Abstract
Background
Robust evidence supports the use of preemptive non-invasive ventilation (NIV) after extubation in selected high-risk patient cohorts. In contrast, current guidelines discourage the use of NIV as a rescue therapy for respiratory failure that develops later after extubation, based on earlier studies indicating a potential increase in hospital mortality due to delayed reintubation. Nonetheless, NIV continues to be employed in this setting. We conducted a post-hoc analysis of a randomized trial to assess the clinical outcomes of rescue NIV for post-extubation respiratory failure.
Methods
In this post-hoc analysis of a randomized trial comparing high-flow with Venturi mask oxygen in hypoxemic patients after extubation, we included those who developed post-extubation respiratory failure according to prespecified criteria; patients who received rescue NIV per physician’s decision were compared to those who received direct re-intubation. Criteria for re-intubation during NIV were prespecified. Odds ratio after inverse probability of treatment weighting and posterior probabilities by Bayesian regression are reported.
Results
Among 494 extubated patients, 147 developed respiratory failure while receiving oxygen therapy, occurring at a median of 37 h [IQR 13–85] after extubation: 83 (57%) were treated with rescue NIV and 64 (43%) received immediate re-intubation. The rate of NIV failure was 58%, without differences between patients with hypoxemic respiratory failure and those with hypercapnia and/or respiratory distress (60% vs. 56%, p = 0.82). In the weighted cohort, the use of rescue NIV, compared to direct re-intubation, was associated with lower intensive care unit mortality (adjusted odds ratio = 0.31 [95%CI: 0.12–0.82], p = 0.019) and similar hospital mortality (adjusted odds ratio = 1.01 [95%CI: 0.43–2.33], p = 0.99). The posterior probability that NIV reduced intensive care unit mortality was > 90% across all priors. The posterior probability that NIV did not increase hospital mortality was 44% under a noninformative prior, 47% under a skeptical prior, and 39% under a pessimistic prior.
Conclusion
Rescue NIV for post-extubation respiratory failure is associated with high failure rates; however, when applied with well-defined criteria for reintubation, it does not appear to be clearly associated with increases in hospital mortality. A randomized trial to re-evaluate the efficacy of rescue NIV for post-extubation respiratory failure is warranted.
Clinical trial registration
Registered at clinicaltrials.gov (NCT02107183) on April 8th, 2014.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13054-025-05689-w.
Keywords: Weaning, Noninvasive ventilation, Post-extubation respiratory failure
Background
Non-invasive ventilation (NIV) is widely used to prevent and treat respiratory failure in critically ill patients. When applied immediately after extubation in selected patients such as those with chronic obstructive pulmonary disease, cardiac insufficiency, obesity, or persistent weaning failure, the benefits of NIV are well established [1–4]. This strategy (i.e. ‘prophylactic’ NIV) reduces the likelihood of re-intubation, shortens intensive care unit stay, and improves outcomes by preventing post-extubation respiratory failure in patients with high risk [5–11].
In contrast, the role of NIV as a ‘rescue’ strategy for post-extubation acute respiratory failure, which develops after initial stability, remains unclear. Unlike prophylactic NIV, this approach is used to treat patients who initially appear stable but subsequently experience deterioration, which may occur in up to 25% of extubated patients [12]. Data supporting the rescue use of NIV are limited, and concerns exist regarding its safety [13]. A pivotal study by Esteban explored the efficacy of rescue NIV compared to conventional oxygen therapy in patients who developed respiratory failure notably after extubation. The results revealed that rescue NIV did not reduce re-intubation rates, and more importantly it was associated with increased mortality [14]. This was attributed to result from a high rate of treatment failure (up to 50%) and delayed intubation [15–19]. Guidelines suggest that NIV should not be used to treat acute respiratory failure that develops notably after extubation in critically ill patients [20]. Nevertheless, in clinical practice, NIV continues to be used in a considerable proportion of patients for this indication, despite the outcomes remaining uncertain. The lack of robust evidence supporting this practice underscores the need for further research to better understand the risks and benefits of NIV.
A recent multicenter randomized trial investigated the use of high-flow nasal oxygen compared to oxygen therapy using a Venturi mask in patients with hypoxemia after extubation. In this trial, the use of NIV was allowed as a rescue strategy in cases of assigned treatment failure, based on the decisions of physicians. NIV has been used as a rescue strategy in a marked proportion of patients [21]. We conducted a post-hoc analysis of this trial to assess the outcomes of rescue NIV in patients who developed acute respiratory failure after extubation. Specifically, we aimed to evaluate whether the association between rescue NIV and increased hospital mortality remains valid in a more modern critical care setting using a pre-specified protocol.
Methods
Study design
This study is a post-hoc analysis of a randomized trial comparing high-flow nasal oxygen with Venturi mask therapy in patients with hypoxemia immediately after extubation in the intensive care unit. This trial was an investigator-initiated, multicenter, randomized, two-arm, open-label study conducted between June 2014 and October 2016 in 13 intensive care unit across Europe [21]. The institutional review board of the coordinator center (Catholic University of The Sacred Heart, Fondazione Policlinico Universitario A. Gemelli IRCCS, Rome, Italy) reviewed and approved the study protocol before trial initiation (n. 12634/13 on December 5th, 2013). By in-site beginning of enrolment, each local ethics committee reviewed and approved the study protocol. All enrolled patients provided written informed consent in accordance with committee recommendations. The study protocol was registered at clinicaltrials.gov (NCT02107183) on April 8th, 2014.
The current analysis focused on a subset of patients from the RINO trial cohort who developed post-extubation acute respiratory failure requiring escalation of respiratory support, provided in the form of either rescue NIV or re-intubation. The primary aim of this post-hoc analysis was to assess clinical outcomes, focusing on the rates of intensive care unit and hospital mortality. No additional patient consent was required for the retrospective review of de-identified data.
Study population
The participants in this analysis were drawn from the original trial population, which included 494 critically ill adult patients who had been intubated and mechanically ventilated for at least 48 hours and extubated following a successful spontaneous breathing trial. Patients in the original trial were included if they developed hypoxemia within 120 minutes after extubation (defined as a PaO2/FiO2 ratio≤300 mmHg or an SpO2/FiO2 ratio≤300% while receiving Venturi mask oxygen). The enrolled patients in the trial were randomized to receive either high-flow or Venturi mask oxygen therapy. Patients who were candidates for prophylactic NIV after extubation according to the pre-specified criteria were not included in the trial. Patients with tracheostomy and pregnant women were excluded from this study.
Throughout the study, clinical teams monitored the patients for signs of respiratory distress, including hypoxemia and hypercapnia, to assess the need for additional interventions. For the post-hoc analysis, we included only those patients who developed post-extubation acute respiratory failure requiring escalation of respiratory support according to predefined criteria. Patients in both treatment groups received rescue NIV or invasive mechanical ventilation based on the predefined clinical criteria if they exhibited signs of respiratory failure; the final decision to apply rescue NIV vs. reintubation was left to the discretion of the attending physician. The possibility of applying rescue NIV was defined by the presence of two or more of the following criteria:
respiratory acidosis, defined as an arterial pH below 7.35 with PaCO2 > 45 mm Hg;
clinical signs suggestive of respiratory muscle fatigue or increased respiratory effort (i.e., use of accessory muscles, intercostal indrawing, or paradoxical motion of the abdomen);
respiratory rate > 35 breaths/min for at least one hour;
hypoxemia (defined as SpO2 < 90% or PaO2 < 80 mmHg with FiO2 > 50%).
