Abstract
We have investigated the role of acetylcholine receptors (AChRs) in an early step of postsynaptic assembly at the neuromuscular synapse, the clustering of postsynaptic proteins induced by nerve-released agrin. To achieve this, we used two variants of C2 myotubes virtually lacking AChRs and C2 cells in which surface AChRs were down-regulated by AChR antibodies. In all cases, agrin caused normal clustering of the agrin receptor component MuSK, α-dystrobrevin and utrophin, but failed to aggregate AChRs, α- and β-dystroglycan, syntrophin isoforms and rapsyn, an AChR–anchoring protein necessary for postsynaptic assembly and AChR clustering. In C2 variants, the stability of rapsyn was decreased, whereas in antibody-treated cells, rapsyn efficiently co-localized with remaining AChRs in microaggregates. Upon ectopic injection into myofibers in vivo, rapsyn did not form clusters in the absence of AChRs. These results show that AChRs and rapsyn are interdependent components of a pre-assembled protein complex that is required for agrin-induced clustering of a full set of postsynaptic proteins, thus providing evidence for an active role of AChRs in postsynaptic assembly.
Keywords: acetylcholine receptor/agrin/neuromuscular junction/postsynaptic apparatus/rapsyn
Introduction
Synaptic transmission requires clustering of neurotransmittor receptors in the postsynaptic density (PSD). This process is thought to be mediated by receptoR–anchoring proteins of the PSD, but it is unclear how such anchoring proteins themselves become synaptically localized and how PSDs are assembled during development.
At the neuromuscular junction (NMJ), acetylcholine receptors (AChRs) are clustered by a closely associated 43 kDa protein, rapsyn, which plays a crucial role in postsynaptic assembly, as rapsyn-deficient mice lack differentiated NMJs, clusters of AChRs and most other postsynaptic proteins (Gautam et al., 1995). Another component of the postsynaptic apparatus of the NMJ is the dystrophin/utrophin glycoprotein complex (D/UGC), which includes α- and β-dystroglycan (DG), utrophin, α- and β2-syntrophin and α-dystrobrevin (DB)-1 and is involved in stabilization of AChR aggregates and aspects of synaptic maturation (Sanes and Lichtman, 1999; Jacobson et al., 2001).
AChR clusters are induced by agrin, a neurally released synaptogenic protein (McMahan, 1990). Agrin-deficient mice lack nerve-associated aggregates of AChRs and other postsynaptic components (Gautam et al., 1996) and agrin causes clustering of several synaptic proteins, including AChRs, when added to cultured myotubes (Wallace, 1989). The receptor for agrin includes MuSK, a receptor tyrosine kinase, which is activated in response to agrin (Glass et al., 1996). Activated MuSK triggers a signaling mechanism that, besides other mediators, also involves Src-family kinases and tyrosine phosphorylation of AChR β subunits (Borges and Ferns, 2001; Mittaud et al., 2001; Mohamed et al., 2001).
However, the actual steps that follow MuSK activation and cause aggregation of postsynaptic proteins are still poorly understood. The available evidence suggests a sequence of events in which MuSK is clustered first by agrin, followed by rapsyn and then other postsynaptic proteins including AChRs (Sanes and Lichtman, 1999). This sequence is based on the observations that, first, MuSK is still clustered at mutant synaptic sites of rapsyn–/– mice (Apel et al., 1997) and that constitutively active MuSK organizes postsynaptic specializations when expressed ectopically in wild-type muscle fibers (Jones et al., 1999), consistent with a role as a primary synaptic scaffold. Secondly, rapsyn induces aggregation of AChRs, DG, MuSK and of itself when expressed in heterologous cells (Froehner et al., 1990; Phillips et al., 1991; Apel et al., 1995; Gillespie et al., 1996), suggesting that rapsyn is a general mediator of protein clustering by forming intracellular aggregates and by recruiting other proteins to the MuSK scaffold. Together, these data suggest that AChRs are passively added to the growing postsynaptic apparatus in a later step.
This sequence of events would predict that AChRs interact with postsynaptic proteins, most notably rapsyn, only in clusters after agrin treatment. However, studies that examined protein interactions with the AChR in myotubes revealed that AChRs bind to rapsyn, tyrosine kinases and other postsynaptic proteins independently of agrin, suggesting that diffusely distributed AChRs can act as a scaffold to pre-assemble postsynaptic components (Fuhrer et al., 1999). Further, AChRs co-localize with rapsyn and some other postsynaptic proteins in post-Golgi transport vesicles in Torpedo electric organ, implying that these proteins are inserted into the plasma membrane (and subsequently into clusters) as a pre-assembled complex (Marchand et al., 2001). These experiments suggest that AChRs are added early to the growing postsynaptic apparatus and imply an active functional role of AChRs in synaptic assembly. Consistent with this, no neuron- or agrin-induced clusters of acetylcholinesterase were observed in rat myotubes in which AChRs were down-regulated by treatment with AChR antibodies (De La Porte et al., 1998).
Therefore, in order to investigate the steps of postsynaptic assembly at the NMJ, we have examined the role of AChRs in agrin-induced clustering of postsynaptic proteins. We used two variants of C2 myotubes virtually lacking AChRs, as well as C2 cells in which surface AChRs were down-regulated by AChR antibodies. In all three cellular systems, agrin failed to cause clustering of AChRs, DGs, syntrophins, phosphotyrosine-containing proteins and rapsyn, whereas normal clustering was observed for MuSK, α-DB-1 and utrophin. In addition, ectopically injected rapsyn did not form clusters in the absence of AChRs in myofibers in vivo. These data show that the AChR is necessary to link together a full set of postsynaptic proteins, most likely by forming pre-assembled complexes with rapsyn, revealing an active role for AChRs in postsynaptic organization.
Results
Characterization of R– cells
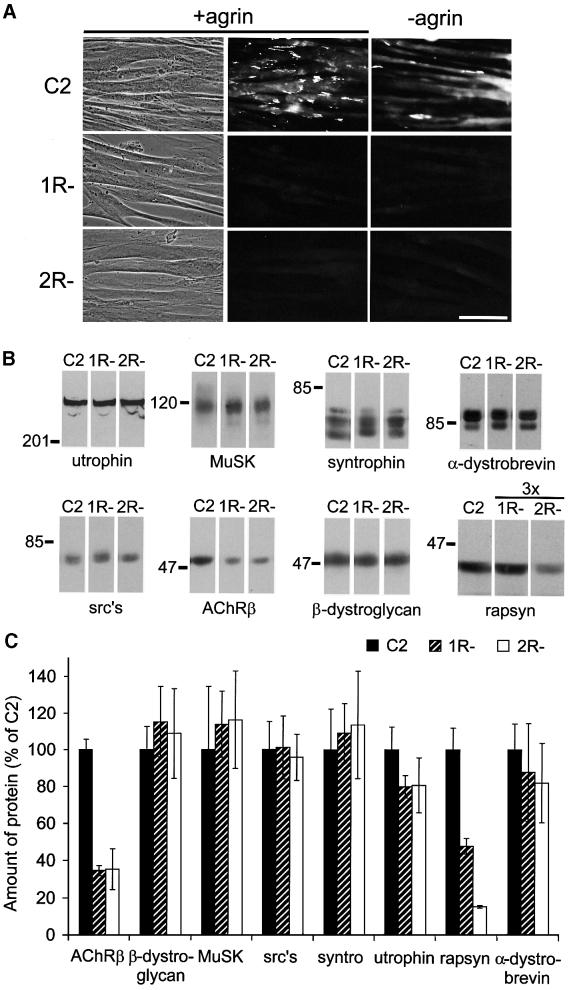
To study agrin-induced clustering of postsynaptic proteins in the absence of AChRs, we first characterized two independent clonal genetic variants of C2, 1R– and 2R– cells. These cells express very low amounts of AChR α subunits, fail to bind α-bungarotoxin (btx) and virtually lack surface AChRs (Black and Hall, 1985; Black et al., 1987). They formed myotubes morphologically indistinguishable from C2, but lacked AChR clusters even after treatment with agrin (Figure 1A). In contrast, agrin induced significant AChR clustering in C2. We analyzed steady-state levels of AChRs by immunoblotting in the variants. Similarly to previous reports (LaRochelle et al., 1989), AChR β subunits were reduced to 35% in R– cells (Figure 1B and C).
