Abstract
The Raf-1 kinase is regulated by phosphorylation, and Ser259 has been identified as an inhibitory phosphorylation site. Here we show that the dephosphorylation of Ser259 is an essential part of the Raf-1 activation process, and further reveal the molecular role of Ser259. The fraction of Raf-1 that is phosphorylated on Ser259 is refractory to mitogenic stimulation. Mutating Ser259 elevates kinase activity because of enhanced binding to Ras and constitutive membrane recruitment. This facilitates the phosphorylation of an activating site, Ser338. The mutation of Ser259 also increases the functional coupling to MEK, augmenting the efficiency of MEK activation. Our results suggest that Ser259 regulates the coupling of Raf-1 to upstream activators as well as to its downstream substrate MEK, thus determining the pool of Raf-1 that is competent for signalling. They also suggest a new model for Raf-1 activation where the release of repression through Ser259 dephosphorylation is the pivotal step.
Keywords: inhibition/MEK/phosphorylation/Raf-1 kinase/Ras
Introduction
The Raf-1 kinase lies at the heart of a signalling network that controls cell proliferation, neoplastic transformation, differentiation and apoptosis (Lewis et al., 1998; Hagemann and Rapp, 1999; Kolch, 2000). Many of these effects are transmitted via the MAPK/ERK pathway, a three-tiered kinase cascade, where Raf-1 phosphorylates and activates MEK, which then phosphorylates and activates ERK. Raf-1 responds to a wide range of extracellular signals and features a complicated regulation, that is still incompletely understood (Kolch, 2000; Avruch et al., 2001; Kerkhoff and Rapp, 2001). This complexity probably reflects the necessity to integrate various upstream signals and translate them into an appropriately dosed response. Most receptors engage Raf-1 by activating Ras. Activated Ras binds to Raf-1 with high affinity, but does not alter the catalytic activity of Raf-1 directly (Avruch et al., 2001; Kerkhoff and Rapp, 2001). Rather, it relocalizes Raf-1 from the cytosol to the plasma membrane where a multistep activation process takes place. Although the initial interaction between the effector domain of Ras and the Ras-binding domain (RBD) of Raf-1 is both necessary and sufficient for membrane translocation, a secondary interaction between the Raf-1 cysteine-rich domain (CRD) and possibly the farnesylated tail of Ras is required for activation to ensue (Luo et al., 1997; Williams et al., 2000).
Raf-1 activation entails interaction with modulatory proteins (Yeung et al., 1999; Li et al., 2000; Morrison, 2001), lipids (Muller et al., 1998; Improta-Brears et al., 1999) and complex changes in phosphorylation. Work with mutants has suggested that the phosphorylation of Ser338 (Diaz et al., 1997; King et al., 1998) and Tyr341 (Fabian et al., 1993; Marais et al., 1995; Mason et al., 1999) is absolutely required and co-operates in Raf-1 activation (Mason et al., 1999). Ser338 phosphorylation is induced by both activated Ras and growth factors (Diaz et al., 1997; Mason et al., 1999). Although it is not a strong activator by itself, it seems to make Raf-1 permissive to further activation. One such event appears to be the phosphorylation of Tyr341. The phosphorylation of both Ser338 and Tyr341 strongly synergizes in Raf-1 activation (Mason et al., 1999). Furthermore, phosphorylation of Thr491 and Ser494 in the activation loop is necessary, but not sufficient for activation. Again, these sites co-operate with Ser338 and Tyr341 (Chong et al., 2001), suggesting that phosphorylation of activating sites may act in a combinatorial way to adjust Raf-1 activation to the proper level. Raf-1 is also negatively regulated by phosphorylation. The cAMP-dependent kinase PKA inhibits Raf-1, phosphorylating Raf-1 on Ser43 (Wu et al., 1993), Ser259 (Dhillon et al., 2002) and Ser621 (Mischak et al., 1996). These sites are phosphorylated in resting cells (Morrison et al., 1993), but are hyperinduced by PKA. All these sites previously have been implicated in the negative regulation of Raf-1 by PKA. Ser43 phosphorylation down-modulates the binding to activated Ras (Wu et al., 1993). Ser621 phosphorylation can down-regulate the activity of the isolated Raf-1 kinase domain (Mischak et al., 1996), but may have an activating role in the context of full-length Raf-1 (Thorson et al., 1998). Ser259 phosphorylation appears to be a main target for inhibitory phosphorylation of full-length Raf-1. The mutation of Ser259 to either alanine or aspartic acid renders Raf-1 largely resistant to PKA inhibition (Dhillon et al., 2002). Ser259 also has been reported to be the target for inhibitory phosphorylation of Raf-1 by Akt/PKB (Zimmermann and Moelling, 1999). Using okadaic acid, an inhibitor of protein phosphatase 2A (PP2A), we have shown previously that the hyperphosphorylation of Ser259 interferes with Raf-1 activation (Abraham et al., 2000). In addition, treatment of cells with general inhibitors of PP1 and PP2A results in the accumulation of inactive 14-3-3–Raf-1 complexes at the plasma membrane (Jaumot and Hancock, 2001). Here, we have investigated the role of Ser259 phosphorylation in detail and show that it plays a pivotal role in Raf-1 activation as well as in the interaction with activators and substrates.
