Abstract
Fc receptors on leukocytes mediate internalization of antibody-containing complexes. Soluble immune complexes are taken up by endocytosis, while large antibody-opsonized particles are internalized by phagocytosis. We investigated the role of ubiquitylation in internalization of the human FcγRIIA receptor by endocytosis and phagocytosis. A fusion of FcγRIIA to green fluorescent protein (GFP) was expressed in ts20 cells, which bear a temperature-sensitive mutation in the E1 ubiquitin-activating enzyme. Uptake of soluble IgG complexes mediated by FcγRIIA–GFP was blocked by incubation at the restrictive temperature, indicating that endocytosis requires ubiquitylation. In contrast, phagocytosis and phagosomal maturation were largely unaffected when ubiquitylation was impaired. FcγRIIA–GFP was ubiquitylated in response to receptor cross-linking. Elimination of the lysine residues present in the cytoplasmic domain of FcγRIIA impaired endocytosis, but not phagocytosis. The proteasomal inhibitor clasto-lactacystin β-lactone strongly inhibited endocytosis, but did not affect phagocytosis. These studies demonstrate a role for ubiquitylation in the endocytosis of immune receptors, and reveal fundamental differences in the mechanisms underlying internalization of a single receptor depending on the size or multiplicity of the ligand complex.
Keywords: antigen presentation/clathrin/phagosome maturation/ts20 cells/ubiquitin
Introduction
Receptors that recognize the Fc portion of antibodies (FcRs) are expressed on virtually all cells of the immune system. Upon interaction with polyvalent antibody– antigen complexes, these receptors trigger a variety of responses in leukocytes, including the release of inflammatory mediators, degranulation, antibody-dependent cellular cytotoxicity and activation of the respiratory burst (Daeron, 1997). A further important function of FcRs is the internalization of antibody-containing complexes. FcRs mediate the uptake of soluble immune complexes by clathrin-dependent endocytosis (Ukkonen et al., 1986). In addition, FcRs are responsible for the phagocytosis of antibody-coated particles by macrophages and neutrophils. Endocytosis and phagocytosis mediated by FcRs are crucial for clearance of immune complexes from the circulation, for efficient antigen presentation and for killing of pathogens.
Both phagocytosis and endocytosis are initiated by cross-linking of FcRs by multivalent ligands. Recent work suggests that endocytosis and phagocytosis make use of several common protein components, including amphiphysin II and dynamin II (Gold et al., 1999, 2000). Moreover, phagosomes and endocytic vesicles undergo similar maturation processes after internalization, progressively acquiring markers of early and late endosomes, and subsequently of lysosomes (Tjelle et al., 2000). Despite these similarities, however, there are fundamental differences between endocytosis and phagocytosis. Most notably, the former process is dependent on clathrin, while the latter depends on the localized assembly of F-actin structures at sites of particle uptake. The molecular events underlying phagocytosis and endocytosis by FcRs and the extent to which they overlap remain incompletely understood. In particular, it is not clear how cross-linking of a single receptor can lead to two mechanistically distinct outcomes, endocytosis or phagocytosis, depending on the size of the cross-linking agent.
A complex array of proteins is involved in initiating and regulating endocytosis via clathrin-coated pits (Schmid, 1997). It has become appreciated that ubiquitylation can play a role in this process in both yeast and mammalian cells. It has long been understood that conjugation of the 79 amino acid polypeptide ubiquitin to cytoplasmic proteins can target them for degradation by the 26S proteasome (Pickart, 2000). More recently, however, it has been realized that ubiquitylation can also function as a signal for internalization and/or targeting of plasma membrane proteins to the endo-lysosomal pathway (Hicke, 1999; Strous and Govers, 1999). In mammalian cells, ubiquitylation has been implicated in the internalization and/or down-regulation of several plasma membrane proteins, including the epithelial sodium channel ENaC (Staub et al., 1997), the growth hormone receptor (GHR) (Strous et al., 1996), the epidermal growth factor receptor (EGFR) (Levkowitz et al., 1998) and the colony-stimulating factor 1 receptor (Lee et al., 1999). Upon cross-linking, these receptors activate signal transduction pathways involving tyrosine phosphorylation.
Because signaling via FcRs similarly involves tyrosine phosphorylation, we sought to define whether ubiquitylation is involved in the initiation of endocytosis and phagocytosis by these receptors. No specific pharmacological means are available at present to block ubiquitylation; however, an alternative approach to investigating the role of ubiquitylation is provided by the ts20 cell line. ts20 is a Chinese hamster cell line that carries a temperature-sensitive mutation in the E1 ubiquitin-activating enzyme, the first step in the ubiquitylation cascade (Kulka et al., 1988). Incubation of cells at temperatures above 40°C results in loss of E1 activity and a consequent block in ubiquitylation. These cells have proven useful in other studies of receptor endocytosis (Strous et al., 1996). Unfortunately, ts20 cells lack endogenous FcRs and are not normally phagocytic. However, we and others have shown previously that heterologous transfection of FcRs can confer phagocytic capacity on non-phagocytic cell types (e.g. Downey et al., 1999). The human FcγRIIA receptor is particularly convenient in this regard. FcγRIIA is unique among the FcRs in that ligand-binding and signaling domains are contained within a single polypeptide chain, which includes within its cytoplasmic domain an immunoreceptor tyrosine activation motif (ITAM) important for phagocytic signaling. In contrast, signaling by the ligand-binding α-subunits of FcγRI and FcγRIIIA requires non-covalent association with separate ITAM-containing FcR γ-subunits. Importantly, expression of FcγRIIA on its own without accessory proteins suffices to allow both endocytosis of soluble immune complexes and phagocytosis of opsonized particles (Indik et al., 1991; Odin et al., 1991). By following the internalization of FcγRIIA expressed in ts20 cells, we have determined that ubiquitylation is required for endocytosis, but not for phagocytosis mediated by this immunoreceptor.
