Abstract
Townes-Brocks syndrome (TBS, MIM#107480) is an autosomal dominant disorder linked to SALL1 alterations and characterized by a clinical triad (anorectal, thumb, and external-ear malformations), along with variable features. Renal failure and deafness can occur at any age, making follow-up essential. Some genotype-phenotype correlations have been suggested but data are limited. We collected clinical and molecular data from 49 patients with a SALL1 (likely) pathogenic variant identified in our laboratory or through collaborations, and reviewed the 207 SALL1 related-TBS patients previously reported in the literature. We performed statistical analysis to study genotype-phenotype correlations based notably on the variant position in relation to the glutamine-rich region. In our series, 25% of individuals presented with the clinical triad compared to 49.7% in the literature. The deafness frequency was similar (65%). Renal failure was diagnosed in 39.6% of our patients compared to 29.3% in the literature. Developmental delay or intellectual disability affected 9% of patients. Of the 22 SALL1 variants in our series, 35% were located upstream of the glutamine-rich region, compared to 6.5% in the literature. Statistical analysis was performed on all patients, of which 26 and 200 carried a variant upstream and downstream of the glutamine-rich region, respectively. A significant increase in deafness, dysplastic ear, and thumb malformations and a significant decrease in renal failure were observed in the individuals carrying a variant located downstream of the region, but the patients were significantly younger. Future studies should aim to elucidate the complex pathophysiological mechanisms and prognosis of TBS, functionally and prospectively.
Subject terms: Disease genetics, Diseases
Introduction
Townes-Brocks syndrome (TBS, MIM#107480) is an autosomal dominant disorder historically characterized by the triad of thumb, external ear and anorectal malformations [1]. Other features have subsequently been described, including deafness, feet malformations, kidney malformations and/or renal failure, congenital heart defects and genital anomalies in males [2–6]. Penetrance is reported to be complete but expressivity, including severity, is highly variable even within the same family [7, 8]. In particular, renal failure and deafness can occur at any age, making the follow-up of TBS patients essential [2, 9, 10].
TBS is due to heterozygous pathogenic variants of the SALL1 (Sal-like 1) gene [11]. The SALL1 protein belongs to the Sal C2H2-type zinc finger protein family, characterized by zinc finger domains along the protein and a glutamine-rich region implicated in self-dimerization and heterodimerization with other Sall proteins [12–14]. SALL1 acts as a repressive transcription factor in developmental processes such as kidney, brain and limb development [13, 15]. Heterozygous Sall1 knock-out mice have no phenotype, whereas ΔZn2-10 Sall1 mice, which produce a truncated protein, show some TBS features such as kidney malformations and hearing loss, suggesting a gain-of-function or dominant-negative effects of SALL1 variants involved in TBS [16–18].
In human disease, most of the SALL1 pathogenic variants involved in TBS are private and truncating [2, 3]. It has been demonstrated that some SALL1 truncating variants were resistant to nonsense-mediated decay in TBS-patient fibroblasts and EBV-transformed B-cells, supporting the hypothesis that expression of a truncated protein is critical in the pathogenesis of TBS [16, 19]. However, few TBS patients carry a partial or complete deletion of SALL1 with or without deletion of contiguous genes, likely resulting in haploinsufficiency. Genotype-phenotype correlations have been suggested but data are limited [2, 3, 20]. Among all the variants, a recurrent one, NM_002968.3:c.826 C > T p.(Arg276*), seems to be associated with a more severe phenotype with a higher prevalence of congenital heart defects [2, 21]. Furthermore, some authors support the association of haploinsufficiency, since some patients carry a SALL1 deletion, with a milder phenotype [20]. It has been suggested that the variants located in the mutational hotspot towards the 5′-end are associated with a more severe phenotype than the variants located towards the 3′-end [2]. Furthermore, Wang et al. analyzed genotype-renal phenotype correlation according to zinc finger domains (n = 81) and concluded that variants affecting the SALL1 gene positions from c.1500 to c.3468 are less likely to cause kidney phenotypes, although there is intrafamilial variability; however no statistical analyses were performed [22]. In a similar manner, genotype-phenotype correlations may be postulated depending on the domains remaining in the truncated-SALL1 protein. In particular, the presence of the glutamine-rich region, that plays a role in the dimerization of Sall proteins [12], has the potential to result in a dominant-negative effect, which may in turn manifest a more severe phenotype.
Here, we describe a new series of 49 patients from 22 families carrying a likely pathogenic or pathogenic heterozygous variant or a deletion of SALL1 gene in order to better characterize TBS. We also review patients carrying a SALL1 variant or deletion from the literature. We perform statistical analyses to establish possible genotype-phenotype correlations according to the position of the truncating variants, in relation to the glutamine-rich region and the mutational hotspot (towards 5′-end/3′-end).
Subjects and methods
Patients
Patients were recruited from the medical files of the laboratory at University Hospital of Lille (France) since 2006, and through calls for collaboration (AnDDI-Rares French network, ERN ITHACA and CRANIO European networks). Data were collected from a detailed form sent to the investigators and from the laboratory files when the analysis was performed locally. The inclusion criterion was the presence of a SALL1 heterozygous truncating variant classified as likely pathogenic or pathogenic according to the ACMG classification [23], or a SALL1 heterozygous deletion. Techniques for the genetic analyses were variable according to their evolution over the years and local diagnostic standards: Sanger sequencing, next generation sequencing, MLPA, chromosomal micro-array, exome sequencing (Table S1; precisions available on request). The variants in families 3, 4, 7, 9 and 17 have already been reported in a previous work from our team, but without detailed clinical description [24].
Literature review
The literature review was performed using the HGMD Pro and Pubmed databases (last assessment October 2023) with the following terms: “Townes Brocks”;”SALL1”. As above, the inclusion criterion was the presence of a SALL1 heterozygous truncating variant or deletion, classified as probably pathogenic or pathogenic. The exclusion criterion was the absence of a phenotype description.
