Abstract
Low-voltage-activated or T-type Ca2+ channels (T-channels) are widely expressed, especially in the central nervous system where they contribute to pacemaker activities and are involved in the pathogenesis of epilepsy. Proper elucidation of their cellular functions has been hampered by the lack of selective pharmacology as well as the absence of generic endogenous regulations. We report here that both cloned (α1G, α1H and α1I subunits) and native T-channels are blocked by the endogenous cannabinoid, anandamide. Anandamide, known to exert its physiological effects through cannabinoid receptors, inhibits T-currents independently from the activation of CB1/CB2 receptors, G-proteins, phospholipases and protein kinase pathways. Anandamide appears to be the first endogenous ligand acting directly on T-channels at submicromolar concentrations. Block of anandamide membrane transport by AM404 prevents T-current inhibition, suggesting that anandamide acts intracellularly. Anandamide preferentially binds and stabilizes T-channels in the inactivated state and is responsible for a significant decrease of T-currents associated with neuronal firing activities. Our data demonstrate that anandamide inhibition of T-channels can regulate neuronal excitability and account for CB receptor-independent effects of this signaling molecule.
Keywords: anandamide/cannabinoid receptor/NG 108-15 cells/pharmacology/T-type calcium channel
Introduction
Voltage-dependent Ca2+ channels are divided into three families: the L-type channels (or CaV1), the neuronal N-, P/Q- and R-type channels (or CaV2) and the T-type channels (or CaV3) (Ertel et al., 2000). T-type Ca2+ channel (T-channel) hallmarks are low-voltage-activated Ca2+ currents, fast inactivation and slow deactivation kinetics, low unitary conductance and strong steady-state inactivation at physiological resting potential (Carbone and Lux, 1984; Armstrong and Matteson, 1985; Nilius et al., 1985; Nowycky et al., 1985; Bean and McDonough, 1998). Three genes encoding the T-channel pore subunits were identified recently and designated α1G (CaV3.1), α1H (CaV3.2) and α1I (CaV3.3) (Cribbs et al., 1998; Perez-Reyes et al., 1998; Klugbauer et al., 1999; Lee et al., 1999; Monteil et al., 2000a,b). T-currents generated by the α1I subunit display slow kinetics that differ markedly from the α1G and α1H currents, which share the typical signature of native T-currents (Lee et al., 1999; Monteil et al., 2000b). Northern blot analyses have shown that the α1G, α1H and α1I mRNAs are expressed in various tissues, including heart and central nervous system (CNS) (Cribbs et al., 1998; Lee et al., 1999; Monteil et al., 2000a,b). In the CNS, T-channels generate low threshold spikes and participate in spontaneous firing (Llinas and Yarom, 1981; Llinas and Jahnsen, 1982; Huguenard, 1996). T-channels are also involved in cardiac pacemaker activity (Hagiwara et al., 1988; Lei et al., 1998), aldosterone secretion (Rossier et al., 1996) and fertilization (Arnoult et al., 1996). Unfortunately, the lack of selective blockers and endogenous ligands targeting T-channels has considerably hampered the elucidation of their functional roles (Huguenard, 1996).
A recent report describing that arachidonic acid (AA) inhibits α1H currents (Zhang et al., 2000) suggested to us that anandamide (N-arachidonoyl-ethanolamine) (Devane et al., 1992) was a potential modulator of T-channel function. Anandamide and 2-arachidonyl glycerol (2-AG) are endogenous ligands of the cannabinoid (CB) receptors, which belong to the G-protein-coupled receptor superfamily. To date, two CB receptors have been cloned, the CB1 receptor expressed primarily in the brain (Matsuda et al., 1990) and the CB2 receptor expressed in the immune system (Munro et al., 1993). CB1 receptor activation inhibits adenylyl cyclase, increases mitogen-activated protein kinase activities, modulates several potassium channel conductances and inhibits N- and P/Q-type Ca2+ channels (Mackie and Hille, 1992; Bouaboula et al., 1995; Mackie et al., 1995; Twitchell et al., 1997; Schweitzer, 2000). Anandamide preferentially binds to the CB1 receptor and mimicks most effects of the major psychoactive component of marijuana, Δ9-tetrahydrocannabinol (Δ9-THC), such as enhancement of sensory perception, alteration in cognition, sedation, catalepsy, analgesia and hypothermia (Di Marzo et al., 1998; Mechoulam et al., 1998; Ameri, 1999). Nevertheless, several physiological roles for anandamide, including modulation of neuronal excitability (Venance et al., 1995; Tognetto et al., 2001), pain (Adams et al., 1998; Vivian et al., 1998) and cardiovascular functions (Lake et al., 1997; Jarai et al., 1999; Zygmunt et al., 1999) are independent of the CB1 receptor activation. Indeed, anandamide can directly activate VR-1 vanilloid receptors (Zygmunt et al., 1999), inhibit Kv1.2 and TASK-1 potassium channels (Poling et al., 1996; Maingret et al., 2001) or act on as yet unidentified targets. In the present study, we provide clear evidence that submicromolar concentrations of anandamide directly block T-type calcium channels, which therefore represent a novel molecular target for this endocannabinoid.
Results
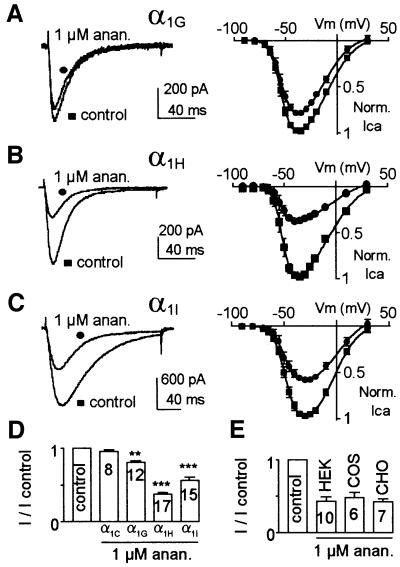
Micromolar concentrations of anandamide inhibited the three human cloned T-channels α1G (CaV3.1), α1H (CaV3.2) and α1I (CaV3.3) expressed in HEK 293 cells (Figure 1). The α1H and α1I currents were blocked strongly by 1 µM anandamide by ∼65 and 45% (I/I control: 0.37 ± 0.02, n = 17 and 0.56 ± 0.04, n = 15, respectively), while α1G currents were inhibited by 20% at this concentration (I/I control: 0.81 ± 0.02, n = 12). These results were observed at every potential (Figure 1A–C) using either extracellular Ca2+ (2 mM) or Ba2+ (5 mM; n = 10). This current inhibition was selective to T-channels since no effect was observed on α1C currents (Figure 1D), even when increasing the anandamide concentration to 10 µM (n = 5, not shown). A similar anandamide inhibition of α1H currents was observed in HEK 293, COS and CHO cells (Figure 1E), indicating further that the reported effects were not restricted to HEK 293 cells.