Results
Complex changes in the phosphorylation of Raf-1 during activation
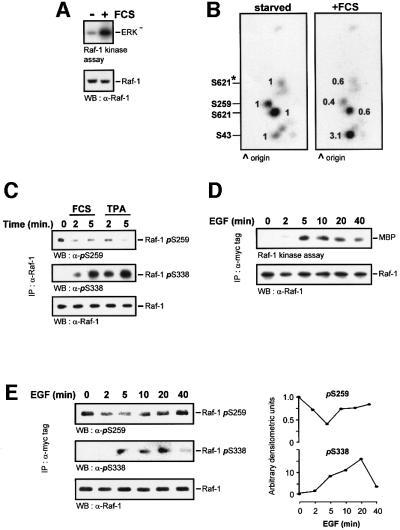
In order to detect phosphorylation events that are associated with activation, we prepared phosphopeptide maps of Raf-1 early after mitogen stimulation, when kinase activation commences (Figure 1A). For this purpose, serum-starved NIH 3T3 cells were labelled with [32P]orthophosphoric acid and treated with fetal calf serum (FCS) for 5 min. Endogenous Raf-1 was immunoprecipitated. One aliquot was assayed for kinase activity (Figure 1A) and another was subjected to two-dimensional tryptic phosphopeptide mapping (Figure 1B). The earliest changes occurred in the phosphorylation of the three basal sites, with Ser43 increasing and Ser259 and Ser621 decreasing. Dephosphorylation of Ser259 in endogenous Raf-1 was also observed in response to stimulation of COS cells with 10% FCS or 12-O-tetradecanoylphorbol-13-acetate (TPA) (Figure 1C). This dephosphorylation correlated with an increase in Ser338 phosphorylation. In order to monitor the changes in Raf-1 phosphorylation in more detail over a time course of stimulation, phosphospecific antibodies were used (Figure 1D and E). Raf-1 was immunoprecipitated and examined for kinase activity (Figure 1D) and changes in phosphorylation (Figure 1E) in response to a defined mitogen, epidermal growth factor (EGF). The activation of the Raf-1 kinase ensued 2 min after treatment, peaked between 5 and 10 min and started to decline after 20 min. Ser338 phosphorylation is required for Raf-1 kinase activation, and its phosphorylation closely coincided with kinase activity. Consistent with the original publication describing Ser338 phosphorylation (Diaz et al., 1997), we found that the phosphopeptide containing Ser338 is hard to detect on two-dimensional phosphopeptide maps, and that a phosphoserine 338 (pSer338) antibody is much more sensitive in this case. Both Ser259 and Ser621 (data not shown) were transiently dephosphorylated, but the dephosphorylation of Ser259 was more pronounced. It ensued when kinase activity started to increase, was maximal when kinase activity peaked, and slowly returned to basal levels as kinase activity declined. Thus, phosphorylation of Ser338 and dephosphorylation of Ser259 showed a close correlation with the catalytic activity of Raf-1. The changes in Ser259 phosphorylation slightly preceded the changes in Ser338 phosphorylation. While the role of Ser338 phosphorylation is well established, these results suggest that Ser259 dephosphorylation and Ser338 phosphorylation may be co-regulated and, more importantly, that the dephosphorylation of Ser259 is also required for Raf-1 activation.
Fig. 1. Changes in Raf-1 phosphorylation during activation. Serum-starved NIH 3T3 cells were labelled with 0.5 mCi/ml [32P]ortho phosphoric acid for 6 h and stimulated with 20% FCS for 5 min. Endogenous Raf-1 was immunoprecipitated, and (A) examined for kinase activity. (B) Another Raf-1 aliquot was digested with trypsin and subjected to two-dimensional phosphopeptide mapping. Equal amounts of Raf were loaded, as evident from the western blot shown in (A). Phosphorylation sites were assigned by comparison with the respective Raf-1 point mutants. The peptide containing Ser621 produces two spots due to partial digestion; the minor spot is labelled by an asterisk. Spots were quantified by phosphoimager, and the changes relative to serum-starved cells are shown. (C) Dephosphoryl ation of endogenous Raf-1 during serum and mitogen stimulation. COS cells were treated with 10% FCS or 100 ng/ml TPA for the indicated times. Raf-1 was immunoprecipitated from the cells with an anti-Raf-1 antibody. The immunoprecipitates were then blotted with antibodies against Raf-1 and phosphoserines 259 and 338. (D) Time course of Raf-1 kinase activation. COS cells were transfected with myc-Raf, serum starved and stimulated with 20 ng/ml EGF for the indicated times. myc-Raf-1 was immunoprecipitated with the 9E10 monoclonal antibody, and catalytic activity was measured using a two-step kinase assay. (E) Myc-Raf immunoprecipitates were blotted with phospho specific antisera against phosphoserines 259 and 338. The blots were quantified by laser densitometry shown in the right hand panels.
The phosphorylation of Ser259 prevents Raf-1 activation
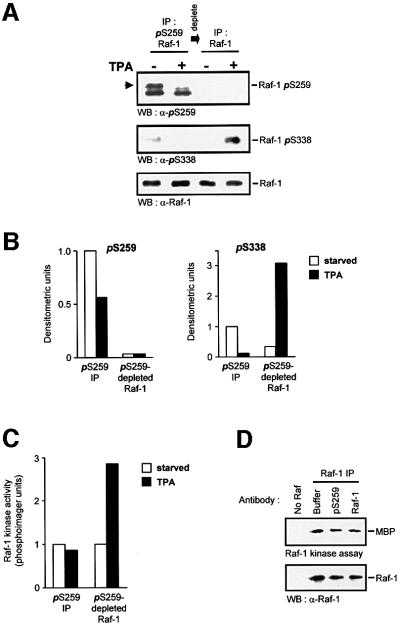
To test this hypothesis directly, we isolated Raf-1 by immunoprecipitation with affinity-purified anti-pSer259 antibodies (Figure 2). For this purpose, cells were lysed in RIPA buffer, which does not inhibit Raf-1 kinase activity, but rids Raf-1 of 14-3-3 protein that is bound to pSer259 and hence would interfere with immunoprecipitation (Michaud et al., 1995). Raf-1 phosphorylated on Ser259 could be depleted quantitatively from cell lysates (Figure 2A). TPA-induced Ser338 phosphorylation was confined to the Raf-1 fraction devoid of Ser259 phosphorylation (Figure 2A and B). Importantly, the kinase activity of this fraction was stimulated by mitogens, whereas the Ser259 phosphorylated pool was completely refractory (Figure 2C). Similar results were obtained with EGF stimulation. To exclude the possibility that the anti-pSer259 antibody had any deleterious impact on the Raf-1 catalytic activity, it was added to a Raf-1 kinase reaction. No inhibition was observed (Figure 2D). Thus, these data strongly indicate that the dephosphorylation of Ser259 is an essential part of the mitogenic activation mechanism. Therefore, we set out to investigate the role of Ser259 in more detail.
Fig. 2. Raf-1 phosphorylated on Ser259 is refractory to mitogen stimulation. Cells were lysed in RIPA buffer and Raf-1 was immunodepleted from serum-starved or TPA-treated (100 ng/ml for 20 min) cells using the affinity-purified anti-pSer259 antibody. The supernatant subsequently was immunoprecipiated with anti-Raf antiserum. Both fractions of Raf-1 were examined for phosphorylation of Ser259 and Ser338, using phosphospecific antisera (A and B), and for kinase activity (C). Raf-1 phosphorylated on Ser259 is indicated by the arrowhead; the lower of the two bands is non-specific. Similar results were obtained with EGF stimulation. For (B), the immunoblots of (A) were quantified by laser densitometry. The pSer259 and pSer338 signals, respectively, were normalized to the signals obtained with a non-discriminatory Raf-1 antibody (lowest panel in A). (D) The Raf-1 Ser259-phosphospecific antiserum does not inhibit kinase activity. COS cells were transfected with CMV5-Raf-1 and treated with 100 ng/ml TPA for 15 min. The cells were lysed in RIPA buffer and Raf-1 was immunoprecipitated with the c-Raf VI antiserum. Aliquots of the immunoprecipitate were incubated with either 2 µg of pSer259-specific or a non-discriminatory Raf-1 antibody (C-12; Santa Cruz) for 2 h. The kinase activities of the immunoprecipitates were then measured.
