Abstract
The ATE1-encoded Arg-transferase mediates conjugation of Arg to N-terminal Asp, Glu, and Cys of certain eukaryotic proteins, yielding N-terminal Arg that can act as a degradation signal for the ubiquitin-dependent N-end rule pathway. We have previously shown that mouse ATE1–/– embryos die with defects in heart development and angiogenesis. Here, we report that the ATE1 Arg-transferase mediates the in vivo degradation of RGS4 and RGS5, which are negative regulators of specific G proteins whose functions include cardiac growth and angiogenesis. The proteolysis of these regulators of G protein signaling (RGS) proteins was perturbed either by hypoxia or in cells lacking ubiquitin ligases UBR1 and/or UBR2. Mutant RGS proteins in which the conserved Cys-2 residue could not become N-terminal were long-lived in vivo. We propose a model in which the sequential modifications of RGS4, RGS5, and RGS16 (N-terminal exposure of their Cys-2, its oxidation, and subsequent arginylation) act as a licensing mechanism in response to extracellular and intracellular signals before the targeting for proteolysis by UBR1 and UBR2. We also show that ATE1–/– embryos are impaired in the activation of extracellular signal-regulated kinase mitogen-activated protein kinases and in the expression of G protein-induced downstream effectors such as Jun, cyclin D1, and β-myosin heavy chain. These results establish RGS4 and RGS5 as in vivo substrates of the mammalian N-end rule pathway and also suggest that the O2-ATE1-UBR1/UBR2 proteolytic circuit plays a role in RGS-regulated G protein signaling in the cardiovascular system.
Keywords: ATE1 R-transferase, G protein signaling, oxidation, ubiquitin, UBR
The ubiquitin (Ub)-dependent N-end rule pathway relates the in vivo half-life of a protein to the identity of its N-terminal residue (1) (Fig. 1A). We previously identified the mouse ATE1 gene encoding Arg-transferase, which conjugates Arg to N-terminal Asp, Glu, and Cys of engineered N-end rule substrates (2, 3), yielding N-terminal Arg that can act as an essential component of N-degron (N-terminal degradation signal). N-degrons can be recognized by Ub ligases (E3s) for protein ubiquitylation. Mouse ATE1–/– embryos died with cardiovascular defects, including ventricular hypoplasia, ventricular septal defect, and impaired late angiogenesis (3), suggesting that the ATE1-dependent proteolysis of unknown substrate(s) is a crucial regulatory mechanism for myocardial growth and blood vessel integrity/maturation. One aim of this study was to identify in vivo ATE1 substrates that are important for cardiovascular functions.
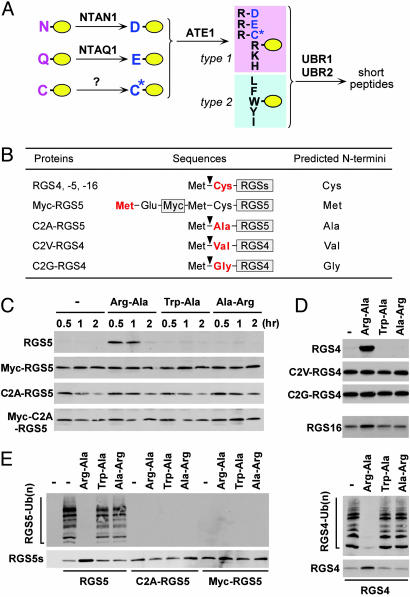
Fig. 1.
In vitro proteolysis of RGS4, RGS5, and RGS16. (A) The organization of mammalian N-end rule pathway, established from previous and current studies. N-terminal Asn and Gln function as pre-N-degrons through their deamidation by NTAN1 and a hypothetical enzyme, NTAQ1, respectively, into Asp and Glu (23). N-terminal Cys also serves as a pre-N-degron through its oxidation into Cys sulfinic acid (CysO2) or cysteic acid (CysO3) (ref. 3 and this study). N-terminal Asp, Glu, and (oxidized) Cys function as pre-N-degrons through their N-terminal arginylation by ATE1-encoded Arg-transferase (2). The resulting N-terminal Arg together with other type 1 and type 2 N-degrons are recognized by specific E3s for subsequent Ub-dependent proteolysis. We have previously characterized UBR1 and UBR2 as such E3s (14, 16). UBR1 and UBR2 are 200 kDa-RING finger E3s that target type 1 (Arg, Lys, and His) and type 2 (Phe, Leu, Trp, Tyr, and Ile) N-degrons (14, 16, 24). In mammals, N-terminal Ala, Ser, or Thr are classified as type 3 N-degron (16), but the E3 that recognizes type 3 N-degron remains elusive. (B) RGS constructs and their expected N-terminal residues. (C–D) RGS proteins were expressed in reticulocyte lysates either in the absence or presence of 2 mM dipeptides, and their degradation was analyzed by using time-course antibiotin Western blotting. Reactions were allowed for 60 min in D. Inhibition of RGS5 proteolysis by Arg-Ala diminished after 120 min perhaps because of instability of dipeptides. (E) RGS proteins were expressed in reticulocyte lysates in the presence of MG132. After 15 min, their ubiquitylation was analyzed by using anti-Ub immunoprecipitation and subsequent antibiotin Western blotting (Upper), while their expression levels were determined by using antibiotin Western blotting (Lower).
