Abstract
Intraerythrocytic survival of the malaria parasite Plasmodium falciparum requires that host cells supply nutrients and dispose of waste products. This solute transport is accomplished by infection-induced new permeability pathways (NPP) in the erythrocyte membrane. Here, whole-cell patch–clamp and hemolysis experiments were performed to define properties of the NPP. Parasitized but not control erythrocytes constitutively expressed two types of anion conductances, differing in voltage dependence and sensitivity to inhibitors. In addition, infected but not control cells hemolyzed in isosmotic sorbitol solution. Both conduct ances and hemolysis of infected cells were inhibited by reducing agents. Conversely, oxidation induced identical conductances and hemolysis in non-infected erythrocytes. In conclusion, P.falciparum activates endogenous erythrocyte channels by applying oxidative stress to the host cell membrane.
Keywords: hemolysis/human red blood cells/malaria/new permeability pathway/patch−clamp
Introduction
In the blood stage of its life cycle, the malaria parasite Plasmodium falciparum multiplies asexually in human erythrocytes. Within 48 h of development, Plasmodium increases its body mass by up to 32-fold. This rapid growth requires supply of nutrients and disposal of waste products, both of which are accomplished by an infection-induced increase in solute transport across the host plasma membrane (Ginsburg and Kirk, 1998). Tracer flux and isosmotic hemolysis experiments indicate that Plasmodium infection induces a broad specificity pathway in the erythrocyte membrane, the so-called new permeability pathway (NPP) which transports a variety of anionic, cationic and neutral solutes such as sorbitol (Ginsburg and Kirk, 1998). Accordingly, infected erythrocytes are hemolyzed in solutions where NaCl is replaced by sorbitol. The NPP is highly selective for Cl– over K+ and blocked by several anion channel inhibitors (Breuer et al., 1987; Kutner et al., 1987; Kirk et al., 1993, 1994; Kirk and Horner, 1995). Recent patch–clamp experiments indeed revealed the induction of an inwardly rectifying anion channel upon Plasmodium infection (Desai et al., 2000).
The present study has been performed to define the properties of the NPP using patch–clamp techniques. Moreover, the study aimed to identify the underlying induction mechanisms. The NPP might be due to Plasmodium-encoded xenoproteins accumulating in the erythrocyte membrane or reflect endogenous erythrocyte proteins activated by Plasmodium. The parasite confers a high oxidative stress on the host erythrocyte (Atamna and Ginsburg, 1993, 1997; Atamna et al., 1994; Becker et al., 1994; Ginsburg and Atamna, 1994). Thus, the possibility has been explored whether oxidative stress could induce an NPP-like conductance in the erythrocyte cell membrane.
Results
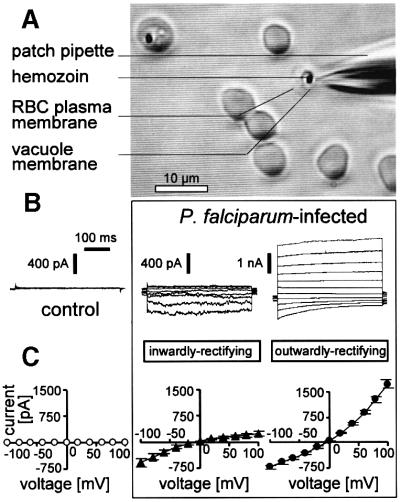
Most control erythrocytes (22 out of 27 cells) exhibited whole-cell currents of <100 pS (70 ± 11 pS; Figure 1B and C, left). Few controls (n = 5) expressed higher conductances in the nS range. These conductances are characterized below (Figure 6A, B and F). In sharp contrast, whole-cell conductances of trophozoite-infected erythrocytes (Figure 1A) were all in the 10 nS range. Two different types of conductances were apparent. Fourteen out of a total of 102 cells exhibited inwardly rectifying currents (Figure 1B, middle) with almost no outward current at positive voltages (Figure 2A) and with a mean conductance of Ginward = 7 ± 1 nS (Figure 1C, middle). The majority of the infected cells (88 cells), however, expressed additionally or predominantly outwardly rectifying currents (Figure 1B, right). The outwardly rectifying current (Goutward = 18 ± 1 nS; Figure 1C; right) exhibited a variable phenotype with respect to rectification, time-dependent activation at depolarization and inactivation at hyperpolarization (compare Figure 1B, right with Figures 2H, left and 6D, left). Due to this inactivation, cells with predominant outwardly rectifying current exhibited only little sustained inward currents (Figure 2H).
Fig. 1. Whole-cell patch–clamp recording in P.falciparum-infected erythrocytes. (A) Light micrograph taken during recording of a trophozoite stage-infected erythrocyte. In addition to the recorded cell, a further trophozoite stage-infected cell (upper left) and several apparently non-infected cells are shown. Note bleaching of the recorded cell due to dialysis of the hemoglobin by the pipet solution. (B) Original current traces recorded in a non-infected human erythrocyte (control; left) and two trophozoite stage-infected cells which expressed inwardly (middle) and outwardly rectifying currents (right), respectively. (C) Current–voltage relationships recorded as in (B) from non-infected control erythrocytes (n = 22; left) and trophozoite-infected cells expressing inwardly (n = 7; middle) and outwardly rectifying currents (n = 35; right).
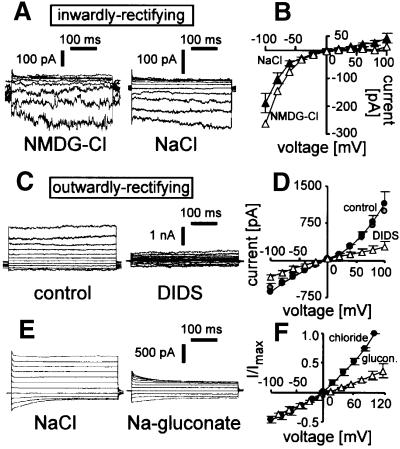
Fig. 6. Inwardly and outwardly rectifying Cl– currents in non-infected cells. (A and B) Inwardly rectifying current. Original traces (A, left) and I–V curve (B) (open symbols) of a spontaneously expressed inwardly rectifying current recorded with NMDG-Cl in the bath and pipet. In addition, in (B) (closed symbols) is the mean I–V curve of one untreated and five oxidized cells expressing inwardly rectifying currents as recorded with the standard NaCl bath and pipet solution. Original current traces of an oxidized cell recorded with NaCl in the bath and pipet are in (A) (right). (C–F) Outwardly rectifying current. Original current traces (C) and mean I–V curves (F) recorded with NaCl bath and pipet solution in oxidized cells before (C, left; and D, black circles) and during bath application of DIDS (100 µM) (C, right; and D, open triangles; n = 4). Gray circles in (D) indicate the averaged I–V curve of all oxidized cells expressing outwardly rectifying currents (n = 13) as recorded in (C) (left). (E) Original current trace of an oxidized cell with outwardly rectifying current recorded in NaCl (left) and Na-gluconate bath solution (right). (F) Mean I–V curves recorded as in (E) first in NaCl (closed circles) and then in Na-gluconate bath solution (open triangle). Shown are the normalized currents of one untreated and two oxidized cells.
