Abstract
Glucocorticoids inhibit the proinflammatory activities of transcription factors such as AP-1 and NF-κB as well as that of diverse cellular signaling molecules. One of these signaling molecules is the extracellular signal-regulated kinase (Erk-1/2) that controls the release of allergic mediators and the induction of proinflammatory cytokine gene expression in mast cells. The mechanism of inhibition of Erk-1/2 activity by glucocorticoids is unknown. Here we report a novel dual action of glucocorticoids for this inhibition. Glucocorticoids increase the expression of the MAP kinase phosphatase-1 (MKP-1) gene at the promoter level, and attenuate proteasomal degradation of MKP-1, which we report to be triggered by activation of mast cells. Both induction of MKP-1 expression and inhibition of its degradation are necessary for glucocorticoid-mediated inhibition of Erk-1/2 activation. In NIH-3T3 fibroblasts, although glucocorticoids up-regulate the MKP-1 level, they do not attenuate the proteasomal degradation of this protein and consequently they are unable to inhibit Erk-1/2 activity. These results identify MKP-1 as essential for glucocorticoid-mediated control of Erk-1/2 activation and unravel a novel regulatory mechanism for this anti-inflammatory drug.
Keywords: cellular signaling/glucocorticoid receptor/mast cells/MKP-1/proteasome
Introduction
Glucocorticoids are well known for their anti-inflammatory, immune suppressive and antiallergic actions (for review see Barnes, 1998). They function by binding to a receptor, the glucocorticoid receptor (GR), which resides in an inactive form in the cytoplasm of target cells. Upon interaction with the hormone, this receptor is transported into the nucleus where it binds to discrete nucleotide sequences to alter the expression of specific genes (Beato et al., 1995). Nuclear accumulation of the GR is a rapid process that occurs with a half-time of ∼5 min, and activation of primary target genes is observable within 30 min of addition of hormone (Webster et al., 1993; Carey et al., 1996). A rapid effect of the receptor (∼30 min to 2 h) has also been reported in negative regulation of action of transcription factors such as AP-1 or NF-κB (König et al., 1992; Nissen and Yamamoto, 2000). As these transcription factors control the expression of numerous proinflammatory genes, the inhibition of their activity by the GR has become a paradigm for the anti-inflammatory and immune suppressive action of glucocorticoids (for review see Cato and Wade, 1996).
A completely different mechanism by which GR might exert its anti-inflammatory effects is the inhibition of signaling pathways that regulate inflammatory processes, in particular the mitogen-activated protein (MAP) kinases pathways. For example, GR-mediated inhibition of c-Jun N-terminal kinase (JNK) activity, leading to inhibition of c-Jun phosphorylation, is proposed as an alternative mode of action of GR for the repression of AP-1 activity (Caelles et al., 1997; Swantek et al., 1997; Hirasawa et al., 1998). The MAP kinase p38 is also a target for negative regulation by glucocorticoids, and its inhibition is involved in the destabilization of cyclooxygenase-2 mRNA by GR (Lasa et al., 2001). Finally, the activity of the extracellular regulated kinases (Erk)-1 and -2 is inhibited by glucocorticoids (Rider et al., 1996; Hulley et al., 1998; González et al., 1999; Gewert et al., 2000). The negative regulation by glucocorticoids of this latter signaling pathway is a particularly good example of how glucocorticoids might contribute to anti-inflammatory processes in mast cells (Rider et al., 1996).
Mast cells play a central role in allergic and inflammatory reactions. They express high affinity IgE receptors (FcεRI) on their surface which, when aggregated, initiate biochemical events leading to the release of inflammatory mediators (Turner and Kinet, 1999). The first demonstrable response after the activation of FcεRI is the rapid activation of cytoplasmic protein tyrosine kinases (PTKs) and effector pathways that control mast cell responses. FcεRI-regulated PTK is also coupled to the activation of RasGTPases, Raf-1 and Erk-1 and -2. The activated Erks are involved in the generation of arachidonic acid via phospholipase A2 and in the production of the inflammatory cytokines tumor necrosis factor-α (TNF-α) and granulocyte–macrophage colony-stimulating factor (GM-CS) (Hirasawa et al., 1995; Zhang et al., 1997; Kimata et al., 2000). The production of these inflammatory mediators has been found to be inhibited by glucocorticoids (Rider et al., 1996; Eklund et al., 1997; Sewell et al., 1998).
In mast cells, although AP-1 and NF-κB control the activity of proinflammatory cytokine genes (Nechushtan and Razin, 1998; Kitaura et al., 2000), upstream signaling molecules have also been shown to be crucial. The expression of several proinflammatory cytokine genes in response to IgE is markedly reduced in embryonic stem cell-derived mast cells with targeted disruption of MEKK-2 (Garrington et al., 2000). Furthermore, JNK (Hirasawa et al., 1998) and Erk-1/2 (Rider et al., 1996; Cissel and Beaven, 2000) have been identified as targets for negative regulation by glucocorticoids. However, in contrast to the rapid response of glucocorticoids in down-regulating the action of AP-1 and NF-κB, the effect of glucocorticoids on the activity of some of these signaling molecules requires prolonged exposure to the hormone. Negative regulation of Erk-1/2 activity by glucocorticoids occurs maximally after 18 h pre-treatment with glucocorticoid (Rider et al., 1996), but the mechanism involved is unclear. It has been suggested that glucocorticoid-mediated disruption of Hsp90 association with an upstream kinase of the Erk pathway, Raf-1 (Cissel and Beaven, 2000), and the inhibition of Raf-1 activity may be responsible for the negative action of this hormone on Erk-1/2 activity (Rider et al., 1996; Cissel and Beaven, 2000). However, these processes occur with kinetics different from those for the inhibition of Erk-1/2 activity. Thus, it is uncertain whether they are related or whether they occur separately.
In this communication, we have shown that the MAP kinase phosphatase-1 (MKP-1) is involved in the inhibition of Erk-1/2 activity by glucocorticoid in mast cells and demonstrated it as a novel mode of action of this hormone in cellular signaling.
Results
Inhibition of phosphorylation of Erk-1 and -2 by glucocorticoids
To investigate the mechanism of down-regulation of activity of Erk-1 and -2 by glucocorticoids, we first assessed the kinetics of the repression in RBL-2H3 rat mast cells by the synthetic glucocorticoid hormone, dexamethasone. Erk-1/2 was activated by cross-linking FcεRI with antigen, and the activation process was detected by immunoblotting using an antibody that recognizes active dual-phosphorylated Erk-1/2. A phosphorylation state-independent anti-Erk-2 antibody was used as control to ensure that any effect observed on Erk-1/2 phosphorylation was not due to differences in the level of these proteins.
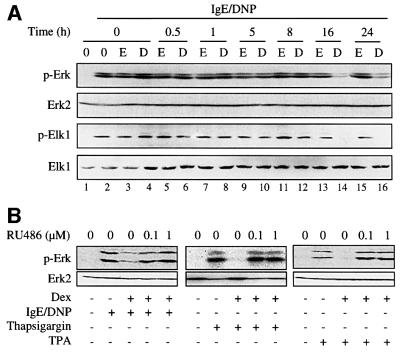
In this assay, dexamethasone inhibited phosphorylation of Erk-1/2 only after 16 or 24 h and not after short-term treatment (0.5–8 h) (Figure 1A). This finding differs from a previously published report where >65% of Erk-1/2 activity was inhibited following 6 h pre-treatment of RBL-2H3 cells with dexamethasone (Rider et al., 1996). The reason for these differences is not known. The kinetics of inhibition of Erk-1/2 phosphorylation in our experiment correlated with the inhibition of phosphorylation of Elk-1, a downstream target of Erk-1/2 (Figure 1A). Phosphorylation of Erk-1/2 was also inhibited by dexamethasone with the same kinetics when the cells were activated with thapsigargin, which increases intracellular free calcium concentration (Hirasawa et al., 1995; Rider et al., 1996), or with the protein kinase C stimulant 12-O-tetradecanoyl phorbol 13-acetate (TPA) (Figure 1B and results not shown). The negative regulatory effect of dexamethasone was abolished by the GR antagonist RU486 at an equimolar concentration or 10-fold molar excess to dexamethasone (Figure 1B), indicating the involvement of the GR in the action of the hormone.
