Abstract
The negative regulator Cbl functions as a ubiquitin ligase towards activated receptor tyrosine kinases and facilitates their transport to lysosomes. Whether Cbl ubiquitin ligase activity mediates its negative regulatory effects on cytoplasmic tyrosine kinases of the Syk/ZAP-70 family has not been addressed, nor is it known whether these kinases are regulated via ubiquitylation during lymphocyte B-cell receptor engagement. Here we show that B-cell receptor stimulation in Ramos cells induces the ubiquitylation of Syk tyrosine kinase which is inhibited by a dominant-negative mutant of Cbl. Intact tyrosine kinase-binding and RING finger domains of Cbl were found to be essential for Syk ubiquitylation in 293T cells and for in vitro Syk ubiquitylation. These same domains were also essential for Cbl-mediated negative regulation of Syk as measured using an NFAT-luciferase reporter in a lymphoid cell. Association with Cbl did not alter the kinase activity of Syk. Altogether, our results support an essential role for Cbl ubiquitin ligase activity in the negative regulation of Syk, and establish that ubiquitylation provides a mechanism of Cbl-mediated negative regulation of cytoplasmic targets.
Keywords: activation/lymphocyte/regulation/tyrosine kinase/ubiquitylation
Introduction
Activation of membrane-associated Src family protein tyrosine kinases (PTKs) followed by recruitment and activation of cytoplasmic PTKs of the Syk/ZAP-70 family is an early event in lymphocyte activation (Bubeck-Wardenburg et al., 1999; van Leeuwen and Samelson, 1999). Activation of Syk and ZAP-70 is essential for signaling downstream of the B- and T-cell antigen receptor, respectively. ZAP-70 is restricted to T lymphocytes and natural killer (NK) cells, and its deficiency in mice or humans results in aberrant T-cell development, with peripheral T cells that are unable to respond to receptor stimulation (Arpaia et al., 1994; Gelfand et al., 1995; Negishi et al., 1995). In addition to B lymphocytes, Syk is also expressed in thymocytes, monocytes, neutrophils and platelets (Chan et al., 1994). While Syk deficiency in mice also results in aberrant B-cell development, the mice exhibit perinatal lethality due to defective platelet function (Cheng et al., 1995; Turner et al., 1995, 1997). Mature B cells lacking Syk are unresponsive to B-cell receptor (BCR) stimulation. Given the vital role of the Syk/ZAP-70 PTKs in initiating lymphocyte activation, appropriate regulation of these kinases is essential to ensure a physiological immune response and to prevent autoimmunity. The biochemical mechanisms that control the function of Syk/ZAP-70 PTKs are not well understood. Recent studies, however, have indicated that the proto-oncogene product Cbl is involved in negative regulation of the Syk/ZAP-70 PTKs.
Cbl is a member of an evolutionarily conserved family of multidomain signaling proteins that have emerged as negative regulators of PTKs (Miyake et al., 1997; Lupher et al., 1999; Thien and Langdon, 2001). Genetic analyses have established that Caenorhabditis elegans and Drosophila Cbl homologs negatively regulate the epidermal growth factor receptor (EGFR)-mediated developmental pathways (Yoon et al., 1995; Meisner et al., 1997). Furthermore, genetic ablation of murine Cbl resulted in hypercellularity and altered development of several organ systems (Murphy et al., 1998; Naramura et al., 1998), whereas Cbl-b deletion led to immune cell hyperproliferation and hyperactivation resulting in autoimmunity (Chiang et al., 2000; Krawczyk et al., 2000).
Structurally, Cbl family proteins share a conserved N-terminal region corresponding to sequences retained in the transforming v-cbl oncogene (Lupher et al., 1999). This region provides a tyrosine kinase-binding (TKB) interface (Lupher et al., 1996), and is itself composed of a four-helical bundle, a calcium-binding EF hand motif and an incomplete SH2 domain (Meng et al., 1999). A second evolutionarily conserved region corresponding to the RING finger (RF) domain recently has been demonstrated to interact with ubiquitin conjugating enzymes (UBCs) (Zheng et al., 2000). Cbl and some of the family members also contain a proline-rich region for interaction with SH3 domain-containing proteins, a C-terminal leucine zipper and multiple tyrosine phosphorylation sites that mediate interactions with SH2 domain-containing proteins (Lupher et al., 1999)
Initial insights into the biochemical basis for the negative regulatory role of Cbl have come from studies of receptor tyrosine kinases (RTKs), such as the platelet-derived growth factor receptor (PDGFR) and the EGFR. These analyses have demonstrated that Cbl binds to activated RTKs via its TKB domain and targets them for ubiquitylation by the RF-associated ubiquitin conjugation (UBC) enzymes. Ubiquitylation in turn enhances the efficiency with which ligand-activated receptors are sorted to lysosomes for degradation by lysosomal enzymes (Levkowitz et al., 1998; Miyake et al., 1998; Joazeiro et al., 1999; Lill et al., 2000). Whether ubiquitylation of the cytoplasmic regions of RTKs also functions as a signal for proteasomal degradation remains unclear, nor is the mechanism of ubiquitin-dependent lysosomal sorting understood at present.
The TKB domain of Cbl directly binds to the negative regulatory phosphorylation site within the SH2–kinase linker region in ZAP-70 (Tyr292) and Syk (Tyr323) PTKs (Lupher et al., 1997, 1998; Deckert et al., 1998). This provides a basis for the selective recruitment of Cbl to activated pools of these PTKs. The tyrosine to phenylalanine mutations of these negative regulatory phosphorylation sites render ZAP-70 and Syk hyperactive in vivo when expressed in lymphoid cells, while the in vitro kinase activity of ZAP-70-Y292F was unchanged (Kong et al., 1996; Zhao and Weiss, 1996; Keshvara et al., 1998). These findings suggested that Cbl functions as a negative regulator of activated Syk/ZAP-70 PTKs. Indeed, overexpression of Cbl in COS cells led to a marked reduction of the kinase-active, phosphorylated pool of co-expressed Syk or ZAP-70 (Lupher et al., 1998; Rao et al., 2000). Similarly, overexpression of Syk in the mast cell line RBL-2H3 led to reduced autophosphorylation of co-expressed Syk and concomitant inhibition of Syk kinase activity (Ota and Samelson, 1997). Importantly, a TKB domain-inactivating mutation (G306E), corresponding to a loss-of-function mutation in the C.elegans Cbl homolog SLI-1, abrogated the effect of Cbl on the Syk/ZAP-70 PTKs in COS cells (Lupher et al., 1998; Rao et al., 2000); conversely, Syk Y323F and ZAP-70 Y292F mutants were resistant to Cbl-induced negative regulation.
Demonstration of the ubiquitin ligase activity of Cbl toward RTKs, together with the requirement of the Cbl RF domain for negative regulation of Syk (Ota et al., 2000), has raised the possibility that cytoplasmic PTKs such as Syk/ZAP-70 may also be targets of Cbl-dependent ubiquitylation. This unresolved question is of importance for a number of reasons. First, ubiquitylation of RTKs, by analogy to yeast transmembrane proteins regulated by ubiquitylation (Hicke, 1997), is thought to facilitate lysosomal sorting, followed by receptor degradation in the lysosome. In contrast, if cytoplasmic PTKs undergo ubiquitylation, these proteins are likely to become direct substrates for proteasome degradation. Secondly, while Cbl-induced reduction of Syk and ZAP-70 protein levels in COS cells is consistent with their being potential targets of Cbl-dependent ubiquitylation, other findings support alternative mechanisms. For example, Ota and Samelson (1997) noted that Cbl overexpression in RBL 2H3 cells did not decrease the Syk protein levels but rather reduced the kinase activity. Another study showed that ZAP-70 levels were reduced upon activation of certain T-cell clones but ascribed this reduction of ZAP-70 to cleavage by calpain, although the role of Cbl in this process was not investigated (Penna et al., 1999). Thirdly, it has been argued that Cbl TKB domain binding to the cytosolic adaptor protein BLNK could compete with the binding of a positive effector protein to an adjacent phosphotyrosine motif on BLNK (Yasuda et al., 2000). An analogous mechanism could be postulated for Cbl regulation of Syk/ZAP-70 since the Cbl TKB domain-binding site in the SH2–kinase linker region is adjacent to other potential effector binding sites. For example, Tyr315 in ZAP-70 and Tyr348 in Syk interact with Vav (Wu et al., 1997), and Tyr319 in ZAP-70 interacts with Lck and phospholipase Cγ1 (Pelosi et al., 1999; Williams et al., 1999). Thus, it remains possible that Cbl-dependent regulation of cytoplasmic PTKs may be fundamentally different from its effects on RTKs. Therefore, it is imperative to examine directly if Cbl-mediated ubiquitylation and degradation underly the negative regulation of Syk/ZAP-70 function by Cbl.
