Abstract
The system N transporter SN1 has been proposed to mediate the efflux of glutamine from cells required to sustain the urea cycle and the glutamine–glutamate cycle that regenerates glutamate and γ-aminobutyric acid (GABA) for synaptic release. We now show that SN1 also mediates an ionic conductance activated by glutamine, and this conductance is selective for H+. Although SN1 couples amino acid uptake to H+ exchange, the glutamine-gated H+ conductance is not stoichiometrically coupled to transport. Protons thus permeate SN1 both coupled to and uncoupled from amino acid flux, providing novel mechanisms to regulate the transfer of glutamine between cells.
Keywords: amino acid transport system N/asparagine/glutamine/H+ conductance/SN1
Introduction
The amino acid glutamine plays a crucial role in nitrogen metabolism. In multicellular organisms, glutamine serves as the principal nitrogen carrier between cells. Glutamine alone constitutes one-fifth of all the amino acids present in plasma and, at ∼0.5 mM, two-thirds of those present in the cerebrospinal fluid (McGale et al., 1977) and brain extracellular space (Hamberger and Nystrom, 1984). Produced by one group of cells and taken up by another, glutamine contributes to multiple physiological processes. In the liver, the release of glutamine from perivenous hepatocytes and the uptake of glutamine by periportal hepatocytes participate in ammonia detoxification and the urea cycle (Haussinger, 1990). In the kidney, glutamine metabolism promotes the secretion of acid (Bender, 1975). Glutamine transfer between distinct cell populations also occurs in the nervous system.
Glutamine participates in a cycle that regenerates amino acid transmitters released during synaptic transmission. Re-uptake by the nerve terminal serves to recycle most classical transmitters as well as to terminate their signalling. However, cloned transporters for the principal excitatory transmitter glutamate generally do not appear at the nerve terminal (Rothstein et al., 1994; Chaudhry et al., 1995), requiring distinct mechanisms to recycle glutamate. After exocytotic release, glutamate is taken up by glia through glutamate transporters (Kanner, 1994; Seal and Amara, 1999). Glutamine synthetase expressed in glia then converts the accumulated glutamate into glutamine (Rothstein and Tabakoff, 1984). After transfer back to neurons, glutamine serves as the direct precursor for glutamate (Hamberger et al., 1979; Thanki et al., 1983). Supporting the importance of this glutamine–glutamate cycle, inhibition of the neuronal enzyme that converts glutamine to glutamate drastically reduces glutamate stores (Conti and Minelli, 1994) and exogenous glutamine can sustain synaptic activity in the absence of glutamate transport and glutamine synthetase activity (Barnett et al., 2000). Inhibition of glutamine synthetase also reduces the levels of γ-aminobutyric acid (GABA) in the nerve terminal (Pow and Robinson, 1994; Laake et al., 1995), consistent with the biosynthesis of GABA from glutamate by glutamic acid decarboxylase. The glutamine–glutamate cycle thus contributes to inhibitory as well as excitatory neurotransmission.
In contrast to the well-characterized transporters involved in glutamate uptake by astrocytes, the mechanisms involved in glutamine transfer from astrocytes to neurons remain less well understood. In previous work, we proposed that the system N transporter SN1 mediates the efflux of glutamine from astrocytes required for the glutamine–glutamate cycle (Chaudhry et al., 1999). SN1 exhibits several properties that suggest a role in glutamine efflux. First, as predicted from classical studies of system N (Kilberg et al., 1980), SN1 preferentially recognizes glutamine. Secondly, astrocytes express SN1 activity (Nagaraja and Brookes, 1996) and SN1 localizes to the plasma membrane of astrocytic processes (Chaudhry et al., 1999). Thirdly, SN1 mediates glutamine efflux under physiological conditions (Chaudhry et al., 1999).
We have shown previously that SN1 transports neutral amino acid and Na+ in exchange for H+ (Chaudhry et al., 1999). We have also observed that the flux mediated by SN1 reverses close to the prevailing extracellular glutamine concentration in the brain (∼0.5 mM); above that concentration, SN1 mediates uptake and, below that concentration, efflux. Since intracellular glutamine is in the low millimolar range, SN1 can thus generate only a shallow concentration gradient of amino acid across the plasma membrane. To account for the shallowness of this gradient, we have suggested that transport by SN1 may be electroneutral. Electroneutrality would eliminate the role of membrane potential as a driving force for Na+-dependent uptake by SN1. Transport by SN1 would then be driven primarily by the Na+ gradient across the plasma membrane, predicting a concentration gradient (in>out) for glutamine that is shallower than if membrane potential were also involved. Supporting this possibility, depolarization did not appear to influence amino acid uptake by SN1 (Chaudhry et al., 1999).
Despite the proposed electroneutrality of transport, SN1 generates currents in Xenopus oocytes (Fei et al., 2000). To reconcile this observation with the apparent electroneutrality of SN1-mediated transport and its reversibility at physiological concentrations of extracellular glutamine, we have now characterized the associated currents. We report that although amino acid and Na+ activate the currents mediated by SN1, the currents are not stoichiometrically coupled to amino acid flux. In addition, the uncoupled conductance is selective for H+. Since transport by SN1 couples to H+ exchange, the uncoupled H+ conductance provides a mechanism to influence the ionic gradients that drive transport by system N.
Results
Transport by SN1 activates uncoupled currents
Since the association of currents with SN1 has suggested that transport by SN1 is not electroneutral, we have used two-electrode voltage clamp in Xenopus oocytes to characterize these currents further. As previously reported (Fei et al., 2000), the addition of system N substrates glutamine, asparagine and histidine (1 mM) to oocytes expressing SN1 generates inward currents (Figure 1A). Uninjected oocytes produce no detectable currents in response to these amino acids (Figure 1B). Glutamine also produces inward currents with a potency similar to the Km for uptake (1–2 mM) (Figure 1C) (Kilberg et al., 1980; Chaudhry et al., 1999). In addition, the inward currents depend on Na+, tolerate substitution of Na+ by Li+ and do not depend on chloride (Figure 1F–H), as previously reported for transport mediated by system N (Kilberg et al., 1980; Chaudhry et al., 1999). Further, low external pH (pHo), which was shown previously to reduce uptake due to the H+ exchange coupling mechanism, also inhibits the currents (Figure 1D). The currents thus exhibit properties very similar to substrate flux, raising the possibility that they reflect electrogenic transport. Indeed, the system A transporters SA1 and 2 that are closely related to SN1 mediate electrogenic rather than electroneutral transport (Reimer et al., 2000; Sugawara et al., 2000; Varoqui et al., 2000; Yao et al., 2000). However, closer examination shows that the currents differ in certain respects from transport by SN1. Asparagine activates currents similar in size to those produced by glutamine (Figure 1A and E), but we have found previously that the same concentration of asparagine (1 mM) causes much smaller increases in intracellular pH (pHi) than 1 mM glutamine (Chaudhry et al., 1999). Unlabelled asparagine also inhibits the uptake of [3H]glutamine by SN1 much less strongly than glutamine (Fei et al., 2000). The currents thus differ from flux in their activation by different amino acid substrates.