Fig. 1. Anandamide inhibits cloned T-channels. (A–C) Effect of 1 µM anandamide (circle) on Ca2+ currents elicited by a –30 mV test pulse (left panel) and current–voltage curves (right panel) for α1G (A), α1H (B) and α1I subunits (C). The holding potential (HP) was –80 mV. (D) Effect of 1 µM anandamide on the α1G, α1H, α1I and α1C currents. Anandamide blocked T-currents but not L-type (α1C) currents. α1C currents were elicited by a +20 mV test pulse applied from an HP of –80 mV. (E) Anandamide (1 µM) produces a similar block of Ba2+ (5 mM) currents when α1H is expressed in HEK 293, CHO or COS cells. For statistical analysis, Student’s t-tests were used with *P <0.05, **P <0.01 and ***P <0.001. The number of cells analysed is indicated on the histogram.
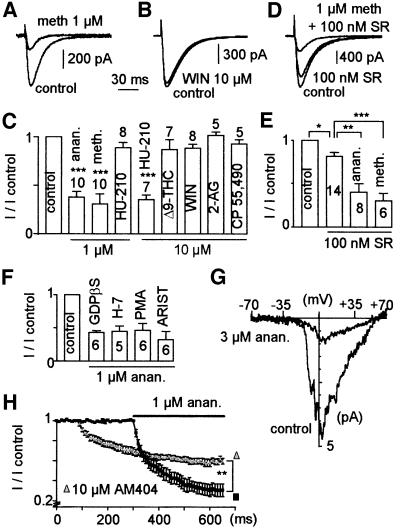
The ability of various cannabinoids to inhibit α1H currents was then investigated (Figure 2A–E). Similarly to anandamide, the non-hydrolysable analogue methanandamide (1 µM) decreased α1H currents by ∼70% (I/I control: 0.31 ± 0.09, n = 10), indicating that anandamide hydrolysis was not involved in the described effects (Figure 2A and C). In contrast, neither of the synthetic CB1/CB2 receptor agonists WIN 55,212-2 (WIN; Figure 2B) and CP 55,490 decreased α1H currents (Figure 2C). Similarly, neither the endocannabinoid 2-AG nor Δ9-THC affected α1H currents (Figure 2C). The CB1/CB2 agonist HU-210 had no significant effect on α1H currents at 1 µM (Figure 2C) but inhibited 65% of the current at 10 µM (I/I control: 0.35 ± 0.05, n = 7). We next tested the ability of the CB1 receptor antagonist SR141716A to prevent inhibition of α1H currents by anandamide. Although 100 nM SR141716A produced a mild inhibition (∼20%) of α1H currents by itself (I/I control: 0.81 ± 0.04, n = 14), it failed to prevent current inhibition by anandamide (51 ± 8%, n = 8) and methanandamide (64 ± 7%, n = 6; Figure 2D and E). An important block of α1H currents was observed using 1 µM SR141716A (70 ± 6%, n = 6; not shown). Activation of CB receptors is known to activate Gi/Go proteins and to regulate various phospholipases (i.e. PLA2, PLC and PLD) and phosphorylation pathways including protein kinases A and C (PKA and PKC). Application through the patch pipette of 1 mM of GDPβS, an inhibitor of G-protein activity, or cell incubation with 50 µM H7, an inhibitor of cyclic nucleotide-dependent protein kinases, did not prevent the inhibition of α1H currents by anandamide (Figure 2F). Similar results were obtained with cells incubated in the presence of 100 nM phorbol 12-myristate 13-acetate (PMA), which affects PLC/PKC but also PLD pathways in HEK-293 cells (reviewed in Exton, 1999), or in the presence of 250 µM aristolochic acid, an inhibitor of PLA2 (Figure 2F). More importantly, inhibition of α1H currents was observed in excised inside-out patches of HEK 293 cells superfused in the presence of 3 µM anandamide (I/I control: 0.15 ± 0.07, n = 6; Figure 2G), suggesting further that anandamide binds directly to T-channels. In addition, experiments in the presence of AM404, which inhibits the membrane transport of anandamide, were performed. Although extracellular application of AM404 (10 µM) produced a mild inhibition (∼30%) of α1H currents by itself (I/I control: 0.69 ± 0.03, n = 5; Figure 2H), it prevented current inhibition by anandamide (I/I control: 0.6 ± 0.03, n = 5), suggesting that anandamide possibly acts at an intracellular site. Overall, these results demonstrate that neither CB receptors, G-protein pathways nor protein kinases and phospholipases are involved in the inhibition of α1H currents by anandamide and strongly suggest that anandamide directly inhibits T-channels by acting at an intracellular site.
Fig. 2. Anandamide directly inhibits α1H currents independently of G-protein-coupled receptors. (A) Methanandamide (meth;1 µM) inhibited α1H currents while 10 µM WIN 55,212-2 (WIN) did not (B). (C) Effects of various agonists of the CB1/CB2 receptors on α1H current amplitude. Concentrations used are indicated on the histogram. (D and E) SR141716A (SR; 100 nM), a CB1 antagonist, did not prevent the α1H current inhibition induced by either anandamide (anan) or methanandamide. (F) Block of T-current by anandamide in the presence of 1 mM GDPβS in the intracellular medium or following 16 h incubation in the culture medium of 50 µM H7, 100 nM PMA or 250 µM aristolochic acid (ARIST). (G) Effect of 3 µM anandamide on α1H currents in inside-out patch recording. The holding potential was –70 mV and currents are stimulated with voltage ramp protocols of 90 ms duration, from –70 to +70 mV. Note that the positive shift for the I–V curve is due to the use of 110 mM barium. (H) Effect of 10 µM AM404 on the time course and amplitude of the anandamide block.
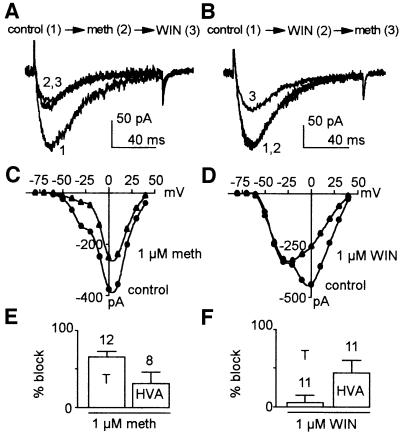
Anandamide block of native T-channels was examined using the neuroblastoma NG108-15 cell line, which expresses well characterized T-channels (Randall and Tsien, 1997) and most of the high-voltage-activated (HVA) channels (Lukyanetz, 1998). Mackie and Hille (1992) demonstrated that in this cell line, activation of CB1 receptors by WIN leads to a pertussis toxin- dependent block of N-currents without any effect on T-currents. As for cloned T-channels, methanandamide but not WIN decreased native T-currents in the NG108-15 cell line (Figure 3A and B). In contrast, both compounds significantly inhibited HVA currents (Figure 3C–F) by acting on CB1 receptors (Mackie and Hille, 1992). The percentages of block by 1 µM methanandamide were 65 ± 6% (n = 12) on T-currents and 31 ± 14% (n = 8) on HVA currents (Figure 3E), while those mediated by 1 µM WIN were 5 ± 9% (n = 11) on T-currents and 43 ± 15% (n = 11) on HVA currents (Figure 3F). The extent of block by methanandamide, as well as its time course (not shown) is similar for cloned and native T-channels. Similar results were obtained using 1 µM anandamide (n = 5, not shown).