Fig. 1. Glucocorticoids inhibit Erk-1/2 activation. RBL-2H3 mast cells were treated (A) for the indicated time with dexamethasone (D, 0.1 µM) or solvent (ethanol, E), or (B) for 16 h with dexamethasone (Dex, 0.1 µM) in the presence of RU486 at the indicated concentrations. The cells were then sensitized with monoclonal anti-DNP IgE, and stimulated with DNP-BSA or activated with thapsigargin or TPA as indicated. Erk-1/2 and Elk-1 phosphorylation (p-Erk, p-Elk-1) was assessed by immunoblotting using phospho-specific antibodies. The membranes were stripped and reprobed with phosphorylation state-independent anti-Erk-2 and anti-Elk-1 antibodies. The results are representative of three different experiments.
Requirement for a protein tyrosine phosphatase
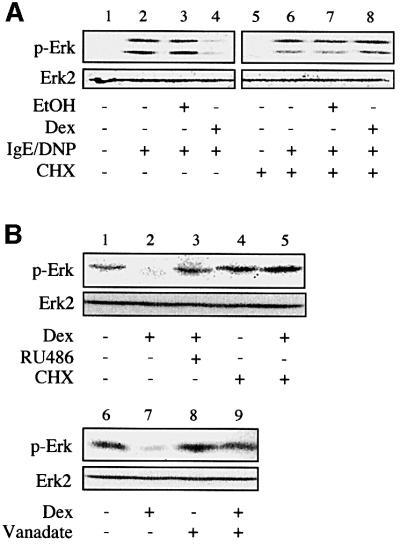
The long-term effect of dexamethasone in the inhibition of Erk-1/2 phosphorylation suggests the involvement of de novo protein synthesis in the action of the hormone. To confirm this, we determined the effect of glucocorticoid on Erk-1/2 phosphorylation in the presence or absence of the protein synthesis inhibitor cycloheximide. While this inhibitor did not have any major effect on the activation of Erk-1/2 by IgE receptor triggering (Figure 2A), it abolished the negative effect of dexamethasone on phosphorylation of Erk-1/2 (Figure 2A). This therefore confirmed the need for new protein synthesis in the glucocorticoid-mediated inhibition of phosphorylation of Erk-1/2.
Fig. 2. Glucocorticoid-mediated inhibition of Erk-1/2 activation involves de novo expression of a tyrosine phosphatase. (A) RBL-2H3 mast cells were treated with dexamethasone (Dex, 0.1 µM) or solvent alone (EtOH) for 16 h in the presence or absence of cycloheximide (CHX), before sensitization with anti-DNP IgE, and activation with DNP-BSA. Phosphorylation of Erk-1/2 (p-Erk) was assessed by immunoblotting using a phospho-specific antibody. The membranes were stripped and reprobed with a phosphorylation state-independent anti-Erk-2 antibody. (B) Phosphorylated Erk-1/2 contained in lysates from IgE-sensitized and DNP-BSA-activated RBL-2H3 cells was subjected to a dephosphorylation assay. This was achieved by incubating the cellular extracts with lysates from RBL-2H3 cells treated for 16 h with dexamethasone (Dex, 0.1 µM) in the presence or absence of RU486 (1 µM) or cycloheximide (CHX). Incubation was performed in the absence (lanes 1–7) or the presence (lanes 8 and 9) of sodium orthovanadate (vanadate, 1 mM). The level of phosphorylation of Erk (p-Erk) was assessed by immunoblotting. The results are representative of three different experiments.
To investigate whether the newly synthesized protein needed for the inhibition of Erk-1/2 phosphorylation is a phosphatase, we performed in vitro dephosphorylation experiments by mixing RBL-2H3 cell lysate after prior activation of Erk-1/2 with lysates of the same cells pre-treated for 16 h with dexamethasone or with solvent alone. Determination of the level of phosphorylation of Erk-1/2 by immunoblotting revealed that the activated Erk was dephosphorylated after incubation with the lysates of the dexamethasone-treated cells, as opposed to the solvent control (Figure 2B). Note that only the intense 42 kDa Erk-2 band was clearly visible in this experiment. The dephosphorylation was prevented by simultaneous treatment of the cells with dexamethasone and RU486 or cycloheximide (Figure 2B). This indicated that the action of dexamethasone involves a GR-mediated enhancement of expression of a dephosphorylating enzyme. Addition of a protein tyrosine phosphatase inhibitor, orthovanadate, to the reaction mixture abrogated the dephosphorylation of Erk-1/2 (Figure 2B), identifying the dephosphorylating enzyme as a protein tyrosine phosphatase.
MKP-1 expression correlates with dexamethasone-mediated inhibition of Erk-1/2 activity
In a search for tyrosine phosphatase genes up-regulated by glucocorticoids in RBL-2H3 mast cells using Affymetrix rat genome U34A arrays, we only identified MKP-1 and MKP-3 as targets for glucocorticoid action. MKP-1 and -3 are dual-specificity protein phosphatases that dephos phorylate and inactivate MAP kinases (Alessi et al., 1993; Sun et al., 1993; Groom et al., 1996). While we could confirm the results of MKP-1, we were unable to demonstrate a glucocorticoid-mediated up-regulation of MKP-3 using three different antibodies that recognize this protein (results not shown). MKP-3 was therefore not studied further, and subsequent analyses were restricted to MKP-1.
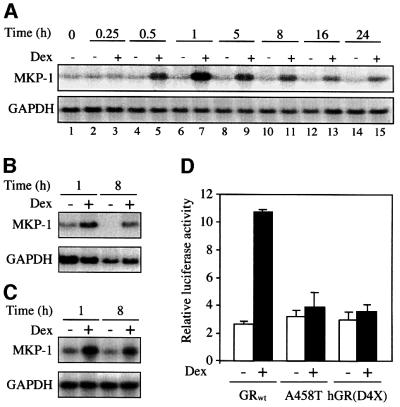
Reassessment of the Affymetrix result by northern blot analyses confirmed that after dexamethasone treatment, MKP-1 transcripts were increased as early as 30 min and remained elevated for 24 h (Figure 3A). RNA from another mast cell line, mouse bone marrow-derived mast cells (BMMCs), also showed an increased expression of MKP-1 as early as 1 h after treatment with dexamethasone (Figure 3B). Interestingly, MKP-1 was up-regulated at the RNA level in mouse NIH-3T3 fibroblasts (Figure 3C) and the promoter activity of this gene was induced ∼4-fold in GR-negative simian kidney COS-7 cells following co-transfection of a human GR expression vector (Figure 3D). Activation of the MKP-1 promoter construct was not observed however when two dimerization-defective and transactivation-negative GR expression vectors A458T and hGR(D4X) (Heck et al., 1994) were used (Figure 3D). Thus, MKP-1 is most probably a classical glucocorticoid-responsive gene, and this conclusion is strengthened by our discovery of several putative glucocorticoid response elements (GREs) on the published MKP-1 promoter sequence (Noguchi et al., 1993).