Fig. 1. SN1 exhibits currents in Xenopus oocytes. (A) Xenopus oocytes injected with SN1 cRNA and held at –50 mV show inward currents on addition of glutamine (Q), asparagine (N), histidine (H) and alanine (A), but not glutamate (E) (each 1 mM) at pH 8. (B) The same amino acids (1 mM) produce no currents in uninjected oocytes held at –50 mV. (C) Increasing concentrations of glutamine (in mM) at pH 8 cause progressively larger inward currents, with half-maximal currents obtained at ∼2 mM glutamine. (D) Extracellular pH (pHo) influences the currents produced by 1 mM glutamine at –50 mV, with a maximum at pHo 8. It also influences the currents produced by 1 mM asparagine, with large currents at pHo 8 and 7 and smaller currents at pHo 9 and 6 (data not shown). Glutamine, asparagine and histidine generate currents in both Na+- (E) and Li+- (F) containing solutions. Other amino acids produce much smaller currents. In addition to the standard code for amino acids, m = MeAIB, c = cystine, g = GABA and t = taurine. The error bars indicate the SEM. (G) Current–voltage relationship of currents produced by 1 mM glutamine. The currents do not tolerate the replacement of Na+ by choline. (H) Substitution of chloride by gluconate has no effect on the glutamine-induced currents.
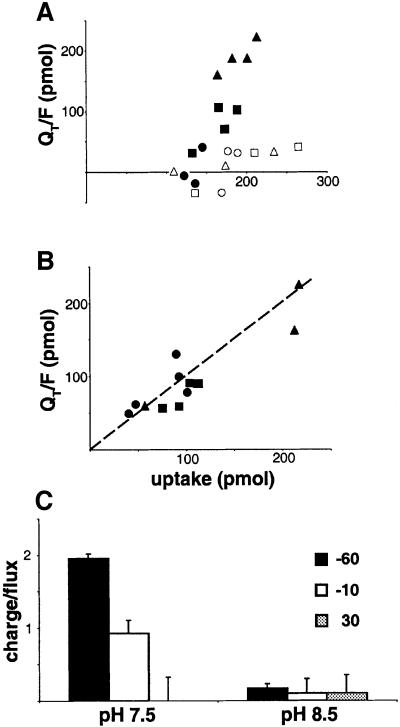
To assess the relationship between currents and amino acid transport mediated by SN1, we determined the ratio of charge movement to amino acid uptake. Since uninjected oocytes exhibit more background uptake of glutamine than asparagine (Taylor et al., 1989), we used [3H]asparagine as a substrate. At pHo 7.5, the largest inward charge movement occurs at –60 mV, and the smallest at +30 mV (Figure 2A). At pHo 8.5, the charge movement is small at all potentials and does not correspond to substrate influx (Figure 2A). Currents induced by asparagine thus differ from those produced by glutamine, which fall off more steeply below pHo 8 than above (Figure 1D). Figure 2C shows the wide variation in charge/flux ratio at pHo 7.5, from ∼2 at –60 mV to essentially zero at +30 mV. At pHo 8.5, the charge/flux ratios all fall close to zero, supporting a dissociation between the currents and transport. Alternatively, the uptake of [3H]asparagine may reflect exchange for cytoplasmic amino acid rather than net flux. The electroneutrality of exchange would then predict low charge/flux ratios. However, charge movement by the closely related system A transporter SA1 is tightly coupled to flux (Figure 2B). In addition, we observed that glutamine produces outward currents at positive potentials (Figure 1G).
Fig. 2. Variable relationship of charge movement to substrate flux mediated by SN1. (A) In oocytes expressing SN1, the charge movement produced over 10 min by 250 µM [3H]asparagine at pH 7.5 (filled symbols) increases at more negative holding potentials. Oocytes held at –60 mV are indicated by triangles, –10 mV by squares and +30 mV by circles. At pHo 8.5 (open symbols), oocytes expressing SN1 exhibit smaller currents at all holding potentials. The currents produced by asparagine thus differ from those produced by glutamine, which fall off more steeply below pHo 8 than above (see Figure 1D). At both pHos, the charge movement caused by asparagine does not correspond to uptake. (B) In contrast, oocytes expressing the related system A transporter SA1 show charge movement that correlates with uptake at –60 mV (triangles), –10 mV (squares) and +30 mV (circles). (C) At pHo 7.5, the charge/flux ratio drops from ∼2 at –60 mV, to ∼1 at –10 and to ∼0 at +30 mV. At pHo 8.5, the currents are all low despite substantial flux, yielding very low charge/flux ratios (∼0.1) at all potentials. Error bars represent the SEM.
If amino acid uptake involves the inward movement of positive charge, the addition of amino acid to the outside of the cell should not promote outward charge movement, even at depolarizing potentials. Thus, outward currents produced by substrate also argue against charge movement coupled to flux. These observations raise the possibility that SN1 mediates an uncoupled current analogous to the anion-selective uncoupled current associated with glutamate transporters (Wadiche et al., 1995; Sonders and Amara, 1996).
Gating and permeation by protons
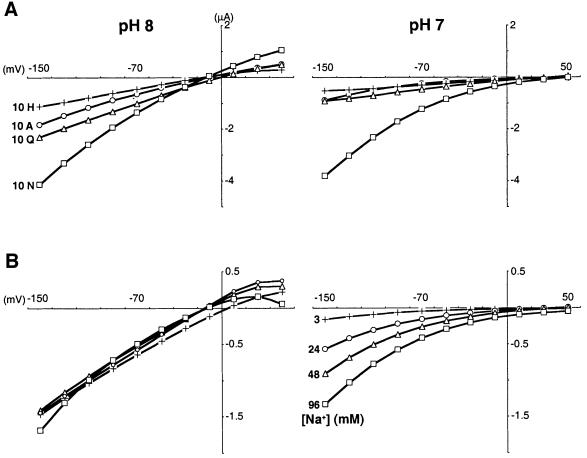
To characterize the uncoupled conductance associated with SN1, we sought to identify the permeant ions. Glutamine activates progressively larger inward currents at polarized potentials and outward currents at depolarized potentials (Figure 3A). The negative reversal potential observed for these currents raised the possibility that they were carried by chloride, but substitution of external chloride by gluconate had no effect on the reversal potential (Figure 1H). Since the reversal potential for H+ is –20 to –30 mV at pHo 8, we also investigated permeation by H+. A reduction in pHo from 8 to 7 has two striking effects on the currents (Figure 3A). First, it shifts the reversal potential by +51.7 ± 2.5 mV (n = 6), as predicted by the Nernst equation for a H+-selective channel. Indeed, we have used the reversal potentials observed at pHo 8 and 7 to calculate a pHi of ∼7.5, consistent with previous reports using Xenopus oocytes (Webb and Nuccitelli, 1981). Thus, SN1 has the properties of a channel selective for H+.