Results
FcγRIIA–GFP mediates phagocytosis and endocytosis when expressed in ts20 cells
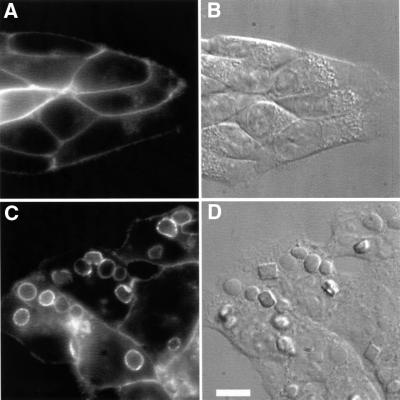
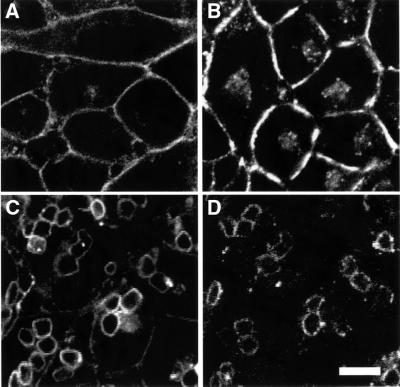
ts20 cells were transfected with human FcγRIIA conjugated to the green fluorescent protein (GFP) to facilitate its detection. Like the endogenous FcRs of myeloid cells, FcγRIIA–GFP was largely targeted to the plasma membrane in ts20 cells, with a small fraction in an intracellular juxtanuclear compartment, probably recycling endosomes (Figure 1A). While ts20 cells are not normally phagocytic, transfection with FcγRIIA–GFP conferred on them the ability to internalize IgG-coated sheep red blood cells (SRBCs) efficiently (Figure 1C and D). The receptor accumulated at sites of SRBC binding and was internalized with the particles, leading to its concentration in the membrane of phagosomes (Figure 1C).
Fig. 1. FcγRIIA–GFP mediates phagocytosis in ts20 cells. GFP fluorescence was visualized in living ts20 cells expressing FcγRIIA–GFP. (A) Fluorescence of cells with no addition of particles. (C) Fluorescence of cells incubated with IgG-coated fixed SRBCs for 30 min at 37°C. (B and D) Corresponding DIC images. Bar = 8 µm.
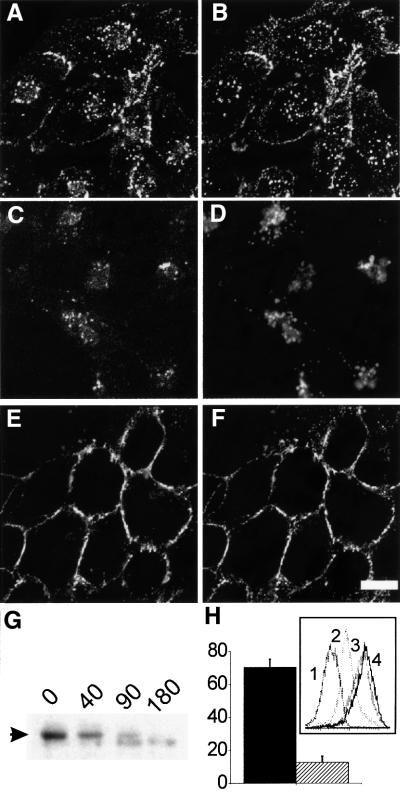
FcγRIIA–GFP also supported receptor-mediated endocytosis of soluble IgG complexes (Figure 2). Upon addition of heat-aggregated human IgG (ag-IgG) to ts20 cells expressing FcγRIIA–GFP, the receptor was internalized and acquired a punctate, perinuclear localization (Figure 2A). Detection of the ag-IgG with fluorescently labeled secondary antibody revealed complete co-localization with the FcγRIIA–GFP, indicating that the receptor and its ligand remained associated after internalization (Figure 2B). To confirm that the punctate fluorescence observed truly represents receptor in internal vesicles and not simply receptor clustering on the cell surface, we verified that cell permeabilization was required for the secondary antibody to gain access to the internalized ag-IgG (not shown). Moreover, FcγRIIA– GFP-containing vesicles acquire the late endosomal/lysosomal marker LAMP-1 (Figure 2C and D), indicating that these endosomes undergo maturation/progression. Accordingly, we also found that the receptors underwent degradation following internalization (Figure 2G), presumably as a result of routing to the lysosomal compartment. The latter observation recapitulates the behavior of the FcRs of mouse macrophages, which undergo lysosomal degradation following internalization of immune complexes (Ukkonen et al., 1986).
Fig. 2. Endocytosis of FcγRIIA–GFP in ts20 cells is blocked by temperature shift. ts20 cells expressing FcγRIIA–GFP were incubated with ag-IgG without (A–D) or with (E and F) prior incubation for 1.75 h at 42°C. (A, C and E) Distribution of FcγRIIA–GFP. (B and F) Distribution of ag-IgG detected with Cy3-anti-human antibody. (D) Distribution of LAMP-1 by immunofluorescence in the same cells as (C). Incubation with ag-IgG was at 37°C for 40 min (A, B, E and F) or 80 min (C and D). Bar = 8 µm. (G) ts20 cells expressing FcγRIIA–GFP were incubated with ag-IgG for 0, 40, 90 or 180 min, then total cell lysates were prepared and analyzed by western blotting with anti-GFP antibodies. The arrow marks the position of FcγRIIA–GFP; the lower band is a non-specific background band. (H) Cells were pre-incubated at 37°C (solid bar) or 42°C (hatched bar) for 1–1.75 h, then endocytosis was analyzed by flow cytometry. Bars indicate internalization of cross-linking antibody as a percentage of initial amount bound (n = 4 experiments, ±SE). Inset: Cy5 fluorescence curves from an individual experiment. Abscissa: relative fluorescence intensity (log scale). Ordinate: number of cells. Curve 1, background (no anti-FcγRII antibody); curve 2, post-cross-linking, cells pre-incubated at 37°C; curve 3, post-cross-linking, cells pre-incubated at 42°C; curve 4, no internalization (cross-linking at 4°C).
The ubiquitin conjugation machinery is required for endocytosis
ts20 cells expressing FcγRIIA–GFP were then incubated at 42°C to inactivate the E1 enzyme. The temperature shift did not significantly alter the plasma membrane localization of FcγRIIA–GFP (not shown). However, pre-incubation of the cells at 42°C blocked internalization of the receptor in response to addition of ag-IgG (Figure 2E). Detection of the ag-IgG with secondary antibody revealed that its internalization was likewise inhibited, but that binding to the receptor was unaffected (Figure 2F). It is noteworthy that while incubation with ag-IgG did not lead to receptor internalization, it did result in the receptor assuming a non-homogeneous distribution in the plasma membrane with concentration at cell–cell interfaces, presumably reflecting receptor clustering.
To obtain a quantitative measure of internalization, we used an alternative method for inducing endocytosis. Monoclonal anti-FcγRII antibody was first allowed to bind to the receptors, which were then cross-linked with goat anti-mouse secondary antibody. Disappearance of the secondary antibody from the cell surface due to internalization was quantified using a Cy5-conjugated anti-goat antibody and flow cytometry (Figure 2H). Using this method, we determined that 70 ± 5% of the IgG–receptor complexes were internalized after 30 min of cross-linking at 37°C. In contrast, after pre-incubation of cells at 42°C, only 13 ± 4% was internalized under the same conditions (Figure 2H).