Genotype-phenotype correlations
Based on the literature data and previous genotype-phenotype studies in TBS, we tested two hypotheses according to the position of the truncating variant (start of the change in the case of a frameshift variant): 1) upstream or within (from c.1 to c.750) versus downstream (from c.751 to c.3975) of the glutamine-rich region (located between c.687 and c.750) [4], with the hypothesis of a dominant-negative effect leading to a more severe phenotype; 2) upstream versus downstream of the middle of the mutational hotspot (c.1164) [4], according to the hypothesis mentioned in the literature [2]. Patients carrying a SALL1 deletion were excluded from this comparative analysis.
We also compared the phenotypes associated with SALL1 deletion with those associated with truncating variants, excluding the patients with the recurrent p.(Arg276*) variant, previously associated with a more severe phenotype [21] to avoid introducing bias.
Statistical analysis
Categorical variables are expressed as numbers (percentage). Continuous variables are expressed as means (standard deviation, SD) in the case of normal distribution or medians [interquartile range] otherwise. Normality of distribution was assessed using histograms and the Shapiro-Wilk test.
Comparative statistical analysis was performed on the data from this study and the literature. Comparisons between two groups relative to the variant location were performed by using Chi-square tests (or Fisher’s exact tests when expected cell frequency was <5) for categorical variables and Mann-Whitney U test for continuous variables. Variables compared were: sex, age, deafness, dysplastic ear, thumb malformation, malformation of lower limb, anorectal malformation, renal malformation, chronic renal failure, genital anomalies, congenital cardiac malformation and endocrinal anomalies.
Statistical testing was done at the two-tailed α level of 0.05. Data was analyzed using SAS software package, release 9.4 (SAS Institute, Cary, NC, USA).
Results
Present series
We recruited 49 TBS patients from 22 families, with a mean age of 23.6 ± 21.8 years old (Table 1, Table S1). The clinical triad (dysplastic ear, anorectal and thumb malformations) was observed in 25% of patients. The most common feature was dysplastic ear (69.4%), often a helix malformation (Fig. 1); thumb malformations were present in 53.1% with mostly triphalangeal and/or duplicated thumbs (Fig. 1); 62.5% of patients had an anorectal malformation. Lower limb malformations were mainly clinodactyly and overlapping toe (Fig. 1). Kidney structural malformations and chronic renal failure affected 53.8% and 39.6% of patients, respectively. Chronic renal failure occurred at any age, mostly in young adults but also in neonates (2 patients) and was often associated with hypo-dysplastic kidneys. Notably, 3 patients had chronic renal failure without abnormalities on kidney ultrasound. End-stage renal failure occurred in 6 patients, mostly adults. Deafness was diagnosed in 65.3% of patients. In most cases, deafness was post lingual, sensorineural, bilateral, mild to moderate. The course was sometimes progressive (4 patients). High frequencies were often affected, but audiograms results were heterogeneous. Six imaging studies of the ear were available: often normal, but incus and external auditory canal anomalies were seen in one patient each. Genital anomalies were observed only in males (6/25, 24%). Of note, 4 patients had hypothyroidism which was sometimes subclinical, and 5 had growth retardation. Regarding ophthalmologic features (16/44, 36.4%), 2/44 individuals had Stilling-Duane anomaly, 3/44 individuals acquired cataract, 8/44 individuals had refractive disorders; no coloboma or epibulbar dermoid were observed. Two patients from the same family (family 18) had tetralogy of Fallot, one of whom died at 3 months. Developmental delay (DD) and/or intellectual disability (ID) affected 6/40 (15%), often mild to moderate. Unfortunately, the intellectual quotient was rarely available (Table S2).
Table 1.
Review of clinical and molecular data from the present series and the literature.
| Present study | Literature | TOTAL | |
|---|---|---|---|
| Clinical data | |||
| Sex ratio (F:M [missing data]) | 0.88 [0] | 0.96 [9] | 0.95 [9] |
| Age (m ± SD [missing data]) | 23.6 ± 21.8 [2] | 15.6 ± 17.3 [114] | 18.3 ± 19.2 [116] |
| Triada (%) | 12/48 (25.0) | 95/191 (49.7) | 107/239 (44.8) |
| Dysplastic ear (%) | 34/49 (69.4) | 162/194 (83.5) | 196/243 (80.7) |
| Thumb malformation (%) | 26/49 (53.1) | 150/202 (74.2) | 176/251 (70.1) |
| Other malformation of upper limb (%) | 8/49 (16.3) | 22/201 (10.9) | 30/250 (12.0) |
| Malformation of lower limb (%) | 21/48 (43.8) | 93/195 (47.7) | 114/243 (46.9) |
| Ano-rectal malformation (%) | 30/48 (62.5) | 130/195 (66.7) | 160/243 (65.8) |
| Deafness (%) | 32/49 (65.3) | 128/197 (65.0) | 160/246 (65.0) |
| Kidney malformation (%) | 21/39 (53.8) | 81/191 (42.4) | 102/230 (44.3) |
| Renal failure (%) | 19/48 (39.6) | 56/191 (29.3) | 75/239 (31.4) |
| Genital anomalies (%) | 7/48 (14.6) | 27/198 (13.6) | 34/246 (13.8) |
| Congenital heart defect (%) | 8/35 (22.9) | 37/196 (18.9) | 45/231 (19.5) |
| Endocrine feature (%) | 11/43 (25.6) | 28/196 (14.3) | 39/239 (16.3) |
| Ophthalmologic features (%) | 16/44 (36.4) | 29/194 (14.9) | 45/238 (18.9) |
| Abnormal cranial nerveb (%) | 1/45 (2.2) | 9/185 (4.9) | 10/230 (4.3) |
| Facial asymmetry (%) | 2/45 (4.4) | 7/185 (3.8) | 9/230 (3.9) |
| Molecular data | |||
| SALL1 variant located upstream or into the glutamine-rich region (%) | 14/40 (35.0) | 12/186 (6.5) | 26/226 (11.5) |
| SALL1 variant located upstream the middle of the mutation hotspot (%) | 29/40 (72.5) | 80/186 (43.0) | 109/226 (48.2) |
| SALL1 deletion (%) | 9/49 (18.4) | 19/205 (9.3) | 28/254 (11.0) |
Triad of thumb, external ear and ano-rectal malformations (in bold).
F, female; M, male; m, mean; SD, standard deviation.
aTriad of thumb, external ear and ano-rectal malformations. bexcluding Stilling-Duane anomaly.