Fig. 1. Characterization of 1R– and 2R– myotubes. (A) Cells were incubated with neural agrin, stained with rhodamine–btx and analyzed by fluorescence microscopy or phase contrast (left). Scale bar, 50 µm. (B) Myotube lysates were analyzed by immunoblotting using antibodies against the indicated proteins. For rapsyn, 3-fold higher protein amounts were analyzed for 1R- and 2R- than for C2 cells, to allow densitometric quantitation. src’s, Src-related kinases. (C) Immunoblots were quantitated by densitometry, using C2 as controls (100%). Data represent mean ± SD of five experiments. Levels of postsynaptic proteins are similar in all three cell lines, apart from a reduction in AChR β and rapsyn in 1R– and 2R– cells.
We then asked whether the defect in the variants concerns only the AChR, by analyzing steady-state levels of several other postsynaptic proteins. Utrophin, MuSK, α-, β1- and β2-syntrophin isoforms, α-DB-1, Src-related kinases and β-DG were all present in normal amounts in R– cells, whereas rapsyn was reduced to 48% in 1R– and to 15% in 2R– cells (Figure 1B and C). The defect in the variants appears thus to be restricted to AChRs and rapsyn. Furthermore, we asked how the synthesis of AChRs and rapsyn is affected in R– cells. As shown previously for the AChR α subunit (Black et al., 1987), synthesis of both AChR α and β (but not creatine phosphokinase) was strongly reduced in 1R– cells, as assessed by [35S]methionine labeling (see Supplementary figure 1 available at The EMBO Journal Online). In contrast, rapsyn was synthesized normally in 1R– cells, but degraded more rapidly than in C2. Finally, in a differential sedimentation assay, mRNA encoding the AChR α subunit sedimented exclusively with the polysomal fraction in 1R– cells, as in C2 (see Supplementary figure 1).
Thus, the defect in 1R– cells appears to reside at some point in translational elongation of AChR polypeptides, leading to the virtual absence of steady-state surface AChRs. Newly synthesized rapsyn is rapidly degraded in variants, providing evidence that AChRs are required to stabilize rapsyn in myotubes.
Protein clustering in R– myotubes
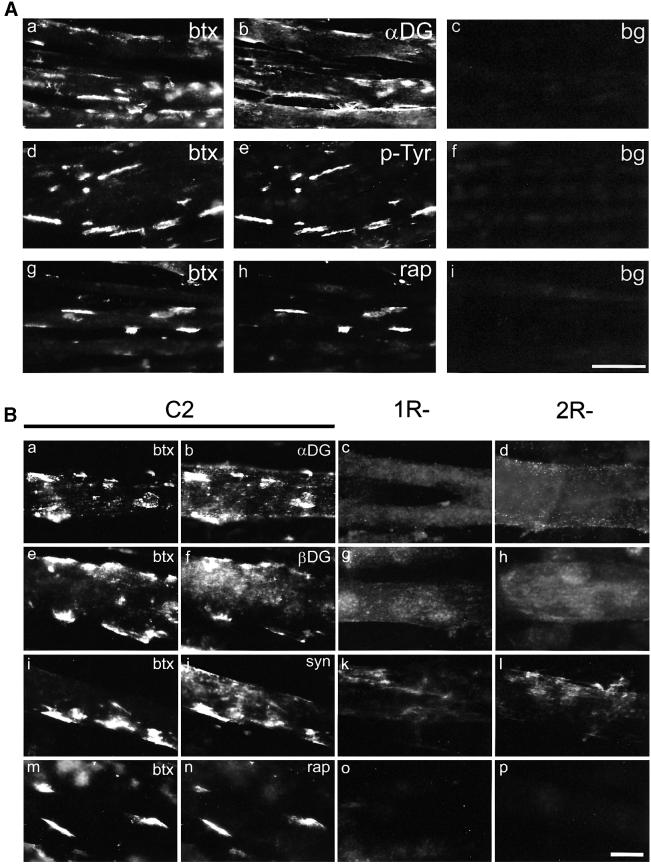
To study clustering of postsynaptic proteins, we next optimized immunocytochemical staining procedures in agrin-treated C2 cells, including binding of the lectin VVAB4, which interacts with synaptic carbohydrates containing N-acetylgalactosamine (Martin and Sanes, 1995). α-DG, β-DG, rapsyn, syntrophin, MuSK, α-DB-1, utrophin and VVAB4-binding proteins all co-localized largely with AChRs in typical bright, large clusters (10–30 µm in length), whereas phosphotyrosine was mostly observed in similar clusters, but occasionally also in less intense and smaller aggregates (∼5 µm) lacking AChRs (Figures 2 and 3A). Quantitation revealed that 74.5 ± 8.0% (mean ± SD, five experiments) of MuSK clusters in agrin-treated cells co-localized with AChRs. For α-DB-1 and utrophin, this degree of co-localization was 77.4 ± 4.0% and 73.3 ± 11.7%, respectively. In C2 myotubes not treated with agrin, postsynaptic proteins were clustered to a significantly lower degree, but still mostly co-localized with AChRs (Figure 3B and data not shown).
Fig. 2. Agrin does not induce clustering of α- and β-DG, syntrophins and rapsyn in 1R– and 2R– myotubes. (A) Agrin-treated C2 myotubes were double-labeled with rhodamine–btx (a, d and g) and antisera against α-DG (b), phosphotyrosine (e) or rapsyn (h), followed by FITC-secondary antisera. For c, f and i, primary antibodies were omitted (bg, background). α-DG, phosphotyrosine and rapsyn co-localize with AChRs in clusters. Scale bar, 50 µm. (B) Agrin-treated cells were double-labeled with rhodamine–btx (a, e, i and m) and antibodies recognizing α-DG (b, c and d), β-DG (f, g and h), syntrophin isoforms (j, k and l) or rapsyn (n, o and p). In 1R– and 2R– myotubes, clusters of DGs, syntrophin isoforms, rapsyn and AChRs (not shown) are not detectable, whereas these proteins co-localize with AChRs in C2 cells. Scale bar, 20 µm.
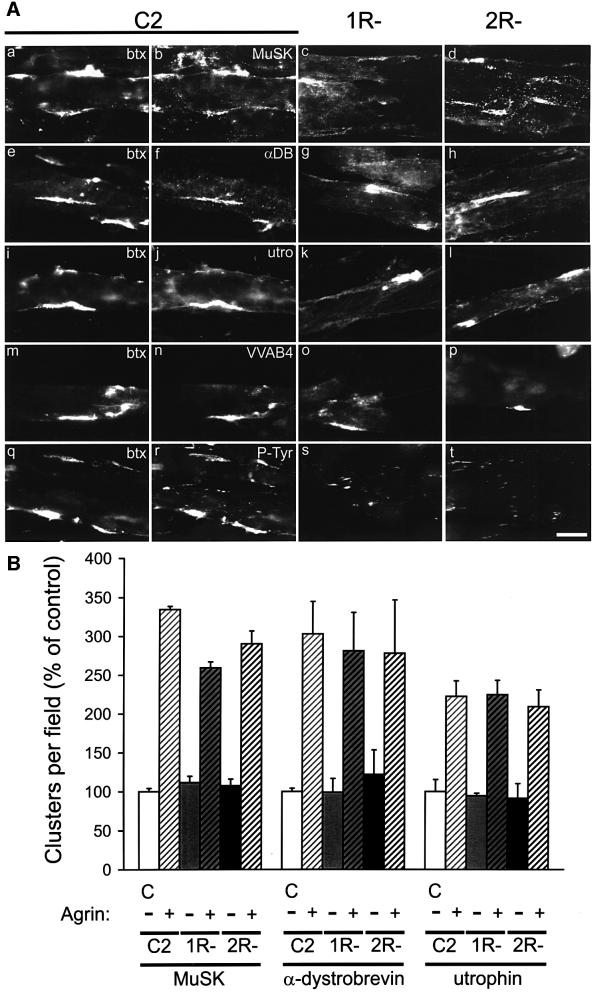
Fig. 3. MuSK, α-DB-1, utrophin and VVAB4-binding proteins are clustered by agrin in 1R– and 2R– myotubes. (A) Agrin-treated myotubes were double-labeled with rhodamine-btx (a, e, i, m and q) and antibodies against MuSK (b, c and d), α-DB-1 (f, g and h), utrophin (j, k and l) or phosphotyrosine (r, s and t). In n, o and p, cells were incubated with FITC-VVAB4 lectin. In 1R– and 2R– myotubes, postsynaptic markers (except phosphotyrosine) form clusters of similar morphology to those in C2, even though AChR aggregates are not detectable (btx not shown). Scale bar, 20 µm. (B) Clusters of MuSK, α-DB-1 and utrophin are shown as % of untreated C2 (C; mean ± SEM of three experiments). Within each staining, values of agrin-treated cells differ significantly from the respective untreated cells (p <0.05, by ANOVA followed by pair-wise Bonferroni’s t-tests), but not from each other (p >0.15).