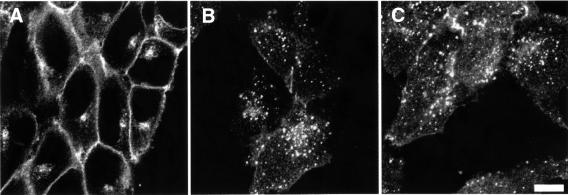
In order to confirm that the effect of incubation at 42°C on endocytosis was due to the mutation in E1 and not to a non-specific effect of temperature, FcγRIIA–GFP was also transfected into E36 cells, the cell line from which the ts20 mutant line was derived. Unlike in ts20 cells, the E1 enzyme in E36 cells remains functional at 42°C. As in ts20 cells, FcγRIIA–GFP in E36 cells localized primarily to the plasma membrane (Figure 3A), and was internalized effectively in response to ag-IgG (Figure 3B). This internalization proceeded unimpaired after pre-incubation at 42°C (Figure 3C). Thus, the block in endocytosis in ts20 cells was due specifically to the temperature-induced defect in the ubiquitin conjugation machinery. Blocking ubiquitylation did not cause a general block in receptor-mediated endocytosis, as uptake of rhodamine-labeled transferrin proceeded normally after temperature shift (data not shown; see also Strous et al., 1996). To rule out the possibility that introduction of the GFP moiety was somehow the cause of the ubiquitin dependence of endocytosis, we also measured endocytosis in ts20 cells transfected with FcγRIIA carrying a small (27 amino acid) myc/His6 epitope tag (FcγRIIA-MH). Endocytosis of FcγRIIA-MH was also blocked by incubation at 42°C (not illustrated).
Fig. 3. Endocytosis of FcγRIIA–GFP in E36 cells. (A) Fluorescence of E36 cells expressing FcγRIIA–GFP prior to addition of ag-IgG. (B and C) Cells were incubated with ag-IgG for 40 min without (B) or with (C) pre-incubation for 1.75 h at 42°C. Representative of five experiments. Bar = 8 µm.
The ubiquitin conjugation machinery is not required for phagocytosis
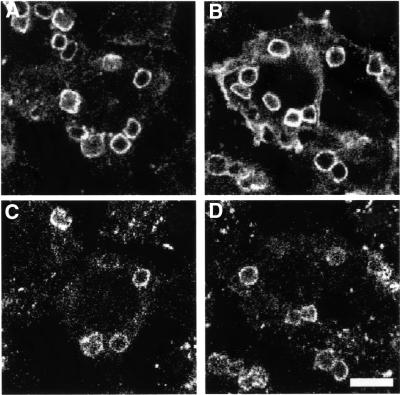
ts20 cells expressing FcγRIIA–GFP were incubated with IgG-opsonized SRBCs after pre-incubation at 42°C to determine whether phagocytosis also depended on ubiquitylation. Notably, in contrast to what we observed for endocytosis under the same conditions, phagocytosis was not blocked upon inactivation of E1 (Figure 4B). When unfixed SRBCs were used as targets in phagocytosis assays, we observed no difference in phagocytic efficiency after pre-incubation of cells at 42°C (110 ± 3% of control; n = 3). When fixed SRBCs were used (which results in a higher overall level of phagocytosis), the phagocytic index showed a moderate decrease after temperature shift (75 ± 2% of control; n = 3).
Fig. 4. Phagocytosis via FcγRIIA–GFP in ts20 cells is not blocked by temperature shift. ts20 cells expressing FcγRIIA–GFP were incubated for 45 min with opsonized SRBCs after pre-incubation for 1.75 h at 37°C (A and C) or 42°C (B and D). (A and B) Distribution of FcγRIIA–GFP. (C and D) Localization of LAMP-1 in corresponding cells. Bar = 8 µm.
After particles are internalized, the phagosome undergoes a maturation process, fusing with early and late endosomes and eventually forming a phagolysosome. It has recently become appreciated that ubiquitylation is involved not only in the internalization of membrane proteins, but also in their intracellular trafficking (Hicke, 2001). Hence, while ubiquitylation did not appear to be necessary for particle internalization, we considered the possibility that it might be required for phagosomal maturation. The acquisition by phagosomes of the late endosome/lysosomal marker LAMP-1 was used as a measure of their maturation (Figure 4C and D). Quantification of LAMP-1 acquisition after pre-incubation of cells at 42°C revealed no difference from control cells (LAMP-1 acquisition as a percentage of control = 108 ± 8%, n = 4). Thus, ubiquitylation does not appear to be essential for either phagocytosis or phagosomal maturation.
Ubiquitylation of FcγRIIA–GFP
The inhibition of FcγRIIA–GFP endocytosis upon inactivation of E1 implied a requirement for ubiquitylation. This prompted us to investigate whether FcγRIIA–GFP itself becomes ubiquitylated upon receptor cross-linking. Analysis of the electrophoretic mobility of FcγRIIA–GFP in whole-cell lysates revealed that following cross-linking with ag-IgG, a fraction of the receptors displayed increased apparent molecular weight on SDS–PAGE (Figure 5A, lanes 2 and 3). The appearance of these high molecular weight forms was abolished by pre-incubation of cells at 42°C (Figure 5A, lane 4). These observations are consistent with conjugation of the receptors with one or more ubiquitin moieties. Only a small fraction of the total expressed receptor appears to be ubiquitylated, as was previously seen for the ubiquitylation of the GHR (Strous et al., 1996). As in that case, this may be attributed to several possible factors: (i) the ubiquitylation may be very short lived during endocytosis; (ii) the binding of ag-IgG to the cell surface is not perfectly synchronized; and (iii) extensive cellular deubiquitylation activity may lead to loss of ubiquitylation during lysate preparation.
