Abstract
Heregulin (HRG)-induced tyrosine phosphorylation of the Gab2 docking protein was enhanced by pretreatment with wortmannin, indicating negative regulation via a PI3-kinase-dependent pathway. This represents phosphorylation by the serine/threonine kinase protein kinase B (PKB), since PKB constitutively associates with Gab2, phosphorylates Gab2 on a consensus phosphorylation site, Ser159, in vitro and inhibits Gab2 tyrosine phosphorylation. However, expression of Gab2 mutated at this site (S159A Gab2) not only enhanced HRG-induced Gab2 tyrosine phosphorylation and association with Shc and ErbB2, but also markedly increased tyrosine phosphorylation of ErbB2 and other cellular proteins and amplified activation of the ERK and PKB pathways. The impact of this negative regulation was further emphasized by a potent transforming activity for S159A Gab2, but not wild-type Gab2, in fibroblasts. These studies establish Gab2 as a proto-oncogene, and a model in which receptor recruitment of Gab2 is tightly regulated via an intimate association with PKB. Release of this negative constraint enhances growth factor receptor signalling, possibly since Gab2 binding limits dephosphorylation and disassembly of receptor-associated signalling complexes.
Keywords: ErbB/Gab2/phosphorylation/protein kinase B/signal transduction
Introduction
Ligand binding to cell surface receptors results in the rapid activation of biochemical pathways and networks ultimately leading to the regulation of a number of critical biological processes including cell growth, differentiation and survival. Understanding the mechanisms by which receptors can amplify signals and coordinate signalling events to elicit distinct biological responses remains the subject of intense investigation.
One such mechanism is through the recruitment of a growing family of scaffolding or docking proteins that lack detectable catalytic activity but provide binding sites for downstream signalling components. This family includes Grb2-associated binders (Gab1–2), Drosophila Daughter of sevenless (DOS), insulin receptor substrates (IRS) 1–4, Downstream of kinases (Dok), Dok-related (Dok-R) and fibroblast growth factor receptor substrate 2 (FRS2) (Holgado-Madruga et al., 1996; Raabe et al., 1996; Kouhara et al., 1997; Yamanashi and Baltimore, 1997; Yenush and White, 1997; Gu et al., 1998; Jones and Dumont, 1998). These proteins all become tyrosine phosphorylated by receptor tyrosine kinases (RTKs) or receptor-associated kinases and subsequently recruit signalling molecules containing src homology 2 (SH2) or phosphotyrosine binding (PTB) domains (Pawson and Scott, 1997), thereby playing a major role in the integration and amplification of diverse signalling pathways and in the targeting of signalling events to particular cellular locations.
Docking proteins, in general, contain a determinant/domain that allows membrane association. Gab1–2, DOS, IRS1–4, DOK and Dok-R all contain N-terminal pleckstrin homology (PH) domains, which are capable of binding specific membrane phospholipids (Lemmon and Ferguson, 2000) whereas FRS2 has a myristoylation site at its N-terminus (Kouhara et al., 1997). These mechanisms localize the docking proteins in close proximity to kinases that phosphorylate them and downstream targets. Another common feature that some docking proteins share is a domain that permits receptor interaction. IRS proteins, FRS2 and Dok all have PTB domains that target specific sites on RTKs. Gab1, however, lacks a PTB domain but contains a proline-rich Met binding domain (MBD), which can mediate binding to the activated MET receptor (Weidner et al., 1996). Receptor association can also occur indirectly through other adaptor proteins such as Grb2 and Shc (Bardelli et al., 1997; Gu et al., 2000). Another key feature is the presence of multiple tyrosine residues that, once phosphorylated, serve as binding sites for SH2 domain-containing proteins.
The Gab/DOS subfamily of docking proteins has three structurally related members to date, Gab1, Gab2 and DOS. Gab1 and Gab2 exhibit significant sequence identity to DOS in the PH domain, and a similar molecular architecture, so are considered higher organism homologues. Genetic analyses demonstrate that DOS functions upstream or independently of Ras1 and downstream of SEV and other RTKs including Torso [platelet-derived growth factor receptor (PDGFR) homologue] and DER [Drosophila epidermal growth factor receptor (EGFR)] and indicate that DOS is a positively acting component in RTK signalling, acting as a substrate for Corkscrew (CSW, the Drosophila homologue of Shp2) (Herbst et al., 1996, 1999; Raabe et al., 1996). Gab1 was originally cloned as a Grb2 binding protein and is tyrosine phosphorylated in response to a wide variety of growth factors and cytokines, and also B- and T-cell antigens (Holgado-Madruga et al., 1996; Takahashi-Tezuka et al., 1998; Lecoq-Lafon et al., 1999). Overexpression of Gab1 enhances EGF-stimulated cell growth (Holgado-Madruga et al., 1996) and is also sufficient to induce hepato cyte growth factor (HGF)-mediated responses such as branching morphogenesis (Weidner et al., 1996). Gab1 interacts with multiple signalling molecules including Shp2, the p85 subunit of phosphatidylinositol (PI) 3-kinase, phospholipase Cγ, Shc and Crk (Holgado-Madruga et al., 1996; Schaeper et al., 2000). Gab1 interaction with Shp2 is essential for biological function in Madine–Darby canine kidney (MDCK) epithelial cells (Schaeper et al., 2000). PI3-kinase acts not only downstream of Gab1 by virtue of p85 association, but also upstream, since the Gab1 PH domain binds the PI3-kinase product PI(3,4,5)P3. This enhances membrane recruitment and receptor coupling (Maroun et al., 1999; Rodrigues et al., 2000; Yart et al., 2001). The more recently cloned family member, Gab2, is tyrosine phosphorylated in response to a variety of cytokines and also antigen receptor stimulation and has a similar array of binding partners to Gab1 (Gu et al., 1998; Nishida et al., 1999). The Gab2–Shp2 interaction controls a novel pathway regulating cytokine-induced immediate-early gene activation that is consistent with Shp2 acting at multiple points in cytokine signalling (Gu et al., 1998). However, although Gab1 and 2 are closely related, they may play non-redundant roles in RTK signalling, since Gab2 exhibits an overlapping but distinct expression pattern when compared with that of Gab1 (Gu et al., 1998) and Gab1–/– and Gab2–/– mice exhibit markedly different phenotypes (Itoh et al., 2000; Sachs et al., 2000; Gu et al., 2001).
To date, the function of Gab2 has been characterized primarily in the context of cytokine responses and relatively little is known about its role in other receptor signalling systems. In this report we demonstrate that Gab2 couples efficiently to heregulin (HRG)-activated ErbB receptors in a manner tightly regulated by protein kinase B (PKB)-mediated negative feedback via phosphorylation of Ser159. Furthermore, we show that release of this feedback constraint (via mutation of the regulatory site) leads to the generation of a Gab2 protein that not only markedly amplifies HRG-induced signalling but also exhibits a potent transforming activity in fibroblasts. These findings lead to a model in which PKB-mediated phosphorylation acts to uncouple Gab2 from ErbB receptors, leading to signal termination. Prevention of Gab2 dissociation leads to increased tyrosine phosphorylation of not only Gab2 but also associated receptors and other signalling molecules, leading to potent amplification of mitogenic signalling pathways.
Results
Coupling of Gab2 to HRG-activated ErbB receptors
As Gab2 signalling has been characterized primarily in the context of cytokine stimulation of haematopoietic cells (Gu et al., 1998; Zhao et al., 1999), but Gab1 couples to a variety of cytokine and growth factor receptors, we were interested in analysing Gab2 complexes following stimulation of ErbB receptor signalling with HRG β1. Following HRG stimulation of MCF-7 cells we detected a marked increase in Gab2 tyrosine phosphorylation accompanied by a significant reduction in Gab2 electrophoretic mobility suggestive of serine/threonine (Ser/Thr) phosphorylation (Figure 1). An increase in co-immunoprecipitating tyrosine phosphorylated proteins was also noted, in particular two prominent bands of ∼52 and 46 kDa. Immunoblotting identified the 52/46 kDa bands as the corresponding Shc isoforms. Besides Shc, tyrosine phosphorylated Gab2 associated with Shp2, p85 and Grb2. The association with Shp2 and Shc was markedly induced following HRG stimulation, whereas the association with p85 and Grb2 was only slightly enhanced above the basal state (Figure 1). This profile of signalling proteins recruited to Gab2 has also been observed upon cytokine/antigen-receptor stimulation (Gu et al., 1998), but there are some notable differences. The constitutive association of Gab2 with Grb2 was noted previously and is attributed to binding of the Grb2 SH3 domains to proline-rich regions of Gab2 (Zhao et al., 1999). The high basal association of p85 differs from that observed in haematopoietic cells, and may reflect increased tyrosine phosphorylation of Gab2 binding sites for the p85 SH2 domain in adherent epithelial cells. Also, the recruitment of Shc to tyrosine phosphorylated Gab2 is particularly marked following HRG stimulation. This difference is probably explained by receptor-specific differences in the mode of assembly of signalling complexes, and is discussed later.