NIV was delivered using a facemask in the pressure-support mode. Positive end-expiratory pressure and pressure support settings were selected by the attending physician, allowing flexibility in response to the evolving clinical status of the patient. However, the suggested settings were a positive end-expiratory pressure of 5–7 cmH2O, and pressure support to achieve a tidal volume of 6–8 mL per kilogram of predicted body weight (up to 12 mL per kilogram in case of respiratory acidosis) but not exceeding 16 cmH2O. The use of both heated moisture exchangers and heated humidifiers was allowed.
In all patients, the final decision to intubate was based predefined criteria, that included the presence of unbearable dyspnea and at least one of the following [22–24]:
hypercapnia with respiratory acidosis (arterial pH ≤ 7.25 with PaCO2 > 45 mmHg);
changes in mental status, making nursing care impossible and requiring sedation;
SpO2 < 85% or PaO2 < 45 mmHg despite oxygen therapy with FiO2 > 50%;
hypotension, with a systolic blood pressure < 70 mmHg for more than 30 min despite fluid resuscitation and/or the use of vasopressors.
copious secretions that were not adequately cleared or were associated with acidosis, hypoxemia, or changes in mental status.
Measurements
All data were recorded in an electronic case report form and managed using a centralized web system (Ferrario Dati, Rome, Italy). Patient demographics were collected at the beginning of the study along with the simplified acute physiologic score II, main comorbidities, cause and length of intensive care unit stay, the reason and duration of invasive mechanical ventilation, the SOFA score on the day of enrollment, physiological parameters certifying successful spontaneous breathing trial, and the oxygenation criteria needed for inclusion in the study. Patients were followed up until hospital discharge. The reasons for NIV and eventual endotracheal re-intubation were recorded. Medical records were reviewed to determine hospital mortality rates. For this analysis, rescue NIV success was defined as a patient who received NIV, was not re-intubated, and was discharged alive from the intensive care unit to a medical or surgical ward. NIV failure was defined as any other condition that required patient re-intubation or death without re-intubation before intensive care unit discharge.
Outcomes
The primary outcome of this post-hoc analysis was hospital and intensive care unit mortality in patients who received NIV versus direct re-intubation as the initial treatment for post-extubation respiratory failure. Secondary outcomes included the duration of intensive care unit and hospital length of stay, the rate of NIV success and failure, that is re-intubation, and the rates of NIV failure in patients who received this treatment for post-extubation respiratory failure associated with hypoxemia vs. hypercapnia or respiratory distress.
Statistical analysis
Data from the original trial were extracted to identify the patients who received rescue NIV for post-extubation acute respiratory failure. Descriptive statistics were used to summarize baseline characteristics and frequency of NIV use. Categorical variables were compared using Chi-square or Fisher’s exact test, whereas continuous variables were analyzed using Student’s t-test or Wilcoxon sum of rank test, as appropriate. Kaplan-Meier survival curves were generated to compare intensive care unit and hospital mortalities between patients treated with rescue NIV and those who received re-intubation without rescue NIV. To account for confounding factors, we used the estimated propensity scores as weights in the inverse probability-weighting model. For this purpose, we estimated the propensity scores for the initial receipt of rescue NIV versus direct re-intubation, accounting for the following variables: age; body mass index; simplified acute physiology score II; SOFA score recorded on the day of extubation; presence of chronic cardiac insufficiency; presence of acute or chronic kidney failure; presence of chronic respiratory failure; acute brain injury as the main cause of mechanical ventilation; duration of mechanical ventilation before extubation; time elapsed from extubation and the development of post-extubation respiratory failure; whether the patient was receiving high-flow or Venturi mask oxygen before the occurrence of post-extubation respiratory failure; and the following conditions at the time of development of post-extubation respiratory failure: hypoxemic respiratory failure, altered mental status, shock, and altered secretion clearance. Among these factors, continuous variables were included as independent predictors in their original form (i.e., as raw, continuous values), assuming a linear relationship between each variable and the log-odds of receiving the treatment, and no transformations were applied to these continuous variables. Goodness of fit of the binary logistic model was assessed with Hosmer and Lemeshow Test. We then used the propensity scores to generate the inverse probability of the treatment weights. Finally, a binary logistic regression on intensive care unit and hospital mortality rates, and linear regression on intensive care unit and hospital length of stay were performed on the weighted cohort, adjusting for the study center; for this purpose, study centers were categorized into high and low volume centers according to whether the number of patients treated with rescue NIV was above or below the median. The same procedure was performed to compare patients who received intubation after rescue NIV failure and those who received re-intubation without rescue NIV, in order to assess whether patients who failed NIV can experience higher mortality than those who were directly intubated, as available literature suggests [14, 20]. To adjust for variance inflation from inverse probability of treatment weighting, all regression models were performed using SPSS’s Complex Samples module with a ‘with replacement’ design and Taylor series linearization to obtain design‐corrected confidence intervals. The results of these weighted analyses are reported as odds ratio (95% confidence intervals) and mean difference (95% confidence intervals), respectively.
To better assess the robustness of our findings under different assumptions, we performed a post-hoc Bayesian analysis to assess the efficacy (ICU mortality) and safety (hospital mortality) of rescue NIV compared with direct endotracheal re-intubation. We conducted a post-hoc Bayesian multivariable logistic regression to estimate the posterior probability of benefit of NIV (compared to direct reintubation) for both ICU and hospital mortality, defined as the probability of observing and odds ratio of less than 1. Models were fitted with death (ICU or hospital) as the primary outcome and NIV as the main predictor. Models were adjusted by the same potential confounder variables used to estimate the propensity scores for the inverse probability of treatment weighting analysis. A binomial distribution with a logit link was used. We employed three different priors in the analysis: noninformative (mean log odds 0, standard deviation 2.5), skeptical (mean log odds 0, standard deviation 1), and pessimistic (mean log odds 0.7, standard deviation 1). The pessimistic prior was selected based on a previous study by Esteban et al., which suggested harm associated with NIV (a relative risk of 1.78, corresponding to an odds ratio of approximately 2 and log odds of 0.7) [14]. All models were run with 100,000 iterations (with 50,000 iterations used for burn-in) and 4 chains. We obtained the posterior distributions of the treatment effects for each set of priors and outcomes, and finally, we plotted the probability distribution curves.
A p-value of ≤ 0.05 was considered statistically significant. Statistical analysis was performed using SPSS version 20.0 (IBM Corp. Released 2011. IBM SPSS Statistics for Windows, Version 26.0. Armonk, NY: IBM Corp) and manuscript figures were prepared using GraphPad Prism version 7.00.
Results
Among the 494 extubated and randomized patients, 147 (30%) developed post-extubation respiratory failure. Among them, 83 patients (57%) were initially treated with rescue NIV compared to 64 (43%) patients who received endotracheal re-intubation (n=64, 43%) (Fig. 1). The use of rescue NIV was common in all the centers involved in the trial (Supplementary figure 1). The demographics, most relevant clinical characteristics, and clinical outcomes of the study population are shown in Table 1 Respiratory failure requiring escalation of respiratory support occurred (median [interquartile range]) 37 [13–85] hours after extubation, without a significant difference between patients treated with rescue NIV and those treated with direct re-intubation (p=0.86) (Supplementary figure 2). NIV was administered because of hypoxemic respiratory failure in 45 patients (54% of the patients who received rescue NIV) and hypercapnia and/or respiratory distress without hypoxemia in 38 patients (46% of the patients who received rescue NIV). The use of rescue NIV rather than direct re-intubation was more frequent among patients previously supported with Venturi mask compared to those supported with high-flow nasal oxygen (64% vs 47%, respectively; p=0.045). However, the time from initiation of oxygen therapy to the diagnosis of post-extubation respiratory failure did not differ significantly between the two groups, with a median of 32 [13–85] in the Venturi mask group and 43 [13–86] hours in the high-flow group (p=0.78); this suggests that this difference in rescue NIV use was not due to delayed recognition of clinical deterioration.