Fig. 3. Protons gate and permeate the uncoupled SN1 conductance. (A and B) The analysis of induced currents (currents in the presence of amino acid from which currents in the absence of amino acid have been subtracted) shows that at pHo 8 (A, left), increasing concentrations of glutamine cause progressively larger inward currents under hyperpolarized conditions in oocytes expressing SN1 (96 mM Na+). However, glutamine also produces increasing outward currents under depolarized conditions, suggestive of a channel rather than a transporter. At pHo 7 (A, right), currents induced by glutamine in the same oocyte are reduced in magnitude. In addition, the reversal potential shifts from approximately –25 to approximately +30 mV, mean difference +51.7 ± 2.5 mV (n = 6 oocytes), as predicted by the Nernst equation for a H+-selective channel. (B) At pHo 8 (left), increasing Na+ concentrations activate progressively larger inward currents at hyperpolarized potentials and outward currents at depolarized potentials in the presence of 1 mM glutamine. Lowering the pHo to 7 (right) again shifts the reversal potential of the same oocyte by approximately +50 mV and reduces the size of the currents. However, the changes in Na+ do not influence reversal potential at either pHo, indicating that the conductance is relatively selective for H+. The left and right panels of (A) and (B) were derived from single oocytes, enabling direct comparison of the results at different pHos. Similar results were obtained in 2–6 experiments. (C) The Km of SN1 for Na+ does not vary with membrane potential. Values around the reversal potential were discarded due to the small size of the currents. In contrast, currents coupled to transport by SA1 exhibit a Km for Na+ that increases progressively with depolarization. The values indicate the mean ± SEM, n = 4 for each.
Secondly, H+ modulates gating of the SN1 conductance. Even though increasing external H+ concentrations (lowering pHo) should increase the size of inward H+ currents through the uncoupled conductance due to the increased driving force, low pHo reduces their magnitude (Figure 3A and B). However, the H+ exchange mechanism for SN1 predicts that just as amino acid and Na+ activate the currents associated with SN1, low pHo should reduce the conductance. The reduced currents at low pHo are thus consistent with gating of the conductance by transport.
To determine whether other cations also permeate the conductance associated with SN1, we varied the concentration of Na+ (Figure 3B). Similarly to glutamine, increasing Na+ concentrations activate outward currents at depolarizing potentials as well as inward currents at hyperpolarizing potentials. Unlike the changes in pHo, however, changes in external Na+ do not alter the reversal potential (Figure 3B), indicating that Na+, although required for transport by SN1, is relatively impermeant through the uncoupled conductance. The increase in current produced by Na+ is therefore attributable to increased activation of the uncoupled conductance rather than to permeation by Na+.
External amino acid substrates are also required to gate the H+ conductance associated with SN1 (Figure 3A). In the absence of external substrate, the reversal potential of oocytes expressing SN1 does not change from pHo 5 to 8 (data not shown). Similarly to glutamine, increasing concentrations of asparagine activate inward currents at negative potentials and outward currents at positive potentials (Figure 4A). A reduction in pHo from 8 to 7 also shifts the reversal potential by +58.3 ± 1.7 mV (n = 6), consistent with an H+-selective channel. Further, the currents produced by asparagine can exceed those produced by glutamine. Unlike the currents produced by glutamine, however, low pHo does not affect the size of currents due to asparagine at physiological Na+ concentrations (Figure 4A). Like glutamine, asparagine requires Na+ to produce the currents (Figure 4B) but the currents evoked by asparagine at pHo 8 saturate at very low concentrations of Na+ <3 mM. At pHo 7, the currents become much less sensitive to activation by Na+. Increasing Na+ concentrations restore the full amplitude of the currents observed at pHo 8 (Figure 4B), suggesting that the interaction between H+ and Na+ during activation of SN1 by asparagine is competitive.
Fig. 4. Asparagine produces a large uncoupled conductance extremely sensitive to Na+. The current–voltage relationship of induced currents shows that (A) asparagine produces larger inward and outward currents than glutamine, alanine or histidine (each 10 mM) at pHo 8 (left) in oocytes expressing SN1. However, the induced currents all reverse at approximately –10 mV. At pHo 7 (right), the reversal potential for the same oocyte shifts by +58.3 ± 1.7 mV (n = 6 oocytes). The lower pHo also reduces the size of the currents produced by glutamine, alanine and histidine, but not those produced by asparagine. (B) At pHo 8 (left), 3 mM Na+ produces saturating, large currents in the presence of 1 mM asparagine (currents in the absence of Na+ were subtracted). At pHo 7 (right), currents obtained from the same oocyte are reduced, and again shift dramatically in reversal potential. Increasing Na+ concentrations restore the size of the currents to those observed at pHo 8, supporting a competitive interaction between H+ and Na+. Na+ concentrations noted in the right panel also apply to the left. In (A) and (B), the left and right panels were derived from single oocytes, enabling direct comparison of the results at pH 8 and 7. Similar results were obtained in 2–6 independent experiments.
Properties of transport by SN1
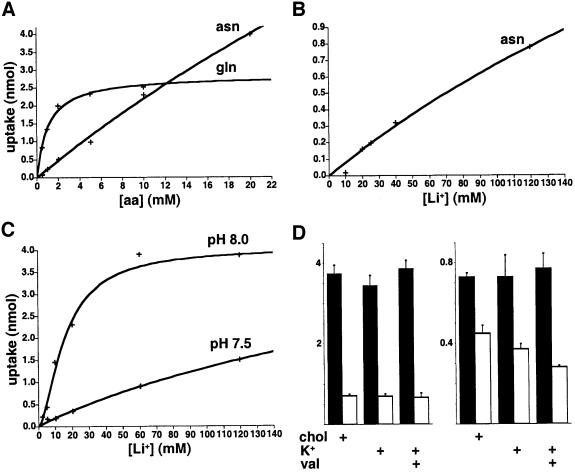
To re-examine the properties of transport by SN1 in light of the H+ conductance, we have measured amino acid uptake. Since Xenopus oocytes exhibit substantial background glutamine uptake (Taylor et al., 1989) and considerable between-batch and within-batch variation, we used PS120 cells stably expressing SN1, which confer highly reproducible transport activity (Chaudhry et al., 1999). Nonetheless, PS120 cells are defective in pH regulation (Pouysségur and Franchi, 1987), so we have confirmed the expression of similar pH-dependent amino acid transport in transiently transfected COS cells (data not shown). In addition, we have taken advantage of Li+ substitution to reduce background uptake by PS120 cells that does not tolerate substitution of Li+ for Na+ (Chaudhry et al., 1999). Figure 5A shows that SN1 transports glutamine with very different kinetics from asparagine. Increasing glutamine concentrations saturate transport by SN1 at much lower levels than asparagine (Figure 5A). Asparagine also exhibits a higher Vmax for uptake than glutamine. In addition, asparagine flux saturates at >120 mM cation (Li+) (Figure 5B) whereas the induced currents saturate at <3 mM Na+ (Figure 4B). [Li+ supports currents similar to those activated by Na+ (data not shown), indicating that this difference in activation does not reflect the use of different alkali cations.] Asparagine flux and currents activated by asparagine thus differ dramatically in their Na+ dependence.
