Abstract
Vrp1 (verprolin, End5) is a Saccharomyces cerevisiae actin-associated protein and is related to mammalian Wiskott–Aldrich syndrome protein (WASP)-interacting protein (WIP). Vrp1-deficient (vrp1Δ) cells are inviable at high temperature, have partially depolarized cortical actin patches and have defects in both actomyosin ring-dependent and Hof1 (Cyk2)-dependent pathways of cytokinesis. We demonstrate here that N-Vrp11–364 and C-Vrp1364–817 are each sufficient to restore viability, actomyosin ring constriction and Hof1 localization at 37°C to vrp1Δ. C-Vrp1, like Vrp1, partially co-localizes with cortical actin patches and restores actin patch polarization to vrp1Δ. Cortical localization of C-Vrp1, but not Vrp1, requires Las17. N-Vrp1 exhibits diffuse cytoplasmic localization and functions in cytokinesis without efficiently restoring polarization of cortical actin patches. N-Vrp1 function is not abolished by mutations affecting the WASP homology 2 (WH2) [verprolin homology (V)] actin-binding domain. N-Vrp1 may function through the type I myosins and actin, while C-Vrp1 may function through both Las17 (Bee1) and type I myosins. The functions of Vrp1 in viability at 37°C and cytokinesis do not require efficient localization to, and function in, the cortical actin cytoskeleton.
Keywords: CAAX box/Cdc15/cell polarity/PSTPIP/Wiskott–Aldrich syndrome
Introduction
In Saccharomyces cerevisiae, the cortical actin cytoskeleton takes the form of F-actin patches and these undergo characteristic changes in distribution through the cell cycle. Actin patches polarize into small emerging buds, are randomly dispersed in the mother and large bud during mitosis, and upon exit from mitosis repolarize to the neck region where they are thought to function in cytokinesis (Adams and Pringle, 1984; Lew and Reed, 1993; reviewed in Winsor and Schiebel, 1997). In addition, F-actin is recruited into a type II myosin (Myo1p)-containing ring at the bud neck in late anaphase. This actomyosin ring undergoes a contraction-like event during cytokinesis and then disappears (Bi et al., 1998; Lippincott and Li, 1998a).
Vrp1p is an S.cerevisiae proline-rich actin-associated protein related to mammalian Wiskott–Aldrich syndrome protein (WASP)-interacting protein (WIP) (Donnelly et al., 1993; Vaduva et al., 1997; 1999; Naqvi et al., 1998). Loss of Vrp1p leads to a partial loss of cortical actin patch polarization during the cell cycle (Donnelly et al., 1993). Moreover, Vrp1p localizes to cortical patches which partially co-localize with cortical actin patches (Vaduva et al., 1997; Naqvi et al., 1998).
Vrp1p interacts with actin through residues 1–70 which contain a WASP homology 2 (WH2)/verprolin homology (V) domain including a single actin-binding motif (KLKKAET) (Vaduva et al., 1997). WH2/V domains are also found in WIP and other mammalian actin monomer-binding proteins such as members of the WASP/Scar/WAVE family (Vaduva et al., 1999; Welch, 1999; Higgs and Pollard, 2001; Martinez-Quiles et al., 2001). The WH2/V domain in WASP/Scar/WAVE family proteins is required for activation of the Arp2/3p complex (Machesky and Insall, 1998). Mutation of the KLKKAET motif in Vrp1p abolishes interaction with actin, and deletion of residues 1–70 has been reported to abolish function (Vaduva et al., 1997). Similarly, the actin-binding motif of human WIP has been reported to be essential for complementation of vrp1-1 (Vaduva et al., 1999).
The C-terminal fragment of Vrp1p interacts with Las17p, the S.cerevisiae homologue of mammalian WASP (Naqvi et al., 1998; Madania et al., 1999; Evangelista et al., 2000), while multiple fragments of Vrp1p have been shown to interact with the SH3 domains of type I myosins Myo3p and Myo5p (Anderson et al., 1998; Evangelista et al., 2000). Both Las17p and the type I myosins localize to cortical patches which partially co-localize with cortical actin patches during the cell cycle, and localization of type I myosins has been shown to be dependent on Vrp1p (Goodson et al., 1996; Li, 1997; Anderson et al., 1998). Both Las17p and type I myosins have a general role in actin patch polarization (Goodson et al., 1996; Karpova et al., 1998) and actin filament assembly through activation of the yeast Arp2/3p complex (Winter et al., 1999; Evangelista et al., 2000; Geli et al., 2000; Lechler et al., 2000). Type I myosins, unlike Las17p, lack a WH2/V domain, and Vrp1p may thus facilitate type I myosin function by supplying actin monomers (Geli et al., 2000).
Vrp1p-deficient cells (vrp1Δ) are viable at 24°C, but not at 37°C (Donnelly et al., 1993), and loss of viability at 37°C is associated with a tight block in cytokinesis and defects in both actomyosin ring constriction and localization of the cytokinesis-specific Hof1p protein to a single ring at the bud neck (Naqvi et al., 2001). Hof1p is the S.cerevisiae homologue of Schizosaccharomyces pombe Cdc15p and is related to the mammalian cleavage furrow protein, proline serine threonine phosphatase-interacting protein (PSTPIP) (Kamei et al., 1998; Lippincott and Li, 1998b; Vallen et al., 2000).
Cell division in S.cerevisiae does not absolutely require the function of the Myo1p-containing actomyosin ring (Bi et al., 1998; Lippincott and Li, 1998a). In the absence of an actomyosin ring (e.g. in myo1Δ) or when actomyosin ring constriction is prevented (e.g. in bni1Δ), another pathway of cytokinesis, which is redundant with the Myo1p pathway and probably involves directed secretion to the bud neck, can mediate septation. This alternative pathway relies on Hof1p function, since loss of both actomyosin ring function and Hof1p function (e.g. in myo1Δ hof1Δ or bni1Δ hof1Δ double mutants) leads to inviability (Vallen et al., 2000). The independence of these two pathways is indicated by the fact that Hof1p relocalization to a single ring at the bud neck does not require Myo1p and that Myo1p ring constriction does not require Hof1p (Vallen et al., 2000).
The subcellular localization of Vrp1p and its role in cortical actin patch polarization suggest that its vital functions may be in cortical actin patches (Vaduva et al., 1997; Smith et al., 2001). Defective cytokinesis may be a consequence of aberrant actin patch polarization. The possibility remains, however, that the functions of Vrp1p in cytokinesis and in cortical actin patches are independent. Here we have addressed this question by demonstrating that both N-Vrp1p1–364 and C-Vrp1p364–817 are sufficient to replace Vrp1p in actomyosin ring constriction and Hof1p localization and, moreover, that N-Vrp1p functions in cytokinesis without either efficiently localizing to cortical actin patches nor fully restoring their polarization. C-Vrp1p localizes to the cortex and restores full cortical actin patch polarization, and localization requires Las17p. The localization and various functions of Vrp1p (and the cytokinesis functions of N-Vrp1p) do not require the WH2/V domain. Both Las17p and type I myosins are important for Hof1p localization to the bud neck during cytokinesis, suggesting a possible general requirement for Arp2/3p-dependent actin polymerization in this process.
Results
N- and C-terminal fragments of Vrp1p can substitute for full-length Vrp1p in restoration of viability at 37°C
During mutational analysis of plasmid-borne VRP1, we noticed that all six mutations we isolated which affect Vrp1p function cause premature chain termination and map to the first 120 codons of VRP1. Amino acid substitution mutations, which were also present in most of these mutagenized plasmids, did not confer phenotypes after separation from the terminator mutations. Further more, extensive site-directed mutagenesis of charged amino acid clusters in plasmid-borne VRP1 did not yield noticeable phenotypes (data not shown).
