Abstract
The bacteriophage T4 AsiA protein is a multifunctional protein that simultaneously acts as both a repressor and activator of gene expression during the phage life cycle. These dual roles with opposing transcriptional consequences are achieved by modification of the host RNA polymerase in which AsiA binds to conserved region 4 (SR4) of σ70, altering the pathway of promoter selection by the holoenzyme. The mechanism by which AsiA flips this genetic switch has now been revealed, in part, from the three-dimensional structure of AsiA and the elucidation of its interaction with SR4. The structure of AsiA is that of a novel homodimer in which each monomer is constructed as a seven-helix bundle arranged in four overlapping helix–loop–helix elements. Identification of the protein interfaces for both the AsiA homodimer and the AsiA–σ70 complex reveals that these interfaces are coincident. Thus, the AsiA interaction with σ70 necessitates that the AsiA homodimer dissociate to form an AsiA–SR4 heterodimer, exchanging one protein subunit for another to alter promoter choice by RNA polymerase.
Keywords: AsiA/NMR/σ70/subunit exchange/three-dimensional structure
Introduction
Bacteriophage T4 development within Escherichia coli is regulated by a controlled program of transcriptional activation and repression (Mosig and Hall, 1994; Sanson and Uzan, 1995). Within a few minutes after infection, the host RNA polymerase (RNAP) holoenzyme (Eσ70) transcribes several early genes of the bacteriophage genome which encode proteins capable of blocking host replication, transcription and translation. To redirect the host Eσ70 to transcribe T4 genes, Eσ70 is modified by both ADP-ribosylation of the α subunits and association of phage-encoded proteins with the Eσ70 (Stitt and Hinton, 1994). Whereas expression of early T4 genes relies on unmodified polymerase, gene expression later in the life cycle requires a modified Eσ70 to transcribe T4 genes effectively.
AsiA, an early gene product, is necessary to ensure the progression of transcription from the T4 early to middle genes. AsiA is a small protein (10.6 kDa) whose amino acid sequence is unrelated to any other protein in either the prokaryotic or eukaryotic sequence databases (Orsini et al., 1993). AsiA strongly represses transcription from host and T4 early gene promoters in vitro (Stevens, 1976; Orsini et al., 1993), although AsiA probably acts in tandem with other phage-encoded factors to achieve complete repression of T4 early gene expression in vivo (Pene and Uzan, 2000). AsiA additionally functions as a co-activator for phage middle gene expression in conjunction with the T4 MotA protein (Ouhammouch et al., 1995; Hinton et al., 1996). These dual regulatory events with opposing transcriptional consequences are the result of specific binding between AsiA and host Eσ70, wherein AsiA interacts with the C-terminal conserved domain of σ70 [termed region 4 (SR4)] (Severinova et al., 1996, 1998; Adelman et al., 1997; Pahari and Chatterji, 1997; Colland et al., 1998; Sharma et al., 1999; Minakhin et al., 2001). Genetic and biochemical experiments have suggested that AsiA interacts with SR4 in a 1:1 complex (Adelman et al., 1997). The interaction with SR4 most probably occurs with at least a portion of the putative DNA recognition helix of SR4 (Pahari and Chatterji, 1997; Colland et al., 1998; Severinova et al., 1998; Minakhin et al., 2001). AsiA is capable of tightly associating with SR4 in the presence or absence of core RNAP (Severinova et al., 1996, 1998; Adelman et al., 1997; Pahari and Chatterji, 1997; Colland et al., 1998) and there may be a preferential association with free σ70 in order to establish the full repressive effect of AsiA on host gene expression (Hinton and Vuthoori, 2000). The consequence of AsiA interaction with SR4 is to block the formation of closed Eσ70 complexes at host promoters which harbor the two conserved σ70 DNA-binding elements centered at –10 and –35. At T4 middle promoters, the σ70 –35 binding element is replaced with a binding site for MotA centered at –30. The presence of a MotA–DNA complex preferentially recruits AsiA-modified Eσ70 to T4 middle promoters to transcribe these genes specifically.
The molecular basis for AsiA engagement with Eσ70 has now been revealed, in part, from the three-dimensional structure of AsiA in solution and elucidation of its binding mechanism for SR4. AsiA is demonstrated to be a novel homodimer in solution in which each monomer is constructed as a seven-helix bundle organized into four overlapping helix–loop–helix (HLH) elements. The interaction of SR4 with AsiA occurs by dissociation of the AsiA dimer to form an AsiA–SR4 complex with a 1:1 stoichiometry. The homodimer and heterodimer interfaces of AsiA are demonstrated by NMR spectroscopy and site-directed mutagenesis to be coincident. Thus, the mechanism by which AsiA engages σ70 is by subunit exchange, exchanging one AsiA monomer for SR4 to form a heteromeric Eσ70–AsiA complex.
Results
AsiA is dimeric in solution
During purification of recombinant AsiA from E.coli, it was observed that AsiA eluted from gel filtration chromatography at approximately twice its molecular weight, suggesting that AsiA was dimeric in solution. Analytical ultracentrifugation of the purified protein confirmed AsiA to be a dimer. van Holde–Weischet analysis (van Holde and Weischet, 1978) of sedimentation velocity experiments conducted at different concentrations (Carruthers et al., 2000) revealed a mixture of monomer and dimer at low concentration (9.8 µM) and mostly dimer at high concentration (68.4 µM). A combined integral distribution plot for the different concentration samples clearly indicates a concentration-dependent, reversible association event, which results in the formation of almost exclusively dimer at the higher concentration. The integral distribution plot of S20,W for the low concentration sample displays the typical half-parabola shape of a self-association event (Demeler et al., 1997) (not shown). Finite element analysis of the high concentration data resulted in random residuals and revealed a mol. wt of 19.64 ± 0.2 kDa, in good agreement with the theoretical molecular weight of an AsiA dimer (21.18 kDa). The frictional coefficient of 3.58 × 10–8 and frictional ratio (f/f0) of 1.13 suggests a mostly globular conformation of the dimer.
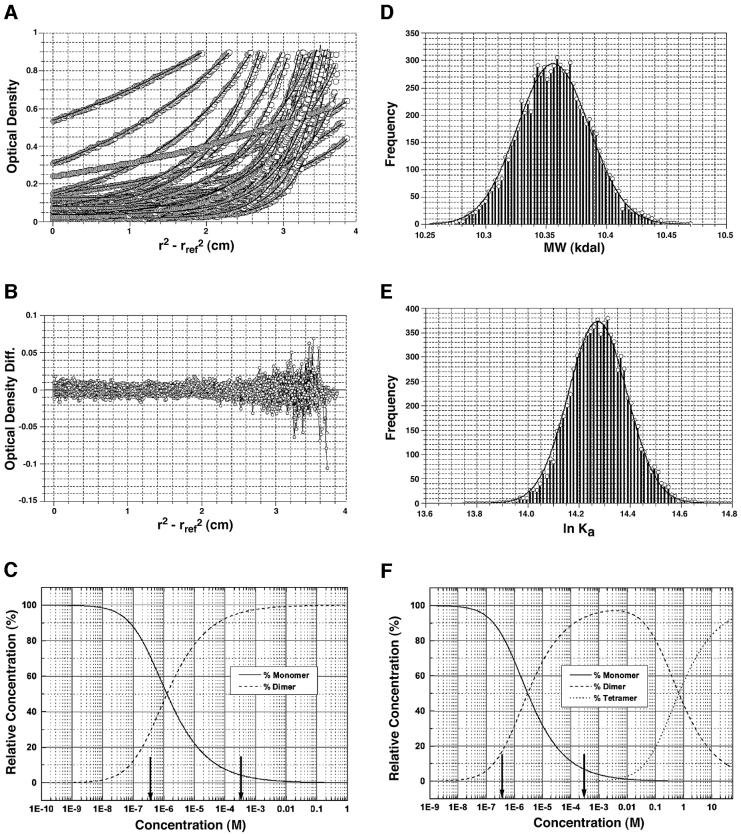
To establish the association properties of AsiA further, sedimentation equilibrium experiments were conducted over a large concentration range and experimental data collected at multiple wavelengths (Figure 1) (Johnson et al., 1981). To analyze the data under multiple rotor speeds, wavelengths and concentrations, the monomer molecular weight and association constant were considered global parameters and forced to be the same for all included data sets (Johnson et al., 1981). The wavelength scans were globally fit to a sum of Gaussian terms, whose width, amplitude and offset were allowed to float but considered global for all scans. For the global equilibrium analysis, 38 equilibrium scans from speeds ranging between 15 000 and 50 000 r.p.m. (Figure 1A and B) and loading concentrations between 0.2 and 0.7 optical density units (ODs) at both 230 and 280 nm were fit to both monomer–dimer and monomer–dimer–tetramer models (Figure 1C and F). Although the monomer–dimer–tetramer fit indicated the presence of small amounts of tetramer, the variance of the fit was reduced insignificantly by adding the additional parameter for the monomer–tetramer association. Thus, the system could be well described by a monomer–dimer equilibrium model, which resulted in random residuals and a monomer mol. wt of 10.36 ± 0.077 kDa (Figure 1D), which is in excellent agreement with the molecular weight derived from the protein sequence (10.59 kDa). The association constant of 1.58 ± 0.404 × 106/M suggests fairly tight binding for the dimer (Figure 1E).