Fig. 5. Kinetics of glutamine and asparagine transport by SN1. (A) Using Krebs–Ringer solution with Li+ substituting for Na+ (120 mM) to reduce background uptake due to other transport systems that do not tolerate Li+ substitution (Kilberg et al., 1980; Chaudhry et al., 1999), PS120 cells stably expressing SN1 exhibit uptake that saturates at much higher concentrations of asparagine (>20 mM) than glutamine (Km 0.96 ± 0.21 mM). Despite the lower apparent affinity for asparagine, the Vmax for asparagine (>>4 nmol/min) also exceeds that for glutamine (4.04 ± 0.74 nmol/min). Representative experiments are shown in (A–C), and the kinetic values in (A) and (B) derive from three independent experiments. (B) In contrast to the very high sensitivity of asparagine currents to Na+ (Figure 4B), the uptake of 1 mM [3H]asparagine saturates only at very high concentrations of Li+ (Km >120 mM). (C) External pH influences the Km of glutamine uptake for Li+. The Vmax does not differ substantially at the two pHos, suggesting that H+ competes with Li+ for activation of transport as well as with Na+ to activate currents (see Table I for kinetic values, the number of independent experiments and experiments at additional pHos). (D) Depolarization does not affect the uptake of [3H]glutamine (left) or [3H]asparagine (right). Using Li+ at 60 mM for all conditions and pHo 8, replacement of 60 mM choline by 60 mM K+ does not reduce glutamine transport, even in the presence of the K+ ionophore valinomycin to ensure depolarization. The black bars represent the PS120 cells expressing SN1 and the open bars untransfected PS120 cells. The values indicate the mean ± SEM, n = 6 for glutamine and n = 3 for asparagine.
We used the transport assay to characterize the interaction of H+ with SN1. An increase in external H+ concentration (decrease in pHo) lowers slightly the Vmax produced by saturating glutamine, from 4.37 ± 0.50 nmol/min at pHo 8 to 3.12 ± 0.65 nmol/min at pHo 7.5 (n = 3), and increases the Km modestly, from 1.12 ± 0.30 mM at pHo 8 to 3.31 ± 0.74 mM at pHo 7.5 (n = 3). When uptake is measured as a function of cation concentration, however, we find that a reduction in pHo from 8.5 to 7.0 progressively increases the Km for cation ∼6-fold without changing the Vmax >2-fold (Figure 5C, Table I), supporting a competition between Na+ and H+ to activate transport by SN1.
Table I. External pH influences the dependence of glutamine transport by SN1 on the cation.
| pHo | Km (mM) | Vmax (nmol/min) | n |
|---|---|---|---|
| 8.5 | 28 ± 12 | 5.6 ± 0.7 | 3 |
| 8.0 | 73 ± 7 | 6.1 ± 0.6 | 4 |
| 7.5 | >120 (269 ± 39) | >2 (2.5 ± 0.5) | 4 |
| 7.0 | >120 (171 ± 59) | >2 (3.0 ± 0.7) | 3 |
[3H]glutamine uptake was measured as a function of Li+ concen tration in PS120 cells expressing SN1, and the results fitted to Michaelis–Menten kinetics. The increasing Km (for Li+) with little change in Vmax (for saturating Li+ in 1 mM [3H]glutamine) at progressively lower pH suggests competition between Li+ and H+ for activation of transport by SN1. Since 120 mM was the highest concentration of Li+ used, saturation considerably above this level (at pHo 7.5 in particular) makes the Km and Vmax less certain. Nonetheless, even the results at low pHo fit Michaelis–Menten kinetics well, producing the kinetic values shown in parentheses. Values represent mean ± SEM.
To determine whether a proportion of the currents associated with SN1 might remain coupled to transport, we examined the effect of membrane potential on amino acid uptake. If transport were stoichiometrically coupled to the movement of charge across the plasma membrane, then amino acid flux should be voltage dependent. However, increased K+, with or without the K+ ionophore valinomycin to ensure depolarization, does not reduce the uptake of [3H]glutamine (Figure 5D, left), consistent with our previous work (Chaudhry et al., 1999). Although SN1 recognizes asparagine differently from glutamine, depolarization also fails to reduce the uptake of [3H]asparagine (Figure 5D, right). Rather, the background uptake of asparagine by endogenous transport systems (open bars) appears sensitive to depolarization. The insensitivity to membrane potential suggests that transport catalysed by SN1 is electroneutral.
To provide an additional test for the role of membrane potential in transport, we examined gating of the uncoupled currents since transport or at least the binding of Na+ and amino acid appears to gate the conductance. If transport is electrogenic and involves charge movement in the same direction as substrate, depolarizing potentials should increase the Km. Electrogenic transport by closely related SA1 indeed exhibits an increase in Km for Na+ with progressively depolarizing potentials (Figure 3C). In contrast, the currents associated with SN1 do not show any significant change in the Km for amino acid or cation at a wide range of membrane potentials, further supporting the electroneutrality of transport.
Simultaneous coupled and uncoupled H+ movement
Although we have used changes in pHi to monitor amino acid transport by SN1, H+ flux down its electrochemical gradient back into the cell may compensate for the efflux of H+ coupled to asparagine uptake, blunting the increase in pHi caused by substrate. To test this possibility, we have determined the relationship between coupled and uncoupled H+ movement by measuring simultaneously both the currents and pHi of oocytes expressing SN1. We used pHo 8 to maximize both currents and amino acid flux. At +30 mV, high concentrations of asparagine produce a small outward current and a large increase in pHi (Figure 6A). Since the current is small, the increase in pHi presumably reflects predominantly H+ efflux coupled to amino acid uptake. At –100 mV, asparagine produces more substantial inward currents and a less dramatic increase in pHi (Figure 6B). The inward H+ current at –100 mV thus reduces the increase in pHi relative to that observed at +30 mV. At –120 mV, the strong inwardly directed current virtually eliminates the rise in pHi (Figure 6C). The uncoupled H+ current can thus oppose the direction of coupled H+ flux.
Fig. 6. Coupled and uncoupled proton movement can flow in opposite directions. Oocytes injected with SN1 cRNA were incubated in BCECF-AM and simultanous recordings made of pHi and currents at pHo 8. (A) At +30 mV, asparagine (10 mM) induces outward currents and strong alkalinization, presumably due to the H+ efflux coupled to amino acid uptake plus the uncoupled H+ conductance. (B) At –100 mV, addition of asparagine to the same oocyte shows alkalinization but inward currents. An inwardly directed uncoupled H+ conductance presumably contributes to the reduced alkalinization by H+ efflux coupled to amino acid uptake. (C) At –120 mV, the oocyte shows even larger inward currents and barely detectable alkalinization after the addition of asparagine. In this case, the magnitude of the uncoupled inward H+ conductance presumably approaches that of the coupled outward H+ flux. V = voltage output of the p100s photomultiplier at 490 nm excitation, normalized to fluorescence at 440 nm.
