Abstract
The osmosensing mechanism of the ATP-binding cassette (ABC) transporter OpuA of Lactococcus lactis has been elucidated for the protein reconstituted in liposomes. Activation of OpuA by osmotic upshift was instantaneous and reversible and followed changes in volume and membrane structure of the proteoliposomes. Osmotic activation of OpuA was dependent on the fraction of anionic lipids present in the lipid bilayer. Also, cationic and anionic lipophilic amphiphiles shifted the activation profile in a manner indicative of an osmosensing mechanism, in which electrostatic interactions between lipid headgroups and the OpuA protein play a major role. Further support for this notion came from experiments in which ATP-driven uptake and substrate-dependent ATP hydrolysis were measured with varying concentrations of osmolytes at the cytoplasmic face of the protein. Under iso-osmotic conditions, the transporter could be activated by high concentrations of ionic osmolytes, whereas neutral ones had no effect, demonstrating that intracellular ionic strength, rather than a specific signaling molecule or water activity, signals osmotic stress to the transporter. The data indicate that OpuA is under the control of a mechanism in which the membrane and ionic strength act in concert to signal osmotic changes.
Keywords: ABC transporter/cell volume regulation/osmosensing/proteoliposomes
Introduction
Maintenance of cell turgor is a prerequisite for almost any form of life, as it provides the mechanical force for expansion of the cell wall. Generally, microorganisms respond to an osmotic upshift by accumulating kosmotropic organic solutes (compatible solutes) to counteract the loss of water and decrease in turgor pressure (Poolman and Glaasker, 1998). Upon osmotic downshift, the cells need to expel the solutes rapidly in order to prevent turgor pressure from becoming too high, which may lead to lysis of the cells. In order to cope with osmotic stress effectively, the primary response to this type of challenge must involve (in)activation of existing transporters, as synthesis of new enzyme systems would take too long to respond. A major topic in the field of cell volume regulation concerns the mechanism(s) underlying the osmotic activation of transport and channel molecules. The potential stimuli for such systems comprise changes in extra- or intracellular osmolality, ionic strength, turgor pressure, molecular crowding, osmolality gradient across the membrane or the physicochemical properties of the membrane (Wood, 1999). Recent work on the osmoregulated ATP-binding cassette (ABC) transporter OpuA from Lactococcus lactis, and the ion-linked transporters ProP from Escherichia coli and BetP from Corynebacterium glutamicum showed that these systems act as both osmosensor and osmoregulator (Racher et al., 1999; Rübenhagen et al., 2000; van der Heide and Poolman, 2000b). These studies excluded turgor as the possible signal for osmotic activation. Activation of OpuA and BetP by charged amphipaths suggested that these transport systems sense osmotic stress via alterations in membrane properties/protein–lipid interactions, but direct effects via changes in the hydration state of the proteins could not be excluded. Several physicochemical properties of the membrane, such as membrane thickness, fluidity, interfacial polarity, membrane charge, hydration of lipid headgroups, acyl chain packing, but also the ‘macroscopic’ membrane folding, are affected upon osmolality shifts. Changes in one or more of these membrane parameters, sometimes described in terms of changes in the lateral pressure profile (Cantor, 1999), could influence the conformation of the transport proteins and thereby their activity.
Lactococcus lactis responds to an osmotic upshift by accumulating glycine betaine via the ABC transporter OpuA, which is osmotically regulated at the level of both expression and transport activity (van der Heide and Poolman, 2000a). The protein is composed of two different polypeptides, i.e. the ATPase and a subunit that comprises both the translocator and ligand-binding domain (van der Heide and Poolman, 2000b). At present, OpuA is one of a few osmoregulated proteins, and the only osmotically activated ABC transporter, that has been characterized in a well-defined proteoliposomal system. Components other than the subunits of OpuA and a lipid membrane are not needed for osmotic regulation of the system. OpuA has the advantage over ion-linked transporters that both translocation and ATPase activity can be followed in a manner that allows discrimination between ‘external’ and ‘cytoplasmic’ hydration effects.
In the present study, we show that the osmotic activation profile of OpuA is set by the bulk charge in the lipid headgroup region of the membrane, indicating that electrostatic interactions between lipids and the transporter are intrinsic to the osmosensing mechanism. It is also demonstrated that the ionic strength at the cytoplasmic face of the OpuA protein, rather than a specific signaling molecule or water activity, signals osmotic stress to the transporter. We thus conclude that cytoplasmic ionic strength serves as an osmotic signal, presumably by affecting lipid–protein interactions.
Results
The response of OpuA towards osmotic stress is instantaneous and reversible
To assess the kinetics and reversibility of osmotic (in)activation of OpuA, the membrane-reconstituted protein was exposed alternately to osmotic up- and downshifts. The internal osmolality was varied between 190 and 380 mosmol/kg. Figure 1 shows that activation of OpuA by osmotic upshift is instantaneous and reversible upon returning to iso-osmotic conditions; the reversibility was not restricted to the particular lipid composition used in this experiment. OpuA was reactivated again by a second osmotic upshift, demonstrating that the integrity of the proteoliposomes was not compromised by the osmotic challenges. Maintenance of liposome integrity was confirmed by internal volume measurements, as shifts between hyper- and iso-osmotic conditions did not result in loss of the enclosed fluorescent dye calcein. It is important to stress here that the internal volume decreased in proportion to the osmotic upshift; the internal and external osmolalities became equal, within the time resolution of the experiment (∼1 s). Thus, upon osmotic upshift, the surface to volume ratio of the proteoliposome increased.
Fig. 1. Kinetics and reversibility of the osmotic activation of OpuA. Uptake of [14C]glycine betaine (final concentration, 76 µM) was assayed in 100 mM KPi pH 7.0 corresponding to 190 mosmol/kg. The proteoliposomes were composed of DOPC/DOPE/DOPG in a 2:1:1 mole ratio. At 105 (circles, squares and triangles) and 285 s (circles), the proteoliposomes were subjected to hyperosmotic conditions by the addition of 100 mM KCl (final osmolality corresponding to 380 mosmol/kg). Iso-osmotic conditions were restored at 180 s (circles) by dilution of the assay mixture with water (plus 76 µM [14C]glycine betaine).
Proteoliposomes form ‘sickle-shaped’ structures when subjected to hyperosmotic stress
To observe the changes in volume of the proteoliposomes upon osmotic upshifts, fluorophore (calcein) self-quenching and cryo-electron microscopy (cryo-EM) studies were performed. It was observed that, at trans-membrane osmotic gradients ranging from 0 to 535 mosmol/kg, the volume of the proteoliposomes decreased proportionally to the increase in the osmolality of the medium (data not shown), thereby dissipating the trans-membrane osmotic gradient. This osmometric behavior was accompanied by changes in ‘macroscopic’ folding of the membrane as visualized by cryo-EM (Figure 2). The proteoliposomes were converted from spherical into ‘sickle-shaped’ structures upon osmotic upshift. In principle, differences in the fraction of anionic lipids could affect the size and ‘macroscopic’ folding of the membrane, and thereby influence the activity of OpuA. The observed macromolecular structures, however, were the same for all lipid mixtures tested (Figure 2 and data not shown). The proteoliposomes with an average diameter of ∼150 nm have a low membrane curvature, but upon osmotic upshift highly curved regions appeared in the proteoliposomes. To exclude the possibility that initial differences in the ‘macroscopic’ curvature had an effect on the osmotic activation of OpuA, proteoliposomes obtained by extrusion through polycarbonate filters with a pore size of 200 and 400 nm were compared. The experiments showed that the dependence of OpuA on the external osmolality (osmotic activation profile) was the same for both preparations (data not shown).