We previously reported that RGS4, a G protein-specific GTPase-activating protein (4), is N-terminally arginylated and degraded by the Ub system in ATP-supplemented reticulocyte extract and that its in vitro degradation is inhibited by the Arg-Ala dipeptide inhibitor of the N-end rule pathway (5). However, previous attempts to verify the functional connection between RGS4 and the N-end rule pathway in vivo (in mammalian cells) were unsuccessful. Nonetheless, RGS4, RGS5, and RGS16, belonging to the R4 subfamily of RGS proteins, were identified, together with other candidate N-end rule substrates through a function-based proteomic approach (I.V.D. and H. Biebuyck, unpublished data). Given that these RGS proteins were implicated as negative regulators of the cardiovascular Gq and Gi signaling pathways (6), we wished to test whether RGS4, RGS5, and RGS16 were, in fact, in vivo substrates of the ATE1-dependent Ub pathway, and whether the corresponding molecular circuits may underlie cardiovascular functions.
Heterotrimeric G proteins are signal transducers that connect the ligand-activated G protein-coupled receptors (GPCRs) to effectors of intracellular signaling pathways (4). Ligand-activated GPCRs stimulate the Gα subunit to exchange GDP to GTP and thereby dissociate the inactive Gαβγ heterotrimer into the active Gα and Gβγ subunits, both of which activate second messenger-producing enzymes and ion channels. Signaling by Gα and Gβγ continues until Gα hydrolyzes GTP and the heterotrimer reassociates. The slow intrinsic rate of GTP hydrolysis by Gα can be strongly increased by RGS proteins (7) that contain the functionally essential ≈120-residue RGS domain. RGS4, RGS5, and RGS16 act as GTPase-activating proteins for Gαq and Gαi and have a crucial role in shutting off G protein-mediated responses in all eukaryotes (7). GTP-bound Gαq activates phospholipase C and mediates IP3-mediated calcium release and diacylglycerol-mediated PKC activation. Gβγ mediates activation of the small GTP-binding protein Ras and initiates a tyrosine kinase, leading to activation of mitogen-activated protein kinases (MAPKs), which in turn activate/induce various downstream transcription factors to promote cell growth (8).
Gq and Gi pathways are critical for proliferation and differentiation of myocardial cells. Angiotensin II, phenylephrine, endothelin, and prostaglandin F2α that activate Gq- or Gi-coupled receptors all stimulated the hypertrophic response in cardiomyocytes (9). Gαq overexpression in cardiac tissue increased heart weight and cardiomyocyte size associated with the expression of cardiac hypertrophic markers such as βMHC (10). Similarly, various studies using cardiomyocytes or transgenic/knockout animals indicated that Gq- or Gi-activated effectors such as PKC, Ras, and MAPKs are critical for regulation of myocardial cell growth (8). Consistent with the biochemical properties of RGS proteins, it is increasingly clear that RGS4, RGS5, and RGS16 act as important negative regulators of the Gq- or Gi-mediated cardiovascular signaling (11). Overexpression of RGS4 mRNA and/or protein was frequently observed in patients or animal models with heart dysfunction (12). RGS4 overexpression reduced endothelin-activated hypertrophic response in cardiomyocytes (6). Transgenic mice overexpressing RGS4 in postnatal ventricular myocardium displayed, upon transverse aortic constriction, left ventricular dilatation, depressed systolic function, and higher postoperative mortality, and failed to induce βMHC expression (13). Although the exact functions of RGS5 and RGS16 in the heart remain to be understood in detail, it is likely, given the above, that RGS4, RGS5, and RGS16 play important roles in cardiovascular homeostasis.
Here, we show that RGS4, RGS5, and RGS16 are degraded by the arginylation branch of the N-end rule pathway in a manner that requires molecular oxygen (O2). We also show that the activity of the Gq/Gi-activated extracellular signal-regulated kinase (ERK) pathway is impaired in ATE1–/– embryos. We propose that this ATE1-dependent proteolytic cascade plays an important role in RGS-regulated G protein signaling in the cardiovascular system and possibly in other biological processes as well.
Materials and Methods
Ubiquitylation and Degradation Assays. For in vitro degradation assay, RGS proteins were expressed and biotin-labeled in the absence or presence of 2 mM dipeptides by using the rabbit reticulocyte lysate TnT system (Promega), followed by time-course Western blotting with horseradish peroxidase-conjugated streptavidin. For biotinylation, biotinylated lysine-tRNA complex (Transcend tRNA, Promega) was added in the reaction. In vitro ubiquitylation of RGS proteins was similarly determined except that the reaction was done in the presence of MG132, followed by anti-Ub immunoprecipitation and subsequent Western blotting with horseradish peroxidase-conjugated streptavidin. Anti-Ub antibody (Biomol International, Plymouth Meeting, PA) recognizes both mono- and multiubiquitylated proteins but not free Ub. Bestatin (Sigma–Aldrich) was added to decrease degradation of dipeptides (14). The RGS4, C2V-RGS4, and C2G-RGS4 constructs were previously described (5) as were the RGS5, Myc-RGS5, and C2A-RGS5 constructs (15). The C2A-RGS5 construct was generated by PCR-mediated mutagenesis. The RGS16 construct was generated by using RT-PCR, subcloning into pENTR vector (Invitrogen), and subsequent recombination into pcDNA-cLumio-DEST vector (Invitrogen). Anti-RGS5 antibody was previously described (15). Anti-RGS4 antibody was purchased (Santa Cruz Biotechnology). Pulse–chase analysis was performed as described (16). ATE1–/– (3), UBR1–/– (14), UBR2–/– (16), and UBR1–/– UBR2–/– (17) embryonic fibroblasts (EFs) were previously described. The ATE1-1 and ATE1-2 constructs were generated by subcloning their ORFs from the yeast expression constructs (2) into pcDNA3 vector (Invitrogen).