Here, we have addressed this hypothesis in the context of Syk by performing analyses in lymphoid cells as well as in a reconstituted 293T cell system, utilizing Cbl and its ubiquitin ligase-deficient mutants. We demonstrate that Syk is indeed a target of ubiquitylation in antigen receptor-stimulated B cells and that Cbl mediates this ubiquitylation. We establish that Cbl TKB domain-mediated association with Syk, as well as a functional Cbl ubiquitin ligase domain are required for Syk ubiquitylation as well as for Cbl-mediated negative regulation of Syk function in a lymphoid cell.
Results
Cbl does not reduce the kinase activity of Syk
While previous studies concur that Cbl functions as a negative regulator of Syk, the mechanistic basis of this regulation has been controversial. Based on our studies in COS and 293T cells demonstrating a loss of Syk protein upon its association with Cbl (Ota et al., 2000; Rao et al., 2000) together with the recent identification of Cbl ubiquitin ligase activity (Joazeiro et al., 1999; Levkowitz et al., 1999), we hypothesized that Cbl may regulate activated Syk via ubiquitylation and degradation. In contrast, studies using vaccinia virus overexpression in RBL-2H3 mast cells concluded that Cbl-induced inhibition of FcεR1-induced Syk activation was due to inhibition of Syk activity without any change in Syk protein levels (Ota and Samelson, 1997). To date, no direct experiments to resolve this disparity have been carried out. As it was our goal to investigate the possible role of ubiquitylation in Cbl-mediated Syk regulation, we considered it important to assess whether Cbl affects the kinase activity of Syk. Since analysis of the kinase activity of the total pool of Syk would show a reduction regardless of the two mechanisms proposed above, we took advantage of the fact that, unlike wild-type Cbl, the Cbl-70Z mutant associates with Syk but does not negatively regulate it (Ota et al., 2000). Therefore, we sought to compare the kinase activity of Syk protein directly associated with wild-type Cbl versus that associated with the Cbl-70Z mutant.
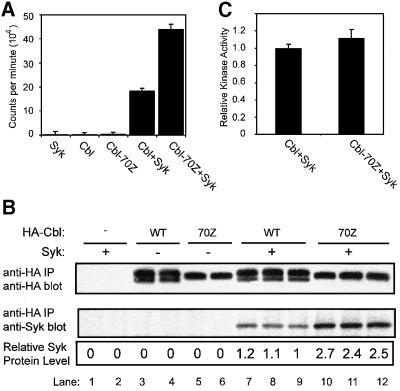
293T cells were transfected with Syk in combination with wild-type Cbl or the Cbl mutant 70Z, and cell lysates were subjected to anti-hemagglutinin (HA) immunoprecipitations. Immunoprecipitates were divided equally; half of each sample was subjected to an in vitro kinase assay and the other half was analyzed by SDS–PAGE followed by immunoblotting to assess the expression of introduced proteins and the levels of Cbl-associated Syk protein. As expected, anti-HA immunoprecipitates from lysates of cells transfected with Syk, Cbl or 70Z alone revealed negligible kinase activity (Figure 1A). However, anti-HA immunoprecipitates from lysates of cells co-transfected with Syk and either Cbl or 70Z exhibited significant kinase activity, with the activity associated with 70Z Cbl ∼2-fold more compared with that associated with Cbl (Figure 1A, mean of 43 617 c.p.m. with Cbl-70Z versus 18 929 c.p.m. for Cbl). As anticipated (Ota et al., 2000), the actual amount of Syk protein co-immunoprecipitated with wild-type Cbl was ∼2.5-fold lower compared with that associated with 70Z (Figure 1B). Normalization of the Syk kinase activity based on the amount of co-immunoprecipitated Syk protein demonstrated that there was no significant difference in Syk kinase activity associated with wild-type Cbl versus Cbl-70Z (Figure 1C). These results strongly indicated that the negative regulatory effect of Cbl on Syk was not a result of the inhibition of Syk kinase activity, and provided a further rationale to assess if Cbl regulates Syk through ubiquitylation.
Fig. 1. Comparable kinase activity of Syk associated with wild-type Cbl and Cbl-70Z mutant. 293T cells were transfected with plasmids encoding wild-type (WT) HA-Cbl, HA-Cbl-70Z (1 µg) or Syk (0.15 µg), as indicated, together with a CD8-ζ (0.5 µg) expression plasmid. The cells were lysed 48 h post-transfection and HA-Cbl proteins were immunoprecipitated (IP) from 1 mg of lysate using the anti-HA antibody. Following washing, half of the immunoprecipitates was used for in vitro kinase assays (A) and the other half was used for anti-HA and anti-Syk immunoblotting (B). The kinase activity was assayed by measuring the incorporation of 32P signal of [γ-32P]ATP into a synthetic substrate (Raytide™). Results in (A) are expressed as the mean ± 1 SD of two or three replicates. Syk protein levels (B, bottom panel) were quantified by densitometry and used to normalize Syk kinase activities in (A). The normalized kinase activity associated with wild-type Cbl versus 70Z is shown in (C). The results shown are representative of three experiments.
Cbl-mediated ubiquitylation of Syk in 293T cells and requirement for an intact Cbl RF domain
Our earlier studies in COS and 293T cells demonstrated that Cbl induces a reduction in Syk protein levels (Lupher et al., 1998; Ota et al., 2000). Therefore, we used this system as a first test to assess if Cbl can induce the ubiquitylation of Syk. 293T cells were co-transfected with Syk and either green fluorescent protein (GFP; control) or GFP-tagged Cbl, together with a plasmid encoding HA-tagged ubiquitin to facilitate detection of ubiquitylated Syk. Prior to harvesting, the cells were treated for 5 h with the proteasome inhibitor MG132 or dimethylsulfoxide (DMSO) control. Cell lysates were subjected to anti-Syk immunoprecipitations and immunoblotted with anti-HA antibody to detect ubiquitylated Syk (Figure 2A).
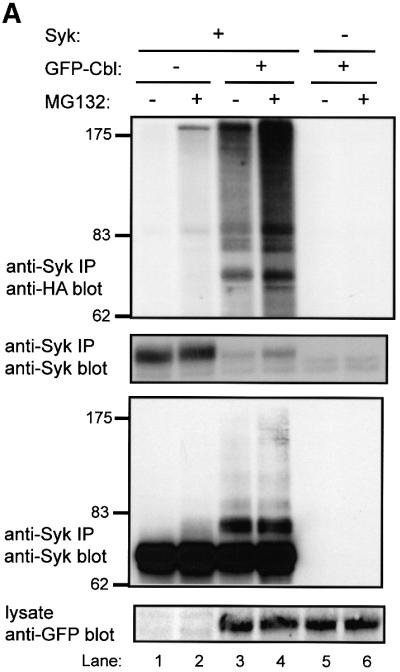
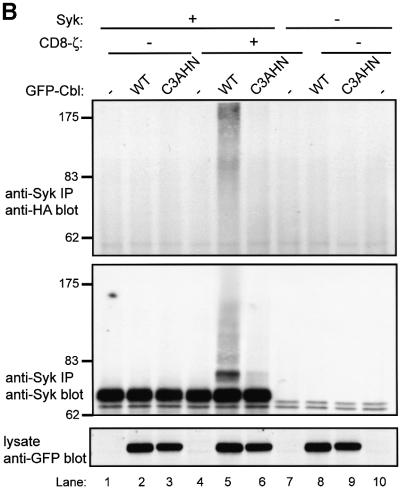
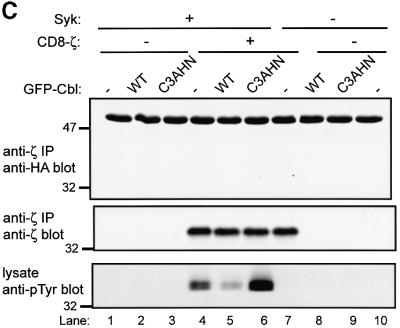
Fig. 2. Cbl-dependent ubiquitylation of Syk in 293T cells and the requirement for an intact Cbl RING finger domain. 293T cells were transfected with 0.15 µg of Syk (+) or vector control (–) together with 3 µg of GFP–Cbl (+) or vector control (–). Each transfection also included plasmids encoding HA-ubiquitin (7 µg) and CD8-ζ (0.5 μg). In (A), the cells were treated with 50 µM MG132 (+) or DMSO control (–) for 5 h prior to harvesting the cells. Anti-Syk IPs from 750 µg aliquots of lysate protein were resolved by SDS–PAGE and immunoblotted with anti-HA antibody (top panel) followed by anti-Syk (second panel), and anti-Syk immunoprecipitates from 1.5 mg aliquots of lysate protein were immunoblotted with anti-Syk (third panel). In (B and C), the cells were transfected with 3 µg of wild-type GFP–Cbl (WT), RING finger mutant C3AHN or vector control (–). In (B), anti-Syk IPs from 750 µg aliquots of lysate protein were resolved by SDS–PAGE and immunoblotted with anti-HA antibody (top panel) followed by anti-Syk antibody (middle panel). Aliquots of cell lysates (25 µg) were immunoblotted directly with an anti-GFP antibody to visualize GFP–Cbl (bottom panel). GFP alone, which runs at a lower molecular weight, is not shown. In (C), anti-ζ IPs from 1.5 mg aliquots of lysate protein were immunoblotted with anti-HA antibody (top panel) followed by anti-ζ antibody (middle panel). Aliquots of cell lysates (25 µg) were immunoblotted directly with an anti-pTyr antibody to visualize phosphorylated CD8-ζ. Size markers (kDa) are indicated on the left.