Fig. 3. Glucocorticoids induce MKP-1 gene expression. RBL-2H3 mast cells (A), mouse bone marrow-derived mast cells (B) and NIH-3T3 fibroblasts (C) were treated for the indicated time with dexamethasone (Dex, 0.1 µM) or solvent alone. Total RNA was extracted and subjected to northern blotting using cDNA probes for MKP-1 and GAPDH. (D) COS-7 cells were transiently co-transfected with an MKP-1 promoter firefly luciferase construct (-1716MKP-1luc) and a Renilla luciferase construct as an internal control, together with either wild-type glucocorticoid receptor (GRwt) or two different dimerization/transactivation-deficient GR mutant [A458T or hGR(D4X)] expression vectors. Treatment with dexamethasone (Dex, 0.1 µM) or solvent alone was performed 4 h after the transfection and the cells were harvested 48 h later for luciferase activity measurements. The results are expressed as the level of MKP-1 promoter-driven firefly luciferase expression after correcting for the transfection efficiency by Renilla luciferase measurements (relative luciferase activity), and are presented as the mean ± SD of three independent experiments.
In agreement with the observed increase in mRNA level, dexamethasone also enhanced MKP-1 protein level with a lag of 5 h, reaching its maximum after 8 h of hormone treatment (Figure 4A). The glucocorticoid-mediated increase in MKP-1 protein level was dose dependent and correlated with the inhibition of phosphorylation of Erk-1/2 (Figure 4B). It occurred with a half-maximal concentration compatible with the dissociation constant of dexamethasone for the GR (6 nM) (Le Van et al., 1997). This suggests an involvement of the GR, a finding confirmed by the fact that RU486 abolished the induction of MKP-1 expression and the inhibition of Erk-1/2 phosphorylation (Figure 4C). Following IgE receptor cross-linking, a decrease in MKP-1 basal level was observed, as depicted in Figure 4B (lanes 3–8) and C (lane 2), which might be due to another regulatory pathway that down-regulates MKP-1 in contrast to the up-regulation by glucocorticoids. This regulatory pathway will be described later.
Fig. 4. Glucocorticoid-induced increase in MKP-1 protein level correlates with inhibition of Erk-1/2 activity. RBL-2H3 mast cells were treated for the indicated time with dexamethasone (Dex, 0.1 µM) or solvent alone (ethanol) (A), for 16 h with dexamethasone (Dex) at the indicated concentration or solvent alone (ethanol, E) (B), or for 16 h with dexamethasone (Dex, 0.1 µM) in the presence of RU486 at the indicated concentration (C). The cells were then sensitized with monoclonal anti-DNP IgE, and activated with DNP-BSA [(B) and (C)]. MKP-1 protein expression was assessed by immunoblotting using a specific anti-MKP-1 antibody, and Erk-1/2 phosphorylation (p-Erk) was assessed by immunoblotting as in Figure 1. (D) Rat recombinant purified activated Erk-2 was subjected to an in vitro dephosphorylation assay, in the presence of RBL-2H3 mast cell lysates that were treated for 16 h with either dexamethasone (Dex, 0.1 µM) or solvent alone (EtOH). The dephosphorylation assay was carried out after depleting MKP-1 from the dexamethasone-treated and control cell extracts by immunoprecipitation using a specific anti-MKP-1 antibody or an isotype control antibody. The level of phosphorylation of Erk-2 (p-Erk) was assessed by immunoblotting as in Figure 1. The membranes were stripped and reprobed with an anti-MKP-1 antibody. An anti-Hsp90 antibody was used for loading control. The results are representative of two different experiments.
A hint that MKP-1 is involved in the inhibition of phosphorylation of Erk-1/2 comes from experiments in which we examined the effect of dexamethasone under conditions of diminished levels of MKP-1. This was achieved by the use of an anti-MKP-1 antibody to decrease the level of this protein in lysates of cells treated with dexamethasone for 16 h prior to their use in the in vitro dephosphorylation experiment. After clearing the lysate by immunoprecipitation with the MKP-1 antibody, it no longer dephosphorylated a pre-activated Erk-2 substrate (Figure 4D). On the contrary, dephosphorylation occurred following the use of a control antibody of the same isotype to clear the lysate (Figure 4D). A control MKP-1 immunoblot confirmed that the level of this protein was elevated following dexamethasone treatment and that the depletion of MKP-1 was effective (Figure 4D). Hsp90 level was determined as a marker for equal loading controls.
Dexamethasone protects MKP-1 from proteasome-mediated degradation
The rapid glucocorticoid-mediated accumulation of MKP-1 transcripts and protein in the presence of dexamethasone cannot explain the late dephosphorylation of Erk-1/2 in the RBL-2H3 cells. Thus, another mechanism must exist to explain the slow kinetics of dephosphorylation of Erk-1/2.
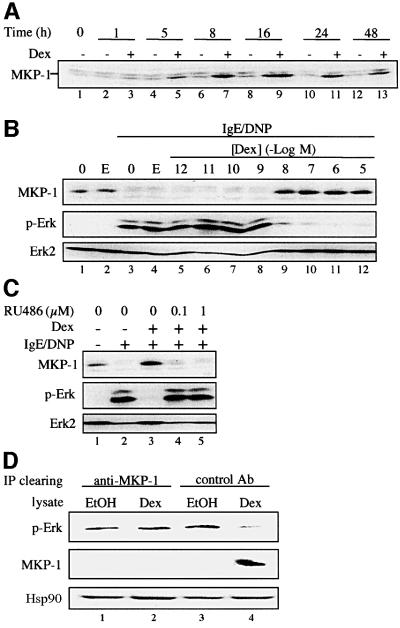
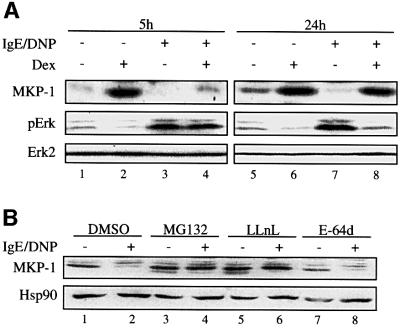
In the absence of IgE receptor cross-linking and Erk-1/2 phosphorylation, MKP-1 expression was induced by dexamethasone after both short- (5–8 h) and long-term treatments (16–24 h) (Figures 4A and 5A for the 5 and 24 h results). Upon IgE receptor cross-linking, MKP-1 was down-regulated (Figure 5A). The down-regulation of MKP-1 level was not affected by a 5 h dexamethasone treatment and, as a result, dexamethasone had no effect on Erk-1/2 phosphorylation (Figure 5A). A different observation was made with cells treated with dexamethasone for 24 h. In this case, dexamethasone prevented the down-regulation of MKP-1 and this correlated with inhibition of Erk-1/2 phosphorylation (Figure 5A). These results were also obtained using 8 h as a short- and 16 h as a long-term treatment with dexamethasone (results not shown). These findings explain the long-term effect of glucocorticoid since it is under these conditions that the down-regulation of MKP-1 is prevented. Activation of the Erk-1/2 pathway by other factors such as TPA or thapsigargin also down-regulated the MKP-1 level and this effect was reversed by a prolonged, but not a short-term treatment with dexamethasone, as described for the IgE receptor cross-linking (results not shown).
