Abstract
In the pathogen Neisseria meningitidis, a completely lipopolysaccharide (LPS)-deficient but viable mutant can be obtained by insertional inactivation of the lpxA gene, encoding UDP-GlcNAc acyltransferase required for the first step of lipid A biosynthesis. To study how outer membrane structure and biogenesis are affected by the absence of this normally major component, inner and outer membranes were separated and their composition analysed. The expression and assembly of integral outer membrane proteins appeared largely unaffected. However, the expression of iron limitation-inducible, cell surface-exposed lipoproteins was greatly reduced. Major changes were seen in the phospholipid composition, with a shift towards phosphatidylethanolamine and phosphatidylglycerol species containing mostly shorter chain, saturated fatty acids, one of which was unique to the LPS-deficient outer membrane. The presence of the capsular polysaccharide turned out to be essential for viability without LPS, as demonstrated by using a strain in which LPS biosynthesis could be switched on or off through a tac promoter-controlled lpxA gene. Taken together, these results can help to explain why meningococci have the unique ability to survive without LPS.
Keywords: capsular polysaccharide/lipopolysaccharide/Neisseria meningitidis/outer membrane proteins/phospholipids
Introduction
The outer membrane of Gram-negative enteric bacteria is an asymmetrical bilayer composed of phospholipids and lipopolysaccharide (LPS) in the inner and outer leaflet, respectively (Kamio and Nikaido, 1976). LPS consists of three moieties: the hydrophobic lipid A, which anchors it to the outer membrane; a generally well-conserved surface-exposed hydrophilic oligosaccharide, designated the core; and a hypervariable polysaccharide, the O-antigen. The O-antigen is lacking in many mucosal surface-colonizing bacteria, such as Neisseria meningitidis. Moreover, the asymmetry of the outer membrane of the Neisseriaceae is less pronounced than in the enteric bacteria since phospholipids are also present in the outer leaflet of the outer membrane (Lysko and Morse, 1981). The core oligosaccharide of LPS is negatively charged, resulting in a strong affinity for divalent cations (Schindler and Osborn, 1979). The resulting network of divalent cations and LPS forms a strong permeability barrier in which outer membrane proteins (OMPs) are inserted to facilitate the influx of nutrients.
Among the major OMPs are the porins, which form pores to allow for the non-specific uptake of small compounds by passive diffusion. The basic structure of these porins is a trimer, in which each monomer forms a β-barrel (Weiss et al., 1991; Cowan et al., 1992). The porins of N.meningitidis are designated PorA and PorB. Additional OMPs are required for the uptake of specific nutrients. For example, growth of N.meningitidis under iron limitation results in the expression of receptors for iron-binding proteins of the host, such as lactoferrin and transferrin. These receptors generally consist of an integral OMP with sequence similarity to the siderophore receptors of Escherichia coli, and a cell surface-exposed lipoprotein (Schryvers and Morris, 1988; Legrain et al., 1993; Pettersson et al., 1994; Pettersson et al., 1998). The outer membrane of N.meningitidis is anchored to the peptidoglycan in the periplasm by the RmpM protein, a homologue of the E.coli OmpA (Klugman et al., 1989). However, in contrast to OmpA, which is anchored in the outer membrane via an N-terminal β-barrel domain (Pautsch and Schulz, 1998), RmpM lacks such a β-barrel domain but is firmly associated with the porins (Jansen et al., 2000) and the iron limitation-inducible OMPs (Prinz and Tommassen, 2000).
The cell surface of many Gram-negative bacteria is covered by a capsular polysaccharide (CPS). In serogroup B N.meningitidis strains, such as our model strain H44/76, this capsule is composed of α-2,8-linked polysialic acid, which is anchored in the outer membrane via a phospholipid at the reducing end of the polysaccharide chain (Gotschlich et al., 1981; Frosch and Muller, 1993). The genes involved in the biosynthesis, regulation and transport of CPS are clustered on the chromosome (Frosch et al., 1989).
Various in vivo and in vitro studies have shown that in E.coli, LPS is required for the correct assembly of OMPs (Koplow and Goldfine, 1974; Ried et al., 1990; Sen and Nikaido, 1991; Laird et al., 1994; de Cock and Tommassen, 1996; de Cock et al., 1999a). Disruption of the genes involved in the early steps of lipid A biosynthesis appears impossible and only conditionally lethal mutants have been described. Therefore, it was suggested that this part of LPS is essential for bacterial growth (Raetz, 1990). However, we have recently isolated an LPS-deficient, but viable mutant of N.meningitidis by insertional inactivation of lpxA, encoding the enzyme required for the first step in lipid A biosynthesis (Steeghs et al., 1998). This mutant still produced an outer membrane, which could be clearly discerned by electron microscopy. Preliminary data did not show major deviations in the OMP profile of this LPS-deficient mutant compared with that of the wild-type strain.
In this study, we have characterized the outer membrane composition of the LPS-deficient mutant in detail. Our results demonstrate that in N.meningitidis, LPS is not essential for correct assembly of integral OMPs, such as the porins, although it is required for biogenesis of cell surface-exposed lipoproteins. Furthermore, the phospholipid composition appears to be altered in the absence of LPS. Interestingly, CPS appears to be required for viability of the LPS-deficient N.meningitidis mutant.
Results
Separation of inner and outer membranes of the LPS-deficient mutant
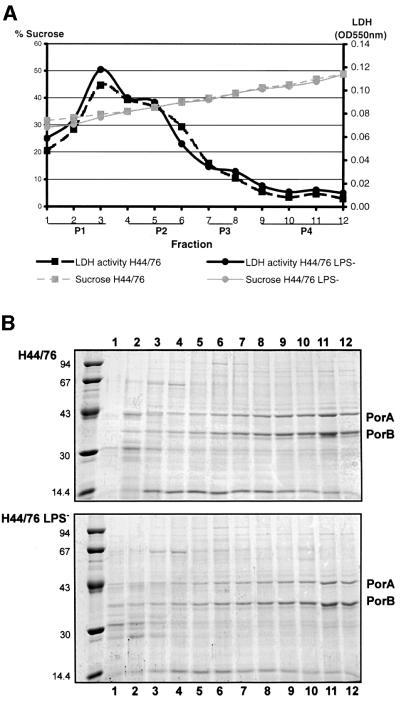
To analyse the cell envelope composition of the LPS-deficient N.meningitidis strain, inner and outer membranes of strain H44/76 and its lpxA mutant derivative were separated by isopycnic sucrose gradient centrifugation. Since it is generally assumed that the higher buoyant density of the outer membranes, as compared with the inner membranes, is due to the presence of LPS, we were not sure whether separation of the membranes of the LPS-deficient mutant was possible by this method. For this analysis, 1 ml gradient fractions were collected and analysed for the presence of lactate dehydrogenase (LDH) activity as a marker for the inner membrane (Osborn et al., 1972), and for the presence of the porins PorA and PorB as outer membrane markers. LDH activity peaked in fraction 3, followed by a gradual decline in fractions 4–6, with no significant difference between the membranes of the two strains (Figure 1A). Thus, the inner membranes of both the wild-type and the mutant strain localized in the low-density region, i.e. fractions 1–6, of the gradient. For both strains, the majority of PorA and PorB was found in the high-density fractions 7–12 (Figure 1B), which contained hardly any LDH activity, while some PorA and PorB was present in the low-density fractions 4–6, which have intermediate LDH activity (Figure 1). Apparently, the absence of LPS did not influence the separation of inner and outer membranes by isopycnic sucrose gradient centrifugation. For further analysis, gradient fractions were pooled in pure inner membrane fraction P1 (fractions 1–3), inner membrane fraction P2 (fractions 4–6), which is slightly contaminated with outer membranes, outer membrane fraction P3 (fractions 7–8), which is slightly contaminated with inner membranes, and pure outer membrane fraction P4 (fractions 9–12), as indicated in Figure 1A.
