Abstract
The fusion of cellular membranes comprises several steps; membrane attachment requires priming of SNAREs and tethering factors by Sec18p/NSF (N-ethylmaleimide sensitive factor) and LMA1. This leads to trans-SNARE pairing, i.e. formation of SNARE complexes between apposed membranes. The yeast vacuole system has revealed two subsequent molecular events: trans-complex formation of V-ATPase proteolipid sectors (V0) and release of LMA1 from the membrane. We have now identified a hetero-oligomeric membrane integral complex of vacuolar transporter chaperone (Vtc) proteins integrating these events. The Vtc complex associates with the R-SNARE Nyv1p and with V0. Subunits Vtc1p and Vtc4p control the initial steps of fusion. They are required for Sec18p/NSF activity in SNARE priming, membrane binding of LMA1 and V0 trans-complex formation. In contrast, subunit Vtc3p is required for the latest step, LMA1 release, but dispensible for all preceding steps, including V0 trans-complex formation. This suggests that Vtc3p might act close to or at fusion pore opening. We propose that Vtc proteins may couple ATP-dependent NSF activity to a subset of V0 sectors in order to activate them for V0 trans-complex formation and/or control fusion pore opening.
Keywords: membrane fusion/priming/Saccharomyces cerevisiae/SNARE/V-ATPase
Introduction
Many components of intracellular membrane fusion are conserved among eukaryotes and among various stations of the secretory and endocytic pathways (Jahn and Südhof, 1999; Pelham, 1999; Guo et al., 2000; Wickner and Haas, 2000). The early phase of this reaction, recognition and membrane attachment, requires SNARE proteins, Rab-like GTPases and complexes of tethering factors, such as the exocyst, TRAPP or the class C Vps complex (HOPS). While Rab proteins and SNAREs are quite conserved, most tethering factors do not display significant sequence similarity. A priming event, driven by the ATPase NSF (N-ethylmaleimide sensitive factor) and its cofactor α-SNAP, activates SNAREs and tethering factors. Tethering factors and Rab-like GTPases can establish an initial SNARE-independent interaction of the membranes. Priming leads to disruption of cis-SNARE complexes, i.e. complexes of SNAREs on the same membrane. In vacuole fusion, LMA1 stabilizes this labile activated state (Wickner and Haas, 2000). LMA1 is a heterodimeric complex of two small cytosolic proteins, thioredoxin and proteinase B inhibitor 2. LMA1 mutants show synthetic phenotypes with Sec18p/NSF mutants. Furthermore, LMA1 is also required for the fusion of endoplasmic reticulum (ER) transport vesicles with the Golgi (Barlowe, 1997), suggesting that it is a general fusion factor. Priming facilitates formation of trans-SNARE complexes, i.e. complexes between SNAREs on opposing primed membranes, which may establish a very close attachment (docking) of the membranes. The systems for priming, tethering and docking interact physically (McBride et al., 1999; Price et al., 2000; Sato et al., 2000; Ungermann et al., 2000; Wang et al., 2000). Tethering factors regulate nucleotide exchange on Rab proteins and, together with the Rab proteins, are required for the formation of trans- SNARE complexes (Eitzen et al., 2000; Sato et al., 2000; Seals et al., 2000; Wang et al., 2000; Wurmser et al., 2000).
The events following docking and trans-SNARE pairing are poorly understood. Trans-SNARE pairing itself may disturb lipid bilayer structure sufficiently to cause fusion (Hanson et al., 1997; Weber et al., 1998; Chen et al., 1999; Grote et al., 2000a). On the other hand, there are indications that trans-SNARE complexes need not be fully assembled for fusion to occur (Xu et al., 1999) and that they can be disassembled before full fusion has occurred without preventing completion of the reaction (Coorssen et al., 1998; Ungermann et al., 1998a, 1999). In yeast exocytosis, Sec1p has a function after trans-SNARE pairing (Grote et al., 2000b) and Munc18, a neuronal Sec1p homolog was implicated in fusion pore opening (Fisher et al., 2001). Several fusion reactions depend on an efflux of Ca2+ from the organellar lumen (Peters and Mayer, 1998; Holroyd et al., 1999; Porat and Elazar, 2000; Pryor et al., 2000), which is triggered by docking. Calmodulin probably acts as a receptor for the calcium efflux on organelles (Peters and Mayer, 1998; Porat and Elazar, 2000; Pryor et al., 2000).
Vacuole fusion shares many features with other intracellular fusion reactions. Most components identified so far, such as Sec18p/NSF, Sec17p/α-SNAP, the SNAREs Vam3p, Nyv1p, Vam7p, Vti1p and Ykt6p, the Rab-GTPase Ypt7p, and the HOPS complex of tethering factors (Vps proteins 11, 16, 18, 33, 39 and 41), have well established roles in recognition and membrane attachment (Wickner and Haas, 2000). Vacuole priming by Sec18p/NSF leads to several events. Cis-SNARE complexes are disrupted, Sec17p/α-SNAP is released from the membrane and LMA1 shifts from a Sec18p/NSF-dependent into a SNARE (Vam3p)-dependent binding state. Priming also partially releases HOPS from the membrane (Eitzen et al., 2000; Price et al., 2000; Seals et al., 2000). After priming, a trans-SNARE complex forms in a HOPS- and Ypt7p-dependent fashion. It leads to docking, i.e. stable vacuole association (Mayer and Wickner, 1997; Ungermann et al., 1998a; Sato et al., 2000). The activity of two Rho–GTPases, Cdc42p and Rho1p, is also required up to this reaction stage (Eitzen et al., 2001; Müller et al., 2001), but their function is still enigmatic. Further factors with a less well explored function are phosphatidylinositol-4,5-bisphosphate (Mayer et al., 2000) and Vac8p, an armadillo repeat protein that becomes palmitoylated during fusion (Fleckenstein et al., 1998; Pan and Goldfarb, 1998; Wang et al., 1998, 2001; Veit et al., 2001).
Docking activates a calcium efflux from the vacuolar lumen (Peters and Mayer, 1998; Eitzen et al., 2000). This enhances calmodulin binding to the membrane (Peters and Mayer, 1998) and is required for V-ATPase V0 sectors from apposed membranes to form trans-complexes (Peters et al., 2001). V0 trans-complex formation depends on Ypt7p action and trans-SNARE pairing. However, once formed, maintenance of V0 trans-complexes is independent of trans-SNARE complexes (Peters et al., 2001), as is the completion of fusion (Ungermann et al., 1998a). This places V0 trans-complex formation as a chemically defined reaction downstream of trans-SNARE pairing and calcium efflux. The fact that V0 is required at this terminal reaction stage can be shown by kinetic studies with affinity-purified antibodies to V0 subunits and by specific V0 mutants (M.J.Bayer, C.Peters and A.Mayer, in preparation). The V0 sector contains a multimeric complex of the proteolipid Vma3p. Purified Vma3p reconstituted into artificial liposomes can generate large pores which open in a Ca2+- and calmodulin-dependent fashion, possibly by lateral separation of the proteolipid subunits and/or radial expansion of the complex (Peters et al., 2001). These properties and the timing of V0 trans-complex formation suggested a role of the V0 sector in membrane fusion, perhaps similar to that proposed in various hypotheses on protein pore-mediated fusion of membranes (Lindau and Almers, 1995; Zimmerberg, 2001). For example, V0 sectors might form continuous proteinaceous channels between the apposed membranes. Radial expansion and lateral separation of their proteolipid subunits could create amphiphilic clefts, permitting the lipids to migrate between the membranes and the bilayers to merge (Peters et al., 2001).
We have now identified the membrane integral vacuolar transporter chaperone (Vtc) complex as a crucial interface between priming and the final stage of vacuole fusion. The Vtc complex consists of four proteins. Vtc1p, Vtc2p and Vtc3p are integral membrane proteins with three predicted transmembrane helices at their C-termini. They localize to vacuoles and, a minor fraction, also to other compartments (Cohen et al., 1999; Ogawa et al., 2000; O.Müller, M.J.Bayer and A.Mayer, submitted). The N-terminal parts of Vtc2p and Vtc3p form large hydrophilic domains exposed to the cytosol and are homologous to Vtc4p, which has no predicted transmembrane spans but behaves like an integral membrane protein (Cohen et al., 1999; O.Müller, M.J.Bayer and A.Mayer, submitted). Vtc1p (14 kDa) and Vtc4p (75 kDa) together are similar in sequence and domain structure to Vtc2p and Vtc3p (95 kDa). The Vtc proteins have homologs in Drosophila and Caenorhabditis elegans and possibly in man (Cohen et al., 1999). However, their molecular function has remained elusive. We have now analyzed the role of the Vtc proteins in vacuole fusion and have discovered striking molecular links between a Vtc complex, the V0 sector and the SNARE/NSF machinery.
