Abstract
In cultured rat dorsal root ganglia neurons, we measured membrane currents, using the patch–clamp whole-cell technique, and the concentrations of free Ca2+ in the cytosol ([Ca2+]i) and in the lumen of the endoplasmic reticulum (ER) ([Ca2+]L), using high- (Fluo-3) and low- (Mag-Fura-2) affinity Ca2+-sensitive fluorescent probes and video imaging. Resting [Ca2+]L concentration varied between 60 and 270 µM. Activation of ryanodine receptors by caffeine triggered a rapid fall in [Ca2+]L levels, which amounted to only 40–50% of the resting [Ca2+]L value. Using electrophysiological depolarization, we directly demonstrate the process of Ca2+-induced Ca2+ release triggered by Ca2+ entry through voltage-gated Ca2+ channels. The amplitude of Ca2+ release from the ER lumen was linearly dependent on ICa.
Keywords: Ca2+-induced Ca2+ release/Ca2+ signalling/Ca2+ stores/endoplasmic reticulum/sensory neurons
Introduction
The endoplasmic reticulum (ER) is an essential intracellular organelle, which also serves as a central intracellular Ca2+ store. The ER membrane forms an intracellular excitable medium, which can produce both synchronous Ca2+ release and propagating Ca2+ waves. The release of Ca2+ from the ER store upon stimulation regulates a variety of cellular events and processes, including excitation–concentration coupling in muscle (Niggli, 1999), excitation–secretion coupling and Ca2+ oscillations in secretory cells (Maruyama et al., 1993; Thorn et al., 1993), regulation of gene expression (Hardingham, 2001), neuronal plasticity (Frenguelli et al., 1996; Rose and Konnerth, 2001), neurotransmitter release and exocytosis (Zucker, 1996), and fertilization signals in oocytes (Galione et al., 1991). In most instances, the release of Ca2+ from the ER follows the generation of a signal at the plasma membrane level: either the G-protein-mediated increase in inositol 1,4,5-trisphosphate production (Berridge, 1993; Petersen et al., 1994) or the entry of Ca2+ through several types of Ca2+-permeable ionic channels (Verkhratsky and Shmigol, 1996).
These second messengers interact with Ca2+ release channels of the ER, triggering Ca2+ release from the ER lumen (Bezprozvanny et al., 1991; Berridge, 1993). The Ca2+-induced Ca2+ release (CICR) is triggered by Ca2+ ions entering the cytoplasm via voltage/ligand-operated plasmalemmal Ca2+ channels and interacting with Ca2+-gated Ca2+ channels (ryanodine receptors; RyRs) present in the ER membrane. The CICR is believed to be significantly involved in shaping neuronal [Ca2+]i transients (Friel and Tsien, 1992; Marrion and Adams, 1992; Hua et al., 1993; Usachev et al., 1993; Llano et al., 1994; Kano et al., 1995; Shmigol et al., 1995; Verkhratsky and Shmigol, 1996) and numerous studies propose that CICR is important for neuronal functions including synaptic transmission and plasticity (Alford et al., 1993; Garaschuk et al., 1997; Emptage et al., 1999; Krizaj et al., 1999; Rose and Konnerth, 2001). Although cytoplasmic Ca2+ recordings from various types of neurons strongly suggest the existence of CICR in physiological conditions, direct demonstration of Ca2+ release activated by Ca2+ entry through plasmalemmal Ca2+ channels has not been achieved. Such a direct demonstration requires real-time simultaneous monitoring of intraluminal free Ca2+ dynamics and transmembrane Ca2+ currents.
The technique for real-time visualization of intraluminal Ca2+ concentration ([Ca2+]L) at a single cell level has been perfected by several groups working with non-excitable cells (Tse et al., 1994; Chatton et al., 1995; Hofer and Schulz, 1996; Park et al., 1999, 2000). Direct measurements of [Ca2+]L are achieved by using low-affinity Ca2+ dyes trapped within the ER; the cytoplasmic portion of the dye is removed by either permeabilization of plasmalemma or by washing it out through the micropipette under a whole-cell patch–clamp configuration. The latter technique was employed successfully in gonadotropes (Tse et al., 1994), hepatocytes (Chatton et al., 1995) and pancreatic acinar cells (Mogami et al., 1998; Park et al., 1999). Here we extended this method to mammalian sensory neurons to characterize directly [Ca2+]L dynamics upon CICR in response to cell depolarization.
