Abstract
Heat stress is a major environmental factor that impairs male fertility by disrupting hormonal regulation, increasing oxidative stress, and inducing germ cell apoptosis. Niacin (vitamin B3) has antioxidant and thermoregulatory properties that may counteract these effects. This study investigated whether oral niacin could protect against chronic heat stress–induced reproductive damage in adult BALB/c male mice. Mice were randomly assigned to Control, Heat Control, and two heat-stressed groups receiving niacin at 100 or 200 mg/kg/day for 30 days, with heat stress applied daily at 36 ± 2 °C for 4 h. Heat stress reduced body weight gain, testis and accessory gland weights, sperm count and motility, acrosome and DNA integrity, testosterone and thyroid hormones, and increased lipid peroxidation and pro-apoptotic gene expression. Niacin supplementation dose-dependently mitigated these effects: total sperm count increased from 9.8 × 105 ± 1.85 × 105 to 3.66 × 106 ± 4.62 × 105, total motility from 49.0 ± 13.6% to 81.7 ± 5.8%, testosterone from 0.89 ± 0.21 to 4.44 ± 0.70 ng/mL, and Bcl2 expression increased 2.7-fold. Fertility improved, with pregnancy risk rising from 50% to 100%, litter size from 3.7 ± 0.3 to 7.2 ± 0.3 pups, and blastocyst formation from 37.8 ± 2.7% to 68.0 ± 2.6%. Niacin also normalized stress-related gene expression, preserved testicular histology, and did not affect liver enzyme activities, highlighting its potential as a dietary supplement to protect against heat-induced male reproductive dysfunction.
Keywords: Niacin, Heat stress, Male fertility, Oxidative stress, Sperm quality, Thermoregulation
Subject terms: Biochemistry, Cell biology, Endocrinology, Physiology, Zoology
Introduction
Heat stress (HS) is a well-documented environmental stressor that negatively impacts male reproductive health in both humans and animal models1. Optimal spermatogenesis requires testicular temperatures 2–4 °C below core body temperature; elevated scrotal temperature leads to reduced sperm count, increased DNA damage, and impaired fertility2,3. Even moderate heat exposure in rodents induces testicular degeneration, germ cell apoptosis, and reduced fertilization success4,5. These effects make rodents a valuable model for studying mechanisms of reproductive impairment relevant to humans and domestic animals6.
The detrimental impact of HS is mediated by multiple mechanisms, with oxidative stress playing a central role. Elevated levels of reactive oxygen species (ROS) damage lipids, proteins, and nucleic acids in germ and Sertoli cells7. Heat stress directly injures testicular tissue and suppresses testosterone synthesis, as shown in pervious research8. Chronic exposure also increases aromatase activity in Leydig cells, shifting the hormonal balance toward estradiol and impairing spermatogenesis9. Key steroidogenic enzymes are downregulated by HS, further disrupting testosterone biosynthesis10.
Endocrine disruption extends beyond the testes. Animal studies, including in cattle, demonstrate that HS reduces circulating triiodothyronine (T3) and thyroxine (T4), indicating thyroid dysfunction under thermal challenge11. Antioxidant intervention studies further support that oxidative stress disrupts endocrine balance12,13. Oxidative stress impairs the hypothalamic–pituitary–thyroid (HPT) axis, leading to decreased T3 and T4 levels. Reduced T3 downregulates steroidogenic acute regulatory (StAR) protein expression in Leydig cells, further compounding testosterone deficiency14.
At the cellular level, HS induces heat shock proteins (e.g., HSP70), activates pro-apoptotic genes (Bax, Caspase-3), and downregulates gap junction proteins such as Connexin 43 (Cx43), collectively impairing testicular integrity and sperm production15. Cx43, expressed in Sertoli and Leydig cells, is essential for intercellular communication during spermatogenesis16. Studies in ruminants show that HS decreases Cx43 mRNA and protein expression, disrupting gap junctional communication, increasing germ cell apoptosis, and impairing sperm development17.
Given the central role of oxidative stress in HS pathology, numerous antioxidant therapies have been investigated. Resveratrol protects rodent testes against HS by restoring antioxidant enzyme activity, reducing apoptosis, improving sperm quality, and normalizing testosterone18. Melatonin decreases oxidative stress, prevents germ cell apoptosis, and preserves testicular function in heat-exposed mice19. Vitamin E, thymoquinone, and selenium enhance antioxidant defenses and preserve sperm viability under subacute HS20,21. Similarly, N-acetylcysteine and quercetin reduce oxidative stress and improve sperm function22–25, while curcumin alleviates spermatogenic disorders in a dose-dependent manner26. Natural extracts such as Nigella sativa and green tea (Camellia sinensis) also improve sperm parameters, oxidative biomarkers, and hormonal levels in heat-exposed mice27,28, while Carob (Ceratonia siliqua L.) fruit or Allium sativum hydro-alcoholic extracts alleviate oxidative stress–induced reproductive toxicity29,30.
Niacin (nicotinic acid) offers a unique dual action. It acts as a potent free radical scavenger and coenzyme precursor in NAD/NADP systems31, and it promotes peripheral vasodilation and skin flushing via prostaglandin release and TRPV1 (transient receptor potential vanilloid 1) channel activation. These mechanisms enhance cutaneous heat dissipation and may help lower body temperature during thermal stress32,33. Clinical studies also report mild hypothermic effects of niacin, especially at high doses or when combined with vasodilators33. While direct evidence for core body temperature reduction in systemic HS models is limited, this dual antioxidant and thermoregulatory potential distinguishes niacin from other compounds such as resveratrol and melatonin. Despite growing interest in niacin’s cardiovascular and metabolic effects, its role in male reproduction under chronic HS remains poorly understood.
Most previous studies have relied on short-term or localized testicular heating models, which fail to capture the full spermatogenic cycle (~ 35 days in mice) and overlook systemic effects of whole-body HS34. To address these limitations, we implemented a 30-day systemic HS protocol to mimic chronic environmental exposure.
Although antioxidants are generally considered safe, their use in reproductive studies warrants monitoring for dose-dependent toxicity. At high pharmacologic doses, niacin has been associated with hepatotoxicity in humans and animals35–37, underscoring the importance of evaluating hepatic enzymes (ALT, AST, ALP) during supplementation. This approach aligns with safety principles applied in studies of vitamin C, coenzyme Q10, and other antioxidants38.
In this study, we investigated whether oral niacin supplementation mitigates reproductive, molecular, and physiological consequences of long-term systemic HS in male mice. Specifically, we assessed testicular histology, sperm parameters, oxidative stress biomarkers (MDA, TAC, SOD), hormone levels (T3, T4, testosterone), stress- and apoptosis-related gene expression (Hspa1a/b, Bax, Bcl2, Caspase-3, Gja1), fertility outcomes, and hepatic safety. These findings will provide novel insights into niacin’s dual role as a thermoregulatory antioxidant and fertility-protective agent, highlighting its potential application against chronic heat stress–induced male reproductive dysfunction.
Results
Body weight
Table 1 presents a summary of the impact of oral niacin administration on the body weight of the mice across various study groups throughout the research. Initially, all subjects had the same body weights. On Days 15 and 30, mice in the Niacin 2 group exhibited a significant higher weight gain compared to the Control group; however, these differences were not statistically significant when compared to the other groups in the study. Over the study, the Control group exhibited no change in body weight, whereas all other groups experienced a significant increase during the initial half of the experiment.
Table 1.
Effects of oral niacin administration on the body weight.
| Study groups | ||||
|---|---|---|---|---|
| Control | Heat Control | Niacin 1 | Niacin 2 | |
| BW1 | 30.31 ± 0.55 Aa | 29.86 ± 0.60 Aa | 30.24 ± 0.54 Aa | 29.89 ± 0.51 Aa |
| BW15 | 30.33 ± 0.80 Aa | 33.20 ± 1.59 Bab | 33.17 ± 0.87 Bab | 34.00 ± 1.46 Bb |
| BW30 | 30.65 ± 0.72 Aa | 33.78 ± 1.60 Bab | 34.40 ± 1.14 Bb | 34.42 ± 1.39 Bb |
A two-way repeated-measures ANOVA was employed to compare various study groups across three distinct time points. To analyze the simple effects related to time and study groups, pairwise comparisons were performed using LSD confidence interval adjustments. Results are expressed as means ± SEM (g) from 15–20 animals per group. Superscript lowercase letters denote statistically significant differences (P ≤ 0.05) within rows, while uppercase letters indicate significant differences across columns. BW1, 15, and 30 refer to body weight measurements taken on specific study days.
Weights of the testis and combined accessory sex glands
Table 2 presents the impact of oral niacin supplementation on the weights of the testis and combined accessory sex glands in mice across various study groups throughout the research period. Oral niacin administration significantly affected testis (TW) and combined accessory sex glands (ASG) weights in male mice under heat stress. At day 15, testis weight in the Heat Control group (121.60 ± 7.35 mg) was not significantly different from that of the Control group (101.60 ± 7.74 mg; P > 0.05), although it was numerically higher. Both Niacin 1 (126.20 ± 5.93 mg) and Niacin 2 (126.00 ± 9.68 mg) groups showed further increases in testis weight compared to Control but were similar to the Heat Control group. By day 30, the Niacin 1 group exhibited the highest testis weight (137.71 ± 6.71 mg), significantly greater than Control (106.00 ± 6.67 mg), Heat Control (98.20 ± 8.88 mg), and Niacin 2 (111.29 ± 11.41 mg) groups (P ≤ 0.05). In contrast, testis weight in the Heat Control group declined compared to Control at this time point. For combined accessory sex glands, weights in the Heat Control group were significantly reduced at day 15 (115.00 ± 5.14 mg) and day 30 (114.00 ± 1.41 mg) compared to Control (137.20 ± 3.12 mg and 136.57 ± 2.89 mg, respectively; P ≤ 0.05). However, Niacin 1 (132.00 ± 2.55 mg at day 15 and 124.71 ± 3.53 mg at day 30) and Niacin 2 (130.00 ± 1.70 mg and 125.43 ± 3.49 mg, respectively) groups maintained accessory gland weights comparable to Control, demonstrating protection against heat-induced reductions.
Table 2.
Effects of oral niacin administration on testis (TW) and combined accessory sex glands weights (ASG).
| Study groups | ||||
|---|---|---|---|---|
| Control | Heat Control | Niacin 1 | Niacin 2 | |
| TW15 | 101.60 ± 7.74 Aa | 121.60 ± 7.35 Aac | 126.20 ± 5.93 Abc | 126.00 ± 9.68 Abc |
| TW30 | 106.00 ± 6.67 Aa | 98.20 ± 8.88 Ba | 137.71 ± 6.71 Abc | 111.29 ± 11.41 Aac |
| ASG15 | 137.20 ± 3.12 Aa | 115.00 ± 5.14 Ab | 132.00 ± 2.55 Aa | 130.00 ± 1.70 Aa |
| ASG30 | 136.57 ± 2.89 Aa | 114 ± 1.41 Ab | 124.71 ± 3.53 Aa | 125.43 ± 3.49 Aa |
A two-way repeated-measures ANOVA was employed to compare various study groups across three distinct time points. To analyze the simple effects related to time and study groups, pairwise comparisons were performed using LSD confidence interval adjustments. Results are expressed as Means ± SEM (mg). Superscript lowercase letters denote statistically significant differences (P ≤ 0.05) within rows, while uppercase letters indicate significant differences across columns. TW and ASG1, 15, and 30 refer to testis and combined accessory sex glands weight measurements taken on specific study days.
Daily water and feed intake
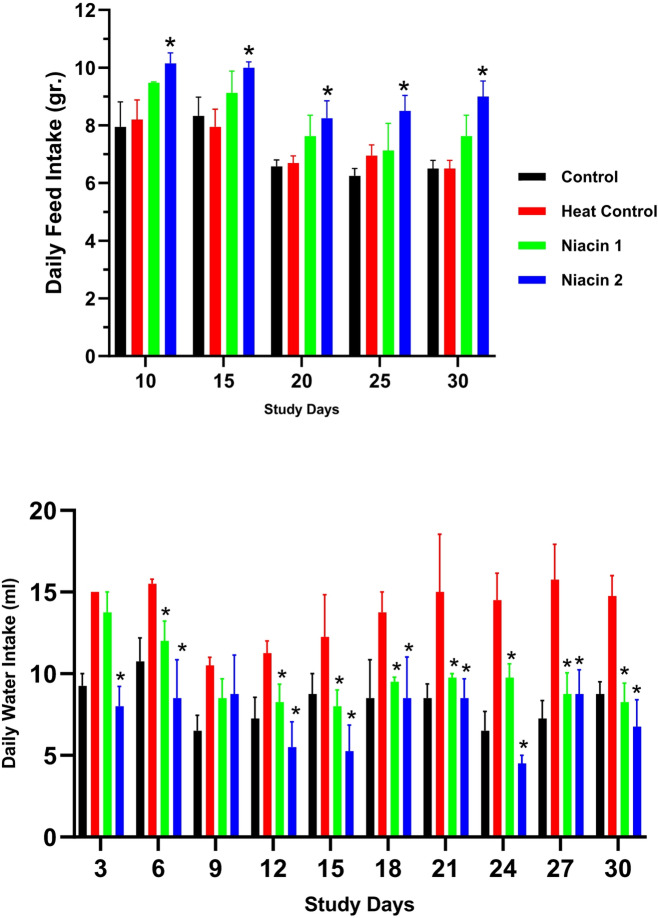
Figure 1 illustrates the effects of niacin supplementation on feed and water intake across different experimental groups during the study period. While heat stress did not significantly affect feed intake, high-dose niacin administration significantly enhanced feed consumption over time. Additionally, heat stress induced a marked increase in water intake; however, niacin supplementation at both dosage levels effectively reduced water consumption to levels comparable to those observed in the control group.
Fig. 1.
A one-way ANOVA followed by LSD confidence interval adjustments was employed to compare values between various study groups on each distinct time points. Asterisks denote statistically significant differences (P ≤ 0.05) between each niacin group and the heat stress group.
Rectal and surface body temperature
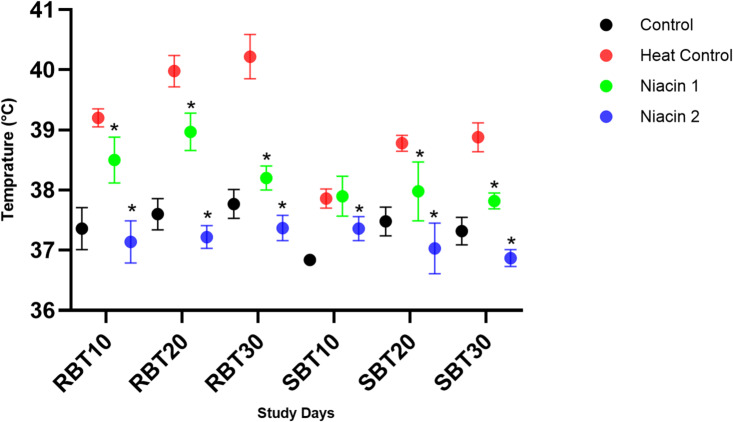
Figure 2 illustrates the effects of niacin supplementation on rectal and surface body temperatures across different experimental groups during the study period. The results indicate that exposure to heat stress significantly increased both rectal and surface body temperatures throughout the study. Surface body temperature values were generally lower than rectal measurements. Niacin administration at both supplementation levels significantly reduced body temperatures in a dose-dependent manner.