Fig. 1. Sedimentation equilibrium analysis of the AsiA dimer. (A) Overlay of 38 wavelength scans and (B) residuals from the global fit of the data as described in Materials and methods. The Monte Carlo distribution of the molecular weight (D) and the association constant (E) (ln Ka) are shown which establish an experimental Mr = 10.36 ± 0.077 kDa for the monomer and an association constant of 1.58 ± 0.404 × 106/M. The equilibrium distribution plots for monomer–dimer (C) and monomer–dimer–tetramer (F) fits reveal that very little tetramer was present at the concentrations at which these measurements were taken (indicated by vertical arrows) and result in low confidence in the monomer–tetramer association constant.
Three-dimensional structure of AsiA
The three-dimensional structure of AsiA was determined from 1353 experimental distance restraints and 209 dihedral angle restraints per monomer employing standard techniques for chemical shift assignment and structure determination. A total of 89 φ and 55 χ1 restraints were derived from 3JNHα, 3JHαHβ and 4JNHβ coupling constants; 65 ψ restraints were determined by chemical shift database analysis with the program TALOS (Cornilescu et al., 1999). The 29 lowest energy structures displayed an r.m.s. coordinate deviation relative to the mean structure of 0.35 Å for backbone atoms and 0.86 Å for all non-hydrogen atoms (Table I).
Table I. Structural statisticsa.
| <SA> | |
|---|---|
| R.m.s.ds from experimental distance restraints per monomer (Å)b | |
| All (1353) | 0.058 ± 0.002 |
| sequential (|i – j| = 1) (350) | 0.035 ± 0.005 |
| short range (1 <|i – j| ≤5) (400) | 0.066 ± 0.003 |
| long range (|i – j|) >5) (230) | 0.077 ± 0.005 |
| intraresidue (283) | 0.053 ± 0.005 |
| H-bonds (90) | 0.062 ± 0.006 |
| R.m.s.ds from experimental distance restraints per dimer (Å)b | |
| All (64) | 0.097 ± 0.008 |
| R.m.s.ds from experimental restraints per monomer | |
| dihedral (°) (209) | 0.74 ± 0.067 |
| 3JNHα coupling constants (Hz) (75) | 0.97 ± 0.04 |
| Deviations from idealized covalent geometry | |
| bonds (Å) | 0.0049 ± 0.0004 |
| angles (°) | 0.64 ± 0.02 |
| impropers (°) | 0.54 ± 0.02 |
| Coordinate precision of dimerc | |
| backbone (residues 2–90) | 0.35 ± 0.04 |
| all non-hydrogen atoms (residues 2–90) | 0.86 ± 0.05 |
| Quality factors for dimerd | |
| % residues in most favorable Ramachandran (5162) | 84.5 |
| Prosa II Z score | –7.2 ± 0.34 |
aR.m.s.ds are calculated relative to the mean coordinates <SA> for the family of 29 simulated annealing structures excluding residue 1.
bNo restraints between protons separated by three bonds were utilized.
cThe precision of the coordinates is defined as the average atomic r.m.s.d. between the 29 individual simulated annealing structures and the mean coordinates <SA> for residues 2–90 in the dimer.
dPROCHECK_NMR (Laskowski et al., 1996) and ProsaII (Sippl, 1993) were used to assess the overall quality of the structures for residues 2–90 in the dimer.
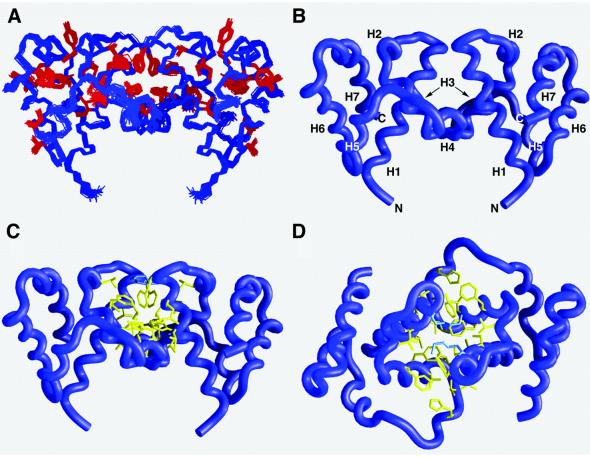
AsiA is an unusual seven-helix bundle composed of overlapping HLH elements (Figure 2A and B). Within each HLH element, the helical axes are oriented antiparallel to one another. The first and last HLH elements are oriented in approximately parallel planes, with the paired helix axes of the two HLH elements orthogonal to one another. Six of the seven helices are amphipathic, with helix H1 displaying an alternation of polar and non-polar surfaces. This alternation is required to form the hydrophobic core of the monomer in the first half of helix H1 (residues 4–12) and the hydrophobic dimer interface in the second half of helix H1 (residues 13–20). Helix H3 is entirely hydrophobic, forming the hydrophobic interior of each monomer and the dimer interface.
Fig. 2. Three-dimensional structure of the AsiA dimer. (A) The superposition of the 29 structures is shown for the non-hydrogen backbone atoms in blue and selected side chains in red. The coordinate precision for the structure family was 0.35 Å r.m.s.d. from the mean for the backbone atoms and 0.86 Å r.m.s.d from the mean for all non-hydrogen atoms. (B) Identical view of the AsiA dimer representing the backbone of the protein as a blue worm. The relative orientation of the helices in each monomer is indicated and illustrates the overlapping helix–loop–helix elements. HLH1 = α-helix H1 (Asn4–Lys20), loop L1 (Phe21–Thr23) and α-helix H2 (Glu24–Glu28). HLH2 = α-helix H3 (Arg30–Gly41) and 310 helix H4 (residues Thr43–Arg47). HLH3 = α-helix H5 (Gln51–Ser59), loop L2 (Glu60–Thr62) and α-helix H6 (Gln63–Glu72). HLH4 = α-helix H6 (Gln63–Glu72) and α-helix H7 (Asn74–Met86). (C and D) Two views of the AsiA dimer interface. In (C), the view is identical to (A) and (B). In (D), the view is rotated 90° about the horizontal axis. Non-polar amino acids are shown in yellow (Thr13, Val14, Ile17, Leu18 and Phe21 of helix H1, Phe33, Phe36, Leu37 and Ile40 of helix H3, and His44), and positively charged amino acids in blue (Lys20).
Approximately 1854 Å2 of accessible surface are buried at the dimer interface. The interface is almost entirely hydrophobic, composed of residues principally located in helices H1 and H3 (Figure 2B, C and D). Thr13, Val14, Ile17, Leu18, Lys20 and Phe21 of helix H1 in one monomer contact Glu24, Phe33, Phe36, Leu37 and Ile40 of helix H3 in the second monomer. At the top and bottom of the interface, charged residues are inferred to form salt bridges to cap the hydrophobic interior.