Results
Imaging of the ER
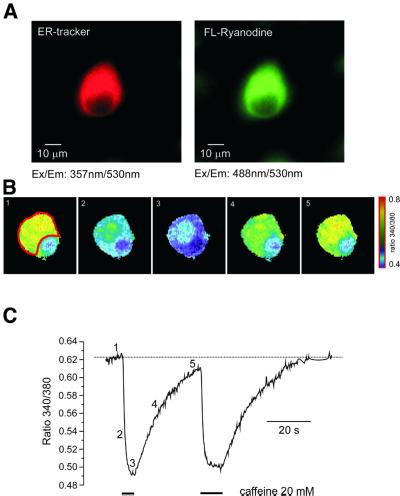
The distribution of the ER within dorsal root ganglia (DRG) neurons was assessed by using fluorescent analogues of ryanodine and thapsigargin (TG) or the ER-Tracker. Figure 1A shows a neuron simultaneously stained with ER-Tracker and green fluorescent ryanodine. The staining by both dyes reveals a similar pattern, indicating that the ER occupies most of the cytoplasm, leaving the nuclear region free of labelling. Imaging with green fluorescent TG, performed separately, has demonstrated essentially the same staining pattern (data not shown). Thus, the DRG neurons have an elaborated ER, with a high density of both RyRs and sarco-endoplasmic reticulum Ca2+ ATPases (SERCA).
Fig. 1. Imaging of the ER in individual DRG neurons. (A) Images of the same DRG neuron stained with ER-Tracker (100 nM, 10 min, left panel) and BODIPY FL-X fluorescent ryanodine (1 µM, 5 min). The fluorescence was excited at 357 (ER-Tracker) and 488 nm (FL-ryanodine) and emitted light was collected at 530 ± 15 nm. (B) Imaging of the Ca2+ concentration within the ER lumen by Mag-Fura-2. The selected ratio (340/380 nm) images were taken from the DRG neuron after the cytoplasmic portion of the dye was washed out via intracellular dialysis with normal intrapipette solution. The images were taken before, during and after the cell was exposed to 20 mM caffeine. The exact times when images were taken are indicated in (C). (C) Caffeine-induced changes in the Mag-Fura-2 ratio taken from the cell shown in (B). Fluorescence was collected from the region of interest (ROI) shown on the first image. Caffeine was applied as indicated on the graph.
As the next step, we performed functional ER imaging by using a low-affinity Ca2+ indicator, Mag-Fura-2. The cells were loaded with the Ca2+ probe by incubation with the membrane-permeable form of Mag-Fura-2, so that the probe was trapped both within intracellular organelles and the cytoplasm. To remove the cytoplasmic portion of the dye, we perfused the cells with normal, dye-free, intrapipette solution.
Mag-Fura-2 is a ratiometric Ca2+ probe that allows accurate calculation of free Ca2+ concentration, which is proportional to the ratio of fluorescent intensities acquired at two different excitation wavelengths, 340 and 380 nm (Grynkiewicz et al., 1985). Hence, the dye present in the cytosol reports low levels of Ca2+ concentration, which is manifested by low values of the 340/380 ratio (R). Indeed, the initial R determined at the very beginning of the intracellular perfusion was low, near the Rmin levels, indicating that the signal derived predominantly from the cytosol. During the course of intracellular perfusion, the R value progressively increased until reaching a steady state in 8–10 min after the whole-cell configuration was established. At this stage, the cell images (at 340 and 380 nm and the ratio images) became very similar to those obtained with ER-Tracker and fluorescent ryanodine and/or TG (Figures 1B and 3A). The resting Ca2+ concentration determined at this point varied between 60 and 270 µM (average level 177 ± 58 µM, mean ± SD, n = 63). We believe that these values are a reflection of the resting Ca2+ concentration within the lumen of the ER.
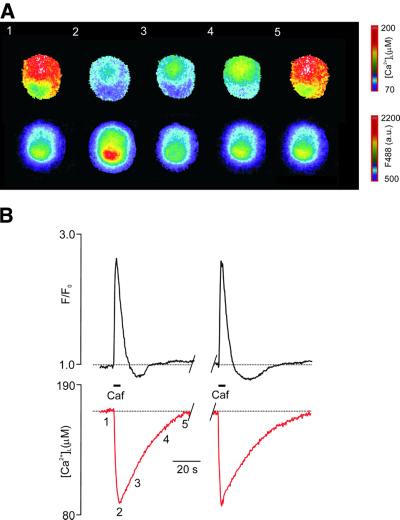
Fig. 3. Simultaneous visualization of [Ca2+]i and [Ca2+]L dynamics in DRG neurons. (A) Selected images of the Mag-Fura-2 ratio (340/380 nm, upper panel) taken simultaneously with images of Fluo-3 fluorescent intensity (488 nm) from the DRG neuron exposed to 20 mM caffeine. The ROI for further measurements is drawn over the first image. (B) Calibrated recordings of [Ca2+]L and normalized Fluo-3 fluorescent intensity (reflecting changes in [Ca2+]i) taken from the cell shown in (A). Caffeine was applied as indicated on the graph; the positions of the images shown in (A) are shown near the [Ca2+]L trace.