O2 Depletion. For in vitro O2 depletion, an N2-containing balloon was connected to a 1.7-cm reaction tube by using a rubber-O-ring cap, and N2 was passed into the tube through a 23-gauge needle connected to a 10-ml syringe. For in vivo hypoxic treatment, +/+ and ATE1–/– EFs were cotransfected with RGS5 and control LacZ plasmids. At 6 h posttransfection, cells were exposed to either normoxia or hypoxia for 24 h. Hypoxia was achieved by exposing cells to 0.1% O2 and 5% CO2 balanced with N2 in an anaerobic incubator (Thermo Electron, Marietta, OH).
Western Blot Analysis, Kinase Assay, and Expression Analysis. To examine the activities of MAPKs, total extracts from +/+ and ATE1–/– embryos and EFs were subjected to immunoblotting by using antibodies against ERK1/2, phospho-ERK1/2, c-Jun N-terminal kinase (JNK) 1, JNK2, phospho-JNK1/2 (Santa Cruz Biotechnology), p38, phospho-p38 (Cell Signaling Technology, Beverly, MA), Gaq (Santa Cruz Biotechnology), and actin (Sigma–Aldrich). In vitro ERK kinase assay was performed by using an assay kit from Upstate Biotechnology (Charlottesville, VA). For Northern blot analysis, total RNA was subjected to hybridization with cDNA fragment probes. Microarray analysis was done by using the GPCR signaling pathway GE array (SuperArray Bioscience, Frederick, MD) with a size of 96 genes.
Results
RGS4, RGS5, and RGS16 Are Degraded in Vitro by the N-End Rule Pathway. We first examined the in vitro proteolysis of RGS4, RGS5, and RGS16 in rabbit reticulocyte lysates. RGS4, RGS5, and RGS16 were all short-lived in vitro, and their degradation was abolished by either MG132 or the type 1 dipeptide Arg-Ala, but not by the type 2 dipeptide Trp-Ala or the control Ala-Arg (Fig. 1 C and D; data not shown), suggesting that these structurally related R4 RGS proteins are all degraded by the N-end rule pathway in reticulocyte lysates. The results with RGS4 (Fig. 1D, first blot) are consistent with previous results (ref. 5; see Introduction), but they show much stronger degradation as well as its specific perturbation by dipeptides. RGS4, RGS5, and RGS16 commonly bear the Cys-2 residue that can be exposed at the N terminus after the removal of N-terminal Met by Metaminopeptidases (MetAPs) (Fig. 1B). We then asked whether the conserved Cys-2 residue was a common degradation determinant. Myc-RGS5, where the original Cys-2 residue cannot be exposed at the N terminus (Fig. 1B), became long-lived (Fig. 1C). C2A-RGS5, Myc-C2A-RGS5, C2V-RGS4, and C2G-RGS4 (Fig. 1B), whose predicted N-terminal residues were Ala, Met, Val, and Gly, respectively, also all became stabilized (Fig. 1C). Furthermore, RGS5 and RGS4 were rapidly ubiquitylated, and their ubiquitylation was inhibited by Arg-Ala but not by Trp-Ala or Ala-Arg, whereas no ubiquitylation of C2A-RGS5 and Myc-RGS5 was detected (Fig. 1E). Thus, the N-end rule-dependent proteolysis of RGS4, RGS5, and RGS16 in reticulocyte lysates strictly requires the conserved Cys-2 residue of these proteins that becomes N-terminal after the (cotranslational) removal of N-terminal Met. Notably, RGS16 was less short-lived than RGS4 and RGS5 (Fig. 1D). Given that the Cys-2 residue of RGS16 can be palmitoylated for plasma membrane localization (18), Cys-2 palmitoylation may be a regulatory mechanism that counter regulates Cys-2-dependent proteolysis.
In Vivo Degradation of RGS4 and RGS5 Is Mediated by ATE1-Encoded Arg-Transferases. Our previous attempts to verify that RGS4 is an in vivo N-end rule substrate in mouse L cells were unsuccessful, which might stem, in hindsight, from inefficient Cys-2 modifications in these cells. Here, we asked whether RGS4 and RGS5 are short-lived in mouse EFs and, if so, whether their degradation is perturbed by ATE1 knockout. RGS4 and RGS5 were transiently expressed in +/+ and ATE1–/– EF cells either in the absence or presence of the proteasome inhibitor MG132, and their steady-state levels were monitored by using anti-RGS immunoblotting. RGS4 and RGS5 were apparently short-lived in +/+ cells in that their levels were strongly increased by either MG132 treatment or ATE1 knockout (Fig. 2A). We then determined the in vivo half-lives of RGS4 and RGS5 in +/+ and ATE1–/– cells by using pulse–chase analysis. Whereas ≈0.2% of RGS5 (compared with the level of Myc-RGS5 at the beginning of chase) remained intact after the 60-min chase in +/+ cells, 71% remained in ATE1–/– cells under the same condition (Fig. 2 B and C). Furthermore, 51% and 45% of C2A-RGS5 and Myc-RGS5, respectively, remained intact even in +/+ cells after the 60-min chase. Similarly, RGS4 was rapidly degraded in +/+ cells, and its degradation was abolished in ATE1–/– cells. Consistent with these results, the level of endogenous RGS4 was significantly higher in ATE1–/– cells than in +/+ cells (Fig. 2D).