Coincidence of the AsiA homo- and heterodimer interfaces
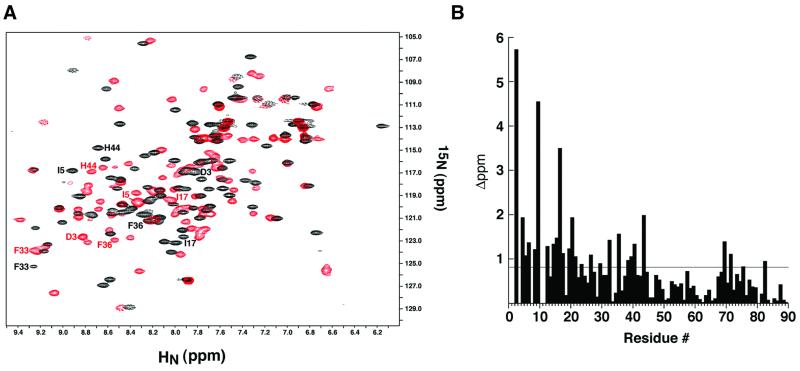
NMR ‘footprinting’ of AsiA bound to SR4 (σ537, residues 537–609; see Materials and methods) enabled identification of a local surface of AsiA which was perturbed upon formation of an AsiA–SR4 complex. Analysis of the combined backbone N and HN chemical shift differences between spectra of the AsiA homodimer and the AsiA–SR4 complex defined a broad ‘footprint’ encompassing many of residues 3–20 and 33–44 (Figure 3). These regions are located within helices H1, H3 and H4, and in the irregular loop between helices H4 and H5, i.e. the segments of the monomer which form the homodimer interface. This suggests that either the homodimer interface is altered upon binding SR4 or the SR4 binding surface is at least partially overlapping with the homodimer interface.
Fig. 3. Analysis of the σ537-binding surface by NMR ‘footprinting’. (A) AsiA homodimer (black) and the AsiA–σ537 complex (red) were analyzed by 15N–1H HSQC spectroscopy with 15N-labeled AsiA and unlabeled σ537. The positions of several residues that move substantially upon addition of σ537 are shown in black for the AsiA homodimer and in red for the AsiA–σ537 complex. (B) A histogram of the composite chemical shift changes (see Materials and methods) defines a broad ‘footprint’ (>0.8 p.p.m. change) of σ537 on AsiA that encompasses many of residues 3–20 and residues 33, 36, 39–40 and 44. These residues reside along the homodimer interface of AsiA.
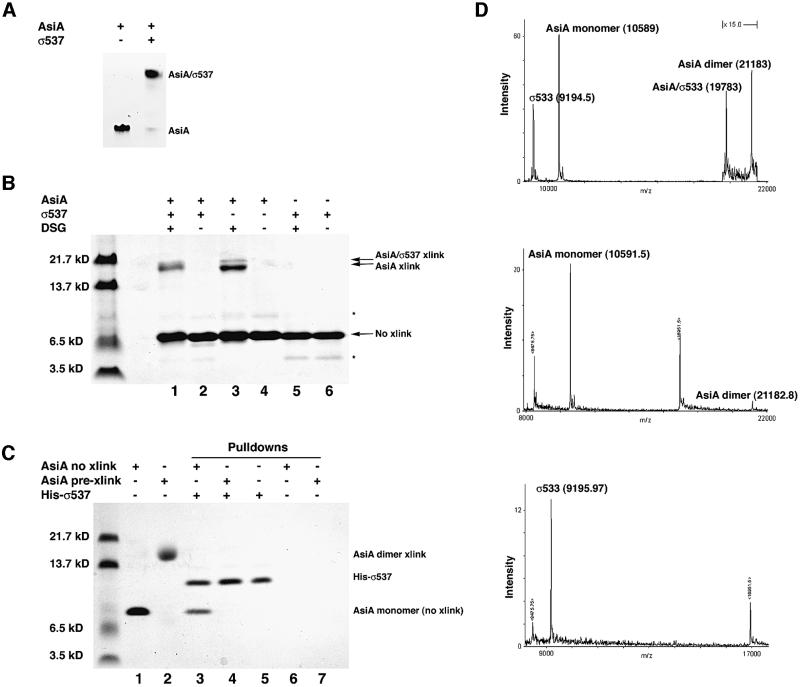
The relative size of the AsiA homodimer and the AsiA–SR4 complex was established by protein cross-linking and mass spectrometry. Cross-linking with disuccinimidyl glutarate (DSG) occurs between primary amines situated within 7.7 Å of one another, therefore DSG is capable of cross-linking both the AsiA homodimer and its complex with SR4, since lysine and arginine residues are present in the vicinity of both the homodimer interface (Lys9, Lys20 and Lys30 in AsiA) and the presumptive AsiA-binding surface of SR4 (Lys593 and Arg596 in SR4; Minakhin et al., 2001). Analysis of the size of the cross-linked species formed with free AsiA revealed that the cross-linked protein was of a size consistent with a homodimer (Figure 4B, lanes 3 and 4). This was in agreement with sedimentation equilibrium data (Figure 1), suggesting that cross-linking with DSG could probe the stoichiometry of AsiA protein complexes. Quite unexpectedly, the apparent size of the cross-linked species formed from the AsiA–SR4 complex was essentially the same as that of cross-linked AsiA alone (Figure 4B, lanes 1 and 2). SR4 itself fails to form cross-links with DSG (Figure 4B, lanes 5 and 6). Gel filtration of the NMR sample of AsiA–SR4 (not shown) also revealed that the AsiA homodimer and the AsiA–SR4 complex were of comparable size. This indicates that the AsiA homodimer is most probably disrupted upon binding SR4 to form the AsiA–SR4 complex.
Fig. 4. AsiA forms a 1:1 complex with SR4. (A) Non-denaturing Tris-alanine PhastGel (Amersham/Pharmacia) at pH 8.8 demonstrating the homogeneity of the AsiA homodimer and AsiA–σ537 complex preparations used for protein cross-linking with DSG. σ537 fails to run into this gel matrix. (B) Denaturing SDS-tricine PAGE of homodimers and AsiA–σ537 complexes following cross-linking with DSG. σ537 fails to form a cross-link with DSG (lanes 5 and 6), in contrast to the AsiA homodimer (lanes 3 and 4) and the AsiA–σ537 complex (lanes 1 and 2). Cross-linking with the AsiA homodimer produces a major and minor species (lane 3), while cross-linking the heterodimer produces a single species of slightly different mobility with apparently the same size as the cross-linked AsiA homodimer. Note that non-denaturing PAGE (A) establishes that the cross-linked species in each case are either pure homodimer (lane 3) or pure complex (lane 1). (C) Pre-cross-linked AsiA does not bind σ537 in an affinity tag pull-down experiment. Solutions of purified AsiA (lane 1) or purified AsiA cross-linked with DSG (lane 2) were probed with N-terminal His6-tagged σ537 immobilized on Ni2+-Sepharose. While uncross-linked AsiA was pulled down by His-σ537 (lane 3), pre-cross-linked AsiA was no longer capable of binding His-σ537 (lane 4). This indicates that an AsiA dimer which cannot dissociate is unable to bind σ537 in solution. Lanes 6 and 7 indicate that neither uncross-linked AsiA (lane 6) nor pre-cross-linked AsiA (lane 7) bind to Ni2+-Sepharose on their own. (D) MALDI of AsiA, σ533 and AsiA–σ533 complexes (see Materials and methods for details). The mass spectra of the AsiA homodimer and σ533 are shown alone in the middle and bottom panels, respectively. The peaks bracketing the main peak are those of the myoglobin calibrant with the +1 ion at 16 951.5 and the +2 ion at 8475.75. The top panel shows the mass spectrum of the AsiA–σ533 mixture with AsiA homodimer. In this spectrum, the masses of both protein complexes are seen, along with each of the monomer species from which they are derived. This demonstrates that the mass of the AsiA–σ533 complex is that of a 1:1 complex with complete subunit dissociation from homodimer to heterodimer.