Ser259 regulates membrane translocation and Ras association
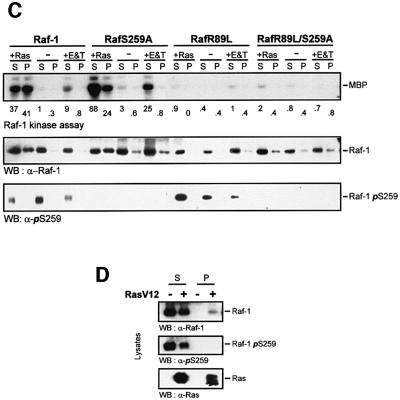
First we tested whether Ser259 phosphorylation regulates the interaction with Ras (Figure 3). As reported previously, the mutation of Ser259 resulted in a modest elevation of the basal kinase activity (Hu et al., 1995; Michaud et al., 1995; Zimmermann and Moelling, 1999). Replacing Ser259 with alanine or the phosphomimetic aspartate made no difference in terms of the behaviour of these mutants, suggesting that the physical presence of a phosphate group is required, rather than merely a negative charge. The constitutive activity of RafS259A could be stimulated further by activated Ras and, in most experiments, to a higher level than the wild-type protein (Figure 3A). This induction was fully dependent on the ability of RafS259A to interact physically with Ras, since it was abolished by a mutation, R89L, which disrupts Ras binding (Fabian et al., 1994). Co-immunoprecipitation experiments revealed that the mutation of Ser259 enhanced the binding of Raf-1 to both endogenous Ras and co-transfected RasV12 (Figure 3B), implicating the phosphorylation of Ser259 in the regulation of Raf-1 membrane recruitment. This hypothesis was examined directly by cell fractionation experiments (Figure 3C and D). Raf-1 was cytosolic, but could be translocated to the membrane fraction by co-expression of activated Ras. Mitogens induced a much more transient membrane translocation of Raf-1, which is not apparent any more at the late time point shown, 30 min after stimulation. This time point was chosen in order to show that, although Raf-1 activation is thought to occur at the membrane, active Raf-1 subsequently is released from the membrane. In contrast, a fraction of RafS259A was constitutively membrane associated even in serum-starved cells. As expected, the R89L mutation, which disrupts the interaction between Ras and the RBD of Raf-1, abolished the membrane recruitment of wild-type Raf-1 as well as kinase activation. The double mutant, RafR89L/S259A, in part still preserved the constitutive membrane localization, but was severely impaired in becoming activated. Importantly, both Ras and mitogen-activated wild-type Raf-1 exhibited a reduction in Ser259 phosphorylation. Curiously, in RafR89L, the phosphorylation of Ser259 was decreased by mitogens, but induced by Ras, suggesting that the kinase that phosphorylates Raf-1 on Ser259 is stimulated by Ras. The anti-pSer259 antibody did not detect the Raf proteins, where Ser259 had been mutated, attesting to the specificity of this antibody. In summary, these results suggest that Ser259 facilitates Raf-1 membrane translocation and activation by enhancing the interaction with Ras. The subsequent deactivation of Raf-1 via Ser259 hyperphosphorylation is also stimulated by Ras, but is independent of binding to Raf-1.
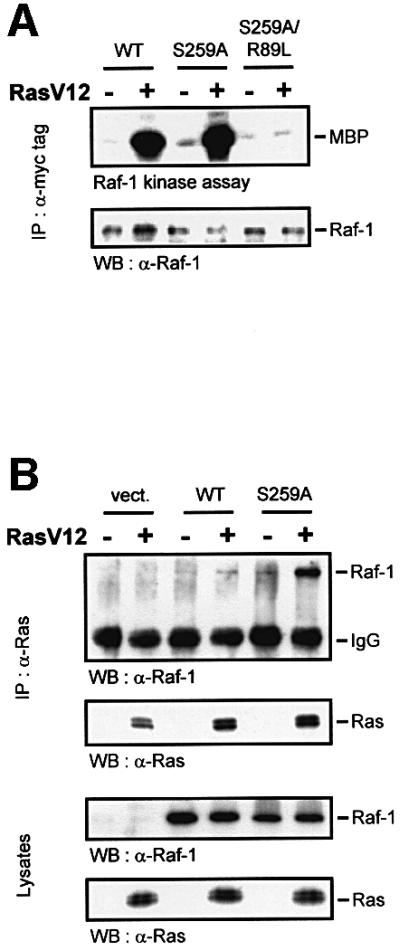
Fig. 3. Ser259 regulates coupling to Ras. (A) Myc-tagged wild-type Raf-1 (WT), RafS259A, and the double mutant RafR89L/S259A were expressed in COS-1 cells either alone or with RasV12 as indicated. Raf-1 immunoprecipitates were examined for kinase activity. (B) The mutation of Ser259 enhances the association with Ras. COS-1 cells were transfected with the indicated expression plasmids. Ras immunoprecipitates were immunoblotted for Ras and associated Raf proteins (upper panel). Crude lysates were immunoblotted to show that all Raf proteins were expressed at comparable levels (lower panel). (C) Cell fractionation. COS cells were transfected with the indicated expression plasmids, serum starved ‘–’ and treated with EGF (20 ng/ml) plus TPA (100 ng/ml), ‘E&T’, for 30 min. Cells were lysed in hypotonic, detergent-free buffer and fractionated into 100 000 g supernatant ‘S’ and pellet ‘P’ containing membranes. The fractions were extracted with 1% Triton X-100, and Raf-1 immunoprecipitates were examined for kinase activity, Ser259 phosphorylation and Raf-1 distribution. Kinase activity was quantified by a phosphoimager and is given below the lanes in arbitrary units. (D) COS cells were transfected with myc-Raf-1 along with RasV12 or vector. The cells were fractionated as described above and equal amounts of whole-cell lysate from each fraction were blotted for Ser259, Raf-1 and Ras levels using the respective antibodies.
