Abstract
The inner membrane of the mitochondrion folds inwards, forming the cristae. This folding allows a greater amount of membrane to be packed into the mitochondrion. The data in this study demonstrate that subunits e and g of the mitochondrial ATP synthase are involved in generating mitochondrial cristae morphology. These two subunits are non-essential components of ATP synthase and are required for the dimerization and oligomerization of ATP synthase. Mitochondria of yeast cells deficient in either subunits e or g were found to have numerous digitations and onion-like structures that correspond to an uncontrolled biogenesis and/or folding of the inner mitochondrial membrane. The present data show that there is a link between dimerization of the mitochondrial ATP synthase and cristae morphology. A model is proposed of the assembly of ATP synthase dimers, taking into account the oligomerization of the yeast enzyme and earlier data on the ultrastructure of mitochondrial cristae, which suggests that the association of ATP synthase dimers is involved in the control of the biogenesis of the inner mitochondrial membrane.
Keywords: ATP synthase oligomer/mitochondria/morphology/yeast
Introduction
The mitochondrion is referred to as the ‘power house’ of the cell, because it is responsible for the synthesis of the majority of ATP under aerobic conditions. The inner membrane of the mitochondrion contains the components of the electron transport chain. Oxidation/reduction reactions along the components of the electron transport chain generate a proton gradient that is used by ATP synthase to phosphorylate ADP, thereby producing ATP. To increase the capacity of the mitochondrion to synthesize ATP, the inner membrane is folded to form cristae. These folds allow a much greater amount of electron transport chain enzymes and ATP synthase to be packed into the mitochondrion. However, until now, little was known about how the inner membrane is able to form cristae. This study provides evidence that subunits of ATP synthase are involved in cristae formation.
ATP synthase, or F1F0 ATP synthase, is composed of a hydrophilic catalytic unit (F1), which is located in the mitochondrial matrix, and a membranous domain (F0), which anchors the enzyme in the inner mitochondrial membrane and mediates the conduction of protons that participate indirectly in ATP synthesis (Fillingame, 1999; Pedersen et al., 2000). Electron microscopy of negatively stained mitochondria revealed 9 nm diameter projections in the mitochondrial matrix (Fernández-Morán, 1962), which were identified as the hydrophilic catalytic units (F1) of the F1F0 ATP synthase (Racker et al., 1965). These projections were observed by electron microscopy to be arranged in a non-random, tightly ordered pattern on tubular cristae in Paramecium multimicronucleatum mitochondria using rapid techniques of freezing followed by fracturing, etching and replication (Allen et al., 1989). In this organism, the F1 complexes are arranged as a double row of particles along the full length of the helically shaped tubular cristae. Allen (1995) proposed a model where the association of ATP synthase dimers promotes a distortion of the plane of the inner mitochondrial membrane, leading to the formation of 50 nm diameter tubular cristae.
Arnold et al. (1998) recently demonstrated the presence of ATP synthase dimers in both Triton X-100 and digitonin extracts of wild-type yeast using blue native polyacrylamide gel electrophoresis (BN–PAGE) (Schägger and von Jagow, 1991). The proximity between yeast ATP synthases has also been shown by the formation in the inner mitochondrial membrane of a disulfide bridge between two subunits 4 (subunit b) belonging to two ATP synthases (Spannagel et al., 1998). Such a dimerization also involves the two ATP synthase-associated subunits e and g (Arnold et al., 1998). These two components are supernumerary subunits that are not essential for cellular growth and are found only in mitochondria (Walker et al., 1991; Higuti et al., 1992; Collinson et al., 1994; Boyle et al., 1999). In Saccharomyces cerevisiae, of the 20 different subunits that compose ATP synthase, four are supernumerary subunits (subunits e, g, i/j and k) that are associated with F0. Inactivation of the corresponding genes does not significantly alter growth on non-fermentable sources (Tokatlidis et al., 1996; Arnold et al., 1997, 1998, 1999; Boyle et al., 1999; Vaillier et al., 1999). The e, g and i subunits are small proteins with a unique transmembrane-spanning segment, and are probably located in the periphery of the yeast enzyme (Soubannier et al., 1999; Paumard et al., 2000). A regulatory role of subunit e in cellular ATP production has been proposed (Levy and Kelly, 1997).
The involvement of at least subunits e and g in the dimerization of the ATP synthase led us to investigate the structure of null mutant ATP synthases and to examine yeast cells devoid of these two subunits by electron microscopy. Surprisingly, there were extensive changes in the morphology of the mitochondrion in mutants lacking subunits e or g. The results of this study indicate that the ATP synthase could be involved, through subunits e and g, in the formation of the cristae by an oligomerization process.
Results
Phenotypic analyses of mutants devoid of ATP synthase-associated subunits e, g and i
The strains used in this study contain null mutations in ATP18, ATP20 or TIM11 genes, encoding subunits i, g and e, respectively. The null mutant in the ATP18 gene was used as a control as it is partially defective for ATP synthesis (Vaillier et al., 1999), yet still contains a stable ATP synthase and thus more closely mimics the mutations in ATP20 and TIM11 genes. A number of biochemical and genetic studies were performed to provide an initial characterization of the mutant strains (Table I). ΔATP20 and ΔTIM11 mutant cells grew using lactate as carbon source either at 28 or 37°C, thus indicating that they were able to generate ATP via oxidative phosphorylation. However, they had generation times longer than that of the wild-type strain. Indeed, 40% of ΔATP20 and ΔTIM11 mutant cells spontaneously converted to rho– cells, which explains both the 44–48% inhibition of ATPase activity by oligomycin and the 34% decrease in the CCCP-stimulated respiration rate. Our interpretation of these data is different from that of Boyle et al. (1999), who showed that the absence of subunit g only reduced cytochrome oxidase activity. The different parental strains used could explain this discrepancy. Our data indicate that although growing on non-fermentable medium, a part of the cellular population, while converting to rho– cells, did not grow but accumulated. As a result, the mitochondrial preparation is a mixture of rho– and rho+ mitochondria. The ATP/O ratio, which is independent of the protein concentration, indicates that the efficiency of the oxidative phosphorylation machinery of ΔATP20 and ΔTIM11 mitochondria was not altered, whereas that of ΔATP18 mitochondria was slightly lowered as a consequence of a proton leak occurring during phosphorylation (Vaillier et al., 1999). As a consequence, ATP synthases of ΔATP20 and ΔTIM11 rho+ mitochondria are fully functional, and the high percentage of rho– cells appearing in cultures is probably the cause of the increase in the generation time of ΔATP20 and ΔTIM11 cell populations. 4,6-diamidino-2-phenylindole staining revealed that ΔATP20 rho– cells still contained mitochondrial DNA (P.Paumard, unpublished observation).
Table I. Phenotypic analyses of yeast strains useda.
| Strains | Doubling time (min) | % of rho– cells in cultures | Uncoupled respiration rate (nmol of O/min/mg of protein)b | ATP/Oc | ATPase activityd |
||
|---|---|---|---|---|---|---|---|
| – oligomycine | + oligomycine (%) | inhibition | |||||
| Wild type | 150 | 0.9 ± 0.2 | 1199 ± 42 | 1.09 ± 0.13 | 4.98 ± 0.10 | 0.67 ± 0.02 | 87 |
| ΔATP20 | 221 | 40.0 ± 2.4 | 795 ± 50 | 1.22 ± 0.04 | 6.61 ± 0.04 | 3.68 ± 0.09 | 44 |
| ΔTIM11 | 229 | 40.9 ± 1.1 | 868 ± 24 | 0.89 ± 0.03 | 5.79 ± 0.29 | 2.99 ± 0.03 | 48 |
| ΔATP18 | 456 | 11.1 ± 0.8 | 1380 ± 130 | 0.79 ± 0.06 | 2.83 ± 0.02 | 2.15 ± 0.06 | 24 |
aYeast cells were grown at 28°C on complete medium containing lactate as carbon source. rho– production in cultures was measured on glycerol plates supplemented with 0.1% glucose. Mitochondria were prepared from protoplasts.
bUncoupled respiration rates were determined in the presence of 3 µM CCCP (carbonyl cyanide m-chlorophenylhydrazone).
cATP/O ratios were determined with NADH as substrate.
dATPase activities and the sensitivity to the F0 inhibitor oligomycin were measured at pH 8.4 in the presence of 0.375% Triton X-100 to remove the natural inhibitor IF1.
eUnits: µmol of Pi/min/mg of protein.