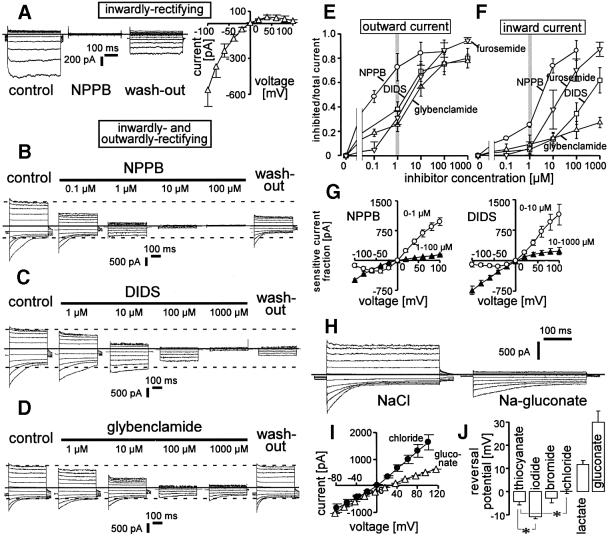
Fig. 2. Whole-cell currents of infected erythrocytes were anion-selective. (A) Original current traces of an infected cell expressing inwardly rectifying currents before (control), during (NPPB) and after (wash-out) application of NPPB (100 µM) to the bath solution (zero current is indicated by solid line). The mean I–V curve in (A) (right) shows the NPPB-sensitive current fraction of n = 4 cells expressing inwardly rectifying currents. (B–D) Original current traces of infected cells expressing both phenotypes recorded before (control; left), during (middle) and after (wash-out; right) applying increasing concentrations (as indicated) of NPPB (B), DIDS (C) and glybenclamide (D) in the bath. Maximal outward and inward currents as well as zero current are indicated by dashed and solid lines. (E and F) Dependence of outward (E) and inward current (F) on the concentration of NPPB (circles), DIDS (squares), furosemide (inverted triangles) and glybenclamide (triangles). Outward and inward currents (n = 5–8) were determined as in (B–D) at +100 mV and –100 mV voltage, respectively. (G) Mean I–V curves (n = 4–6) of the current fractions inhibited by increasing concentrations of NPPB (0–1 µM, circles; 1–100 µM, triangles; n = 4) and DIDS (0–10 µM, circles; 10–1000 µM, triangles; n = 6). The sensitive current fractions were recorded as shown in (B–D). (H) Original traces of the outwardly rectifying current recorded first with NaCl (left) and then with Na-gluconate bath solution (right; zero current is indicated by the solid line). (I) Mean I–V relationships of mixed outwardly and inwardly rectifying currents recorded in paired experiments in NaCl (circles) and Na-gluconate (triangles; n = 13). (J) Mean reversal potential of the currents recorded with different anions in the bath as indicated (n = 3–17; *P ≤0.05).
The inverse voltage dependence of the outward and inward rectifying current allowed simultaneous determination of individual blocker sensitivities in cells with both current phenotypes (Figure 2B–D). The reported blockers of the NPP, 5-nitro-2-(3-phenyl-propylamino)-benzoic acid (NPPB), 4,4′-diisothiocyano-stilbene-2,2′-disulfonic acid (DIDS), furosemide and glybenclamide (Kirk et al., 1994; Ginsburg and Kirk, 1998), inhibited the outward current at +100 mV with IC50s in the range of 100 nM (NPPB) and 1–10 µM (DIDS, glybenclamide and furosemide; Figure 2E). In contrast, the IC50s of the inward current at –100 mV were >1 µM (NPPB), >100 µM (DIDS), >1 mM (glybenclamide) and >10 µM (furosemide; Figure 2F), indicating lower sensitivities to those inhibitors of the inward rectifier as compared with the outward rectifier: accordingly, the current–voltage (I–V) curve of the current fractions inhibited by low concentrations of NPPB (1 µM) and DIDS (10 µM) rectified outwardly (Figure 2G, open circles) while the current fractions further inhibited by increasing the blocker concentrations to 100 µM and 1 mM, respectively, rectified inwardly (Figure 2G, closed triangles).
Substitution of Cl– in the bath with gluconate decreased the outward current and shifted the reversal potential of the I–V curve, with the change of ECl indicating anion selectivity (Figure 2H and I). In addition, applying Na-gluconate in the bath apparently decreased the time-dependent inactivating inward current and increased the time constant of inactivation (Figure 2H; see also Figure 6E). This modification of inactivation kinetics might suggest an open channel block by gluconate. The permselectivity of the overall (i.e. inwardly and outwardly rectifying) current was I–>SCN–≈Br–≥Cl– >lactate>gluconate as deduced from the reversal potential under bi-ionic conditions (Figure 2J).
To identify the influence of the redox state on the infection-induced conductances, reducing agents were applied during continuous whole-cell recording. The reducing agent dithioerythritol (DTE, 100 µM) added to the bath irreversibly inactivated inwardly and outwardly rectifying currents of infected cells within 5 min of incubation (Figure 3A–D). Furthermore, applying reduced glutathione (GSH; 10 mM) in the pipet solution induced a run-down of both currents, while oxidized glutathione (GSSG; 10 mM) had no effect (Figure 3E–H). In all cells with comparable amounts of both currents (n = 3), the GSH-induced run down of the inwardly rectifying current appeared before that of the outward rectifier. The inwardly rectifying current ran down within 3.0 ± 0.5 min (n = 5) while the decrease of outwardly rectifying current equilibrated within 7 ± 1 min (n = 9). This might suggest a difference in redox sensitivity between both induced currents.
Fig. 3. Infection-induced currents were sensitive to reduction. (A–D) Original traces of inwardly (A) and outwardly (C) rectifying currents before (left) and after incubation with DTE (100 µM; right). In (B) and (D), the corresponding mean DTE-sensitive current fractions (n = 3 and n = 6, respectively) are plotted against the voltage. (E–H) Run-down of infection-induced currents by reduced glutathione (GSH; 10 mM) added to the pipet solution. Original traces of inwardly (E) and outwardly (G) rectifying currents during continuous whole-cell recording. The incubation time beginning with dialysis of the cytosol by the pipet solution upon achievement of whole-cell recording mode is indicated. In (F) (n = 5; open triangles) and (H) (open circles; n = 9) are the I–V curves of the corresponding GSH-sensitive current fractions. In addition, the I–V plots in (F and H) (closed symbols) show that no current change was induced within 8 ± 1.5 min (n = 7) of recording when oxidized glutathione (GSSG; 10 mM) was added to the pipet solution as recorded in unpaired control experiments from cells expressing inwardly and outwardly rectifying currents (n = 3 and n = 4), respectively.
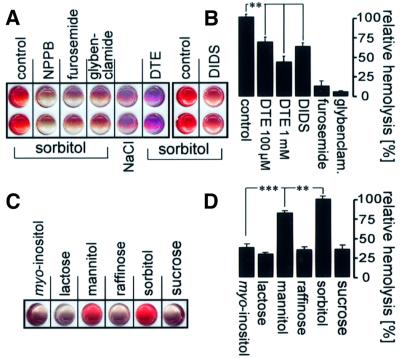
Similarly to the currents, hemolysis of infected erythrocytes in isosmotic sorbitol (10 min of incubation) was sensitive to NPPB, furosemide, glybenclamide, DIDS (each 100 µM) and to reduction (DTE, 100 µM and 1 mM, respectively; Figure 4A and B). DIDS, furosemide and glybenclamide inhibited 37 ± 4% (n = 16), 87 ± 7% (n = 3) and 94 ± 1% (n = 3), respectively, of the sorbitol-induced hemolysis fraction that was inhibitable by NPPB (100 µM; Figure 4B). In addition, hemolysis of infected cells in isosmotic concentrations of different carbohydrates revealed a permeability rank order of sorbitol>mannitol>myo-inositol≈lactose≈sucrose≈raffinose (Figure 4C and D). Interestingly, the hemolysis of infected cells was significantly smaller in isosmotic lactose (0.54 ± 0.04, n = 6) and in isosmotic sorbitol in the presence of NPPB (0.65 ± 0.01, n = 3) as compared with that in NaCl in the absence of NPPB, suggesting significant NaCl entry and hemolysis in infected cells exposed to isotonic saline.