Fig. 1. Ear dysplasia and limb malformations in 18 patients from 12 families in the present series.
Ear dysplasia consisted mainly of abnormally folded helix and auricular tag or pit (arrow) (patient F20P40 was considered unaffected for ear dysplasia). Upper limb malformations mainly included triphalangeal thumb (patients F1P1, F5P9, F6P10, F6P13, F13P26, F18P32, F18P34, F19P36), duplicated thumb (patients F4P7, F5P9, F6P13, F9P16, F18P32, F18P34, F20O47), broad thumb (patients F4P8 and F20P47) (patients F6P12 and F8P15 were considered unaffected for upper limb malformations).
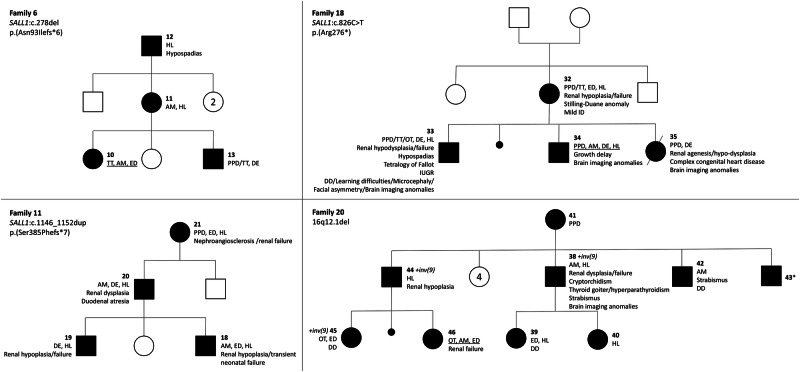
Individuals with TBS show highly variable intrafamilial expressivity (Fig. 2). For example, there was no common clinical feature between all affected relatives in the three-generation family 6 (Fig. 2). In addition, the clinical spectrum may be mild, such as in patient F20P42 who had an anorectal malformation, non-specific moderate speech delay and strabismus, making the clinical diagnosis of TBS sometimes challenging. In the same family, patient F20P43 had no phenotypic features. However, the initial assessment was incomplete as it did not include renal and cardiac assessment.
Fig. 2. Pedigrees of 4 families in the present series.
The first row refers to the four main signs: thumb features (preaxial polydactyly (PPD)/triphalangeal thumb (TT)/other thumb abnormality (OT)), anorectal malformation (AM), ear dysplasia (ED) and hearing loss (HL). The clinical triad is underlined when present. The numbers refer to the patient numbers given in Table S1, where the clinical and molecular characteristics are detailed. *No external abnormalities; cardiac and renal examinations were not performed. DD, developmental delay; del, deletion; dup, duplication; ID, intellectual disability; inv, inversion; IUGR, intra-uterine growth retardation.
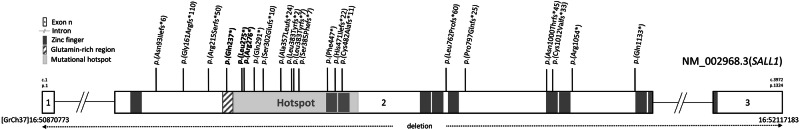
Among the molecular data (Fig. 3), 9 members from one family carried a large 16q12.1 deletion (1.2 Mb) encompassing SALL1 and HNRPNA1L3 (absent from the OMIM database) protein-coding genes. Forty patients from 21 families carried frameshift or nonsense variants of which 18 had not been previously reported in the literature. Of note, one family (family 18) carried the recurrent variant c.826 C > T,p.(Arg276*) [21]. The variants c.709 C > T,p.(Gln237*) (family 12) and c.824 T > G;p.(Leu275*) (family 17) were reported once each [25, 26]. Among all, 14/40 (35%) patients carried a variant located upstream or within the glutamine-rich region, and 29/40 (72.5%) upstream of the middle of the mutational hotspot. No somatic mosaicism for a variant in the SALL1 gene was identified in our series.
Fig. 3. Schematic representation of the truncating variants and deletion of SALL1 gene from the present series.
All variants were located in exon 2. Fifty-two percent (11/21) of variants were located in the mutation hot-spot defined by Botzenhart et al. in 2007 [4]. Nineteen percent (4/21) of variants were located upstream or within the glutamine-rich region [4, 12], and 72.5% (29/40) of variants were located upstream of the middle of the mutation hotspot [4]. Three variants (bold) have been reported in other families in the literature [21, 25, 26]. The deletion involved SALL1 and HNRPNA1L3 genes.
Literature review
A total of 207 patients from 130 families (60 reports) were included in the literature review (Table S3). The mean age was 15.6 ± 17.3 years (missing data for 114 individuals). The clinical triad was identified in 95/191 (49.7%) of patients with dysplastic ears being the most common sign (83.5%), followed by thumb malformations (74.2%) and anorectal malformation (66.7%). Lower limb malformations were variable and affected 93/195 (47.7%) of patients. Kidney structural malformations and chronic renal failure affected 81/191 (42.4%) and 56/191 (29.3%) of patients (9.5% of which progressed to end-stage renal failure), respectively. Additionally, 128/197 (65.0%) of patients were diagnosed with deafness, which was often sensorineural and mild to moderate. A genital anomaly was observed in 24/96 (25%) of males and in only 3/93 (3.23%) of females. Endocrinological features were present in 28/196 (14.3%) of patients with 5.6% having hypothyroidism and 4% having growth retardation. Various ocular anomalies were observed: notably, 5 individuals had Stilling-Duane anomaly, 5 coloboma, 3 acquired or congenital cataract, and 3 epibulbar dermoid. Nearly 19% (37/196) of individuals had congenital heart defect. We undertook a review of patients with DD and/or ID and found a proportion of 8% (14/175) having DD/ID, after excluding patients with a double genetic diagnosis (one patient with mosaic trisomy 8), prematurity, neonatal resuscitation or only mild DD. For DD, when data was available, the severity is often mild; some patients have had good outcomes, particularly in the case of speech delay associated with deafness. The review of TBS patients with DD and/or ID from our series and the literature is available in Table S2.
Molecular data analysis revealed a deletion encompassing SALL1 in 19/205 (9.3%), a truncating SALL1 variant located upstream or within the glutamine-rich region in 12/186 (6.5%), a variant upstream of the middle of the mutational hotspot in 80/186 (43.0%) of patients.