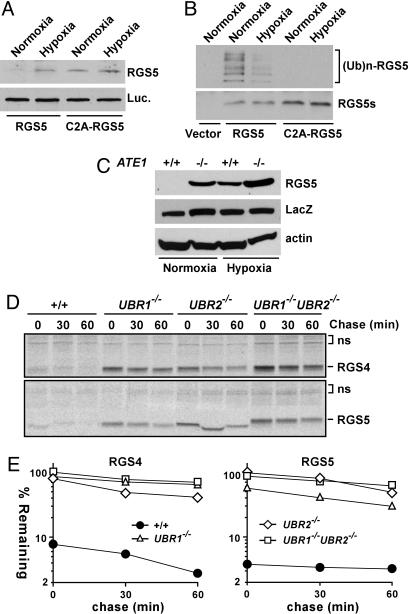
Fig. 2.
In vivo proteolysis of RGS4 and RGS5. (A) Immunoblotting of RGS4 and RGS5 transiently expressed in +/+ and ATE1–/– cells either in the absence and presence of MG132. (B) Pulse–chase analysis of RGS5, C2A-RGS5, Myc-RGS5, and RGS4. The transfected cells were labeled for 12 min with [35S]Met/[35S]Cys, followed by anti-RGS4 or RGS5 immunoprecipitation, SDS/PAGE analysis, and autoradiography. (C) Quantitation of data shown in B using PhosphorImager. (D) Immunoblotting of endogenous RGS4 in +/+ and ATE1–/– EFs. Anti-RGS5 and RGS16 antibodies did not detect endogenous proteins in either +/+ or ATE1–/– cells. (E) An ATE1 isoform and an RGS protein were coexpressed in ATE1–/– cells, followed by anti-RGS immunoblotting. A total of 4 μg of the plasmid mixture (RGS4 or RGS5, 2 μg; ATE1-1 or ATE1-2, 0, 0.25, 0.5, or 2 μg, respectively; pcDNA3, 2, 1.75, 1.5, 0 μg, respectively) was transfected into cells in a six-well plate.
We previously identified two ATE1-encoded isoforms of Arg-transferase, termed ATE1-1 and ATE1-2, containing either of two similar 41-residue domains encoded by a pair of alternative exons (2). We then asked whether and to what degree each ATE1 isoform promotes the proteolysis of RGS4 and RGS5. A combination of RGS proteins and increasing amounts of either of ATE1-1 or ATE1-2 were coexpressed in transiently transfected ATE1–/– cells, where RGS4 and RGS5 were stabilized (Fig. 2B), followed by anti-RGS4 and RGS5 immunoblotting. RGS4 was destabilized by both ATE1-1 and ATE1-2, whereas RGS5 was prominently destabilized by ATE1-1 (Fig. 2E). These results (Fig. 2) collectively suggest that RGS4 and RGS5 (and perhaps RGS16 as well) are in vivo substrates of the (ATE1-dependent) arginylation branch of the mammalian N-end rule pathway.
O2 Depletion Perturbs the in Vitro and In Vivo Proteolysis of RGS5. We previously observed that the molecular mass of the Cys-2 residue of arginylated RGS4 purified from mouse L cells was increased by 48 Da. We proposed that the N-terminal Cys-2 residue of RGS4 (exposed at the N terminus by MetAPs) can be oxidized into CysO2 or CysO3 (3). However, it remains to be tested whether the 48-Da modification is indeed oxidation and, if so, whether this modification is relevant (inhibitory or stimulatory) to the in vivo proteolysis of RGS4. Therefore, we tested whether O2 depletion would perturb the degradation of newly synthesized RGS5 in reticulocyte lysate and, if so, whether the Cys-2 residue is essential for the O2-dependent RGS5 proteolysis. The degradation of normally short-lived RGS5 was significantly decreased upon O2 depletion (Fig. 3A), in contrast to the level of the long-lived C2A-RGS5 mutant, which was not affected by O2 depletion. These results suggest that O2 may be an essential cofactor for RGS5 proteolysis. Consistent with these findings, the ubiquitylation of RGS5 was also significantly reduced upon O2 depletion (Fig. 3B). To address the role of O2 in the in vivo degradation of RGS5, it was expressed through transient transfection in +/+ and ATE1–/– cells under normoxic or hypoxic (0.1% O2) conditions, followed by anti-RGS5 immunoblotting. Consistent with the in vitro results (Fig. 3 A and B), the in vivo degradation of RGS5 was strongly inhibited by a hypoxic treatment (Fig. 3C). In our previous Edman sequencing of N-terminally arginylated RGS4 (3), the internal Cys-12 residue was detected as alkylated Cys, whereas Cys-2 could not be identified, suggesting that the Cys-2 residue of RGS4 (and, thus, RGS5 and RGS16 as well) is oxidized before alkylation. Whereas the role of O2 in Cys-2-dependent proteolysis of short-lived proteins (including RGS proteins) remains to be further investigated in vivo, these results (Fig. 3 A–C) suggest that the Cys-2 oxidation of RGS proteins may be a rate-limiting, regulated step in this arginylation-dependent process.