However, the precision of this [Ca2+]L evaluation is not absolute. First, Mag-Fura-2 could have accumulated not only in the ER, but also in other organelles with high levels of free Ca2+ ions. Secondly, our calibration procedure was based on measuring the fluorescence of the dye present in intracellular organelles and the cytoplasm, which may interfere with the precise determination of K*. As the actual KD of the dye may be much higher within the store as compared with the cytoplasm (Golovina and Blaustein, 1997), it is possible that we may in fact underestimate the [Ca2+]L values.
With all these reservations, the [Ca2+]L value in large DRG neurons varied between 50 and 300 µM, which agrees well with the [Ca2+]L levels determined with low-affinity Ca2+ probes in other cell types [pancreatic acinar cells, ∼150 µM (Park et al., 1999); hepatocytes, 630 µM (Chatton et al., 1995); gonadotrophes, 60–200 µM (Tse et al., 1994); fibroblasts, 539 µM (Hofer and Schulz, 1996); and astrocytes, 153 µM (Golovina and Blaustein, 1997, 2000)].
Caffeine-induced [Ca2+]L dynamics
As caffeine represents a widely accepted tool for inducing Ca2+ release in nerve cells, we investigated its action on [Ca2+]L in more detail. For this purpose, we studied the dynamics of [Ca2+]L changes in response to caffeine, used at high (20 mM) concentration. At this concentration, caffeine activates RyRs in a Ca2+-independent manner (Sitsapesan and Williams, 1990). Brief (3–10 s) application of 20 mM caffeine in Ca2+-free solution resulted in a rapid decrease of [Ca2+]L, which remained low as long as caffeine was present in the bath (Figure 1B and C). On washout of the drug, [Ca2+]L started to increase back towards the pre-stimulation level.
The amplitude of the caffeine-induced [Ca2+]L drop varied between different cells, ranging from 20 to 120 µM. Interestingly, we never observed a complete depletion of the store in response to short caffeine application. On average, caffeine diminished the resting [Ca2+]L value by 39 ± 11% (n = 41).
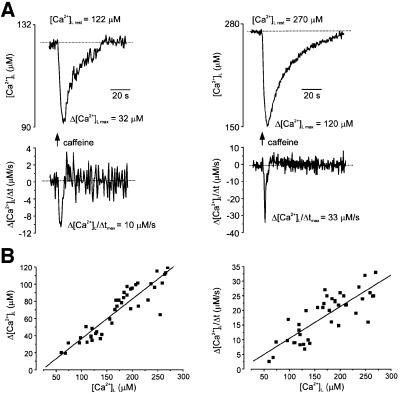
The amplitude of [Ca2+]L decrease was directly proportional, in a linear fashion, to the pre-stimulation (‘resting’) [Ca2+]L level: the higher the [Ca2+]L, the larger the amplitude of [Ca2+]L decrease (Figure 2A and B). A similar relationship was observed for the maximal rate of [Ca2+]L decrease, which ranged between 6 and 33 µM/s (Figure 2B). On washout, the [Ca2+]L recovered to the resting level, with a half-time recovery constant of 36 ± 9 s (mean ± SD, n = 41).
Fig. 2. Caffeine-induced [Ca2+]L decrease is regulated by the pre-stimulated level of [Ca2+]L. (A) Two examples of caffeine (20 mM)-induced [Ca2+]L responses obtained from different DRG neurons with different resting [Ca2+]L. Upper traces show calibrated [Ca2+]L changes whereas their first derivatives are shown at the bottom. The drug was administered for 5 s as indicated on the graph. Note the difference in amplitudes and maximal rate of [Ca2+]L decrease. (B) Amplitudes (Δ[Ca2+]L, left panel) and maximal rate of fall of [Ca2+]L (Δ[Ca2+]L/Δt, right panel) of [Ca2+]L transients induced by 5 s of 20 mM caffeine applications plotted as a function of resting intraluminal Ca2+ concentration. Every point represents an individual cell. The red line shows a linear regression fit.
Finally, we directly compared the changes in luminal and cytoplasmic Ca2+ induced by caffeine. In these experiments, [Ca2+]i was visualized by use of the low-affinity Ca2+ indicator Fluo-3 loaded into the cytoplasm via the patch pipette (see Materials and methods). Application of caffeine triggered co-ordinated changes in [Ca2+]i and [Ca2+]L, i.e. a drop in ER Ca2+ was mirrored by an elevation of [Ca2+]i (Figure 3).
Ca2+ reuptake into the ER store is regulated by [Ca2+]L
The recovery of [Ca2+]L after the removal of caffeine reflects the SERCA-dependent Ca2+ reuptake into the ER lumen as demonstrated by the fact that this recovery could be completely prevented by addition of 5 µM TG immediately after the end of caffeine application (n = 7; data not shown).