Fig. 1. Analysis of fractions 1–12, collected after isopycnic sucrose gradient centrifugation, to separate the membranes of wild-type strain H44/76 and its LPS-deficient mutant. (A) LDH per milligram of protein and sucrose concentration of the fractions. P1–4 indicate the fractions that were pooled for detailed analysis. (B) SDS–PAGE analysis of the fractions. Positions of molecular weight standard proteins are indicated on the left in kDa; positions of the porins PorA and PorB are indicated on the right.
Assembly of outer membrane proteins in the LPS-deficient mutant
LPS has been implicated in the biogenesis of Gram-negative bacterial porins, and therefore defects in the assembly of PorA and PorB in the LPS-deficient strain were to be expected. The oligomeric state of PorA in outer membrane fractions P3 and P4 was analysed by SDS–PAGE under denaturing and non-denaturing conditions followed by western blotting. Analysis of outer membranes of the LPS-deficient strain under denaturing conditions revealed, besides the full-length protein of the expected apparent molecular weight, a partial degradation product of PorA with a slightly higher electrophoretic mobility (Figure 2A, lanes a). This degradation product was detected with monoclonal antibody (mAb) MN5C11G (Figure 2A) and MN23G2.38 (results not shown), which recognize epitopes in the putative cell surface-exposed loops 4 and 3 of PorA, respectively. No reaction was found with mAb MN14C11G, which recognizes loop 1, indicating that proteolysis had occurred at the N-terminal end of the protein (results not shown). In spite of this partial degradation, trimerization of PorA appeared unaffected in the LPS-deficient mutant. Both the PorA and its degradation product migrated as trimers with the same electrophoretic mobility as the trimers in the wild-type strain, when the samples were not boiled before electrophoresis (Figure 2A, lanes b). The PorA trimers from the H44/76 membranes have been shown to remain associated with the RmpM protein during non-denaturing SDS–PAGE (Jansen et al., 2000). Western blot analysis with the RmpM-specific mAb MN2D6D confirmed the presence of RmpM in the trimeric porin complexes of both the wild-type and the mutant strain (Figure 2B). Thus, porin assembly is not affected in the LPS-deficient strain. In addition, no effect was found on expression and assembly of other integral OMPs, such as the opacity proteins and the outer membrane phospholipase A (results not shown). Expression of the secretin PilQ could also be clearly demonstrated in the LPS-deficient mutant, although apparently at a slightly lower level as compared with the wild-type strain (Figure 3). However, no difference in pili expression was found for the LPS-deficient mutant when compared with the wild type as measured by a dot-blot assay with the pilin-specific mAb SM1 (M.Christoudoulides, unpublished observation).
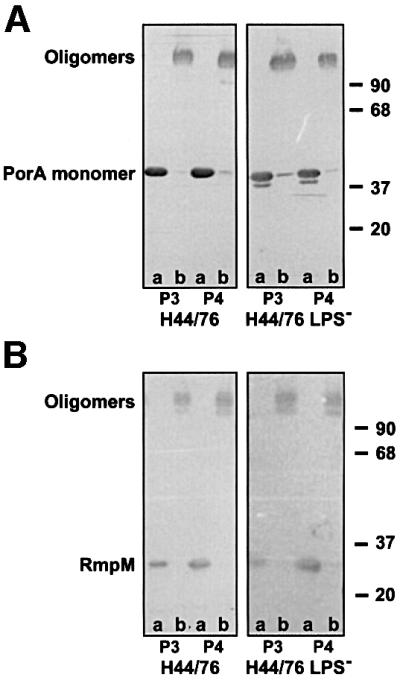
Fig. 2. Western immunoblot of outer membrane fractions P3 and P4 of the wild-type strain H44/76 and of its LPS-deficient mutant, incubated with anti-PorA mAb MN5C11G (A) and anti-RmpM mAb MN2D6D (B). The samples were incubated at 100°C (lanes a) or room temperature (lanes b) in the presence of 2% SDS before SDS–PAGE. The positions of the molecular weight markers (kDa), the PorA-containing oligomeric complexes, and the PorA and the RmpM monomer are indicated.

Fig. 3. Western immunoblot of membrane fractions P1–P4 of the wild-type strain H44/76 and its LPS-deficient mutant, incubated with anti-PilQ monoclonal antibody.
We also studied the expression of the cell surface-exposed lipoproteins LbpB and TbpB, which are parts of the lactoferrin and transferrin receptors, respectively. To express these receptors, strain H44/76 and the LPS-deficient mutant were grown under iron limitation, and the membranes were isolated by isopycnic sucrose gradient centrifugation. Western blot analysis did not reveal any significant differences between the two strains with respect to the expression level of the integral membrane component LbpA of the lactoferrin receptor (Figure 4A). In contrast, the amounts of the lipoproteins LbpB and TbpB were drastically reduced in the outer membranes of the mutant (Figure 4A). The LbpB and TbpB proteins did not accumulate in the inner membrane fractions P1 and P2, in the soluble fraction or in the extracellular medium (results not shown) and are probably degraded upon mislocalization. In contrast, no difference was found in the expression of other outer membrane-associated lipoproteins, such as CtrA (Figure 4B), presumed to be part of the CPS transport complex (Frosch et al., 1992). Similarly, expression of the lipoprotein H.8 associated with the periplasmic side of the outer membrane appeared unaffected (R.Moxon and J.Richards, unpublished observation). In conclusion, these results show that the biogenesis of cell surface-exposed lipoproteins is clearly disturbed in the LPS-deficient mutant, whereas the biogenesis of integral OMPs, RmpM and other lipoproteins is not affected.
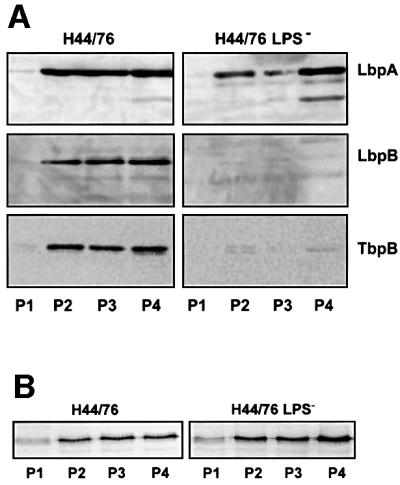
Fig. 4. Western immunoblot of membrane fractions P1–P4 of the wild-type strain H44/76 and its LPS-deficient mutant, incubated with anti-LbpA antiserum, anti-LbpB antiserum and anti-TbpB antiserum (A) and anti-CtrA mAb 2619 (B).
