Abstract
Ligand-induced desensitization of the epidermal growth factor receptor (EGFR) is controlled by c-Cbl, a ubiquitin ligase that binds multiple signaling proteins, including the Grb2 adaptor. Consistent with a negative role for c-Cbl, here we report that defective Tyr1045 of EGFR, an inducible c-Cbl docking site, enhances the mitogenic response to EGF. Signaling potentiation is due to accelerated recycling of the mutant receptor and a concomitant defect in ligand-induced ubiquitylation and endocytosis of EGFR. Kinetic as well as morphological analyses of the internalization-defective mutant receptor imply that c-Cbl-mediated ubiquitylation sorts EGFR to endocytosis and to subsequent degradation in lysosomes. Unexpectedly, however, the mutant receptor displayed significant residual ligand-induced ubiquitylation, especially in the presence of an overexpressed c-Cbl. The underlying mechanism seems to involve recruitment of a Grb2 c-Cbl complex to Grb2-specific docking sites of EGFR, and concurrent acceleration of receptor ubiquitylation and desensitization. Thus, in addition to its well-characterized role in mediating positive signals, Grb2 can terminate signal transduction by accelerating c-Cbl-dependent sorting of active tyrosine kinases to destruction.
Keywords: growth factor/SH2 domain/signal transduction/tyrosine kinase/ubiquitin ligase
Introduction
Polypeptide growth factors mediate cell-to-cell interactions by initiating an ordered cascade of membranal and cytoplasmic events culminating in altered gene expression (van der Geer et al., 1994). While events involved in signal generation and maintenance are extensively characterized, our understanding of processes that terminate signaling is relatively limited. The role for such negative signaling pathways extends beyond the ability to terminate intracellular signals. For example, analyses of one of the major signaling pathways, the mitogen-activated protein kinase (MAPK) cascade, led to the realization that the amplitude and duration of MAPK activation critically determine not only the kinetics but also the identity of cellular responses to hormonal signals (Marshall, 1995). The ErbB family of growth factor receptors exemplifies the importance of negatively acting regulatory pathways and their significance to human diseases (reviewed in Yarden and Sliwkowski, 2001). The four ErbB proteins bind a large group of growth factors all sharing an epidermal growth factor (EGF) domain. Interestingly, the four receptors differ in their signaling potency in accordance with distinct mechanisms that negatively regulate the receptor’s fate. For example, only ErbB-1 (also called EGFR) is strongly coupled to the c-Cbl adaptor protein, and this receptor, unlike other ErbB members, is effectively targeted to lysosomal degradation (Levkowitz et al., 1998). Similarly, the ortholog of ErbB proteins in Caenorhabditis elegans, LET-23, is negatively regulated by SLI-1, the ortholog of mammalian Cbl proteins (Jongeward et al., 1995). Recent studies that made use of an in vitro ubiquitylation system uncovered the role of c-Cbl as an E3 ubiquitin ligase that recruits ubiquitin-loaded E2 enzymes to ligand-activated receptors (Joazeiro et al., 1999; Levkowitz et al., 1999; Waterman et al., 1999; Yokouchi et al., 1999). Apparently, Cbl proteins bind ligand-activated receptor tyrosine kinases through their N-terminally located phosphotyrosine-binding domain, whereas the flanking RING finger enables close apposition of an E2 enzyme, permitting transfer of ubiquitin to target proteins.
Exactly how c-Cbl-induced poly-ubiquitylation of EGFR regulates delivery to the lysosome remains an open question. Internalization of yeast membrane proteins is initiated by protein mono-ubiquitylation (reviewed by Hicke, 2001). In line with the possibility that a similar mechanism operates in mammalian cells, internalization of the macrophage growth factor receptor is retarded in c-Cbl-defective cells (Lee et al., 1999). However, although overexpression of c-Cbl enhanced ubiquitylation of EGFR, no concurrent increase in receptor internalization rate could be demonstrated (Levkowitz et al., 1998; Thien et al., 2001). Likewise, it is unclear whether c-Cbl is recruited to EGFR at the plasma membrane or only when the receptors reach the early endosome (Levkowitz et al., 1998; Lee et al., 1999; Stang et al., 2000). To address the function of c-Cbl in negative signaling, we made use of a mutant EGFR, the c-Cbl docking site of which—Tyr1045—was defective (Levkowitz et al., 1999). Here we provide evidence that this site restricts the mitogenic action of EGFR by enabling recruitment of c-Cbl to the plasma membrane-localized receptors, thereby accelerating their internalization and delivery to lysosomes. By employing the defective receptor, we uncovered a secondary pathway that allows indirect coupling of c-Cbl to activated receptors. This surrogate pathway involves the adaptor function of Grb2. Thus, our studies help resolve the role of c-Cbl in receptor desensitization and reveal a previously uncharacterized negative function of Grb2.
Results
An EGFR mutant defective at Tyr1045 elicits stronger mitogenic signals
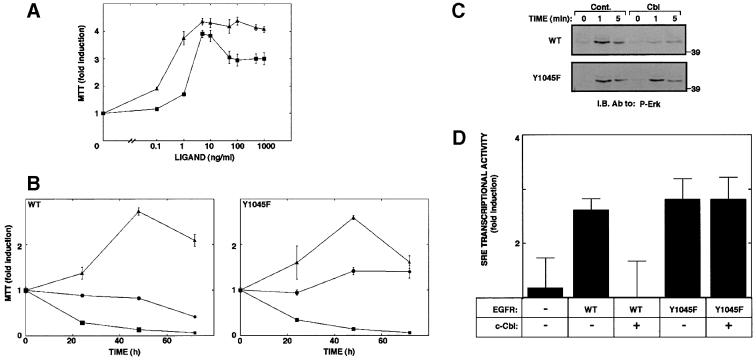
Replacement of Tyr1045 of EGFR with a phenylalanine reduced ligand-induced down-regulation in living cells and significantly reduced receptor ubiquitylation (Levkowitz et al., 1999). To resolve the relationship between receptor ubiquitylation and signaling capacity, we stably expressed the mutant receptor (Y1045F) in interleukin-3 (IL-3)-dependent 32D myeloid cells. These cells are originally devoid of any ErbB protein, but ectopic expression of ErbB receptors confers mitogenic responsiveness to the respective ligands. Cell lines expressing comparable numbers of wild-type and mutant receptors were established by drug selection and their growth examined using the MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide] assay. When cultured for 24 h in the absence of IL-3, but in the presence of increasing concentrations of EGF, cells expressing a wild-type (wt) EGFR generated a moderate mitogenic signal (Figure 1A). However, the mutant receptor defective in c-Cbl binding elicited significantly higher proliferative signals. The relatively potent mitogenic potential of the mutant receptor was also reflected in a long-term cell growth assay. Whereas EGF only slightly extended survival of cells expressing wt-EGFR, ligand stimulation of 32D cells expressing Y1045F-EGFR exerted marked cell proliferation, albeit lower than the effect observed with IL-3 (Figure 1B). To examine the possibility that Y1045F-EGFR is endowed with relatively potent mitogenic signaling because it escapes inhibition by c-Cbl, we tested the effect of an ectopic c-Cbl on signaling downstream of EGFR (Figure 1C and D). The biochemical assays employed were MAPK activation and transcription of a reporter gene controlled by the serum response element (SRE). The assays were performed with Chinese hamster ovary (CHO) cells, because these cells express no endogenous EGFR. As expected, stimulation of wt-EGFR with EGF resulted in marked activation of MAPK, but introduction of an exogenous c-Cbl significantly reduced the effect of EGF. Likewise, transcription from the SRE was markedly elevated by EGF and almost completely suppressed when c-Cbl was overexpressed. The Y1045F mutant mediated comparable MAPK activation, as well as marked transcription from the SRE, but both activities were unaffected by an ectopically overexpressed c-Cbl. Taken together with the results of the cell growth assays, these observations indicate that c-Cbl acts as a suppressor of EGFR signaling.