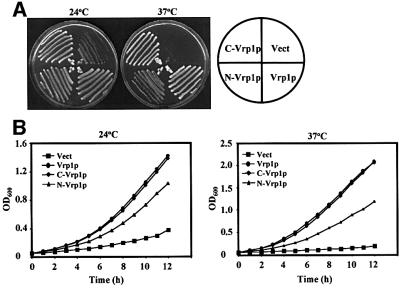
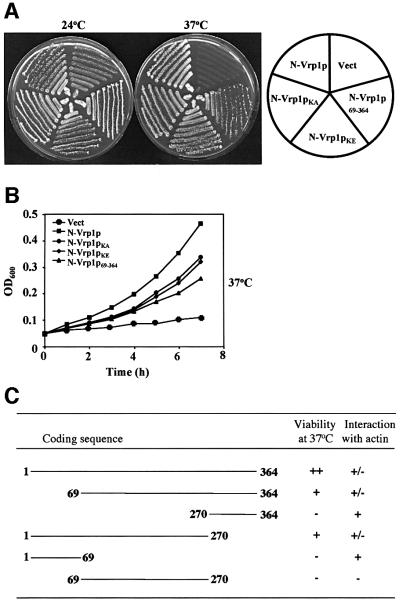
Interestingly, subsequent testing of the ability of individual fragments of Vrp1p to rescue the defects of vrp1Δ revealed that the N-terminal (1–364) (N-Vrp1p) and C-terminal (364–817) (C-Vrp1p) fragments are each functional. Centromeric plasmids expressing either N-Vrp1p or C-Vrp1p from the endogenous VRP1 promoter, but not empty vector, were each able to improve viability at 24°C significantly and restore viability at 37°C when introduced into vrp1Δ. Restoration of viability by N-Vrp1p and C-Vrp1p was observed both on solid (Figure 1A) and in liquid (Figure 1B) media. C-Vrp1p was significantly more efficient than N-Vrp1p in each case and restored a doubling time to vrp1Δ comparable with that of full-length Vrp1p (Figure 1B).
Fig. 1. Both N-Vrp1p and C-Vrp1p restore viability to vrp1Δ. (A) Viability at 24 and 37°C of vrp1Δ (AMY88) carrying YCplac111 vector (Vect), pAM228 (Vrp1p), pAM232 (N-Vrp1p) and pAM236 (C-Vrp1p). Each strain was streaked for single colonies on YPUAD solid medium, incubated at either 24 or 37°C, and photographed after 3 days. (B) Growth rate of vrp1Δ (AMY88) carrying YCplac111 vector (Vect), pAM228 (Vrp1p), pAM232 (N-Vrp1p) or pAM236 (C-Vrp1p). A YPUAD culture of each strain was grown at 24°C, diluted to an OD600 of 0.05 in fresh YPUAD medium and incubated at either 24 or 37°C. The OD600 was monitored at 1 h intervals.
The complementation of vrp1Δ by N-Vrp1p and C-Vrp1p is not due to the spontaneous appearance of suppressor mutations in our vrp1Δ strain. We observed strong complementation by N-Vrp1p or C-Vrp1p, but not by the empty vector, in many experiments involving the use of more than one vrp1Δ mutant strain. Comple mentation is also not dependent on the genetic background, since similar results were obtained using three other vrp1Δ strains: Y15246 (from Euroscarf, Frankfurt, Germany), T65-1D (Zoladek et al., 1995) and vrp1Δ derivatives of YEF1986 (Vallen et al., 2000) and YEF1698 (Bi et al., 1998) constructed in our laboratory (data not shown).
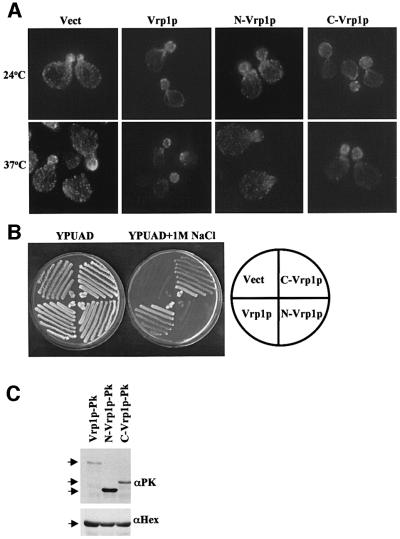
Further analysis revealed that C-Vrp1p is able to restore full polarization of cortical actin patches to vrp1Δ, while N-Vrp1p restores viability without correcting this defect efficiently (Figure 2A, Table I). That N-Vrp1p is not able to correct the cytoskeletal defects of vrp1Δ efficiently was supported further by the observation that the sensitivity of vrp1Δ strains to 1 M NaCl at 24°C was not corrected by N-Vrp1p, although, in contrast, C-Vrp1p was able to correct this defect (Figure 2B).
Fig. 2. C-Vrp1p, but not N-Vrp1p, restores full polarization of cortical actin patches to vrp1Δ. (A) Cortical actin patch polarization of vrp1Δ (AMY88) carrying YCplac111 vector (Vect), pAM228 (Vrp1p), pAM232 (N-Vrp1p) or pAM236 (C-Vrp1p). Cells were grown in YPUAD to exponential phase at 24°C and then either left at 24°C or shifted to 37°C for 2 h. The cells were fixed, permeabilized and F-actin stained with Alexa-488-conjugated phalloidin. Stained cells were viewed by fluorescence microscopy. Fields containing small-budded cells are shown to compare the polarization of cortical actin patches at this stage of the cell cycle. All panels are at equal magnification. (B) Salt sensitivity of vrp1Δ (AMY88) carrying YCplac111 vector (Vect), pAM228 (Vrp1p), pAM232 (N-Vrp1p) or pAM236 (C-Vrp1p). Each strain was streaked for single colonies on YPUAD solid medium containing 1 M sodium chloride, incubated at 24°C and photographed after 5 days. (C) Total protein extracts from vrp1Δ (AMY88) carrying pAM249 (Vrp1p-Pk), pAM250 (N-Vrp1p-Pk) or pAM251 (C-Vrp1p-Pk) after growth to exponential phase in YPUAD at 24°C were resolved by SDS–PAGE, transferred to a PVDF membrane and immunoblotted with anti-Pk antisera (αPK). We also immunoblotted with α-hexokinase as a loading control (αHex). The band intensities were quantified and full-length Vrp1p-Pk was normalized to 1. C-Vrp1p-Pk was 3.5 while N-Vrp1p-Pk was 12.7.
Table I. Actin patch polarization in vrp1Δ cells carrying vector, or plasmids expressing various forms of Vrp1p.
| Plasmids | 24°C |
37°C |
||
|---|---|---|---|---|
| Fully polarized | Depolarized | Fully polarized | Depolarized | |
| Vector | 1 | 99 | 0 | 100 |
| Vrp1p | 94 | 6 | 90.5 | 9.5 |
| Vrp1pKA | 91.5 | 8.5 | 92.5 | 7.5 |
| Vrp1pKE | 93.5 | 6.5 | 91.5 | 8.5 |
| Vrp1p69–817 | 92.5 | 7.5 | 91 | 9 |
| C-Vrp1p | 86.5 | 13.5 | 61.5 | 38.5 |
| N-Vrp1p | 17.5 | 82.5 | 19 | 81 |
| N-Vrp1p-CAAX | 37 | 63 | 41 | 59 |
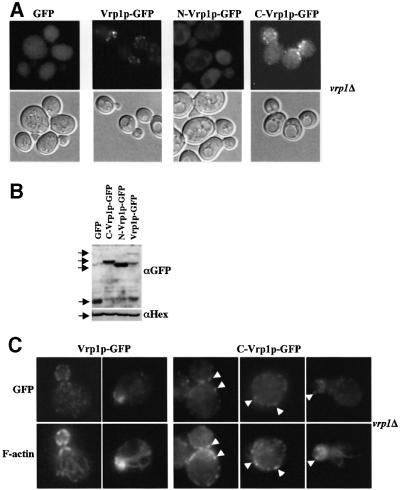
The difference in functionality between N-Vrp1p and C-Vrp1p is not due to differences in expression level or protein stability. Equivalent plasmids expressing Pk epitope-tagged full-length Vrp1p, N-Vrp1p or C-Vrp1p were introduced into vrp1Δ. Immunoblotting of extracts showed that N-Vrp1p-Pk is significantly more abundant than C-Vrp1p-Pk, and that C-Vrp1p-Pk is slightly more abundant than full-length Vrp1p-Pk in vivo (Figure 2C). Similar results were obtained with green fluorescent protein (GFP)-tagged constructs (Figure 6B).
Fig. 6. C-Vrp1p localizes to cortical patches which partially co-localize with actin patches. (A) vrp1Δ (AMY88) carrying pAM237 (GFP), pAM162 (Vrp1p–GFP), pAM239 (N-Vrp1p–GFP) or pAM241 (C-Vrp1p–GFP) were grown in YPUAD to exponential phase at 24°C and GFP was visualized in living cells by fluorescence microscopy. Upper panels: FITC fluorescence optics. Lower panels: DIC optics. All panels are at equal magnification. (B) Total protein extracts from vrp1Δ (AMY88) carrying pAM237 (GFP), pAM162 (Vrp1p–GFP), pAM239 (N-Vrp1p–GFP) or pAM241 (C-Vrp1p–GFP) after growth to exponential phase in YPUAD at 24°C were resolved by SDS–PAGE, transferred to a PVDF membrane and immunoblotted with anti-GFP antisera (αGFP). We also immunoblotted with α-hexokinase as a loading control (αHex). (C) vrp1Δ (AMY88) carrying pAM162 (Vrp1p–GFP) or pAM241 (C-Vrp1p–GFP) were grown in YPUAD to exponential phase at 24°C, fixed, permeabilized and GFP was visualized by immunofluorescence (GFP). Cortical actin patches were visualized in the same cells by staining with Alexa-488-conjugated phalloidin (Actin). Stained cells were viewed by fluorescence microscopy using FITC- and rhodamine-specific light filters. Arrowheads: Vrp1p–GFP and C-Vrp1p–GFP patches which co-localize with cortical actin patches. All panels are at equal magnification.