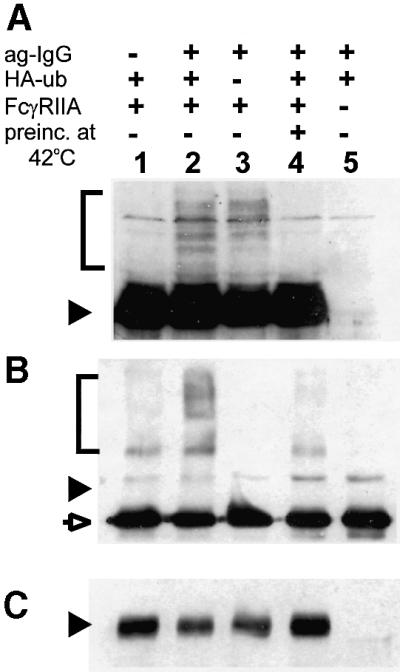
Fig. 5. FcγRIIA–GFP is ubiquitylated upon receptor aggregation. (A) Total cell lysates were analyzed by SDS–PAGE and immunoblotting with polyclonal anti-GFP antibody. Lane 2: cells stably transfected with FcγRIIA–GFP and transiently transfected with HA-ubiquitin were incubated with ag-IgG at 37°C for 10 min before preparation of lysates. Conditions for other lanes were the same as for lane 2, with the following exceptions: lane 1, no addition of ag-IgG; lane 3, no transfection with HA-ubiquitin; lane 4, cells were pre-incubated at 42°C for 1.75 h before addition of ag-IgG; lane 5, no transfection with FcγRIIA–GFP. (B) FcγRIIA–GFP was immunoprecipitated from the lysates used in (A) with mouse anti-GFP and was analyzed by SDS–PAGE and immunoblotting with anti-HA. Lanes correspond to those in (A). (C) The blot from (B) was reprobed with rabbit anti-GFP antibody to compare total amounts of receptor loaded. The position of the 66 kDa marker is indicated by a filled arrowhead on each blot. The brackets indicate the region in which polyubiquitylated forms of FcγRIIA–GFP migrate. The open arrow indicates the immunoprecipitating antibody. The band seen at the position of the 66 kDa marker in (B) is present in all lanes and thus reflects non-specific binding of antibodies.
To confirm that the molecular weight shift of the receptor reflected ubiquitylation, the cells were transfected with a vector encoding a hemagglutinin (HA)-tagged form of ubiquitin (Treier et al., 1994). FcγRIIA–GFP was cross-linked with ag-IgG, and cell lysates were prepared under denaturing conditions. The receptors were then immunoprecipitated from these lysates using a monoclonal anti-GFP antibody, and the immunoprecipitates were analyzed by SDS–PAGE followed by western blotting with anti-HA to detect receptor-associated ubiquitin (Figure 5B). A high molecular weight smear corresponding to polyubiquitylated receptor was observed (lane 2). Its appearance was induced by the addition of ag-IgG (lane 2 versus lane 1), was abrogated by pre-incubation of the cells at 42°C (lane 4) and was absent in cells not transfected with FcγRIIA–GFP (lane 5) or with HA-ubiquitin (lane 3). Note that in cells not transfected with HA-ubiquitin, high molecular weight receptor bands are nevertheless observed with anti-GFP due to conjugation of endogenous ubiquitin, as expected (Figure 5A, lane 3).
Cytoplasmic lysine residues of FcγRIIA are important for endocytosis, but not phagocytosis
Ubiquitin is covalently attached to lysine residues of target proteins, and five such lysines are present in the cytosolic tail of FcγRIIA. To investigate the role of ubiquitylation of the receptor during endocytosis, a mutant receptor in which all five cytoplasmic lysines of FcγRIIA were substituted with arginine (FcγRIIA/5KR–GFP) was expressed in ts20 cells. Analysis of receptor migration by SDS–PAGE revealed, interestingly, that some ubiquitylation nevertheless still occurred in response to ag-IgG, albeit less than that observed for wild-type FcγRIIA–GFP, as judged by a smaller molecular weight shift (not shown). This observation implies that some attachment of ubiquitin must be directed to lysine residues in the attached GFP protein. We proceeded to test whether the presence of the five endogenous lysines of FcγRIIA is important for endocytosis by comparing uptake of ag-IgG by FcγRIIA/5KR–GFP with that observed with wild-type FcγRIIA–GFP. After addition of ag-IgG, relatively little FcγRIIA/5KR–GFP was observed in perinuclear vesicles. Instead, receptor aggregation led to its accumulation at areas of cell–cell contact, as was seen when cells expressing the wild-type receptor were incubated at the non-permissive temperature (Figure 6B, compare with 6A). When assayed by flow cytometry, the initial rate of endocytosis of FcγRIIA/5KR–GFP was markedly inhibited. Internalization of FcγRIIA/5KR–GFP measured during the first 10 min of uptake was only 12 ± 3% of wild type (mean ± SE; n = 3.). At later times, however, endocytosis proceeded, such that, after 30 min, internalization of the mutant receptor was comparable to the wild-type control (not shown). This indicates that the endogenous lysines of FcγRIIA are necessary for optimal endocytosis, but are not strictly required for this process to occur.
Fig. 6. FcγRIIA/5KR is deficient in endocytosis, but not in phagocytosis. (A) Fluorescence of ts20 cells expressing FcγRIIA/5KR–GFP. (B and C) Fluorescence after incubation for 40 min at 37°C with ag-IgG (B) or IgG-coated fixed SRBCs (C). Images in (A) and (B) were acquired with identical gain settings on the confocal microscope. (D) Distribution of LAMP-1 in the same cells as (C). Images reflect at least four experiments. Bar = 8 µm.
In contrast to the deficiencies observed in endocytosis, FcγRIIA/5KR–GFP effectively supported phagocytosis (Figure 6C). Internalization of SRBCs proceeded with equal efficiency to that observed with the wild-type receptor (phagocytic index as a percentage of wild type = 96 ± 6%, n = 3). Phagosomes formed in cells expressing FcγRIIA/5KR also acquired LAMP-1 (Figure 6D). These results are consistent with the conclusion reached earlier that ubiquitylation is not required for either phagocytosis or phagosome maturation.
Inhibition of the proteasome blocks endocytosis, but not phagocytosis
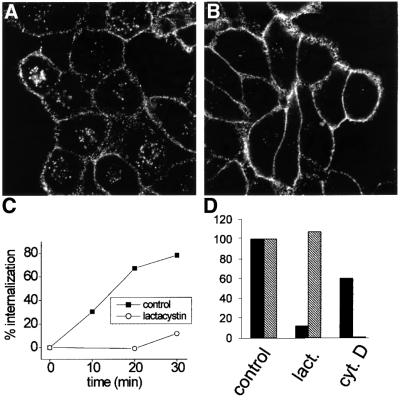
It has been shown that proteasome inhibitors impair endocytosis and/or intracellular trafficking of several surface receptors (van Kerkhof et al., 2000, 2001; Rocca et al., 2001). To test whether this is the case for FcγRIIA, we measured endocytosis in the presence of the specific proteasomal inhibitor clasto-lactacystin β-lactone. Uptake of ag-IgG was largely blocked in the presence of 20 µM inhibitor (Figure 7A and B). Analysis of endocytosis by flow cytometry confirmed this strong inhibitory effect (Figure 7C). Phagocytosis, however, was unaffected by clasto-lactacystin β-lactone treatment under the same conditions (Figure 7D). Conversely, treatment with 10 µM cytochalasin D to disrupt the actin cytoskeleton had only a moderate inhibitory effect on endocytosis, while leading to phagocytosis being completely abolished (Figure 7D), again highlighting distinct requirements for the two internalization processes.