Fig. 2.
A one-way ANOVA followed by LSD confidence interval adjustments was employed to compare values between various study groups on each distinct time points. Asterisks denote statistically significant differences (P ≤ 0.05) between each niacin group and the heat stress group. RBT, Rectal Body Temperature; SB, Surface Body Temperature. 10, 20, and 30 indicate the days after the start of the heat stress period.
Andrology parameters
Total epidydimal sperm count
Table 3 presents the effects of oral niacin administration on total epididymal sperm count in male mice subjected to chronic heat stress. At both time points assessed (TES15 and TES30), the heat control group showed a marked reduction in sperm count compared to the untreated control group, confirming the detrimental impact of heat stress on spermatogenesis. In contrast, oral niacin supplementation resulted in significant improvements in total epididymal sperm counts, particularly in the Niacin 1 group (lower dose) at TES15, which showed the highest recovery (6.09 × 106 ± 1.41 × 106). By TES30, the Niacin 2 group (higher dose) showed the most notable improvement (3.66 × 106 ± 4.62 × 105), suggesting a time- and dose-dependent protective effect.
Table 3.
Effects of oral niacin administration on total epidydimal sperm count.
| Study groups | ||||
|---|---|---|---|---|
| Control | Heat Control | Niacin 1 | Niacin 2 | |
| TES15 | 3.12 × 106 ± 1.03 × 106 | 1.38 × 106 ± 1.98 × 105 | 6.09 × 106 ± 1.41 × 106 | 3.83 × 106 ± 4.89 × 105 |
| TES30 | 2.48 × 106 ± 2.43 × 105 | 9.80 × 105 ± 1.85 × 105 | 1.65 × 106 ± 2.92 × 105 | 3.66 × 106 ± 4.62 × 105 |
A two-way repeated-measures ANOVA was employed to compare various study groups across three distinct time points. To analyze the simple effects related to time and study groups, pairwise comparisons were performed using LSD confidence interval adjustments. Results are expressed as Means ± SEM (mg). Superscript lowercase letters denote statistically significant differences (P ≤ 0.05) within rows, while uppercase letters indicate significant differences across columns. TES 15, and 30 refer to the total epidydimal sperm counts on specific study days.
Epidydimal sperm motion kinematics
The Table 4 compares various sperm motility and kinematic parameters across four study groups over the course of the heat stress period. The data clearly show that heat stress significantly impairs sperm motility and kinematic parameters, as seen in the Heat Control group, where values for total motility (TM2: 49.03%), progressive motility (PM2: 14.43%), curvilinear velocity (VCL2: 44.64 μm/s), average path velocity (VAP2: 20.01 μm/s), and straight-line velocity (VSL2: 12.37 μm/s) were significantly lower than those in the Control group (TM2: 92.91%, PM2: 44.39%, VCL2: 109.70 μm/s, VAP2: 53.05 μm/s, VSL2: 37.51 μm/s). Supplementation with niacin, especially in the Niacin 1 group, substantially mitigated these negative effects; for instance, TM2 improved to 85.20%, PM2 to 33.35%, VCL2 to 91.98 μm/s, VAP2 to 42.48 μm/s, and VSL2 to 27.48 μm/s. In some cases, such as VCL1 (118.70 μm/s) and VAP1 (54.52 μm/s), Niacin 1 even exceeded the Control values, highlighting a strong restorative or even enhancing effect. The Niacin 2 group also showed notable improvements compared to Heat Control, though generally slightly less pronounced than Niacin 1. Other parameters like straightness (STR), linearity (LIN), and wobble (WOB) were moderately affected by heat stress but still showed partial recovery with niacin treatment. Overall, niacin supplementation effectively counteracts heat-induced impairments in sperm quality, with the higher dose (Niacin 1) yielding the most consistent and robust improvements across both motility and kinematic indices.
Table 4.
Effects of oral niacin administration on epidydimal sperm motion kinematics.
| Parameter | Unit | Study groups | |||
|---|---|---|---|---|---|
| Control | Heat control | Niacin 1 | Niacin 2 | ||
| TM1 | % | 87.62 ± 5.74 Aa | 72.21 ± 0.81 Ab | 87.88 ± 4.66 Aa | 87.09 ± 2.80 Aa |
| TM2 | % | 92.91 ± 4.28 Aa | 49.03 ± 13.63 Bb | 85.20 ± 2.54 Aa | 81.74 ± 5.80 Aa |
| PM1 | % | 36.89 ± 4.60 Aa | 21.97 ± 0.96 Abc | 34.73 ± 5.93 Aa | 29.39 ± 2.46 Aac |
| PM2 | % | 44.39 ± 7.80 Aa | 14.43 ± 5.99 Abc | 33.35 ± 2.44 Aa | 29.06 ± 5.04 Aac |
| VCL1 | µm/s | 86.41 ± 13.49 Aab | 70.83 ± 3.85 Ab | 118.70 ± 15.97 Aa | 94.05 ± 7.14 Aab |
| VCL2 | µm/s | 109.70 ± 13.00 Aa | 44.64 ± 20.00 Ab | 91.98 ± 8.93 Aab | 95.55 ± 13.90 Aab |
| VAP1 | µm/s | 41.70 ± 6.74 Aab | 30.84 ± 1.84 Ab | 54.52 ± 8.69 Aa | 41.02 ± 3.55 Aab |
| VAP2 | µm/s | 53.05 ± 6.92 Aa | 20.01 ± 9.12 Ab | 42.48 ± 4.16 Aab | 41.74 ± 6.90 Aab |
| VSL1 | µm/s | 27.10 ± 4.81 Aab | 19.06 ± 1.34 Ab | 36.07 ± 7.30 Aa | 25.21 ± 2.50 Aab |
| VSL2 | µm/s | 37.51 ± 6.15 Aa | 12.37 ± 6.05 Ab | 27.48 ± 3.10 Aab | 26.98 ± 5.49 Aab |
| STR1 | % | 64.31 ± 1.52 Aa | 61.68 ± 1.02 Aa | 64.38 ± 2.86 Aa | 61.25 ± 1.09 Aa |
| STR2 | % | 69.98 ± 3.10 Aa | 56.55 ± 5.10 Ab | 64.03 ± 1.23 Aab | 62.70 ± 2.45 Aab |
| LIN1 | % | 31.05 ± 2.30 Aa | 26.86 ± 0.85 Aab | 29.32 ± 2.06 Aab | 26.64 ± 0.67 Ab |
| LIN2 | % | 33.89 ± 3.00 Aa | 24.63 ± 3.37 Ab | 29.55 ± 0.80 Aab | 27.44 ± 2.24 Aab |
| WOB1 | % | 48.17 ± 2.84 Aa | 43.51 ± 0.68 Ab | 45.33 ± 1.19 Aab | 43.49 ± 0.50 Aab |
| WOB2 | % | 48.25 ± 2.33 Aa | 43.01 ± 2.32 Aa | 46.12 ± 0.54 Aa | 43.38 ± 2.02 Aa |
A two-way repeated-measures ANOVA was employed to compare various study groups across three distinct time points. Pairwise comparisons were performed using LSD confidence interval adjustments to analyze the simple effects related to time and study groups. Results are expressed as Means ± SEM. Superscript lowercase letters denote statistically significant differences (P ≤ 0.05) within rows, while uppercase letters indicate significant differences across columns.
Effects of oral niacin administration on epidydimal sperm quality measures
Table 5 shows the effects of oral niacin administration on epidydimal sperm quality measures (Fig. 3). On Day 15, heat-stressed mice showed a decline in several sperm parameters compared to the control, including a marked reduction in DNA integrity (63.60 ± 1.86 vs. 84.17 ± 1.45) and increased DNA fragmentation (21.60 ± 1.21 vs. 10.50 ± 1.61). Niacin supplementation counteracted these effects, particularly Niacin 2, which showed higher DNA integrity (86.20 ± 1.98) and the lowest DNA fragmentation (7.20 ± 1.16). Sperm viability was also improved by both treatments (Niacin 1: 93.80 ± 1.46; Niacin 2: 83.40 ± 2.80) relative to heat control (75.20 ± 2.73). On Day 30, the detrimental effects of heat stress were more pronounced, with viability in the heat-stressed group significantly declining (48.20 ± 3.99) compared to the control (79.86 ± 1.98), accompanied by higher DNA fragmentation (24.00 ± 5.79). In contrast, both niacin treatments preserved sperm quality, particularly Niacin 2, which showed improved viability (82.00 ± 1.81), DNA integrity (85.60 ± 1.63), and low fragmentation (10.60 ± 1.69). Between-day comparisons revealed that while sperm quality in the heat-stressed group deteriorated over time (e.g., viability dropped from 75.20 to 48.20, and CTCF from 94.50 to 83.75), niacin-treated groups maintained or even improved parameters such as viability (Niacin 2: 83.40 → 82.00) and DNA integrity (Niacin 1 SCDAL: 73.40 → 83.00). Acrosomal integrity (CTCF) was significantly better preserved in niacin groups than in the heat-stressed group at both time points. Furthermore, chromatin maturity (AOG) remained high in Niacin 1 and 2 (96.60 ± 1.72 and 92.60 ± 3.20, respectively), while it dropped in the heat control group (70.00 ± 2.74). These findings demonstrate that heat stress progressively impairs sperm viability, acrosome integrity, and chromatin structure, whereas niacin, particularly at the higher dose, provides time-dependent and dose-dependent protection, mitigating heat-induced reproductive damage.
Table 5.
Effects of oral niacin administration on epidydimal sperm quality measures.
| Study groups | ||||
|---|---|---|---|---|
| Control | Heat Control | Niacin 1 | Niacin 2 | |
| VIB1 | 74.25 ± 9.44 Aa | 75.20 ± 2.73 Aa | 93.80 ± 1.46 Abc | 83.40 ± 2.80 Aac |
| VIB2 | 79.86 ± 1.98 Aac | 48.20 ± 3.99 Bb | 75.14 ± 1.97 Ba | 82.00 ± 1.81 Ac |
| HOST1 | 57.00 ± 4.60 Aa | 61.80 ± 8.94 Aa | 85.40 ± 2.50 Ab | 82.00 ± 2.21 Ab |
| HOST2 | 56.17 ± 2.60 Aa | 55.20 ± 11.77 Aa | 67.71 ± 4.25 Aa | 71.14 ± 5.36 Aa |
| CBB1 | 87.71 ± 1.48 Aa | 82.60 ± 1.50 Aa | 89.80 ± 0.66 Ab | 90.40 ± 1.63 Ab |
| CBB2 | 82.14 ± 2.40 Aa | 68.60 ± 2.44 Ba | 90.00 ± 1.18 Ab | 93.43 ± 0.95 Ab |
| CTCF1 | 98.14 ± 0.77 Aab | 94.50 ± 0.29 Aa | 100.00 ± 0.00 Ab | 99.25 ± 0.75 Ab |
| CTCF2 | 90.86 ± 0.86 Ba | 83.75 ± 1.25 Bb | 94.60 ± 1.29 Ba | 92.57 ± 1.29 Ba |
| CTCB1 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| CTCB2 | 2.71 ± 0.87 Aa | 6.25 ± 1.25 Bb | 1.20 ± 0.49 Aa | 3.14 ± 0.74 Ba |
| CTCAR1 | 1.86 ± 0.77 Aab | 5.50 ± 0.29 Aa | 0.00 ± 0.00 Ab | 0.75 ± 0.75 Ab |
| CTCAR2 | 6.29 ± 1.25 Ba | 10.00 ± 0.00 Ba | 4.20 ± 1.07 Bb | 4.29 ± 0.75 Bb |
| SCDAL1 | 84.17 ± 1.45 Aac | 63.60 ± 1.86 Ab | 73.40 ± 2.98 Abc | 86.20 ± 1.98 Aa |
| SCDAL2 | 72.00 ± 2.55 Aac | 71.00 ± 5.79 Aa | 83.00 ± 1.22 Bbc | 85.60 ± 1.63 Ab |
| SCDAS1 | 10.50 ± 1.61 Aac | 21.60 ± 1.21 Ab | 15.60 ± 1.29 Abc | 7.20 ± 1.16 Aa |
| SCDAS2 | 23.00 ± 2.55 Aab | 24.00 ± 5.79 Aa | 12.00 ± 1.22 Ab | 10.60 ± 1.69 Ab |
| SCDAN1 | 5.33 ± 0.99 Aa | 14.80 ± 1.56 Aa | 10.20 ± 2.63 Aa | 6.60 ± 1.17 Aa |
| SCDAN2 | 5.00 ± 0.00 Aa | 5.00 ± 0.00 Ba | 5.00 ± 0.00 Ba | 3.80 ± 0.97 Aa |
| AOG1 | 87.86 ± 4.86 Aa | 84.00 ± 4.00 Ab | 94.60 ± 1.63 Aa | 96.60 ± 1.89 Aa |
| AOG2 | 96.25 ± 1.25 Aa | 70.00 ± 2.74 Ab | 96.60 ± 1.72 Aa | 92.60 ± 3.20 Aa |
| AOY1 | 7.14 ± 2.14 Aa | 11.00 ± 2.45 Ab | 5.40 ± 1.63 Aa | 3.40 ± 1.89 Aa |
| AOY2 | 3.75 ± 1.25 Aa | 30.00 ± 2.74 Ab | 3.40 ± 1.72 Aa | 7.40 ± 3.20 Aa |
| AOR1 | 5.00 ± 3.27 Aa | 5.00 ± 3.16 Aa | 0.00 ± 0.00 Aa | 0.00 ± 0.00 Aa |
| AOR2 | 0.00 ± 0.00 A | 0.00 ± 0.00 B | 0.00 ± 0.00 A | 0.00 ± 0.00 A |
Values are expressed as mean ± standard error. Statistical analysis was performed using one-way repeated measures ANOVA with LSD post hoc test, with significance set at P < 0.05. Different uppercase superscripts within the same row indicate statistically significant differences among groups at each time point, while lowercase superscripts within the same column denote significant differences over time within the same group. VIB – Viability; HOST – Hypo-osmotic swelling test; CTC – Chlortetracycline assay (CTCF: non-capacitated sperm with intact acrosome, CTCB: capacitated sperm with intact acrosome, CTCAR: reacted or damaged acrosome); SCD – Sperm chromatin dispersion assay (SCDAL: cells with large halo, SCDAS: cells with small halo, SCDAN: cells without halo); AO – Acridine orange staining.
Fig. 3.
Representative images of mouse gametes and embryos. (A) Bulged oviducts after superovulation containing expanded cumulus–oocyte complexes (arrowheads). (B) Early and expanded blastocysts on day 5 of in vitro culture compared with retarded or degenerated embryos. (C) 4-cell stage embryos on day 2 of culture. (D,E) Chlortetracycline-stained sperm showing non-capacitated (F pattern) and capacitated (B pattern) cells. (F–H) Acridine orange-stained sperm highlighting mature (green) versus immature or protamine-deficient (yellow/reddish) nuclei. (I,J) Eosin–nigrosin-stained sperm illustrating live (colorless heads) versus dead (red heads) cells. (K) Coomassie Brilliant Blue-stained sperm showing acrosome-intact (blue line, arrowheads) versus acrosome-reacted/damaged spermatozoa. (L) Sperm chromatin dispersion assay displaying non-fragmented DNA with large halos around nuclei.