Phospholipid composition of inner and outer membranes in the LPS-deficient mutant
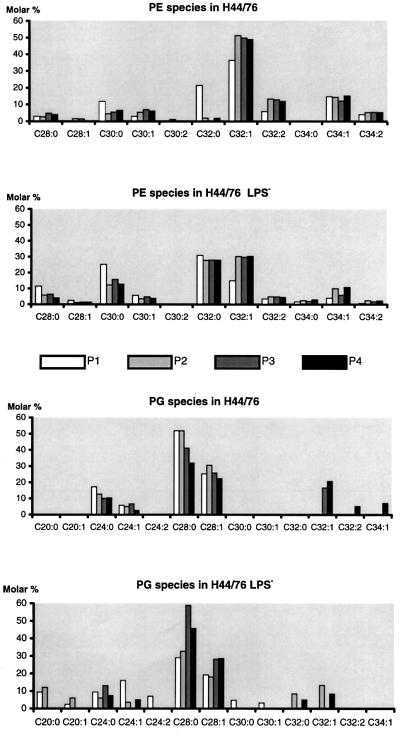
To study the effect of LPS deficiency on the phospholipid composition of the cell envelope, phospholipids were isolated from crude membrane fractions and analysed by thin-layer chromatography (TLC). The majority of the phospholipids found in both H44/76 and the LPS-deficient mutant were phosphatidylethanolamine (PE) and, to a lesser extent, phosphatidylglycerol (PG). Only trace amounts of cardiolipin (CL) and no detectable amounts of lysophospholipids were present in both strains (results not shown). In both strains, the distribution of these phospholipid classes in inner and outer membranes was similar. However, analysis of the fatty acyl chains of PE in the isolated membrane fractions P1–P4 showed a remarkable difference between the two strains (Figure 5). In both inner membranes (fractions P1 and P2) and outer membranes (fractions P3 and P4) of the LPS-deficient strain, an increase in the relative amounts of the saturated PE species PE(C30:0) [PE(C30:0) indicates a saturated phosphatidylethanolamine in which the total number of carbons for both fatty acyl residues is 30] and PE(C32:0), and a concomitant decrease in the unsaturated PE species PE(C32:1), PE(C32:2), PE(C34:1) and PE(C34:2), was apparent. The reduced amount of unsaturated PE was most striking for PE(C32:1), representing 50% of the PE species in H44/76 outer membranes (fractions P3 and P4), but only 30% in the outer membrane of the mutant strain. Remarkably, PE(C32:0), which was barely detectable in outer membranes of the wild-type strain, represented 30% of the PE species in those of the mutant.
Fig. 5. Distribution of the PE and PG species in membrane fractions P1–P4 of the wild-type strain H44/76 and its LPS-deficient mutant. The estimated error in the measurements of the fatty acid contents of the membrane phospholipids is 10%. Example of abbrevations of phospholipids: PE(C32:0) indicates a phosphatidyl ethanolamine in which the total number of carbons for both fatty acyl residues is 32.
A similar analysis of the PG content revealed that the fatty acyl chain length in both strains was generally shorter than in the major PE species: PG(C28) versus PE(C32) (Figure 5). As for PE, an increase in saturated fatty acids was apparent in PG in the outer membranes of the LPS-deficient mutant, but this was not the case in the inner membrane. PG(C28:0), representing 50 and 35% of the PG species in inner and outer membranes of the wild-type strain, respectively, was reduced to 30% and increased to 50%, respectively, in those of the mutant. In conclusion, these data show that the LPS-deficient mutant preferentially incorporates saturated PE and PG species with shorter fatty acyl chains in its cell envelope, and of these phospholipids, PE(C32:0) is uniquely present in the LPS-deficient outer membrane.
CPS is essential for viability of the LPS-deficient mutant
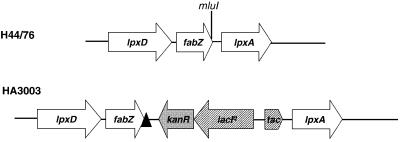
The expression of the CPS in the LPS-deficient mutant was comparable to CPS expression in wild-type H44/76, as measured by whole-cell enzyme-linked immunosorbent assay (ELISA) with a CPS-specific antibody. Capsule-deficient mutants of the wild-type strain H44/76 could readily be obtained by complete deletion of the cps cluster or by insertional inactivation of the siaD gene, encoding the polysialyl transferase involved in multimerization of the polysialic B CPS as described previously (Edwards et al., 1994). However, we were not able to isolate a CPS-deficient mutant of the lpxA mutant strain in either way. This raised the possibility that the CPS is essential for viability of the LPS-deficient mutant. To test this hypothesis, we constructed a derivative of strain H44/76, designated HA3003, which carries an isopropyl-β-d-thiogalactopyranoside (IPTG)-inducible lpxA gene on the chromosome, allowing for depletion of LPS (Figure 6).
Fig. 6. Schematic representation of the chromosomal arrangement of the lpxD-fabZ-lpxA locus of wild-type strain H44/76 and of the lacIq-tac-regulated lpxA strain HA3003. The transcriptional terminator is indicated by a black triangle.
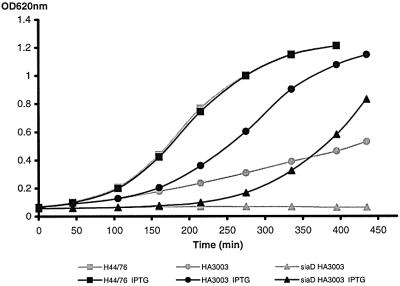
To test for lacIq-tac control of lpxA in strain HA3003, LPS was extracted from lysates of whole cells, which were grown in Mueller–Hinton broth, and the LPS was analysed by Tricine–SDS–PAGE (Figure 7). Surprisingly, no difference in LPS expression was observed between HA3003 grown with or without IPTG. Possibly, the casein hydrolysate present in Mueller–Hinton broth contained enough lactose to derepress the tac promoter. Therefore, other growth media, i.e. GC agar and Meningococcal Medium, neither of which contains casein, were tested as well. As expected, LPS was barely, if at all, detected when HA3003 was grown in either of these media without IPTG, but its biosynthesis was restored to the wild-type level upon induction with IPTG (Figure 7). Furthermore, the growth properties of HA3003 were compared with those of the wild-type strain and the lpxA mutant in Meningoccocal Medium with and without IPTG (Figure 8). The reduced growth rate previously reported for the lpxA mutant (Steeghs et al., 1998) was again observed (results not shown) and was also seen, even to a stronger degree, for HA3003 in the absence of IPTG. However, the growth rate of HA3003 was largely restored upon addition of IPTG (Figure 8), confirming that a tight regulation of lpxA was achieved in this strain.
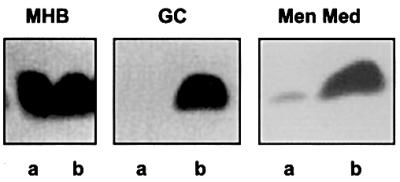
Fig. 7. Silver-stained Tricine–SDS–PAGE gel showing the LPS pattern of proteinase K-treated whole-cell lysates of HA3003 grown in the absence (a) or presence (b) of 1 mM IPTG on Mueller–Hinton broth (MHB), Gonococcal Agar (GC) or Meningococcal Medium (Men Med).
Fig. 8. Growth curves of the lacIq-tac-regulated lpxA strain HA3003 (circles), its CPS-deficient derivative HA2104 (triangles) and wild-type strain H44/76 (squares) in Meningococcal Medium in the absence (grey lines) or presence (black lines) of 1 mM IPTG.