Fig. 4. Hemolysis of infected cells in isosmotic sorbitol solution. (A and B) Hemolysis was sensitive to reduction, NPPB, furosemide, glybenclamide and DIDS. Enriched trophozoite-infected erythrocytes were suspended in isosmotic sorbitol or, for control, in NaCl and incubated in the presence and absence of DIDS, NPPB, furosemide, glybenclamide (each 100 µM) and DTE (100 µM and 1 mM), respectively. Incubation was stopped by centrifugation, and hemolysis was indicated by hemoglobin in the supernatant. In (A) are the scanned images of supernatants from two individual experiments performed in duplicate. (B) Mean hemolysis in isosmotic sorbitol in the absence (control) and presence of DTE (100 µM and 1 mM, respectively), DIDS, furosemide and glybenclamide (each 100 µM), respectively. The hemoglobin concentration of the supernatants was determined photometrically and data were expressed as a percentage of that fraction of total hemolysis that could be inhibited by NPPB (n = 3–16; **P ≤0.01). (C and D) Substrate dependence of the infection-induced isosmotic hemolysis. In (C) are the imaged supernatants (individual experiment) and in (D) the mean relative hemolysis of cells incubated in different isosmotic carbohydrate solutions as indicated (n = 8–9; **P ≤0.01; ***P ≤0.001).
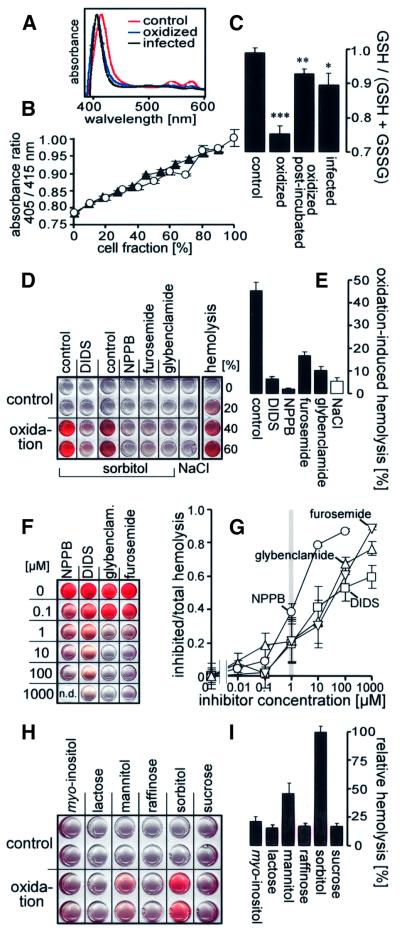
Since both infection-induced currents and hemolysis in isosmotic sorbitol were sensitive to reduction, and P.falciparum is known to confer oxidative stress on the host cell membrane (Atamna and Ginsburg, 1993), oxidation might be directly involved in the induction of the NPP. To test this possibility, non-infected erythrocytes (in NaCl solution) were treated with t-butylhydroxyperoxide (t-BHP; 1 mM for 15 min), resuspended in NaCl and post-incubated for a further 2.5 h before analysis. In order to estimate whether this in vitro oxidation mimics the oxidative stress in parasitized erythrocytes, methemoglobin (metHb) and the ratio between reduced and total (i.e. reduced and oxidized) glutathione [GSH/(GSH + GSSG) ratio] was determined in both conditions (Figure 5A–C). Hemoglobin of oxidized/post-incubated and trophozoite/schizont-infected cells (90% enriched) exhibited a spectral light absorbance similar to that of metHb, while hemoglobin of control cells had that of oxyHb, suggesting profound metHb formation in both infected and oxidized erythrocytes (Figure 5A). The metHb/oxyHb ratio increased to a similar extent with increasing fraction of infected and oxidized cells, respectively (Figure 5B), suggesting a similar degree of oxidation of hemoglobin in infected and oxidized cells.
Fig. 5. Oxidation-induced isosmotic hemolysis of non-infected cells. (A) Absorbance spectrum of the hemolysate of control, oxidized and infected erythrocytes, respectively. (B) Oxidized (open circles; n = 6) and highly enriched (90%) trophozoite/schizont-infected erythrocytes (closed triangles; n = 6) were mixed with control cells, hemolyzed and the absorbance was determined at the Soret bands of metHb (405 nm) and oxyHb (415 nm; Sakai et al., 2000). The 405/415 nm absorbance ratio is plotted against the fraction of oxidized and infected cells (in % of total cells), respectively. (C) The redox state [GSH/(GSH + GSSG) ratio] of control (n = 43), infected (n = 19) and oxidized erythrocytes was determined photometrically using the sulfhydryl group-reactive dye DTNB. The oxidized cells were investigated either directly after oxidation (oxidation; n = 17) or after a further 2.5 h period of post-incubation (oxidized post-incubated; n = 12). The reduced sulfhydryl groups were determined in the supernatant of heat-denaturated, centrifuged erythrocytes before (GSH) and after reduction by KHB4 (GSH + GSSG; *P ≤0.05, **P ≤0.01, ***P ≤0.001). (D) Imaged supernatants of untreated (control; lines 1 and 2) and oxidized erythrocytes (oxidation; lines 3 and 4) after incubation in sorbitol (or, for control, in NaCl) in the absence (control, NaCl) or presence of different blockers as indicated (shown is an individual experiments performed in duplicate). (E) Mean oxidation-induced hemolysis in sorbitol (closed columns; n = 8–15) and NaCl solution (open column; n = 8) in the absence (control, NaCl) and presence of the blockers (all 100 µM). (F and G) Sensitivity of oxidation-induced sorbitol hemolysis to various concentrations of NPPB (circles), DIDS (squares), furosemide (inverted triangles) and glybenclamide (triangles). Imaged supernatants (F; individual experiments; n.d. = not determined) and (G) mean dose–response curves (n = 4–15). (H and I) Substrate dependence of the oxidation-induced isosmotic hemolysis. Imaged supernatants (H; individual experiment in duplicate) and (I) mean relative oxidation-induced hemolysis of cells in different isosmotic carbohydrate solutions (n = 7–10).
In control erythrocytes, the mean GSH/(GSH + GSSG) ratio was not different from 1.0 (Figure 5C). This ratio decreased to 0.75 ± 0.02 (n = 17) within 15 min of exposure to t-BHP (1 mM) and approached 0.93 ± 0.01 (n = 12) within the following 2.5 h of incubation in NaCl solution, i.e. at the time when hemolysis was determined and patch–clamp experiments were performed (Figure 5C). Highly enriched trophozoite/schizont-infected cells exhibited a GSH/(GSH + GSSG) ratio of 0.90 ± 0.03 (n = 19), which was not significantly different from that of oxidized, post-incubated cells (Figure 5C), suggesting a similar state of GSH oxidation in trophozoite/schizont-infected erythrocytes and cells oxidized in vitro.
