Abstract
Background
Many meta-analyses and systematic reviews have explored the impact of omega-3 supplementation on clinical outcomes in individuals with gastrointestinal (GI) cancers. Thus, this study aimed to capture the effects of omega-3 supplementation on GI cancers and associated complications.
Methods
This umbrella study was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. A comprehensive advanced search was executed across Scopus, PubMed, and Web of Science until 25 January 2025. Data were pooled by using random-effects models based on heterogeneity. The entire statistical analysis was performed via RStudio and R. The statistical analysis results are presented as the mean difference (MD), standard mean difference (SMD), and relative risk (RR) in conjunction with their 95% confidence intervals (CIs).
Results
Eight meta-analysis papers were included in our umbrella review. Omega-3 fatty acid supplementation improved the serum concentrations of tumor necrosis factor alpha (TNF-α) (SMD: −0.34; 95% CI: −0.56, −0.11), interleukin-6 (IL-6) (SMD: −0.30; 95% CI: −0.49, −0.12; MD: −4.96; 95% CI: −6.62, −3.30), and C-reactive protein (CRP) (MD: −5.46; 95% CI: −10.06, −0.87). Omega-3 supplementation improved the CD4+/CD8+ ratio (SMD: 0.48; 95% CI: 0.26, 0.71) and reduced the length of hospitalization (MD: −2.45 d; 95% CI: −3.11, −1.80). Omega-3 supplementation was associated with a 24% significant reduction in the risk of overall complications (RR: 0.76; 95% CI: 0.67, 0.86).
Conclusion
Omega-3 supplementation may reduce the risk of overall complications and length of hospitalization in individuals suffering from GI cancers. Additionally, supplementation with omega-3 may alleviate the levels of pro-inflammatory cytokines such as TNF-α and IL-6, and acute-phase proteins such as CRP.
Keywords: gastrointestinal cancer, omega-3 fatty acid, inflammation, cancer complications
Introduction
Cancers of the gastrointestinal (GI) tract, such as those of the stomach, liver, esophagus, pancreas, and colon, account for more than a quarter of all cancers, and their prevalence is increasing steadily [1, 2]. Pre-pandemic data suggest that there were >5 million new cases of gastrointestinal cancer (GC) in 2018, associated with >3 million related deaths [3]. In Iran, 49.8% of fatal malignancies are GCs, including those of the stomach, colon, liver, pancreas, and esophagus [4].
The symptoms of GI cancers can range from asymptomatic to symptoms such as swallowing difficulties, abdominal pain, and bleeding, depending on the location within the gastrointestinal system [5]. The affected area determines the diagnosis of these tumors, which can be made via techniques such as endoscopy, colonoscopy, and endoscopic retrograde cholangiopancreatography [6]. Depending on the patient’s condition and disease stage, treatment for these malignancies may involve medication, chemotherapy, surgery, and alternative therapies [7, 8]. Radiotherapy is a crucial component of comprehensive cancer treatment strategies [9], but it often leads to adverse effects that can significantly impact patients’ overall well-being and quality of life (QOL) [10]. In an effort to better manage adverse effects and enhance patient outcomes, there has been a recent surge in interest in investigating alternative medicines. The use of dietary supplements that include omega-3 polyunsaturated fatty acids (PUFAs), which are known for their anti-inflammatory qualities and possible protective effects against oxidative stress, is an intriguing strategy [11]. Omega-3 PUFAs, particularly eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), have demonstrated beneficial effects across various clinical contexts, including the prevention and management of cardiovascular disease, inflammation reduction, and mood regulation [12]. In patients with cancer, the anti-inflammatory properties of omega-3 PUFAs may offer protection against radiation-induced tissue damage and facilitate recovery post radiotherapy [13]. Omega-3 PUFAs may help reduce inflammation, which is often exacerbated by radiation exposure [14]. Furthermore, previous research has suggested that omega-3 PUFA supplementation could alleviate symptoms associated with chemoradiotherapy, such as fatigue, nausea, and cognitive dysfunction. However, more extensive investigations, particularly those focused on radiotherapy patients, are needed to draw definitive conclusions about the efficacy of omega-3 PUFA supplementation for improving QOL [15].
Therefore, this umbrella review aimed to synthesize evidence from existing meta-analyses on the effects of omega-3 fatty acid supplementation on clinical outcomes—including inflammatory markers, immune parameters, hospitalization duration, and complications—in patients with GCs.
Methods
In accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) criteria, an umbrella review employing all related meta-analyses and systematic reviews was carried out [16].
Search strategy
A comprehensive advanced search was executed across three databases, namely Scopus, PubMed, and Web of Science, to identify meta-analyses concentrating on omega-3 supplementation in individuals diagnosed with GI cancer published from inception to 15 February 2025. The search methodology involved the utilization of specific Medical Subject Heading (MeSH) terms and keywords to expand an effective search strategy: ((((“Fatty Acids, Omega-3”[Mesh] OR “Eicosapentaenoic Acid”[Mesh]) OR “Docosahexaenoic Acids”[Mesh]) OR ((((((“omega 3”[Title/Abstract]) OR (“ω − 3”[Title/Abstract])) OR (“eicosapentaenoic acid”[Title/Abstract])) OR (“EPA”[Title/Abstract])) OR (“Docosahexaenoic acid”[Title/Abstract])) OR (“DHA”[Title/Abstract]))) AND (((((((((((((“cancer”[Title/Abstract]) OR (“carcinoma”[Title/Abstract])) OR (“Radiotherapy”[Title/Abstract])) OR (“chemotherapy”[Title/Abstract])) OR (“neoplasm”[Title/Abstract])) OR (“adenoma”[Title/Abstract])) OR (“malignan”[Title/Abstract])) OR (“sarcoma”[Title/Abstract])) OR (“metastas”[Title/Abstract])) OR (“leukemia”[Title/Abstract])) OR (“Lymphoma”[Title/Abstract])) OR (“glioma”[Title/Abstract])) OR ((“Neoplasms”[MeSH]) OR “Carcinoma”[MeSH]))) AND (((“Systematic Review” [Publication Type]) OR “Meta-Analysis” [Publication Type]) OR ((“meta-analysis”[Title/Abstract]) OR (“systematic review”[Title/Abstract]))). Additionally, to increase the sensitivity of the search strategies, the wild-card “*” was utilized. The PubMed alert was activated by the first author (H.A.) to ensure the article’s retrieval and prevent its omission. Moreover, an exhaustive review of the citations within the qualifying papers was carried out to minimize the likelihood of missing critical research.