Dimerization of subunit 4 in mitochondria devoid of subunits g or e
In a previous study, we demonstrated the dimerization of the ATP synthase by monitoring the formation of the disulfide bond between two subunit 4 molecules containing the mutation D54C, a position located in the inter-membrane space (Spannagel et al., 1998). Subunit 4 is the product of the ATP4 gene and is homologous to the bovine subunit b. It is present as only one per ATP synthase molecule, and constitutes an essential component of the second stalk (Collinson et al., 1994, 1996; Spannagel et al., 1998; Bateson et al., 1999). As a result, the disulfide bond between subunit 4 molecules is probably due to the dimerization of ATP synthases. Since subunits e and g are also involved in the dimerization of the yeast ATP synthases (Arnold et al., 1998), we analysed the dimerization state of subunit 4 in either the ΔATP20 or the ΔTIM11 context. Figure 1A shows the presence of a subunit 4 dimer in ΔATP20-4D54C and ΔTIM11-4D54C mitochondria, thus showing that ATP synthase dimers are probably present in the inner mitochondrial membrane lacking subunits g or e. The efficiency of the spontaneous dimerization was quantified. Subunit 4–subunit 4 dimer ratios were found to be 1.6, 1.3 and 1.9 in 4D54C, ΔATP20-4D54C and ΔTIM11-4D54C mitochondria, respectively. The presence of such dimers is apparently inconsistent with the results of Arnold et al. (1998), who did not find any ATP synthase dimers in Triton X-100 extracts of mitochondria isolated from either ΔATP20 or ΔTIM11 mutant strains. However, our experimental conditions were different from those of Arnold et al. (1998), as we analysed whole mitochondrial membranes by SDS–PAGE and not native solubilized complexes.
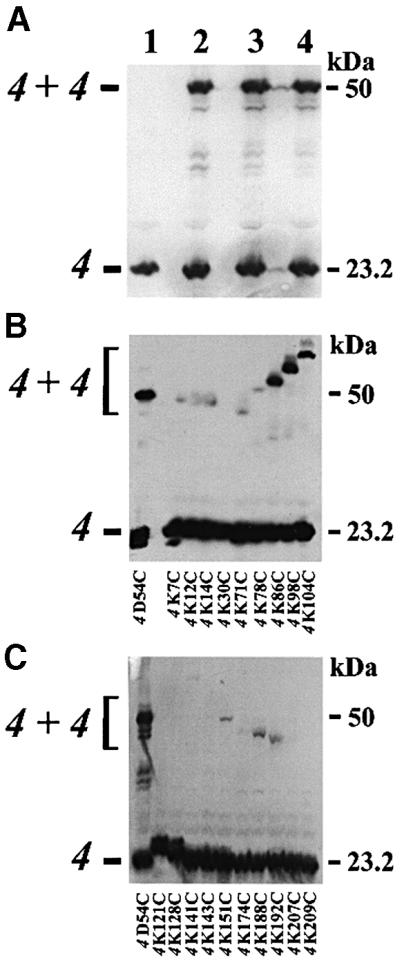
Fig. 1. Dimerization of subunit 4. Mutant and wild-type mitochondria (50 µg of protein) were incubated with 40 mM NEM, dissolved in sample buffer containing SDS and submitted to western blot analysis using polyclonal antibodies against subunit 4 of the ATP synthase. (A) Mitochondria were isolated from wild-type yeast (lane 1), and from 4D54C (lane 2), ΔATP20-4D54C (lane 3) and ΔTIM11-4D54C (lane 4) mutant strains. (B and C) Mitochondria isolated from cysteine mutants of subunit 4 were analysed as above.
Other sites in subunit 4 are involved in the dimerization of subunit 4
Cysteine mutants replacing lysine residues have been built to determine the environment of subunit 4 (Soubannier et al., 1999). Spontaneous subunit 4 dimer formation was found in mitochondrial membranes of mutants modified in the matricial C-terminal domain of the subunit. In addition to position 54, three other positions gave strong subunit 4 dimers, i.e. positions 86, 98 and 104, while positions 14, 71, 151, 188 and 192 gave low intensity adducts (Figure 1B and C). Surprisingly, the apparent molecular weight of subunit 4 dimers varied, probably reflecting the shape of the SDS-solubilized covalently linked molecules. With a stoichiometry of one subunit 4 per ATP synthase, the C-terminal domain of subunit 4 probably participates in an interface involved in the dimerization of ATP synthases. The identification of subunit 4 dimers was not performed by two-dimensional analysis of oxidized ATP synthases, unlike in a previous study with mutant 4D54C (Spannagel et al., 1998). However, as for mutant 4D54C, we observed that Triton X-100 extraction destabilized the subunit 4 dimers that were separated from the solubilized enzyme during centrifugation of extracts and were recovered in the pellet (not shown).
Digitonin extracts of yeast mitochondria contain oligomers of ATP synthases
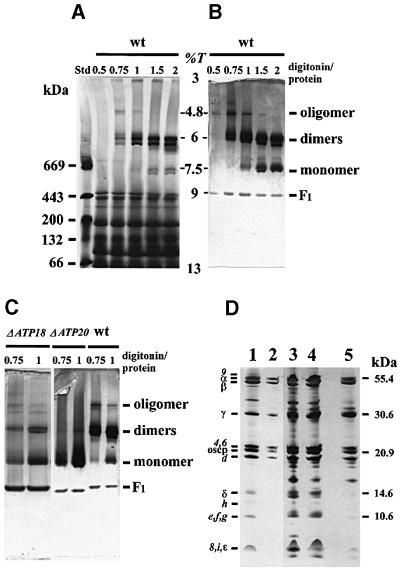
The association of ATP synthases was studied by BN–PAGE. Digitonin was used as detergent, because it solubilizes the enzyme without significantly altering interactions between the mitochondrial complexes (Schägger and Pfeiffer, 2000). After digitonin extraction and centrifugation of the extract, the proteins contained in the supernatant were separated under native conditions on a 3–13% linear gradient acrylamide gel. The slab gel was either stained with Coomassie Blue (Figure 2A) or analysed for ATPase activity. Four bands showing an ATPase activity migrated at acrylamide concentrations of 9, 7.5, 6 and 4.8% (Figure 2B). The band running at an acrylamide concentration of 9% with a relative mol. wt of ∼450 kDa corresponds to free F1, while the band at 7.5% (650 kDa) corresponds to the monomeric form of the enzyme devoid of subunits e and g (Arnold et al., 1998). With detergent/protein ratios <1 g/g, the monomer was found in a small amount. The two bands migrating at an acrylamide concentration of 6% correspond to the dimeric form of the enzyme and displayed the most intense ATPase activity. Why the dimeric form was a doublet is not clear. Either this doublet reflects different associations of ATP synthases or different compositions in subunits and associated lipids. Indeed, SDS–PAGE analysis of the two bands revealed some slight differences in the low molecular weight range (Figure 2D). Additional analyses will solve this issue and allow the identification of 14–20 kDa-associated proteins that are present in either the dimeric or the monomeric forms (Figure 2D, lanes 3–5).