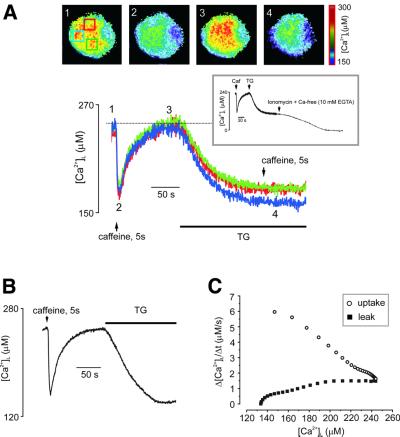
We also used TG to compare the process of caffeine-induced Ca2+ release with another, less specific, Ca2+ release process, i.e. the passive Ca2+ leakage from the store that is unmasked in the presence of TG, when Ca2+ leakage across the ER membrane is not counterbalanced by SERCA pumps (Mogami et al., 1998). The typical experimental protocol employed is shown in Figure 4. First, the cell was stimulated with caffeine for 5 s, which triggered a rapid decrease in [Ca2+]L. After caffeine washout and recovery of the [Ca2+]L levels, the cell was then exposed to 5 µM of TG. This resulted in a relatively slow [Ca2+]L decrease that became even slower as the store continued to empty. As the steady-state level was reached, the caffeine/TG-sensitive store was fully depleted, as further application of caffeine failed to release more [Ca2+]L (Figure 4A). It was notable that TG depleted the ER to the same extent as caffeine (Figure 4A and B). Moreover, both caffeine and TG decreased [Ca2+]L rather homogeneously throughout the ER-rich part of the cell (Figure 4A), further indicating the overlap between the caffeine- and TG-sensitive ER compartments.
Fig. 4. Ca2+ release, Ca2+ reuptake and Ca2+ leak from the ER store: dependence on [Ca2+]L. (A) [Ca2+]L was measured separately from three ROIs from the neuron, images of which and the ROI positions (distinguished by colours) are shown on the top. The lower panel shows [Ca2+]L traces, colour coded according to the respective ROI. Initial application of caffeine triggered rapid Ca2+ release followed by [Ca2+]L reuptake after removal of agonist. When [Ca2+]L recovered to the pre-stimulated level, 5 µM thapsigargin was added to block the SERCA pumps. This resulted in a slow decrease in [Ca2+]L due to an unopposed leakage from the store. Note that caffeine and TG reduced [Ca2+]L to the same extent. The inset shows that application of ionomycin in Ca2+-free, EGTA-containing solution following TG led to a complete depletion of the store. (B) [Ca2+]L trace taken from another DRG neuron employing the protocol described in (A). (C) The relationship between Ca2+ transport rates and [Ca2+]L derived from the data shown in (B). The uptake rate was leak-corrected.
The residual level of [Ca2+]L that remained after the stores were discharged either by caffeine or TG most probably represents the free Ca2+ remaining in the ER, since application of 5 µM of carbonylcyanide m-chlorophenylhydrazone (CCCP) after caffeine or TG did not trigger further changes in [Ca2+]L (n = 5 and 3, respectively; data not shown). Furthermore, application of ionomycin in Ca2+-free solutions following TG brought [Ca2+]L to zero (Rmin) level (n = 5, Figure 4A, inset).
The experimental protocol described above was used to derive the relationship between the actual [Ca2+]L and the rates of Ca2+ reuptake and Ca2+ leakage, as shown in Figure 4C, that were calculated from the trace shown in Figure 4B. The rate of Ca2+ uptake, measured after the removal of caffeine, was steeply dependent on [Ca2+]L, being very high when the store was maximally depleted and gradually decreasing as the ER refilled. The rate of Ca2+ leakage showed an opposite dependence on [Ca2+]L: it was negligible when the store was depleted and increased before saturating at resting [Ca2+]L.
The maximal rate of uptake was 6 ± 0.85 µM/s (mean ± SD, n = 6; all experiments were performed on neurons with resting [Ca2+]L ranging between 230 and 270 µM) and the maximal leak rate was 1.48 ± 0.17 µM/s (mean ± SD; n = 6).
Direct demonstration of CICR in sensory neurons
Most of the experimental support currently available for the existence of physiological CICR (i.e. CICR triggered by Ca2+ entry during depolarization) in neurons is based on indirect evidence, originating from measurements of cytoplasmic, not luminal, Ca2+ concentration (see for example Friel and Tsien, 1992; Marrion and Adams, 1992; Hua et al., 1993; Usachev et al., 1993; Shmigol et al., 1995; Garaschuk et al., 1997; Hernandez-Cruz et al., 1997). Using the combination of low- and high-affinity probes that independently report Ca2+ changes within cytosolic and intra-ER compartments, we were able to address directly the question of whether Ca2+ entry through voltage-gated plasmalemmal channels can indeed trigger release of Ca2+ from the ER lumen.