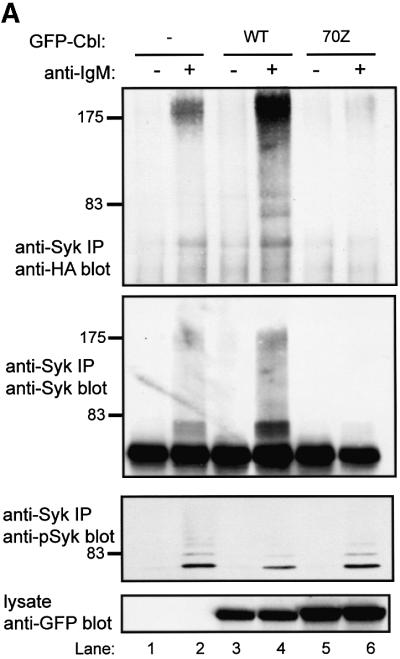
Relatively little ubiquitin signal was observed on Syk in the absence of co-transfected Cbl. In contrast, co-expression of GFP–Cbl led to easily detectable Syk ubiquitylation, which was accompanied by an expected decrease in the level of Syk protein (Figure 2A, compare lane 1 with lane 3). Pre-treatment of cells with MG132 prior to lysis resulted in marked accumulation of ubiquitylated Syk and an increase in the level of Syk protein (Figure 2A, second panel, compare lane 3 with lane 4). No ubiquitin signal was observed in the absence of transfected Syk. In order to determine whether the higher molecular weight, ubiquitin-reactive Syk species correlated with anti-Syk immunoreactivity, Syk immunoprecipitations from larger aliquots of cell lysate were prepared and immunoblotted with anti-Syk antibody. This revealed that the higher molecular weight, ubiquitin-reactive Syk species were indeed reactive with Syk antibody (Figure 2A, third panel). Similar results were obtained in other experiments involving Syk (Figures 3, 5 and 6; and data not shown). The relatively low reactivity of the highest molecular weight species with anti-Syk antibody apparently is not a consequence of masking of the epitopes (as immunoprecipitations were done with the same antibody), but probably due to amplification of the anti-ubiquitin signal by polyubiquitylation. Equivalent expression of GFP-tagged Cbl protein in the appropriate lysates was confirmed by anti-GFP immunoblotting of whole-cell lysates (Figure 2A, bottom panel). Thus, wild-type Cbl can target Syk for ubiquitylation in 293T cells.
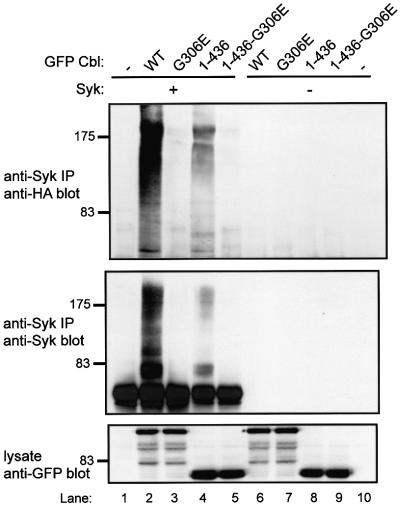
Fig. 3. The Cbl TKB and RF domains are sufficient for Cbl-dependent ubiquitylation of Syk in 293T cells. 293T cells were transfected with the indicated expression plasmids together with HA-ubiquitin and CD8-ζ plasmids as in Figure 2. Anti-Syk IP and serial anti-HA and anti-Syk immunoblotting, and anti-GFP immunoblotting of whole-cell lysates were also as in Figure 2. The transfected GFP–Cbl construct designations are: GFP-full length Cbl (WT) and its G306E mutants (G306E); GFP–Cbl amino acids 1–436 (1–436) and its corresponding G306E mutant (1–436-G306E).
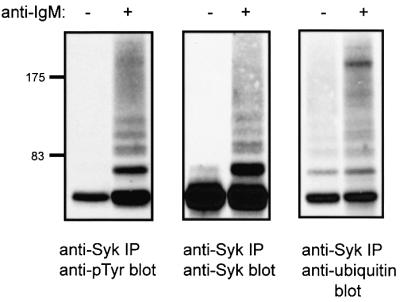
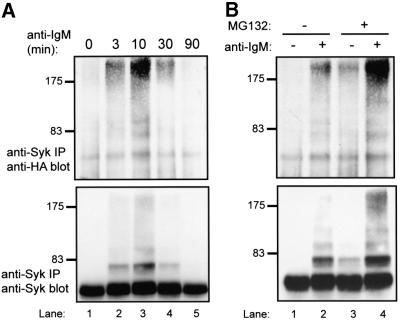
Fig. 5. Ubiquitylation of Syk upon BCR stimulation in Ramos B cells. Ramos B cells were resuspended in medium at 25 × 106/ml and either left unstimulated or stimulated with goat anti-human IgM antibody. Anti-Syk IPs from 1 mg aliquots of lysate protein were resolved by SDS–PAGE and immunoblotted with anti-pTyr antibody (left panel). The blot was stripped and serially reprobed with anti-Syk (middle panel) and anti-ubiquitin antibodies (right panel).
Fig. 6. BCR-induced Syk ubiquitylation in Ramos-T cells and its enhancement by MG132 treatment. The SV40 T-antigen-expressing Ramos B-cell line (Ramos-T) was transiently transfected by electroporation with 10 µg of a plasmid encoding HA-ubiquitin. At 48 h post-transfection, cells were washed and stimulated as in Figure 5 for the indicated times (A) or for 10 min (B). Anti-Syk IPs from 1 mg aliquots of lysate protein were subjected to serial immunoblotting with anti-HA followed by anti-Syk antibody. In (B), cells were treated with 50 µM MG132 (+) or DMSO control (–) 5 h prior to stimulation.
Next, we examined if the RF domain of Cbl, which is required for loss of Syk protein in COS cells (Ota et al., 2000) and mediates the ubiquitin ligase activity (Joazeiro et al., 1999; Levkowitz et al., 1999), was required for Cbl-induced Syk ubiquitylation. For this purpose, we analyzed the RF domain C3AHN mutant with four substitutions predicted to abrogate coordination of the two zinc atoms (Ota et al., 2000). This mutant was unable to induce a reduction in Syk levels in COS or 293T cells. In contrast to wild-type Cbl, the RF domain mutant was unable to mediate Syk ubiquitylation (Figure 2B, top and middle panels) despite expression levels comparable with wild-type Cbl (Figure 2B, bottom panel). Thus an intact Cbl RF domain is necessary for Cbl-induced Syk ubiquitylation in vivo.
Furthermore, in this reconstitution system, Syk is only active and sensitive to Cbl-mediated ubiquitylation when co-expressed with CD8-ζ (Figure 2B, compare lanes 2 and 5). It has been reported recently that Cbl, recruited via ZAP-70, can induce ubiquitylation of the ζ chain in a 293T cell expression system (Wang et al., 2001). We did not detect any ubiquitylated CD8-ζ under our experimental conditions (Figure 2C, top panel), which were optimized to detect Syk ubiquitylation, even though CD8-ζ phosphorylation was easily detectable when co-expressed with Syk (Figure 2C, bottom panel).