Fig. 2. The yeast ATP synthase exists in an oligomeric form. Wild-type, ΔATP18 and ΔATP20 mitochondria were solubilized with the indicated digitonin/protein ratios (g/g). After centrifugation, the mitochondrial complexes were separated by BN–PAGE and the gels were either stained with Coomassie Blue (A) or incubated with ATP-Mg2+ and Pb2+ to reveal the ATPase activity (B and C). The figures shown in (B) and (C) are the negatives of original gels. Bands in (B) revealed by the ATPase activity were cut and submitted to SDS–PAGE (D), and the slab gel was then silver stained. Lane 1, purified yeast wild-type ATP synthase; lane 2, oligomer (digitonin/protein ratio of 0.75 g/g); lanes 3 and 4, higher and lower bands of dimers, respectively (digitonin/protein ratio of 2 g/g); lane 5, monomer (digitonin/protein ratio of 2 g/g). Std, standard proteins; wt, wild type; %T, acrylamide concentration.
This result also demonstrates the existence of oligomeric forms of ATP synthase in mitochondrial digitonin extracts. The major oligomer migrated at an acrylamide concentration of 4.8%. A higher form was also detected, but the reproducibility was poor. The oligomer contained the major components of F1 and F0 as detected by SDS–PAGE (Figure 2D, lane 2), and was active, as shown by the ATPase activity detected in the gel at this position (Figure 2B and C). The oligomer was the major form of the ATP synthase in extracts obtained with a digitonin/protein ratio of 0.5 g/g. Quantification from the activity resulted in 54 and 18% of the oligomeric and dimeric forms, respectively, with a digitonin/protein ratio of 0.5 g/g, and 32 and 57% of oligomeric and dimeric forms, respectively, with a digitonin/protein ratio of 0.75 g/g. Digitonin/protein ratios >1 g/g destabilized the oligomeric forms. The major oligomeric form could correspond at least to a tetrameric form of the enzyme. Usually, the estimation of the molecular weight of proteins migrating on pore gradient electrophoresis is determined from the relationship between the log (molecular weight) and log (%T) (acrylamide concentration) (Poduslo and Rodbard, 1980). However, in our experiments, the absence of protein standards with molecular weights of several million Daltons led to a weight estimation of the oligomer that was probably erroneous. Whatever the molecular weight of the oligomer, this result shows that oligomeric forms of the yeast ATP synthase with a molecular weight higher than that of the dimeric form are present in digitonin extracts.
In agreement with Arnold et al. (1998), detergent/protein ratios of 0.75 or 1 g/g led to digitonin extracts of ΔATP20 mitochondria containing the monomeric form of the ATP synthase (Figure 2C). However, a very faint band of the dimeric form was observed only by using the ATPase activity assay, whereas the oligomeric forms of the enzyme were absent. Similar observations were made with digitonin extracts of ΔTIM11 mitochondria (not shown). On the other hand, digitonin extracts of ΔATP18 mitochondria contained the dimeric and oligomeric forms of ATP synthase. The high amount of F1 present in ΔATP18 extracts may be correlated with the low oligomycin-sensitive ATPase activity due to the lack of subunit i (Table I).
The mitochondrial morphology in cells devoid of subunits e or g is altered
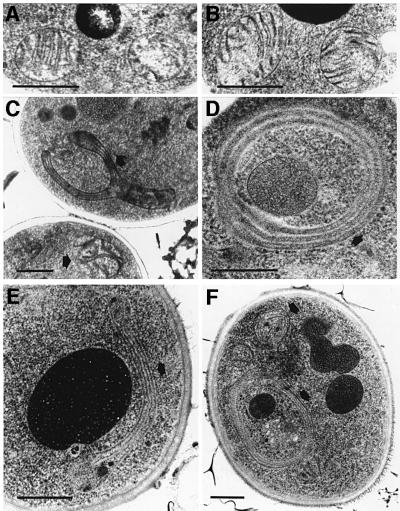
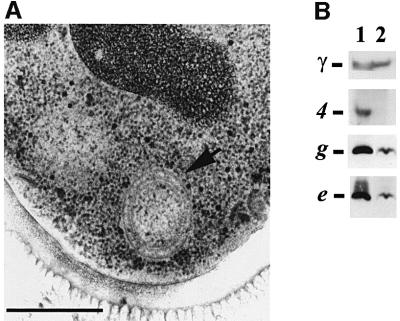
Transmission electron micrographs of yeast cell sections were performed to determine the effect of the loss of subunits e and g on the ultrastructure of the mitochondrial membrane. Mitochondria from either wild-type or ΔATP18 mutant cells appeared normal, with numerous cristae (Figure 3A and B). Transmission electron micrographs of null mutant cells devoid of either subunits e or g showed mitochondria with much different morphologies, i.e. onion-like structures (Figure 3D and F), and large digitations surrounding other organelles (Figure 3C and E). These abnormal structures were observed with cells harvested during both exponential and stationary growth phases. This kind of data has not previously been reported with mutant strains deficient in α- or β-subunits or in F1 assembly. Indeed, mitochondria of atp1, atp2 and atp12 mutants appear to be small and entirely devoid of cristae (J.P.diRago, unpublished result). The interpretation of this is difficult, owing to the defect in energy production of strains devoid of components of the ATP synthase catalytic domain. Nevertheless, in the absence of subunit 4 (subunit b), a structural component of the stator, ΔATP4 cells displayed mitochondria showing very small onion-like structures (Figure 4A). The ATP synthase of ΔATP4 mitochondria is not functional, although it contains an active catalytic sector F1, but it is devoid of the F0 sector (Paul et al., 1989). Subunits e and g were detected in ΔATP4 mitochondrial membranes, albeit in low concentrations (Figure 4B), showing that subunit 4 and therefore a functional ATP synthase are involved in generating mitochondrial cristae morphology.
Fig. 3. Yeast cells devoid of either subunit e or g have abnormal mitochondria. Samples were prepared as described in Materials and methods. They were observed by transmission electron microscopy. The arrows indicate abnormal mitochondria. (A) Wild-type, (B) ΔATP18, (C and D) ΔATP20 and (E and F) ΔTIM11. Bars indicate 0.5 µm.
Fig. 4. ΔATP4 yeast cells have abnormal mitochondria. (A) Cells were grown with 2% galactose as carbon source, and observed by transmission electron microscopy. The arrow indicates abnormal mitochondria. Bar indicates 0.5 µm. (B) Wild-type (lane 1) and ΔATP4 mitochondria (lane 2) were dissociated and samples (50 µg of protein) were submitted to western blot analysis. Blots were probed with polyclonal antibodies raised against subunits γ, 4, g and e.