N-Vrp1p and C-Vrp1p each function in cytokinesis by restoring actomyosin ring constriction and Hof1p relocalization to a single ring at 37°C
We next tested the ability of the Vrp1p fragments to rescue the actomyosin ring constriction and Hof1p localization defects at 37°C of vrp1Δ (Naqvi et al., 2001). Plasmids expressing full-length Vrp1p, N-Vrp1p, C-Vrp1p or the empty vector were introduced into vrp1Δ strains expressing either a functional Myo1p–GFP fusion protein or a functional Hof1p–GFP fusion protein. The Myo1p–GFP and Hof1p–GFP fusion proteins were visualized by fluorescence microscopy in living cells after shifting to 37°C for 3 h (Figure 3A and B, respectively, and Table II). The majority of Myo1p–GFP rings appeared uncontracted at 37°C in vrp1Δ carrying empty vector, as previously reported (Naqvi et al., 2001). Myo1p–GFP ring contraction at 37°C was restored by expression of either N-Vrp1p, C-Vrp1p or full-length Vrp1p (Figure 3A, Table II). Full-length Vrp1p and C-Vrp1p were approximately equally efficient, and both were more efficient than N-Vrp1p (Table II).
Fig. 3. N-Vrp1p and C-Vrp1p each function in both actomyosin ring constriction and Hof1p localization to a single ring at the bud neck prior to cytokinesis. (A) Actomyosin ring constriction in vrp1Δ expressing a functional Myo1p–GFP fusion protein (AMY114) and carrying YCplac111 vector (Vect), pAM228 (Vrp1p), pAM232 (N-Vrp1p) or pAM236 (C-Vrp1p). Cells were grown in YPUAD to exponential phase at 24°C and shifted to 37°C for 3 h. GFP was visualized in living cells by fluorescence microscopy. The arrows point to constricted Myo1p–GFP rings present at the neck of large-budded cells. Upper panels: fluorescence optics. Lower panels: DIC optics. All panels are at equal magnification. (B) Hof1p localization to a single ring at the bud neck at 37°C in vrp1Δ expressing a functional Hof1p–GFP fusion protein (AMY147) and carrying YCplac111 vector (Vect), pAM228 (Vrp1p), pAM232 (N-Vrp1p) or pAM236 (C-Vrp1p). Cells were grown in YPUAD to exponential phase at 24°C and shifted to 37°C for 3 h. GFP was visualized in living cells by fluorescence microscopy. Arrows: Hof1p–GFP single rings present at the neck of large-budded cells. Upper panels: FITC fluorescence optics. Lower panels: DIC optics. All panels are at equal magnification.
Table II. Hof1p–GFP relocalization and Myo1p–GFP ring constriction in large-budded vrp1Δ cells carrying vector, or plasmids expressing Vrp1p, N-Vrp1p, C-Vrp1p or N-Vrp1p-CAAX.
| Vector | Vrp1p | N-Vrp1p | C-Vrp1p | N-Vrp1p-CAAX | |
|---|---|---|---|---|---|
| Hof1p–GFP | |||||
| Single ring | NDa | 59 | 32 | 61 | 51 |
| Double ring | NDa | 41 | 68 | 39 | 49 |
| Myo1p–GFP | |||||
| Constricted | 1 | 47.5 | 33 | 52 | 65.5 |
| Unconstricted | 99 | 52.5 | 67 | 48 | 34.5 |
aMost cells had diffuse cytoplasmic fluorescence after shifting to 37°C for 3 h.
In this vrp1Δ strain (AMY147), we observed stronger Hof1p mislocalization than we previously reported for RH2892 (Naqvi et al., 2001) (Figure 3B). In AMY147, we found mainly diffuse Hof1p–GFP fluorescence in large-budded cells after 3 h at 37°C, and in only very few large-budded cells did we observe Hof1p–GFP in two rings at the bud neck. In agreement with our previous report (Naqvi et al., 2001), however, we observed no cells with Hof1p–GFP single rings under these conditions. Hof1p– GFP localization to double rings and subsequent relocalization to single rings at the bud neck was restored by expression of N-Vrp1p, C-Vrp1p or full-length Vrp1p, but not by empty vector (Figure 3B, Table II). Full-length Vrp1p and C-Vrp1p were approximately equally efficient, and both were more efficient than N-Vrp1p (Table II).
The functions of N-Vrp1p in cytokinesis do not require the conserved WH2/V domain containing the actin-binding motif
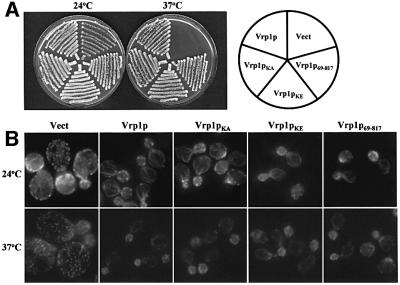
The finding that C-Vrp1p is fully functional suggests that the N-terminal actin-binding motif (KLKKAET) in the WH2/V domain may not be essential, in disagreement with a previous report (Vaduva et al., 1997). To re-examine the importance of the actin-binding motif, we made a construct expressing full-length Vrp1p in which K45 and K46 of the KLKKAET motif are substituted with alanine (Vrp1pKA) or glutamate (Vrp1pKE). Previously, substitution of K45 and K46 with glutamate was shown to abolish two-hybrid interaction between a fragment comprising residues 1–70 of Vrp1p and actin (Vaduva et al., 1997). We also detected a two-hybrid interaction between this fragment of Vrp1p and actin. Also consistent with the findings of Vaduva et al. (1997), this interaction was abolished by substitution of K45 and K46 (data not shown). Both Vrp1pKA and Vrp1pKE were analysed for their ability to restore viability (Figure 4A) and full polarization of cortical actin patches (Figure 4B) to vrp1Δ. Neither mutation significantly affected the function of Vrp1p in these processes. Previously, a mutant Gal4– Vrp1p fusion protein in which Vrp1p residues 1–70 were deleted was found to be unable to restore viability to vrp1-1 (Vaduva et al., 1997). We made a construct expressing residues 69–817 of Vrp1p (Vrp1p69–817) which is similar to the construct tested by Vaduva et al. (1997) except that in our construct VRP1 was not fused to GAL4. Vrp1p69–817 was as functional as full-length Vrp1p in restoring viability at 37°C and actin patch polarization to vrp1Δ (Figure 4A and B).
Fig. 4. The conserved WH2/V domain of Vrp1p is not essential for viability at 37°C or cortical actin patch polarization. (A) Viability at 24 and 37°C of vrp1Δ (AMY88) carrying YCplac111 vector (Vect), pAM228 (Vrp1p), pAM229 (Vrp1pKA), pAM230 (Vrp1pKE) or pAM231 (Vrp1p69–817). Each strain was streaked for single colonies on YPUAD solid medium, incubated at either 24 or 37°C and photographed after 3 days. (B) Cortical actin patch polarization in vrp1Δ (AMY88) carrying YCplac111 vector (Vect), pAM228 (Vrp1p), pAM229 (Vrp1pKA), pAM230 (Vrp1pKE) or pAM231 (Vrp1p69–817). Cells were grown in YPUAD to exponential phase at 24°C and either left at 24°C or shifted to 37°C for 2 h. The cells were fixed, permeabilized and F-actin stained with Alexa-488-conjugated phalloidin. Stained cells were viewed by fluorescence microscopy. Fields containing small-budded cells are shown to compare the polarization of cortical actin patches. All panels are at equal magnification.
We next tested whether the KLKKAET actin-binding motif is essential for the functions of N-Vrp1p. Centro meric plasmids expressing forms of N-Vrp1p with the K45A K46A (N-Vrp1pKA) or K45E K46E (N-Vrp1pKE) actin-binding site mutations or with residues 1–68 deleted (Vrp1p69–364) were constructed and tested for their ability to restore viability to vrp1Δ at 24 and 37°C on solid medium (Figure 5A) and in liquid medium (Figure 5B). The K45A K46A and K45E K46E mutations did not significantly affect the function of N-Vrp1p. Deletion of residues 1–68 of N-Vrp1p, while not completely abolishing function, may have caused some loss of activity compared with the other mutations (Figure 5B). Thus, the WH2/V domain is not required, even for the function of the N-Vrp1p fragment.