Fig. 1. A mutant EGFR defective at Tyr1045 elicits potent signals and is refractory to c-Cbl. (A) Sublines of 32D cells that express either a wt-EGFR (squares), or a Tyr1045 mutant (Y1045F; triangles) were deprived of IL-3 and plated at a density of 5 × 105 cells/ml in media containing serial dilutions of EGF. The MTT assay was performed 24 h later. The results obtained are presented as fold induction relative to a control culture maintained in the absence of added growth factor. (B) The indicated sublines of 32D cells (5 × 105 cells/ml) were incubated for various time intervals with EGF at 100 ng/ml (circles). Control cultures were incubated in the absence (squares) or presence of IL-3 (triangles). Cell growth was determined daily by using the colorimetric MTT assay, and compared with the signal observed at time zero. The data presented are the mean ± SD of four determinations. (C) CHO cells were transiently transfected with plasmids encoding either a wt-EGFR (WT) or a Tyr1045 mutant (Y1045F). Alongside, we used a vector encoding c-Cbl or a control empty vector. Cell monolayers were stimulated with EGF (100 ng/ml) for the indicated time intervals at 37°C. Whole-cell lysates were analyzed with an antibody specific to the active form of MAPK. (D) CHO cells were co-transfected in triplicates as in (C), along with a reporter plasmid (SRE-luc). Thirty-six hours later cells were untreated or treated with EGF (20 ng/ml). Following an additional 12 h, cells were harvested for a luciferase assay. Signals obtained were normalized to protein concentrations and are presented as average ± SD.
Abrogation of c-Cbl’s interaction with EGFR retards receptor endocytosis and accelerates recycling
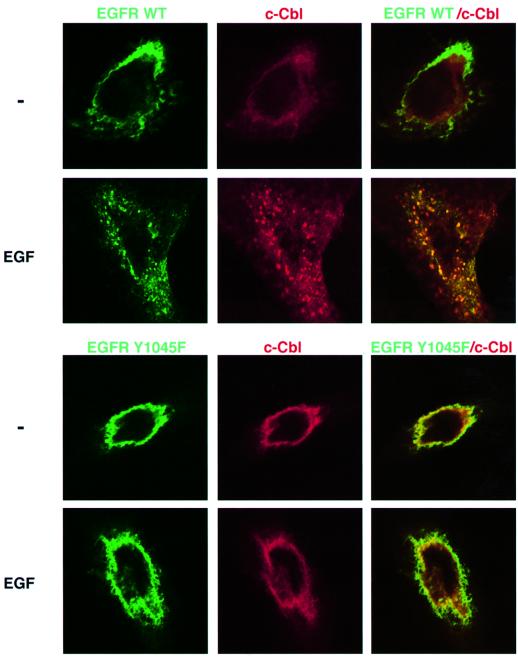
To address the relationships between c-Cbl recruitment and receptor endocytosis, we made use of a previously described fusion protein comprised of a full-length EGFR fused to green fluorescence protein (GFP) (Brock et al., 1999). A mutation was introduced in the codon corresponding to Tyr1045 and the plasmid used to co-transfect CHO cells. Consistent with previous reports, the plasma membrane-associated wt-EGFR translocated into large endocytic vesicles upon short exposure of cells to EGF (Figure 2). Prior to stimulation with a ligand, c-Cbl was localized primarily to the cytoplasm (Wang et al., 1999). However, shortly after stimulation with EGF, a large fraction of c-Cbl translocated into cytoplasmic vesicles, many of which contained the GFP–EGFR. These results are consistent with observations made with a GFP–Cbl fusion protein (Levkowitz et al., 1998). The behavior of GFP–Y1045F revealed remarkable differences: upon stimulation with EGF, this mutant underwent only limited translocation into endosomal vesicles. Furthermore, in line with a major defect in internalization, the mutant receptor was unable to induce translocation of c-Cbl.
Fig. 2. The c-Cbl docking site of EGFR is necessary for ligand-induced receptor endocytosis and for translocation of c-Cbl to endocytic vesicles. CHO cells transiently expressing HA-tagged c-Cbl along with a wild-type GFP–EGFR (upper panel), or a similar fusion protein containing a mutation at Tyr1045 (Y1045F; lower panel), were grown on cover slips. Cells were incubated for 15 min at 37°C without or with EGF (100 ng/ml). To visualize c-Cbl, cells were fixed, permeabilized and incubated with an anti-HA antibody, followed by incubation with a Cy3-conjugated secondary antibody (red, middle column). The GFP–EGFR fluorescence is represented in the left column (green). The right column presents the overlay of GFP and Cy3 fluorescence, generating a yellow color in areas of co-localization.
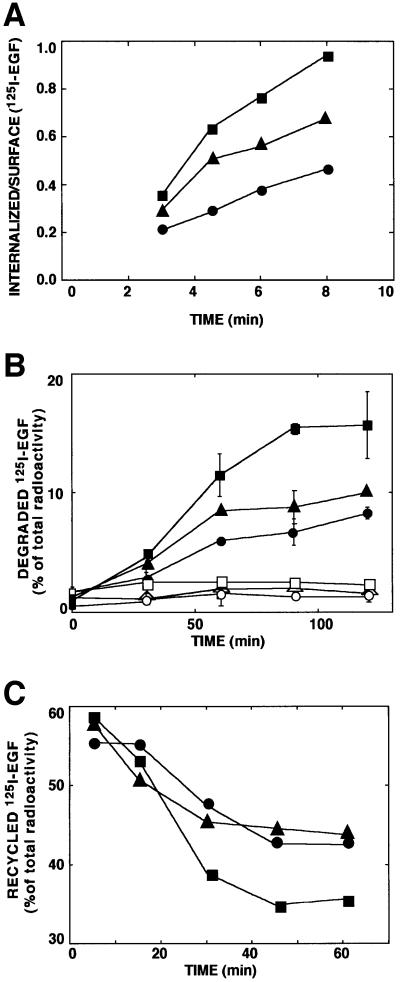
To elucidate the defect in internalization of the Y1045F mutant receptor, we performed several kinetic assays. For a reference mutant whose ligand-induced internalization is defective we used an EGFR devoid of tyrosine kinase activity due to a mutation in the ATP-binding site (K721A) (Chen et al., 1989; Felder et al., 1990). A short-term ligand internalization assay confirmed the relatively slow internalization rate of EGF by K721A (Figure 3A). In the same assay Y1045F displayed an intermediate rate of ligand endocytosis. Likewise, whereas the wt-EGFR mediated rapid ligand degradation, which was completely inhibited by an inhibitor of lysosomal hydrolases, both mutant receptors mediated significantly slower rates of EGF degradation (Figure 3B). In concordance with the internalization assay, Y1045F was slightly more effective than the kinase-dead mutant, but even the latter mediated ligand destruction, probably because it can enter the basal route of endocytosis (Wiley et al., 1991). In agreement with previous reports that documented a defect in translocation of a kinase-defective EGFR to late endosomal compartments (Felder et al., 1990; Hopkins et al., 1990), this mutant recycled EGF more efficiently (Figure 3C). Interestingly, Y1045F was as effective as the kinase-dead receptor in recycling an intact ligand. In conclusion, Tyr1045-mediated interaction of EGFR with c-Cbl seems essential for rapid endocytosis of ligand-receptor complexes and their translocation to lysosomal sites of EGF degradation.