To better characterize the unusual mitochondrial morphology of mutant strains, immunolocalization studies were performed with a polyclonal antibody raised against the β-subunit of the ATP synthase. The gold particles were mainly found in cristae of wild-type mitochondria (Figure 5A), but also in the digitations and onion-like structures of null mutant cells (Figure 5B–D). A statistical analysis of the location of gold particles revealed that 82% of them were associated with digitations and onion-like structures (Table II). Thus, these membranes clearly contain ATP synthase and the organelles are in fact mitochondria with an altered morphology. Similarly, an immunolocalization study was performed with a monoclonal antibody raised against the yeast mitochondrial porin (Figure 6). Eighty percent of the gold particles were found in the periphery of wild-type mitochondria and were restricted to the outermost membranes of onion-like structures (Table II). These observations point to a role of subunits e and g in cristae formation. The ΔATP18 mutant, which is devoid of the ATP synthase-associated subunit i, did not display such morphological modifications (Figure 3B). Consistent with the role of subunits e and g in cristae formation, western blot analysis of mitochondrial membranes devoid of subunit i indicated the presence of both subunits e and g (not shown). The morphological modifications described above were also investigated by fluorescence microscopy (not shown). ΔATP20 yeast cells were 1.5-fold larger than wild-type cells. Mitochondria were labelled with a matrix-targeted green fluorescent protein (Okamoto et al., 1998). A peripheral distribution of mitochondria was observed in wild-type and ΔATP20 yeast cells, but the latter also displayed diffuse large mitochondria that invaded the cells.
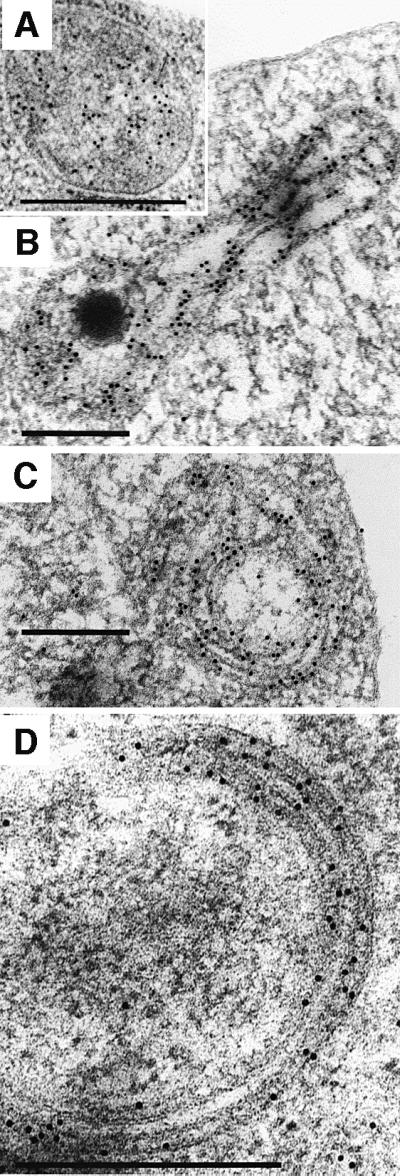
Fig. 5. Immunological detection in yeast cells of β-subunit of yeast ATP synthase Immunogold electron microscopy was carried as described in Materials and methods. The pictures are representative of experiments performed with wild-type (A) and ΔATP20 (B–D) cells. Bars indicate 0.5 µm.
Table II. Location of immunogold particles in yeast cellsa.
| Strains | Cytoplasm and plasma membrane |
Membrane-associated |
Inside organellesd |
|||
|---|---|---|---|---|---|---|
| anti-βATPase | anti-porin | anti-βATPaseb | anti-porinc | anti-βATPase | anti-porin | |
| Wild type | 187 (21%) | 32 (12%) | 629 (71%) | 223 (84%) | 69 (8%) | 11 (4%) |
| ΔATP20 | 564 (15%) | 63 (14%) | 3056 (83%) | 354 (80%) | 80 (2%) | 27 (6%) |
aGold particles corresponding to β and porin immunocomplexes were counted from electron micrographs.
bMitochondria or onion-like structures.
cOutermost membranes of mitochondria or onion-like structures.
dMitochondria or onion-like structures, but not clearly associated with membranes.
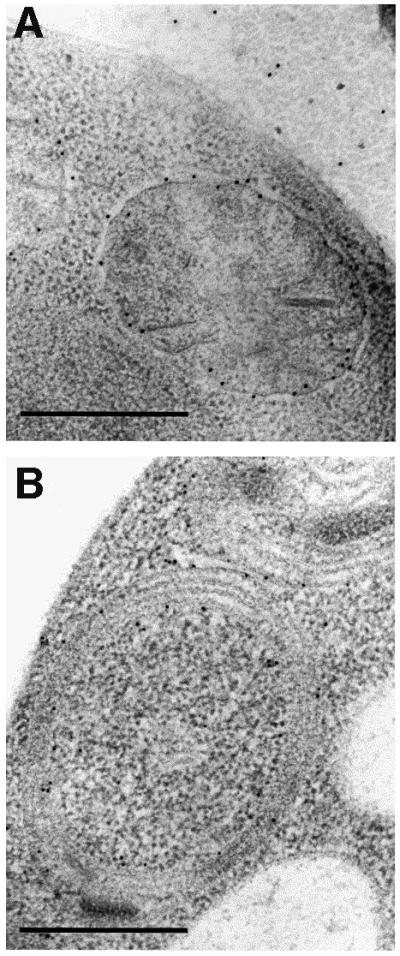
Fig. 6. Immunological detection in yeast cells of yeast mitochondrial porin. Immunogold electron microscopy was carried out as described in Materials and methods. The images are representative of experiments performed with wild-type (A) and ΔATP20 (B) cells. Bars indicate 0.5 µm.
Discussion
As discussed by Frey and Mannela (2000), the formation of tubular cristae and cristae junctions at the inter-membrane compartment level probably has important functional implications in terms of efficiency of oxidative phosphorylation by providing high surface-to-volume ratios. This would limit the diffusion of ions and substrates involved in ATP synthesis. The ultrastructural data shown above indicate that the ATP synthase-associated subunits e and g are indispensable for the biogenesis of the mitochondrial cristae. These observations lead to the conclusion that in the absence of either subunits e or g, the cristae are also absent, even though the inner membrane is conspicuously present. Thus, in mutant strains devoid of either subunit e or g, the inner membrane is organized differently than it is in wild-type mitochondria. Immunogold electron microscopy showed onion-like structures composed of two to three concentric layers of double leaflets of membranes containing a material dense to electrons. Figure 3D clearly displays three of these dense layers that are separated by two white spaces. The dense layers might correspond to the matrix space, as gold particles that localize F1 are mainly associated with membranes, which are therefore the inner mitochondrial membrane (Figure 5D). The white spaces could correspond to the inter-membrane space. Many gold particles that localize F1 are also associated with the most external double leaflet, so this membrane is also the inner mitochondrial membrane. However, gold particles that localize porin, a main component of the outer membrane, are also located in the periphery of wild-type mitochondria and are associated with the outermost membrane of onion-like structures, thus showing that the outer membrane of abnormal mitochondria constitutes a continuous envelope. The abnormal mitochondrial structure can be interpreted as the consequence of an uncontrolled biogenesis of the inner mitochondrial membrane. As a result, the folding of the inner membrane inside the organelle appears in cell sections as alternations of matricial and inter-membrane spaces. Digitations and onion-like structures are probably the same objects observed under different cross-sections. From immunolocalization studies it is clear that the inner mitochondrial membrane is contained inside a continuous envelope of outer membrane, a feature that is different from the mitochondrial Nebenkern occurring during Drosophila melanogaster spermatogenesis (Hales and Fuller, 1997).