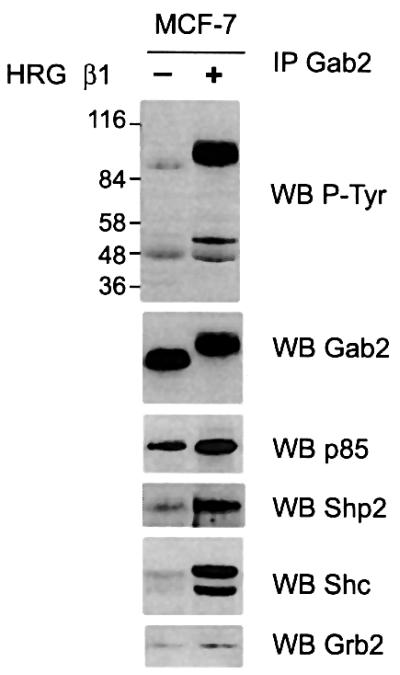
Fig. 1. HRG stimulation leads to assembly of a Gab2-associated signalling complex. Gab2 immunoprecipitates (IP, 1.25 mg total protein) derived from serum-starved or HRG-stimulated MCF-7 breast cancer cells were western blotted (WB) with antibodies as indicated.
Gab2 phosphorylation is regulated by a wortmannin-sensitive kinase
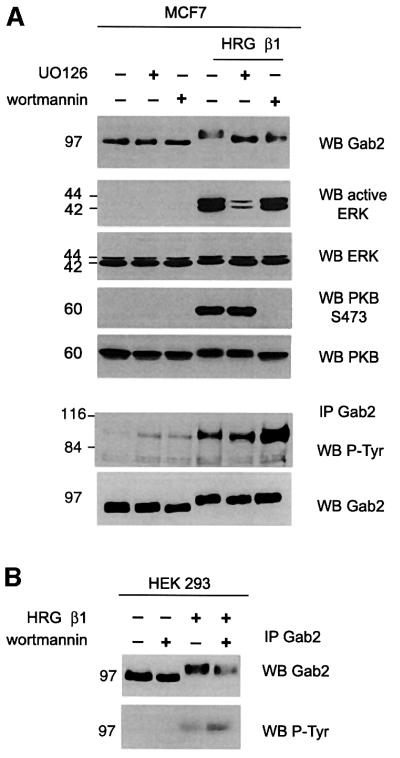
In order to characterize the HRG-stimulated pathways that regulate Gab2 Ser/Thr phosphorylation, we incubated MCF-7 cells with different kinase inhibitors before HRG stimulation and the effect on Gab2 tyrosine phosphorylation and mobility were investigated. Pre-incubation with UO126, a potent mitogen-activated protein kinase/ERK-activating kinase (MEK) inhibitor, markedly reduced HRG-induced ERK activation, whereas treatment with the PI3-kinase inhibitor wortmannin completely abolished PKB activation as detected by Ser473 phosphorylation (Figure 2A). Upon longer exposure a low basal activation of the ERKs and PKB, sensitive to the appropriate inhibitors, could be detected in serum-starved cells. Pre-incubation with either inhibitor increased Gab2 electrophoretic mobility under HRG-stimulated conditions (Figure 2A). However, the levels of HRG-induced Gab2 tyrosine phosphorylation were significantly increased (at least 3-fold) following pretreatment with wortmannin but not U0126 when compared with cells treated with vehicle alone (Figure 2A). An effect of either inhibitor on basal Gab2 tyrosine phosphorylation was not reproducibly observed. This effect of PI3-kinase inhibition on HRG-induced Gab2 tyrosine phosphorylation was also seen in HEK 293 cells (Figure 2B). These data indicate that both MEK- and PI3-kinase-dependent pathways are involved in regulating Gab2 Ser/Thr phosphorylation and that a PI3-kinase-dependent pathway negatively regulates HRG-induced Gab2 tyrosine phosphorylation.
Fig. 2. HRG-induced Gab2 tyrosine phosphorylation is inhibited by a PI3-kinase-dependent pathway. Serum-starved MCF-7 (A) or HEK 293 (B) cells were incubated with the inhibitors indicated before HRG stimulation. Cell lysates [(A), upper panels] or Gab2 immunoprecipitates (IP, 0.5 mg total protein) were then western blotted with antibodies as indicated.
Gab2 is a PKB target
Since we knew that the kinase responsible was sensitive to the PI3-kinase inhibitor wortmannin, PKB, a kinase that acts downstream of PI3-kinase (Lawlor and Alessi, 2001), seemed a plausible candidate. An alignment of the mouse, rat and human Gab2 sequences revealed the presence of a single conserved serine residue (Ser160, Ser160 and Ser159, respectively; RERKSSA in single letter code) that conforms to a consensus PKB phosphorylation motif [RXRXX(S/T)Hyd] (Obata et al., 2000; Lawlor and Alessi, 2001).
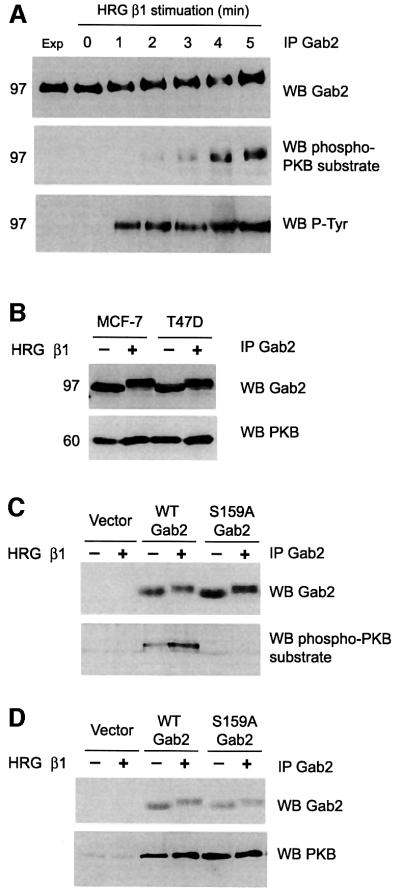
To investigate whether this site was phosphorylated following HRG treatment we stimulated MCF-7 cells with HRG for varying times and blotted Gab2 immunoprecipitates with a phosphorylation state-specific antibody that recognizes PKB substrates when phosphorylated on Ser or Thr in the context of the RXRXX(S/T) motif. Whereas tyrosine phosphorylation of Gab2 occurred within 1 min (Figure 3A), Gab2 was recognized by the phospho-specific antibody at between 2 and 5 min, correlating with the decrease in Gab2 electrophoretic mobility (Figure 3A). This indicates that Gab2 is phosphorylated by PKB in a time-dependent manner following HRG stimulation as part of a feedback mechanism, and that this contributes to the mobility shift.
Fig. 3. Gab2 is a PKB target. (A) Gab2 is recognized as a PKB substrate following HRG stimulation. MCF-7 cells were serum starved overnight before HRG β1 stimulation for the times indicated. Gab2 was immunoprecipitated from cell lysates and blotted with either a Gab2 antibody (upper panel), a phosphospecific antibody selective for PKB substrates (middle panel) or for phosphotyrosine (lower panel). The mobility shift is not evident in the lower panel due to gel running conditions. (B) PKB associates with Gab2 in vivo. Gab2 immunoprecipitates derived from serum-starved or HRG-stimulated MCF-7 and T-47D breast cancer cells were western blotted for either Gab2 or PKB. (C) Recognition of Gab2 as a PKB substrate is dependent on Ser159. HEK 293 cells transiently transfected with expression vector alone or expression constructs for wild-type (WT) or S159A Gab2 were serum-starved and stimulated with HRG β1 as indicated. Gab2 immunoprecipitates were then subjected to western blotting with antibodies as indicated. (D) Association of PKB with Gab2 is independent of Ser159. Gab2 was immunoprecipitated from the cell lysates described in (C) and then western blotted for either Gab2 or PKB. Gab2 was detected in the vector control lanes on longer exposures.