Fig. 5. The conserved WH2/V domain is not essential for the function of N-Vrp1p in restoring viability at 37°C. (A) Viability at 24 and 37°C of vrp1Δ (AMY88) carrying YCplac111 vector (Vect), pAM232 (N-Vrp1p), pAM233 (N-Vrp1pKA), pAM234 (N-Vrp1pKE) or pAM235 (Vrp1p69–364). Each strain was streaked for single colonies on YPUAD solid medium, incubated at either 24 or 37°C and photographed after 3 days. (B) Growth rate of vrp1Δ (AMY88) carrying YCplac111 vector (Vect), pAM232 (N-Vrp1p), pAM233 (N-Vrp1pKA), pAM233 (N-Vrp1pKE) or pAM235 (Vrp1p69–364). A YPUAD culture of each strain was grown at 24°C, diluted to an OD600 of 0.05 in fresh YPUAD medium and incubated at 37°C. The OD600 was monitored at 1 h intervals. (C) Fragments of N-Vrp1p were tested for complementation of vrp1Δ (AMY88) and two-hybrid interaction with actin.
We next carried out a more extensive deletion analysis on N-Vrp1p to identify sequences that are important for its function, as assayed by formation of colonies at 37°C (Figure 5C). Like deletion of residues 1–68, deletion of residues 270–364 did not abolish function, but may have reduced the activity of N-Vrp1p somewhat. A combination of both the 1–68 and 270–364 deletions, in contrast, did completely abrogate colony formation at 37°C. This result suggests that several regions of N-Vrp1p may have the ability to contribute, perhaps in similar ways, to the function of this fragment.
C-Vrp1p localizes to cortical patches which partially co-localize with actin patches in a mechanism dependent on Las17p, while N-Vrp1p does not localize
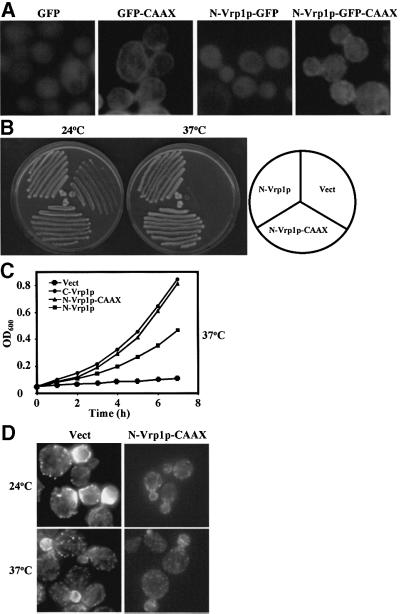
To investigate whether either fragment of Vrp1p contains sufficient information for localization to cortical patches, we tagged full-length Vrp1p, N-Vrp1p and C-Vrp1p with GFP and expressed them from the VRP1 promoter carried on a centromeric plasmid. As a control, we also made a construct expressing GFP under the control of the VRP1 promoter. The GFP-tagged Vrp1p fragments, but not GFP itself, were partially functional in complementation tests when expressed in vrp1Δ (data not shown). Live cell imaging showed that both full-length Vrp1p–GFP and C-Vrp1p–GFP localized predominantly to punctate cortical structures, while N-Vrp1p–GFP showed a diffuse cytoplasmic fluorescence pattern similar to that of GFP alone (Figure 6A). Lack of strong cortical localization of N-Vrp1p–GFP is not due to poor expression or instability of this fusion protein. Immunoblotting experiments showed that N-Vrp1p–GFP is expressed at somewhat higher levels than C-Vrp1p–GFP, and C-Vrp1p–GFP is expressed at somewhat higher levels than full-length Vrp1p–GFP (Figure 6B). Immunofluorescence localization revealed that C-Vrp1p–GFP, like full-length Vrp1p– GFP, partially co-localizes with cortical actin patches (Figure 6C).
Next, we tested whether full-length Vrp1p–GFP or C-Vrp1p–GFP require Las17p for localization to cortical patches and for partial co-localization with cortical actin patches. Full-length Vrp1p–GFP localized efficiently to the cortex in Las17p-deficient cells (Figure 7A) and still partially co-localized with cortical F-actin patches, although these patches were delocalized (Figure 7B). In contrast, C-Vrp1p–GFP exhibited only diffuse cytoplasmic fluorescence similar to GFP alone (Figure 7A) in cells lacking Las17p. The region comprising the C-terminal 36 residues of Vrp1p interacts with Las17p (Madania et al., 1999). Deletion of this region (residues 760–817) in C-Vrp1p completely abolished its ability to complement vrp1Δ (data not shown). This suggests that the function of C-Vrp1p requires interaction with Las17p.
Fig. 7. Localization of C-Vrp1p, but not full-length Vrp1p, to cortical patches requires Las17p. (A) las17Δ (IDY166) carrying pAM237 (GFP), pAM162 (Vrp1p–GFP) or pAM241 (C-Vrp1p–GFP) were grown in YPUAD to exponential phase at 24°C and GFP fluorescence was visualized in living cells by fluorescence microscopy. Upper panels: FITC fluorescence optics. Lower panels: DIC optics. All panels are at equal magnification. (B) las17Δ (IDY166) carrying pAM162 (Vrp1p–GFP) was grown in YPUAD to exponential phase at 24°C, fixed, permeabilized and GFP was visualized by immunofluorescence (GFP). Cortical actin patches were visualized in the same cells by staining with Alexa-488-conjugated phalloidin (Actin). Stained cells were viewed by fluorescence microscopy. Arrowheads: Vrp1p–GFP patches which co-localize with cortical actin patches. All panels are at equal magnification.
N-Vrp1p-CAAX localizes to the cortex and exhibits improved function in cytokinesis
We asked whether by artificially directing N-Vrp1p to the cortex we would restore any additional Vrp1p-dependent functions. Addition of a CAAX box to the C-terminus has been used to confer covalent lipid modification and strong cortical localization to proteins which do not normally localize to the cortex (Golsteyn et al., 1997). We con structed centromeric plasmids expressing from the VRP1 promoter forms of N-Vrp1p in which the C-terminus of the fragment was modified by addition of either the CAAX box of S.cerevisiae Ras1p (Powers et al., 1984) (N-Vrp1p-CAAX) or both GFP and the CAAX box (N-Vrp1p–GFP-CAAX). The Ras1p CAAX box was functional in these experiments since it conferred efficient cortical localization to GFP (Figure 8A). N-Vrp1p–GFP-CAAX was able to localize to the cortex, although the distribution was diffuse at the cortex without predominant patches, similar to GFP–CAAX (Figure 8A). N-Vrp1p-CAAX was able to restore growth at 37°C on solid media (Figure 8B) and in liquid media (Figure 8C) better than N-Vrp1p when expressed in vrp1Δ. N-Vrp1p-CAAX was approximately as functional in these assays as either C-Vrp1p or full-length Vrp1p (Figure 1A and B). The improved growth at 37°C correlated with improved ability of N-Vrp1p-CAAX, compared with N-Vrp1p alone, to restore actomyosin ring constriction and Hof1p–GFP localization to single rings at the bud neck during cytokinesis (Table II). Interestingly, the improved function of N-Vrp1p-CAAX in cytokinesis was not associated with full restoration of cortical actin patch polarization, although some improvement in actin patch polarization was observed relative to N-Vrp1p alone (Figure 8D, Table I). Consistent with the lack of a fully polarized actin cytoskeleton, vrp1Δ cells expressing N-Vrp1p-CAAX were also not viable on media containing 1 M NaCl (data not shown).
Fig. 8. Addition of a CAAX box to N-Vrp1p (N-Vrp1p-CAAX) confers cortical localization and improved ability to restore viability to vrp1Δ. (A) vrp1Δ (AMY88) carrying either pAM237 (GFP), pAM242 (GFP–CAAX), pAM239 (N-Vrp1p–GFP) or pAM243 (N-Vrp1p–GFP-CAAX) were grown in YPUAD to exponential phase at 24°C and GFP fluorescence was visualized in living cells by fluorescence microscopy. All panels are at equal magnification. (B) Viability at 24 and 37°C of vrp1Δ (AMY88) carrying YCplac111 vector (Vect), pAM232 (N-Vrp1p) or pAM244 (N-Vrp1p-CAAX). Each strain was streaked for single colonies on YPUAD solid medium, incubated at either 24 or 37°C and photographed after 3 days. (C) Growth rate of vrp1Δ (AMY88) carrying YCplac111 vector (Vect), pAM232 (N-Vrp1p), pAM236 (C-Vrp1p) or pAM244 (N-Vrp1p-CAAX). A YPUAD culture of each strain was grown at 24°C, diluted to an OD600 of 0.05 in fresh YPUAD medium and incubated at 37°C. The OD600 was monitored at 1 h intervals. (D) Cortical actin patch distribution in vrp1Δ (AMY88) carrying YCplac111 vector (Vect) or pAM244 (N-Vrp1p-CAAX). Cells were grown in YPUAD to exponential phase at 24°C and either left at 24°C or shifted to 37°C for 2 h. The cells were fixed, permeabilized and F-actin stained with Alexa-488-conjugated phalloidin. Stained cells were viewed by fluorescence microscopy. Fields containing small-budded cells are shown to compare the polarization of cortical actin patches. All panels are at equal magnification.