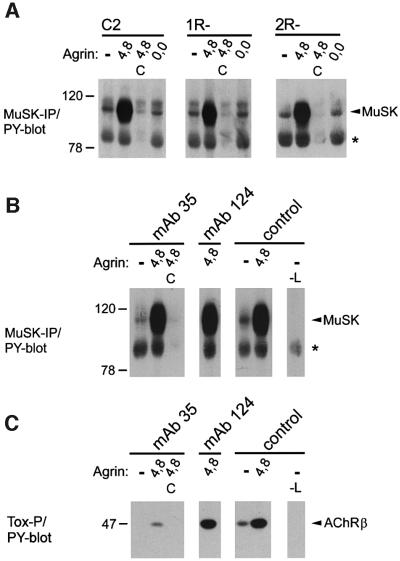
We then applied these procedures to R– myotubes and found no detectable clusters of α- and β-DG, syntrophins or rapsyn in agrin-treated 1R– and 2R– cells (Figure 2B). In contrast, similarly to C2, 1R– and 2R– myotubes formed agrin-induced aggregates of MuSK, α-DB-1, utrophin and VVAB4-binding proteins, even though the variants lacked any detectable AChR clusters (Figure 3A). The size, morphology and intensity of these clusters were indistinguishable from C2. In the variants, as occasionally in C2, phosphotyrosine-containing proteins were detected in small aggregates, ∼5 µm in length. Quantitation revealed that the number of spontaneous and agrin-induced aggregates of MuSK, α-DB-1 and utrophin per field was not significantly different between 1R–, 2R– and C2 cells (Figure 3B), indicating that agrin fully maintained its effect on clustering of these proteins in variant cells. To investigate whether agrin signaling is affected in R– cells, we examined MuSK activation by agrin. Neural agrin caused identical tyrosine phosphorylation of MuSK in R– cells when compared with C2 (Figure 4A).
Fig. 4. Agrin causes normal tyrosine phosphorylation of MuSK in R- and mAb35-treated C2 myotubes. Cells were treated for 40 min with 0.5 nM neural (4,8) or muscle (0,0) agrin. Lysates were split into two parts and analyzed either by MuSK immunoprecipitation (A and B) or by precipitation with biotinylated btx (C), followed by phosphotyrosine immunoblotting. (A) In 1R– and 2R– cells, MuSK is activated by neural, but not muscle, agrin as in C2. As controls, MuSK antibodies were omitted (C). The asterisk denotes a protein band originating from MuSK antibodies. (B) C2 myotubes were incubated with mAb35, mAb124 or control media, followed by addition of agrin as in (A). As controls, the lysate (–L) or MuSK-precipitating antibody (C) was omitted. MuSK is activated equally by neural agrin in cells treated with or without antibodies. (C) Significant AChR β subunit phosphorylation is observed in cells treated with mAb35 and neural agrin. As controls, the lysate (–L) was omitted, or an excess of free btx was added (C).
Together, these results show that in 1R– and 2R– cells, AChRs are virtually absent and the stability of rapsyn is substantially decreased. Under these circumstances, agrin causes normal MuSK activation and MuSK, α-DB-1, utrophin and VVAB4-binding proteins form normal spontaneous and agrin-induced clusters. In contrast, comparable aggregates of phosphotyrosine, DGs, syntrophins and rapsyn are lacking.
C2 cells treated with mAb35 do not form agrin-induced AChR clusters
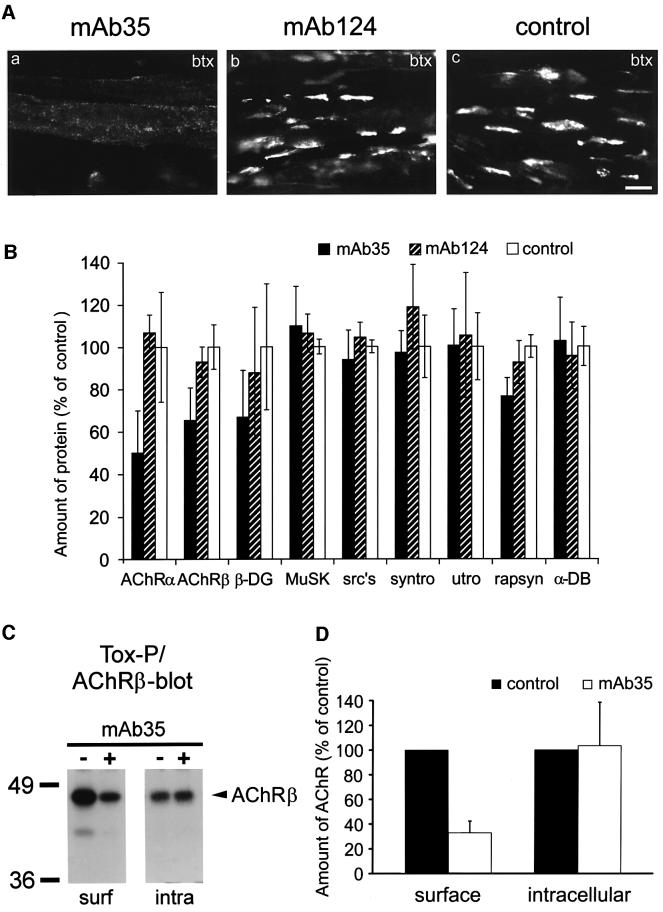
We intended to analyze agrin-induced protein clustering in the absence of aggregated AChRs by an independent alternative approach. For this purpose, surface AChRs of C2 cells were down-regulated by a monoclonal antibody, mAb35, which reacts with the main immunogenic region of the AChR α subunit and causes internalization and degradation of AChRs (De La Porte et al., 1998). In mAb35-treated C2, no agrin-induced AChR clusters were visible, while AChR aggregation was normal in the presence of mAb124, a control monoclonal antibody reactive with the intracellular portion of the AChR β subunit (Figure 5A). The remaining surface AChRs in mAb35-treated cells, if detectable, were visible as round microaggregates of <1 µm in size.
Fig. 5. mAb35-treated C2 myotubes do not form agrin-induced AChR clusters. (A) Antibody-treated and control C2 myotubes were incubated with agrin and stained with rhodamine–btx. In mAb35-treated cells, agrin fails to induce AChR clustering and the remaining surface AChRs are often organized in microaggregates of <1 µm in size. (B) Quantitation of postsynaptic proteins in cellular extracts of antibody-treated cells by immunoblotting (mean ± SD, six experiments). mAb35 reduces the cellular content of the AChR α and β subunits, without strongly affecting other proteins. (C) Surface or intracellular AChRs were isolated and analyzed by AChR β immunoblotting and densitometric scanning. (D) Signals of surface and intracellular AChR pools of untreated cells were set to 100% (mean ± SEM, three experiments). mAb35 reduces surface, but not intracellular, AChRs. Scale bar, 20 µm.
To further establish the consequences of mAb35 treatment, we analyzed steady-state levels of postsynaptic components by immunoblotting. As expected, AChR α and β subunits were reduced in mAb35-treated cells (to 50% and 65% of controls, respectively), whereas rapsyn was marginally affected (76% remaining; Figure 5B). All other proteins examined were not significantly affected. Since rapsyn is an abundant component of myotubes, occurring in roughly equimolar amounts with the AChR (LaRochelle and Froehner, 1987), its residual level in mAb35-treated cells represents a high molar amount of protein. To examine whether mAb35 reduces surface or intracellular AChRs, we isolated either surface or intracellular AChRs from antibody-treated cells as described in Materials and methods. Control experiments established that the size of the intracellular AChR pool in untreated cells is ∼35% of the surface pool (not shown). After mAb35 treatment, surface AChRs were strongly reduced to 33%, whereas levels of intracellular receptors were not affected (Figure 5C and D). These intracellular AChRs most likely represent newly synthesized receptors on their way to the surface, because intracellular AChRs are found mainly in the endoplasmic reticulum and Golgi apparatus (Gu et al., 1989).
To assess the fate of mAb35-bound surface AChRs, cells were treated with the antibody and then with cycloheximide to prevent new protein synthesis. Under these circumstances, no surface AChRs, not even microaggregates, were visible (see Supplementary figure 2A), showing that mAb35-bound surface AChRs are targeted for endocytosis and degradation. The microaggregates seen after normal mAb35 treatment (Figure 5A) thus appear to reflect AChRs redistributed to undergo endocytosis as well as newly synthesized AChRs inserted into the plasma membrane. Indeed, after blocking the surface with btx, newly inserted receptors (visualized by rhodamine–btx) appeared as microaggregates (see Supplementary figure 2B).