Having demonstrated that Gab2 represents a potential PKB substrate we investigated whether Gab2 could interact with PKB in cells. Gab2 was immunoprecipitated from both MCF-7 and T-47D cells that had been serum starved or stimulated for 5 min with HRG, and association with endogenous PKB was analysed by immunoblotting with PKB-specific antibodies. As shown in Figure 3B, PKB co-immunoprecipitates with Gab2 in both cell lines and association was unaffected by HRG stimulation. These data indicate that Gab2 constitutively associates with PKB in vivo, which is likely to enhance its phosphorylation by this enzyme (Lawlor and Alessi, 2001).
We next tested whether PKB-mediated phosphorylation of Gab2 was dependent on Ser159. For this purpose we generated a Gab2 mutant by site-directed mutagenesis in which S159 was converted to alanine. HEK 293 cells were transiently transfected with mutant (S159A) or wild-type (WT) Gab2, HRG stimulated and then Gab2 immunoprecipitates were analysed by immunoblotting with the phospho-specific PKB substrate antibody (Figure 3C). Wild-type Gab2 was recognized as a PKB substrate even in the absence of stimulation and this was increased at least 2-fold following treatment with HRG. However, although S159A Gab2 was expressed at slightly higher levels than the wild-type protein, reactivity with the phospho-specific PKB substrate antibody was completely abolished by the presence of the S159A mutation. To determine whether this single amino acid change would affect the formation of Gab2–PKB complexes in vivo, we immunoprecipitated Gab2 from the same cell extracts and analysed the interaction using PKB-specific antibodies. Co-immunoprecipitation of PKB with Gab2 was unaffected by the S159A mutation (Figure 3D). This indicates that PKB binding to Gab2 is independent of the serine residue in the consensus phosphorylation motif.
In order to confirm that PKB could phosphorylate Gab2 in vitro, Gab2 was immunoprecipitated from transiently transfected COS-7 cells and incubated with a constitutively active form of PKB, myr-PKBα (Andjelkovic et al., 1997). The immunoprecipitates used in these reactions were extensively washed with radioimmune precipitation assay (RIPA) buffer to reduce the likelihood of associated or contaminating kinases contributing to the phosphorylation reaction. Gab2 was effectively phosphorylated by PKB and phosphorylation was only detected in the presence of myr-PKB. However, when S159A Gab2 was used as the substrate, phosphorylation by PKB was completely abolished, indicating that Ser159 represents the only target for PKB-mediated phosphorylation (Figure 4).
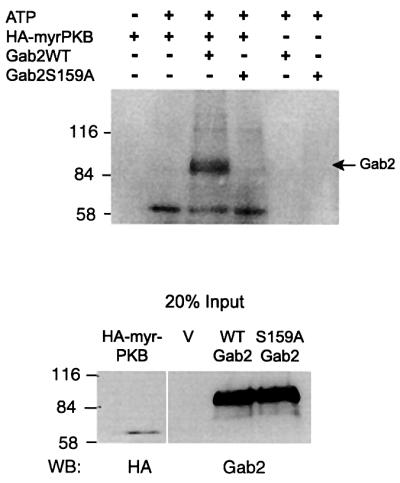
Fig. 4. Gab2 is phosphorylated in vitro by PKB in a Ser159-dependent manner. COS-7 cells were transiently transfected with expression constructs for wild-type Gab2, S159A Gab2 or a HA-tagged myristoylated PKB (HA-myrPKB). Immunoprecipitations were performed from cell extracts using Gab2 polyclonal antisera or anti-HA monoclonal antibodies. Equal amounts of precipitated Gab2 proteins were subjected to an in vitro kinase assay by immunoprecipitated activated PKB as described in Materials and methods. The top panel shows an autoradiograph of the reaction products following separation by SDS–PAGE. Control western blots for levels of protein input are shown (bottom panel).
PKB-mediated phosphorylation regulates Gab2 tyrosine phosphorylation
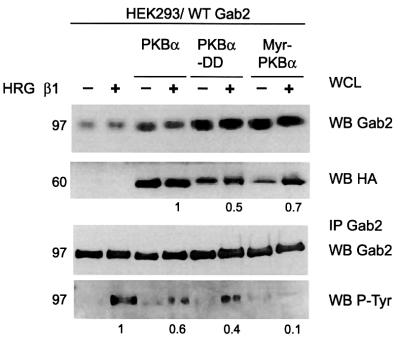
To investigate whether expression of PKB was sufficient to inhibit HRG-induced tyrosine phosphorylation of Gab2, we transiently expressed Gab2 in HEK 293 cells with wild-type or two constitutively active forms of PKBα (Alessi et al., 1996; Andjelkovic et al., 1997) and analysed the effects on HRG-stimulated Gab2 tyrosine phosphorylation. Co-expression with PKB or the T308D/S473D mutant reduced Gab2 tyrosine phosphorylation in the HRG-stimulated samples by 40 and 60%, respectively (Figure 5). The T308D/S473D mutant was therefore more effective at inhibiting Gab2 tyrosine phosphorylation than the wild-type enzyme, considering that it was expressed at 2-fold lower levels. Interestingly, co-expression with a different constitutively active form of PKBα, a myristoylated version, reduced Gab2 tyrosine phosphorylation by ∼90%. The difference in potency of the constitutively active PKB proteins probably reflects their subcellular localization, i.e. myr-PKBα is targeted to the plasma membrane where it is likely to be in closer proximity to Gab2. These data demonstrate that PKB-mediated Gab2 phosphorylation negatively regulates Gab2 tyrosine phosphorylation downstream of ErbB receptors.
Fig. 5. PKB inhibits HRG-induced Gab2 tyrosine phosphorylation. HEK 293 cells were transiently transfected with a wild-type Gab2 expression vector alone or together with expression constructs for HA-tagged wild-type PKBα or two activating mutants of PKBα: double mutant T308D: S473D (PKBαDD) and a myristoylated form (myrPKBα). Cells were serum starved and stimulated with HRG β1. Cell extracts were immunoprecipitated with Gab2 polyclonal antisera (in the experiment shown the amount of antibody used was limiting) and the immunoprecipitates were then western blotted with antibodies as indicated. In the second panel the relative expression of different PKB enzymes in HRG-stimulated lysates is expressed relative to that for wild type. In the fourth panel Gab2 tyrosine phosphorylation, normalized for Gab2 protein levels, is expressed relative to that for vector transfected cells.
Removal of Ser159-mediated negative feedback amplifies HRG signalling
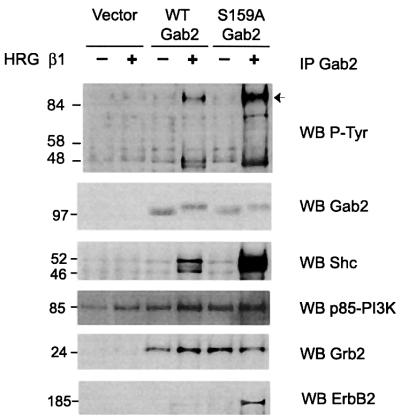
Having shown that PKB can modulate Gab2 tyrosine phosphorylation we investigated the role of the Ser159 phosphorylation site. HEK 293 cells were transiently transfected with constructs driving the expression of wild-type or S159A Gab2, stimulated with HRG and the effect on Gab2 tyrosine phosphorylation analysed. Introduction of the S159A mutation markedly enhanced HRG-stimulated tyrosine phosphorylation of Gab2 at least 4-fold (Figure 6). Furthermore, a significant increase in co-immunoprecipitation of two tyrosine phosphorylated proteins of 52 and 46 kDa was also noted. These associating proteins corresponded to Shc isoforms as revealed by immunoblotting, since introduction of the S159A mutation increased their ligand-stimulated association at least 4-fold. Furthermore, HRG-induced association with the receptor ErbB2 was also markedly enhanced by the S159A mutation. Association with p85 and Grb2 was largely independent of HRG stimulation and was unaffected by the S159A mutation. Increased association of S159A Gab2 with ErbB2 suggests that phosphorylation of Ser159 is responsible for uncoupling the Gab2–receptor complex and attenuating receptor signalling.
Fig. 6. S159A Gab2 exhibits increased HRG-induced tyrosine phosphorylation and association with Shc and ErbB2. HEK 293 cells transiently transfected with expression vector alone or expression constructs for wild-type or S159A Gab2 were serum starved and stimulated with HRG β1 as indicated. Cell extracts were prepared and Gab2 immunoprecipitates were then western blotted with the antibodies indicated. An arrow indicates the position of Gab2. Gab2 was detected in the vector control lanes on longer exposures.