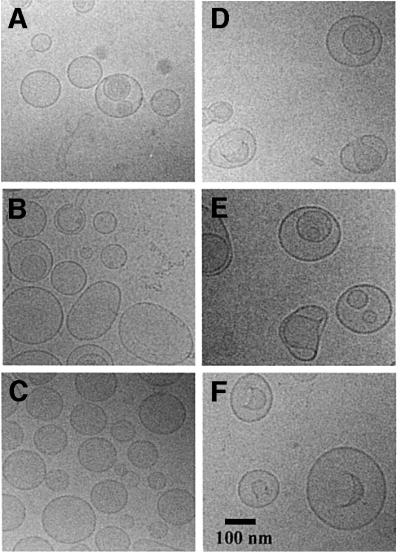
Fig. 2. Morphology of proteoliposomes. Cryo-EM was used to study liposomes composed of mixtures of DOPC, DOPE and DOPG (20 mg/ml, prepared in 100 mM KPi pH 7.0). Samples were prepared under iso- and hyperosmotic conditions; the latter was effected by the addition of 200 mM KCl. The iso- and hyperosmotic conditions correspond to 190 and 535 mosmol/kg, respectively. (A and D) Liposomes composed of DOPC/DOPE in a 1:1 mole ratio, under iso- and hyperosmotic conditions, respectively. (B and E) Liposomes composed of DOPC/DOPE/DOPG in a 3:4:1 mole ratio, under iso- and hyperosmotic conditions, respectively. (C and F) Liposomes composed of DOPC/DOPE/DOPG in a 1:4:3 mole ratio, under iso- and hyperosmotic conditions, respectively. The vast majority of the proteoliposomes were unilamellar, and the appearance of a spherical shape inside a vesicle (E and F) generally represents a top view of an invaginated liposome.
Membrane-permeable versus membrane-impermeable osmolytes
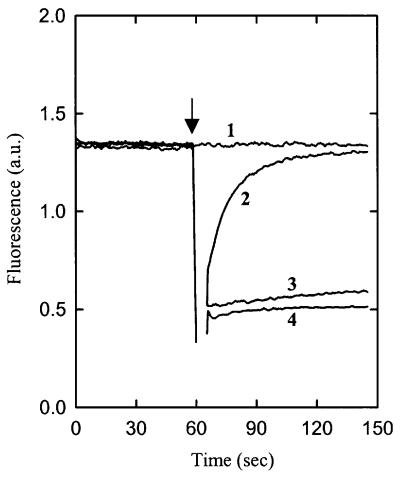
It has been argued that membrane-impermeable osmolytes such as KCl and sucrose activate osmoregulated transporters by a mechanism different from that of membrane-permeable osmolytes, e.g. glycerol and low molecular weight polyethylene glycols (PEGs) (Racher et al., 2001). To test this suggestion experimentally, the effects of glycerol on the kinetics of osmotic activation of OpuA and on the changes in volume and macroscopic structure of the proteoliposomes were determined. In the presence of 270 mM glycerol, the amount of glycine betaine taken up after 30 s was 30% higher than that of the iso-osmotic control sample. The stimulation by glycerol was not observed when the osmolyte was added 1 min prior to the transport measurements (data not shown). Thus, glycerol elicited a transient activation of OpuA that paralleled the time dependence of the shrinkage of the (proteo)liposomes (Figure 3). The transient changes in liposome volume were confirmed by cryo-EM (data not shown). The kinetics of OpuA activation by glycerol reflect the transient osmotic stress imposed by this highly membrane-permeable osmolyte. We conclude that the mechanism underlying osmotic activation of OpuA is not dependent on the type of osmolyte used to challenge the system.
Fig. 3. The effect of membrane-permeable versus membrane-impermeable osmolytes on the internal volume of proteoliposomes. Calcein quenching was assayed in 100 mM KPi pH 7.0. The proteoliposomes were composed of 25 mol% DOPC, 50 mol% DOPE and 25 mol% DOPG. Iso-osmotic (1) and hyperosmotic conditions correspond to 190 and 390 mosmol/kg, respectively. To impose hyperosmotic conditions, 200 mM KCl (4), 270 mM glycerol (2) or 327 mM sucrose (3) were added to the sample after 1 min pre-incubation (indicated by an arrow).
Effect of anionic lipids on the activation profile of OpuA
To investigate whether osmotic activation of OpuA is dependent on a particular lipid composition, lipids were used that varied in headgroup size (lamellar versus non-lamellar lipids), charge (anionic versus zwitterionic/ neutral lipids), acyl chain length (14–22 carbon atoms) and position and configuration of the unsaturated bond in the acyl chain. The lipids predominantly used were phosphatidylglycerol (PG), phosphatidylserine (PS), phosphatidylcholine (PC) and phosphatidylethanolamine (PE).
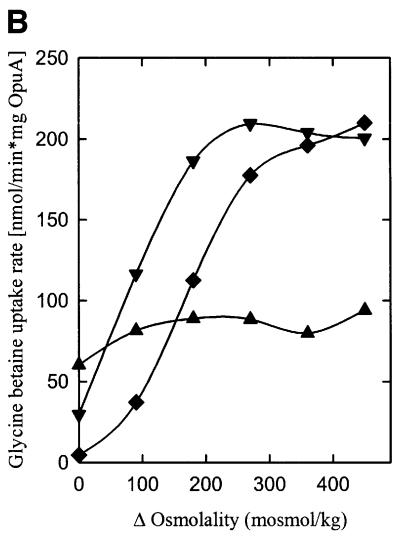
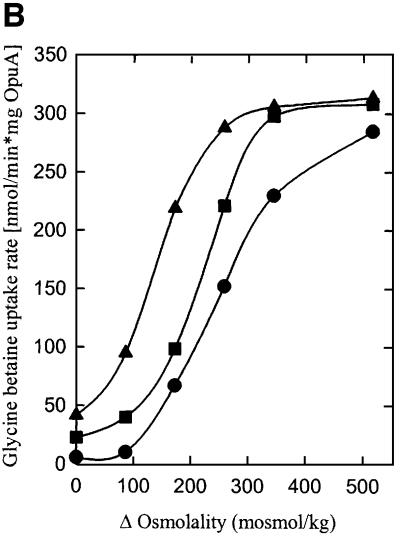
To determine the specific effects of anionic lipids on OpuA activity, the protein was reconstituted in proteoliposomes composed of the dioleoyl (18:1 Δ9 cis) derivatives DOPC (zwitterionic, lamellar), DOPE (zwitterionic, non-lamellar) and DOPG or DOPS (anionic, lamellar) in the mole ratios 8:8:0, 7:8:1, 6:8:2, 4:8:4, 2:8:6 and 0:8:8, yielding proteoliposomes with 0, 6, 13, 25, 38 and 50 mol% of anionic lipid, respectively. Figure 4A shows that OpuA was active under iso-osmotic conditions and that an osmotic upshift did not stimulate the activity with 6–13 mol% of anionic lipid present (only the data for 13 mol% DOPG are shown). OpuA had low or no activity under iso-osmotic conditions when the membrane contained ≥25 mol% DOPG. From 25 to 50 mol% anionic lipid, the activation threshold (trans-membrane osmotic gradient needed for activation) of OpuA was shifted to higher values. The same trend was observed for the value of the trans-membrane osmotic gradient at which maximal activity was reached. The fact that these effects were observed with both DOPG and DOPS (data not shown) suggests that the activation threshold is determined by the fraction of anionic lipids rather than by a specific lipid requirement. No activity was observed in proteoliposomes composed of DOPC or DOPC plus DOPE.