The Cbl TKB and RF domain are sufficient for Syk ubiquitylation in 293T cells
The Cbl TKB domain and its cognate binding site (Tyr323) on Syk are essential for Cbl–Syk association and Cbl-mediated loss of Syk in transfected COS cells (Lupher et al., 1998). We have shown that the C-terminal region of Cbl is dispensable for negative regulation of Syk, and the Cbl residues 1–436 comprising the TKB and RF domains were sufficient for this activity (Ota et al., 2000). Co-transfection of GFP–Cbl 1–436 with Syk revealed that these two domains were indeed sufficient to induce Syk ubiquitylation (Figure 3, lane 4). Mutation of the TKB domain in Cbl 1–436 (1–436 G306E) completely abrogated its ability to induce Syk ubiquitylation (Figure 3, lane 5). Additionally, mutation of the Cbl TKB domain-binding site on Syk (Y323F) also resulted in abrogation of Syk ubiquitylation (data not shown). Together, these data demonstrate that the Cbl TKB and RF domains are necessary and sufficient for Syk ubiquitylation in the 293T cell system, as they are for reduction of Syk protein levels.
In vitro ubiquitylation of Syk by recombinant Cbl and the requirement for Cbl TKB and RF domains
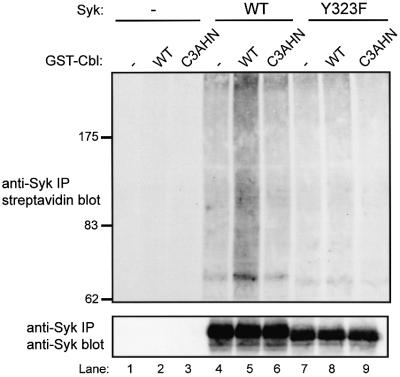
Next, we tested if Cbl functions directly as a ubiquitin ligase towards Syk. For this purpose, we tested the ability of recombinant GST–Cbl fusion proteins to mediate in vitro ubiquitylation of immunopurified Syk. The validity of this assay was established by demonstrating the expected GST–Cbl-induced, ATP-dependent ubiquitylation of the EGFR immunoprecipitated from EGFR-overexpressing A431 cells (Levkowitz et al., 1999), and lack of such activity with GST–Cbl-G306E and GST–Cbl-C3AHN (data not shown). When in vitro ubiquitylation reactions were carried out in the absence of Cbl, a relatively low level of Syk ubiquitylation was detected (Figure 4, top panel, lane 4). However, inclusion of recombinant Cbl in the reaction resulted in a marked increase in Syk ubiquitylation (Figure 4, top panel, compare lane 4 with lane 5). In contrast, the Cbl RF mutant C3AHN was unable to mediate the ubiquitylation of Syk. Furthermore, the Syk Y323F mutant, which is unable to interact with the Cbl TKB domain (Lupher et al., 1998), was resistant to Cbl-mediated ubiquitylation (Figure 4, top panel, lanes 7–9). These in vitro studies provide strong evidence that Syk is a direct target of Cbl ubiquitin ligase activity and that functional Cbl TKB and RF domains are required for this activity.
Fig. 4. In vitro ubiquitylation of Syk by recombinant Cbl and requirement for an intact Cbl RING finger domain and TKB domain-binding site on Syk. 293T cells were transiently transfected with vector (–), wild-type Syk (WT) or Y323F mutant (Y323F), together with CD8-ζ plasmid, as in Figure 2. Anti-Syk IPs carried out from 1 mg aliquots of lysate protein were incubated with the indicated GST–Cbl fusion proteins and biotin-labeled ubiquitin in an in vitro ubiquitylation reaction. The bead-bound Syk protein was resolved by SDS–PAGE and immunoblotted with streptavidin–horseradish peroxidase to visualize in vitro ubiquitylated Syk (top panel). The membrane was stripped and reprobed with an anti-Syk antibody (bottom panel). GST–Cbl fusion protein designations are: GST alone (–), Cbl wild-type (WT) and C3AHN RING finger mutant (C3AHN).
Syk is ubiquitylated upon antigen receptor stimulation in Ramos B cells
While the analyses presented above clearly demonstrated the Cbl-dependent Syk ubiquitylation in the 293T cell system, we considered it important to establish its direct relevance to lymphoid cells. For this purpose, we used the human IgM+ B-lymphocytic cell line Ramos that has been widely used as a model to examine BCR signaling. First, we wished to establish whether endogenous Syk is targeted for ubiquitylation in untransfected Ramos B cells. For this purpose, Ramos cells were stimulated with an anti-IgM antibody, and anti-Syk immunoprecipitates from these lysates were subjected to anti-ubiquitin immunoblotting.
Anti-IgM stimulation induced rapid and prominent phosphorylation of Syk (Figure 5, left panel), together with the appearance of several slower migrating species. These slower migrating species were also reactive with anti-Syk antibody (Figure 5, middle panel). Reblotting with an anti-ubiquitin antibody revealed several bands corresponding to slower migrating Syk species as well as a smear extending to the top of the gel (Figure 5, right panel), a characteristic of ubiquitylated cytoplasmic proteins including non-receptor PTKs (Winberg et al., 2000). Thus, endogenous Syk protein is ubiquitylated upon BCR stimulation in a B-lymphoid cell system.
Cbl-mediated Syk ubiquitylation upon antigen receptor stimulation in Ramos B cells is dependent on the Cbl RF domain and enhanced by proteasome inhibitor treatment
Next, we wished to assess if Cbl played a role in the ubiquitylation of endogenous Syk in Ramos B cells. Previous analyses using distal readouts of lymphocyte activation have shown that Cbl mutant 70Z functions in a dominant-negative manner in lymphoid cells (van Leeuwen et al., 1999). Therefore, we chose to introduce dominant-negative Cbl mutants into Ramos cells and then assess if Syk ubiquitylation was inhibited. However, the relative inefficiency with which Ramos cells can be transiently transfected presented a substantial impediment to these analyses (data not shown). To circumvent this problem, we utilized an SV40 T-antigen-expressing derivative of the Ramos cell line, Ramos-T, which can be transiently transfected with high efficiency (Ota et al., 2000). To assess Syk ubiquitylation in Cbl transfected cells selectively and to enhance the detection of ubiquitin signals, we co-transfected these cells with an HA-ubiquitin plasmid. Transfected Ramos-T cells were either left unstimulated or stimulated for various times with anti-IgM antibody, and anti-Syk immunoprecipitates were immunoblotted with anti-HA antibody to detect ubiquitylation signals. Using this approach, activation-dependent Syk ubiquitylation was easily detectable, with maximal ubiquitylation at 10 min post-stimulation (Figure 6A). When HA-ubiquitin-transfected Ramos-T cells were pre-treated with a proteasome inhibitor for 5 h prior to BCR stimulation, a substantial enhancement of Syk ubiquitylation was observed (Figure 6B). Again, the ubiquitin signal was observed primarily after receptor engagement, although some basal ubiquitylation could be seen in MG132-treated cells (Figure 6B). Thus, the Ramos-T cell system appeared suitable for analyses using dominant-negative Cbl mutants.
To assess the effect of introduced Cbl proteins, Ramos-T cells were transfected with GFP vector, GFP–Cbl or GFP–Cbl-70Z, together with the HA-ubiquitin plasmid, and anti-Syk immunoprecipitates were subjected to anti-HA immunoblotting. As expected (Figure 7A), activation-induced ubiquitylation was observed in cells transfected with GFP alone (Figure 7A, upper panel, lanes 1 and 2). Co-expression of wild-type GFP–Cbl markedly enhanced this signal, which was still largely activation dependent; in contrast, introduction of the Cbl-70Z RF mutant led to a marked decrease in the level of Syk ubiquitylation compared with GFP-transfected cells (Figure 7A, upper panel, compare lane 2 with lanes 4 and 6). Although we did not observe a decrease in Syk protein levels, co-expression of GFP–Cbl did cause a decrease in the levels of activated Syk detected by blotting with a phospho-Syk antibody specific for the activation loop phosphorylation site of Syk (pY525) (Figure 7A, third panel). The specificity of the phospho-Syk antibody was verified by immunoblotting wild-type and mutant Syk proteins (Y525F, Y526F) as well as phosphatase treatment (www.cellsignal.com). The inhibition of activation-induced Syk ubiquitylation by a dominant-negative Cbl mutant strongly supports the role of endogenous Cbl in mediating Syk ubiquitylation in lymphoid cells.