Subsequently, a CPS-deficient mutant of strain HA3003 was constructed in the presence of IPTG, by transformation and allelic replacement with plasmid pMF32.35::T5 (Frosch et al., 1989) carrying a Tn1725 insertion in the siaD gene. Absence of the capsule in one of the transformants was confirmed by ELISA with a CPS-specific antibody and this strain was designated HA2104. This strain was then used to study the influence of CPS deficiency in an LPS-deficient background. Whereas a siaD mutant derivative of the wild-type strain H44/76 grew like its parental strain in the presence or absence of IPTG (results not shown), HA2104 did not grow at all when IPTG was absent, whereas growth was restored in the presence of IPTG (Figure 8). Thus, it can be concluded that the capsule is essential for the viability of an LPS-deficient N.meningitidis mutant.
Outer membrane permeability barrier
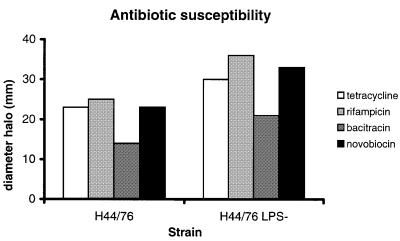
In order to determine the effect of LPS deficiency on the barrier function of the outer membrane, the susceptibility of the LPS-deficient mutant to four hydrophobic antibiotics was determined (Figure 9). Larger growth inhibition zones around filter discs containing these antibiotics were observed for the LPS-deficient mutant than for the wild-type strain, indicating that the barrier function of the outer membrane is compromised.
Fig. 9. Antibiotic susceptibility of wild-type strain H44/76 and its LPS-deficient mutant. The diameter of the inhibition zone around filter paper discs containing each of four different antibiotics is shown. The data shown represent one of three experiments.
Discussion
In contrast to most Gram-negative bacteria, N.meningitidis is viable without LPS (Steeghs et al., 1998). Since LPS determines the asymmetry of the outer membrane and many important functions in membrane biogenesis have been assigned to this molecule, one might expect that the composition of the LPS-deficient mutant outer membrane is drastically changed and/or that the role of LPS is taken over by other components. Therefore, we studied the outer membrane composition of the LPS-deficient mutant in detail. Inner and outer membranes were separated by isopycnic sucrose gradient centrifugation. Although it is generally assumed that the presence of LPS determines the higher buoyant density of the outer membrane as compared with the inner membrane (Osborn et al., 1972), the buoyant density of the LPS-deficient outer membranes appeared unchanged. Apparently, other outer membrane components contribute to the higher buoyant density of the N.meningitidis outer membrane. In this respect, the CPS, which is anchored in the outer membrane via a phospholipid tail (Gotschlich et al., 1981; Frosch and Muller, 1993) and peptidoglycan, which is presumably associated to the outer membrane via the RmpM protein (Klugman et al., 1989), are the most likely candidates.
Analysis of the phospholipid composition of the separated membranes revealed a remarkable shift in both inner and outer membranes of the LPS-deficient mutant towards phospholipids with shorter and saturated fatty acyl chains. This effect was most pronounced for the major phospholipid PE. The preference for the shorter fatty acids in the phospholipids in the outer membrane of the lpxA mutant can be rationalized by the presence of generally shorter fatty acids in LPS than in phospholipids. For the hydrophobic matching with the integral outer membrane proteins, those lipidic components replacing LPS should not affect the overall thickness of the outer membrane. Furthermore, the fatty acyl chains of LPS are generally saturated. Thus, the increased saturation of the fatty acyl chains of the phospholipids might compensate for the membrane fluidity changes invoked by the LPS deficiency. The observed increase in susceptibility to hydrophobic antibiotics is consistent with such a replacement of the missing LPS by phospholipids.
The failure to disrupt CPS synthesis suggested an essential role for the CPS in the LPS-deficient mutant strain. To verify this, strain HA3003 carrying the lpxA gene under the control of an IPTG-inducible promoter was constructed. When this strain was grown in the absence of IPTG, LPS was barely detectable and growth was even more impaired then that of the lpxA knock-out strain. A possible explanation for this observation is that the insertion of a transcriptional terminator immediately downstream of fabZ has affected its expression and/or regulation either at the transcriptional or translational level. Consequently, expression of FabZ, an essential enzyme involved in fatty acid biosynthesis (Mohan et al., 1994), might be reduced, with a negative effect on the biosynthesis of phospholipids. Furthermore, we can not exclude the existence of secondary compensating mutations in the lpxA knock-out strain resulting in a higher growth rate of this strain compared with strain HA3003. Upon induction with IPTG, LPS synthesis was restored to the wild-type level in HA3003, but there was still a slight growth defect apparent. HA2104, a siaD mutant of HA3003, was not viable in the absence of IPTG, directly demonstrating that CPS is essential for viability in an LPS-deficient N.meningitidis mutant. This would explain our unsuccessful attempts to construct an LPS-deficient mutant of the closely related bacterium Neisseria gonorrhoeae, which lacks a CPS (our unpublished observation). Interestingly, it has been reported that glycosphingolipids that are present in the outer membrane of the naturally LPS-deficient Gram-negative rod Sphingomonas paucimobilis exhibit a high degree of similarity to LPS in their contribution to outer membrane function (Wiese et al., 1996). Like the CPS of H44/76, these glycosphingolipids contain two fatty acyl chains covalently linked to a sugar moiety, in contrast to the six or seven fatty acyl chains normally present in LPS. In this respect, CPS seems structurally and functionally analogous to sphingolipids. Furthermore, CPS could possibly maintain the typical asymmetry of the outer membrane, by which it can be distinguished from the inner membrane, thus allowing for insertion of outer membrane proteins in the correct membrane. In this way, the role of LPS in the biogenesis of porins, which has been described previously in E.coli and Salmonella typhimurium (Koplow and Goldfine, 1974; Ried et al., 1990; Sen and Nikaido, 1991; Laird et al., 1994; de Cock and Tommassen, 1996; de Cock et al., 1999a), might be taken over by CPS. In deep-rough LPS mutants of E.coli and S.typhimurium, a reduction in the amounts of OMPs has been observed (Koplow and Goldfine, 1974). In contrast, such a reduction was not observed in a comparable heptose-deficient rfaC mutant of N.meningitidis (P.van der Ley, unpublished data), already indicating that OMP assembly proceeds differently in this organism. Moreover, our data also show that the lipid A moiety of LPS is not required for assembly of integral outer membrane proteins, such as the porins. Although a minor degradation product of PorA was detected, the expression and assembly of the major OMPs appeared unaffected in the N.meningitidis LPS-deficient mutant. This degradation product was also observed in the rfaC mutant of N.meningitidis (P.van der Ley, unpublished observation), pointing to a proteolytic cleavage site within PorA that becomes accessible in the absence of the complete oligosaccharide core. If the meningococcal porins do indeed need a glycolipid for their assembly, the CPS would be the obvious candidate.