Results
A Vtc complex is associated with the R-SNARE Nyv1p and the V0 sector
Several observations suggested that the Vtc proteins might be involved in vacuole fusion. Together with V0 components, they can be cross-linked and co-immuno- precipitated with calmodulin (Peters et al., 2001), which acts as a calcium sensor in vacuole fusion. Furthermore, a vtc1 mutant suppresses the lethality of V-ATPase mutations (Cohen et al., 1999), suggesting a functional relationship to the V-ATPase. Therefore, we explored the interactors of Vtc proteins in detail.
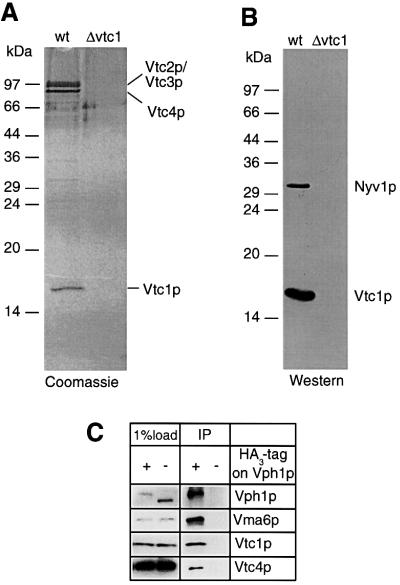
We generated antibodies to Vtc1p and immunoprecipitated Vtc1p from vacuolar membranes solubilized in the detergent CHAPS. Three major bands co-fractionated with Vtc1p in roughly stoichiometric amounts (Figure 1A). They could be precipitated from wild-type vacuoles only and not from vacuoles of vtc1 deletion mutants. The major co-precipitated bands were identified by MALDI mass spectrometry as Vtc2p, Vtc3p and Vtc4p. Immuno- precipitation against Vtc2p (HA tagged), Vtc3p (HA tagged) or Vtc4p yielded similar patterns (not shown). This indicates that all four Vtc proteins form a complex, extending the initial proposal of a Vtc1p–Vtc4p interaction, which was based on the observation that deletion of VTC1 leads to a severe reduction of Vtc4p levels (Cohen et al., 1999). Several minor bands were also co-precipitated specifically from wild-type membranes, but their amounts did not permit direct identification. Western blotting revealed that one of those minor bands was Nyv1p, an R-SNARE essential for vacuolar fusion (Figure 1B). The cognate Q-SNARE Vam3p could not be found, although the sensitivity of immunodecoration would have allowed us to detect amounts of Vam3p at least an order of magnitude below those of Nyv1p (not shown). We also performed immunoprecipitation with an HA-tagged version of Vph1p, an integral component of the V0 sector of the V-ATPase. Vtc1p and Vtc4p were recovered in association with Vph1p (Figure 1C). Co-precipitation was specific, because it was not observed with untagged Vph1p. Furthermore, a peptide tag on Vph1p showed lower proteolytic sensitivity (in vivo) in vtc1 and vtc3 deletion mutants than in the wild type (O.Müller, M.J.Bayer and A.Mayer, submitted; compare with Figure 7B). This indicates an altered conformation of Vph1p in vtc mutants and indicates an interaction of V0 with the Vtc complex in living cells. The observation is complemented by genetic evidence of suppression of V-ATPase mutations by a vtc1 mutant (Cohen et al., 1999). Collectively, the data suggest a physical link of V0 to Vtc proteins, which can influence V0 conformation.
Fig. 1. Co-immunoprecipitation with Vtc1p. (A) Vacuoles from wild type (OMY1) or a vtc1Δ strain (OMY2) were solubilized in CHAPS and incubated with immobilized affinity-purified antibodies to Vtc1p. Proteins eluted from the column were resolved by SDS–PAGE and stained with colloidal Coomassie Blue. The bands indicated were excised and identified by MALDI mass spectrometry. (B) An aliquot of the samples in (A) was used for western blotting with antibodies to Vtc1p and Nyv1p. (C) Vacuoles from strain SBY119 (chromosomally expressing Vph1p-His6-HA3) or from BJ3505 (non-tagged) were solubilized in Triton X-100 and incubated with monoclonal antibodies to HA and protein G–Sepharose. Absorbed proteins were analyzed by SDS–PAGE and western blotting with the antibodies indicated.

Fig. 7. Vtc3p affects a step between V0 trans-complex formation and LMA1 release. (A) Effect of Sec18p and LMA1 on fusion of vtc1Δ and vtc3Δ vacuoles. Vacuoles prepared from vtc3Δ (strains OMY3/OMY6) or vtc1Δ (strains OMY2/OMY5) were used in standard fusion reactions with the indicated additions of Sec18p (15 µg/ml) and LMA1 (0.5 µg/ml). After 70 min at 27°C, fusion activity was assayed. Fusion of the mutant vacuoles without Sec18p and LMA1 was set as the 1× value for each strain (compare with Figure 2B; n = 3). (B) V0 trans-complex formation was assayed as in Figure 5E, but without Sec18p and LMA1, using wild-type (OMY8/OMY9) or vtc3Δ (OMY12/OMY13) vacuoles.
The Vtc proteins are novel fusion factors
In order to test whether Vtc proteins are involved in fusion, we generated deletion strains for each of the VTC genes. All strains were viable on YPD at 30°C, although a vtc4 deletion could only be obtained in a different strain background through sporulation of a diploid, heterozygous cell. The vtc3Δ mutant showed a slow-growth phenotype, whereas the vtc1Δ, vtc2Δ and vtc4Δ mutants grew almost at wild-type rates (compare with Cohen et al., 1999). A genomic functional analysis approach showed that an insertion mutation in VTC1 leads to slow growth (Smith et al., 1996). Overexpression of the protein is lethal in Schizosaccharomyces pombe (Murray and Johnson, 2000). Vacuoles in all deletion strains except vtc2Δ showed abnormal morphology, indicating that vacuole biogenesis was disturbed. Wild-type cells typically had three to five smaller vacuoles, whereas the vtc1Δ mutant tended to have one enlarged vacuole (Figure 2A). This phenotype, which was also observed with vtc4Δ mutants (not shown), is the same as reported for an nyv1Δ (R-SNARE) mutant (Nichols et al., 1997). It correlates with the preferential association between Vtc1p and Nyv1p in co-immunoprecipitation experiments (Figure 1) and supports a functional interaction. Vacuoles of the vtc3Δ mutant were fragmented into multiple smaller vesicles, similar to in the majority of mutants for vacuolar fusion components (Wickner and Haas, 2000). In contrast to vam3Δ (Q-SNARE) or ypt7Δ (Rab-GTPase) mutants, in which vacuolar fragments are dispersed throughout the cytosol, fragments in vtc3Δ mutants were clustered. Clustered vacuole fragments were also described for temperature-sensitive alleles of calmodulin or protein phosphatase 1 (Glc7p), both of which are involved in the post-docking phase of vacuole fusion (Peters and Mayer, 1998; Peters et al., 1999), and for Vac8p (Fleckenstein et al., 1998; Pan and Goldfarb, 1998; Wang et al., 1998).
Fig. 2. The Vtc proteins are required for vacuole fusion. (A) Vacuoles from the wild type (DKY 6281) or vtc1Δ (OMY5) or vtc3Δ (OMY6) were labeled with the fluorescent dye FM4-64 at 30°C (Peters and Mayer, 1998) and visualized by fluorescence microscopy. Nomarski and fluorescence images were overlaid. (B) Standard fusion reactions without cytosol were performed with vacuoles from the wild type (OMY1/DKY6281 or SBY86/SBY85, respectively) or the indicated deletion mutants. Fusion of the wild-type vacuoles ranged from 3.5 to 6.2 U and was set to 100%. Three independent experiments were averaged. (C) Antibody inhibition. Standard fusion reactions without cytosol were incubated (10 min, 0°C) with the indicated amounts of affinity-purified antibodies to Vtc4p. All samples had been supplemented with control antibodies (protein G-purified from a non-immune serum) to the same final IgG concentration in every sample. After 70 min incubation at 27°C, fusion was assayed (n = 3). The fusion signal of the samples without specific antibodies (2.3–2.7 U) was set to 100%.