For this purpose, we monitored [Ca2+]i and [Ca2+]L in a voltage-clamped neuron as shown in Figure 5. First, we probed the ER store with caffeine, which was applied for 5 s. As also shown in Figure 3, this application induced an increase in [Ca2+]i and a decrease in the concentration of [Ca2+]L. After complete recovery of the Ca2+ concentration in both compartments, we depolarized the cell from –70 to 0 mV, evoking substantial Ca2+ current (ICa). The depolarization resulted in a significant [Ca2+]i elevation as well as a transient decrease in [Ca2+]L. The amplitude of [Ca2+]L decrease was smaller than that evoked by caffeine, ranging between 5 and 30 µM. The amplitude of this depolarization-evoked [Ca2+]L drop correlated linearly with the resting [Ca2+]L: i.e. at higher resting [Ca2+]L there was a larger [Ca2+]L drop in response to depolarization (Figure 5, inset). When Ca2+ current was blocked by Ca2+ removal from extracellular solution, both [Ca2+]i and [Ca2+]L responses disappeared completely (n = 4; data not shown). Therefore, these observations demonstrate directly that plasmalemmal Ca2+ entry triggers Ca2+ release from the ER lumen.
Fig. 5. Direct visualization of Ca2+-induced Ca2+ release in DRG neurons. Simultaneous recordings of cytoplasmic Ca2+ (normalized Fluo-3 fluorescent intensity, upper trace) and [Ca2+]L dynamics recorded from the same neuron are presented. The cell was challenged subsequently by an application of caffeine (20 mM) and by a 3 s step depolarization from –70 to 0 mV (the corresponding Ca2+ current is shown in the inset on the top of the Fluo-3 trace). Note that depolarization triggers an increase in cytoplasmic Ca2+ and a decrease in [Ca2+]L. Selected images of the Mag-Fura-2 ratio are shown near the [Ca2+]L trace. The inset in the right lower corner shows a linear relationship between resting luminal Ca2+ concentration and the amplitude of depolarization-induced [Ca2+]L decrease.
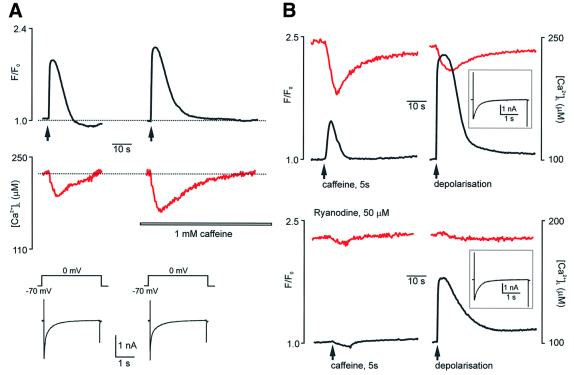
A definite association between the depolarization-evoked decrease in [Ca2+]L and the RyRs required further experiments, using established modulators of these receptors. It is known (Sitsapesan and Williams, 1990; Usachev and Thayer, 1997) that at low concentrations (0.5–1 mM), caffeine sensitizes RyRs to cytosolic Ca2+ ions. In our experiments, the application of 1 mM caffeine to the DRG neuron, which on its own did not affect [Ca2+]L or [Ca2+]i, significantly increased the amplitude of the [Ca2+]L drop in response to the Ca2+ current evoked by 3 s depolarization (Figure 6A). This potentiation of Ca2+ release occurred despite the fact that the amplitude of ICa (and hence the amount of Ca2+ ions entering the cell) remained unchanged. On average, the amplitude of ICa-induced [Ca2+]L decrease was 87 ± 8% (n = 7) larger in the presence of 1 mM of caffeine as compared with the control.
Fig. 6. Pharmacological manipulation with CICR. (A) A low (1 mM) caffeine concentration enhances the CICR. The [Ca2+]i (black traces) and [Ca2+]L (red traces) were measured from the same DRG neuron. The neuron was stimulated by 3 s depolarizations in control conditions and in the presence of 1 mM caffeine (the instants of depolarizations are indicated by arrows). Note the significant rise in the amplitude of the [Ca2+]L decrease in the presence of caffeine. The corresponding Ca2+ currents are shown on the lower panel at a higher time resolution. (B) Ryanodine completely inhibits both caffeine- and Ca2+-induced Ca2+ release. The upper panel shows control [Ca2+]i and [Ca2+]L traces (depicted in black and red, respectively) in response to 20 mM caffeine and 3 s depolarization. The lower panel demonstrates the same experiment performed on another neuron from the same culture after 10 min incubation with 50 µM ryanodine. Ca2+ currents are shown in the insets.