The only group of OMPs whose expression was greatly reduced in the LPS-deficient mutant encompasses the cell surface-exposed lipoproteins, such as LbpB and TbpB. These lipoproteins did not accumulate in the inner membrane and were not detected in the soluble fraction or extracellular medium. These proteins are probably degraded when they are not properly localized. In E.coli, the LolA-E system is required for the sorting of lipoproteins from the inner membrane to the outer membrane (Yokota et al., 1999; Yakushi et al., 2000). Homologues of the Lol system are present in the N.meningitidis chromosome (Tettelin et al., 2000), indicating that a similar route for the translocation of outer membrane lipoproteins exists in N.meningitidis. Since normal expression of the lipoproteins CtrA and H.8, both of which are associated with the outer membrane but are not fully cell surface exposed, was found in the LPS-deficient mutant, it can be concluded that lipoprotein transporting to the outer membrane by the Lol system is not affected. However, no component of the Lol system involved in the translocation of the surface-exposed lipoproteins from the periplasmic side of the outer membrane to the cell surface has yet been identified, a step which is not known to occur in E.coli. LPS could be involved in this step by binding these lipoproteins on their way to the outer leaflet of the outer membrane. Another possibility is that these lipoproteins do not correctly insert and/or are easily released and degraded, due to the altered biophysical properties of the outer membrane.
OMPs are considered important candidates for the development of a vaccine against N.meningitidis. However, the presence of LPS, also known as endotoxin, is a drawback in the use of outer membrane vesicles for immunization. With the availability of an LPS-deficient strain, this problem may be circumvented. However, we have previously shown that the immunogenicity of outer membrane complexes of an LPS-deficient N.meningitidis mutant is greatly reduced (Steeghs et al., 1999). Furthermore, the requirement for the presence of the polysialic acid CPS, which is homologous to host antigens, constitutes another drawback in the use of this LPS-deficient N.meningitidis mutant for a whole-cell or vesicle vaccine. However, by using the newly constructed strain HA3003 with an IPTG-inducible lpxA gene, it might be feasible to find an optimal balance between endotoxin content and immunogenicity even when the CPS is not present.
Neisseria meningitidis is the only Gram-negative bacterium described so far in which the synthesis of LPS can be disrupted completely. The viability of the mutant is apparently related to the fact that N.meningitidis carries a CPS, which is anchored in the outer membrane via a phospholipid. The structure of the E.coli K1 capsule is similar to the N.meningitidis B capsule (Kasper et al., 1973) and it would be interesting to investigate whether an LPS-deficient E.coli K1 mutant is also viable. On the other hand, in contrast to E.coli, N.meningitidis and also N.gonorrhoeae contain phospholipids in the outer leaflet of the outer membrane (Lysko and Morse, 1981), indicating that the typical asymmetry of the outer membrane normally found in Gram-negative bacteria is less pronounced in the Neisseriaceae. Consequently, Neisseriaceae are apparently more flexible than other bacterial species in accepting lipidic components other than LPS in the outer leaflet of the outer membrane. Nevertheless, Sprong et al. (2001) recently demonstrated that the LPS content of N.meningitidis strain H44/76 is similar to that previously reported for E.coli. It can thus be ruled out that LPS normally represents only a very minor component of the outer membrane of N.meningitidis, and would therefore be more easily replaced by phospholipids than in E.coli. However, as compared with E.coli, greater heterogeneity of the membrane phospholipids has been found in N.meningitidis and N.gonorrhoeae (Rahman et al., 2000). In particular, the pool of neisserial phospholipids having short-chain fatty acyl substituents, i.e. C12:0, C14:0 and C14:1, is much greater than previously reported for E.coli (Wilkinson, 1988). This diversity of meningococcal phospholipid acyl chains presumably results from the different substrate specificities of its multiple lysophosphatidic acid (LPA) acyltransferases. At least two LPA acyltransferases were demonstrated in N.meningitidis (Shih et al., 1998), in contrast to only a single one in E.coli (Coleman, 1992). Regulation of these LPA acyltransferases in N.meningitidis might help to compensate for defects occurring after the loss of LPS. Taken together, the ability of N.meningitidis to synthesize such a heterogeneous phospholipid pool and the existence of the CPS, being a glycolipid itself, apparently permit this bacterium to survive without LPS.
Materials and methods
Bacterial strains and growth conditions
Escherichia coli NM522 (Promega), used for the propagation of plasmids, was grown in LB medium containing ampicillin or kanamycin (100 µg/ml) at 37°C. Neisseria meningitidis strains were grown overnight at 37°C on GC medium base (Difco Laboratories) supplemented with IsoVitaleX (Becton Dickinson) in a humid atmosphere containing 5% CO2. For the isolation of inner and outer membranes, bacteria were grown in Meningococcal Medium (van der Ley et al., 1993), supplemented, if appropriate, with 39 µM ethylene-diamine-dihydroxyphenyl-acetic acid (Sigma) to impose iron restriction. Bacteria were grown until late log phase and inactivated by incubation for 2 h with 200 µg/ml tetracycline in 2% ethanol (final concentration) at 37°C under aeration. Transformation of N.meningitidis was carried out as described previously (van der Ley and Poolman, 1992) with selection on kanamycin (100 µg/ml) or chloramphenicol (10 µg/ml). To induce the tac promoter, strains were grown in Mueller–Hinton broth or Meningococcal Medium in the presence of 1 mM IPTG (Roche).
Construction of plasmids
Plasmids were constructed by using standard recombinant DNA techniques (Sambrook et al., 1989). Plasmid DNA was isolated using the Wizard kit (Promega). Plasmid pLA19 is a pUC18 derivative carrying a 1.9 kb insert, containing nucleotides 656–2472 of the lpxD-fabZ-lpxA locus of strain H44/76 (Steeghs et al., 1997). This plasmid was the basis for the construction of plasmid pLSK10, containing lpxA under an IPTG-inducible promoter. First, a 64mer linker named Termlink1 (5′-CGC GTGGTTTGAGTGAAGTTAAGGGATGCATAAACTGCATCCCTT AACTTGTTTTTTCGCGAA-3′), which possesses MluI overhangs and contains the last 12 bp (including stop codon) of fabZ, a transcriptional terminator and a unique NruI site, was cloned into the MluI site located 12 bp upstream of the stop codon of fabZ (Figure 6), resulting in plasmid pLS7. In this way, the complete fabZ open reading frame was maintained, while a transcriptional terminator was introduced 3 bp downstream of the fabZ stop codon. The orientation of Termlink1 in pLS7 was verified by sequence analysis with an Applied Biosystems automatic sequencer 310 on double-stranded plasmid DNA templates with a cycle sequencing protocol and primers hybridizing with fabZ and lpxA. Subsequently, the lacIq-tac control region released from plasmid pJF118EH (Fürste et al., 1986) by a NruI–SmaI digest was inserted into the unique NruI site in pLS7 upstream of lpxA, resulting in plasmid pLS8. The orientation of the lacIq-tac control region was checked by restriction analysis with NruI, which had to be maintained after this cloning procedure. pLS8 was then used to insert a kanamycin-resistance gene, released from plasmid pUC4K (Vieira and Messing, 1982) by HindII digestion, in the opposite transcriptional orientation of the tac promoter to create pLSK10. This plasmid was then used to transform N.meningitidis H44/76 (van der Ley and Poolman, 1992) to create strain HA3003. Plasmid pMF32.35::T5, containing a Tn1725 insertion element in the siaD gene, was a generous gift of Dr M.Frosch (Universität Würzburg, Germany).