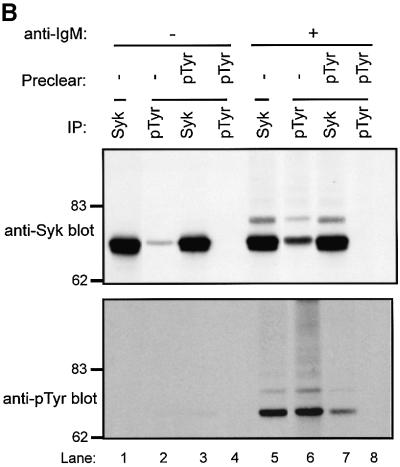
Fig. 7. BCR-induced Syk ubiquitylation in Ramos-T cells is inhibited by a dominant-negative Cbl RING finger mutant. Ramos-T cells were transfected by electroporation with 10 µg of plasmid encoding HA-ubiquitin along with GFP (–), GFP–Cbl (WT) or GFP–Cbl-70Z (70Z). At 48 h post-transfection, the cells were washed and either left unstimulated (–) or stimulated (+) for 10 min with goat anti-human IgM. In (A), anti-Syk IPs from 1.5 mg aliquots of lysate protein were subjected to immunoblotting with anti-HA antibody (top panel) followed by anti-Syk antibody (second panel) and then anti-phospho-Syk antibody (third panel). Aliquots of the lysates (30 µg) were immunoblotted with anti-GFP antibody to visualize GFP–Cbl proteins (bottom panel). In (B), anti-Syk or anti-pTyr IPs from unstimulated or stimulated Ramos-T cells were either mock treated (–) or pre-cleared with anti-Syk or anti-pTyr antibodies prior to serial immunoblotting with anti-Syk (top panel) and anti-pTyr antibodies (bottom panel).
The inability to observe a reduction in Syk levels upon receptor stimulation in Ramos cells could be due to the relatively small pool of Syk that is phosphorylated downstream. To address this question, lysates of unstimulated and anti-IgM-stimulated Ramos cells were either mock-treated or pre-cleared with anti-Syk or anti-pTyr antibodies. The lysates were then subjected to anti-Syk and anti-pTyr immunoprecipitations, and serially immunoblotted using anti-Syk and anti-pTyr antibodies. As expec ted, phosphorylation of Syk was dependent on stimulation (Figure 7B, compare lanes 1 and 2 with lanes 5 and 6, respectively). Importantly, direct anti-pTyr immunoprecipitations from lysates of activated cells showed that only a small fraction (∼10%) of Syk was phosphorylated (Figure 7B, compare lane 5 with lane 6). Furthermore, under conditions that resulted in pre-clearing of >80% of phosphorylated Syk, relatively minor loss of Syk signal was observed (compare lane 5 with lane 7). These data support the idea that lack of apparent reduction in Syk levels in IgM-stimulated Ramos cells reflects the recruitment of a small pool of Syk. Nonetheless, Cbl-dependent loss of Syk does occur, as shown by reduction in levels of the activated pool of Syk (Figure 7A, third panel).
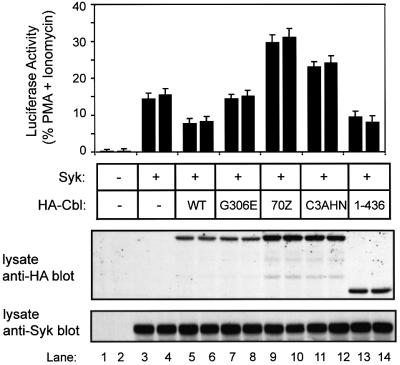
The TKB and RF domains are essential for Cbl-mediated negative regulation of Syk-dependent lymphocyte activation
Since our studies in 293T cells demonstrated that the TKB and RF domains of Cbl are essential for Syk ubiquitylation, we wished to determine if this structure– function relationship held true for Cbl-mediated negative regulation of Syk in lymphoid cells. The requirement for these domains would strongly support the role of ubiquitin ligase activity in Cbl-mediated regulation of Syk in lymphoid cells. For this purpose, we utilized a transfected nuclear factor of activated T cells (NFAT)-luciferase reporter, which has been widely used as a downstream readout of Syk/ZAP-70-mediated antigen receptor signaling (Williams et al., 1998). To demonstrate directly that the effects of Cbl proteins reflected their influence on Syk, we utilized the p116 Jurkat mutant, which is Syk and ZAP-70 negative (Williams et al., 1998). Previous studies have demonstrated that reconstitution of these cells with Syk activates the T-cell receptor (TCR) signaling program, albeit in a TCR ligation-independent manner (Williams et al., 1998). To facilitate transient expression analyses, we generated and utilized an SV40 T-antigen-expressing derivative of the p116 cell line, designated p116-T. These cells were transfected with Syk alone or in combination with various Cbl constructs, and a plasmid encoding the NFAT-luciferase reporter. Each transfection was performed in duplicate and luciferase assays were performed in replicates of five. The values are expressed as a percentage of maximal luciferase activity induced by phorbol 12-myristate 13-acetate (PMA) plus ionomycin treatment.
As expected, the vector-transfected p116-T cells exhibited no NFAT-luciferase activity (Figure 8, upper panel). Introduction of Syk into these cells led to substantial induction of NFAT-luciferase activity. Co-transfection of Cbl or Cbl 1–436 with Syk resulted in nearly a 50% decrease in NFAT-luciferase activity compared with that seen in Syk-transfected cells. Notably, the TKB domain mutant of Cbl (Cbl-G306E) had no effect on Syk-dependent NFAT-luciferase activity. Importantly, the Cbl RF mutants, 70Z or C3AHN, were unable to reduce the Syk-dependent NFAT activity and in fact enhanced the activity compared with that observed with Syk alone. Co-expression of Cbl did not lead to a detectable decrease in Syk levels, apparently reflecting the activation of only a small pool of Syk. Expression of Syk and Cbl proteins was confirmed in these transfections by immunoblot analyses (Figure 8, middle and lower panels). Overall, these results provided direct evidence that the TKB and RF domains are required for the negative regulatory effect of Cbl on Syk function in a lymphoid cell.
Fig. 8. Inhibition of Syk-dependent NFAT-luciferase activity by Cbl and the requirement for the TKB and RING finger domains. Jurkat cells, p116-T, were transfected with plasmids encoding the NFAT-luciferase reporter (10 µg) and the indicated plasmids encoding Syk (5 µg) or various Cbl proteins (15 µg). The HA-Cbl constructs used were: wild-type (WT), its TKB domain mutant (G306E), RING finger mutants 70Z-Cbl (70Z) and C3AHN (C3AHN), and a truncation construct encoding amino acids 1–436 (1–436). Transfections were performed in duplicate and cells were analyzed in replicates of five. Cells were either left unstimulated or stimulated with PMA plus ionomycin. Mean luciferase activity was expressed as a percentage of values obtained with PMA plus ionomycin treatment. The combined standard error of the mean is displayed. Data are representative of three independent experiments. Aliquots of cell lysates (20 µg) were subjected to serial anti-HA (middle panel) and anti-Syk immunoblotting.
Discussion
Cbl family proteins have emerged as evolutionarily conserved negative regulators that associate with a number of cellular PTKs following their activation. Recent analyses of RTK targets of Cbl, such as the PDGFR and EGFR, have demonstrated that Cbl functions as a ubiquitin ligase and induces the ubiquitylation of activated species of these transmembrane proteins (Levkowitz et al., 1998, 1999; Miyake et al., 1998; Joazeiro et al., 1999). Ubiquitylation subsequently facilitates the transport of these receptors to lysosomes where they are degraded. In contrast, the mechanism and location of Cbl-mediated negative regulation of cytoplasmic PTK targets have been less clear. The Syk/ZAP-70 family of cytoplasmic PTKs is a prominent target of Cbl-mediated negative regulation. While analyses in model cell systems, such as COS and 293T cells, indicated that Cbl facilitates the degradation of these tyrosine kinases (Lupher et al., 1998; Ota et al., 2000; Rao et al., 2000), analyses in mast cells led others to suggest that Cbl inhibits Syk kinase activity without affecting its protein levels (Ota and Samelson, 1997). Furthermore, several studies using mutant Cbl proteins (70Z) have implicated the C-terminal regions, including the phosphorylation sites and the leucine zipper region, to be important for Cbl’s effect on Syk/ZAP-70 tyrosine kinases (Deckert et al., 1998; Feshchenko et al., 1998). Thus, without formal evidence that Cbl can indeed induce ubiquitylation of the Syk/ZAP-70 family of PTKs and demonstration of this pathway in lymphoid cells, it has remained unclear whether Cbl-mediated negative regulation of Syk/ZAP-70 PTKs relies on the same mechanisms that mediate Cbl’s effects on RTKs. Here, we provide evidence that Syk is indeed a direct target of Cbl ubiquitin ligase activity, and that the basic Cbl ubiquitin ligase module, consisting of the Syk-tethering TKB domain and the UBC-binding RF domain, is sufficient for the negative regulation of Syk in B lymphocytes. Furthermore, we demonstrate that Cbl-mediated negative regulation is due to loss of Syk protein and not due to inhibition of its kinase activity.