Ser259 regulates the coupling to MEK
In the course of these experiments, we noted that RafS259 mutants displayed only a modest activation of kinase activity, but were very efficient in activating MEK. RafS259D expressed in Sf-9 insect cells exhibited a 2-fold elevation of kinase activity when measured with MEK as substrate (Figure 4A). However, when the kinase activity of co-expressed glutathione S-transferase (GST)–MEK was determined, it was increased 11-fold. Thus, although the mutation of Ser259 had a small effect on catalytic activity, it appeared to enhance greatly the ability of Raf-1 to activate MEK in cells. Alternatively, this could be due to a non-linear relationship between Raf-1 activity and MEK activation. This possibility was ruled out by transfecting COS cells with different amounts of Raf-1 and RafS259D in order to generate comparable levels of Raf kinase activity (Figure 4B). Raf-1 had to be overexpressed 2- to 3-fold over RafS259D to achieve equal activities in Raf kinase assays. Under these conditions, co-expressed MEK was still activated much better by RafS259D than by Raf-1, indicating that Ser259 regulates the functional interaction between Raf-1 and MEK. In order to test whether this occurred through modulation of the physical interaction between Raf and MEK, we performed co-immunoprecipitation experiments (Figure 4C). RafS259 mutants showed a clear decrease of MEK association. This result was unexpected, because we would have anticipated that the increase in MEK activation would be paralleled by an increase in binding. However, the co-immunoprecipitation assay represents a snapshot of a dynamic equilibrium. It, therefore, could reflect a faster turnover of the MEK substrate by the RafS259 mutants. Here, the phosphorylation of MEK would lower the affinity of MEK for Raf-1, favouring the dissociation of the complex. This indeed seems to be the case (Figure 4D). When a GST–Raf-1–MEK complex was isolated and incubated with ATP, the release of MEK was stimulated, suggesting that phosphorylated MEK does not remain bound to Raf-1. This interpretation is in keeping with the observation that although MEK activation is thought to occur at the cell membrane (Traverse et al., 1993; Stokoe et al., 1994), MEK is not retained at the membrane, but found in the cytosol (Zheng and Guan, 1994; Moriguchi et al., 1995; Lenormand et al., 1998).
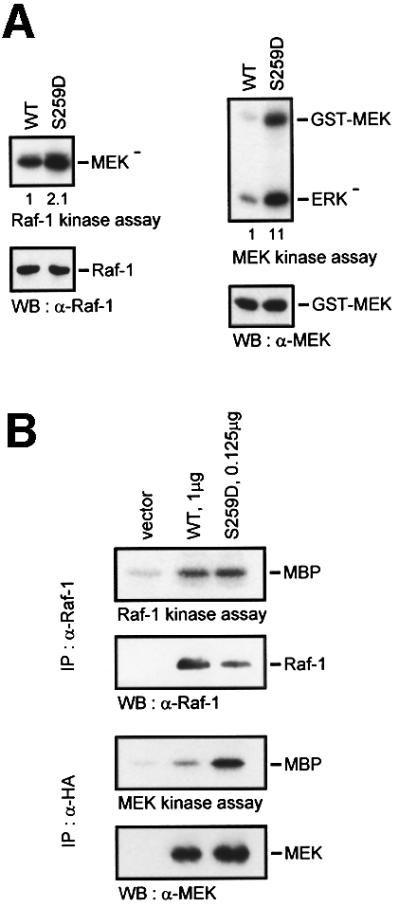
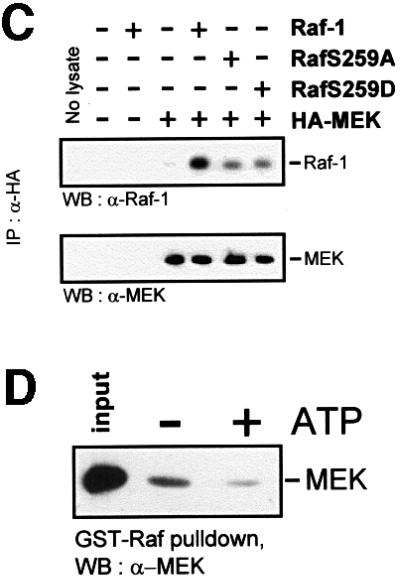
Fig. 4. Ser259 regulates the coupling of Raf-1 to MEK. (A) GST–MEK was co-expressed with Raf-1 or RafS259D, respectively, in Sf-9 cells. Raf-1 proteins were immunoprecipitated and examined for kinase activity with kinase-negative MEK as substrate. GST–MEK was isolated by adsorption to glutathione–Sepharose beads and assayed for kinase activity using kinase-negative ERK (ERK–) as substrate. (B) COS cells were transfected with different amounts of Raf-1 and RafS259D in order to achieve a comparable Raf kinase activity. A constant amount of HA-MEK was co-transfected and assayed for kinase activity with kinase-negative ERK as substrate. (C) Co-immunoprecipitation between Raf and MEK. HA-MEK was co-expressed with Raf-1 or RafS259 mutants, respectively, in COS cells. Lysates were immunoprecipitated and subsequently blotted with the indicated antibodies. (D) Phosphorylation diminishes the Raf-1–MEK association. GST–Raf-1 was expressed in Sf-9 cells and purified by affinity adsorption to glutathione–Sepharose beads. GST–Raf-1 beads were incubated in vitro with purified kinase-negative MEK-1 produced in Escherichia coli in the absence or presence of 100 µM ATP. The beads were washed and bound MEK was detected by immunoblotting.
Ser259 phosphorylation determines the composition of Raf-1 signalling complexes
The results presented above suggest a pivotal role for Ser259 in regulating the interaction of Raf-1 with upstream activators and downstream substrates. There fore, we examined how the mutation of Ser259 affects the assembly and dynamics of Raf-1 signalling complexes (Figure 5). For this purpose, Raf-1 and RafS259A immunoprecipitates were probed for various associated proteins. Confirming the above results, RafS259A featured a decrease in MEK association and an increase in binding of activated RasV12. Interestingly, the co-expression of RasV12 reduced MEK binding to both wild-type Raf-1 and RafS259A. Since RasV12 induces a robust activation of MEK (Figure 3A and C), this result supports the interpretation that this reduction of Raf–MEK binding reflects a faster turnover and hence enhanced activation of MEK. RasV12 also reduced the phosphorylation of Ser259 and the binding of 14-3-3. RafS259A was severely compromised in 14-3-3 binding, but RasV12 did not reduce it further. The residual binding is due to pSer621, since a Raf double mutant where both Ser259 and Ser621 were replaced was devoid of 14-3-3 binding (data not shown). These observations do not necessarily indicate that pSer259 is the major 14-3-3 docking site, because 14-3-3 binding is co-operative (Yaffe et al., 1997). Thus, the loss of one binding site may lead to the disproportional reduction of overall binding. They suggest, however, that RasV12 selectively reduces the interaction of 14-3-3 with Ser259 presumably by facilitating Ser259 dephosphorylation. Ras also induced the phosphorylation of Ser338 in both wild-type Raf-1 and RafS259A, although in RafS259A this induction was smaller. This was due to a higher constitutive phosphorylation of Ser338 in RafS259A and a lesser peak induction. Such dampening of the maximal induction is often seen in situations where activated mutants trigger negative feedback circuits. Additionally, Raf-1 seems to be exposed to negative feedback regulation, since the inhibition of MEK by drugs (Alessi et al., 1995; Weiss et al., 1998) or expression of dominant-negative mutants (Alessandrini et al., 1996) enhances Raf-1 activity. In summary, this experiment provides a synopsis of the regulatory effects exerted by Ser259 phosphorylation, demonstrating that Ser259 regulates crucial aspects of Raf-1 activation and signalling.