Effects of oral niacin administration on endocrinologic factors and hepatic function
Oral niacin administration influenced serum thyroid hormones, testosterone levels, and hepatic enzyme activities in heat-stressed animals over 30 days (Table 6). At 15 days (Time 1), the Heat Control group had significantly lower serum T3 (56.67 ± 3.28 ng/dL) compared to Control (92.67 ± 6.69 ng/dL), while Niacin 1 (87.67 ± 3.48) and Niacin 2 (105.67 ± 6.44) groups did not differ significantly from Control. At 30 days (Time 2), T3 levels did not differ significantly across groups. Serum T4 levels were similar at Time 1; however, at Time 2, Niacin 1 (4.17 ± 0.10 ng/mL) and Niacin 2 (4.52 ± 0.65) were significantly higher than Control (3.24 ± 0.37). Serum testosterone at Time 1 was significantly lower in the Heat Control group (0.88 ± 0.15 ng/mL) than in Control (2.51 ± 0.40), Niacin 1 (2.32 ± 0.49), and Niacin 2 (3.29 ± 0.72). At Time 2, serum testosterone levels in Niacin 1 (4.07 ± 0.55) and Niacin 2 (4.44 ± 0.70) were significantly higher than both Control (1.47 ± 0.18) and Heat Control (0.89 ± 0.21). Tissue testosterone at Time 1 was significantly higher in Niacin 1 (9.29 ± 1.94) and Niacin 2 (11.50 ± 1.95) than in Heat Control (3.11 ± 0.47), with Control at 5.21 ± 0.78. At Time 2, Niacin 2 (12.30 ± 1.21) was significantly higher than all other groups, and Niacin 1 (8.57 ± 1.29) was significantly higher than Control (4.08 ± 0.32) and Heat Control (3.29 ± 0.29). AST and ALT levels showed no significant differences across groups at each time point. However, ALT decreased significantly from Time 1 to Time 2 in Niacin 1 (39.40 ± 4.77 to 29.38 ± 3.23 U/L) and Niacin 2 (48.63 ± 6.22 to 37.90 ± 4.98). ALP levels did not differ significantly among groups at each time point but showed a significant decrease in Heat Control from Time 1 (253.33 ± 44.05) to Time 2 (167.50 ± 21.80). Different uppercase superscripts indicate differences among groups within time points (columns); different lowercase superscripts indicate differences within groups over time (rows).
Table 6.
Effects of oral niacin administration on endocrinologic factors and hepatic function.
| Study Groups | ||||
|---|---|---|---|---|
| Parameter (Unit) | Control | Heat Control | Niacin 1 | Niacin 2 |
| T3-1 (ng/dL) | 92.67 ± 6.69 Aa | 56.67 ± 3.28 Ab | 87.67 ± 3.48 Aab | 105.67 ± 6.44 Aa |
| T3-2 (ng/dL) | 93.00 ± 10.44 Aa | 99.50 ± 4.50 Ba | 75.00 ± 11.06 Aa | 70.00 ± 6.08 Aa |
| T4-1 (ng/mL) | 4.41 ± 0.15 Aa | 4.43 ± 0.18 Aa | 4.51 ± 0.38 Aa | 4.49 ± 0.52 Aa |
| T4-2 (ng/mL) | 3.24 ± 0.37 Aa | 3.80 ± 0.16 Aab | 4.17 ± 0.10 Ab | 4.52 ± 0.65 Ab |
| ST 1 (ng/mL) | 2.51 ± 0.40 Aa | 0.88 ± 0.15 Ab | 2.32 ± 0.49 Aa | 3.29 ± 0.72 Aa |
| ST 2 (ng/mL) | 1.47 ± 0.18 Aa | 0.89 ± 0.21 Aa | 4.07 ± 0.55 Bb | 4.44 ± 0.70 Bb |
| TT 1 (ng/g) | 5.21 ± 0.78 Aac | 3.11 ± 0.47 Aa | 9.29 ± 1.94 Abc | 11.50 ± 1.95 Aa |
| TT 2 (ng/g) | 4.08 ± 0.32 Aa | 3.29 ± 0.29 Aa | 8.57 ± 1.29 Ab | 12.30 ± 1.21 Bc |
| AST 1 (U/L) | 186.50 ± 21.61 Aa | 144.25 ± 23.30 Aa | 168.20 ± 21.60 Aa | 130.00 ± 22.52 Aa |
| AST 2 (U/L) | 155.60 ± 16.71 Ba | 185.00 ± 21.27 Ba | 120.38 ± 11.12 Ba | 141.70 ± 20.77 Ba |
| ALT 1 (U/L) | 43.38 ± 6.04 Aa | 58.88 ± 11.80 Aa | 39.40 ± 4.77 Aa | 48.63 ± 6.22 Aa |
| ALT 2 (U/L) | 38.20 ± 2.99 Aac | 33.78 ± 5.52 Aa | 29.38 ± 3.23 Bb | 37.90 ± 4.98 Bc |
| ALP 1 (U/L) | 223.20 ± 37.46 Aa | 253.33 ± 44.05 Aa | 266.40 ± 29.81 Abc | 276.00 ± 25.52 Aa |
| ALP 2 (U/L) | 247.40 ± 11.06 Bac | 167.50 ± 21.80 Abc | 206.50 ± 27.92 Ab | 237.80 ± 37.35 Aa |
Data are presented as mean ± standard error. Statistical analysis was performed using one-way repeated measures ANOVA with LSD post hoc test. Different uppercase superscripts within the same column indicate statistically significant differences among groups at each time point (P < 0.05). Different lowercase superscripts within the same row indicate significant differences over time within the same group (P < 0.05). T3 – Triiodothyronine; T4 – Thyroxine; ST – Serum testosterone; TT – Tissue testosterone; AST – Aspartate aminotransferase; ALT – Alanine aminotransferase; ALP – Alkaline phosphatase.
Effects of oral niacin administration on oxidative stress markers
Oral niacin supplementation significantly modulated oxidative stress parameters in heat-stressed males, with distinct effects observed between groups at each time point and across days (Table 7). On Day 15, serum MDA-1 concentrations were elevated in the Heat Control group compared to Control, Niacin 1, and Niacin 2 groups (P < 0.05), whereas no differences were noted among the latter three. By Day 30, serum MDA-2 remained elevated in Heat Control animals and was highest in the Niacin 2 group (P < 0.05). Notably, in Niacin 2 animals, serum MDA increased over time despite niacin supplementation, an unexpected finding that contrasts with its anticipated antioxidant effect. Over time, serum MDA levels declined in the Control group but increased significantly in Niacin 2 animals (P < 0.05). In testicular tissue, MDA-1 values were greater in Niacin 1 compared to Niacin 2 on Day 15 (P < 0.05), with Control and Heat Control showing intermediate, nonsignificant values. On Day 30, testicular MDA-2 was lower in Heat Control animals relative to Control (P < 0.05), with no significant differences between the niacin-treated groups. A temporal decline in testicular MDA was detected only in the Heat Control group (P < 0.05). Total antioxidant capacity (TAC) at Day 15 was reduced in Niacin 1 animals compared to Control (P < 0.05), while Heat Control and Niacin 2 showed intermediate values. By Day 30, TAC was significantly higher in Niacin 1 compared to all other groups (P < 0.05), whereas Niacin 2 animals exhibited the lowest TAC. TAC declined from Day 15 to Day 30 in Control, Heat Control, and Niacin 2 animals but increased in Niacin 1 animals (P < 0.05). SOD-1 activity at Day 15 did not differ among groups; however, by Day 30, SOD-2 activity was significantly greater in Niacin 2 animals relative to Control, Heat Control, and Niacin 1 (P < 0.05). SOD activity increased significantly over time in all groups (P < 0.05), indicating an overall adaptive antioxidant response.
Table 7.
Effects of oral niacin administration on oxidative stress markers.
| Study Groups | ||||
|---|---|---|---|---|
| Parameter (Unit) | Control | Heat Control | Niacin 1 | Niacin 2 |
| MDA-1 (nmol/mL) | 27.00 ± 7.96 Aa | 86.83 ± 22.62 Ab | 20.50 ± 4.00 Aa | 19.50 ± 1.33 Aa |
| MDA-2 (nmol/mL) | 18.50 ± 1.05 Aa | 21.75 ± 3.48 Ba | 22.00 ± 3.36 Aa | 41.75 ± 5.43 Ab |
| Testicular MDA-1 (nmol/g tissue) | 65.25 ± 9.61 Aab | 70.00 ± 13.05 Aab | 100.50 ± 18.03 Aa | 55.50 ± 4.77 Ab |
| Testicular MDA-2 (nmol/g tissue) | 57.00 ± 8.86 Aa | 28.75 ± 6.10 Bb | 40.75 ± 3.02 Bab | 50.69 ± 10.10 Aa |
| TAC-1 (mmol Trolox equiv./L) | 0.55 ± 0.03 Aa | 0.48 ± 0.01 Aac | 0.43 ± 0.04 Abc | 0.49 ± 0.05 Aab |
| TAC-2 (mmol Trolox equiv./L) | 0.37 ± 0.04 Bab | 0.33 ± 0.02 Bab | 0.42 ± 0.04 Aa | 0.25 ± 0.04 Bb |
| SOD-1 (U/ml) | 48.68 ± 1.50 Aa | 51.82 ± 2.12 Aac | 49.99 ± 0.93 Aa | 54.25 ± 1.60 Abc |
| SOD-2 (U/ml) | 58.87 ± 1.41 Ba | 61.28 ± 1.07 Ba | 65.60 ± 3.35 Ba | 71.12 ± 1.10 Bb |
Data are expressed as mean ± standard error. Statistical analysis was performed using one-way repeated measures ANOVA followed by LSD post hoc test. Different uppercase superscript letters within the same column indicate significant differences among groups at each time point (P < 0.05), while different lowercase superscript letters within the same row indicate significant differences between Day 15 and Day 30 within the same group (P < 0.05).
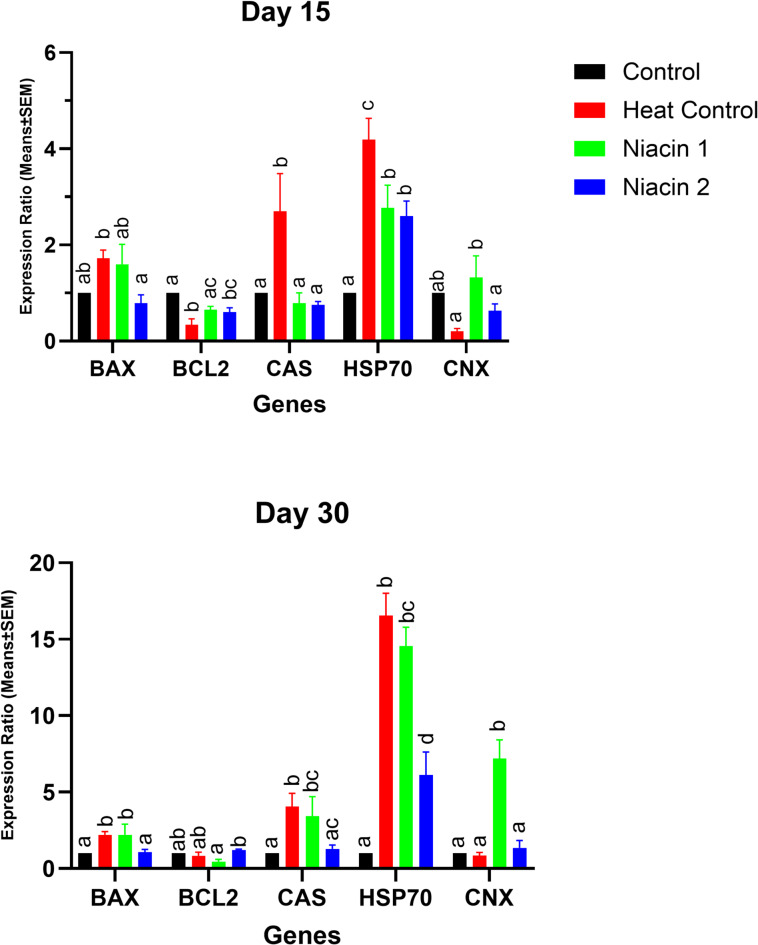
Effects of oral niacin administration on the testicular gene expression patterns
At day 15, mice exposed to chronic heat stress (Heat Control) showed significantly increased mRNA expression of HSP70 (5.2-fold vs. Control, p < 0.01), Bax (3.1-fold, p < 0.05), and Caspase-3 (2.8-fold, p < 0.05), while Connexin 43 (Gja1) was significantly decreased (0.45-fold, p < 0.05). Bcl2 expression did not differ significantly (p > 0.05). Both niacin-treated groups exhibited reduced expression of HSP70 (Niacin 1: 2.0-fold; Niacin 2: 1.6-fold vs. Control, p < 0.05), Bax, and Caspase-3 relative to the Heat Control group (p < 0.05 for each), with no significant change in Bcl2.
By day 30, the Heat Control group exhibited a marked upregulation of HSP70 (~ 17-fold vs. Control, p < 0.001), along with sustained elevation of Bax (3.5-fold, p < 0.01) and Caspase-3 (3.2-fold, p < 0.01) and suppression of Connexin 43 (0.30-fold, p < 0.01). Niacin supplementation significantly reversed these changes, with Niacin 2 showing the strongest effect (HSP70: 2.1-fold vs. Control, p < 0.01; Bax and Caspase-3 reduced to near-control levels, p < 0.05). In addition, Bcl2 expression was significantly elevated in both niacin groups (Niacin 1: 2.5-fold; Niacin 2: 2.7-fold vs. Heat Control, p < 0.05). (Fig. 4 ).
Fig. 4.
A one-way ANOVA followed by LSD confidence interval adjustments was employed to compare values between various study groups on each distinct time points. Asterisks denote statistically significant differences (P ≤ 0.05) between each niacin group and the heat stress group.
Effects of oral niacin administration on in vivo fertility
Long-term heat stress markedly impaired reproductive outcomes in mice, as evidenced by a significant reduction in pregnancy risk (50%), litter size (3.67 ± 0.33), and a substantial increase in stillbirth risk (47.22 ± 12.11%) compared to the control group (pregnancy risk: 83.3%; litter size: 5.40 ± 0.24; stillbirth risk: 0%). Niacin supplementation significantly mitigated these adverse effects, with both treated groups (Niacin 1 and Niacin 2) achieving 100% pregnancy risk, significantly higher litter sizes (6.83 ± 0.40 and 7.17 ± 0.31, respectively), and complete elimination of stillbirths. Birthweights of both male and female offspring were significantly reduced under heat stress but were restored to higher levels with niacin treatment (P < 0.05). No significant differences were observed among groups in sex ratio (P = 0.478) (Table 8).
Table 8.