Type I myosins and Las17p are required at 37°C for Hof1p localization during cytokinesis
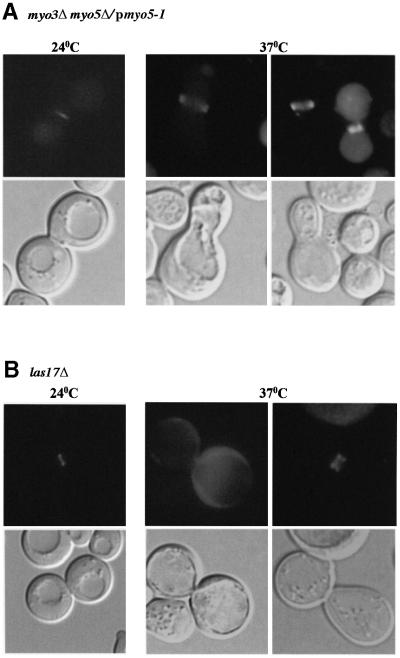
The type I myosins Myo3p and Myo5p interact with both N-Vrp1p and C-Vrp1p (Anderson et al., 1998; Evangelista et al., 2000; our unpublished results). Las17p has been shown to interact with C-Vrp1p (Naqvi et al., 1998; Madania et al., 1999; Evangelista et al., 2000) and is required for efficient cytokinesis (Li, 1997). To investigate whether type I myosins are required at 37°C for localization of Hof1p during cytokinesis, we made use of a strain carrying deletions of both MYO3 and MYO5 genes and with a plasmid-borne copy of a myo5-ts allele (myo5-1) (Geli and Riezman, 1996). To test the requirement for Las17p, a las17Δ strain was used. A plasmid expressing Hof1p–GFP was introduced into these strains and then the cells were grown to exponential phase at 24°C, and either left at 24°C or shifted to 37°C for 3 h. The localization of Hof1p–GFP was then examined in living cells by fluorescence microscopy (Figure 9). In large-budded myo3Δ myo5Δ/pmyo5-1 (Figure 9A) or las17Δ (Figure 9B) cells left at 24°C, we observed either two Hof1p–GFP rings or a single Hof1p–GFP ring at the bud neck similar to what we find in wild-type cells. In contrast, after shift to 37°C, we observed either diffuse Hof1p–GFP fluorescence or occasionally two Hof1p–GFP rings in the bud neck region in large-budded cells. In large-budded las17Δ cells shifted to 37°C, we also observed mainly diffuse Hof1p–GFP fluorescence, although in a few cells double Hof1p–GFP rings were visible. In both strains, the Hof1p–GFP rings were often mislocalized away from the bud neck as observed in vrp1Δ (Naqvi et al., 2001). In neither mutant did we observe any single Hof1p–GFP rings after shift to 37°C.
Fig. 9. Type I myosins and Las17p are required at 37°C for Hof1p localization to a single ring at the bud neck prior to cytokinesis. (A) myo3Δ myo5Δ carrying a myo5-ts allele on a centromeric plasmid (RH3383) and (B) las17Δ (IDY166) were both transformed with a plasmid (pAM220) expressing a functional Hof1p–GFP fusion protein. Cells were grown in YPUAD to exponential phase at 24°C and shifted to 37°C for 3 h. GFP was visualized in living cells by fluorescence microscopy. Upper panels: FITC fluorescence optics. Lower panels: DIC optics. All panels are at equal magnification.
Discussion
We have demonstrated here that Vrp1p comprises two functional modules defined by residues 1–364 (N-Vrp1p) and 364–817 (C-Vrp1p). Each module has the ability, at moderate levels of expression, to substitute for full-length Vrp1p in restoration of viability at 37°C. C-Vrp1p also restores polarization of cortical actin patches, whereas N-Vrp1p restores viability at 37°C without efficiently restoring this cortical actin cytoskeletal function. The discovery that N-Vrp1p and C-Vrp1p constitute independent functional modules with a partial duplication of activities was quite unexpected. N-Vrp1p and C-Vrp1p share only limited stretches of sequence homology and, furthermore, the actin-binding WH2/V domain, which is the sequence most highly conserved between Vrp1p and its mammalian orthologue, WIP, resides only in N-Vrp1p (Ramesh et al., 1997; Vaduva et al., 1997, 1999; Naqvi et al., 1998). To our knowledge, this is the first report to demonstrate the ability of two non-homologous halves of any protein to function in place of the full-length protein in vivo.
While the molecular basis for the slow growth of vrp1Δ at 24°C and its inviability at 37°C is not yet fully understood, both actomyosin ring constriction and Hof1p relocalization to a single ring at the bud neck during cytokinesis are affected at 37°C (Naqvi et al., 2001). Since actomyosin ring function and Hof1p function in S.cerevisiae are independent and either is sufficient for completion of cytokinesis (Vallen et al., 2000), part of the explanation for why N-Vrp1p and C-Vrp1p rescue viability at 37°C may lie in their ability to restore some cellular activity such that either or both of these cell cycle events is restored. We have shown here that rescue of viability at 37°C by N-Vrp1p and C-Vrp1p is accompanied in each case by correction of both the actomyosin ring constriction and Hof1p relocalization defects of vrp1Δ.
We have shown that neither mutation nor deletion of the KLKKAET actin-binding motif in the WH2/V domain strongly reduces the activity of Vrp1p (Figure 4A). Neither do mutation or deletion of this motif abolish the activity of N-Vrp1p (Figure 5A). One explanation for the difference between our results and those reported by Vaduva et al. (1997) is that we constructed our mutations and deletion in Vrp1p, whereas in the previous study a Gal4–Vrp1p fusion construct was used. The full-length Gal4–Vrp1p fusion protein, although functional in complementation tests using vrp1-1, localizes mainly to the nucleus (Gal4p has a nuclear localization sequence) (Vaduva et al., 1997). It is quite likely that a similar mislocalization of the Gal4– Vrp1p fusion protein lacking residues 1–70 contributes to the reported loss of complementation of this deletion construct. In agreement with the results of Vaduva et al. (1997), we find that this truncated Gal4–Vrp1p fusion construct fails to complement vrp1Δ, although the full-length Gal4–Vrp1p fusion also complements vrp1Δ less well than full-length Vrp1p alone (see the figure in the Supplementary data available at The EMBO Journal Online). The observation that Vrp1p functions without the WH2/V domain is consistent with our earlier finding that the minimal fragment of VRP1 necessary for complementation of end5-1/vrp1-E5 does not include codons 1–363 (Munn et al., 1995).
It has also been reported that expression of human WIP in yeast can restore both colony formation at 37°C and fluid-phase endocytosis in vrp1-1 mutant cells. Activity is lost when the actin-binding site in WIP is deleted or when the conserved lysines (K47 and K48) are mutated to alanine (Vaduva et al., 1999). This result suggests that either WIP is intrinsically more reliant on the actin-binding motif than Vrp1p, or perhaps that this apparent reliance is due to an enhanced sensitivity to alterations in the protein when it is expressed in a heterologous organism.
Perhaps the actin-binding motifs in the WH2/V domains of Vrp1p and WIP are mechanistically important but non-essential due to the presence of a related and potentially redundant actin-binding site in the WH2/V domain of Las17p. Interestingly, deletion of a C-terminal fragment of Las17p which includes the WH2/V domain does not lead to a strong phenotype in otherwise wild-type cells (Winter et al., 1999), but cells deficient in both Vrp1p and Las17p are inviable (Naqvi et al., 1998). Another possibility is that Vrp1p may have another actin-binding domain(s) which acts redundantly with the WH2/V domain. In support of this, residues 270–364 of Vrp1p also exhibit two-hybrid interactions with actin (Figure 5C). Finally, the actin-binding WH2/V domain in Vrp1p may have a more regulatory function, as recently suggested for the WH2/V domain of Scar/WAVE (Westphal et al., 2000).