Membrane separation
Inner membranes and outer membranes were isolated and separated by sucrose density gradient centrifugation as described (Masson and Holbein, 1983). Briefly, bacteria were harvested by centrifugation in a Sorvall SS34 rotor at 6000 r.p.m. at 4°C, and pellets were washed three times with phosphate-buffered saline (PBS). Pellets were resuspended in 50 mM Tris–HCl pH 8.0, and 50 µg/ml RNase (Roche) and DNase (Roche) were added. Bacteria were passed twice through a French pressure cell at 15 000 p.s.i. Unbroken cells were removed by centrifugation in a Sorvall SS34 rotor at 6000 r.p.m. at 4°C, and the supernatant was loaded on top of a discontinuous sucrose gradient consisting of a 6 ml 55% (w/w) sucrose cushion and a 9 ml 15% (w/w) sucrose top layer, both in 3 mM EDTA pH 8.0. After centrifugation for 2 h at 50 000 r.p.m. in a Beckman Ti60 rotor at 4°C, the crude membrane fraction was collected from the top of the cushion with a J-shaped Pasteur pipette. The sucrose concentration of this crude membrane fraction was lowered to 30% (w/w) sucrose with 3 mM EDTA pH 8.0, followed by separation on a second discontinuous sucrose gradient, consisting of 2.9 ml layers of 45, 40 and 35% (w/w) sucrose on top of a 1.9 ml 50% (w/w) sucrose cushion. The gradient was centrifuged for 36 h at 33 200 r.p.m. in an SW41 Beckman rotor at 4°C, and fractionated in 12 1-ml fractions from top to bottom. Sucrose concentrations in the fractions were determined by using a refractometer. Subsequently, the sucrose concentration of either the individual fractions or pooled fractions as depicted in Figure 1 was lowered to <10% (w/w) sucrose with 3 mM EDTA pH 8.0. Subsequently, membranes were collected by centrifugation for 2 h at 50 000 r.p.m. in a Beckman Ti60 rotor at 4°C and resuspended in buffer L (50 mM triethanolamine acetate pH 7.5, 250 mM sucrose, 1 mM dithiothreitol). The protein content of the membranes was determined using a bicinchronic acid protein assay reagent (Pierce Chemical Co.) with bovine serum albumin as a standard. Membranes were stored at –20°C.
Lactate dehydrogenase activity assay
Lactate dehydrogenase (LDH) activity was measured as described (Osborn et al., 1972).
SDS–PAGE and western blotting
Prior to electrophoresis, fractions were incubated in sample buffer (Lugtenberg et al., 1975) containing 2% SDS (final concentration). Incubations were performed for 10 min at room temperature or 100°C. SDS–polyacrylamide gels were prepared according to Lugtenberg et al. (1975). The gels were run at 20 mA in a temperature-controlled room at 4°C to prevent denaturation of various folded forms of the proteins as a result of heating of the gels during electrophoresis. Western blotting was performed as described (de Cock et al., 1999b). Prior to the blotting procedure, gels were heated under steam (Brok et al., 1995) to denature folded proteins. The PorA-specific mAbs used were MN5C11G (P1.16) or MN14C11.6 (P1.7) (Scholten et al., 1993). The RmpM-specific mAb used was MN2D6D (Poolman et al., 1985). The PilQ-specific antiserum was raised in mice against purified PilQ. The LbpA-specific mouse antiserum was directed against loop 2 of LbpA (Prinz et al., 1999). The LbpB-specific antiserum was raised in rabbits (A.Pettersson, P.Voet, P.Nadin, J.T.Poolman and J.Tommassen, in preparation). The TbpB-specific rabbit antiserum was a generous gift of Dr Anthony Schryvers (University of Calgary, Canada).
Phospholipid analysis
Total lipids were isolated from membrane fractions as described (Bligh and Dyer, 1959). Phospholipids were separated on TLC plates (HPTLC silicagel 60, Merck) with chloroform/methanol/acetic acid in a ratio of 65:25:10. Lipids were visualized by spraying with 50% sulfuric acid followed by charring. The fatty acyl content of PE and PG species was determined by using fast atom bombardment neutral loss tandem mass spectrometry (Cole and Enke, 1991). The specific loss of 141 u and 172 u was used to assign specific ions as those derived from PE and PG lipids, respectively.
LPS analysis
Tricine–SDS–PAGE was performed in 4% stacking and 16% separating gels as described (Lesse et al., 1990). Proteinase K-treated, boiled bacterial cells were used as samples (Apicella et al., 1994). The gels were run for 17 h at a constant current of 20 mA and silver stained (Tsai and Frasch, 1982).
Whole-cell ELISA
Expression of CPS and LPS was tested by whole-cell ELISA (Abdillahi and Poolman, 1987). The group B CPS- and LPS-specific mAbs used were 101C11 (Hurpin et al., 1992) and Mn4A8B2 (Scholten et al., 1994), respectively.
Determination of antibiotic sensitivity
Meningococcal strains were grown overnight on GC agar plates. Three colonies of each strain were resuspended in 200 µl of Meningococcal Medium and 175 µl of this solution were spread on fresh GC plates. Filter paper discs (Oxoid) containing rifampicin (5 µg), bacitracin (10 U), tetracycline (10 µg) or novobiocin (30 µg) were placed on the agar surface. After overnight incubation at 37°C, the halo of growth inhibition around each disc was measured.
Acknowledgments
Acknowledgements
We gratefully acknowledge Dr M.Frosch (Universität Würzburg, Germany) for providing plasmid pMF32.35::T5 and anti-CtrA mAb 2619, and Dr Anthony Schryvers (University of Calgary, Canada) for providing the TbpB-specific rabbit antiserum.