To test for the involvement of oxidative processes in the induction of the NPP, oxidized cells (t-BHP/15 min) were suspended in isosmotic sorbitol or NaCl solution. Within 2.5 h of incubation, almost 50% of these erythrocytes hemolyzed in sorbitol but only 5% in NaCl solution, suggesting the induction of a sorbitol permeability by oxidation (Figure 5D and E). The oxidation-induced hemolysis in sorbitol was inhibited by NPPB, furosemide and glybenclamide (all 100 µM) by 93 ± 5% (n = 21), 61 ± 6% (n = 23) and 71 ± 1% (n = 20; Figure 5E), respectively, values similar to the inhibitory effect of those drugs on the infection-induced hemolysis in isosmotic sorbitol (Figure 4B). Also similarly to hemolysis of infected cells, oxidation-induced hemolysis in sorbitol in the presence of NPPB was less than in NaCl (Figure 5E), suggesting oxidation-induced net uptake of NaCl by the erythrocyte.
DIDS (100 µM) inhibited the oxidation-induced hemolysis in sorbitol (Figure 5E) more efficiently than the infection-induced hemolysis (Figure 4B). Since this difference might be due to the different incubation times (2.5 h versus 10 min) applied in oxidation- and infection-induced hemolysis, the effect of DIDS upon acute exposure was studied with a modified hemolysis protocol. Acute addition (10 min) of DIDS (100 µM) inhibited hemolysis in oxidized cells by 48 ± 6% (n = 18; Figure 5F and G), a value not significantly different from acute inhibition of hemolysis in infected cells (37 ± 4%, Figure 4B).
NPPB inhibited oxidation-induced hemolysis in sorbitol with an IC50 of ∼1 µM, while DIDS (acute application), glybenclamide and furosemide exerted half-maximal inhibition at a concentration of ∼10–30 µM (Figure 5F and G). The IC50s resembled those for the outwardly rectifying current (Figure 2E), but were very different from those for the inwardly rectifying current (Figure 2F).
Oxidation-induced hemolysis in isosmotic solutions of different carbohydrates indicated a permeability rank order of sorbitol>mannitol>myo-inositol≈lactose≈sucrose≈ raffinose identical to that of the infection-induced hemolysis (Figure 5H and I).
Taken together, these experiments indicated that the induction of hemolysis in isosmotic sorbitol by P.falciparum infection was mimicked by oxidation of non-infected cells.
While most non-infected, untreated control cells exhibited whole-cell conductances <100 pS, two and three (out of 27 cells) spontaneously expressed inwardly and outwardly rectifying currents, respectively. The inwardly rectifying current was Cl–-sensitive, as demonstrated in an experiment where the impermeable cation N-methyl-d-glutamine (NMDG) was used in bath and pipet solution (Figure 6A, left; and B, open symbols). In a further cell with outwardly rectifying current, bath replacement of Cl– by gluconate reversibly decreased outward currents (Figure 6F), indicating Cl– selectivity of this current. Thus, these spontaneous currents were similar to those of infected cells, suggesting that endogenous erythrocyte proteins generated the infection-induced conductances.
To identify the electrophysiological correlate of the oxidation-induced sorbitol permeability, and to test whether the endogenous erythrocyte channels were indeed activated by oxidation, non-infected erythrocytes were oxidized with t-BHP (15 min), post-incubated (2.5 h) and analyzed by whole-cell recording. The oxidized cells (n = 18) expressed inwardly (n = 5; Figure 6A, right; and B, closed symbols) and outwardly rectifying currents (n = 13; Figure 6C–F). The outwardly rectifying current of non-infected cells was inhibited irreversibly by DIDS (100 µM; Figure 6C and D). In addition, bath replacement of Cl– by gluconate reversibly decreased outward currents (n = 2), indicating Cl– selectivity of the oxidation-induced outwardly rectifying current (Figure 6E and F). In summary, currents of non-infected cells resemble those of infected cells in rectification behavior, Cl– selectivity and (in the case of outwardly rectifying currents) DIDS sensitivity.
Discussion
Increasing resistance of P.falciparum against conventional chemotherapy demands new pharmacological targets for the treatment of malaria. The NPP is required for the development and survival of the parasite, and blockage of the NPP results in inhibition of Plasmodium growth in vitro (Kutner et al., 1987; Kirk et al., 1993; Kirk and Horner, 1995; our own unpublished results). Thus, the NPP might be an excellent target for pharmacological treatment and prevention of malaria (Pasvol, 2001).
Functionally, the NPP is a Cl– channel with significant permeability for organic osmolytes and cations (Ginsburg and Kirk, 1998). In the present study, oxidized cells hemolyzed in isosmotic carbohydrate solutions with a substrate specificity identical to that of infected cells. In addition, hemolysis of both infected and oxidized cells in sorbitol was inhibited by NPPB, glybenclamide and furosemide, all blockers which have been reported to inhibit NPP-associated hemolysis or tracer fluxes of infected cells (Ginsburg and Kirk, 1998). Moreover, in the present and a previous study (Kirk et al., 1994), DIDS (100 µM) inhibited ∼40% of the NPPB-inhibitable infection-induced hemolysis in sorbitol and ∼40–50% of the NPP-associated choline uptake by parasitized erythrocytes, respectively. Futhermore, DTE attenuated hemolysis of infected cells in sorbitol. In addition, DTE and GSH inhibited in infected cells, and oxidation induced in non-infected cells, two anion conductances that were inhibited by NPPB, DIDS, furosemide and glybenclamide with different efficacies. Although not proven directly, these data strongly suggest that the oxidation- and infection-induced (and reduction-inhibited) permeability of sorbitol (i.e. the NPP) was generated by the oxidation- and infection-induced (and reduction-inhibited) anion channels.
NPPB, furosemide, DIDS and glybenclamide blocked the inwardly rectifying current with IC50s of >1 µM, >10 µM, >100 µM and >1 mM, respectively. The outwardly rectifying current, however, proved to be highly sensitive to all four blockers (IC50 0.1–10 µM). The inhibitor sensitivity of the oxidation-induced hemolysis in isosmotic sorbitol was similar to that of the outwardly rectifying current but only partially to that of the inward rectifier. This suggests that the outwardly rectifying current participates in the generation of the sorbitol pathway.
Besides the blocker sensitivities and voltage dependence, the inwardly and outwardly rectifying currents differed in their permselectivities. For the inwardly rectifying current, a permeability sequence of SCN–>I–>Cl– has been reported (Desai et al., 2000), while the mixed current of the present study exhibited a permeability rank order of I–>SCN–>Cl–. This further suggests that both currents were generated by different channel types (i.e. proteins/protein complexes) and also suggests that the NPP represents more than one type of protein (protein complex). Moreover, the outwardly rectifying current of the present study displayed a variable phenotype with respect to rectification and time-dependent activation/inactivation at high depolarizing/hyperpolarizing voltages. This variability raises the possibility that the outwardly rectifying current itself was generated by more than one channel type, a possibility which cannot be ruled out by the present study.