Fig. 7. Inhibition of the proteasome impairs endocytosis, but not phagocytosis. (A and B) ts20 cells expressing FcγRIIA-MH were incubated with DMSO vehicle (A) or 20 µM clasto-lactacystin β-lactone (B) for 1 h, then ag-IgG was added for 40 min to induce endocytosis. Ag-IgG was detected with Cy3-anti-human secondary antibody after cell permeabilization. (C) The kinetics of endocytosis of immune complexes via FcγRIIA–GFP were analyzed by flow cytometry in cells pre-treated for 1 h with DMSO (control; solid squares) or 20 µM clasto-lactacystin β-lactone (open circles). Values are the means of two experiments. (D) Cells were pre-incubated with 20 µM clasto-lactacystin β-lactone (lact.) for 1 h or with 10 µM cytochalasin D (cyt. D) for 10 min. Inhibitors were also present during the uptake assays, except for clasto-lactacystin β-lactone during endocytosis. Endocytosis (solid bars) and phagocytosis (hatched bars) were assayed for 30 min at 37°C by flow cytometry or by scoring phagocytic index. Levels are expressed as a percentage of control (DMSO vehicle alone) and reflect means of two experiments.
Discussion
This work provides the first indication that ubiquitylation is important in the internalization of an immune receptor. Our results in ts20 cells indicate that an active ubiquitin conjugation system is required for endocytosis of FcγRIIA. Biochemical analysis reveals that the receptor itself is ubiquitylated in response to aggregation, and the effects of mutation of cytoplasmic lysines in the receptor suggest that this ubiquitylation is involved in receptor endocytosis.
It has been observed previously that subunits of the T-cell receptor become ubiquitylated in response to CD3 cross-linking (Cenciarelli et al., 1996). There is also evidence that the FcR γ-chain is ubiquitylated in response to cross-linking of its associated FcεRI or FcγRI α-chains (Paolini and Kinet, 1993; Duchemin et al., 1994; Lafont and Simons, 2001). However, the functional significance of ubiquitylation in these cases was not studied. Our results suggest that the ligand-induced ubiquitylation associated with these other ITAM-containing immune receptors may also be required for their endocytosis. Receptor-mediated endocytosis of immune complexes is important for efficient presentation of extracellular antigens to the adaptive immune system (Lanzavecchia, 1990). Thus, it appears that ubiquitylation may play two distinct roles in antigen presentation. On the one hand, ubiquitylation is required to target cytosolic proteins to the proteasome for degradation, generating peptides that are presented on class I major histocompatibility complex (MHC) molecules (Rock and Goldberg, 1999). Ubiquitylation also appears to be required for efficient endocytosis by FcRs of extracellular antigens to enable their processing in endosomes and loading and presentation on class II MHC molecules.
Ubiquitylation functions at multiple subcellular locations to effect down-regulation of membrane proteins (Hicke, 2001). It can function during endocytosis of proteins from the plasma membrane, but has also been implicated in intracellular sorting of membrane proteins into multivesicular bodies (Katzmann et al., 2001; Reggiori and Pelham, 2001). For FcγRIIA, it appears that ubiquitylation is required during the endocytosis step, since neither the receptor nor its bound ligand are internalized in the absence of ubiquitylation. However, it cannot be excluded that in the absence of ubiquitylation, FcγRIIA is still internalized, but is recycled rapidly to the plasma membrane without release of its ligand, resulting in no net internalization of either receptor or ligand. It is also possible that ubiquitylation is involved both in the endocytosis of FcγRIIA and in the subsequent maturation of FcγRIIA-containing endosomes; the requirement for ubiquitylation in the early endocytic step clearly will complicate the analysis of any role it may have at subsequent stages.
Inhibitors of proteasome function have been shown to inhibit endocytosis of FcγRIIA (this study) and the GHR (van Kerkhof et al., 2000). Proteasomal inhibitors also impair the intracellular targeting of the GHR and the interleukin-2 receptor to late endosomes (Rocca et al., 2001; van Kerkhof et al., 2001). Moreover, they have been noted to inhibit the degradation of a number of other surface receptors. While direct degradation of these receptors by the proteasome was initially invoked, the data may instead reflect defects in receptor trafficking to late endosomes. What are the substrates of proteasomal degradation that influence endocytosis and intracellular sorting? One possibility is that a negative regulatory component of the endocytic or sorting machinery must be removed via proteasomal degradation. Another possibility is that the effect of inhibiting the proteasome is indirect. Inhibition of the proteasome may cause depletion of cellular levels of free ubiquitin, thereby leading to defects in ubiquitin-dependent endocytosis and sorting (van Kerkhof et al., 2001).
While mutation of the endogenous lysines of FcγRIIA inhibited the initial rate, endocytosis did proceed at longer times. Also, the fraction of FcγRIIA–GFP that was recovered in ubiquitylated form in lysates was low. This may largely reflect loss of ubiquitin during lysate preparation. Alternatively, these results together may indicate that stoichiometric ubiquitylation of the receptor itself is not essential for uptake, and that ubiquitylation of other associated proteins or components of the endocytic machinery can suffice to allow endocytosis. Studies on the endocytosis of the GHR in mammalian cells (Govers et al., 1999) and of a Ste2p–ubiquitin chimera in yeast (Dunn and Hicke, 2001) have indicated that, in both cases, ubiquitylation of proteins distinct from the receptors themselves is involved during endocytosis.
Ubiquitylation of target proteins generally involves specific recognition by ubiquitin-conjugating (E2) and/or ubiquitin-ligating (E3) enzymes (Rotin et al., 2000). What E2 and/or E3 enzymes are involved in the ubiquitylation required for FcγRIIA endocytosis is an open question, but candidate E3 enzymes include the proto-oncoprotein Cbl and its relatives. Cbl is a negative regulator of immune signaling that becomes tyrosine phosphorylated in response to cross-linking of several immune receptors, including FcγRIIA (Izadi et al., 1998; Saci et al., 1999). Cbl has been shown recently to possess ubiquitin ligase activity, and it induces ubiquitylation of the EGFR via the E3 activity of its RING finger domain, causing EGFR down-regulation (Levkowitz et al., 1999). One might envisage complementary roles for Cbl and Syk. The former would induce ubiquitylation and endocytosis, while Syk kinase, which itself is negatively regulated by Cbl, would mediate phagocytosis (Ota and Samelson, 1997). The balance between these activities may determine whether endocytosis or phagocytosis ensues.