Fig. 3.
Role of O2, UBR1, and UBR2 in proteolysis of RGS4 and RGS5. (A) RGS5 and C2A-RGS5 were coexpressed with luciferase in reticulocyte lysates under a normoxic or hypoxic condition, followed by anti-RGS5 or biotin-based Western blotting. (B Upper) RGS5 and C2A-RGS5 were expressed in reticulocyte lysates in the presence of MG132 either under a normoxic or hypoxic condition, followed by anti-Ub immunoprecipitation and a subsequent antibiotin Western blotting. (B) (Bottom) Comparison of RGS5 expression using anti-biotin Western blotting. (C) RGS5 was coexpressed with LacZ in +/+ and ATE1–/– EFs in normoxic and hypoxic (0.1% O2) condition, followed by anti-RGS5, LacZ, and actin immunoblotting. (D) Pulse–chase analysis of RGS4 and RGS5 in +/+, UBR1–/–, UBR2–/–, and UBR1–/–UBR2–/– cells. The transfected cells were labeled for 12 min with [35S]Met/[35S]Cys, followed by anti-RGS4 or RGS5 immunoprecipitation, SDS/PAGE analysis, and autoradiography. (E) Quantitation of data in D using PhosphorImager.
UBR1 and UBR2 Are Ubiquitin Ligases Mediating the ATE1-Dependent Degradation of RGS4 and RGS5. We also examined the identity of E3s that are required for the proteolysis of RGS4 and RGS5. Given that the Cys-2 residue of RGS4 and RGS5 is a degradation determinant for ATE1-dependent proteolysis (Figs. 1 and 2), the posttranslationally conjugated N-terminal Arg of RGS4 and RGS5 is likely to be an N-degron that mediates their ubiquitylation. We recently observed that the Ub ligases UBR1 and UBR2 directly bind to N-terminal Arg as well as to other type 1 and type 2 N-degrons (of a model substrate), and we proposed that UBR1 and UBR2 are functionally overlapping E3s for type 1 and type 2 N-degrons (16). To test whether UBR1 and UBR2 participate in the proteolysis of RGS4 and RGS5, we examined the in vivo half-lives of RGS4 and RGS5 in +/+, UBR1–/–, UBR2–/–, and double-mutant UBR1–/–UBR2–/– EFs. Whereas <4% of RGS5 (compared with the level in UBR1–/–UBR2–/– cells at the end of pulse) remained after the 60-min chase in +/+ cells, 30%, 49%, and 61%, respectively, remained in UBR1–/–, UBR2–/–, and UBR1–/–UBR2–/– cells under the same condition (Fig. 3 D and E). Furthermore, the in vivo degradation of RGS4 was similarly inhibited in these UBR mutant cells. The strong stabilization of RGS4 and RGS5 in UBR1–/– and UBR2–/– single knockout cells may be because RGS4 and RGS5 are ubiquitylated by an E3 complex containing UBR1 and UBR2 or because the N-end rule pathway is saturated by overexpression of RGS proteins. These findings indicate that RGS4 and RGS5 (and perhaps RGS16 as well) are physiological substrates of the functionally overlapping Ub ligases UBR1 and UBR2 of the mammalian N-end rule pathway.
ATE1–/– Embryos and Cells Are Impaired in the Activation of the ERK MAPK Pathway. A variety of evidence indicates that RGS4, RGS5, and RGS16 act as negative regulators of the Gq- and Gi-activated signaling pathways in the cardiovascular system and probably in other systems as well (see Introduction). Consistent with the function of ATE1 in the proteolysis of RGS proteins, we previously observed that ATE1–/– embryos die with massive cardiovascular defects (3). Gq and Gi execute their functions by activating, in particular, a complex network of MAPKs: extracellular signal-regulated protein kinases (ERK1 and ERK2), JNKs, and p38 MAPKs. To test whether the ATE1-dependent proteolysis plays a role in MAPK pathways, we examined the amounts of active (phosphorylated) MAPKs in ATE1–/– and littermate +/+ embryos at 12.5 and 13.5 days postcoitum (dpc). Total extracts from these embryos were subjected to immunoblotting with antibodies against MAPKs (inactive form), phospho-MAPKs (active form), and Gαq. The amounts of Gαq and inactive ERK1/ERK2, JNKs, and p38 MAPKs were not significantly affected in ATE1–/– embryonic extracts (Fig. 4A). Notably, the amount of phospho-ERK2 was significantly decreased in ATE1–/– embryos (Fig. 4A, first blot), whereas the amounts of phospho-JNKs and phospho-p38 MAPKs were not significantly altered in the same embryos. Consistent with these results, total kinase activities of ERK1 and ERK2, as determined by using a kinase assay with myelin basic protein as a substrate, was ≈5-fold lower in ATE1–/– embryonic extracts than in +/+ extracts (Fig. 4B). ERK1 and ERK2 activate cell growth and proliferation in response to various extracellular signals including growth factors (19). These results suggest that the functional ATE1 gene is required for appropriate activation of the ERK–MAPK pathway in normally developing mouse embryos.