References
- Abdillahi H. and Poolman,J. (1987) Whole cell ELISA for typing Neisseria meningitidis with monoclonal antibodies. FEMS Microbiol. Lett., 48, 367–371. [PubMed] [Google Scholar]
- Apicella M.A., Griffiss,J.M. and Schneider,H. (1994) Isolation and characterization of lipopolysaccharides, lipooligosaccharides, and lipid A. Methods Enzymol., 235, 242–252. [DOI] [PubMed] [Google Scholar]
- Bligh E.G. and Dyer,W.J. (1959) A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol., 37, 911–917. [DOI] [PubMed] [Google Scholar]
- Brok R.G., Dekker,N., Gerrits,N., Verheij,H.M. and Tommassen,J. (1995) A conserved histidine residue of Escherichia coli outer-membrane phospholipase A is important for activity. Eur. J. Biochem., 234, 934–938. [DOI] [PubMed] [Google Scholar]
- Cole M.J. and Enke,C.G. (1991) Direct determination of phospholipid structures in microorganisms by fast atom bombardment triple quadrupole mass spectrometry. Anal. Chem., 63, 1032–1038. [DOI] [PubMed] [Google Scholar]
- Coleman J. (1992) Characterization of the Escherichia coli gene for 1-acyl-sn-glycerol-3-phosphate acyltransferase (plsC). Mol. Gen. Genet., 232, 295–303. [DOI] [PubMed] [Google Scholar]
- Cowan S.W., Schirmer,T., Rummel,G., Steiert,M., Ghosh,R., Pauptit,R.A., Jansonius,J.N. and Rosenbusch,J.P. (1992) Crystal structures explain functional properties of two E.coli porins. Nature, 358, 727–733. [DOI] [PubMed] [Google Scholar]
- de Cock H. and Tommassen,J. (1996) Lipopolysaccharides and divalent cations are involved in the formation of an assembly-competent intermediate of outer-membrane protein PhoE of E.coli. EMBO J., 15, 5567–5573. [PMC free article] [PubMed] [Google Scholar]
- de Cock H., Brandenburg,K., Wiese,A., Holst,O. and Seydel,U. (1999a) Non-lamellar structure and negative charges of lipopolysaccharides required for efficient folding of outer membrane protein PhoE of Escherichia coli. J. Biol. Chem., 274, 5114–5119. [DOI] [PubMed] [Google Scholar]
- de Cock H., Schafer,U., Potgeter,M., Demel,R., Muller,M. and Tommassen,J. (1999b) Affinity of the periplasmic chaperone Skp of Escherichia coli for phospholipids, lipopolysaccharides and non-native outer membrane proteins. Role of Skp in the biogenesis of outer membrane protein. Eur. J. Biochem., 259, 96–103. [DOI] [PubMed] [Google Scholar]
- Edwards U., Muller,A., Hammerschmidt,S., Gerardy-Schahn,R. and Frosch,M. (1994) Molecular analysis of the biosynthesis pathway of the α-2,8 polysialic acid capsule by Neisseria meningitidis serogroup B. Mol. Microbiol., 14, 141–149. [DOI] [PubMed] [Google Scholar]
- Frosch M. and Muller,A. (1993) Phospholipid substitution of capsular polysaccharides and mechanisms of capsule formation in Neisseria meningitidis. Mol. Microbiol., 8, 483–493. [DOI] [PubMed] [Google Scholar]
- Frosch M., Weisgerber,C. and Meyer,T.F. (1989) Molecular characteriz ation and expression in Escherichia coli of the gene complex encoding the polysaccharide capsule of Neisseria meningitidis group B. Proc. Natl Acad. Sci. USA, 86, 1669–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frosch M., Muller,D., Bousset,K. and Muller,A. (1992) Conserved outer membrane protein of Neisseria meningitidis involved in capsule expression. Infect. Immun., 60, 798–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fürste J.P., Pansegrau,W., Frank,R., Blocker,H., Scholz,P., Bagdasarian,M. and Lanka,E. (1986) Molecular cloning of the plasmid RP4 primase region in a multi-host-range tacP expression vector. Gene, 48, 119–131. [DOI] [PubMed] [Google Scholar]
- Gotschlich E.C., Fraser,B.A., Nishimura,O., Robbins,J.B. and Liu,T.Y. (1981) Lipid on capsular polysaccharides of gram-negative bacteria. J. Biol. Chem., 256, 8915–8921. [PubMed] [Google Scholar]
- Hurpin C.M., Carosella,E.D. and Cazenave,P.A. (1992) Bactericidal activity of two IgG2a murine monoclonal antibodies with distinct fine specificities for group B Neisseria meningitidis capsular polysaccharide. Hybridoma, 11, 677–687. [DOI] [PubMed] [Google Scholar]
- Jansen C., Wiese,A., Reubsaet,L., Dekker,N., de Cock,H., Seydel,U. and Tommassen,J. (2000) Biochemical and biophysical characterization of in vitro folded outer membrane porin PorA of Neisseria meningitidis. Biochim. Biophys. Acta, 1464, 284–298. [DOI] [PubMed] [Google Scholar]
- Kamio Y. and Nikaido,H. (1976) Outer membrane of Salmonella typhimurium: accessibility of phospholipid headgroups to phospholipase C and cyanogen bromide activated dextran in the external medium. Biochemistry, 15, 2561–2570. [DOI] [PubMed] [Google Scholar]
- Kasper D.L., Winkelhake,J.L., Zollinger,W.D., Brandt,B.L. and Artenstein,M.S. (1973) Immunochemical similarity between polysaccharide antigens of Escherichia coli 07: K1(L):NM and group B Neisseria meningitidis. J. Immunol., 110, 262–268. [PubMed] [Google Scholar]
- Klugman K.P., Gotschlich,E.C. and Blake,M.S. (1989) Sequence of the structural gene (rmpM) for the class 4 outer membrane protein of Neisseria meningitidis, homology of the protein to gonococcal protein III and Escherichia coli OmpA, and construction of meningococcal strains that lack class 4 protein. Infect. Immun., 57, 2066–2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koplow J. and Goldfine,H. (1974) Alterations in the outer membrane of the cell envelope of heptose-deficient mutants of Escherichia coli. J. Bacteriol., 117, 527–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird M.W., Kloser,A.W. and Misra,R. (1994) Assembly of LamB and OmpF in deep rough lipopolysaccharide mutants of Escherichia coli K-12. J. Bacteriol., 176, 2259–2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legrain M., Mazarin,V., Irwin,S.W., Bouchon,B., Quentin-Millet,M.J., Jacobs,E. and Schryvers,A.B. (1993) Cloning and characterization of Neisseria meningitidis genes encoding the transferrin-binding proteins Tbp1 and Tbp2. Gene, 130, 73–80. [DOI] [PubMed] [Google Scholar]
- Lesse A.J., Campagnari,A.A., Bittner,W.E. and Apicella,M.A. (1990) Increased resolution of lipopolysaccharides and lipooligosaccharides utilizing tricine–sodium dodecyl sulfate–polyacrylamide gel electrophoresis. J. Immunol. Methods, 126, 109–117. [DOI] [PubMed] [Google Scholar]
- Lugtenberg B., Meijers,J., Peters,R., van der Hoek,P. and van Alphen,L. (1975) Electrophoretic resolution of the ‘major outer membrane protein’ of Escherichia coli K12 into four bands. FEBS Lett., 58, 254–258. [DOI] [PubMed] [Google Scholar]
- Lysko P.G. and Morse,S.A. (1981) Neisseria gonorrhoeae cell envelope: permeability to hydrophobic molecules. J. Bacteriol., 145, 946–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masson L. and Holbein,B.E. (1983) Physiology of sialic acid capsular polysaccharide synthesis in serogroup B Neisseria meningitidis. J. Bacteriol., 154, 728–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan S., Kelly,T.M., Eveland,S.S., Raetz,C.R. and Anderson,M.S. (1994) An Escherichia coli gene (fabZ) encoding (3R)-hydroxymyristoyl acyl carrier protein dehydrase. Relation to fabA and suppression of mutations in lipid A biosynthesis. J. Biol. Chem., 269, 32896–32903. [PubMed] [Google Scholar]
- Osborn M.J., Gander,J.E., Parisi,E. and Carson,J. (1972) Mechanism of assembly of the outer membrane of Salmonella typhimurium. Isolation and characterization of cytoplasmic and outer membrane. J. Biol. Chem., 247, 3962–3972. [PubMed] [Google Scholar]
- Pautsch A. and Schulz,G.E. (1998) Structure of the outer membrane protein A transmembrane domain. Nature Struct. Biol., 5, 1013–1017. [DOI] [PubMed] [Google Scholar]
- Pettersson A., Maas,A. and Tommassen,J. (1994) Identification of the iroA gene product of Neisseria meningitidis as a lactoferrin receptor. J. Bacteriol., 176, 1764–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersson A., Prinz,T., Umar,A., van der Biezen,J. and Tommassen,J. (1998) Molecular characterization of LbpB, the second lactoferrin-binding protein of Neisseria meningitidis. Mol. Microbiol., 27, 599–610. [DOI] [PubMed] [Google Scholar]
- Poolman J.T., Wientjes,F.B., Hopman,C.T.P. and Zanen,H.C. (1985) Influence of the length of lipopolysaccharide molecules on the surface exposure of class 1 and 2 outer membrane proteins of Neisseria meningitidis strain 2996 (B:2b:P1.2). In Schoolnik,G.K. (ed.), The Pathogenic Neisseria. American Society for Microbiology, Washington, DC, pp. 562–570.