Fig. 4. The effect of anionic lipids on the osmotic activation of OpuA. (A) Uptake of [14C]glycine betaine (final concentration, 76 µM) was assayed in 100 mM KPi pH 7.0. The proteoliposomes were composed of 50 mol% DOPE and 0 (circles), 13 (upright triangles), 25 (inverted triangles), 38 (diamonds) or 50 mol% DOPG (squares) plus 50, 37, 25, 12 or 0 mol% DOPC, respectively. (B) The proteoliposomes were composed of 50 mol% DOPE and 13 (upright triangles), 25 (inverted triangles) or 38 mol% DOPG (diamonds) plus 37, 25 or 12 mol% DOPC, respectively, and were obtained via fusion of proteoliposomes (DOPE/DOPG, 1:1 mole ratio) with liposomes of the appropriate composition in a 1:1 mole ratio. The osmolality of the medium was varied with KCl. Δ Osmolality refers to the difference in external and internal osmolality.
To exclude the possibility that differences in activity were due to variations in reconstitution efficiencies, proteoliposomes consisting of DOPC/DOPE/DOPG (1:2:1 mole ratio) were fused in a 1:1 ratio with liposomes composed of DOPC/DOPE (1:1 mole ratio), DOPC/DOPE/DOPG (1:2:1 mole ratio) and DOPE/DOPG (1:1 mole ratio), resulting in proteoliposomes containing 50 mol% DOPE plus 13, 25 and 38 mol% DOPG, respectively. These fusion experiments yielded activation profiles similar to those observed with the membrane reconstitutions of OpuA in the separate lipid mixtures (Figure 4B). Fusion of proteoliposomes composed of DOPC or DOPC plus DOPE with DOPG liposomes did not result in significant transport activity despite the fact that all OpuA protein was associated with the vesicles. We conclude that the absence of anionic lipids in the reconstitution process results in trapping of the protein into an inactive state.
Finally, comparable activation profiles of OpuA were observed with ionic (KCl, NaCl, KPi or K2SO4) and neutral osmolytes (sucrose) used to vary the external osmolality (data not shown). This indicates that the change in the external ionic strength is not responsible for the observed effects, but that changes in the activation of OpuA are due solely to osmotic effects. It is important to stress here that the majority of the experiments were performed at a relatively high ionic strength (iso-osmotic conditions correspond to 190 mosmol/kg or 100 mM KPi, pH 7.0, externally).
Effect of non-bilayer lipids on the activation profile of OpuA
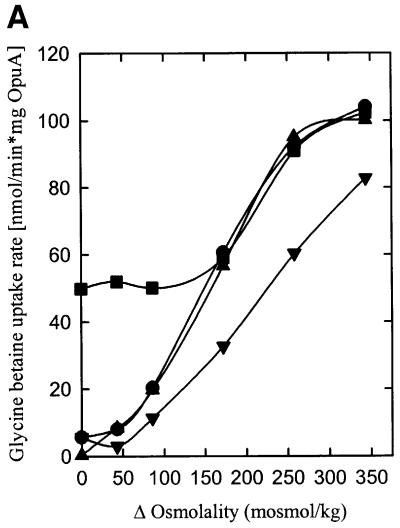
To determine the effects of non-bilayer lipids on OpuA activity, the fraction of DOPE was varied between 0 and 50 mol% with DOPG at a constant 38 mol% (Figure 5). From these experiments, it is concluded that DOPE is essential for high activity of OpuA, but the non-bilayer lipid does not affect the activation threshold of OpuA or the value of the trans-membrane osmotic gradient at which maximal activity is reached. To assess further the requirement of OpuA for non-bilayer lipids, the effect of one (mono-methyl-DOPE) or two (di-methyl-DOPE) additional methyl groups on the ethanolamine headgroup of DOPE was investigated. The larger the size of the headgroup, the higher the propensity to form stable bilayer structures. It was observed that DOPE, mono-methyl and di-methyl DOPE, in combination with a fixed concentration of DOPG, are decreasingly effective in stimulating OpuA activity (data not shown). These observations confirm the notion that non-bilayer-forming lipids are needed for maximal activity of OpuA but do not affect the activation mechanism.
Fig. 5. The effect of non-lamellar lipids (DOPE) on the osmotic activation of OpuA. The proteoliposomes were composed of 38 mol% DOPG and 6 (circles), 13 (squares), 25 (upright triangles), 38 (inverted triangles) or 50 mol% DOPE (diamonds) plus 56, 49, 37, 24 or 12 mol% DOPC, respectively, and were obtained via fusion of proteoliposomes (DOPC/DOPE/DOPG, 2:1:1 mole ratio) with liposomes of the appropriate composition in a 1:3 mole ratio. Experimental details are as described in the legend to Figure 4.
Effect of acyl chain length and configuration/position of the unsaturated bond on the activity of OpuA
Besides the headgoup region, the hydrophobic core of the lipid bilayer could influence the activity and/or the osmotic activation profile of OpuA. To determine the effect of the acyl chain length (membrane thickness) on OpuA activity, membrane reconstitution was performed with liposomes consisting of 25 mol% DOPG, 25 mol% DOPE and 50 mol% PC with an acyl chain length of either 14, 16, 18, 20 or 22 carbon atoms (cis-unsaturated at the Δ9 position). The activation profiles of OpuA were not affected by the differences in acyl chain length, but the absolute activity reached a maximum in the composition with a PC acyl chain of 18 carbon atoms (data not shown). The osmotic activation profile of OpuA was also not significantly affected when PC (18:1 trans-unsaturated at the Δ9 position), PG (18:1 trans-unsaturated at the Δ9 position) or PE (18:1 cis-unsaturated at the Δ6 position) were used instead of the corresponding dioleoyl (all 18:1 cis-unsaturated at the Δ9 position) lipids. Taken together, this data set indicates that changes in the physicochemical properties of the hydrophobic core of the lipid bilayer are not a determining factor in the osmotic activation mechanism of OpuA.
Effect of amphiphilic molecules on OpuA activity
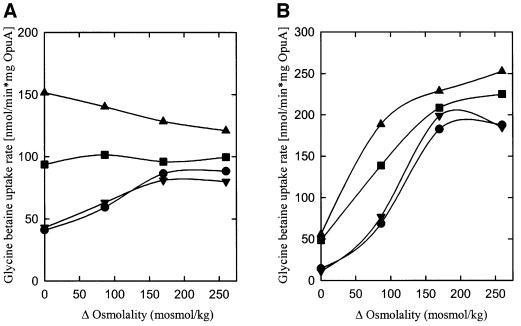
Since activation of OpuA is strongly dependent on the fraction of anionic lipids present in the membrane, experiments were performed with small charged amphiphilic molecules that partition into the lipid bilayer. Insertion of a cationic or anionic amphipath would lead to a decrease or increase, respectively, in the bulk negative charge at the membrane surface, while neutral amphipaths are predicted to have little effect. With proteoliposomes composed of DOPC/DOPE/DOPG in the mole ratios 2:1:1 and 0:1:1, low concentrations of the cationic amphipath tetracaine in the assay medium indeed decreased the activation threshold of OpuA (Figure 6A and B; data from experiments with other lipid compositions and/or tetracaine concentrations not shown). The synergy between ionic strength and the presence of tetracaine was more pronounced at high than low mole fractions of DOPG (e.g. compare Figure 6A and B), suggesting that the effects of tetracaine on OpuA activity may be due not only to perturbation of the membrane surface charge. Figure 6B shows that pre-loading of the proteoliposomes with tetracaine resulted in a more pronounced shift of the activation threshold of OpuA than when the amphipath was added 1 min prior to the initiation of the transport reaction. This most probably reflects the time needed for tetracaine to equilibrate over the two membrane leaflets. Importantly, and opposite to the effects of tetracaine, the anionic amphipath capric acid (n-decanoic acid) shifted the activation threshold of OpuA to higher osmolalities (Figure 6A).