Fig. 5. Dexamethasone inhibits MKP-1-targeted degradation by the proteasome in RBL-2H3 mast cells. RBL-2H3 mast cells were treated for the indicated time with dexamethasone (Dex, 0.1 µM) or solvent alone (ethanol) (A). Cells were also treated for 3 h with solvent alone (DMSO), the proteasome inhibitors MG132 or LLnL, or the cysteine proteinase inhibitor E-64d (B). The cells were sensitized with anti-DNP IgE, then stimulated with DNP-BSA [(A) and (B)]. Phosphorylation of Erk-1/2 (p-Erk) and expression of MKP-1 were assessed by immunoblotting as described in Materials and methods. The results are representative of two different experiments.
As MKP-1 is degraded via the proteasome pathway (Brondello et al., 1999) or by cysteine proteinases (Torres et al., 2000), we wondered whether these contributed to the down-regulation of this protein in our study. We therefore treated the RBL-2H3 mast cells with the proteasome inhibitors MG132 and LLnL as well as the cysteine proteinase inhibitor E-64d prior to IgE receptor cross-linking. MG132 and LLnL but not E-64d prevented the down-regulation of MKP-1 (Figure 5B), indicating that MKP-1 is degraded via the proteasome pathway following activation of mast cells. A calpain inhibitor, calpeptin (up to 50 µM), had no effect on the degradation of MKP-1 (results not shown), confirming the specificity of the effect of the two proteasome inhibitors used. Similar results to those shown above were obtained when the same set of inhibitors was used before activation of mast cells with thapsigargin or TPA (results not shown).
Both the attenuation of proteasomal degradation and up-regulation of MKP-1 expression are essential for glucocorticoid-mediated inhibition of Erk-1/2 activity
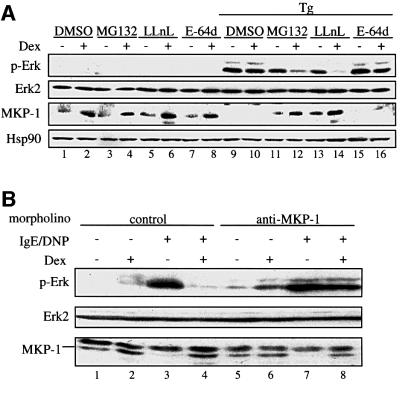
To assess the contribution of the proteasomal degradation of MKP-1 to the down-regulation of Erk-1/2 phosphorylation by glucocorticoids, we examined the effect of this hormone in the presence and absence of proteasome inhibitors. Mast cells were treated for 5 h with dexamethasone to enhance MKP-1 expression in the absence or presence of the proteasome inhibitors MG132 or LLnL, or in the presence of solvent alone (dimethylsulfoxide; DMSO) or E-64d as a control, prior to activation with thapsigargin, which leads to phosphorylation of Erk-1/2. In this study, as already shown, the MKP-1 level was enhanced following 5 h dexamethasone treatment in the absence of activation with thapsigargin (Figure 6A, lanes 1–8). Upon activation, the MKP-1 level was down-regulated in both the dexamethasone-treated and untreated samples (Figure 6A, lanes 9 and 10). The proteasome inhibitors MG132 and LLnL, but not E-64d, prevented the degradation of MKP-1, and the effect of dexamethasone was again evident (Figure 6A, lanes 11–16). Under these conditions of glucocorticoid-mediated increase in MKP-1 levels and in the presence of reduced proteasomal degradation, phosphorylation of Erk-1/2 was repressed even after 5 h of treatment with dexamethasone. Similar results were obtained with TPA activation instead of thapsigargin (results not shown). Thus, the inhibition of the proteasomal degradation of MKP-1 is important for the down-regulation of Erk-1/2 phosphorylation.
Fig. 6. Attenuation of proteasomal degradation and induction of the expression of MKP-1 are involved in dexamethasone-mediated dephosphorylation of Erk-1/2. (A) RBL-2H3 mast cells were treated for 5 h with dexamethasone (Dex, 0.1 µM) or solvent alone (ethanol), and for 3 h with solvent alone (DMSO), the proteasome inhibitors MG132 or LLnL, or the cysteine proteinase inhibitor E-64d, prior to activation with thapsigargin (Tg). Phosphorylation of Erk-1/2 (p-Erk) and expression of MKP-1 were assessed by immunoblotting as described in Materials and methods. An anti-Hsp90 antibody was used for loading control. (B) RBL-2H3 mast cells were loaded with an anti-MKP-1 antisense morpholino-oligonucleotide or a control morpholino-oligonucleotide before treatment with either solvent alone or dexamethasone (0.1 µM) for 16 h. The cells were sensitized with anti-DNP IgE and stimulated with DNP-BSA. Phosphorylation of Erk-1/2 (p-Erk) was assessed by immunoblotting using an anti-phosphoErk antibody. The membranes were stripped and reprobed with an anti-MKP-1 antibody and with the anti-Erk-2 antibody. The results are representative of two different experiments.
To determine whether the up-regulation of MKP-1 expression by glucocorticoid was also necessary for the inhibition of Erk-1/2 phosphorylation, we used morpholino-oligonucleotides to dissociate the up-regulation of MKP-1 expression by glucocorticoid from the inhibition of its degradation. In mast cells treated with control morpholino-oligonucleotides, dexamethasone after 16 h application inhibited Erk-1/2 phosphorylation induced by IgE receptor triggering (Figure 6B). MKP-1 levels were also increased following dexamethasone treatment (Figure 6B, lanes 1 and 2), and the degradation of MKP-1 observed after IgE receptor triggering was prevented by the hormone (Figure 6B, compare lane 4 with lane 3). Note that the batch of MKP-1 antibody used in this assay also interacted with another protein with a higher molecular weight. On the other hand, mast cells treated with a specific anti-MKP-1 oligonucleotide no longer responded to glucocorticoid by the down-regulation of Erk-1/2 phosphorylation (Figure 6B, lanes 7 and 8). Interestingly, within the time frame of exposure of the cells to the antisense MKP-1 oligonucleotide, the basal level of MKP-1 was not altered, suggesting that under basal conditions MKP-1 is a stable protein in mast cells. Most importantly, the glucocorticoid-mediated up-regulation of MKP-1 was totally abolished (Figure 6B, lanes 5 and 6). Under these conditions of complete inhibition of inducibility of MKP-1 expression, the proteosomal degradation following IgE receptor triggering still persisted and dexamethasone prevented this process by maintaining the basal level (Figure 6B, lanes 7 and 8). Thus, under conditions of absence of the up-regulation of MKP-1 expression by glucocorticoid, the inhibition of degradation still occurred; nonetheless, it did not suffice to down-regulate Erk-1/2 phosphorylation. These results together confirm that both the glucocorticoid-mediated inhibition of degradation and the increased expression of MKP-1 are necessary for down-regulating Erk-1/2 phosphorylation.