Since the increased association of S159A Gab2 with ErbB2 might prolong receptor signalling we investigated the effect of S159A Gab2 expression on HRG-induced tyrosine phosphorylation of other cellular proteins. Expression of wild-type Gab2 slightly increased the tyrosine phosphorylation of a subset of cellular proteins in response to HRG stimulation (Figure 7A). However, a global amplification of HRG-induced tyrosine phosphorylation was observed in cells expressing S159A Gab2. In particular, proteins at ∼185, 115, 97, 60, 52 and 46 kDa exhibited markedly enhanced tyrosine phosphorylation when compared with vector and wild-type Gab2 controls. We have previously identified the 97 kDa protein as Gab2 (Figure 6). Due to the increased association of S159A Gab2 with ErbB2 and the concomitant increase in HRG-induced tyrosine phosphorylation of a 185 kDa protein we examined whether ErbB2 tyrosine phosphorylation was also increased in these cells. ErbB2 immunoprecipitates were therefore analysed by immunoblotting with anti-phosphotyrosine. HRG-induced tyrosine phosphorylation of ErbB2 was increased 2-fold in cells expressing S159A Gab2 compared with wild-type and vector controls (Figure 7B). Consequently, ErbB2 association with a Gab2 protein insensitive to PKB-mediated negative feedback results in potentiation of receptor-mediated signalling.
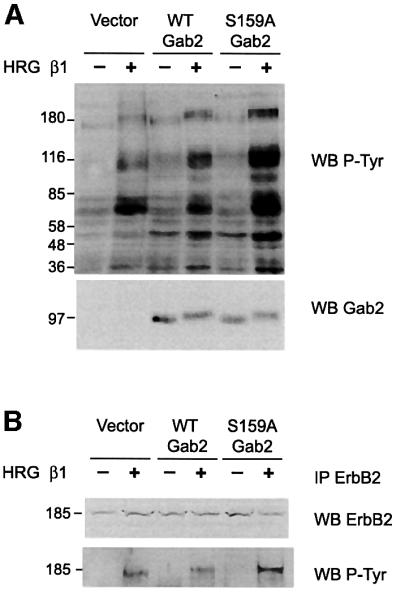
Fig. 7. S159A Gab2 enhances HRG-induced tyrosine phosphorylation of cellular proteins, including ErbB2. HEK 293 cells were trans fected and treated as for Figure 6. Cell extracts (A) or ErbB2 immunoprecipitates (B) were western blotted with the antibodies indicated.
We next investigated whether this upregulation of receptor signalling was reflected in the activation of downstream signalling pathways such as ERK and PKB activation. Activation of these kinases was monitored using phosphorylation state-specific antibodies. HRG stimulation resulted in the phosphorylation of PKB on Ser473 and in the activation of the ERKs (Figure 8). Expression of wild-type Gab2 had no effect on the levels of activation of either of these protein kinases. However, expression of S159A Gab2 further increased the level of activation by HRG at least 4-fold (Figure 8). This confirms that Gab2, when unable to be phosphorylated on Ser159, acts to potentiate signal transduction downstream of the ErbB family of RTKs.
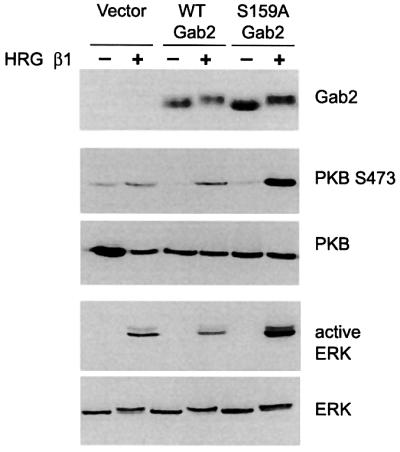
Fig. 8. S159A Gab2 amplifies HRG-induced activation of PKB and the ERKs. HEK 293 cells were transfected and treated as for Figure 6. Cell extracts were then western blotted with the antibodies indicated.
Removal of Ser159-mediated negative feedback releases a potent transforming activity in NIH 3T3 fibroblasts
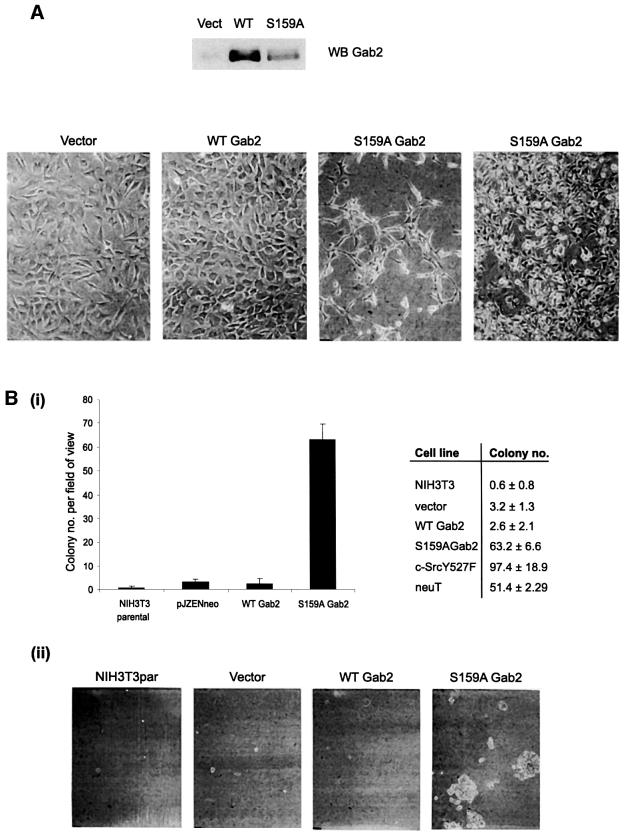
Having shown that Ser159 is an important site for the regulation of Gab2 function, we were interested to see what effect S159A Gab2 had on cell proliferation. For this purpose we generated stable pools of NIH 3T3 fibroblasts expressing wild-type or S159A Gab2 by retroviral infection. Expression levels of wild-type or S159A Gab2 were analysed by immunoblotting (Figure 9A). Both wild-type and S159A Gab2 were expressed at levels several-fold greater than vector control cells; however, wild-type Gab2 was expressed at significantly higher levels than the mutant. Changes in cell morphology and a loss of contact inhibition of growth are among the hallmarks of cell transformation in vitro. The vector control cells displayed a normal fibroblast morphology, as did those cells expressing wild-type Gab2 (Figure 9A). Both cell populations also retained contact inhibition of growth at confluence. However, expression of S159A Gab2 resulted in spindle-shaped refractile cells at low density, indicative of cell transformation. Furthermore, at higher densities these had a tendency to overgrow neighbouring cells and pile up due to loss of contact inhibition of growth. Acquisition of anchorage-independent growth (AIG) is often used as a measurement of cell transformation in vitro. We therefore investigated whether expression of S159A Gab2 could permit AIG, measured by the ability to form colonies in soft agar (Figure 9B). The parental, vector control and wild-type Gab2 cell lines were unable to form colonies in soft agar above a background level, indicating that wild-type Gab2 expression is unable to confer AIG in vitro. However, cells expressing S159A Gab2 formed large numbers of colonies, comparable with the positive controls (active src and transforming neu) (Figure 9B). These results provide evidence that tight negative-feedback regulation of Gab2 by PKB is required for normal cellular growth and proliferation and that release of this feedback constraint causes potent and prolonged amplification of receptor-mediated signalling, resulting in a transformed phenotype.
Fig. 9. S159A Gab2 exhibits a potent transforming activity in NIH 3T3 fibroblasts. (A) Effects of expression of wild-type Gab2 and S159A Gab2 on cellular morphology (lower panel). Phase contrast micrographs show morphology for the neoR control cell line (vector), the wild-type Gab2 cell line and two different cell densities of the mutant S159A Gab2 cell line grown in complete growth medium (×10 magnification). Upper panel shows level of expression, determined by western blotting, of wild-type and S159A Gab2 compared with the neoR control cell line (Vect). (B) (i) Effects of expression of wild-type Gab2 and S159A Gab2 on AIG. Data shown compare growth of NIH 3T3 parental, vector, wild-type Gab2 and S159A Gab2 cell lines in 0.3% agarose for 14 days as described in Materials and methods. Colonies were visualized with p-INT-violet and counted [error bars and ± are the SD (n = 5)]. Assays were performed with mutant c-src (Y527F c-src) and mutant neu (neuT) for comparison (right). (ii) Phase contrast micrographs showing colony formation and morphology for the parental cell line, the neoR cell line (vector), the wild-type Gab2 and S159A Gab2 cell lines (×10 magnification).