Fig. 1.
Study flow diagram
Table 1.
Characteristics of patients (original cohort) and clinical outcome of patients who experienced post-extubation respiratory failure, according to the use of NIV or endotracheal intubation as first-line treatment (data with p<0.05 are considered statistically different between groups)
| Characteristic |
All patients (n = 147) |
Rescue NIV (n = 83) |
Direct re-intubation (n = 64) |
p value |
|---|---|---|---|---|
| Age – years | 65 (56–73) | 66 (58–73) | 63 (54–72) | 0.10 |
| Female sex – no. (%) | 46 (31) | 26 (31) | 20 (31) | > 0.999 |
| Body Mass index§ | 26 (23–30) | 26 (23–31) | 25 (23–29) | 0.34 |
| SAPSII† | 40 (33–48) | 40 (34–53) | 40 (32–44) | 0.14 |
| SOFA at extubation | 5 (3–7) | 6 (3–8) | 4 (3–7) | 0.036 |
| Type of admission in the intensive care unit – no. (%) | ||||
| Medical | 98 (67) | 61 (74) | 37 (58) | 0.053 |
| Surgical/trauma | 49 (33) | 22 (26) | 27 (42) | 0.053 |
| Current smoking | 32 (22) | 20 (24) | 12 (19) | 0.54 |
| Comorbidities – no. (%) | ||||
| Cardiac failure | 20 (14) | 15 (18) | 5 (8) | 0.09 |
| Acute myocardial infarction | 9 (6) | 5 (6) | 4 (6) | > 0.999 |
| Renal failure | 18 (12) | 16 (19) | 2 (3) | 0.004 |
| Chronic respiratory failure | 23 (16) | 14 (17) | 9 (14) | 0.82 |
| Liver cirrhosis | 15 (10) | 9 (11) | 6 (9) | > 0.999 |
| HIV | 5 (3) | 3 (4) | 2 (3) | > 0.999 |
| Multiple transfusions | 4 (3) | 3 (4) | 1 (2) | 0.63 |
| Use of corticosteroids | 13 (9) | 8 (10) | 5 (8) | 0.78 |
| Chemotherapy | 8 (5) | 5 (6) | 3 (5) | > 0.999 |
| Initial reason for mechanical ventilation – no. (%) | ||||
| Acute respiratory failure | 79 (54) | 50 (60) | 29 (45) | 0.07 |
| Brain Injury/Altered consciousness | 22 (15) | 7 (8) | 15 (23) | 0.011 |
| Surgery | 25 (17) | 12 (15) | 13 (20) | 0.35 |
| Cardiac arrest | 6 (4) | 5 (6) | 1 (2) | 0.35 |
| Circulatory failure | 10 (7) | 7 (8) | 3 (5) | 0.57 |
| Trauma | 5 (3) | 2 (2) | 3 (5) | 0.77 |
| Duration of mechanical ventilation before extubation – days | 6 (3–10) | 6 (3–10) | 6 (4–9) | 0.84 |
| Arterial blood gases immediately after extubation | ||||
| pH | 7.45 (7.42–7.48) | 7.46 (7.42–7.49) | 7.45 (7.42–7.48) | 0.07 |
| PaO2:FiO2 – mmHg | 219 (194–247) | 220 (194–248) | 217 (193–248) | 0.94 |
| PaCO2 – mmHg | 38 (32–43) | 38 (32–42) | 38 (32–43) | 0.81 |
| Respiratory rate immediately after extubation, breaths/minute | 22 (18–25) | 22 (19–25) | 21 (18–26) | 0.55 |
| Time elapsed from extubation to post-extubation respiratory failure – hours | 37 (13–85) | 34 (12–74) | 39 (14–96) | 0.86 |
| Treatment received after extubation – no. (%) | ||||
| High-flow nasal oxygen | 66 (45) | 31 (37) | 35 (55) | 0.045 |
| VenturiMask oxygen | 81 (55) | 52 (63) | 29 (45) | 0.045 |
| Duration of intensive care unit stay before development of post-extubation respiratory failure – days | 9 (6–13) | 9 (5–15) | 9 (6–13) | 0.89 |
| Causes of post-extubation respiratory failure – no (%) | ||||
| Hypoxemia | 75 (51) | 51 (61) | 24 (38) | 0.007 |
| Hypercapnia | 23 (16) | 16 (19) | 7 (11) | 0.25 |
| Dyspnea/respiratory muscle fatigue | 123 (84) | 73 (88) | 50 (78) | 0.12 |
| Tachypnea | 82 (56) | 48 (58) | 34 (53) | 0.62 |
| Altered mental status | 24 (16) | 0 | 24 (38) | < 0.001 |
| Altered secretion clearance | 37 (25) | 0 | 37 (58) | < 0.001 |
| Circulatory failure/shock | 8 (5) | 0 | 8 (13) | 0.001 |
| Clinical outcome | ||||
| Intensive care unit mortality – no. (%) | 27 (18) | 10 (12) | 17 (27) | 0.032 |
| Duration of stay in the intensive care unit – days | 10 (5–19) | 9 (4–18) | 12 (7–20) | 0.58 |
| Hospital mortality – no. (%) | 49 (33) | 27 (33) | 22 (34) | 0.86 |
| Duration of stay in the hospital – days | 23 (12–47) | 24 (13–47) | 22 (11–47) | 0.85 |
| 90-day mortality – no. (%) | 45 (31) | 25 (30) | 20 (31) | > 0.999 |
* Data are expressed as median (interquartile range), if not otherwise specified
§ The body-mass index is the weight in kilograms divided by the square of the height in meters
† The Simplified Acute Physiology Score (SAPS) II was calculated from 17 variables at enrolment, information about previous health status and information obtained at admission. Scores range from 0 to 163, with higher scores indicating more severe disease
NIV outcome
Overall, NIV was successful in 35 (42%) patients and failed in 48 (58%). In failing patients, endotracheal re-intubation was performed 64 hours [95% confidence interval: 37–90] after NIV initiation. The demographics, clinical characteristics, and clinical outcomes of succeeding and failing patients are reported in Table 2; no patient-related factors were associated with the success or failure of rescue NIV. The rate of rescue NIV failure was 60% in patients who received NIV because of hypoxemic respiratory failure and 56% in patients who received NIV because of hypercapnia and/or respiratory distress without hypoxemia, a difference that was not statistically significant (p=0.82) (Fig. 2). Among patients who received NIV, the failure rate was 60% in those previously supported with Venturi mask and 55% in those previously supported with high-flow nasal oxygen (p=0.82), indicating no significant difference in NIV outcomes based on the preceding oxygenation strategy. Causes of re-intubation (direct and after NIV) are shown in Supplementary figure 3.
Table 2.