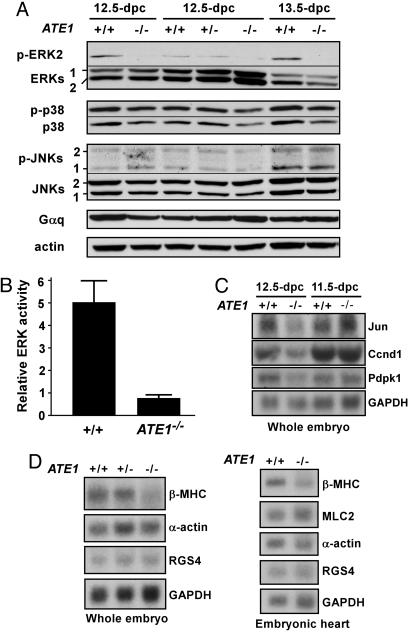
Fig. 4.
Analysis of the Gq- or Gi-activated downstream effectors in +/+ and ATE1–/– embryos. (A) The +/+ and ATE1–/– embryonic extracts were subjected to immunoblotting with antibodies against MAPKs, phospho-MAPKs, Gaq, and actin. As expected, phospho-ERK1 was not detected in mouse embryonic extracts. (B) The 12.5-dpc +/+ and ATE1–/– embryonic extracts were subjected to in vitro ERK kinase assay. (C) Total RNAs from +/+ and ATE1–/– embryos at 11.5 and 12.5 dpc were subjected to Northern blotting. (D) Total RNAs from 12.5-dpc +/+ and ATE1–/– embryos and embryonic hearts were subjected to Northern blotting.
The activated GPCR pathways, including the MAPK pathways, promote cell growth via transcriptional induction of down-stream effectors. To test whether ATE1 has a role in the induction of GPCR-regulated gene expression in normally growing mouse embryos, we used microarray analysis of 96 GPCR-induced genes by using total RNAs from 12.5-dpc +/+ and ATE1–/– embryos. The mRNA levels of several MAPK-activated downstream effectors, including Jun, Fos, Pdpk1, and cyclin D1, were ≈2-fold down-regulated in 12.5-dpc ATE1–/– cells (data not shown), which was in part confirmed by Northern blot analysis (Fig. 4C). Myocardial growth through stimulation of the G protein signaling is accompanied by activation of ventricular embryonic genes such as those for βMHC and skeletal α-actin (10). To examine the role of ATE1 in myocardial growth, we performed Northern blot analysis of these genes by using 12.5-dpc +/+ and ATE1–/– embryos and embryonic hearts. The mRNA level of βMHC, but not of MLC2, RGS4, or β-actin, was ≈2-fold decreased in ATE1–/– embryos and embryonic hearts (Fig. 4D). Taken together, these results (Fig. 4 C and D) suggest that altered expression of a rather broad spectrum of cardiovascular downstream effectors that are induced by the G protein pathways may collectively underlie the observed cardiovascular phenotypes of ATE1–/– embryos.
Discussion
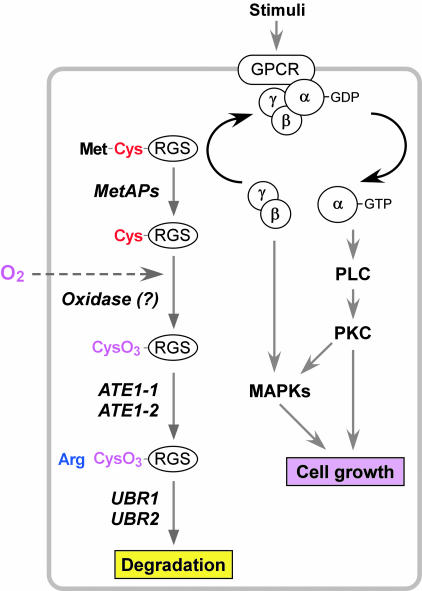
We propose a model (Fig. 5) in which the MetAPs-O2-ATE1-UBR1/UBR2 proteolytic system is required to maintain appropriate concentrations of RGS4, RGS5, and RGS16. Given that Cys-2 is a degradation determinant of RGS4 and RGS5 (Fig. 1) and that the Cys-2 residue of RGS4 can be arginylated in mammalian cells (3), MetAPs are likely to be essential components for the in vivo degradation of RGS4, RGS5, and RGS16, and thereby of other short-lived proteins bearing Cys-2 as well. MetAP2 is a target of angiogenic inhibitors such as fumagillin and its related drugs (20). The results from this study and our previous finding that ATE1–/– embryos suffer from defective angiogenesis (3) together suggest that these drugs may function in part through the stabilization of RGS4, RGS5, and RGS16. In this model, the treatment of fumagillin may inhibit the N-terminal Met cleavage in RGS4, RGS5, and RGS16, and thus their proteolysis as well, which in turn may suppress the RGS-regulated GPCR signaling during angiogenesis. Consistent with this model, RGS5 is strongly and specifically expressed in vascular smooth muscle cells and pericytes (21) and has been implicated in capillary growth and angiogenesis (11).
Fig. 5.
A model for the role of the N-end rule pathway in the RGS-regulated G protein pathways.