Vacuoles from vtc1Δ, vtc3Δ and vtc4Δ mutants did not fuse in vitro, whereas the activity of vtc2Δ vacuoles was only moderately affected (Figure 2B). Since vacuole fusion requires a proton motive force up to docking (Mayer et al., 1996; Mayer and Wickner, 1997; Ungermann et al., 1999) and the Vtc proteins have a genetic relationship to V-ATPase function (Cohen et al., 1999), we checked the H+-pump activity of the mutants. Under conditions of our fusion assay, vtc1Δ and vtc3Δ mutants showed only minor deviations in H+-pump activity from the wild type, which cannot explain their fusion deficiency (O.Müller, M.J.Bayer and A.Mayer, submitted). A direct involvement of Vtc proteins in vacuole fusion could also be demonstrated with affinity purified antibodies to Vtc4p. They inhibited fusion (IC50 = 5 µM; Figure 2C) but did not influence pump activity (O.Müller, M.J.Bayer and A.Mayer, submitted). This rules out the possibility that the fusion defect arose as a secondary effect of the vtc4 mutation. The IC50 value of the antibodies to Vtc4p is significantly higher than the IC50 of antibodies to many other fusion factors, which are usually <1 µM (Haas et al., 1995; Haas and Wickner, 1996; Mayer et al., 1996; Price et al., 2000). This may be due to the abundance of Vtc proteins on the vacuole, which is similar to that of the V-ATPase and higher than that of SNAREs (data not shown). Furthermore, the antibodies used are polyclonal, i.e. directed against many different epitopes of Vtc4p. The subclass of antibodies responsible for the inhibitory effect may only be a minor fraction among the total anti-Vtc4p–IgGs. Antibodies to Vtc1p had no effect on fusion at comparable concentrations (data not shown), probably because this small protein is embedded in the membrane and is barely accessible (Cohen et al., 1999; O.Müller, M.J.Bayer and A.Mayer, submitted). Monospecific antibodies to Vtc2p and Vtc3p have not been obtained so far. Taken together, Vtc1p, Vtc3p and Vtc4p are required for vacuole fusion and Vtc2p can be deleted without major effects.
We assayed the relative amounts of fusion relevant proteins on vacuoles from vtc deletion mutants. These vacuoles contained wild-type levels of all fusion relevant proteins tested, except for the LMA1 complex, a heterodimer of Trx1p and Pbi2p (showing no changes in V0 subunits; Figure 3; O.Müller, M.J.Bayer and A.Mayer, submitted). LMA1 undergoes a cycle of Sec18p/NSF-dependent membrane binding, transfer to a Vam3p (Q-SNARE)-dependent site and release from the membrane in the final phase of fusion (Xu et al., 1998). vtc3Δ vacuoles carried significantly more LMA1 than those from the wild type. In contrast, vtc2Δ vacuoles had normal amounts and vtc1Δ or vtc4Δ vacuoles had no detectable LMA1. Thus, the fusion incompetence of vtc1Δ, vtc4Δ and vtc3Δ vacuoles (Figure 2B) correlated with their abnormal levels of LMA1. If integrated into the LMA1 cycle, these data suggest that Vtc1p and Vtc4p are required for the initial binding of LMA1 and that Vtc3p is involved in LMA1 release in the final stage of fusion.

Fig. 3. LMA1 binding to vacuoles of different deletion strains. Vacuoles (30 µg) from the indicated strains were TCA precipitated and subjected to SDS–PAGE and western blotting with different antibodies. All strains were deleted for PEP4.
Vtc1p and Vtc4p are required for NSF activity
We combined kinetic studies with antibodies and molecular assays for defined sub-reactions to characterize Vtc protein function further. Three assays for priming are available: Sec17p/α-SNAP release (Mayer et al., 1996), disruption of cis-SNARE complexes (abolishing Vam3p– Nyv1p co-immunoprecipitation; Ungermann et al., 1998b) and partial release of the HOPS complex (monitored via the HOPS component Vps41p/Vam2p; Price et al., 2000). All three sub-reactions were defective in vacuoles from vtc1Δ and vtc4Δ mutants, in comparison with the corresponding wild-type vacuoles for each mutation. Note that the efficiency of HOPS release was strain dependent. The corresponding wild type to vtc4Δ showed a more efficient HOPS release than the corresponding wild type to vtc1Δ (Figure 4A, rows A and E). In vacuoles from a vtc2Δ mutant all the priming reactions occurred normally (Figure 4A and C). Sec17p/α-SNAP release was also prevented by antibodies to Vtc4p (Figure 4B), underscoring the direct role of Vtc4p in priming. Equivalent concentrations of non-immune antibodies (Figure 4B) or antibodies to the V-ATPase subunit Vph1p (M.J.Bayer, C.Peters and A.Mayer, in preparation) had no effect. We conclude that Vtc1p and Vtc4p are required for Sec18p/NSF-mediated SNARE and HOPS activation.
Fig. 4. Priming depends on Vtc1p and Vtc4p. Vacuoles were prepared from different vtc deletion mutants [strains OMY2 (B), OMY3 (C), OMY4 (D) and SBY83 (F)] and from their corresponding wild types [OMY1 (A) and SBY86 (E), respectively]. (A) Sec17p/α-SNAP release. Standard fusion reactions (3-fold volume) without cytosol were incubated (70 min) without ATP on ice (–fusion) or with ATP at 27°C (+fusion). Reactions were chilled on ice and centrifuged (10 000 g, 10 min, 4°C). The supernatants (S) were recovered and the pellets (P) resuspended in 90 µl of PS buffer. All samples were TCA-precipitated and processed for SDS–PAGE and western blotting with the antibodies indicated. Alkaline phosphatase (ALP) serves as a membrane integral vacuolar marker. (B) Effect of anti-Vtc4p on Sec17p release. Vacuoles (BJ3505) were used in standard fusion reactions without cytosol supplemented with affinity-purified antibodies to Vtc4p or with control antibodies (protein G-purified from a non-immune serum). After 10 min on ice, the ATP-regenerating system was added and the samples incubated for 15 min on ice (–fusion) or at 27°C (+fusion). The vacuoles were diluted with 0.5 ml of PS, 150 mM KCl, 1× PIC, 0.5 mM PMSF, re-isolated (10 000 g, 10 min, 4°C), resuspended in 0.2 ml of the same buffer, TCA-precipitated and analyzed as in (A). Antibody concentrations were 45 µM. (C) SNARE complex disruption. Standard fusion reactions (15-fold volume) without cytosol were incubated without the ATP-regenerating system on ice or with the ATP-regenerating system at 27°C for 10 min. SNARE complexes were recovered by immunoprecipitation and analyzed by SDS–PAGE and western blotting.
vtc1 and vtc4 mutant vacuoles were deficient for Sec18p/NSF-driven priming, although they had normal amounts of this protein (Figure 3). This suggests that the endogenous membrane-bound Sec18p/NSF might be inactive. We tested this hypothesis by asking whether vtc mutant vacuoles could be rescued by adding purified recombinant Sec18p/NSF and/or LMA1. LMA1, which is lacking on vtc1Δ and vtc4Δ vacuoles (Figure 3), binds to Sec18p/NSF and moderately stimulates its ATPase activity (Xu et al., 1997, 1998). The activity of both recombinant proteins had been verified in separate tests. The two factors synergically stimulated fusion of salt-washed vacuoles (data not shown; compare with Xu et al., 1997), as well as ER to Golgi trafficking in vitro (A.Spang, personal communication; compare with Barlowe, 1997). Addition of LMA1 alone did not reactivate vtc1Δ vacuoles at all (Figure 5A), even at concentrations 20 times higher than those sufficient to activate vacuoles from which LMA1 had been removed by salt washing (compare with Xu et al., 1997, 1998). The fusion defect of vtc1Δ vacuoles was therefore not due to the lack of LMA1 in the assay. Addition of Sec18p/NSF alone rescued fusion of vtc1Δ vacuoles to 20–30% of wild-type activity (Figure 5B). The rescued reaction was sensitive to established inhibitors of vacuole fusion, such as BAPTA, GTPγS or microcystin LR, demonstrating that it followed the authentic reaction pathway (data not shown). Equivalent results were obtained with vtc4 mutant vacuoles (data not shown). When added in addition to Sec18p/NSF, LMA1 only led to a very minor stimulation of fusion (3–5%, Figure 5A). Rescue to more than 20–30% of wild-type activity was not possible, even when both components were titrated over broad concentration ranges in various combinations (Figure 5A and B and data not shown). The fact that inactivation of Vtc1p and Vtc4p by mutation or antibodies blocks priming suggests that these proteins are important cofactors of endogenous Sec18p/NSF on the vacuole.
Fig. 5. Rescue of vtc1Δ vacuoles by exogenous Sec18p. (A) Fusion activity. Standard fusion reactions without cytosol were performed with vacuoles from vtc1Δ (OMY2, OMY5) or corresponding wild-type cells (OMY1, DKY 6281). The samples had been supplemented with 15 µg/ml Sec18p and the indicated amounts of LMA1. One hundred per cent fusion was 6.40 U. (B) As in (A), but the reactions were supplemented with the indicated amounts of Sec18p. (C and D) Priming. Vacuoles from strain OMY2 (vtc1Δ) were incubated in fusion reactions without cytosol, but supplemented with 15 µg/ml purified Sec18p and 0.5 µg/ml LMA1 or with control buffer only. Vacuoles were analyzed for SNARE complex disruption (C) after 10 min at 27°C as in Figure 4C. Sec17p and HOPS (Vps41p) release (D) were assayed at the end of the fusion reaction as in Figure 4A. (E) V0 trans-complex formation was assayed as described (Peters et al., 2001). Fusion reactions (1 ml) without cytosol were incubated (27°C, 45 min) in the presence or absence of Sec18p (15 µg/ml) and LMA1 (0.5 µg/ml). They contained a mixture of vacuoles bearing either Vph1p-His6-HA3 or Vph1p-AU1 [strains OMY8/OMY9 (wt); OMY10/OMY11 (vtc1Δ)]. Vph1p-His6- HA3 was immunoprecipitated. Co-immunoprecipitated Vph1p-AU1 was analyzed by western blotting.