Fig. 3. The c-Cbl docking site of EGFR is essential for rapid ligand endocytosis and degradation, and it enables sequestration from the recycling pathway. (A) CHO cells transiently expressing a wt-EGFR (squares), a kinase-defective mutant receptor (circles) and a Tyr1045 mutant (Y1045F; triangles) were incubated at 37°C with a radiolabeled EGF (2 ng/ml). At the indicated times, cell monolayers were acid-washed to remove surface-bound EGF. Radioactivity present in the acidic fraction was quantified in triplicates and designated surface-associated ligand. The remaining cell-associated radioactivity (internalized) was similarly quantified following cell solubilization. The ratio obtained at each time point is presented (average ± SD). (B) Monolayers of CHO cells transfected as in (A) were incubated for 1 h at 20°C with a radiolabeled EGF. Sister monolayers were pre-incubated for 30 min with chloroquine (0.2 mM; open symbols). Thereafter, cells were washed and maintained at 37°C for the indicated time intervals. Media were then collected, and the cells were solubilized. The trichloroacetic acid-soluble radioactivity was determined and the fraction of degraded ligand presented. (C) CHO cells transiently expressing wt-EGFR (squares), the Y1045F mutant receptor (circles) or a kinase-defective mutant (triangles) were allowed to internalize a radiolabeled EGF (1 ng/ml) for the indicated time periods. The remaining surface bound ligand was removed by mild acid-washing. The EGF-loaded cells were then incubated with an unlabeled EGF at 4°C, followed by 1 h at 37°C. Intact radioactive ligand was harvested from the medium following a 1 h chase period. Similarly, the fraction of surface-bound radioactivity was determined and the combined fractions designated recycled ligand. The fraction of recycled ligand is presented as average ± SD (bars) of triplicate determinations.
Grb2 enhances a surrogate mechanism of c-Cbl-dependent receptor ubiquitylation
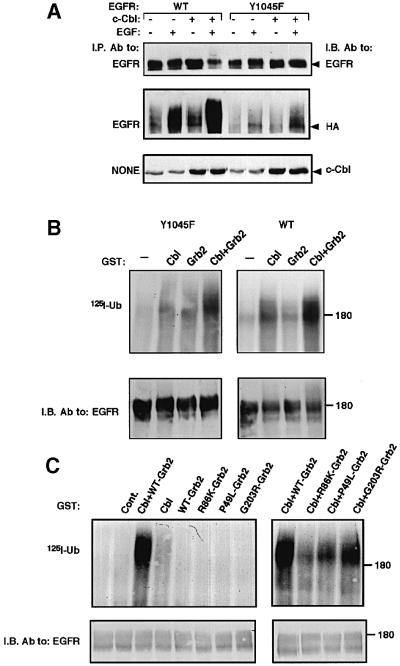
Several results we obtained with the Y1045F mutant receptor raised the possibility that ligand-induced endocytosis of the mutant receptor is only partially impaired. Assuming that c-Cbl and receptor ubiquitylation are essential for the residual ligand-dependent endocytosis of Y1045F, we examined the status of ubiquitylation of this mutant receptor upon high-level ectopic expression of c-Cbl. The results depicted in Figure 4A confirmed the ability of an overexpressed c-Cbl to enhance ligand-induced ubiquitylation and degradation of wt-EGFR. Likewise, faint but reproducible ubiquitylation of Y1045F was detectable upon short stimulation with EGF, and this modification was further enhanced when c-Cbl was overexpressed. In line with weak ligand-induced ubiquitylation, Y1045F underwent undetectable degradation following stimulation with EGF (Figure 4A). These results suggest the existence of a secondary mechanism of c-Cbl-induced receptor ubiquitylation. Because the surrogate pathway is sensitive to EGF but independent of Tyr1045, it seemed likely that another tyrosine phosphorylation site of EGFR is engaged.
Fig. 4. An alternative receptor ubiquitylation pathway independent of Tyr1045 may involve Grb2. (A) CHO cells were transfected with plasmids encoding a wt-EGFR (WT) or the Y1045F mutant, along with vectors encoding c-Cbl and an HA-tagged ubiquitin. Following 48 h, cell monolayers were treated for 10 min at 37°C without or with EGF (100 ng/ml) and cell lysates subjected to immunoprecipitation (I.P.) and immunoblotting (I.B.) with the indicated antibodies. Whole-cell lysates were also analyzed (lower panel). (B) wt-EGFR or the Y1045F mutant receptor were immunopurified from transfected HEK-293 cells. Receptor immunoprecipitates were subjected to an in vitro ubiquitylation assay with a radiolabeled ubiquitin. c-Cbl, Grb2 or a combination of the two proteins was added to the reaction mixtures in the form of GST fusion proteins. Receptor immunoprecipitates were resolved by electrophoresis and proteins transferred to filters, which were first autoradiographed (upper panel, 125I-Ub) and then immunoblotted with anti-EGFR antibodies (lower panel). (C) Immuno precipitates of Y1045F were subjected to an in vitro ubiquitylation assay in the presence of one or two of the indicated GST fusion proteins. As a control, GST was added alone (Cont.) or it was omitted from the reaction (unlabeled lane).
The above results led us to suspect that a c-Cbl-associated protein, which is capable of binding a phosphotyrosine distinct from Tyr1045, is involved in recruiting c-Cbl to Y1045F. Because two autophosphorylation sites of EGFR can bind Grb2 (Batzer et al., 1994; Okutani et al., 1994), and this adaptor is constitutively and stoichiometrically bound to c-Cbl (Meisner and Czech, 1995; Donovan et al., 1996), we reasoned that Grb2 may be involved in the surrogate pathway of EGFR ubiquitylation. To test this prediction, we utilized a previously described in vitro ubiquitylation assay (Levkowitz et al., 1999; Waterman et al., 1999). Figure 4B shows that incubation of an immuno-affinity purified wt-EGFR with reticulocyte lysate in the presence of a radiolabeled ubiquitin resulted in faint receptor ubiquitylation. However, addition of c-Cbl strongly promoted receptor ubiquitylation, as has been reported previously (Joazeiro et al., 1999; Levkowitz et al., 1999; Waterman et al., 1999; Yokouchi et al., 1999). In contrast, a recombinant Grb2 protein was ineffective, but its combination with c-Cbl moderately enhanced receptor ubiquitylation. This synergistic effect of Grb2 and c-Cbl was more conspicuous when the Y1045F mutant receptor was used in vitro as a substrate (Figure 4B). To test which domains of Grb2 are involved in Y1045F ubiquitylation, we used proteins carrying partially inactivating point mutations at each of the three domains of Grb2. Of the three mutants we tested, a protein mutated at the single SH2 domain (mutant denoted R86K-Grb2) was severely impaired in its ability to ubiquitylate Y1045F (Figure 4C), in line with binding to a phosphotyrosine of EGFR. On the other hand, a Grb2 protein mutated at the C-terminal SH3 domain (G203R-Grb2) was almost as active as wild-type Grb2, but a mutation within the N-terminal SH3 domain (mutant denoted P49L-Grb2) partly inactivated Grb2. Taken together, these results support recruitment of c-Cbl to Y1045F by simultaneous binding of Grb2 to c-Cbl (primarily via the N-terminal SH3 domain) and EGFR (via the SH2 domain).