Characteristics of patients (original cohort) treated with first-line NIV for post-extubation respiratory failure, according to the occurrence of NIV success or failure (data with p<0.05 are considered statistically different between groups)
| Characteristic |
NIV success (n = 35) |
NIV failure (n = 48) |
p value |
|---|---|---|---|
| Age – years | 67 (56–71) | 66 (58–75) | 0.52 |
| Female sex – no. (%) | 10 (29) | 16 (33) | 0.81 |
| Body Mass index§ | 28 (23–31) | 26 (23–31) | 0.99 |
| SAPSII† | 41 (30–54) | 40 (34–49) | 0.78 |
| SOFA at extubation | 7 (3–8) | 5 (3–8) | 0.80 |
| Type of admission in the intensive care unit – no. (%) | |||
| Medical | 28 (80) | 33 (69) | 0.32 |
| Surgical/trauma | 7 (20) | 15 (31) | 0.32 |
| Current smoking | 12 (34) | 8 (17) | 0.07 |
| Comorbidities – no. (%) | |||
| Cardiac failure | 4 (11) | 11 (23) | 0.25 |
| Myocardial infarction | 3 (9) | 2 (4) | 0.65 |
| Renal failure | 5 (14) | 11 (23) | 0.41 |
| Chronic respiratory failure | 8 (23) | 6 (13) | 0.25 |
| Liver cirrhosis | 4 (11) | 5 (10) | > 0.999 |
| HIV | 1 (3) | 2 (4) | > 0.999 |
| Multiple transfusions | 3 (1) | 2 (4) | > 0.999 |
| Use of corticosteroids | 4 (11) | 4 (8) | 0.72 |
| Chemotherapy | 4 (11) | 1 (2) | 0.16 |
| Initial reason for mechanical ventilation – no. (%) | |||
| Acute respiratory failure | 22 (63) | 28 (58) | 0.17 |
| Brain Injury/Altered consciousness | 4 (11) | 3 (6) | 0.70 |
| Surgery | 4 (11) | 8 (17) | 0.50 |
| Cardiac arrest | 1 (3) | 4 (8) | 0.30 |
| Circulatory failure | 3 (9) | 4 (8) | 0.97 |
| Trauma | 1 (3) | 1 (2) | 0.82 |
| Duration of mechanical ventilation before extubation – days | 6 (3–9) | 5 (3–11) | 0.61 |
| Arterial blood gases immediately after extubation | |||
| pH | 7.45 (7.41–7.49) | 7.46 (7.42–7.49) | 0.73 |
| PaO2:FiO2 – mmHg | 219 (191–257) | 221 (201–239) | 0.81 |
| PaCO2 – mmHg | 38 (32–44) | 37 (33–42) | 0.65 |
| Respiratory rate immediately after extubation, breaths/minute | 22 (20–25) | 23 (18–25) | 0.92 |
| Time elapsed from extubation to post-extubation respiratory failure – hours | 34 (14–69) | 33 (9–91) | 0.30 |
| Duration of intensive care unit stay before development of post-extubation respiratory failure – days | 8 (5–13) | 9 (5–16) | 0.15 |
| Causes of post-extubation respiratory failure – no (%) | |||
| Hypoxemia | 19 (54) | 32 (67) | 0.27 |
| Hypercapnia | 5 (14) | 11 (23) | 0.41 |
| Dyspnea/respiratory muscle fatigue | 34 (97) | 31 (81) | 0.039 |
| Tachypnea | 20 (57) | 28 (58) | > 0.999 |
| Clinical outcome | |||
| Intensive care unit mortality, no (%) | 0 (0) | 10 (21) | 0.004 |
| Duration of stay in the intensive care unit – days | 5 (3–7) | 14 (9–26) | < 0.002 |
| Hospital mortality, no (%) | 8 (23) | 19 (40) | 0.16 |
| Duration of stay in the hospital – days | 19 (12–38) | 28 (17–50) | 0.42 |
| 90-day mortality, no (%) | 8 (23) | 17 (35) | 0.24 |
* Data are expressed as median (interquartile range), if not otherwise specified
§ The body-mass index is the weight in kilograms divided by the square of the height in meters
† The Simplified Acute Physiology Score (SAPS) II was calculated from 17 variables at enrolment, information about previous health status and information obtained at admission. Scores range from 0 to 163, with higher scores indicating more severe disease
Fig. 2.

Kaplan Meier tables depicting the cumulative proportion of patients experiencing rescue NIV failure in the intensive care unit
Clinical outcome
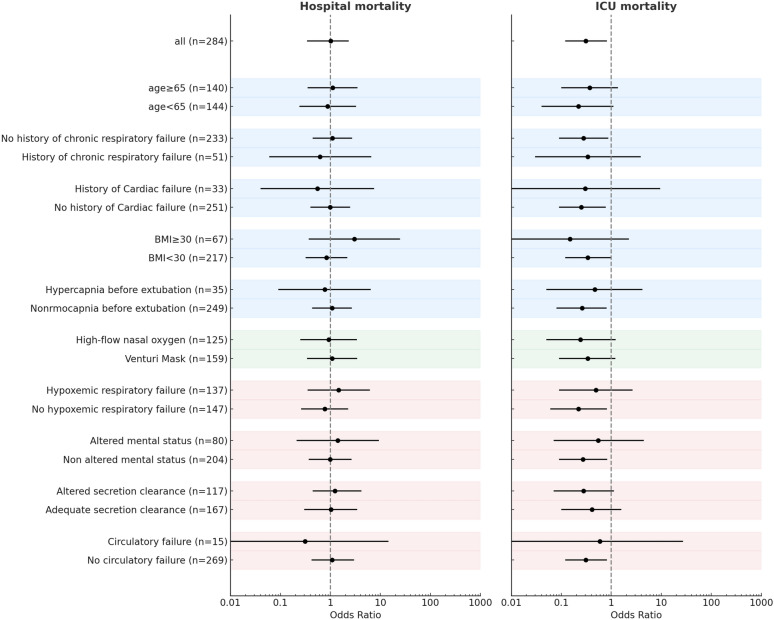
The results are displayed in Tables 1 and 2. In-intensive care unit mortality was 12% in patients who received rescue NIV and 27% in patients who were re-intubated without receiving NIV. Hospital mortality was 33% in patients who received rescue NIV and 34% in patients who were re-intubated without receiving NIV, (Fig. 3). The binary logistic regression model to calculate propensity scores for the initial receipt of rescue NIV vs. endotracheal re-intubation had acceptable goodness of fit (Hosmer-Lemeshow test p=0.16 – Supplementary material and Supplementary figure 4). After applying inverse probability of treatment weighting based on propensity scores, a weighted cohort of 284 patients was obtained: the demographics, most relevant clinical characteristics, and clinical outcomes of this cohort are shown in Supplementary Table 1. In the weighted cohort, the use of rescue NIV was associated with reduced intensive care unit mortality, with an adjusted odds ratio of 0.31 [95% confidence interval: 0.12–0.82] (p=0.019). Hospital mortality was not statistically different between patients who received rescue NIV vs. those who were directly re-intubated, with an adjusted odds ratio of 1.01 [95%CI: 0.43–2.33.43.33], p=0.99) (Fig. 3). In the logistic regression model, there was no evidence of interaction between treatment strategy (rescue NIV vs direct re-intubation) and prior oxygenation modality (high flow vs Venturi mask) on both intensive care unit hospital mortality (p=0.92). The effect of the interventions was consistent across all patient groups (all p for interaction>0.05) (Fig. 4).
Fig. 3.
Kaplan Meier tables depicting the cumulative proportion of patients discharged alive from the intensive care unit (left) and patients’ survival to hospital discharge (right)
Fig. 4.
Forest plots of hospital and intensive care unit mortality across predefined subgroups. Each point represents the odds ratio for mortality (hospital, left panel; intensive care unit, right panel) associated with rescue NIV versus direct reintubation, with 95% confidence intervals. Results were consistent across all subgroups, with all interaction p-values > 0.05. Shaded background colors indicate clinical domains: blue for patient-related baseline characteristics, green for post-extubation management strategy, and red for clinical features of post-extubation respiratory failure
Among patients who were discharged from the intensive care unit (n=120 in the original cohort), hospital mortality was 23% (17 out of 73 discharged patients) in patients who were discharged after having received rescue NIV (with or without subsequent re-intubation), and 11% (5 out of 47 discharged patients) in patients who were discharged after having received endotracheal re-intubation without rescue NIV (p=0.09).
The intensive care unit length of stay was not different between patients initially treated with NIV or endotracheal re-intubation, with an adjusted mean difference of 0 day (95% confidence interval: −6–7, p=0.89). The length of hospital stay did not differ between patients initially treated with NIV or endotracheal re-intubation, with an adjusted mean difference of 6 days (95% confidence interval: −9–20, p=0.41).