Cross-linking and gel filtration of the AsiA–σ537 complex suggested that the AsiA homodimer completely dissociated to form the AsiA–σ537 complex. Thus, an undissociable form of AsiA would be expected to be unable to bind σ537. This indeed is the case, as demonstrated in Figure 4C. A covalently cross-linked AsiA dimer is unable to associate with σ537 in a His6 tag affinity pull-down experiment (Figure 4C, lanes 2 and 4). In contrast, uncross-linked AsiA retains the ability to bind σ537 in this assay system (Figure 4C, lanes 1 and 3). In conjunction with NMR footprinting, cross-linking of the AsiA–σ537 complex and gel filtration, the data support the notion that the AsiA homodimer must dissociate to permit σ537 to bind.
Stoichiometry of the AsiA–SR4 complex
The stoichiometry of the AsiA–SR4 complex was established by matrix-assisted laser desorption ionization (MALDI) mass spectrometry. Mass analysis was conducted on AsiA, SR4 (σ533, residues 533–609) and on a mixture of AsiA–σ533 complex and AsiA homodimer. Figure 4D demonstrates that the individual masses of the AsiA homodimer and AsiA–σ533 complex (21 183 and 19 783 kDa, respectively) are equal to the expected sum of the measured masses of the AsiA monomer (measured 10 591.5 ± 3 kDa, theoretical 10 590.5 kDa) with itself or the expected sum of AsiA monomer plus σ533 (measured 9196.0 ± 3 kDa, theoretical 9196 kDa). Thus, the AsiA–σ533 complex is formed by complete dissociation of the AsiA dimer, resulting in a 1:1 heterodimeric complex.
Characterization of the SR4-binding surface of AsiA
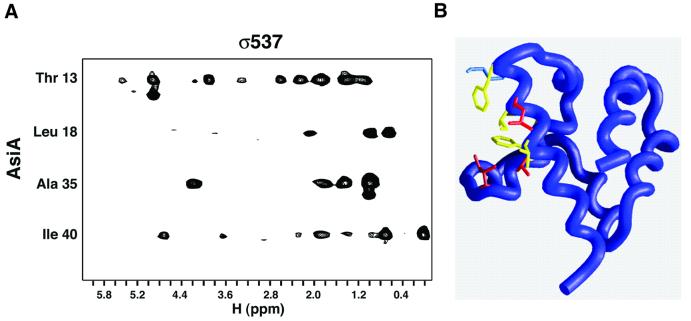
Interfacial nuclear Overhauser effects (NOEs) were observed between AsiA and SR4 and assigned to specific residues in the AsiA sequence to characterize the SR4-binding surface of AsiA further. These residues are found only at the homodimer interface, reinforcing the notion of complete subunit exchange from homodimer to heterodimer. Figure 5 displays a partial map of the AsiA residues that form direct contacts with SR4. These residues, Thr13, Leu18, Ala35 and Ile40, clearly encompass the surface of the AsiA monomer that forms the homodimer interface (compare Figure 2C and D with Figure 5B).
Fig. 5. The homodimer and heterodimer interfaces of AsiA are coincident. (A) The AsiA–σ537 complex was labeled asymmetrically with 13C/15N incorporation into AsiA and no labeling in σ537. A partial map of the AsiA–σ537 interface was visualized by F1-filtered/F3-edited 13C-NOESY (Zwahlen et al., 1997) and AsiA residues at the interface assigned by standard techniques. (B) The identified residues at the AsiA–σ537 interface are mapped onto the AsiA monomer, which represents the right monomer in Figure 2B. Residues at the homodimer interface are shown in yellow and blue as described in Figure 2C; residues that participate in both the homo- and heterodimer interfaces are shown in red. Ala35 is obscured in this view and cannot be seen.
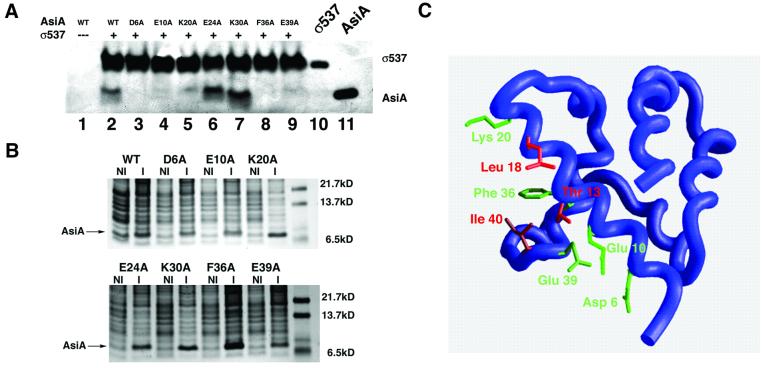
The presumptive AsiA–SR4 interface was analyzed further by site-directed mutagenesis. Alanine substitution at Asp6, Glu10, Lys20, Phe36 and Glu39, all residues that participate in the homodimer interface, appear to disrupt completely the interaction with SR4 in a pull-down assay (Figure 6A) employing crude E.coli extracts of the AsiA proteins (Figure 6B). Several of these positions, Glu10, Lys20 and Phe36, also displayed significant changes in chemical shift by NMR ‘footprinting’ (Figure 3B), although it is not theoretically possible to correlate the extent of the NMR chemical shift changes with the relative binding affinity seen for these mutants in a pull-down assay. Alanine mutagenesis at Glu24 and Lys30 does not appear to disrupt binding with SR4 in this assay (Figure 6A). In accordance with the mutagenesis data, these positions were relatively unperturbed by ‘footprinting’ analysis (Figure 3B).
Fig. 6. Mutagenesis of the AsiA homodimer interface. (A) The indicated mutant proteins (lanes 2–9) were analyzed for their ability to bind σ537 immobilized on chelating Sepharose 4B. Lane 1 represents a pull-down assay with beads alone (i.e. Ni2+ beads lacking σ537). Lane 10 represents purified σ537 that has not been exposed to AsiA. Lane 11 is purified wild-type AsiA that has not been exposed to σ537. Alanine substitution of charged residues that reside at the AsiA homodimer interface (Asp6, Glu10 and Glu39) results in mutant proteins that fail to bind σ537. Alanine substitutions at two other residues, Glu24 and Lys30, retain the ability to bind AsiA in this assay. (B) Expression of wild-type and mutant AsiAs. Uninduced (NI) and induced (I) expression of wild-type and mutant AsiAs visualized by Coomassie Blue staining. The position of the induced band representing the wild-type or mutant proteins is indicated. (C) Mapping of the heterodimer interface onto the AsiA monomer structure. Red residues (Thr13, Leu18, Ala35 and Ile40) display intermolecular NOEs to SR4 in an AsiA–σ537 complex. Green residues (Asp6, Glu10, Lys20, Phe36 and Glu39) fail to bind SR4 in a pull-down assay when mutated to alanine. Ala35 is obscured in this view and cannot be seen.
Discussion
AsiA was the first protein to have been reported to possess an anti-σ activity (Stevens and Rhoton, 1975; Stevens, 1976), although its primary role is now recognized to be both an anti-σ/transcriptional repressor of host genes and a co-activator for T4 middle gene expression. The action of AsiA is to bind the housekeeping σ factor, σ70, at SR4 and alter the pathway of Eσ70 recruitment to promoter DNA. An AsiA–Eσ70 complex does not display a DNase I footprint on promoters that possess both –10 and –35 σ-binding elements such as the E.coli lac UV5 (Adelman et al., 1997) or T7 A2 promoters (Severinova et al., 1998). This suggests that AsiA plays a direct role in precluding Eσ70–DNA interaction and formation of closed complex at promoters bearing σ-binding elements. Clearly, the interruption of Eσ70–DNA interaction is restricted to a subset of promoters, since the AsiA–Eσ70 complex displays a ‘typical’ open complex footprint on strong T4 promoters such as P15.0 (Adelman et al., 1997) and on E.coli promoters that lack a –35 element (Severinova et al., 1998). Thus, AsiA’s role as both repressor and activator of gene expression is to redirect the host transcription apparatus to transcribe specifically the subset of T4 genes that are dependent on both MotA and AsiA for activation. AsiA, like the bacteriophage φ29 p4 protein (Monsalve et al., 1997), exploits the presence or absence of a –35 element in a promoter to convert a repressive configuration into an activating configuration for transcription.