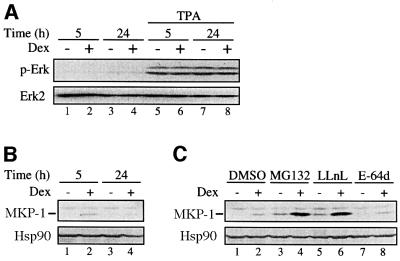
A further hint that both regulations of MKP-1 are necessary for down-regulating Erk-1/2 phosphorylation is demonstrated by studies in NIH-3T3 fibroblasts. In these cells, phosphorylation of Erk-1/2 was not inhibited by glucocorticoid (Figure 7A) although dexamethasone enhanced MKP-1 mRNA levels (Figure 3C). This discrepancy could possibly be explained at the level of MKP-1 protein degradation and the effect of glucocorticoid in this process. In both control and glucocorticoid-treated NIH-3T3 cells, MKP-1 protein was barely detected (Figure 7B). Basal MKP-1 protein levels and the enhancement by dexamethasone were clearly seen only in the presence of the proteasome inhibitors MG132 and LLnL, but not in the presence of the cysteine proteinase inhibitor E-64d (Figure 7C). Thus, in the NIH-3T3 fibroblastic cell line, MKP-1 protein is most likely to be degraded very rapidly by the proteasome pathway, as previously described by other investigators in CCL39 hamster fibroblasts (Brondello et al., 1999). The degradation of MKP-1 in NIH-3T3 cells did not require activation of the cells with TPA, as is the case in mast cells where compounds that activated Erk-1/2 led to the degradation of MKP-1. Furthermore, MKP-1 degradation was not blocked in the NIH-3T3 cells by dexamethasone even after 24 h treatment (Figure 7B). These results together demonstrate that both the glucocorticoid-mediated up-regulation of MKP-1 gene expression and the attenuation of MKP-1 protein degradation are required for the down-regulation of Erk-1/2 phosphorylation by glucocorticoids.
Fig. 7. Dexamethasone does not inhibit MKP-1 degradation by the proteasome in NIH-3T3 fibroblasts. NIH-3T3 fibroblasts were treated for the indicated time [(A and B)] or for 5 h (C) with dexamethasone (Dex, 0.1 µM) or solvent alone (ethanol). For the last 3 h of the treatment period, solvent alone (DMSO), the proteasome inhibitors MG132 or LLnL, or the cysteine proteinase inhibitor E-64d were added (C). The cells were then stimulated with TPA as indicated (A). Expression of MKP-1 and phosphorylation of Erk-1/2 (p-Erk) were assessed by immunoblotting as described in Materials and methods. The results are representative of two different experiments.
Discussion
In this work, we have shown that glucocorticoids require ongoing protein synthesis to inhibit phosphorylation of Erk-1/2 in mast cells. We demonstrated that increased expression of a tyrosine phosphatase is required, and identified MKP-1 as the phosphatase necessary for the inhibition of activated Erk-1/2. We also report that upon activation of mast cells by IgE receptor cross-linking or by thapsigargin or TPA, MKP-1 is degraded via the proteasome pathway and this process is inhibited by prolonged treatment with glucocorticoid. Both induction of MKP-1 expression and inhibition of its degradation play a crucial role in the glucocorticoid-mediated attenuation of Erk-1/2 phosphorylation.
Phosphorylation of Erk-1/2 is necessary for the generation of inflammatory mediators in mast cells (arachidonic acid release, prostanoid biosynthesis and the expression of proinflammatory cytokines) (Hirasawa et al., 1995; Zhang et al., 1997; Kimata et al., 2000). Thus, the negative regulation of this process described in our study may be of relevance to the anti-inflammatory action of glucocorticoids. Previous reports have suggested that the ability of the GR to suppress transactivation by the transcription factors AP-1, NF-κB or NF-AT are hallmarks of the anti-inflammatory action of glucocorticoids (Cato and Wade, 1996; Barnes, 1998). In the present study, we describe a novel regulatory action of the GR that consists of an up-regulation of MKP-1 gene expression and an inhibition of the degradation of this phosphatase. We do not know the mechanism(s) of inhibition of MKP-1 degradation by glucocorticoids. It might be due to inhibition of proteasome activity by this hormone, or result from an inhibition by glucocorticoids of the transduction pathway targeting MKP-1 for proteasomal degradation. In contrast, we have shown that the enhanced expression of the MKP-1 gene in the presence of glucocorticoid is a primary response of the GR at the level of increased promoter activity. This is consistent with the fact that at least three putative GREs are present in the MKP-1 promoter (Noguchi et al., 1993).
The anti-inflammatory action of glucocorticoids may therefore not only be due to negative regulation by the GR, as suggested by several studies, but also involves positive regulation by the receptor. This finding is consistent with increasing evidence of the use of positive regulatory mechanisms by the GR for its anti-inflammatory function. For example, glucocorticoids positively regulate the gene coding for secretory leukocyte protease inhibitor, which protects from damage to healthy lung tissues by factors produced by leukocytes during airway inflammatory diseases (Abbinante-Nissen et al., 1995). Furthermore, positive regulation by glucocorticoids has been reported for the thymosin β-4-sulfoxide gene, which encodes a factor with potent anti-inflammatory action on monocytes and macrophages (Young et al., 1999). We can now add the increased expression of MKP-1 by glucocorticoids to this list of positive functions of the receptor potentially involved in the anti-inflammatory action of glucocorticoids.
Several protein tyrosine phosphatases have been described in mast cells that influence IgE receptor signaling, such as HePTP, SHP-1 and -2 as well as CD45 (Berger et al., 1994; Swieter et al., 1995; Lienard et al., 1999; Xie et al., 2000). The balance between the action of these phosphatases and kinases activated by IgE receptor cross-linking regulates the extent of tyrosine phosphorylation and signal transduction cascades leading to degranulation and the release of inflammatory mediators. Increased expression of any of these phosphatases by glucocorticoids could, in principle, alter the balance in such a way that dephosphorylation is favored. This could result in the attenuation of the phosphorylation signals generated by aggregation of the IgE receptor. With the exception of MKP-1, we failed to identify any of the phosphatases listed above in our screen of genes that were clearly up-regulated by glucocorticoids in mast cells. Kinetic analyses of the regulation of expression of HePTP and SHP-1 by glucocorticoid in our mast cells by northern blot analysis were also negative (unpublished results). Thus, the mechanism of glucocorticoid-mediated inhibition of activation of the Erk-1/2 signal in mast cells appears to be rather specific for MKP-1.
Other MAP kinases such as JNK or p38 have also been identified as targets for negative regulation by glucocorticoids (Caelles et al., 1997; Swantek et al., 1997; Hirasawa et al., 1998; Lasa et al., 2001). The mechanisms used by the GR in these regulatory processes are, however, diverse. Glucocorticoid-mediated inhibition of JNK occurs after a short time of hormone treatment (Caelles et al., 1997; Hirasawa et al., 1998), does not require new protein synthesis and is independent of the transactivation function of the GR (Caelles et al., 1997). The inhibition of p38, on the other hand, occurs after a short time of treatment with glucocorticoids but requires de novo protein expression (Lasa et al., 2001). Our finding that the inhibition of Erk-1/2 by glucocorticoids occurs via a dual function of the GR re-emphasizes the diversity of regulatory possibilities used by the GR to modulate cellular signaling events.
In this study, we have identified MKP-1 as a specific glucocorticoid-regulated gene that may play an important role in the mediation of the anti-inflammatory action of glucocorticoids in mast cells. So far, targeted disruption of the MKP-1 gene in mice has not revealed any developmental defects (Dorfman et al., 1996). These animals are viable and appear healthy, but they have not been analyzed for their response to glucocorticoid after various inflammatory stimuli. These experiments are now in progress and will, hopefully, in agreement with our cell culture results, establish a role for MPK-1 in the mediation of the anti-inflammatory action of glucocorticoid.
Materials and methods
Plasmid constructs
The MKP-1 promoter construct -1716MKP-1luc was generated by linearizing the plasmid pUC18erp7 (a gift from R.Bravo containing >7 kb of MKP-1 promoter sequence) with the restriction enzyme BsiEI. The BsiEI site was blunted by T4 DNA polymerase (Amersham Pharmacia Biotech, Freiburg, Germany) followed by digestion with BamHI to generate a 2.7 kb fragment. This fragment was then inserted into the BamHI–EcoRV site of a TOPO TA cloning vector (Invitrogen, Groningen, The Netherlands). A 1800 bp fragment containing the promoter region of the MKP-1 gene was recovered by digestion with Asp718–XhoI and cloned into the Asp718–XhoI sites of pGL3 basic vector (Promega, Mannheim, Germany) such that the MKP-1 promoter now controls the expression of the luciferase gene. The wild-type human GR expression vector and the dimerization-defective mutants A458T and hGR(D4X) have been described previously (Heck et al., 1994).