SN1 transport in astrocytes
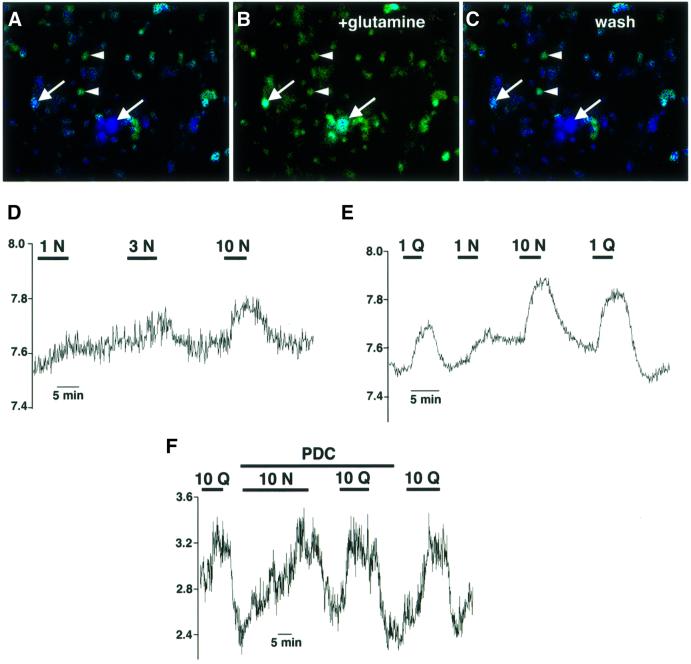
To determine whether SN1 regulates glutamine transport in native tissue as well as by heterologous expression, we used hippocampal cultures. Figure 7A–C demonstrates that glutamine reversibly increases the pHi of large, flat, presumably astrocytic cells, consistent with the H+ exchange mechanism of transport by SN1 and its expression by astrocytes (Nagaraja and Brookes, 1996; Chaudhry et al., 1999). Other cells in the culture including neurons (identified by their round soma and characteristic processes) do not show this alkalinization. In addition, the glutamate transport blocker PDC (l-trans-pyrrolidine-2,4-dicarboxylic acid; 300 µM) does not reduce the increases in pHi caused by substrate (Figure 7F), excluding a role for plasma membrane glutamate transporters in the pHi changes produced by glutamine and asparagine. At low concentrations, asparagine produces a smaller increase in pHi than glutamine (Figure 7D–F), as expected for SN1. Further, the effects of glutamine and asparagine appear to saturate at different concentrations (Figure 7D–F). The relative increase in pHi from 1 to 10 mM asparagine exceeds the difference between 1 and 10 mM glutamine. Saturation by asparagine at higher concentrations than glutamine parallels the saturation of SN1-mediated currents and flux by the two amino acids. SN1 expressed by astrocytes thus exhibits the same properties observed in heterologous expression systems.
Fig. 7. Different effects of asparagine and glutamine on the pHi of astrocytes implicate SN1. (A–C) A subset of hippocampal cells loaded with BCECF show an increase in pHi (arrows) on addition of 1 mM glutamine (A and B). Removal of glutamine restores the pHi to baseline (C). The large, flat and polygonal shape of these cells (revealed most clearly by phase contrast microscopy) indicates that they are astrocytes. Neuronal cells distinguished by their round soma and characteristic processes (arrowheads) do not change pHi in response to glutamine. (D and E) Asparagine (1 mM) increases the pHi of cultured astrocytes less than glutamine. However, in contrast to the changes in pHi produced by glutamine which saturate at lower concentrations, higher concentrations of asparagine (up to 10 mM) produce larger increases in pHi, consistent with the properties of SN1 we have observed in heterologous expression systems (Figures 4 and 5). (F) The glutamate transport blocker PDC (300 µM) has no effect on the pHi changes caused by asparagine or glutamine. Fluorescence (440/490 ratio) reflects the relative pHi.
Discussion
The results show that SN1 exhibits currents not stoichiometrically coupled to amino acid transport. The ratio of charge movement to substrate flux varies considerably, and approaches zero at high pHo, where transport is still robust. The uptake of radiolabelled asparagine may reflect only exchange with cytoplasmic amino acid, rather than net uptake. Since exchange is electroneutral, this could account for the low charge/flux ratios observed. However, closely related SA1 shows a fixed charge/flux ratio under all conditions tested. The addition of external amino acid to cells expressing SN1 also produces outward currents at depolarizing potentials, which is incompatible with inward charge movement accompanying amino acid uptake. Indeed, another report interpreting the currents associated with SN1 as electrogenic transport has already shown that glutamine produces outward currents at depolarizing potentials (Fei et al., 2000). SN1 thus exhibits an ionic conductance uncoupled from transport.
Protons permeate the uncoupled conductance associated with SN1. A reduction in pHo by 1 unit shifts the reversal potential by +52–58 mV, as predicted by the Nernst equation for an H+-selective channel. Consistent with this selectivity, changes in external Na+ and Cl– have no effect on the reversal potential. SN1 thus mediates the movement of H+ across the plasma membrane that is both coupled to and uncoupled from transport. The uncoupled as well as coupled H+ movement distinguishes SN1 from other transporters which exhibit channel-like function. Excita tory amino acid transporters, for example, exhibit an uncoupled conductance for Cl–, but Cl– is not stoichiometrically coupled to glutamate transport (Sonders and Amara, 1996). On the other hand, these carriers have been shown to exhibit a H+ conductance in the presence of arachidonic acid (Fairman et al., 1998; Tzingounis et al., 1998), and glutamate uptake is driven by the co-transport of H+ as well as Na+, setting a precedent for H+ permeation both coupled to and uncoupled from transport.
Although uncoupled from transport, the H+ conductance associated with SN1 appears to be gated by the transport cycle. No H+ conductance is detected in the absence of amino acid substrate. Na+ activates the conductance and, in addition to carrying the uncoupled currents, external H+ inhibits them. In addition, we have observed that different amino acid substrates gate the SN1 conductance to different extents. At high concentrations, asparagine produces larger currents than glutamine. Indeed, asparagine saturates transport by SN1 with a Km >10-fold higher than the Km for glutamine, and with a higher Vmax as well. The difference in currents produced by the two amino acids thus appears to parallel differences in the rates of amino acid flux. However, at low concentrations (1 mM), asparagine produces currents similar to glutamine despite lower levels of transport. Asparagine may therefore activate the currents more potently than glutamine, independently of transport rate. A similar but less dramatic difference exists between glutamate and aspartate in the gating of an uncoupled anion conductance associated with the plasma membrane excitatory amino acid transporters (Wadiche et al., 1995).
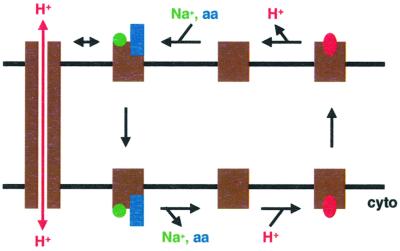
The currents produced by asparagine show a striking sensitivity to Na+. Less than 3 mM Na+ fully activates these currents. In contrast, Li+ activates asparagine uptake with a Km >120 mM. Since we have not observed any substantial difference in the activation of currents by Li+ and Na+ (data not shown), these differences in activation of transport and current suggest a fundamental difference between the activation of flux and conductance. In particular, the extreme Na+ sensitivity of asparagine-induced currents suggests that they associate with the state of the transport cycle after Na+ and amino acid have bound to SN1, but before translocation has occurred (Figure 8). Indeed, the intracellular Na+ concentration (∼10 mM) exceeds the low millimolar concentrations of Na+ that produce maximal asparagine-gated currents. Neither the relatively low asparagine concentration (1 mM) nor the low external Na+ could thus provide a driving force for amino acid uptake by SN1. The anion conductance observed with excitatory amino acid transporters also appears to associate with the state of the transport cycle with substrates bound to the external face of the carrier (Wadiche et al., 1995). Since low concentrations of asparagine (1 mM) induce currents similar to equal concentrations of glutamine despite lower rates of flux, SN1 may spend more time in this particular stage of the cycle with asparagine bound than with glutamine. The lower apparent affinity of asparagine uptake for both amino acid and cation may indeed reflect the slow rate of this transition. At high concentrations, asparagine causes larger currents than glutamine, presumably because SN1 transports asparagine more rapidly than glutamine when saturated with amino acid substrate.