Finally, in mAb35-treated cells, neural agrin caused normal tyrosine phosphorylation of MuSK (Figure 4B) and also significant phosphorylation of the remaining AChR β subunits (Figure 4C). Thus, using mAb35, we had developed a system to specifically down-regulate surface AChRs to prevent agrin-induced clustering of AChRs in C2 myotubes, without interfering with agrin signaling and without dramatically affecting the protein amounts of other postsynaptic components.
Protein clustering in mAb35-treated cells
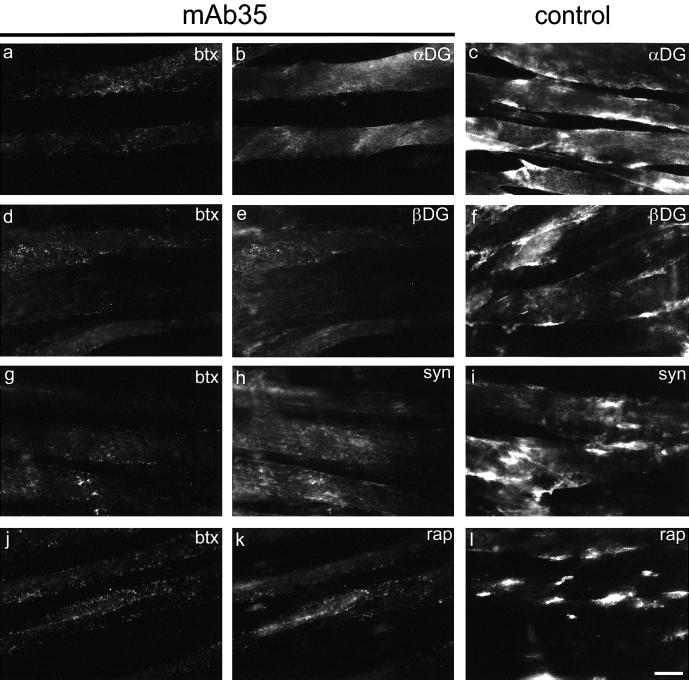
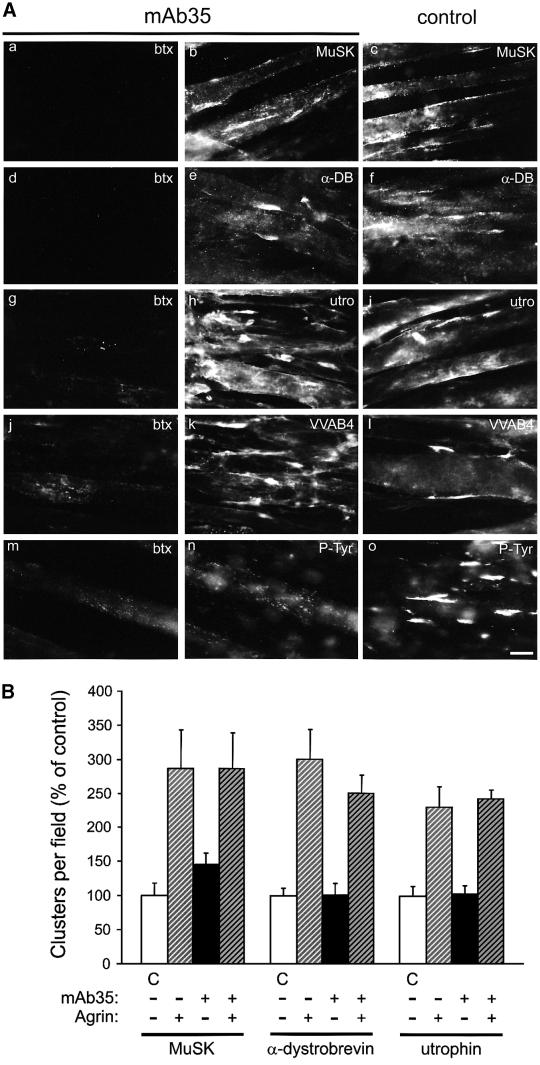
We applied mAb35 to analyze agrin-induced clustering of postsynaptic proteins in the absence of aggregated AChRs in C2 myotubes. Under these circumstances, no agrin-induced clusters of rapsyn, α- and β-DG, syntrophin isoforms or tyrosine phosphorylated proteins were detected (Figures 6 and 7A). After mAb35 treatment, rapsyn and, to a less pronounced degree, DGs, tyrosine phosphorylated proteins and syntrophins, were sometimes found in microaggregates of similar size and distribution to AChR microaggregates. In contrast, mAb35-treated cells, although lacking AChR clusters, were still able to form large agrin-induced aggregates of MuSK, α-DB-1, utrophin and VVAB4-binding proteins (Figure 7A). The size, morphology and intensity of these clusters were indistinguishable from controls. Furthermore, the numbers of spontaneous and agrin-induced clusters of MuSK, α-DB-1 and utrophin were identical in antibody-treated and control cells (Figure 7B), demonstrating that agrin fully maintained its effect on clustering of these proteins in mAb35-treated cells.
Fig. 6. mAb35-treated C2 myotubes do not form agrin-induced clusters of α- and β-DG, syntrophins and rapsyn. Antibody-treated and control C2 cells were incubated with neural agrin. Surface AChRs and postsynaptic markers were detected by double-labeling with rhodamine-btx and the respective antisera. The middle and left sections indicate the same cells under fluorescein and rhodamine optics (btx), respectively. No clusters of α-DG (b), β-DG (e), syntrophins (h) or rapsyn (k) are detected in mAb35-treated cells, unlike control cells, which contain co-aggregates of these proteins (c, f, i and l) and AChRs (btx not shown). Scale bar, 20 µm.
Fig. 7. MuSK, α-DB-1, utrophin and VVAB4-binding proteins form agrin-induced clusters in mAb35-treated C2 myotubes. (A) mAb35-treated and control cells were incubated with agrin and double-labeled with rhodamine-btx (btx) and the respective antibodies or VVAB4. Even though mAb35-treated cells lack AChR clusters (a, d, g, j and m), they form clusters of MuSK (b), α-DB-1 (e), utrophin (h) and VVAB4-binding proteins (k). Scale bar, 20 µm. (B) Clusters are shown as mean ± SEM of three experiments (C, control). Within each staining, values of agrin-treated samples differ significantly from untreated cells (p <0.05, by ANOVA followed by pair-wise Bonferroni’s t-tests), but not from each other (p >0.4).
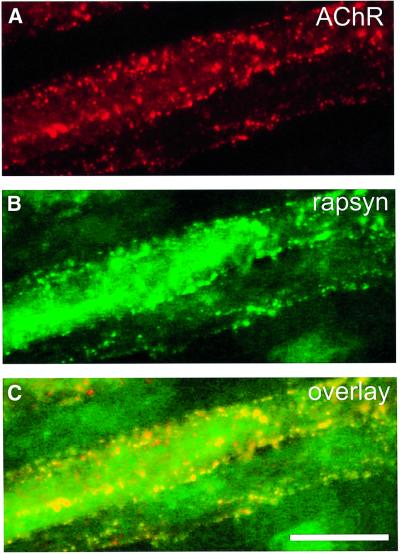
To determine whether the dotted distribution of rapsyn in mAb35-treated cells (Figure 6) represents true co-localization with remaining AChRs in microaggregates, we merged digitized pictures. In the resulting overlays, microaggregates of rapsyn indeed co-localized to a high degree with AChRs and therefore appeared yellow (Figure 8).
Fig. 8. AChRs and rapsyn co-localize in microaggregates in mAb35-treated C2 myotubes. Cells were treated with mAb35 and agrin, double-labeled for AChRs (A) and rapsyn (B) and examined at higher magnification. An overlay of both pictures (C) reveals a high degree of co-localization in microaggregates, as shown in yellow. Scale bar, 20 µm.
These results show that clustering of the AChR is necessary for DGs, syntrophins, tyrosine phosphorylated proteins and rapsyn to form agrin-induced aggregates. In contrast, MuSK, α-DB-1, utrophin and VVAB4-binding proteins cluster independently of AChR aggregation. Interestingly, in mAb35-treated cells, rapsyn, although present in abundant amounts, is not clustered by agrin but follows the distribution of AChRs.