Subunit exchange of non-homologous proteins as a prerequisite for transcriptional activation has not been observed previously for a transactivating protein in prokaryotes. Thus, one must consider whether the AsiA homodimer described herein is relevant to the activity of AsiA in a T4-infected cell. Clearly, AsiA overexpressed in E.coli is capable of forming a dimer in vivo as that is how the recombinant protein used in this study was isolated. Analytical centrifugation, NMR spectroscopy, gel filtration, protein cross-linking and mass spectrometry have confirmed the predominance of the AsiA homodimer in solution. The chemical composition of the dimer interface also argues for a potential role for the homodimer in AsiA function. This interface is composed almost entirely of non-polar amino acids (Thr13, Val14, Ile17, Leu18, Ile19, Phe21, Phe33, Phe36 and Ile40), suggesting that the monomer would have limited solubility in solution. In vivo, comparison of the relative amounts of AsiA and σ70 20 min post-T4-infection suggests that AsiA is present in ∼25% excess (Kolesky et al., 1999). Thus, under physiological conditions, there is a pool of unbound AsiA present such that the homodimer could form depending on the in vivo concentration.
In order for subunit exchange to be a relevant mechanism in vivo, the affinity of AsiA for σ70 must greatly exceed that of AsiA binding to itself. Preferential association with σ70 is expected since the repression of host gene expression is evident within a few minutes following infection. An estimate of the AsiA–σ70 equilibrium dissociation constant (KD) can be made from recent studies with SR4-derived peptides in which the KD of a region 4.2–AsiA complex was estimated to be 20–100 nM (Minakhin et al., 2001). This indicates that σ70 binds AsiA 10–30 times more tightly than AsiA binds itself, consistent with a model in which σ70 could compete effectively for binding sites at the AsiA homodimer interface.
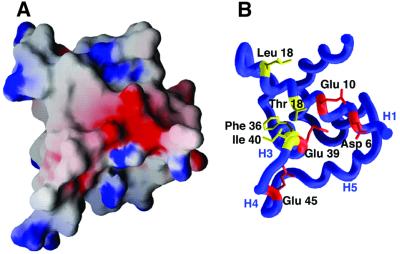
A total of 927 Å2 per monomer are buried at the homodimer interface. At least three-quarters of this surface must also be buried at the interface of the AsiA–SR4 complex given the extent of the heterodimer interface identified by NMR and mutagenesis. In the vicinity of the N-terminus of AsiA, there is a patch of negative charge formed by residues at the N-terminus of helix H1, the C-terminus of helix H3, helix H4 and the irregular loop between helices H4 and H5 (Figure 7). This may explain the observation that the AsiA–SR4 complex does not migrate into pH 8.8 native PAGE as far as the AsiA dimer itself (Figure 4A). SR4 may partially bury this negative surface, reducing the mobility of the AsiA–SR4 complex. Mutagenesis at three of these positions (Asp6, Glu10 and Glu39) abolished AsiA binding to SR4 in a pull-down assay (Figure 6A), consistent with this hypothesis. The balance of the SR4-binding surface is uncharged, resulting in an unusual interface in which a non-polar surface is contiguous with a negatively charged surface. These characteristics should be reflected in the complementary elements of SR4 that come into contact with AsiA. A distinctly positively charged surface at the C-terminus of the putative DNA-binding helix of SR4 (Lys593 and Arg596) has already been identified (Minakhin et al., 2001). Presumably, this region of SR4 engages the negatively charged surface of AsiA described above.
Fig. 7. Characteristics of the SR4-binding surface of AsiA. (A) Electrostatic potential map (drawn at ±9 kT) of the AsiA monomer viewed from the homodimer interface (Nicholls et al., 1991). Negatively charged surfaces are shown in red and positively charged surfaces in blue. (B) The negative patch seen in the center of the electrostatic surface is created by a collection of acidic residues (Asp6, Glu10, Glu39 and Glu45). These residues are hypothesized to be at least partially buried at the AsiA–SR4 interface. Hydrophobic amino acids which also make up part of the SR4 binding surface are shown in yellow.
Given that the stoichiometry of an SR4–AsiA complex is 1:1 (Adelman et al., 1997; this report), it was unexpected to find that AsiA was dimeric in solution. Many SR4-binding activators and at least one other anti- σ factor, SpoIIAB (Campbell and Darst, 2000), are also dimeric, and several dimeric activators contact SR4 asymmetrically. The λ cI dimer, for example, binds to adjacent half-sites in the λ PRM promoter, with one monomer binding to a DNA site that overlaps with the –35 binding site for SR4. The λ cI monomer nearest –35 contacts SR4 at residue 596 (Li et al., 1994), in the vicinity of the putative AsiA interaction site of SR4 (residues 587–596) (Minakhin et al., 2001). As observed in AsiA itself, the SR4 contact surface of λ cI is rich in negatively charged amino acids (Hochschild et al., 1983; Bushman et al., 1989). Similarly, the arabinose-activated form of AraC is dimeric and contacts DNA in the vicinity of –35 and forms a specific contact between the promoter-proximal C-terminal domain of one AraC monomer and residue 596 of SR4 (Hu and Gross, 1985). The same observations have been made for the dimeric malT activator (Hu and Gross, 1985). Thus, the core segment proposed to form the AsiA interaction site in SR4 (residues 587–596) may be a generalized docking site for prokaryotic activators that stimulate Eσ70 via SR4 (Severinova et al., 1996, 1998; Adelman et al., 1997; Pahari and Chatterji, 1997; Colland et al., 1998; Sharma et al., 1999; Minakhin et al., 2001). These dimeric activators that bind SR4 simultaneously with DNA remain dimeric due to their interaction with DNA rather than their interaction with Eσ70. The architecture of the AsiA–SR4 interaction, therefore, is not unlike these activators since all of these homodimeric proteins appear to utilize only one of their potential interaction surfaces to stimulate Eσ70 via SR4 interaction.
The SR4 interaction surface observed in AsiA is a striking mix of hydrophobic and charged surfaces, reminiscent of the recent analysis of the activation surface of phosphorylated CREB bound to the co-activator CBP/p300 (Radhakrishnan et al., 1997). The activation surface of CREB binds CBP/p300 via a helical segment that is largely hydrophobic, with a pair of negatively charged residues at one end of the helix. This is not unlike the activation surface of AsiA, in which the negatively charged surface formed by Asp6, Glu10 and Glu39 clusters at one end of the SR4-binding site and hydrophobic residues Thr18, Leu18, Phe36 and Ile40 cluster at the other end (Figure 7). Although the structures of the activation surfaces of CREB and AsiA are dissimilar, the distributions of charge along the recognition surfaces in these examples do bear a relationship to one another. Interspersed negatively charged and hydrophobic residues are also optimal for activation by the λ cI repressor at its interaction surface for SR4 (Bushman et al., 1989).
What remains to be understood is the detailed interaction between AsiA and SR4, in order to explain the ability of AsiA to preclude DNA recruitment of Eσ70 and block formation of open complexes at –10/–35 promoters. More importantly, the interplay between AsiA-modified Eσ70 and MotA remains unclear, leaving open the question of how AsiA may alter the structure of Eσ70 such that the modified polymerase preferentially associates with MotA– DNA complexes at T4 middle promoters. The ability to determine the structure and assess the functional consequences of the AsiA–SR4 complex now opens an avenue to address these questions in molecular detail.