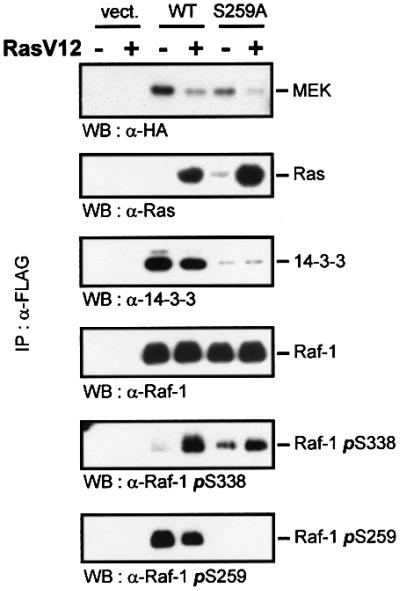
Fig. 5. The effects of Ser259 mutation and Ras on the composition of Raf-1 signalling complexes. COS cells were transfected with FLAG-Raf-1, HA-MEK1 and RasV12 as indicated. Raf proteins were immunoprecipitated with FLAG antibodies and immunoblotted using the indicated antibodies.
Discussion
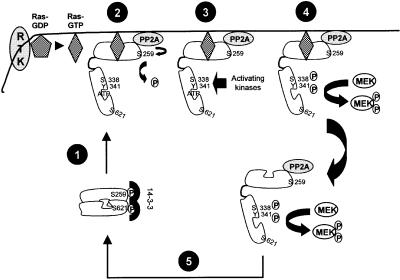
The mutation of Ser259 causes a modest activation of Raf-1 (Morrison et al., 1993; Michaud et al., 1995; Zimmermann and Moelling, 1999), and Ser259 has been identified as an inhibitory phosphorylation site (Abraham et al., 2000; Dhillon et al., 2002). However, the mechanism by which Ser259 regulates Raf-1 function is unknown. Our results allow us to define the role of Ser259 as a key regulator and to devise a conceptually new model of Raf-1 activation (Figure 6).
Fig. 6. Model for the role of Ser259 in Raf-1 activation. The steps designated in the figure are: (1) Raf-1 membrane translocation and Ras binding. (2) Derepression by PP2A-mediated dephosphorylation of Ser259. (3) Phosphorylation of Raf-1 by activating kinases on Ser338 and Tyr341. (4) Phosphorylation of the substrate MEK. (5) Re-phosphorylation of Ser259, dephosphorylation of activating sites and transition into a quiescent state. See text for details.
Ser259 impinges on Raf-1 activation on several levels. The dephosphorylation of Ser259 closely parallels the kinetics of Raf-1 activation. As we have demonstrated earlier, preventing the dephosphorylation of Ser259 by inhibiting PP2A severely impairs Raf-1 activation (Abraham et al., 2000). Here we show directly that the fraction of Raf-1 phosphorylated on Ser259 cannot be activated. Mutating Ser259 enhances the binding of Raf-1 to Ras as well as activation. Both the constitutive and induced activation of RafS259 are compromised by mutating Arg89, which abolishes the binding of Raf-1 to the Ras effector domain (Fabian et al., 1994). Raf-1 membrane translocation is also accompanied by a reduction of 14-3-3 binding, and RafS259A exhibits a very low capacity of 14-3-3 interaction, which, however, does not change upon membrane translocation. These results are consistent with the proposal that Ras displaces 14-3-3 from the N-terminus of Raf-1 (Rommel et al., 1996), and the observation that 14-3-3 dissociates from Raf-1 at the cell membrane (Thorson et al., 1998). Since pSer259 is the main 14-3-3 docking site in the Raf-1 N-terminus (Michaud et al., 1995; Muslin et al., 1996), it appears plausible that its mutation can facilitate Ras binding. Our findings are also compatible with the suggestion that the phosphorylation of Ser259 interferes with the interaction of Ras with the Raf-1 CRD (Sendoh et al., 2000). This interaction is needed for the activation of Raf-1 by Ras, but is dependent on the prior interaction between Ras and the Raf-1 RBD. Thus, we conclude that the dephosphorylation of Ser259 promotes the recruitment of Raf-1 to the membrane as well as its subsequent activation. This interpretation is supported by studies which show that the inhibition of PP2A prevents Ser259 dephosphorylation and membrane recruitment of Raf-1. (M.Kubicek and M.Baccarini, personal communication).
A crucial event in Ras-triggered activation of Raf-1 is the phosphorylation of Ser338. While not sufficient to imbue full activation, Ser338 seems to be essential for activation (Diaz et al., 1997; King et al., 1998; Mason et al., 1999). Recent results show that this phosphorylation must occur at the cell membrane in order to lead to activation (Chiloeches et al., 2001). Our results suggest that Ser259 can cross-regulate the phosphorylation of Ser338. During mitogenic stimulation, the dephosphorylation of Ser259 slightly precedes the phosphorylation of Ser338, and the re-phosphorylation of Ser259 is followed by a sharp drop in Ser338 phosphorylation. Further, RafS259 mutants are constitutively hyperphosphorylated on Ser338, whereas the hyperphosphorylation of Ser259 induced by the cAMP-activated protein kinase PKA reduces Ser338 phosphorylation and Raf-1 activation (Dhillon et al., 2002). The simplest explanation is that pSer259 interferes with the phosphorylation of Ser338. Thus, the dephosphorylation of Ser259 followed by the phosphorylation of Ser338 appear to constitute the priming steps in Raf-1 activation at the cell membrane.
Curiously, Ras also may limit Raf-1 activation by initiating the re-phosphorylation of Ser259. This would provide a direct feedback mechanism to adjust the amplitude and duration of Raf-1 signalling. The re-phosphorylation of Ser259 may serve to release Raf-1 from the cell membrane back into the cytosol and promote the re-binding of 14-3-3. This configuration, cytosolic location and a 14-3-3 dimer clamped to pSer259 and pSer621, is thought to represent an inactive conformation of Raf-1 (Tzivion et al., 1998). The identity of the Ras-stimulated Ser259 kinase currently is under investigation, as we have ruled out Akt/PKB, which in our cells does not cross-regulate Raf-1. An obvious candidate is PKA, which cross-talks to the Raf→MEK→ERK pathway on several levels and can be activated indirectly by ERK (Houslay and Kolch, 2000). Another candidate is Rsk, a downstream effector of the Ras→Raf→MEK→ERK cascade, which has been reported to phosphorylate Ser259 in in-gel kinase assays (Kinuya et al., 2000). Rsk already has been described at the effector end of a feedback loop that terminates Ras activation by phosphorylating the guanine nucleotide exchange factor SOS (Douville and Downward, 1997).
