Abstract
Background
This is an updated version of the original review published in Issue 4, 2004. The use of concurrent chemotherapy and radiotherapy in non‐small cell lung cancer (NSCLC) might be seen as a way of increasing the effectiveness of radical radiotherapy at the same time as reducing the risks of metastatic disease.
Objectives
To determine the effectiveness of concurrent chemoradiotherapy as compared to radiotherapy alone with regard to overall survival, tumour control and treatment‐related morbidity. To determine the effectiveness of concurrent versus sequential chemoradiotherapy.
Search methods
For this update we ran a new search in October 2009, using a search strategy adapted from the design in the original review. We searched: CENTRAL (accessed through The Cochrane Library, 2009, Issue 4), MEDLINE (accessed through PubMed), and EMBASE (accessed through Ovid).
Selection criteria
Randomised trials of patients with stage I‐III NSCLC undergoing radical radiotherapy and randomised to receive concurrent chemoradiotherapy versus radiotherapy alone, or concurrent versus sequential chemoradiotherapy.
Data collection and analysis
Study selection, data extraction and assessment of risk of bias was performed independently by two authors. Pooled hazard ratios and relative risks were calculated according to a random‐effects model.
Main results
Nineteen randomised studies (2728 participants) of concurrent chemoradiotherapy versus radiotherapy alone were included. Chemoradiotherapy significantly reduced overall risk of death (HR 0.71, 95% CI 0.64 to 0.80; I2 0%; 1607 participants) and overall progression‐free survival at any site (HR 0.69, 95% CI 0.58 to 0.81; I2 45%; 1145 participants). Incidence of acute oesophagitis, neutropenia and anaemia were significantly increased with concurrent chemoradiation. Six trials (1024 patients) of concurrent versus sequential chemoradiation were included. A significant benefit of concurrent treatment was shown in overall survival (HR 0.74, 95% CI 0.62 to 0.89; I2 0%; 702 participants). This represented a 10% absolute survival benefit at 2 years. More treatment‐related deaths (4% vs 2%) were reported in the concurrent arm without statistical significance (RR 2.02, 95% CI 0.90 to 4.52; I2 0%; 950 participants). There was increased severe oesophagitis with concurrent treatment (RR 4.96, 95%CI 2.17 to 11.37; I2 66%; 947 participants).
Authors' conclusions
This update of the review published in 2004 incorporates additional trials and more mature data. It demonstrates the benefit of concurrent chemoradiation over radiotherapy alone or sequential chemoradiotherapy. Patient selection is an important consideration in view of the added toxicity of concurrent treatment. Uncertainty remains as to how far this is purely due to a radiosensitising effect and whether similar benefits could be achieved by using modern radiotherapy techniques and more dose intensive accelerated and/ or hyperfractionated radiotherapy regimens.
Keywords: Humans; Antineoplastic Combined Chemotherapy Protocols; Antineoplastic Combined Chemotherapy Protocols/therapeutic use; Carcinoma, Non-Small-Cell Lung; Carcinoma, Non-Small-Cell Lung/drug therapy; Carcinoma, Non-Small-Cell Lung/radiotherapy; Combined Modality Therapy; Combined Modality Therapy/adverse effects; Combined Modality Therapy/methods; Combined Modality Therapy/mortality; Lung Neoplasms; Lung Neoplasms/drug therapy; Lung Neoplasms/radiotherapy; Radiation-Sensitizing Agents; Radiation-Sensitizing Agents/therapeutic use; Randomized Controlled Trials as Topic
Plain language summary
Concurrent chemoradiotherapy reduces risk of death at two years compared to sequential chemoradiotherapy or radiotherapy alone in patients with stage III non small cell lung cancer
The use of chemotherapy concurrent with radiotherapy in locally advanced non‐small cell lung cancer may enhance the benefits of radiotherapy in terms of local and regional control and thus improve survival. A total of twenty‐five randomised studies (including 3752 patients) were included in this updated review: nineteen trials (2728 patients) comparing concurrent chemoradiotherapy with radiotherapy alone and six trials (1024 patients) comparing concurrent with sequential chemoradiotherapy. Both comparisons demonstrated significant reduction in risk of death with use of concurrent chemoradiation, with an associated increase in incidence of acute oesophagitis.
Background
This review is an update of a previously published review in The Cochrane Database of Systematic Reviews Issue 4, 2004 (Rowell 2004).
For patients of good performance status with locally advanced NSCLC (i.e. stage IIIA and selected cases with stage IIIB) whose disease can be encompassed within an appropriate treatment volume, radical (as opposed to palliative) radiotherapy is regarded as the treatment of choice. Higher doses of conventionally fractionated radiotherapy (60 Gy in 30 daily fractions compared to 40 Gy to 50 Gy in 20 to 25 fractions) have been found to be more effective in terms of local control but not survival (Perez 1987). The use of twice daily fractionation to a total dose of 69.6 Gy, in 58 fractions, resulted in improved local control and survival compared to 60 Gy conventionally fractionated (Cox 1990). Furthermore, a trial of continuous hyperfractionated accelerated radiotherapy (CHART: 54 Gy in 36 fractions, over 12 days) in stages I‐III NSCLC showed an improvement in two‐year survival from 20% to 29% when compared to 60 Gy conventionally fractionated (Saunders 1999). These studies provide compelling evidence of improved survival and local control with more intensive regimens of radiotherapy.
In a meta‐analysis of adjuvant chemotherapy in NSCLC (NSCLCCG 1995; NSCLCCG 2000), there was a 13% reduction in the risk of death with an absolute benefit in two‐year survival of 4% (95% CI 1% to 7%) in patients receiving radical radiotherapy; that review specifically excluded trials in which chemotherapy and radiotherapy were given concurrently (NSCLCCG 1995; NSCLCCG 2000). On this evidence sequential chemotherapy and radiotherapy was generally seen as the standard of care for locally advanced NSCLC at the time this review was originally published in 2004. The optimal timing of chemotherapy relative to radiation was not established and although many of the trials in the original meta‐analysis examined the addition of chemotherapy after radiation (described variously as 'consolidation' or 'adjuvant'), the most common practice in 2004 and indeed the updated trials have used 'sequential' chemotherapy meaning induction chemotherapy followed by radiation.
The rationale for combining chemotherapy and radiotherapy is to combine the benefits of radiotherapy in terms of local regional control with the benefits of chemotherapy in terms of reducing the risks of metastatic disease. With concurrent chemoradiation there is the potential for chemotherapy, given during a course of radiotherapy, to enhance the effectiveness of radiotherapy (i.e. radiosensitisation).. This is now a standard approach in other tumour types (e.g. head and neck, cervical, anal and oesophageal cancer). A number of randomised trials have compared concurrent chemoradiotherapy to radiotherapy alone in NSCLC but the majority have failed to show a significant benefit. In recent years there have in addition been trials of concurrent chemoradiotherapy versus sequential treatment but individual trials have been underpowered or closed early or not reported in full.
The purpose of this review was to determine whether the effectiveness of radical radiotherapy may be improved by the use of concurrent chemoradiotherapy and whether any benefit is at the expense of increased treatment‐related morbidity. At the outset it was anticipated that few, if any, trials would include data on quality of life.
Objectives
To determine the effectiveness in patients with NSCLC of concurrent chemoradiotherapy as compared to radiotherapy alone, in terms of overall survival and progression‐free survival, treatment‐related mortality and morbidity.
To determine the effectiveness in patients with NSCLC of concurrent chemoradiotherapy as compared to sequential chemoradiotherapy, in terms of overall survival and progression‐free survival, treatment‐related mortality and morbidity.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials.
Types of participants
Patients of any age and any performance status with pathologically confirmed NSCLC but without distant metastases (i.e. stage I‐III).
Types of interventions
Patients undergoing radical radiotherapy either randomised to receive concurrent chemoradiotherapy or radical radiotherapy alone, or randomised to concurrent or sequential chemoradiotherapy.
Radical radiotherapy was defined as a minimum dose of 50 Gy in 25 daily fractions, or its radiobiological equivalent (Jones 2001), and had to be the same in both arms of the study.
Concurrent chemotherapy was defined as chemotherapy given on radiotherapy treatment days (whether before or after each fraction of radiotherapy). Sequential chemoradiotherapy involved chemotherapy given before or after a course of radiotherapy but not during. Chemotherapy agents and doses had to be equivalent in both arms of the study.
Trials were eligible for inclusion if additional chemotherapy had been given prior to radiotherapy, following radiotherapy, or both, provided this was the same in both treatment arms.
Types of outcome measures
Primary outcomes
Overall survival (OS).
Secondary outcomes
Progression‐free survival (progression at any site whether locoregional or distant; PFS). Locoregional progression‐free survival (local PFS).
Overall survival at two years. Progression‐free survival (progression at any site whether locoregional or distant) at two years. Locoregional progression‐free survival at two years.
Treatment morbidity ‐ acute The incidence in each treatment arm of: (1) lung damage, acute (pneumonitis), grade 3 or worse; (2) oesophagitis, grade 3 or worse ; (3) neutropenia, grade 3 or worse; (4) anaemia, any grade. Treatment morbidity ‐ late The incidence in each treatment arm of: (1) lung damage, late (fibrosis), grade 3 or worse; (2) oesophageal damage, grade 3 or worse; (3) radiation myelopathy;
(4) quality of life.
Search methods for identification of studies
We ran a search in October 2009 to update the original completed review. In this update we adapted the original searches to search the following databases: Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2009, Issue 4), MEDLINE (accessed through PubMed), and EMBASE (accessed through Ovid). We include in the Appendix 1 the search strategies used and the results obtained. We also report in the Appendix 2 the text published for this section in the original review, and the search strategies designed.
We additionally checked the reference lists from relevant studies to identify further eligible studies. On‐going trials were searched for on the metaRegister of Controlled Trials (Current Controlled Trials) . Only one trial was identified which fulfilled the criteria for this review ‐ the NCRI SOCCAR study (NCRI SOCCAR)‐ which was converted from a randomised phase III to a phase II study after slow recruitment and is now closed with results pending.
Data collection and analysis
Selection of studies
References identified by the search strategy were screened independently by two authors (NOR, MR) to assess eligibility for inclusion in the review and a list of trials eligible for inclusion was agreed. In case of discrepancy, consensus was reached by discussion and the input of a third reviewer was sought if necessary.
Data extraction and management
For each of the included trials, details of treatment given and outcomes were recorded independently by two authors (NOR, NF or MR) and any disparity resolved by discussion.
Assessment of risk of bias in included studies
Two review authors (NOR or NF, and MR) independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2009). Any disagreement was resolved by discussion or involving a third assessor. From the risk of bias domains listed on the handbook, the authors assessed three (sequence generation, allocation concealment and incomplete outcome data). A fourth domain wasn't assessed (blinding of participants, personnel and outcome assessors) due to the practical difficulties or impossibility to blind participants and health providers given the differences in schedule of the interventions. Although outcome assessors could have been blinded in the studies, it was not deemed necessary given the objectivity of outcomes and the generalized use of standardized outcome assessment methods.
(1) Sequence generation (checking for possible selection bias)
We described for each included study the methods used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups. We assessed the methods as:
adequate (any truly random process e.g. random number table; computer random number generator),
inadequate (any non random process e.g. odd or even date of birth; hospital or clinic record number) or
unclear.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal the allocation sequence in sufficient detail and determine whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment. We assessed the methods as:
adequate (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
inadequate (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear.
(3) Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations)
We described for each included study and for each outcome or class of outcomes the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. We assessed whether each study was free of attrition bias:
yes;
no;
unclear.
Measures of treatment effect
The effect of treatment was estimated by hazard ratios (HR) and risks ratios (RR), with their corresponding confidence intervals (CI). HR were computed for time‐to‐event variables (overall survival, progression‐free survival at any site, locoregional progression‐free survival). When the papers did not report HR, they were computed following the formulae of Parmar (Parmar 1998) implemented in a freely‐available spreadsheet (Tierney 2007).
Risk ratios were computed for dichotomous variables (time‐to‐event variables assessed at 2 years and treatment morbidity) in preference to odds ratio as being less open to misinterpretation. Percentages of OS, PFS and local PFS at 2 years were estimated from Kaplan Meier graphics when they were not presented in the text. Two trials (Bonner 1998; Trovo 1992) had minimum follow‐up <24 months and thus the estimations from survival curves are slightly unreliable. Absolute survival differences at 2 years were computed as risk differences for overall survival at 2 years. These estimations of absolute survival benefit approximate estimations obtained with methods based on hazard ratios, with the advantage to fully use the data provided by the included trials, since 2 years survival data was available for all or them but not hazard ratios.
Assessment of heterogeneity
Global estimates for each variable effect were computed by conducting a meta‐analysis of single effect measures of the study (HR for time‐to‐event variables and RR for dichotomous variables) applying a random‐effects model (DerSimonian 1986). Prior to calculating estimates of effect, the presence and degree of heterogeneity was assessed by means of I2.
Data synthesis