Cell culture
RBL-2H3 mast cells were cultured at 37°C in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 15% fetal calf serum (FCS). Simian kidney COS-7 cells and NIH-3T3 fibroblasts were cultured in DMEM supplemented with 10% FCS. Murine BMMCs were generated from 5- to 10-week-old C57Bl/6 mice. The mice were sacrificed by cervical dislocation, intact femurs and tibias were removed and bone marrow cells were harvested by repeated flushing with minimal Eagle’s medium (MEM). The cells were cultured at a density of 3 × 106 cells/ml in Iscove’s modified Dulbecco’s medium (IMDM), supplemented with 10% FCS, 2 mM l-glutamine, 1 mM pyruvate, 100 U/ml penicillin, 100 µg/ml streptomycin, 20 U/ml murine interleukin-3 (mIL-3) and 50 U/ml mIL-4. Non-adherent cells were transferred to fresh culture plates every 2–3 days for a total of at least 21 days to remove adherent macrophages and fibroblasts. Fluorescence-activated cell sorting (FACS) analyses using an anti-CD13 antibody and IgE + anti-IgE mAb as well as May–Grünwald–Giemsa and toluidine blue staining revealed that the resulting cell population consisted of ∼99% BMMCs (Stassen et al., 2001).
Treatments and activation of cells
Confluent RBL-2H3 and NIH-3T3 cells were treated for various time periods with different concentrations of dexamethasone (Sigma, Deisenhofen, Germany), RU486 (Sigma) or solvent alone (ethanol) diluted in the culture medium. In some experiments, the cells were treated with dexamethasone or solvent together with cycloheximide (5 µg/ml; Sigma). Mast cells were sensitized with monoclonal anti-dinitrophenyl (DNP) IgE (0.5 µg/ml; Sigma) for 1 h at 37°C. They were then washed four times with HEPES buffer (137 mM NaCl, 2.7 mM KCl, 1.0 mM CaCl2, 1.0 mM MgCl2, 0.4 mM NaH2PO4, 5.6 mM glucose, 10 mM HEPES pH 7.4) to remove unbound IgE, and equilibrated in HEPES buffer containing 0.1% bovine serum albumin (BSA) for 10 min at 37°C. They were then stimulated with DNP-BSA (0.5 µg/ml; Sigma) for 15 min at 37°C and the reaction terminated by washing three times in ice-cold HEPES buffer. Stimulation of the cells was also performed with thapsigargin (150 nM; Sigma) or TPA (80 ng/ml; Sigma) for 15 min at 37°C and the reaction terminated as stated above. Treatment of cells with proteasome inhibitors was performed by exposing the cells for 3 h to the proteasome inhibitors MG132 (30 µM, carbobenzoxy-l-leucyl-l-leucyl-l-leucinal; Calbiochem, Schwalbach, Germany), LLnL (100 µM, N-acetyl-l-leucyl-l-leucyl-l-norleucinal; Calbiochem), the cysteine proteinase inhibitor E-64d [30 µM, (2S,3S)-trans-epoxysuccinyl-l-leucylamido-3-methylbutane-ethyl ester; Calbiochem] or solvent alone (DMSO).
In experiments with antisense oligonucleotides, mast cells were loaded with an anti-MKP-1 morpholino antisense oligonucleotide (5′-GCCAGCACCAGCGACCCCAGCGTG-3′) or a control morpholino-oligonucleotide (5′-CCTCTTACCTCAGTTACAATTTATA-3′) (Gene Tools, Corvallis, OR) by the scrape delivery procedure (Partridge et al., 1996). Briefly, morpholino-oligonucleotides were added to the culture medium at a concentration of 20 µM, and the cells were scraped gently with a rubber blade cell scraper (Gene Tools). The cells were allowed to recover for 16 h before dexamethasone treatment and IgE receptor triggering as described above.
Transfection of cells
Two hundred thousand COS-7 cells in 3.5 cm culture plates were transiently transfected with 1.2 µg of -1716MKP-1luc reporter construct, together with 0.2 µg of GR expression plasmids, either GRwt or dimerization-defective mutant A458Tor hGR(D4X), and 0.3 µg of Renilla luciferase vector as an internal standard, using Fugene6™ (Roche diagnostics, Mannheim, Germany) according to the manufacturer’s instructions. Four hours after transfection, the cells were treated for 48 h with dexamethasone (0.1 µM) or solvent alone. The transfected cells were harvested thereafter and luciferase assay measurements were performed with the dual luciferase assay system of Promega according to the manufacturer’s instructions.
Immunoblots
Cells were lysed in RIPA buffer containing 1 mM phenylmethylsulfonyl fluoride (PMSF), 1 mg/ml leupeptin, 1 mg/ml aprotinin, 1 mM β-glycerophosphate, 1 mM sodium fluoride and 1 mM sodium orthovanadate, and the lysates were clarified by centrifugation at 4°C, 13 000 g for 15 min. Proteins from the cell lysates (50–100 µg) were separated by SDS–PAGE and transferred onto PVDF ImmobilonP membranes (Millipore, Eschborn, Germany). Erk-1 and -2 and Elk-1 phosphorylation were assessed using anti-phosphoMAP kinase and anti-phosphoElk-1 antibodies (New England Biolabs, Frankfurt am Main, Germany). The membranes were stripped and reprobed with phosphorylation state-independent anti-Erk-2 (Santa Cruz Biotechnology, Heidelberg, Germany) or anti-Elk-1 (New England Biolabs) antibodies. MKP-1 expression was assessed with an anti-MKP-1 antibody (M18; Santa Cruz). An anti-Hsp90 antibody (a generous gift from E.E.Baulieu, Paris, France) was used for loading control. The signals were detected by enhanced chemiluminescence (Amersham Pharmacia Biotech) after incubation with the appropriate secondary antibodies.
Gene expression array analysis
RBL-2H3 cells were treated with dexamethasone (0.1 µM) or solvent alone for 5, 16 or 24 h before total RNA extraction using PeqGOLD TriFast™ (Peqlab Biotechnologie, Erlangen, Germany). Poly(A)+ RNA was enriched from total RNA samples by two rounds of affinity chromatography on oligo(dT) cellulose (mRNA Purification kit; Amersham Pharmacia Biotech). A 3 µg aliquot of poly(A)+ RNA was used for first and second strand cDNA synthesis using an oligo(dT) primer harboring a T7 polymerase promoter sequence (Superscript Choice System; Life Technologies, Karlsruhe, Germany). Aliquots of the cDNA were subjected to T7 polymerase in vitro transcription in the presence of biotinyl-UTP and biotinyl-CTP (MEGAscript T7 kit; Ambion, Austin, TX). The biotinylated cRNA obtained was purified from unincorporated nucleotides (RNeasy Mini kit; Qiagen, Hilden, Germany) and subjected to partial alkaline fragmentation. Hybridization using 15 µg of fragmented cRNA to Rat Genome U34A arrays (Affymetrix Inc., Santa Clara, CA) as well as staining and data collection were carried out as previously described (Mahadevappa and Warrington, 1999). The results from two independent experiments were correlated using proprietary software to identify coherent mRNA level changes induced by dexamethasone over several time points.