Materials and methods
Preparation of proteins
AsiA cDNA was inserted in pET3a and the protein expressed in modified BL21(DE3) cells in which the T7 RNAP gene was under the control of a salt-inducible promoter. Modified BL21(DE3) cells were grown by adaptive control fermentation (Werner et al., 2001) employing 15NH4Cl and/or [13C]glucose, and induced for 2 h by addition of NaCl and isopropyl-β-d-thiogalactopyranoside (IPTG) to a final concentration of 150 and 0.4 mM, respectively. The protein was extracted by French press and purified by a combination of anion exchange (Q-Sepharose) and gel filtration (Sephacryl S-100HR) chromatographies in pH 8 Tris–HCl. The purity and homogeneity of the protein product were determined by SDS–PAGE and MALDI mass spectrometry. A cDNA encoding either residues 533–609 (σ533) or 537–609 (σ537) of E.coli σ70 was inserted into pET30b and expressed in HMS174(DE3) cells in the presence of 0.5 mM IPTG for 2 h. The two σ70 constructs were used interchangably without functional distinction. These constructs removed a C-terminal phenylalanine residue that reduced the solubility of the AsiA–σ537 or AsiA–σ533 complex without altering the binding properties for AsiA (Severinov and Muir, 1998). The σ proteins were purified from inclusion bodies by a combination of cation exchange (SP-Sepharose) chromatography in the presence of 50 mM Tris–HCl, 4 M urea pH 7.5, followed by cation exchange (Source 15S) chromatography under non-denaturing conditions.
The dimer interface was analyzed by asymmetrical labeling in which 13C/15N-labeled and unlabeled AsiA were denatured and mixed in a ratio of 1:1.05 to create a sample in which only one monomer was 13C-enriched. The extent of labeling was determined by integration of an isolated methyl group resonance in one-dimensional NMR spectra in which the 1JCH coupling was either active or suppressed by broadband 13C decoupling during acquisition.
Complexes of AsiA and σ537 or σ533 for NMR studies were prepared in two ways with identical results. First, denatured σ537 or σ533 was diluted rapidly into a solution of AsiA in 10 mM sodium phosphate, 50 mM NaCl pH 6.2 to a final guanidine HCl (GnHCl) concentration of 0.1 M, followed by dialysis to remove the residual GnHCl. Secondly, complexes were prepared by placing both AsiA and σ537 or σ533 in 6 M GnHCl in phosphate buffer followed by gradual removal of the GnHCl by dialysis. Complexes of AsiA and σ537 for cross-linking studies were prepared by first removing denaturant from stock solutions of σ537 (0.6 mg/ml) and subsequently adding a stoichiometric quantity of AsiA. Complexes of AsiA and σ533 for mass spectrometry studies were prepared similarly without the aid of denaturant. In all cases, the protein complex solutions were concentrated by centrifugal ultrafiltration.
Analytical ultracentrifugation
All sedimentation velocity and equilibrium experiments were performed with a Beckman Optima XL-I. Velocity, equilibrium and Monte Carlo analyses were performed with UltraScan version 5.0 (Demeler, 2001). Hydrodynamic corrections for buffer conditions were made according to data published by Laue et al. (1992), and as implemented in UltraScan. The partial specific volume of AsiA was estimated according to the method of Cohn and Edsall (1943), and as implemented in UltraScan. Monte Carlo analyses were calculated on a 40 processor Linux Beowulf cluster running Slackware Linux version 7.0. All samples were analyzed in a buffer containing 10 mM sodium phosphate pH 6.2 with 50 mM NaCl. Sedimentation velocity experiments were performed at 60 000 r.p.m. and 20°C. The samples were spun with double sector aluminum centerpieces in the AN-60-TI rotor. Scans were collected at either 230 or 280 nm in continuous radial mode with 0.001 cm steps and no averaging. Sedimentation equilibrium experiments were performed at 20°C and speeds ranging between 15 000 and 50 000 r.p.m. Samples were spun in double sector epon/charcoal centerpieces in the AN-50-TI rotor. Scans were collected at equilibrium at either 230 or 280 nm in radial step mode with 0.001 cm steps and 50 point averaging. Multiple loading concentrations ranging between 0.2 and 0.7 OD were measured at the given wavelength; data exceeding 0.9 OD were excluded from the fit.
NMR spectroscopy
Samples of 13C- and/or 15N-enriched AsiA were concentrated to 1 mM in 10 mM sodium phosphate, 50 mM NaCl, 1 mM benzamidine HCl pH 6.2 and assignments conducted at 25°C using standard techniques on Bruker DMX 500 and DMX 600 spectrometers. 3JNHα, 3JNHβ, 3JCγN, 3JCγCO coupling constants were measured by quantitative J correlation spectroscopy (Vuister et al., 1999). 15N- and 13C-edited three- and four-dimensional NOE spectroscopy was conducted with mixing times of 110 (15N) and 100 ms (13C), respectively. Secondary structure elements were identified from a combination of secondary 13Cα and 13Cβ shifts using the chemical shift index (Wishart et al., 1992) as well as the pattern and intensity of NOEs observed in 15N-edited NOESY (Wüthrich, 1986). The dimer interface was identified by 13C-F1-filtered/F3-edited NOESY (Zwahlen et al., 1997) and 13C-edited NOESY using an asymmetrically labeled sample as described. The changes in N and HN chemical shifts which defined the ‘footprint’ were calculated according to the following equation: Δp.p.m. = [(Nbound – Nfree)2 + (HN bound – HN free)2]1/2 (Nagata et al., 2001).
Cross-linking
Purified AsiA or AsiA–σ537 complexes were examined for homogeneity on non-denaturing 20% Tris-alanine PAGE pH 8.8, at a concentration of 0.5–0.6 mg/ml in sodium phosphate pH 6.2, 50 mM NaCl. A 15 µg aliquot of protein or protein complex was mixed with DSG at a final [DSG] = 67 µM and reacted for 30 min on ice in a final volume of 21 µl. The reaction was quenched with 1 µl of 3 M Tris–HCl pH 8 for 15 min followed by the addition of SDS loading dye. The cross-linked species were analyzed on 16% Tris-tricine gels.
Pull-down assay with cross-linked AsiA
Purified AsiA (0.5 mg/ml) was cross-linked with 10 µl of 2 mM DSG in dimethylsulfoxide (DMSO) and incubated for 30 min at room temperature. The reaction was quenched with 5 µl of 0.3 M Tris–HCl pH 8.0 buffer. The pull-down assay was performed by cloning σ537 into pET28b (Novagen) with an N-terminal His6 affinity tag and immobilizing the expressed protein on Ni2+-Sepharose using standard procedures. The σ537-loaded beads (20 µl) were then incubated with either AsiA or pre-cross-linked AsiA in 150 µl of a binding buffer containing 10 mM sodium phosphate, 150 mM NaCl, 0.2% NP-40 (w/v), 5% glycerol (w/v), 1 mM benzamidine HCl, 50 mM imidazole. After 2 h incubation at 4°C, the beads were pelleted, washed twice with 200 µl of binding buffer, eluted with SDS–PAGE loading dye and visualized by Coomassie Blue staining following 16% Tris-tricine PAGE. Control reactions were performed identically using Ni2+-Sepharose beads lacking σ537.
Mass spectrometry
AsiA, σ533 enriched with 15N and a mixture of an AsiA–σ533 complex with AsiA homodimer were analyzed using sinapinic acid (Sigma) as a matrix. Solvent conditions were optimized to prevent disruption of labile contacts (Cohen et al., 2000). The sinapinic acid matrix was prepared as a saturated solution in a 2:1 (v/v) mixture of water and acetonitrile. Samples at a concentration of 0.6–3.4 mg/ml were diluted 1:10 to 1:30 in matrix solution. Apomyoglobin (Sigma) at 400 nM was used as an internal mass calibrant. A small aliquot (0.5 µl) of protein–matrix solution was spotted onto the sample plate using an ultra-thin layer method (Cadene and Chait, 2000).
All mass measurements were performed on a Voyager DE-STR (Perseptive Biosystems, Foster City, CA) MALDI-TOF mass spectrometer, operating in positive linear, delayed extraction mode. This instrument is equipped with a nitrogen laser delivering pulses of UV light (wavelength 337 nm) at 3.5 Hz to the matrix spot. Spectra from 200 individual laser shots were averaged (using a 1 ns data channel width) with software provided by the manufacturer. The spectra were calibrated externally and internally and analyzed further using the program M-over-Z (http://www.proteometrics.com, http://prowl.rockefeller.edu).