The review's main analysis was an "available data" analysis, where data for each included trial was analyzed as provided by the study authors, either per protocol or by intention‐to‐treat.
Subgroup analysis and investigation of heterogeneity
Two planned subgroup analyses were performed:
‐ According to chemotherapy with platinum‐based regimens
‐ According to frequency of chemotherapy administration
Other non‐planned subgroup analysis were also performed:
‐ According to total dose of cisplatin or carboplatin. In the analysis by platinum dose, trials were arbitrarily divided into those in which the planned total dose of cisplatin was above or below 150 mg/m2; or the total dose of carboplatin was 700 mg/m2 and greater, or less than 700 mg/m2. Carboplatin was prescribed on mg/m2 basis except in two trials where it had been prescribed as a total dose (Jeremic 1995; Jeremic 1996); for the purposes of this analysis total dose was converted to mg/m2 by assuming a typical body surface area of 1.7m2.
‐ Comparing once or twice daily radiotherapy fractionation
‐ According to radiotherapy dose. In the analysis by radiotherapy dose, trials were divided into those who received a low dose (50‐60 Gy total dose) or a high dose (more than 60 Gy).
‐ According to duration of follow‐up.
There were two three‐arm trials (Jeremic 1995; Schaake‐Koning 1992) which contained a single control arm and two treatment arms with different frequency of chemotherapy. For most analyses, the data from the two treatment arms were combined and treated as a single treatment group. In the subgroup analysis of frequency of chemotherapy administration (which differed between the treatment arms), the control arm was included with the relevant treatment arm in each subgroup but data from the subgroups were not combined to give an overall relative risk.
Statistical comparison of subgroups was performed with the method implemented in RevMan for fixed‐effect analyses based on the inverse‐variance method. The procedure is based on the test for heterogeneity chi‐squared statistics (see section 9.6.3.1 in Higgins 2009).
Sensitivity analysis
Several sensitivity analyses were performed to assess how robust the estimate of the global effect was regarding the:
missing data (performing an intention‐to‐treat analysis with all randomised patients under a "worst case" scenario, where data was imputed for all withdrawn participants assuming they did not respond or presented an adverse outcome. When it was not known the distribution of withdrawn participants among treatment arms, they were divided equally among arms;
trials not fully assessed (excluding from the analysis trials published only as abstracts);
statistical model (performing a meta‐analysis with a fixed‐effect model).
Dichotomous variables related to adverse effects were not considered in the ITT imputation sensitivity analysis, since the authors considered it wrong to attribute unintended (adverse) effects to a treatment that somebody did not receive (Higgins 2009b). Whenever number of lost patients was known but it was not known its distribution by treatment arm, the losses were attributed to the chemoradiotherapy arm in the comparison against radiotherapy alone, and equally distributed in the comparison of concurrent vs sequential. The only trial where no information about losses was available was excluded from this sensitivity analysis (Curran 2003).
Results
Description of studies
In the first publication of the review, twenty‐nine apparently randomised trials of concurrent chemoradiotherapy versus radiotherapy alone were identified. Twelve trials were excluded (Ball 1997;Chan 1976; Furuse 1999; Guschall 2000; Isakovic‐Vidovic2002; Japan ACNU 1989; Johnson 1990; Koca 1996; Komaki 2002; LePar 1967; Sarihan 2002; Ulutin 2000) and seventeen trials were included (Ball 1999; Blanke 1995; Bonner 1998; Cakir 2004; Clamon 1999; Curran 2003; Fournel 2001; Groen 1999; Huber 2003; Jeremic 1995; Jeremic 1996; Landgren 1974; Manegold 2003; Schaake‐Koning 1992; Soresi 1988; Trovo 1992; Zatloukal 2003), of which three were then published only in abstract form. In this update, a four‐arm included trial (Ball 1999) was considered as two separate trials of once daily and twice daily radiotherapy each with and without chemotherapy (Ball 1999 once daily; Ball 1999 twice daily), inflating the number of trials by one in the text and analyses.
The update of the bibliographic search identified 501 unique references. Of those, nine new trials were included (Atagi 2005; Gouda 2006; Li 2008; Lu 2005; Rao 2007; Reinfuss 2005; Wu 2006; Yadav 2005; Zhang 2006), five trials previously included had a more complete publication (Fournel 2001; Groen 1999; Huber 2003; Manegold 2003; Zatloukal 2003) and three trials were excluded (Belderbos 2007; DasGupta 2006; Misirlioglu 2006). One of the included trials was later labelled as awaiting assessment and excluded from the review because it was not possible to retrieve a paper copy of it (Zhang 2006).
A total of 25 studies (3752 participants) were included in this updated review (Table 1): 19 trials (2728 participants, Table 2) comparing concurrent chemoradiotherapy versus radiotherapy alone and six trials (1024 participants, Table 3) comparing concurrent versus sequential chemoradiotherapy. Duration of follow‐up is shown in Table 1.
1. Recruitment and duration of follow‐up for individual trials.
| author | study name | N analized / randomized | date accrued | minimum follow‐up | comparators |
| Atagi 2005 | JCOG9812 | 46/46 | 1999‐2001 | unclear | concurrent v RT alone |
| Ball 1999 | Australian multicentre | 204/208 | 1989‐1995 | 32 months | concurrent v RT alone |
| Blanke 1995 | Hoosier Oncology Group | 215/240 | 1986‐1992 | 26 months | concurrent v RT alone |
| Bonner 1998 | North Central Cancer Treatment Group | 99/110 | 1992‐1993 | 18 months | concurrent v RT alone |
| Cakir 2004 | Turkey (Samsun and Ankara) | 176/185 | 1997‐1999 | 36 months | concurrent v RT alone |
| Clamon 1999 | CALGB/ECOG | 250/283 | >1991 | uncertain | concurrent v RT alone |
| Gouda 2006 | Egypt | 60/60 | 1998‐2000 | uncertain | concurrent v RT alone |
| Groen 1999 | Netherlands | 160/160 | 1994‐1998 | uncertain | concurrent v RT alone |
| Huber 2003 | BROCAT | 212/219 | not stated | 3 years (except for 2 patients lost) |
concurrent v RT alone |
| Jeremic 1995 | Kragujevac (single centre) | 169/178 | 1988‐1989 | uncertain | concurrent v RT alone |
| Jeremic 1996 | Kragujevac (single centre) | 131/165 | 1990‐1991 | uncertain | concurrent v RT alone |
| Landgren 1974 | MDAnderson | 53/54 | 1970‐1971 | 24 months | concurrent v RT alone |
| Li 2008 | China | 58/60 | not stated | (median 24 months) | concurrent v RT alone |
| Lu 2005 | China | 85/92 | 2001‐2003 | uncertain | concurrent v RT alone |
| Manegold 2003 | European multicentre (8 centres) | 98/98 | 1999‐2001 | uncertain | concurrent v RT alone |
| Schaake‐Koning 1992 | EORTC | 331/331 | 1984‐1989 | 22 months | concurrent v RT alone |
| Soresi 1988 | Milan | 93/95 | 1986‐1987 | uncertain (median 12 months) |
concurrent v RT alone |
| Trovo 1992 | GOCCNE | 167/173 | 1987‐1991 | 6 months | concurrent v RT alone |
| Yadav 2005 | India | 30/30 | 2002‐2003 | uncertain (median 10 months) |
concurrent v RT alone |
| Curran 2003 | RTOG 94‐10 | 402/402 | 1994‐1998 | 48 months | concurrent v sequential |
| Fournel 2001 | GLOT‐GFPC NPC 95‐01 | 201/212 | 1996‐2000 | (median 4.8 years) | concurrent v sequential |
| Rao 2007 | China | 53/55 | not stated | 4 months | concurrent v sequential |
| Reinfuss 2005 | Poland | 173/173 | 2001‐2004 | 12 months | concurrent v sequential |
| Wu 2006 | China | ‐‐/80 | not stated | uncertain | concurrent v sequential |
| Zatloukal 2003 | Czech Lung Cancer Group | 102/102 | not stated | 18 months | concurrent v sequential |
2. Concurrent chemoradiotherapy versus radiotherapy alone: treatment details.
| trial | induction chemo | RT dose & fractions | chemo frequency | chemo details | total platinum dose | chemo post RT |
| Atagi 2005 | no |
60Gy in 30 fractions in 6 weeks | daily | Daily carboplatin 30mg/m2 for first 20 fractions | 600mg/m2 carboplatin in CRT arm | no |
| Ball 1999 ‐ once daily | no | 60Gy in 30 daily fractions over 6 weeks | 4‐weekly | carboplatin 70mg/m2 days 1‐5 weeks 1 & 5 | 700mg/m2 | no |
| Ball 1999 ‐ twice daily | no | 60Gy in 30 fractions (twice daily) over 3 weeks | 3‐weekly | carboplatin 70mg/m2 days 1‐5 week 1 | 350mg/m2 | no |
| Blanke 1995 | no | 60‐65Gy in daily fractions of 1.8‐2Gy (6‐7 weeks) | 3‐weekly | cisplatin 70mg/m2 weeks 1,4 & 7 | 210mg/m2 | no |
| Bonner 1998 | no | 60Gy in 40 fractions of 1.5Gy (twice daily) split over 6 weeks | 4‐weekly | cisplatin 30mg/m2 + etoposide 100mg/m2 days 1‐3, weeks 1 & 5 | 180mg/m2 | no |
| Cakir 2004 | no | 64Gy in 32 fractions over 6.5 weeks | 3‐weekly | cisplatin 20mg/m2 days 1‐5 weeks 2 & 6 | 200mg/m2 | no |
| Clamon 1999 | cisplatin 100mg/m2 days 1 & 29 + vinblastine 5mg/m2 days 1,8,15,22,29 | 60Gy in 30 daily fractions over 6 weeks | weekly | carboplatin 100mg/m2 weekly | 600mg/m2 | no |
| Gouda 2006 | No |
60 Gy at "conventional fractionation" in 30 fractions | 2 to 4‐weekly | Paclitaxel175mg/m2 Carbo AUC6 D1 and D28 concur with RT |
Carbo AUC6 x2 in group B | No |
| Groen 1999 | no | 60Gy in 30 daily fractions over 6 weeks | continuous infusion | carboplatin 840mg/m2 continuous infusion over 6 weeks | 840mg/m2 | no |
| Huber 2003 | carboplatin AUC6 + paclitaxel 200mg/m2; 3‐weekly, 2 cycles | 60Gy in 30 daily fractions over 6 weeks | weekly | paclitaxel 60mg/m2 weekly | ‐ | no |
| Jeremic 1995 | no | 64.8Gy in 54 fractions of 1.2Gy (twice daily) over 5.5 weeks | weekly OR alternate weeks | carboplatin 100mg total dose days 1,2 + etoposide 100mg total dose days 1‐3 each week OR carboplatin 200mg total dose days 1,2 + etopside 100mg total dose days 1‐5 weeks 1,3, & 5 | 1200mg total (approx 700mg/m2) | no |
| Jeremic 1996 | no | 69.6Gy in 58 fractions of 1.2Gy (twice daily) over 6 weeks | daily | carboplatin 50mg total dose + etoposide 50mg total dose daily | 1450mg total (approx 850mg/m2) | no |
| Landgren 1974 | no | 60Gy in 20 daily fractions split over 8 weeks | daily | hydroxyurea 30mg/kg daily | ‐ | no |
| Li 2008 | no | 1.2 Gy bd. AP/PA to 38.4 Gy then changed field angles to continue to total RT dose. 62.4‐67.2 GY | Daily | Hydroxycomphothecin 10 mg/d for 1st and last week of radiotherapy | ‐ | no |
| Lu 2005 | 2 cycles both arms |
40 Gy/20 AP/PA with subsequent boost to tumour avoiding cord to total dose 60‐65Gy | 2 to 4‐weekly | Vinorelbine 15‐18 mg/m2 D1 and D8 Cisplatin 60mg/m2 D1 q 3w | 4‐6 cycles 240‐360mg/m2 | 2‐4 cycles chemo |
| Manegold 2003 | cisplatin 40mg/m2 days 1,2 + docetaxel 85mg/m2 day 1; 3‐weekly, 2 cycles | 60Gy in 30 daily fractions over 6 weeks | weekly | docetaxel 20mg/m2 weekly | ‐ | no |
| Schaake‐Koning 1992 | no | 55Gy in 20 daily fractions split over 7 weeks | weekly OR daily | cisplatin 30mg/m2 weekly OR cisplatin 6mg/m2 daily | 120mg/m2 | no |
| Soresi 1988 | no | 50.4Gy in 28 fractions over 5.5 weeks | weekly | cisplatin 15mg/m2 weekly | 90mg/m2 | no |
| Trovo 1992 | no | 45Gy in 15 daily fractions over 3 weeks | daily | cisplatin 6mg/m2 daily | 90mg/m2 | no |
| Yadav 2005 | No |
50 Gy in 25 # AP/PA with cord shielding last five fractions ? same treatment both arms |
weekly | cisplatin 30mg/m2 IV weekly | 150mg/m2 | no |
3. Concurrent versus sequential chemoradiotherapy : treatment details.
| trial | chemotherapy | RT dose & fractions | concurrent RT | sequential RT | notes |
| Curran 2003 | cisplatin 100mg/m2 days 1,29 + vinblastine 5mg/m2 days1,8,15,22,29 | 63 Gy once daily fractions | RT starting day 1 | RT starting day 50 | |
| Fournel 2001 | sequential: cisplatin 120mg/m2 days 1,29,57 + vinorelbine 30mg/m2 weekly; concurrent: cisplatin 20mg/m2 + etoposide days 1‐5 & 29‐33 followed by cisplatin 80mg/m2 days 78 & 106 + vinorelbine weekly days 78‐127 | 66 Gy in 33 daily fractions over 6.5 weeks | RT starting day 1 | RT starting day 85 | total cisplatin dose same in both arms |
| Rao 2007 | vinorel 25mg/m2 D1, D8 cisplatin 25mg/m2 D1‐3 q 3w | 36 Gy/18 AP/PA with tumour boost 26‐34Gy avoiding cord | RT starting day 1 | RT starting after 2 cycles chemo approx D42 | |
| Reinfuss 2005 | cisplatin 100mg/m2 D1, vinorel 20mg/m2 D1,D8 q4wks 2 cycles | 50.4 Gy/28# with boost 19.8 Gy/11# same schedule both arms | RT starting day 1 | RT starting day 8 second course | cisplatin 200mg/m2 both arms |
| Wu 2006 | vinorelbine 12.5mg/m2 and cisplatin 40mg/m2 D1, 8, 29 and 36 during RT | 40 Gy/20‐22 AP/PA then avoiding cord to total dose of 60Gy/30‐33 | RT starting day 1 | RT starting D1 with chemo given subsequent to RT | |
| Zatloukal 2002,2003 | cisplatin 80mg/m2 day 1 + vinorelbine 25mg/m2 days 1,8,15 (12.5mg/m2 in cycles 2 & 3) 4‐weekly cycles x4 | 60 Gy in 30 daily fractions over 6 weeks | RT starting day 4 cycle 2 | RT starting day 113 (approx) |
Of the 19 randomised studies of concurrent chemoradiotherapy versus radiotherapy alone, sixteen used platinum‐based chemotherapy, in combination with etoposide in three; three used concurrent docetaxel or paclitaxel; one used hydroxyurea and one hydroxycomptothecin. Chemotherapy was administered on each radiotherapy treatment day in seven studies (by continuous infusion in one of these); once‐weekly in six; and two‐ to four‐weekly in eight (a total of 21 different intervention groups). The radiotherapy dose was most commonly 60 Gy given in 30 fractions over six weeks (seven studies). There were four studies using accelerated or hyperfractionated regimes and four employing a split course.
Two three‐arm trials had their two intervention arms combined for most analyses (Jeremic 1995; Schaake‐Koning 1992). In time‐to‐event analyses, Jeremic 1995 appears twice because it presented separated HR for each of its two‐intervention arms compared to its control arm. As a consequence, in these time‐to‐event analyses the number of trials is artificially inflated by one. A sensitivity analysis excluding one or the other HR for this trial didn't cause relevant changes in metanalysis results.
Risk of bias in included studies
Overall, risk of bias in the 19 trials of concurrent chemoradiotherapy vs radiotherapy alone was moderate. Selection bias was not adequately addressed since most trials were unclear on whether the sequence generation was adequate or not, and whether there was allocation concealment or not. On the other hand, attrition bias was mostly adequately addressed, with all but two trials providing data on the losses and exclusions or participants as well as the reasons for exclusion. The level of missing data is low with regard to the total number of randomised participants, limiting the extent to which attrition bias limits our confidence in the results.
Overall, risk of bias in the six trials of concurrent vs sequential choradiotherapy was moderate. Selection bias was not adequately addressed (allocation concealment unclear or inadequate for all but one trials). Attrition bias was moderate since incomplete data was addressed adequately in half of the trials and the overall level of missing data was low with regard to the number of randomised patients. Nevertheless, one of the included trials was only published as an abstract and this limits the confidence on their results.
See individual and summarized results of risk of bias assessment in Figure 1 and Figure 2.
1.

Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
2.

Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Allocation
Sequence generation Of the 19 trials of concurrent chemoradiotherapy vs radiotherapy alone, the sequence generation was adequately generated in 8 trials (Atagi 2005; Ball 1999 once daily; Ball 1999 twice daily; Blanke 1995; Bonner 1998; Groen 1999; Huber 2003; Yadav 2005), and unclear in 11 trials.
Of the 5 fully published trials of concurrent vs sequential chemoradiotherapy, the sequence generation was adequately generated in 3 trials (Rao 2007; Reinfuss 2005; Wu 2006) and unclear in 2.
Allocation concealment Of the 19 trials of concurrent chemoradiotherapy vs radiotherapy alone, allocation was adequately concealed in 5 trials (Ball 1999 once daily; Ball 1999 twice daily; Bonner 1998; Groen 1999; Huber 2003; Schaake‐Koning 1992), inadequately concealed in 1 trial and unclear in 13 trials.
Of the 5 fully published trials of concurrent vs sequential chemoradiotherapy, allocation was adequately concealed in 1 trial (Zatloukal 2003), inadequate in 3 trials and unclear in 1.
Incomplete outcome data
Of the 19 trials of chemoradiotherapy versus radiotherapy alone, risk of bias from incomplete outcome data was low on seventeen trials and unclear in 2 (Li 2008; Lu 2005).
Of the 5 fully published trials of concurrent vs sequential chemoradiotherapy, risk of bias from incomplete outcome data was low on 3 trials (Fournel 2001; Reinfuss 2005; Zatloukal 2003), high in one trial, unclear in 2.
Eight trials of chemoradiotherapy versus radiotherapy alone (Cakir 2004; Gouda 2006; Groen 1999; Landgren 1974; Manegold 2003; Schaake‐Koning 1992;Yadav 2005), and two trials of concurrent vs sequential chemoradiotherapy (Reinfuss 2005; Zatloukal 2003) analyzed all randomized patients, either because they had no losses or because they did an intention‐to‐treat analysis. Three trials stated an intention‐to‐treat analysis of the eligible patients, but not of the randomised patients (Ball 1999 once daily; Ball 1999 twice daily; Blanke 1995). On the 24 fully published trials, 125 patients (3.3%) were excluded from analysis due to ineligibility, loss to follow‐up and other reasons.
Other potential sources of bias
Other potential biases identified were the early stop of recruitment in two trials (Groen 1999; Zatloukal 2003), and the lack of full publication in one trial published as abstract (Curran 2003), which stated neither details of the randomisation procedure nor data analysis.
Effects of interventions
NOTE: In all time‐to‐event analyses, Jeremic 1995 appears twice, corresponding the two comparisons defined by its three arms. Across the whole review, Ball 1999 is considered as two separate RCT, thus inflating the number of trials by one.
Concurrent chemoradiotherapy versus radiotherapy alone
Overall survival (comparisons 1.1 and 1.2)
Meta‐analysis of nine trials (based on 1607 evaluable participants) showed that the addition of concurrent chemotherapy to radical radiotherapy reduced the overall risk of death with no evidence of heterogeneity (HR 0.71; 95% CI 0.64 to 0.80, I2 0%). The analysis of risk of death at two years also showed a significant benefit of chemoradiotherapy with moderate heterogeneity (RR 0.91; 95% CI 0.86 to 0.97, 20 trials, 2587 evaluable participants; I2 38%). Absolute survival benefit at 2 years is 8% (RD ‐0.08; 95% CI ‐0.12 to ‐0.03; I2 39%).
Subgroup analyses of 2‐year survival rates
‐ According to chemotherapy with platinum‐based regimens (comparison 3.1):
Sixteen trials administering platinum‐based regimens (2173 participants) showed a benefit of concurrent chemoradiotherapy in two‐year survival with moderate heterogeneity (RR 0.92; 95% CI 0.86 to 0.98; I2 41%). Two trials administering taxane‐containing regimens (301 participants) showed a benefit of concurrent chemoradiotherapy in two‐year survival (RR 0.82; 95% CI 0.71 to 0.94; I2 0%). Two trials (113 participants) administering other regimens failed to show differences in two‐year survival (RR 0.88; 95% CI 0.56 to 1.36; I2 70%). A test for differences between subgroups was not significant (P = 0.25).
‐ According to frequency of chemotherapy administration (comparison 3.2):
Analysis by chemotherapy frequency showed that the benefit of addition of two‐ to four‐weekly chemotherapy was similar to that achieved by weekly chemotherapy. The results in the group of 7 trials of daily chemotherapy (840 participants) were not significant and contained relevant statistical heterogeneity (RR 0.94; 95% CI 0.84 to 1.05; I2 55%). Seven trials with weekly chemotherapy (1013 participants) showed a benefit of chemoradiotherapy in two‐year survival, with homogeneous results (RR 0.90; 95% CI 0.84 to 0.96; I2 0%). Eight trials with 2‐ to 4‐weekly chemotherapy (909 participants) showed a benefit of chemoradiotherapy in two‐year survival, with moderate heterogeneity (RR 0.90; 95% CI 0.81 to 0.99; I2 41%). A test for differences between subgroups was not significant (P = 0.36).
To explore further the relationship between frequency of chemotherapy and outcome, a meta‐analysis was undertaken using data from the two treatment arms in each of the two three‐arm trials (325 patients) that directly compared frequency of administration to the same total dose (Jeremic 1995; Schaake‐Koning 1992). No differences were observed in survival at 2 years (RR 0.91; 95% CI 0.80 to 1.03; I2 0%).
‐ According to total dose of cisplatin or carboplatin (comparison 3.3):
Analysis by total platinum dose resulted in increased heterogeneity and loss of power in the two subgroups of trials (high dose and low dose). No differences were apparent in the results obtained by the two subgroups. Nine trials with 1145 participants in the high dose subgroup reached results on the verge of significance (RR 0.91; 95% CI 0.84 to 1.00; I2 40%), while seven trials with 1028 participants in the low dose subgroup failed to show significant differences (RR 0.93; 95% CI 0.84 to 1.03; 48%). A test for differences between subgroups was not significant (P = 0.45).
‐ Comparing once or twice daily radiotherapy fractionation (comparison 3.4):
In the subgroup analysis by radiotherapy fractionation, the benefit of chemoradiotherapy persisted in the subgroup with once daily fractionation (RR 0.91; 95% CI 0.85 to 0.97; I2 41%; 15 trials, 2065 participants) but not in the twice daily (RR 0.92; 95% CI 0.79 to 1.08; I2 40%; 5 trials, 522 participants). A test for differences between subgroups was not significant (P = 0.81).
‐ According to radiotherapy dose (comparison 3.5):
A subgroup analysis was performed to investigate the possibility that effectiveness of concurrent chemotherapy was related to radiotherapy dose (up to or more than 60 Gy). Thirteen trials used low doses of radiotherapy from 50 Gy to 60 Gy on 1691 participants (RR 0.93; 95% CI 0.86 to 1.01; I2 44%), while seven trials used high doses of radiotherapy from 60 Gy to 69.6 Gy on 896 participants (RR 0.88; 95% CI 0.81 to 0.95; I2 21%). Although a moderate level of heterogeneity can be observed among subgroups (I2 46.7), a test for differences between subgroups was not significant (P = 0.17).
‐ According to duration of follow‐up (comparison 3.6):
A sensitivity analysis was performed by dividing trials into three subgroups: where the minimum follow‐up duration was 22 months or more, less than 22 months, or where the duration of follow‐up was uncertain (Table 1). The hypothesis was that data from trials with longer follow‐up might be considered more reliable. The treatment effect estimates were similar for the subgroups with long (RR 0.90; 95% CI 0.83 to 0.97; I2 39%; 7 trials, 1191 participants) or uncertain follow‐up (RR 0.89; 95% CI 0.79 to 0.99; I2 40%; 9 trials, 1037 participants), but that of the subgroup with short follow‐up was higher (RR 0.99; 95% CI 0.85 to 1.15; I2 24%;4 trials, 359 participants), suggesting that any bias due to short follow‐up was, if anything, reducing rather than increasing the magnitude of the treatment effect. A test for differences between subgroups was not significant (P = 0.14).
Progression‐free survival (comparisons 1.3, 1.4, 1.5 and 1.6)
A minority of trials reported progression‐free survival for recurrence at any site (9 trials, 1405 participants), or locoregional progression‐free survival (5 trials, 872 participants). Concurrent chemoradiotherapy resulted in significant improvements in overall progression‐free survival at any site (i.e. distant or locoregional), (HR 0.69; 95% CI 0.58 to 0.81; I2 45%) as well as in overall locoregional progression‐free survival (HR 0.67; 95% CI 0.54 to 0.82; I2 60%). Results at two years corroborate these results both for PFS (RR 0.91; 95% CI 0.86 to 0.97; I2 43%) and locorregional PFS (RR 0.84; 95% CI 0.72 to 0.98; I2 59%).
Treatment‐related mortality and morbidity (comparison 1.7)
All trials reported treatment‐related deaths and some aspect of acute treatment‐related morbidity. There were 6 treatment‐related deaths in the radiotherapy alone group (966 participants) and 11 in the chemoradiotherapy group (1109 participants) (RR 1.38; 95% CI 0.51 to 3.72; I2 0%; 14 trials, 2075 participants). The incidence of acute pneumonitis (grade 3 or worse) ranged from 0% to12% in those receiving radiotherapy alone to 1% to 16% in those receiving concurrent treatment (RR 1.06; 95% CI 0.58 to 1.93; I2 0%; 9 trials, 1179 participants). The incidence of grade 3 or worse acute oesophagitis ranged from 0% to 33% in those receiving radiotherapy alone to 0% to 48% in those receiving concurrent treatment (RR 1.76; 95% CI 1.34 to 2.31; I2 0%; 17 trials, 2227 participants). Fewer trials reported pulmonary fibrosis (4 trials, 596 participants; RR 1.27; 95% CI 0.34 to 4.64; I2 50%) or late oesophageal damage (2 trials, 300 participants; RR 1.72; 95% CI 0.32 to 9.33; I2 23%); neither appeared statistically significantly increased and confidence intervals were wide. A single case of radiation myelopathy was reported in a patient receiving radiotherapy alone (Landgren 1974). Neutropenia (grade 3 or worse) was reported in 7 trials (837 participants), 3 of which used induction chemotherapy. Neutropenia was significantly more common with combined treatment (RR 3.53; 95% CI 1.84 to 6.77; I2 0%) but this result owed much to the greater incidence of neutropenia in two trials (Atagi 2005; Clamon 1999). Similarly, anaemia (grade 3 or worse) was much more frequently reported in one trial (Clamon 1999). Although considering all trials in this subgroup (5 trials, 822 patients) severe anaemia was more frequent with concurrent chemotherapy (RR 4.17; 95% CI 1.13 to 15.35; I2 15%), significance disappears when the effect measure is risk differences and the analysis then takes into account the study by Ball with zero events. In contrast, anaemia of any grade appeared more frequent with concurrent chemoradiotherapy (RR 1.99; 95% CI 1.49 to 2.64; I2 0%; 9 trials, 887 patients).
Concurrent versus sequential chemoradiotherapy
Overall survival (comparison 2.1 and 2.2)
Meta‐analysis is based on a total of 5 trials of concurrent versus sequential treatment in 937 participants. Results indicated a significant benefit of concurrent over sequential treatment with respect to overall survival (HR 0.74; 95% CI 0.62 to 0.89; I2 0%; 3 trials, 702 participants) as well as survival at two years (RR 0.87; 95% CI 0.78 to 0.97; I2 37%; 5 trials, 937 participants). Absolute survival benefit at 2 years is 10% (RD ‐0.10; 95% CI ‐0.18 to ‐0.02; I2 41%). All the trials analyzed used cisplatin‐based regimes and once daily radiotherapy (Table 3).
Subgroup analyses of 2‐year survival
The low number of trials included in this comparison precluded deriving strong conclusions from the differences observed between groups. Results in each subgroup lose power and differences in significance cannot be reliably interpreted . Estimation of effect was similar among all subgroups.
‐ According to radiotherapy dose (comparison 4.1):
The results in the single trial using low dose radiotherapy (RR 0.76; 95% CI 0.61 to 0.95; 102 participants) were similar to those of the 4 trials (835 participants) using high dose radiotherapy (RR 0.89; 95% CI 0.79 to 1.01; I2 32%). A test for differences between subgroups was not significant (P = 0.17).
‐ According to duration of follow‐up (comparison 4.2):
Results in the 2 trials (607 participants) that had minimum follow‐up of 22 or more months (RR 0.88; 95% CI 0.79 to 0.99; I2 0%) were similar to those of the 3 trials (330 participants) that had a minimum follow‐up of 22 or less months (RR 0.84; 95% CI 0.66 to 1.06; I2 64%). A test for differences between subgroups was not significant (P = 0.99).
Progression‐free survival (comparison 2.3, 2.4 and 2.5)
Only two trials (378 participants) reported progression‐free survival, which was not significantly different between the two groups. Neither overall PFS (HR 0.67; 95% CI 0.30 to 1.50; I2 0%; 1 trial, 201 participants) nor results at 2 years were significant (RR 0.92; 95% CI 0.78 to 1.09; I2 69%; 2 trials, 378 participants). Only one trial (402 participants) has thus far reported locoregional progression‐free survival at two years, which was not significantly different between the two groups (RR 0.84; 95% CI 0.64 to 1.10).
Treatment‐related mortality and morbidity (comparison 2.6)
More deaths (4% vs 2%) were reported in the concurrent arm but this did not reach statistical significance (RR 2.02; 95% CI 0.90 to 4.52; I2 0%; 5 trials, 950 participants). There were no significant differences in acute pneumonitis (grade 3 or worse) with concurrent treatment compared to sequential treatment (RR 0.99; 95% CI 0.51 to 1.91; I2 41%; 5 trials, 947 participants). There were more acute oesophagitis (grade 3 or worse) with concurrent treatment (range 8% to 47%) compared to sequential treatment (range 0% to 25%; RR 4.96; 95% CI 2.17 to 11.37; I2 66%; 5 trials, 947 participants). The incidence of neutropenia (grade 3 or worse) was similar, overall, in both arms but there was significant heterogeneity between trials (RR 1.18; 95% CI 0.90 to 1.55; I2 77%; 5 trials, 947 participants). The incidence of anaemia (grade 3 or worse) was reported in 2 trials (292 participants) without differences among treatment arms (RR 0.95; 95% CI 0.41 to 2.21; I2 42%). No data was presented on late morbidity in any study.
Sensitivity analyses
‐ Fixed‐effect meta‐analysis
Results changed minimally when a fixed‐effect analysis was performed, and no change in significance was observed in any outcome.
‐ ITT analysis of dichotomous efficacy variables
Data was imputed for the 125 ineligible patients excluded from the main analysis, assuming all of them presented the outcome of interest (death or local/distant progression).
Results changed minimally when an ITT analysis with data imputed was performed, and no change in significance related to the random effect models was observed in any outcome .
‐ Restriction to trials fully published
This analysis was limited to the concurrent vs sequential comparison, where there was the only study published as abstract (Curran 2003). Results changed minimally when the study published as abstract was excluded, and no change in significance was observed in any outcome.
Discussion
Summary of main results
In 2004 as a result of the meta‐analysis of chemotherapy in NSCLC (NSCLCCG 1995), the combination of (sequential) chemotherapy and radical radiotherapy had become the standard of care for patients of good performance status with stage III NSCLC whose disease could be encompassed within a radical radiotherapy volume. The original version of this review concluded that with concurrent chemoradiotherapy there was a 14% reduction in risk of death at two years compared to sequential chemoradiotherapy but only a 7% reduction compared to radiotherapy alone. With only short follow‐up, particularly in the concurrent versus sequential comparison, the review concluded that sequential treatment should remain the standard of care while mature data and further trials were awaited. This update of the review has identified additional trials but benefits also from full publication of the previous concurrent versus sequential comparisons and this has consolidated the evidence for concurrent chemoradiation. Of the 25 trials in both comparisons, only four included patients with stage I/II disease, accounting for only a very small number of patients, so conclusions should be regarded as relating only to patients with stage III disease.
The differences in risk of death at two years are similar to the original review: an 8% reduction in risk for concurrent chemoradiation compared to radiotherapy alone and a 13% reduction in risk with the use of concurrent rather than sequential chemoradiation . This update also describes overall survival benefit in terms of hazard ratios for concurrent treatment compared to radiotherapy alone (HR 0.71, 95%CI 0.64 to 0.80) and for concurrent compared to sequential chemoradiation (HR 0.74, 95%CI 0.62 to 0.89). In absolute survival terms the difference at 2 years is 10% benefit (RD ‐0.10; 95% CI ‐0.18 to ‐0.02) for concurrent compared to sequential treatment.
With sequential chemoradiation the added survival benefit from use of chemotherapy is presumed to be due to reduction in distant metastases whereas for concurrent chemoradiation there should additionally be improved local control by sensitising the tumour to radiation. Only five trials reported locoregional progression‐free survival in the comparison of concurrent treatment against radiation alone. There was a significant benefit from concurrent therapy, which also held for the analysis at two years but there was substantial between‐study heterogeneity in this analysis. In contrast only one trial reported locoregional progression‐free survival for the concurrent versus sequential comparison and this was not different between the two treatment groups. The assumption remains that the added value of concurrent chemotherapy is achieved by radiosensitisation leading to improved local control but the data in this review are inadequate to prove this.
Overall completeness and applicability of evidence
This review includes over 3700 patients treated within randomised controlled trials. We have used strict inclusion criteria to limit comparisons to the effect of adding concurrent chemotherapy to radical radiotherapy in the treatment of NSCLC and in so doing have excluded trials with different radiotherapy or different chemotherapy regimens between the two arms. This is not an individual patient data meta‐analysis and therefore the comparison of absolute survival at 2 years is based on the number of deaths at 2 years computed as pooled risk differences. The lack of individual patient data and short follow‐up in some trials mean that it is difficult to comment on effect of treatment beyond two years. Nonetheless the hazard ratios and confidence intervals on survival demonstrate a statistically significant survival benefit for the use of concurrent chemoradiation.