Desai et al. (2000) have reported the inwardly rectifying anion current in P.falciparum-infected human erythrocytes. In the present study, several infected cells similarly expressed only the inwardly rectifying current. The majority of infected cells, however, exhibited additionally or predominantly the outwardly rectifying current. Specific experimental conditions, such as host cell swelling, erythrocyte age and pathogen strain, might have specifically induced this additional channel in the present study. However, swelling and shrinkage of infected cells did not modulate the outwardly rectifying current (data not shown). Furthermore, the outwardly rectifying Cl– channel was observed in both infected banked and infected fresh erythrocytes, and was not only observed after infection with the BINH P.falciparum strain but also with the P.falciparum strain FCR-3 (see Materials and methods). Since inwardly and outwardly rectifying currents might differ in their redox sensitivity (as suggested by the present study), parasite stage-induced differences in oxidation states might account, in theory, for the heterogeneous phenotype of the infected cells observed in the present study. However, it is unlikely that the differences between the present study and that of Desai et al. (2000) result from differences in parasite developmental stages since even in synchronized cultures several developmental stages are present that can hardly be distinguished in the recorded cell by phase contrast microscopy. It is noteworthy that the Cl– channels described by Desai et al. (2000) were blocked by 100 µM glybenclamide, a property of the outwardly rectifying Cl– channels in the present study. This might hint at the possibility that a mixed current phenotype might also have been recorded at least in some experiments of this previous study.
Evidence for the presence of Cl– channels in non-infected human erythrocytes has been reported before (Freedman and Miller, 1984; Schwarz et al., 1989; Freedman et al., 1994; Freedman and Novak, 1997; Huber et al., 2001). Specifically, a DIDS-sensitive (Freedman and Miller, 1984) and a hyperpolarization-induced, DIDS-insensitive Cl– conductance (Freedman et al., 1994; Freedman and Novak, 1997) have been demonstrated by indirect means. Besides the GARDOS K+ channel (Grygorczyk and Schwarz, 1983) and a 30 pS non-selective cation channel (recorded with symmetrical 150 mM KCl; Kaestner et al., 2000), a 6 pS channel with assumed Cl– selectivity has been identified on the single-channel level in non-infected human erythrocytes (Schwarz et al., 1989). The 6 pS channel has a linear I–V relationship and is active at negative membrane potential, while channel opening becomes a very rare event at positive membrane potentials. Desai et al. (2000) observed a Cl–-selective channel with a conductance of <10 pS in infected but not in control human erythrocytes. Similarly to the 6 pS channel, this channel exhibits a linear I–V relationship and a high open probability at negative but not at positive membrane potentials, and most probably generates the infection-induced inwardly rectifying anion current.
The present study showed significant hemolysis of infected and oxidized cells in NaCl solution, suggesting the entry of NaCl which requires the operation of Na+-permeable cation channels in parallel to the Cl– channels. A low non-selective cation conductance has been demonstrated to be activated in non-infected human erythrocytes by oxidative stress (Duranton et al., 2002), and increased activity of non-selective cation channels has indeed been observed in Plasmodium-infected human (Desai et al., 1996) and chicken erythrocytes (Thomas et al., 2001).
Our data reveal that the inwardly and outwardly rectifying anion channels of parasitized erythrocytes were dependent on oxidative stress because opening of the channels by P.falciparum was reversed by addition of reduced GSH, but not by oxidized GSSG, to the pipet solution. Plasmodium falciparum produces H2O2 by digestion of host hemoglobin and, therefore, oxidizes the host cell membrane (Atamna and Ginsburg, 1993). Thus, oxidation can account for activation of the channels and, indeed, infected erythrocytes exhibited an oxidation state similar to that of non-infected cells oxidized in vitro. This was concluded in the present study from a similar extent of metHb formation and comparable decline of the GSH/(GSH + GSSG) ratio in both conditions. A comparable decline in the GSH/(GSH + GSSG) ratio from almost 1.00 (control) to 0.96 (parasitized erythrocyte) has been reported previously (Atamna and Ginsburg, 1997).
The present study demonstrates that the inwardly and outwardly rectifying Cl– channels induced by P.falciparum are endogenous erythrocyte channels. In the absence of oxidative stress, the whole-cell conductance of intact human erythrocytes is in the range of few pS (Hoffman, 1992), indicating inactivity of the endogenous anion channels in mature cells. This is in good agreement with the very low whole-cell conductance of non-infected control cells reported previously (Desai et al., 2000) which was also true for the majority of control cells in the present study. Accordingly, sorbitol-induced hemolysis of the present study was minimal in untreated erythrocytes. Some control cells, however, expressed large whole-cell currents, suggesting channel activity in non-infected cells not exposed to t-BHP. Aging erythrocytes increase their state of oxidation with time (Dumaswala et al., 1999) and might reach oxidation levels sufficient to induce activity of both channel types. This is supported by further experiments where stored erythrocytes increased spontaneous but blockable hemolysis in isosmotic sorbitol with time of storage (our own unpublished results).
In principle, oxidative stress can induce plasma membrane ion conductances either by triggering of signal transduction cascades or by direct modification (or formation) of channel protein complexes. The latter has been demonstrated for the heterodimeric amino acid transporter LAT1/4F2hc: cross-linking of LAT1 and 4F2hc via a cytoplasmatic disulfide bridge by oxidative stress induces a non-selective cation conductance (Wagner et al., 2000). Mature human erythrocytes express a variety of membrane transporters such as, for example, the Na+ and Ca2+ pump, the Cl–/HCO3– exchanger (band-3; AE1), the Na+ (K+)/H+ exchanger, the Na+K+2Cl– and KCl co-transporter, and choline, monocarboxylate, glucose, amino acid and nucleoside carriers (Ginsburg and Kirk, 1998). Indirect or direct modifications of, for example, band-3 by oxidative stress have been reported [including tyro sine phosphorylation (Zipser et al., 1997), clustering (Dumaswala et al., 1999; Hornig and Lutz, 2000) and methyl esterification (Ingrosso et al., 2000)]. Consistently, similar AE1 alterations occur upon Plasmodium infection (Giribaldi et al., 2001). Furthermore, some AE1 isoforms (trout AE1 but not human AE1) behave as a bifunctional protein with both anion exchange and Cl–/organic osmolytes channel functions (Motais et al., 1997). Therefore, oxidation-induced modification of erythrocyte transporters, especially of AE1, might result in infection-induced Cl– conductances. The present study, of course, does not rule out further mechanisms in addition to oxidation participating in the activation of the Cl– conductances.
In conclusion, infection of human erythrocytes with P.falciparum induces two types of endogenous erythrocyte Cl– channels, an inwardly and an outwardly rectifying channel. Both channels in concert are suggested to generate the infection-induced NPP of the erythrocyte membrane. Oxidation of non-infected erythrocytes elicits and reduction of infected erythrocytes abolishes the channel activities, indicating that oxidative processes are involved in the induction of the NPP.
Materials and methods
Parasite culture
An isolate of P.falciparum BINH (Binh et al., 1997), obtained from a diseased traveler after a visit to Kenya, was used. A separate series of patch–clamp experiments was performed with the P.falciparum strain FCR-3. The parasites were cultured according to the modified method of Trager and Jensen (Trager and Jensen, 1976; Cranmer et al., 1997). In brief, RPMI medium 1640 was supplemented with 25 mM HEPES/NaOH pH 7.4, 20 µg/ml gentamicin sulfate, 2 mM glutamine, 200 µM hypoxanthine and 0.5% AlbumaxII (Gibco-BRL). Washed human erythrocytes of blood group O+ (banked and fresh) were added to a hematocrit of 5%. Parasites were maintained at a parasitemia of 2–5% in an atmosphere of 90% N2/5% O2/5% CO2 at 37°C. Synchronization of ring stage-infected erythrocytes was performed by sorbitol treatment (Lambros and Vanderberg, 1979), and trophozoite-infected erythrocytes were enriched by Gelafundin (Braun-Melsungen, Melsungen, Germany) separation (Jensen and Trager, 1978). In addition, late-stage trophozoites and schizonts were enriched magnetically using MACS technology (Staalsoe et al., 1999). In brief, parasite cultures were centrifuged, re-suspended in phosphate-buffered saline (PBS)/2% bovine serum albumin (BSA) and applied to a CS column in a SuperMACS (Miltenyi Biotech, Germany). The flow-through contained uninfected red blood cells and ring stages, whereas the eluted fraction had a parasitemia of up to 90% trophozoites. All reagents were purchased from Sigma (Deisenhofen, Germany) if not stated otherwise.