Another test was the use of a specific inhibitor of CICR, ryanodine, which at high concentrations (50–100 µM) completely inhibits RyRs, and hence blocks the caffeine-induced Ca2+ release as well as CICR. In these experiments (Figure 6B), we first measured the caffeine- and depolarization-induced responses of [Ca2+]i and [Ca2+]L in control conditions. Then the same coverslip was incubated with 50 µM ryanodine for 10 min. We found that such a treatment completely blocked caffeine-induced responses of both [Ca2+]i and [Ca2+]L in all cells studied (n = 9). Ryanodine also completely inhibited CICR, as judged by the absence of [Ca2+]L changes in response to depolarization (n = 9). The Ca2+ current as well as depolarization-induced [Ca2+]i elevation, however, remained.
CICR is directly proportional to the amount of Ca2+ entry
Previous investigations of neuronal CICR suggested that significant release through RyRs requires a large Ca2+ entry, i.e. CICR became apparent only when a certain threshold of ‘trigger’ Ca2+ in the cytosol is reached (Llano et al., 1994; Shmigol et al., 1995). We addressed this question directly, by investigating the relationship between [Ca2+]L dynamics and Ca2+ entry via voltage-gated channels.
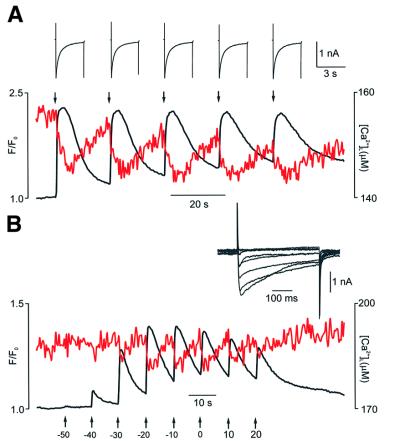
First, stimulation of DRG neurons by 3 s depolarizations separated by 20 s intervals triggered almost identical events of Ca2+ release from the ER. As shown in Figure 7A, every Ca2+ current evoked a drop in [Ca2+]L with an amplitude of 10–11 µM. Thus, identical Ca2+ entries trigger similar release of Ca2+ from the ER lumen.
Fig. 7. CICR is graded by Ca2+ entry (1). (A) [Ca2+]L and [Ca2+]i dynamics (red and black traces, respectively) recorded from the DRG neuron stimulated by five consecutive depolarizations (moments of depolarizations are indicated by arrows) from –70 to 0 mV. Ca2+ currents are shown above at a higher time resolution. (B) Similarly to the experiment described above, [Ca2+]L and [Ca2+]i (red and black traces) were recorded in response to step depolarizations of increasing amplitude. Holding potential –70 mV, step increment 10 mV.
Different responses occurred when Ca2+ entry was graded. First, we stimulated the DRG neurons by a standard I–V protocol, when the cell was depolarized from –70 mV by a series of 400 ms steps with increasing amplitude. This stimulation resulted in a characteristic bell-shaped pattern of [Ca2+]i transients, which were mirrored by transient [Ca2+]L decreases (Figure 7B).
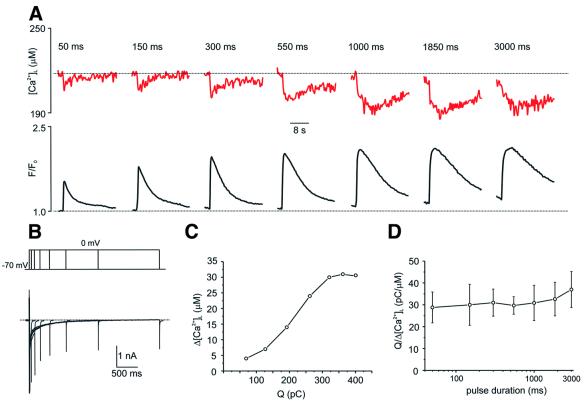
A similar relationship was also observed when we graded Ca2+ entry by varying the duration of ICa between 50 and 3000 ms (Figure 8). When stimulating the neurons, we found that the shortest ICa (50 ms) already induced Ca2+ release, as shown by a [Ca2+]L transient decrease. The increase in the duration of the ICa resulted in an almost linear increase in the amplitude of Ca2+ release (Figure 8A). This linear relationship is illustrated quantitatively in Figure 8C, which plots the amplitude of [Ca2+]L decrease against the actual charge (Q) carried by the Ca2+ current, which directly reflects the number of Ca2+ ions entering the cell. From this type of data, we can derive the ‘unitary CICR potency’ of the Ca2+ current by dividing the charge carried by a given current by the amplitude of the corresponding [Ca2+]L transient decrease. This ‘unitary CICR potency’ would thus be a measure of how many pC carried by a Ca2+ current are required to release 1 µM of Ca2+ from the store. Figure 8D shows the average Q/Δ[Ca2+]L values plotted against ICa duration obtained for five different experiments analogous to that shown on Figure 8A. For these experiments, we have chosen five different cells with similar levels of resting [Ca2+]L (range 230–270 µM). The plot shows that the ‘unitary CICR potency’ of Ca2+ current remains constant for currents of different length, thus demonstrating the graded relationship between Ca2+ entry and Ca2+ release in neuronal cells.