Aside from genetic inactivation of the two proteins necessary for LMA1 binding (Vtc1p and Vtc4p), LMA1 can also be removed from wild-type vacuoles by a salt wash (Xu et al., 1997, 1998). Such wild-type vacuoles cannot fuse, but they retain Vtc1p and Vtc4p (O.Müller, M.J.Bayer, and A.Mayer, submitted). They can be strongly stimulated by readdition of low concentrations of LMA1 (Xu et al., 1997, 1998). vtc1Δ vacuoles do not show such stimulation (Figure 5A). This is not due to incomplete activation of SNAREs or HOPS. Both sub-reactions, monitored by SNARE complex disruption (Figure 5C) and HOPS release (Figure 5D), can be completely rescued by adding the optimal concentrations of exogenous Sec18p/NSF and LMA1 determined in Figure 5A. Rescue also worked with Sec18p/NSF alone (data not shown). Sec17p/α-SNAP release was not fully rescued (Figure 5D). This is probably due to unspecific binding of excess exogenous Sec18p/NSF to the vacuoles (J.Kunz and A.Mayer, unpublished observation), which may result in partial retention of Sec17p/α-SNAP on the membrane. The findings that, despite complete priming rescue, fusion was still inefficient suggest that there may be an aspect of fusion that is independent of SNARE and HOPS activation but depends on LMA1, Vtc1p and Vtc4p. This hypothesis is strengthened by an analysis of V0 trans-complex formation (Figure 5E). V0 is involved in the final stage of fusion, as demonstrated by the specific effects of different V0 mutant vacuoles and by kinetic studies with antibodies to V0 subunits (M.J.Bayer, C.Peters and A.Mayer, in preparation). Furthermore, V0 trans-complex formation is intimately tied into the reaction sequence. It requires docking and precedes full fusion (Peters et al., 2001). In vtc1Δ vacuoles, Sec18p/NSF and LMA1 reactivated V0 trans-complex formation, but to a significantly lower extent than observed with wild-type vacuoles (Figure 5E). In contrast, SNARE and HOPS priming had been rescued completely (Figure 5C and D). This is consistent with the view that, in addition to controlling Sec18p/NSF activity on vacuoles, Vtc1p and Vtc4p enhance the efficiency of V0 trans-complex formation.
We explored this possiblity further by kinetic assays, monitoring when the reaction became resistant to anti-Vtc4p (Figure 6). The majority of vacuoles complete distinct reaction steps within defined intervals and become resistant to inhibitors of these steps (Conradt et al., 1994; Mayer et al., 1996), revealing the sequence of events in vacuole fusion. Inhibitors or control buffer were added to an ongoing fusion reaction at different times and the samples were incubated further until the end of a standard fusion period (70 min). Another aliquot was chilled to stop fusion at that time and was used to monitor progression of the reaction. All inhibitors abolished fusion when added at the start of the reaction (Figure 6). After 15 min, the reaction was resistant to anti-Sec18p, i.e. priming was completed. After 35 min, the reaction was resistant to Gdi1p, which extracts the Rab-GTPase Ypt7p from the vacuolar membrane (Wickner and Haas, 2000), and to antibodies to the R-SNARE Nyv1p. Resistance to these two reagents indicates the completion of docking (Mayer and Wickner, 1997; Ungermann et al., 1998a). The inhibition curve for anti-Vtc4p overlapped with those of Gdi1p and anti-Nyv1p. Thus, the Vtc4p requirement extends up to the docking stage. We want to stress that this type of kinetic analysis indicates only the last step requiring the respective component. Functions in earlier reaction steps do not become apparent in this approach. Hence the result is consistent with and complements the molecular assays above. Taken together, the data suggest that Vtc1p and Vtc4p control SNARE and HOPS priming, are required for the LMA1 dependent part of fusion and have an accessory role in V0 trans-complex formation.
Fig. 6. Time course of inhibition by antibodies to Vtc4p. Standard fusion reactions without cytosol were started. After the indicated times at 27°C, inhibitors or control buffer only were added. The samples were left on ice for 10 min and then transferred to 27 or 0°C for the rest of the 70 min reaction period. Finally, fusion activity was assayed. Inhibitors used were 2 µM anti-Sec18p, 5 µM Gdi1p, 2 µM anti-Nyv1p and 80 µM anti-Vtc4p.
Vtc3p controls the last known step of vacuole fusion
vtc3Δ vacuoles did not fuse and, in contrast to vtc1Δ vacuoles, could not be stimulated by Sec18p/NSF (Figure 7A). They were fully competent for all priming reactions, such as Sec17p/α-SNAP release (Figure 4A), HOPS release (Figure 4A) and disruption of cis-SNARE complexes (Figure 4C). They even formed V0 trans-complexes very efficiently (Figure 7B). V0 trans-complex formation is followed by two events, a GTPγS- and a micro cystin LR-sensitive step (Peters et al., 2001). The latter is prerequisite for LMA1 release (Xu et al., 1998), which by inference is the last molecularly defined event in fusion. vtc3Δ vacuoles carried significantly higher amounts of LMA1 on their membrane (Figure 3), consistent with a defect in LMA1 release. These observations place the Vtc3p requirement after V0 trans-complex formation, but prior to LMA1 release.
In summary, the Vtc complex is physically and functionally connected to two systems of crucial importance to different stages of the reaction: two of its subunits, Vtc1p and Vtc4p, control the capacity of NSF to prime SNAREs and the HOPS tethering factors, a key event for membrane recognition and attachment. Furthermore, they are associated with V0 and promote V0 trans-complex formation during the transition from docking to full fusion. Vtc3p controls LMA1 release and progression of the reaction from V0 trans-complex formation to full fusion, i.e. the terminal step of the reaction (Xu et al., 1998; Peters et al., 2001).
Discussion
Recent studies implicated Vtc proteins in very different functions: as polyphosphate synthases (Ogawa et al., 2000); regulators of V-ATPase activity (Cohen et al., 1999); vacuolar transporter chaperones (vtc) influencing the distribution of membrane proteins (Cohen et al., 1999; Nelson et al., 2000) and proteins that are required for endocytosis (Murray and Johnson, 2000). We found molecularly defined physical and functional relations of these proteins to components involved in membrane fusion. This function in fusion could be the basis for several of the physiological effects of vtc mutations. It is compatible with the observed effects on endocytosis and with a potential function in the trafficking of membrane proteins, such as the plasma membrane ATPase (Cohen et al., 1999; Murray and Johnson, 2000). Blocked endocytic traffic (Murray and Johnson, 2000) might also explain why vtcΔ mutants do not show the phosphate overplus phenomenon, i.e. why they do not form poly- phosphate inside vacuoles upon shift from phosphate depletion to high phosphate media (Ogawa et al., 2000). Phosphate transfer into the vacuole might occur via endocytosis, similarly as shown for fluorescent dyes or acidic buffer (Munn and Riezman, 1994). The proposed function as a polyphosphate synthase is unlikely. All parts of the Vtc proteins except for their transmembrane domains are facing the cytosol (Cohen et al., 1999; O.Müller, M.J.Bayer and A.Mayer, submitted). However, an enzyme synthesizing polyphosphate in the vacuole would be expected to face the vacuolar lumen. In addition, deletion of the VTC4 homolog in S.pombe led to a similar enlarged vacuole phenotype as in Saccharomyces cerevisiae, but not to polyphosphate deficiency (J.Armstrong, personal communication).
In S.cerevisiae, vtc1Δ and vtc4Δ mutant phenotypes are largely equivalent. These correlate with the observation that deletion of VTC1 leads to a loss of most of Vtc4p and vice versa, and indicate a close functional cooperation (Cohen et al., 1999 and Figure 3 in this study). This may reflect the fact that S.cerevisiae Vtc4p (75 kDa) has no predicted transmembrane helices, unlike its close relatives Vtc2p (95 kDa, three transmembrane domains) and Vtc3p (95 kDa, three transmembrane domains). Vtc1p (14 kD) consists only of these three transmembrane domains. It may thus provide the transmembrane domains required for Vtc4p function (Cohen et al., 1999). Surprisingly, an nrf1Δ (VTC1 = NRF1 in S.pombe) mutant was reported not to have enlarged vacuoles (Murray and Johnson, 2001) and not to have a vacuole fusion defect. The latter result is hard to interpret because fusion incompetent mutants were not included as negative controls. However, a plausible explanation is offered by the fact that S.pombe Vtc4p is longer and itself contains three transmembrane domains (J.Armstrong, personal communication). Function of the Vtc complex in S.pombe may therefore be less dependent on Nrf1p/Vtc1p. Resolution of this issue will require closer analysis of the S.pombe complex.