How do N-Vrp1p and C-Vrp1p function? Both N- and C-terminal Vrp1p fragments, including Vrp1p1–200 and Vrp1p195–817, interact with the SH3 domains of both type I myosins Myo3p and Myo5p (Anderson et al., 1998). Vrp1p1–200, Vrp1p211–437 (Evangelista et al., 2000) and Vrp1p465–817 (our unpublished results) interact with the Myo3p SH3 domain. In fact, a consensus sequence for binding to the SH3 domain of Myo3p has been proposed (PXXXXPXXP), and there are 17 copies of this motif in Vrp1p: 10 in N-Vrp1p and seven in C-Vrp1p (Evangelista et al., 2000). This suggests that N-Vrp1p may function through interaction with type I myosins. Given the existence of a second actin-binding site, however, it is still possible that N-Vrp1p functions through interaction with actin. C-Vrp1p, but not N-Vrp1p, also interacts with Las17p, suggesting that it may function through interaction with type I myosin and/or Las17p (Naqvi et al., 1998; Evangelista et al., 2000). Based on these known interactions of N-Vrp1p and C-Vrp1p, we propose that both Vrp1p fragments function through different protein– protein interactions to stabilize an actin–Vrp1p–Las17p– type I myosin–Arp2/3p complex. The activity or localization of this complex may differ between vrp1Δ strains expressing different Vrp1p fragments. This role for Vrp1p would be consistent with the finding that Vrp1p is required for in vitro stimulation of actin polymerization by type I myosins (Geli et al., 2000) and the observation that overexpression of Las17p can suppress defects in end5-1/vrp1-E5 mutant cells (Naqvi et al., 1998). It is possible that the actin–Vrp1p–Las17p–type I myosin–Arp2/3p complex plays a general role in actomyosin ring constriction and Hof1p relocalization. This would be consistent with previous reports that implicate Las17p, type I myosin and Arp2p in cytokinesis (Moreau et al., 1996; Li, 1997; Anderson et al., 1998) and our present finding that Hof1p relocalization at 37°C requires Las17p and type I myosins.
We have shown here that N-Vrp1p does not localize efficiently to the cortex and the localization of C-Vrp1p to the cortex is absolutely dependent on Las17p. This suggests that the ability of N-Vrp1p to interact with actin is insufficient to confer cortical localization. It is possible that KLKKAET is a G-actin-binding motif (Vaduva et al., 1997) and does not confer localization to cortical actin patches, which consist predominantly of F-actin. Our results also suggest that interaction with type I myosins may be insufficient to confer localization on either N-Vrp1p or C-Vrp1p. In fact, type I myosins have been reported to depend on Vrp1p for their localization (Anderson et al., 1998). Introduction of a CAAX box into N-Vrp1p (N-Vrp1p-CAAX) caused efficient localization to the cortex and enhanced function in restoring viability and cytokinesis. Significantly, addition of a CAAX box to N-Vrp1p did not fully restore polarization of cortical actin patches, although it did improve actin patch polarization somewhat (Figure 8D, Table I).
The results presented here demonstrate that restoration of viability and cytokinesis by N-Vrp1p can occur despite the inability of this Vrp1p fragment to localize efficiently or function efficiently in cortical actin patch polarization. This provides the first evidence that Vrp1p’s function in cytokinesis is independent of its function in organization of cortical actin patches. Vrp1p may function in delivery of actomyosin ring components and Hof1p to the bud neck, processes which may not be strictly dependent on a cortical localization. In support of this idea, Vrp1p has already been implicated in cytoplasmic delivery of mRNA to mitochondria (Zoladek et al., 1995), and the human orthologue, WIP, functions in cytoplasmic movement of virus particles (Moreau et al., 2000). It is possible that other components of the actin–Vrp1p–Las17p–type I myosin–Arp2/3p complex may remain associated with the mislocalized N-Vrp1p and facilitate its function in the cytoplasm. We cannot exclude, however, the possibility that restoration of function is dependent on some residual N-Vrp1p present at the cortex. Localization of the bulk of N-Vrp1p to the cortex using a CAAX box does indeed enhance its function. This may be due to more effective movement due to restriction to a two-dimensional surface or, alternatively, if Vrp1p functions in vesicle movement the CAAX box may help N-Vrp1p to localize to the correct vesicles.
In conclusion, we have used the observation that the N- and C-terminal fragments of Vrp1p have a partial duplication of activities despite their differential ability to localize to the cortex and restore cortical actin patch polarization to show that the roles of Vrp1p in actomyosin ring constriction and Hof1p relocalization: (i) do not require strong cortical localization of Vrp1p; (ii) are independent of the role of Vrp1p in cortical actin patch polarization; and (iii) appear to be important for viability at 37°C. Finally, we have shown that type I myosins and Las17p, which interact with N-Vrp1p and C-Vrp1p and are known activators of the Arp2/3p complex, are also required for efficient Hof1p localization to a single ring at the bud neck prior to cytokinesis.
Materials and methods
Strains, plasmids, media and reagents
The yeast strains and plasmids used in this study are listed in Tables III and IV. YPUAD is YPD supplemented with 40 µg/ml adenine and 20 µg/ml uracil. SD minimal medium and YPD are described in Adams et al. (1997). Yeast strain PJ69-4A was a gift from Philip James, University of Wisconsin. Zymolyase 20T was from US Biologicals (Swampscott, MA). The rabbit polyclonal GFP-specific antiserum was a gift from J.Kahana and P.Silver, Dana Farber Cancer Center, Boston, MA. Alexa-488-conjugated phalloidin and Alexa-594-conjugated secondary antibodies were from Molecular Probes (Eugene, OR). The anti-Pk monoclonal antibody was from Serotec (Oxford, UK). Polyvinylidene fluoride membranes (Immobilon-PSQ) were from Millipore (Bedford, MA).
Table III. Yeast strains used in this study.
| Strain | Genotype | Reference |
|---|---|---|
| RH1201 | MATa/MATα his4/his4 leu2/leu2 ura3/ura3 lys2/lys2 bar1/bar1 | Riezman laboratory strain |
| AMY145 | As RH1201 except vrp1Δ::KanMx/VRP1 | this study |
| AMY88 | MATa his4 leu2 ura3 lys2 vrp1Δ::KanMx bar1 (haploid segregant of AMY145) | this study |
| IDY166 | MATa his3 leu2 ura3 trp1 las17Δ::URA3 | Naqvi et al. (1998) |
| PJ69-4A | MATa his3 leu2 ura3 trp1 gal4Δ gal80Δ met2::GAL7-lacZ GAL2-ADE2 LYS2::GAL1-HIS3 | James et al. (1996) |
| YEF1698 | MATa/MATα his3/his3 leu2/leu2 ura3/ura3 trp1/trp1 lys2/lys2 MYO1-GFP::KanMx/ MYO1-GFP::KanMx | Vallen et al. (2000) |
| AMY112 | As YEF1698 except vrp1Δ::URA3/VRP1 | this study |
| AMY114 | MATa his3 leu2 ura3 trp1 lys2 MYO1-GFP::KanMx vrp1Δ::URA3 (haploid segregant of AMY112) | this study |
| YEF1986 | MATa/MATα his3/his3 leu2/leu2 ura3/ura3 trp1/trp1 lys2/lys2 HOF1-GFP::KanMx/ HOF1-GFP::KanMx | Vallen et al. (2000) |
| AMY146 | As YEF1986 except vrp1Δ::URA3/VRP1 | this study |
| AMY147 | MATa his3 leu2 ura3 trp1 lys2 HOF1-GFP::KanMx vrp1Δ::URA3 (haploid segregant of AMY146) | this study |
| RH3383 | MATa his3 leu2 ura3 trp1 ade2 myo3Δ::HIS3 myo5Δ::TRP1 bar1 pmyo5-1::URA3 | Geli and Riezman (1996) |
Table IV. Plasmids used in this study.