Fig. 8. CICR is graded by Ca2+ entry (2). (A) Changes in [Ca2+]L (upper panel, red traces) and [Ca2+]i (lower panel, black traces) in response to step depolarizations from –70 mV to 0 mV of increasing duration. (B) Voltage protocol and Ca2+ currents from the experiment shown in (A). (C) The relationship between the charge carried by the Ca2+ current (Q) and the amplitude of the [Ca2+]L decrease (Δ[Ca2+]L) derived from the traces shown in (A) and (B). (D) The ratio between the charge carried by ICa and the amplitude of [Ca2+]L decrease plotted against duration of corresponding Ca2+ currents. Data are mean ± SD derived from five independent experiments similar to those shown in (A).
Discussion
CICR in sensory neurons
The existence of ER Ca2+ stores in nerve cells with a full complement of Ca2+ release channels has been firmly established (for initial observations see Lipscombe et al., 1988; Thayer et al., 1988; Brorson et al., 1991). Nonetheless, the functional dynamics of Ca2+ movements between store lumen and cytoplasm upon physiological stimulation of nerve cells remains completely unexplored. The current evidence for a functional CICR (i.e. CICR triggered by Ca2+ entry via plasmalemmal Ca2+ channels) in neurons is based only on an indirect approach, measuring the cytosolic Ca2+ changes, from which it is difficult to distinguish the extra- and intracellular components of the Ca2+ transients that follow the activation of the plasmalemmal Ca2+ currents. The only attempt at measuring [Ca2+]L in neuron-like preparations was performed recently by transfecting PC12 pheochromocytoma cells with ER-targeted aequorin (Alonso et al., 1999). These recordings, however, measured average [Ca2+]L changes in the whole culture, and the experimental limitations precluded both ICa monitoring and cell stimulation in physiologically relevant time frames. By combining Ca2+ probes with different affinities and spectral properties, we describe here fluorescence measurements that can differentiate between two distinct intracellular compartments, the ER and the cytoplasm.
Using this direct approach, we show, for the first time, that the dynamics of Ca2+ concentration changes within the ER in response to physiological stimulation of single neurons. This allowed us to demonstrate clearly that Ca2+ entry via voltage-gated Ca2+ channels triggers Ca2+ loss from the ER, thus proving unequivocally the existence of physiological CICR in nerve cells.
Activation of voltage-gated Ca2+ currents following a depolarization pulse resulted in changes in the opposite direction in the fluorescence signals derived from the cytoplasm and the ER lumen; an increase in [Ca2+]i was associated with a simultaneous decrease of [Ca2+]L. This decrease reflects the process of CICR, since the manipulations of the RyRs, which mediate CICR, selectively affected [Ca2+]L dynamics in response to ICa. Thus, sensitization of RyRs by incubation with low (1 mM) concentrations of caffeine increased the CICR amplitude almost 2-fold, although the Ca2+ current remained unaffected. Also, blockade of RyRs by 50 µM ryanodine completely inhibited the CICR-related decrease in [Ca2+]L, again without any apparent effect on Ca2+ current parameters.
An important issue in neuronal CICR activation is the relationship between Ca2+ entry and the activation of Ca2+ release. Most of the previous studies on neuronal CICR showed a non-linear relationship between the amount of Ca2+ entry (as measured by the Ca2+ current integral) and the corresponding rise in the amplitude of the [Ca2+]i transient (Hua et al., 1993, 1994; Llano et al., 1994; Shmigol et al., 1995). These observations suggested a relatively high threshold for CICR activation in neurons as compared with heart muscle cells. For example, in DRG neurons, CICR became apparent (Shmigol et al., 1995) only with an ICa activation time in excess of 200 ms (compare with 4 ms for cardiac muscle; Han et al., 1994).
Direct monitoring of [Ca2+]L changes showed that the degree of CICR activation, as measured by the decrease in [Ca2+]L, correlated linearly with the amount of Ca2+ entering the cytoplasm. This was apparent when Ca2+ entry was graded by varying either the amplitude of the depolarization step (Figure 7) or the length of the Ca2+ current (Figure 8). Moreover, by calculating the ratio between the charge carried by ICa and the amplitude of [Ca2+]L decrease (Figure 8D), we found that the amount of Ca2+ ions required to release 1 µM of Ca2+ from the store remained constant for a wide range of ICa duration. Only at very long ICa (3 s) did the CICR show a tendency for saturation, presumably reflecting an equilibrium between Ca2+ entry into the cytoplasm (from both extra- and intracellular sources) and Ca2+ buffering/extrusion, so that no further recruitment of RyRs occurs.