Oxidation of non-infected erythrocytes
Non-infected erythrocytes of volunteers (different blood groups) were washed and suspended in NaCl solution (140 mM NaCl, 10 mM HEPES/NaOH pH 7.4, 5 mM KCl, 2.5 mM CaCl2, 1 mM MgCl2) to a hematocrit of 5% and stored at 8°C for 1–7 days. Aliquots of the erythrocyte suspension (500 µl) were oxidized by adding 1 ml of NaCl solution containing t-BHP (1 mM final concentration) and centrifuged (15 min total incubation time). For patch–clamp recording, determination of metHb formation and GSH/(GSH + GSSG) ratio, and for some hemolysis experiments, cell pellets (∼30–40 µl) were suspended in NaCl solution (400 µl) and re-incubated at 37°C for 2.5 h (hematocrit ∼6%). Then, cells were centrifuged and resuspended in standard patch–clamp bath solution (see below), NaCl solution [metHb and GSH/(GSH + GSSG) ratio] and in 400 µl of isosmotic sorbitol solution (hemolysis experiments), respectively (see below). For most hemolysis experiments and some determinations of GSH/(GSH + GSSG) ratio, pellets (∼30–40 µl) of oxidized cells (1 mM t-BHP for 15 min) were suspended directly in different isosmotic hemolysis solutions (400 µl) or in NaCl solution [400 µl; GSH/(GSH + GSSG) ratio]. In a few hemolysis experiments, oxidized cells (1 mM t-BHP for 15 min) were washed twice in 1.5 ml of NaCl solution before resuspending in isosmotic sorbitol solution. Non-oxidized control cells were prepared with identical protocols but in the absence of the oxidant.
Patch–clamp recordings
Human erythrocytes were recorded at room temperature. Continuous superfusion (1 ml/min) was applied through a flow system inserted into the dish which reduced the bath volume to ∼200 µl. The bath was grounded via a bridge filled with standard bath solution (see below). Borosilicate glass pipets (6–9 MΩ pipet resistance; GC150 TF-10, Clark Medical Instruments, Pangbourne, UK) manufactured by a microprocessor-driven DMZ puller (Zeitz, Augsburg, Germany) were used in combination with an MS314 electrical micromanipulator (MW, Märzhäuser, Wetzlar, Germany). Currents were recorded in fast whole-cell, voltage-clamp mode, and 3 kHz low-pass filtered by an EPC-9 amplifier (Heka, Lambrecht, Germany) using Pulse software (Heka) and an ITC-16 Interface (Instrutech, Port Washington, NY). After Giga ohm seal formation, rupture of the aspirated membrane and entry in whole-cell recording configuration was indicated by a minute increase in capacitance and a simultaneous bleaching of the erythrocyte due to fast dialysis of hemoglobin by the pipet solution (Figure 1A). The liquid junction potentials ΔE between the bridge (filled with standard bath solution) and the other bath solutions applied were estimated according to Barry and Lynch (1991). Data were corrected for the estimated ΔE values. Whole-cell currents were evoked by 11 voltage pulses from –30 mV (–10 mV) holding potential to voltages between –100 mV and +100 mV. Original whole-cell current traces are depicted after 500 Hz low-pass filtering. Currents of the individual voltage square pulses are superimposed. Applied voltages refer to the cytoplasmic face of the membrane with respect to the extracellular space. Inward currents, defined as flow of positive charge from the extracellular to the cytoplasmic membrane face, are negative currents and are depicted as downward deflections of the original current traces. Current values were analyzed by averaging the whole-cell currents between 320 and 370 ms of each square pulse.
Records were obtained from trophozoite stage-infected, non-infected and oxidized post-incubated erythrocytes, respectively. For controls, apparently non-infected erythrocytes of the P.falciparum culture (Figure 1A) and erythrocytes freshly taken from volunteers with different blood groups were used. Since no differences in whole-cell currents were apparent between the cultured and fresh control cells, data were pooled. The standard NaCl bath solution contained 115 mM NaCl, 20 mM HEPES/NaOH pH 7.4, 5 mM CaCl2, 10 mM MgCl2. The standard NaCl pipet solution was 115 mM NaCl, 20 mM HEPES/NaOH pH 7.4, 10 mM MgCl2 and 0.5 mM EGTA. These solutions which were similar to those of a previous patch–clamp study on P.falciparum-infected human erythrocytes (Desai et al., 2000) were chosen because (i) the results could be compared directly with the recently published data (Desai et al., 2000); and (ii) the high divalent cation concentrations provided high seal resistances. Whole-cell currents were characterized further with the standard NaCl bath and pipet solution by substituting the NaCl bath with solutions containing 140 mM Na-X, 20 mM HEPES/NaOH pH 7.4, where X was SCN–, I–, Br–, lactate and gluconate, respectively. In addition, DIDS, furosemide, glybenclamide, NPPB or DTE (0.01–1000 µM) was applied to the standard NaCl bath solution. In another set of experiments, a pipet solution containing reduced (GSH) or oxidized glutathione (GSSG; both 10 mM) was used. In further records, Na+ in the bath and pipet solution was replaced by the impermeable cation NMDG+. In general, records were obtained from banked erythrocytes infected with P.falciparum BINH. Infection of banked erythrocytes with P.falciparum FCR-3 and infection of fresh erythrocytes with P.falciparum BINH led to an identical current phenotype with a similar conductance [Goutward = 16 ± 2 nS (n = 10) and Goutward = 21 ± 2 nS (n = 8), respectively] as infection of banked erythrocytes with P.falciparum BINH (Goutward = 18 ± 1 nS; n = 35; Figure 1B).
Hemolysis of infected cells
Enriched trophozoite-infected erythrocytes (parasitemia 8–30%; hematocrit 5%; 1.5 ml) were spun down and resuspended in 400 µl of isosmotic sorbitol solutions (290 mM sorbitol/5 mM HEPES/NaOH pH 7.4) or, for control, in NaCl solution, and hemolyzed for 10 min at 37°C in the presence and absence of DIDS, NPPB, furosemide, glybenclamide (100 µM each) and DTE (100 µM or 1 mM), respectively. After centrifugation, the hemoglobin color of the supernatants (350 µl) was recorded qualitatively by image scanning and the hemoglobin concentration was determined quantitatively by photometry (absorbance at 546 nm after oxidation to cyanomet-hemoglobin). Since the hemolysis in sorbitol/NPPB (100 µM) and di-/trisaccharides was even lower than that in the NaCl controls (see Results), the sorbitol-induced hemolysis in Figure 4B was defined as that fraction of hemolysis that was inhibitable by NPPB (100 µM). To characterize the substrate specificity, cells were hemolyzed in isosmotic solutions of various carbohydrates (290 mM X/5 mM HEPES/NaOH pH 7.4 with X = myo-inositol, lactose, mannitol, raffinose, sorbitol and sucrose, respectively).
metHb formation
Control, highly enriched trophozoite/schizont-infected and oxidized cells (in NaCl solution) were hemolyzed in O2-saturated H2O. Cell membranes and, in the case of the infected cells, parasitophorous vacuoles bearing parasites were spun down (12 000 r.p.m./5 min). The supernatant was diluted further with O2-saturated H2O, and the absorbance spectrum of hemoglobin was determined photometrically. In addition, the 405/415 nm absorbance ratio (Soret bands of metHb and oxyHb, respectively) was assessed in supernatants obtained from various mixtures of enriched infected or oxidized cells with control erythrocytes.