Given the findings that O2 depletion perturbs the proteolysis of RGS5 (Fig. 3 A–C) and that Cys-2 of RGS4 is converted into CysO3 in vivo (3), the N-terminal Cys-2 may act as a pre-N-degron. During oxidation of Cys-2 to CysO3, there may be a transiently oxidized form, CysO2, whose structure is similar to Asp (3). Notably, the oxidized Cys structurally mimics Asp, an ATE1 substrate, for recognition by ATE1 Arg-transferase. Therefore, the Cys-2 oxidation of RGS proteins may be a mechanism that is essential and limiting for Cys-2 arginylation. We propose that O2 acts as a cofactor for the GPCR signaling by diffusing across the plasma membrane of the target cell and oxidizing RGS4, RGS5, and RGS16, and thereby provides a vital regulatory mechanism for the cardiovascular GPCR pathway (Fig. 5). If this were true, the (unknown) oxidase that mediates Cys-2 oxidation of RGS proteins may function as an O2 sensor, like prolyl-4-hydroxylases that oxidize HIF1α in a normoxic condition (22). Our findings also suggest that a broad range of proteins bearing N-terminal Cys may be regulated by the same N-terminal Cys oxidation, opening up a new field in studies of the role of protein oxidation in various biological processes.
The findings that the in vivo degradation of RGS4 and RGS5 requires ATE1-encoded Arg-transferases (Fig. 2) and that the N-terminal Cys-2 of RGS4 can be conjugated with Arg (3) suggest that arginylation of the N-terminal Cys-2 of RGS4, RGS5, and RGS16 is essential and limiting for subsequent ubiquitylation by E3s. Mammalian genome encodes ≈350 Cys-2-bearing proteins (F. Du and A. Varshavsky, personal communication). It remains to be determined which proteins bearing Cys-2 are arginylated for proteolytic and nonproteolytic processes. Together, our results suggest that the proteolysis of RGS4, RGS5, and RGS16 may be tightly controlled by a balance between proproteolytic (Cys-2 exposure, oxidation, and arginylation) and antiproteolytic (Cys-2 palmitoylation) mechanisms. These complicated Cys-2 modifications may act as a licensing mechanism, in response to various physiological states before their irreversible degradation by the Ub machinery. Because ATE1-dependent arginylation requires Arg-tRNAArg, an essential component for the protein synthesis, ATE1 may act as a translation sensor.
The findings that the in vivo degradation of RGS4 and RGS5 is perturbed in cells deficient in UBR1 and/or UBR2 (Fig. 3 D and E) and that UBR1 and UBR2 bind to N-terminal Arg (16) suggest that Cys-2-arginylated RGS4, RGS5, and RGS16 are ubiquitylated by two functionally overlapping E3s, UBR1 and UBR2. Notably, RGS4 and RGS5 were degraded more rapidly in UBR1–/–UBR2–/– cells (Fig. 3E) than in ATE1–/– cells (Fig. 2C), predicting the presence of additional E3s for RGS4 and RGS5. Consistent with this prediction, we recently isolated two proteins, termed UBR4 and UBR5, that can bind to N-terminal Arg (17) and found that RGS4 is more strongly stabilized in UBR1–/–UBR2–/–UBR4RNAi cells than in UBR1–/–UBR2–/– cells (M.J.L., T.T., and Y.T.K., unpublished data).
We propose a model (Fig. 5) in which the ATE1-dependent proteolytic pathway acts as a positive regulatory mechanism for the RGS-regulated G protein pathway in the heart, vasculature, and other organs as well. Consistent with this model are the results, by us and by others, that (i) ATE1–/– embryos are impaired in myocardial growth and vascular integrity/maturation (3); (ii) RGS4, RGS5, and RGS16 act as negative regulators of Gq and Gi pathways (see Introduction); (iii) RGS4 is implicated in myocardial proliferation (see Introduction); (iv) the pathways composed of Gq, Gi, their agonists, and the downstream signal transducers such as PKC, Ras, MEK, and MAPKs are critical for cardiovascular function (see Introduction); and (v) ATE1 knockout reduces the activation of the GPCR-regulated ERK pathway and the mRNA expression of several GPCR-induced proteins (Fig. 4). In conclusion, our results indicate that the regulated proteolysis of RGS4, RGS5, and RGS16 by the MetAPs-O2-ATE1-UBR1/UBR2 proteolytic pathway may be important for the optimal function of the cardiovascular GPCR-signaling pathway.
Acknowledgments
We thank Hans Biebuyck for providing us with the results from a functional proteomic approach for N-end rule substrates, which prompted us to initiate this study; Yong Jun Lee for allowing us to use the hypoxia chamber; Jai Wha Seo for technical help; and the National Institutes of Health and the American Heart Association for funding.
Author contributions: M.J.L. and Y.T.K. designed research; T.T., K.M., S.K., and I.V.D. contributed new reagents/analytic tools; M.J.L., T.T., and J.Y.A. performed research; M.J.L., T.T., K.M., J.Y.A., S.K., I.V.D., and Y.T.K. analyzed data; and Y.T.K. wrote the paper.
Abbreviations: dpc, days postcoitum; EF, embryonic fibroblast; ERK, extracellular signal-regulated kinase; GPCR, G protein-coupled receptor; JNK, c-Jun N-terminal kinase; MAPK, mitogen-activated protein kinase; MetAP, Met-aminopeptidase; N-degron, N-terminal degradation signal; RGS, regulator of G protein signaling; Ub, ubiquitin.