The TKB and RF domains are the most highly conserved regions of Cbl proteins through evolution. Our previous studies demonstrated that these domains were required as well as sufficient for the negative regulation of Syk in a COS cell reconstitution system (Ota et al., 2000). Importantly, we established here that the TKB and RF domains are essential as well as sufficient for Cbl-induced ubiquitylation of Syk. Furthermore, we demonstrated that these two domains are also sufficient to cause negative regulation of Syk-mediated signaling in a lymphoid cell line. These results strongly support the role of ubiquitylation in Cbl-mediated negative regulation of activated Syk. Indeed, we demonstrate that BCR stimulation induces the ubiquitylation of endogenous Syk, and introduction of an RF domain-defective, dominant-negative Cbl mutant, Cbl-70Z, abrogated this ubiquitylation. Thus, our data strongly favor the model that Cbl-mediated negative regulation of Syk/ZAP-70 PTKs is mediated via ubiquitylation rather than inhibition of their kinase activity, as suggested by others (Ota and Samelson, 1997). This notion is strengthened further by our direct quantification of the kinase activity of the pool of Syk associated with Cbl, which was comparable with the activity of Syk associated with a mutant Cbl, 70Z, which does not induce negative regulation of Syk.
The likely role of ubiquitylation as a mechanism to regulate the levels of activated Syk/ZAP-70 PTKs is supported further by the requirement for an ITAM-bearing chain (CD8-ζ) for Cbl-induced Syk ubiquitylation and degradation (Figure 2B). A phosphorylated ITAM chain is known to be required for recruitment and activation of Syk (Latour et al., 1996), and CD8-ζ was indeed phosphorylated in our system (Figure 2C, bottom panel). Interest ingly, under our experimental conditions where we co-express CD8-ζ and Syk, we did not detect ubiquitylation of CD8-ζ itself; however, a recent report suggests that under suitable experimental conditions, ZAP-70 can serve as a linker to mediate ζ chain ubiquitylation by Cbl (Wang et al., 2001). It remains possible that stimulation through the CD8-ζ chimeric receptor might enhance ubiquitylation of the ζ chain itself. Moreover, one recent study demonstrated that both Syk and ZAP-70 are ubiquitylated and degraded upon CD16 engagement in human NK cells (Paolini et al., 2001). As discussed in this study, it remains to be determined whether the observed ubiquitylation is Cbl mediated. Furthermore, recent findings with viral protein modulators of the immune response reveal that Syk was targeted for ubiquitylation, further supporting the role of ubiquitylation in regulating activated Syk/ZAP-70 PTKs. Winberg et al. (2000) showed that latent membrane protein 2A of the Epstein–Barr virus binds to E3 ubiquitin ligases, possibly Nedd4-like proteins (Ikeda et al., 2000), and enhances the ubiquitylation of B-cell PTKs including Syk. However, it remains possible that other mechanisms of Syk/ZAP-70 degradation also operate in cells. For example, it has been reported that ZAP-70 protein levels decreased in T cells and that this decrease occurred via calpain-mediated proteolytic cleavage (Penna et al., 1999).
Our studies indicate that the basic mechanism of Cbl-mediated negative regulation of Syk/ZAP-70 cytoplasmic PTKs is similar to that of RTK regulation by Cbl, in that the activated species of both families of PTKs are targeted for ubiquitylation. However, the fate of ubiquitylated Syk/ZAP-70 PTKs is likely to be vastly different from that of ubiquitylated RTKs. While Cbl-mediated ubiquitylation of RTKs targets them to lysosomes, resulting in receptor degradation via lysosomal enzymes, ubiquitylated Syk is likely to be targeted to the proteasome. Consistent with this suggestion, Cbl–ZAP-70 complexes in activated T cells were found predominantly in the cytosolic fraction (Fournel et al., 1996). Moreover, we showed directly that inhibition of proteasome function with MG132 led to accumulation of ubiquitylated Syk in both 293T cells and Ramos B cells, and stabilization of the Syk protein levels in 293T cells, even though this treatment was carried out for a relatively short period of time. MG132 treatment, however, did not lead to any detectable increase in Syk protein levels in Ramos B cells; this result is not surprising given that only a small fraction of the total cellular pool of Syk protein is recruited to the BCR under such conditions and becomes phosphorylated (Figure 7B) (Latour et al., 1996). In contrast, the larger, activated pool of Syk in the 293T reconstitution system (Zoller et al., 1997) is subject to Cbl-mediated ubiquitylation for a longer time period, resulting in a detectable loss of the Syk protein. In additional experiments, we have noted stabilization of the tyrosine-phosphorylated Syk signals when Cbl-transfected Ramos cells were treated with MG132 (data not shown), as well as a Cbl-dependent decrease in the pool of activated Syk detected using a phospho-specific antibody directed at the phosphorylated kinase activation loop (Figure 7A). An elevation of the tyrosine-phosphorylated pool of ZAP-70 was also observed in thymocytes of Cbl–/– mice even though the overall levels of ZAP-70 protein were similar to those in wild-type mice. (Thien et al., 1999). These data support a model whereby the activated pools of Syk/ZAP-70 kinases selectively associate with the ubiquitin ligase Cbl, as a result of the Cbl TKB domain binding to phosphorylation sites within the linker regions of these kinases (Lupher et al., 1997, 1998; Deckert et al., 1998). This model also provides a basis for enhanced ZAP-70- (van Leeuwen et al., 1999) or Syk-mediated (Figure 8) NFAT-luciferase reporter activity when RF mutants of Cbl are introduced into lymphoid cells. Apparently, these mutant Cbl proteins bind to activated Syk/ZAP-70 proteins but are unable to mediate their ubiquitylation (Figures 2B, 4 and 7). Indeed, the inactivation of the TKB domain by the G306E mutation, which abrogates Cbl binding to Syk/ZAP-70, led to the inability of Cbl-70Z to enhance downstream signals in lymphoid cells (van Leeuwen et al., 1999).
In conclusion, our results provide evidence that Cbl functions as a ubiquitin ligase towards Syk and that ubiquitylated Syk is targeted for proteasomal degradation. We show that BCR stimulation targets Syk for ubiquitylation, and that Cbl mediates this effect. Finally, the core motifs of Cbl required for the negative regulation of Syk in lymphocytes correspond to those required for Cbl to function as a ubiquitin ligase. Thus, Cbl-dependent ubiquitylation is likely to provide a key mechanism of negative regulation of Syk-mediated immune cell activation.
Materials and methods
Cells
293T human embryonic kidney epithelial cells and the human IgM+ B lymphocytic cell line Ramos and its SV40 T-antigen-expressing derivative, Ramos-T, were maintained as described (Andoniou et al., 2000; Ota et al., 2000). The p116 T-antigen-expressing cell line was maintained as previously described (Rao et al., 2000) with the addition of 500 µg/ml G418.
Antibodies and expression plasmids
The following antibodies were used: monoclonal antibody (mAb) 12CA5 (anti-influenza HA epitope tag; IgG2b); mAb 4G10 (anti-pTyr; IgG2a); mAb anti-ubiquitin (IgG1, MMS-258R) from Covance; mAb anti-Syk (IgG2a, sc-1240) from Santa Cruz Biotechnology Inc.; rabbit polyclonal antibody (pAb) anti-GFP (RDI-GRNFPabR) from Research Diagnostics; pAb anti-phospho-Syk (2711) from Cell Signaling; and goat anti-human IgM from Zymed. The pSRαNeo-CD8-ζ chimera, HA-tagged Cbl, GFP–Cbl, HA-ubiquitin and Syk expression constructs have been described previously (Lupher et al., 1998; Lill et al., 2000; Ota et al., 2000). The EcoRI and SalI fragments of the respective pAlterMAX-HA-Cbl constructs were cloned into pGEX4T-2-GST-Cbl-N (Lupher et al., 1996) digested with EcoRI and XhoI to generate GST–Cbl constructs.
Transient transfection and preparation of cell lysates
293T cells were transfected as previously described (Andoniou et al., 2000). Ramos-T cells were transfected by electroporation as previously described (Ota et al., 2000). Ramos and Ramos-T cells were stimulated at 37°C through their BCR for the indicated times using 10 µg/ml goat-anti human IgM (Zymed). Cell lysates were prepared in lysis buffer (Andoniou et al., 2000) containing 0.1% SDS and 0.5% deoxycholate.
Immunoblotting
Immunoprecipitations, SDS–PAGE, transfer to polyvinylidine difluoride (PVDF) membranes (NEN) and immunoblotting were performed as described (Lupher et al., 1996). Densitometry was carried out on directly scanned images using ScionImage for Windows™ software.