Fig. 6. The effect of membrane-active lipophilic compounds on OpuA activity. (A) Proteoliposomes were composed of DOPC/DOPE/DOPG in a 2:1:1 mole ratio. Transport activity was assayed without (circles) or with tetracaine (squares; cationic; 2 mM), decane (upright triangles; neutral; 4.2 mM) or capric acid (inverted triangles; anionic; 0.7 mM) present in the assay medium. (B) Proteoliposomes were composed of DOPE/DOPG in a 1:1 mole ratio. Transport activity was assayed in proteoliposomes, non-loaded (circles, squares) or pre-loaded (triangles) with 2 mM tetracaine, in the presence (squares, triangles) or absence (circles) of 2 mM tetracaine in the assay medium. In the case of the non-loaded proteoliposomes, the lipophilic compounds were added to the proteoliposomes 1 min prior to the initiation of the transport assay. Experimental details are as described in the legend to Figure 4.
Tetracaine, at a concentration (2 mM) that increased the iso-osmotic activity ∼8-fold (Figure 6A), did not affect the morphology or surface to volume ratio of the proteoliposomes (data not shown). In these experiments, OpuA thus is not activated via direct changes in water activity, and vesicle shrinkage is not occurring. Since the cationic amphipath tetracaine remains mostly at the phospholipid headgroup level, it is conceivable that the charged form of the anesthetic altered the existing intra- and intermolecular electrostatic interactions (Boulanger et al., 1981). It is plausible that the same is true for the anionic amphipath n-decanoic acid, which shifted the activation profile to higher osmolalities, comparable with an increased fraction of anionic lipids in the membrane. The opposite effects, displayed by cationic and anionic amphipaths, support the view that electrostatic interactions between protein and membrane lipids are intrinsic to the mechanism of osmotic activation. The neutral amphipath lyso-PC and the aliphatic hydrocarbon n-decane (Figure 6A) did not affect the activation profile of OpuA. This suggests that the physical insertion of amphipaths in the lipid bilayer is not sufficient for osmotic activation of OpuA. Additionally, it is known that compounds such as tetracaine and n-decane increase the membrane fluidity (Salesse et al., 1982; Ueda and Yoshida, 1999), which on the basis of our work does not seem critical for osmotic activation of OpuA. Furthermore, the tetracaine-induced activation of glycine betaine transport is paralleled by a corresponding increase in the ATPase activity (data not shown), which indicates that the coupling between transport and ATP hydrolysis is maintained.
High ionic strength at the cytoplasmic face of OpuA activates the transporter under iso-osmotic conditions
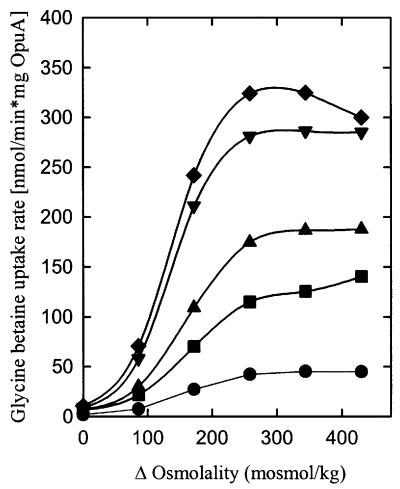
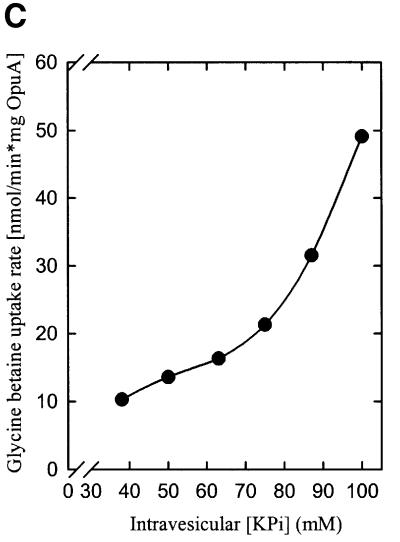
Since the volume of proteoliposomes decreases upon osmotic upshift, the luminal concentrations of osmolytes increase. If ionic interactions between lipids and protein are intrinsic to the osmosensing mechanism of OpuA, then increases in the intravesicular concentration of ionic osmolytes should affect the activation profile. To discriminate between ionic strength, osmolality and specific osmolyte effects, the intravesicular composition and concentration were varied with the compounds NaCl, KCl, sucrose, fructose, KPi (pH 7.0), NaPi (pH 7.0), K2SO4 and Na2SO4. Each of these osmolytes was included in the vesicle lumen at a concentration corresponding to 100 mosmol/kg and was present in addition to the standard components (50 mM KPi pH 7.0, plus the ATP-regenerating system), resulting in a total internal osmolality of 290 mosmol/kg. Figure 7A and B shows that each of the ions stimulated OpuA activity, whereas the neutral osmolytes had no effect. Representative data for the osmotic activation profiles of OpuA in proteoliposomes with 17 (Figure 7A) and 25 mol% (Figure 7B) DOPG are shown. Equi-osmolar concentrations of KPi and K2SO4 proved to be more effective in stimulating OpuA activity than the monovalent KCl and NaCl; K+ and Na+ proved to be equally effective as cation. To determine the effect of ionic strength on the iso-osmotic activity of OpuA, increasing concentrations of potassium phosphate were enclosed in the proteoliposomal lumen, thereby varying the internal osmolality from 170 to 290 mosmol/kg. Figure 7C shows the dependence of the iso-osmotic activity of OpuA on the internal potassium phosphate concentration. A number of conclusions can be drawn from these experiments. First, OpuA seems activated specifically by high ionic strength in the vesicle lumen, which indicates that OpuA does not sense water activity or a specific signaling. Secondly, the shifts in the activation profile of OpuA are similar to those observed when the surface charge of the membrane is altered by varying the fraction of anionic phospholipids or the insertion of charged amphiphiles, which strongly suggests that OpuA senses osmotic stress via alterations in the ionic interactions between protein and bilayer lipids.
Fig. 7. The effect of ionic strength on the osmotic activation of OpuA. Uptake of [14C]glycine betaine (final concentration, 76 µM) was assayed in 100 (172 mosmol/kg) or 150 mM (258 mosmol/kg) KPi pH 7.0. Proteoliposomes were composed of DOPC/DOPE/DOPG in a 58:25:17 (A) and 50:25:25 (B) mole ratio. The standard components (circles, 172 mosmol/kg) plus additional KPi (upright triangles), KCl (squares) or sucrose (inverted triangles) (total of 258 mosmol/kg) were enclosed in the proteoliposomal lumen. (C) Uptake of [14C]glycine betaine (final concentration, 76 µM) was assayed in KPi pH 7.0 (equi-osmolal to the intravesicular osmolality). Proteoliposomes were composed of DOPC/DOPE/DOPG in a 50:25:25 mole ratio. Potassium phosphate pH 7.0 was enclosed in the proteoliposomal lumen at the concentrations indicated.