Abnormal mitochondrial morphologies have already been described in yeast mutants (for reviews see Hermann and Shaw, 1998; Yaffe, 1999). The proteins of the outer mitochondrial membrane Mmm1p, Mdm10p and Mdm12p are required to maintain normal mitochondrial shape, mitochondrial segregation and mitochondrial DNA stability (Burgess et al., 1994; Sogo and Yaffe, 1994; Berger et al., 1997). In their absence, mitochondria appear as giant, spherical organelles under the fluorescence microscope. A striking loss of normal inner-membrane organization was also observed in the absence of Mmm1p. The tubular-shaped cristae were absent and the inner membrane was collapsed, forming stacks of membrane sheets inside the organelle (Aiken Hobbs et al., 2001). The maintenance of the mitochondrial reticulum in S.cerevisiae also requires opposing fission and fusion events that regulate organelle morphology and number. Fusion is regulated by the Fzo1p transmembrane GTPase (Hermann et al., 1998). Conversely, the dynamin-related GTPase, Dnm1p, associated with Mdv1p forms a complex associated with the outer mitochondrial membrane that controls mitochondrial morphology in yeast by regulating mitochondrial fission (Otsuga et al., 1998; Bleazard et al., 1999; Mozdy et al., 2000; Tieu and Nunnari, 2000). A second dynamin-related GTPase, Mgm1p, is also involved in genome maintenance and morphology (Shepard and Yaffe, 1999). The Mgm1 mutant is unable to grow with non-fermentable sources. Mgm1p plays a role in inner membrane remodelling events in yeast cells. In co-ordination with Dnm1p-dependent outer membrane fission, it regulates inner membrane division. It has recently been proposed that Mgm1p may help to form and/or stabilize inner membrane cristae or regulate inner membrane fission (Wong et al., 2000). The phenotypic alterations due to the loss of subunits e or g appear to be different from those described in the null mutants cited above. In the absence of subunits e or g, cells still grew on non-fermentable sources. rho– cells accumulated, but they still possessed mitochondrial DNA. Finally the large onion-like structures have not yet been observed in yeast mutants having a mitochondrial morphology deficiency. However, we cannot exclude a relationship between the C-terminal part of subunit e (amino acid residues 26–96) located in the inter-membrane space and other proteins involved in the maintenance of morphology, such as the inter-membrane protein Mgm1p, which is peripherically associated with the inner membrane. Cross-linking experiments will be performed to address this issue.
Earlier work concerning the yeast chondriome of wild-type yeast grown aerobically with glucose in the late S phase sometimes showed mitochondria to have small onion-like structures evoking the structures described in this paper (Yotsuyanagi, 1962a). It has also been reported that cross-sections of rho– cells display small, spherical mitochondria, the major alteration of which is the loss of cristae. Most of these mitochondria have no discernible internal organization, but sometimes display small onion-like structures (Yotsuyanagi, 1962b; Stevens, 1981). It should be remembered that rho– mitochondria do not have a functional F0 and are also devoid of subunits e and g (Arnold et al., 1998). Although some small onion-like structures have been observed in rho– cells, they cannot account for the extensive mitochondrial morphological alterations described in this paper. In fact, cross-sections of rho– cells isolated from the ΔATP20 strain also display small, spherical mitochondria without discernible internal organization (data not shown). It has also been reported that yeast mutants in apocytochrome b and cytochrome oxidase are characterized by mitochondria displaying normal-looking but less numerous cristae compared with normal respiring yeasts (Yotsuyanagi, 1988). Therefore, it is most likely the absence of either subunit e or g and the presence of a functional ATP synthase that are the cause of such large onion-like structures seen in the ΔATP20 and ΔTIM11 mutant strains. For these reasons we conclude that ATP synthase is also an essential element for normal mitochondrial morphology, and propose an alternative in the way mitochondrial cristae are generated.
Model of ATP synthase organization in mitochondrial cristae
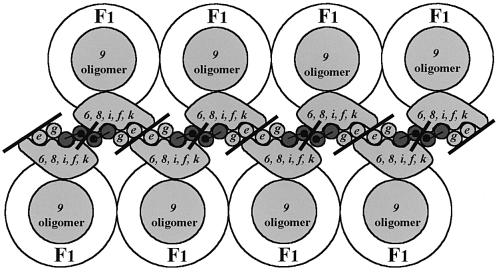
The main purpose of this paper is to provide the link between the dimerization of the mitochondrial ATP synthase and the biogenesis of cristae. Allen (1995) proposed an exciting hypothesis that described the association of ATP synthase dimers as generating the tubular cristae. The association of identical ATP synthase complexes approximating truncated cones in overall shape might offer the potential to form a rigid arc, thus leading to a protrusion of the inner mitochondrial membrane, which in turn might amplify to form tubules upon association of additional complexes during mitochondrial biogenesis. The crucial point is the dimerization of the enzyme. Subunits e and g are involved in the latter process, since ATP synthase is not dimerized and cristae are not formed in their absence. The second point is the association of ATP synthase dimers to form higher complexes. In this paper we show such associations in digitonin extracts of wild-type mitochondria. Figure 7 shows a proposed model consisting of the association of F1F0 ATP synthase dimers. Such an association requires two different interfaces. The first is composed of subunits e and g (Arnold et al., 1998). The proximity between two subunits e and between subunits e and g has already been described in the bovine enzyme (Belogrudov et al., 1996). Indeed, the C-terminal part of subunit e (amino acids residues 26–60) has the potential for a coiled-coil configuration in the inter-membrane space, probably in association with another subunit e (Belogrudov et al., 1996). The model suggests intermolecular interactions between subunits e of two adjacent ATP synthase complexes. We now propose a second intermolecular interaction involving subunit b (subunit 4) (Velours et al., 2000), since spontaneous dimers of the subunit were found in mitochondrial membranes of cysteine mutants of subunit 4 in either the presence or absence of subunits e and g. The model also takes into account the intramolecular interaction of subunit g and the N-terminal part of subunit 4 (Soubannier et al., 1999). Subunits e, g and 4 are at least responsible for the association of ATP synthases through their F0 portions.
Fig. 7. Schematic representation of associations of F1F0 ATP synthase dimers. The grey circles and blocks represent the membranous parts of yeast F0 components as observed from the intra-cristae space. Subunit 4 (subunit b) is represented by two dark grey circles (the two membrane-spanning segments) that are linked by a line corresponding to the inter-membrane hydrophilic loop (amino acid residues 46–56). The black dots represent the mutation 4D54C. This model displays two interfaces (black bars). One is mediated by subunit 4 and the other is mediated at least by subunits e and g.
An apparent paradox was the dimerization of subunit 4 in ΔATP20 and ΔTIM11 membranes. The postulated existence of two different interfaces at the F0 level reconciles the observations of Arnold et al. (1998) with ours, because interactions could occur between two distinct faces in the membrane, one composed of subunits 4 and the other composed of subunits e and g. Thus, it is possible to obtain subunit 4 dimerization in the membrane in the absence of subunits e or g.
However, we have not yet been able to increase the oligomer amount in digitonin extracts by cross-linking experiments involving subunits 4 as targets. Although subunit 4 dimers were obtained, they were mainly present in the pellets after centrifugation of the digitonin extracts, thus precluding the observation of cross-linked ATP synthase complexes by BN–PAGE. In addition, an increase in the F1 concentration was observed in digitonin supernatant extracts, thus showing a destabilization of ATP synthase complexes upon cross-linking (not shown). We have already reported the lack of solubility of subunit 4 dimers in detergents other than SDS. The covalent linkage between subunits 4 of two different complexes resulted in a disconnection between the F1 and F0 sectors, leading to a decrease in oligomycin sensitivity (Spannagel et al., 1998). At present, a digitonin/protein ratio of 0.75 g/g is the best ratio to obtain a high amount of ATP synthase oligomer under native conditions. This probably reflects the lability of the interface involving the association of dimers in our experimental conditions.
