003) Biochem. Biophys. Res. Commun. 308, 885-891. [DOI] [PubMed] [Google Scholar]
- 15.McKenzie, E., Young, K., Hircock, M., Bennett, J., Bhaman, M., Felix, R., Turner, P., Stamps, A., McMillan, D., Saville, G., et al. (2003) Biochem. J. 373, 423-435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nevo, E. (1999) Mosaic Evolution of Subterranean Mammals: Regression, Progression, and Global Convergence (Oxford Univ. Press, Oxford).
- 17.Nevo, E., Ivanitskaya, E. & Beiles, A. (2001) Adaptive Radiation of Blind Subterranean Mole Rats (Backhuys, Leiden, The Netherlands).
- 18.Nevo, E. (1991) Evol. Biol. 25, 1-125. [Google Scholar]
- 19.Widmer, H. P., Hoppeler, H., Nevo, E., Taylor, C. R. & Weibel, E. W. (1997) Proc. Natl. Acad. Sci. USA 94, 2062-2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shams, I., Avivi, A. & Nevo, E. (2004) Proc. Natl. Acad. Sci. USA 26, 9698-9703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Edoute, Y., Arieli, R. & Nevo, E. (1988) J. Comp. Physiol. 158, 575-582. [DOI] [PubMed] [Google Scholar]
- 22.Weibel, E. R., Federspiel, W. J., Fryder-Doffey, F., Hsia, C. W., Konig, M., Stalder-Navarro, V. & Vock, R. (1993) Respir. Physiol. 93, 125-149. [DOI] [PubMed] [Google Scholar]
- 23.Arieli, R. (1990) in Evolution of Subterranean Mammals at Organismal and Molecular Levels, eds. Nevo, E. & Reig, O. (Wiley-Liss, New York), pp. 251-268.
- 24.Avivi, A., Resnick, M. B., Nevo, E., Joel, A. & Levy, A. P. (1999) FEBS Lett. 452, 133-140. [DOI] [PubMed] [Google Scholar]
- 25.Avivi, A., Shams, I., Joel, A., Lache, O., Levy, A. P. & Nevo, E. (2005) FASEB J. 19, 1314-1316. [DOI] [PubMed] [Google Scholar]
- 26.Ashur-Fabian, O., Avivi, A., Trakhtenbrot, L., Adamsky, K., Cohen, M., Kajakaro, G., Joel, A., Amariglio, N., Nevo, E. & Rechavi, G. (2004) Proc. Natl. Acad. Sci. USA 101, 12236-12241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Avivi, A., Ashur-Fabian, O., Amariglio, N., Nevo, E. & Rechavi, G. (2005) Cell Cycle 4, 368-372. [DOI] [PubMed] [Google Scholar]
- 28.Koshikawa, N., Iyozumi, A., Gassmann, M. & Takenaga, K. (2003) Oncogene 22, 6717-6724. [DOI] [PubMed] [Google Scholar]
- 29.Kimura, M. (1983) The Neutral Theory of Molecular Evolution (Cambridge Univ. Press, Cambridge, U.K.).
- 30.Goldshmidt, O., Zcharia, E., Abramovitch, R., Metzger, S., Aingorn, H., Friedmann, Y., Mitrani, E. & Vlodavsky, I. (2002) Proc. Natl. Acad. Sci. USA 99, 10031-10036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goldshmidt, O., Zcharia, E., Aingorn, H., Guatta-Rangini, Z., Atzmon, R., Michal, I., Pecker, I., Mitrani, E. & Vlodavsky, I. (2001) J. Biol. Chem. 276, 29178-29187. [DOI] [PubMed] [Google Scholar]
- 32.Miao, H.-Q., Elkin, M., Aingorn, E., Ishai-Michaeli, R., Stien, C. A. & Vlodavsky, I. (1999) Int. J. Cancer 83, 424-431. [DOI] [PubMed] [Google Scholar]
- 33.Friedmann, Y., Vlodavsky, I., Aingorn, H., Aviv, A., Peretz, T., Pecker, I. & Pappo, O. (2000) Am. J. Pathol. 157, 1167-1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zetser, A., Levy-Adam, F., Kaplan, V., Gingis-Velitski, S., Bashenko, Y., Schubert, S., Flugelman, M. Y., Vlodavsky, I. & Ilan, N. (2004) J. Cell Sci. 117, 2249-2258. [DOI] [PubMed] [Google Scholar]
- 35.Zetser, A., Bashenko, Y., Miao, H.-Q., Vlodavsky, I. & Ilan, N. (2003) Cancer Res. 63, 7733-7741. [PubMed] [Google Scholar]
- 36.Miao, H.-Q., Navarro, E., Patel, S., Sargent, D., Koo, H., Wan, H., Plata, A., Zhou, Q., Ludwig, D., Bohlen, P. & Kussie, P. (2002) Protein Expression Purif. 26, 425-431. [DOI] [PubMed] [Google Scholar]
- 37.Dong, J., Kukula, A. K., Toyoshima, M. & Nakajima, M. (2000) Gene 253, 171-178. [DOI] [PubMed] [Google Scholar]
- 38.Levy-Adam, F., Abboud-Jarrous, G., Guerrini, M., Beccati, D., Vlodavsky, I. & Ilan, N. (2005) J. Biol. Chem. 280, 20457-20466. [DOI] [PubMed] [Google Scholar]
- 39.Vlodavsky, I., Fuks, Z., Bar-Ner, M., Ariav, Y. & Schirrmacher, V. (1983) Cancer Res. 43, 2704-2711. [PubMed] [Google Scholar]
- 40.Avivi, A., Albrecht, U., Oster, H., Joel, A., Beiles, A. & Nevo, E. (2001) Proc. Natl. Acad. Sci. USA 98, 13751-13756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Avivi, A., Oster, H., Joel, A., Beiles, A., Albrecht, U. & Nevo, E. (2002) Proc. Natl. Acad. Sci. USA 98, 11718-11723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sanyal, S., Jansen, H. G., de Grip, W. G., Nevo, E. & de Jong, W. W. (1990) Invest. Ophthalmol. Visual Sci. 31, 1398-1404. [PubMed] [Google Scholar]
- 43.Simizu, S., Ishida, K., Wierzba, M. K. & Osada, H. (2004) J. Biol. Chem. 279, 2697-2703. [DOI] [PubMed] [Google Scholar]
- 44.Ast, G. (2004) Nat. Rev. Genet. 5, 773-782. [DOI] [PubMed] [Google Scholar]
- 45.Boue, S., Letunic, I. & Bork, P. (2003) BioEssays 25, 1031-1034. [DOI] [PubMed] [Google Scholar]