The purpose in undertaking this review was to determine whether addition of concurrent chemotherapy to radical radiation would improve survival. The conclusions however are limited by the trial data available and by the treatments given within those trials. Although we have limited inclusion to radiotherapy regimens delivering more than 50Gy, the trials date back to 1974 and there is no detail on radiotherapy technique or formal radiotherapy quality assurance. The delivery of radical radiotherapy has improved substantially in recent years with improved imaging, PET staging and definition of treatment volumes and continually evolving techniques of conformal, 4D and intensity modulated radiotherapy. It is not clear by how much these techniques alone may improve survival and, if so, whether the added benefit of concurrent chemotherapy is less or not. It is interesting that the absolute survival benefit of 10% at 2 years for concurrent chemoradiation compared to sequential is of the same magnitude as the benefit at 2 years for CHART radiotherapy over conventional fractionation. Both approaches enhance the effectiveness of local regional control, but at the expense of increased local toxicity.
An increased risk of acute oesophagitis was convincingly demonstrated in this review for both comparisons for the use of concurrent chemoradiation. In one study (Reinfuss 2005) 21% of patients in the concurrent arm had their treatment discontinued due to severe oesophagitis and in that study there was no difference in outcome between the concurrent and sequential arms. Across all trials there was not found to be increased risk of acute pneumonitis with the concurrent schedule but in one study (Atagi 2005) there were four treatment‐related deaths from acute pneumonitis in the first 46 patients entered, leading to early closure of the trial. For both neutropenia and anaemia there was increased incidence with concurrent chemoradiation compared to radiation alone but no difference in the comparison of concurrent versus sequential therapy consistent with the expectation that these toxicities arise from the use of chemotherapy, regardless of scheduling. In both comparisons there were more treatment‐related deaths (approximately twice as many) in the concurrent group but the difference did not achieve statistical significance.
Subgroup analyses were used to investigate other factors which might influence the results, but no significant differences were found. The sensitivity analysis indicated that in the group with short follow‐up (i.e. where there is the greatest potential for bias) there appeared to be no benefit from the concurrent treatment over radiotherapy alone. Therefore, any bias resulting from including trials with short follow‐up would tend to have underestimated the benefits of concurrent chemoradiotherapy rather than the reverse.
The majority of trials in this systematic review used platinum‐based chemotherapy although there was considerable clinical heterogeneity in terms of frequency of administration and total dose. Some trials used cisplatin, others carboplatin. Total doses ranged from 90 to 210 mg/m2 cisplatin and 350 to 850mg/m2 carboplatin. In comparison with doses of cisplatin used in concurrent regimes for head and neck cancer (up to 300 mg/m2 over a 7 week course of radiotherapy), these doses appear relatively modest. The optimal frequency of chemotherapy administration remains uncertain with the combined analysis of data from the two three‐armed trials (Jeremic 1995; Schaake‐Koning 1992). The principle underlying concurrent chemotherapy is that local control can be improved by radiosensitisation and that in reducing micrometastatic disease the risk of systemic relapse is also reduced. Low dose chemotherapy might be adequate to achieve radiosensitisation without the increase in toxicity seen with full dose combination chemotherapy but there is too little data for comparison of full dose with radiosensitising doses of chemotherapy.
The other possible approach to enhancing the effectiveness of radiotherapy is to optimise the schedule of radiation in terms of dose, fractionation and overall treatment time. One trial excluded from this review was EORTC 08972 (Belderbos 2007) using hypofractionated accelerated radiotherapy with concurrent low dose cisplatin which produced favourable survival figures but the study closed early and was underpowered. The CHART trial of hyperfractionated accelerated radiotherapy gave a 9% increase in 2‐year survival from altered fractionation alone, raising the possiblity of further enhanced results if this were combined with chemotherapy.
Quality of the evidence
Since the original publication of this review the new format for Cochrane reviews has incorporated risk of bias tables. This has enabled a more structured assessment of the methodological quality of the studies included in the review. The trials included in this update show overall moderate risk of selection and attrition bias and these biases could cause the interventions tested to appear more beneficial and clinically relevant than they really are. Nonetheless, the number of trials and participants included and the consistency of their results with regard to overall survival supports the confidence on the review conclusions.
Agreements and disagreements with other studies or reviews
Individual patient data meta‐analyses can estimate treatment effects with greater precision. Two such analyses were conducted by Rolland et al (Rolland 2007) and Auperin et al (Auperin 2003, Auperin 2007, Auperin 2010). Rolland reported on 16 trials comparing concurrent chemoradiation with radiation alone, demonstrating significant improvement with concurrent treatment in overall survival (HR = 0.88) and in progression‐free survival (HR = 0.81). Auperin undertook comparison of concurrent with sequential treatment, including 6 trials . The conclusion was that concurrent improved overall survival (HR, 0.84; 95% CI, 0.74 to 0.95; P = 0.004), as well as locoregional progression‐free survival (HR, 0.77; 95% CI, 0.62 to 0.95; P = 0.01) but with no difference in distant progression between the two arms. There are differences between the inclusion criteria set for the present review and these meta‐analyses with only two overlapping trials in the concurrent versus sequential comparison which may account in part for the difference in results. Median follow‐up in the Auperin meta‐analysis is longer than in this review which may also contribute to the lower absolute survival benefit seen..
The largest single trial listed in this review is the Curran report on RTOG 9410 with 402 patients. Unfortunately this trial has only ever been published as an abstract in 2003 and this was a limiting factor in our analysis of these patients. However data from this trial were included in the Auperin meta‐analysis.
It is interesting that in both the IPD meta‐analyses and in the current review the magnitude of survival benefit observed with concurrent chemoradiation is similar whether compared against sequential chemoradiation or against radiation alone. There are several factors which may contribute to the lack of efficacy of the sequential schedule. Firstly there is a risk of disease progression during induction chemotherapy such that patients do not complete the scheduled treatment. In the Zatloukal trial only 58% of patients in the sequential group completed chemotherapy compared to 83% in the concurrent group (Zatloukal 2003). More importantly only 64% of the sequential group received radiotherapy treatment against 94% of concurrent group. Similarly in the Fournel trial less than 60% of the sequential patients received more than 60 Gy radiotherapy compared to 88% in the concurrent group (Fournel 2001). In these trials therefore the benefit achieved by concurrent chemoradiation might be due less to radiosensitisation than to the fact that in the sequential arm many patients did not get radiotherapy at all.
A further consideration with sequential treatment is that if there is a delay before starting radiotherapy, tumour regrowth has been observed following completion of neoadjuvant chemotherapy which is at a rate more rapid than prior to treatment (El Sharouni 2003). It is possible that this accelerated repopulation may render tumours more resistant to subsequent irradiation, further contributing to the difference in effectiveness of concurrent and sequential chemoradiotherapy. It is possible that longer conventional fractionation schedules over 6 to 7 weeks (in contrast to accelerated regimens of 4 weeks or less) may be particularly vulnerable to accelerated repopulation.
In summary, concurrent chemoradiotherapy appears more effective than radiotherapy alone or sequential chemoradiotherapy. The optimal chemotherapy regimen remains unclear, in particular, it is unknown whether platinum‐ or taxane‐based chemotherapy is more effective and whether dose and frequency of administration have a significant impact on outcome. Equally the optimal radiotherapy schedule is unclear. The majority of trials in this review used conventional fractionation over 6‐7 weeks with the presumed benefit of concurrent chemoradiation derived from radiosensitisation at this schedule. Severe oesophagitis is signficantly increased with concurrent chemoradiation and there is an increase in treatment related mortality. The focus for future practice and research should be on appropriate selection of patients for concurrent chemoradiation regimes, addressing ways of reducing toxicity and evaluating the optimal chemotherapy combinations and optimal radiotherapy schedules, using modern, quality assured radiotherapy techniques.
Authors' conclusions
Implications for practice.
The trials reported in this review demonstrate a survival benefit for concurrent chemoradiation but with increased toxicity compared to radiation alone or sequential chemoradiation. For patients of good performance status with locally advanced NSCLC which can be encompassed within a radical radiotherapy volume concurrent chemoradiotherapy should be considered. It is important to note however that the effects of altered radiotherapy fractionation or technique may similarly enhance local regional control and survival and the optimal radiotherapy schedule remains uncertain.
Implications for research.
Further trials are required to address the optimal chemotherapy regime to use in combination with radiotherapy. Trials should be designed to: * investigate the combination of chemotherapy with alternative radiotherapy fractionation schedules such as 55 Gy in 20 fractions over 4 weeks, or CHART;
*incorporate optimal radiotherapy techniques with formal quality assurance of radiotherapy treatment * investigate the impact of total dose of chemotherapy delivered during radiotherapy; * compare more frequent versus less frequent chemotherapy administration; * compare platinum‐ and taxane‐based chemotherapy; * investigate the impact of anaemia on the benefits of chemoradiotherapy.
What's new
| Date | Event | Description |
|---|---|---|
| 13 January 2010 | New citation required and conclusions have changed | A search was run and nine new studies were identified with updated publications on five studies included in original review. Conclusions changed. Contact author changed. |
History
Protocol first published: Issue 1, 2000 Review first published: Issue 4, 2004
| Date | Event | Description |
|---|---|---|
| 18 September 2008 | Amended | Converted to new review format. |
| 2 July 2004 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
The authors acknowledge the key contribution to Dr Nick Rowell in the design and conduct of the first published version of the review.
The authors are grateful to Ms Juan and Dr Ali Sever for translations, to Dr David Ball and Dr Harry Groen for additional data and to colleagues for support and comment. They also acknowledge the useful contributions of Gian Luca Di Tanna and Mia Schmidt‐Hansen, who peer‐reviewed the manuscript. The original version of the review was possible with the support of Maidstone & Tunbridge Wells NHS Trust and Beatson Oncology Centre.
Appendices
Appendix 1. Updated search strategy
CENTRAL (The Cochrane Library 2009, Issue 4)
#1 MeSH descriptor Antineoplastic Agents explode all trees 28644
#2 MeSH descriptor Antineoplastic Protocols explode all trees 8440
#3 MeSH descriptor Chemotherapy, Adjuvant explode all trees 2443
#4 chemotherap* 27875
#5 adjuvant:ti AND therapy:ti 1328
#6 (#1 OR #2 OR #3 OR #4 OR #5) 45628
#7 cisplatin* 5852
#8 etoposide 2148
#9 vinblastine 1150
#10 mitomycin* 1820
#11 vindesine 545
#12 gemcitabine 926
#13 paclitaxel 1919
#14 docetaxel 1037
#15 carboplatin 1861
#16 cyclophosphamide 6123
#17 ifos*amide 810
#18 fluorouracil 5248
#19 pemetrexed 85
#20 (#6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19) 50158
#21 MeSH descriptor Carcinoma, Non‐Small‐Cell Lung explode all trees 1570
#22 non small cell lung 3733
#23 nsclc 1850
#24 (#21 OR #22 OR #23) 3876
#25 MeSH descriptor Radiotherapy explode all trees 3989
#26 radiotherap* 11360
#27 irradiat* 4696
#28 (#25 OR #26 OR #27) 13583
#29 (#20 AND #24 AND #28) 677
#30 (#20 AND #24 AND #28), from 2004 to 2009 206 (117 in Clinical Trials)
MEDLINE (PubMed 15. October. 2009)
#5 Search "Carcinoma, Non‐Small‐Cell Lung"[Mesh] 19937
#6 Search non small cell lung[tw] 24487
#7 Search nsclc[tw] 10435
#8 Search ((#5) OR (#6)) OR (#7) 24808
#9 Search "Radiotherapy"[Mesh] 109762
#10 Search radiotherap*[tw] 199013
#11 Search irradiat*[tw] 159619
#12 Search ((#9) OR (#10)) OR (#11) 321865
#13 Search "Chemotherapy, Adjuvant"[Mesh] 20902
#14 Search "Antineoplastic Agents"[Mesh] 201197
#15 Search chemotherap*[tw] 241706
#16 Search adjuvant[ti] AND therapy[ti] 3830
#17 Search "Antineoplastic Protocols"[Mesh] 78967
#18 Search ((((#13) OR (#14)) OR (#15)) OR (#16)) OR (#17) 384400
#19 Search ((#8) AND (#12)) AND (#18) 2833
#20 Search (randomized controlled trial [pt] OR controlled clinical trial [pt] OR randomized [tiab] OR placebo [tiab] OR clinical trials as topic [mesh: noexp] OR randomly [tiab] OR trial [ti]) NOT (animals[mh] NOT (humans[mh] AND animals[mh])) 619556
#21 Search (#19) AND (#20) 932 (310)
EMBASE (Ovid 15. October. 2009)
1 exp lung non small cell cancer/ (23516)
2 (non small cell adj3 lung).mp. (26227)
3 nsclc.mp. (9972)
4 1 or 2 or 3 (26470)
5 exp radiotherapy/ (162634)
6 radiotherap*.mp. (130110)
7 irradiat*.mp. (120294)
8 (radiation adj2 therapy).mp. (34853)
9 8 or 6 or 7 or 5 (266901)
10 exp chemotherapy/ (196505)
11 exp antineoplastic agent/ (764918)
12 chemotherap*.mp. (265018)
13 (adjuvant adj3 therapy).mp. (41008)
14 11 or 13 or 10 or 12 (857609)
15 4 and 9 and 14 (4547)
16 Clinical trial/ (558947)
17 Randomized controlled trials/ (174578)
18 Random Allocation/ (27087)
19 Single‐Blind Method/ (8587)
20 Double‐Blind Method/ (74328)
21 Cross‐Over Studies/ (21843)
22 Placebos/ (132301)
23 Randomi?ed controlled trial$.tw. (35268)
24 RCT.tw. (2950)
25 Random allocation.tw. (647)
26 Randomly allocated.tw. (10529)
27 Allocated randomly.tw. (1372)
28 (allocated adj2 random).tw. (565)
29 Single blind$.tw. (7704)
30 Double blind$.tw. (86977)
31 ((treble or triple) adj blind$).tw. (142)
32 Placebo$.tw. (113396)
33 Prospective Studies/ (86261)
34 33 or 32 or 21 or 26 or 17 or 22 or 18 or 30 or 16 or 23 or 29 or 25 or 27 or 28 or 20 or 24 or 19 or 31 (733789)
35 Case study/ (6429)
36 Case report.tw. (123578)
37 Abstract report/ or letter/ (513341)
38 35 or 36 or 37 (640898)
39 34 not 38 (708199)
40 animal/ not human/ (14494)
41 39 not 40 (708103)
42 synchronous.ti,ab. (13207)
43 concurrent.ti,ab. (43027)
44 concomitant.ti,ab. (82547)
45 sequential.ti,ab. (61371)
46 45 or 44 or 42 or 43 (196219)
47 46 and 15 (990)
48 41 and 47 (604)
Appendix 2. Old Search strategy
The databases and search strategies used were the same than in the previous version of this review. We include here the text published in Rowell 2004: Trials were identified by electronic searching of the Cochrane Central Register of Controlled Trials (CENTRAL) using a search strategy, and by electronic searching of MEDLINE 1966 to 2004 (OVID version 4.1.1). We also searched EMBASE and CINAHL (1980 to 2004) (See Appendix 1). Titles, abstracts and, where necessary, full papers were examined to determine whether chemotherapy had been given sequentially or concurrently with radiotherapy. Further studies were sought from references cited in the initial list. Handsearching of published abstracts of major meetings (ASCO, ESTRO and WCLC) published since 1998 was also carried out.
MEDLINE
1. exp carcinoma, bronchogenic/ 2. exp carcinoma, small cell/ 3. 1 not 2 4. carcinoma, non‐small‐cell lung/ 5. 3 or 4 6. exp radiotherapy/ 7. radiotherapy.ti,ab 8. irradiation.ti,ab 9. 6 or 7 or 8 10. exp antineoplastic agents/ 11. chemotherapy adjuvant/ 12. chemotherapy.ti,ab 13. 10 or 11 or 12 14. 5 and 9 and 13 15. synchronous.ti,ab 16. concurrent.ti,ab 17. concomitant.ti,ab 18. 15 or 16 or 17 19. 14 and 18 20. exp randomized controlled trials/ 21. exp random allocation/ 22. 20 or 21 23. 19 and 22 24. postoperative.ti,ab 25. preoperative.ti,ab 26. palliative.ti,ab 27. (phase 1 or phase I).ti,ab 28. 24 or 25 or 26 or 27 29. 23 not 28 30. sequential.ti,ab 31. 29 and 30
EMBASE
1. CHEMOTHERAPY 2. CHEMOTHERAPY‐ADJUVANT*:ME 3. DRUG‐THERAPY*:ME 4. ANTINEOPLASTIC‐AGENTS*:ME 5. CISPLATIN* 6. ETOPOSIDE 7. VINBLASTINE 8. MITOMYCIN* 9. VINDESINE 10. GEMCITABINE 11. PACLITAXEL 12. DOCETAXEL 13. CARBOPLATIN 14. CYCLOPHOSPHAMIDE 15. IFOS*AMIDE 16. FLUOROURACIL 17. or/1‐16 18. LUNG and CANCER 19. NON‐SMALL and CELL 20. #18 and #19 21. CARCINOMA‐NON‐SMALL‐CELL‐LUNG*:ME 22. BRONCHOGENIC and CARCINOMA 23. CARCINOMA‐BRONCHOGENIC*:ME 24. or/20‐23 25. CARCINOMA‐SMALL‐CELL*:ME 26. #24 not #25 27. RADIOTHERAPY*:ME 28. RADIOTHERAPY 29. #27 or #28 30. #17 and #26 and #29
Data and analyses
Comparison 1. Concurrent chemoradiotherapy vs Radiotherapy alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall survival | 9 | 1607 | Hazard Ratio (Random, 95% CI) | 0.71 [0.64, 0.80] |
| 2 Overall survival 2‐years | 20 | 2587 | Risk Difference (M‐H, Random, 95% CI) | ‐0.08 [‐0.12, ‐0.03] |
| 3 Progression‐free survival | 7 | 1145 | Hazard Ratio (Random, 95% CI) | 0.69 [0.58, 0.81] |
| 4 Progression‐free survival 2‐years | 9 | 1405 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.86, 0.97] |
| 5 Locoregional progression‐free survival | 2 | 345 | Hazard Ratio (Fixed, 95% CI) | 0.67 [0.54, 0.82] |
| 6 Locoregional progression‐free survival 2‐years | 5 | 872 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.72, 0.98] |
| 7 Toxicity | 19 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 Treatment‐related deaths | 14 | 2075 | Risk Ratio (M‐H, Random, 95% CI) | 1.38 [0.51, 3.72] |
| 7.2 Acute pneumonitis | 9 | 1179 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.58, 1.93] |
| 7.3 Acute oesophagitis | 17 | 2227 | Risk Ratio (M‐H, Random, 95% CI) | 1.76 [1.34, 2.31] |
| 7.4 Pulmonary fibrosis | 4 | 596 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [0.34, 4.64] |
| 7.5 Late oesophagitis | 2 | 300 | Risk Ratio (M‐H, Random, 95% CI) | 1.72 [0.32, 9.33] |
| 7.6 Neutropenia | 7 | 837 | Risk Ratio (M‐H, Random, 95% CI) | 3.53 [1.84, 6.77] |
| 7.7 Anaemia (grade 3 to 4) | 5 | 822 | Risk Ratio (M‐H, Random, 95% CI) | 4.17 [1.13, 15.35] |
| 7.8 Anaemia (grade 1 to 4) | 9 | 887 | Risk Ratio (M‐H, Random, 95% CI) | 1.99 [1.49, 2.64] |
1.1. Analysis.