Discussion
The role of the Gab2 docking protein has been investigated primarily in the context of cytokine and T- and B-cell antigen receptor signalling in haematopoietic cells, where it functions to regulate activation of PI3-kinase and the ERKs, and also induction of early gene expression (Gu et al., 1998; Pratt et al., 2000). In this study we demonstrate for the first time efficient coupling of Gab2 to HRG-activated ErbB receptors in epithelial cells, but in a manner tightly regulated by a negative feedback loop involving PKB and a single Gab2 phosphorylation site, Ser159. We also identify a novel capacity for Gab2, when released from this constraint via mutation of the phosphorylation site, to mediate global amplification of HRG-induced signalling. The mitogenic potential of this ‘unharnessed’ form of Gab2 is emphasized by its potent transforming activity in fibroblasts. Gab2 therefore represents a newly identified proto-oncogene.
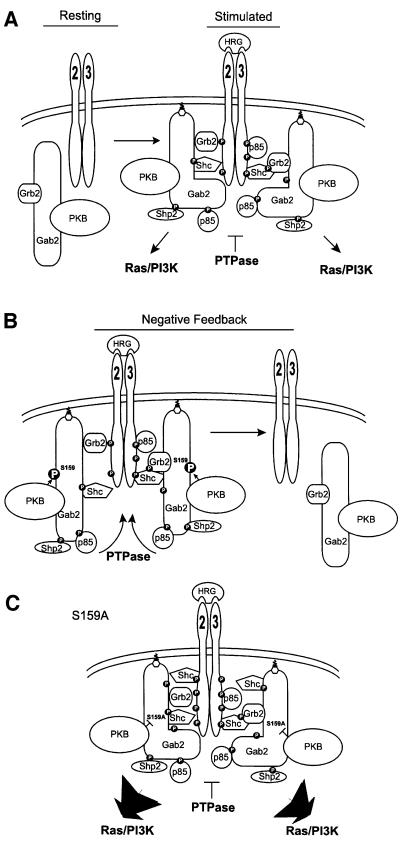
HRG stimulation of MCF-7 (Figure 1), T-47D (not shown) and HEK 293 (Figure 6) cells resulted in a marked tyrosine phosphorylation of Gab2 and assembly of a complex including Shc, Grb2, Shp2 and the p85 subunit of PI3-kinase. A co-immunoprecipitating tyrosine phosphorylated band of ∼185 kDa was also detectable following prolonged exposures (data not shown), which is likely to represent associated ErbB receptors. HRG stimulation of these cell lines would be expected to induce formation of ErbB2–ErbB3 heterodimers, and indeed, one of the co-precipitating receptors was identified as ErbB2 (Figure 6). We hypothesize that there may be at least two modes of Gab2 recruitment to HRG-activated ErbB receptors. First, via Grb2, which binds via its SH2 domain to phosphorylated Y1139 of ErbB2 (Ricci et al., 1995) and predominantly via its C-terminal SH3 domain to Gab2 (Zhao et al., 1999) (Figure 10). This is supported by the observation that binding of Gab1 to the EGFR can be competed by phosphopeptides corresponding to Y1068 and Y1086, docking sites for the Grb2 SH2 domain (Rodrigues et al., 2000). Also, antisense studies have implicated Grb2 in regulation of PKB activity in HRG-transfected or ErbB2-overexpressing breast cancer cells, which could be due to Grb2-mediated receptor recruitment of Gab1/2 (Lim et al., 2000). Secondly, an elaboration on this mode of binding is formation of a receptor–Shc–Grb2–Gab2 complex (Figure 10), as described by Gu et al. (2000) for the IL-3 receptor β common chain. This is possible since Shc can bind the ErbB2–ErbB3 heterodimer via either SH2 or PTB domain-mediated interactions (Prigent et al., 1995; Ricci et al., 1995), and tyrosine phosphorylated Shc binds the SH2 domain of Grb2. This interaction may contribute to the association of tyrosine phosphorylated Shc with Gab2 detected upon HRG stimulation (Figures 1 and 6). However, we also note that there are consensus sites for Shc SH2 domain binding at Tyr192, 476 and 614 of Gab2, suggesting that Shc may bind directly to this docking protein (Songyang et al., 1994; Zhao et al., 1999) (Figure 10).
Fig. 10. Schematic representation of Gab2 coupling to HRG-activated ErbB receptors. (A) Gab2 is recruited to HRG-activated ErbB2 and 3 through a Shc–Grb2 complex or directly via Grb2. The Gab2 PH domain is also assumed to contribute to membrane localization through binding to PI(3,4,5)P3. The action of PTPases on particular receptor and Gab2 phosphorylation sites is blocked by the formation of this complex. (B) PKB-mediated phosphorylation of Gab2 on Ser159 causes a conformational change in Gab2, allowing PTPases to access and dephosphorylate the receptor, Gab2 and their targets, resulting in complex disassembly. (C) Mutation of Ser159 to alanine blocks PKB-mediated phosphorylation. Gab2 continues to shield the active signalling complex from the action of PTPases, resulting in hyperphosphorylation of both the receptor and Gab2, in turn resulting in prolonged and amplified signalling.
The enhancement of HRG-induced Gab2 tyrosine phosphorylation following pretreatment with wortmannin or introduction of the S159A mutation (Figures 2 and 6) and its inhibition upon expression of activated PKB constructs (Figure 5), clearly establish a negative feedback role for PI3-kinase/PKB in regulation of Gab2 signalling. Since pretreatment of MCF-7 cells with wortmannin also increases EGF-induced Gab2 tyrosine phosphorylation (data not shown), this regulatory mechanism is not restricted to HRG-stimulated ErbB receptor signalling. This role for PI3-kinase contrasts with that observed for EGFR signalling via Gab1. In this signalling system, PI3-kinase acts not only downstream of Gab1 but also upstream in a positive feedback mode to enhance Gab1 tyrosine phosphorylation and subsequent activation of the Ras pathway (Rodrigues et al., 2000; Yart et al., 2001). This is due to binding of the Gab1 PH domain to the PI3-kinase product PI(3,4,5)P3 increasing membrane recruitment of the docking protein and presumably juxtaposition with the activated EGFR (Rodrigues et al., 2000). This positive feedback also appears to regulate HRG-induced tyrosine phosphorylation of Gab1, since this is decreased by pretreatment of MCF-7 cells with wortmannin (data not shown). Currently, the binding selectivity of the Gab2 PH domain has not been characterized, although its sequence is closely related to that of Gab1, including the β1–β2 loop region important for binding selectivity (Gu et al., 1998). In the context of coupling to the PI3 kinase/PKB pathway, the critical difference between the two proteins may therefore be the absence of a consensus PKB phosphorylation site in Gab1. If the Gab2 PH domain does bind PI3-kinase products, then PKB-mediated negative feedback must override this regulatory event, since treatment with wortmannin clearly amplifies HRG-induced Gab2 tyrosine phosphorylation (Figure 2). This is the first demonstration of a clear difference in the mechanism of regulation of Gab1 versus Gab2.
In addition to its well characterized tyrosine phosphorylation sites, Gab2 contains in excess of 50 potential Ser/Thr phosphorylation sites for a wide variety of protein kinases including protein kinase A, protein kinase C (PKC), PKB and ERKs. Ser/Thr phosphorylation of docking proteins is commonly utilized to regulate their function. For example, phosphorylation of IRS-1 by PKCs (Chin et al., 1993, 1994; Liu et al., 2001), the ERKs (De Fea and Roth, 1997) and c-Jun N-terminal kinase (Aguirre et al., 2000) inhibits insulin-induced tyrosine phosphorylation, and Ser/Thr phosphorylation of IRS-1 can inhibit association with the insulin receptor (Paz et al., 1997). Phosphorylation of Gab1 by PKCα and β1 inhibits its HGF-induced tyrosine phosphorylation, and enhanced Gab1 Ser/Thr phosphorylation induced by okadaic acid blocks HGF-induced cell scattering (Gual et al., 2001). Positive regulation of docking proteins by Ser/Thr kinases has also been reported. Gab1 associates with ERK2 (Roshan et al., 1999), and ERK-mediated phosphorylation of Gab1 enhances Gab1–p85 association and PI3-kinase activation (Yu et al., 2001). Also, PKB has been reported to positively regulate IRS1 tyrosine phosphorylation by inducing conformational changes that inhibit the action of protein tyrosine phosphatases (PTPases) (Paz et al., 1999).