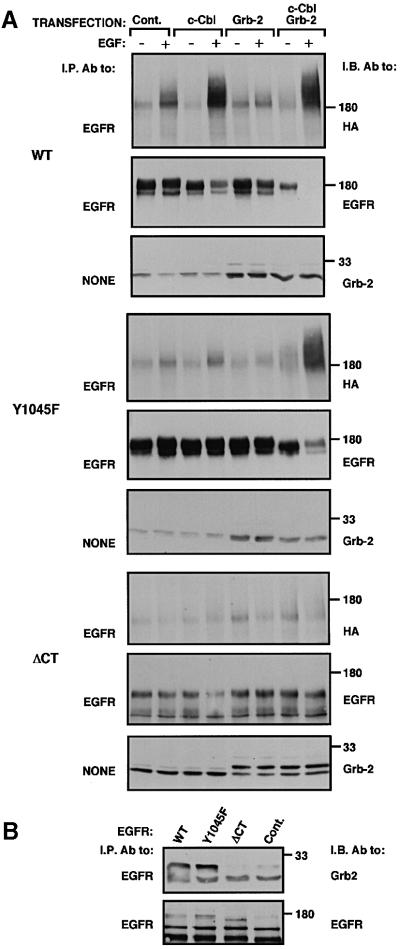
The synergistic in vitro effect of Grb2 and c-Cbl prompted us to examine their combined action on EGFR in living cells. Overexpression of c-Cbl exerted only a moderate effect on ubiquitylation of the wt-EGFR (Figure 5A). However, co-expression of Grb2 and c-Cbl enhanced receptor ubiquitylation and significantly increased its degradation. Moreover, when singly overexpressed, neither c-Cbl nor Grb2 could strongly enhance EGF-dependent ubiquitylation and degradation of Y1045F, but their combination effectively enhanced both activities (Figure 5A). Because the effect of Grb2 was especially strong when cells were stimulated with EGF, we assumed that at least one of the two Grb2 association sites of EGFR [tyrosines 1068 and 1086 (Batzer et al., 1994; Okutani et al., 1994)] is involved in recruiting a complex of Grb2 and c-Cbl. This possibility was indirectly supported by the inability of a combination of Grb2 and c-Cbl to reconstitute ligand-induced ubiquitylation of a receptor lacking the whole C-terminus (ΔCT, residues 1–972), including all Grb2 and Shc association sites (Figure 5A). In line with loss of ligand-induced ubiquitylation, we observed no co-immunoprecipitation of the tail-less mutant receptor with Grb2 (Figure 5B), but both Y1045F and the wt-EGFR displayed physical association with the adaptor. In other experiments we analyzed EGFR mutants whose seven (mutant denoted F7) or eight (F8) potential phosphorylation sites, including Tyr1045, were replaced by phenylalanines. Whereas F8 lost ligand-induced ubiquitylation, F7 displayed some EGF-induced modification (data not shown), probably because its Tyr1114, a known Grb2 and Shc docking site, was intact.
Fig. 5. Grb2 enhances c-Cbl-dependent ubiquitylation and degradation of EGFR in living cells. (A) Monolayers of CHO cells were transiently transfected with expression vectors encoding the indicated forms of EGFR. Alongside, plasmids encoding c-Cbl, Grb2, HA-ubiquitin and control empty vectors were used as indicated. Cell monolayers were treated for 10 min at 37°C without or with EGF (100 ng/ml). Subsequently, cell lysates were directly subjected to immunoblotting (I.B.; panels labeled NONE). Alternatively, EGFR was first isolated, and the immunoprecipitates analyzed with the indicated antibodies. (B) CHO cells were transfected with plasmids encoding the indicated forms of EGFR, along with plasmids encoding Grb2 and c-Cbl. A control culture was treated with an empty expression vector. Following stimulation with EGF (100 ng/ml; 10 min at 37°C), EGFR was isolated from cell lysates and the immuno-complexes analyzed by using antibodies to either Grb2 or EGFR.
Grb2 and c-Cbl co-operatively accelerate down-regulation and endocytosis of a mutant EGFR
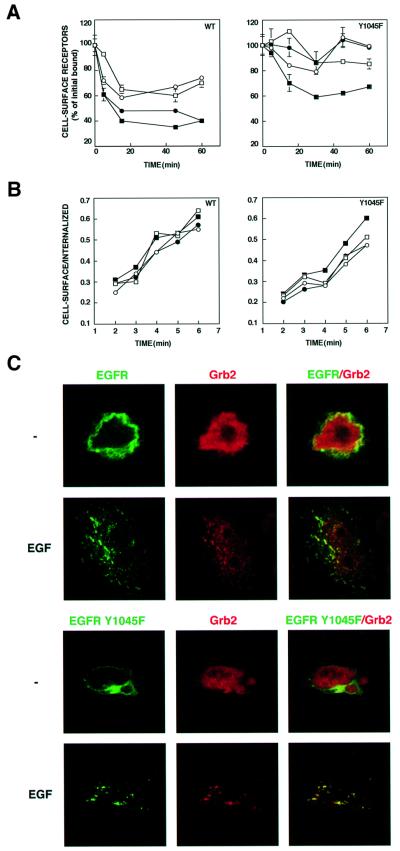
To substantiate the combined effect of Grb2 and c-Cbl, we examined receptor down-regulation and endocytosis in CHO cells ectopically expressing the combination. As is evident from Figure 6A, overexpression of Grb2 alone exerted no significant effect on the extent of ligand-induced down-regulation of the wt-EGFR. This contrasts with c-Cbl, whose overexpression enhanced receptor down-regulation, in line with previous reports (Levkowitz et al., 1998; Yokouchi et al., 1999; Lill et al., 2000). However, co-transfection of Grb2 and c-Cbl slightly enhanced receptor down-regulation (Figure 6A). The synergistic effect was more significant in the case of Y1045F, as this mutant underwent almost no ligand-induced down-regulation upon overexpression of either c-Cbl or Grb2 (Figure 6A). A complementary short-term ligand internalization assay reflected the lower rate of Y1045F internalization relative to wt-EGFR (Figures 3A and 6B). As noted previously (Levkowitz et al., 1998; Thien et al., 2001), we observed no significant effect of an overexpressed c-Cbl on the rate of internalization of the wt-EGFR. Likewise, Grb2 exerted no effect on internalization of either receptor form. However, the combination of Grb2 and c-Cbl reproducibly accelerated the rate of Y1045F internalization (Figure 6B). Taken together, these results extend the combined effect of c-Cbl and Grb2 to receptor endocytosis, implying causative relationships between receptor ubiquitylation and endocytosis.
Fig. 6. Grb2 accelerates internalization and down-regulation of the Y1045F mutant EGFR. (A) CHO cells were transfected with expression vectors encoding wt-EGFR (WT) or the Y1045F mutant. Alongside we used plasmids encoding c-Cbl (closed circles), Grb-2 (open squares), a combination of Grb2 and c-Cbl (closed squares) or an empty control vector (open circles). Forty-eight hours post-transfection, cultures were incubated at 37°C with EGF (25 ng/ml) for the indicated periods of time. Cell-bound ligand was removed, and the level of surface receptors was determined by binding of a radiolabeled EGF at 4°C. The average ± SD (bars) of triplicate determinations is shown for each time point. (B) Monolayers of CHO cells were transfected as in (A) with vectors driving expression of the indicated version of EGFR. After 48 h, cells were incubated at 37°C with a radiolabeled EGF (2 ng/ml). At the indicated time points, monolayers were acid-washed to remove surface-bound ligand. Radioactivity present in the acidic fraction was designated surface-associated ligand. The remaining cell-associated radioactivity was determined in triplicates following cell solubilization and designated internalized ligand. Symbols are as in (A). (C) CHO cells transiently overexpressing HA-tagged c-Cbl and histidine-tagged Grb2, along with a GFP–EGFR, or a fusion protein containing a mutation at Tyr1045 (Y1045F), were grown on cover slips for 48 h after transfection. Thereafter, cells were incubated for 15 min at 37°C without or with EGF (100 ng/ml). To visualize Grb2, cells were fixed, permeabilized, and incubated with a rabbit anti-His6 followed by a Cy3-conjugated secondary antibody (red). The GFP–EGFR fluorescence is shown in the left column (green). The right column presents the overlay of GFP and Cy3 fluorescence; a yellow color is seen in areas of co-localization.
The ability of Grb2 to enhance ligand-induced internalization of Y1045F was supported by fluorescence microscopy. As is shown in Figure 2, EGF and c-Cbl exerted only a small effect on endocytosis of GFP–Y1045F. However, expression of an ectopic Grb2 together with c-Cbl enhanced ligand-induced endocytosis of GFP–Y1045F (Figure 6C). Interestingly, we observed partial co-localization of Grb2 with the internalized EGFR (note the yellow figures in the merge panels of Figure 6C). Indeed, a recent report utilizing fluorescence energy transfer detected physical association between Grb2 and EGFR in endosomes (Sorkin et al., 2000). Together with the biochemical lines of evidence, our morphological analyses support the existence of a dual mechanism of c-Cbl-induced endocytosis: a major pathway mediated by Tyr1045 and c-Cbl, and a second route that involves recruitment of Grb2, presumably pre-complexed with c-Cbl.