Comparison 1 Concurrent chemoradiotherapy vs Radiotherapy alone, Outcome 1 Overall survival.
1.2. Analysis.

Comparison 1 Concurrent chemoradiotherapy vs Radiotherapy alone, Outcome 2 Overall survival 2‐years.
1.3. Analysis.

Comparison 1 Concurrent chemoradiotherapy vs Radiotherapy alone, Outcome 3 Progression‐free survival.
1.4. Analysis.

Comparison 1 Concurrent chemoradiotherapy vs Radiotherapy alone, Outcome 4 Progression‐free survival 2‐years.
1.5. Analysis.
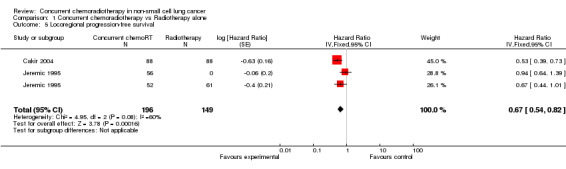
Comparison 1 Concurrent chemoradiotherapy vs Radiotherapy alone, Outcome 5 Locoregional progression‐free survival.
1.6. Analysis.

Comparison 1 Concurrent chemoradiotherapy vs Radiotherapy alone, Outcome 6 Locoregional progression‐free survival 2‐years.
1.7. Analysis.

Comparison 1 Concurrent chemoradiotherapy vs Radiotherapy alone, Outcome 7 Toxicity.
Comparison 2. Concurrent vs Sequential chemoradiotherapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall survival | 3 | 702 | Hazard Ratio (Random, 95% CI) | 0.74 [0.62, 0.89] |
| 2 Overall survival 2‐years | 5 | 937 | Risk Difference (M‐H, Random, 95% CI) | ‐0.10 [‐0.18, ‐0.02] |
| 3 Progression‐free survival | 1 | 201 | Hazard Ratio (Random, 95% CI) | 0.67 [0.30, 1.50] |
| 4 Progression‐free survival 2‐years | 2 | 378 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.78, 1.09] |
| 5 Locoregional PFS 2‐years | 1 | 402 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.64, 1.10] |
| 6 Toxicity | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 Treatment‐related deaths | 5 | 950 | Risk Ratio (M‐H, Random, 95% CI) | 2.02 [0.90, 4.52] |
| 6.2 Acute pneumonitis | 5 | 947 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.51, 1.91] |
| 6.3 Acute oesophagitis | 5 | 947 | Risk Ratio (M‐H, Random, 95% CI) | 4.96 [2.17, 11.37] |
| 6.4 Neutropenia | 5 | 947 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.90, 1.55] |
| 6.5 Anaemia | 2 | 292 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.41, 2.21] |
2.1. Analysis.

Comparison 2 Concurrent vs Sequential chemoradiotherapy, Outcome 1 Overall survival.
2.2. Analysis.

Comparison 2 Concurrent vs Sequential chemoradiotherapy, Outcome 2 Overall survival 2‐years.
2.3. Analysis.

Comparison 2 Concurrent vs Sequential chemoradiotherapy, Outcome 3 Progression‐free survival.
2.4. Analysis.
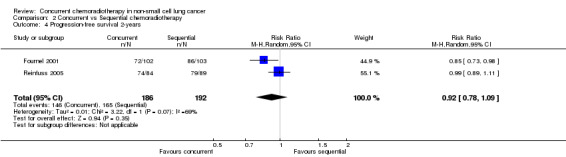
Comparison 2 Concurrent vs Sequential chemoradiotherapy, Outcome 4 Progression‐free survival 2‐years.
2.5. Analysis.

Comparison 2 Concurrent vs Sequential chemoradiotherapy, Outcome 5 Locoregional PFS 2‐years.
2.6. Analysis.

Comparison 2 Concurrent vs Sequential chemoradiotherapy, Outcome 6 Toxicity.
Comparison 3. Subgroup analysis Chemoradiotherapy vs Radiotherapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Chemotherapy regime | 20 | 2587 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.86, 0.97] |
| 1.1 Platinum‐containing regimes | 16 | 2173 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.86, 0.98] |
| 1.2 Taxane‐containing regimes | 2 | 301 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.71, 0.94] |
| 1.3 Other regimes | 2 | 113 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.56, 1.36] |
| 2 Frequency of chemotherapy administration | 20 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Daily administration | 7 | 840 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.84, 1.05] |
| 2.2 Weekly administration | 7 | 1013 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.84, 0.96] |
| 2.3 Two‐ to four‐weekly administration | 8 | 909 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.81, 0.99] |
| 3 Platinum dose | 16 | 2173 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.86, 0.98] |
| 3.1 High dose | 9 | 1145 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.84, 1.00] |
| 3.2 Low dose | 7 | 1028 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.84, 1.03] |
| 4 Radiotherapy fractionation | 20 | 2587 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.86, 0.97] |
| 4.1 Once daily fractionation | 15 | 2065 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.85, 0.97] |
| 4.2 Twice daily fractionation | 5 | 522 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.79, 1.08] |
| 5 Dose of radiotherapy | 20 | 2587 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.86, 0.97] |
| 5.1 Low dose | 13 | 1691 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.86, 1.01] |
| 5.2 High dose | 7 | 896 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.81, 0.95] |
| 6 Duration of follow‐up | 20 | 2587 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.86, 0.97] |
| 6.1 Minimum follow‐up 22 months or more | 7 | 1191 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.83, 0.97] |
| 6.2 Minimum follow‐up 18 months or less | 4 | 359 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.85, 1.15] |
| 6.3 Duration of follow‐up uncertain | 9 | 1037 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.79, 0.99] |
3.1. Analysis.

Comparison 3 Subgroup analysis Chemoradiotherapy vs Radiotherapy, Outcome 1 Chemotherapy regime.
3.2. Analysis.

Comparison 3 Subgroup analysis Chemoradiotherapy vs Radiotherapy, Outcome 2 Frequency of chemotherapy administration.
3.3. Analysis.

Comparison 3 Subgroup analysis Chemoradiotherapy vs Radiotherapy, Outcome 3 Platinum dose.
3.4. Analysis.

Comparison 3 Subgroup analysis Chemoradiotherapy vs Radiotherapy, Outcome 4 Radiotherapy fractionation.
3.5. Analysis.
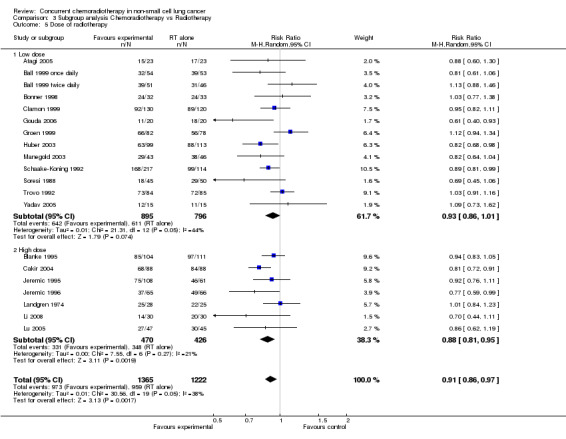
Comparison 3 Subgroup analysis Chemoradiotherapy vs Radiotherapy, Outcome 5 Dose of radiotherapy.
3.6. Analysis.
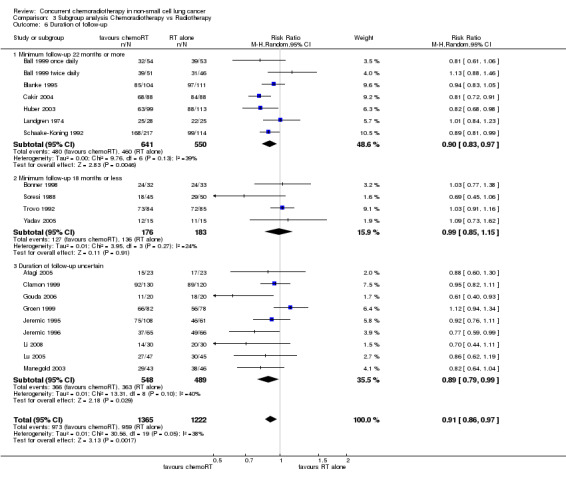
Comparison 3 Subgroup analysis Chemoradiotherapy vs Radiotherapy, Outcome 6 Duration of follow‐up.
Comparison 4. Subgroup analysis Concurrent vs Sequential.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Dose of radiotherapy | 5 | 937 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.78, 0.97] |
| 1.1 Low dose | 1 | 102 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.61, 0.95] |
| 1.2 High dose | 4 | 835 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.79, 1.01] |
| 2 Duration of follow‐up | 5 | 937 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.78, 0.97] |
| 2.1 Minimum follow‐up 22 months or more | 2 | 607 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.79, 0.99] |
| 2.2 Minimum follow‐up 18 months or less | 3 | 330 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.66, 1.06] |
| 2.3 Duration of follow‐up uncertain | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
4.1. Analysis.

Comparison 4 Subgroup analysis Concurrent vs Sequential, Outcome 1 Dose of radiotherapy.
4.2. Analysis.

Comparison 4 Subgroup analysis Concurrent vs Sequential, Outcome 2 Duration of follow‐up.
Comparison 5. More frequent versus less frequent chemotherapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Frequency of chemotherapy | 2 | 325 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.80, 1.03] |
5.1. Analysis.
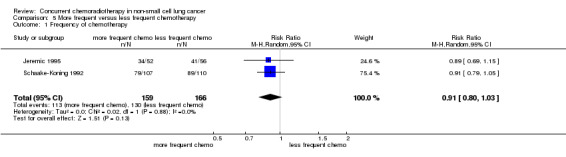
Comparison 5 More frequent versus less frequent chemotherapy, Outcome 1 Frequency of chemotherapy.
Comparison 6. Sensitivity fixed: Concurrent vs Radiotherapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall survival | 9 | Hazard Ratio (Fixed, 95% CI) | 0.71 [0.64, 0.80] | |
| 2 Overall survival 2‐years | 20 | 2587 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.86, 0.94] |
| 3 Progression‐free survival | 7 | Hazard Ratio (Fixed, 95% CI) | 0.70 [0.62, 0.79] | |
| 4 Progression‐free survival 2‐years | 9 | 1405 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.87, 0.95] |
| 5 Locoregional progression‐free survival | 2 | Hazard Ratio (Fixed, 95% CI) | 0.67 [0.54, 0.82] | |
| 6 Locoregional progression‐free survival 2‐years | 5 | 872 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.76, 0.91] |
| 7 Toxicity | 19 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 Treatment‐related deaths | 14 | 2075 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.51 [0.60, 3.79] |
| 7.2 Acute pneumonitis | 9 | 1179 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.57, 1.74] |
| 7.3 Acute oesophagitis | 17 | 2227 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.96 [1.50, 2.57] |
| 7.4 Pulmonary fibrosis | 4 | 596 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.56, 2.60] |
| 7.5 Late oesophagitis | 2 | 300 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.94 [0.53, 7.14] |
| 7.6 Neutropenia | 7 | 837 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.29 [2.28, 8.07] |
| 7.7 Anaemia (grade 3 to 4) | 5 | 822 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.96 [1.82, 13.51] |
| 7.8 Anaemia (grade 1 to 4) | 9 | 887 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.95 [1.46, 2.61] |
6.1. Analysis.

Comparison 6 Sensitivity fixed: Concurrent vs Radiotherapy, Outcome 1 Overall survival.
6.2. Analysis.

Comparison 6 Sensitivity fixed: Concurrent vs Radiotherapy, Outcome 2 Overall survival 2‐years.
6.3. Analysis.

Comparison 6 Sensitivity fixed: Concurrent vs Radiotherapy, Outcome 3 Progression‐free survival.
6.4. Analysis.

Comparison 6 Sensitivity fixed: Concurrent vs Radiotherapy, Outcome 4 Progression‐free survival 2‐years.
6.5. Analysis.

Comparison 6 Sensitivity fixed: Concurrent vs Radiotherapy, Outcome 5 Locoregional progression‐free survival.
6.6. Analysis.

Comparison 6 Sensitivity fixed: Concurrent vs Radiotherapy, Outcome 6 Locoregional progression‐free survival 2‐years.
6.7. Analysis.

Comparison 6 Sensitivity fixed: Concurrent vs Radiotherapy, Outcome 7 Toxicity.
Comparison 7. Sensitivity fixed: Concurrent vs Sequential.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall survival | 3 | Hazard Ratio (Fixed, 95% CI) | 0.74 [0.62, 0.89] | |
| 2 Overall survival 2‐years | 5 | 937 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.81, 0.96] |
| 3 Progression‐free survival | 1 | Hazard Ratio (Fixed, 95% CI) | 0.67 [0.30, 1.50] | |
| 4 Progression‐free survival 2‐years | 2 | 378 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.83, 1.00] |
| 5 Locoregional progression‐free survival 2‐years | 1 | 402 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.64, 1.10] |
| 6 Toxicity | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Treatment‐related deaths | 5 | 950 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.09 [0.95, 4.57] |
| 6.2 Acute pneumonitis | 5 | 947 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.60, 1.46] |
| 6.3 Acute oesophagitis | 5 | 947 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.97 [3.28, 7.53] |
| 6.4 Neutropenia | 5 | 947 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.97, 1.20] |
7.1. Analysis.

Comparison 7 Sensitivity fixed: Concurrent vs Sequential, Outcome 1 Overall survival.
7.2. Analysis.

Comparison 7 Sensitivity fixed: Concurrent vs Sequential, Outcome 2 Overall survival 2‐years.
7.3. Analysis.

Comparison 7 Sensitivity fixed: Concurrent vs Sequential, Outcome 3 Progression‐free survival.
7.4. Analysis.

Comparison 7 Sensitivity fixed: Concurrent vs Sequential, Outcome 4 Progression‐free survival 2‐years.
7.5. Analysis.

Comparison 7 Sensitivity fixed: Concurrent vs Sequential, Outcome 5 Locoregional progression‐free survival 2‐years.
7.6. Analysis.

Comparison 7 Sensitivity fixed: Concurrent vs Sequential, Outcome 6 Toxicity.
Comparison 8. Sensitivity ITT: Concurrent vs Radiotherapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall survival 2‐years | 20 | 2691 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.87, 0.97] |
| 2 Progression‐free survival 2‐years | 9 | 1458 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.86, 0.98] |
| 3 Locoregional progression‐free survival 2‐years | 5 | 902 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.74, 0.99] |
8.1. Analysis.
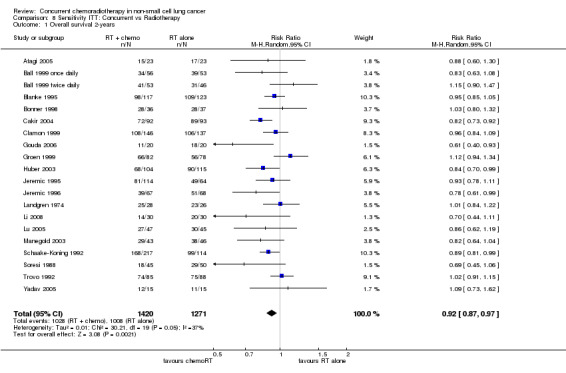
Comparison 8 Sensitivity ITT: Concurrent vs Radiotherapy, Outcome 1 Overall survival 2‐years.
8.2. Analysis.

Comparison 8 Sensitivity ITT: Concurrent vs Radiotherapy, Outcome 2 Progression‐free survival 2‐years.
8.3. Analysis.
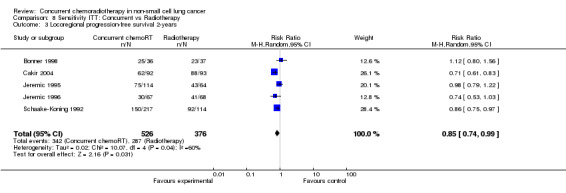
Comparison 8 Sensitivity ITT: Concurrent vs Radiotherapy, Outcome 3 Locoregional progression‐free survival 2‐years.
Comparison 9. Sensitivity ITT: Concurrent vs Sequential.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall survival 2‐years | 5 | 944 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.78, 0.97] |
| 2 Progression‐free survival 2‐years | 2 | 385 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.79, 1.08] |
9.1. Analysis.
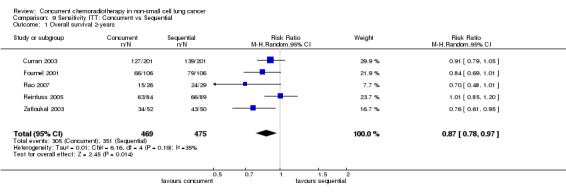
Comparison 9 Sensitivity ITT: Concurrent vs Sequential, Outcome 1 Overall survival 2‐years.
9.2. Analysis.

Comparison 9 Sensitivity ITT: Concurrent vs Sequential, Outcome 2 Progression‐free survival 2‐years.
Comparison 10. Sensitivity fully published: Concurrent vs Sequential.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall survival | 2 | Hazard Ratio (Random, 95% CI) | 0.63 [0.44, 0.90] | |
| 2 Overall survival 2‐years | 4 | 535 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.72, 0.99] |
| 3 Toxicity | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Treatment‐related deaths | 4 | 548 | Risk Ratio (M‐H, Random, 95% CI) | 2.45 [0.81, 7.43] |
| 3.2 Acute pneumonitis | 4 | 545 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [0.57, 2.65] |
| 3.3 Acute oesophagitis | 4 | 545 | Risk Ratio (M‐H, Random, 95% CI) | 4.85 [1.52, 15.45] |
| 3.4 Neutropenia | 4 | 545 | Risk Ratio (M‐H, Random, 95% CI) | 1.35 [0.79, 2.32] |
10.1. Analysis.

Comparison 10 Sensitivity fully published: Concurrent vs Sequential, Outcome 1 Overall survival.
10.2. Analysis.