Unlike IRS1, our data indicate that PKB has a negative role in Gab2 function by way of a feedback loop. Moreover, removal of this regulatory mechanism by mutation of Ser159 to alanine has a global effect on cellular signalling, whereas the Ser/Thr phosphorylation of other docking proteins only affects their tyrosine phosphorylation and receptor association. Specifically, in addition to the marked increase in Gab2 tyrosine phosphorylation and its association with ErbB2 (Figure 6), tyrosine phosphorylation of ErbB2 and other receptor substrates including Shc isoforms is also enhanced (Figures 6 and 7) and this is reflected further in the increased activation of the downstream ERK and PKB pathways (Figure 8). These results indicate the following model for regulation of Gab2 signalling by PKB. Phosphorylation of Gab2 on Ser159 by PKB induces a conformational change (Figure 10B), allowing PTPases to access the activated receptor complex, resulting in receptor dephosphorylation, complex disassembly and ultimately signal termination. Prevention of this feedback mechanism by mutation of the phosphorylation site allows Gab2 to act as a shield, blocking access of PTPases to the receptor complex (Figure 10C). The resulting receptor complex is therefore resistant to down-modulation, resulting in prolonged and amplified signalling. Other mechanisms by which S159A Gab2 may amplify signalling are effects on compartmentalization of receptors and their substrates, and in the longer term, attenuation of receptor down-regulation from the cell surface. These and the PTPase model are not mutually exclusive. However, effects on receptor internalization may be less significant for HRG-activated ErbB receptors, which are endocytosed slowly and then recycled (Baulida et al., 1996; Pinkas Kramarski et al., 1996; Waterman et al., 1998).
The impact of such a global amplification of growth factor receptor-mediated signalling is demonstrated by the markedly transformed phenotype induced in NIH 3T3 cells following moderate (∼5-fold) overexpression of S159A Gab2 (Figure 9). Interestingly, more extreme (at least 10-fold) overexpression of wild-type Gab2 did not result in detectable differences in morphology or growth properties, emphasizing the potency of S159-mediated negative feedback. Since the assays described were performed in 10% serum, we do not know the origin(s) of the growth factor receptor signal amplified by S159A Gab2 and contributing to cellular transformation. However, at least in MCF-7 human breast cancer cells, tyrosine phosphorylation of Gab2 is induced not only by HRG treatment but also by addition of EGF, insulin or bFGF (R.J.Daly et al., manuscript submitted), indicating that multiple RTK types can couple to this docking protein. These findings suggest that aberrant signalling via Gab2 could occur in human cancers and contribute to tumour progression. Mutations or deletions at the Ser159 phosphorylation site so that it is no longer phosphorylated by PKB, or alterations in Gab2 regions that mediate association with PKB and hence lower the Km for Ser159 phosphorylation, could lead to potentiation of mitogenic signalling downstream of growth factor receptors via the mechanisms described above. These hypotheses are currently under investigation.
Materials and methods
DNA constructs/expression plasmids
A partial human GAB2 cDNA (designated KIAA0571, clone HH02388) was obtained from the Kazusa DNA Research Institute (Chiba, Japan). Assembly of the open reading frame according to Zhao et al. (1999) will be described in detail elsewhere. The GAB2 cDNA was cloned into Bluescript BSKII(+) (Stratagene, La Jolla, CA) as a XhoI–NotI fragment. Site-directed mutagenesis was performed using the Quickchange site-directed mutagenesis kit (Stratagene) and correct incorporation of the mutation was confirmed by DNA sequence analysis (Promega, Annandale, NSW, Australia). Primer details can be provided upon request. Both wild-type Gab2 and S159A Gab2 constructs were subcloned into pcDNA3.1(+) (Invitrogen, Groningen, The Netherlands) as a XhoI–XbaI fragment or into the retroviral expression vector pJZENneo as a XhoI–HpaI fragment. PKB constructs (HA-PKBα/pCMV5, HA-PKBαDD/pCMV5, Myr-HA-PKBα/pECE) were obtained from Dr J.Tavaré (Bristol, UK) and Dr D.Alessi (Dundee, UK); c-srcY527F/pBabepuro was obtained from Dr W.Langdon (Perth, Australia) and neuT/pINA was obtained from Dr P.Edwards (Cambridge, UK).
Cell culture and treatments
MCF-7 and T-47D cells were maintained in RPMI-1640 containing 10% (v/v) fetal calf serum (FCS), 2 mM glutamine, 100 µg/ml penicillin and 100 µg/ml streptomycin (all Gibco-BRL, Melbourne, Australia). HEK 293 cells were maintained in minimal essential medium (MEM) with Hanks’ buffered salts solution containing 10% (v/v) FCS, 2 mM glutamine, 100 µg/ml penicillin, 100 µg/ml streptomycin and supplemented with 0.05% (w/v) sodium bicarbonate (Gibco-BRL). COS-7 cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% (v/v) FCS, 2 mM glutamine, 100 µg/ml penicillin and 100 µg/ml streptomycin, and NIH 3T3 cells in DMEM containing 10% (v/v) newborn calf serum (NBCS), 2 mM glutamine, 100 µg/ml penicillin and 100 µg/ml streptomycin. All cells were cultured in an atmosphere of 5% (v/v) CO2/air in a humidified incubator at 37°C. Human recombinant HRG β1 was obtained from R&D systems and was used at a concentration of 10 nM (80 ng/ml). Cells were stimulated for 5 min unless stated otherwise. For inhibitor studies cells were treated for 30 min before stimulation or lysis with 10 µM MEK inhibitor U0126 (Promega) or 100 nM wortmannin (Sigma, Castle Hill, NSW, Australia). Cells were serum starved in full medium without serum for 16–20 h.
Transfection of mammalian cells
HEK 293 or COS-7 cells were grown to 60–80% confluence in 10 cm dishes and then transfected using Fugene 6 (Roche Molecular Biochemicals, Castle Hill, NSW, Australia) transfection reagent. Briefly, 2–5 µg of plasmid DNA were mixed with dilute Fugene 6 (transfection reagent:DNA ratio 3:1) following manufacturer’s instructions. Twenty-four hours post-transfection, cells were serum starved for 16–20 h before treatment with inhibitors and/or growth factors.
Immunoprecipitation and immunoblotting
Cells were lysed on ice in ice-cold normal lysis buffer (NLB: 1% v/v Triton X-100, 50 mM HEPES pH 7.5, 150 mM NaCl, 10% v/v glycerol, 1.5 mM MgCl2, 1 mM EGTA, 10 mM NaPPi, 20 mM NaF) containing 1 mM phenylmethylsulfonyl fluoride (PMSF), 1 mM sodium orthovanadate, 10 µg/ml aprotinin and 10 µg/ml leupeptin for 10 min. Insoluble material was removed by centrifugation (17 000 g for 10 min at 4°C). Protein concentration was determined using a Bradford dye-based assay (Bio-Rad, Hercules, CA). Lysates containing 0.5–1 mg of protein were immunoprecipitated with antibodies specific for Gab2 at a concentration of 2 µg/mg protein for at least 1 h at 4°C. Antibody–protein immune complexes were incubated with 100 µl of a 1:1 suspension of rec-protein G–Sepharose 4B (Zymed, San Francisco, CA, USA) for 1 h at 4°C. Immunoprecipitates were washed three times in NLB, resuspended and boiled in Laemmli sample buffer and subjected to SDS–PAGE followed by immunoblotting with appropriate antibodies at recommended dilutions. Protein detection was performed using Renaissance chemiluminescence reagent (NEN Life Science Products Inc., Boston, MA). For whole cell lysates 20 µg of protein were loaded. Densitometry was performed using IP Lab Gel software (Signal Analytics).
Commercial antibodies
The following monoclonal antibodies and polyclonal antisera were used: Gab2 (Santa Cruz Biotechnology, Santa Cruz, CA), phospho-PKB substrate, PKB, phospho-S473 PKB, mitogen-activated protein kinase (MAPK), phospho-MAPK (all New England Biolabs, Beverly, CA), HRP-RC20, Shc, Grb2 (all Transduction Laboratories, Lexington, KY), p85-PI3K and Shp2 (both Upstate Biotechnologies Inc., Lake Placid, NY). Mouse monoclonal HA antibodies were prepared from 12CA5 cell culture supernatants by protein A affinity chromatography.