The Vtc complex influences several key aspects of the fusion reaction. It binds to V0 and is preferentially associated with the R-SNARE Nyv1p. Strikingly, a small subset of V0 sectors is preferentially associated with the cognate Q-SNARE Vam3p (Peters et al., 2001). These physical associations correlate with several functional interactions. Both the V0–Vam3p and Vtc–Nyv1p complexes are intimately connected to the LMA1 cycle. Vtc1p and Vtc4p are required for LMA1 binding to the membrane and for the priming activity of endogenous Sec18p/NSF. This activity transfers LMA1 from its initial Sec18p/NSF-dependent binding state to an intermediate binding site on a Vam3p-containing complex (which need not be Vam3p itself) from which it is finally released in a reaction sensitive to the phosphatase inhibitor microcystin LR (Xu et al., 1998). Hence, LMA1 release must follow the phosphatase dependent step and also V0 trans-complex formation, which is not inhibited by microcystin LR (Peters et al., 2001). This terminal event in vacuole fusion depends on Vtc3p. The Vtc complex might therefore be a crucial interface coupling the initial activating events with the terminal steps initiating bilayer mixing.
Sec18p/NSF action disrupts cis-SNARE complexes, which can be expected to coincide with significant conformational changes of Sec18p/NSF (Hanson et al., 1997) and SNAREs (Fasshauer et al., 1997). The functional and physical association of the NSF/SNARE system with the Vtc complex and V0 provides reason to speculate that priming may comprise more than SNARE activation. An NSF function independent of SNARE complex disassembly was postulated in the fusion of mitotic Golgi fragments (Müller et al., 1999). Mutant NSF proteins that had lost their ability to disrupt SNARE complexes and their ATPase function support this reaction. It was hypothesized that mitotic Golgi fragments contain a sufficient supply of primed SNAREs to make SNARE complex disruption dispensible. However, the nature of the additional NSF function, which is still required, has not yet been determined. In vacuole fusion, the Vtc complex is a prime candidate to mediate potential additional tasks of Sec18p/NSF. For example, part of the energy provided by NSF-dependent ATP hydrolysis might be transduced via the Vtc complex and/or SNAREs to V0 sectors. It is obvious that V0 sectors must be activated for trans-complex formation. Only a small subset of V0 sectors on the vacuoles participates in this reaction and it does so only as a consequence of priming and SNARE-dependent docking (Peters et al., 2001). This is the subset bound to the Q-SNARE Vam3p.
Priming by Sec18p/NSF has actually been shown to produce an activated labile state (Mayer and Wickner, 1997; Xu et al., 1997, 1998). This activated state is stabilized by LMA1 and decays rapidly and non-productively in its absence. Priming transfers LMA1 from a Sec18p/NSF-dependent binding site to the Vam3p-containing complex from which it is released once the final, phosphatase-dependent step of fusion has occurred (Xu et al., 1998). If Vam3p is inactivated, this transfer fails, resulting in premature release of LMA1 from the membrane and in a block of fusion. These effects are remarkably complementary with our observations on the Vtc complex and V0 trans-complex formation. Inactivation of Vtc1p or Vtc4p blocks priming by endogenous Sec18p/NSF and prevents binding of LMA1 to the membrane. If priming is rescued by exogenous Sec18p/NSF, a basic level of fusion activity (∼20–30%) can be rescued, but the LMA1-dependent aspect of the reaction cannot be recovered. On the other hand, Vtc3p inactivation permits priming and V0 trans-complex formation, but selectively interferes with subsequent LMA1 release. These observations can be reconciled best by assuming that Vtc1p/Vtc4p and LMA1 may be required for generating and preserving the activated state and Vtc3p might regulate its productive consumption. Recent observations that the Vtc proteins influence the conformation of the V0 subunit Vph1p (O.Müller, M.J.Bayer and A.Mayer, submitted), which is also required in the terminal phase of vacuole fusion (M.J.Bayer, C.Peters and A.Mayer, in preparation), link V0 to this activation process.
Available data can then be integrated into the following working hypothesis: SNAREs, the V0 sector and LMA1 act as an integrated network. We propose that ATP hydrolysis by Sec18p/NSF not only disrupts cis-SNARE complexes, but also activates associated V0 sectors for trans-complex formation and the Ca2+/calmodulin-dependent initiation of fusion. Vtc1p/Vtc4p and LMA1 may participate in this process and help to conserve the activated state. Vtc3p may regulate the transition from V0 trans-complexes to fusion, which involves LMA1 release and requires a dephosphorylation. In line with proteinaceous pore models of fusion (Lindau and Almers, 1995; Peters et al., 2001; Zimmerberg, 2001), this step could coincide with fusion pore opening. For example, radial opening of a proteinaceous pore (Lindau and Almers, 1995) would occur when the subunits of a pore spanning both membranes could move apart to create amphiphilic clefts facilitating transfer of lipids between the two membranes. This should require energy that could be derived partially from NSF- and Vtc-dependent priming. To test this hypothesis, we try to develop assays to detect conformational changes of SNAREs, V0 and the Vtc proteins at different stages of fusion.
Vacuole fusion is an excellent model reaction to test the potential of different components to mediate bilayer fusion. Its favourable kinetic properties permit resolution of processes after the docking stage that have not yet become accessible in other systems. However, several parallels to other organelles exist. Like vacuole fusion (Peters and Mayer, 1998), many other fusion reactions on intracellular compartments, such as intra-Golgi and ER to Golgi transport, lysosome fusion, endosome fusion and endosome-lysosome fusion require Ca2+ (Holroyd et al., 1999; Porat and Elazar, 2000; Pryor et al., 2000), probably via an efflux from the vesicle lumen, which is triggered by membrane docking. Most of these systems also require calmodulin (Peters and Mayer, 1998; Holroyd et al., 1999; Porat and Elazar, 2000; Pryor et al., 2000), which may act as a receptor for the Ca2+ efflux (Peters and Mayer, 1998). Finally, LMA1 is not restricted to vacuole fusion but is clearly required also for ER to Golgi transport (Barlowe, 1997). These similarities provide some reason to speculate that the mechanisms revealed in vacuolar fusion also operate on other organelles, perhaps via other components with properties similar to the V0/Vtc system. Further identification and analysis of relevant fusion proteins in these compartments will be required to resolve this issue.
Materials and methods
General procedures
Vacuole isolation, cytosol preparation, fusion, antibodies, inhibitors and FM4-64 staining were as described (Peters and Mayer, 1998; Peters et al., 1999). PS buffer is 10 mM PIPES–KOH pH 6.8, 200 mM sorbitol. Protease inhibitor cocktail (1× PIC) contained 100 µM pefabloc SC, 100 ng/ml leupeptin, 50 µM O-phenanthroline and 500 ng/ml pepstatin A. LMA1 and Sec18p were purified as described (Xu et al., 1998).
Strains
Standard fusion strains were S.cerevisiae BJ3505 and DKY6281. SBY119 was described previously (Peters et al., 2001). For the convenient construction of different deletion mutants in a background suitable for the vacuole fusion assay, we made the strain OMY1, which is deficient in vacuolar proteinases. The genes for PEP4 and PRB1, respectively, were replaced by URA3 and TRP1 markers in strain BY4732 (MATα his3Δ200 met15Δ0 trp1Δ63 ura3Δ0) using PCR-generated cassettes from plasmids pRS306 (URA3) and pRS304 (TRP1) (Brachmann et al., 1998). The following derivatives of OMY1 were created in the same manner by replacing the indicated genes with the HIS3 marker from plasmid pRS303: OMY2 (vtc1Δ), OMY3 (vtc3Δ) and OMY4 (vtc2Δ). The same genes were deleted in DKY6281 using the HIS3 marker resulting in the following strains: OMY5 (vtc1Δ), OMY6 (vtc3Δ) and OMY7 (vtc2Δ).
To obtain deletion mutants in VTC4, one allele of the gene was replaced by a loxP–kanMX–loxP cassette as described (Sattler and Mayer, 2000) in the diploid strain BY262 (MATa/α TRP/TRP Gal2/gal2 ura3-52/ura3-52 lys2-801a/lys2-801a ade2-107a/ade2-107a his3Δ200/his3Δ200 leu2-Δ1/leu2-Δ1; B.Andrews, Toronto). After sporulation, the resulting strain (MATa vtc4::loxP-kanMX-loxP) and a corresponding wild-type strain were deleted for either PEP4 (using plasmid pAR2; J.Shaw, Salt Lake City, UT) or PHO8 (using plasmids pTS17 or pTS15; T.Stevens, Eugene, OR) resulting in strains SBY86 (MATa pep4Δ), SBY85 (MATa pho8Δ), SBY83 (MATa pep4Δ vtc4Δ) and SBY82 (MATa pho8Δ vtc4Δ). Deletions were verified by western blotting and/or PCR.