Fig. 8. Relationship of the uncoupled conductance to coupled transport by SN1. Uptake by SN1 involves the binding of external Na+ and amino acid (aa) to the outwardly facing conformation of the transporter, followed by translocation across the membrane, release of Na+ and amino acids into the cytoplasm, and reorientation of the carrier to the extracellular face of the membrane by H+. Since very low Na+o concentrations (lower than Na+i), which do not support uptake, suffice to activate maximal currents in the presence of asparagine, the uncoupled H+ conductance (left) appears to associate with the step of the cycle in which Na+ and amino acids have bound but not yet translocated. The recognition of asparagine with low apparent affinity relative to glutamine may account for the more potent activation of currents than substrate flux at low concentrations of asparagine, due to a slower transition through the step of the transport cycle in which substrate translocates across the membrane. At high concentrations, asparagine produces larger currents than glutamine, presumably because the maximal turnover of the entire transport cycle is faster for asparagine than glutamine. The cycle is clearly reversible under physiological conditions, but the arrows indicate uptake since uptake rather than efflux appears to be associated with the uncoupled H+ conductance.
Although the currents associated with SN1 are not coupled stoichiometrically to transport, transport nonetheless may generate coupled charge movement that contributes to the observed currents. Indeed, the dependence of SN1 on Na+ may also be cooperative (Fei et al., 2000), suggesting a stoichiometry for Na+ of 2 or more that would make transport electrogenic if only one H+ were exchanged. Asparagine has also been suggested to produce inward currents even at depolarizing potentials in oocytes expressing SN1 (Fei et al., 2000), more consistent with electrogenic transport than an uncoupled conductance. However, we have found that, consistent with the predominantly uncoupled nature of the conductance, asparagine invariably produces outward currents at depolarizing potentials and pHo 8 (n >10). It is of course much more difficult to detect outward currents at pHo 7 due to the shift in reversal potential. Further supporting the electroneutrality of transport by SN1, we show that for SN1, depolarization does not reduce amino acid uptake. This does not exclude the possibility of electrogenic transport by SN1, because an electroneutral step may limit the rate of an electrogenic transport cycle. On the other hand, the closely related but electrogenic system A transporters SA1 and 2 are inhibited by depolarization (data not shown). In addition, the Km for SN1 appears to be independent of membrane potential, as expected for an electroneutral transporter. This also fails to exclude the possibility of a rate-limiting electroneutral step in an electrogenic transport cycle, but again the Km of closely related SA1 does vary with membrane potential. SN1 may thus be electroneutral.
Elimination of membrane potential from the driving force for uptake would contribute to the shallowness of the amino acid gradients produced by SN1. Indeed, we have observed flux reversal by SN1 at 400 µM external glutamine (Chaudhry et al., 1999), close to the level of extracellular glutamine observed in vivo (McGale et al., 1977; Hamberger and Nystrom, 1984). Assuming an intracellular concentration of 5–8 mM glutamine (Schousboe et al., 1979; Patel and Hunt, 1985; Storm-Mathisen et al., 1992), flux reversal thus occurs at a 12- to 20-fold gradient, consistent with the magnitude of the Na+ concentration gradient across the plasma membrane. However, the movement of one charge would add ∼14-fold to the concentration gradient of substrate accumulated at –70 mV (for a final gradient of ∼160-fold), making the observed efflux at resting potential unlikely. Similarly, a Na+ stoichiometry >1 would be difficult to reconcile with the observed flux reversal. However, more than one Na+ may be co-transported with amino acid (Fei et al., 2000), and the actual stoichiometry of Na+ and H+ coupling by SN1 remains unknown.
In contrast to SN1, the closely related Na+-dependent system A transporters SA1 and 2 do not couple H+ movement to amino acid flux. In addition, the system A transporters exhibit charge/flux ratios of ∼1.0–1.4 at all membrane potentials, and depolarization inhibits uptake (Chaudhry et al., 2001), indicating electrogenic transport. The loss of H+ coupling by SA1 and 2 may therefore contribute to their electrogenic nature, which in turn restricts efflux to depolarization far above resting potential. System A transporters are thus more likely to mediate the uptake of glutamine into cells than SN1, and the expression of SA1 and 2 on neurons together with SN1 on astrocytes presumably contributes to the directional transfer of glutamine from astrocytes to neurons required for the glutamine–glutamate cycle. The coupling of system N to both H+ exchange and Na+ co-transport further suggests that it may be an evolutionary intermediate between the related vesicular GABA transporter which is driven only by H+ exchange, and the system A transporters driven only by Na+ co-transport.
The results suggest that H+ competitively inhibits the activation of SN1 by Na+. Increasing Na+ concentrations restore both the glutamine- and asparagine-induced currents inhibited by low pHo. In addition, H+ competes with Li+ in the activation of asparagine uptake by SN1. We have also found that the closely related system A transporters exhibit competition between H+ and Na+ (Chaudhry et al., 2001). This family of proteins may therefore use a similar binding site for both cations. However, interaction with the two ions has very different functional consequences. In the case of SN1, Na+ binding promotes amino acid uptake. In contrast, H+ binding promotes amino acid efflux.
We have taken advantage of the observations in heterologous expression systems to assess the potential function of SN1 in brain astrocytes. Both asparagine and glutamine increase astrocyte pHi, as predicted by the H+ exchange mechanism of transport by SN1. The changes in astrocyte pHi also saturate at higher concentrations of asparagine than glutamine. Glial cells thus exhibit the same differential response to asparagine and glutamine as heterologous cell systems expressing SN1, consistent with the known expression of SN1 activity, mRNA and protein by astrocytes (Nagaraja and Brookes, 1996; Chaudhry et al., 1999).
What is the physiological significance of uncoupled as well as coupled H+ movement by SN1? Unlike Na+, K+ and Cl–, which form large (high mM), stable concentration gradients across the plasma membrane, H+ occurs at much lower concentrations (100 nM at pH 7). Transport coupled to H+ as well as Na+ may therefore drive changes in cell pH, and the high rates of amino acid uptake catalysed by SN1 do indeed increase pHi. However, the uncoupled conductance associated with SN1 allows H+ to run down its electrochemical gradient back into the cell and replenish the H+ lost through amino acid uptake. Supporting this role, we have found that inward currents carried by H+ at polarizing potentials blunt the increases in pHi produced by amino acid. The uncoupled inward movement of H+ may therefore serve to buffer changes in pHi produced by coupled H+ efflux. If the uncoupled currents were present constitutively, however, the cell might acidify excessively. Activation by amino acid substrate, which increases pHi through coupled H+ efflux, thus confers the appropriate regulation. Further, inward H+ flux through the uncoupled conductance would help to drive glutamine uptake by the coupled mechanism of H+ exchange. In times of abundant glutamine, this function would produce higher intracellular concentrations of glutamine. Conversely, inactivation of the conductance at low external glutamine concentrations would promote the efflux of glutamine required for nitrogen metabolism by the liver and for glutamate production by neurons.