Our results indicate that a dependence on ubiquitylation is a characteristic of endocytosis of soluble ligands by FcγRIIA, but not of phagocytosis of particles by the same receptor. In both cases, internalization is initiated by an equivalent signal, namely receptor aggregation. The differences observed between endocytosis and phagocytosis imply that aggregating a large number of receptors can lead to a qualitatively different outcome from that which occurs using a smaller number of the same receptors. If the fundamental signaling unit in both cases is a receptor dimer or other low-order oligomer (as, for example, a transphosphorylation model would imply), the cell must have some means of integrating signals generated by many such units in order to trigger phagocytosis-specific events such as actin cup formation. Perhaps the local concentration of activated kinases such as Syk must reach some threshold to initiate phagocytosis. The differences between endocytosis and phagocytosis also raise the converse question: is endocytosis-specific machinery excluded or inactive during phagocytosis, or is the phagocytic machinery simply overlaid on top of a frustrated attempt by the cells to endocytose opsonized particles that are too large for clathrin-coated pits to effect internalization? The observation that clathrin does accumulate at sites of phagocytosis suggests that elements of the endocytic machinery may indeed still be recruited during phagocytosis (Aggeler and Werb, 1982). Even if these endocytic components do not function in the particle internalization step, they may play a role in initiating vesicle budding to remove proteins from the phagosomal membrane during phagosome maturation. Additional experiments will be required to resolve these alternatives.
Materials and methods
Reagents and antibodies
Fetal bovine serum and α-modified Earle’s medium (MEM) were from Wisent (St Bruno, QC). G418 was from Mediatech (Hernden, VA). Mounting medium for fluorescence microscopy was from DAKO. Anti-HA monoclonal antibody was from BabCo. Rabbit anti-GFP and Alexa350 anti-rabbit antibodies were from Molecular Probes. Mouse anti-GFP was from Santa Cruz Biotechnology. SRBCs and rabbit anti-SRBC antibody were from ICN Biomedicals. Latex beads were from Bangs Beads. Cy3- and Cy5-conjugated secondary antibodies were from Jackson Immunoresearch Laboratories. Horseradish peroxidase (HRP)-conjugated secondary antibodies and Ultralink-immobilized protein G were from Pierce. Anti-hamster LAMP-1 antibody UH-1 was from the Development Studies Hybridoma Bank (University of Iowa). Goat anti-mouse F(ab′)2 was from Kierkegaard and Perry Laboratories (Gaithersburg, MD). Phosphate-buffered saline (PBS), HEPES-buffered RPMI (HPMI), protease inhibitors, human IgG, clasto-lactacstin β-lactone and all other reagents were from Sigma Aldrich.
DNA constructs
The plasmid encoding FcγRIIA–GFP was constructed by cloning a cDNA of FcγRIIA into pEGFP-N1 (Clontech) at HindIII and SacII sites. The plasmid encoding FcγRIIA/5KR–GFP was constructed by overlap PCR. Plasmid pMT123 encoding HA-ubiquitin was kindly provided by Dr D.Bohmann (EMBL, Heidelberg).
Cell culture and transfection
ts20 and E36 cells were kindly provided by Dr A.Schwartz (Washington University School of Medicine, St Louis, MO). ts20 cells were grown in α-MEM at 34°C in 5% CO2. For inactivation of the E1 enzyme, cells were incubated in this medium at 42–43°C for 1–1.75 h. Transfections were performed with FuGene 6 (Roche Molecular Biochemicals) according to the manufacturer’s instructions. Stable lines expressing FcγRIIA–GFP were selected with G418 at 0.5 mg/ml.
Phagocytosis assays
SRBCs or latex beads were used as particles for phagocytosis assays. For most experiments, the SRBCs were fixed overnight in 4% paraformaldehyde (PFA) in PBS before opsonization, a procedure that we found empirically to result in higher levels of phagocytosis. SRBCs were opsonized with rabbit anti-SRBC antibody (1:50 dilution) in PBS for 1 h at 37°C, then washed in PBS; latex beads were opsonized with 3.75 mg/ml human IgG for 1.5 h at 37°C. ts20 cells grown on glass coverslips were overlaid with IgG-coated SRBCs (50 µl of a 10% suspension of SRBCs added per 25 mm coverslip) or latex beads. After incubation to allow phagocytosis (30–45 min at 34–37°C), SRBCs that had not been internalized were lysed by a brief exposure to water (for unfixed SRBC) or particles were labeled by incubating with Cy3- or Alexa350-anti-rabbit (1:1000 in HPMI) at 4°C (for fixed SRBCs or latex beads). For scoring phagocytic efficiency, cells were incubated with particles for 30 min at 37°C. The phagocytic index was defined as the number of phagosomes per 100 cells. For comparison of phagocytic efficiency of FcγRIIA–GFP versus FcγRIIA/5KR–GFP, phagocytosis was measured in transient transfectants of ts20 cells. For quantitation of LAMP-1 acquisition, cells were incubated with fixed SRBCs for 15 min, at which point external SRBCs were labeled with antibody, followed by a chase at 37°C for an additional 20 min. LAMP-1 acquisition was then scored in those phagosomes that had closed within the first 15 min.
Endocytosis assays
Human IgG was aggregated by heating at 62°C for 20 min at a concentration of 10 mg/ml in PBS, followed by centrifugation at 16 000 g for 10 min to remove insoluble aggregates. The supernatant was used at 1:50–1:100 dilution to induce receptor cross-linking and endocytosis, by incubation for 40 min at 34–37°C. For quantification of endocytosis, a modification of the protocol of Odin et al. (1991) was used. ts20 cells growing on tissue culture dishes were incubated with 0.56 µg/ml of mouse anti-FcγRII antibody IV.3 for 20 min at 4°C, washed, incubated with 5 µg/ml goat anti-mouse F(ab′)2 at 4°C for 20 min, then warmed rapidly to 37°C for various times. Cells were then washed, incubated for 30 min at 4°C with 3.5 µg/ml Cy5-goat anti-mouse and washed again. All washes were with cold PBS + 1 mM CaCl2, 1 mM MgCl2. All the incubations with antibodies were in HPMI; in some experiments, 5% donkey serum was added as a blocking agent. For experiments comparing endocytosis with and without pre-incubation of cells at 42°C, the cross-linking with goat anti-mouse was performed for 30 min at 37°C without pre-binding at 4°C, in order to minimize the time the cells spent at low temperature. After labeling with Cy5-goat anti-mouse, cells were detached from the plastic with PBS + 2 mM EDTA, dispersed by passing through a 25 gauge needle and fixed with 2% PFA. Cy5 fluorescence was analyzed by flow cytometry. The mean background fluorescence observed when anti-FcγRIIA antibody was omitted was subtracted from fluorescence values. The fluorescence observed when cells were maintained at 4°C throughout the procedure was used as the reference for no endocytosis.