Ectopically injected rapsyn does not form clusters in myofibers in the absence of AChRs
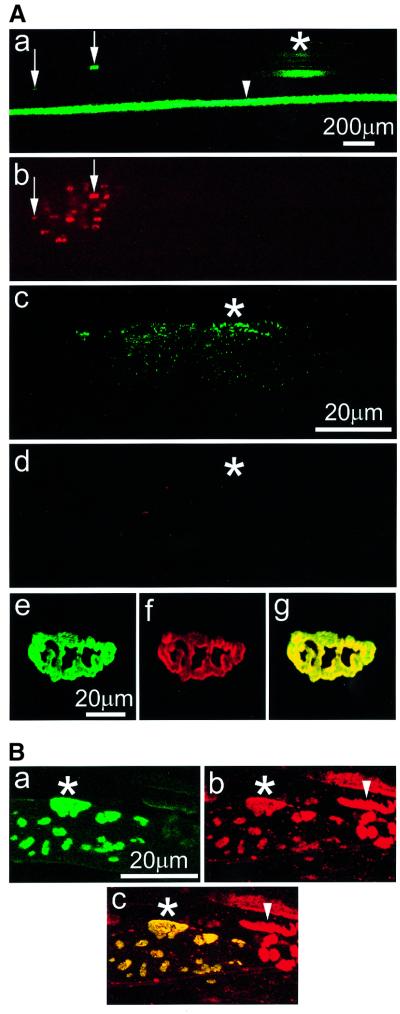
To confirm these data in vivo, we ectopically injected adult rat muscle fibers with a rapsyn–green fluorescent protein (GFP) expression construct as described by Sander et al. (2000). At the injection site, rapsyn–GFP was expressed but failed to form typical clusters as assessed by confocal microscopy (Figure 9A, part c). However, rapsyn–GFP was transported from the injection site to the NMJ of the injected fibers, where it was efficiently incorporated into AChR clusters (Figure 9A, parts a and e).
Fig. 9. Ectopically expressed rapsyn–GFP does not form clusters in the absence of AChRs in myofibers in vivo. (A) Rat muscle fibers were injected ectopically with a rapsyn–GFP expression construct. After 21 days, fibers were excised and stained with rhodamine–btx. From five rats, 44 fibers expressing rapsyn–GFP were analyzed, first at low magnification in fluorescein (a) and rhodamine (b) channels. Rapsyn–GFP is detectable at the injection site (a, asterisk) and is transported to the NMJs of the injected fibers (a, arrows) where it co-localizes with AChRs (b, arrows). A parallel control fiber was injected with nGFP DNA (a, arrowhead). nGFP diffuses widely, showing that ectopically injected gene products can be transported over long distances within myofibers. At the ectopic injection site of rapsyn–GFP, integration of a series of confocal images taken at higher magnification shows that neither rapsyn (c) nor AChRs (d) form clusters (asterisks). In contrast, at NMJs of these injected fibers, confocal analysis shows co-localization of rapsyn–GFP (e) and AChRs (f; g is an overlay of e and f). (B) Rat muscle fibers were injected ectopically with rapsyn–GFP and agrin expression constructs and analysed by confocal microscopy as above. At the injection site (asterisks), rapsyn–GFP is found in clusters (a) that co-localize with AChRs (b). In the overlay, these clusters appear yellow (c). AChR clusters in adjacent fibers, produced through agrin secretion from the injected fibers, do not contain rapsyn–GFP (arrowheads in b and c).
In parallel studies, we co-injected myofibers ectopically with expression constructs for both rapsyn–GFP and agrin (Figure 9B). Ectopically expressed agrin is known to induce expression and clustering of AChRs, even in innervated muscle (Jones et al., 1997). Accordingly, we observed typical ectopic clusters of AChRs that co-localized with rapsyn–GFP clusters in the injected fibers (Figure 9B). These data demonstrate that in the absence of AChRs, rapsyn cannot form clusters in myofibers in vivo, similarly to our findings with cultured myotubes. When synthesis and clustering of AChRs are induced by agrin, however, rapsyn–GFP is recruited into AChR clusters.
Discussion
In this study, we show that agrin-induced clustering of a full set of postsynaptic proteins requires the presence of clustered complexes of AChRs and rapsyn at the surface of myotubes. Whereas MuSK, α-DB-1, utrophin and VVAB4- binding proteins cluster independently of the AChR, aggregation of α-DG, β-DG, syntrophin isoforms, phosphotyrosine-containing proteins and rapsyn requires the presence of AChRs, indicating a functional role of AChRs in postsynaptic assembly.
R– cells as model systems
1R– and 2R– cell variants are valuable to study the role of AChRs in protein clustering, as they virtually lack surface AChRs due to a specific defect in AChR translation. All mRNAs examined are normal in the variants and recruited into polysomes, but R– cells fail to synthesize AChR subunits. As a consequence, levels of AChR subunits are reduced in steady state as shown by immunoblotting, and functional AChRs are virtually absent from the cell surface as shown by btx binding (Black and Hall, 1985). The specificity in the defect is further illustrated by rapsyn, which is synthesized normally but degraded rapidly in 1R– cells. This suggests that functional AChRs are necessary to stabilize rapsyn. Moreover, all other postsynaptic proteins examined were present in normal amounts. Finally, the fact that two independent cell lines, 1R– and 2R–, behaved identically in all of our clustering assays strongly suggests that our findings originate from the common primary defect of the cells (a reduction in AChRs) and not secondary defects.
While the absence of AChRs leads to enhanced degradation of rapsyn, AChRs are likewise metabolically stabilized by rapsyn (Wang et al., 1999). Since the two proteins can be co-precipitated and associate independently of agrin signaling in C2 myotubes (Fuhrer et al., 1999), at least some AChRs and rapsyn are always interdependent components of a pre-assembled protein complex, thereby stabilizing each other.
Crucial role of AChR–rapsyn complexes
To reconfirm the results obtained from R– cells, we treated C2 myotubes with mAb35, which caused specific and reversible down-regulation of surface AChRs. We observed identical agrin-induced clustering of postsynaptic proteins in mAb35-treated C2 myotubes to those in 1R– and 2R– cells: in all three cellular systems, rapsyn, α-DG, β-DG, syntrophin isoforms and phosphotyrosine-containing proteins did not form agrin-induced aggregates, whereas MuSK, α-DB-1, utrophin and VVAB4-binding proteins formed clusters independently of the AChR.
Is this reduction in clustering of postsynaptic proteins due to the absence of AChRs or rather rapsyn? Our results suggest that the critical parameter is the absence of clusters of pre-assembled AChR–rapsyn complexes at the surface of myotubes. Thus, the common element between 1R–, 2R– and mAb35-treated C2 myotubes is, in fact, the absence of these clusters. Further, our present and previous data provide strong evidence that rapsyn exists in a free pool as well as in an AChR–associated pool and that free rapsyn cannot form clusters in the absence of AChRs. The free pool of rapsyn is illustrated, first, by the fact that in R– cells, AChR α subunits and therefore functional oligomeric AChRs are almost completely absent (5% remaining; Black et al., 1987), while the amount of rapsyn is significantly higher (15–48% remaining). Thus, rapsyn can exist in the absence of AChRs in myotubes, although it is less stable. Secondly, treatment with mAb35 reduced the amount of functional AChRs more strongly (50% remaining) than the amount of rapsyn (76% remaining), suggesting that about half of the total rapsyn pool is associated with the AChR. Finally, we did not observe all of rapsyn in a complex with AChRs in co-precipitation experiments (Fuhrer et al., 1999). However, the free pool of rapsyn cannot form agrin-induced clusters in R– and mAb35-treated cells. Therefore, the crucial pool of rapsyn that triggers its own clustering (and clustering of other proteins) is that associated with the AChR and these pre-assembled complexes of AChR and rapsyn appear to be nucleation sites for clusters of postsynaptic proteins.
Accumulating evidence suggests that these pre-assembled complexes are co-transported through part of the secretory pathway and inserted into the plasma membrane as a unit. For example, rapsyn and AChRs are co-isolated in post-Golgi vesicles of Torpedo electric organ (Marchand et al., 2001) and we have recently observed biochemical interactions between rapsyn and intracellular AChRs (M.Moransard and C.Fuhrer, unpublished observations). Furthermore, rapsyn and AChRs co-localize in microaggregates at the surface of mAb35-treated myotubes and part of these AChR microaggregates reflect newly inserted receptors. If pre-assembled AChR–rapsyn complexes at the surface of myotubes are abolished, as is the case in R– cells or in mAb35-treated cells, the overall amount of rapsyn is reduced, although not as strongly as AChRs, and clustering of a full set of postsynaptic proteins is no longer observed.
This reveals a fundamental difference in the mechanism of rapsyn clustering in heterologous cells as opposed to endogenous rapsyn in myotubes. In heterologous systems, rapsyn clusters itself independently of AChRs and forms co-extensive aggregates with the AChR when both proteins are expressed, suggesting that rapsyn first multimerizes to form aggregates and then recruits AChRs into these clusters (Froehner et al., 1990; Phillips et al., 1991). In contrast, our studies lead to the conclusion that in myotubes, AChRs and rapsyn are interdependent components of a protein complex in which both are necessary for optimal stability and for agrin-induced clustering. Consistent with this, ectopically expressed rapsyn is not able to form clusters in the absence of AChRs in myofibers in vivo.