The RING finger and the regulatory region of c-Cbl, but not the SH2 domain, are required for Grb2-mediated receptor ubiquitylation
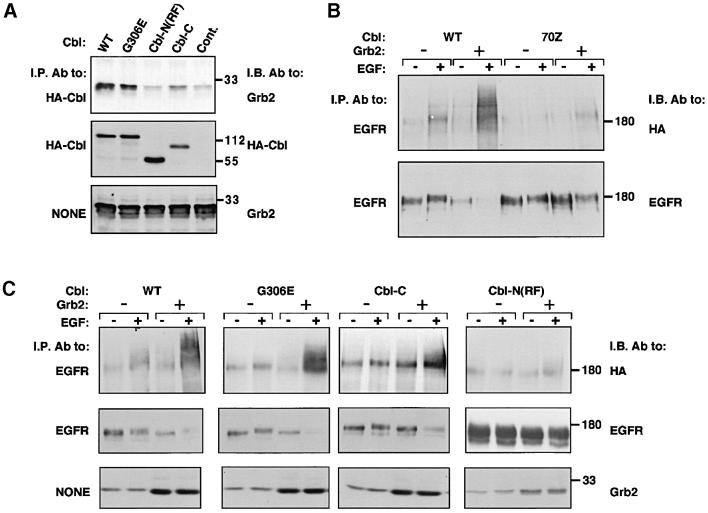
We next analyzed the domains of c-Cbl that are necessary for interaction with Grb2 and for the associated increase in Y1045F ubiquitylation. Three mutants of c-Cbl were used: a protein whose SH2 domain is defective due to a point mutation [G306E (Bonita et al., 1997)] and two deletion mutants lacking either the N-terminal or the C-terminal half [Cbl-C and Cbl-N(RF), respectively]. Both deletion mutants carried an intact RING finger, but only Cbl-N(RF), whose SH2 domain is intact, could support ubiquitylation of wt-EGFR (data not shown; Lill et al., 2000). In contrast, this mutant displayed only weak co-immunoprecipitation with Grb2 (Figure 7A), and when co-expressed together with the adaptor it mediated no increase in ubiquitylation and degradation of Y1045F (Figure 7C). Thus, recruitment of Grb2 to the C-terminus of c-Cbl appears to be essential for the surrogate pathway of EGFR ubiquitylation, but the presence of the SH2 domain is not critical. Indeed, a mutant containing an intact poly-proline domain, the site of Grb2 binding (Meisner and Czech, 1995; Donovan et al., 1996), but lacking the SH2 domain (Cbl-C), retained binding to Grb2 (Figure 7A) and partially enhanced Y1045F ubiquitylation and degradation (Figure 7C). In experiments that are not presented, we examined the relative ability of native c-Cbl and Cbl-N(RF) to enhance ubiquitylation and degradation of wt-EGFR. As expected, the mutant was inferior to c-Cbl, probably due to evading the surrogate pathway. By analyzing a natural mutant of the RING finger, namely 70Z-Cbl, we learned that this domain is essential for the surrogate pathway of receptor ubiquitylation (Figure 7B). In contrast, by using the G306E mutant, we confirmed that the SH2 domain of c-Cbl is not involved in the surrogate pathway. In conclusion, unlike the major pathway of receptor ubiquitylation, which requires binding of the SH2 domain of c-Cbl to a specific phosphotyrosine of the receptor, the surrogate pathway is independent of the c-Cbl’s SH2 domain. In addition to the RING finger, this pathway involves the C-terminal half of c-Cbl, probably because this portion of the molecule contains several proline-rich sites that recruit Grb2.
Fig. 7. Subdomains of c-Cbl involved in receptor ubiquitylation. (A) CHO cells were transfected with plasmids encoding EGFR, Grb2, the indicated HA peptide-tagged mutants of c-Cbl or a control empty vector. Whole-cell extracts were analyzed by immunoblotting either directly or after immunoprecipitation of c-Cbl. The following mutants of c-Cbl were used: a protein the SH2 domain of which is defective (G306E); and deletion mutants containing either the N-terminal half [Cbl-N(RF), residues 1–429] or the C-terminal half (Cbl-C, residues 338 to end). (B) Monolayers of CHO cells were transiently transfected with the Y1045F mutant of EGFR, along with wild type (WT) or the 70Z mutant of c-Cbl, in the presence or absence of a Grb2 expression vector. Receptor ubiquitylation was detected using a HA-tagged ubiquitin expression vector. Forty-eight hours post-transfection, monolayers were incubated without or with EGF (100 ng/ml). Cell lysates were subjected to immunoprecipitation (I.P.) with an anti-EGFR antibody and immunoblotting (I.B.) with either an antibody to HA-ubiquitin or an antibody recognizing EGFR. (C) CHO cells were co-transfected with vectors driving expression of the indicated c-Cbl mutants [see (A)], along with a plasmid encoding the Y1045F mutant receptor. As indicated, transfections were performed with a plasmid encoding Grb2 or a control empty vector. All transfections were carried out in the presence of a plasmid directing expression of a HA-tagged ubiquitin. Cells were treated for 10 min at 37°C without or with EGF (100 ng/ml). Whole-cell lysates were analyzed by immunoblotting (I.B.) either directly (panels labeled NONE) or following immunoprecipitation (I.P.).
Discussion
Receptor endocytosis terminates signal transduction
Several previous attempts to block EGFR internalization, by either receptor mutagenesis (Wells et al., 1990) or a mutant of dynamin (Vieira et al., 1996), concluded that endocytosis terminates the mitogenic activity of EGF. Nevertheless, the effect of receptor endocytosis on signaling potency remained a matter of some controversy (Di Guglielmo et al., 1994; Emlet et al., 1997; Haugh et al., 1999). In part, this issue is complicated by the existence of at least two routes of receptor endocytosis: the ligand-activated route requires the intrinsic tyrosine kinase activity of EGFR. In contrast, the slower pathway is constitutive, and unlike the inducible pathway it primarily recycles receptors back to the cell surface (Wiley et al., 1991). By resolving the role of c-Cbl in receptor endocytosis and sorting to destruction, our results favor the notion that receptor endocytosis terminates mitogenic signaling. Thus, a c-Cbl’s site mutant receptor (Y1045F) with defective endocytosis (Figures 2 and 3A) and increased recycling (Figure 3C) is endowed with enhanced signaling capacity (Figure 1).
Previous attempts to construct an internalization-defective mutant of EGFR led to the realization that the 213 C-terminal amino acids of EGFR include an inhibitory domain and an internalization region (amino acids 973–1022). It is interesting to note that Tyr1045 is located outside of the mapped internalization region. However, consistent with its role in endocytosis, genetic analyses of vulva formation in C.elegans attributed an inhibitory role to the respective residue (Yoon et al., 2000). In addition, mutagenesis of EGFR has identified residues 1022–1123 as a region required for lysosomal degradation (Kornilova et al., 1996). Consistent with the association of internalization with decreased mitogenic signaling, virulence of several strains of the oncogenic avian erythroblastosis virus, which encodes an ortholog of EGFR, share deletions of a region encompassing the c-Cbl-specific docking site (Shu et al., 1991). Likewise, this site is missing in oncogenic forms of the human EGFR, which are frequently found in brain tumors (Ekstrand et al., 1992). In a complementary way, the transforming activity of some ubiquitylation-defective forms of c-Cbl depends on binding to EGFR (Thien et al., 2001), and targeted inactivation of c-Cbl sensitizes macrophages to the colony stimulating factor-1 (Lee et al., 1999).