GSH/(GSH + GSSG) ratio
Thiols in protein-free extract of erythrocytes were assessed photometrically by the reduction-sensitive dye 5,5′-dithio-bis(2-nitrobenzoic acid) (DTNB) according to Jocelyn (1972). Since GSH accounts for almost all of the erythrocyte non-protein thiols, DTNB reactivity directly reflects GSH concentration. Control, oxidized, oxidized and post-incubated, and highly enriched trophozoite/schizont-infected erythrocytes (in 400 µl of NaCl solution) were heated (15 min/96°C) for protein denaturation and centrifuged (17 000 r.p.m., 30 min, 4°C). Supernatants were aliquoted (2 × 100 µl) and one aliquot was stored on ice (for determination of reduced sulfhydryls) while the second was reduced (for determination of total sulfhydryls) by KBH4 (0.2 mM for 15 min at room temperature). After reduction, the sample pH, when alkaline, was titrated with HCl to pH 7.0. Both aliquots were then diluted with 0.1 M Na-phosphate buffer (pH 7.0; 550 µl final volume), DTNB (1.5 mM/200 µl) was added and samples were incubated for 10 min at room temperature, before measuring the absorbance at 430 nm.
Hemolysis of oxidized non-infected cells
Pelleted oxidized cells (and, for control, untreated cells) were resuspended in 400 µl of isosmotic carbohydrate solutions (see above) or NaCl solution (∼6% hematocrit) and incubated (37°C/2.5 h) in the presence and absence of DIDS, NPPB, furosemide and glybenclamide, respectively (0.01–1000 µM each). Incubation was stopped by centrifugation and the hemoglobin concentration of the supernatants (200 µl aliquots) was determined as above. The incubation time necessary for hemolysis of oxidized cells exceeded that of infected cells (see above). To test whether this slow hemolysis was due to poor permeability or to a late appearance of sorbitol entry into oxidized cells, pelleted oxidized cells were suspended in NaCl, incubated for 2.5 h, spun down and resuspended in sorbitol. Using this protocol, hemolysis in sorbitol equilibrated within 10 min (T = 2.5 ± 0.9 min; n = 3), suggesting that (i) the induced sorbitol permeabilities in infected and oxidized cells were similarly high and (ii) that the development of the sorbitol permeability was dependent on a further incubation period in the sorbitol- (or NaCl)-diluted oxidant. The latter was confirmed by a third type of experiment where the oxidized cells were washed in order to eliminate the oxidant before resuspending in sorbitol, which yielded only 2–3% of hemolyzed cells within 2.5 h of sorbitol incubation (not shown). In separate experiments investigating the acute effect of DIDS on oxidation-induced hemolysis, oxidized (1 mM t-BHP/15 min) and post-incubated (2.5 h in NaCl) cells were resuspended in isosmotic sorbitol and hemolysis was determined after 10 min of incubation in the presence or absence of DIDS (0.1–1000 µM).
Data analysis and statistics
Data are means ± SE; n = number of cells (patch–clamp) or experiments (hemolysis, metHb, GSH). Differences between means were estimated by unpaired t-test (two-tailed) or Welch approximation (two-tailed) using Instat software (Graphpad, San Diego, CA).
Acknowledgments
Acknowledgements
The authors wish to thank Silvelia Grummes and Uta Hamacher for expert technical assistance, and Professor Helmut Heinle for technical advice. The experiments have been supported by the Deutsche Forschungsgemeinschaft (DFG, La 315/4-3) and the fortune program (838-1-0) of the University of Tübingen. C.D. has been supported by a grant of the Alexander von Humboldt foundation. A preliminary account of this work has been published in abstract form (Pflugers Arch., 441, Suppl: R137 and R246, 2001).
References
- Atamna H. and Ginsburg,H. (1993) Origin of reactive oxygen species in erythrocytes infected with Plasmodium falciparum. Mol. Biochem. Parasitol., 61, 231–241. [DOI] [PubMed] [Google Scholar]
- Atamna H. and Ginsburg,H. (1997) The malaria parasite supplies glutathione to its host cell—investigation of glutathione transport and metabolism in human erythrocytes infected with Plasmodium falciparum. Eur. J. Biochem., 250, 670–679. [DOI] [PubMed] [Google Scholar]
- Atamna H., Pascarmona,G. and Ginsburg,H. (1994) Hexose-monophosphate shunt activity in intact Plasmodium falciparum-infected erythrocytes and in free parasites. Mol. Biochem. Parasitol., 67, 79–89. [DOI] [PubMed] [Google Scholar]
- Barry P.H. and Lynch,J.W. (1991) Liquid junction potentials and small cell effects in patch-clamp analysis. J. Membr. Biol., 121, 101–117. [DOI] [PubMed] [Google Scholar]
- Becker K., Gui,M., Traxler,A., Kirsten,C. and Schirmer,R.H. (1994) Redox processes in malaria and other parasitic diseases. Determination of intracellular glutathione. Histochemistry, 102, 389–395. [DOI] [PubMed] [Google Scholar]
- Binh V.Q., Luty,A.J. and Kremsner,P.G. (1997) Differential effects of human serum and cells on the growth of Plasmodium falciparum adapted to serum-free in vitro culture conditions. Am. J. Trop. Med. Hyg., 57, 594–600. [DOI] [PubMed] [Google Scholar]
- Breuer W.V., Kutner,S., Sylphen,J., Ginsburg,H. and Cabantchik,Z.I. (1987) Covalent modification of the permeability pathways induced in the human erythrocyte membrane by the malarial parasite Plasmodium falciparum. J. Cell Physiol., 133, 55–63. [DOI] [PubMed] [Google Scholar]
- Cranmer S.L., Magowan,C., Liang,J., Coppel,R.L. and Cooke,B.M. (1997) An alternative to serum for cultivation of Plasmodium falciparum in vitro. Trans. R. Soc. Trop. Med. Hyg., 91, 363–365. [DOI] [PubMed] [Google Scholar]
- Desai S.A., McCleskey,E.W., Schlesinger,P.H. and Krogstad,D.J. (1996) A novel pathway for Ca++ entry into Plasmodium falciparum-infected blood cells. Am. J. Trop. Med. Hyg., 54, 464–470. [DOI] [PubMed] [Google Scholar]
- Desai S.A., Bezrukov,S.M. and Zimmerberg,J. (2000) A voltage-dependent channel involved in nutrient uptake by red blood cells infected with the malaria parasite. Nature, 406, 1001–1005. [DOI] [PubMed] [Google Scholar]
- Dumaswala U.J., Zhuo,L., Jacobsen,D.W., Jain,S.K. and Sukalski,K.A. (1999) Protein and lipid oxidation of banked human erythrocytes: role of glutathione. Free Radical Biol. Med., 27, 1041–1049. [DOI] [PubMed] [Google Scholar]
- Duranton C., Huber,S.M. and Lang,F. (2002) Oxidation induces a Cl-dependent cation conductance in human red blood cells. J. Physiol., in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman J.C. and Miller,C. (1984) Membrane vesicles from human red blood cells in planar lipid bilayers. Ann. NY Acad. Sci., 435, 541–544. [Google Scholar]
- Freedman J.C. and Novak,T.S. (1997) Electrodiffusion, barrier and gating analysis of DIDS-insensitive chloride conductance in human red blood cells treated with valinomycin or gramicidin. J. Gen. Physiol., 109, 201–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman J.C., Novak,T.S., Bisognano,J.D. and Pratap,P.R. (1994) Voltage dependence of DIDS-insensitive chloride conductance in human red blood cells treated with valinomycin or gramicidin. J. Gen. Physiol., 104, 961–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsburg H. and Atamna,H. (1994) The redox status of malaria-infected erythrocytes: an overview with an emphasis on unresolved problems. Parasite, 1, 5–13. [DOI] [PubMed] [Google Scholar]
- Ginsburg H. and Kirk,K. (1998) Membrane transport in malaria-infected erythrocyte. In Sherman,I.W. (ed.), Malaria: Parasite Biology, Pathogenesis and Protection. American Society for Microbiology, Washington, DC, pp. 219–232.