The oligonucleotides used for generation of deletion cassettes and for control PCR are given in Table I.
Table I. Oligonucleotides used for the generation of deletion cassettes and for control PCR.
| Oligonucleotide | Sequence |
|---|---|
| 5′PEP4 DEL | 5′-TGGTCAGCGCCAACCAAGTTGCTGCAAAAGTCCACAAGGCAGATTGTACTGAGAGTGCAC-3′ |
| 3′PEP4 DEL | 5′-AATCGTAAATAGAATAGTATTTACGCAAGAAGGCATCACCCTGTGCGGTATTTCACACCG-3′ |
| 5′PEP4 CONTROL | 5′-GCTTGAAAGCATTATTGCCATTGGCC-3′ |
| 3′PEP4 CONTROL | 5′-GGCCAAACCAACCGCATTGTTGCCC-3′ |
| PRB1.5′DEL | 5′-CAATAAAAAAACAAACTAAACCTAATTCTAACAAGCAAAGAGATTGTACTGAGAGTGCAC-3′ |
| PRB1.3′DEL | 5′-AAGAAAAAAAAAAGCAGCTGAAATTTTTCTAAATGAAGAACTGTGCGGTATTTCACACCG-3′ |
| PRB1.FORW.CONTROL | 5′-GTCCCGTTATATTGGAGTTCTTCCC-3′ |
| PRB1.REV.CONTROL | 5′-CTCCGACTTGTAACCTCGAGACGCC-3′ |
| YER072w(VTC1) 5′DEL | 5′-GTCTTCAGCACCATTATTACAAAGAACACCTGGGAAAAAGATCGAGATTGTACTGAGAGTG CAC-3′ |
| YER072w(VTC1) 3′DEL | 5′-TCATAACTTAGTGTTAGCGTCATTGTACTTCAATCTTAATATAAAGCATCTGTGCGGTATTTC ACACC-3′ |
| YER072w(VTC1) FORW.CONTROL | 5′-CCATATTTCCATAACCAGCGAC-3′ |
| YER072w(VTC1) REV.CONTROL | 5′-GCTCCTGAAACGTGAATATTT-3′ |
| YPL019c(VTC3) 5′DEL | 5′-GCGAACAGCAGAATTTGTCCTTGGTTTTCAGAGTTTGAAAAGATTGTACTGAGAGTGCAC-3′ |
| YPL019c(VTC3) 3′DEL | 5′-ACTTGTGTAATATATGTGTATATAAAAAATATACATGTTCCTGTGCGGTATTTCACACCG-3′ |
| YPL019c(VTC3) FORW.CONTROL | 5′-TTATTATCACTATAGGTCATTTGGC-3′ |
| YPL019c(VTC3) REV.CONTROL | 5′-ACAATTAATTAATTATGATAGTAAC-3′ |
| YFL004w(VTC2) 5′DEL | 5′-AAAGAACGACTACACCTCAACATAACGACACTTTTTTGACAGATTGTACTGAGAGTGCAC-3′ |
| YFL004w(VTC2) 3′DEL | 5′-AACATAAAAACACATGGTCTCAGTAGATAAGTACATATTCTGTGCGGTATTTCACACCG-3′ |
| YFL004w(VTC2) FORW.CONTROL | 5′-ATAGGTATAAACAGATAAAATAGAC-3′ |
| YFL004w(VTC2) REV.CONTROL | 5′-TTTCTAAAAGTAAGCAATTGTTAGC-3′ |
| YJL012c(VTC4) 5′DEL | 5′-GCTAACAATCAAATCGGCCAATAAAAGAGCATAACAAGGCCAGCTGAAGCTTCGTACGC-3′ |
| YJL012c(VTC4) 3′DEL | 5′-GGAAGTAATAAAGAACCCAATGGAGCCAATCATGGCTGTTGCATAGGCCACTAGTGGATC TG-3′ |
| YJL012c(VTC4) FORW.CON | 5′-GGCAATCAGAAAGAAGTTACAGGC-3′ |
| YJL012c(VTC4) REV.CON | 5′-CCATAACAGTACGTATCAACACAG-3′ |
For analysis of V0 sector trans-complex formation His6-HA3 and AU1 tags were chromosomally integrated at the 3′ end of Vph1p as described (Peters et al., 2001) in strains OMY1 (wt), OMY2 (vtc1Δ) and OMY3 (vtc3Δ), resulting in strains OMY8 (wt, Vph1-His6-HA3), OMY9 (wt, Vph1-AU1), OMY10 (vtc1Δ, Vph1-His6-HA3), OMY11 (vtc1Δ, Vph1-AU1), OMY12 (vtc3Δ, Vph1p-His6-HA3) and OMY13 (vtc3Δ, Vph1-AU1).
Co-immunoprecipitation with anti-Vtc1p
Affinity purified antibodies to Vtc1p (500 µg) were coupled via their carbohydrate groups to 2 ml of Carbolink™ resin (Pierce). Vacuoles (1 mg) were solubilized (15 min, 4°C) in 1.5 ml of buffer A (10 mM PIPES–KOH pH 6.8, 200 mM sorbitol, 150 mM KCl, 500 µM MnCl2, 100 µM CaCl2, 40 mM CHAPS, 1× PIC) and centrifuged (100 000 g, 30 min, 4°C). Antibody matrix (300 µl) was added to the supernatants. Samples were rotated for 45 min at 4°C. The matrices were packed into columns and washed with 5 ml of buffer A (with 10 mM CHAPS). Proteins were eluted with 1 ml of 0.1 M glycine pH 2.75/150 mM NaCl, trichloroacetic acid (TCA) precipitated and analyzed by SDS–PAGE.
Production of antisera
To raise antibodies to Vtc1p and Vtc4p, the proteins were expressed in Escherichia coli BL21. Vtc1p was expressed as a glutathione S-transferase (GST) fusion protein. The coding region of the VTC1 ORF was amplified by PCR using primers YER–GST-5′, 5′-CGGGAATTCGGGATGTCTTCAGCACCATTATT-3′ and YER– GST-3′, 5′-CGGCTCGAGCTATCATAACTTAGTGTTAGCGT-3′, and genomic DNA as a template. The primers introduced an EcoRI restriction site at the 5′ end and an XhoI restriction site at the 3′ end. Via these restriction sites, the fragment was cloned into pGEX-6P-1 (Pharmacia). The resulting plasmid, pGST-YER, was transformed in E.coli BL21. Expression was induced with 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) at 37°C for 2 h. Phenylmethylsulfonyl fluoride (PMSF; 1 mM) had to be added to the growth medium to prevent degradation of the full-length fusion protein. Cells were broken by a freeze–thaw cycle and sonification in PBS/1 mM PMSF. Triton X-100 was added to a final concentration of 1% (w/v) and the lysate was mixed for 30 min at 4°C to complete solubilization. Under these conditions the fusion protein was partially soluble. The lysate was cleared (100 000 g, 20 min, 4°C) and binding to GSH–Sepharose (Pharmacia) was carried out for 4 h at 4°C. The beads were packed into a column and washed with 20 column volumes of cleavage buffer [50 mM Tris–HCl pH 7.0, 150 mM NaCl, 1 mM EDTA, 1 mM dithiothreitol (DTT)]. The fusion protein was cleaved on column by adding PreScission™ Protease (Pharmacia; 80 U/ml GSH–Sepharose) in cleavage buffer and incubation at 4°C for 4 h.
Vtc4p was expressed with a His6 tag. The coding region of the VTC4 open reading frame was amplified by PCR using primers HIS-YJL-1 (5′-CGCGGATCCATGAGAGGATCGCATCACCATCACCATCACAAGTTTGGTGAGCACTTGAGCAAGTCT-3′) and HIS-YJL-2 (5′-CGGAAGCTTTTAATAAAGTAGTGGAGACACCGCCCA-3′) and genomic DNA as a template. The primers introduced a BamHI restriction site followed by a start codon and the codons for the RGS-His6 tag at the 5′ end as well as a HindIII restriction site at the 3′ end. Via these restriction sites the fragment was cloned into pQE-50 (Qiagen). The plasmid was transformed in E.coli M15[pREP4]. Expression was induced with 1 mM IPTG at 25°C for 4 h. PMSF (1 mM) was added to the growth medium to prevent degradation of the full-length protein. Cells were broken by two freeze–thaw cycles and sonification in buffer A [2× phosphate-buffered saline (PBS) buffer pH 8.0, 1 mM PMSF]. The protein was partially soluble. The lysate was centrifuged (100 000 g, 20 min, 4°C) and 10 mM imidazole was added to the supernatant. The protein was bound to Ni2+-NTA resin. The beads were packed into a column and washed with 20 column volumes of 20 mM imidazole/0.5% (w/v) Triton X-100 in buffer A and 5 column volumes of the same buffer without Triton X-100. Bound protein was eluted with 250 mM imidazole in buffer A.