A linkage between receptor ubiquitylation and endocytosis
Previous attempts to resolve the relationships between receptor ubiquitylation and endocytosis reported no effect of an overexpressed c-Cbl on the rate of EGFR endocytosis (Levkowitz et al., 1998; Thien et al., 2001). This observation and the ligand-induced co-translocation of c-Cbl and EGFR to endosomes led us to suggest that the effect of c-Cbl on receptor sorting occurs at the early endosome (Levkowitz et al., 1998). By using a receptor mutant incapable of c-Cbl recruitment (Y1045F), we identified an earlier effect of c-Cbl. Apparently, ubiquitylation by c-Cbl occurs at the plasma membrane or very close to it, and it determines the rate of receptor endocytosis. It is conceivable that c-Cbl-mediated ubiquitylation of a cell surface-localized EGFR dictates sorting to the invaginating clathrin-coated pit. This conclusion is consistent with the ability of a mutant dynamin to block endocytosis, but not ubiquitylation of EGFR (Stang et al., 2000).
Collectively, the observations made with an overexpressed c-Cbl and the Y1045F mutant receptor lead us to conclude that sorting of internalized EGFRs is a progressive process. It probably initiates at the cell surface upon limited c-Cbl-mediated ubiquitylation, which accelerates endocytosis (de Melker et al., 2001). However, ubiquitylation seems to proceed en route to the late endosome/pre-lysosome, because c-Cbl and EGFR remain physically associated (Levkowitz et al., 1998), and blocking receptor internalization prevents maximal ubiquitylation (Stang et al., 2000). The emerging model is thus reminiscent of the mechanism regulating endocytosis in yeast (reviewed in Hicke, 2001). Mono-ubiquitylation seems sufficient to signal internalization of several yeast proteins into primary endocytic vesicles. Apparently, ubiquitin itself carries an internalization signal, because fusion of a lysine-less ubiquitin to several yeast and mammalian proteins enhances their rate of internalization (Nakatsu et al., 2000 and references therein). It is therefore possible that c-Cbl enhances the rate of EGFR endocytosis by appending an internalization signal intrinsic to ubiquitin.
The dual role of Grb2
The Grb2 adaptor protein is involved in many signal transduction pathways, including those initiated by growth factors, antigens and antibodies. Its single SH2 domain allows inducible binding to tyrosine-phosphorylated proteins, whereas the two flanking SH3 domains recruit signaling proteins, including the guanine nucleotide exchange protein Sos and the E3 ubiquitin ligase, c-Cbl (Fukazawa et al., 1995). The interaction with Sos is relatively well characterized; it recruits the exchange protein to the plasma membrane and activates the Ras pathway. However, although a complex of c-Cbl and Grb2 is abundant in many cell types and its dissociation may be regulated (Buday et al., 1996; Donovan et al., 1996), the functional consequences of recruiting a c-Cbl–Grb2 complex to activated receptors is currently unknown. The results presented in this study indicate that one function of Grb2–Cbl interaction is to negatively regulate signaling by mediating receptor degradation. Interestingly, genetic evidence raised the possibility that Grb2 recruits another negative regulator of EGFR in worms, namely Ark-1 (Hopper et al., 2000). However, unlike c-Cbl/Sli-1, the mechanism underlying the Ark-1 pathway of receptor desensitization remains unknown.
Although our conclusions rely on overexpression, and therefore they must be confirmed by additional approaches, it is conceivable that two distinct mechanisms underlie inducible degradation of EGFR. The major one is mediated by Tyr1045, which directly binds to the SH2 domain of c-Cbl. The secondary mechanism seems to involve one of the Grb2-binding sites of EGFR, and indirect recruitment of c-Cbl through its constitutive interactions with an SH3 domain of the adaptor. The relative contribution of the two pathways to EGFR desensitization will require further investigation; according to a previous report a c-Cbl mutant incapable of Grb2 binding is as effective as the wild-type protein (Lill et al., 2000), but the data we presented and additional preliminary results suggest that in some cell lines, coupling to Grb2 may contribute to the overall extent of receptor degradation.
Additional open questions relate to the possible involvement of the many other c-Cbl-associated proteins, like the adaptors Nck, Shc and Crk. The involvement of Grb2, however, was predictable because of two lines of evidence. First, microinjection of a recombinant SH2 domain of Grb2 inhibited endocytosis of EGFR (Wang and Moran, 1996). Secondly, deletion of the Grb2-binding poly-proline motif of SLI-1 significantly affected vulva formation in transgenic worms (Yoon et al., 2000). Together with the results we have presented, these lines of evidence attribute to Grb2 a dual role in signaling; along with the extensively characterized stimulatory activity of the Ras pathway through binding to SOS, the adaptor also initiates the process of receptor degradation by recruiting c-Cbl. Because c-Cbl and SOS do not co-immunoprecipitate (Meisner et al., 1995; Fukazawa et al., 1996), they seem to form exclusive complexes with Grb2. Thus, it is conceivable that by alternative interaction with SOS and c-Cbl, Grb2 integrates both positive and negative inputs to signaling pathways.
In summary, c-Cbl critically controls receptor fate: its close apposition to and phosphorylation by EGFR initiate receptor ubiquitylation. Apparently, ubiquitylation of EGFR identifies it for rapid endocytosis, which starts the process of homologous desensitization. However, c-Cbl remains associated with the receptor throughout the endocytic compartment, probably in order to allow effective sorting to degradation. Evidently, several mechanisms of c-Cbl recruitment exist in cells: the major route involves the SH2 domain of c-Cbl and autophosphorylation at Tyr1045 of EGFR, which is situated within a lysosome-targeting motif. In contrast, the surrogate route bypasses the lysosome-targeting motif. Apparently, c-Cbl is recruited to this route in a complex with the Grb2 adaptor, which engages with EGFR through several tyrosine autophosphorylation sites. Future studies will address functional characteristics of each route of receptor ubiquitylation, as well as the relationships to positive signaling through the adaptors Grb2 and Shc.
Materials and methods
Materials
Rabbit antibodies to c-Cbl (C-15), a His6 tag and EGFR were from Santa Cruz Biotechnology (Santa Cruz, CA). A monoclonal antibody (mAb) to Grb2 was from Transduction Laboratories (Lexington, KY) and an anti-hemagglutinin (HA) mAb was purchased from Boehringer Mannheim. A mAb to the active doubly phosphorylated form of Erk was from Sigma. The composition of buffers was as described previously (Waterman et al., 1999).
Plasmid construction and transfection of expression vectors
Mammalian expression plasmids for wild-type and mutant EGFR, c-Cbl, glutathione S-transferase (GST)–Cbl and Cbl-C have been described previously (Levkowitz et al., 1999). The Cbl-N(RF) deletion mutant was prepared by introducing a stop codon next to the codon encoding amino acid 429. GST–Grb2 mutants were obtained from Dr Jan Sap (New York University). A His6 tag was inserted at the C-terminus of the human Grb2 cDNA by PCR amplification. Expression vectors were introduced to CHO cells by using the LipofectAMINE transfection method (Gibco-BRL). The total amount of DNA in each transfection was normalized with the pCDNA3 plasmid.
Cell proliferation and SRE transcription assays
Cells were washed free of IL-3, resuspended in RPMI 1640 medium at 5 × 105 cells/ml, and treated without or with growth factors or IL-3. Cell survival was determined by using the MTT assay (Mosman, 1983). The SRE transcription assay was performed as described previously (Waterman et al., 1999).
Lysate preparation, immunoprecipitation and western blotting
Following stimulation, cells were extracted in solubilization buffer, and lysates cleared by centrifugation. The proteins in the lysate supernatants were immunoprecipitated for 2 h at 4°C. The immunoprecipitates were washed three times with HNTG buffer (20 mM HEPES pH 7.5, 150 mM NaCl, 0.1% Triton X-100 and 10% glycerol), resolved by electrophoresis and electrophoretically transferred to a nitrocellulose membrane. Membranes were blocked for 1 h in Tris–HCl-buffered saline containing 1% milk, blotted for 2 h with a primary antibody (1 µg/ml), followed by a secondary antibody (0.5 µg/ml) linked to horseradish peroxidase. Immunoreactive protein bands were detected using the enhanced chemiluminescence reagent (Pharmacia-Amersham).