Fig. 3. Methanandamide blocks native T and HVA currents of NG108-15 cells while WIN only affects HVA currents. (A) Effects on T-currents of 1 µM methanandamide applied first (2) followed by 1 µM WIN (3). (B) Effects on T currents of 1 µM WIN applied first (2) and 1 µM methanandamide applied after (3). (C and D) Corresponding current–voltage relationships (I–V curves) of the calcium currents presented in (A) and (B), respectively. Drugs were applied at HP –80 mV while I–V curves were performed just after at HP –110 mV in order to record maximal T-currents. (E and F) Percentage block of T- and HVA currents by 1 µM methanandamide (E) and by 1 µM WIN (F). To avoid contamination of T currents by HVA currents (and reciprocally), the peak of T-currents was measured at –30 mV while HVA currents were measured 100 ms after the beginning of the test pulse at +10 mV. In all experiments, HP was –80 mV and 10 mM Ba2+ was used as charge carrier.
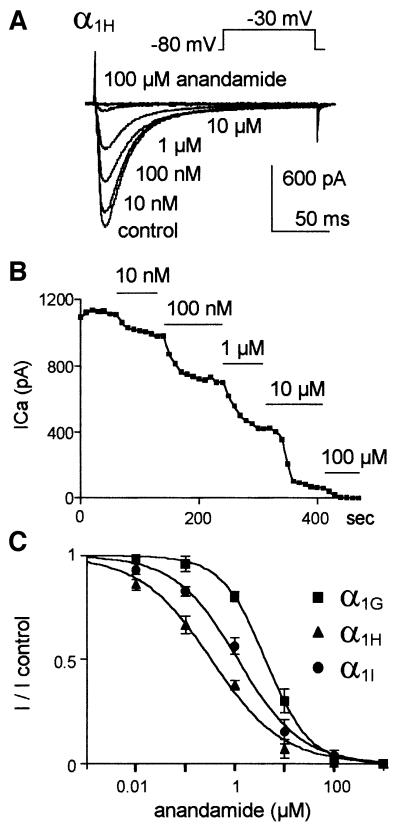
Inhibition of Ca2+ currents generated by the three cloned T-channels was concentration dependent (Figure 4). Using a holding potential (HP) of –80 mV, submicromolar concentration of anandamide as low as 10 nM significantly decreased α1H and α1I currents, while concentrations higher than 100 nM were required to inhibit α1G currents significantly (Figure 4A and C). Dose–response curves indicated IC50 values of 330 ± 66 nM, n = 8 for α1H, 1.10 ± 0.05 µM, n = 8 for α1I and 4.15 ± 0.03 µM, n = 8 for α1G; and Hill slope factors were 0.60 ± 0.05, n = 8 for α1H, 0.68 ± 0.05, n = 8 for α1I and 0.98 ± 0.02, n = 8 for α1G (Figure 4C). The time course of the α1H current block by increasing concentrations of anandamide was fast and reached steady state in <1 min (Figure 4B). Current inhibition by anandamide was partially reversible. Recovery was ∼15% of the total current after 10 min of washout of the drug (n = 5, not shown). Anandamide had little effect on membrane capacitance (7 ± 14%, n = 8, not shown), indicating that its effects could not be explained by simple membrane-disrupting mechanisms.
Fig. 4. Submicromolar concentrations of anandamide inhibit α1H and α1I currents but not α1G currents. (A) Effects of increasing concentrations of anandamide on calcium currents generated by the α1H subunit. (B) Time course of the inhibition of α1H currents by increasing concentrations of anandamide. (C) Dose–response curves of anandamide for the three human cloned T-channels. The curves were fitted with a sigmoidal relationship where I/Icontrol = 1/(1 + 10[(LogEC50 – log[anandamide]) × Hillslope]).
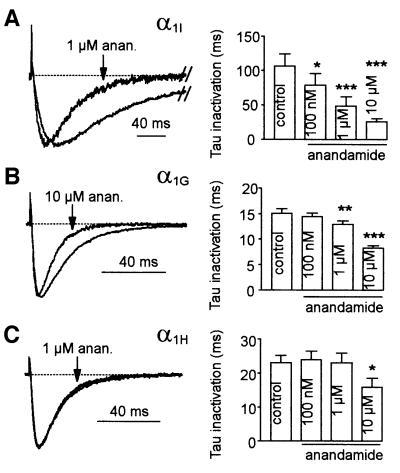
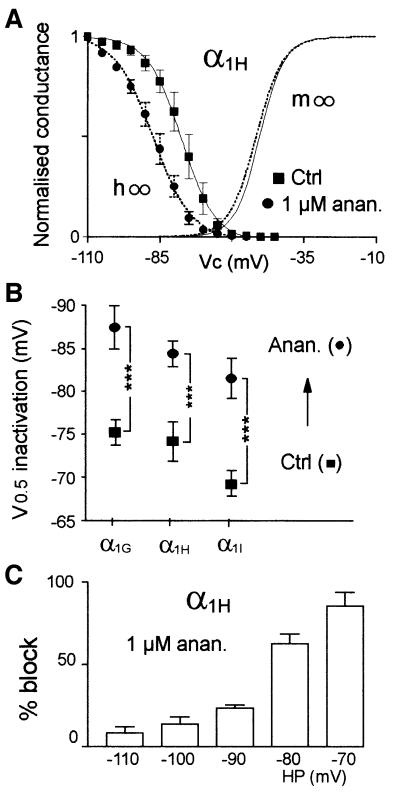
Closer inspection of T-currents revealed that anandamide also affects their kinetics (Figure 5). Anandamide accelerated inactivation kinetics of the three cloned T-channels in a concentration-dependent way. This effect was most pronounced for α1I currents (significant at 100 nM; Figure 5A), while faster kinetics for α1G and α1H currents were observed at higher concentrations (1 and 10 µM, respectively; Figure 5B and C). Anandamide-induced acceleration of kinetics occurred at all voltages, especially in the negative range of potentials (not shown). Although 1 µM anandamide produced strong effects on inactivation kinetics, it also significantly accelerated the activation kinetics of α1G and α1I currents for potentials lower than –10 mV (P <0.01). Activation kinetics were faster by 1.4- (n = 9), 1.1- (n = 6) and 2-fold (n = 8) at –40 mV for α1G, α1H and α1I, respectively (not shown). It is interesting to note that the α1I currents, which display the slowest kinetics, are the most markedly modulated by anandamide. As a consequence, the sustained component of α1I current measured 200 ms after the beginning of the test pulse was abolished in the presence of 1 µM anandamide (Figure 5A). Anandamide-induced acceleration of the inactivation kinetics suggested that it could also modulate steady-state inactivation of T-channels. In order to test this hypothesis, steady-state inactivation protocols were performed in the presence of anandamide concentrations that approximately blocked 50% of T-currents at –80 mV [the half-potential value (V0.5) of steady-state inactivation]. Anandamide shifted the steady-state inactivation curves (h∞) of T-channels (Figure 6A and B) towards more negative potentials (–12.2 ± 2.5 mV, n = 8 for α1G, –10.2 ± 2.2 mV, n = 8 for α1H, and –12.1 ± 2.1 mV, n = 8 for α1I). No shift was observed for steady-state activation curves (m∞). Consequently, the window current generated by the overlapping steady-state activation and inactivation curves was decreased markedly (Figure 6A). Such a negative shift of the steady-state inactivation curve would suggest that anandamide binds and stabilizes T-channels in the inactivated state. A direct method to evaluate anandamide’s binding to inactivated T-channels is to measure the current inhibition at various HPs. We therefore examined the inhibition of α1H currents by 1 µM anandamide for HPs ranging from –110 to –70 mV (Figure 6C). Expectedly, anandamide strongly blocked T-currents by 85 ± 8% (n = 7) at –70 mV, while at –110 mV anandamide modestly reduced α1H currents by 8 ± 3% (n = 7).