Aggregation of postsynaptic proteins in the absence of clustered AChRs
Formation of the postsynaptic apparatus is likely to be a multi-step process, in which some proteins play a role while diffusely distributed (e.g. by signaling), while others need to be aggregated to be functional. We can analyze these issues by comparing mAb35-treated myotubes, where some proteins are present but not clustered, with knockout mice lacking these proteins. For example, DG is not clustered at NMJs of rapsyn-deficient mice (Gautam et al., 1995) as well as in mAb35-treated C2 myotubes, indicating that clustering of DG not only requires the presence of rapsyn, but clusters of rapsyn and AChRs. Conversely, in DG-deficient myotubes, agrin-induced aggregation of AChRs is still observed (Grady et al., 2000) and DG appears to act downstream in AChR clustering, by linking some components of the D/UGC to AChR aggregates (Jacobson et al., 2001). α-DG also recruits perlecan and acetylcholinesterase to AChR clusters (Jacobson et al., 2001), consistent with the observation that acetylcholinesterase is not aggregated in 1R– myotubes (De La Porte et al., 1998).
Similarly, syntrophin requires clustered AChR–rapsyn complexes to form aggregates, as shown by the absence of syntrophin clusters in our experiments and at NMJs of rapsyn-deficient mice (Gautam et al., 1995). Conversely, AChRs are still concentrated at NMJs of mice lacking α-syntrophin (Adams et al., 2000). Utrophin, in contrast, formed clusters in our assays, but is not clustered at mutant NMJs of mice lacking rapsyn or α-syntrophin, nor in agrin-treated myotubes lacking DG (Gautam et al., 1995; Adams et al., 2000). Clustering of utrophin thus requires the presence, but not aggregation, of these three proteins. Similarly, α-DB-1 was clustered in our assays but not in cultured DG–/– myotubes (Grady et al., 2000), showing that α-DB-1 requires the presence, but not clusters, of DG to form aggregates. AChR–independent clustering of utrophin and α-DB-1 may occur at the plasma membrane, implying another membrane anchor for the UGC than β-DG. Alternatively, as utrophin and α-DB-1 are part of the subsynaptic cytoskeleton, their AChR–independent aggregation may occur at some distance from the membrane.
Besides subsynaptic proteins, extracellular components can also aggregate independently of clustered AChRs, as visualized by staining with VVAB4 lectin. We do not know which glycoproteins are recognized in clusters by VVAB4 in our cultures, but can rule out α-DG, which is known to bind to VVAB4 (Grow et al., 1999), based on the diffuse distribution of DG in variant and mAb35-treated myotubes.
MuSK, in turn, was clustered in our assays and even in the absence of rapsyn, as seen in rapsyn–/– mice (Apel et al., 1997), consistent with its role as a primary synaptic scaffold. Activation of MuSK leads to autophosphorylation and to tyrosine phosphorylation of AChRs. The absence of large phosphotyrosine clusters in R– cells suggests that phosphotyrosine clustering mostly reflects phosphorylated AChRs. However, as agrin-treated R– cells contain many small phosphotyrosine clusters (5 µm), further proteins seem to be tyrosine phosphorylated due to agrin and may play a role in formation of large AChR clusters.
Recently, results partially consistent with ours were reported by Grow and Gordon (2000). In these studies, 1R– cells had a different morphology from that of C2 and displayed low-frequency clustering of MuSK and β-DG which was not increased by a 6 h agrin treatment. In contrast, our 1R– cultures were morphologically indistinguishable from C2 and treated with agrin for at least 15 h. While we cannot rule out that these differences might have contributed to the different experimental outcome, it is noteworthy to emphasize that our results were reconfirmed in 2R– cells, as well as by using mAb35-treated C2 cells.
Roles of AChRs in synaptic development
Several lines of evidence suggest that our in vitro observations also pertain to synaptic development in vivo. First, ectopically expressed rapsyn is not able to form clusters in the absence of AChRs in myofibers in vivo, whereas it is efficiently recruited to AChR clusters at existing NMJs (Figure 9), similar to our findings with cultured myotubes. Furthermore, in zebrafish mutants lacking functional AChRs, NMJs are formed and appear ultrastructurally normal (Westerfield et al., 1990), but lack synaptic rapsyn (Ono et al., 2001), showing that AChRs are required for synaptic clustering of rapsyn. In addition, in AChR ε subunit-deficient mice aged 2 months, AChRs and rapsyn are strongly reduced in parallel at NMJs, whereas agrin, α-DB and utrophin are still efficiently clustered at these mutant NMJs (Schwarz et al., 2000). Thus, the synaptic densities of α-DB and utrophin, but not rapsyn, can be regulated independently of AChRs at agrin-containing NMJs, similar to the clustering of these proteins in our agrin-treated myotubes. Finally, proteins that require AChRs to form agrin-induced clusters in cultured myotubes (i.e. rapsyn, β-DG and syntrophin) are co-transported with AChRs to the postsynaptic membrane in post-Golgi transport vesicles in Torpedo electric organ (Marchand et al., 2001). Dystrophin and α-DB are not found on these vesicles and thus reach the postsynaptic membrane independently of AChRs and the secretory pathway in Torpedo (Marchand et al., 2001), in analogy with the AChR–independent clustering of utrophin and α-DB in cultured myotubes.
In summary, our findings demonstrate that AChRs play an early and active role in agrin-induced postsynaptic organization, at least in part by forming pre-assembled complexes with rapsyn. Due to agrin, these complexes are most likely linked to a primary synaptic scaffold formed by MuSK. The resulting AChR aggregates enable co-clustering of DGs, syntrophins and acetylcholinesterase. These clusters may then interact with AChR–independent clusters of utrophin and α-DB via β-DG or another membrane anchor. The results also raise the possibility that AChRs are actively involved in the assembly of the synaptic UGC, in agreement with the differential requirement of AChR–containing secretory vesicles for synaptic targeting of D/UGC components in Torpedo.
Our findings unravel parallels to inhibitory brain synapses, where GABAA receptor subtypes are localized through their interaction with gephyrin (Kneussel et al., 1999). Gephyrin, on the other hand, is not clustered at postsynaptic sites of mice lacking GABAA receptor γ2 subunits, showing that gephyrin requires the presence of GABAA receptors to form aggregates (Essrich et al., 1998). Thus, neurotransmitter receptors of the NMJ and inhibitory CNS synapses are actively involved in clustering of their anchoring proteins and in postsynaptic organization and it remains to be investigated what other components, in particular signal transducing molecules, interact with these receptors and participate in postsynaptic assembly.
Materials and methods
Expression of agrin and cell culture
Soluble agrin (C-Ag12,4,8 and C-Ag12,0,0) was produced and cells (C2, 1R– and 2R–) were grown as described previously (Black et al., 1987; Fuhrer et al., 1997). To induce clustering, myotubes were incubated with 0.1 nM neural agrin for 15–20 h. To down-regulate AChRs, 40 nM of mAb35 was added to myotubes for 2 days. For agrin-induced clustering upon AChR down-regulation, C2 myotubes were first treated for 1 day with mAb35; agrin was then added, along with new antibodies and staining was performed after 15–20 h.
Antibody production
mAb35-producing hybridoma cells were obtained from ATTC (Manassas, VA) and grown in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal bovine serum and 2 mM glutamine. At high cell density, cells were switched to serum-free DMEM and IgG fractions were isolated by precipitation with 50% ammonium sulfate followed by dialysis against phosphate-buffered saline (PBS; pH 7.5). IgGs were sterile-filtered and quantitated using the BCA protein assay (Pierce, Socochim, Lausanne, Switzerland). mAb124 was produced accordingly.
Immunoblot analysis and precipitation assays
Lysates of myotubes were prepared as described (Fuhrer et al., 1999) and identical protein amounts analysed by immunoblotting. Syntrophins, β-DG, MuSK, Src-related kinases, AChR β subunits, utrophin and rapsyn were detected as described earlier (Fuhrer et al., 1999). α-DB-1 was visualized by α1CT-FP, an antibody that reacts with α-DB-1 forms and detects two prominent protein bands (Nawrotzki et al., 1998). Immunoblot quantitation was performed by scanning films with a computerized densitometer (Nikon Scantouch 210) and using the NIH Image J 1.04b software (National Institutes of Health, MD).
AChRs were precipitated from cell lysates with 200 nM biotin-conjugated btx, followed by streptavidin-coupled agarose beads (Molecular Probes, Eugene, ON; Mittaud et al., 2001). To examine agrin-induced tyrosine phosphorylation events, lysates were split into two parts and analyzed either by MuSK or AChR precipitation, followed by phosphotyrosine immunoblotting.