Mutagenesis
Site-specific mutants of AsiA were introduced using the Stratagene Quik-Change kit according to the manufacturer’s instructions. The mutant plasmids were transformed into salt-inducible BL21(DE3) cells and expressed as described above. Crude lysate containing the expressed AsiA protein was prepared by sonication in 10 mM sodium phosphate pH 6.2, 150 mM NaCl, 10 mM benzamidine HCl, 20 µg/ml phenylmethylsulfonyl fluoride (PMSF), 0.25% NP-40, 5% glycerol. A 1 ml aliquot of crude lysate was then mixed with 100 µl of His6-σ537 immobilized on Chelating Sepharose Fast Flow (Amersham/Pharmacia). The binding reaction was carried out at 4°C for 3 h followed by 2× 1 ml washes of the Sepharose beads in the same buffer. The proteins were eluted from the beads with SDS–PAGE loading dye and visualized by Coomassie Blue staining.
Structure determination
NOEs within the protein were grouped into four distance ranges as described previously (Omichinski et al., 1997). Distances involving methyl groups, aromatic ring protons and non-stereospecifically assigned methylene protons were represented as a (Σr–6)–1/6 sum. φ, χ1 and χ2 angles were derived from 3J coupling constants and qualitative analysis of heteronuclear NOEs as described previously (Vuister et al., 1999). Protein backbone hydrogen bonding restraints (rNH–O = 1.5–2.8 Å, rN–O = 2.4–3.5 Å) within areas of regular secondary structure were introduced during the final stages of refinement. The minimum ranges employed for φ, χ1 and χ2 torsion angle restraints were ±30°. The structures were calculated with the program X-PLOR-3.843 (Brünger, 1992) adapted to incorporate pseudo-potentials for 3JNHα coupling constants (Garrett et al., 1994), Cα and Cβ chemical shifts (Kuszewski et al., 1995) and a conformational database potential (Kuszewski et al., 1997) employing a hybrid protocol as described previously (O’Donoghue et al., 1996; Omichinski et al., 1997). There were no hydrogen bonding, electrostatic or 6–12 Lennard–Jones empirical potential energy terms in the target function. Structure quality was assessed with PROCHECK_NMR (Laskowski et al., 1996) and Prosa II (Sippl, 1993) (Table I).
Acknowledgments
Acknowledgements
The authors are grateful to Debbie Hinton for providing cDNA for AsiA, and Marius Clore, Richard Ebright, Ann Hochschild and Debbie Hinton for useful discussions. The coordinates of the 29 AsiA dimer structures have been deposited in the Protein Data Bank with accession code 1KA3. This work was supported by the NIH grant GM63793 to M.H.W. and NCRR grant RR00862-28 to Brian T.Chait. M.H.W. is a Distinguished Young Scholar of the W.M.Keck Foundation.
References
- Adelman K., Orsini,G., Kolb,A., Graziani,L. and Brody,E.N. (1997) The interaction between the AsiA protein of bacteriophage T4 and the σ70 subunit of Escherichia coli RNA polymerase. J. Biol. Chem., 272, 27435–27443. [DOI] [PubMed] [Google Scholar]
- Brünger A.T. (1992) X-PLOR Manual, Version 3.1. Yale University Press, New Haven, CT.
- Bushman F.D., Shang,C. and Ptashne,M. (1989) A single glutamic acid residue plays a key role in the transcriptional activation function of λ repressor. Cell, 58, 1163–1171. [DOI] [PubMed] [Google Scholar]
- Cadene M. and Chait,B.T. (2000) A robust, detergent-friendly method for mass spectrometric analysis of integral membrane proteins. Anal. Chem., 72, 5655–5658. [DOI] [PubMed] [Google Scholar]
- Campbell E.A. and Darst,S.A. (2000) The anti-σ factor SpoIIAB forms a 2:1 complex with σF, contacting multiple conserved regions of the σ factor. J. Mol. Biol., 300, 17–28. [DOI] [PubMed] [Google Scholar]
- Carruthers L.M., Schirf,V.R., Demeler,B. and Hansen,J.C. (2000) Sedimentation velocity analysis of macromolecular assemblies. Methods Enzymol., 321, 66–80. [DOI] [PubMed] [Google Scholar]
- Cohen S.L., Padovan,J.C. and Chait,B.T. (2000) Mass spectrometric analysis of mercury incorporation into proteins for X-ray diffraction phase determination. Anal. Chem., 72, 574–579. [DOI] [PubMed] [Google Scholar]
- Cohn E.J. and Edsall,J.T. (1943) Proteins, Amino Acids and Peptides as Ions and Dipolar Ions. Reinhold, New York, NY.
- Colland F., Orsini,G., Brody,E.N., Buc,H. and Kolb,A. (1998) The bacteriophage T4 AsiA protein: a molecular switch for σ70 dependent promoters. Mol. Microbiol., 27, 819–829. [DOI] [PubMed] [Google Scholar]
- Cornilescu G., Delaglio,F. and Bax,A. (1999) Protein backbone angle restraints from searching a database for chemical shift and sequence homology. J. Biomol. NMR, 13, 289–302. [DOI] [PubMed] [Google Scholar]
- Demeler B. (2001) UltraScan 5.0: An Integrated Data Analysis Software Package for Sedimentation Experiments. Department of Biochemistry, University of Texas Health Science Center at San Antonio, TX.
- Demeler B., Saber,H. and Hansen,J.C. (1997) Identification and interpretation of complexity in sedimentation velocity boundaries. Biophys. J., 72, 397–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett D.S., Kuszewski,J., Hancock,T.J., Lodi,P.J., Vuister,G.W., Gronenborn,A.M. and Clore,G.M. (1994) The impact of direct refinement against three-bond HN-CαH coupling constants on protein structure determination by NMR. J. Magn. Reson. B, 104, 99–103. [DOI] [PubMed] [Google Scholar]
- Hinton D.M. and Vuthoori,S. (2000) Efficient inhibition of E.coli RNA polymerase by the bacteriophage T4 AsiA protein requires that AsiA binds first to free σ70. J. Mol. Biol., 304, 731–739. [DOI] [PubMed] [Google Scholar]
- Hinton D.M., March-Amegadzie,R., Gerber,J.S. and Sharma,M. (1996) Bacteriophage T4 middle transcription system: T4-modified RNA polymerase; AsiA, a σ70 binding protein; and transcriptional activator MotA. Methods Enzymol., 274, 43–57. [DOI] [PubMed] [Google Scholar]
- Hochschild A., Irwin,N. and Ptashne,M. (1983) Repressor structure and the mechanism of positive control. Cell, 32, 319–325. [DOI] [PubMed] [Google Scholar]
- Hu J.C. and Gross,C.A. (1985) Mutations in the σ subunit of E.coli RNA polymerase which affect positive control of transcription. Mol. Gen. Genet., 199, 7–13. [DOI] [PubMed] [Google Scholar]
- Johnson M.L., Correia,J.J., Yphantis,D.A. and Halvorson,H.R. (1981) Analysis of data from the analytical ultracentrifuge by nonlinear least squares techniques. Biophys. J., 36, 575–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolesky S., Ouhammouch,M., Brody,E.N. and Geiduschek,E.P. (1999) σ competition: the contest between bacteriophage T4 middle and late transcription. J. Mol. Biol., 291, 267–281. [DOI] [PubMed] [Google Scholar]
- Kuszewski J., Qin,J., Gronenborn,A.M. and Clore,G.M. (1995) The impact of direct refinement against 13Cα and 13Cβ chemical shifts on protein structure determination by NMR. J. Magn. Reson. B, 106, 92–96. [DOI] [PubMed] [Google Scholar]
- Kuszewski J., Gronenborn,A.M. and Clore,G.M. (1997) Improvements and extensions in the conformational database potential for the refinement of NMR and X-ray structures of proteins and nucleic acids. J. Magn. Reson. B, 125, 171–177. [DOI] [PubMed] [Google Scholar]
- Laskowski R.A., Rullmann,J.A.C., MacArthur,M.W., Kaptein,R. and Thornton,J.M. (1996) AQUA and PROCHECK-NMR: programs for checking the quality of protein structures solved by NMR. J. Biomol. NMR, 8, 477–486. [DOI] [PubMed] [Google Scholar]
- Laue T.M., Shah,B.D., Ridgeway,T.M. and Pelletier,S.L. (1992) Computer-aided interpretation of analytical sedimentation data for proteins. In Harding,S.E., Rowe,A.J. and Horton,J.C. (eds), Analytical Ultracentrifugation in Biochemistry and Polymer Science. Royal Society of Chemistry, Cambridge, UK, pp. 90–125.