Comparison 10 Sensitivity fully published: Concurrent vs Sequential, Outcome 2 Overall survival 2‐years.
10.3. Analysis.
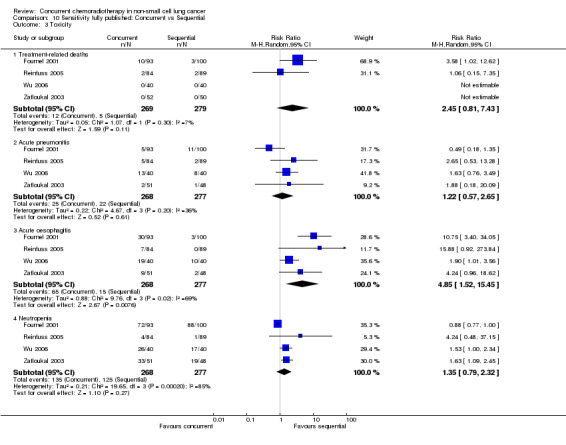
Comparison 10 Sensitivity fully published: Concurrent vs Sequential, Outcome 3 Toxicity.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Atagi 2005.
| Methods | Randomised | |
| Participants | 46 patients with stage III NSCLC | |
| Interventions | RT to 60 Gy in 30 fractions alone vs with daily carboplatin 30mg/m2 for first 20 fractions | |
| Outcomes | Median survival 428 vs 554 days | |
| Notes | Closed early after 4 treatment‐related deaths | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | "By minimization method balancing PS, stage and institution" |
| Allocation concealment? | Unclear risk | Not reported. |
| Incomplete outcome data addressed? All outcomes | Low risk | All 46 randomised patients were analysed. |
Ball 1999 once daily.
| Methods | Randomised | |
| Participants | 208 patients with stage I‐IIIB | |
| Interventions | RT to 60Gy in 30 fractions randomised between once and twice daily fractionation v RT once or twice daily with weekly carboplatin | |
| Outcomes | Overall 31% 2‐year survival; no significant difference between arms | |
| Notes | Subset treated with once daily irradiation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | ”Randomisation within each stratum was based on an adaptative biased coin procedure weighted to achieve approximate balance between the four treatment arms. However, because fo the large number of strata, the total numbers of patients in the four arms were slightly imbalanced.” |
| Allocation concealment? | Low risk | Randomised by telephone to the central trial office. |
| Incomplete outcome data addressed? All outcomes | Low risk | 208 patients were randomised. ”All patients were analysed according to their randomised treatment arm (“intention‐to‐treat”), except for the exclusion from the toxicity analysis of three patients who received no treatment and one patient who received only 6 Gy. “ “Response rates were calculated as percentages of all randomised patients.””All patients were followed up to January 6, 1998 (close‐out data)” “Four patients were deemed to be ineligible after review of their records revealed that two (one R3, one R6C) had pleural effusions at the time of randomisation. A third (R3) had a pleural effusion evident 1 day after randomisation and a fourth (R6C) was noted to have cervical lymph node involvement 3 days after randomisation”. “ this left 204 patients available for analysis.” |
Ball 1999 twice daily.
| Methods | Randomised | |
| Participants | 208 patients with stage I‐IIIB | |
| Interventions | RT to 60Gy in 30 fractions randomised between once and twice daily fractionation v RT once or twice daily with weekly carboplatin | |
| Outcomes | Overall 31% 2‐year survival; no significant difference between arms | |
| Notes | Subset treated with twice daily irradiation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | ”Randomisation within each stratum was based on an adaptative biased coin procedure weighted to achieve approximate balance between the four treatment arms. However, because fo the large number of strata, the total numbers of patients in the four arms were slightly imbalanced.” |
| Allocation concealment? | Low risk | Randomised by telephone to the central trial office. |
| Incomplete outcome data addressed? All outcomes | Low risk | 208 patients were randomised. ”All patients were analysed according to their randomised treatment arm (“intention‐to‐treat”), except for the exclusion from the toxicity analysis of three patients who received no treatment and one patient who received only 6 Gy. “ “Response rates were calculated as percentages of all randomised patients.””All patients were followed up to January 6, 1998 (close‐out data)” “208 patients were randomised. Four patients were deemed to be ineligible after review of their records revealed that two (one R3, one R6C) had pleural effusions at the time of randomisation. A third (R3) had a pleural effusion evident 1 day after randomisation and a fourth (R6C) was noted to have cervical lymph node involvement 3 days after randomisation”. “ this left 204 patients available for analysis.” |
Blanke 1995.
| Methods | Randomised | |
| Participants | 240 patients with stage I‐IIIB | |
| Interventions | Daily RT to 60‐65Gy v RT with three‐weekly cisplatin | |
| Outcomes | 1‐year survival 45% v 43%; 2‐year survival 13% v 18%. | |
| Notes | Improved progression‐free survival for non‐squamous cancers | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | “Patients were stratified”. “Permuted‐blocks randomization was used to assign patients within the strata”. |
| Allocation concealment? | Unclear risk | Not reported. |
| Incomplete outcome data addressed? All outcomes | Low risk | Although they claim an ITT analysis, only assessable patients are included in the analysis. Description of the flow of patients and reasons for ineligibility is providedn in the paper, although no reasons are provided for non‐assessable patients. ”the intent‐to‐treat principle was adhered to throughout for eligible patients.” "240 patients were enrolled". “Thirteen patients (six on the thoracic XRT arm and seven on the combination arm) were ineligible and 12 additional patients (six on each arm) were not assessable for reasons listen in Table 1“ |
Bonner 1998.
| Methods | Randomised | |
| Participants | 110 patients with stage IIIA/B | |
| Interventions | Twice daily RT to 60Gy v RT with cisplatin and etoposide | |
| Outcomes | 2 year survival 27% v 25%. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | ”Randomization to one of the three treatment arms was performed by a dynamic allocation procedure that balanced the marginal distributions of the stratification factors among the study arms. “ |
| Allocation concealment? | Low risk | "Patients were randomized at a centralized NCCTG randomization center.” |
| Incomplete outcome data addressed? All outcomes | Low risk | "110 patients entered the study. Eleven patients were declared ineligible on the basis of the eligibility criteria listed above, leaving 99 patients with analyzable data.” |
Cakir 2004.
| Methods | Randomised | |
| Participants | 185 patients with stage IIIA/B | |
| Interventions | Daily RT to 64Gy v RT with cisplatin days 1‐5 in weeks 2 and 6 | |
| Outcomes | 2‐year survival 5% v 23% | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | ”A stratified randomisation procedure was performed; the stages (III‐A,III‐B) were used as a stratifying factor. The patients having these two stages allocated to treatment arms equally.” |
| Allocation concealment? | Unclear risk | Not reported. |
| Incomplete outcome data addressed? All outcomes | Low risk | ”Of the 185 pt who underwent randomisation, 9 patients (4 assigned to radiotherapy plus cisplatin and 5 assigned to radiotherapy alone) were subsequently found to be ineligible and were excluded. The reasons for exclusion were the patient’s admission in another protocol, and presence of other malignant conditions, or patient’s refusal to participate in the study. Of the remaining 176 stage III patients, 88 were randomly assigned to radiotherapy plus cisplatin and 88 to radiotherapy alone”. |
Clamon 1999.
| Methods | Randomised | |
| Participants | 283 patients with stage IIIA/B | |
| Interventions | Induction chemotherapy then daily RT to 60Gy v induction chemo and RT with weekly carboplatin | |
| Outcomes | 1‐year survival 54% v 56%; 2‐year survival 26% v 29%. | |
| Notes | In‐field failure rate 69% v 59% | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | ”Randomization was stratified by stage so that an equal proportion of clinical stage IIIA and IIIB patients would be assigned to each treatment arm” |
| Allocation concealment? | Unclear risk | Not reported. |
| Incomplete outcome data addressed? All outcomes | Low risk | ”Of the 283 patients registered onto the trial, 137 were randomized to receive induction chemotherapy and radiation therapy, and 146 were randomized to receive the same therapy but with the addition of weekly carboplatin during the radiation treatment. One patient in each treatment group left the study before receiving any protocol therapy. “ 16 patients in the first group and 15 in the carboplatin group were considered ineligible after review, and reasons of inegibility are described. |
Curran 2003.
| Methods | Randomised | |
| Participants | 402 patients with stage II‐IIIB | |
| Interventions | Chemotherapy with vinblastine and cisplatin plus daily RT to 60Gy commencing day 1 (concurrent) or day 50 (sequential) | |
| Outcomes | 1‐year survival 63% v 57%; 2‐year survival 37% v 31% | |
| Notes | Third arm of study included hyperfractionated RT (total number of patients in study = 610). This study was published as an abstract. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not reported. |
| Allocation concealment? | Unclear risk | Not reported. |
| Incomplete outcome data addressed? All outcomes | Unclear risk | Numbers are presented, although causes of patients loss and groups they belong to aren't. "610 enrolled patients, 595 analyzable patients". |
Fournel 2001.
| Methods | Randomised | |
| Participants | 212 patients with stage IIIAN2/IIIB | |
| Interventions | Chemotherapy with vinblastine and etoposide plus daily RT to 66Gy commencing day 1 followed by cisplatin and vinorelbine (concurrent) or chemotherapy with cisplatin and vinorelbine followed by RT alone commencing approx week 13 (sequential) | |
| Outcomes | 1‐year survival 56% v 56%; 2‐year survival 35% v 23% | |
| Notes | Although etoposide used concurrently with RT in place of vinorelbine; study designed to deliver the same total dose of cisplatin in both arms | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | It’s not clear how patients were randomised. “Patients were stratified by stage (IIIA‐N2/IIIB) and were then randomly assigned to receive sequential or concurrent therapy.” |
| Allocation concealment? | Unclear risk | It’s not clear whether randomisation was centralized. “The central office stratified patients according to institute and stage (IIIAN2/IIIB).” |
| Incomplete outcome data addressed? All outcomes | Low risk | “212 patients were enrolled” “Seven patients were not eligible after panel file revue (three in the sequential arm and four in the concurrent arm); six patients had stage IV disease, and one had pleural effusion.” “Thus, 205 patients (103 in the sequential arm and 102 in the concurrent arm) were assessable for survival, and 193, for toxicity. Four patients were lost to follow‐up.” 201 eligible patients. |
Gouda 2006.
| Methods | Randomised | |
| Participants | 40 patients with stage III NSCLC | |
| Interventions | 60Gy at conventional fractionation vs RT with 2 cycles concurrent carboplatin/taxol | |
| Outcomes | 2y survival 10% RT vs 45% concurrent chemoRT | |
| Notes | Only two arms (n=40) are analyzed in this review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not reported. |
| Allocation concealment? | Unclear risk | Not reported. |
| Incomplete outcome data addressed? All outcomes | Low risk | All 60 randomised patients were analysed. |
Groen 1999.
| Methods | Randomised | |
| Participants | 160 patients with stage IIIA/B | |
| Interventions | Daily RT to 60Gy v RT with carboplatin continuous infusion | |
| Outcomes | 2‐year survival 28% v 20% | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | "Patients were stratified according to stage, performance status and hospital. Block randomization was used “ |
| Allocation concealment? | Low risk | ” treatment allocation was performed by telephone into either carboplatin and radiation, or radiation alone.” |
| Incomplete outcome data addressed? All outcomes | Low risk | ”After randomizing 160 patients, /../ the trial was closed. “ ”Five patients were ineligible after data review because of pretreatment distant metastases: three in the carboplatin arm and two in the radiotherapy alone arm. This report is about these 160 patients.” |
Huber 2003.
| Methods | Randomised | |
| Participants | 219 patients with stage IIIA/IIIB | |
| Interventions | Induction chemotherapy then daily RT to 60Gy v induction chemo and RT with weekly paclitaxel | |
| Outcomes | 2‐year survival 27% v 35% | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | ”Random assignment after response evaluation allocated patients to radiotherapy alone or to chemoradiotherapy by stratification according to center and stage using computer‐generated random lists of permuted blocks of varying size.” |
| Allocation concealment? | Low risk | ”The study center in Munich, Germany, was responsible for central random assignment based on patient details the centers had submitted.” |
| Incomplete outcome data addressed? All outcomes | Low risk | ”The analysis of time to progression and overall survival was based on the intent‐to‐treat population.” “Two hundred nineteen patients were randomly assigned (115 patients to radiotherapy alone and 104 patients to simultaneous chemoradiotherapy). After careful review, five patients were excluded (three patients were randomly assigned despite progressive disease, and two patients showed brain metastasis before the start of treatment), leading to 214 patients (radiotherapy alone: n = 113; simultaneous chemoradiotherapy: n = 101). Two patients were completely lost from follow‐up. Survival analyses are limited to 212 patients (99 receiving chemoradiotherapy and 113 receiving radiotherapy alone; Fig 1).” |
Jeremic 1995.
| Methods | Randomised | |
| Participants | 178 patients with stage IIIA/IIIB | |
| Interventions | Twice daily RT to 64.8Gy v RT with weekly carboplatin and etoposide v RT with carboplatin and etoposide alternate weeks | |
| Outcomes | 1‐year survival 39% v 73% v 50%; 2‐year survival 25% v 35% v 27%; 3‐year survival 6.6% v 23% v 16%. | |
| Notes | More toxicity in alternate week chemotherapy arm resulting in more treatment interruptions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not reported. |
| Allocation concealment? | Unclear risk | Not reported. |
| Incomplete outcome data addressed? All outcomes | Low risk | 178 randomised patients, 169 patients assessable. “Nine patients (six in group Iand three in group III) “ were excluded. “No patient was lost for follow‐up evaluation.” |
Jeremic 1996.
| Methods | Randomised | |
| Participants | 135 patients with stage IIIA/IIIB | |
| Interventions | Twice daily RT to 69.6Gy v RT with daily carboplatin and etoposide | |
| Outcomes | 1‐year survival 68% v 74%; 2‐year survival 26% v 43%; 3‐year survival 11% v 23%. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not reported. |
| Allocation concealment? | Unclear risk | Not reported. |
| Incomplete outcome data addressed? All outcomes | Low risk | ”Four patients (two in each group) were subsequently found to have /.../ and data for them were excluded from further analysis.” “All patients completed treatment as planned, and no patient was lost for follow‐up evaluation.” 135 enrolled, 131 assessd. |
Landgren 1974.
| Methods | Randomised | |
| Participants | 54 patients with unresectable NSCLC | |
| Interventions | RT to 60Gy in 20 fractions split over 8 weeks v RT with daily hydroxyurea | |
| Outcomes | 1‐year survival 48% v 46%; 2‐year survival 12% v 11%. | |
| Notes | Only 64% completed RT as planned | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not reported. |
| Allocation concealment? | Unclear risk | Not reported. |
| Incomplete outcome data addressed? All outcomes | Low risk | 54 patients were randomised “All patients (except one from the irradiation‐alone group /.../ were considered evaluable.” |
Li 2008.
| Methods | Randomised | |
| Participants | 60 patients with stage IIIA‐IIIB | |
| Interventions | RT 62.4Gy treating bd over 6 weeks v same RT with daily hydroxycomptothecin on first and last week of RT | |
| Outcomes | 2‐year survival 54.4% in concurrent vs 33.9% radiotherapy | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not reported. |
| Allocation concealment? | Unclear risk | Not reported. |
| Incomplete outcome data addressed? All outcomes | Unclear risk | 60 patients randomised. Two patients lost to follow‐up, but they don't specify the group they belong to. |
Lu 2005.
| Methods | Randomised | |
| Participants | 92 patients with stage III disease | |
| Interventions | 2 cycles induction cis/vin then 60‐65Gy either alone follwed by 4 more cycles or with 2 concurrent cycles and 2 subsequent | |
| Outcomes | 2y survival 42.5% concurrent vs 33.3% RT alone | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not reported. |
| Allocation concealment? | Unclear risk | Randomisation "using envelopes” . It does not specify whether envelopes sealed or opaque. |
| Incomplete outcome data addressed? All outcomes | Unclear risk | 92 patients randomised. 7 patients lost to follow‐up, but they don’t specify the group they belong to. |