In re-intubated patients, hospital mortality was not significantly different between patients who were intubated after failing NIV and those who were intubated at the occurrence of post-extubation respiratory failure without receiving rescue NIV (40% vs. 34%), with an adjusted odds ratio for rescue NIV failure after inverse probability of treatment weighting of 1.30 [95% confidence interval: 0.50–3.38.50.38 (p=0.58) (Supplementary figure 5).
Posterior estimates
In the original cohort, under a noninformative prior, the posterior probability that rescue NIV reduced intensive care unit mortality compared to direct endotracheal re-intubation was estimated at 98%. When a skeptical prior was employed, the probability decreased to 97%, while a pessimistic prior led to a posterior probability of NIV efficacy of 93%. Under noninformative and skeptical priors, the probability that rescue NIV did not increase hospital mortality compared to direct endotracheal re-intubation was estimated at 44% and 47%, respectively. This decreased to 39% using a pessimistic prior (Fig. 5).
Fig. 5.
Probability distributions for the expected effect of rescue NIV for ICU and hospital mortality under noninformative (mean log odds 0, standard deviation 2.5), skeptical (mean log odds 0, standard deviation 1), and pessimistic (mean log odds 0.7, standard deviation 1) priors. The pessimistic prior was selected based on a previous study by Esteban et al., which suggested harm on hospital mortality by rescue NIV (a relative risk of 1.78, corresponding to an odds ratio of approximately 2 and log odds of 0.7) [14]
Discussion
The findings of this post-hoc analysis of a multicenter randomized trial on the post-extubation management of critically ill patients can be summarized as follows:
Rescue NIV was frequently used to treat post-extubation respiratory failure that occurs during oxygen therapy, regardless of whether the failure is because of hypoxemia or hypercapnia/respiratory muscle fatigue.
The failure rate of rescue NIV was approximately 60%, with no significant difference between the patients with hypoxemic respiratory failure and those with hypercapnia/respiratory distress. When applied using standardized criteria for both initiation and re-intubation, failure typically occurred within 2–3 days.
The use of rescue NIV instead of direct endotracheal re-intubation was associated to an increased likelihood of intensive care unit discharge; however, this finding should be interpreted with caution, as it does not necessarily translate into improved hospital survival.
Rescue NIV did not appear to be clearly associated with either harm or benefit in terms of hospital survival, highlighting the uncertainty of its impact on hospital mortality.
Robust evidence supports the use of NIV immediately after extubation in patients at high risk of weaning failure [5–11]. However, recent data clarifying the outcomes of rescue NIV for the treatment of respiratory failure that develops significantly after extubation are lacking. After the trial by Esteban et al. [14], the management of post-extubation respiratory failure is a rather poorly addressed topic in the field of respiratory critical care. In that study, the use of rescue NIV was associated with similar re-intubation and higher mortality rates compared to oxygen therapy alone. A subsequent meta-analysis confirmed the lack of benefits of rescue NIV in the management of post-extubation respiratory failure [25]. Hence, most recent guidelines recommend against the use of rescue NIV in this indication [20]. Nevertheless, it continues to be used in a barely quantifiable proportion of patients who develop notable respiratory failure after extubation, and this topic has gained renewed interest [12]. The findings of the present study provide a nuanced perspective on the role of rescue NIV in the management of post-extubation respiratory failure. When rescue NIV was applied using standardized criteria for both initiation and re-intubation, it was not clearly associated with increased hospital mortality. This differs from previous observations, such as those by Esteban et al. [14], whose results significantly shaped the most recent guideline recommendations against its routine use in this context [20]. Our results, therefore, suggest that, under more controlled and protocolized conditions, the use of rescue NIV may not yield the same detrimental impact on survival. In Esteban’s trial, most patients experienced post-extubation failure quite early (on average just 9 hours after extubation), likely due to unresolved respiratory failure. In our study, by contrast, respiratory failure occurred later (median >30 hours), pointing to a potential different clinical scenario. These discrepancies may also reflect broader changes in practice over time: current criteria for assessing extubation readiness and conducting spontaneous breathing trials may differ from those of early 2000s. As a result, the patients that are extubated today may differ significantly in terms of physiological reserve and risk profile, which could influence how they respond to rescue NIV in case of post-extubation respiratory failure. Also, while rescue NIV was permitted for all patients meeting pre-specified criteria, the decision to apply it ultimately rested with the treating clinician. It is possible that, being fully aware of the potential risks, clinicians selectively used rescue NIV in patients they believed had a high likelihood of success: this element of judgment of clinicians with expertise on the topic may have increased the perceived benefit in our study. Overall, while not definitive, these findings are intended to generate hypotheses and underscore the need for prospective trials to re-evaluate the safety and efficacy of rescue NIV in modern critical care settings.
Our data indicate that rescue NIV is associated with a high failure rate of approximately 60%, with no significant differences between patients with hypoxemia and those with hypercapnia/respiratory distress. This is consistent with a recent investigation [12], and suggests inherent challenges in using NIV to manage respiratory failure after extubation, where patients are already compromised, rather than its use as a preventive measure. Comparable failure rates in patients with hypoxemia and hypercapnia further indicate that rescue NIV may fail to meet different physiological needs in a considerable proportion of patients [13]. In addition, it suggests that the definition of post-extubation respiratory failure based on gas exchange is not the best way to track pathophysiological differences, if any, in this group of patients and may ultimately indicate that post-extubation respiratory failure may have distinct pathophysiological mechanisms than de novo acute and hypercapnic respiratory failures, warranting further investigation.
In our study, the choice of rescue NIV was higher use in patients previously treated with Venturi mask than in those treated with high-flow nasal oxygen. Despite theoretical concerns [26], high-flow nasal oxygen was not associated with delayed recognition of respiratory failure, as timing from extubation to diagnosis of post-extubation respiratory failure was similar between groups. This may also be since predefined, protocol-based criteria were systematically used to identify post-extubation respiratory failure. Our findings suggest that clinical perception of high-flow nasal oxygen as a more advanced support may reduce the likelihood of escalation to NIV [27, 28], as previously reported in the original trial [29]. Importantly, in our trial, there was no interaction between the type of prior oxygenation support (high-flow or Venturi mask) and either the clinical outcome of rescue NIV or its effect on hospital mortality, suggesting that the observed treatment effects are consistent regardless of the preceding support strategy.
Our findings revealed that rescue NIV may facilitate discharge from the intensive care unit by avoiding intubation in some patients. However, this result must be interpreted with caution as it does not directly translate into improved survival after hospital discharge. This discrepancy in clinical outcomes is attributable to the fact that some patients who initially respond positively to rescue NIV and are subsequently discharged from the intensive care unit, may eventually experience further complications in the hospital ward. This sequence emphasizes that, although NIV may provide temporary respiratory stability sufficient for intensive care unit discharge, it may not ensure sustained recovery once patients leave the close monitoring environment of the intensive care unit. These findings would yield inherent considerations for critical care teams. Although NIV can offer short-term benefits by alleviating respiratory distress, it may not address the underlying conditions that contribute to mortality later in the hospital stay. Indeed, if confirmed in future prospective randomized studies, the ability of rescue NIV to facilitate discharge from the intensive care unit may yield important implications for resource management. The judicious use of NIV with pre-specified failure criteria as a temporary respiratory support strategy may be a valuable tool for optimizing intensive care capacity and meeting the fluctuating demands of a high-acuity patient population [30]. However, the absence of a marked impact on the overall hospital mortality raises questions about the clinical scenarios in which rescue NIV should be employed over immediate re-intubation, particularly when considering the possible risks for certain patients once transferred from the intensive care unit.