Fertility outcomes in mice under long-term heat stress and niacin supplementation.
| Study group | No. of females | No. of males | Pregnancy risk (%)* | Litter size | Stillbirth risk (%) | Sex (M/F) ratio (%) # | Male offspring birthweight (gr) @ | Female Offspring Birthweight (gr) @ | Overall Offspring Birthweight (gr) @ |
|---|---|---|---|---|---|---|---|---|---|
| Control | 6 | 3 | 83.3 | 5.40 ± 0.24 a | 0.00 a | 51.33 ± 5.33 | 1.48 ± 0.03 a | 1.42 ± 0.02 a | 1.45 ± 0.02 a |
| Heat Control | 6 | 3 | 50 | 3.67 ± 0.33 b | 47.22 ± 12.11 b | 36.11 ± 7.35 | 1.35 ± 0.04 b | 1.34 ± 0.02 b | 1.34 ± 0.02 b |
| Niacin 1 | 6 | 3 | 100 | 6.83 ± 0.40 c | 0.00 a | 47.72 ± 4.94 | 1.59 ± 0.02 c | 1.57 ± 0.02 c | 1.58 ± 0.02 c |
| Niacin 2 | 6 | 3 | 100 | 7.17 ± 0.31 c | 0.00 a | 55.28 ± 4.57 | 1.56 ± 0.02 c | 1.59 ± 0.03 c | 1.57 ± 0.02 c |
*A Chi-Square test was used to compare pregnancy risk between the study groups, Pearson Chi-Square (df = 3, N = 24, p = 0.066), Likelihood Ratio (df = 3, N = 24, p = 0.048). #A Pearson Chi-Square test was used to compare pregnancy risk between the study groups (df = 3, N = 24, p = 0.478). @A one-way ANOVA test followed by LSD confidence interval adjustments was employed to compare values between various study groups.
Effects of oral niacin administration on in vitro fertility
Heat stress significantly reduced in vitro fertility, with the heat control group showing lower two-cell (74.50 ± 1.53%), morula (52.05 ± 1.08%), and blastocyst (37.81 ± 2.72%) rates compared to the non-stressed control group (91.40 ± 1.42%, 81.83 ± 1.12%, and 73.73 ± 1.52%, respectively; P ≤ 0.05). Oral administration of niacin at two different doses improved these outcomes, with both Niacin 1 and Niacin 2 groups demonstrating significantly higher fertilization and embryo development rates than the heat control group. Two-cell rates in niacin-treated groups (86.55 ± 1.87% and 88.20 ± 1.90%) were comparable to controls, while morula and blastocyst rates were also elevated (morula: 72.80 ± 3.07% and 77.90 ± 3.42%; blastocyst: 64.68 ± 3.52% and 68.00 ± 2.61%), indicating a partial to near-complete recovery of embryo development impaired by heat stress. The higher niacin dose tended to yield better results, particularly for morula and blastocyst rates, though differences between doses were not always statistically significant (Table 9).
Table 9.
In vitro fertility outcomes in mice under long-term heat stress and niacin supplementation.
| Repeats | Total number of COCs | Two-cell rate*(%) | Morula rate** (%) | Blastocyst Rate*** (%) | ||
|---|---|---|---|---|---|---|
| Groups | Control | 3 | 150 | 91.40 ± 1.42 a | 81.83 ± 1.12 a | 73.73 ± 1.52 a |
| Heat Control | 3 | 160 | 74.50 ± 1.53 b | 52.05 ± 1.08 b | 37.81 ± 2.72 b | |
| Niacin 1 | 3 | 156 | 86.55 ± 1.87 a | 72.80 ± 3.07c | 64.68 ± 3.52 c | |
| Niacin 2 | 3 | 146 | 88.20 ± 1.90 a | 77.90 ± 3.42 ac | 68.00 ± 2.61 ac | |
A one-way ANOVA followed by LSD post hoc test was used to make comparisons between groups. Different superscript letters indicate significant differences between groups (P ≤ 0.05). Data are expressed as mean ± standard error of mean. *, the percentage of COCs developed to two-cell embryonic stage, as a measure of fertilization rate, **, The percentage two-cell embryos developed to morula stage, ***, The percentage of two-cell embryos developed to blastocyst stage.
Effects of oral niacin administration on testicular histomorphometry
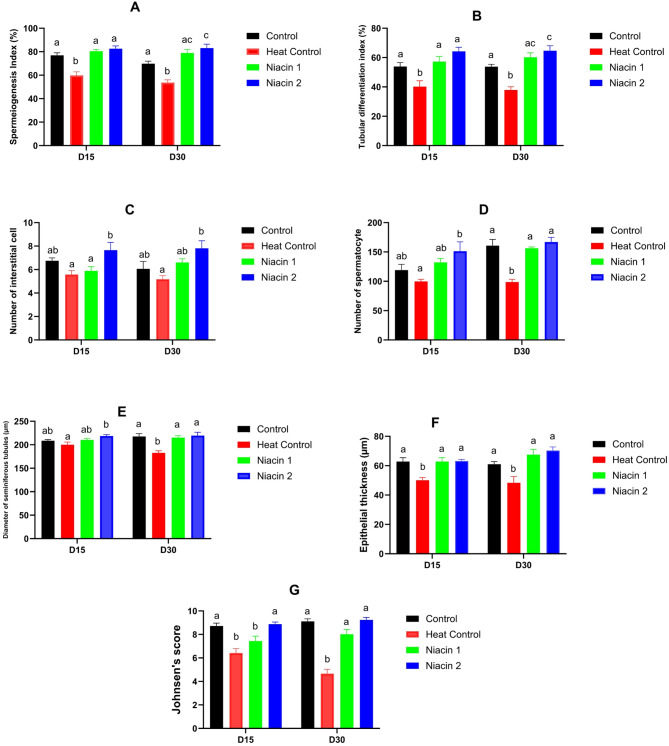
Histomorphometric evaluation of testicular tissue showed that heat stress (Heat Control) caused significant structural damage and impaired spermatogenesis at both 15 and 30 days compared to the Control group (Figs. 5, 6 and 7). Specifically, the thickness of the seminiferous tubule epithelium in the Heat Control group was significantly reduced at both time points (P < 0.05), whereas the Niacin 1 and Niacin 2 groups showed no significant difference from the Control and maintained higher epithelial thickness. The diameter of the seminiferous tubules in the Heat Control group was significantly decreased at day 30 compared to the Control (P < 0.01). In contrast, both Niacin 1 and Niacin 2 groups had significantly larger tubule diameters than Heat Control at both 15 and 30 days (P < 0.01). The number of spermatocytes was lowest in the Heat Control group, showing a highly significant reduction compared to Control at day 30 (P < 0.001), while the Niacin 1 and Niacin 2 groups exhibited significantly increased spermatocyte counts relative to Heat Control at both time points (P < 0.05 to P < 0.001), with Niacin 2 displaying the highest counts at day 30. Interstitial cell counts followed a similar pattern: Heat Control had the lowest numbers, and Niacin 2 had the highest at both time points, significantly exceeding Heat Control (P < 0.01), though no differences were observed among the other groups. Functional indices of spermatogenesis were also adversely affected by heat stress. The spermiogenesis index (SI) was significantly lower in Heat Control compared to Control at both time points (P < 0.05), while Niacin 1 and Niacin 2 groups had significantly higher SI values than Heat Control (P < 0.001), with Niacin 2 exceeding Control values at day 30 (P < 0.05). The tubular differentiation index (TDI) also decreased in the Heat Control group versus Control (P < 0.05), but was highest in the Niacin 2 group at both time points, significantly surpassing Heat Control (P < 0.001) and Control at day 30 (P < 0.05). Finally, Johnsen’s score, an overall measure of spermatogenic quality, was significantly reduced in the Heat Control group compared to Control at both time points (P < 0.001), while Niacin 1 and Niacin 2 groups showed significantly higher scores than Heat Control (P < 0.001), with no significant difference between the two niacin groups. These results collectively indicate that heat stress severely compromises testicular histology and spermatogenesis, whereas niacin supplementation, especially at the higher dose, effectively protects and restores seminiferous tubule structure and spermatogenic function.
Fig. 5.
Effects of chronic heat stress and oral niacin supplementation on histological and morphometric parameters of testicular tissue in mice at Days 15 and 30. (A) Spermatogenesis index (%), (B) Tubular differentiation index (%), (C) Number of interstitial cells, (D) Number of spermatocytes, (E) Diameter of seminiferous tubules (µm), (F) Epithelial thickness (µm), and (G) Johnsen’s score were evaluated in four groups: Control (black), Heat Control (red), Niacin 1 (green; lower dose), and Niacin 2 (blue; higher dose).Bars represent mean ± SEM. Columns with different letters (a, b, c) are significantly different at p < 0.05.
Fig. 6.
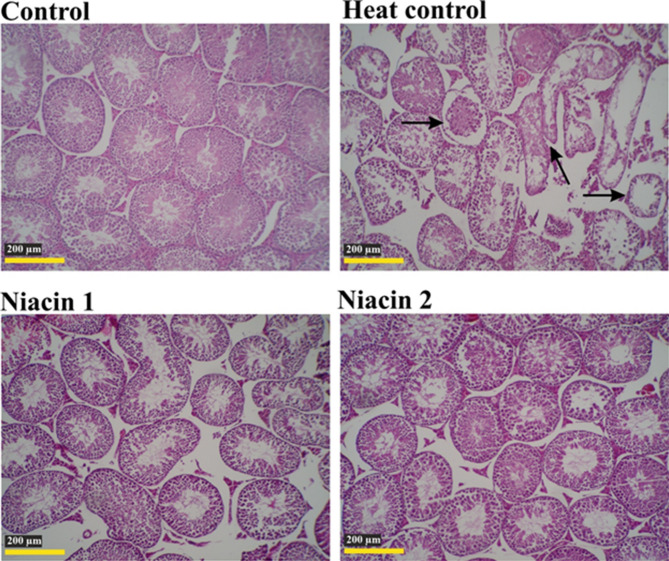
Histological structure of the testis in different groups during the 15-day study period (H&E staining). Degenerated seminiferous tubules (arrows), disruption of cellular structural organization, and a marked reduction in epithelial thickness and seminiferous tubule diameter are evident in the HC group compared to the other study groups.
Fig. 7.
Histological structure of the testis in different groups during the 30-day study period (H&E staining). Degenerated seminiferous tubules (arrows), disruption of cellular structural organization, and a marked reduction in epithelial thickness and seminiferous tubule diameter are evident in the H group compared to the other study groups.
Discussion
This study demonstrates that oral niacin supplementation mitigates the deleterious effects of chronic heat stress (HS) on male reproductive health in mice. By integrating physiological, endocrine, molecular, oxidative, and fertility endpoints, our findings highlight niacin’s multi-modal protective actions. Several antioxidant interventions have previously been evaluated in rodent HS models. Some compounds such as resveratrol, melatonin, quercetin, N-acetylcysteine, vitamin E, thymoquinone, selenium, and curcumin have been shown to reduce oxidative stress, suppress germ cell apoptosis, improve sperm function, and partially restore hormonal balance18,19,22,23,26. Likewise, natural extracts—including Nigella sativa, green tea (Camellia sinensis), have improved sperm quality, antioxidant status, and endocrine function in heat-exposed animals27,28. Our findings complement these reports but also extend them, as niacin provides a broader spectrum of protection: beyond its antioxidant properties, it uniquely promotes thermoregulation through peripheral vasodilation and supports cellular metabolism via its role as a precursor of NAD/NADP coenzymes essential for mitochondrial energy production and redox balance. This unique combination of antioxidant, thermoregulatory, and metabolic actions distinguishes niacin from previously tested compounds and underpins the novelty of our study.
Oxidative stress is widely recognized as the central mechanism through which heat stress impairs male fertility. Excess reactive oxygen species (ROS) disrupt testicular homeostasis, causing lipid peroxidation, DNA fragmentation, and apoptosis in germ and Sertoli cells1,7,10. In our study, heat-stressed animals displayed elevated serum and testicular malondialdehyde (MDA) and diminished superoxide dismutase (SOD) activity and total antioxidant capacity (TAC), confirming oxidative imbalance.
Niacin supplementation significantly restored redox homeostasis, especially by increasing SOD (Niacin 2 group) and TAC (Niacin 1 group), and reducing MDA levels, underscoring its antioxidant efficacy. These findings are consistent with prior in vitro evidence, where niacin derivatives reduced ROS levels and DNA damage in porcine oocytes exposed to heat stress39. The observed oxidative stress improvements likely reflect niacin’s role in NAD/NADP-dependent redox reactions, mitochondrial biogenesis, and modulation of antioxidant enzyme systems7,31. The stronger TAC response in Niacin 1 and SOD elevation in Niacin 2 suggest dose-specific regulatory pathways, possibly involving differential activation of sirtuins or Nrf2-dependent antioxidant genes40. These effects are consistent with in vitro evidence showing that niacin-related compounds can restore mitochondrial function and cytoskeletal integrity in heat-stressed reproductive cells39.
Comparison with earlier studies reveals that various antioxidants have shown partial efficacy against heat-induced testicular injury. Resveratrol (20 mg/kg/day) reduced testicular lipid peroxidation and germ cell apoptosis in heat-exposed mice41. Melatonin (10 mg/kg/day) was shown to enhance sperm chromatin integrity and testosterone levels while modulating Bax and Bcl2 expression42. Vitamin E, selenium and thymoquinone protected sperm viability and histoarchitecture in subacute HS 20,21. Similarly, quercetin and N-acetylcysteine (NAC) restored spermatogenesis via antioxidant and anti-apoptotic pathways43–45. Curcumin has also demonstrated dose-dependent protective effects in mice exposed to scrotal heat stress, significantly improving testicular histology, sperm count and motility, and oxidative stress markers. Natural extracts such as Nigella sativa, green tea, and also demonstrated improvements in oxidative stress biomarkers and hormonal levels27,28. These studies collectively support the principle that antioxidants can mitigate HS-induced testicular damage, though most only partially restored reproductive parameters.
Antioxidant efficacy is not limited to thermal stress models. In chemically or environmentally induced reproductive toxicities, compounds such as Carob (Ceratonia siliqua L.) fruit extract, Allium sativum, L-proline with fulvic acid, glutathione, 3,4-dihydroxyphenylglycol with hydroxytyrosol, hesperidin, and rutin have shown protective effects against oxidative sperm and testicular damage12,29,30,46–51. These findings highlight the broad utility of antioxidants in preserving male fertility under diverse oxidative challenges and provide mechanistic support for niacin’s effectiveness.
An unexpected observation, however, was the significant elevation of serum MDA at day 30 in the Niacin 2 group compared with all other groups. This result appears inconsistent with the antioxidant hypothesis. Several explanations are possible: variability in systemic oxidative markers at later time points may have exaggerated differences in a subset of animals; niacin’s metabolic and vasodilatory effects at higher doses may have transiently increased lipid mobilization and turnover, thereby elevating circulating MDA without reflecting a true increase in testicular oxidative damage. Importantly, testicular MDA values did not mirror this serum pattern, and Niacin 2 consistently improved sperm quality, testosterone levels, histomorphometric indices, and fertility outcomes. This suggests that the serum MDA rise may not indicate functional impairment. Nevertheless, further studies incorporating broader markers of lipid peroxidation (e.g., 4-HNE, isoprostanes) and mitochondrial ROS production are warranted to clarify whether this increase represents a dose-specific systemic effect of niacin or a statistical anomaly.
The thermoregulatory effect of niacin also impacted feeding and drinking behavior. Heat-stressed animals showed increased water intake, reflecting compensatory thermolysis mechanisms, and reduced appetite, consistent with heat-induced anorexia. Niacin-treated mice demonstrated enhanced feed consumption and normalized water intake, likely due to improved thermal comfort through peripheral vasodilation and cutaneous heat loss, whereas other antioxidants, such as resveratrol, quercetin, and β-Cryptoxanthin, did not modulate thermoregulation in similar models41,45,52,53.