- Li M., Moyle,H. and Susskind,M.M. (1994) Target of the transcriptional activation function of the phage λ cI protein. Science, 263, 75–77. [DOI] [PubMed] [Google Scholar]
- Minakhin L., Camarero,J.A., Holford,M., Parker,C., Muir,T.W. and Severinov,K. (2001) Mapping the molecular interface between σ70 subunit of E.coli RNA polymerase and T4 AsiA. J. Mol. Biol., 306, 631–642. [DOI] [PubMed] [Google Scholar]
- Monsalve M., Calles,B., Mencia,M., Salas,M. and Rojo F. (1997) Transcription activation or repression by phage φ29 protein p4 depends on the strength of the RNA polymerase–promoter interactions. Mol. Cell, 1, 99–107. [DOI] [PubMed] [Google Scholar]
- Mosig G. and Hall,D.H. (1994) Gene expression: a paradigm of integrated circuits. In Karam,J.D. (ed.), Molecular Biology of Bacteriophage T4. American Society for Microbiology Press, Washington, DC, pp. 127–131.
- Nagata T. and Werner,M.H. (2001) Functional mutagenesis of AML1/RUNX1 and PEBP2β/CBFβ define distinct, non-overlapping sites for DNA recognition and heterodimerization by the Runt domain. J. Mol. Biol., 308, 191–203. [DOI] [PubMed] [Google Scholar]
- Nicholls A., Sharp,K. and Honig,B. (1991) Protein folding and association: insights from the interfacial and thermodynamic properties of hydrocarbons. Proteins, 11, 281–296. [DOI] [PubMed] [Google Scholar]
- O’Donoghue S.I., King,G.F. and Nilges,M. (1996) Calculation of symmetric multimer structures from NMR data using a priori knowledge of the monomer structure, co-monomer restraints and interface mapping: the case of leucine zippers. J. Biomol. NMR, 8, 193–206. [DOI] [PubMed] [Google Scholar]
- Omichinski J.G., Pedone,P.V., Felsenfeld,G., Gronenborn,A.M. and Clore,G.M. (1997) The solution structure of a specific GAGA factor–DNA complex reveals a modular binding mode. Nature Struct. Biol., 4, 122–132. [DOI] [PubMed] [Google Scholar]
- Orsini G., Ouhammouch,M., Le Caer,J.P. and Brody,E.N. (1993) The asiA gene of bacteriophage T4 codes for the anti-σ70 protein. J. Bacteriol., 175, 85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouhammouch M., Adelman,K., Harvey,S.R., Orsini,G. and Brody,E.N. (1995) Bacteriophage T4 MotA and AsiA proteins suffice to direct Escherichia coli RNA polymerase to initiate transcription at T4 middle promoters. Proc. Natl Acad. Sci. USA, 92, 1451–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pahari S. and Chatterji,D. (1997) Interaction of bacteriophage T4 AsiA protein with Escherichia coli σ70 and its variant. FEBS Lett., 411, 60–62. [DOI] [PubMed] [Google Scholar]
- Pene C. and Uzan,M. (2000) The bacteriophage T4 anti-σ factor AsiA is not necessary for the inhibition of early promoters in vivo. Mol. Microbiol., 35, 1180–1191. [DOI] [PubMed] [Google Scholar]
- Radhakrishnan I., Pérez-Alvarado,G.C., Parker,D., Dyson,J., Montminy,M.R. and Wright,P.E. (1997) Solution structure of the KIX domain of CBP bound to the transactivation domain of CREB: a model for activator:coactivator interactions. Cell, 91, 741–752. [DOI] [PubMed] [Google Scholar]
- Sanson B. and Uzan,M. (1995) Post-transcriptional controls in bacteriophage T4: roles of the sequence-specific endoribonuclease RegB. FEMS Microbiol. Rev., 17, 141–150. [DOI] [PubMed] [Google Scholar]
- Severinov K. and Muir,T.W. (1998) Expressed protein ligation, a novel method for studying protein–protein interactions in transcription. J. Biol. Chem., 273, 16205–16209. [DOI] [PubMed] [Google Scholar]
- Severinova E., Severinov,K., Fenyo,D., Marr,M., Brody,E.N., Roberts,J.W., Chait,B.T. and Darst,S.A. (1996) Domain organization of the Escherichia coli RNA polymerase σ70 subunit. J. Mol. Biol., 263, 637–647. [DOI] [PubMed] [Google Scholar]
- Severinova E., Severinov,K. and Darst S.A. (1998) Inhibition of Escherichia coli RNA polymerase by bacteriophage T4 AsiA. J. Mol. Biol., 279, 9–18. [DOI] [PubMed] [Google Scholar]
- Sharma M., Marshall,P. and Hinton,D.M. (1999) Binding of the bacteriophage T4 transcriptional activator, MotA, to T4 middle promoter DNA: evidence for both major and minor groove contacts. J. Mol. Biol., 290, 905–915. [DOI] [PubMed] [Google Scholar]
- Sippl M.J. (1993) Recognition of errors in three-dimensional structures of proteins. Proteins, 17, 355–362. [DOI] [PubMed] [Google Scholar]
- Stevens A. (1976) A salt-promoted inhibitor of RNA polymerase isolated from T4 phage-infected E.coli. In Losick,R. and Chamberlin,M. (eds), RNA Polymerase. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, pp. 617–627.
- Stevens A. and Rhoton,J.C. (1975) Characterization of an inhibitor causing potassium chloride sensitivity of an RNA polymerase from T4 phage-infected Escherichia coli. Biochemistry, 14, 5074–5079. [DOI] [PubMed] [Google Scholar]
- Stitt B. and Hinton,D. (1994) Regulation of middle-mode transcription. In Karam,J.D. (ed.), Molecular Biology of Bacteriophage T4. American Society for Microbiology Press, Washington, DC, pp. 142–160.
- van Holde K.E. and Weischet,W.O. (1978) Boundary analysis of sedimentation velocity experiments with monodisperse and paucidisperse solutes. Biopolymers, 17, 1387–1403. [Google Scholar]
- Vuister G.W., Tessari,M., Karini-Nejad,Y. and Whitehead,B. (1999) Pulse sequences for measuring coupling constants. In Krishna,N.R. and Berliner,L.J. (eds), Biological Magnetic Resonance 16. Kluwer Press, New York, NY, pp. 195–259.
- Werner M.H., Gupta,V., Lambert,L.J. and Nagata,T. (2001) Uniform 13C/15N labeling of DNA by tandem repeat amplification. Methods Enzymol., 338, 283–305. [DOI] [PubMed] [Google Scholar]
- Wishart D.S., Sykes,B.D. and Richards,F.M. (1992) The chemical shift index: a fast and simple method for the assignment of protein secondary structure through NMR spectroscopy. Biochemistry, 31, 1647–1651. [DOI] [PubMed] [Google Scholar]
- Wüthrich K. (1986) NMR of Proteins and Nucleic Acids. John Wiley and Sons, New York, NY.
- Zwahlen C., Legault,P., Vincent,S.J.F., Greenblatt,J., Konrat,R. and Kay,L.E. (1997) Methods for measurement of intermolecular NOEs by multinuclear NMR spectroscopy: application to a bacteriophage λ N-peptide/boxB RNA complex. J. Am. Chem. Soc., 119, 6711–6721. [Google Scholar]