Our Bayesian analysis confirmed a robust benefit of NIV on intensive care unit mortality, with posterior probabilities exceeding 90% for a reduction in intensive care unit mortality, regardless of the prior assumptions employed. In contrast, the analysis of hospital mortality revealed considerable uncertainty; under a neutral prior, the probability that NIV does not increase hospital mortality was only around 45%, with only modest shifts observed under both skeptical and pessimistic priors. This divergence suggests that, overall, the impact of rescue NIV on hospital mortality remains unclear. Unlike the 2004 findings of Esteban et al., who showed that rescue NIV for post-extubation respiratory failure could increase mortality [14], our results suggest that rescue NIV may not be directly associated with higher in-hospital mortality, even among patients who eventually require re-intubation. This difference in mortality outcomes compared with the findings of Esteban et al. (2004) may be explained by an evolving awareness within the critical care community regarding both the benefits and limitations of rescue NIV, leading to more judicious application [31, 32]. Over the past two decades, intensivists have become increasingly aware of the specific risks associated with delayed intubation in patients failing NIV, which has likely driven a more selective approach in its use [2, 7, 17, 19]. Additionally, advances in clinical protocols now allow for a more appropriate application of NIV, with prespecified criteria guiding clinicians on when to escalate to intubation in cases of NIV failure [22, 33, 34]. These criteria help ensure that patients who do not respond adequately to NIV are promptly intubated, potentially mitigating the increased mortality risk associated with delayed respiratory support, which likely contributed to worse outcomes in earlier studies. Collectively, these factors suggest that safer and more strategic NIV applications in recent years may have mitigated the detrimental effects of NIV failure on all-cause hospital mortality. However, with a pessimistic prior, which is consistent with available data on NIV effect on overall mortality, the posterior probability that NIV does not increase hospital mortality dropped to 39%. This confirms the high level of uncertainty of both our frequentist and Bayesian analyses regarding hospital mortality, which would underscore the need for prospective, randomized trials to definitively re-evaluate and eventually establish the efficacy and safety of NIV in this setting [35].
An additional insight from the analysis is that in experienced centers, rescue NIV failure generally occurs within 2–3 days. Although this time window was significantly longer than previous reports [14], NIV failure did not seem to affect patient survival. This provides a clinically relevant period for evaluating the effectiveness of NIV and deciding whether to continue or escalate intervention.
Our study has several limitations. First, this was a non-prespecified post hoc analysis of the original randomized controlled trial; hence, the results should be interpreted as hypothesis generating rather than conclusive; detailed physiological data at the time of respiratory failure were not available, preventing adjustment for the severity of illness at the moment of clinical deterioration. While we applied inverse probability of treatment weighting to account for measured baseline differences, unmeasured confounding cannot be excluded. Second, owing to the trial design, detailed information about patient-specific conditions at the start of NIV was lacking, limiting the ability to draw conclusions about specific patient populations. Third, data about ventilator settings and treatment duration were not available, hampering any analysis on risk factors related to NIV failure and success. Fourth, criteria for prophylactic NIV have been widened since the trial was conducted: it is possible that some patients who developed post-extubation respiratory failure in our cohort might have avoided progression to respiratory distress had they received earlier NIV intervention, thereby increasing the perceived benefit by NIV in this study. Finally, NIV was applied with pre-specified criteria for treatment initiation and defining failure in the context of a clinical trial, which may limit the generalizability of these findings. This underscores the critical role of accurate monitoring in the success of noninvasive treatments. This may also indicate that with the right protocols and oversight, which are detailed in this manuscript, experienced teams can use rescue NIV without significantly compromising patient safety. Although this would facilitate the reproducibility of our findings in other clinical settings, whether these results are applicable to real-life scenarios remains uncertain.
Conclusions
The use of rescue NIV for the treatment of post-extubation respiratory failure was associated with high failure rates. Overall, however, when applied in selected patients with well-defined failure criteria, it was not directly linked to clear increases in hospital mortality. A randomized trial to re-evaluate the efficacy of rescue NIV for post-extubation respiratory failure appears warranted.
Supplementary Information
Additional file 1: Figure 1. Use of rescue NIV compared to direct re-intubation in the 13 centers involved in the clinical trial. Figure 2. Kaplan Meier tables depicting the cumulative occurrence of post-extubation respiratory failure, according to whether patients were treated with rescue NIV or direct re-intubation. Figure 3. Distribution of re-intubation causes among patients who failed noninvasive ventilationversus those who were directly re-intubated without a trial of NIV. The chart shows the percentage of patients within each group for the most frequently reported clinical indications for re-intubation: hypoxemia, altered mental status, altered secretion clearance, circulatory failure/shock, hypercapnia, and NIV intolerance – those defined as predefined criteria in the original trial. Red bars represent patients in the NIV failure group; dark blue bars represent those in the direct re-intubation group. Figure 4 Love plot showing the standardized mean differencesfor each covariate before and after inverse probability of treatment weighting. Covariates are ordered by baseline imbalance. The vertical dashed line at 0.1 represents the conventional threshold for acceptable balance. Bars represent SMDs beforeand afterweighting. Please note that caution is warranted when interpreting SMDs for binary variables with null prevalence in one treatment group, as SMDs may underestimate imbalance in these settings. For this reason, absolute mean differences are reported in Supplementary Table 1. Figure 5. Kaplan Meier tables depicting patients’ survival to hospital discharge according to whether they belonged to the group of NIV failure, success or direct re-intubation
Acknowledgements
Acknowledgements This study was supported by Italian Ministry of Health (Ministero della Salute-Ricerca corrente 2025).
Rino trial study group (collaborative authorship): Daniele Natalini, Eleni Ischaki, Danielle Reuter, Indalecio Morán, Béatrice La Combe, Andrea De Gaetano, V. Marco Ranieri, Yassir Aarab, Elie Azoulay, Fouad Belafia, Guillaume Berquier, Matthieu Conseil, Andrea Costamagna, Laurence Dangers, Audrey De Jong, Julie Delemazure, Francesco Della Corte, Stèphane Gaudry, Francesca Grossi, Giovanni Carmine Iovino, Sylvain Jean-Baptiste, Mohammed Laissi, Romaric Larcher, Matthieu Le Meur, Clèment Leclaire, Paula Andrea Lopez, Sotirios M Malachias, Marilena Matteo, Julienne Mayaux, Jonathan Messika, Clement Monet, Elise Morawiec, Laurent Papazian, Francisco Parrilla, Laura Platon, Damien Roux, Maria Teresa Santantonio, Juan Carlos Suarez, Eloisa Sofia Tanzarella, Flavia Toni, Luca Salvatore Menga.
Abbreviation
- NIV
Noninvasive ventilation
Author contributions
DLG, MA and SMM designed the study. DLG conducted statistical analysis. DLG and SMM interpreted the data and wrote the first draft of the manuscript. All authors contributed to data acquisition and critically revised the manuscript. SMM organized the study as an overall supervisor. JM contributed significantly to conceptual design and study mentalization. All the authors reviewed the final draft of the manuscript and agreed on submitting it to Critical Care.
Funding
The study was funded by an unrestricted research grant by Fisher and Paykel Healthcare and was supported by Italian Ministry of Health (Ministero della Salute-Ricerca corrente 2025). Fisher and Paykel,Unrestricted research grant
Data availability
DLG and SMM takes responsibility for (is the guarantor of) the content of the manuscript, including the data and analysis. The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Human ethics and consent to participate
The institutional review board of the coordinator center (Catholic University of The Sacred Heart, Fondazione Policlinico Universitario A. Gemelli IRCCS, Rome, Italy) reviewed and approved the study protocol before trial initiation (n. 12634/13 on December 5th, 2013). By in-site beginning of enrolment, each local ethics committee reviewed and approved the study protocol. All enrolled patients provided written informed consent in accordance with committee recommendations.