Thyroid and gonadal hormones are highly susceptible to thermal disruption. Heat stress suppressed serum T3 and testosterone, consistent with impaired hypothalamic–pituitary–thyroid (HPT) and hypothalamic–pituitary–gonadal (HPG) axes9,11,54. Niacin supplementation reversed these trends, with Niacin 2 maintaining T3 and significantly elevating T4 at Day 30. Testosterone levels, both in serum and testis, were significantly increased in niacin-treated groups, likely due to preserved Leydig cell function and reduced oxidative stress. Reduced T3 has been shown to downregulate StAR protein expression, impairing testosterone synthesis54. The restoration of T3 and testosterone in our study supports niacin’s role in endocrine resilience under HS.
Niacin supplementation conferred significant protection against heat-induced reductions in male reproductive organ weights, as reflected in both testis and combined accessory sex gland measurements. Although testis weight in heat-stressed mice was numerically higher than controls at day 15, possibly due to acute edema, this increase was not statistically significant. By day 30, however, prolonged heat exposure led to a marked decline in testis weight compared to controls, suggesting progressive testicular degeneration. In contrast, niacin-treated groups—especially Niacin 1—exhibited preserved and even enhanced testis weights over time, with Niacin 1 showing the highest values by day 30. This suggests a dose-responsive protective effect on testicular tissue integrity. Similarly, heat stress significantly reduced accessory sex gland weights at both time points, indicating impaired androgenic stimulation. Yet, both Niacin 1 and Niacin 2 maintained glandular weights comparable to controls, further supporting niacin’s role in preserving androgen-dependent tissue growth. These findings align with the observed restoration of testosterone levels and reflect niacin’s capacity to safeguard structural reproductive parameters under thermal stress conditions.
Interestingly, by day 30, the Niacin 1 group exhibited a significantly higher testis weight than all other groups, including Niacin 2. This finding appears inconsistent with other reproductive parameters, since superior improvements in sperm quality, endocrine balance, and fertility were generally observed in the Niacin 2 group. The increased testis weight in Niacin 1 may therefore not reflect enhanced spermatogenic capacity but could instead be attributable to dose-specific effects such as transient interstitial fluid retention, vascular changes, or localized hypertrophy of Leydig cells. Although circulating and tissue testosterone levels were elevated in both niacin-treated groups, the disproportionate increase in testis weight at the lower dose may indicate an adaptive rather than strictly functional change. Another factor to consider is the relatively higher variability (SEM) observed in the Niacin 2 group, which may have reduced statistical power and prevented detection of differences despite a comparable mean testis weight. Thus, the apparent superiority of Niacin 1 in this parameter may partly reflect random variation rather than a true biological advantage. Given that fertility outcomes and histomorphometric indices did not show parallel superiority in Niacin 1, this isolated increase in testis weight should be interpreted cautiously. Further studies incorporating stereological analysis and assessment of interstitial volume, edema, or Leydig cell morphology are warranted to clarify the biological significance of this observation.
Despite concerns regarding niacin-induced hepatotoxicity at high doses in clinical settings35,55, our findings demonstrated no hepatocellular enzyme elevation. On the contrary, ALT levels decreased over time in both niacin groups. AST and ALP levels also remained within physiological ranges. These findings align with rodent studies using oral niacin at similar doses, showing no hepatic damage and even hepatoprotective effects in oxidative models56. Thus, our data confirm that 4 mg/day of niacin is safe and non-hepatotoxic in mice under HS.
Molecular assessments revealed significant alterations in gene expression, with heat stress upregulating pro-apoptotic Bax and Caspase-3 and HSP70 (Hspa1a/b) while downregulating Cx43 (Gja1), confirming germ cell apoptosis and loss of Sertoli-Leydig intercellular communication; these findings are consistent with prior reports demonstrating that antioxidant interventions, such as Allium sativum extract30 and β-Cryptoxanthin53, mitigated oxidative stress and modulated apoptotic gene expression. Niacin reversed these gene changes—particularly at Day 30—reducing apoptosis markers and increasing Bcl2 and Cx43. Connexin 43 (Cx43) is a key gap junction protein mediating Sertoli–germ cell communication, which is essential for synchronized spermatogenesis and sperm quality; its restoration has been linked to improved post-thaw sperm quality and antioxidant status in other species57. Unlike antioxidants such as melatonin or NAC, which improved apoptotic profiles44, niacin’s additional impact on Cx43 offers a novel therapeutic angle in testicular preservation under heat stress. These transcriptomic effects may be linked to the modulation of heat shock proteins, as previously suggested by niacin-induced upregulation of HSP70 and HSP27 in heat-stressed bovine reproductive cells58.
The improvements in both in vitro and in vivo fertility outcomes further validate niacin’s effectiveness. Although female reproductive factors can contribute to fertility variation under environmental stress, all females in our study were kept under thermoneutral conditions, housed under identical husbandry management, and selected for close genetic similarity, body weight, and age. Therefore, the observed differences in pregnancy rates, litter sizes, and offspring viability can be attributed to male reproductive performance. Heat-stressed males exhibited reduced pregnancy rates, litter sizes, and increased stillbirths—outcomes reversed entirely with niacin treatment. These findings are consistent with evidence from other species, where antioxidant supplementation, such as glutathione in turkeys, improved post-thawed semen quality, oxidative stress parameters, and fertilization and hatching potential47, highlighting the translational potential of antioxidant interventions, including niacin, for preserving male fertility across species. Both Niacin 1 and 2 groups achieved 100% pregnancy success and higher birthweights. These outcomes reflect the compound effect of improved sperm quality, DNA integrity, acrosome status, and hormonal balance. Prior antioxidant therapies have shown improvements in sperm parameters but not complete recovery of fertility indices18,42–44,59. Niacin’s unique multi-modal effects likely contributed to the full fertility restoration observed here.
Sperm DNA integrity is crucial for fertilization, embryonic development, and offspring viability. During epididymal maturation, histones are replaced with protamines, leading to chromatin condensation and enhanced DNA stability. This compaction renders sperm DNA highly resistant to damage. However, heat stress disrupts this maturation process, primarily through ROS overproduction, which induces DNA strand breaks and impairs protamine incorporation. Elevated DNA fragmentation and immature chromatin structure have been reported in various species following scrotal or systemic hyperthermia60. In our study, the HS group showed significantly increased sperm DNA fragmentation and abnormal chromatin condensation, corroborating oxidative and testicular damage. Notably, niacin supplementation—particularly at the higher dose (Niacin 2)—significantly improved both DNA integrity and chromatin maturity. While antioxidants like vitamin E or folic acid have shown partial improvements in DNA stability, niacin’s simultaneous effects on oxidative status, hormonal milieu, and thermoregulation likely explain its superior efficacy. These results emphasize niacin’s unique capacity to protect nuclear quality and support complete spermatogenic competence under chronic HS conditions.
Sperm motility and kinematic parameters—such as total and progressive motility, curvilinear velocity (VCL), average path velocity (VAP), and straight-line velocity (VSL)—are crucial predictors of male fertility, as they reflect flagellar function and mitochondrial activity required for oocyte navigation and penetration. Improvements in these parameters by antioxidant agents have been demonstrated across different stress models, including cryopreservation studies where niacin supplementation in cooling and freezing extenders enhanced stallion semen motility, membrane integrity, and overall functional quality61.
In the present study, chronic heat stress caused significant declines across nearly all motion kinematics, consistent with previous reports in rabbit and livestock demonstrating thermal damage to axonemal structures and mitochondrial membranes62–64. Our data confirm these findings, with heat-stressed mice exhibiting dramatic reductions in TM, PM, VCL, and VAP values. Notably, niacin supplementation—particularly in the Niacin 1 group—robustly restored these indices, in some cases surpassing control values. These improvements likely reflect preserved mitochondrial function, reduced lipid peroxidation of sperm membranes, and protection of axonemal proteins. While prior antioxidant treatments such as resveratrol or melatonin have partially improved motility under HS 18, few have demonstrated such comprehensive recovery in kinematic profiles. The restoration of sperm motility parameters by niacin provides mechanistic support for its observed improvements in fertilization and pregnancy outcomes and underscores its potential as a fertility-protective agent in thermally stressed males.
Histopathological analysis aligned with these findings. Heat stress caused seminiferous tubule atrophy, epithelial thinning, and decreased Johnsen’s scores, as reported in other heat models65,66. Niacin treatment preserved seminiferous morphology, maintained spermatocyte counts, and normalized histological scores. This histological protection complements molecular and functional findings, confirming niacin’s tissue-level efficacy.
Conclusion
In conclusion, this study is the first to show that oral niacin supplementation mitigates heat-induced reproductive, endocrine, oxidative, and molecular impairments in male mice while maintaining hepatic safety. Through thermoregulation, antioxidation, hormonal preservation, and gene expression modulation, niacin presents as a powerful protective agent against environmental HS-induced male infertility. Future research should explore niacin’s effects in large animals, determine long-term outcomes, and evaluate combinatory approaches with other therapeutic agents to enhance male fertility under thermal stress.
Study limitations and future directions
While our findings offer strong evidence of niacin’s protective effects against chronic heat stress, several limitations should be acknowledged. First, although our study fills a critical gap by being the first to investigate oral niacin in an in vivo heat stress model, broader generalizability remains limited. The use of a murine model, while informative, may not fully capture the complexity of reproductive physiology in larger mammals or humans. Follow-up studies in livestock or primate models are warranted to support translational application.
Second, while we observed robust changes in gene expression of apoptosis-related and junctional proteins, we did not assess downstream protein-level changes. Techniques such as Western blotting or immunohistochemistry would enhance mechanistic clarity, especially regarding Connexin 43, Bcl2/Bax ratios, and heat shock proteins previously shown to be modulated by niacin-related compounds in vitro.
Third, our investigation was limited to short-term reproductive outcomes. Longitudinal assessment of offspring health, transgenerational effects, and sustained fertility outcomes post-recovery from heat stress would provide critical insights into the long-term safety and efficacy of niacin intervention.
Finally, while we demonstrated the efficacy of two niacin dosages, the optimal timing, dosing schedule, and potential synergistic effects with other antioxidants (e.g., vitamin E, melatonin, betaine) remain unknown. Given prior evidence suggesting timing-dependent protection and multi-modal mechanisms, future studies should explore combinatory strategies and refine therapeutic windows to enhance fertility preservation under environmental heat stress.
Materials and methods
Animals and ethics
This study was conducted using adult inbred BALB/c mice obtained from a certified breeding colony. Eight-week-old male mice (mean ± SD body weight: 30 ± 2 g) and adult female mice (mean ± SD body weight: 26 ± 2 g) were used. All animals were housed under standard laboratory conditions, including a 12:12 h light-dark cycle controlled by an automatic lighting system and a thermoneutral temperature range of 21–23 °C maintained by automated air-conditioning units. Males and females were housed in separate rooms but under identical husbandry conditions. All animal procedures were approved by the Ethics Committee of the School of Veterinary Medicine, Shiraz University (ethics code: 4687/63) and complied with the European Council Directive 2010/63/EU on the protection of animals used for scientific purposes. The study was designed, conducted, and reported in accordance with the ARRIVE guidelines (Animal Research: Reporting of In Vivo Experiments). At the end of the experimental period, mice were euthanized by gradual-fill CO₂ inhalation (20–30% chamber volume per minute) in a dedicated CO₂ euthanasia chamber, followed by cervical dislocation performed by trained personnel to ensure death, in accordance with the AVMA Guidelines for the Euthanasia of Animals (2020).
Experimental design
A total of 120 adult male BALB/c mice were randomly allocated into four experimental groups (n = 30 per group; 10 mice per cage): Control, Heat Control, Niacin 1, and Niacin 2. Group sizes were determined based on previous studies evaluating testicular damage and fertility outcomes under heat or reproductive stress conditions, ensuring adequate statistical power to detect meaningful differences22,67 and consistent with similar experimental designs in reproductive stress studies68. Sample sizes for each analysis were selected according to expected effect sizes and variability reported in inbred BALB/c mice: biochemical, hormonal, and oxidative stress assays (n = 7 per group) allow detection of ~ 25% differences with SD’s of 10–15% at ≥ 80% power and α = 0.05; molecular analyses (n = 6 per group) detect 1.5–2 fold changes with SDs of 0.3–0.4; histological and sperm assessments (n = 7 per group) detect ≥ 15% differences in motility or ≥ 1.5-unit changes in Johnsen scores; in vivo and in vitro fertility endpoints (n = 3 males per group) were based on previous reports showing that two females per male or ≥ 50 oocytes per male are sufficient to detect biologically meaningful differences under standardized conditions. The study included two sampling time points, on days 15 and 30 of systemic heat stress exposure. This design enabled temporal assessment of physiological, molecular, and fertility-related parameters under different treatment conditions.
All groups received a daily oral gavage of 200 µL sterile phosphate-buffered saline (PBS; calcium- and magnesium-free, Kalazist Company, Iran). In the Niacin 1 and Niacin 2 groups, PBS was supplemented with niacin (nicotinic acid; Sigma, NO761-100G) at doses of 100 mg/kg/day (Niacin 1) and 200 mg/kg/day (Niacin 2), corresponding to approximately 4 mg and 8 mg per mouse per day based on average body weights (20–40 g). These doses were selected using allometric scaling from human equivalent doses (500–1000 mg/day for a 65 kg adult) and guided by previous rodent studies demonstrating dose-dependent reproductive effects and safety69,70.
Heat stress was applied to the Heat Control, Niacin 1, and Niacin 2 groups by exposing the animals to 36 ± 2 °C and 55% relative humidity for 4 h daily (10:00–14:00) over 30 consecutive days71, following methodologies validated in rodent and rat studies assessing testicular ischemia-reperfusion and reproductive stress models25,50,72,73. Heat exposure began 30 min after gavage. The selected temperature was well above the thermoneutral zone to ensure exposure to heat stress, while the 4-hour duration was sub-lethal, sufficient to induce chronic stress without causing acute mortality. The Control group and all female mice were maintained under thermoneutral conditions throughout the study. Female mice were used exclusively under thermoneutral conditions for both in vitro and in vivo fertility evaluations.
Animal monitoring
All mice had ad libitum access to commercial pelleted feed (21-Beiza Company, Fars, Iran) and fresh water provided via graduated bottles. Feed and water intake were recorded daily to monitor nutritional status and detect any changes due to treatment or heat exposure. To assess physiological responses to heat stress, body temperature was measured daily at 12:00, the midpoint of the heat exposure period. Rectal temperature was recorded using a pediatric digital thermometer with a flexible tip (Carent©), and abdominal surface temperature was measured using a non-contact infrared thermometer (NEUTRAL DT-8806©, CEM Instruments, Shenzhen, China) held 2 cm above the skin. All measurements were performed under minimal-stress conditions to avoid confounding effects.