Eligibility criteria
In this umbrella review, only studies meeting the following conditions were considered: (i) systematic reviews and meta-analyses; and (ii) papers investigating the impact of omega-3 supplementation on individuals with GI cancer. Papers of an observational nature, quasi-experimental designs, case reports, animal studies, letters, reviews, and commentaries were excluded from our umbrella review. The PICO structure used in this study was as follows: population/patients (P: individuals with GI cancer); intervention (I: omega-3 fatty acid supplementation); comparison (C: placebo or standard treatment); and outcome (O: inflammatory markers, immune system, serum concentrations of albumin, length of hospitalization, and total complication risk).
Study selection
Using EndNote software, duplicate papers were excluded by an investigator (H.A.). Following the PICO structure and eligibility criteria, the titles/abstracts and full texts of the remaining publications were separately reviewed by the authors (S.N., Z.R.J., and A.P.). The papers were subsequently assessed by the first and corresponding authors (H.A. and S.D.).
Methodological quality assessment
One of the researchers (H.A.) carried out an entire evaluation of the meta-analyses and systematic analyses that were entered into this paper, which was subsequently inspected by the corresponding author (S.D.). Quality assessment of the methodology of the selected papers was conducted via the Assessment of Multiple Systematic Reviews (AMSTAR)-2 tool. The aforementioned tool is particularly designed for evaluating the quality of meta-analyses and systematic reviews, comprising 7 critical domains with 16 questions. The responses to these questions in the AMSTAR-2 tool are classified as “No meta-analysis,” “No,” “Partial Yes,” or “Yes,” and the overall quality of the selected papers is classified as “High,” “Low,” “Moderate,” or “Critically low” [17].
Data extraction
One researcher (Z.M.) collected vital details, which were then inspected by another researcher (H.A.). Information such as country, publication year, study duration, outcomes, quality assessment scales, first author of the selected papers, and basic characteristics of participants (including sample size, age, ranges for duration and dose of omega-3 supplementation, mean dosage, and duration of omega-3 supplementation) were written in a predesigned table.
Data synthesis and statistical analysis
For the statistical analysis, the mean difference (MD), standard mean difference (SMD), and relative risk (RR) were used in conjunction with their 95% confidence intervals (CIs). Relying on the I2 and Q statistics, either a fixed-effect model or a random-effect model was selected. High and low heterogeneity were defined as I2 > 75% and I2 ≤ 40%, respectively [18]. Subgroup analysis of the included papers was conducted to explore sources of heterogeneity according to the following factors: age, AMSTAR score, number of included studies, and participants. Graphical funnel plots and trim-and-fill methods were drawn to assess the publication bias of the included papers. The entire statistical analysis was performed via RStudio version 2023.03.1 and R version 4.3.2 simultaneously, with a significance threshold set at P ≤ 0.05.
Results
Study selection
In accordance with the PRISMA guidelines, a total of 1,347 records were initially identified through the mentioned databases, of which 166 duplicates were removed. Among the 1,181 studies whose titles and abstracts were screened, 1,167 were excluded. Of the remaining 14 studies, 6 were removed because they did not explicitly mention GCs, leaving 8 studies that were eligible for assessment in the current umbrella review (Figure 1). Table 1 provides detailed information about the studies included in the umbrella review.
Figure 1.
Study-selection process for the umbrella review on omega-3 supplementation and GCs.
Table 1.
Detailed information about the studies included in the umbrella review
| Study, date, reference number | Number of included studies | Location, duration | Number of participants | Age (years) | Intervention (range and mean of dose and duration) | Quality assessment scale | Outcomes |
|---|---|---|---|---|---|---|---|
| Yan et al., 2019 [19] | 11 | China, 2008–2017 | 755 |
|
|
Cochrane 5.3/7 |
|
| Lu et al., 2022 [23] | 10 | China, 2012–2015 | 663 |
|
|
Cochrane 6/7 |
|
| Liu et al., 2023 [24] | 19 | China, 2002–2020 | 179 |
|
|
Cochrane 6/7 |
|
| Yue et al., 2022 [26] | 20 | China, 2002–2021 | 1,761 |
|
|
Cochrane 6/7 |
|
| De Castro et al., 2022 [22] | 6 | Brazil, 2008–2018 | 278 |
|
|
Cochrane 5.7–7 |
|
| Xie et al., 2016 [21] | 11 | China, 1966–2016 | 694 | Range: 50–71 years (60.5) |
|
Cochrane 5.7/7 |
|
| Ma et al., 2015 [25] | 15 | China, 1995–2014 | 1,297 |
|
|
Cochrane 5.5/7 |
|
| Jamali et al., 2024 [20] | 28 | Iran, 1999–2022 | 1,561 |
|
|
|
|
CD: cluster of differentiation, CRP: C-reactive protein, IG: immunoglobulin, IL: interleukin, LOS: length of hospital stay, SIRS: systemic inflammatory response syndrome, TNF-α: tumor necrosis factor α, CRP = C-reactive protein, I = intervention, C = control, wk = week, NS = non-significant, S = significant.
Methodological quality assessment
In accordance with the AMSTAR-2 tool, the methodological quality results of the meta-analyses are summarized in Table 2. In this umbrella review, five included meta-analyses were rated as high and the three remaining meta-analyses were rated as moderate.
Table 2.