High ionic strength at the cytoplasmic face of OpuA increases the ATPase activity
A caveat of the experiments in which the luminal contents of the proteoliposomes were varied is that separately prepared proteoliposome samples were compared. To characterize the ionic strength dependence of OpuA further, glycine betaine-dependent ATPase activity was measured. Since a fraction of OpuA is inserted into the proteoliposomes in the ‘inside-out’ orientation, we used this population of molecules to monitor the ATPase activity of the system as a function of external osmolality and ionic strength. Table I shows that the ATPase activity of OpuA, in proteoliposomes consisting of DOPC/DOPE/DOPG in a 2:1:1 mole ratio, required glycine betaine (in the vesicle lumen with this ‘inside-out’ system) and a relatively high ionic strength on the outside (the cytoplasmic face of the protein). The ATPase activity increased up to a KPi concentration of 200 mM (Table I). Above 200 mM, KPi became inhibitory in the ATPase assay, a phenomenon also observed when transport activity was measured (data not shown). The activation of ATPase by ionic osmolytes confirms the observations made with the transport assays, and indicates that in vivo osmotic stress is signaled to the protein via alterations in the intracellular ionic strength. A tight coupling between ATPase and transport activity is suggested by the requirement for (internal) glycine betaine when (external) ATPase activity was assayed.
Table I. ATPase activity of membrane-reconstituted OpuAa as a function of ionic osmolyte concentration.
| Osmolytes | Glycine betaine (0.5 mM) | Osmolality (mosmol/kg) | ATPase activities (nmol/min*mg OpuA) |
|---|---|---|---|
| KPi 50 mM | + | 90 | 18 ± 7 [10%] |
| KPi 100 mM | + | 180 | 23 ± 7 [13%] |
| KPi 150 mM | + | 270 | 117 ± 16 [65%] |
| KPi 200 mM | + | 360 | 180 ± 20 [100%] |
| KPi 200 mM | – | 360 | 5 ± 6 [3%] |
| KPi 250 mM | + | 450 | 146 ± 15 [81%] |
| KPi 350 mM | + | 630 | 124 ± 15 [69%] |
| KPi (50 mM) + K2SO4 (160 mM) | + | 450 | 148 ± 16 [82%] |
| KPi (50 mM) + KCl (200 mM) | + | 450 | 99 ± 12 [55%] |
| KPi (50 mM) + NaCl (200 mM) | + | 450 | 95 ± 14 [53%] |
| KPi (50 mM) + sucrose (290 mM) | + | 450 | 9 ± 4 [5%] |
aProteoliposomes were composed of 50 mol% DOPC, 25 mol% DOPE and 25 mol% DOPG. The osmolality of the assay medium was varied by the addition of salt or sugar as indicated under osmolytes.
Discussion
Here we describe the mechanism of osmotic activation of an ABC transporter for glycine betaine. Using a proteoliposomal system with purified protein components and synthetic lipids, and by assaying for ATP-dependent uptake and substrate-dependent ATPase activity, we show that osmotic stress most probably is transduced to the OpuA protein via changes in the intracellular concentrations of ionic osmolytes. The actual osmotic activation profile depends not only on the cytoplasmic ionic strength but also on the fraction of ionic lipids in the membrane, suggesting that both parameters are interrelated through specific ionic interactions between lipids and protein. Changes in ionic strength are likely to be instantaneous with osmotic (up)shifts, and a mechanism involving (in)activation via changes in the protein–lipid interactions will provide a rapid response to counteract the osmotic imbalance.
With regard to our experimental system, a few aspects are worth emphasizing. First, the osmotic activation of OpuA in proteoliposomes mimics the regulation in vivo, which may seem surprising as the vesicles do not withstand turgor, whereas the cell turgor is several atmospheres (Wood, 1999). However, there is increasing evidence that in bacteria, turgor exists only across the cell wall (and outer membrane in the case of Gram-negative bacteria) and not across the cytoplasmic membrane (Cayley et al., 2000). This implies that osmosensing devices present in the cytoplasmic membrane cannot respond to changes in turgor; rather, they must sense the consequences of the water influx or efflux, e.g. changes in ionic strength. The consequences of osmotic shifts that OpuA is experiencing in our membrane model system thus may be similar to those in the in vivo situation. Secondly, a few other (binding protein-dependent) ABC transport systems have been studied in proteoliposomes, but the coupling between ATPase and translocation activity is generally poor (Ambudkar et al., 1997; Chen et al., 2001). Although the details of the coupling between ATP hydrolysis and translocation activity by OpuA will be presented elsewhere, it is important to note that, in experiments such as those presented in Table I, ATP hydrolysis is strictly dependent on the presence of substrate. Moreover, the system needs the appropriate ‘osmotic stimulus’, i.e. high salt when the protein is embedded in a membrane with ≥25 mol% of anionic lipids, before ATP hydrolysis takes place. This osmotic stimulus is not required at low concentrations of anionic lipids (e.g. 6–13 mol% DOPG or DOPS). These dependencies match the observations made in the translocation assays.
Kinetics of osmotic activation
In order to cope effectively with osmotic stress, cells need osmotically controllable systems in the membrane at all times, as synthesis takes too long to respond. Here, we show that OpuA is activated instantaneously upon raising the medium osmolality, i.e. when the external medium is made hyperosmotic relative to the inside. Activation is elicited by ionic and non-ionic osmolytes, provided the molecules do not equilibrate across the membrane on the time scale of the transport measurements (van der Heide and Poolman, 2000b). Since (proteo)liposomes behave osmometrically, i.e. water diffuses across the membrane in response to the osmotic difference between the inner compartment and the outside medium, proteoliposomes are expected to decrease their volume to surface ratio when the outside osmolality is increased. The changes in membrane structure and lumen contents (osmolyte concentration) in osmotically stressed (proteo)liposomes may be compared with those in cells that are in a state of plasmolysis. Proteoliposomes with an average diameter of ∼150 nm changed their shape from spherical to sickle-like structures, as shown by the cryo-EM experiments. These morphological changes occurred within milliseconds, i.e. on a time scale much shorter than the interval over which transport was measured. Upon lowering the outside osmolality to the initial value, yielding iso-osmotic conditions again, the vesicles regained their spherical shape and the transporter was deactivated (Figure 1). Thus, osmotic activation and inactivation of OpuA is entirely reversible, occurs on a time scale of seconds or less and follows the shape and volume changes of the liposomes.
For the proton-linked co-transporter ProP, it has been argued recently that surface hydration of the protein acts as a regulator of ProP activity in addition to osmotic effects that are transduced via the membrane. This suggestion is based on the observation that membrane-permeable osmolytes such as glycerol and low molecular weight PEGs activated ProP to some extent, whereas these compounds did not affect the volume of the proteoliposomes (Racher et al., 2001). As membrane-permeant osmolytes rapidly equilibrate across the membrane, one expects the volume decrease as a result of water efflux to be transient, as is shown experimentally here. Racher et al. (2001) have measured the volume of osmotically challenged liposomes 1 min after the addition of the membrane-permeant osmolyte. At this point of time, the volume of the proteoliposomes had already returned to its initial value when glycerol or low molecular weight PEGs were applied (see Figure 3). Since the activity of ProP was measured within seconds after the addition of permeant osmolytes, rather than after 1 min (kinetic resolution of the volume measurements), the system was in the activated state for a short period of time, resulting in the observed low uptake. Thus, differences in kinetic resolution of the transport and volume measurements may have masked an apparent relationship between the two observations, thereby weakening the argument that ProP activity is regulated via changes in surface hydration of the protein.