Andrology assessments
Sample collection, timing, and organ dissection
Andrological parameters were evaluated at two time points: day 15 and day 30 of the experimental period. On each day, seven male mice from each group were randomly selected and euthanized by CO₂ inhalation followed by cervical dislocation at 08:00 AM, prior to daily heat exposure. Immediately postmortem, the testes were exposed, and the cauda epididymides were carefully excised and transferred to sperm analysis medium for evaluation. The abdominal cavity was then opened through a midline incision, and the reproductive tract was exposed. The seminal vesicles and prostate glands were identified and dissected under a stereomicroscope. The seminal vesicles were isolated intact, avoiding rupture of the glandular contents, and the entire prostate complex (including ventral, dorsal, and lateral lobes) was excised with minimal surrounding connective tissue. Additionally, the left testis was carefully removed after blunt dissection of the surrounding fat and epididymis. All tissues were briefly rinsed in ice-cold phosphate-buffered saline (PBS) to remove blood residues, then gently blotted on sterile filter paper to eliminate surface moisture. Wet combined weights of the seminal vesicles and prostate, as well as left testis were immediately measured using an analytical balance with 0.1 mg precision. The weights of each organ were recorded individually, and the combined weight of the seminal vesicles and prostate was also calculated74.
Total epididymal sperm count
For sperm collection, each cauda epididymis was finely minced into multiple pieces using sterile scissors and placed into sterile multi-well ELISA plates containing 100 µL of pre-warmed (37 °C) sperm analysis medium. The medium consisted of Tris (3.025 g), citrate (1.7 g), glucose (1.25 g), crystalline penicillin G (150,000 U), and crystalline streptomycin sulfate (150 mg), dissolved in distilled water to a final volume of 100 mL (pH = 6.76; osmolarity = 330 mosmol/kg H₂O), following methods previously validated for sperm viability and motility in small mammals75. Samples were incubated undisturbed on a 37 °C heating stage for 15 min before further analysis. Total sperm count was then determined using a hemocytometer under a light microscope.
Epididymal sperm motion kinematics
Sperm motility and motion characteristics were assessed using a computer-assisted semen analysis (CASA) system (HFT™ CASA System, HFT Co., Tehran, Iran) coupled with an Olympus bright-field microscope (Olympus, Tokyo, Japan) at 100× magnification. Approximately 5 µL of the sperm suspension was transferred into a pre-warmed (37 °C) counting chamber (Sperm 360 labs™, India) and immediately analyzed. At least five fields per sample were examined, with a minimum of 400 sperm cells analyzed across four random microscopic fields. Parameters evaluated included total motility (TM, %), progressive motility (PM, %), curvilinear velocity (VCL, µm/s), straight-line velocity (VSL, µm/s), average path velocity (VAP, µm/s), linearity (LIN, %), wobble (WOB, %), straightness (STR, %), mean angular displacement (MAD, °), amplitude of lateral head displacement (ALH, µm), and beat cross frequency (BCF, Hz). Sperm cells with a VAP < 10 μm/s were considered immotile. The CASA system operated at a frame rate of 50 Hz. Detailed technical settings used for the CASA analysis are provided in Table 10. All CASA parameters were adapted and validated specifically for BALB/c mouse sperm. Pilot experiments were conducted to optimize motility and velocity thresholds, ensuring accurate classification of motile, progressive, and immotile sperm. Calibration with reference sperm samples confirmed reproducibility, and all technical settings were applied consistently across experimental groups. This validation ensures that CASA measurements accurately reflect mouse-specific sperm motility and motion characteristics.
Table 10.
CASA technical settings.
| Function | Parameter | Value |
|---|---|---|
| Sperm detection | Minimum sperm area | 8 pixels |
| Maximum sperm area | 40 pixels | |
| Mean sperm area | 20 pixels | |
| Activity classification | Motile minimum speed 1 | 9.9 μm/s |
| Motile minimum speed 2 | 12 μm/s | |
| Motile maximum speed | 250 μm/s | |
| VSL low | 5 μm/s | |
| VSL high | 10 μm/s | |
| LIN low | 15% | |
| LIN high | 30% |
VAP, average path velocity; LIN, linearity.
Sperm viability
Sperm viability was assessed using Eosin-Nigrosin (EN) staining. Ten microliters of sperm suspension were mixed with an equal volume of 1% eosin Y and 10% nigrosin in 2.9% sodium citrate solution, smeared on a clean glass slide, air-dried, and examined under a light microscope at 1000× magnification. Live sperm excluded eosin and appeared white, while dead sperm absorbed eosin and appeared pink or red. At least 200 spermatozoa were counted per slide.
Membrane functional integrity
The hypo-osmotic swelling test (HOST) was used to assess sperm plasma membrane functional integrity. Ten microliters of sperm suspension were incubated in 100 µL of hypo-osmotic solution (75 mOsm/kg; sodium citrate 7.35 g/L and fructose 13.51 g/L) at 37 °C for 30 min. Afterwards, a smear was prepared and evaluated under light microscopy. Sperm exhibiting swollen or coiled tails were considered HOST-positive. A minimum of 200 sperm were assessed per sample.
Acrosome integrity (coomassie brilliant blue staining)
To evaluate acrosome integrity, air-dried sperm smears were fixed in methanol for 10 min, then stained with 0.22% Coomassie Brilliant Blue G-250 in 50% methanol and 10% glacial acetic acid for 2 min. Slides were rinsed and examined at 1000× magnification. Intact acrosomes stained blue over the apical region, while damaged acrosomes showed partial or no staining.
Acrosome status (CTC staining)
Capacitation and acrosome reaction status were evaluated using chlortetracycline (CTC) staining. Spermatozoa were fixed in 4% paraformaldehyde and incubated in CTC solution (750 µM CTC, 130 mM NaCl, 5 mM cysteine, 20 mM Tris-HCl; pH 7.8) for 20 min in the dark. Slides were examined using a fluorescence microscope. Based on fluorescence patterns, sperm were classified as: F pattern: non-capacitated (uniform bright fluorescence over head), B pattern: capacitated (fluorescence-free band in post-acrosomal region) AR pattern: acrosome-reacted (dim or absent fluorescence over head).
DNA integrity (sperm chromatin dispersion assay)
DNA fragmentation was evaluated using the sperm chromatin dispersion assay (SCDA), modified for light microscopy with Diff-Quik staining. Briefly, spermatozoa were embedded in low-melting-point agarose on pre-coated glass slides and allowed to solidify. Slides were then incubated in an acid denaturation solution (0.08 N HCl) for 7 min at room temperature, followed by a lysis solution (containing 2.5 M NaCl, 100 mM EDTA, 10 mM Tris-HCl, and 1% SDS; pH 7.4) for 25 min. After washing and drying, the slides were stained with Diff-Quik (HFT Co., Tehran, Iran) according to the manufacturer’s protocol. Spermatozoa were examined under a light microscope at 1000× magnification. Nuclei with large and dispersed halos of chromatin around the core were classified as non-fragmented (intact DNA), whereas those with small or no halos were considered fragmented. A minimum of 200 sperm cells were evaluated per sample.
DNA maturation (acridine orange staining)
Sperm nuclear protamine deficiency was assessed using acridine orange (AO) staining. Air-dried smears were fixed in Carnoy’s solution (methanol: acetic acid, 3:1) for 2 h, stained with AO solution (0.19 mg/mL AO in phosphate-citrate buffer, pH 2.5) for 5 min, and immediately examined under a fluorescence microscope. Mature sperm chromatin fluoresced green (double-stranded DNA), while immature or protamine-deficient sperm showed yellow to red fluorescence (single-stranded or denatured DNA).
Endocrinology and hepatic function tests
Sample collection and preparation
Immediately upon sacrifice, blood samples were collected from all animals via direct collection from the heart chambers at Days 15 and 30 into sterile tubes without anticoagulants. After clotting for 30 min at room temperature, samples were centrifuged at 3000 rpm for 15 min to separate serum, which was aliquoted and stored at − 20 °C. Testis tissues were excised immediately after euthanasia, rinsed with cold phosphate-buffered saline (PBS), blotted dry, and stored at − 80 °C. For testosterone assays, tissues were thawed, homogenized in ice-cold PBS (1:10 w/v), centrifuged at 12,000 × g for 15 min at 4 °C, and the supernatants stored at − 20 °C.
Biochemical analysis of hepatic enzymes
Serum activities of AST, ALT, and ALP were measured using Bio-Rex commercial kits following the manufacturer’s instructions. AST and ALT activities were determined by monitoring NADH oxidation spectrophotometrically at 340 nm after enzymatic substrate conversion. ALP activity was assessed by measuring the hydrolysis of p-nitrophenyl phosphate to p-nitrophenol at 405 nm. Quality controls and calibrators were included in each run.
Measurement of thyroid hormones (T3 and T4)
Serum T3 and T4 concentrations were quantified using Denazist ELISA kits. Samples, standards, and controls (100 µL/well) were added in duplicate to antibody-coated microplates and incubated at 37 °C for 60 min. After washing, biotinylated detection antibodies and streptavidin-HRP conjugate were sequentially added with incubation steps. Color development was achieved using TMB substrate, stopped with acid, and absorbance measured at 450 nm. Hormone levels were calculated from standard curves.
Determination of serum and testis testosterone
Testosterone in serum and testis homogenates was measured using the Accubind Testosterone ELISA kit. Samples and standards (100 µL/well) were added to testosterone antibody-coated wells and incubated for 60 min at room temperature. Following washes, HRP-labeled enzyme conjugate and TMB substrate were added with appropriate incubations. The reaction was stopped with acid, and absorbance read at 450 nm. Concentrations were determined via standard curves. All assays were run in duplicates with intra- and inter-assay variability below 10%.
Oxidative stress biomarker assessments
Blood and testicular tissue samples were collected from male mice (n = 6 per group) on Days 15 and 30. Mice were euthanized using CO₂ gas followed by cervical dislocation at 08:00 prior to heat stress exposure. Blood was collected by cardiac puncture and allowed to clot for 30 min at room temperature. Serum was separated by centrifugation at 3000 × g for 10 min and stored at − 80 °C. Testes were excised, washed with ice-cold saline, weighed, and homogenized in cold phosphate-buffered saline (10% w/v). Homogenates were centrifuged at 10,000 × g for 15 min at 4 °C, and supernatants stored at − 80 °C. All oxidative stress markers were measured using commercial ZellBio GmbH assay kits (Ulm, Germany), strictly following the manufacturer’s protocols.
Malondialdehyde (MDA) assay
Serum and testicular MDA levels were quantified using the ZellBio TBARS (thiobarbituric acid reactive substances) assay kit (Catalog No. ZB-MDA96). For serum, 100 µL of each sample or standard was mixed with 100 µL of thiobarbituric acid (TBA) reagent in microcentrifuge tubes. For testicular tissue, excised testes were washed in ice-cold saline, weighed, and homogenized in cold phosphate-buffered saline (10% w/v). Homogenates were centrifuged at 10,000 × g for 15 min at 4 °C and the supernatant collected. Then, 100 µL of testicular supernatant was mixed with 100 µL TBA reagent. Samples were incubated at 95 °C for 40 min to allow formation of MDA-TBA adduct, cooled on ice, centrifuged at 4000 × g for 10 min, and absorbance measured at 535 nm using a microplate reader. MDA concentration was determined from a standard curve and expressed as nmol/mL for serum and nmol/g tissue for testis.
Total antioxidant capacity (TAC) assay
TAC was measured using the ZellBio TAC assay kit (Catalog No. ZB-TAC96), based on the Trolox equivalent antioxidant capacity method. Twenty microliters of serum were added into each well of a 96-well plate, followed by 200 µL of pre-mixed TAC reaction solution (containing chromogenic substrate and assay buffer). The plate was incubated at room temperature for 10 min protected from light. Absorbance was read at 593 nm. Trolox standards provided in the kit were used to generate a calibration curve. TAC values were expressed as mmol Trolox equivalents per liter of serum.
Superoxide dismutase (SOD) activity assay
SOD activity was assessed with the ZellBio SOD assay kit (Catalog No. ZB-SOD96), which uses a colorimetric method based on the inhibition of formazan dye formation by superoxide radicals. Twenty microliters of serum were added to 200 µL of WST-1 working solution and enzyme working solution in a 96-well plate according to the kit protocol. After incubation at 37 °C for 20 min, absorbance was measured at 450 nm. SOD activity was calculated based on the percentage inhibition of WST-1 reduction compared to a blank control, and expressed in units per milliliter (U/mL), where one unit corresponds to 50% inhibition of superoxide-induced formazan dye formation. All samples were analyzed in duplicate. Standard curves and blank controls were included in each assay run. Intra-assay coefficients of variation were maintained below 10%. Assays were performed with reagents and samples equilibrated to room temperature to ensure consistency.
Molecular assay
RNA isolation, cDNA synthesis, and quantitative real-time PCR
Immediately after sacrifice, testicles were snap-frozen in liquid nitrogen and stored at − 80 °C until use. For RNA extraction, tissues were thawed on ice in 500 µl of sterile, calcium- and magnesium-free phosphate-buffered saline (PBS; molecular grade, Shellmax, Cat. No. P1530-100 ml). Approximately 50 mg of tissue was transferred into sterile 1.5 ml RNase-free microcentrifuge tubes and homogenized on ice using a mechanical homogenizer with sterile plastic tips. One milliliter of ice-cold RNX™-PLUS reagent (CinnaGen, Iran) was added to each homogenized sample. Tubes were vortexed for 5–10 s and incubated at room temperature for 5 min. Then, 200 µl of chloroform was added, the tubes were gently shaken for 15 s (without vortexing), and incubated on ice or at 4 °C for 5 min. Samples were centrifuged at 12,000 × g for 15 min at 4 °C to achieve phase separation. The upper aqueous phase was carefully transferred to a new RNase-free tube, mixed with an equal volume of absolute ethanol, and applied in two steps to RNA spin columns (Pars Tous Co., Iran). The remaining steps were performed according to the manufacturer’s instructions. The purified RNA was eluted in 30 µl of nuclease-free water and stored at − 80 °C until use. RNA concentration and purity were assessed using a NanoDrop™ spectrophotometer (Thermo Fisher Scientific, USA) by measuring absorbance at 260 and 280 nm. cDNA synthesis was carried out using 600–700 ng of total RNA and the Easy cDNA Synthesis Kit (Pars Tous Co., Iran), utilizing both oligo(dT) and random hexamer primers, following the manufacturer’s protocol. Quantitative real-time PCR (qPCR) was performed to assess the expression levels of key genes involved in apoptosis (Bax, Bcl2, Casp3), heat stress response (Hspa1a/b), and cell communication (Gja1), with Actb used as the reference gene for normalization, following methods similar to those already described in oxidative-stressed testicular tissue and testicular stress models in rodents72,76. Gene-specific primers were designed using NCBI Primer-BLAST and synthesized commercially. Primer sequences, product sizes, and melting temperatures are provided in Table 11. qPCR was carried out using a SYBR Green detection system in 25 µl reactions containing 12.5 µl of RealQ Plus 2× Master Mix Green (Ampliqon™, Denmark), forward and reverse primers, 4 µl of diluted cDNA (15 ng/µl), and nuclease-free water. Amplification was performed using a Rotor-Gene Q real-time PCR cycler (Qiagen, Germany) under the following cycling conditions: initial denaturation at 95 °C for 30 s, followed by 40 cycles of 95 °C for 5 s and 60 °C for 45 s. A melting curve analysis was conducted from 55 °C to 95 °C with 0.2 °C increments every 2 s to confirm the specificity of the amplified products. All samples were analyzed in triplicate. No-template and no-RT controls were included in each run to monitor for contamination and genomic DNA carryover. Relative expression levels were calculated using the 2^−ΔΔCt method. Primer efficiency was assessed using standard curves and confirmed to fall within the acceptable range of 90–110%.