Results of the selected papers according to AMSTAR-2
| Study, date, reference number | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | Q10 | Q11 | Q12 | Q13 | Q14 | Q15 | Q16 | Overall |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yan et al., 2019 [19] | Y | Y | Y | Y | Y | Y | Y | Y | N | Y | Y | Y | Y | Y | PY | Y | Moderate |
| Lu et al., 2022 [23] | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | PY | Y | High |
| Liu et al., 2023 [24] | Y | Y | Y | Y | Y | Y | PY | Y | Y | Y | Y | Y | Y | PY | PY | Y | Moderate |
| Yue et al., 2022 [26] | Y | PY | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | PY | Y | High |
| De Castro et al., 2022 [22] | Y | PY | Y | Y | Y | Y | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | High |
| Xie et al., 2016 [21] | Y | Y | Y | PY | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | PY | Y | Moderate |
| Ma et al., 2016 [25] | Y | PY | Y | PY | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | PY | Y | High |
| Jamali et al., 2024 [20] | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | High |
Y = Yes, PY = Partially Yes, N = No. Questions: Q1: Did the research questions and inclusion criteria for the review include the components of PICO? Q2: Did the report of the review contain an explicit statement that the review methods were established prior to the conduct of the review, and did the report justify any significant deviations from the protocol? Q3: Did the review authors explain their selection of the study designs for inclusion in the review? Q4: Did the review authors use a comprehensive literature search strategy? Q5: Did the review authors perform study selection in duplicate? Q6: Did the review authors perform data extraction in duplicate? Q7: Did the review authors provide a list of excluded studies and justify the exclusions? Q8: Did the review authors describe the included studies in adequate detail? Q9: Did the review authors use a satisfactory technique for assessing risk of bias (RoB) in individual studies that were included in the review? Q10: Did the review authors report on the sources of funding for the studies included in the review? Q11: If meta-analysis was performed, did the review authors use appropriate methods for the statistical combination of results? Q12: If meta-analysis was performed, did the review authors assess the potential impact of RoB in individual studies on the results of the meta-analysis or other evidence synthesis? Q13: Did the review authors account for RoB in individual studies when interpreting/discussing the review results? Q14: Did the review authors provide a satisfactory explanation for and discussion of any heterogeneity observed in the review results? Q15: If they performed quantitative synthesis, did the review authors conduct an adequate investigation of publication bias (small-study bias) and discuss its likely impact on the review results? Q16: Did the review authors report any potential sources of conflict of interest, including any funding they received for conducting the review?
Impact of omega-3 supplementation on inflammatory markers in papers reporting SMD
The selected papers that used SMD [19–22] were included in our umbrella analysis. Omega-3 fatty acid supplementation significantly alleviated tumor necrosis factor alpha (TNF-α) (SMD: −0.34; 95% CI: −0.56, −0.11; Figure 2A) and interleukin-6 (IL-6) (SMD: −0.30; 95% CI: −0.49, −0.12; Figure 2B) levels, with moderately significant and slightly insignificant heterogeneity, respectively (I2 = 63%, P = 0.05; I2 = 6%, P = 0.38). On the other hand, the C-reactive protein (CRP) concentration did not significantly change after omega-3 supplementation (SMD: −0.08; 95% CI: −0.24, 0.09; Figure 2C), with low heterogeneity (I2 = 0%, P = 0.69). With respect to subgroup analysis, in papers on colorectal cancer (CC), the impact of omega-3 supplementation on serum TNF-α and IL-6 concentrations was significant in those with a study duration of >7 years and in studies with durations of both <5 years and >5 years (Table 3). Despite the serum concentrations of TNF-α and IL-6, the combined SMD for the serum CRP concentration did not change even after subgroup analysis. Graphical inspection of the funnel plots for those inflammatory markers revealed the presence of publication bias (Figure 2D–F). Trim-and-fill analysis for TNF-α, IL-6, and CRP was applied, and the results showed no small-studies effect.
Figure 2.
Impact of omega-3 supplementation on inflammatory markers in patients with GCs. Meta-analysis of randomized–controlled trials that evaluated the effect of omega-3 supplementation on serum levels of TNF-α, IL-6, and CRP using SMD with 95% CI. (A) Forest plot showing the pooled SMD in TNF-α levels across four studies. (B) Forest plot showing the pooled SMD in IL-6 levels across the same six studies. (C) Forest plot showing the pooled SMD in CRP levels across the same three studies. (D–F) Funnel plots corresponding to (A–C), respectively, assessing potential publication bias. Egger’s test results were non-significant, indicating no major publication bias. IL-6 = interleukin-6, CRP = C-reactive protein, SD = standard deviation, I2 = I-squared statistic for heterogeneity, τ2 = tau-squared, between-study variance, P = statistical significance.
Table 3.