Microscopy and immunofluorescence
Cells were either viewed live in HPMI with a Leica DM-IRB microscope, or were fixed in 4% PFA, washed, mounted and analyzed by confocal microscopy using an LSM 510 laser scanning confocal microscope (Zeiss) with a 100× oil immersion objective. GFP and Cy3 were examined using the standard laser excitation and filter sets. For detection of LAMP-1 or ag-IgG, cells were fixed, washed in PBS, permeabilized in methanol at –20°C and washed in PBS. For LAMP-1 immunofluorescence, cells were then blocked with 4% non-fat dry milk in PBS and treated with anti-LAMP-1 UH1 antiserum at a 1:1 dilution in blocking buffer for 40 min at room temperature. Samples were then washed, incubated with Cy3-anti-mouse antibody (1:1000 in blocking buffer) for 30 min at room temperature, washed and mounted. For detection of ag-IgG, Cy3-anti-human antibody (1:1000 in blocking buffer) was added directly after permeabilization.
Protein analysis
ts20 cells growing in 6-well plates were lysed by adding boiling 1% SDS in PBS (200 µl/well). Lysates were transferred to Eppendorf tubes and boiled for 5 min. They were then sheared by passing 10× through a 25 gauge needle, and insoluble material was precipitated by centrifugation at 16 000 g for 10 min. Supernatants were frozen until further use. For immunoprecipitations, lysates were diluted into buffer with a final composition of 1% Triton X-100, 0.25% deoxycholate, 0.5% SDS, 0.5% bovine serum albumin (BSA), 1 mM EDTA, 20 mM NaF in PBS with added protease inhibitors (IP buffer). Mouse anti-GFP was pre-bound to protein G beads blocked with BSA. Diluted lysates were incubated with these beads for 2 h at 4°C, and then the beads were washed twice with cold IP buffer, then twice with cold PBS. For analysis, samples were solubilized in Laemmli’s sample buffer, resolved by SDS–PAGE and transferred to nitrocellulose membranes. The membranes were blocked for 1 h at room temperature in 4% non-fat dry milk, 0.1% Triton X-100, 0.01% SDS in PBS, then probed overnight at 4°C with rabbit anti-GFP antibody (1:4000 in blocking buffer) or mouse anti-HA antibody (1:5000 in blocking buffer). Blots were then washed in 0.1% Triton X-100, 0.01% SDS in PBS, incubated with secondary antibodies conjugated to HRP at 1:20 000 dilution in blocking buffer for 45 min, then washed again. Blots were exposed using enhanced chemiluminescence (Amersham).
Acknowledgments
Acknowledgements
We thank Sheyun Zhao and Rahim Kaba for technical assistance, and Dr Daniela Rotin for helpful discussions. This work was supported by the Canadian Institutes of Health Research, the Arthritis Society of Canada, the National Sanatorium Association and by the NIH. J.W.B. is the recipient of a fellowship from the Canadian Cystic Fibrosis Foundation. S.G. is an International Scholar of the Howard Hughes Medical Institute and is the current holder of the Pitblado Chair in Cell Biology.
References
- Aggeler J. and Werb,Z. (1982) Initial events during phagocytosis by macrophages viewed from outside and inside the cell: membrane–particle interactions and clathrin. J. Cell Biol., 94, 613–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cenciarelli C., Wilhelm,K.G.,Jr, Guo,A. and Weissman,A.M. (1996) T cell antigen receptor ubiquitination is a consequence of receptor-mediated tyrosine kinase activation. J. Biol. Chem., 271, 8709–8713. [DOI] [PubMed] [Google Scholar]
- Daeron M. (1997) Fc receptor biology. Annu. Rev. Immunol., 15, 203–234. [DOI] [PubMed] [Google Scholar]
- Downey G.P., Botelho,R.J., Butler,J.R., Moltyaner,Y., Chien,P., Schreiber,A.D. and Grinstein,S. (1999) Phagosomal maturation, acidification and inhibition of bacterial growth in nonphagocytic cells transfected with FcγRIIA receptors. J. Biol. Chem., 274, 28436–28444. [DOI] [PubMed] [Google Scholar]
- Duchemin A.M., Ernst,L.K. and Anderson,C.L. (1994) Clustering of the high affinity Fc receptor for immunoglobulin G (FcγRI) results in phosphorylation of its associated γ-chain. J. Biol. Chem., 269, 12111–12117. [PubMed] [Google Scholar]
- Dunn R. and Hicke,L. (2001) Multiple roles for Rsp5p-dependent ubiquitination at the internalization step of endocytosis. J. Biol. Chem., 276, 25974–25981. [DOI] [PubMed] [Google Scholar]
- Gold E.S., Underhill,D.M., Morrissette,N.S., Guo,J., McNiven,M.A. and Aderem,A. (1999) Dynamin 2 is required for phagocytosis in macrophages. J. Exp. Med., 190, 1849–1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold E.S., Morrissette,N.S., Underhill,D.M., Guo,J., Bassetti,M. and Aderem,A. (2000) Amphiphysin IIm, a novel amphiphysin II isoform, is required for macrophage phagocytosis. Immunity, 12, 285–292. [DOI] [PubMed] [Google Scholar]
- Govers R., ten Broeke,T., van Kerkhof,P., Schwartz,A.L. and Strous,G.J. (1999) Identification of a novel ubiquitin conjugation motif, required for ligand-induced internalization of the growth hormone receptor. EMBO J., 18, 28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicke L. (1999) Gettin’ down with ubiquitin: turning off cell-surface receptors, transporters and channels. Trends Cell Biol., 9, 107–112. [DOI] [PubMed] [Google Scholar]
- Hicke L. (2001) A new ticket for entry into budding vesicles—ubiquitin. Cell, 106, 527–530. [DOI] [PubMed] [Google Scholar]
- Indik Z., Kelly,C., Chien,P., Levinson,A.I. and Schreiber,A.D. (1991) Human FcγRII, in the absence of other Fcγ receptors, mediates a phagocytic signal. J. Clin. Invest., 88, 1766–1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izadi K.D., Erdreich-Epstein,A., Liu,Y. and Durden,D.L. (1998) Characterization of Cbl–Nck and Nck–Pak1 interactions in myeloid FcγRII signaling. Exp. Cell Res., 245, 330–342. [DOI] [PubMed] [Google Scholar]