Ser259 also regulates the physical and functional interaction with MEK. Preventing Ser259 phosphorylation seems to enhance MEK activation by increasing the turnover of Raf-1–MEK complexes. Given the lack of an X-ray structure for Raf-1, several possibilities are conceivable, which are being investigated further. Ser259 dephosphorylation could have an allosteric effect on the MEK-binding site in the Raf-1 kinase domain. It also could promote MEK turnover by enhancing the catalytic activity of Raf-1. Alternatively, Ser259 could be involved in the binding of modulating proteins in a phosphorylation-dependent manner. When phosphorylated, it can bind 14-3-3, which is thought to stabilize an inactive conformation (Tzivion et al., 1998). However, when dephosphorylated, it could bind a factor that enhances the rate of MEK phosphorylation. The cloning of proteins, such as Ksr and RKIP, which modulate the Raf-1–MEK association (Yeung et al., 1999, 2000; Morrison, 2001), testifies that this interface is used to regulate signal transmission.
In summary, Ser259 appears to have a central role in the coupling of Raf-1 to upstream activators, such as Ras, as well as downstream substrates, such as MEK. This explains why Raf-1 requires the de-phosphorylation of Ser259 in order for activation and downstream signalling to proceed. These results further suggest that, in the first instance, Raf-1 is regulated by release from repression.
Materials and methods
Cells culture and transfection
NIH 3T3 and COS-1 cells were grown in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 2 mM glutamine and 10% FCS. COS-1 cells were transfected in 6-well plates with 0.5–1 µg of total plasmid DNA/well or in 10 cm dishes with 1.5–2 µg of total plasmid DNA using Effectene (Qiagen). NIH 3T3 cells were transfected as described previously (Kortenjann et al., 1994). Cells were serum starved overnight prior to treatment with mitogens.
Plasmids
The pCEFL-Ras, pCis-RasV12 and pCHA-MEK plasmids were kindly provided by Silvio Gutkind and Michael Weber, respectively. pCMV5-Raf-1 constructs were described previously (Häfner et al., 1994) and mutants were derived by site-directed mutagenesis. pEFmycRaf, pEFmycRafS259A and pcDNA3-FLAG Raf were generous gifts from Richard Marais and Deborah Morrison, respectively. All other Raf constructs were made by site-directed mutagenesis using the Stratagene Quick Change kit.
Antibodies
The anti-myc tag (clone 9E10) antibody was from Roche, anti-Raf-1 and anti-Ras monoclonal antibodies were from Transduction Labs, the Raf-1 pSer259 antibody was either affinity purified by ourselves (see below) or purchased from NEB, pSer338 was purchased from UBI, anti-v-H-Ras (Y13-238) was from Oncogene Sciences, FLAG M2 antibodies and FLAG agarose were from Sigma and haemagglutinin (HA) antibodies used for immunoprecipitations and western blotting were from Roche and Santa Cruz, respectively. Antibodies used for immunoprecipitation of Raf-1 phosphorylated on Ser259 were affinity purified from sheep injected with the phosphopeptide RST(p)STPNC coupled to keyhole limpet haemocyanin (KLH). Briefly, serum was clarified by centrifugation and desalted using PD10 columns (Amersham Pharmacia Biotech). Following crude purification of the IgG on a protein G column (Amersham Biotech), antiserum specific for pSer259 was obtained by first passing the purified IgG over a column coupled to a corresponding non-phosphopeptide QRQRSTSTPNVHC, then passing the flowthrough over a column coupled to the RST(p)STPNC phosphopeptide. The phospho-specific antibodies were eluted with 0.1 M glycine pH 2.7, immediately neutralized with 1 M Tris pH 8 and then desalted.
Immunoprecipitations
For the pSer259 immunodepletion experiments, COS-1 cells were serum starved overnight (0.1% FCS/DMEM) prior to stimulation with TPA (100 ng/ml, 15 min). For each treatment, lysates were prepared from three wells of a 6-well plate in RIPA buffer (50 mM Tris pH 7.4, 150 mM NaCl, 1 mM EDTA, 1% NP-40, 0.25% Na-deoxycholate) containing inhibitors of protease (1 mM phenylmethylsulfonyl fluoride, 5 µg/ml leupeptin, 2 µg/ml aprotinin) and phosphatase (2 mM NaF, 0.2 mM Na-pyrophosphate, 10 mM β-glycerophosphate, 0.1 µM okadaic acid) and pooled. Lysis in RIPA buffer does not affect Raf kinase activity, but dissociates bound 14-3-3 from Raf proteins (Michaud et al., 1995). The lysates were pre-cleared with 25 µl of protein G–agarose beads (Roche) for 2 h, then incubated overnight with 80 µg of pSer259 antiserum coupled to protein G–agarose beads. The beads were washed three times with phosphate-buffered saline–0.1% Triton X-100 and snap-frozen. The supernatant was then passed six times over a mini-pSer259– protein G–agarose column (80 µg) and the flowthrough was incubated with 2 µg of Raf-1 monoclonal antibody (Transduction labs) coupled to protein G–agarose beads. The beads were washed as above and snap-frozen. For all other kinase assays and Ras immunoprecipitations, COS-1 cells from one well of a 6-well plate were lysed in kinase lysis buffer (Barnard et al., 1998) and incubated with 1 µg of anti-Ras antibody (Y13-238, Oncogene Sciences). For all other co-immunoprecipitations, cells from a 10 cm dish were lysed in buffer containing 20 mM HEPES pH 7.5, 5 mM EGTA, 150 mM NaCl and 0.5% NP-40 plus protease and phosphatase inhibitors. The lysates were incubated overnight at 4°C with 20 µl of FLAG–agarose beads (Sigma), and then washed four times with lysis buffer containing 0.1% NP-40.
Kinase assays and western blotting
Raf-1 activity was assayed using a linked assay, where Raf immunoprecipitates are incubated with recombinant MEK-1 and kinase-dead ERK-2 proteins. This assay measures the ability of Raf-1 to phosphorylate and activate MEK. MEK activation is detected by the phosphorylation of kinase-negative ERK. ERK activation was assayed with myelin basic protein (MBP) as substrate. In some experiments, Raf-1 activity was measured using a more sensitive two-step kinase assay exactly as described (Barnard et al., 1998). In this assay, Raf proteins are immunoprecipitated and used in the first step to activate recombinant MEK and ERK. In the second step, an aliquot of the reaction is incubated with MBP in the presence of [γ-32P]ATP to measure the activity of ERK. Western blotting was performed using the La Roche Diagnostic chemoluminescence kit as recommended by the supplier. Primary antibodies were used at concentrations specified by the suppliers. Densitometric evaluation was performed with NIH image.