References
- 1.Bachmair, A., Finley, D. & Varshavsky, A. (1986) Science 234, 179–186. [DOI] [PubMed] [Google Scholar]
- 2.Kwon, Y. T., Kashina, A. S. & Varshavsky, A. (1999) Mol. Cell. Biol. 19, 182–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kwon, Y. T., Kashina, A. S., Davydov, I. V., Hu, R. G., An, J. Y., Seo, J. W., Du, F. & Varshavsky, A. (2002) Science 297, 96–99. [DOI] [PubMed] [Google Scholar]
- 4.Neves, S. R., Ram, P. T. & Iyengar, R. (2002) Science 296, 1636–1639. [DOI] [PubMed] [Google Scholar]
- 5.Davydov, I. V. & Varshavsky, A. (2000) J. Biol. Chem. 275, 22931–22941. [DOI] [PubMed] [Google Scholar]
- 6.Tamirisa, P., Blumer, K. J. & Muslin, A. J. (1999) Circulation 99, 441–447. [DOI] [PubMed] [Google Scholar]
- 7.Berman, D. M., Wilkie, T. M. & Gilman, A. G. (1996) Cell 86, 445–452. [DOI] [PubMed] [Google Scholar]
- 8.Molkentin, J. D. & Dorn, I. G., II (2001) Annu. Rev. Physiol. 63, 391–426. [DOI] [PubMed] [Google Scholar]
- 9.Adams, J. W., Migita, D. S., Yu, M. K., Young, R., Hellickson, M. S., Castro-Vargas, F. E., Domingo, J. D., Lee, P. H., Bui, J. S. & Henderson, S. A. (1996) J. Biol. Chem. 271, 1179–1186. [DOI] [PubMed] [Google Scholar]
- 10.D'Angelo, D. D., Sakata, Y., Lorenz, J. N., Boivin, G. P., Walsh, R. A., Liggett, S. B. & Dorn, G. W., II (1997) Proc. Natl. Acad. Sci. USA 94, 8121–8126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wieland, T. & Mittmann, C. (2003) Pharmacol. Ther. 97, 95–115. [DOI] [PubMed] [Google Scholar]
- 12.Mittmann, C., Chung, C. H., Hoppner, G., Michalek, C., Nose, M., Schuler, C., Schuh, A., Eschenhagen, T., Weil, J., Pieske, B., et al. (2002) Cardiovasc. Res. 55, 778–786. [DOI] [PubMed] [Google Scholar]
- 13.Rogers, J. H., Tamirisa, P., Kovacs, A., Weinheimer, C., Courtois, M., Blumer, K. J., Kelly, D. P. & Muslin, A. J. (1999) J. Clin. Invest. 104, 567–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kwon, Y. T., Xia, Z., Davydov, I. V., Lecker, S. H. & Varshavsky, A. (2001) Mol. Cell. Biol. 21, 8007–8021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou, J., Moroi, K., Nishiyama, M., Usui, H., Seki, N., Ishida, J., Fukamizu, A. & Kimura, S. (2001) Life Sci. 68, 1457–1469. [DOI] [PubMed] [Google Scholar]
- 16.Kwon, Y. T., Xia, Z., An, J. Y., Tasaki, T., Davydov, I. V., Seo, J. W., Sheng, J., Xie, Y. & Varshavsky, A. (2003) Mol. Cell. Biol. 23, 8255–8271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tasaki, T., Mulder, L., Iwamatsu, A., Lee, M. J., Davydov, I. V., Varshavsky, A., Muesing, M. & Kwon, Y. T. (2005) Mol. Cell. Biol. 25, 7120–7136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Druey, K. M., Ugur, O., Caron, J. M., Chen, C. K., Backlund, P. S. & Jones, T. L. (1999) J. Biol. Chem. 274, 18836–18842. [DOI] [PubMed] [Google Scholar]
- 19.Boulton, T. G., Nye, S. H., Robbins, D. J., Ip, N. Y., Radziejewska, E., Morgenbesser, S. D., DePinho, R. A., Panayotatos, N., Cobb, M. H. & Yancopoulos, G. D. (1991) Cell 65, 663–675. [DOI] [PubMed] [Google Scholar]
- 20.Sin, N., Meng, L., Wang, M. Q., Wen, J. J., Bornmann, W. G. & Crews, C. M. (1997) Proc. Natl. Acad. Sci. USA 94, 6099–6103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cho, H., Kozasa, T., Bondjers, C., Betsholtz, C. & Kehrl, J. H. (2003) FASEB J. 17, 440–442. [DOI] [PubMed] [Google Scholar]
- 22.Ivan, M., Kondo, K., Yang, H., Kim, W., Valiando, J., Ohh, M., Salic, A., Asara, J. M., Lane, W. S. & Kaelin, W. G., Jr. (2001) Science 292, 464–468. [DOI] [PubMed] [Google Scholar]
- 23.Kwon, Y. T., Balogh, S. A., Davydov, I. V., Kashina, A. S., Yoon, J. K., Xie, Y., Gaur, A., Hyde, L., Denenberg, V. H. & Varshavsky, A. (2000) Mol. Cell. Biol. 20, 4135–4148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Varshavsky, A. (1996) Proc. Natl. Acad. Sci. USA 93, 12142–12149. [DOI] [PMC free article] [PubMed] [Google Scholar]