Genotype-phenotype correlations
Statistical analysis was performed on data from the present study (Table S1) and the literature (Table S3), comparing the clinical features of two groups defined by the localization of the truncating variant in relation to the glutamine-rich region: upstream and within (n = 26) or downstream (n = 200) of the region (Table 2). The prevalence of deafness (p = 0.037), dysplastic ear (p = 0.003) and thumb malformations (p = 0.003) was significantly higher when the variant was located downstream of the glutamine-rich region, whereas the prevalence of renal failure (p = 0.04) was significantly lower. Importantly, the patients were significantly younger and there were more missing data in the group “downstream of the glutamine-rich region”.
Table 2.
Clinical comparisons of TBS patients carrying variants located upstream (n = 26) or downstream (n = 200) of the glutamine-rich region, and carrying variants located upstream (n = 109) or downstream (n = 117) of the middle of the mutational hotspot, from the present series and the literature.
| Variable | Variant location | ||||||
|---|---|---|---|---|---|---|---|
| Upstream or into the glutamin-rich domain region | Downstream of the glutamin-rich domain region | p value | Upstream of the middle of the mutational hotspot | Downstream of the middle of the mutational hotspot | p value | ||
| Sex | Male (%) | 13/25 (52.0) | 99/193 (51.3) | 0.95 | 61/105 (58.1) | 51/113 (45.1) | 0.056 |
| Female (%) | 12/25 (48.0) | 94/193 (48.7) | 44/105 (41.9) | 62/113 (54.9) | |||
| Age | Available data | 20/26 | 99/200 | 0.033* | 70/109 | 49/117 | 0.36 |
| Median [Q1;Q3] | 30.50 [4.000;44.00] | 8.000 [2.670;28.00] | 6.900 [2.250;35.00] | 13.00 [5.000;31.00] | |||
| Clinical features (%) | Deafness | 11/24 (45.8) | 130/193 (67.4) | 0.037* | 80/107 (74.8) | 61/110 (55.5) | 0.003* |
| Dysplastic ear | 14/24 (58.3) | 162/190 (85.3) | 0.003* | 88/105 (83.8) | 88/109 (80.7) | 0.56 | |
| Thumb malformation | 13/26 (50.0) | 146/196 (74.5) | 0.009* | 81/109 (74.3) | 78/113 (69.0) | 0.38 | |
| Malformation of lower limb | 11/25 (44.0) | 94/189 (49.7) | 0.59 | 62/107 (57.9) | 43/107 (40.2) | 0.009* | |
| Ano-rectal malformation | 15/24 (62.5) | 127/191 (66.5) | 0.70 | 71/106 (67.0) | 71/109 (65.1) | 0.78 | |
| Kidney malformation | 6/17 (35.3) | 88/188 (46.8) | 0.36 | 48/99 (48.5) | 46/106 (43.4) | 0.46 | |
| Chronic renal failure | 12/24 (50.0) | 55/188 (29.3) | 0.040* | 39/105 (37.1) | 28/107 (26.2) | 0.086 | |
| Genital anomalies | 2/26 (7.7) | 30/191 (15.7) | 0.38 | 23/108 (21.3) | 9/109 (8.3) | 0.007* | |
| Congenital heart defect | 1/18 (5.6) | 39/188 (20.7) | 0.21 | 24/99 (24.2) | 16/107 (15.0) | 0.092 | |
| Endocrine feature | 7/23 (30.4) | 29/190 (15.3) | 0.079 | 18/106 (17.0) | 18/107 (16.8) | 0.975 | |
Bold values indicate significant results.
*p < 0,05.
Q1;Q3, first and third interquartile; SD, standard deviation.
Additionally, we conducted a statistical analysis based on the mutational hotspot, comparing variants located upstream (n = 109) and downstream (n = 117) of the middle of the mutational hotspot (Table 2). We found a significant increase in deafness (p = 0.003), lower limb malformations (p = 0.009) and genital anomalies (p = 0,007) (sex ratio with p = 0.056) in individuals carrying a variant located upstream of c.1164.
Death occurred at 3 months in a patient (F18P35) with a severe phenotype associating complex congenital heart defect (pulmonary atresia, ventricular septal defect, major aortopulmonary collateral arteries, tetralogy of Fallot), unilateral kidney agenesis and multiple cystic changes in both anterior horns of the lateral ventricles with mild ventriculomegaly. She carried the recurrent variant c.826 C > T,p.(Arg276*) inherited from her mother. The phenotype associated with this variant is known to be more severe due to an increased frequency of congenital heart defects [21], estimated here at 46.2% (12/26), whereas only 15.6% (28/180) of individuals carrying other genotype (excluding SALL1 deletions) were affected. Notably, this is the only variant associated with tetralogy of Fallot in 3 patients reported in the literature, one of whom also died [21, 27].
A total of 22 individuals were found to carry a SALL1 deletion without any other OMIM-morbid gene(s) involved (including 9 patients from 1 family in our series), while 6 individuals carried a larger deletion. When the deletion only involved SALL1 as OMIM-morbid gene, the proportions of all variables appeared to be decreased compared to individuals who carried a SALL1 variant (excluding the recurrent variant c.826 C > T,p.(Arg276*) associated to a more severe phenotype), except for DD/ID which affected 6/20 (30%) patients.
Discussion
We present a novel series of TBS involving 49 patients from 22 families, and compared their phenotypes to the previously reported patients. Whereas we observed similar complete penetrance and highly variable expressivity [2, 4–6, 8, 11] in both groups, the clinical triad was found in only 25% of our series compared with 49.7% of patients from the literature. Yan et al. [28] demonstrated that patients with typical-TBS are more prevalent in the second generation than in the first, possibly due to a mosaic state in the first generation. However, we do not support this observation because when evidence of de novo occurrence of a SALL1 variant was provided (parental genetic analysis performed), the index case presented the characteristic clinical triad in our series. Furthermore, the pedigrees of the four families shown in Figure 2 suggest a highly variable intra-familial expressivity, rather than a generational effect possibly linked to somatic mosaicism in the first affected generation.