The model in Figure 7 is still speculative and originates from the observation of the regular association of ATP synthases on the tubular cristae of P.multimicronucleatum mitochondria. Such an investigation has not yet been carried out on yeast cristae. Another point concerns the oligomerization of ATP synthase molecules. The question is whether digitonin extracts contain an association of ATP synthase dimers or ATP synthase aggregates, as with the detergent-solubilized chloroplast ATP synthase (Böttcher and Gräber, 2000). The observation by BN–PAGE of distinct bands having higher molecular weights than that of the dimeric form of the enzyme, and the absence of such bands in digitonin extracts of ΔATP20 and ΔTIM11 mitochondria while they are present in ΔATP18 extracts, are all characteristics not in favour of aggregates.
From the above data it appears that the F1F0 ATP synthase, the main role of which is to provide energy to the cell, is also involved in mitochondrial morphology. We suggest that in the absence of dimerization involving F0 interfaces, the role of ATP synthase in the organization of the tubular cristae is abolished and the inner membrane is produced without control, leading to onion-like structures, although small, similar objects are present in ΔATP4 mutant cells and sometimes in rho– cells. The small size of the onion-like structures of ΔATP4 and rho– mutants can result in a decrease in the biogenesis of mitochondrial membranes, due to a dramatic loss in energy. For instance, rho– cells are devoid of F0 and respiratory complexes and the energy source of rho– mitochondria is a transmembrane potential ΔΨ provided by ATP hydrolysis by F1, coupled with the adenine nucleotide carrier (Giraud and Velours, 1997). Similarly, ΔATP4 mutant is also devoid of F0 and a dramatic decrease in mitochondrial respiratory complexes has been reported previously (Paul et al., 1989). Experiments are now being performed to identify all the components involved in protein interfaces between ATP synthase complexes and allowing the oligomers of yeast ATP synthase to control tubular cristae biogenesis.
Subunits e and g have only been found as associated components of ATP synthase in organelles with cristae. We suggest an involvement of subunits e and g in the mitochondrial morphology of eukaryotic cells other than yeast, even if the cristae are not always tubular, since flattened lamellar cristae have been observed in brown adipose tissue and Neurospara crassa mitochondria (Perkins et al., 1998; Nicastro et al., 2000). This raises the question of the presence and stoichiometry of ATP synthase-associated proteins in various tissues and organisms, which may vary according to the metabolic state of the cell.
Materials and methods
Yeast strains
The S.cerevisiae strain D273–10B/A/H/U (MATα, met6, ura3, his3) (Paul et al., 1989) was the wild-type strain. Strain ΔATP18 has been described previously (Vaillier et al., 1999). The null mutant ΔATP20 and ΔTIM11 genes were constructed by a PCR-based mutagenesis (Güldener et al., 1996).
Biochemical procedures
Cells were grown aerobically at 28°C in a complete liquid medium containing 2% lactate as carbon source (Arselin de Chateaubodeau et al., 1976) and harvested in logarithmic growth phase. rho– production in cultures was measured on glycerol plates supplemented with 0.1% glucose. Mitochondria were prepared according to Guérin et al. (1979). Protein amounts were determined according to Lowry et al. (1951) in the presence of 5% SDS using bovine serum albumin as standard. Oxygen consumption rates were measured with NADH as substrate (Rigoulet and Guérin, 1979). The ATPase activity was measured at pH 8.4 (Velours et al., 2001).
BN–PAGE experiments were carried out as described previously (Schägger and von Jagow, 1991; Schägger et al., 1994). The mitochondrial digitonin extracts were centrifuged at 4°C for 15 min at 40 000 g and immediately loaded on the top of a 3–13% polyacrylamide slab gel. After electrophoresis the gel was either stained with Coomassie Blue or incubated in a solution of 5 mM ATP, 5 mM MgCl2, 0.05% lead acetate, 50 mM glycine-NaOH pH 8.6 to detect the ATPase activity (Grandier-Vazeille and Guérin, 1996). The bands were cut and analysed in a second dimension by SDS–PAGE (Schägger and von Jagow, 1987). The slab gel was then silver stained. Protein standards were thyroglobulin (669 kDa), apoferritin (443 kDa), β-amylase (200 kDa) and serum albumin (132 and 66 kDa). Nitrocellulose membranes (Membrane Protean BA83, 0.2 µm; Schleicher et Schuell) were used for western blot analyses. Polyclonal antibodies raised against subunits e and g were kindly provided by Drs R.Stuart and W.Neupert (Institut für Physiologishe Chemie der Universität Munchen, Germany). Antibodies against subunit 4, e and g subunits were used with dilutions of 1:10 000. Membranes were incubated with peroxidase-labelled antibodies and visualized with the enhanced chemiluminescence reagent (ECL; Amersham Pharmacia).
Freezing and freeze-substitution for ultrastructural studies
The yeast pellet was placed on the surface of copper EM grids (3 mm diameter, 400 mesh) that had been coated with formvar. Each loop was quickly submersed in pre-cooled liquid propane kept at –180°C by liquid nitrogen. The loops were then transferred to a pre-cooled solution of 4% osmium tetroxide in dry acetone and maintained at –82°C for 48–72 h. Specimens were stained for 1 h in 2% uranyl acetate in acetone at 4°C and infiltrated with Araldite (epoxy resin; Fluka). Ultra-thin sections were stained with lead citrate and were viewed in a Philips CM10 electron microscope (80 kV) or on a Philips Tecnai 12 Biotwin (120 kV).
Immunogold electron microscopy
Yeast cells were cryofixed as above and substituted with acetone plus 0.1% glutaraldehyde or methanol plus 0.5% uranyl acetate for 3 days at –80°C. Samples were rinsed with acetone and ethanol at –20°C and embedded progressively at –20°C in LR Gold acrylic resin (Electron Microscopy Sciences, Fort Washington, PA). Resin polymerization was carried out at –20°C for 3 days under UV illumination. Ultra-thin sections were collected on nickel grids coated with formvar. Sections were first incubated for 5 min with 1 mg/ml glycine, and 10 min with fetal calf serum (dilution 1:20). The grids were incubated for 45 min at room temperature with either polyclonal antibodies raised against the β-subunit of the yeast ATP synthase (dilution 1:3000) or monoclonal anti-yeast mitochondrial porin (Molecular Probes) (dilution 1:100). They were then incubated for 45 min at room temperature with anti-rabbit or anti-mouse IgG conjugated to 10 nm gold particles (Biocell). The sections were rinsed with distilled water and contrasted for 10 min with 2% uranyl acetate in water, followed by 1% lead citrate for 1 min.
Acknowledgments
Acknowledgements
We are grateful to Drs Y.Yotsuyanagi, N.Camougrand, S.Manon, X.Grandier-Vazeilles, C.Napias and M.Aigle for stimulating discussions. P.P. and V.S. hold a research grant from the Ministère de la Recherche et de la Technologie. This research was supported by the Centre National de la Recherche Scientifique, the Ministère de la Recherche et de l’Enseignement supérieur, the Université Victor Ségalen, Bordeaux 2, the Etablissement Public Régional d’Aquitaine and by grant GM44412 to D.M.M. from the National Institute of Health.