Mechanism of osmosensing
Osmotic activation of membrane transport proteins may be triggered through a change in the hydration state of the protein, resulting from the altered water activity (aw), or the signal may be transmitted to the protein via a specific signaling molecule or a change in the physicochemical properties of the surrounding membrane. Since OpuA is activated not only by osmotic upshift but also by the insertion of cationic amphipaths in the membrane, it seems plausible that the membrane transduces the activation. Osmotic upshift yields sickle-shaped vesicle structures, but these morphological changes are not important per se for the activation of the transporter, as they do not occur upon insertion of amphipaths into the membrane. However, at the molecular level, physical properties such as membrane fluidity, bilayer thickness, hydration state of lipid headgroups, interfacial polarity and charge, and/or lateral pressure may vary with changes in the medium osmolality. To define the osmotic signaling process in more detail, OpuA was incorporated into liposomes of different lipid composition, thereby varying one or more of these parameters. We show that the fraction of anionic (charged) lipids is of major importance for the osmosensing mechanism, whereas variations in acyl chain length, position and configuration (cis/trans) of the double bond and the fraction of non-bilayer lipids have relatively minor effects. By varying the fraction of anionic lipids (DOPG or DOPS) from <6 to 13, to 25%, OpuA is converted from an inactive (I) to an ‘constitutively’ active (C) to an osmotically controllable (R) state. Moreover, at 25 mol% DOPG, OpuA can be converted from R to C by adding cationic amphipaths, whereas the anionic amphipath decanoic acid mimics an increase of anionic lipids in the membrane. This suggests that the overall charge of the headgroup region of the membrane lipids determines the activity (kinetic state) of the transporter. The possibility that high salt concentrations also interfere with the hydrogen bonding between anionic lipids and OpuA cannot be excluded.
The suggestion that ionic interactions between lipids and OpuA protein determine the activity is supported by the experiments in which the osmolality and osmolyte composition at the cytoplasmic face of the transporter were varied (Figure 7; Table I). The experiments show that OpuA is activated at iso-osmotic conditions simply by an increase in the concentration of ionic osmolytes. K+ and Na+ proved to be equally efficient and there is no additional regulation via K+ as proposed for KdpD (Jung et al., 2000). The more efficient stimulation with phosphate and sulfate compared with chloride as anion is consistent with the higher ionic strength at the same salt concentration. The work also indicates that cytoplasmic ionic strength rather than internal osmolality or a specific signaling molecule switches the system between different kinetic states. Because the effects of ionic strength vary with the fraction of anionic lipids in the membrane, and the threshold for osmotic activation is lowered by cationic and raised by anionic amphipaths, the data strongly suggest that the bilayer in which the protein is embedded mediates the osmotic signaling.
Charged amphipaths have been used previously to study osmoregulated systems under conditions where a membrane potential is present across the membrane (Martinac et al., 1990; Rübenhagen et al., 2000), which results in an asymmetric distribution of the molecules over the inner and outer leaflet of the membrane. In our experimental system, there is no membrane potential and the ultimate distribution will be symmetric. Upon addition of tetracaine to a liposome suspension, the amphipath will insert into the outer leaflet of the membrane and from there the molecule will flop–flip to the inner leaflet. The shift to lower osmolalities of the osmotic activation profile of OpuA was most pronounced when tetracaine was given the time to equilibrate, i.e. to fill the inner membrane leaflet (Figure 6B). This is consistent with the idea that the important ionic interactions between protein and lipids reside at the cytoplasmic face of the membrane, allowing internal ionic strength specifically to regulate the activity of OpuA.
Osmotic signal
Our work indicates that a change in intracellular ionic strength serves as a primary signal of osmotic stress for OpuA. We propose that this signal is not sensed by the protein directly, i.e. via changes in surface hydration of the protein or direct effects of the ions on the protein (allosteric site); rather, the membrane in which the protein is embedded serves as mediator. Changes in cellular ionic strength are likely to alter specific interactions between (ionic) lipids and the protein, thereby affecting the transport activity. It remains to be established whether or not the physicochemical parameters of the inner and outer leaflet of the lipid bilayer are equally important for osmotic activation of OpuA. The effects of ionic strength, however, are exerted only at the cytoplasmic face of the protein.
Why would the cell use ionic strength rather than intracellular osmolality (affecting protein hydration) or a specific signaling molecule (allosteric regulatory site on the protein)? When the medium osmolality is raised, the initial change in cytoplasmic water activity depends on the elasticity of the cell wall. Contrary to what is often thought, the cell wall of bacteria is not rigid but actually quite elastic (Csonka and Hanson, 1991; Doyle and Marquis, 1994). Consequently, even at a turgor pressure above zero, the cytoplasmic volume decreases with increasing external osmolality, and the ion (osmolyte) concentrations increase accordingly. The increase in ionic strength accompanying the volume decrease is undesirable as too high concentrations of electrolytes interfere with macromolecular functioning in eubacteria as well as in higher organisms (Yancey et al., 1982). As best documented for E.coli (Record et al., 1998), eubacteria expel ionic compounds in the event that the electrolyte concentration becomes too high and replace these molecules with neutral osmolytes such as glycine betaine to balance the cellular osmolality. The increase in electrolyte concentration (or ionic strength) upon a modest decrease in turgor pressure would thus represent an excellent trigger (‘osmotic signal’) for the activation of any osmoregulated transporter for neutral compatible solutes such as OpuA. Actually, it would prevent the osmotic stress from turning into ‘electrolyte stress’.
Why is the increase in intracellular osmolality less suitable as the osmotic signal? In order to maintain a relatively constant turgor pressure at different external osmolalities, the cell will have to switch on OpuA and to take up glycine betaine with maximal activity at different internal osmolalities. In other words, the ability of (the majority of) microorganisms to grow at maximal rate over a wide range of medium osmolalities implies that cellular processes function optimally over a wide range of intracellular osmolalities. Finally, the cell could use the osmotic upshift-dependent change in concentration of a specific molecule as the signal, but ionic strength seems a more general signal for osmo-responsive systems, including signal transduction pathways to control the expression of osmoregulated genes.
Concluding remarks
How general are the observations and concepts reported here for the osmoregulated ABC transporter OpuA? Although similar detailed in vitro studies have not yet been reported for other osmoregulated transporters, there is substantial evidence that the gating of the mechanosensitive channel MscL is mediated by membrane stretch (Hamill and Martinac, 2001). MscL responds to hypo-osmotic stress and expels osmolytes when the turgor pressure becomes too high. This system has to be operative under osmotic conditions opposite to those required for OpuA, and a different osmosensing mechanism may therefore be expected. Well-studied transporters with a similar function, but structurally unrelated to OpuA, are the ion-linked transporters ProP from E.coli and BetP from C.glutamicum (Racher et al., 1999; Rübenhagen et al., 2000). We have already argued that one does not need to invoke surface hydration of ProP as a regulatory mechanism (and thus internal osmolality as a signal) to explain the observations. Moreover, in vivo studies on this protein have indicated that activation of ProP requires the presence of K+ in the medium (Koo et al., 1991). Since K+ is accumulated rapidly in the initial response of E.coli to hyperosmotic stress, the requirement for K+ is consistent with a role for intracellular ionic strength in the regulation of ProP, but other factors such as an increase in the intracellular pH may have contributed as well (Poolman and Glaasker, 1998). Similarly to the observations made for OpuA, the optimum of osmotic stimulation of BetP from C.glutamicum shifts to higher values of osmolality with increasing amounts of PG lipids in the membrane, pointing towards a role for the membrane in transducing ionic changes to the protein (Rübenhagen et al., 2000). Also, for the osmotic regulation of gene expression, there is evidence that intracellular ionic strength serves as a signal (Higgins et al., 1987). Thus, altogether there are clear indications that nature has used similar principles for the evolution of different types of osmoregulatory circuit.