Besides, renal failure was more common in our series, perhaps due to an older age compared to the literature data and/or a better follow-up due to a better knowledge of the TBS course. Chronic renal failure is observed at any age, mostly in young adults but also in neonates. Whereas hypo-dysplastic kidneys are often associated, we noted, in 3 patients of our series, the occurrence of chronic renal failure without obvious kidney malformation, suggesting that a renal follow-up throughout the life is essential for TBS patients. SALL1 variants have also been reported in patients with isolated kidney disease, highlighting the wide variability in the expression of SALL1-associated phenotypes [29].
Deafness associated with TBS appears to be mostly post lingual, sensorineural, bilateral, mild to moderate. The course was sometimes progressive. CT scan are more often normal but, as we noted incus and external auditory canal anomalies in 2 patients, Yan et al. recently reported malformations in the middle ear and enlargement of the vestibular system in one patient [28].
Cognitive impairment was reported in 20/215 (9%) of documented TBS patients. Unfortunately, the outcomes for most patients affected by DD and the intellectual quotient were rarely available. As Sall1 has been implicated in neurogenesis in mice [30], this modest increase in DD/ID risk suggests that other factors are involved in the cognitive phenotype of TBS patients. Fifty-seven percent of patients in our series have prenatal ultrasounds findings, with intrauterine growth retardation, renal, limb and cardiac malformations. With the improvement of ultrasound scans and the increased use of prenatal genetic analyses [31], family counselling in relation to the prognosis becomes central. In this respect, it is essential to precise the outcome of TBS patients, which is still poorly known. Further prospective studies are therefore needed, in particular to clarify the neurodevelopmental trajectory of TBS patients, such as large-scale genetic analysis (exome or genome sequencing), since a significant proportion of patients may have a double genetic diagnosis [32, 33].
Regarding genotype-phenotype correlations, we hypothesized that the ability of the truncated protein to dimerize through the conservation of the glutamine-rich region may cause a more severe phenotype. This could be due to the escape from the nonsense-mediated mRNA decay process and a dominant negative effect. Here, we found a significantly higher prevalence of deafness, dysplastic ear and thumb malformations when the SALL1 variant is located downstream of the glutamine-rich region in patients who were significantly younger (more missing data on age in this group), whereas deafness occurs with age. Kidney malformations seem to be more frequent also but without reaching statistical significance. Since the glutamine-rich region seems to play a role in the dimerization of Sal proteins, the above features could be partly due to a dominant negative effect, as suggested by other authors [12, 17]. Furthermore, Kiefer et al. showed that the truncated Sall1 protein is sufficient to cause preaxial polydactyly and triphalangeal thumb in transgenic mice (expression under the control of a limb-specific promoter), with this phenotype being more likely to manifest when a wild-type Sall1 is co-expressed [16]. Besides, the prevalence of renal failure is significantly lower when the variant is located downstream of the glutamine-rich region. Importantly, patients were significantly younger and there were more missing data on age in this group, which could lead to a confounding bias as renal failure occurs with age. Nevertheless, some data support the hypothesis that a stable truncated Sall1 protein, which is able to dimerize, may also have a partial function in kidneys later in life. Sall1 is required during development [15, 34], but its expression is also reactivated after kidney injury [35]. Interestingly, Hirsch et al. observed that a mouse model expressing a truncated mutant of Sall1, containing the glutamine-rich region, is less susceptible to acute kidney injury than wild-type and knock-out Sall1 mice [36]. This phenomenon could explain the better renal function in TBS patients with a SALL1 variant located downstream of the glutamine-rich domain, expressing a more stable truncated Sall1 protein possibly capable of dimerization. We also performed a statistical analysis between two groups based on the localization of the variant in the mutational hotspot (upstream or downstream of the middle of the mutational hotspot, c.1164). As reported in the previous statistical analysis, we found a significant increase in the frequency of deafness in individuals carrying a variant located upstream of c.1164, suggesting that the pathophysiology of deafness may be related to nucleotide positions from c.751 to c.1164. We also confirmed that the recurrent p.(Arg276*) variant is associated with a more frequent congenital heart defect, including tetralogy of Fallot [21, 27]. In our series, this specific malformation had only been observed with this recurrent variant (family 18). Besides, Innoceta et al. mention a correlation between haploinsufficiency (i.e. intragenic deletion or large deletion involving only the SALL1 gene, n = 10) and a milder phenotype, except for DD/ID. They compared patients carrying deletion to GeneReviews data [2], and patients carrying the recurrent p.(Arg276*) variant (n = 20). In this previous paper, a significant difference was seen only for dysplastic ears and congenital heart defects when compared to patients carrying the p.(Arg276*) variant, possibly due to lack of statistical power [20]. Thus, these previous and current genotype-phenotype correlations do not appear to be robust enough to be used in patient follow-up, especially as there is considerable intra-family variability (Fig. 2), but remain interesting for the study of TBS pathophysiology. Taken together these results suggest that SALL1 variants could cause TBS either by affecting the ability of SALL1 to interact with SALL proteins and DNA through a resistance to nonsense-mediated decay and a dominant negative effect (truncating variants located downstream of the glutamine-rich domain), or through a dosage effect (deletion or truncating variants located upstream of the glutamine-rich domain).
To date, there is no international consensus on the management of TBS. With regard to the data collected in the literature review, our French multidisciplinary working group proposes an initial evaluation and follow-up of TBS patients regardless of the SALL1 variant identified [37] (Table 3). Of note, in addition to the annual audiological and renal function monitoring previously recommended by J. Kohlhase [2], we advise annual monitoring of thyroid function, the usefulness of which will need to be reassessed.
Table 3.