| Plasmid | Description | Reference |
|---|---|---|
| YCplac111 | LEU2 CEN ARS plasmid | Gietz and Sugino (1988) |
| YCplac33 | URA3 CEN ARS plasmid | Gietz and Sugino (1988) |
| YCplac22 | TRP1 CEN ARS plasmid | Gietz and Sugino (1988) |
| pACT2 | LEU2 vector with Gal4p activation domain | Clontech |
| pAM162 | YCplac111 with full-length VRP1-GFP | Naqvi et al. (1998) |
| pAM220 | YCplac111 with full-length HOF1-GFP | Naqvi et al. (2001) |
| pAM228 | YCplac111 with full-length VRP1 | this study |
| pAM229 | YCplac111 with full-length vrp1 (K45A K46A) | this study |
| pAM230 | YCplac111 with full-length vrp1 (K45E K46E) | this study |
| pAM231 | YCplac111 with vrp1(1–68)Δ | this study |
| pAM232 | YCplac111 with vrp1(365–817)Δ | this study |
| pAM233 | YCplac111 with vrp1(365–817)Δ (K45A K46A) | this study |
| pAM234 | YCplac111 with vrp1(365–817)Δ (K45E K46E) | this study |
| pAM235 | YCplac111 with vrp1(1–68)Δ, (365–817)Δ | this study |
| pAM236 | YCplac111 with vrp1(1–363)Δ | this study |
| pAM237 | YCplac111 with GFP under VRP1 promoter | this study |
| pAM239 | YCplac111 with vrp1(365–817)Δ-GFP | this study |
| pAM241 | YCplac111 with vrp1(1–363)Δ-GFP | this study |
| pAM242 | Same as pAM237 except GFP-CAAX | this study |
| pAM243 | YCplac111 with vrp1(365–817)Δ-GFP-CAAX | this study |
| pAM244 | YCplac111 with vrp1(365–817)Δ-CAAX | this study |
| pAM249 | YCplac33 with full-length VRP1-Pk | this study |
| pAM250 | YCplac33 with vrp1(365–817)Δ-Pk | this study |
| pAM251 | YCplac33 with vrp1(1–363)Δ-Pk | this study |
| pAM252 | YCplac22 with GAL4BD-ACT1 | this study |
| pAM253 | pACT2 with vrp1(71–817)Δ | this study |
| pAM254 | pACT2 with vrp1(71–817)Δ (K45A K46A) | this study |
Yeast genetic techniques
Tetrad dissections were performed as described (Adams et al., 1997). Tetrads were dissected on YPUAD and spore germination was at 24°C. Plasmid DNA was introduced into yeast cells using a modification of the lithium acetate protocol (Munn et al., 1995). Yeast two-hybrid interactions were tested using PJ69-4A (James et al., 1996).
DNA techniques and plasmid construction
Standard DNA techniques were performed as described in Sambrook et al. (1989). PCR was carried out using Pfu polymerase (Roche, Mannheim, Germany).
Fragments of Vrp1p were expressed from a YCplac111- or YCplac33-based low copy number plasmid (Gietz and Sugino, 1988) under the control of the VRP1 promoter (nucleotides –240 to the VRP1 ATG start codon). Sequences encoding N-Vrp1p (codons 1–364) or C-Vrp1p (codons 364–817) were inserted into this plasmid. For expression of N-Vrp1p, a termination codon was introduced after codon 364, and, for expression of C-Vrp1p, an ATG was introduced before codon 364. In constructs expressing GFP- or Pk-epitope- (Craven et al., 1998) tagged Vrp1p fragments, the tags were introduced at the C-terminus of the fragment. N-Vrp1p-CAAX, GFP–CAAX and N-Vrp1p–GFP-CAAX include the Ras1p CAAX box (GCCIIC) (Powers et al., 1984) at the C-terminus. Site-directed mutagenesis was carried out using a Unique Site Elimination kit (Amersham-Pharmacia Biotech, Uppsala, Sweden) and the mutated VRP1 sequences were used to replace wild-type sequences in the plasmids described above. Further details of the constructions are available upon request.
Generation of a vrp1Δ strain
The VRP1 deletion construct removes the entire open reading frame (ORF) (–177 upstream to +9 downstream of the VRP1 ORF) and replaces it with KanMx (Longtine et al., 1998). One copy of VRP1 was disrupted in diploid strain RH1201 and the resulting diploid (AMY145) was sporulated, tetrads were dissected and haploid segregants were scored for the presence of the KanMx marker. AMY88 was a vrp1Δ::KanMx spore obtained from this dissection. vrp1Δ cells were viable at 24°C but not at 37°C, in agreement with previous reports (Naqvi et al., 1998).
Visualization of Vrp1p subcellular localization
For Vrp1p–GFP localization, cells growing exponentially at 24°C were applied to a microscope slide and the GFP signal was visualized by fluorescence microscopy using a fluorescein isothiocyanate (FITC)-specific light filter.
For indirect immunofluorescence experiments, exponentially growing cells were fixed with 3.7% formaldehyde for 30 min, washed three times with phosphate-buffered saline (PBS) pH 8.3 and digested with Zymolyase 20T in the presence of β-mercaptoethanol. Digested cells were washed once with PBS containing 1% Triton X-100 and then washed three times with PBS. The cells were resuspended in PBAL (PBS with 1% bovine serum albumin and 100 mM lysine). The cells were incubated with rabbit polyclonal anti-GFP antibodies for 3 h, washed three times with PBAL and the GFP was visualized with the aid of Alexa-594-labelled anti-rabbit IgG secondary antibodies.
Visualization of F-actin
Yeast cells were grown in YPUAD to exponential phase at 24°C and then either left at 24°C or shifted to 37°C for 2 h and fixed by direct addition of 3.7% formaldehyde to the culture and incubation for 30 min at the temperature of growth. Fixed cells were permeabilized using 1% Triton-X-100 in PBS, stained with Alexa-488-conjugated phalloidin (Molecular Probes), and F-actin was visualized using fluorescence microscopy as described (Chowdhury et al., 1992). The percentage of small-budded cells with depolarized actin patches was estimated by scoring a total of 200 cells in each sample. When the mother cell exhibited >10 actin patches, the cell was classified as having a depolarized actin patch phenotype.
Hof1p–GFP and Myo1p–GFP localization
Cells (AMY114 or AMY147) were grown to exponential phase at 24°C, shifted to 37°C for 3 h and Myo1p–GFP or Hof1p–GFP were visualized by fluorescence microscopy. The percentages of large-budded cells with single or duplicated Hof1p–GFP rings and constricted or unconstricted Myo1p–GFP rings were estimated in each case by scoring 200 cells in each sample.
Light microscopy
Light microscopy was performed using a Leica DMLB fluorescence microscope (Leica, Singapore). Images were captured using an Optronix DEI-470T cooled charge-coupled device camera and QWIN software (Leica).
Supplementary data
Supplementary data for this paper are available at The EMBO Journal Online.
Acknowledgments
Acknowledgements
We thank M.Geli, V.Boulton, S.Oliferenko and V.Wachtler for reading and offering valuable suggestions on the manuscript, R.Tsien and the Howard Hughes Medical Institute, University of California, San Diego, CA for permission to use the S65T mutant form of GFP, and H.Riezman, M.Geli, B.Winsor, J.Kahana, P.Silver, G.Vaduva, A.Hopper, E.Bi, D.Mitchell and G.Sprague for sending strains, constructs and antisera. Funding from the National Science and Technology Board of Singapore is gratefully acknowledged.