To precipitate intracellular AChRs, surface receptors were first blocked in living cells with 100 µM btx for 1 h at 37°C. After washing, cell lysates were incubated with 200 nM biotin-conjugated btx for 1 h at 4°C. For precipitation of surface AChRs, cells were directly incubated with 200 nM biotin-conjugated btx for 1 h at 4°C and washed several times with cold PBS prior to lysis. In both cases, receptors were precipitated using streptavidin-coupled agarose beads.
Immunocytochemical staining
AChRs were visualized by incubating myotubes grown on chamberslides with 100 nM tetramethylrhodamine-conjugated btx (Molecular Probes) in fusion medium for 1 h at 37°C, prior to fixation and double-staining for other postsynaptic markers. In the case of MuSK and VVAB4 stains, btx was added to cells along with the MuSK antibody or VVAB4 lectin, respectively. All secondary and tertiary antibodies, as well as biotinylated antibodies or DTAF-conjugated streptavidin, were purchased from Jackson ImmunoResearch Laboratories (Milan Analytica, La Roche, Switzerland).
To stain DGs and syntrophins, cells were fixed shortly at room temperature (RT) with 4% paraformaldehyde (PFA; Sigma, Fluka Chemie AG, Buchs, Switzerland) in K-phosphate buffer (pH 7.4) containing 11% sucrose. The respective proteins were detected using antibodies against α-DG (clone VIA4-1, Upstate Biotech), β-DG (clone 43DAG1/8D5, Novocastra, UK) or syntrophin (mAb1351, a gift from Dr S.C.Froehner, University of Washington, Seattle), followed by FITC- conjugated goat anti-mouse secondary antibodies.
For detection of rapsyn, cells were fixed with 2% PFA at RT and incubated with mAb1234, a monoclonal antiserum and gift of Dr S.C.Froehner. As secondary and tertiary sera, we used FITC-conjugated goat anti-mouse and rabbit anti-goat antibodies. For binding of Vicia villosa Isolectin B4, cells were incubated with 2 µg/ml FITC-conjugated VVAB4 (Sigma) for 1 h at 37°C, followed by fixation with 4% PFA in K-phosphate buffer containing 11% sucrose.
Staining of MuSK was performed using a MuSK antiserum, provided by Dr M.A.Rüegg (University of Basel, Switzerland; Meier et al., 1997), for 2 h at RT. Fixation and detection were continued as described below for utrophin. For detection of α-DB-1 and utrophin, cells were fixed shortly in methanol at –20°C and incubated with α1CT-FP sera and URD40, respectively. The latter is an affinity-purified, dystrophin-pre-absorbed polyclonal antibody reactive specifically with utrophin. Signals were visualized with biotinylated goat anti-rabbit secondary antibodies and DTAF-conjugated streptavidin.
To stain tyrosine phosphorylated proteins, cells were fixed at –20°C and incubated with antibody PY20 (Transduction Laboratories) followed by FITC-conjugated goat anti-mouse antibodies, all in the presence of 1 mM Na–orthovanadate. Finally, cells were mounted in a solution containing glycerol and p-phenylenediamine (Sigma).
Fluorescence microscopy and quantitation of clusters
Myotubes were examined at 200- or 400-fold magnification with a fluorescence microscope (Axioskop II, Zeiss, Germany) and representative images were taken and processed with a cooled 3CCD Camera (Hamamatsu, Japan). In Figures 1A and 2A, 200-fold magnification was used to obtain overview pictures. For Figures 2B and 3A, parts of images taken at 400× were enlarged to illustrate the size and morphology of individual clusters. mAb35-treated myotubes were photographed at 400× without such enlargement (Figures 6 and 7A). To analyze microaggregates, pictures were taken at 400×, followed by strong image enlargement (Figure 8).
To quantitate clusters, random fields of myotubes (chosen under phase contrast) were photographed at 400×. Signals were counted as clusters if their intensity was clearly distinguishable from diffuse staining of myotubes, if signal intensity was strongly above background levels and if clusters were at least 10 µm in length. Within each experiment, 10 fields per treatment were counted and the number of clusters averaged. Data from at least three such experiments were used to calculate mean ± SEM.
Muscle fiber injection and histological analysis
Rapsyn–GFP and neural agrin (cAgrin 7A4B8) expression vectors were obtained from Drs J.B.Cohen (Harvard Medical School, Boston) and M.A.Rüegg, respectively (Denzer et al., 1995; Ramarao and Cohen, 1998). The nGFP expression vector encodes GFP with a nuclear localization signal, which nevertheless leads to cytosolic GFP expression (Sander et al., 2000).
Muscle fibers were ectopically injected as described previously (Jones et al., 1997; Sander et al., 2000). Soleus muscles of anesthetized 4- to 5-week-old Sprague–Dawley rats were surgically exposed and individual myofibers were pressure-injected, using micropipets filled with 90 mM KCl, 15 mg/ml Fast Green FCF (Sigma) and 100 ng/µl of each plasmid.
Injected myofibers were analyzed 21 days later as described by Sander et al. (2000). Rats were killed to excise DNA-injected fibers, which were incubated for 1 h with 2 µg/ml rhodamine-btx. Labeled AChRs and GFP fluorescence were viewed at low magnification with a Zeiss Axioplan microscope. For confocal microscopy, a thin layer of PFA-fixed myofibers was mounted onto glass coverslips. Confocal image series were recorded on a Leica confocal laser scanning unit, TCS NT, which was coupled to a Leica DM IRB microscope. The image series was processed using the TCS NT software (Leica, Bensheim, Germany).
Supplementary data
Supplementary data for this paper are available at The EMBO Journal Online.
Acknowledgments
Acknowledgements
We are very grateful to Dr V.Witzemann for helpful advice with in vivo injection experiments. Drs J.R.Sanes, M.E.Schwab, M.Gesemann and R.A.McKinney, as well as members of the Fuhrer laboratory, are acknowledged for comments on the manuscript. Further thanks go to R.Schöb, B.Stierli, Dr J.-M.Fritschy and Dr S.Kröger. This work was supported by the Dr Eric Slack-Gyr Foundation, the NCCR on Neural Plasticity and Repair and by grants from the Swiss National Science Foundation and the Swiss Foundation for Research on Muscle Diseases (to C.F.). D.J.B. is a Wellcome Trust Senior Fellow.