References
- Aiken Hobbs A.E., Srinivasan,M., McCaffery,J.M. and Jensen,R.E. (2001) Mmm1p, a mitochondrial outer membrane protein, is connected to mitochondrial DNA (mtDNA) nucleoids and required for mtDNA stability. J. Cell Biol., 152, 401–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen R.D. (1995) Membrane tubulation and proton pumps. Protoplasma, 189, 1–8. [Google Scholar]
- Allen R.D., Schroeder,C.C. and Fok,A.K. (1989) An investigation of mitochondrial inner membranes by rapid-freeze deep-etch techniques. J. Cell Biol., 108, 2233–2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold I., Bauer,M.F., Brunner,M., Neupert,W. and Stuart,R.A. (1997) Yeast mitochondrial F1F0-ATPase: the novel subunit e is identical to Tim11. FEBS Lett., 411, 195–200. [DOI] [PubMed] [Google Scholar]
- Arnold I., Pfieffer,K., Neupert,W., Stuart,R.A. and Schägger,H. (1998) Yeast mitochondrial F1F0-ATP synthase exists as a dimer: identification of three dimer-specific subunits. EMBO J., 17, 7170–7178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold I., Pfeiffer,K., Neupert,W., Stuart,R.A. and Schägger,H. (1999) ATP synthase of yeast mitochondria—Isolation of subunit j and disruption of the ATP18 gene. J. Biol. Chem., 274, 36–40. [DOI] [PubMed] [Google Scholar]
- Arselin de G., Guérin,M. and Guérin,B. (1976) Perméabilité de la membrane interne des mitochondries de levure: étude des relations entre structure et activité. Biochimie (Paris), 58, 601–610. [DOI] [PubMed] [Google Scholar]
- Bateson M., Devenish,R.J., Nagley,P. and Prescott,M. (1999) Single copies of subunits d, oligomycin-sensitivity conferring protein and b are present in the Saccharomyces cerevisiae mitochondrial ATP synthase. J. Biol. Chem., 274, 7462–7466. [DOI] [PubMed] [Google Scholar]
- Belogrudov G.I., Tomich,J.M. and Hatefi,Y. (1996) Membrane topography and near-neighbor relationships of the mitochondrial ATP synthase subunits e, f and g. J. Biol. Chem., 271, 20340–20345. [DOI] [PubMed] [Google Scholar]
- Berger K.H., Sogo,L.F. and Yaffe,M.P. (1997) Mdm12p, a component required for mitochondrial inheritance that is conserved between budding and fission yeast. J. Cell Biol., 136, 545–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleazard W., McCaffery,J.M., King,E.J., Bale,S., Mozdy,A., Tieu,Q., Nunnari,J. and Shaw,F.M. (1999) The dynamin-related GTPase Dnm1 regulates mitochondrial fission in yeast. Nature Cell Biol., 1, 298–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böttcher B. and Gräber,P. (2000) The structure of the H+-ATP synthase from chloroplasts and its subcomplexes as revealed by electron microscopy. Biochim. Biophys. Acta, 1458, 404–416. [DOI] [PubMed] [Google Scholar]
- Boyle G.M., Roucou,X., Nagley,P., Devenish,R.J. and Prescott,M. (1999) Identification of subunit g of yeast mitochondrial F1F0-ATP synthase, a protein required for maximal activity of cytochrome c oxidase. Eur. J. Biochem., 262, 315–323. [DOI] [PubMed] [Google Scholar]
- Burgess S.M., Delannoy,M. and Jensen,R.E. (1994) MMM1 encodes a mitochondrial outer membrane protein essential for establishing and maintaining the structure of yeast mitochondria. J. Cell Biol., 126, 1375–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collinson I.R., Runswick,M.J., Buchana,S.K., Fearnley,I.M., Skehel,J.M., van Raaij,M.J., Griffiths,D.E. and Walker,J.E. (1994) Fo membrane domain of ATP synthase from bovine heart mitochondria: purification, subunit composition and reconstitution with F1-ATPase. Biochemistry, 33, 7971–7978. [DOI] [PubMed] [Google Scholar]
- Collinson I.R., Skehel,J.M., Fearnley,I.M., Runswick,M.J. and Walker,J.E. (1996) The F1F0-ATPase complex from bovine heart mitochondria: the molar ratio of the subunits in the stalk region linking the F1 and F0 domains. Biochemistry, 35, 12640–12646. [DOI] [PubMed] [Google Scholar]
- Fernández-Morán H. (1962) Cell-membrane ultrastructure. Low-temperature electron microscopy and X-ray diffraction studies of lipoprotein components in lamellar systems. Circulation, 26, 1039–1065. [DOI] [PubMed] [Google Scholar]
- Fillingame R.H. (1999) Molecular rotary motors. Science, 286, 1687–1688. [DOI] [PubMed] [Google Scholar]
- Frey T.G. and Mannela,C.A. (2000) The internal structure of mitochondria. Trends Biochem. Sci., 25, 319–324. [DOI] [PubMed] [Google Scholar]
- Giraud M.F. and Velours,J. (1997) The absence of the mitochondrial ATP synthase δ subunit promotes a slow growth phenotype of rho– cells by lack of assembly of the catalytic sector. Eur. J. Biochem., 245, 813–818. [DOI] [PubMed] [Google Scholar]
- Grandier-Vazeille X. and Guérin,M. (1996) Separation by blue native and colorless native polyacrylamide gel electrophoresis of the oxidative phosphorylation complexes of yeast mitochondria solubilized by different detergents: specific staining of the different complexes. Anal. Biochem., 242, 248–254. [DOI] [PubMed] [Google Scholar]
- Guérin B., Labbe,P. and Somlo,M. (1979) Preparation of yeast mitochondria (Saccharomyces cerevisiae) with good P/O and respiratory control ratios. Methods Enzymol., 55, 149–159. [DOI] [PubMed] [Google Scholar]
- Güldener U., Heck,S., Fiedler,T., Beinhauer,J. and Hegemann,J.H. (1996) A new efficient gene disruption cassette for repeated use in budding yeast. Nucleic Acids Res., 24, 2519–2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hales K.G. and Fuller,M.T. (1997) Developmentally regulated mitochondrial fusion mediated by a conserved, novel, predicted GTPase. Cell, 90, 121–129. [DOI] [PubMed] [Google Scholar]
- Hermann G.J. and Shaw,J.M. (1998) Mitochondrial dynamics in yeast. Annu. Rev. Cell Dev. Biol., 14, 265–303. [DOI] [PubMed] [Google Scholar]
- Hermann G.J., Thatcher,J.W., Mills,J.P., Hales,K.G., Fuller,M.T., Nunnari,J. and Shaw,J.M. (1998) Mitochondrial fusion in yeast requires the transmembrane GTPase Fzo1p. J. Cell Biol., 143, 359–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuti T., Kuroiwa,K., Kawamura,Y. and Yoshihara,Y. (1992) Complete amino acid sequence of subunit e of rat liver mitochondrial H+-ATP synthase. Biochemistry, 31, 12451–12454. [DOI] [PubMed] [Google Scholar]
- Levy F.H. and Kelly,D.P. (1997) Regulation of ATP synthase subunit e expression by hypoxia: cell differentiation stage-specific control. Am. J. Physiol., 272, C457–C465. [DOI] [PubMed] [Google Scholar]
- Lowry O.H., Rosebrough,N.J., Farr,A.L. and Randall,R.J. (1951) Protein measurement with the folin reagent. J. Biol. Chem., 193, 265–275. [PubMed] [Google Scholar]
- Mozdy A.D., McCaffery,J.M. and Shaw,J.M. (2000) Dnm1p GTPase-mediated mitochondrial fission is a multi-step process requiring the novel integral membrane component Fis1p. J. Cell Biol., 151, 367–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicastro D., Frangakis,A.S., Typke,D. and Baumeister,W. (2000) Cryo-electron tomography of neurospora mitochondria. J. Struct. Biol., 129, 48–56. [DOI] [PubMed] [Google Scholar]
- Okamoto K., Perlman,P.S. and Butow,R.A. (1998) The sorting of mitochondrial DNA and mitochondrial proteins in zygotes: Preferential transmission of mitochondrial DNA to the mediated bud. J. Cell Biol., 142, 613–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuga D., Keegan,B.R., Brisch,E., Thatcher,J.W., Hermann,G.J., Bleazard,W. and Shaw,J.M. (1998) The dynamin-related GTPase, Dnm1p, controls mitochondrial morphology in yeast. J. Cell Biol., 143, 333–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul M.F., Velours,J., Arselin de Chateaubodeau,G., Aigle,M. and Gurérin,B. (1989) The role of subunit 4, a nuclear-encoded protein of the Fo sector of yeast mitochondrial ATP synthase, in the assembly of the whole complex. Eur. J. Biochem., 185, 163–171. [DOI] [PubMed] [Google Scholar]
- Paumard P., Vaillier,J., Napias,C., Arselin,G., Brethes,D., Graves,P.V. and Velours,J. (2000) Environmental study of subunit i, a Fo component of the yeast ATP synthase. Biochemistry, 39, 4199–4205. [DOI] [PubMed] [Google Scholar]
- Pedersen P.L., Ko,Y.H. and Hong,S. (2000) ATP synthases in the year 2000: evolving views about the structures of these remarkable enzyme complexes. J. Bioenerg. Biomembr., 32, 325–332. [DOI] [PubMed] [Google Scholar]
- Perkins G.A., Song,T.Y., Tarsa,L., Deerinck,T.J., Ellisman,M.H. and Frey,T.G. (1998) Electron tomography of mitochondria from brown adipocytes reveals crista junctions. J. Bioenerg. Biomembr., 30, 431–442. [DOI] [PubMed] [Google Scholar]
- Poduslo J.F. and Rodbard,D. (1980) Molecular weight estimation using sodium dodecyl sulfate–pore gradient electrophoresis. Anal. Biochem., 101, 394–406. [DOI] [PubMed] [Google Scholar]
- Racker E., Tyler,D.D., Estabrook,R.W., Conover,T.E., Parsons,D.F. and Chance,B. (1965) Correlations between electron-transport activity, ATP-ase and morphology of submitochondrial particles. In King,T.E., Mason,H.S. and Morisson,M. (eds), Oxidases and Related Redox Systems. J.Wiley & Sons, New York, NY, pp. 1077–1101.