Fig. 5. Anandamide accelerates inactivation kinetics of T-currents. (A–C) Normalized currents in the presence or absence of anandamide (left panel) and effects of increasing concentration of anandamide on inactivation kinetics (right panel) for the α1G (A, n = 5), α1H (B, n = 6) and α1I subunit (C, n = 5). Currents were elicited by a –30 mV test pulse from an HP of –80 mV.
Fig. 6. Effect of anandamide on T-currents is state dependent. (A) Anandamide shifts the steady-state inactivation curve (circle) without a corresponding effect on the activation curve (dotted line) of the α1H subunit. Steady-state activation (normalized conductance m∞) was deduced from the I–V curves presented in Figure 1 (V0.5 = –50.3 ± 1.7 mV, k = 5.4 ± 0.4 mV and V0.5 = –52.6 ± 1.5 mV, k =0 5.6 ± 0.5 mV (n = 17) in the absence and presence of 1 µM anandamide, respectively). In order to visualize window currents better, corresponding symbols were omitted. Steady-state inactivation curves were obtained by stepping the membrane potential to –30 mV after holding the membrane for 10 s at potentials ranging from –110 to –45 mV. The normalized peak current amplitude (h∞) was plotted as a function of the HP. (B) Effects of 1 µM anandamide (circle) on the V0.5 of inactivation for α1H and α1I subunits and of 10 µM anandamide on the V0.5 of inactivation for the α1G subunit. No significant difference in the slope factor was observed (C) Block of α1H currents by anandamide was dependent on the HP (n = 7 in each condition).
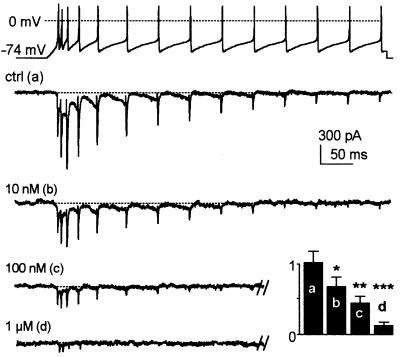
In order to appreciate better the physiological impact of anandamide’s block of T-currents, we performed voltage clamp experiments using a thalamo-cortical relay cell firing activity as waveform. The α1I currents strongly participate in the Ca2+ entry during sustained neuronal activities because of their slow inactivation kinetics (Figure 7). Anandamide decreased the amplitude and accelerated the decay of α1I currents in a concentration- dependent manner (Figure 7). Remarkably, 100 nM anandamide completely abolished the α1I current remaining after the seventh spike. To quantify these effects, the integral of the current was measured for the various anandamide concentrations (Figure 7, inset). Since 100 nM anandamide blocked ∼50% of the Ca2+ influx, these data clearly indicated that anandamide blocks T-channels more efficiently during neuronal activities than during conventional square step stimulations (IC50 ∼1 µM, Figure 4).
Fig. 7. Anandamide blocks α1I currents during thalamic relay cell-like activities. A firing activity typical of those of thalamic relay neurons was used as waveform (upper panel), and the resulting α1I currents are presented in the absence [ctrl (a)] and presence of increasing concentrations of anandamide [10 nM (b), 100 nM (c) and 1 µM (d)]. The normalized integral of total current (arbitrary units) is shown as an inset (n = 6).
Discussion
This study demonstrates that submicromolar concentrations of the endocannabinoid anandamide blocks cloned α1H and α1I channels (and to a lesser extent α1G channels) as well as native T-channels. The block is independent of CB receptors, G-proteins, PLA2, PLC, PLD, cyclic nucleotide-dependent protein kinases and PKC. The block is observed in excised inside-out patch configuration, indicating that inhibition of T-currents by anandamide is probably direct. In addition, block of anandamide membrane transport by AM404 (Beltramo et al., 1997) suggests an intracellular action of anandamide. The pharmacological profile of the endocannabinoid block of T-channels is specific for anandamide and further confirms that this molecule acts directly on the T-channels. First, the classical CB1/CB2 agonists WIN and CP 55,490 are inactive and the CB1 receptor antagonist SR141716A does not prevent inhibition of T-currents by anandamide. Secondly, other natural CB1/CB2 agonists, including the endocannabinoid 2-AG and Δ9-THC, also fail to affect T-currents. Finally, the non-hydrolysable analogue methanandamide also inhibits T-currents, demonstrating that anandamide hydrolysis to AA is not involved in the inhibition of T-channels. The reported effects are therefore distinct from the recently described inhibition of α1H currents by AA (Zhang et al., 2000). Similarly to cloned channels, native T-channels are blocked potently by anandamide and methanadamide. The NG108-15 cell line, which displays typical T-currents but also N-, P/Q-, R- and L-type currents (Randall and Tsien, 1997; Lukyanetz, 1998), was used previously by Mackie and Hille (1992) to demonstrate that activation of CB1 receptors by WIN leads to the inhibition of N-currents, without affecting T-currents. Using this cell line, we demonstrate that anandamide, but not WIN, is a potent blocker of native T-currents, confirming that this inhibition is independent of CB receptors.