Northern blots
A 20 µg aliquot of total RNA extracted using PeqGOLD TriFast™ was denatured and fractionated on a 1% agarose gel and transferred onto a Hybond-N+ membrane (Amersham Pharmacia Biotech). The filter was hybridized with randomly primed radioactively labeled cDNA fragments for MKP-1 (clone AI096961; IMAGE Consortium, Resource Center of the German Human Genome Project, Berlin, Germany) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Fort et al., 1985).
In vitro Erk dephosphorylation assay
RBL-2H3 mast cells were treated for 16 h with dexamethasone (0.1 µM), RU486 (1 µM) or solvent alone, in the presence or absence of cycloheximide (5 µg/ml) to prepare treated cell lysates. Untreated cells were sensitized with IgE and activated with DNP-BSA, as described above, to prepare activated cell lysates. The cells were harvested in phosphatase assay buffer (10 mM EDTA, 10 mM EGTA, 50 mM HEPES pH 7.6, 1 mM PMSF, 10 mg/ml leupeptin, 10 mg/ml aprotinin) and lysed by four cycles of freezing in liquid N2–thawing at 4°C, followed by disruption through a 24 gauge needle. The lysates were clarified by centrifugation at 4°C, 13 000 g for 15 min. In some experiments, the lysates from cells treated with dexamethasone or solvent were depleted of MKP-1 by immunoprecipitation with the anti-MKP-1 antibody or isotype control antibody. To assess the dephosphorylation of Erk, two different sources of pre-activated Erk were used: activated cell lysates or rat recombinant purified activated Erk-2 (Calbiochem). Pre-activated Erk (100 µg of total protein from activated cell lysates, or 0.5 ng of rat recombinant purified activated Erk-2) was incubated for 15 min at 30°C in the presence of 100 µg of total protein from treated cell lysates or post-immunoprecipitation supernatants of treated cell lysates. In some experiments, the incubation was performed in the presence of the protein tyrosine phosphatase inhibitor sodium orthovanadate (1 mM). The reactions were stopped by placing the mixtures on ice and adding Laemmli sample buffer. The level of phosphorylation of Erk was assessed by immunoblotting as described above.
Acknowledgments
Acknowledgements
We thank Daniela Schilling (Berlin) for performing the gene expression array sample preparation and hybridization, and Hartwig Hennekes and Heike Schaecke (Berlin) for suggestions and helpful criticisms. O.K. was partly supported by a fellowship from the French–German Science Foundation’s exchange programme (Institut National de la Santé et de la Recherche Médicale–Deutsche Forschungsgemeinschaft). This work was partly supported by a grant from Schering AG, Berlin.
References
- Abbinante-Nissen J.M., Simpson,L.G. and Leikauf,G.D. (1995) Corticosteroids increase secretory leukocyte protease inhibitor transcript levels in airway epithelial cells. Am. J. Physiol., 268, 601–606. [DOI] [PubMed] [Google Scholar]
- Alessi D.R., Smythe,C. and Keyse,S.M. (1993) The human CL100 gene encodes a Tyr/Thr-protein phosphatase which potently and specifically inactivates MAP kinase and suppresses its activation by oncogenic ras in Xenopus oocyte extracts. Oncogene, 8, 2015–2020. [PubMed] [Google Scholar]
- Barnes P.J. (1998) Antiinflammatory action of glucocorticoids: molecular mechanisms. Clin. Sci., 94, 557–572. [DOI] [PubMed] [Google Scholar]
- Beato M., Herrlich,P. and Schutz,G. (1995) Steroid hormone receptors: many actors in search of a plot. Cell, 83, 851–857. [DOI] [PubMed] [Google Scholar]
- Berger S.A., Mak,T.W. and Paige,C.J. (1994) Leukocyte common antigen (CD45) is required for immunoglobulin E-mediated degranulation of mast cells. J. Exp. Med., 180, 471–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brondello J.-M., Pouysségur,J. and McKenzie,F.R. (1999) Reduced MAP kinase phosphatase-1 degradation after p42/p44MAPK-dependent phosphorylation. Science, 286, 2514–2517. [DOI] [PubMed] [Google Scholar]
- Caelles C., González-Sancho,J.M. and Muñoz,A. (1997) Nuclear hormone receptor antagonism with AP-1 by inhibition of the JNK pathway. Genes Dev., 11, 3351–3364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey K.L., Richards,S.A., Lounsbury,K.M. and Macara,I.G. (1996) Evidence using a green fluorescent protein–glucocorticoid receptor chimera that the RAN/TC4 GTPase mediates an essential function independent of nuclear protein import. J. Cell Biol., 133, 985–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cato A.C.B. and Wade,E. (1996) Molecular mechanisms of antiinflammatory action of glucocorticoids. BioEssays, 18, 371–378. [DOI] [PubMed] [Google Scholar]
- Cissel D.S. and Beaven,M.A. (2000) Disruption of Raf-1/heat shock protein 90 complex and Raf signaling by dexamethasone in mast cells. J. Biol. Chem., 275, 7066–7070. [DOI] [PubMed] [Google Scholar]
- Dorfman K., Carrasco,D., Gruda,M., Ryan,C., Lira,S.A. and Bravo,R. (1996) Disruption of the erp/mkp-1 gene does not affect mouse development: normal MAP kinase activity in ERP/MKP-1-deficient fibroblasts. Oncogene, 13, 925–931. [PubMed] [Google Scholar]
- Eklund K.K., Humphries,D.E., Xia,Z., Ghildyal,N., Friend,D.S., Gross,V. and Stevens,R.L. (1997) Glucocorticoids inhibit the cytokine-induced proliferation of mast cells, the high affinity IgE receptor-mediated expression of TNF-α and the IL-10-induced expression of chymases. J. Immunol., 158, 4373–4380. [PubMed] [Google Scholar]
- Fort P., Marty,L., Piechaczyk,M., el Sabrouty,S., Dani,C., Jeanteur,P. and Blanchard,J.M. (1985) Various rat adult tissues express only one major mRNA species from the glyceraldehyde-3-phosphate-dehydrogenase multigenic family. Nucleic Acids Res., 13, 1431–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrington T.P. et al. (2000) MEKK2 gene disruption causes loss of cytokine production in response to IgE and c-Kit ligand stimulation of ES cell-derived mast cells. EMBO J., 19, 5387–5395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gewert K., Hiller,G. and Sundler,R. (2000) Effects of dexamethasone on mitogen-activated protein kinases in mouse macrophages: implications for the regulation of 85 kDa cytosolic phospholipase A(2). Biochem. Pharmacol., 60, 545–551. [DOI] [PubMed] [Google Scholar]
- González M.V., Gonzalez-Sancho,J.M., Caelles,C., Munoz,A. and Jimenez,B. (1999) Hormone-activated nuclear receptors inhibit the stimulation of the JNK and ERK signalling pathways in endothelial cells. FEBS Lett., 459, 272–276. [DOI] [PubMed] [Google Scholar]
- Groom L.A., Sneddon,A.A., Alessi,D.R., Dowd,S. and Keyse,S.M. (1996) Differential regulation of the MAP, SAP and RK/p38 kinases by Pyst1, a novel cytosolic dual-specificity phosphatase. EMBO J., 15, 3621–3632. [PMC free article] [PubMed] [Google Scholar]