Proposal for initial assessment and follow-up in patients with TBS.
| Concern | Initial assessment | Follow-up |
|---|---|---|
| Constitutional | • Psychomotor development, measurement of growth parameters | • Classical follow-up: psychomotor development, measurement of growth parameters, etc |
| Genetics |
• Personal and familial past medical history • Clinical examination: features suggestive of TBS and differential diagnoses • Genetic analysis |
• Genetic counselling according to patient and physician expectations • No systematic follow-up |
| Orthopaedic |
• Clinical examination: thumb malformation and clubfeet, mobility and joint stability • Hand X-rays |
• According to initial assessment • No systematic follow-up |
| Hearing |
• Audiological evaluation • +/− Ear CT-scan and/or MRI if deafness |
• According to initial assessment • Systematic: annual audiological evaluation |
| Gastro-intestinal | • Clinical examination: anorectal malformation |
• According to initial assessment • No systematic follow-up |
| Renal |
• Clinical examination: urinary functional signs, measurement of growth parameters and blood pressure • Blood tests: creatininemia, estimation of glomerular filtration rate +/− complementary cystanine C • Urine tests: proteinuria, creatinuria • Renal ultrasound • +/− Cystography if suspected uropathy • +/− Scintigraphy or uro-MRI |
• According to initial assessment • Systematic: - Annual: measurement of growth parameters and blood pressure, blood tests (creatininemia, estimation of glomerular filtration rate), urine tests (proteinuria, microalbuminuria, creatinuria) - Every two years: renal ultrasound |
| Cardiac |
• Clinical examination: signs of cardiac insufficiency, heart auscultation • Heart ultrasound • +/− ECG, pulmonary X-rays |
• According to initial assessment • No systematic follow-up |
| Endocrine |
• Clinical examination: measurement of growth parameters, external genitalia • Blood tests: TSH • +/− Neonatal and kinetics during mini-puberty hormonal assessments, SRY, karyotype if bilateral cryptorchidism or posterior hypospadias |
• According to initial assessment • Systematic: - Clinical examination: measurement of growth parameters, hypothyroidism symptoms - Blood tests: TSH annually |
| Eyes | • Ophthalmologic assessment: visual behavior/acuity, refraction examination under cycloplegia, slit lamp and fundus examination, study of oculomotor skills |
• According to initial assessment • No systematic follow-up |
From Protocole National de Diagnostic et de Soins-Syndrome de Townes-Brocks, Haute Autorité de Santé [38].
CT-scan, Computed Tomography; MRI, Magnetic Resonance Imaging; TBS, Townes-Brocks syndrome.
The proportion of patients with typical TBS but no molecular diagnosis has not been studied here as there are no consensus criteria in the literature for assessing clinical diagnosis. The literature reports a proportion of 75% of molecular diagnoses in typical TBS patients (classic triad) [2]. This gap may be explained by differential diagnoses such as Duane-radial ray syndrome (MIM #607323), branchio-oto-renal syndrome (MIM #113650) or VACTERL association (MIM #192350). Besides, DACT1 variants have been associated with a phenotype known as Townes-Brocks syndrome 2 (MIM #617466), which causes mostly congenital anomalies of the kidney and urinary tract, possibly associated with skeletal, anorectal and genital anomalies [38, 39]. In addition, alteration of SALL1 regulatory elements may be involved in molecular negative cases. In support of this hypothesis, C.A. Stevens et al. [40] found a deletion upstream of SALL1 in a propositus with anorectal malformation, congenital anomalies of the kidney and urinary tract, broad thumbs and hearing loss compatible with TBS. Genome sequencing and functional studies should allow to elucidate non-coding molecular alterations and increase the diagnostic yield in TBS.
In conclusion, we found a genotype-phenotype correlation according to the variant location relative to the glutamine-rich region, involved in the dimerization of Sal proteins, for features that may appear at any age, such as deafness, and, with the bias of a significant difference in age and missing data, renal failure. In the future, functional and prospective studies are needed to elucidate the pathophysiological mechanisms and the outcomes of TBS in order to better monitor patients and treat them as early as possible to improve prognosis.
Supplementary information
Supplementary Table S1: Clinical and molecular characteristics of 49 TBS patients from the present series.
Supplementary Table S2: Review of TBS patients with DD and/or ID from our series and the literature.
Supplementary Table S3: Clinical and molecular characteristics of 207 TBS patients from the literature.
Acknowledgements
The authors would like to thank the patients and their families who participated in the study. The authors thank the French Rare diseases organizations AnDDI-Rares and SENSGENE, European Rare Networks Ithaca and CRANIO, and the patients’ organization TINTAMARRE for their data sharing initiatives. The authors thank the editorial and reading committee for the national diagnosis and care protocol: Olivia DOMANSKI, Vasily SMIRNOV, Laetitia CLABAUT, Véronique DEBARGE, Charlotte CELERIER, Franck-Noël FITOUSSI, Laurence HEIDET, Célia CRETOLLE, Marielle ISTER, Nathalie QUINTERO, Stéphanie MOUMEIN, Sandrine MARLIN, and Célia CARDOSO.
Author contributions
F.L. and C.V. designed the work and analyzed the data. F.L., P.B., F.E., L.B., S.M.H., C.V.D., F.P., and C.V. contributed to data acquisition. Rare Disease networks gathers the clinical and biological geneticists who provided additional clinical and biological data about their TBS patients. NR performed the statistical analysis. L.G., C.L., A.M., C.S., R.S., P.T., F.L., FP., P.B., C.V.D., L.B. and C.V. contributed to the redaction of clinical guidelines for T.B.S. F.L. wrote the first draft of the manuscript. C.V., P.B., F.P., N.R. and S.N. revised the manuscript. All authors read and approved the final version on the manuscript.
Funding
This study received no specific funding. Open access funding provided by Centre Hospitalier Universitaire de Lille.
Data availability
Molecular data have been submitted to the ClinVar database (accessions from SCV005880107 to SCV005880127). The datasets generated and analyzed in the current study are available from the corresponding author upon reasonable request.
Competing interests
The authors declare no competing interests.
Ethical approval
This is an observational, multicenter, medical data study that does not require ethical approval. In accordance with the French law, a non-opposition form for data collection was obtained from each patient and/or their parents for minors. An informed consent for photography publication was obtained from patient and/or their parents for minor. The data collection design was approved by the data protection officer of the University Hospital of Lille.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
A list of authors and their affiliations appears at the end of the paper.
Contributor Information
Fiona Leduc, Email: fiona.leduc@chu-lille.fr.