- Katzmann D.J., Babst,M. and Emr,S.D. (2001) Ubiquitin-dependent sorting into the multivesicular body pathway requires the function of a conserved endosomal protein sorting complex, ESCRT-I. Cell, 106, 145–155. [DOI] [PubMed] [Google Scholar]
- Kulka R.G., Raboy,B., Schuster,R., Parag,H.A., Diamond,G., Ciechanover,A. and Marcus,M. (1988) A Chinese hamster cell cycle mutant arrested at G2 phase has a temperature-sensitive ubiquitin-activating enzyme, E1. J. Biol. Chem., 263, 15726–15731. [PubMed] [Google Scholar]
- Lafont F. and Simons,K. (2001) Raft-partitioning of the ubiquitin ligases Cbl and Nedd4 upon IgE-triggered cell signaling. Proc. Natl Acad. Sci. USA, 98, 3180–3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanzavecchia A. (1990) Receptor-mediated antigen uptake and its effect on antigen presentation to class II-restricted T lymphocytes. Annu. Rev. Immunol., 8, 773–793. [DOI] [PubMed] [Google Scholar]
- Lee P.S., Wang,Y., Dominguez,M.G., Yeung,Y.G., Murphy,M.A., Bowtell,D.D. and Stanley,E.R. (1999) The Cbl protooncoprotein stimulates CSF-1 receptor multiubiquitination and endocytosis and attenuates macrophage proliferation. EMBO J., 18, 3616–3628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levkowitz G., Waterman,H., Zamir,E., Kam,Z., Oved,S., Langdon,W.Y., Beguinot,L., Geiger,B. and Yarden,Y. (1998) c-Cbl/Sli-1 regulates endocytic sorting and ubiquitination of the epidermal growth factor receptor. Genes Dev., 12, 3663–3674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levkowitz G. et al. (1999) Ubiquitin ligase activity and tyrosine phosphorylation underlie suppression of growth factor signaling by c-Cbl/Sli-1. Mol. Cell, 4, 1029–1040. [DOI] [PubMed] [Google Scholar]
- Odin J.A., Edberg,J.C., Painter,C.J., Kimberly,R.P. and Unkeless,J.C. (1991) Regulation of phagocytosis and [Ca2+]i flux by distinct regions of an Fc receptor. Science, 254, 1785–1788. [DOI] [PubMed] [Google Scholar]
- Ota Y. and Samelson,L.E. (1997) The product of the proto-oncogene c-cbl: a negative regulator of the Syk tyrosine kinase. Science, 276, 418–420. [DOI] [PubMed] [Google Scholar]
- Paolini R. and Kinet,J.P. (1993) Cell surface control of the multiubiquitination and deubiquitination of high-affinity immunoglobulin E receptors. EMBO J., 12, 779–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickart C.M. (2000) Ubiquitin in chains. Trends Biochem. Sci., 25, 544–548. [DOI] [PubMed] [Google Scholar]
- Reggiori F. and Pelham,H.R. (2001) Sorting of proteins into multivesicular bodies: ubiquitin-dependent and -independent targeting. EMBO J., 20, 5176–5186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocca A., Lamaze,C., Subtil,A. and Dautry-Varsat,A. (2001) Involvement of the ubiquitin/proteasome system in sorting of the interleukin 2 receptor β chain to late endocytic compartments. Mol. Biol. Cell, 12, 1293–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock K.L. and Goldberg,A.L. (1999) Degradation of cell proteins and the generation of MHC class I-presented peptides. Annu. Rev. Immunol., 17, 739–779. [DOI] [PubMed] [Google Scholar]
- Rotin D., Staub,O. and Haguenauer-Tsapis,R. (2000) Ubiquitination and endocytosis of plasma membrane proteins: role of Nedd4/Rsp5p family of ubiquitin–protein ligases. J. Membr. Biol., 176, 1–17. [DOI] [PubMed] [Google Scholar]
- Saci A., Pain,S., Rendu,F. and Bachelot-Loza,C. (1999) Fc receptor-mediated platelet activation is dependent on phosphatidylinositol 3-kinase activation and involves p120(Cbl). J. Biol. Chem., 274, 1898–1904. [DOI] [PubMed] [Google Scholar]
- Schmid S.L. (1997) Clathrin-coated vesicle formation and protein sorting: an integrated process. Annu. Rev. Biochem., 66, 511–548. [DOI] [PubMed] [Google Scholar]
- Staub O., Gautschi,I., Ishikawa,T., Breitschopf,K., Ciechanover,A., Schild,L. and Rotin,D. (1997) Regulation of stability and function of the epithelial Na+ channel (ENaC) by ubiquitination. EMBO J., 16, 6325–6336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strous G.J. and Govers,R. (1999) The ubiquitin–proteasome system and endocytosis. J. Cell Sci., 112, 1417–1423. [DOI] [PubMed] [Google Scholar]
- Strous G.J., van Kerkhof,P., Govers,R., Ciechanover,A. and Schwartz,A.L. (1996) The ubiquitin conjugation system is required for ligand-induced endocytosis and degradation of the growth hormone receptor. EMBO J., 15, 3806–3812. [PMC free article] [PubMed] [Google Scholar]
- Tjelle T.E., Lovdal,T. and Berg,T. (2000) Phagosome dynamics and function. BioEssays, 22, 255–263. [DOI] [PubMed] [Google Scholar]
- Treier M., Staszewski,L.M. and Bohmann,D. (1994) Ubiquitin-dependent c-Jun degradation in vivo is mediated by the delta domain. Cell, 78, 787–798. [DOI] [PubMed] [Google Scholar]
- Ukkonen P., Lewis,V., Marsh,M., Helenius,A. and Mellman,I. (1986) Transport of macrophage Fc receptors and Fc receptor-bound ligands to lysosomes. J. Exp. Med., 163, 952–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kerkhof P., Govers,R., Alves dos Santos,C.M. and Strous,G.J. (2000) Endocytosis and degradation of the growth hormone receptor are proteasome-dependent. J. Biol. Chem., 275, 1575–1580. [DOI] [PubMed] [Google Scholar]
- van Kerkhof P., dos Santos,C.M., Sachse,M., Klumperman,J., Bu,G. and Strous,G.J. (2001) Proteasome inhibitors block a late step in lysosomal transport of selected membrane but not soluble proteins. Mol. Biol. Cell, 12, 2556–2566. [DOI] [PMC free article] [PubMed] [Google Scholar]