References
- Adams A.E. and Pringle,J.R. (1984) Relationship of actin and tubulin distribution to bud growth in wild-type and morphogenetic-mutant Saccharomyces cerevisiae. J. Cell Biol., 98, 934–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams A., Gottschling,D.E., Kaiser,C.A. and Stearns,T. (1997) Methods in Yeast Genetics. A Cold Spring Harbor Laboratory Course Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Anderson B.L., Boldogh,I., Evangelista,M., Boone,C., Greene,L.A. and Pon,L.A. (1998) The Src homology domain 3 (SH3) of a yeast type I myosin, Myo5p, binds to verprolin and is required for targeting to sites of actin polarization. J. Cell Biol., 141, 1357–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi E., Maddox,P., Lew,D.J., Salmon,E.D., McMillan,J.N., Yeh,E. and Pringle,J.R. (1998) Involvement of an actomyosin contractile ring in Saccharomyces cerevisiae cytokinesis. J. Cell Biol., 142, 1301–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury S., Smith,K.W. and Gustin,M.C. (1992) Osmotic stress and the yeast cytoskeleton: phenotype-specific suppression of an actin mutation. J. Cell Biol., 118, 561–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craven R.A., Griffiths,D.J., Sheldrick,K.S., Randall,R.E., Hagan,I.M. and Carr,A.M. (1998) Vectors for the expression of tagged proteins in Schizosaccharomyces pombe. Gene, 221, 59–68. [DOI] [PubMed] [Google Scholar]
- Donnelly S.F., Pocklington,M.J., Pallotta,D. and Orr,E. (1993) A proline-rich protein, verprolin, involved in cytoskeletal organization and cellular growth in the yeast Saccharomyces cerevisiae. Mol. Microbiol., 10, 585–596. [DOI] [PubMed] [Google Scholar]
- Evangelista M., Klebl,B.M., Tong,A.H., Webb,B.A., Leeuw,T., Leberer,E., Whiteway,M., Thomas,D.Y. and Boone,C. (2000) A role for myosin-I in actin assembly through interactions with Vrp1p, Bee1p, and the Arp2/3 complex. J. Cell Biol., 148, 353–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geli M.I. and Riezman,H. (1996) Role of type I myosins in receptor-mediated endocytosis in yeast. Science, 272, 533–535. [DOI] [PubMed] [Google Scholar]
- Geli M.I., Lombardi,R., Schmelzl,B. and Riezman,H. (2000) An intact SH3 domain is required for myosin I-induced actin polymerization. EMBO J., 19, 4281–4291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gietz R.D. and Sugino,A. (1988) New yeast–Escherichia coli shuttle vectors constructed with in vitro mutagenized yeast genes lacking six-base pair restriction sites. Gene, 74, 527–534. [DOI] [PubMed] [Google Scholar]
- Golsteyn R.M., Beckerle,M.C., Koay,T. and Friederich,E. (1997) Structural and functional similarities between the human cytoskeletal protein zyxin and the ActA protein of Listeria monocytogenes. J. Cell Sci., 110, 1893–1906. [DOI] [PubMed] [Google Scholar]
- Goodson H.V., Anderson,B.L., Warrick,H.M., Pon,L.A. and Spudich,J.A. (1996) Synthetic lethality screen identifies a novel yeast myosin I gene (MYO5): myosin I proteins are required for polarization of the actin cytoskeleton. J. Cell Biol., 133, 1277–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgs H.N. and Pollard,T.D. (2001) Regulation of actin filament network formation through Arp2/3 complex: activation by a diverse array of proteins. Annu. Rev. Biochem., 70, 649–676. [DOI] [PubMed] [Google Scholar]
- James P., Halladay,J. and Craig,E.A. (1996) Genomic libraries and a host strain designed for highly efficient two-hybrid selection in yeast. Genetics, 144, 1425–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamei T., Tanaka,K., Hihara,T., Umikawa,M., Imamura,H., Kikyo,M., Ozaki,K. and Takai,Y. (1998) Interaction of Bnr1p with a novel Src homology 3 domain-containing Hof1p. Implication in cytokinesis in Saccharomyces cerevisiae. J. Biol. Chem., 273, 28341–28345. [DOI] [PubMed] [Google Scholar]
- Karpova T.S., McNally,J.G., Moltz,S.L. and Cooper,J.A. (1998) Assembly and function of the actin cytoskeleton of yeast: relationships between cables and patches. J. Cell Biol., 142, 1501–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechler T., Shevchenko,A., Shevchenko,A. and Li,R. (2000) Direct involvement of yeast type I myosins in Cdc42-dependent actin polymerization. J. Cell Biol., 148, 363–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew D.J. and Reed,S.I. (1993) Morphogenesis in the yeast cell cycle: regulation by Cdc28 and cyclins. J. Cell Biol., 120, 1305–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R. (1997) Bee1, a yeast protein with homology to Wiscott–Aldrich syndrome protein, is critical for the assembly of cortical actin cytoskeleton. J. Cell Biol., 136, 649–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippincott J. and Li,R. (1998a) Sequential assembly of myosin II, an IQGAP-like protein, and filamentous actin to a ring structure involved in budding yeast cytokinesis. J. Cell Biol., 140, 355–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippincott J. and Li,R. (1998b) Dual function of Cyk2, a cdc15/PSTPIP family protein, in regulating actomyosin ring dynamics and septin distribution. J. Cell Biol., 143, 1947–1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longtine M.S., McKenzie,A., Demarini,D.J., Shah,N.G., Wach,A., Brachat,A., Philippsen,P. and Pringle,J.R. (1998) Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast, 14, 953–961. [DOI] [PubMed] [Google Scholar]
- Machesky L.M. and Insall,R.H. (1998) Scar1 and the related Wiskott–Aldrich syndrome protein, WASP, regulate the actin cytoskeleton through the Arp2/3 complex. Curr. Biol., 8, 1347–1356. [DOI] [PubMed] [Google Scholar]
- Madania A., Dumoulin,P., Grava,S., Kitamoto,H., Scharer-Brodbeck,C., Soulard,A., Moreau,V. and Winsor,B. (1999) The Saccharomyces cerevisiae homologue of human Wiskott–Aldrich syndrome protein Las17p interacts with the Arp2/3 complex. Mol. Biol. Cell, 10, 3521–3538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Quiles N. et al. (2001) WIP regulates N-WASP-mediated actin polymerization and filopodium formation. Nature Cell Biol., 3, 484–491. [DOI] [PubMed] [Google Scholar]
- Moreau V., Madania,A., Martin,R.P. and Winsor,B. (1996) The Saccharomyces cerevisiae actin-related protein Arp2 is involved in the actin cytoskeleton. J. Cell Biol., 134, 117–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau V., Frischknecht,F., Reckmann,I., Vincentelli,R., Rabut,G., Stewart,D., and Way,M. (2000) A complex of N-WASP and WIP integrates signalling cascades that lead to actin polymerization. Nature Cell Biol., 2, 441–448. [DOI] [PubMed] [Google Scholar]
- Munn A.L., Stevenson,B.J., Geli,M.I. and Riezman,H. (1995) end5, end6, and end7: mutations that cause actin delocalization and block the internalization step of endocytosis in Saccharomyces cerevisiae. Mol. Biol. Cell, 6, 1721–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi S.N., Zahn,R., Mitchell,D.A., Stevenson,B.J. and Munn,A.L. (1998) The WASp homologue Las17p functions with the WIP homologue End5p/verprolin and is essential for endocytosis in yeast. Curr. Biol., 8, 959–962. [DOI] [PubMed] [Google Scholar]
- Naqvi S.N., Feng,Q., Boulton,V.J., Zahn,R. and Munn,A.L. (2001) Vrp1p functions in both actomyosin ring-dependent and Hof1p-dependent pathways of cytokinesis. Traffic, 2, 189–201. [DOI] [PubMed] [Google Scholar]
- Powers S., Kataoka,T., Fasano,O., Goldfarb,M., Strathern,J., Broach,J. and Wigler,M. (1984) Genes in S.cerevisiae encoding proteins with domains homologous to the mammalian ras proteins. Cell, 36, 607–612. [DOI] [PubMed] [Google Scholar]
- Ramesh N., Anton,I.M., Hartwig,J.H. and Geha,R.S. (1997) WIP, a protein associated with Wiskott–Aldrich syndrome protein, induces actin polymerization and redistribution in lymphoid cells. Proc. Natl Acad. Sci. USA, 94, 14671–14676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J., Fritsch,E.F. and Maniatis,T. (1989) Molecular Cloning: A Laboratory Manual, 2nd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Smith M.G., Swamy,S.R. and Pon,L.A. (2001) The life cycle of actin patches in mating yeast. J. Cell Sci., 114, 1505–1513. [DOI] [PubMed] [Google Scholar]
- Vaduva G., Martin,N.C. and Hopper,A.K. (1997) Actin-binding verprolin is a polarity development protein required for the morphogenesis and function of the yeast actin cytoskeleton. J. Cell Biol., 139, 1821–1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaduva G., Martinez-Quiles,N., Anton,I.M., Martin,N.C., Geha,R.S., Hopper,A.K. and Ramesh,N. (1999) The human WASP-interacting protein, WIP, activates the cell polarity pathway in yeast. J. Biol. Chem., 274, 17103–17108. [DOI] [PubMed] [Google Scholar]
- Vallen E.A., Caviston,J. and Bi,E. (2000) Roles of Hof1p, Bni1p, Bnr1p, and Myo1p in cytokinesis in Saccharomyces cerevisiae. Mol. Biol. Cell, 11, 593–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch M.D. (1999) The world according to Arp: regulation of actin nucleation by the Arp2/3 complex. Trends Cell Biol., 9, 423–427. [DOI] [PubMed] [Google Scholar]
- Westphal R.S., Soderling,S.H., Alto,N.M., Langeberg,L.K. and Scott,J.D. (2000) Scar/WAVE-1, a Wiskott–Aldrich syndrome protein, assembles an actin-associated multi-kinase scaffold. EMBO J., 19, 4589–4600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winsor B. and Schiebel,E. (1997) Review: an overview of the Saccharomyces cerevisiae microtubule and microfilament cytoskeleton. Yeast, 13, 399–434. [DOI] [PubMed] [Google Scholar]
- Winter D., Lechler,T. and Li,R. (1999) Activation of the yeast Arp2/3 complex by Bee1p, a WASP-family protein. Curr. Biol., 9, 501–504. [DOI] [PubMed] [Google Scholar]
- Zoladek T., Vaduva,G., Hunter,L.A., Boguta,M., Go,B.D., Martin,N.C. and Hopper,A.K. (1995) Mutations altering the mitochondrial– cytoplasmic distribution of Mod5p implicate the actin cytoskeleton and mRNA 3′ ends and/or protein synthesis in mitochondrial delivery. Mol. Cell. Biol., 15, 6884–6894. [DOI] [PMC free article] [PubMed] [Google Scholar]