Table 11.
Technical specifications of the PCR primers.
| Gene Symbol | Full Gene Name | Accession No. | Product Size (bp) | Primer Type | Sequence (5′→3′) | Length (nt) | Tm (°C) | GC% |
|---|---|---|---|---|---|---|---|---|
| Bax | BCL2-associated X protein | NM_007527.4 | 181 | Forward | AGCAAACTGGTGCTCAAGGC | 20 | 61.46 | 55.0 |
| Reverse | CCACAAAGATGGTCACTGTC | 20 | 56.36 | 50.0 | ||||
| Bcl2 | B-cell leukemia/lymphoma 2 | NM_009741.5 | 205 | Forward | GTGGTGGAGGAACTCTTCAG | 20 | 57.54 | 55.0 |
| Reverse | GTTCCACAAAGGCATCCCAG | 20 | 59.11 | 55.0 | ||||
| Casp3 | Caspase 3, apoptosis-related cysteine peptidase | XM_030243266.1 | 122 | Forward | TGACTGGAAAGCCGAAACTC | 20 | 58.12 | 50.0 |
| Reverse | AGCCTCCACCGGTATCTTCT | 20 | 60.03 | 55.0 | ||||
| Actb | Beta-actin | NM_007393.5 | 120 | Forward | AGTGTGACGTTGACATCCGT | 20 | 59.61 | 50.0 |
| Reverse | TGCTAGGAGCCAGAGCAGTA | 20 | 60.03 | 55.0 | ||||
| Hspa1a/b | Heat shock protein family A (Hsp70) member 1 A / 1B | NM_010479.2 / NM_010478.2 | 237 | Forward | GAAGGTGCTGGACAAGTGC | 19 | 59.05 | 57.9 |
| Reverse | GCCAGCAGAGGCCTCTAATC | 20 | 60.25 | 60.0 | ||||
| Gja1 | Gap junction protein alpha 1 (Connexin 43) | XM_036155617.1 | 106 | Forward | ACAGCGGTTGAGTCAGCTTG | 20 | 60.88 | 55.0 |
| Reverse | GAGAGATGGGGAAGGACTTGT | 21 | 58.81 | 52.4 |
In vitro fertility assay
Preparation of media
All culture media (T6, KSOM, and PB1; Table 12) were prepared in advance, sterile filtered, and equilibrated in a 5% CO₂ incubator at 37 °C for at least 4 h before use. All procedures involving gametes and embryos were performed under mineral oil (Sigma-Aldrich) in sterile 35-mm petri dishes (Nunc, Denmark) using a stereomicroscope in a laminar flow hood.
Table 12.
Component concentrations in T6, KSOM, and PB1 Media.
| Component | T6 | KSOM | PB1 |
|---|---|---|---|
| NaCl | 117.20 mM | 95.00 mM | 137.00 mM |
| KCl | 4.80 mM | 2.50 mM | 2.70 mM |
| KH₂PO₄ | 1.19 mM | 0.35 mM | 1.80 mM |
| Na₂HPO₄·2 H₂O | — | — | 10.00 mM |
| MgSO₄·7 H₂O | 0.81 mM | 0.20 mM | — |
| CaCl₂·2 H₂O | 1.71 mM | 1.71 mM | — |
| NaHCO₃ | 25.07 mM | 25.00 mM | — |
| Glucose | 5.56 mM | 0.20 mM | 5.56 mM |
| Sodium Pyruvate | 0.33 mM | 0.20 mM | — |
| Sodium DL-Lactate | 21.4 mM | 10.0 mM | — |
| EDTA (Na₂) | – | 0.01 mM | — |
| L-Glutamine | 1.00 mM | 1.00 mM | — |
| Phenol Red | 10 µg/mL | 10 µg/mL | — |
| BSA (fatty acid free) | 4 mg/mL | 4 mg/mL | 4 mg/mL |
| pH | 7.3–7.4 | 7.3–7.4 | 7.2–7.4 |
| Osmolality | ~ 285 mOsm/kg | 275–285 mOsm/kg | — |
Sperm collection and capacitation
Upon sacrifice, male mice were placed in a sterile dissecting area. The cauda epididymides were immediately removed and placed in a sterile dish containing a 500 µl drop of pre-equilibrated T6 medium under mineral oil. Small incisions were made in the cauda regions using fine scissors and 26G needles to transfer the extracted sperm clot into the medium drop. The sperm suspension was incubated for at least 1.5 h at 37 °C in 5% CO₂ to allow for capacitation. Only the peripheral zone of the capacitation drop was used to collect motile sperm for IVF.
Superovulation and oocyte collection
Female mice were hormonally superovulated with 15 IU of human menopausal gonadotropin (HMG; Humegnan, Iran) administered intraperitoneally (i.p.) in a 100 µL volume at 5:00 PM, followed 50 h later by 10 IU of human chorionic gonadotropin (hCG; Gonarex, Iran) injected i.p. in 100 µL at 7:00 PM. Fifteen to sixteen hours after hCG administration, the females were euthanized using CO₂ inhalation. Both oviducts were carefully excised and placed in pre-warmed PB1 medium. The ampullary region was punctured using sterile fine-tipped needles to release cumulus-oocyte complexes (COCs), which were gently aspirated using finely drawn glass pipettes and transferred into pre-equilibrated 100 µL drops of T6 medium under mineral oil. COCs were washed 3–4 times and held in the incubator at 37 °C with 5% CO₂ until in vitro fertilization (IVF).
Fertilization procedure
Up to 50 COCs were placed in each 100 µL drop of T6 medium under mineral oil. Capacitated sperm were diluted in pre-equilibrated T6 to achieve a final concentration of 1 × 106 sperm/mL, following IVF protocols previously used for rodent sperm functional and fertility assessment77. A calculated volume of this sperm suspension was added to each fertilization drop containing COCs. The gametes were co-incubated at 37 °C in 5% CO₂ for 6 hours. After fertilization, cumulus cells were gently pipetted to aid removal if necessary. Presumptive zygotes were washed 3–5 times in clean pre-equilibrated KSOM drops to completely eliminate excess sperm and debris. Embryos were then transferred into fresh 100 µL KSOM drops under mineral oil for further development.
In vitro culture and developmental assessment
Embryos were cultured continuously for 5 days in the same conditions (37 °C, 5% CO₂, high humidity) without media change. Embryonic development was assessed at the following time points: 24 h post-insemination (Day 1): two-cell embryos (indicative of successful fertilization), 72 h post-insemination (Day 3): morula stage, 120 h post-insemination (Day 5): blastocyst stage. The number of embryos at each developmental stage was recorded, and representative images were captured using an inverted phase-contrast microscope equipped with a digital camera (e.g., Olympus IX71 with DP27 camera). Embryo development was expressed as a percentage of the initial number of oocytes cultured per group.
In vivo fertility assay
The in vivo fertility assay was conducted at the end of a 30-day treatment period using adult BALB/c mice. Female mice were synchronized using a modified hormonal protocol consisting of two intraperitoneal injections of PGF₂α (Dinoprost tromethamine, 10 µg/mouse) administered three days apart, with a single injection of progesterone (2 mg/mouse, s.c.) administered 24 h after the first PGF₂α dose, as described previously78. Vaginal cytology was performed daily using saline lavage and methylene blue staining to determine estrous stages. Only females identified in the estrus stage were selected for mating13. These females were housed with males from their respective treatment groups (2 females per 1 male) and cohabited for three consecutive days. The presence of a vaginal plug was checked each morning and considered evidence of mating. Plug-positive females were separated and monitored individually for pregnancy and delivery outcomes. Reproductive parameters recorded included pregnancy rate, litter size, stillbirth rate, and offspring sex ratio. Pup birthweights were measured within 24 h post-partum using a precision digital balance, and sex was determined based on external genital morphology.
Testicular histomorphometry
Sample collection and histological slide Preparation
At two designated time points (Day 15 and Day 30), five male mice from each experimental group were euthanized by CO₂ inhalation at 8:00 AM, prior to daily heat stress exposure. Immediately after euthanasia, the abdominal cavity was opened and both testes were carefully excised. Excess connective and adipose tissue were removed, and the testes were weighed using a precision analytical balance (± 0.001 g accuracy). The testes were then fixed in 10% neutral buffered formalin (pH 7.4) at room temperature for 72 h to preserve tissue architecture. The fixed testes were washed in PBS, dehydrated through graded ethanol, cleared in xylene, and embedded in paraffin using an automated processor. Paraffin blocks were trimmed and sectioned at 5 μm thickness, floated on a 40 °C water bath, mounted on slides, and dried overnight. Slides were then deparaffinized, rehydrated, stained with hematoxylin and eosin, dehydrated, cleared, and mounted with DPX (Di-styrene, Plasticizer, and Xylene).
Histomorphometry analyses
All stained slides were examined under a light microscope (Olympus BX51, Tokyo, Japan) connected to a digital camera (Dino-Lite, Taiwan) and a computer equipped with ImageJ software (version 1.53, NIH, USA) for morphometric measurements. For each testis, 25 randomly selected, round seminiferous tubule cross-sections with clear basement membranes and minimal distortion were chosen for analysis. The parameters measured included79:
• Seminiferous tubule diameter Two perpendicular diameters of each tubule were measured, and their mean was calculated to account for shape irregularities.
• Epithelial Thickness Measured as the linear distance from the basement membrane to the luminal border of the seminiferous epithelium at three equidistant points around each tubule and averaged.
• Spermatocyte Counts The number of spermatocytes per tubule cross-section was counted manually within the seminiferous epithelium.
• Interstitial Cell Counts Leydig and other interstitial cells were counted in at least twenty-five random, non-overlapping fields per testis at 400× magnification.
Histological Evaluation of Spermatogenic Function.
Three histological indices were used to assess spermatogenic function and quality79:
• Spermiogenesis Index (SI) Defined as the percentage of seminiferous tubules containing mature spermatids relative to the total number of tubules examined.
• Tubular Differentiation Index (TDI) Calculated as the percentage of seminiferous tubules containing more than three layers of differentiated germ cells, including spermatocytes and spermatids.
• Johnsen’s Score Each seminiferous tubule was assigned a score from 1 to 10 based on the degree of spermatogenic maturation and cellular organization, according to the Johnsen scoring system:
Score 10: Complete spermatogenesis with abundant spermatozoa.
Score 1: Absence of germ cells and complete tubular atrophy.
At least 100 seminiferous tubules were evaluated per animal, and mean values were calculated for each experimental group. Data were recorded using Microsoft Excel and subsequently analyzed statistically.
Statistical analyses
All statistical analyses were performed using SPSS software version 25 (IBM Corp., Armonk, NY, USA). Data were expressed as mean ± standard error of the mean (SEM). Normality of data distribution was assessed using the Shapiro-Wilk test. Depending on the experimental design, one-way or two-way repeated measures ANOVA was employed to evaluate differences among groups and across time points. When significant main effects or interactions were detected, post hoc comparisons were conducted using the least significant difference (LSD) test. Non-parametric variables, such as pregnancy rates and sex ratios, were analyzed using the Chi-square test or Fisher’s exact test as appropriate80. Statistical significance was set at P < 0.05 for all analyses.
Acknowledgements
We would like to express our gratitude to Prof. Behbahani (Medical Virology Lab, Shiraz University of Paramedical Sciences) and his cooperative staff members for their invaluable technical assistance with the molecular assays conducted in this research.
Author contributions
M.R.D. conceived the study. M.R.D., Z.M., G.J., and S.A.J. developed the methodology. M.R.D., Z.M., D.E., and A.M.A. conducted the investigation. M.R.D. performed the formal analysis. M.R.D. and Z.M. wrote the original draft. M.R.D., Z.M., H.R.M., G.J., and S.A.J. reviewed and edited the manuscript. H.R.M. performed the histological analysis. S.A.J. provided technical support for IVF. M.R.D. supervised the project. All authors reviewed and approved the final manuscript.Project administration: Mohammad Reza Divar.
Funding
This work was completely supported by Shiraz University [grant number: 2GCB3M369962]. The funding source had no involvement in the study design, collection, analysis and interpretation of data, writing of the report, or decision to submit the article for publication.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Setchell, B. The effects of heat on the testes of mammals. Anim. Reprod. (AR). 3, 81–91 (2018). [Google Scholar]
- 2.Hansen, P. J. Effects of heat stress on mammalian reproduction. Philosophical Trans. Royal Soc. B: Biol. Sci.364, 3341–3350 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mieusset, R. & Bujan, L. Testicular heating and its possible contributions to male infertility: a review. Int. J. Androl.18, 169–184 (1995). [DOI] [PubMed] [Google Scholar]
- 4.Rockett, J. C. et al. Effects of hyperthermia on spermatogenesis, apoptosis, gene expression, and fertility in adult male mice. Biol. Reprod.65, 229–239 (2001). [DOI] [PubMed] [Google Scholar]
- 5.Rizzoto, G., Boe-Hansen, G., Klein, C., Thundathil, J. & Kastelic, J. Acute mild heat stress alters gene expression in testes and reduces sperm quality in mice. Theriogenology158, 375–381 (2020). [DOI] [PubMed] [Google Scholar]
- 6.Sheikholeslami, S. A., Soleimanzadeh, A., Rakhshanpour, A. & Shirani, D. The evaluation of lycopene and cysteamine supplementation effects on sperm and oxidative stress parameters during chilled storage of canine semen. Reprod. Domest. Anim.55, 1229–1239 (2020). [DOI] [PubMed] [Google Scholar]
- 7.Agarwal, A., Virk, G., Ong, C. & Du Plessis, S. S. Effect of oxidative stress on male reproduction. World J. Men’s Health. 32, 1 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shahat, A. M., Rizzoto, G. & Kastelic, J. Amelioration of heat stress-induced damage to testes and sperm quality. Theriogenology158, 84–96 (2020). [DOI] [PubMed] [Google Scholar]
- 9.Ko, S. H. Effects of heat stress-induced sex hormone dysregulation on reproduction and growth in male adolescents and beneficial foods. Nutrients16, 3032 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Monageng, E., Offor, U., Takalani, N. B., Mohlala, K. & Opuwari, C. A review on the impact of oxidative stress and medicinal plants on Leydig cells. Antioxidants12, 1559 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aggarwal, A., Upadhyay, R., Aggarwal, A. & Upadhyay, R. Heat stress and hormones. Heat Stress Anim. Productivity 27–51 (2013).