- Rigoulet M. and Guérin,B. (1979) Phosphate transport and ATP synthesis in yeast mitochondria: effect of a new inhibitor: the tribenzylphosphate. FEBS Lett., 102, 18–22. [DOI] [PubMed] [Google Scholar]
- Schägger H. and von Jagow,G. (1987) Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal. Biochem., 166, 368–379. [DOI] [PubMed] [Google Scholar]
- Schägger H. and von Jagow,G. (1991) Blue native electrophoresis for isolation of membrane protein complexes in enzymatically active form. Anal. Biochem., 199, 223–231. [DOI] [PubMed] [Google Scholar]
- Schägger H. and Pfeiffer,K. (2000) Supercomplexes in the respiratory chains of yeast and mammalian mitochondria. EMBO J., 19, 1777–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schägger H., Cramer,W.A. and von Jagow,G. (1994) Analysis of molecular masses and oligomeric states of protein complexes by blue native electrophoresis and isolation of membrane protein complexes by two-dimensional native electrophoresis. Anal. Biochem., 217, 220–230. [DOI] [PubMed] [Google Scholar]
- Shepard K.A. and Yaffe,M.P. (1999) The yeast dynamin-like protein, Mgm1p, functions on the mitochondrial membrane to mediate mitochondrial inheritance. J. Cell Biol., 144, 711–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sogo L.F. and Yaffe,M.P. (1994) Regulation of mitochondrial morphology and inheritance by Mdm10p, a protein of the mitochondrial outer membrane. J. Cell Biol., 126, 1361–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soubannier V. et al. (1999) The second stalk of the yeast ATP synthase complex: identification of subunits showing cross-links with known positions of subunit 4 (subunit b). Biochemistry, 38, 15017–15024. [DOI] [PubMed] [Google Scholar]
- Spannagel C., Vaillier,J., Arselin,G., Graves,P.V., Grandier-Vazeille,X. and Velours,J. (1998) Evidence of a subunit 4 (subunit b) dimer in favor of the proximity of ATP synthase complexes in yeast inner mitochondrial membrane. Biochim. Biophys. Acta, 1414, 260–264. [DOI] [PubMed] [Google Scholar]
- Stevens B. (1981) Mitochondrial structure. In Strathern,J.N., Jones,E.W. and Broach,J.R. (eds), The Molecular Biology of the Yeast Saccharomyces cerevisiae. Life Cycle and Inheritance. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, pp. 471–504.
- Tieu Q. and Nunnari,J. (2000) Mdv1p is a WD repeat protein that interacts with the dynamin-related GTPase, Dnm1p, to trigger mitochondrial division. J. Cell Biol., 151, 353–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokatlidis K., Junne,T., Moes,S., Schatz,G., Glick,B.S. and Kronidou,N. (1996) Translocation of an intramitochondrial sorting signal next to Tim11 at the inner-membrane import site. Nature, 384, 585–588. [DOI] [PubMed] [Google Scholar]
- Vaillier J., Arselin,G., Graves,P.V., Camougrand,N. and Velours,J. (1999) Isolation of supernumerary yeast ATP synthase subunits e and i. Characterization of subunit i and disruption of its structural gene ATP18. J. Biol. Chem., 274, 543–548. [DOI] [PubMed] [Google Scholar]
- Velours J., Paumard,P., Soubannier,V., Spannagel,C., Vaillier,J., Arselin,G. and Graves,P.V. (2000) Organisation of the yeast ATP synthase F0: a study based on cysteine mutants, thiol modification and cross-linking reagents. Biochim. Biophys. Acta, 1458, 443–456. [DOI] [PubMed] [Google Scholar]
- Velours J., Vaillier,J., Paumard,P., Soubannier,V., Lai-Zhang,J. and Mueller,D.M. (2001) Bovine coupling factor 6, with just 14.5% shared identity, replaces subunit h in the yeast ATP synthase. J. Biol. Chem., 276, 8602–8607. [DOI] [PubMed] [Google Scholar]
- Walker J.E., Lutter,R., Dupuis,A. and Runswick,M.J. (1991) Identification of the subunits of F1F0-ATPase from bovine heart mitochondria. Biochemistry, 30, 5369–5378. [DOI] [PubMed] [Google Scholar]
- Wong E.D., Wagner,J.A., Gorsich,S.W., McCaffery,M., Shaw,J.M. and Nunnari,J. (2000) The dynamin-related GTPase, Mgm1p, is an intermembrane space protein required for maintenance of fusion competent mitochondria. J. Cell Biol., 151, 341–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe M.P. (1999) The machinery of mitochondrial inheritance and behavior. Science, 283, 1493–1497. [DOI] [PubMed] [Google Scholar]
- Yotsuyanagi Y. (1962a) Études sur le chondriome de la levure. I. Variations de l’ultrastructure du chondriome au cours du cycle de la croissance aérobie. J. Ultrastruct. Res., 7, 121–140. [DOI] [PubMed] [Google Scholar]
- Yotsuyanagi Y. (1962b) Études sur le chondriome de la levure. II. Chondriomes des mutants à déficience respiratoire. J. Ultrastruct. Res., 7, 141–158. [DOI] [PubMed] [Google Scholar]
- Yotsuyanagi Y. (1988) Fibrous component of yeast mitochondria. J. Ultrastruct. Mol. Struct. Res., 98, 254–266. [DOI] [PubMed] [Google Scholar]