Previous studies have described that other signaling molecules modulate native T-currents. Nociceptin, an endogenous ligand of the opioid receptor ORL1, mediates inhibition of T-currents from dorsal root ganglion neurons independently of G-proteins (Abdulla and Smith, 1997). This effect is prevented by the κ3 antagonist, nalbzoh, indicating that nociceptin inhibition of T-currents involves receptor activation. Similarly, the 5-hydroxytryptamine inhibition of T-currents in sensory neurons of Xenopus larvae is mediated by a functional domain of the receptor that is distinct from that which couples to G-proteins (Sun and Dale, 1999). In contrast, modulation of T-channels in adrenal glomerulosa cells by angiotensin AII and dopamine D1 receptors occurs through G-protein receptor-coupled pathways (McCarthy et al., 1993; Drolet et al., 1997). Overall, anandamide appears to be one of the first endogenous ligands ever described capable of directly modulating T-channels. The IC50 values for T-current inhibition (330 nM for α1H channels) are equivalent to those obtained for the CB1 receptor activation (Felder et al., 1995), suggesting that T-channels could be one of the principal targets of anandamide. Direct effects of anandamide on ionic channels have already been reported. Indeed, anandamide inhibits Kv1.2 potassium channels independently of CB1 receptor activation with an IC50 of 2.7 µM (Poling et al., 1996), and directly activates vallinoid VR1 receptors at even higher concentrations (Zygmunt et al., 1999; Smart et al., 2000). In addition, it was shown recently that submicromolar concentrations of anandamide directly inhibit the potassium channel TASK-1, which underlies the background current IKso (Maingret et al., 2001). Altogether, besides its classical action on CB receptors, anandamide can act directly on several ionic channels, including T-channels, to produce its powerful pharmacological and physiological effects.
Inhibition of T-currents by anandamide is highly dependent on the inactivation state of the channel. Indeed, the block is negligible for HPs lower than –100 mV and reaches ∼85% at –70 mV, an HP at which T-channels are strongly inactivated. In addition, anandamide induces an approximately –10 mV shift of the steady-state inactivation curves of each cloned T-channel, but not of the activation curves. This results in a net decrease of the window current component of T-channels. Overall, these data strongly indicate that anandamide binds and stabilizes T-channels in the inactivated state. Binding to the inactivated T-channels is an important feature of the anandamide block, since T-channels are strongly inactivated at physiological resting potentials. This property could also contribute to tissue selectivity of the anandamide effects. For example, dihydropyridines that bind preferentially to the inactivated state of L-type Ca2+ channels are useful as anti-hypertensive drugs by acting on smooth muscle while having little effect on the heart (Triggle, 1992). Another interesting feature of the block of T-currents by anandamide is the acceleration of current kinetics. T-currents generated by the α1I subunit inactivate slowly (Lee et al., 1999; Monteil et al., 2000b), leading during long test pulses to a sustained current component that is abolished by anandamide. Acceleration of current decay was observed before the decrease in the current amplitude (not shown), suggesting that anandamide could act as an open channel blocker (Bezprozvanny and Tsien, 1995). In that respect, the appearance of a faster inactivation would be due to an increase in the open to blocked transition, which would also explain apparent faster activation kinetics. A similar state-dependent block was described recently for other T-channel blockers such as the antiepileptic drugs methyl-phenylsuccinimide and ethosuximide (Gomora and Perez-Reyes, 2001). Speed up of current decay is also observed for α1G and α1H subunits but at higher anandamide concentrations, possibly due to their faster inactivation kinetics, as reported for the mibefradil block of HVA Ca2+ channels (Bezprozvanny and Tsien, 1995).
By stimulating cells overexpressing α1I channels with thalamic firing activities, we demonstrate that anandamide block of T-currents is ∼10 times more potent during neuronal firing activity (IC50 ∼100 nM for α1I channels). This suggests that anandamide could be an important physiological regulator of T-channels. What could be the physiological relevance of the blockade of T-currents by anandamide? First, the fact than anandamide preferentially binds and stabilizes T-channels in the inactivated state suggests that it could significantly decrease T-currents induced by low-threshold spike (LTS). LTS results from deinactivation of T-channels after inhibitory synaptic input, leading to a Ca2+-dependent depolarization, which activates sodium channels (Huguenard, 1996). This phenomenon is well known in the thalamus where T-channels are thought to mediate action potentials and to control the frequency and time course of repetitive firing (McCormick and Huguenard, 1992). In the thalamus, T-channels are involved in slow wave sleep (Steriade et al., 1993) and in the pathogenesis of epilepsy (Tsakiridou et al., 1995). It is important to note that a high level of anandamide was detected in the thalamus (Felder et al., 1996) while CB1 receptors are expressed at low levels (Herkenham et al., 1991). It would be of great interest to test on these neurons whether anandamide could modify their activities via non-CB receptor mechanisms. Inhibition of T-channels could also participate in the antinociceptive effects of anandamide. On one hand, the α1H subunit is highly expressed in dorsal root ganglion neurons (Talley et al., 1999) and neuropathic pain alters T-current properties (Hogan et al., 2000). On the other hand, anandamide-induced antinociception is not fully prevented by the CB1 receptor antagonist SR141716A (Adams et al., 1998; Smith et al., 1998; Vivian et al., 1998). In addition, the antinociceptive response attributed to tonic activation of CB1 receptors (Chapman, 1999) could be related to the SR141716A block of T-currents described here. Another important property of the anandamide block is the abolition of the window current component of T-channels. The window current occurs near the resting potential of many excitable cells. It contributes to the control of basal levels of Ca2+ (Chemin et al., 2000) and potentially participates in cell proliferation and differentiation processes (Bijlenga et al., 2000). The window current is also suspected to participate in the control of the cardiac pacemaker activity (Lei et al., 1998). In the heart, high levels of anandamide but few CB receptors were detected (Felder et al., 1996; Herkenham et al., 1991). It will therefore be important to determine whether direct inhibition of pacemaker T-channels by anandamide or SR141716A also participates in the bradycardic effect of these compounds (Lake et al., 1997). Altogether, anandamide inhibition of T-channels represents a novel non-CB receptor mechanism that can contribute to a wide variety of anandamide’s effects. This strong regulation of T-channels should therefore be taken into account in further studies investigating the physiological roles of anandamide.
Materials and methods
Cell culture and transfection protocols
HEK-293, COS-7, CHO and NG 108-15 cells were cultured using standard techniques. Transfection was performed as previously described (Chemin et al., 2001) with a DNA mix containing 10% of a GFP plasmid and 90% of either of the pBK-CMV plasmid constructs that code for human α1Ga, α1Ia and α1H T-channel isoforms (Cribbs et al., 1998; Monteil et al., 2000b) or a 1:1:1 ratio of α1C, β1b and α2δ1b subunits (Tomlinson et al., 1993). When indicated, 50 µM of the kinase inhibitor 1-(5-isoquinolinesulfonyl)-2-methyl-piperazine (H7, Sigma), 100 nM PMA (Sigma) or 250 µM aristolochic acid (Sigma) were added in the culture medium 16 h prior to the electrophysiological recordings. For NG 108-15 experiments, cells between 10 and 17 passages were plated at low confluence (∼0%) and differentiation was induced 5–10 days before recordings by decreasing the serum content in the medium to 1% and by adding 1 mM cAMP (Sigma).