The purified proteins were subjected to SDS–PAGE and blotted to nitrocellulose. Protein bands were excised, dissolved in dimethylsulfoxide and injected into rabbits (Vtc1p) or goats (Vtc4p) using Freund’s adjuvant. Antibodies were affinity purified with the respective recombinant proteins immobilized on CH–Sepharose 4B (Pharmacia). Antibodies were eluted with 0.1 M glycine pH 2.75 and 150 mM NaCl, and the pH was immediately neutralized by addition of 1 M Tris–HCl pH 8.8. The antibodies were dialyzed into PS buffer (10 mM PIPES–KOH pH 6.8, 200 mM sorbitol) containing 150 mM KCl, concentrated by ultrafiltration in centricon-30 (Amicon) and stored at –20°C.
Mass spectrometry
Protein bands were excised from the gels and digested by trypsin. Two percent of the supernatant was analyzed by MALDI mass spectrometry on a Bruker REFLEX instrument (Bruker Daltonics, Bremen, Germany). Resulting peptide mass maps were searched in a non-redundant sequence database using PeptideSearch software developed by P.Mortensen and M.Mann. Hits were verified using second pass searches taking account of chemical modifications.
Co-immunoprecipitation with Vph1p-HA
Vacuoles (900 µg) were solubilized (10 min, 4°C) in buffer B [1% (w/v) Triton X-100, 100 mM Tris–HCl pH 7.4, 110 mM NaCl, 5 mM EDTA, 1× PIC] and centrifuged (100 000 g, 10 min, 4°C). One percent of the supernatant was extracted with chloroform/methanol to serve as input control. The rest of the supernatant was rotated (1 h, 4°C) with 25 µg of monoclonal anti-HA (Babco) and 15 µl of protein G–agarose (Roche). The beads were washed six times with 1 ml of buffer B [with 0.1% (w/v) Triton X-100], transferred to a fresh tube and eluted with non-reducing SDS sample buffer.
Assay for SNARE complex disruption
Vacuoles were diluted with 1 ml of cold PS/150 mM KCl, centrifuged (10 000 g, 10 min, 4°C), solubilized in 600 µl of buffer S (40 mM CHAPS, 100 mM Tris–HCl pH 7.4, 110 mM NaCl, 5 mM EDTA, 1 mM PMSF, 1× PIC) and incubated for 10 min at 4°C on a mixer. The lysates were cleared (125 000 g, 10 min, 4°C) and 60 µl were TCA-precipitated to serve as a 10% input control. Affinity purified anti-Vam3p (5 µg) and 20 µl protein A–Sepharose beads were added to the rest of the lysates and rotated (45 min, 4°C). The beads were washed twice with 1 ml of buffer S (with 10 mM CHAPS). Proteins were eluted in a fresh tube with 50 µl of non-reducing SDS sample buffer and boiled for 5 min.
Acknowledgments
Acknowledgements
We thank Christa Baradoy and Alexandra Glatz for assistance, and William Wickner, Gabriele Fischer von Mollard, Tom Vida, Tom Stevens and Brenda Andrews for providing antibodies, strains and plasmids. This work was supported by the Deutsche Forschungsgemeinschaft (SFB446; A.M.), the Boehringer-Ingelheim-Foundation (A.M.) and the Boehringer-Ingelheim-Fonds (O.M.).
References
- Barlowe C. (1997) Coupled ER to Golgi transport reconstituted with purified cytosolic proteins. J. Cell Biol., 139, 1097–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brachmann C.B., Davies,A., Cost,G.J., Caputo,E., Li,J., Hieter,P. and Boeke,J.D. (1998) Designer deletion strains derived from Saccharomyces cerevisiae S288C: a useful set of strains and plasmids for PCR-mediated gene disruption and other applications. Yeast, 14, 115–132. [DOI] [PubMed] [Google Scholar]
- Chen Y.A., Scales,S.J., Patel,S.M., Doung,Y.-C. and Scheller,R.H. (1999) SNARE complex formation is triggered by Ca2+ and drives membrane fusion. Cell, 97, 165–174. [DOI] [PubMed] [Google Scholar]
- Cohen A., Perzov,N., Nelson,H. and Nelson,N. (1999) A novel family of yeast chaperones involved in the distribution of V-ATPase and other membrane proteins. J. Biol. Chem., 274, 26885–26893. [DOI] [PubMed] [Google Scholar]
- Conradt B., Haas,A. and Wickner,W. (1994) Determination of four biochemically distinct, sequential stages during vacuole inheritance in vitro. J. Cell Biol., 126, 99–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coorssen J.R., Blank,P.S., Tahara,M. and Zimmerberg,J. (1998) Biochemical and functional studies of cortical vesicle fusion: the SNARE complex and Ca2+ sensitivity. J. Cell Biol., 143, 1845–1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eitzen G., Will,E., Gallwitz,D., Haas,A. and Wickner,W. (2000) Sequential action of two GTPases to promote vacuole docking and fusion. EMBO J., 19, 6713–6720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eitzen G., Thorngren,N. and Wickner,W. (2001) Rho1 and Cdc42p act after Ypt7p to regulate vacuole docking. EMBO J., 20, 5650–5656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasshauer D., Otto,H., Eliason,W.K., Jahn,R. and Brunger,A.T. (1997) Structural changes are associated with soluble N-ethylmaleimide-sensitive fusion protein attachment protein receptor complex formation. J. Biol. Chem., 272, 28036–28041. [DOI] [PubMed] [Google Scholar]
- Fisher R.J., Pevsner,J. and Burgoyne,R.D. (2001) Control of fusion pore dynamics during exocytosis by Munc18. Science, 291, 875–878. [DOI] [PubMed] [Google Scholar]
- Fleckenstein D., Rohde,M., Klionsky,D.J. and Rüdiger,M. (1998) Yel013p (Vac8p), an armadillo repeat protein related to plakoglobin and importin α is associated with the yeast vacuole membrane. J. Cell Sci., 111, 3109–3118. [DOI] [PubMed] [Google Scholar]
- Grote E., Baba,M., Ohsumi,Y. and Novick,P.J. (2000a) Geranylgeranylated SNAREs are dominant inhibitors of membrane fusion. J. Cell Biol., 151, 453–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grote E., Carr,C.M. and Novick,P.J. (2000b) Ordering the final events in yeast exocytosis. J. Cell Biol., 151, 439–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W., Sacher,M., Barrowman,J., Ferro-Novick,S. and Novick,P. (2000) Protein complexes in transport vesicle targeting. Trends Cell Biol., 10, 251–255. [DOI] [PubMed] [Google Scholar]
- Haas A. and Wickner,W. (1996) Homotypic vacuole fusion requires Sec17p (yeast α-SNAP) and Sec18p (yeast NSF). EMBO J., 15, 3296–3305. [PMC free article] [PubMed] [Google Scholar]
- Haas A., Scheglmann,D., Lazar,T., Gallwitz,D. and Wickner,W. (1995) The GTPase Ypt7p of Saccharomyces cerevisiae is required on both partner vacuoles for the homotypic fusion step of vacuole inheritance. EMBO J., 14, 5258–5270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson P.I., Roth,R., Morisaki,H., Jahn,R. and Heuser,J.E. (1997) Structure and conformational changes in NSF and its membrane receptor complexes visualized by quick-freeze/deep-etch electron microscopy. Cell, 90, 523–535. [DOI] [PubMed] [Google Scholar]
- Holroyd C., Kistner,U., Annaert,W. and Jahn,R. (1999) Fusion of endosomes involved in synaptic vesicle recycling. Mol. Biol. Cell, 10, 3035–3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn R. and Südhof,T.C. (1999) Membrane fusion and exocytosis. Annu. Rev. Biochem., 68, 863–911. [DOI] [PubMed] [Google Scholar]
- Lindau M. and Almers,W. (1995) Structure and function of fusion pores in exocytosis and ectoplasmic membrane fusion. Curr. Opin. Cell Biol., 7, 509–517. [DOI] [PubMed] [Google Scholar]
- Mayer A. and Wickner,W. (1997) Docking of yeast vacuoles is catalyzed by the ras-like GTPase Ypt7p after symmetric priming by Sec18p (NSF). J. Cell Biol., 136, 307–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer A., Wickner,W. and Haas,A. (1996) Sec18p (NSF) driven release of Sec17p (α-SNAP) can precede docking and fusion of yeast vacuoles. Cell, 85, 83–94. [DOI] [PubMed] [Google Scholar]
- Mayer A., Scheglmann,D., Dove,S., Glatz,A., Wickner,W. and Haas,A. (2000) Phosphatidylinositol-4,5-bisphosphate controls two steps of homotypic vacuole fusion. Mol. Biol. Cell, 11, 807–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride H.M., Rybin,V., Murphy,C., Giner,A., Teasdale,R. and Zerial,M. (1999) Oligomeric complexes link Rab5 effectors with NSF and drive membrane fusion via interactions between EEA1 and syntaxin 13. Cell, 98, 377–386. [DOI] [PubMed] [Google Scholar]