Materials and methods
Bacterial strains, growth conditions and isolation of membrane vesicles
Lactococcus lactis strain NZ9000 (de Ruyter et al., 1996) was cultivated semi-anaerobically at 30°C in M17 broth pH 6.5, supplemented with 1.0% (w/v) glucose (GM17 medium) and 5 µg/ml chloramphenicol when carrying pNZopuAhis or derivatives. For the isolation of membranes, cells were grown in a 10 l pH-regulated fermentor to an OD660 of 2, after which transcription from the nisA promoter was switched on by the addition of 0.2% (v/v) culture supernatant of the nisin A-producing strain NZ9700 (de Ruyter et al., 1996). The final concentration of nisin A was ∼2 ng/ml. The cells were harvested after 1 h of induction and inside-out membrane vesicles were prepared by lysing the bacteria (20 mg/ml) with a high pressure homogenizer (Kindler type NN2002; single passage at 10 000 p.s.i.), following (partial) digestion of the cell wall with 10 mg/ml lysozyme for 30 min at 30°C (Poolman et al., 1983). The membrane preparations were stored in liquid nitrogen.
Purification of OpuA
Membranes were resuspended in buffer A (50 mM KPi pH 8.0, 200 mM KCl, 20% glycerol) to a final concentration of 5 mg protein/ml and solubilized with 0.5% n-dodecyl-β-d-maltoside (DDM) for 30 min on ice. Following centrifugation, the solubilized material was incubated with Ni2+-NTA resin (0.5 ml of resin/10 mg of membrane protein) for 2 h at 4°C in the presence of 15 mM imidazole. Subsequently, the resin was washed with 20 column volumes of buffer A supplemented with 0.05% Triton X-100 and 15 mM imidazole. The histidine-tagged proteins were eluted from the column with three column volumes of buffer A supplemented with 0.05% Triton X-100 and 200 mM imidazole.
Membrane reconstitution of OpuA
Liposomes composed of the desired lipids were prepared, and membrane reconstitution was performed, essentially as described by Knol et al. (1996). Briefly, pre-formed liposomes (4 mg/ml) were destabilized by titration with Triton X-100, and the turbidity of the suspension at 540 nm was used to monitor the physical state of the liposomes. Unless stated otherwise, liposomes destabilized to a point just beyond ‘detergent-saturation’ (Knol et al., 1998) were mixed with purified OpuA in a 100:1 ratio (w/w), and incubated for 30 min at room temperature under gentle agitation. To remove the detergent, polystyrene beads (Biobeads SM2) were added at a wet weight of 40 mg/ml and the sample was incubated for another 15 min. Fresh Biobeads SM2 (40 mg/ml) were added to the sample four times and the incubations were continued at 4°C for 15 min, 30 min, overnight and 2 h, respectively. Finally, the proteoliposomes were collected by centrifugation, washed twice with 50 mM KPi pH 7.0 and stored in liquid nitrogen.
Transport assays
ATP-driven uptake in proteoliposomes. An ATP-regenerating system, consisting of creatine kinase (2.4 mg/ml), ATP (6 mM), MgSO4 (9 mM) and creatine phosphate (24 mM), was enclosed in the proteoliposomes by three freeze–thaw cycles. Following extrusion of the proteoliposomes through a polycarbonate filter (200 nm pore size), the proteoliposomes were washed twice and resuspended in 100 mM KPi (iso-osmolar with the intraliposomal medium) pH 7.0 to a concentration of 80 mg lipid/ml. Prior to transport, the proteoliposomes were diluted to a lipid concentration of 3.6 mg/ml in 100 mM potassium phosphate pH 7.0 (total volume 50 µl). To impose hyperosmotic conditions, additional salt or sugar was added to the medium. Unless stated otherwise, proteoliposomes were pre-incubated at 30°C for 1 min, after which transport was initiated by the addition of radiolabeled substrate. After given time intervals, the samples were diluted with 2 ml of ice-cold buffer of the same composition and osmolality as the assay medium, except that [14C]glycine betaine was omitted. The samples were filtered rapidly through 0.45 µm pore size cellulose nitrate filters (Schleicher and Schuell GmbH, Dassel, Germany) and washed once more with 2 ml of stop buffer. The radioactivity on the filters was determined by liquid scintillation spectrometry. Initial rates of transport were obtained from the linear increase in glycine betaine uptake over the first 30 s, and triplicate samples were taken for analysis. Each experiment was repeated at least twice.
Cryo-EM
A small droplet of phospholipid vesicle suspension (20 mg/ml) was placed on a glow-discharged holey carbon-coated grid. The excess liquid was blotted away with a filter paper and the grid was vitrified in liquid ethane. To avoid evaporation during the procedure, the grid was placed in a closed chamber oversaturated with water. This was achieved by using an ultrasonic vaporizer, which creates a cloud around the specimen (D.H.W.Hubert, P.H.H.Bomans and P.M.Frederik, in preparation). The grids were examined in a Philips (Eindhoven, The Netherlands) CM120 cryo-electron microscope operating at 120 kV equipped with a Gatan cryo-stage (model 626). Images were recorded under low-dose conditions with a Gatan (model 794) slow-scan CCD camera.
Internal volume measurements
The fluorophore calcein was prepared at a concentration of 100 mM in 50 mM potassium phosphate and the pH was adjusted to 7.0 using KOH. Calcein (final concentration: 10 mM calcein in 85 mM KPi pH 7.0, corresponding to 190 mosmol/kg) was enclosed in the (proteo)liposomes by three freeze–thaw cycles. Following extrusion of the (proteo)liposomes through a polycarbonate filter (200 nm pore size), the external calcein was removed by applying the sample to a NAP-10 column (Pharmacia), after which the (proteo)liposomes were eluted, washed twice and resuspended in 100 mM KPi pH 7.0 (190 mosmol/kg). A calibration curve for calcein quenching was made by preparing a range of (proteo)liposomes in which calcein was enclosed at different concentrations but keeping the internal osmolality constant at 190 mosmol/kg. Fluorescence measurements were made with an Aminco SPF-500 spectrofluorometer at an exitation wavelength of 495 nm and an emission wavelength of 520 nm (with slit widths of 10 nm). The fluorescence measurements were started by diluting 4 µl of calcein-loaded (proteo)liposomes (20 mg/ml lipid) into 1 ml of 100 mM KPi pH 7.0. To impose hyperosmotic conditions, additional osmolytes were added to the sample after 1 min pre-incubation. Samples were stirred and the fluorescence measurements were performed at 25°C. The maximal unquenched calcein fluorescence signal was determined by solubilizing the liposomes with 0.1% Triton X-100.
ATPase assay
Proteoliposomes were used in which OpuA was reconstituted in a 1:50 ratio (w/w). The proteoliposomes were frozen and thawed twice with or without glycine betaine (1 mM) present. Following extrusion of the proteoliposomes through a polycarbonate filter (200 nm pore size), the proteoliposomes were washed twice and resuspended in 50 mM KPi (iso-osmolar with the intraliposomal medium) pH 7.0 to a concentration of 100 mg lipid/ml. The assay was initiated by diluting the liposomes 2-fold with an iso-osmotic phosphate buffer containing ATP (6 mM) and MgSO4 (6 mM). To impose hyperosmotic conditions, additional salt or sugar was added to the medium. At given time intervals, samples were drawn and diluted 500-fold with 50 mM KPi pH 7.0 and assayed for ATP by using the ATPLite™-M system (Packard).
Materials
M17 broth was obtained from Difco. Ni2+-NTA resin was obtained from Qiagen Inc., Biobeads SM-2 from Bio-Rad, DDM from Sigma and Triton X-100 from Boehringer Mannheim. Total E.coli lipid extracts, l-α-phosphatidylcholine from egg yolk and synthetic lipids were obtained from Avanti Polar Lipids. Radiolabeled [N-methyl-14C]- and [N-methyl-3H]choline chloride (55 and 80 Ci/mmol, respectively) were obtained from Amersham (Buckinghamshire, UK), and these precursors were used to synthesize [N-methyl-14C]glycine betaine and [N-methyl-3H]glycine betaine as described (Landfald and Strom, 1986). Creatine kinase, creatine phosphate and tetracaine were obtained from Sigma Chemical Co. All other chemicals were of reagent grade and obtained from commercial sources.