Phosphopeptide mapping
Metabolic labelling of NIH 3T3 cells with [32P]orthophosphoric acid was carried out as described (Häfner et al., 1994). Raf-1 was immunoprecipitated, separated by SDS–PAGE and autoradiographed. Raf bands were cut out and subjected to tryptic digestion and two-dimensional peptide mapping as described (Häfner et al., 1994). pH 8.9 buffer was used for the first, and phosphochromatography buffer for the second dimension. Phosphopeptides were quantified with a Fuji phosphoimager.
Acknowledgments
Acknowledgements
We thank Drs S.Gutkind, M.Weber, R.Marais and D.Morrison for providing reagents, and V.Cleghon and J.A.Wyke for critical reading of the manuscript. This work was supported by the Cancer Research Campaign.
References
- Abraham D. et al. (2000) Raf-1-associated protein phosphatase 2A as a positive regulator of kinase activation. J. Biol. Chem., 275, 22300–22304. [DOI] [PubMed] [Google Scholar]
- Alessandrini A., Greulich,H., Huang,W.D. and Erikson,R.L. (1996) Mek1 phosphorylation site mutants activate Raf-1 in NIH 3T3 cells. J. Biol. Chem., 271, 31612–31618. [DOI] [PubMed] [Google Scholar]
- Alessi D.R., Cuenda,A., Cohen,P., Dudley,D.T. and Saltiel,A.R. (1995) PD 098059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. J. Biol. Chem., 270, 27489–27494. [DOI] [PubMed] [Google Scholar]
- Avruch J., Khokhlatchev,A., Kyriakis,J.M., Luo,Z., Tzivion,G., Vavvas, D. and Zhang,X.F. (2001) Ras activation of the Raf kinase: tyrosine kinase recruitment of the MAP kinase cascade. Recent Prog. Horm. Res., 56, 127–155. [DOI] [PubMed] [Google Scholar]
- Barnard D., Diaz,B., Clawson,D. and Marshall,M. (1998) Oncogenes, growth factors and phorbol esters regulate Raf-1 through common mechanisms. Oncogene, 17, 1539–1547. [DOI] [PubMed] [Google Scholar]
- Chiloeches A., Mason,C.S. and Marais,R. (2001) S338 phosphorylation of raf-1 is independent of phosphatidylinositol 3-kinase and pak3. Mol. Cell. Biol., 21, 2423–2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong H., Lee,J. and Guan,K.L. (2001) Positive and negative regulation of Raf kinase activity and function by phosphorylation. EMBO J., 20, 3716–3727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhillon A.S., Pollock,C., Steen,H., Shaw,P.E., Mischak,H. and Kolch,W. (2002) The cAMP dependent kinase regulates the Raf-1 kinase mainly by phosphorylation of serine 259. Mol. Cell. Biol., in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz B., Barnard,D., Filson,A., MacDonald,S., King,A. and Marshall,M. (1997) Phosphorylation of Raf-1 serine 338 serine 339 is an essential regulatory event for Ras-dependent activation and biological signaling. Mol. Cell. Biol., 17, 4509–4516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douville E. and Downward,J. (1997) EGF induced SOS phosphorylation in PC12 cells involves P90 RSK-2. Oncogene, 15, 373–383. [DOI] [PubMed] [Google Scholar]
- Fabian J.R., Daar,I.O. and Morrison,D.K. (1993) Critical tyrosine residues regulate the enzymatic and biological activity of Raf-1 kinase. Mol. Cell. Biol., 13, 7170–7179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabian J.R., Vojtek,A.B., Cooper,J.A. and Morrison,D.K. (1994) A single amino acid change in Raf-1 inhibits Ras binding and alters Raf-1 function. Proc. Natl Acad. Sci. USA, 91, 5982–5986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häfner S. et al. (1994) Mechanism of inhibition of Raf-1 by protein kinase A. Mol. Cell. Biol., 14, 6696–6703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagemann C. and Rapp,U.R. (1999) Isotype-specific functions of Raf kinases. Exp. Cell Res., 253, 34–46. [DOI] [PubMed] [Google Scholar]
- Houslay M.D. and Kolch,W. (2000) Cell-type specific integration of cross-talk between extracellular signal-regulated kinase and cAMP signaling. Mol. Pharmacol., 58, 659–668. [PubMed] [Google Scholar]
- Hu C.D., Kariya,K., Tamada,M., Akasaka,K., Shirouzu,M., Yokoyama,S. and Kataoka,T. (1995) Cysteine-rich region of Raf-1 interacts with activator domain of post-translationally modified Ha-Ras. J. Biol. Chem., 270, 30274–30277. [DOI] [PubMed] [Google Scholar]
- Improta-Brears T., Ghosh,S. and Bell,R.M. (1999) Mutational analysis of Raf-1 cysteine rich domain: requirement for a cluster of basic amino acids for interaction with phosphatidylserine. Mol. Cell. Biochem., 198, 171–178. [DOI] [PubMed] [Google Scholar]
- Jaumot M. and Hancock,J.F. (2001) Protein phosphatases 1 and 2A promote Raf-1 activation by regulating 14-3-3 interactions. Oncogene, 20, 3949–3958. [DOI] [PubMed] [Google Scholar]
- Kerkhoff E. and Rapp,U.R. (2001) The Ras–Raf relationship: an unfinished puzzle. Adv. Enzyme Regul., 41, 261–267. [DOI] [PubMed] [Google Scholar]
- King A.J., Sun,H., Diaz,B., Barnard,D., Miao,W., Bagrodia,S. and Marshall,M.S. (1998) The protein kinase Pak3 positively regulates Raf-1 activity through phosphorylation of serine 338. Nature, 396, 180–183. [DOI] [PubMed] [Google Scholar]
- Kinuya M., Takishima,K. and Mamiya,G. (2000) Detection of kinases that phosphorylate 14-3-3 binding sites of Raf-1 using in situ gel kinase assay. Biol. Pharm. Bull., 23, 1158–1162. [DOI] [PubMed] [Google Scholar]
- Kolch W. (2000) Meaningful relationships: the regulation of the Ras/Raf/MEK/ERK pathway by protein interactions. Biochem. J., 351, 289–305. [PMC free article] [PubMed] [Google Scholar]
- Kortenjann M., Thomae,O. and Shaw,P.E. (1994) Inhibition of v-raf-dependent c-fos expression and transformation by a kinase-defective mutant of the mitogen-activated protein kinase Erk2. Mol. Cell. Biol., 14, 4815–4824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenormand P., Brondello,J.M., Brunet,A. and Pouyssegur,J. (1998) Growth factor-induced p42/p44 MAPK nuclear translocation and retention requires both MAPK activation and neosynthesis of nuclear anchoring proteins. J. Cell Biol., 142, 625–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis T.S., Shapiro,P.S. and Ahn,N.G. (1998) Signal transduction through MAP kinase cascades. Adv. Cancer Res., 74, 49–139. [DOI] [PubMed] [Google Scholar]