In vitro kinase assay
In vitro kinase assays were carried out as previously described (Ota et al., 2000). Briefly, anti-HA immunoprecipitations of 293T cell lysates were prepared, and the beads were resuspended in 20 µl of kinase assay buffer. The level of phosphorylated Raytide substrate was measured using a liquid scintillation counter.
Luciferase assay
p116-T cells were transfected by electroporation, as previously described (Rao et al., 2000). The cells were cultured for 16 h and stimulated for 7 h with media alone or 50 ng/ml PMA plus 1 µg/ml ionomycin (Sigma). Luciferase activity was determined on equal protein aliquots using a Monolight 3010C luminometer (Analytical Bioluminescence Laboratory Inc.) and Luciferin Reagent (Promega).
In vitro ubiquitylation assay
GST fusion proteins of Cbl or its mutants were affinity purified on glutathione–Sepharose beads from Escherichia coli lysate and eluted. In vitro ubiquitylation assays were performed as described (Levkowitz et al., 1999) on protein A–Sepharose-bound Syk protein obtained by immunoprecipitation from lysates of transiently transfected 293T cells. The reactions were carried out for 1 h at 30°C.
Acknowledgments
Acknowledgements
We thank Dr Dirk Bohmann (EMBO, Heidelberg, Germany) for the HA-ubiquitin expression construct, and Dr Robert Abraham (Department of Immunology, Mayo Clinic) for the p116 Jurkat T-cell line. This work was supported by grants to H.B. from the National Institutes of Health (CA76118, CA87986 and CA75075) and the American Cancer Society (066-04-CIM). N.R. is a Howard Hughes Medical Institute Predoctoral Fellow. A.G. is a US Department of Defense Breast Cancer Research Program Postdoctoral Fellow (DAMD 17-98-1-8032).
References
- Andoniou C.E., Lill,N.L., Thien,C.B., Lupher,M.L.,Jr, Ota,S., Bowtell,D.D., Scaife,R.M., Langdon,W.Y. and Band,H. (2000) The Cbl proto-oncogene product negatively regulates the Src-family tyrosine kinase Fyn by enhancing its degradation. Mol. Cell. Biol., 20, 851–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arpaia E., Shahar,M., Dadi,H., Cohen,A. and Roifman,C.M. (1994) Defective T cell receptor signaling and CD8+ thymic selection in humans lacking zap-70 kinase. Cell, 76, 947–958. [DOI] [PubMed] [Google Scholar]
- Bubeck-Wardenburg J., Wong,J., Futterer,K., Pappu,R., Fu,C., Waksman,G. and Chan,A.C. (1999) Regulation of antigen receptor function by protein tyrosine kinases. Prog. Biophys. Mol. Biol., 71, 373–392. [DOI] [PubMed] [Google Scholar]
- Chan A.C., van Oers,N.S., Tran,A., Turka,L., Law,C.L., Ryan,J.C., Clark,E.A. and Weiss,A. (1994) Differential expression of ZAP-70 and Syk protein tyrosine kinases and the role of this family of protein tyrosine kinases in TCR signaling. J. Immunol., 152, 4758–4766. [PubMed] [Google Scholar]
- Cheng A.M., Rowley,B., Pao,W., Hayday,A., Bolen,J.B. and Pawson,T. (1995) Syk tyrosine kinase required for mouse viability and B-cell development. Nature, 378, 303–306. [DOI] [PubMed] [Google Scholar]
- Chiang Y.J. et al. (2000) Cbl-b regulates the CD28 dependence of T-cell activation. Nature, 403, 216–220. [DOI] [PubMed] [Google Scholar]
- Deckert M., Elly,C., Altman,A. and Liu,Y.C. (1998) Coordinated regulation of the tyrosine phosphorylation of Cbl by Fyn and Syk tyrosine kinases. J. Biol. Chem., 273, 8867–8874. [DOI] [PubMed] [Google Scholar]
- Feshchenko E.A., Langdon,W.Y. and Tsygankov,A.Y. (1998) Fyn, Yes and Syk phosphorylation sites in c-Cbl map to the same tyrosine residues that become phosphorylated in activated T cells. J. Biol. Chem., 273, 8323–8331. [DOI] [PubMed] [Google Scholar]
- Fournel M., Davidson,D., Weil,R. and Veillette,A. (1996) Association of tyrosine protein kinase Zap-70 with the protooncogene product p120c-cbl in T lymphocytes. J. Exp. Med., 183, 301–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelfand E.W., Weinberg,K., Mazer,B.D., Kadlecek,T.A. and Weiss,A. (1995) Absence of ZAP-70 prevents signaling through the antigen receptor on peripheral blood T cells but not on thymocytes. J. Exp. Med., 182, 1057–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicke L. (1997) Ubiquitin-dependent internalization and down-regulation of plasma membrane proteins. FASEB J., 11, 1215–1226. [DOI] [PubMed] [Google Scholar]
- Ikeda M., Ikeda,A., Longan,L.C. and Longnecker,R. (2000) The Epstein–Barr virus latent membrane protein 2A PY motif recruits WW domain-containing ubiquitin-protein ligases. Virology, 268, 178–191. [DOI] [PubMed] [Google Scholar]
- Joazeiro C.A., Wing,S.S., Huang,H., Leverson,J.D., Hunter,T. and Liu,Y.C. (1999) The tyrosine kinase negative regulator c-Cbl as a RING-type, E2-dependent ubiquitin-protein ligase. Science, 286, 309–312. [DOI] [PubMed] [Google Scholar]
- Keshvara L.M., Isaacson,C.C., Yankee,T.M., Sarac,R., Harrison,M.L. and Geahlen,R.L. (1998) Syk- and Lyn-dependent phosphorylation of Syk on multiple tyrosines following B cell activation includes a site that negatively regulates signaling. J. Immunol., 161, 5276–5283. [PubMed] [Google Scholar]
- Kong G., Dalton,M., Wardenburg,J.B., Straus,D., Kurosaki,T. and Chan,A.C. (1996) Distinct tyrosine phosphorylation sites in ZAP-70 mediate activation and negative regulation of antigen receptor function. Mol. Cell. Biol., 16, 5026–5035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krawczyk C. et al. (2000) Cbl-b is a negative regulator of receptor clustering and raft aggregation in T cells. Immunity, 13, 463–473. [DOI] [PubMed] [Google Scholar]
- Latour S., Chow,L.M.L. and Veillette,A. (1996) Differential intrinsic enzymatic activity of Syk and Zap-70 protein-tyrosine kinases. J. Biol. Chem., 271, 22782–22790. [DOI] [PubMed] [Google Scholar]
- Levkowitz G., Waterman,H., Zamir,E., Kam,Z., Oved,S., Langdon,W.Y., Beguinot,L., Geiger,B. and Yarden,Y. (1998) c-Cbl/Sli-1 regulates endocytic sorting and ubiquitination of the epidermal growth factor receptor. Genes Dev., 12, 3663–3674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levkowitz G. et al. (1999) Ubiquitin ligase activity and tyrosine phosphorylation underlie suppression of growth factor signaling by c-Cbl/Sli-1. Mol. Cell, 4, 1029–1040. [DOI] [PubMed] [Google Scholar]
- Lill N.L., Douillard,P., Awwad,R.A., Ota,S., Lupher,M.L.,Jr, Miyake,S., Meissner-Lula,N., Hsu,V.W. and Band,H. (2000) The evolutionarily conserved N-terminal region of Cbl is sufficient to enhance down-regulation of the epidermal growth factor receptor. J. Biol. Chem., 275, 367–377. [DOI] [PubMed] [Google Scholar]
- Lupher M.L. Jr, Reedquist,K.A., Miyake,S., Langdon,W.Y. and Band,H. (1996) A novel phosphotyrosine-binding domain in the N-terminal transforming region of Cbl interacts directly and selectively with ZAP-70 in T cells. J. Biol. Chem., 271, 24063–24068. [DOI] [PubMed] [Google Scholar]
- Lupher M.L. Jr, Songyang,Z., Shoelson,S.E., Cantley,L.C. and Band,H. (1997) The Cbl phosphotyrosine-binding domain selects a D(N/D)XpY motif and binds to the Tyr292 negative regulatory phosphorylation site of ZAP-70. J. Biol. Chem., 272, 33140–33144. [DOI] [PubMed] [Google Scholar]
- Lupher M.L. Jr, Rao,N., Lill,N.L., Andoniou,C.E., Miyake,S., Clark,E.A., Druker,B. and Band,H. (1998) Cbl-mediated negative regulation of the Syk tyrosine kinase. A critical role for Cbl phosphotyrosine-binding domain binding to Syk phosphotyrosine 323. J. Biol. Chem., 273, 35273–35281. [DOI] [PubMed] [Google Scholar]