Manegold 2003.
| Methods | Randomised | |
| Participants | 89 patients with stage IIIA/IIIB | |
| Interventions | Induction chemo followed by randomisation to daily RT to 60Gy v RT with weekly docetaxel | |
| Outcomes | Median survival 14.0m v 14.9m | |
| Notes | Insufficient follow‐up; 2‐year survival not published | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not described although “patients stratified according to centre and disease stage.” |
| Allocation concealment? | Unclear risk | Not reported. |
| Incomplete outcome data addressed? All outcomes | Low risk | ”The primary objective was overall response rate (ORR) at study end (i.e. 12 weeks from randomisation) for the intent‐to‐treat (ITT) population.” “108 patients were enrolled “ “Eighty‐nine patients were subsequently randomised to local treatment (ITT population). Reasons for treatment discontinuation among the remaining 19 patients were: PD (n=10), protocol deviation (n=1), adverse event (n=2), death (n=3) and other (n=3: investigator decision, metastasis, massive decay of lesions (a contraindication for radiotherapy owing to the high risk of developing life‐threatening pulmonary haemorrhage) (one patient each)).” |
Rao 2007.
| Methods | Randomised | |
| Participants | 55 patients with stage III NSCLC | |
| Interventions | 2 cycles cis/vin induction then 62‐70Gy alone followed by up to 4 more cycles or induction and concurrent followed by up to 2 more cycles | |
| Outcomes | 2y survival 17.2% sequential vs 42.3% concurrent | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Randomisation by number table. |
| Allocation concealment? | High risk | Randomisation by number table. |
| Incomplete outcome data addressed? All outcomes | Unclear risk | 55 patients randomised. ”Two patients who were lost in the follow‐up were deemed as dead. The follow‐up rate ws 95.65%” It’s not clear to which group did they pertain. |
Reinfuss 2005.
| Methods | Randomised | |
| Participants | 173 patients with stage III disease | |
| Interventions | 2 cycles cis/vin then 70.2Gy vs same RT concurr with 2 cis/vin | |
| Outcomes | 2y survival 26% sequential vs 25% concurrent | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Used randomisation tables. |
| Allocation concealment? | High risk | Used randomisation tables. |
| Incomplete outcome data addressed? All outcomes | Low risk | All 173 patients randomised were analysed. |
Schaake‐Koning 1992.
| Methods | Randomised | |
| Participants | 331 patients with stage I‐IIIB NSCLC | |
| Interventions | RT to 55Gy in 20 fractions split over 7 weeks versus RT with weekly cisplatin v RT with daily cisplatin | |
| Outcomes | 1‐year survival 46% v 44% v 54%; 2‐year survival 13% v 19% v 26%; 3‐year survival 2% v 13% v 16%. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not reported. |
| Allocation concealment? | Low risk | ”randomisation performed centrally” |
| Incomplete outcome data addressed? All outcomes | Low risk | 331 patients were enrolled. “Twenty‐two patients were later found to be ineligible. All ineligible patients were included in the survival analyses.” Numbers for inegibility are listed with the groups they belonged to, but causes are given aggregated by trial. “Sixty‐three patients could not be evaluated for response to treatment, and 45 could not be evaluated for toxic effects”. |
Soresi 1988.
| Methods | Randomised | |
| Participants | 95 patients with stage IIIA/B NSCLC | |
| Interventions | Daily RT to 50.4Gy v RT with weekly cisplatin | |
| Outcomes | 2‐year survival 23% v 39% | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not reported. |
| Allocation concealment? | Unclear risk | Not reported. |
| Incomplete outcome data addressed? All outcomes | Low risk | 95 randomised patients ”Two patients in the RT group were lost too early to follow‐up /.../ so they were excluded from analysis of progression‐free interval and survival.” |
Trovo 1992.
| Methods | Randomised | |
| Participants | 173 patients with stage IIIA/B NSCLC | |
| Interventions | RT 45Gy in 15 fractions over 3 weeks v RT with daily cisplatin | |
| Outcomes | 2‐year survival 14% v 15%. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not reported. |
| Allocation concealment? | Unclear risk | Not reported. |
| Incomplete outcome data addressed? All outcomes | Low risk | 173 randomised patients. “four patients (three in the RT group and one in the combined ttm group) were found to be ineligible”. Reasons are described, but only aggregated numbers per group they belong to. Patients evaluable for response and toxicity: 73 per group (146). 167 patients ealuable for survival. |
Wu 2006.
| Methods | Randomised | |
| Participants | 80 unresectable stage III NSCLC | |
| Interventions | RT60Gy /30‐33 concurr with cis/vin followed by 3 more cycles or RT alone followed by 4‐5 cycles cis/vin | |
| Outcomes | No survival data accrued yet ‐ just toxicity data | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Randomisation table |
| Allocation concealment? | High risk | Randomisation table |
| Incomplete outcome data addressed? All outcomes | High risk | Inadequate follow‐up so far to report any outcome data |
Yadav 2005.
| Methods | Randomised | |
| Participants | 30 patients with stage III NSCLC | |
| Interventions | 50Gy/25 alone vs with weekly cisplatin | |
| Outcomes | 2y survival 25% RT alone vs 19% concurrent chemoRT | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Randomisation by table of Tippet (random numbers table). |
| Allocation concealment? | High risk | Randomisation by table of Tippet (random numbers table). |
| Incomplete outcome data addressed? All outcomes | Low risk | 30 randomised patients. Response rate was quoted for all 30 patients in study suggesting complete follow‐up at least to time of response assessment. Response assessment on CXR alone and immediately at end of treatment |
Zatloukal 2003.
| Methods | Randomised | |
| Participants | 102 patients with stage IIIA/B | |
| Interventions | 4 cycles of cisplatin and vinorelbine with daily RT to 60Gy starting week 5 (concurrent) or following completion of chemo approx week 17 (sequential) | |
| Outcomes | 1‐year survival 69% v 53%; 2‐year survival 34% v 14% | |
| Notes | "Due to the slow accrual and results of interim analysis, which demonstrated statistical difference in survival in favor of concurrent arm, the study was prematurely terminated." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not described, although randomisation was by envelope method. |
| Allocation concealment? | Low risk | "Randomisation by envelope method was used” |
| Incomplete outcome data addressed? All outcomes | Low risk | "102 eligible patients were enrolled in the study" ”All eligible patients at study entry were included in the intent‐to‐treat (ITT) population for efficacy” “Four patients were not enrolled for the response analysis.” Reasons were provided for the 4 patients not enrolled. “Of the 101 patients qualified for safety analysis, 99 were assessable”. Reasons provided for the 2 non‐assessable patients. |
Patients with stage I/II disease were those considered medically inoperable. Ball 1999 once daily and Ball 1999 twice daily refer both to the same study Ball 1999
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ball 1997 | Radiation dose less than 50Gy (20Gy in 5 fractions) |
| Belderbos 2007 | Different chemotherapy in the two study arms ; sequential had gemcitabine/cisplatin combination and concurrent had cisplatin alone. Also very variable radiation doses 49‐94Gy |
| Chan 1976 | Radiation dose less than 50Gy (20Gy in 5 fractions, 3‐week gap then further 20Gy in 5 fractions) |
| DasGupta 2006 | Different radiotherapy doses in the two arms 65Gy in sequential arm and 50 Gy in concurrent arm. |
| Furuse 1999 | Radiation fractionation different between arms (56Gy in 28 fractions in both arms but included 10 day gap in concurrent arm, continuous in sequential arm) |
| Guschall 2000 | Concurrent chemotherapy arm had total of 6 cycles of chemotherapy (2 concurrent, 4 sequential) versus none in RT alone arm so comparison could not distinguish how much benefit was from concurrent treatment and how much was due to additional sequential chemotherapy. |
| Isakovic‐Vidovic2002 | Planned radiation dose differed between two arms (60Gy in 30 fractions in RT alone arm and 55Gy in 20 fractions split over 6 weeks in concurrent arm) |
| Japan ACNU 1989 | 25/73 patients had stage IV disease |
| Johnson 1990 | Post‐radiotherapy chemotherapy differed in 2 arms: 29/104 in RT alone arm crossed over to receive vindesine while responders in concurrent arm continued vindesine for up to 6 months |
| Koca 1996 | Post‐radiotherapy chemotherapy differed in 2 arms; concurrent arm also received 6 cycles of chemotherapy after radiotherapy |
| Komaki 2002 | Both arms had concurrent chemotherapy, one with daily RT, the other twice‐daily; the once‐daily arm also received induction chemotherapy (ie both sequential and concurrent) |
| LePar 1967 | Trial may have included patients with stage IV disease; one patient (of 26) had small cell lung cancer |
| Misirlioglu 2006 | The concurrent chemotherapy arm also has consolidation chemotherapy. |
| Sarihan 2002 | Planned radiation dose differed between two arms (63Gy in RT alone arm and 59.Gy in concurrent arm) |
| Ulutin 2000 | Not randomised: accrual into treatment groups by "consecutive registration" |
Characteristics of studies awaiting assessment [ordered by study ID]
Zhang 2006.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | This study is pending translation. |
Characteristics of ongoing studies [ordered by study ID]
NCRI SOCCAR.
| Trial name or title | SOCCAR Trial Cisplatin and Vinorelbine for NSCLC (LUWOS‐012/1) |
| Methods | Randomised phase III trial |
| Participants | Patients with inoperable stage III NSCLC and good performance status. |
| Interventions | Sequential arm ‐ patients receive 4 x 21 day cycle of vinorelbine and cisplatin, followed by radical radiotherapy. Concurrent arm ‐ patients receive vinorelbine concurrently with fractions 1, 6, 15 and 20 of radical radiotherapy and cisplatin with fractions 1‐4 and 16‐19. Four weeks after concurrent treatment is completed patients receive 2 x 21 day cycle of vinorelbine and cisplatin. |
| Outcomes | Overall survival; progression‐free survival; local progression‐free survival; hematological, pulmonary, esophageal, and neurological toxicities; response; quality of life; cost‐effectiveness |
| Starting date | 01/12/2005 |
| Contact information | Dr Joseph Maguire Clatterbridge Centre for Oncology Bebington Wirral Liverpool United Kingdom CH63 4JY |
| Notes |
Contributions of authors
NOR screened the search results, extracted data for all new and updated trials, and drafted the manuscript.
MR screened the search results, assessed risk of bias data for trials included in the original version of the review, extracted data for updated trials, extracted time‐to‐event data for all trials, performed the statistical analysis.
NF extracted data for all new trials included in the update.
FM helped with interpretation of the results and in drafting the manuscript.
All authors commented on the manuscript. NOR and Nick Rowell developed the original version of the review.
Sources of support
Internal sources
Beatson Oncology Centre, UK.
External sources
-
National Institute for Health Research, UK.
2009 CochraneReview Incentive Scheme. Funding awarded to the completion of the update of this review
Declarations of interest
None known.
Edited (conclusions changed)
References
References to studies included in this review
Atagi 2005 {published data only}
- Atagi S, Kawahara M, Tamura T, Noda K, Watanabe K, Yokoyama A, et al. Standard thoracic radiotherapy with or without concurrent daily low‐dose carboplatin in elderly patients with locally advanced non‐small cell lung cancer: A phase III trial of the Japan Clinical Oncology Group (JCOG9812). Japanese Journal of Clinical Oncology 2005;35(4):195‐201. [DOI] [PubMed] [Google Scholar]
Ball 1999 once daily {published data only}
- Ball D, Bishop J, Smith J, O'Brien P, Davis S, Ryan G, et al. A randomised phase II study of accelerated or standard fraction radiotherapy with or without concurrent carboplatin in inoperable non‐small cell lung cancer: final report of an Australian multi‐centre trial. Radiotherapy and Oncology 1999;52(2):129‐36. [DOI] [PubMed] [Google Scholar]
Ball 1999 twice daily {published data only}
- Ball D, Bishop J, Smith J, O'Brien P, Davis S, Ryan G, et al. A randomised phase II study of accelerated or standard fraction radiotherapy with or without concurrent carboplatin in inoperable non‐small cell lung cancer: final report of an Australian multi‐centre trial. Radiotherapy and Oncology 1999;52(2):129‐36. [DOI] [PubMed] [Google Scholar]
Blanke 1995 {published data only}
- Ansari R, Tokars R, Fisher W, Pennington K, Mantravadi R, O'Connor T, et al. A phase III study of thoracic irradiation with or without concomitant cisplatin in locoregional unresectable non small cell lung cancer: a Hoosier Oncology Group Protocol. Proceedings of the American Society of Clinical Oncology 1991;10:241. [DOI] [PubMed] [Google Scholar]
- Blanke C, Ansari R, Mantravadi R, Gonin R, Tokars R, Fisher W, et al. Phase III trial of thoracic irradiation with or without cisplatin for locally advanced unresectable non‐small‐cell lung cancer: a Hoosier Oncology Group Protocol. Journal of Clinical Oncology 1995;13(6):1425‐9. [DOI] [PubMed] [Google Scholar]
Bonner 1998 {published data only}
- Bonner JA, McGinnis WL, Stella PJ, Marschke FR, Sloan JA, Shaw EG, et al. The possible advantage of hyperfractionated thoracic radiotherapy in the treatment of locally advanced non‐small lung carcinoma. Cancer 1996;82(6):1037‐48. [PubMed] [Google Scholar]
Cakir 2004 {published data only}
- Cakir S, Egehan I. A randomised clinical trial of radiotherapy plus cisplatin versus radiotherapy alone in stage III non‐small cell lung cancer. Lung Cancer 2004;43:309‐16. [DOI] [PubMed] [Google Scholar]
Clamon 1999 {published data only}
- Clamon G, Herndon J, Cooper R, Chang AY, Rosenman J, Green MR. Radiosensitization with carboplatin for patients with unresectable stage III non‐small‐cell lung cancer: a phase III trial of the Cancer and Leukaemia Group B and the Eastern Cooperative Oncology Group. Journal of Clinical Oncology 1999;17(1):4‐11. [DOI] [PubMed] [Google Scholar]
Curran 2003 {published and unpublished data}
- Curran WJ, Scott CB, Langer CJ, Komaki R, Lee JS, Hauser S, et al. Long‐term benefit is observed in a phase III comparison of sequential vs concurrent chemoradiation for patients with unresected stage III NSCLC: RTOG 9410. Proceedings of the American Society of Clinical Oncology 2003;22:621. [Google Scholar]
Fournel 2001 {published data only}
- Fournel P, Perol M, Robinet G, Thomas P, Souquet JP, Lena H, et al. A randomized phase III trial of sequential chemo‐radiotherapy versus concurrent chemo‐radiotherapy in locally advanced non small cell lung cancer (NSCLC) (GLOT‐GFPC NPC 95‐01 study) [abstract]. Proceedings of the American Society of Clinical Oncology 2001;20:312. [Google Scholar]
- Fournel P, Robinet G, Thomas P, Souquet PJ, Lena H, Vergnenegre A, et al. Randomized phase III trial of sequential chemoradiotherapy compared with concurrent chemoradiotherapy in locally advanced non‐small‐cell lung cancer: Groupe Lyon‐Saint‐Etienne d'Oncologie Thoracique‐Groupe Francais de Pneumo‐Cancerologie NPC 95‐01 Study. Journal of Clinical Oncology 2005;23(25):5910‐7. [DOI] [PubMed] [Google Scholar]
- Vergnenegre A, Combescure C, Fournel P, Bayle S, Gimenez C, Souquet PJ, et al. Cost‐minimization analysis of a phase III trial comparing concurrent versus sequential radiochemotherapy for locally advanced non‐small‐cell lung cancer (GFPC‐GLOT 95‐01). Annals of Oncology 2006;17(8):1269‐74. [DOI] [PubMed] [Google Scholar]
Gouda 2006 {published data only}
- Gouda YS, Kohail HM, Eldeeb NA, Omar AM, El‐Geneidy MM, Elkerm YM. Randomized study of concurrent carboplatin, paclitaxel, and radiotherapy with or without prior induction chemotherapy in patients with locally advanced non‐small cell lung cancer. Journal of the Egyptian National Cancer Institute 2006;18(1):73‐81. [PubMed] [Google Scholar]
Groen 1999 {published and unpublished data}
- Groen HJ, Leest AH, Fokkema E, Timmer PR, Nossent GD, Smit WJ, et al. Continuously infused carboplatin used as radiosensitizer in locally unresectable non‐small‐cell lung cancer: a multicenter phase III study. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO 2004;15(3):427‐32. [DOI] [PubMed] [Google Scholar]
- Groen HJM, Leest AHW, Snoek WJ, Nossent GD, Oosterhuis B, Nabers H, et al. Phase III study of continuous infusion carboplatin over 6 weeks with radiation versus radiation alone in stage III non‐small cell lung cancer [abstract]. Proceedings of the American Society of Clinical Oncology 1999;18:466. [Google Scholar]