Electrophysiology
Whole-cell currents were recorded at room temperature as previously described (Chemin et al., 2001). Extracellular solution contained (in mM): 2 CaCl2, 160 TEACl and 10 HEPES (pH to 7.4 with TEAOH). Pipettes have a resistance of 1–3 MΩ when filled with a solution containing (in mM): 110 CsCl, 10 EGTA, 10 HEPES, 3 Mg-ATP and 0.6 GTP (pH to 7.2 with CsOH). When indicated, 0.6 mM GTP was substituted with 1 mM GDPβS (Sigma). For inside-out patch experiments, a sylgard-coated pipette has a resistance of 8–13 MΩ when filled with a solution containing (in mM): 100 BaCl2, 10 HEPES (pH to 7.2 with TEAOH). Membrane potential was reduced towards 0 mV by bathing the cells in solution containing (in mM): 140 K gluconate, 10 EGTA, 10 glucose, 1 MgCl2, 10 HEPES (pH to 7.3 with KOH). The sampling frequency for acquisition was 10 kHz and data were filtered at 2 kHz. For action potential clamp studies, a thalamo-cortical relay cell firing activity generated by the NEURON model (Hines and Carnevale, 1997) was used in the configuration of a three-compartment model of burst behaviour (Destexhe et al., 1998) available at Yale University database (http://senselab.med.yale.edu/senselab/neurondb). With this model, a firing activity produced by a 0.3 nA current injection in the soma during 700 ms was converted into a pCLAMP stimulation file. In this case, leak and capacitive currents were subtracted using a P/–5 procedure. Electrophysiological analysis was performed as previously described (Chemin et al., 2001). Student’s t-tests were used to compare the different values, and were considered significant at P <0.05. Results are presented as the mean ± SEM, and n is the number of cells used.
Pharmacological agents
Anandamide solution (10 mg/ml) and its vehicle were obtained from Tocris. WIN 55,212-2, CP 55,490 and HU-210 (Tocris) were dissolved at 50 mM in dimethylsulfoxide (DMSO) and AM404 (Tocris) was dissolved at 50 mM in ethanol. 2-AG, R(+)-methanandamide (RBI) and Δ9-THC (Sigma) were dissolved at 20 mM in ethanol. SR141716A (10 mM in DMSO) was a gift from Sanofi Recherche (Montpellier, France). Dilutions were prepared daily from these stocks. Solvents were included in all control solutions. Drugs were applied to cells by a gravity-driven home-made perfusion device, controlled by solenoid valves.
Acknowledgments
Acknowledgements
We are grateful to E.Bourinet for help with excised patch clamp recordings and stimulating discussions, O.Manzoni, D.Robbe, M.Mangoni, F.Rassendren and S.Dubel for helpful discussions and comments on the manuscript, C.Barrère, S.Chaumont and C.Altier for technical support, and T.P.Snutch for providing α1C, β1b and α2δ1b cDNAs. We are also grateful to Sanofi Recherche (Montpellier, France) for the gift of SR141716A. This work was supported in part by the CNRS, the Association pour la Recherche contre le Cancer (ARC) and Association Française contre les myopathies (AFM).
References
- Abdulla F.A. and Smith,P.A. (1997) Nociceptin inhibits T-type Ca2+ channel current in rat sensory neurons by a G-protein-independent mechanism. J. Neurosci., 17, 8721–8728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams I.B., Compton,D.R. and Martin,B.R. (1998) Assessment of anandamide interaction with the cannabinoid brain receptor: SR 141716A antagonism studies in mice and autoradiographic analysis of receptor binding in rat brain. J. Pharmacol. Exp. Ther., 284, 1209–1217. [PubMed] [Google Scholar]
- Ameri A. (1999) The effects of cannabinoids on the brain. Prog. Neurobiol., 58, 315–348. [DOI] [PubMed] [Google Scholar]
- Armstrong C.M. and Matteson,D.R. (1985) Two distinct populations of calcium channels in a clonal line of pituitary cells. Science, 227, 65–67. [DOI] [PubMed] [Google Scholar]
- Arnoult C., Cardullo,R.A., Lemos,J.R. and Florman,H.M. (1996) Activation of mouse sperm T-type Ca2+ channels by adhesion to the egg zona pellucida. Proc. Natl Acad. Sci. USA, 93, 13004–13009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean B.P. and McDonough,S.I. (1998) Two for T. Neuron, 20, 825–828. [DOI] [PubMed] [Google Scholar]
- Beltramo M., Stella,N., Calignano,A., Lin,S.Y., Makriyannis,A. and Piomelli,D. (1997) Functional role of high-affinity anandamide transport, as revealed by selective inhibition. Science, 277, 1094–1097. [DOI] [PubMed] [Google Scholar]
- Bezprozvanny I. and Tsien,R.W. (1995) Voltage-dependent blockade of diverse types of voltage-gated Ca2+ channels expressed in Xenopus oocytes by the Ca2+ channel antagonist mibefradil (Ro 40-5967). Mol. Pharmacol., 48, 540–549. [PubMed] [Google Scholar]
- Bijlenga P., Liu,J.H., Espinos,E., Haenggeli,C.A., Fisher-Lougheed,J., Bader,C.R. and Bernheim,L. (2000) T-type α1H Ca2+ channels are involved in Ca2+ signaling during terminal differentiation (fusion) of human myoblasts. Proc. Natl Acad. Sci. USA, 97, 7627–7632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouaboula M., Poinot-Chazel,C., Bourrie,B., Canat,X., Calandra,B., Rinaldi-Carmona,M., Le Fur,G. and Casellas,P. (1995) Activation of mitogen-activated protein kinases by stimulation of the central cannabinoid receptor CB1. Biochem. J., 312, 637–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone E. and Lux,H.D. (1984) A low voltage-activated, fully inactivating Ca channel in vertebrate sensory neurones. Nature, 310, 501–502. [DOI] [PubMed] [Google Scholar]