Biochemical analyses of endocytosis
Receptor down-regulation was performed as described previously (Levkowitz et al., 1998). To follow receptor recycling and to monitor EGF internalization, we used the previously described protocols of Kornilova et al. (1996) and Sorkin et al. (1993), respectively. Ligand degradation was assayed as follows: conditioned media containing radiolabeled ligands were assayed by the addition of cold trichloroacetic acid (TCA) (10% final concentration). Samples were precipitated after 1 h at 2°C by centrifugation. Radioactivity present in the supernatants and pellets (TCA-insoluble fraction) was determined.
In vitro assay for receptor ubiquitylation
Receptors were immunoprecipitated from cleared lysates of transfected HEK-293T cells. Following isolation, agarose beads were extensively washed with HNTG followed by an additional wash with ubiquitin wash buffer (5 mM MgCl2, 40 mM Tris–HCl pH 7.5). Agarose beads were then resuspended in buffer containing 40 mM Tris–HCl (pH 7.5), 5 mM MgCl2, 2 mM dithiothreitol, 2 mM ATP-γ-S and 3 µg/ml radiolabeled ubiquitin supplemented with crude rabbit reticulocyte lysate (5 µl; Promega) and the indicated recombinant proteins (5 µg), and incubated for 1 h at 30°C. The beads were then washed extensively and EGFR eluted with gel sample buffer.
Immunofluorescence
Transfected cells grown on cover slips were incubated for 15 min at 37°C with or without EGF (100 ng/ml). The cells were then fixed for 15 min with 3% paraformaldehyde in phosphate-buffered saline (PBS). Fixed cells were washed, and permeabilized for 10 min at 22°C with PBS containing 1% albumin and 0.2% Triton X-100. For labeling, cover slips were incubated for 1 h at room temperature with an anti-HA antibody or a rabbit antibody to His6 peptide tag. After extensive washing in PBS, the cover slips were incubated for an additional 1 h with a Cy3-conjugated secondary antibody. The cover slips were mounted in mowiol. Confocal microscopy was performed using a Zeiss Axiovert 100 TV microscope (Oberkochen, Germany) with a 63X/1.4 plan-Apochromat objective, attached to the Bio-Rad Radiance 2000 laser scanning system, operated by LaserSharp software. Figures were taken from middle sections of cells.
Acknowledgments
Acknowledgements
We thank Drs Wallace Langdon, Jan Sap, Stan Lipkowitz and Dirk Bohmann for plasmids. This work was supported by grants from the National Cancer Institute (grant CA72981) and the US Army (grant DAMD 17-00-1-0499).
References
- Batzer A.G., Rotin,D., Urena,J.M., Skolnik,E.Y. and Schlessinger,J. (1994) Hierarchy of binding sites for Grb2 and Shc on the epidermal growth factor receptor. Mol. Cell. Biol., 14, 5192–5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonita D.P., Miyake,S., Lupher,M.L.,Jr, Langdon,W.Y. and Band,H. (1997) Phosphotyrosine binding domain-dependent upregulation of the platelet-derived growth factor receptor alpha signaling cascade by transforming mutants of Cbl: implications for Cbl’s function and oncogenicity. Mol. Cell. Biol., 17, 4597–4610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock R., Hamelers,I.H. and Jovin,T.M. (1999) Comparison of fixation protocols for adherent cultured cells applied to a GFP fusion protein of the epidermal growth factor receptor. Cytometry, 35, 353–362. [DOI] [PubMed] [Google Scholar]
- Buday L., Khwaja,A., Sipeki,S., Farago,A. and Downward,J. (1996) Interactions of Cbl with two adapter proteins, Grb2 and Crk, upon T cell activation. J. Biol. Chem., 271, 6159–6163. [DOI] [PubMed] [Google Scholar]
- Chen W.S. et al. (1989) Functional independence of the epidermal growth factor receptor from a domain required for ligand-induced internalization and calcium regulation. Cell, 59, 33–43. [DOI] [PubMed] [Google Scholar]
- de Melker A.A., van der Horst,G., Calafat,J., Jansen,H. and Borst,J. (2001) c-Cbl ubiquitinates the EGF receptor at the plasma membrane and remains receptor associated throughout the endocytic route. J. Cell Sci., 114, 2167–2178. [DOI] [PubMed] [Google Scholar]
- Di Guglielmo G.M., Baass,P.C., Ou,W.J., Posner,B.I. and Bergeron,J.J. (1994) Compartmentalization of SHC, GRB2 and mSOS and hyperphosphorylation of Raf-1 by EGF but not insulin in liver parenchyma. EMBO J., 13, 4269–4277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan J.A., Ota,Y., Langdon,W.Y. and Samelson,L.E. (1996) Regulation of the association of p120cbl with Grb2 in Jurkat T cells. J. Biol. Chem., 271, 26369–26374. [DOI] [PubMed] [Google Scholar]
- Ekstrand A.J., Sugawa,N., James,C.D. and Collins,V.P. (1992) Amplified and rearranged epidermal growth factor receptor genes in human glioblastomas reveal deletions of sequences encoding portions of the N- and/or C-terminal tails. Proc. Natl Acad. Sci. USA, 89, 4309–4313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emlet D.R., Moscatello,D.K., Ludlow,L.B. and Wong,A.J. (1997) Subsets of epidermal growth factor receptors during activation and endocytosis. J. Biol. Chem., 272, 4079–4086. [DOI] [PubMed] [Google Scholar]
- Felder S., Miller,K., Moehren,G., Ullrich,A., Schlessinger,J. and Hopkins,C.R. (1990) Kinase activity controls the sorting of the epidermal growth factor receptor within the multivesicular body. Cell, 61, 623–634. [DOI] [PubMed] [Google Scholar]
- Fukazawa T., Reedquist,K.A., Trub,T., Soltoff,S., Panchamoorthy,G., Druker,B., Cantley,L., Shoelson,S.E. and Band,H. (1995) The SH3 domain-binding T cell tyrosyl phosphoprotein p120. Demonstration of its identity with the c-cbl protooncogene product and in vivo complexes with Fyn, Grb2 and phosphatidylinositol 3-kinase. J. Biol. Chem., 270, 19141–19150. [DOI] [PubMed] [Google Scholar]
- Fukazawa T., Miyake,S., Band,V. and Band,H. (1996) Tyrosine phosphorylation of Cbl upon epidermal growth factor (EGF) stimulation and its association with EGF receptor and downstream signaling proteins. J. Biol. Chem., 271, 14554–14559. [DOI] [PubMed] [Google Scholar]
- Haugh J.M., Schooler,K., Wells,A., Wiley,H.S. and Lauffenburger,D.A. (1999) Effect of epidermal growth factor receptor internalization on regulation of the phospholipase C-γ1 signaling pathway. J. Biol. Chem., 274, 8958–8965. [DOI] [PubMed] [Google Scholar]
- Hicke L. (2001) Protein regulation by monoubiquitin. Nature Rev. Mol. Cell Biol., 2, 195–201. [DOI] [PubMed] [Google Scholar]
- Hopkins C.R., Gibson,A., Shipman,M. and Miller,K. (1990) Movement of internalized ligand–receptor complexes along a continuous endosomal reticulum. Nature, 346, 335–339. [DOI] [PubMed] [Google Scholar]