Consent for publication
Not applicable.
Competing interests
DLG has received payments for travel expenses by Getinge, Draeger and Hamilton, research grants by Fisher and Paykel and GE, and non-financial support for research by Intersurgical, Dimar and Harol. SJ reports receiving consulting fees from Draeger, Medtronic, Baxter, Fresenius-Xenios, and Fisher & Paykel. A Demoule reports grants from the French Ministry of Health, Lungpacer, Respinor, Liberate Medical, consulting fees from Respinor, Liberate Medical, SAT Lutech, payment or honoraria for lectures, presentations from Fisher & Paykel, Astra, support for attending meetings and/or travel from Respinor, outside the submitted work JDR reports consulting fees, covering of travel expenses and equipment support by Fisher & Paykel. PN research laboratory has received equipment and grants from Draeger, Gilead and Intersurgical S.p.A. He also received honoraria/speaking fees from Gilead, GSK, Shionogi, Getinge, Mindray. He contributed to the development of the helmet Next, whose license for patent belongs to Intersurgical S.P.A. and receives royalties for that invention. SH reports speaking fees by MSD, Shionogi, Advanz Pharma Biomerieux. JPF reports having received grants from the French Ministry of Health, outside the submitted work; grants, personal fees and non-financial support from Fisher & Paykel HealthCare, outside the submitted work; personal fees and non-financial support from SOS Oxygène, outside the submitted work. VL belongs to a research group which received fees from Gilead, Pfizer, Alexion, Sanofi, MSD and personal fees and covering for travel expenses by Biomerieux. LB’S laboratory has received support for research by Covidien (PAV), Air Liquide (CPR), Philips (equipment for sleep), Fisher & Paykel (high flow therapy) and GE healthcare. MA has received payments for Board participation from Menarini, and Shionogi, and a research grant by General Electric Healthcare. And Fisher & Paykel SMM discloses having received speaking fees by GE Healthcare, Masimo, Getinge and Aspen, fees for advisory board participation by Sanofi, and a research grant, support for travel expenses and receipt of materials by Fisher and Paykel.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Domenico Luca Grieco, Email: domenicoluca.grieco@unicatt.it.
the RINO trial study group:
Daniele Natalini, Eleni Ischaki, Danielle Reuter, Indalecio Morán, Béatrice La Combe, Andrea De Gaetano, V. Marco Ranieri, Yassir Aarab, Elie Azoulay, Fouad Belafia, Guillaume Berquier, Matthieu Conseil, Andrea Costamagna, Laurence Dangers, Audrey De Jong, Julie Delemazure, Francesco Della Corte, Stèphane Gaudry, Francesca Grossi, Giovanni Carmine Iovino, Sylvain Jean-Baptiste, Mohammed Laissi, Romaric Larcher, Matthieu Le Meur, Clèment Leclaire, Paula Andrea Lopez, Sotirios M. Malachias, Marilena Matteo, Julienne Mayaux, Jonathan Messika, Clement Monet, Elise Morawiec, Laurent Papazian, Francisco Parrilla, Laura Platon, Damien Roux, Maria Teresa Santantonio, Juan Carlos Suarez, Eloisa Sofia Tanzarella, Flavia Toni, and Luca Salvatore Menga
References
- 1.Ferreyro BL, De Jong A, Grieco DL. How to use facemask noninvasive ventilation. Intensive Care Med. 2024;50:1346–9. [DOI] [PubMed] [Google Scholar]
- 2.Fernando SM, Tran A, Sadeghirad B, Burns KEA, Fan E, Brodie D, et al. Noninvasive respiratory support following extubation in critically ill adults: a systematic review and network meta-analysis. Intensive Care Med. 2022;48:137–47. [DOI] [PubMed] [Google Scholar]
- 3.Ferrer M, Esquinas A, Arancibia F, Bauer TT, Gonzalez G, Carrillo A, et al. Noninvasive ventilation during persistent weaning failure: a randomized controlled trial. Am J Respir Crit Care Med. 2003;168:70–6. [DOI] [PubMed] [Google Scholar]
- 4.Hernández G, Vaquero C, Colinas L, Cuena R, González P, Canabal A, et al. Effect of Postextubation High-Flow Nasal Cannula vs Noninvasive Ventilation on Reintubation and Postextubation Respiratory Failure in High-Risk Patients: A Randomized Clinical Trial. JAMA [Internet]. 2016 316 1565 74. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26975498 [DOI] [PubMed]
- 5.Thille AW, Coudroy R, Nay M-A, Gacouin A, Decavèle M, Sonneville R, et al. Beneficial Effects of Noninvasive Ventilation after Extubation in Obese or Overweight Patients: A Post Hoc Analysis of a Randomized Clinical Trial. Am J Respir Crit Care Med [Internet]. 2022 205 440 9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/34813391 [DOI] [PubMed]
- 6.Thille AW, Muller G, Gacouin A, Coudroy R, Decavèle M, Sonneville R, et al. Effect of postextubation high-flow nasal oxygen with noninvasive ventilation vs high-flow nasal oxygen alone on reintubation among patients at high risk of extubation failure: a randomized clinical trial. JAMA. 2019;322:1465–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hernández G, Paredes I, Moran F, Buj M, Colinas L, Rodríguez ML, et al. Effect of postextubation noninvasive ventilation with active humidification vs high-flow nasal cannula on reintubation in patients at very high risk for extubation failure: a randomized trial. Intensive Care Med. 2022;48:1751–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nava S, Ambrosino N, Clini E, Prato M, Orlando G, Vitacca M, et al. Noninvasive mechanical ventilation in the weaning of patients with respiratory failure due to chronic obstructive pulmonary disease: a randomized, controlled trial. Ann Intern Med. 1998;128:721–8. [DOI] [PubMed] [Google Scholar]
- 9.Nava S, Gregoretti C, Fanfulla F, Squadrone E, Grassi M, Carlucci A, et al. Noninvasive ventilation to prevent respiratory failure after extubation in high-risk patients. Crit Care Med. 2005;33:2465–70. [DOI] [PubMed] [Google Scholar]
- 10.De Jong A, Bignon A, Stephan F, Godet T, Constantin J-M, Asehnoune K, et al. Effect of non-invasive ventilation after extubation in critically ill patients with obesity in France: a multicentre, unblinded, pragmatic randomised clinical trial. Lancet Respir Med. 2023;11:530–9. [DOI] [PubMed] [Google Scholar]
- 11.Rochwerg B, Einav S, Chaudhuri D, Mancebo J, Mauri T, Helviz Y, et al. The role for high flow nasal cannula as a respiratory support strategy in adults: a clinical practice guideline. Intensive Care Med. 2020;46:2226–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thille AW, Monseau G, Coudroy R, Nay M-A, Gacouin A, Decavèle M, et al. Non-invasive ventilation versus high-flow nasal oxygen for postextubation respiratory failure in ICU: a post-hoc analysis of a randomized clinical trial. Crit Care. 2021;25:221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keenan SP, Powers C, McCormack DG, Block G. Noninvasive positive-pressure ventilation for postextubation respiratory distress: A randomized controlled trial. JAMA. 2002;287:3238–44. [DOI] [PubMed] [Google Scholar]
- 14.Esteban A, Frutos-Vivar F, Ferguson ND, Arabi Y, Apezteguía C, González M, et al. Noninvasive positive-pressure ventilation for respiratory failure after extubation. N Engl J Med. 2004;350:2452–60. [DOI] [PubMed] [Google Scholar]