- Li W., Han,M. and Guan,K.L. (2000) The leucine-rich repeat protein SUR-8 enhances MAP kinase activation and forms a complex with ras and Raf. Genes Dev., 14, 895–900. [PMC free article] [PubMed] [Google Scholar]
- Luo Z.J., Diaz,B., Marshall,M.S. and Avruch,J. (1997) An intact Raf zinc finger is required for optimal binding to processed Ras and for Ras-dependent Raf activation in situ. Mol. Cell. Biol., 17, 46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marais R., Light,Y., Paterson,H.F. and Marshall,C.J. (1995) Ras recruits Raf-1 to the plasma membrane for activation by tyrosine phosphorylation. EMBO J., 14, 3136–3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason C.S., Springer,C.J., Cooper,R.G., Superti-Furga,G., Marshall,C.J. and Marais,R. (1999) Serine and tyrosine phosphorylations cooperate in Raf-1, but not B-Raf activation. EMBO J., 18, 2137–2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaud N.R., Fabian,J.R., Mathes,K.D. and Morrison,D.K. (1995) 14-3-3 is not essential for Raf-1 function: identification of Raf-1 proteins that are biologically activated in a 14-3-3- and Ras-independent manner. Mol. Cell. Biol., 15, 3390–3397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mischak H., Seitz,T., Janosch,P., Eulitz,M., Steen,H., Schellerer,M., Philipp,A. and Kolch,W. (1996) Negative regulation of Raf-1 by phosphorylation of serine 621. Mol. Cell. Biol., 16, 5409–5418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriguchi T., Gotoh,Y. and Nishida,E. (1995) Activation of two isoforms of mitogen-activated protein kinase kinase in response to epidermal growth factor and nerve growth factor. Eur. J. Biochem., 234, 32–38. [DOI] [PubMed] [Google Scholar]
- Morrison D.K. (2001) KSR: a MAPK scaffold of the Ras pathway? J. Cell Sci., 114, 1609–1612. [DOI] [PubMed] [Google Scholar]
- Morrison D.K., Heidecker,G., Rapp,U.R. and Copeland,T.D. (1993) Identification of the major phosphorylation sites of the Raf-1 kinase. J. Biol. Chem., 268, 17309–17316. [PubMed] [Google Scholar]
- Muller G., Storz,P., Bourteele,S., Doppler,H., Pfizenmaier,K., Mischak,H., Philipp,A., Kaiser,C. and Kolch,W. (1998) Regulation of Raf-1 kinase by TNF via its second messenger ceramide and cross-talk with mitogenic signalling. EMBO J., 17, 732–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muslin A.J., Tanner,J.W., Allen,P.M. and Shaw,A.S. (1996) Interaction of 14-3-3 with signalling proteins is mediated by the recognition of phosphoserine. Cell, 84, 889–897. [DOI] [PubMed] [Google Scholar]
- Rommel C., Radziwill,G., Lovric,J., Noeldeke,J., Heinicke,T., Jones,D., Aitken,A. and Moelling,K. (1996) Activated Ras displaces 14-3-3 protein from the amino terminus of c-Raf-1. Oncogene, 12, 609–619. [PubMed] [Google Scholar]
- Sendoh H. et al. (2000) Role of Raf-1 conserved region 2 in regulation of Ras-dependent Raf-1 activation. Biochem. Biophys. Res. Commun., 271, 596–602. [DOI] [PubMed] [Google Scholar]
- Stokoe D., Macdonald,S.G., Cadwallader,K., Symons,M. and Hancock,J.F. (1994) Activation of Raf as a result of recruitment to the plasma membrane. Science, 264, 1463–1467. [DOI] [PubMed] [Google Scholar]
- Thorson J.A., Yu,L.W.K., Hsu,A.L., Shih,N.Y., Graves,P.R., Tanner,J.W., Allen,P.M., Piwnica-Worms,H. and Shaw,A.S. (1998) 14-3-3 proteins are required for maintenance of raf-1 phosphorylation and kinase activity. Mol. Cell. Biol., 18, 5229–5238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traverse S., Cohen,P., Paterson,H., Marshall,C., Rapp,U. and Grand,R.J. (1993) Specific association of activated MAP kinase kinase kinase (Raf) with the plasma membranes of ras-transformed retinal cells. Oncogene, 8, 3175–3181. [PubMed] [Google Scholar]
- Tzivion G., Luo,Z. and Avruch,J. (1998) A dimeric 14-3-3 protein is an essential cofactor for Raf kinase activity. Nature, 394, 88–92. [DOI] [PubMed] [Google Scholar]
- Weiss R.H., Maga,E.A. and Ramirez,A. (1998) MEK inhibition augments Raf activity, but has variable effects on mitogenesis, in vascular smooth muscle cells. Am. J. Physiol., 274, C1521–C1529. [DOI] [PubMed] [Google Scholar]
- Williams J.G., Drugan,J.K., Yi,G.S., Clark,G.J., Der,C.J. and Campbell,S.L. (2000) Elucidation of binding determinants and functional consequences of RAS/RAF-CRD interactions. J. Biol. Chem., 275, 22172–22179. [DOI] [PubMed] [Google Scholar]
- Wu J., Dent,P., Jelinek,T., Wolfman,A., Weber,M.J. and Sturgill,T.W. (1993) Inhibition of the EGF-activated MAP kinase signaling pathway by adenosine 3′,5′-monophosphate. Science, 262, 1065–1069. [DOI] [PubMed] [Google Scholar]
- Yaffe M.B., Rittinger,K., Volinia,S., Caron,P.R., Aitken,A., Leffers,H., Gamblin,S.J., Smerdon,S.J. and Cantley,L.C. (1997) The structural basis for 14-3-3:phosphopeptide binding specificity. Cell, 91, 961–971. [DOI] [PubMed] [Google Scholar]
- Yeung K. et al. (1999) Suppression of Raf-1 kinase activity and MAP kinase signalling by RKIP. Nature, 401, 173–177. [DOI] [PubMed] [Google Scholar]
- Yeung K., Janosch,P., McFerran,B., Rose,D.W., Mischak,H., Sedivy,J.M. and Kolch,W. (2000) Mechanism of suppression of the Raf/MEK/extracellular signal-regulated kinase pathway by the raf kinase inhibitor protein. Mol. Cell. Biol., 20, 3079–3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng C.F. and Guan,K.L. (1994) Cytoplasmic localization of the mitogen-activated protein kinase activator MEK. J. Biol. Chem., 269, 19947–19952. [PubMed] [Google Scholar]
- Zimmermann S. and Moelling,K. (1999) Phosphorylation and regulation of raf by akt (protein kinase B). Science, 286, 1741–1744. [DOI] [PubMed] [Google Scholar]