- Hopper N.A., Lee,J. and Sternberg,P.W. (2000) ARK-1 inhibits EGFR signaling in C. elegans. Mol. Cell, 6, 65–75. [PubMed] [Google Scholar]
- Joazeiro C.A., Wing,S.S., Huang,H., Leverson,J.D., Hunter,T. and Liu,Y.C. (1999) The tyrosine kinase negative regulator c-Cbl as a RING-type, E2-dependent ubiquitin–protein ligase. Science, 286, 309–312. [DOI] [PubMed] [Google Scholar]
- Jongeward G.D., Clandinin,T.R. and Sternberg,P.W. (1995) sli-1, a negative regulator of let-23-mediated signaling in C. elegans. Genetics, 139, 1553–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornilova E., Sorkina,T., Beguinot,L. and Sorkin,A. (1996) Lysosomal targeting of epidermal growth factor receptors via a kinase-dependent pathway is mediated by the receptor carboxyl-terminal residues 1022–1123. J. Biol. Chem., 271, 30340–30346. [DOI] [PubMed] [Google Scholar]
- Lee P.S., Wang,Y., Dominguez,M.G., Yeung,Y.G., Murphy,M.A., Bowtell,D.D. and Stanley,E.R. (1999) The cbl protooncoprotein stimulates CSF-1 receptor multiubiquitination and endocytosis and attenuates macrophage proliferation. EMBO J., 18, 3616–3628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levkowitz G., Waterman,H., Zamir,E., Kam,Z., Oved,S., Langdon, W.Y., Beguinot,L., Geiger,B. and Yarden,Y. (1998) c-Cbl/Sli-1 regulates endocytic sorting and ubiquitination of the epidermal growth factor receptor. Genes Dev., 12, 3663–3674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levkowitz G. et al. (1999) Ubiquitin ligase activity and tyrosine phosphorylation underlie suppression of growth factor signaling by c-Cbl/Sli-1. Mol. Cell, 4, 1029–1040. [DOI] [PubMed] [Google Scholar]
- Lill N.L., Douillard,P., Awwad,R.A., Ota,S., Lupher,M.L.,Jr, Miyake,S., Meissner-Lula,N., Hsu,V.W. and Band,H. (2000) The evolutionarily conserved N-terminal region of Cbl is sufficient to enhance down-regulation of the epidermal growth factor receptor. J. Biol. Chem., 275, 367–377. [DOI] [PubMed] [Google Scholar]
- Marshall C.J. (1995) Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell, 80, 179–185. [DOI] [PubMed] [Google Scholar]
- Meisner H. and Czech,M.P. (1995) Coupling of the proto-oncogene product c-Cbl to the epidermal growth factor receptor. J. Biol. Chem., 270, 25332–25335. [DOI] [PubMed] [Google Scholar]
- Meisner H., Conway,B.R., Hartley,D. and Czech,M.P. (1995) Interactions of Cbl with Grb2 and phosphatidylinositol 3′- kinase in activated Jurkat cells. Mol. Cell. Biol., 15, 3571–3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosman T. (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods, 65, 55–63. [DOI] [PubMed] [Google Scholar]
- Nakatsu F., Sakuma,M., Matsuo,Y., Arase,H., Yamasaki,S., Nakamura,N., Saito,T. and Ohno,H. (2000) A di-leucine signal in the ubiquitin moiety. Possible involvement in ubiquitination-mediated endocytosis. J. Biol. Chem., 275, 26213–26219. [DOI] [PubMed] [Google Scholar]
- Okutani T., Okabayashi,Y., Kido,Y., Sugimoto,Y., Sakaguchi,K., Matuoka,K., Takenawa,T. and Kasuga,M. (1994) Grb2/Ash binds directly to tyrosines 1068 and 1086 and indirectly to tyrosine 1148 of activated human epidermal growth factor receptors in intact cells. J. Biol. Chem., 269, 31310–31314. [PubMed] [Google Scholar]
- Shu H.K., Pelley,R.J. and Kung,H.J. (1991) Dissecting the activating mutations in v-erbB of avian erythroblastosis virus strain R. J. Virol., 65, 6173–6180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorkin A., Di Fiore,P.P. and Carpenter,G. (1993) The carboxyl terminus of epidermal growth factor receptor/erbB-2 chimerae is internalization impaired. Oncogene, 8, 3021–3028. [PubMed] [Google Scholar]
- Sorkin A., McClure,M., Huang,F. and Carter,R. (2000) Interaction of EGF receptor and grb2 in living cells visualized by fluorescence resonance energy transfer (FRET) microscopy. Curr. Biol., 10, 1395–1398. [DOI] [PubMed] [Google Scholar]
- Stang E., Johannessen,L.E., Knardal,S.L. and Madshus,I.H. (2000) Polyubiquitination of the epidermal growth factor receptor occurs at the plasma membrane upon ligand-induced activation. J. Biol. Chem., 275, 13940–13947. [DOI] [PubMed] [Google Scholar]
- Thien C.B.F., Walker,F. and Langdon,W.Y. (2001) Ring finger mutations that abolish c-Cbl-directed polyubiquitination and downregulation of the EGF receptor are insufficient for cell transformation. Mol. Cell, 7, 355–365. [DOI] [PubMed] [Google Scholar]
- van der P., Hunter,T. and Lindberg,R.A. (1994) Receptor protein-tyrosine kinases and their signal transduction pathways. Annu. Rev. Cell. Biol., 10, 251–337. [DOI] [PubMed] [Google Scholar]
- Vieira A.V., Lamaze,C. and Schmid,S.L. (1996) Control of EGF receptor signaling by clathrin-mediated endocytosis. Science, 274, 2086–2088. [DOI] [PubMed] [Google Scholar]
- Wang Y., Yeung,G.-Y. and Stanley,E.-R. (1999) CSF-1 stimulated multiubiquitination of the CSF-1 receptor and of Cbl follows their tyrosine phosphorylation and association with other signaling proteins. J. Cell. Biochem., 72, 119–134. [PubMed] [Google Scholar]
- Wang Z. and Moran,M.F. (1996) Requirement for the adapter protein GRB2 in EGF receptor endocytosis. Science, 272, 1935–1938. [DOI] [PubMed] [Google Scholar]
- Waterman H., Levkowitz,G., Alroy,I. and Yarden,Y. (1999) The RING finger of c-Cbl mediates desensitization of the epidermal growth factor receptor. J. Biol. Chem., 274, 22151–22154. [DOI] [PubMed] [Google Scholar]
- Wells A., Welsh,J.B., Lazar,C.S., Wiley,H.S., Gill,G.N. and Rosenfeld,M.G. (1990) Ligand-induced transformation by a non-internalizing epidermal growth factor receptor. Science, 247, 962–964. [DOI] [PubMed] [Google Scholar]
- Wiley H.S., Herbst,J.J., Walsh,B.J., Lauffenburger,D.A., Rosenfeld, M.G. and Gill,G.N. (1991) The role of tyrosine kinase activity in endocytosis, compartmentation and down-regulation of the epidermal growth factor receptor. J. Biol. Chem., 266, 11083–11094. [PubMed] [Google Scholar]
- Yarden Y. and Sliwkowski,M.X. (2001) Untangling the ErbB signalling network. Nature Rev. Mol. Cell Biol., 2, 127–137. [DOI] [PubMed] [Google Scholar]
- Yokouchi M., Kondo,T., Houghton,A., Bartkiewicz,M., Horne,W.C., Zhang,H., Yoshimura,A. and Baron,R. (1999) Ligand-induced ubiquitination of the epidermal growth factor receptor involves the interaction of the c-Cbl RING finger and UbcH7. J. Biol. Chem., 274, 31707–31712. [DOI] [PubMed] [Google Scholar]
- Yoon C.H., Chang,C., Hopper,N.A., Lesa,G.M. and Sternberg,P.W. (2000) Requirements of multiple domains of SLI-1, a Caenorhabditis elegans homologue of c-Cbl and an inhibitory tyrosine in LET-23 in regulating vulval differentiation. Mol. Biol. Cell, 11, 4019–4031. [DOI] [PMC free article] [PubMed] [Google Scholar]