Materials and methods
Oocyte expression
Methods for Xenopus laevis oocyte expression were as previously described (Quick and Lester, 1994). Briefly, adult frogs were anaesthetized with cold 3-aminobenzoic acid ethyl ester. Unfertilized stage V and VI oocytes were surgically removed from the ovaries and gently agitated for 30–45 min at room temperature in OR-Mg solution (82 mM NaCl, 2 mM KCl, 20 mM MgCl2 and 5 mM HEPES pH 7.4) containing 2 mg/ml collagenase A. The defolliculated oocytes were then washed in enzyme-free OR-Mg, and injected with 1–15 ng of capped SN1 cRNA (prepared from the rat SN1 cDNA in the pGEMHE vector; Liman et al., 1992) or similar volumes of diethylpyrocarbonate (DEPC)-treated water. The oocytes were then incubated at 18°C for 2–6 days in ND96 (96 mM NaCl, 2 mM KCl, 1.8 mM CaCl2, 1 mM MgCl2 and 5 mM HEPES pH 7.5) containing 50 µg/ml gentamycin, 2.5 mM sodium pyruvate, 5% heat-inactivated horse serum and 5 mM theophylline.
Electrophysiology
Two-electrode voltage clamp recordings were performed at room temperature using GeneClamp 500B. All voltage clamp experiments used ND96 as perfusate, except where noted in the text. In addition, choline chloride was used to maintain the osmolarity and the chloride concentration of NaCl and LiCl below 96 mM. Voltage pulse protocols started from a holding potential of –50 mV and involved 1 s steps ranging from –150 to +50 mV in 20 mV increments, with 1.5 s interpulse intervals. Signals were filtered using a four-pole low-pass Bessel filter set at a 100 Hz cut-off prior to sampling at 1000 Hz. Water-injected and uninjected oocytes were used as controls, and underwent the same preparation as transcript-injected oocytes. To determine the total charge moved in response to asparagine application, the mean asparagine-induced current was multiplied by the time of application (10 min). Representative traces and current–voltage relationships are shown from experiments performed on at least three oocytes.
pHi measurement
Stably transfected PS120 cells expressing SN1 were grown and imaged as previously described (Chaudhry et al., 1999). Briefly, the cells were loaded for 10 min with 5 mM BCECF-AM, washed for 20 min in Krebs–Ringer solution pH 7.4 (120 mM NaCl, 4.7 mM KCl, 2.2 mM CaCl2, 1.2 mM MgSO4, 1.2 mM KH2PO4, 10 mM HEPES, 0.18% glucose) and pHi determined by ratiometric imaging at 440 and 490 nm excitation. Calibration was performed using 50 µM nigericin in Krebs– Ringer solution that contains 90 mM KCl (Krizaj and Copenhagen, 1998). The hippocampal cultures were loaded with BCECF, imaged and calibrated similarly. Xenopus oocytes were imaged at 490 nm excitation, subtracting background fluorescence at 440 nm (which did not change during amino acid addition).
Transport assays
Transport by PS120 cells was measured as previously described (Chaudhry et al., 1999). Briefly, cells expressing SN1 were pre-incubated for 10 min at 37°C in Krebs–Ringer solution at pH 6.5 with choline chloride replacing NaCl, then incubated in Krebs–Ringer at pH 8.0 containing LiCl (in place of choline chloride) and radiolabelled amino acid, except where indicated in the text. Choline chloride was also used to maintain the osmolarity and the chloride concentration of NaCl and LiCl below 120 mM. Kinetic measurements were all made during the early phase of linear amino acid uptake (usually at 1 min). The reactions were terminated by two cold washes in Krebs–Ringer solution pH 8 containing choline, the cells lysed in 1% SDS and the radioactivity was measured by scintillation counting in 2.5 ml of Ecolume (ICN). Transport was measured in duplicate on at least three independent occasions, and the uptake by untransfected PS120 cells under the same conditions subtracted from each value to determine the SN1-mediated flux. In a single experiment, the averaged uptake by untransfected cells was subtracted from the averaged uptake by SN1-expressing cells, and the values obtained from multiple experiments then used to obtain the mean and standard error. The flux measurements were fit to Michaelis–Menten kinetics using Ultrafit (Biosoft). Oocyte transport was measured at different holding potentials in ND96 (at different pHos) containing 250 µM [3H]asparagine, and the oocytes were solubilized in 1% SDS before scintillation counting. After subtracting the asparagine uptake by uninjected oocytes, the charge/flux ratio was determined by dividing the total current induced by asparagine (measured as above) by the SN1-mediated asparagine flux.
Acknowledgments
Acknowledgements
We thank A.Gray, M.Dresser, N.Zerangue, D.Schmitz, S.-E.Jordt and the members of the Edwards, Copenhagen and Kavanaugh laboratories for helpful discussion, D.Barber for the PS120 cells, E.Schnell for hippocampal cultures, and the Norwegian Research Council (F.A.C., J.S.-M.), Research to Prevent Blindness (D.R.C.), the NEI (D.R.C.), the Wheeler Center for the Study of Addiction (D.R.C., R.H.E.) and NINDS (D.R.C., R.H.E.) for their support.
References
- Barnett N.L., Pow,D.V. and Robinson,S.R. (2000) Inhibition of Müller cell glutamine synthetase rapidly impairs the retinal response to light. Glia, 30, 64–73. [DOI] [PubMed] [Google Scholar]
- Bender D.A. (1975) Amino Acid Metabolism. John Wiley & Sons, London.