Subgroup analysis of the impact of omega-3 supplementation on inflammatory markers (TNF-α, IL-6, and CRP) and CD4+/CD8+ ratio in studies reporting SMD
| Subgroup variable | Effect size (N) | SMD (95% CI) | I 2 (%) | P-heterogeneity |
|---|---|---|---|---|
| TNF-α | ||||
| Overall | 4 | −0.34 (−0.56, −0.11) | 63 | 0.05 |
| Type | ||||
| CC | 2 | −0.39 (−0.64, −0.14) | 0 | 0.83 |
| Other GCs | 2 | −0.29 (−0.73, 0.14) | 87 | <0.01 |
| Duration (years) | ||||
| ≥7 | 2 | −0.47 (−0.63, −0.11) | 0 | 0.44 |
| <7 | 2 | −0.19 (−0.52, 0.14) | 44 | 0.18 |
| IL-6 | ||||
| Overall | 6 | −0.30 (−0.49, −0.12) | 6 | 0.38 |
| Type | ||||
| CC | 2 | −0.36 (−0.62, −0.10) | 0 | 1.00 |
| GC | 3 | −0.29 (−0.62, 0.05) | 55 | 0.11 |
| EC | 1 | −0.03 (−0.67, 0.61) | ||
| Duration (years) | ||||
| ≥7 | 3 | −0.41 (−0.62, −0.21) | 0 | 0.39 |
| <7 | 3 | −0.14 (−0.40, 0.11) | 0 | 0.65 |
| Included studies | ||||
| ≥5 | 2 | −0.42 (−0.83, −0.12) | 40 | 0.20 |
| <5 | 4 | −0.24 (−0.43, −0.04) | 0 | 0.56 |
| CRP | ||||
| Overall | 3 | −0.08 (−0.24, 0.09) | 0 | 0.69 |
| Type | ||||
| DC | 1 | −0.06 (−0.26, 0.14) | ||
| GC | 2 | −0.11 (−0.42, 0.19) | 0 | 0.41 |
| Duration (years) | ||||
| ≥16 | 2 | −0.11 (−0.42, 0.19) | 0 | 0.41 |
| <16 | 1 | −0.06 (−0.26, 0.14) | ||
| Included studies | ||||
| ≥7 | 2 | −0.09 (−0.27, 0.09) | 0 | 0.48 |
| <7 | 1 | 0.04 (–0.43, 0.51) | ||
| CD4+/CD8+ ratio | ||||
| Overall | 3 | 0.48 (0.26, 0.71) | 28 | 0.25 |
| Type | ||||
| CC | 2 | 0.55 (0.27, 0.83) | 33 | 0.22 |
| GC | 1 | 0.35 (0.04, 0.66) | ||
| Duration (years) | ||||
| ≥6 | 2 | 0.52 (0.21, 0.71) | 55 | 0.14 |
| <6 | 1 | 0.36 (−0.04, 0.76) | ||
| Included studies | ||||
| ≥9 | 1 | 0.66 (0.40, 0.92) | ||
| <9 | 2 | 0.35 (0.11, 0.60) | 0 | 0.97 |
N represents the number of included studies in each subgroup analysis.
Impact of omega-3 supplementation on inflammatory markers in papers reporting MD
Three selected papers [23–25] explored the positive influence of omega-3 supplementation on the serum concentrations of CRP (MD: −5.46; 95% CI: −10.06, −0.87; Figure 3A) and TNF-α (MD: −0.43; 95% CI: −0.71, −0.16; Figure 3B). Moreover, highly significant heterogeneity was detected (I2 = 13%, P = 0.28). Furthermore, a significant reduction in the serum IL-6 concentration was detected in two studies (MD: −4.96; 95% CI: −6.62, −3.30; Figure 3C). Subgroup analysis of CRP revealed that omega-3 supplementation significantly decreased CRP concentrations in meta-analyses of patients with gastric cancer and those with fewer than six included studies (Table 4). In contrast, the CRP levels did not change after subgroup analysis. Visual inspection of the funnel plots for the CRP, TNF-α, and IL-6 levels confirmed their asymmetric shapes (Figure 3D–F, respectively). To evaluate the impact of small papers, a trim-and-fill analysis for CRP was performed and the results showed no small-studies effect.
Figure 3.
Impact of omega-3 supplementation on inflammatory markers in patients with GCs. Meta-analysis of randomized–controlled trials assessed the effect of omega-3 supplementation on serum levels of CRP, TNF-α, and IL-6 using MD with 95% CI. (A) Forest plot showing the pooled MD in CRP levels across three studies. (B) Forest plot showing the pooled MD in TNF-α levels across two studies. (C) Forest plot showing the pooled MD in IL-6 levels across two studies. (D–F) Funnel plots corresponding to (A–C), respectively, assessing potential publication bias. SD = standard deviation, I2 = I-squared statistic for heterogeneity, τ2 = tau-squared, between-study variance, P = statistical significance.
Table 4.
Subgroup analysis of the impact of omega-3 supplementation on inflammatory markers (TNF-α, IL-6, and CRP) and length of hospital stay in studies reporting MD
| Subgroup variable | Effect size (N) | MD (95% CI) | I 2 (%) | P-heterogeneity |
|---|---|---|---|---|
| LOS | ||||
| Overall | 4 | −2.45 (−3.11, −1.80) | 72 | 0.01 |
| Type | ||||
| CC | 2 | 2.71 (−9.26, 14.68) | 91 | <0.01 |
| Other GCs | 2 | −2.48 (−3.20, −1.76) | 0 | 0.95 |
| Duration (years) | ||||
| ≥12 | 3 | 0.51 (−6.43, 7.46) | 81 | <0.01 |
| <12 | 1 | −2.54 (−4.39, −0.69) | ||
| Included studies | ||||
| ≥6 | 2 | −2.55 (−3.25, −1.85) | 0 | 0.64 |
| <6 | 2 | 2.88 (−8.74, 14.50) | 90 | <0.01 |
| CRP | ||||
| Overall | 3 | −5.46 (−10.06, −0.87) | 83 | <0.01 |
| Type | ||||
| CC | 1 | −2.41 (−5.45, 0.63) | ||
| GC | 2 | −7.07 (−12.92, −1.21) | 81 | 0.02 |
| Duration (years) | ||||
| ≥18 | 2 | −6.16 (−13.55, 1.23) | 91 | <0.01 |
| <18 | 1 | −3.97 (−7.87, −0.07) | ||
| Included studies | ||||
| ≥6 | 1 | −2.41 (−5.45, 0.63) | ||
| <6 | 2 | −7.07 (−12.92, −1.21) | 81 | 0.02 |
LOS = length of hospital stay. N represents the number of included studies in each subgroup analysis.