- 12.Shakouri, N., Soleimanzadeh, A., Rakhshanpour, A. & Bucak, M. N. Antioxidant effects of supplementation of 3, 4-dihydroxyphenyl glycol on sperm parameters and oxidative markers following cryopreservation in canine semen. Reprod. Domest. Anim.56, 1004–1014 (2021). [DOI] [PubMed] [Google Scholar]
- 13.Nourian, A., Soleimanzadeh, A., Jalali, A. S. & Najafi, G. in Veterinary research forum. 341. [PMC free article] [PubMed]
- 14.Manna, P. R., Tena-Sempere, M. & Huhtaniemi, I. T. Molecular mechanisms of thyroid hormone-stimulated steroidogenesis in mouse Leydig tumor cells: involvement of the steroidogenic acute regulatory (StAR) protein. J. Biol. Chem.274, 5909–5918 (1999). [DOI] [PubMed] [Google Scholar]
- 15.Cai, H., Qin, D. & Peng, S. Responses and coping methods of different testicular cell types to heat stress: overview and perspectives. Biosci. Rep.41, BSR20210443 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weider, K., Bergmann, M. & Brehm, R. Connexin 43: its regulatory role in testicular junction dynamics and spermatogenesis. Histol. Histopathol.26(10) (2011). [DOI] [PubMed]
- 17.Hassanpour, H. et al. in Veterinary Research Forum. 125. [PMC free article] [PubMed]
- 18.Hafez Hafez, M. et al. Resveratrol mitigates heat stress-induced testicular injury in rats: enhancing male fertility via antioxidant, antiapoptotic, pro-proliferative, and anti-inflammatory mechanisms. Naunyn-Schmiedeberg’s Archives Pharmacology 1–15 (2025). [DOI] [PubMed]
- 19.Zhang, P. et al. Melatonin protects the mouse testis against heat-induced damage. Mol. Hum. Reprod.26, 65–79 (2020). [DOI] [PubMed] [Google Scholar]
- 20.Kaur, S. & Bansal, M. Protective role of dietary-supplemented selenium and vitamin E in heat‐induced apoptosis and oxidative stress in mice testes. Andrologia47, 1109–1119 (2015). [DOI] [PubMed] [Google Scholar]
- 21.Al-Zahrani, S., Mohany, M., Kandeal, S. & Badr, G. Thymoquinone and vitamin E supplementation improve the reproductive characteristics of heat stressed male mice. J. Med. Plants Res.6, 493–499 (2012). [Google Scholar]
- 22.Adel, O., El-Sherbiny, H. R., Shahat, M., Ismail, S. T. & A. & N‐Acetylcysteine supplementation improves testicular Haemodynamics, testosterone Levels, seminal antioxidant capacity and semen quality in Heat‐Stressed goat bucks. Reprod. Domest. Anim.59, e14709 (2024). [DOI] [PubMed] [Google Scholar]
- 23.Naseer, Z., Ahmad, E., Aksoy, M. & Epikmen, E. Impact of Quercetin supplementation on testicular functions in summer heat-stressed rabbits. World Rabbit Sci.28, 19–27 (2020). [Google Scholar]
- 24.Naseer, Z. et al. Dietary Quercetin maintains the semen quality in rabbits under summer heat stress. Theriogenology122, 88–93 (2018). [DOI] [PubMed] [Google Scholar]
- 25.Zolfaghari, S., Soleimanzadeh, A. & Baqerkhani, M. The synergistic activity of Fisetin on Quercetin improves testicular recover in ischemia-reperfusion injury in rats. Sci. Rep.15, 12053 (2025). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin, C. et al. Curcumin dose-dependently improves spermatogenic disorders induced by scrotal heat stress in mice. Food Funct.6, 3770–3777 (2015). [DOI] [PubMed] [Google Scholar]
- 27.Abshenas, J., Babaei, H., Allahbakhshi, Z. A. R. E. I. M. H. & Sharififar, F. A. (Veterinary research forum).
- 28.Mohajeri, D. Effects of Nigella sativa on heat-induced testis damage in mouse. Bratisl. Lek. Listy. 116, 264–269 (2015). [DOI] [PubMed] [Google Scholar]
- 29.Soleimanzadeh, A., Kian, M., Moradi, S. & Mahmoudi, S. Carob (Ceratonia siliqua L.) fruit hydro-alcoholic extract alleviates reproductive toxicity of lead in male mice: evidence on sperm parameters, sex hormones, oxidative stress biomarkers and expression of Nrf2 and iNOS. Avicenna J. Phytomedicine. 10, 35 (2020). [PMC free article] [PubMed] [Google Scholar]
- 30.Soleimanzadeh, A., Mohammadnejad, L. & Ahmadi, A. in Veterinary Res. Forum 265 . [DOI] [PMC free article] [PubMed]
- 31.Bogan, K. L. & Brenner, C. Nicotinic acid, nicotinamide, and nicotinamide riboside: a molecular evaluation of NAD + precursor vitamins in human nutrition. Annu. Rev. Nutr.28, 115–130 (2008). [DOI] [PubMed] [Google Scholar]
- 32.Stephenson, L. A. & Kolka, M. A. Increased skin blood flow and enhanced sensible heat loss in humans after nicotinic acid ingestion. J. Therm. Biol. 20, 409–423 (1995). [Google Scholar]
- 33.Benyó, Z. et al. GPR109A (PUMA-G/HM74A) mediates nicotinic acid–induced Flushing. J. Clin. Investig.115, 3634–3640 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wechalekar, H. et al. Effects of whole-body heat on male germ cell development and sperm motility in the laboratory mouse. Reprod. Fertility Dev.28, 545–555 (2016). [DOI] [PubMed] [Google Scholar]
- 35.Habibe, M. N. & Kellar J. Z. Niacin Toxic. (2020).
- 36.Ellsworth, M. A., Anderson, K. R., Hall, D. J., Freese, D. K. & Lloyd, R. M. Acute liver failure secondary to niacin toxicity. Case Reports in Pediatrics 692530 (2014). [DOI] [PMC free article] [PubMed]
- 37.Rader, J. I., Calvert, R. J. & Hathcock, J. N. Hepatic toxicity of unmodified and time-release preparations of niacin. Am. J. Med.92, 77–81 (1992). [DOI] [PubMed] [Google Scholar]
- 38.Kabirian, A., Batavani, R. A., Asri-Rezaei, S. & Soleimanzadeh, A. in Veterinary Research Forum. 217. [DOI] [PMC free article] [PubMed]
- 39.Song, X., He, Y. & Zhou, Y. Nicotinamide improves developmental competence and reduces oxidative stress of Porcine oocytes exposed to heat stress. Theriogenology184, 38–47 (2022). [Google Scholar]
- 40.Cantó, C. & Auwerx, J. Targeting Sirtuin 1 to improve metabolism: all you need is NAD+? Pharmacol. Rev.64, 166–187 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shen, Y., Xu, L. & Zhang, X. Resveratrol attenuates testicular damage in mice with experimental varicocele: role of ROS and apoptosis. Int. J. Mol. Sci.17, 918 (2016).27294923 [Google Scholar]
- 42.Zhang, P. et al. &. Melatonin improves testicular function in mice with experimental cryptorchidism by inhibiting oxidative stress and apoptosis. Oxid. Med. Cell. Longev.2014, 703516 (2014).
- 43.Jannatifar, R., Parivar, K., Roodbari, N. H. & Nasr-Esfahani, M. H. Effects of N-acetyl-cysteine supplementation on sperm quality, chromatin integrity and level of oxidative stress in infertile men. Reproductive Biology Endocrinol.17, 24 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Khouri, N. A. & El-Khatib, A. R. N-Acetylcysteine protects against heat stress–induced testicular damage in rats. Toxicol. Rep.5, 1176–1183. 10.1016/j.toxrep.2018.10.004 (2018). [Google Scholar]
- 45.Zhou, Y., Zhang, Y. & Wang, X. Protective effects of Quercetin on heat stress–induced testicular damage in mice. Andrologia5210.1111/and.13503 (2020).
- 46.Farjami, A., Soleimanzadeh, A. & Ayen, E. Evaluation of co-supplementation of Rutin in simmental bull semen extender: evaluation of kinetic parameters, sperm quality. Veterinary Res. Biol. Prod.37, 79–88 (2024). [Google Scholar]
- 47.Izanloo, H., Soleimanzadeh, A., Bucak, M., Imani, M. & Zhandi, M. The effects of glutathione supplementation on post-thawed Turkey semen quality and oxidative stress parameters and fertilization, and hatching potential. Theriogenology179, 32–38 (2022). [DOI] [PubMed] [Google Scholar]
- 48.Ramazani, N. et al. The influence of L-proline and fulvic acid on oxidative stress and semen quality of Buffalo bull semen following cryopreservation. Veterinary Med. Sci.9, 1791–1802 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sadeghirad, M., Soleimanzadeh, A., Shalizar-Jalali, A. & Behfar, M. Synergistic protective effects of 3, 4-dihydroxyphenylglycol and Hydroxytyrosol in male rats against induced heat stress-induced reproduction damage. Food Chem. Toxicol.190, 114818 (2024). [DOI] [PubMed] [Google Scholar]
- 50.Behnejad, G., Mohammadi, T. & Soleimanzadeh, A. Allicin and hesperidin protect sperm production from environmental toxins in mice. Sci. Rep.15, 14224 (2025). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Istanbullugil, F. R. Natural Products/Bioactive Compounds as a Source of Anticancer Drugs (2023).
- 52.Shahat, A. M. et al. Bovine spermatozoa: emergent indicators of testicular function and fertility. Anim. Reprod. Sci.211, 106228. 10.1016/j.anireprosci.2019.106228 (2020). [Google Scholar]
- 53.Mohammadnejad, K., Mohammadi, R., Soleimanzadeh, A., Shalizar-Jalali, A. & Sarrafzadeh-Rezaei, F. The effect of β-Cryptoxanthin on testicular Ischemia-Reperfusion injury in a rat model: evidence from testicular histology. Iran J. Vet. Surg10 (2024).
- 54.Maran, R. R., Arunakaran, J. & Aruldhas, M. M. T3 modulates LH-stimulated testosterone production in adult rat Leydig cells by affecting steroidogenic acute regulatory protein expression. Endocrine13, 55–60 (2000).11051047 [Google Scholar]
- 55.Xie, Y., Liu, Y. & Pan, Y. Toxic effects of high doses of niacin on liver in mice. BMC Pharmacol. Toxicol.21, 25. 10.1186/s40360-020-00410-7 (2020).32293547 [Google Scholar]
- 56.Ganji, S. H., Qin, S., Zhang, L., Kamanna, V. S. & Kashyap, M. L. Niacin inhibits fat accumulation and oxidative stress in hepatocytes and hepatoma cells. Mol. Cell. Biochem.393, 203–213 (2014). [Google Scholar]
- 57.Ramazani, N. et al. Reducing oxidative stress by κ-carrageenan and C60HyFn: the post-thaw quality and antioxidant status of Azari water Buffalo bull semen. Cryobiology111, 104–112 (2023). [DOI] [PubMed] [Google Scholar]
- 58.Xiao, Y., Yang, X. & Wang, Y. Nicotinamide protects bovine granulosa cells from heat stress-induced apoptosis by maintaining mitochondrial function and inhibiting oxidative stress. Reproductive Biology Endocrinol.15, 61 (2017). [Google Scholar]
- 59.Othman, A. I. et al. Melatonin controlled apoptosis and protected the testes and sperm quality against bisphenol A-induced oxidative toxicity. Toxicol. Ind. Health. 32, 1537–1549 (2016). [DOI] [PubMed] [Google Scholar]
- 60.Rao, M., Zhao, X. & Yang, J. Effect of transient scrotal hyperthermia on sperm parameters, seminal plasma biochemical markers, and oxidative stress in men. Asian J. Androl.18, 543–548 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bahrami, A., Divar, M. R., Azari, M. & Kafi, M. Nicotinic acid (Niacin) supplementation in cooling and freezing extenders enhances stallion semen characteristics. J. Equine Veterinary Sci.94, 103236 (2020). [DOI] [PubMed] [Google Scholar]
- 62.Gong, Y. et al. Heat stress reduces sperm motility via activation of glycogen synthase kinase-3α and Inhibition of mitochondrial protein import. Front. Physiol.8, 718 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Capela, L., Leites, I., Romão, R., Lopes-da-Costa, L. & Pereira, R. M. L. N. Impact of heat stress on bovine sperm quality and competence. Animals12, 975 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sabés-Alsina, M., Tallo-Parra, O., Mogas, M. T., Morrell, J. M. & Lopez-Bejar, M. Heat stress has an effect on motility and metabolic activity of rabbit spermatozoa. Anim. Reprod. Sci.173, 18–23 (2016). [DOI] [PubMed] [Google Scholar]
- 65.Paul, C., Teng, S. & Saunders, P. T. A single, mild, transient scrotal heat stress causes hypoxia and oxidative stress in mouse testes, which induces germ cell death. Biol. Reprod.80, 913–919 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Thanh, T. N., Van, P. D., Cong, T. D., Minh, L., Vu, Q. H. & T. & N. Assessment of testis histopathological changes and spermatogenesis in male mice exposed to chronic scrotal heat stress. J. Anim. Behav. Biometeorol.8, 174–180 (2020). [Google Scholar]
- 67.Kim, K. H., Park, K. H. & Kim, J. S. Resveratrol protects against heat-induced damage in the testes of adult mice. Korean J. Urol.54, 860–864. 10.4111/kju.2013.54.12.860 (2013). [Google Scholar]
- 68.Baqerkhani, M., Soleimanzadeh, A. & Mohammadi, R. Effects of intratesticular injection of hypertonic mannitol and saline on the quality of Donkey sperm, indicators of oxidative stress and testicular tissue pathology. BMC Vet. Res.20, 99 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nair, A. & Jacob, S. (2016).
- 70..
- 71.Škop, V. et al. Mouse thermoregulation: introducing the concept of the thermoneutral point. Cell Reports 31 (2020). [DOI] [PMC free article] [PubMed]
- 72.Mohammadnehjad, K., Mohammadi, R., Soleimanzadeh, A., Shalizar-Jalali, A. & Sarrafzadeh-Rezaei, F. in Veterinary Res. Forum 277 . [DOI] [PMC free article] [PubMed]
- 73.Mostahsan, Z., Azizi, S. & Soleimanzadeh, A. & Shalizar-Jalali, A. in Veterinary Research Forum. 665. [DOI] [PMC free article] [PubMed]
- 74.Bahmyari, S. et al. The effects of wharton’s jelly MSCs secretomes for restoring busulfan-induced reproductive toxicity in male mice. Hum. Exp. Toxicol.43, 09603271241269019 (2024). [DOI] [PubMed] [Google Scholar]
- 75.Heydari, M., Rakhshanpour, A., Khameneh, R. M. & Azad, A. S. in Veterinary Research Forum. 691. [DOI] [PMC free article] [PubMed]
- 76.Soleimanzadeh, A., Kian, M., Moradi, S. & Malekifard, F. Protective effects of hydro-alcoholic extract of Quercus Brantii against lead-induced oxidative stress in the reproductive system of male mice. Avicenna J. Phytomedicine. 8, 448 (2018). [PMC free article] [PubMed] [Google Scholar]
- 77.Afsar, M., Soleimanzadeh, A., Khaki, A. & Ayen, E. Improvement of Post-Thaw quality and in vivo fertility of simmental bull spermatozoa using ferulic acid. Veterinary Med. Sci.10, e70064 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pallares, P. & Gonzalez-Bulnes, A. A new method for induction and synchronization of oestrus and fertile ovulations in mice by using exogenous hormones. Lab. Anim.43, 295–299 (2009). [DOI] [PubMed] [Google Scholar]
- 79.Moradi, H. R. et al. Protective effects of wheat sprout extract on acrylamide-induced toxicity in testis, prostate gland and sperm parameters of rats. J. Mol. Histol.56, 1–12 (2025). [DOI] [PubMed] [Google Scholar]
- 80.Izanloo, H., Soleimanzadeh, A., Bucak, M. N., Imani, M. & Zhandi, M. The effects of varying concentrations of glutathione and trehalose in improving microscopic and oxidative stress parameters in Turkey semen during liquid storage at 5 C. Cryobiology101, 12–19 (2021). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.