- Chaudhry F.A., Lehre,K.P., van Lookeren Campagne,M., Otterson,O.P., Danbolt,N.C. and Storm-Mathisen,J. (1995) Glutamate transporters in glial plasma membranes: highly differentiated localizations revealed by quantitative ultrastructural immunocytochemistry. Neuron, 15, 711–720. [DOI] [PubMed] [Google Scholar]
- Chaudhry F.A., Reimer,R.J., Krizaj,D., Barber,D., Storm-Mathisen,J., Copenhagen,D.R. and Edwards,R.H. (1999) Molecular analysis of system N suggests novel physiological roles in nitrogen metabolism and synaptic transmission. Cell, 99, 769–780. [DOI] [PubMed] [Google Scholar]
- Chaudhry F.A., Schmitz,D., Reimer,R.J., Larsson,P., Gray,A.T., Nicoll,R., Kavanaugh,M. and Edwards,R.A. (2001) Glutamine uptake by neurons: interaction of protons with System A transporters. J. Neurosci., in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti F. and Minelli,A. (1994) Glutamate immunoreactivity in rat cerebral cortex is reversibly abolished by 6-diazo-5-oxo-l-norleucine. J. Histochem. Cytochem., 42, 717–726. [DOI] [PubMed] [Google Scholar]
- Fairman W.A., Sonders,M.S., Murdoch,G.H. and Amara,S.G. (1998) Arachidonic acid elicits a substrate-gated proton current associated with the glutamate transporter EAAT4. Nature Neurosci., 1, 105–113. [DOI] [PubMed] [Google Scholar]
- Fei Y.J., Sugawara,M., Nakanishi,T., Huang,W., Wang,H., Prasad,P.D., Leibach,F.H. and Ganapathy,V. (2000) Primary structure, genomic organization and functional and electrogenic characteristics of human system N1, a Na+- and H+-coupled glutamine transporter. J. Biol. Chem., 275, 23707–23717. [DOI] [PubMed] [Google Scholar]
- Hamberger A. and Nystrom,B. (1984) Extra- and intracellular amino acids in the hippocampus during development of hepatic encephalopathy. Neurochem. Res., 9, 1181–1192. [DOI] [PubMed] [Google Scholar]
- Hamberger A.C., Chiang,G.H., Nylen,E.S., Scheff,S.W. and Cotman,C.W. (1979) Glutamate as a CNS transmitter. I. Evaluation of glucose and glutamine as precursors for the synthesis of preferentially released glutamate. Brain Res., 168, 513–530. [DOI] [PubMed] [Google Scholar]
- Haussinger D. (1990) Nitrogen metabolism in the liver: structural and functional organization and physiological relevance. Biochem. J., 267, 281–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanner B.I. (1994) Sodium-coupled neurotransmitter transport: structure, function and regulation. J. Exp. Biol., 196, 237–249. [DOI] [PubMed] [Google Scholar]
- Kilberg M.S., Handlogten,M.E. and Christensen,H.N. (1980) Characteristics of an amino acid transport system in rat liver for glutamine, asparagine, histidine and closely related analogs. J. Biol. Chem., 255, 4011–4019. [PubMed] [Google Scholar]
- Krizaj D. and Copenhagen,D.R. (1998) Compartmentalization of calcium extrusion mechanisms in the outer and inner segments of photoreceptors. Neuron, 21, 249–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laake J.H., Slyngstad,T.A., Haug,F.M. and Ottersen,O.P. (1995) Glutamine from glial cells is essential for the maintenance of the nerve terminal pool of glutamate: immunogold evidence from hippocampal slice cultures. J. Neurochem., 65, 871–881. [DOI] [PubMed] [Google Scholar]
- Liman E.R., Tytgat,J. and Hess,P. (1992) Subunit stoichiometry of a mammalian K+ channel determined by construction of multimeric cDNAs. Neuron, 9, 861–871. [DOI] [PubMed] [Google Scholar]
- McGale E.H., Pye,I.F., Stonier,C., Hutchinson,E.C. and Aber,G.M. (1977) Studies of the inter-relationship between cerebrospinal fluid and plasma amino acid concentrations in normal individuals. J. Neurochem., 29, 291–297. [DOI] [PubMed] [Google Scholar]
- Nagaraja T.N. and Brookes,N. (1996) Glutamine transport in mouse cerebral astrocytes. J. Neurochem., 66, 1665–1674. [DOI] [PubMed] [Google Scholar]
- Patel A.J. and Hunt,A. (1985) Concentration of free amino acids in primary cultures of neurones and astrocytes. J. Neurochem., 44, 1816–1821. [DOI] [PubMed] [Google Scholar]
- Pouysségur J. and Franchi,A. (1987) Use of tritium-labeled precursors to select mutants. Methods Enzymol., 151, 131–139. [DOI] [PubMed] [Google Scholar]
- Pow D.V. and Robinson,S.R. (1994) Glutamate in some retinal neurons is derived solely from glia. Neuroscience, 60, 355–366. [DOI] [PubMed] [Google Scholar]
- Quick M.W. and Lester,H.A. (1994) Methods for expression of excitability proteins in Xenopus oocytes. In Conn,P.M. (ed.) Methods in Neuroscience. Vol. 19. Academic Press, San Diego, CA, pp. 261–279.
- Reimer R.J., Chaudhry,F.A., Gray,A.T. and Edwards,R.H. (2000) Amino acid transport system A resembles system N in sequence but differs in mechanism. Proc. Natl Acad. Sci. USA, 97, 7715–7720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein J.D. and Tabakoff,B. (1984) Alteration of striatal glutamate release after glutamine synthetase inhibition. J. Neurochem., 43, 1438–1446. [DOI] [PubMed] [Google Scholar]
- Rothstein J.D., Martin,L., Levey,A.I., Dykes-Hoberg,M., Jin,L., Wu,D., Nash,N. and Kuncl,R.W. (1994) Localization of neuronal and glial glutamate transporters. Neuron, 13, 713–725. [DOI] [PubMed] [Google Scholar]
- Schousboe A., Hertz,L., Svenneby,G. and Kvamme,E. (1979) Phosphate activated glutaminase activity and glutamine uptake in primary cultures of astrocytes. J. Neurochem., 32, 943–950. [DOI] [PubMed] [Google Scholar]
- Seal R.P. and Amara,S.G. (1999) Excitatory amino acid transporters: a family in flux. Annu. Rev. Pharmacol. Toxicol., 39, 431–456. [DOI] [PubMed] [Google Scholar]
- Sonders S. and Amara,S.G. (1996) Channels in transporters. Curr. Opin. Neurobiol., 6, 294–302. [DOI] [PubMed] [Google Scholar]
- Storm-Mathisen J., Danbolt,N.C., Rothe,F., Torp,R., Zhang,N., Aas,J.E., Kanner,B.I., Langmoen,I. and Ottersen,O.P. (1992) Ultrastructural immunocytochemical observations on the localization, metabolism and transport of glutamate in normal and ischemic brain tissue. Prog. Brain Res., 94, 225–241. [DOI] [PubMed] [Google Scholar]
- Sugawara M., Nakanishi,T., Fei,Y.-J., Huang,H., Ganapathy,M., Leibach,F.H. and Ganapathy,V. (2000) Cloning of an amino acid transporter with functional characteristics and tissue expression pattern identical to that of system A. J. Biol. Chem., 275, 16473–16477. [DOI] [PubMed] [Google Scholar]
- Taylor P.M., Hundal,H.S. and Rennie,M.J. (1989) Transport of glutamine in Xenopus laevis oocytes: relationship with transport of other amino acids. J. Membr. Biol., 112, 149–157. [DOI] [PubMed] [Google Scholar]
- Thanki C.M., Sugden,D., Thomas,A.J. and Bradford,H.F. (1983) In vivo release from cerebral cortex of [14C]glutamate synthesized from [U-14C]glutamine. J. Neurochem., 41, 611–617. [DOI] [PubMed] [Google Scholar]
- Tzingounis A.V., Lin,C.L., Rothstein,J.D. and Kavanaugh,M.P. (1998) Arachidonic acid activates a proton current in the rat glutamate transporter EAAT4. J. Biol. Chem., 273, 17315–17317. [DOI] [PubMed] [Google Scholar]
- Varoqui H., Zhu,H., Yao,D., Ming,H. and Erickson,J.D. (2000) Cloning and functional identification of a neuronal glutamine transporter. J. Biol. Chem., 275, 4049–4054. [DOI] [PubMed] [Google Scholar]
- Wadiche J.I., Amara,S.G. and Kavanaugh,M.P. (1995) Ion fluxes associated with excitatory amino acid transport. Neuron, 15, 721–728. [DOI] [PubMed] [Google Scholar]
- Webb D.J. and Nuccitelli,R. (1981) Direct measurement of intracellular pH changes in Xenopus eggs at fertilization and cleavage. J. Cell Biol., 91, 562–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao D., Mackenzie,B., Ming,H., Varoqui,H., Zhu,H., Hediger,M.A. and Erickson,J.D. (2000) A novel system A isoform mediating Na+/neutral amino acid cotransport. J. Biol. Chem., 275, 22790–22797. [DOI] [PubMed] [Google Scholar]