Impact of omega-3 supplementation on the immune system
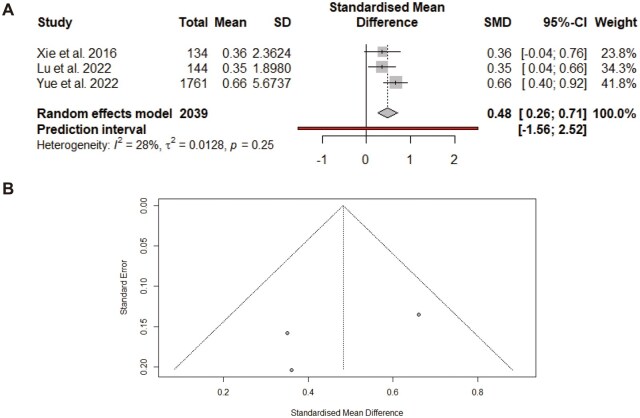
In the three selected papers that evaluated the effect of omega-3 supplementation on the CD4+/CD8+ ratio, a significant increase (SMD: 0.48; 95% CI: 0.26, 0.71; Figure 4A) was found, with insignificant heterogeneity (I2 = 28%, P = 0.25). Subgroup analysis revealed that a significant increase in the CD4+/CD8+ ratio was detected in those papers that were on CC and had a duration of >6 years and <9 years (Table 3). The funnel plot was asymmetrical (Figure 4B). After the trim-and-fill method was applied, the results remained similar, confirming the robustness of the findings.
Figure 4.
Effect of omega-3 supplementation on CD4+/CD8+ T-cell ratio in patients with GCs. Meta-analysis of randomized–controlled trials evaluated the impact of omega-3 supplementation on immune function, specifically the CD4+/CD8+ ratio, using SMD with 95% CI. (A) Forest plot showing the pooled SMD for the CD4+/CD8+ ratio across included studies. Heterogeneity and prediction interval values are reported to assess consistency across studies. (B) Funnel plot corresponding to (A), used to evaluate potential publication bias. SD = standard deviation, I2 = I-squared statistic for heterogeneity, τ2 = tau-squared, between-study variance, P = statistical significance.
Impact of omega-3 supplementation on the serum ALB concentration
Impact of omega-3 supplementation on the serum albumin concentration in papers reporting SMD
Two studies [22, 26] that investigated the effect of omega-3 supplementation on the level of albumin included in the umbrella analysis revealed an insignificant increase in the albumin level (SMD: 0.16; 95% CI: −0.38, 0.71; Figure 5A), with highly significant heterogeneity among the papers (I2 = 83%, P = 0.02). The obtained funnel plot did not reject the absence of publication bias (Figure 5B).
Figure 5.
Effect of omega-3 supplementation on serum albumin concentration in patients with GCs. Meta-analysis of randomized–controlled trials evaluated the impact of omega-3 supplementation on serum albumin using SMD with 95% CI. (A) Forest plot showing the pooled SMD for the serum albumin across the included studies. Heterogeneity and prediction interval values are reported to assess consistency across studies. (B) Funnel plot corresponding to (A), used to evaluate potential publication bias. SD = standard deviation, I2 = I-squared statistic for heterogeneity, τ2 = tau-squared, between-study variance, P = statistical significance.
Impact of omega-3 supplementation on the serum albumin concentration in papers reporting MD
In two meta-analyses [23, 24], serum albumin concentrations were reported to have an insignificant impact on omega-3 supplementation (SMD: 0.36; 95% CI: −0.02, 0.74; Figure 6A), with low, meaningless between-paper heterogeneity (I2 = 0%, P = 0.50). The absence of publication bias was not confirmed due to the asymmetric shape of the funnel plot (Figure 6B).
Figure 6.
Effect of omega-3 supplementation on serum albumin concentration in patients with GCs. Meta-analysis of randomized–controlled trials evaluated the impact of omega-3 supplementation on serum albumin using MD with 95% CI. (A) Forest plot showing the pooled MD for the serum albumin across the included studies. Heterogeneity and prediction interval values are reported to assess consistency across studies. (B) Funnel plot corresponding to (A), used to evaluate potential publication bias. SD = standard deviation, I2 = I-squared statistic for heterogeneity, τ2 = tau-squared, between-study variance, P = statistical significance.
Impact of omega-3 supplementation on the length of hospital stay
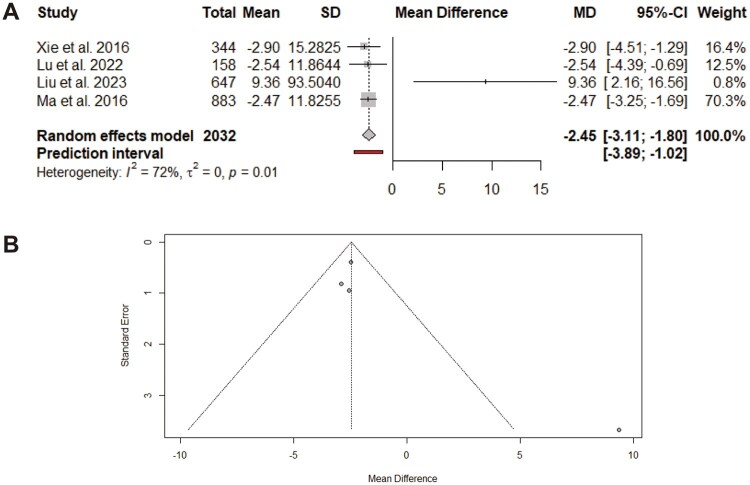
On the basis of four meta-analyses [21, 23–25], omega-3 supplementation eventually led to a significant reduction in the length of hospital stay (MD: −2.45; 95% CI: −3.11, −1.80; Figure 7A). However, moderate between-paper heterogeneity was observed (I2 = 72%, P = 0.01). Subgroup analysis revealed a significant reduction in papers that involved patients with GC and those with more than six included studies (Table 4). Visual inspection of the funnel plot revealed asymmetry (Figure 7B). After small papers were omitted, a trim-and-fill analysis indicated no change in the pooled MD.
Figure 7.
Effect of omega-3 supplementation on length of hospital stay in patients with GCs. Meta-analysis of randomized–controlled trials evaluated the impact of omega-3 supplementation on length of hospital stay using MD with 95% CI. (A) Forest plot showing the pooled MD for the length of hospital stay across the included studies. Heterogeneity and prediction interval values are reported to assess consistency across studies. (B) Funnel plot corresponding to (A), used to evaluate potential publication bias. SD = standard deviation, I2 = I-squared statistic for heterogeneity, τ2 = tau-squared, between-study variance, P = statistical significance.