References
- Adams M.E., Kramarcy,N., Krall,S.P., Rossi,S.G., Rotundo,R.L., Sealock,R. and Froehner,S.C. (2000) Absence of α-syntrophin leads to structurally aberrant neuromuscular synapses deficient in utrophin. J. Cell Biol., 150, 1385–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apel E.D., Roberds,S.L., Campbell,K.P. and Merlie,J.P. (1995) Rapsyn may function as a link between the acetylcholine receptor and the agrin-binding dystrophin-associated glycoprotein complex. Neuron, 15, 115–126. [DOI] [PubMed] [Google Scholar]
- Apel E.D., Glass,D.J., Moscoso,L.M., Yancopoulos,G.D. and Sanes,J.R. (1997) Rapsyn is required for MuSK signaling and recruits synaptic components to a MuSK-containing scaffold. Neuron, 18, 623–635. [DOI] [PubMed] [Google Scholar]
- Black R.A. and Hall,Z.W. (1985) Use of a replica technique to isolate muscle cell lines defective in expressing the acetylcholine receptor. Proc. Natl Acad. Sci. USA, 82, 124–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black R., Goldman,D., Hochschwender,S., Lindstrom,J. and Hall,Z.W. (1987) Genetic variants of C2 muscle cells that are defective in synthesis of the α subunit of the acetylcholine receptor. J. Cell Biol., 105, 1329–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borges L.S. and Ferns,M. (2001) Agrin-induced phosphorylation of the acetylcholine receptor regulates cytoskeletal anchoring and clustering. J. Cell Biol., 153, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De La Porte S., Chaubourt,E., Fabre,F., Poulas,K., Chapron,J., Eymard,B., Tzartos,S. and Koenig,J. (1998) Accumulation of acetylcholine receptors is a necessary condition for normal accumulation of acetylcholinesterase during in vitro neuromuscular synaptogenesis. Eur. J. Neurosci., 10, 1631–1643. [DOI] [PubMed] [Google Scholar]
- Denzer A.J., Gesemann,M., Schumacher,B. and Ruegg,M.A. (1995) An amino-terminal extension is required for the secretion of chick agrin and its binding to extracellular matrix. J. Cell Biol., 131, 1547–1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essrich C., Lorez,M., Benson,J.A., Fritschy,J.M. and Luscher,B. (1998) Postsynaptic clustering of major GABAA receptor subtypes requires the γ2 subunit and gephyrin. Nature Neurosci., 1, 563–571. [DOI] [PubMed] [Google Scholar]
- Froehner S.C., Luetje,C.W., Scotland,P.B. and Patrick,J. (1990) The postsynaptic 43K protein clusters muscle nicotinic acetylcholine receptors in Xenopus oocytes. Neuron, 5, 403–410. [DOI] [PubMed] [Google Scholar]
- Fuhrer C., Sugiyama,J.E., Taylor,R.G. and Hall,Z.W. (1997) Association of muscle-specific kinase MuSK with the acetylcholine receptor in mammalian muscle. EMBO J., 16, 4951–4960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrer C., Gautam,M., Sugiyama,J.E. and Hall,Z.W. (1999) Roles of rapsyn and agrin in interaction of postsynaptic proteins with acetylcholine receptors. J. Neurosci., 19, 6405–6416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautam M., Noakes,P.G., Mudd,J., Nichol,M., Chu,G.C., Sanes,J.R. and Merlie,J.P. (1995) Failure of postsynaptic specialization to develop at neuromuscular junctions of rapsyn-deficient mice. Nature, 377, 232–236. [DOI] [PubMed] [Google Scholar]
- Gautam M., Noakes,P.G., Moscoso,L., Rupp,F., Scheller,R.H., Merlie, J.P. and Sanes,J.R. (1996) Defective neuromuscular synaptogenesis in agrin-deficient mutant mice. Cell, 85, 525–535. [DOI] [PubMed] [Google Scholar]
- Gillespie S.K., Balasubramanian,S., Fung,E.T. and Huganir,R.L. (1996) Rapsyn clusters and activates the synapse-specific receptor tyrosine kinase MuSK. Neuron, 16, 953–962. [DOI] [PubMed] [Google Scholar]
- Glass D.J. et al. (1996) Agrin acts via a MuSK receptor complex. Cell, 85, 513–523. [DOI] [PubMed] [Google Scholar]
- Grady R.M., Zhou,H., Cunningham,J.M., Henry,M.D., Campbell,K.P. and Sanes,J.R. (2000) Maturation and maintenance of the neuromuscular synapse: genetic evidence for roles of the dystrophin–glycoprotein complex. Neuron, 25, 279–293. [DOI] [PubMed] [Google Scholar]
- Grow W.A. and Gordon,H. (2000) Acetylcholine receptors are required for postsynaptic aggregation driven by the agrin signalling pathway. Eur. J. Neurosci., 12, 467–472. [DOI] [PubMed] [Google Scholar]
- Grow W.A., Ferns,M. and Gordon,H. (1999) A mechanism for acetylcholine receptor clustering distinct from agrin signaling. Dev. Neurosci., 21, 436–443. [DOI] [PubMed] [Google Scholar]
- Gu Y., Ralston,E., Murphy-Erdosh,C., Black,R.A. and Hall,Z.W. (1989) Acetylcholine receptor in a C2 muscle cell variant is retained in the endoplasmic reticulum. J. Cell Biol., 109, 729–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson C., Cote,P., Rossi,S., Rotundo,R. and Carbonetto,S. (2001) The dystroglycan complex is necessary for stabilization of acetylcholine receptor clusters at neuromuscular junctions and formation of the synaptic basement membrane. J. Cell Biol., 152, 435–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G., Meier,T., Lichtsteiner,M., Witzemann,V., Sakmann,B. and Brenner,H.R. (1997) Induction by agrin of ectopic and functional postsynaptic-like membrane in innervated muscle. Proc. Natl Acad. Sci. USA, 94, 2654–2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G., Moore,C., Hashemolhosseini,S. and Brenner,H.R. (1999) Constitutively active MuSK is clustered in the absence of agrin and induces ectopic postsynaptic-like membranes in skeletal muscle fibers. J. Neurosci., 19, 3376–3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kneussel M., Brandstatter,J.H., Laube,B., Stahl,S., Muller,U. and Betz,H. (1999) Loss of postsynaptic GABA(A) receptor clustering in gephyrin-deficient mice. J. Neurosci., 19, 9289–9297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaRochelle W.J. and Froehner,S.C. (1987) Comparison of the postsynaptic 43kDa protein from muscle cells that differ in acetylcholine receptor clustering activity. J. Biol. Chem., 262, 8190–8195. [PubMed] [Google Scholar]
- LaRochelle W.J., Ralston,E., Forsayeth,J.R., Froehner,S.C. and Hall,Z.W. (1989) Clusters of 43 kDa protein are absent from genetic variants of C2 muscle cells with reduced acetylcholine receptor expression. Dev. Biol., 132, 130–138. [DOI] [PubMed] [Google Scholar]
- Marchand S., Stetzkowski-Marden,F. and Cartaud,J. (2001) Differential targeting of components of the dystrophin complex to the postsynaptic membrane. Eur. J. Neurosci., 13, 221–229. [PubMed] [Google Scholar]
- Martin P.T. and Sanes,J.R. (1995) Role for a synapse-specific carbohydrate in agrin-induced clustering of acetylcholine receptors. Neuron, 14, 743–754. [DOI] [PubMed] [Google Scholar]
- McMahan U.J. (1990) The agrin hypothesis. Cold Spring Harb. Symp. Quant. Biol., 55, 407–418. [DOI] [PubMed] [Google Scholar]
- Meier T., Hauser,D.M., Chiquet,M., Landmann,L., Ruegg,M.A. and Brenner,H.R. (1997) Neural agrin induces ectopic postsynaptic specializations in innervated muscle fibers. J. Neurosci., 17, 6534–6544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittaud P., Marangi,P.A., Erb-Vögtli,S.E. and Fuhrer,C. (2001) Agrin-induced activation of AChR–bound Src family kinases requires rapsyn and correlates with AChR clustering. J. Biol. Chem., 276, 14505–14513. [DOI] [PubMed] [Google Scholar]
- Mohamed A.S., Rivas-Plata,K.A., Kraas,J.R., Saleh,S.M. and Swope,S.L. (2001) Src-class kinases act within the agrin/MuSK pathway to regulate acetylcholine receptor phosphorylation, cytoskeletal anchoring and clustering. J. Neurosci., 21, 3806–3818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawrotzki R., Loh,N.Y., Ruegg,M.A., Davies,K.E. and Blake,D.J. (1998) Characterisation of α-dystrobrevin in muscle. J. Cell Sci., 111, 2595–2605. [DOI] [PubMed] [Google Scholar]
- Ono F., Higashijima,S., Shcherbatko,A., Fetcho,J.R. and Brehm,P. (2001) Paralytic zebrafish lacking acetylcholine receptors fail to localize rapsyn clusters to the synapse. J. Neurosci., 21, 5439–5448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips W.D., Kopta,C., Blount,P., Gardner,P.D., Steinbach,J.H. and Merlie,J.P. (1991) ACh receptoR–rich membrane domains organized in fibroblasts by recombinant 43kDa protein. Science, 251, 568–570. [DOI] [PubMed] [Google Scholar]
- Ramarao M.K. and Cohen,J.B. (1998) Mechanism of nicotinic acetylcholine receptor cluster formation by rapsyn. Proc. Natl Acad. Sci. USA, 95, 4007–4012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander A., Guth,A., Brenner,H.R. and Witzemann,V. (2000) Gene transfer into individual muscle fibers and conditional gene expression in living animals. Cell Tissue Res., 301, 397–403. [DOI] [PubMed] [Google Scholar]
- Sanes J.R. and Lichtman,J.W. (1999) Development of the vertebrate neuromuscular junction. Annu. Rev. Neurosci., 22, 389–442. [DOI] [PubMed] [Google Scholar]
- Schwarz H., Giese,G., Muller,H., Koenen,M. and Witzemann,V. (2000) Different functions of fetal and adult AChR subtypes for the formation and maintenance of neuromuscular synapses revealed in epsilon-subunit-deficient mice. Eur. J. Neurosci., 12, 3107–3116. [DOI] [PubMed] [Google Scholar]
- Wallace B.G. (1989) Agrin-induced specializations contain cytoplasmic, membrane and extracellular matrix-associated components of the postsynaptic apparatus. J. Neurosci., 9, 1294–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z.Z., Mathias,A., Gautam,M. and Hall,Z.W. (1999) Metabolic stabilization of muscle nicotinic acetylcholine receptor by rapsyn. J. Neurosci., 19, 1998–2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerfield M., Liu,D.W., Kimmel,C.B. and Walker,C. (1990) Pathfinding and synapse formation in a zebrafish mutant lacking functional acetylcholine receptors. Neuron, 4, 867–874. [DOI] [PubMed] [Google Scholar]