Generation of ecotropic retrovirus and infection of NIH 3T3 cells
The retroviral producer cell line Phoenix-Eco was transfected with 5 µg of plasmid DNA using Fugene transfection reagent in 100 mm dishes. After 24 h, the medium was removed and replaced with 5 ml of fresh DMEM containing 10% FCS. The cells were then incubated for 24 h and virus-containing medium removed and filtered using a 0.45 µm Millex HV Durapore filter (Millipore, Bedford, MA). For retroviral infection 3 × 105 NIH 3T3 cells were seeded in 60 mm dishes in 5 ml of DMEM/10% NBCS containing 8 µg/ml polybrene. After 24 h, 2.5 ml of virus-containing supernatant were added. After a further 24 h, virus-containing medium was removed and replaced with fresh DMEM/10% NBCS containing 2 µl/ml Fungizone/amphotericin B and selection agent.
AIG assay
AIG was assayed by the ability to form colonies and grow in soft agar. The bottom agar contained 10% (v/v) NBCS, DMEM and 0.5% low melting point (LMP) agarose (Seaplaque; FMC BioProducts, Rockland, ME). Five hundred cells were resuspended in DMEM containing 10% (v/v) NBCS, 2 µl/ml Fungizone/amphotericin B and 0.3% LMP agarose and plated on top of the bottom agar. All assays were performed in six-well plates in triplicate and were incubated for 2 weeks. Colonies were then stained overnight with p-iodonitrotetrazolium (INT)-violet and counted using a dissecting microscope.
Gab2 in vitro phosphorylation assay
COS-7 cells transiently transfected with constructs for wild-type Gab2, S159A Gab2 or HA-myrPKBα were serum starved overnight. Cells were washed twice with ice-cold phosphate-buffered saline (PBS), lysed in RIPA buffer (0.5% w/v sodium deoxycholate, 150 mM NaCl, 1% v/v NP-40, 50 mM Tris–HCl pH 8.0, 0.1% w/v SDS, 10% v/v glycerol, 5 mM EDTA, 1 mM sodium orthovanadate, 1 mM PMSF, 10 µg/ml aprotinin, 10 µg/ml leupeptin) and insoluble material was removed by centrifugation at 12 000 g for 10 min at 4°C. Supernatants containing 2 mg of cellular protein were subjected to immunoprecipitation with either 5 µl of mouse monoclonal HA antibodies, 2 µg of goat polyclonal Gab2 antibodies or appropriate controls lacking antibody for 2 h at 4°C. The resulting immune complexes were incubated with 100 µl of a 1:1 suspension of rec-protein G–Sepharose 4B for 1 h at 4°C. Immunoprecipitates were washed five times with RIPA buffer and a further three times in kinase buffer (100 mM HEPES, 5 mM MnCl2 pH 7.0). PKB immunoprecipitates were split into five aliquots and resuspended in 10 µl of kinase buffer. Gab2 immune complexes and the minus antibody control beads were resuspended in 50 µl of kinase buffer and 50 µl of the slurry added to the PKB immune complexes. Reactions were started by the addition of 1 µl (10 µCi) of [γ-32P]ATP (NEN, 10 mCi/ml), incubated for 10 min at 30°C with agitation and terminated by the addition of 3× Laemmli sample buffer. Reactions were then subjected to SDS–PAGE followed by autoradiography.
Acknowledgments
Acknowledgements
We thank Dr Takahiro Nagase and his colleagues at the Kazusa DNA Research Institute for the GAB2 cDNA clone, Dr J.Tavare, Dr B.Hemmings and Dr D.Alessi for providing PKB expression constructs, Ruth Lyons for cloning the full-length GAB2 cDNA and Dr Chris Mitchell and Jason Carroll for helpful discussions and critical reading of the manuscript. The National Health and Medical Research Council of Australia and the New South Wales State Cancer Council supported this work.
References
- Aguirre V., Uchida,T., Yenush,L., Davis,R. and White,M.F. (2000) The c-Jun NH2-terminal kinase promotes insulin resistance during association with insulin receptor substrate-1 and phosphorylation of Ser(307). J. Biol. Chem., 275, 9047–9054. [DOI] [PubMed] [Google Scholar]
- Alessi D.R., Andjelkovic,M., Caudwell,B., Cron,P., Morrice,N., Cohen,P. and Hemmings,B.A. (1996) Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J., 15, 6541–6551. [PMC free article] [PubMed] [Google Scholar]
- Andjelkovic M. et al. (1997) Role of translocation in the activation and function of protein kinase B. J. Biol. Chem., 272, 31515–31524. [DOI] [PubMed] [Google Scholar]
- Bardelli A., Longati,P., Gramaglia,D., Stella,M.C. and Comoglio,P.M. (1997) Gab1 coupling to the HGF/Met receptor multifunctional docking site requires binding of Grb2 and correlates with the transforming potential. Oncogene, 15, 3103–3111. [DOI] [PubMed] [Google Scholar]
- Baulida J., Kraus,M.H., Alimandi,M., Di Fiore,P.P. and Carpenter,G. (1996) All ErbB receptors other than the epidermal growth factor receptor are endocytosis impaired. J. Biol. Chem., 271, 5251–5257. [DOI] [PubMed] [Google Scholar]
- Chin J.E., Dickens,M., Tavare,J.M. and Roth,R.A. (1993) Overexpression of protein kinase C isoenzymes α, βI, γ and ε in cells overexpressing the insulin receptor. Effects on receptor phosphorylation and signaling. J. Biol. Chem., 268, 6338–6347. [PubMed] [Google Scholar]
- Chin J.E., Liu,F. and Roth,R.A. (1994) Activation of protein kinase Cα inhibits insulin-stimulated tyrosine phosphorylation of insulin receptor substrate-1. Mol. Endocrinol., 8, 51–58. [DOI] [PubMed] [Google Scholar]
- De Fea K. and Roth,R.A. (1997) Modulation of insulin receptor substrate-1 tyrosine phosphorylation and function by mitogen-activated protein kinase. J. Biol. Chem., 272, 31400–31406. [DOI] [PubMed] [Google Scholar]
- Gu H., Pratt,J.C., Burakoff,S.J. and Neel,B.G. (1998) Cloning of p97/Gab2, the major SHP2-binding protein in hematopoietic cells, reveals a novel pathway for cytokine-induced gene activation. Mol. Cell, 2, 729–740. [DOI] [PubMed] [Google Scholar]
- Gu H., Maeda,H., Moon,J.J., Lord,J.D., Yoakim,M., Nelson,B.H. and Neel,B.G. (2000) New role for Shc in activation of the phosphatidylinositol 3-kinase/Akt pathway. Mol. Cell. Biol., 20, 7109–7120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu H. et al. (2001) Essential role for Gab2 in the allergic response. Nature, 412, 186–190. [DOI] [PubMed] [Google Scholar]
- Gual P., Giordano,S., Anguissola,S., Parker,P.J. and Comoglio,P.M. (2001) Gab1 phosphorylation: a novel mechanism for negative regulation of HGF receptor signaling. Oncogene, 20, 156–166. [DOI] [PubMed] [Google Scholar]
- Herbst R., Carroll,P.M., Allard,J.D., Schilling,J., Raabe,T. and Simon,M.A. (1996) Daughter of sevenless is a substrate of the phosphotyrosine phosphatase Corkscrew and functions during sevenless signaling. Cell, 85, 899–909. [DOI] [PubMed] [Google Scholar]
- Herbst R., Zhang,X., Qin,J. and Simon,M.A. (1999) Recruitment of the protein tyrosine phosphatase CSW by DOS is an essential step during signaling by the sevenless receptor tyrosine kinase. EMBO J., 18, 6950–6961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holgado-Madruga M., Emlet,D.R., Moscatello,D.K., Godwin,A.K. and Wong,A.J. (1996) A Grb2-associated docking protein in EGF- and insulin-receptor signalling. Nature, 379, 560–564. [DOI] [PubMed] [Google Scholar]
- Itoh M., Yoshida,Y., Nishida,K., Narimatsu,M., Hibi,M. and Hirano,T. (2000) Role of Gab1 in heart, placenta and skin development and growth factor- and cytokine-induced extracellular signal-regulated kinase mitogen-activated protein kinase activation. Mol. Cell. Biol., 20, 3695–3704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones N. and Dumont,D.J. (1998) The Tek/Tie2 receptor signals through a novel Dok-related docking protein, Dok-R. Oncogene, 17, 1097–1108. [DOI] [PubMed] [Google Scholar]