Impact of omega-3 supplementation on total complication risk
In three meta-analyses [21, 24, 25], the effect of omega-3 supplementation on the risk of total complications was explored and the results revealed a 24% significant reduction (RR: 0.76; 95% CI: 0.67, 0.86; Figure 8A), with low, insignificant between-paper heterogeneity (I2 = 0%, P = 0.48). Apart from CC, other subgroup analyses demonstrated a significant reduction in the total complication risk (Table 5). Graphical inspection revealed an asymmetric funnel plot (Figure 8B). The results of the trim-and-fill analysis did not change, even after the omission of small papers.
Figure 8.
Effect of omega-3 supplementation on total complication risk in patients with GCs. Meta-analysis of randomized–controlled trials evaluated the impact of omega-3 supplementation on total complication risk using RR with 95% CI. (A) Forest plot showing the pooled RR for the total complication risk across the included studies. Heterogeneity and prediction interval values are reported to assess consistency across studies. (B) Funnel plot corresponding to (A), used to evaluate potential publication bias.SD = standard deviation, I2 = I-squared statistic for heterogeneity, τ2 = tau-squared, between-study variance, P = statistical significance.
Table 5.
Subgroup analysis of the impact of omega-3 supplementation on total complication risk
| Subgroup variable | Effect size ( N ) | RR (95% CI) | I 2(%) | P-heterogeneity |
|---|---|---|---|---|
| Total complications | ||||
| Overall | 6 | 0.76 (0.67, 0.86) | 0 | 0.48 |
| Type | ||||
| CC | 3 | 0.87 (0.64, 1.17) | 0 | 0.54 |
| Other GCs | 3 | 0.73 (0.64, 0.84) | 13 | 0.32 |
| Duration (years) | ||||
| ≥13 | 3 | 0.71 (0.59, 0.84) | 0 | 0.73 |
| <13 | 3 | 0.80 (0.66, 0.98) | 29 | 0.24 |
| Included studies | ||||
| ≥8 | 4 | 0.76 (0.60, 0.96) | 0 | 0.41 |
| <8 | 2 | 0.76 (0.63, 0.92) | 38 | 0.20 |
N represents the number of included studies in each subgroup analysis.
Discussion
It is crucial to optimize the intake of key nutrients in a way that greatly inhibits carcinogenesis and improves the treatment response to achieve the most effective and efficient nutritional intervention in cancer treatment. Over the past few decades, the results of clinical studies on omega-3 supplementation among GI cancer patients undergoing radiotherapy have been ambiguous. As an umbrella review study, we have therefore compiled the findings of excellent systematic reviews and meta-analyses. In the present study, supplementation with omega-3 fatty acids was associated with improvements in TNF-α and IL-6 levels. IL-6 is one of the most sensitive and critical inflammatory agents. During surgery, IL-6 drives the liver to generate the acute-phase protein CRP, which increases the phagocytic activity of neutrophils and macrophages [27]. The release of IL-6 and the presence of CRP in the body can reflect the body’s stress status. Recent research has demonstrated that omega-3 PUFAs can lower the levels of IL-6 and TNF-α in cancer patients following surgery, shorten the duration of ventilator use and hospital stay in patients undergoing major abdominal surgery, and enhance immune function [28]. These findings are consistent with the results of our umbrella review. Studies have shown that the dietary support of omega-3 PUFAs reduces inflammatory situations in cancer patients in the digestive system. Many preclinical studies indicate that inflammation is a key step in the initiation and progression of cancer [29, 30]. Omega-3 PUFAs play a critical role in lowering inflammation and facilitating tissue homeostasis recovery in various diseases, including certain gastrointestinal ailments [31]. This is likely attributed to their strong anti-inflammatory and antioxidant properties, as well as their ability to promote the early epithelialization of ulcers. However, other studies reported no associations between the levels of inflammatory factors, such as IL-6, IL-10, and CRP, and the intake of omega-3 supplements [32]. The anti-inflammatory effect may be dose- and duration-dependent in terms of supplement intake.
Moreover, the improvement in the CD4+/CD8+ ratio in individuals receiving radiation therapy for gastrointestinal malignancies was another finding of the study. Studies have demonstrated increases in immune function in GC patients taking omega-3 supplements [26]. The majority of GCs are in an immune-suppressive state, which further lowers immunity as a result of decreased appetite, digestive and absorptive abnormalities, tumor self-suppression, and the decreased generation of immune-suppressive substances. By increasing the total number of T cells and CD4+ T cells in the peripheral blood, omega-3 PUFAs increase the immunological response [33]. A small number of studies on postoperative complications of gastrointestinal surgeries have reported no significant correlation between omega-3 supplementation and decreased infection rates. The type of study, sample size, mode of supplement delivery, and length of supplementation can all significantly affect the outcome [34]. Another significant finding of this study was the association between omega-3 supplement use and reduced hospitalization duration in patients with GCs receiving chemotherapy. Studies by Tsekos et al. [35] in 2004 and Lu et al. [23] in 2022 also obtained similar results. Pre-albumin, albumin, and retinol-binding protein are protein components of total plasma protein and are significant markers for evaluating patients’ nutritional health. Under stressful and critical conditions following surgery, a decrease in plasma proteins is typically observed [36]. In the present study, supplementation with omega-3 support was associated with improvements in albumin levels in patients receiving radiotherapy for GCs. However, a study by Lu et al. [23] in 2022 did not draw such a conclusion. The heterogeneity and small size of that study may be potential biases in the interpretation of the results.