- Chapman V. (1999) The cannabinoid CB1 receptor antagonist, SR141716A, selectively facilitates nociceptive responses of dorsal horn neurones in the rat. Br. J. Pharmacol., 8, 1765–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chemin J., Monteil,A., Briquaire,C., Richard,S., Perez-Reyes,E., Nargeot,J. and Lory,P. (2000) Overexpression of T-type calcium channels in HEK-293 cells increases intracellular calcium without affecting cellular proliferation. FEBS Lett., 478, 166–172. [DOI] [PubMed] [Google Scholar]
- Chemin J., Monteil,A., Bourinet,E., Nargeot,J. and Lory,P. (2001) Alternatively spliced α1G (CaV3.1) intracellular loops promote specific T-type Ca2+ channel gating properties. Biophys. J., 80, 1238–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cribbs L.L. et al. (1998) Cloning and characterization of α1H from human heart, a member of the T-type Ca2+ channel gene family. Circ. Res., 83, 103–109. [DOI] [PubMed] [Google Scholar]
- Destexhe A., Neubig,M., Ulrich,D. and Huguenard,J. (1998) Dendritic low-threshold calcium currents in thalamic relay cells. J. Neurosci., 18, 3574–3588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devane W.A. et al. (1992) Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science, 258, 1946–1949. [DOI] [PubMed] [Google Scholar]
- DiMarzo V., Melck,D., Bisogno,T. and De Petrocellis,L. (1998) Endocannabinoids: endogenous cannabinoid receptor ligands with neuromodulatory action. Trends Neurosci., 12, 521–528. [DOI] [PubMed] [Google Scholar]
- Drolet P., Bilodeau,L., Chorvatova,A., Laflamme,L., Gallo-Payet,N. and Payet,M.D. (1997) Inhibition of the T-type Ca2+ current by the dopamine D1 receptor in rat adrenal glomerulosa cells: requirement of the combined action of the Gβγ protein subunit and cyclic adenosine 3′,5′-monophosphate. Mol. Endocrinol., 11, 503–514. [DOI] [PubMed] [Google Scholar]
- Ertel E.A. et al. (2000) Nomenclature of voltage-gated calcium channels. Neuron, 25, 533–535. [DOI] [PubMed] [Google Scholar]
- Exton J.H. (1999) Regulation of phospholipase D. Biochim. Biophys. Acta, 1439, 121–133. [DOI] [PubMed] [Google Scholar]
- Felder C.C., Joyce,K.E., Briley,E.M., Mansouri,J., Mackie,K., Blond,O., Lai,Y., Ma,A.L. and Mitchell,R.L. (1995) Comparison of the pharmacology and signal transduction of the human cannabinoid CB1 and CB2 receptors. Mol. Pharmacol., 48, 443–450. [PubMed] [Google Scholar]
- Felder C.C. et al. (1996) Isolation and measurement of the endogenous cannabinoid receptor agonist, anandamide, in brain and peripheral tissues of human and rat. FEBS Lett., 393, 231–235. [DOI] [PubMed] [Google Scholar]
- Gomora J.C. and Perez-Reyes,E. (2001) State dependent block of three human T-type cloned channels by anti-epileptics Biophys. J., 80, 621a (abstract). [Google Scholar]
- Hagiwara N., Irisawa,H. and Kameyama,M. (1988) Contribution of two types of calcium currents to the pacemaker potentials of rabbit sino-atrial node cells. J. Physiol., 395, 233–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herkenham M., Lynn,A.B., Johnson,M.R., Melvin,L.S., de Costa,B.R. and Rice,K.C. (1991) Characterization and localization of cannabinoid receptors in rat brain: a quantitative in vitro autoradiographic study. J. Neurosci., 11, 563–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hines M.L. and Carnevale,N.T. (1997) The NEURON simulation environment. Neural Comput., 9, 1179–1209. [DOI] [PubMed] [Google Scholar]
- Hogan Q.H., McCallum,J.B., Sarantopoulos,C., Aason,M., Mynlieff,M., Kwok,W.M. and Bosnjak,Z.J. (2000) Painful neuropathy decreases membrane calcium current in mammalian primary afferent neurons. Pain, 86, 43–53. [DOI] [PubMed] [Google Scholar]
- Huguenard J.R. (1996) Low-threshold calcium currents in central nervous system neurons. Annu. Rev. Physiol., 58, 329–348. [DOI] [PubMed] [Google Scholar]
- Jarai Z. et al. (1999) Cannabinoid-induced mesenteric vasodilation through an endothelial site distinct from CB1 or CB2 receptors. Proc. Natl Acad. Sci. USA, 96, 14136–14141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klugbauer N., Marais,E., Lacinova,L. and Hofmann,F. (1999) A T-type calcium channel from mouse brain. Pflugers Arch., 437, 710–715. [DOI] [PubMed] [Google Scholar]
- Lake K.D., Martin,B.R., Kunos,G. and Varga,K. (1997) Cardiovascular effects of anandamide in anesthetized and conscious normotensive and hypertensive rats. Hypertension, 29, 1204–1210. [DOI] [PubMed] [Google Scholar]
- Lee J.H., Daud,A.N., Cribbs,L.L., Lacerda,A.E., Pereverzev,A., Klockner,U., Schneider,T. and Perez-Reyes,E. (1999) Cloning and expression of a novel member of the low voltage-activated T-type calcium channel family. J. Neurosci., 19, 1912–1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei M., Brown,H. and Noble,D. (1998) What role do T-type calcium channels play in cardiac pacemaker activity? In Tsien,R.W., Clozel,J.P. and Nargeot,J. (eds), Low-voltage-activated T-type Calcium Channels. ADIS International, Chester, UK, pp. 103–109.