- Müller J.M.M., Rabouille,C., Newman,R., Shorter,J., Freemont,P., Schiavo,G., Warren,G. and Shima,D.T. (1999) An NSF function distinct from ATPase-dependent SNARE disassembly is essential for Golgi membrane fusion. Nature Cell Biol., 1, 335–340. [DOI] [PubMed] [Google Scholar]
- Müller O., Johnson,D.I. and Mayer,A. (2001) Cdc42p functions at the docking stage of yeast vacuole fusion. EMBO J., 20, 5657–5665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munn A.L. and Riezman,H. (1994) Endocytosis is required for the growth of vacuolar H+-ATPase-defective yeast: identification of six new END genes. J. Cell Biol., 127, 373–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray J.M. and Johnson,D.I. (2000) Isolation and characterization of Nrf1p, a novel negative regulator of the Cdc42p GTPase in Schizosaccharomyces pombe. Genetics, 154, 155–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray J.M and Johnson,D.I. (2001) The Cdc42p GTPase and its regulators Nrf1p and Scd1p are involved in endocytic trafficking in the fission yeast Schizosaccharomyces pombe. J. Biol. Chem., 276, 3004–3009. [DOI] [PubMed] [Google Scholar]
- Nelson N., Perzov,N., Cohen,A., Hagai,K., Padler,V. and Nelson,H. (2000) The cellular biology of proton-motive force generation by V-ATPases. J. Exp. Biol., 203, 89–95. [DOI] [PubMed] [Google Scholar]
- Nichols B.J., Ungermann,C., Pelham,H.R.B., Wickner,W.T. and Haas,A. (1997) Homotypic vacuolar fusion mediated by t- and v-SNAREs. Nature, 387, 199–202. [DOI] [PubMed] [Google Scholar]
- Ogawa N., DeRisi,J. and Brown,P.O. (2000) New components of a system for phosphate accumulation and polyphosphate metabolism in Saccharomyces cerevisiae revealed by genomic expression analysis. Mol. Biol. Cell, 11, 4309–4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan X. and Goldfarb,D.S. (1998) YEB3/VAC8 encodes a myristylated armadillo protein of the Saccharomyces cerevisiae vacuolar membrane that functions in vacuole fusion and inheritance. J. Cell Sci., 111, 2137–2147. [DOI] [PubMed] [Google Scholar]
- Pelham H.R. (1999) SNAREs and the secretory pathway—lessons from yeast. Exp. Cell Res., 247, 1–8. [DOI] [PubMed] [Google Scholar]
- Peters C. and Mayer,A. (1998) Ca2+/calmodulin signals the completion of docking and triggers a late step of vacuole fusion. Nature, 396, 575–580. [DOI] [PubMed] [Google Scholar]
- Peters C., Andrews,P.D., Stark,M.J.R., Cesaro-Tadic,S., Glatz,A., Podtelejnikov,A., Mann,M. and Mayer,A. (1999) Control of the terminal step of membrane fusion by protein phosphatase 1. Science, 285, 1084–1087. [DOI] [PubMed] [Google Scholar]
- Peters C., Bayer,M.J., Bühler,S., Andersen,J.S., Mann,M. and Mayer,A. (2001) Trans-complex formation of proteolipid channels in the terminal phase of membrane fusion. Nature, 409, 581–588. [DOI] [PubMed] [Google Scholar]
- Porat A. and Elazar,Z. (2000) Regulation of intra-Golgi transport by calcium. J. Biol. Chem., 275, 29233–29237. [DOI] [PubMed] [Google Scholar]
- Price A., Seals,D., Wickner,W. and Ungermann,C. (2000) The docking stage of yeast vacuole fusion requires the transfer of proteins from a cis-SNARE complex to a Rab/Ypt protein. J. Cell Biol., 148, 1231–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryor P.R., Mullock,B.M., Bright,N.A., Gray,S.R. and Luzio,J.P. (2000) The role of intraorganellar Ca2+ in late endosome–lysosome heterotypic fusion and in the reformation of lysosomes from hybrid organelles. J. Cell Biol., 149, 1053–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T.K., Rehling,P., Peterson,M.R. and Emr,S.D. (2000) Class C Vps protein complex regulates vacuolar SNARE pairing and is required for vesicle docking/fusion. Mol. Cell, 6, 661–671. [DOI] [PubMed] [Google Scholar]
- Sattler T. and Mayer,A. (2000) Cell-free reconstitution of microautophagic vacuole invagination and vesicle formation. J. Cell Biol., 151, 529–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seals D.F., Eitzen,G., Margolis,N., Wickner,W.T. and Price,A. (2000) A Ypt/Rab effector complex containing the Sec1 homolog Vps33p is required for homotypic vacuole fusion. Proc. Natl Acad. Sci. USA, 97, 9402–9407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith V., Chou,K.N., Lashkari,D., Botstein,D. and Brown,P.O. (1996) Functional analysis of the genes of yeast chromosome V by genetic footprinting. Science, 274, 2069–2074. [DOI] [PubMed] [Google Scholar]
- Ungermann C., Sato,K. and Wickner,W. (1998a) Defining the functions of trans-SNARE pairs. Nature, 396, 543–548. [DOI] [PubMed] [Google Scholar]
- Ungermann C., Nichols,B.J., Pelham,H.R.B. and Wickner,W. (1998b) A vacuolar v-t-SNARE complex, the predominant form in vivo and on isolated vacuoles, is disassembled and activated for docking and fusion. J. Cell Biol., 140, 61–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungermann C., Wickner,W. and Xu,Z.Y. (1999) Vacuole acidification is required for trans-SNARE pairing, LMA1 release, and homotypic fusion. Proc. Natl Acad. Sci. USA, 96, 11194–11199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungermann C., Price,A. and Wickner,W. (2000) A new role for a SNARE protein as a regulator of the Ypt7/Rab-dependent stage of docking. Proc. Natl Acad. Sci. USA, 97, 8889–8891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veit M., Laage,R., Dietrich,L., Wang,L. and Ungermann,C. (2001) Vac8p release from the SNARE complex and its palmitoylation are coupled and essential for vacuole fusion. EMBO J., 20, 3145–3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Sacher,M. and Ferro-Novick,S. (2000) TRAPP stimulates guanine nucleotide exchange on Ypt1p. J. Cell Biol., 151, 289–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y.X., Catlett,N.L. and Weisman,L.S. (1998) Vac8p, a vacuolar protein with armadillo repeats, functions in both vacuole inheritance and protein targeting from the cytoplasm to vacuole. J. Cell Biol., 140, 1063–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y.X., Kauffman,E.J., Duex,J.E. and Weisman,L.S. (2001) Fusion of docked membranes requires the armadillo repeat protein Vac8p. J. Biol. Chem., 276, 35133–35140. [DOI] [PubMed] [Google Scholar]
- Weber T., Zemelman,B.V., McNew,J.A., Westermann,B., Gmachl,M., Parlati,F., Sollner,T.H. and Rothman,J.E. (1998) SNAREpins: minimal machinery for membrane fusion. Cell, 92, 759–772. [DOI] [PubMed] [Google Scholar]
- Wickner W. and Haas,A. (2000) Yeast homotypic vacuole fusion: a window on organelle trafficking mechanisms. Annu. Rev. Biochem., 69, 247–275. [DOI] [PubMed] [Google Scholar]
- Wurmser A.E., Sato,T.K. and Emr,S.D. (2000) New component of the vacuolar class C-Vps complex couples nucleotide exchange on the Ypt7p GTPase to SNARE-dependent docking and fusion. J. Cell Biol., 151, 551–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T., Rammner,B., Margittai,M., Artalejo,A.R., Neher,E. and Jahn,R. (1999) Inhibition of SNARE complex assembly differentially affects kinetic components of exocytosis. Cell, 99, 713–722. [DOI] [PubMed] [Google Scholar]
- Xu Z., Mayer,A., Muller,E. and Wickner,W. (1997) A heterodimer of thioredoxin and IB2 cooperates with Sec18p (NSF) to promote yeast vacuole inheritance. J. Cell Biol., 136, 299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z.Y., Sato,K. and Wickner,W. (1998) LMA1 binds to vacuoles at Sec18p (NSF), transfers upon ATP hydrolysis to a t-SNARE (Vam3p) complex, and is released during fusion. Cell, 93, 1125–1134. [DOI] [PubMed] [Google Scholar]
- Zimmerberg J. (2001) How can proteolipids be central players in membrane fusion? Trends Cell Biol., 11, 233–235. [DOI] [PubMed] [Google Scholar]