- Lupher M.L. Jr, Rao,N., Eck,M.J. and Band,H. (1999) The Cbl protooncoprotein: a negative regulator of immune receptor signal transduction. Immunol. Today, 20, 375–382. [DOI] [PubMed] [Google Scholar]
- Meisner H., Daga,A., Buxton,J., Fernandez,B., Chawla,A., Banerjee,U. and Czech,M.P. (1997) Interactions of Drosophila Cbl with epidermal growth factor receptors and role of Cbl in R7 photoreceptor cell development. Mol. Cell. Biol., 17, 2217–2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng W., Sawasdikosol,S., Burakoff,S.J. and Eck,M.J. (1999) Structure of the amino-terminal domain of Cbl complexed to its binding site on ZAP-70 kinase. Nature, 398, 84–90. [DOI] [PubMed] [Google Scholar]
- Miyake S., Lupher,M.L.,Jr, Andoniou,C.E., Lill,N.L., Ota,S., Douillard,P., Rao,N. and Band,H. (1997) The Cbl protooncogene product: from an enigmatic oncogene to center stage of signal transduction. Crit. Rev. Oncogen., 8, 189–218. [DOI] [PubMed] [Google Scholar]
- Miyake S., Lupher,M.L.,Jr, Druker,B. and Band,H. (1998) The tyrosine kinase regulator Cbl enhances the ubiquitination and degradation of the platelet-derived growth factor receptor α. Proc. Natl Acad. Sci. USA, 95, 7927–7932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy M.A., Schnall,R.G., Venter,D.J., Barnett,L., Bertoncello,I., Thien,C.B., Langdon,W.Y. and Bowtell,D.D. (1998) Tissue hyperplasia and enhanced T-cell signalling via ZAP-70 in c-Cbl-deficient mice. Mol. Cell. Biol., 18, 4872–4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naramura M., Kole,H.K., Hu,R.J. and Gu,H. (1998) Altered thymic positive selection and intracellular signals in Cbl-deficient mice. Proc. Natl Acad. Sci. USA, 95, 15547–15552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negishi I., Motoyama,N., Nakayama,K., Senju,S., Hatakeyama,S., Zhang,Q., Chan,A.C. and Loh,D.Y. (1995) Essential role for ZAP-70 in both positive and negative selection of thymocytes. Nature, 376, 435–438. [DOI] [PubMed] [Google Scholar]
- Ota S., Hazeki,K., Rao,N., Lupher,M.L.,Jr, Andoniou,C.E., Druker,B. and Band,H. (2000) The RING finger domain of Cbl is essential for negative regulation of the Syk tyrosine kinase. J. Biol. Chem., 275, 414–422. [DOI] [PubMed] [Google Scholar]
- Ota Y. and Samelson,L.E. (1997) The product of the proto-oncogene c-cbl: a negative regulator of the Syk tyrosine kinase. Science, 276, 418–420. [DOI] [PubMed] [Google Scholar]
- Paolini R., Molfetta,R., Piccoli,M., Frati,L. and Santoni,A. (2001) Ubiquitination and degradation of Syk and ZAP-70 protein tyrosine kinases in human NK cells upon CD16 engagement. Proc. Natl Acad. Sci. USA, 98, 9611–9616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelosi M., Di Bartolo,V., Mounier,V., Mege,D., Pascussi,J.M., Dufour,E., Blondel,A. and Acuto,O. (1999) Tyrosine 319 in the interdomain B of ZAP-70 is a binding site for the Src homology 2 domain of Lck. J. Biol. Chem., 274, 14229–14237. [DOI] [PubMed] [Google Scholar]
- Penna D., Muller,S., Martinon,F., Demotz,S., Iwashima,M. and Valitutti,S. (1999) Degradation of ZAP-70 following antigenic stimulation in human T lymphocytes: role of calpain proteolytic pathway. J. Immunol., 163, 50–56. [PubMed] [Google Scholar]
- Rao N., Lupher,M.L.,Jr, Ota,S., Reedquist,K.A., Druker,B.J. and Band,H. (2000) The linker phosphorylation site Tyr292 mediates the negative regulatory effect of Cbl on ZAP-70 in T cells. J. Immunol., 164, 4616–4626. [DOI] [PubMed] [Google Scholar]
- Thien C.B. and Langdon,W.Y. (2001) Cbl: many adaptations to regulate protein tyrosine kinases. Nat. Rev. Mol. Cell. Biol., 2, 294–307. [DOI] [PubMed] [Google Scholar]
- Thien C.B., Bowtell,D.D. and Langdon,W.Y. (1999) Perturbed regulation of ZAP-70 and sustained tyrosine phosphorylation of LAT and SLP-76 in c-Cbl-deficient thymocytes. J. Immunol., 162, 7133–7139. [PubMed] [Google Scholar]
- Turner M., Mee,P.J., Costello,P.S., Williams,O., Price,A.A., Duddy,L.P., Furlong,M.T., Geahlen,R.L. and Tybulewicz,V.L. (1995) Perinatal lethality and blocked B-cell development in mice lacking the tyrosine kinase Syk. Nature, 378, 298–302. [DOI] [PubMed] [Google Scholar]
- Turner M., Gulbranson-Judge,A., Quinn,M.E., Walters,A.E., MacLennan,I.C. and Tybulewicz,V.L. (1997) Syk tyrosine kinase is required for the positive selection of immature B cells into the recirculating B cell pool. J. Exp. Med., 186, 2013–2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Leeuwen J.E. and Samelson,L.E. (1999) T cell antigen-receptor signal transduction. Curr. Opin. Immunol., 11, 242–248. [DOI] [PubMed] [Google Scholar]
- van Leeuwen J.E., Paik,P.K. and Samelson,L.E. (1999) The oncogenic 70Z Cbl mutation blocks the phosphotyrosine binding domain-dependent negative regulation of ZAP-70 by c-Cbl in Jurkat T cells. Mol. Cell. Biol., 19, 6652–6664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H.Y., Altman,Y., Fang,D., Elly,C., Dai,Y., Shao,Y. and Liu,Y.C. (2001) Cbl promotes ubiquitination of the T cell receptor ζ through an adaptor function of Zap-70. J. Biol. Chem., 276, 26004–26011. [DOI] [PubMed] [Google Scholar]
- Williams B.L., Schreiber,K.L., Zhang,W., Wange,R.L., Samelson,L.E., Leibson,P.J. and Abraham,R.T. (1998) Genetic evidence for differential coupling of Syk family kinases to the T-cell receptor: reconstitution studies in a ZAP-70-deficient Jurkat T- cell line. Mol. Cell. Biol., 18, 1388–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams B.L. et al. (1999) Phosphorylation of Tyr319 in ZAP-70 is required for T-cell antigen receptor-dependent phospholipase C-γ1 and Ras activation. EMBO J., 18, 1832–1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winberg G., Matskova,L., Chen,F., Plant,P., Rotin,D., Gish,G., Ingham,R., Ernberg,I. and Pawson,T. (2000) Latent membrane protein 2A of Epstein–Barr virus binds WW domain E3 protein-ubiquitin ligases that ubiquitinate B-cell tyrosine kinases. Mol. Cell. Biol., 20, 8526–8535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J., Zhao,Q., Kurosaki,T. and Weiss,A. (1997) The Vav binding site (Y315) in ZAP-70 is critical for antigen receptor-mediated signal transduction. J. Exp. Med., 185, 1877–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda T., Maeda,A., Kurosaki,M., Tezuka,T., Hironaka,K., Yamamoto,T. and Kurosaki,T. (2000) Cbl suppresses B cell receptor-mediated phospholipase C (PLC)-γ2 activation by regulating B cell linker protein–PLC-γ2 binding. J. Exp. Med., 191, 641–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon C.H., Lee,J., Jongeward,G.D. and Sternberg,P.W. (1995) Similarity of sli-1, a regulator of vulval development in C.elegans, to the mammalian proto-oncogene c-cbl. Science, 269, 1102–1105. [DOI] [PubMed] [Google Scholar]
- Zhao Q. and Weiss,A. (1996) Enhancement of lymphocyte responsiveness by a gain-of-function mutation of ZAP-70. Mol. Cell. Biol., 16, 6765–6774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng N., Wang,P., Jeffrey,P.D. and Pavletich,N.P. (2000) Structure of a c-Cbl–UbcH7 complex: RING domain function in ubiquitin-protein ligases. Cell, 102, 533–539. [DOI] [PubMed] [Google Scholar]
- Zoller K.E., MacNeil,I.A. and Brugge,J.S. (1997) Protein tyrosine kinases Syk and ZAP-70 display distinct requirements for Src family kinases in immune response receptor signal transduction. J. Immunol., 158, 1650–1659. [PubMed] [Google Scholar]