- Llinas R. and Jahnsen,H. (1982) Electrophysiology of mammalian thalamic neurones in vitro. Nature, 297, 406–408. [DOI] [PubMed] [Google Scholar]
- Llinas R. and Yarom,Y. (1981) Properties and distribution of ionic conductances generating electroresponsiveness of mammalian inferior olivary neurones in vitro. J. Physiol., 315, 569–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukyanetz E.A. (1998) Diversity and properties of calcium channel types in NG108-15 hybrid cells. Neuroscience, 87, 265–274. [DOI] [PubMed] [Google Scholar]
- Mackie K. and Hille,B. (1992) Cannabinoids inhibit N-type calcium channels in neuroblastoma-glioma cells. Proc. Natl Acad. Sci. USA, 89, 3825–3829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackie K., Lai,Y., Westenbroek,R. and Mitchell,R. (1995) Canna binoids activate an inwardly rectifying potassium conductance and inhibit Q-type calcium currents in AtT20 cells transfected with rat brain cannabinoid receptor. J. Neurosci., 15, 6552–6561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maingret F., Patel,A.J., Lazdunski,M. and Honore,E. (2001) The endocannabinoid anandamide is a direct and selective blocker of the background K(+) channel TASK-1. EMBO J., 20, 47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda L.A., Lolait,S.J., Brownstein,M.J., Young,A.C. and Bonner,T.I. (1990) Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature, 346, 561–564. [DOI] [PubMed] [Google Scholar]
- McCarthy R.T., Isales,C. and Rasmussen,H. (1993) T-type calcium channels in adrenal glomerulosa cells: GTP-dependent modulation by angiotensin II. Proc. Natl Acad. Sci. USA. 90, 3260–3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick D.A. and Huguenard,J.R. (1992) A model of the electrophysiological properties of thalamocortical relay neurons. J. Neurophysiol., 68, 1384–1400. [DOI] [PubMed] [Google Scholar]
- Mechoulam R., Fride,E. and Di Marzo,V. (1998) Endocannabinoids. Eur. J. Pharmacol., 359, 1–18. [DOI] [PubMed] [Google Scholar]
- Monteil A., Chemin,J., Bourinet,E., Mennessier,G., Lory,P. and Nargeot,J. (2000a) Molecular and functional properties of the human α(1G) subunit that forms T-type calcium channels. J. Biol. Chem., 275, 6090–6100. [DOI] [PubMed] [Google Scholar]
- Monteil A., Chemin,J., Leuranguer,V., Altier,C., Mennessier,G., Bourinet,E., Lory,P. and Nargeot,J. (2000b) Specific properties of T-type calcium channels generated by the human α1I subunit. J. Biol. Chem., 275, 16530–16535. [DOI] [PubMed] [Google Scholar]
- Munro S., Thomas,K.L. and Abu-Shaar,M. (1993) Molecular characterization of a peripheral receptor for cannabinoids. Nature, 365, 61–65. [DOI] [PubMed] [Google Scholar]
- Nilius B., Hess,P., Lansman,J.B. and Tsien,R.W. (1985) A novel type of cardiac calcium channel in ventricular cells. Nature, 316, 443–446. [DOI] [PubMed] [Google Scholar]
- Nowycky M.C., Fox,A.P. and Tsien,R.W. (1985) Three types of neuronal calcium channel with different calcium agonist sensitivity. Nature, 316, 440–443. [DOI] [PubMed] [Google Scholar]
- Perez-Reyes E., Cribbs,L.L., Daud,A., Lacerda,A.E., Barclay,J., Williamson,M.P., Fox,M., Rees,M. and Lee,J.H. (1998) Molecular characterization of a neuronal low-voltage-activated T-type calcium channel. Nature, 391, 896–900. [DOI] [PubMed] [Google Scholar]
- Poling J.S., Rogawski,M.A., Salem,N.,Jr and Vicini,S. (1996) Anandamide, an endogenous cannabinoid, inhibits Shaker-related voltage-gated K+ channels. Neuropharmacology, 35, 983–991. [DOI] [PubMed] [Google Scholar]
- Randall A.D. and Tsien,R.W. (1997) Contrasting biophysical and pharmacological properties of T-type and R-type calcium channels. Neuropharmacology, 36, 879–893. [DOI] [PubMed] [Google Scholar]
- Rossier M.F., Burnay,M.M., Vallotton,M.B. and Capponi,A.M. (1996) Distinct functions of T- and L-type calcium channels during activation of bovine adrenal glomerulosa cells. Endocrinology, 137, 4817–4826. [DOI] [PubMed] [Google Scholar]
- Schweitzer P. (2000) Cannabinoids decrease the K(+) M-current in hippocampal CA1 neurons. J. Neurosci., 20, 51–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart D., Gunthorpe,M.J., Jerman,J.C., Nasir,S., Gray,J., Muir,A.I., Chambers,J.K., Randall,A.D. and Davis,J.B. (2000) The endogenous lipid anandamide is a full agonist at the human vanilloid receptor (hVR1). Br. J. Pharmacol., 129, 227–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith F.L., Fujimori,K., Lowe,J. and Welch,S.P. (1998) Character ization of Δ9-tetrahydrocannabinol and anandamide antinociception in nonarthritic and arthritic rats. Pharmacol. Biochem. Behav., 60, 183–191. [DOI] [PubMed] [Google Scholar]
- Steriade M., McCormick,D.A. and Sejnowski,T.J. (1993) Thalamo cortical oscillations in the sleeping and aroused brain. Science, 262, 679–685. [DOI] [PubMed] [Google Scholar]
- Sun Q.Q. and Dale,N. (1999) G-proteins are involved in 5-HT receptor-mediated modulation of N- and P/Q- but not T-type Ca2+ channels. J. Neurosci., 19, 890–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talley E.M., Cribbs,L.L., Lee,J.H., Daud,A., Perez-Reyes,E. and Bayliss,D.A. (1999) Differential distribution of three members of a gene family encoding low voltage-activated (T-type) calcium channels. J. Neurosci., 19, 1895–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tognetto M., Amadesi,S., Harrison,S., Creminon,C., Trevisani,M., Carreras,M., Matera,M., Geppetti,P. and Bianchi,A. (2001) Anand amide excites central terminals of dorsal root ganglion neurons via vanilloid receptor-1 activation. J. Neurosci., 21, 1104–1109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlinson W.J., Stea,A., Bourinet,E., Charnet,P., Nargeot,J. and Snutch,T.P (1993) Functional properties of a neuronal class C L-type calcium channel. Neuropharmacology, 32, 1117–1126. [DOI] [PubMed] [Google Scholar]
- Triggle D.J. (1992) Calcium-channel antagonists: mechanisms of action, vascular selectivities and clinical relevance. Clev. Clin. J. Med., 59, 617–627. [DOI] [PubMed] [Google Scholar]
- Tsakiridou E., Bertollini,L., de Curtis,M., Avanzini,G. and Pape,H.C. (1995) Selective increase in T-type calcium conductance of reticular thalamic neurons in a rat model of absence epilepsy. J. Neurosci., 15, 3110–3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twitchell W., Brown,S. and Mackie,K. (1997) Cannabinoids inhibit N- and P/Q-type calcium channels in cultured rat hippocampal neurons. J. Neurophysiol., 78, 43–50. [DOI] [PubMed] [Google Scholar]
- Venance L., Piomelli,D., Glowinski,J. and Giaume,C. (1995) Inhibition by anandamide of gap junctions and intercellular calcium signalling in striatal astrocytes. Nature, 376, 590–594. [DOI] [PubMed] [Google Scholar]
- Vivian J.A., Kishioka,S., Butelman,E.R., Broadbear,J., Lee,K.O. and Woods,J.H (1998) Analgesic, respiratory and heart rate effects of cannabinoid and opioid agonists in rhesus monkeys: antagonist effects of SR 141716A. J. Pharmacol. Exp. Ther., 286, 697–703. [PubMed] [Google Scholar]
- Zhang Y., Cribbs,L.L. and Satin,J. (2000) Arachidonic acid modulation of α1H, a cloned human T-type calcium channel. Am. J. Physiol., 278, 184–193. [DOI] [PubMed] [Google Scholar]
- Zygmunt P.M., Petersson,J., Andersson,D.A., Chuang,H., Sorgard,M., Di Marzo,V., Julius,D. and Hogestatt,E.D. (1999) Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature, 400, 452–457. [DOI] [PubMed] [Google Scholar]