Huber 2003 {published and unpublished data}
- Huber RM, Flentje M, Schmidt M, Pöllinger B, Gosse H, Willner J, et al. Simultaneous chemoradiotherapy compared with radiotherapy alone after induction chemotherapy in inoperable stage IIIA or IIIB non‐small‐cell lung cancer: study CTRT99/97 by the Bronchial Carcinoma Therapy Group. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2006;24(27):4397‐404. [DOI] [PubMed] [Google Scholar]
- Huber RM, Schmidt M, Flentje M, Poellinger B, Gosse H, Willner J, et al for the BROCAT group. Induction chemotherapy and following simultaneous radio/chemotherapy versus induction chemotherapy and radiotherapy alone in inoperable NSCLC (stage IIIA/IIIB). Proceedings of the American Society of Clinical Oncology 2003;22:622. [DOI] [PubMed] [Google Scholar]
Jeremic 1995 {published data only}
- Jeremic B, Shibamoto Y, Acimovic L, Djuric L. Randomized trial of hyperfractionated radiation therapy with or without concurrent chemotherapy for stage III non‐small‐cell lung cancer. Journal of Clinical Oncology 1995;13(2):452‐8. [DOI] [PubMed] [Google Scholar]
Jeremic 1996 {published data only}
- Jeremic B, Shibamoto Y, Acimovic L, Milisavljevic S. Hyperfractionated radiation therapy with or without concurrent low‐dose daily carboplatin / etoposide for stage III non‐small‐cell lung cancer: a randomized study. Journal of Clinical Oncology 1996;14(4):1065‐70. [DOI] [PubMed] [Google Scholar]
Landgren 1974 {published data only}
- Landgren RC, Hussey DH, Barkley HT, Samuels ML. Split‐course irradiation compared to split‐course irradiation plus hydroxyurea in inoperable bronchogenic carcinoma ‐ a randomized study of 53 patients. Cancer 1974;34:1598‐1601. [DOI] [PubMed] [Google Scholar]
Li 2008 {published data only}
- Li GZ, Zhang SH, Zhang Z, Song WG, Wang RL, Fu ZZ. The clinical comparative study on hydroxycomptothecin combined with cocurrent hyperfractionated radiotherapy for unresectable stage III non‐small cell lung cancer. Tumor 2008;28(2):156‐8. [Google Scholar]
Lu 2005 {published data only}
- Lu C, Liu J, Wang J, Wang C, Guo J, Pan F, et al. Analysis of concurrent chemoradiotherapy in patients with stage III non‐small cell lung cancer after two cycles of induction chemotherapy. Chinese Journal of Lung Cancer 2005;8(1):48‐50. [DOI] [PubMed] [Google Scholar]
Manegold 2003 {published data only}
- Manegold C, Debus J, Buchholz E, Scagliotti GV, Ricardi U, Cardenal F, et al. A phase II randomized study comparing docetaxel/cisplatin induction therapy followed by thoracic radiotherapy with or without weekly docetaxel in unresectable stage IIIA‐IIIB non‐small cell lung cancer. European Journal of Cancer 2003;1 Suppl(5):248‐9. [Google Scholar]
- Ramlau R, Scagliotti GV, Manegold C, Buchholz E, Szczesna A, Cardenal F, et al. Radiotherapy and concurrent weekly docetaxel following cisplatin‐docetaxel induction chemotherapy in unresectable stage IIIA‐B non‐small‐cell lung cancer: a randomized phase II trial. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO 2002;13(suppl 5):134. [Google Scholar]
- Scagliotti GV, Manegold C, Bucholtz E, Zierhut D, Szczesna A, Kaczmarek Z, et al. Randomized phase II study evaluating the feasibility of thoracic radiotherapy with or without weekly docetaxel (Taxotere) following induction chemotherapy with cisplatin (DDP) and docetaxel in unresectable stage IIIA/B non‐small cell lung cancer. Proceedings of the American Society of Clinical Oncology 2002;21:320a (abstr 1279). [Google Scholar]
- Scagliotti GV, Szczesna A, Ramlau R, Cardenal F, Mattson K, Zandwijk N, et al. Docetaxel‐based induction therapy prior to radiotherapy with or without docetaxel for non‐small‐cell lung cancer. British Journal of Cancer 2006;94:1375‐82. [DOI: 10.1038/sj.bjc.6603115] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rao 2007 {published data only}
- Rao CZ. Study of concurrent versus sequential chemoradiotherapy with vinorelbine and cisplatin is stage III non‐small cell lung cancer. Chinese Journal of Cancer Prevention and Treatment 2007;14(12):942‐3. [Google Scholar]
Reinfuss 2005 {published data only}
- Reinfuss M, Skolyszewski J, Kowalska T, Glinski B, Dymek P, Walasek T, et al. Evaluation of efficacy of combined chemoradiotherapy in locoregional advanced, inoperable Non‐Small Cell Lung Cancer (clinical randomized trial). Nowotwory 2005;55(3):200‐6. [Google Scholar]
Schaake‐Koning 1992 {published data only}
- Schaake‐Koning C, Bogert W, Dalesio O, Festen J, Hoogenhout J, Houtte P, et al. Effects of concomitant cisplatin and radiotherapy on inoperable non‐small‐cell lung cancer. The New England Journal of Medicine 1992;326(8):524‐30. [DOI] [PubMed] [Google Scholar]
Soresi 1988 {published data only}
- Soresi E, Clerici M, Grilli R, Borghini U, Zucali R, Leoni M, et al. A randomized clinical trial comparing radiation therapy v radiation therapy plus cis‐dichlorodiammine platinum (II) in the treatment of locally advanced non‐small cell lung cancer. Seminars in Oncology 1988;15 Suppl 7(6):20‐5. [PubMed] [Google Scholar]
Trovo 1992 {published data only}
- Trovo MG, Minatel E, Franchin G, Boccieri MG, Nascimben O, Bolzicco G, et al. Radiotherapy versus radiotherapy enhanced by cisplatin in stage III non‐small cell lung cancer. International Journal of Radiation Oncology, Biology, Physics 1992;24(1):11‐5. [DOI] [PubMed] [Google Scholar]
Wu 2006 {published data only}
- Wu H, Zhou D, Wu Q, Cai Y, Liu Y, Jiang J. [Clinical trial of concurrent low‐dose chemotherapy plus radiation vs sequential chemoradiotherapy for unresectable stage III non‐small cell lung cancer]. Chinese Journal of Lung Cancer 2006;9(3):283‐5. [DOI] [PubMed] [Google Scholar]
Yadav 2005 {published data only}
- Yadav B S, Ghoshal S, Sharma SC, Behera D, Kapoor V. Radiotherapy versus weekly concomitant cisplatin in locally advanced non‐small cell lung cancer ‐ a pilot study. Bulletin, Postgraduate Institute of Medical Education & Research, Chandigarh 2005;39(2):75‐80. [Google Scholar]
Zatloukal 2003 {published data only}
- Zatloukal P, Petruzelka L, Zemanova M, Havel L, Janku F, Judas L, et al. Concurrent versus sequential chemoradiotherapy with cisplatin and vinorelbine in locally advanced non‐small cell lung cancer: A randomized study. Lung Cancer 2004;46(1):87‐98. [DOI] [PubMed] [Google Scholar]
- Zatloukal P, Petruzelka L, Zemanova M, Havel L, Pecen L, Judas L. Concurrent versus sequential radiochemotherapy with vinorelbine plus cisplatin (V‐P) in locally advanced non‐small cell lung cancer. A randomized phase II study ‐ long‐term follow‐up data. Lung Cancer 2003;41 Suppl(2):76. [Google Scholar]
- Zatloukal P, Petruzelka L, Zemanova M, Havel L, Pecen L, Krepela E, et al. Concurrent versus sequential radiochemotherapy with vinorelbine plus cisplatin (V‐P) in locally advanced non‐small cell lung cancer. A randomized phase II study [abstract]. Proceedings of the American Society of Clinical Oncology 2002;21:290. [Google Scholar]
References to studies excluded from this review
Ball 1997 {published data only}
- Ball D, Smith J, Olver I, Davis S, O'Brien P, Bernshaw D, et al. A phase III study of radiotherapy with or without continuous‐infusion fluorouracil as palliation for non‐small‐cell lung cancer. British Journal of Cancer 1997;75(5):690‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Belderbos 2007 {published data only}
- Belderbos J, Uitterhoeve L, Zandwijk N, Belderbos H, Rodrigus P, Vaart P, et al. Randomised trial of sequential versus concurrent chemo‐radiotherapy in patients with inoperable non‐small cell lung cancer (EORTC 08972‐22973). European Journal of Cancer 2007;43(1):114‐21. [DOI] [PubMed] [Google Scholar]
Chan 1976 {published data only}
- Chan PYM, Byfield JE, Kagan AR, Aronstam EM. Unresectable squamous cell carcinoma of the lung and its management by combined bleomycin and radiotherapy. Cancer 1976;37(6):2671‐6. [DOI] [PubMed] [Google Scholar]
DasGupta 2006 {published data only}
- Dasgupta A, Dasgupta C, Basu S, Majumdar A. A prospective and randomized study of radiotherapy, sequential chemotherapy radiotherapy and concomitant chemotherapy‐radiotherapy in unresectable non small cell carcinoma of the lung. Journal of cancer research and therapeutics 2006;2(2):47‐51. [DOI] [PubMed] [Google Scholar]
Furuse 1999 {published data only}
- Furuse K, Fukuoka M, Kawahara M, Nishikawa H, Takada Y, Kudoh S, et al. Phase III study of concurrent versus sequential radiotherapy in combination with mitomycin, vindesine and cisplatin in unresectable stage III non‐small cell lung cancer. Journal of Clinical Oncology 1999;17(9):2692‐9. [DOI] [PubMed] [Google Scholar]
Guschall 2000 {published data only}
- Gurschall W‐R, Schneemann‐Heinze S, Floegel R, Liebetrau G, Dittrich I. The therapy of the inoperable non small cell lung cancer: radiotherapy vs radio‐chemotherapy. Lung Cancer 2000;29 Suppl(1):115‐6. [Google Scholar]
Isakovic‐Vidovic2002 {published data only}
- Isakovic‐Vidovic S, Radosevic‐Jelic L, Borojevic N. Combined chemoradiotherapy in the treatment of locally advanced non‐small cell lung cancer. Journal of B.U.ON: official journal of the Balkan Union of Oncology 2002;7(1):47‐51. [PubMed] [Google Scholar]
Japan ACNU 1989 {published data only}
- Japan Radiation‐ACNU Study Group. A randomized prospective study of radiation versus radiation plus ACNU in inoperable non‐small cell carcinoma of the lung. Cancer 1989;63(2):249‐54. [DOI] [PubMed] [Google Scholar]
Johnson 1990 {published data only}
- Johnson DH, Einhorn LH, Bartolucci A, Birch R, Omura G, Perez CA, et al. Thoracic radiotherapy does not prolong survival in patients with locally advanced, unresectable non‐small cell lung cancer. Annals of Internal Medicine 1990;113(1):33‐8. [DOI] [PubMed] [Google Scholar]
Koca 1996 {published data only}
- Koca S, Oner‐Dincbas F, Serdengecti S, Yaman M, Ongen G, Dimirci S, et al. Inoperabl kucuk hucreli disi akciger kanserinde hiperfraksiyone radyoterapi ile cisplatin+5FU kombinasyonunun yeri: randomize prospektif klinik calisma [Radiotherapy alone vs combined chemotherapy and radiotherapy in nonresectable non‐small cell lung cancer: a phase III randomized clinical trial]. Cerrahpasa J Med 1996;27:63‐69. [Google Scholar]
Komaki 2002 {published data only}
- Komaki R, Seiferheld W, Ettinger D, Lee JS, Mosvas B, Sause W. Randomized phase II chemotherapy and radiotherapy trial for patients with locally advanced inoperable non‐small‐cell lung cancer: long‐term follow‐up of RTOG 92‐04. International Journal of Radiation Oncology, Biology, Physics 2002;53(3):548‐57. [DOI] [PubMed] [Google Scholar]
LePar 1967 {published data only}
- LePar E, Faust DS, Brady LW, Beckloff GL. Clinical evaluation of the adjunctive use of hydroxyurea (NSC‐32065) in radiation therapy of carcinoma of the lung. Radiologia Clinica et Biologica 1967;36:32‐40. [PubMed] [Google Scholar]
Misirlioglu 2006 {published data only}
- Misirlioglu CH, Erkal H, Elgin Y, Ugur I, Altundag K. Effect of concomitant use of pentoxifylline and alpha‐tocopherol with radiotherapy on the clinical outcome of patients with stage IIIB non‐small cell lung cancer: A randomized prospective clinical trial. Medical Oncology 2006;23(2):185‐9. [DOI] [PubMed] [Google Scholar]
Sarihan 2002 {published data only}
- Sarihan S, Kayisogullari U, Sozer N, Ercan I, Ozkan L, Engin K. A phase III study: results of radiotherapy alone versus radiotherapy with paclitaxel concomitantly in non‐small cell lung cancer. Radiotherapy and Oncology 2002;64 Suppl(1):258. [Google Scholar]
Ulutin 2000 {published data only}
- Ulutin HC, Guden M, Oysul K, Surenkok S, Pak Y. Split‐course radiotherapy with or without concurrent or sequential chemotherapy in non‐small cell lung cancer. Radiation Medicine 2000;18:93‐6. [PubMed] [Google Scholar]
References to studies awaiting assessment
Zhang 2006 {published data only}
- Zhang Y, Gao X. [The clinical research on 10‐hydroxycamplothecin (HCPT) for radiosensitization on local advanced non‐small cell lung cancer]. Chinese Journal of Clinical Oncology 2006;33(8):458‐61. [Google Scholar]
References to ongoing studies
NCRI SOCCAR {published data only}
- SOCCAR Trial Cisplatin and Vinorelbine for NSCLC (LUWOS‐012/1). Ongoing study 01/12/2005.
Additional references
Auperin 2003
- Auperin A, Péchoux C. Meta‐analysis of randomized trials evaluating cisplatin or carboplatin‐based concomitant chemoradiation versus radiotherapy alone in locally advanced non‐small cell lung cancer (NSCLC). Lung Cancer 2003;41 Suppl(2):70. [Google Scholar]
Auperin 2007
- Auperin A, Rolland E, Curran WJ, Furuse K, Fournel P, Belderbos J, et al. Concomitant radio‐chemotherapy (RT‐CT) versus sequential RT‐CT in locally advanced non‐small cell lung cancer (NSCLC): a meta‐analysis using individual patient data (IPD) from randomised clinical trials (RCTs). Journal of Thoracic Oncology 2007;2(8):suppl 4 S310. [Google Scholar]
Auperin 2010
Ball 1999
- Ball D, Bishop J, Smith J, O'Brien P, Davis S, Ryan G, et al. A randomised phase II study of accelerated or standard fraction radiotherapy with or without concurrent carboplatin in inoperable non‐small cell lung cancer: final report of an Australian multi‐centre trial. Radiotherapy and Oncology 1999;52(2):129‐36. [DOI] [PubMed] [Google Scholar]
Cox 1990
- Cox JD, Azarnia N, Byhardt RW, Shin KH, Emami B, Pajak TF. A randomized phase I/II trial of hyperfractionated radiation therapy with total doses of 60.0Gy to 79.2Gy: possible survival benefit with greater than or equal to 69.6Gy in favorable patients with Radiation Therapy Oncology Group stage III non‐small cell lung carcinoma: report of Radiation Therapy Oncology Group 83‐11. Journal of Clinical Oncology 1990;8:1543‐55. [DOI] [PubMed] [Google Scholar]
Current Controlled Trials
- Biomed Central. Current Controlled Trials. www.controlled‐trials.com (accessed 19 October 2009).
DerSimonian 1986
- DerSimonian R. Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. [DOI] [PubMed] [Google Scholar]
El Sharouni 2003
- Sharouni SY, Kal HB, Battermann JJ. Accelerated regrowth of non‐small cell lung tumours after induction chemotherapy. British Journal of Cancer 2003;89(12):2184‐89. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2009
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2. [updated September 2009]. The Cochrane Collaboration, 2009. [Google Scholar]
Higgins 2009b
- Higgins JPT, Deeks JJ, Altman DG (editors). Chapter 16: Special topics in statistics. Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2 (updated September 2009). The Cochrane Collaboration, 2009. [Google Scholar]
Jones 2001
- Jones B, Dale RG, Deehan C, Hopkins KI, Morgan DA. The role of biologically effective dose (BED) in clinical oncology. Clinical Oncology 2001;13(2):71‐81. [DOI] [PubMed] [Google Scholar]
NSCLCCG 1995
- Non‐small Cell Lung Cancer Collaborative Group. Chemotherapy in non‐small cell lung cancer: a meta‐analysis using updated data on individual patients from 52 randomised clinical trials. BMJ 1995;311:899‐909. [PMC free article] [PubMed] [Google Scholar]
NSCLCCG 2000
- Non‐small Cell Lung Cancer Collaborative Group. Chemotherapy for non‐small cell lung cancer. Cochrane Database of Systematic Reviews 2000, Issue 2. [DOI: 10.1002/14651858.CD002139] [DOI] [PMC free article] [PubMed] [Google Scholar]
Parmar 1998
- Parmar MKB, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17:2815‐34. [DOI] [PubMed] [Google Scholar]
Perez 1987
- Perez CA, Pajak TF, Rubin P, Simpson JR, Mohiuddin M, Brady LW, et al. Long‐term observations of the patterns of failure in patients with unresectable non‐oat cell carcinoma of the lung treated with definitive radiotherapy. Cancer 1987;59:1874‐81. [DOI] [PubMed] [Google Scholar]
Rolland 2007
- Rolland E, Chevalier T, Auperin A, Burdett S, Pignon JP on behalf of the NSCLC Collaborative Group. Sequential radio‐chemotherapy(RT‐CT) versus radiotherapy alone (RT) and concomitant RT‐CT versus RTalone in locally advanced non‐small cell lung cancer (NSCLC): two metaanalyses using individual patient data (IPD) from randomised clinical trials(RCTs). Journal of Thoracic Oncology 2007;2(8):Suppl 4 S309‐10. [Google Scholar]
Saunders 1999
- Saunders M, Dische S, Barrett A, Harvey A, Griffiths G, Parmar M (on behalf of the CHART steering committee). Continuous hyperfractionated accelerated radiotherapy (CHART) versus conventional radiotherapy in non‐small cell lung cancer: mature data from the randomised multicentre trial. Radiotherapy and Oncology 1999;52:137‐48. [DOI] [PubMed] [Google Scholar]
Tierney 2007
- Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time‐to‐event data into meta‐analysis. Trials 2007;8:1‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Rowell 2004
- Rowell NP, O'Rourke NArt. No.: CD002140. DOI: 10.1002/14651858.CD002140.pub2. Concurrent chemoradiotherapy in non‐small cell lung cancer. Cochrane Database of Systematic Reviews 2004, Issue 4. [Art. No.: CD002140. DOI: 10.1002/14651858.CD002140.pub2] [DOI] [PubMed] [Google Scholar]