- Kouhara H., Hadari,Y.R., Spivak Kroizman,T., Schilling,J., Bar Sagi,D., Lax,I. and Schlessinger,J. (1997) A lipid-anchored Grb2-binding protein that links FGF-receptor activation to the Ras/MAPK signaling pathway. Cell, 89, 693–702. [DOI] [PubMed] [Google Scholar]
- Lawlor M.A. and Alessi,D.R. (2001) PKB/Akt: a key mediator of cell proliferation, survival and insulin responses? J. Cell Sci., 114, 2903–2910. [DOI] [PubMed] [Google Scholar]
- Lecoq-Lafon C., Verdier,F., Fichelson,S., Chretien,S., Gisselbrecht,S., Lacombe,C. and Mayeux,P. (1999) Erythropoietin induces the tyrosine phosphorylation of GAB1 and its association with SHC, SHP2, SHIP and phosphatidylinositol 3-kinase. Blood, 93, 2578–2585. [PubMed] [Google Scholar]
- Lemmon M.A. and Ferguson,K.M. (2000) Signal-dependent membrane targeting by pleckstrin homology (PH) domains. Biochem. J., 350, 1–18. [PMC free article] [PubMed] [Google Scholar]
- Lim S.J., Lopez Berestein,G., Hung,M.C., Lupu,R. and Tari,A.M. (2000) Grb2 downregulation leads to Akt inactivation in heregulin-stimulated and ErbB2-overexpressing breast cancer cells. Oncogene, 19, 6271–6276. [DOI] [PubMed] [Google Scholar]
- Liu Y.F. et al. (2001) Insulin stimulates PKCζ-mediated phosphorylation of insulin receptor substrate-1 (IRS-1). A self-attenuated mechanism to negatively regulate the function of IRS proteins. J. Biol. Chem., 276, 14459–14465. [DOI] [PubMed] [Google Scholar]
- Maroun C.R., Holgado-Madruga,M., Royal,I., Naujokas,M.A., Fournier,T.M., Wong,A.J. and Park,M. (1999) The Gab1 PH domain is required for localization of Gab1 at sites of cell–cell contact and epithelial morphogenesis downstream from the met receptor tyrosine kinase. Mol. Cell. Biol., 19, 1784–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida K. et al. (1999) Gab-family adapter proteins act downstream of cytokine and growth factor receptors and T- and B-cell antigen receptors. Blood, 93, 1809–1816. [PubMed] [Google Scholar]
- Obata T., Yaffe,M.B., Leparc,G.G., Piro,E.T., Maegawa,H., Kashiwagi, A., Kikkawa,R. and Cantley,L.C. (2000) Peptide and protein library screening defines optimal substrate motifs for AKT/PKB. J. Biol. Chem., 275, 36108–36115. [DOI] [PubMed] [Google Scholar]
- Pawson T. and Scott,J.D. (1997) Signaling through scaffold, anchoring and adaptor proteins. Science, 278, 2075–2080. [DOI] [PubMed] [Google Scholar]
- Paz K., Hemi,R., LeRoith,D., Karasik,A., Elhanany,E., Kanety,H. and Zick,Y. (1997) A molecular basis for insulin resistance. Elevated serine/threonine phosphorylation of IRS-1 and IRS-2 inhibits their binding to the juxtamembrane region of the insulin receptor and impairs their ability to undergo insulin-induced tyrosine phosphorylation. J. Biol. Chem., 272, 29911–29918. [DOI] [PubMed] [Google Scholar]
- Paz K., Liu,Y., Shorer,H., Hemi,R., LeRoith,D., Quan,M., Kanety,H., Seger,R. and Zick,Y. (1999) Phosphorylation of insulin receptor substrate-1 (IRS-1) by protein kinase B positively regulates IRS-1 function. J. Biol. Chem., 274, 28816–28822. [DOI] [PubMed] [Google Scholar]
- Pinkas Kramarski R. et al. (1996) Diversification of Neu differentiation factor and epidermal growth factor signaling by combinatorial receptor interactions. EMBO J., 15, 2452–2467. [PMC free article] [PubMed] [Google Scholar]
- Pratt J.C., Igras,V.E., Maeda,H., Baksh,S., Gelfand,E.W., Burakoff,S.J., Neel,B.G. and Gu,H. (2000) Cutting edge: gab2 mediates an inhibitory phosphatidylinositol 3′-kinase pathway in T cell antigen receptor signaling. J. Immunol., 165, 4158–4163. [DOI] [PubMed] [Google Scholar]
- Prigent S.A., Pillay,T.S., Ravichandran,K.S. and Gullick,W.J. (1995) Binding of Shc to the NPXY motif is mediated by its N-terminal domain. J. Biol. Chem., 270, 22097–22100. [DOI] [PubMed] [Google Scholar]
- Raabe T., Riesgo Escovar,J., Liu,X., Bausenwein,B.S., Deak,P., Maroy,P. and Hafen,E. (1996) DOS, a novel pleckstrin homology domain-containing protein required for signal transduction between sevenless and Ras1 in Drosophila. Cell, 85, 911–920. [DOI] [PubMed] [Google Scholar]
- Ricci A., Lanfrancone,L., Chiari,R., Belardo,G., Pertica,C., Natali,P.G., Pelicci,P.G. and Segatto,O. (1995) Analysis of protein–protein interactions involved in the activation of the Shc/Grb-2 pathway by the ErbB-2 kinase. Oncogene, 11, 1519–1529. [PubMed] [Google Scholar]
- Rodrigues G.A., Falasca,M., Zhang,Z., Ong,S.H. and Schlessinger,J. (2000) A novel positive feedback loop mediated by the docking protein Gab1 and phosphatidylinositol 3-kinase in epidermal growth factor receptor signaling. Mol. Cell. Biol., 20, 1448–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roshan B., Kjelsberg,C., Spokes,K., Eldred,A., Crovello,C.S. and Cantley,L.G. (1999) Activated ERK2 interacts with and phosphorylates the docking protein GAB1. J. Biol. Chem., 274, 36362–36368. [DOI] [PubMed] [Google Scholar]
- Sachs M., Brohmann,H., Zechner,D., Muller,T., Hulsken,J., Walther,I., Schaeper,U., Birchmeier,C. and Birchmeier,W. (2000) Essential role of Gab1 for signaling by the c-Met receptor in vivo. J. Cell Biol., 150, 1375–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeper U., Gehring,N.H., Fuchs,K.P., Sachs,M., Kempkes,B. and Birchmeier,W. (2000) Coupling of Gab1 to c-Met, Grb2 and Shp2 mediates biological responses. J. Cell Biol., 149, 1419–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Songyang Z. et al. (1994) Specific motifs recognized by the SH2 domains of Csk, 3BP2, fps/fes, GRB-2, HCP, SHC, Syk and Vav. Mol. Cell. Biol., 14, 2777–2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi-Tezuka M., Yoshida,Y., Fukada,T., Ohtani,T., Yamanaka,Y., Nishida,K., Nakajima,K., Hibi,M. and Hirano,T. (1998) Gab1 acts as an adapter molecule linking the cytokine receptor gp130 to ERK mitogen-activated protein kinase. Mol. Cell. Biol., 18, 4109–4117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterman H., Sabanai,I., Geiger,B. and Yarden,Y. (1998) Alternative intracellular routing of ErbB receptors may determine signaling potency. J. Biol. Chem., 273, 13819–13827. [DOI] [PubMed] [Google Scholar]
- Weidner K.M., Di Cesare,S., Sachs,M., Brinkmann,V., Behrens,J. and Birchmeier,W. (1996) Interaction between Gab1 and the c-Met receptor tyrosine kinase is responsible for epithelial morphogenesis. Nature, 384, 173–176. [DOI] [PubMed] [Google Scholar]
- Yamanashi Y. and Baltimore,D. (1997) Identification of the Abl- and rasGAP-associated 62 kDa protein as a docking protein, Dok. Cell, 88, 205–211. [DOI] [PubMed] [Google Scholar]
- Yart A. et al. (2001) A critical role for phosphoinositide 3-kinase upstream of Gab1 and SHP2 in the activation of ras and mitogen-activated protein kinases by epidermal growth factor. J. Biol. Chem., 276, 8856–8864. [DOI] [PubMed] [Google Scholar]
- Yenush L. and White,M.F. (1997) The IRS-signalling system during insulin and cytokine action. BioEssays, 19, 491–500. [DOI] [PubMed] [Google Scholar]
- Yu C.F., Roshan,B., Liu,Z.X. and Cantley,L.G. (2001) Erk regulates the hepatocyte growth factor-mediated interaction of gab1 and the phosphatidylinositol 3-kinase. J. Biol. Chem., 276, 32552–32558. [DOI] [PubMed] [Google Scholar]
- Zhao C., Yu,D.H., Shen,R. and Feng,G.S. (1999) Gab2, a new pleckstrin homology domain-containing adapter protein, acts to uncouple signaling from ERK kinase to Elk-1. J. Biol. Chem., 274, 19649–19654. [DOI] [PubMed] [Google Scholar]