- Giribaldi G., Ulliers,D., Mannu,F., Arese,P. and Turrini,F. (2001) Growth of Plasmodium falciparum induces stage-dependent haemichrome formation, oxidative aggregation of band 3, membrane deposition of complement and antibodies and phagocytosis of parasitized erythrocytes. Br. J. Haematol., 113, 492–499. [DOI] [PubMed] [Google Scholar]
- Grygorczyk R. and Schwarz,W. (1983) Properties of the Ca2+-activated K+ conductance of human red cells as revealed by the patch-clamp technique. Cell Calcium, 4, 499–510. [DOI] [PubMed] [Google Scholar]
- Hoffman J.F. (1992) The band 3 proteins: anion transporters, binding proteins and senescent antigens. In Bamberg,E. and Passow,H. (eds), Progress in Cell Research. Vol. 2. Elsevier, Amsterdam, The Netherlands, pp. 173–178.
- Hornig R. and Lutz,H.U. (2000) Band 3 protein clustering on human erythrocytes promotes binding of naturally occurring anti-band 3 and anti-spectrin antibodies. Exp. Gerontol., 35, 1025–1044. [DOI] [PubMed] [Google Scholar]
- Huber S.M., Gamper,N. and Lang,F. (2001) Chloride conductance and volume-regulatory nonselective cation conductance in human red blood cell ghosts. Pflugers Arch., 441, 551–558. [DOI] [PubMed] [Google Scholar]
- Ingrosso D., D’Angelo,S., di Carlo,E., Perna,A.F., Zappia,V. and Galletti,P. (2000) Increased methyl esterification of altered aspartyl residues in erythrocyte membrane proteins in response to oxidative stress. Eur. J. Biochem., 267, 4397–4405. [DOI] [PubMed] [Google Scholar]
- Jensen J.B. and Trager,W. (1978) Plasmodium falciparum in culture: establishment of additional strains. Am. J. Trop. Med. Hyg., 27, 743–746. [DOI] [PubMed] [Google Scholar]
- Jocelyn P.C. (1972) Biochemistry of the SH Group. Academic Press, London, UK.
- Kaestner L., Christophersen,P., Bernhardt,I. and Bennekou,P. (2000) The non-selective voltage-activated cation channel in the human red blood cell membrane: reconciliation between two conflicting reports and further characterisation. Bioelectrochemistry, 52, 117–125. [DOI] [PubMed] [Google Scholar]
- Kirk K. and Horner,H.A. (1995) In search of a selective inhibitor of the induced transport of small solutes in Plasmodium falciparum-infected erythrocytes: effects of arylaminobenzoates. Biochem. J., 311, 761–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk K., Horner,H.A., Spillett,D.J. and Elford,B.C. (1993) Glibenclamide and meglitinide block the transport of low molecular weight solutes into malaria-infected erythrocytes. FEBS Lett., 323, 123–128. [DOI] [PubMed] [Google Scholar]
- Kirk K., Horner,H.A., Elford,B.C., Ellory,J.C. and Newbold,C.I. (1994) Transport of diverse substrates into malaria-infected erythrocytes via a pathway showing functional characteristics of a chloride channel. J. Biol. Chem., 269, 3339–3347. [PubMed] [Google Scholar]
- Kutner S., Breuer,W.V., Ginsburg,H. and Cabantchik,Z.I. (1987) On the mode of action of phlorizin as an antimalarial agent in in vitro cultures of Plasmodium falciparum. Biochem. Pharmacol., 36, 123–129. [DOI] [PubMed] [Google Scholar]
- Lambros C. and Vanderberg,J.P. (1979) Synchronization of Plasmodium falciparum erythrocytic stages in culture. J. Parasitol., 65, 418–420. [PubMed] [Google Scholar]
- Motais R., Fievet,B., Borgese,F. and Garcia-Romeu,F. (1997) Association of the band 3 protein with a volume-activated, anion and amino acid channel: a molecular approach. J. Exp. Biol., 200, 361–367. [DOI] [PubMed] [Google Scholar]
- Pasvol G. (2001) Targeting voracious appetite of malaria-infected red-blood cell. Lancet, 357, 408–410. [DOI] [PubMed] [Google Scholar]
- Sakai H., Onuma,H., Umeyama,M., Takeoka,S. and Tsuchida,E. (2000) Photoreduction of methemoglobin by irradiation in the near-ultraviolet region. Biochemistry, 39, 14595–14602. [DOI] [PubMed] [Google Scholar]
- Schwarz W., Grygorczyk,R. and Hof,D. (1989) Recording single-channel currents from human red cells. Methods Enzymol., 173, 112–121. [DOI] [PubMed] [Google Scholar]
- Staalsoe T., Giha,H.A., Dodoo,D., Theander,T.G. and Hviid,L. (1999) Detection of antibodies to variant antigens on Plasmodium falciparum-infected erythrocytes by flow cytometry. Cytometry, 35, 329–336. [DOI] [PubMed] [Google Scholar]
- Thomas S.L., Egee,S., Lapaix,F., Kaestner,L., Staines,H.M. and Ellory,J.C. (2001) Malaria parasite Plasmodium gallinaceum up-regulates host red blood cell channels. FEBS Lett., 500, 45–51. [DOI] [PubMed] [Google Scholar]
- Trager W. and Jensen,J.B. (1976) Human malaria parasites in continuous culture. Science, 193, 673–675. [DOI] [PubMed] [Google Scholar]
- Wagner C.A., Broer,A., Albers,A., Gamper,N., Lang,F. and Bröer,S. (2000) The heterodimeric amino acid transporter 4F2hc/LAT1 is associated in Xenopus oocytes with a non-selective cation channel that is regulated by the serine/threonine kinase sgk-1. J. Physiol., 526, 35–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipser Y., Piade,A. and Kosower,N.S. (1997) Erythrocyte thiol status regulates band 3 phosphotyrosine level via oxidation/reduction of band 3-associated phosphotyrosine phosphatase. FEBS Lett., 406, 126–130. [DOI] [PubMed] [Google Scholar]