- 15.Carrillo A, Gonzalez-Diaz G, Ferrer M, Martinez-Quintana ME, Lopez-Martinez A, Llamas N, et al. Non-invasive ventilation in community-acquired pneumonia and severe acute respiratory failure. Intensive Care Med. 2012;38:458–66. [DOI] [PubMed] [Google Scholar]
- 16.Bellani G, Laffey JG, Pham T, Madotto F, Fan E, Brochard L, et al. Noninvasive ventilation of patients with acute respiratory distress syndrome. Insights from the LUNG SAFE study. Am J Respir Crit Care Med. 2017;195:67–77. [DOI] [PubMed] [Google Scholar]
- 17.Yoshida T, Fujino Y, Amato MBP, Kavanagh BP. Fifty years of research in ARDS. Spontaneous breathing during mechanical ventilation. Risks, mechanisms, and management. Am J Respir Crit Care Med. 2017;195:985–92. [DOI] [PubMed] [Google Scholar]
- 18.Grieco DL, Menga LS, Eleuteri D. Patient self-inflicted lung injury: Implications for acute hypoxemic respiratory failure and ARDS patients on non-invasive support. Minerva Anestesiol. 2019;85:1014–23 (Antonelli M.). [DOI] [PubMed] [Google Scholar]
- 19.Brochard L, Slutsky A, Pesenti A. Mechanical ventilation to minimize progression of lung injury in acute respiratory failure. Am J Respir Crit Care Med. 2017;195:438–42. [DOI] [PubMed] [Google Scholar]
- 20.Rochwerg B, Brochard L, Elliott MW, Hess D, Hill NS, Nava S, et al. Official ERS/ATS clinical practice guidelines: noninvasive ventilation for acute respiratory failure. Eur Respir J. 2017. 10.1183/13993003.02426-2016. [DOI] [PubMed] [Google Scholar]
- 21.Maggiore SM, Jaber S, Grieco DL, Mancebo J, Zakynthinos S, Demoule A, et al. High-flow versus venturimask oxygen therapy to prevent reintubation in hypoxemic patients after extubation: a multicenter randomized clinical trial. Am J Respir Crit Care Med. 2022;206:1452–62. [DOI] [PubMed] [Google Scholar]
- 22.Frat J-P, Thille AW, Mercat A, Girault C, Ragot S, Perbet S, et al. High-flow oxygen through nasal cannula in acute hypoxemic respiratory failure. N Engl J Med [Internet]. 2015;372:2185–96. 10.1056/NEJMoa1503326. [DOI] [PubMed] [Google Scholar]
- 23.Patel BK, Wolfe KS, Pohlman AS, Hall JB, Kress JP. Effect of noninvasive ventilation delivered by helmet vs face mask on the rate of endotracheal intubation in patients with acute respiratory distress syndrome: a randomized clinical trial. JAMA. 2016;315:2435–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Antonelli M, Conti G, Rocco M, Bufi M, De Blasi RA, Vivino G, et al. A comparison of noninvasive positive-pressure ventilation and conventional mechanical ventilation in patients with acute respiratory failure. N Engl J Med. 1998;339:429–35. [DOI] [PubMed] [Google Scholar]
- 25.Lin C, Yu H, Fan H, Li Z. The efficacy of noninvasive ventilation in managing postextubation respiratory failure: A meta-analysis. Heart Lung. 2014;43:99–104. [DOI] [PubMed] [Google Scholar]
- 26.Kang BJ, Koh Y, Lim C-M, Huh JW, Baek S, Han M, et al. Failure of high-flow nasal cannula therapy may delay intubation and increase mortality. Intensive Care Med. 2015;41:623–32. 10.1007/s00134-015-3693-5. [DOI] [PubMed] [Google Scholar]
- 27.Frat J-P, Grieco DL, De Jong A, Gibbs K, Carteaux G, Roca O, et al. Noninvasive respiratory supports in ICU. Intensive Care Med. 2025;51:1476–89. 10.1007/s00134-025-08036-3. [DOI] [PubMed] [Google Scholar]
- 28.Maggiore SM, Grieco DL, Lemiale V. The use of high-flow nasal oxygen. Intensive Care Med [Internet]. 2023;33:65–72. 10.1007/s00134-023-07067-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maggiore SM, Jaber S, Grieco DL, Mancebo J, Zakynthinos S, Demoule A, et al. High-Flow Versus VenturiMask Oxygen Therapy to Prevent Reintubation in Hypoxemic Patients after Extubation: A Multicenter Randomized Clinical Trial. Am J Respir Crit Care Med [Internet]. 2022 206:1452–62. Available from: http://www.ncbi.nlm.nih.gov/pubmed/35849787 [DOI] [PubMed]
- 30.Menga LS, Berardi C, Ruggiero E, Grieco DL, Antonelli M. Noninvasive respiratory support for acute respiratory failure due to COVID-19. Curr Opin Crit Care. 2022;28:25–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Demoule A, Chevret S, Carlucci A, Kouatchet A, Jaber S, Meziani F, et al. Changing use of noninvasive ventilation in critically ill patients: trends over 15 years in francophone countries. Intensive Care Med. 2016;42:82–92. [DOI] [PubMed] [Google Scholar]
- 32.Demoule A, Girou E, Richard J-C, Taille S, Brochard L. Benefits and risks of success or failure of noninvasive ventilation. Intensive Care Med. 2006;32:1756–65. [DOI] [PubMed] [Google Scholar]
- 33.Grieco DL, Menga LS, Cesarano M, Spadaro S, Bitondo MM, Berardi C, et al. Phenotypes of patients with COVID-19 who have a positive clinical response to helmet noninvasive ventilation. Am J Respir Crit Care Med. 2022;205:360–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grieco DL, Menga LS, Cesarano M, Rosà T, Spadaro S, Bitondo MM, et al. Effect of helmet noninvasive ventilation vs high-flow nasal oxygen on days free of respiratory support in patients with COVID-19 and moderate to severe hypoxemic respiratory failure: the HENIVOT randomized clinical trial. JAMA. 2021;325:1731–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cutuli SL, Grieco DL, Michi T, Cesarano M, Rosà T, Pintaudi G, et al. Personalized respiratory support in ARDS: a physiology-to-bedside review. J Clin Med. 2023;12:4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Figure 1. Use of rescue NIV compared to direct re-intubation in the 13 centers involved in the clinical trial. Figure 2. Kaplan Meier tables depicting the cumulative occurrence of post-extubation respiratory failure, according to whether patients were treated with rescue NIV or direct re-intubation. Figure 3. Distribution of re-intubation causes among patients who failed noninvasive ventilationversus those who were directly re-intubated without a trial of NIV. The chart shows the percentage of patients within each group for the most frequently reported clinical indications for re-intubation: hypoxemia, altered mental status, altered secretion clearance, circulatory failure/shock, hypercapnia, and NIV intolerance – those defined as predefined criteria in the original trial. Red bars represent patients in the NIV failure group; dark blue bars represent those in the direct re-intubation group. Figure 4 Love plot showing the standardized mean differencesfor each covariate before and after inverse probability of treatment weighting. Covariates are ordered by baseline imbalance. The vertical dashed line at 0.1 represents the conventional threshold for acceptable balance. Bars represent SMDs beforeand afterweighting. Please note that caution is warranted when interpreting SMDs for binary variables with null prevalence in one treatment group, as SMDs may underestimate imbalance in these settings. For this reason, absolute mean differences are reported in Supplementary Table 1. Figure 5. Kaplan Meier tables depicting patients’ survival to hospital discharge according to whether they belonged to the group of NIV failure, success or direct re-intubation
Data Availability Statement
DLG and SMM takes responsibility for (is the guarantor of) the content of the manuscript, including the data and analysis. The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.