- Heck S., Kullmann,M., Gast,A., Ponta,H., Rahmsdorf,H.J., Herrlich,P. and Cato,A.C.B. (1994) A distinct modulating domain in glucocorticoid receptor monomers in the repression of activity of the transcription factor AP-1. EMBO J., 13, 4087–4095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirasawa N., Santini,F. and Beaven,M.A. (1995) Activation of the mitogen-activated protein kinase/cytosolic phospholipase A2 pathway in a rat mast cell line. J. Immunol., 154, 5391–5402. [PubMed] [Google Scholar]
- Hirasawa N., Sato,Y., Fujita,Y., Mue,S. and Ohuchi,K. (1998) Inhibition by dexamethasone of antigen-induced c-Jun N-terminal kinase activation in rat basophilic leukemia cells. J. Immunol., 161, 4939–4943. [PubMed] [Google Scholar]
- Hulley P.A., Gordon,F. and Hough,F.S. (1998) Inhibition of mitogen-activated protein kinase activity and proliferation of an early osteoblast cell line (MBA 15.4) by dexamethasone: role of protein phosphatases. Endocrinology, 139, 2423–2431. [DOI] [PubMed] [Google Scholar]
- Kimata M., Inagaki,N., Kato,T., Miura,T., Serizawa,I. and Nagai,H. (2000) Roles of mitogen-activated protein kinase pathways for mediator release from human cultured mast cells. Biochem. Pharmacol., 60, 589–594. [DOI] [PubMed] [Google Scholar]
- Kitaura J., Asai,K., Maeda-Yamamoto,M., Kawakami,Y., Kikkawa,U. and Kawakami,T. (2000) Akt-dependent cytokine production in mast cells. J. Exp. Med., 192, 729–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- König H., Ponta,H., Rahmsdorf,H.J. and Herrlich,P. (1992) Interference between pathway-specific transcription factors: glucocorticoids antagonize phorbol ester-induced AP-1 activity without altering AP-1 site occupancy in vivo. EMBO J., 11, 2241–2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasa M., Brook,M., Saklatvala,J. and Clark,A.R. (2001) Dexamethasone destabilizes cyclooxygenase 2 mRNA by inhibiting mitogen-activated protein kinase p38. Mol. Cell. Biol., 21, 771–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Van T.D., Behr,F.D., Adkins,K.K., Miesfeld,R.L. and Bloom,J.W. (1997) Glucocorticoid receptor signaling in a bronchial epithelial cell line. Am. J. Physiol., 272, L838–L843. [DOI] [PubMed] [Google Scholar]
- Lienard H., Bruhns,P., Malbec,O., Fridman,W.H. and Daeron,M. (1999) Signal regulatory proteins negatively regulate immunoreceptor-dependent cell activation. J. Biol. Chem., 274, 32493–32499. [DOI] [PubMed] [Google Scholar]
- Mahadevappa M. and Warrington,J.A. (1999) A high-density probe array sample preparation method using 10- to 100-fold fewer cells. Nature Biotechnol., 17, 1134–1136. [DOI] [PubMed] [Google Scholar]
- Nechushtan H. and Razin,E. (1998) Deciphering the early-response transcription factor network in mast cells. Trends Immunol., 19, 441–444. [DOI] [PubMed] [Google Scholar]
- Nissen R.M. and Yamamoto,K.R. (2000) The glucocorticoid receptor inhibits NFκB by interfering with serine-2 phosphorylation of the RNA polymerase II carboxy-terminal domain. Genes Dev., 14, 2314–2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi T., Metz,R., Chen,L., Mattéi,M.-G., Carrasco,D. and Bravo,R. (1993) Structure, mapping and expression of erp, a growth factor-inducible gene encoding a nontransmembrane protein tyrosine phosphase and effect of erp on cell growth. Mol. Cell. Biol., 13, 5195–5205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partridge M., Vincent,A., Matthews,P., Puma,J., Stein,D. and Summerton,J. (1996) A simple method for delivering morpholino antisense oligos into the cytoplasm of cells. Antisense Nucleic Acid Drug Dev., 6, 169–175. [DOI] [PubMed] [Google Scholar]
- Rider L.G., Hirasawa,N., Santini,F. and Beaven,M.A. (1996) Activation of the mitogen-activated protein kinase cascade is suppressed by low concentrations of dexamethasone in mast cells. J. Immunol., 157, 2374–2380. [PubMed] [Google Scholar]
- Sewell W.A., Scurr,L.L., Orphanides,H., Kinder,S. and Ludowyke,R.I. (1998) Induction of interleukin-4 and interleukin-5 expression in mast cells is inhibited by glucocorticoids. Clin. Diagn. Lab. Immunol., 5, 18–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stassen M., Müller,C., Arnold,M., Hültner,L., Klein-Hessling,S., Neudörfl,C., Reineke,T., Serfling,E. and Schmitt,E. (2001) IL-9 and IL-13 production by activated mast cells is strongly enhanced in the presence of lipopolysaccharide: NF-κB is decisively involved in the expression of IL-9. J. Immunol., 166, 4391–4398. [DOI] [PubMed] [Google Scholar]
- Sun H., Charles,C.H., Lau,L.F. and Tonks,N.K. (1993) MKP-1 (3CH134), an immediate early gene product, is a dual specificity phosphatase that dephosphorylates MAP kinase in vivo. Cell, 75, 487–493. [DOI] [PubMed] [Google Scholar]
- Swantek J.L., Cobb,M.H. and Geppert,T.D. (1997) Jun-N-terminal kinase/stress-activated protein kinase (JNK/SAPK) is required for lipopolysaccharide stimulation of tumor necrosis factor α (TNF-α) translation: glucocorticoids inhibit TNF-α translation by blocking JNK/SAPK. Mol. Cell. Biol., 17, 6274–6282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swieter M., Berenstein,E.H., Swaim,W.D. and Siraganian,R.P. (1995) Aggregation of IgE receptors in rat basophilic leukemia 2H3 cells induces tyrosine phosphorylation of the cytosolic protein-tyrosine phosphatase HePTP. J. Biol. Chem., 270, 21902–21906. [DOI] [PubMed] [Google Scholar]
- Torres C., Li,M., Walter,R. and Sierra,F. (2000) Modulation of the ERK pathway of signal transduction by cysteine proteinase inhibitors. J. Cell. Biochem., 80, 11–23. [DOI] [PubMed] [Google Scholar]
- Turner H. and Kinet,J.-P. (1999) Signalling through the high-affinity IgE receptor FcεRI. Nature, 402, B24–B30. [DOI] [PubMed] [Google Scholar]
- Webster M.K., Goya,L., Ge,Y., Maiyar,A.C. and Firestone,G.L. (1993) Characterization of sgk, a novel member of the serine/threonine protein kinase gene family which is transcriptionally induced by glucocorticoids and serum. Mol. Cell. Biol., 13, 2031–2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z.-H., Zhang,J. and Siraganian,R.P. (2000) Positive regulation of c-Jun N-terminal kinase and TNF-α production but not histamine release by SHP-1 in RBL-2H3 mast cells. J. Immunol., 164, 1521–1528. [DOI] [PubMed] [Google Scholar]
- Young J.D., Lawrence,A.J., MacLean,A.G., Leung,B.P., McInnes,I.B., Canas,B., Pappin,D.J.C. and Stevenson,R.D. (1999) Thymosin β4 sulfoxide is an anti-inflammatory agent generated by monocytes in the presence of glucocorticoids. Nature Med., 5, 1424–1427. [DOI] [PubMed] [Google Scholar]
- Zhang C., Baumgartner,R.A., Yamada,K. and Beaven,M.A. (1997) Mitogen-activated protein (MAP) kinase regulates production of tumor necrosis factor-α and release of arachidonic acid in mast cells. J. Biol. Chem., 272, 13397–13402. [DOI] [PubMed] [Google Scholar]