Rare Disease networks:
Catheline Vilain, Sebastian Neuens, Julie Soblet, Elise Schaefer, Olivia Boyer, Radka Stoeva, Alissandre Lecordier, Mathilde Nizon, Bertrand Isidor, Solène Conrad, Laëtitia Lambert, Mélanie Berard-Cloteau, Maria K Haanpää, Minna Toivonen, Sahar Mansour, Mohamed Wafik, Shereen Tadros, Abid Sharif, Lewis Darnell, Khaoula Zaafrane-Khachnaoui, Lucile Riera-Navarro, Fanny Morice-Picard, Klaus Dieterich, Alicia Coudert, Sophie Blesson, Anne-Marie Guerrot, Sacha Weber, Kara Ranguin, Sabine Sigaudy, Olga Glazunova, Geneviève Baujat, Sarah Grotto, Sébastien Moutton, Audrey Putoux, and Hélène Vallin
Supplementary information
The online version contains supplementary material available at 10.1038/s41431-025-01855-4.
References
- 1.Townes PL, Brocks ER. Hereditary syndrome of imperforate anus with hand, foot, and ear anomalies. J Pediatr. 1972;81:321–6. [DOI] [PubMed] [Google Scholar]
- 2.Kohlhase J. Townes-Brocks Syndrome. In: Adam MP, Everman DB, Mirzaa GM, Pagon RA, Wallace SE, Bean LJ, et al., editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993 [cited 2023 Jan 14]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK1445/.
- 3.Miller EM, Hopkin R, Bao L, Ware SM. Implications for genotype-phenotype predictions in Townes-Brocks syndrome: case report of a novel SALL1 deletion and review of the literature. Am J Med Genet A 2012;158A:533–40. [DOI] [PubMed] [Google Scholar]
- 4.Botzenhart EM, Bartalini G, Blair E, Brady AF, Elmslie F, Chong KL, et al. Townes-Brocks syndrome: twenty novel SALL1 mutations in sporadic and familial cases and refinement of the SALL1 hot spot region. Hum Mutat. 2007;28:204–5. [DOI] [PubMed] [Google Scholar]
- 5.Botzenhart EM, Green A, Ilyina H, König R, Lowry RB, Lo IFM, et al. SALL1 mutation analysis in Townes-Brocks syndrome: twelve novel mutations and expansion of the phenotype. Hum Mutat. 2005;26:282. [DOI] [PubMed] [Google Scholar]
- 6.Marlin S, Blanchard S, Slim R, Lacombe D, Denoyelle F, Alessandri JL, et al. Townes-Brocks syndrome: detection of a SALL1 mutation hot spot and evidence for a position effect in one patient. Hum Mutat. 1999;14:377–86. [DOI] [PubMed] [Google Scholar]
- 7.Kosaki R, Fujimaru R, Samejima H, Yamada H, Izumi K, Iijima K, et al. Wide phenotypic variations within a family with SALL1 mutations: Isolated external ear abnormalities to Goldenhar syndrome. Am J Med Genet A 2007;143A:1087–90. [DOI] [PubMed] [Google Scholar]
- 8.Sudo Y, Numakura C, Abe A, Aiba S, Matsunaga A, Hayasaka K. Phenotypic variability in a family with Townes-Brocks syndrome. J Hum Genet. 2010;55:550–1. [DOI] [PubMed] [Google Scholar]
- 9.Beaudoux O, Lebre AS, Doco Fenzy M, Spodenkiewicz M, Canivet E, Colosio C, et al. Adult diagnosis of Townes-Brocks syndrome with renal failure: Two related cases and review of literature. Am J Med Genet A 2021;185:937–44. [DOI] [PubMed] [Google Scholar]
- 10.Faguer S, Pillet A, Chassaing N, Merhenberger M, Bernadet-Monrozies P, Guitard J, et al. Nephropathy in Townes-Brocks syndrome (SALL1 mutation): imaging and pathological findings in adulthood. Nephrol Dial Transpl Publ Eur Dial Transpl Assoc - Eur Ren Assoc. 2009;24:1341–5. [DOI] [PubMed] [Google Scholar]
- 11.Kohlhase J, Wischermann A, Reichenbach H, Froster U, Engel W. Mutations in the SALL1 putative transcription factor gene cause Townes-Brocks syndrome. Nat Genet. 1998;18:81–3. [DOI] [PubMed] [Google Scholar]
- 12.Sweetman D, Smith T, Farrell ER, Chantry A, Munsterberg A. The conserved glutamine-rich region of chick csal1 and csal3 mediates protein interactions with other spalt family members. Implications for Townes-Brocks syndrome. J Biol Chem. 2003;278:6560–6. [DOI] [PubMed] [Google Scholar]
- 13.de Celis JF, Barrio R. Regulation and function of Spalt proteins during animal development. Int J Dev Biol. 2009;53:1385–98. [DOI] [PubMed] [Google Scholar]
- 14.Kohlhase J, Schuh R, Dowe G, Kühnlein RP, Jäckle H, Schroeder B, et al. Isolation, Characterization, and Organ-Specific Expression of Two Novel Human Zinc Finger Genes Related to theDrosophilaGenespalt. Genomics 1996;38:291–8. [DOI] [PubMed] [Google Scholar]
- 15.Sweetman D, Münsterberg A. The vertebrate spalt genes in development and disease. Dev Biol. 2006;293:285–93. [DOI] [PubMed] [Google Scholar]
- 16.Kiefer SM, Robbins L, Barina A, Zhang Z, Rauchman M. SALL1 truncated protein expression in Townes-Brocks syndrome leads to ectopic expression of downstream genes. Hum Mutat. 2008;29:1133–40. [DOI] [PubMed] [Google Scholar]
- 17.Kiefer S, Ohlemiller K, Yang J, McDill B, Kohlhase J, Rauchman M. Expression of a truncated Sall1 transcriptional repressor is responsible for Townes-Brocks syndrome birth defects. Hum Mol Genet. 2003;12:2221–7. [DOI] [PubMed] [Google Scholar]
- 18.Nishinakamura R, Matsumoto Y, Nakao K, Nakamura K, Sato A, Copeland NG, et al. Murine homolog of SALL1 is essential for ureteric bud invasion in kidney development. Dev Camb Engl. 2001;128:3105–15. [DOI] [PubMed] [Google Scholar]
- 19.Furniss D, Critchley P, Giele H, Wilkie AOM. Nonsense-mediated decay and the molecular pathogenesis of mutations in SALL1 and GLI3. Am J Med Genet A 2007;143A:3150–60. [DOI] [PubMed] [Google Scholar]