Strengths and limitations
The current umbrella review has several strengths, including the use of comprehensive search strategies with various publications and databases, rigorous quality assessment (risk of bias assessment), and precise statistical analysis. As meta-analyses were pooled in the current studies, they can provide promising and robust evidence on this issue. Moreover, publication-bias assessment and different subgroup analyses contributed to the reliability of the findings. However, there were several limitations, such as the small number of studies, which could have affected the generalizability of the findings. This umbrella review of meta-analyses faced the following limitations: (i) the low number of meta-analyses included in this study affects generalizability to patients struggling with GCs. Also, although funnel plots were presented, the limited number of included studies restricts their interpretability regarding publication bias; (ii) the selected meta-analyses did not separate the various types of omega-3 fatty acids, such as EPA and DHA; (ii) few included studies mentioned the type of omega-3 fatty acids used for patients with GCs; (iv) most of the selected meta-analyses did not designate which omega-3 fatty acids used in their studies are for which stage of cancer treatment, encompassing radiation therapy, chemotherapy, and surgery; and (v) serum proteins, which are used in the present study, are not reliable markers of nutritional status, as inflammation usually affects their levels.
Conclusions
The current umbrella review highlights the potential benefits of omega-3 supplementation for individuals with different GCs. Our findings indicated that omega-3 supplementation is associated with improvements in inflammatory markers (TNF-α, IL-6, and CRP) and immune function (increased CD4+/CD8+ ratio), a reduced hospital length of stay, and a decreased risk of complications.
While these findings are promising, more longitudinal studies are warranted to identify the optimal dosage and proper duration of omega-3 supplementation in GC patients. Moreover, more comprehensive, long-term trials are necessary to validate the accurate benefits and potential associated risks. Overall, omega-3 supplementation can enhance the QOL of individuals with GCs, provided that future studies validate our conclusions.
Acknowledgements
We thank the participants and our colleagues at Shahid Beheshti Medical Science University for their cooperation.
Contributor Information
Hamid Abbasi, Student Research Committee, Faculty of Nutrition and Food Technology, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
Majid Kamali, Nutrition and Food Sciences Research Center, School of Nutrition and Food Sciences, Shiraz University of Medical Sciences, Shiraz, Iran.
Alireza Eftekhar, Department of Medical Physics, Ahvaz Jondy Shapour University of Medical Sciences, Ahvaz, Iran.
Faezeh Tejareh, Tehran Medical Branch, Islamic Azad University, Tehran, Iran.
Amin Paydareh, Faculty of Nutrition Sciences and Food Technology, Department of Clinical Nutrition and Dietetics, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
Mohammad Hassan Naji, Department of Nutrition, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
Zahra Rangraz, Department of Nutrition, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
Zahra Mohamadiyan, Faculty of Medical Sciences, Science and Research Branch, Department of Nutrition, Islamic Azad University, Tehran, Iran.
Farnush Bakhshimoghaddam, Department of Nutrition, School of Allied Medical Sciences, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran.
Ali Shamsi-Goushki, Department of Nutrition, School of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran.
Barbod Alhouei, Department of Community Nutrition, School of Nutrition and Food Sciences, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
Saeid Doaei, Cancer Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran; Unit of Nutrition and Cancer, Cancer Epidemiology Research Program, Catalan Institute of Oncology, Bellvitge Biomedical Research Institute (IDIBELL), L'Hospitalet de Llobregat, Barcelona, Spain.
Marjan Ajami, Department of Food and Nutrition Policy and Planning Research, National Nutrition and Food Technology Research Institute, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
Maryam Gholamalizadeh, Unit of Nutrition and Cancer, Cancer Epidemiology Research Program, Catalan Institute of Oncology, Bellvitge Biomedical Research Institute (IDIBELL), L'Hospitalet de Llobregat, Barcelona, Spain; National Nutrition and Food Technology Research Institute, Faculty of Nutrition Sciences and Food Technology, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
Data availability
The datasets used and/or examined in the present investigation may be obtained from the corresponding author upon reasonable request.
Authors’ contributions
H.A. was responsible for the study’s design, collection and analysis of data, data curation, and drafting of the manuscript. M.K., A.E., M.H.N., Z.R., A.P., F.T., M.A., M.G.H., and S.D. were responsible for the study’s design. Z.M., F.B., B.A., A.S.H.K., and S.D. contributed to the study’s design and provided a critical review of the manuscript. The final manuscript was reviewed and approved by all the authors.
Funding
This study was derived from a project approved by the Ethics Committee of Shahid Beheshti University of Medical Sciences, Tehran, Iran (Ethics code: IR.SBMU.CRC.REC.1403.020). Shahid Beheshti University of Medical Sciences, Tehran, Iran, provided financial support for the investigation [Code 43011537].
Conflicts of interest
The authors affirm that they do not possess any conflicting interests.
References
- 1. Sung H, Ferlay J, Siegel RL et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021;71:209–49. [DOI] [PubMed] [Google Scholar]
- 2. Arnold M, Abnet CC, Neale RE et al. Global burden of 5 major types of gastrointestinal cancer. Gastroenterology 2020;159:335–49. e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bray F, Ferlay J, Soerjomataram I et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- 4. Ebrahimi P, Karami M, Delavari S et al. Investigating the mortality trend of gastrointestinal cancers in Babol, North Iran (2013–2021). BMC Gastroenterol 2024;24:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rubin G, Walter F, Emery J et al. Reimagining the diagnostic pathway for gastrointestinal cancer. Nat Rev Gastroenterol Hepatol 2018;15:181–8. [DOI] [PubMed] [Google Scholar]
- 6. Johnson KD, Perisetti A, Tharian B et al. Endoscopic retrograde cholangiopancreatography-related complications and their management strategies: a “scoping” literature review. Dig Dis Sci 2020;65:361–75. [DOI] [PubMed] [Google Scholar]
- 7. Numico G, Longo V, Courthod G et al. Cancer survivorship: long-term side-effects of anticancer treatments of gastrointestinal cancer. Curr Opin Oncol 2015;27:351–7. [DOI] [PubMed] [Google Scholar]
- 8. Fearon K, Jenkins J, Carli F et al. Patient optimization for gastrointestinal cancer surgery. Br J Surg 2013;100:15–27. [DOI] [PubMed] [Google Scholar]
- 9. Valentini V, Boldrini L, Mariani S et al. Role of radiation oncology in modern multidisciplinary cancer treatment. Mol Oncol 2020;14:1431–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Majeed H, Gupta V. Adverse effects of radiation therapy. 2020. [PubMed]
- 11. Nabavi SF, Bilotto S, Russo GL et al. Omega-3 polyunsaturated fatty acids and cancer: lessons learned from clinical trials. Cancer Metastasis Rev 2015;34:359–80. [DOI] [PubMed] [Google Scholar]
- 12. Djuricic I, Calder PC. Beneficial outcomes of omega-6 and omega-3 polyunsaturated fatty acids on human health: An update for 2021. Nutrients 2021;13:2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kumar S, Malini SS. Omega 3 fish oil suppress radiation induced hepato and renal toxicity in mice through modulation in Wnt canonical pathway combined with NHEJ and Intrinsic Apoptotic pathway. bioRxiv 2023;2023.02.05.527226.