- Prinz T. and Tommassen,J. (2000) Association of iron-regulated outer membrane proteins of Neisseria meningitidis with the RmpM (class 4) protein. FEMS Microbiol. Lett., 183, 49–53. [DOI] [PubMed] [Google Scholar]
- Prinz T., Meyer,M., Pettersson,A. and Tommassen,J. (1999) Structural characterization of the lactoferrin receptor from Neisseria meningitidis. J. Bacteriol., 181, 4417–4419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raetz C.R. (1990) Biochemistry of endotoxins. Annu. Rev. Biochem., 59, 129–170. [DOI] [PubMed] [Google Scholar]
- Rahman M.M., Kolli,V.S., Kahler,C.M., Shih,G., Stephens,D.S. and Carlson,R.W. (2000) The membrane phospholipids of Neisseria meningitidis and Neisseria gonorrhoeae as characterized by fast atom bombardment mass spectrometry. Microbiology, 146, 1901–1911. [DOI] [PubMed] [Google Scholar]
- Ried G., Hindennach,I. and Henning,U. (1990) Role of lipopolysaccharide in assembly of Escherichia coli outer membrane proteins OmpA, OmpC, and OmpF. J. Bacteriol., 172, 6048–6053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J., Fritsch,E.F. and Maniatis,T. (1989) Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Schindler M. and Osborn,M.J. (1979) Interaction of divalent cations and polymixin B with lipopolysaccharide. Biochemistry, 18, 4425–4430. [DOI] [PubMed] [Google Scholar]
- Scholten R.J., Bijlmer,H.A., Poolman,J.T., Kuipers,B., Caugant,D.A., van Alphen,L., Dankert,J. and Valkenburg,H.A. (1993) Meningococcal disease in The Netherlands, 1958–1990: a steady increase in the incidence since 1982 partially caused by new serotypes and subtypes of Neisseria meningitidis. Clin. Infect. Dis., 16, 237–246. [DOI] [PubMed] [Google Scholar]
- Scholten R.J., Kuipers,B., Valkenburg,H.A., Dankert,J., Zollinger,W.D. and Poolman,J.T. (1994) Lipo-oligosaccharide immunotyping of Neisseria meningitidis by a whole-cell ELISA with monoclonal antibodies. J. Med. Microbiol., 41, 236–243. [DOI] [PubMed] [Google Scholar]
- Schryvers A.B. and Morris,L.J. (1988) Identification and characterization of the transferrin receptor from Neisseria meningitidis. Mol. Microbiol., 2, 281–288. [DOI] [PubMed] [Google Scholar]
- Sen K. and Nikaido,H. (1991) Lipopolysaccharide structure required for in vitro trimerization of Escherichia coli OmpF porin. J. Bacteriol., 173, 926–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih G.C., Kahler,C.M., Swartley,J.S., Rahman,M.M., Coleman,J., Carlson,R.W. and Stephens,D.S. (1998) Multiple lysophosphatidic acid acyltransferases in Neisseria meningitidis. Mol. Microbiol., 32, 874–880. [DOI] [PubMed] [Google Scholar]
- Sprong T., Stikkelbroeck,N., van der Ley,P., Steeghs,L., van Alphen,L., Klein,N., Netea,M.G., van der Meer,J.W.M. and van Deuren,M. (2001) The relative contribution of lipopolysaccharide and non-LPS components of Neisseria meningitidis to the proinflammatory cytokine response. J. Leukoc. Biol., 70, 283–288. [PubMed] [Google Scholar]
- Steeghs L., Jennings,M.P., Poolman,J.T. and van der Ley,P. (1997) Isolation and characterization of the Neisseria meningitidis lpxD-fabZ-lpxA gene cluster involved in lipid A biosynthesis. Gene, 190, 263–270. [DOI] [PubMed] [Google Scholar]
- Steeghs L., den Hartog,R., den Boer,A., Zomer,B., Roholl,P. and van der Ley,P. (1998) Meningitis bacterium is viable without endotoxin. Nature, 392, 449–450. [DOI] [PubMed] [Google Scholar]
- Steeghs L., Kuipers,B., Hamstra,H.J., Kersten,G., van Alphen,L. and van der Ley,P. (1999) Immunogenicity of outer membrane proteins in a lipopolysaccharide-deficient mutant of Neisseria meningitidis: influence of adjuvants on the immune response. Infect. Immun., 67, 4988–4993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tettelin H. et al. (2000) Complete genome sequence of Neisseria meningitidis serogroup B strain MC58. Science, 287, 1809–1815. [DOI] [PubMed] [Google Scholar]
- Tsai C.M. and Frasch,C.E. (1982) A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal. Biochem., 119, 115–119. [DOI] [PubMed] [Google Scholar]
- van der Ley P. and Poolman,J.T. (1992) Construction of a multivalent meningococcal vaccine strain based on the class 1 outer membrane protein. Infect. Immun., 60, 3156–3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Ley P., van der Biezen,J., Hohenstein,P., Peeters,C. and Poolman,J.T. (1993) Use of transformation to construct antigenic hybrids of the class 1 outer membrane protein in Neisseria meningitidis. Infect. Immun., 61, 4217–4224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J. and Messing,J. (1982) The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene, 19, 259–268. [DOI] [PubMed] [Google Scholar]
- Weiss M.S., Abele,U., Weckesser,J., Welte,W., Schiltz,E. and Schulz,G.E. (1991) Molecular architecture and electrostatic properties of a bacterial porin. Science, 254, 1627–1630. [DOI] [PubMed] [Google Scholar]
- Wiese A., Reiners,J.O., Brandenburg,K., Kawahara,K., Zahringer,U. and Seydel,U. (1996) Planar asymmetric lipid bilayers of glycosphingolipid or lipopolysaccharide on one side and phospholipids on the other: membrane potential, porin function, and complement activation. Biophys. J., 70, 321–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson S.G. (1988) Gram-negative bacteria. In Ratledge,C. and Wilkinson,S.G. (eds), Microbial Lipids. Academic Press, London, UK, pp. 299–488.
- Yakushi T., Masuda,K., Narita,S., Matsuyama,S. and Tokuda,H. (2000) A new ABC transporter mediating the detachment of lipid-modified proteins from membranes. Nature Cell Biol., 2, 212–218. [DOI] [PubMed] [Google Scholar]
- Yokota N., Kuroda,T., Matsuyama,S. and Tokuda,H. (1999) Characterization of the LolA–LolB system as the general lipoprotein localization mechanism of Escherichia coli. J. Biol. Chem., 274, 30995–30999. [DOI] [PubMed] [Google Scholar]