- 20.Innoceta AM, Olivucci G, Parmeggiani G, Scarano E, Pragliola A, Graziano C. Chromosomal Microarray Analysis Identifies a Novel SALL1 Deletion, Supporting the Association of Haploinsufficiency with a Mild Phenotype of Townes–Brocks Syndrome. Genes 2023;14:258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kohlhase J, Liebers M, Backe J, Baumann-Müller A, Bembea M, Destrée A, et al. High incidence of the R276X SALL1 mutation in sporadic but not familial Townes-Brocks syndrome and report of the first familial case. J Med Genet. 2003;40:e127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Z, Sun Z, Diao Y, Wang Z, Yang X, Jiang B, et al. Identification of two novel SALL1 mutations in chinese families with townes-brocks syndrome and literature review. Orphanet J Rare Dis. 2023;18:250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med J Am Coll Med Genet. 2015;17:405–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jourdain AS, Petit F, Odou MF, Balduyck M, Brunelle P, Dufour W, et al. Multiplex targeted high-throughput sequencing in a series of 352 patients with congenital limb malformations. Hum Mutat. 2020;41:222–39. [DOI] [PubMed] [Google Scholar]
- 25.Lugli L, Rossi C, Ceccarelli PL, Calabrese O, Bedetti L, Miselli F, et al. Townes-Brocks syndrome with craniosynostosis in two siblings. Eur J Med Genet. 2022;65:104642. [DOI] [PubMed] [Google Scholar]
- 26.Liberalesso PBN, Cordeiro ML, Karuta SCV, Koladicz KRJ, Nitsche A, Zeigelboim BS, et al. Phenotypic and genotypic aspects of Townes-Brock syndrome: case report of patient in southern Brazil with a new SALL1 hotspot region nonsense mutation. BMC Med Genet. 2017;18:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ilhan O, Gumus E, Hakan N, Istar H, Harmandar B, Olgun H, et al. A Genotyped Case of Townes–Brocks Syndrome with Absent Pulmonary Valve Syndrome from Turkey. J Pediatr Genet [Internet]. 2021 Dec 10 [cited 2023 Jan 27]; Available from: http://www.thieme-connect.de/DOI/DOI?10.1055/s-0041-1740371. [DOI] [PMC free article] [PubMed]
- 28.Yan X, Wang J, Yang W, Li L, Shen T, Geng J, et al. Molecular diagnosis, clinical evaluation and phenotypic spectrum of Townes-Brocks syndrome: insights from a large Chinese hearing loss cohort. J Med Genet. 2024;61:459–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stein Q, Vostrizansky A, Magay Y, Jandeska S, Westemeyer M, Hendricks E, et al. Townes-Brocks Syndrome Revealed by Kidney Gene Panel Testing. Kidney Int Rep. 2024;9:1810–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harrison SJ, Nishinakamura R, Jones KR, Monaghan AP. Sall1 regulates cortical neurogenesis and laminar fate specification in mice: implications for neural abnormalities in Townes-Brocks syndrome. Dis Model Mech. 2012;5:351–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tran Mau-Them F, Delanne J, Denommé-Pichon AS, Safraou H, Bruel AL, Vitobello A, et al. Prenatal diagnosis by trio exome sequencing in fetuses with ultrasound anomalies: A powerful diagnostic tool. Front Genet. 2023;14:1099995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Monies D, Abouelhoda M, AlSayed M, Alhassnan Z, Alotaibi M, Kayyali H, et al. The landscape of genetic diseases in Saudi Arabia based on the first 1000 diagnostic panels and exomes. Hum Genet. 2017;136:921–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meng L, Pammi M, Saronwala A, Magoulas P, Ghazi AR, Vetrini F, et al. Use of Exome Sequencing for Infants in Intensive Care Units: Ascertainment of Severe Single-Gene Disorders and Effect on Medical Management. JAMA Pediatr. 2017;171:e173438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Basta JM, Robbins L, Denner DR, Kolar GR, Rauchman M. A Sall1-NuRD interaction regulates multipotent nephron progenitors and is required for loop of Henle formation. Development 2017;144:3080–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hosoe-Nagai Y, Hidaka T, Sonoda A, Sasaki Y, Yamamoto-Nonaka K, Seki T, et al. Re-expression of Sall1 in podocytes protects against adriamycin-induced nephrosis. Lab Investig J Tech Methods Pathol. 2017;97:1306–20. [DOI] [PubMed] [Google Scholar]
- 36.Hirsch S, El-Achkar T, Robbins L, Basta J, Heitmeier M, Nishinakamura R, et al. A mouse model of Townes-Brocks syndrome expressing a truncated mutant Sall1 protein is protected from acute kidney injury. Am J Physiol-Ren Physiol. 2015;309:F852–63. [DOI] [PubMed] [Google Scholar]
- 37.Haute Autorité de Santé [Internet]. [cited 2024 Mar 30]. Syndrome de Townes – Brocks. Available from: https://www.has-sante.fr/jcms/p_3493442/fr/syndrome-de-townes-brocks.
- 38.Webb BD, Metikala S, Wheeler PG, Sherpa MD, Houten SM, Horb ME, et al. Heterozygous Pathogenic Variant in DACT1 Causes an Autosomal-Dominant Syndrome with Features Overlapping Townes-Brocks Syndrome. Hum Mutat. 2017;38:373–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Christians A, Kesdiren E, Hennies I, Hofmann A, Trowe MO, Brand F, et al. Heterozygous variants in the DVL2 interaction region of DACT1 cause CAKUT and features of Townes-Brocks syndrome 2. Hum Genet. 2023;142:73–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stevens CA, May KM. Deletion upstream of SALL1 producing Townes-Brocks syndrome. Am J Med Genet A 2016;170:2476–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table S1: Clinical and molecular characteristics of 49 TBS patients from the present series.
Supplementary Table S2: Review of TBS patients with DD and/or ID from our series and the literature.
Supplementary Table S3: Clinical and molecular characteristics of 207 TBS patients from the literature.
Data Availability Statement
Molecular data have been submitted to the ClinVar database (accessions from SCV005880107 to SCV005880127). The datasets generated and analyzed in the current study are available from the corresponding author upon reasonable request.