- 14. Little-Letsinger SE, Turner ND, Ford JR et al. Omega-3 fatty acid modulation of serum and osteocyte tumor necrosis factor-α in adult mice exposed to ionizing radiation. J Appl Physiol (1985) 2021;130:627–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Morsy BM, El Domiaty S, Meheissen MA et al. Omega-3 nanoemulgel in prevention of radiation-induced oral mucositis and its associated effect on microbiome: a randomized clinical trial. BMC Oral Health 2023;23:612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Moher D, Liberati A, Tetzlaff J et al. ; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009;151:264–9, W64. [DOI] [PubMed] [Google Scholar]
- 17. Shea BJ, Reeves BC, Wells G et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomized or nonrandomized studies of healthcare interventions, or both. BMJ 2017;358:j4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Higgins JPT, Altman DG, Gøtzsche PC et al. ; Cochrane Statistical Methods Group. The Cochrane Collaboration’s tool for assessing risk of bias in randomized trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yan S, Li M, Yang D et al. Associations between omega-3 fatty acid supplementation and anti-inflammatory effects in patients with digestive system cancer: a meta-analysis. Nutr Cancer 2020;72:1098–114. [DOI] [PubMed] [Google Scholar]
- 20. Jamali M, Zarezadeh M, Jamilian P et al. The effect of n-3 polyunsaturated fatty acids on inflammation status in cancer patients: updated systematic review and dose-and time-response meta-analysis. PharmaNutrition 2024;27:100372. [Google Scholar]
- 21. Xie H, Chang Y-N Omega-3 polyunsaturated fatty acids in the prevention of postoperative complications in colorectal cancer: a meta-analysis. Onco Targets Ther 2016;9:7435–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. de Castro GS, Andrade MF, Pinto FCS et al. Omega-3 fatty acid supplementation and its impact on systemic inflammation and body weight in patients with Cancer Cachexia—A systematic review and Meta-analysis. Front Nutr 2021;8:797513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lu S, Yang Z, Tang H et al. Associations between omega-3 polyunsaturated fatty acids supplementation and surgical prognosis in patients with gastrointestinal cancer: A systematic review and meta-analysis. Food Chem (Oxf) 2022;4:100099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liu H, Chen J, Shao W et al. Efficacy and safety of Omega-3 polyunsaturated fatty acids in adjuvant treatments for colorectal cancer: a meta-analysis of randomized controlled trials. Front Pharmacol 2023;14:1004465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ma Y-J, Liu L, Xiao J et al. Perioperative ω-3 polyunsaturated fatty acid nutritional support in gastrointestinal cancer surgical patients: a systematic evaluation. Nutr Cancer 2016;68:568–76. [DOI] [PubMed] [Google Scholar]
- 26. Yue T, Xiong K, Deng J et al. Meta-analysis of omega-3 polyunsaturated fatty acids on immune functions and nutritional status of patients with colorectal cancer. Front Nutr 2022;9:945590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tantri AR, Sukmono RB, Tobing SDAL et al. Comparing the effect of classical and modified thoracolumbar interfascial plane block on postoperative pain and IL-6 level in posterior lumbar decompression and stabilization surgery. Anesth Pain Med 2022;12:e122174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yu J, Liu L, Zhang Y et al. Effects of omega-3 fatty acids on patients undergoing surgery for gastrointestinal malignancy: a systematic review and meta-analysis. BMC Cancer 2017;17:271–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet 2001;357:539–45. [DOI] [PubMed] [Google Scholar]
- 30. Coussens LM, Werb Z. Inflammation and cancer. Nature 2002;420:860–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lessa RC, Alves FDA, Fortunati E et al. Oral mucositis in cancer and potential use of omega-3 free fatty acids in its management: a review. Biomedicines 2021;9:1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bakker N, van den Helder RS, Stoutjesdijk E et al. Effects of perioperative intravenous ω-3 fatty acids in colon cancer patients: a randomized, double-blind, placebo-controlled clinical trial. Am J Clin Nutr 2020;111:385–95. [DOI] [PubMed] [Google Scholar]
- 33. Marano L, Porfidia R, Pezzella M et al. Clinical and immunological impact of early postoperative enteral immunonutrition after total gastrectomy in gastric cancer patients: a prospective randomized study. Ann Surg Oncol 2013;20:3912–8. [DOI] [PubMed] [Google Scholar]
- 34. Klek S, Kulig J, Sierzega M et al. The impact of immunostimulating nutrition on infectious complications after upper gastrointestinal surgery: a prospective, randomized, clinical trial. Ann Surg 2008;248:212–20. [DOI] [PubMed] [Google Scholar]
- 35. Tsekos E, Reuter C, Stehle P et al. Perioperative administration of parenteral fish oil supplements in a routine clinical setting improves patient outcome after major abdominal surgery. Clin Nutr 2004;23:325–30. [DOI] [PubMed] [Google Scholar]
- 36. Wu J-M, Ho T-W, Lai I-R et al. Parenteral glutamine supplementation improves serum albumin values in surgical cancer patients. Clin Nutr 2021;40:645–50. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or examined in the present investigation may be obtained from the corresponding author upon reasonable request.