Miscellaneous
The osmolalities of media and buffers were measured by freezing point depression with an Osmostat 030 (Gonotec, Berlin, Germany). The protein concentration was determined by the method of Lowry et al. (1951), using bovine serum albumin as a standard.
Acknowledgments
Acknowledgements
We thank Eric R.Geertsma for critical reading of the manuscript. This research was supported by grants from The Netherlands Foundation of Life Sciences, which is subsidized by The Netherlands Organization for Scientific Research (NWO) and the National Leading Research Institute ‘Materials Science Centerplus’.
References
- Ambudkar S.V., Cardarelli,C.O., Pashinsky,I. and Stein,W.D. (1997) Relation between the turnover number for vinblastine transport and for vinblastine-stimulated ATP hydrolysis by human P-glycoprotein. J. Biol. Chem., 272, 21160–21166. [DOI] [PubMed] [Google Scholar]
- Boulanger Y., Schreier,S. and Smith,I.C. (1981) Molecular details of anesthetic–lipid interaction as seen by deuterium and phosphorus-31 nuclear magnetic resonance. Biochemistry, 20, 6824–6830. [DOI] [PubMed] [Google Scholar]
- Cantor R.S. (1999) Lipid composition and the lateral pressure profile in bilayers. Biophys. J., 76, 2625–2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cayley D.S., Guttman,H.J. and Record,M.T.,Jr (2000) Biophysical characterization of changes in amounts and activity of Escherichia coli cell and compartment water and turgor pressure in response to osmotic stress. Biophys. J., 78, 1748–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Sharma,S., Quiocho,F.A. and Davidson,A.L. (2001) Trapping the transition state of an ATP-binding cassette transporter: evidence for a concerted mechanism of maltose transport. Proc. Natl Acad. Sci. USA, 98, 1525–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csonka L.N. and Hanson,A.D. (1991) Prokaryotic osmoregulation: genetics and physiology. Annu. Rev. Microbiol., 45, 569–606. [DOI] [PubMed] [Google Scholar]
- de Ruyter P.G., Kuipers,O.P. and de Vos,W.M. (1996) Controlled gene expression systems for Lactococcus lactis with the food-grade inducer nisin. Appl. Environ. Microbiol., 62, 3662–3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle R.J. and Marquis,R.E. (1994) Elastic, flexible peptidoglycan and bacterial cell wall properties. Trends Microbiol., 2, 57–60. [DOI] [PubMed] [Google Scholar]
- Hamill O.P. and Martinac,B. (2001) Molecular basis of mechanotransduction in living cells. Physiol. Rev., 81, 685–740. [DOI] [PubMed] [Google Scholar]
- Higgins C.F., Cairney,J., Stirling,D.A., Sutherland,L. and Booth,I.R. (1987) Osmotic regulation of gene expression: ionic strength as an intracellular signal? Trends Biochem. Sci., 12, 339–344. [Google Scholar]
- Jung K., Veen,M. and Altendorf,K. (2000) K+ and ionic strength directly influence the autophosphorylation activity of the putative turgor sensor KdpD of Escherichia coli. J. Biol. Chem., 275, 40142–40147. [DOI] [PubMed] [Google Scholar]
- Knol J., Veenhoff,L., Liang,W.J., Henderson,P.J., Leblanc,G. and Poolman,B. (1996) Unidirectional reconstitution into detergent-destabilized liposomes of the purified lactose transport system of Streptococcus thermophilus. J. Biol. Chem., 271, 15358–15366. [DOI] [PubMed] [Google Scholar]
- Knol J., Sjollema,K. and Poolman,B. (1998) Detergent-mediated reconstitution of membrane proteins. Biochemistry, 37, 16410–16415. [DOI] [PubMed] [Google Scholar]
- Koo S.P., Higgins,C.F. and Booth,I.R. (1991) Regulation of compatible solute accumulation in Salmonella typhimurium: evidence for a glycine betaine efflux system. J. Gen. Microbiol., 137, 2617–2625. [DOI] [PubMed] [Google Scholar]
- Landfald B. and Strom,A.R. (1986) Choline–glycine betaine pathway confers a high level of osmotic tolerance in Escherichia coli. J. Bacteriol., 165, 849–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry O.H., Rosebrough,N.J., Farr,A.L. and Randall,R.J. (1951) Protein measurement with the Folin phenol reagent. J. Biol. Chem., 165, 265–275. [PubMed] [Google Scholar]
- Martinac B., Adler,J. and Kung,C. (1990) Mechanosensitive ion channels of E.coli activated by amphipaths. Nature, 348, 261–263. [DOI] [PubMed] [Google Scholar]
- Poolman B. and Glaasker,E. (1998) Regulation of compatible solute accumulation in bacteria. Mol. Microbiol., 29, 397–407. [DOI] [PubMed] [Google Scholar]
- Poolman B., Konings,W.N. and Robillard,G.T. (1983) The location of redox-sensitive groups in the carrier protein of proline at the outer and inner surface of the membrane in Escherichia coli. Eur. J. Biochem., 135, 41–46. [DOI] [PubMed] [Google Scholar]
- Racher K.I. et al. (1999) Purification and reconstitution of an osmosensor: transporter ProP of Escherichia coli senses and responds to osmotic shifts. Biochemistry, 38, 1676–1684. [DOI] [PubMed] [Google Scholar]
- Racher K.I., Culham,D.E. and Wood,J.M. (2001) Requirements for osmosensing and osmotic activation of transporter ProP from Escherichia coli. Biochemistry, 40, 7324–7333. [DOI] [PubMed] [Google Scholar]
- Record M.T. Jr, Courtenay,E.S., Cayley,S. and Guttman,H.J. (1998) Biophysical compensation mechanisms buffering E.coli protein–nucleic acid interactions against changing environments. Trends Biochem. Sci., 23, 190–194. [DOI] [PubMed] [Google Scholar]
- Rübenhagen R., Ronsch,H., Jung,H., Krämer,R. and Morbach,S. (2000) Osmosensor and osmoregulator properties of the betaine carrier BetP from Corynebacterium glutamicum in proteoliposomes. J. Biol. Chem., 275, 735–741. [DOI] [PubMed] [Google Scholar]
- Salesse R., Garnier,J., Leterrier,F., Daveloose,D. and Viret,J. (1982) Modulation of adenylate cyclase activity by the physical state of pigeon erythrocyte membrane. 1. Parallel drug-induced changes in the bilayer fluidity and adenylate cyclase activity. Biochemistry, 21, 1581–1586. [DOI] [PubMed] [Google Scholar]
- Ueda I. and Yoshida,T. (1999) Hydration of lipid membranes and the action mechanisms of anesthetics and alcohols. Chem. Phys. Lipids, 101, 65–79. [DOI] [PubMed] [Google Scholar]
- van der Heide T. and Poolman,B. (2000a) Glycine betaine transport in Lactococcus lactis is osmotically regulated at the level of expression and translocation activity. J. Bacteriol., 182, 203–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Heide T. and Poolman,B. (2000b) Osmoregulated ABC-transport system of Lactococcus lactis senses water stress via changes in the physical state of the membrane. Proc. Natl Acad. Sci. USA, 97, 7102–7106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood J.M. (1999) Osmosensing by bacteria: signals and membrane-based sensors. Microbiol. Mol. Biol. Rev., 63, 230–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yancey P.H., Clark,M.E., Hand,S.C., Bowlus,R.D. and Somero,G.N. (1982) Living with water stress: evolution of osmolyte systems. Science, 217, 1214–1222. [DOI] [PubMed] [Google Scholar]