Thus, we conclude that the ER represents an important Ca2+ storage organelle in DRG neurons. It occupies most of the cytoplasm and contains a high (>100–200 µM) concentration of free, releasable Ca2+. A proportion of this Ca2+ is readily available for Ca2+ signals activated by cell depolarization. The CICR in DRG neurons is graded linearly by Ca2+ entry and is limited to ∼10% of the stored Ca2+.
Luminal Ca2+ controls Ca2+ release and Ca2+ uptake
Release of Ca2+ ions from the ER is controlled not only by the changes in cytosolic Ca2+ but also by the level of [Ca2+]L. By analysing the [Ca2+]L transients induced by caffeine application in cells that had different levels of [Ca2+]L, we found that [Ca2+]L controls the amplitude and maximal rate of Ca2+ release in a linear fashion. This shows that when all RyRs are activated in the presence of maximal caffeine concentration, Ca2+ release is governed by the electrochemical driving force between the ER lumen and cytosol. Similarly, the rate of resting Ca2+ leak from the ER depends on [Ca2+]L, with the leak being maximal when the stores are full.
In addition, the [Ca2+]L level controls the refilling of the store after it has been discharged. The rate of Ca2+ uptake was maximal when the caffeine/TG stores were maximally depleted. In this respect, DRG neurons show a behaviour similar to that found in non-excitable pancreatic acinar cells (Mogami et al., 1998). The notable difference was that the actual rates of Ca2+ uptake/leak were substantially higher in neurons. That is, in pancreatic cells, the maximal rates of leak and uptake were 19 and 95 µM/min, respectively, whereas in neurons they were 90 and 360 µM/min, respectively. This implies a relatively high resting turnover of Ca2+ ions across the ER membrane.
Finally, we found that neither caffeine nor TG are able to deplete the neuronal intracellular Ca2+ store fully: in both conditions, [Ca2+]L remained, even after full agent-dependent depletion, at a relatively high level (∼50–60% of the pre-stimulated value). Obviously, this might reflect a limitation of the method, suggesting that part of the signal comes from an intracellular compartment not connected with the caffeine- or TG-sensitive portion of the ER (although not from mitochondria, as CCCP does not affect the degree of store depletion). Alternatively, such a limitation of the release could be an intrinsic property of the store, since a fall in luminal Ca2+ content decreases the availability and open probability of RyRs (Lukyanenko et al., 1996; Shmigol et al., 1996). Such a mechanism, which will prevent a severe depletion of the store, may be an important factor in preserving the integrity of the ER (Paschen, 2001; Petersen et al., 2001).
Materials and methods
Simultaneous measurements of Ca2+ in the store and in the cytoplasm
DRG neurons were isolated enzymatically from neonatal (1–3 days old) Sprague–Dawley rats using a conventional treatment with 0.1% protease (type XIV) in HEPES-buffered minimum essential Eagle’s medium (MEM) for 8 min at 37°C. Individual cells were separated mechanically and plated on poly-l-ornithine- (1 mg/ml) and laminin (0.01 mg/ml)-covered glass coverslips. Neurons were maintained in culture media [Dulbecco’s modified Eagle’s medium (DMEM), supplemented with 10% horse serum, 50 U/ml penicillin/streptomycin mixture and 6 µg/ml insulin] at 37°C in an atmosphere of 95% CO2 + 5% O2 for 1–2 days prior to the experiment.
For [Ca2+]L recordings, we used Mag-Fura-2 (KD ∼25–50 µM) suitable for detecting high intraluminal [Ca2+] levels (Park et al., 1999). The neurons were incubated with 5 µM Mag-Fura-2 for 30 min at 37°C and washed at 37°C for 1 h prior to the experiment (loading at 37°C promotes dye compartmentalization within the ER lumen; Golovina and Blaustein, 1997; Thomas et al., 2000).
The whole-cell patch–clamp configuration was established on cells loaded with Mag-Fura-2. In the present study, we investigated only large (proprioceptive) neurons with somas >35 µm in diameter. Before establishing the whole-cell configuration, Mag-Fura-2 was evenly distributed between organelles and the cytosol, as judged by fluorescence imaging. Immediately after establishing the whole-cell configuration, the dye started to diffuse into the pipette, decreasing the fluorescence signal until a stable level was achieved ∼10 min after the beginning of the intracellular dialysis. At this stage, the nuclear region, with significantly less fluorescence, became clearly visible (Figures 1, 3 and 4).
To measure simultaneously [Ca2+]L and cytoplasmic Ca2+ concentration ([Ca2+]i), the intrapipette solution was supplemented with 0.05 mM of the high-affinity Ca2+ indicator Fluo-3K5. Cell perfusion with Fluo-3K5 did not significantly affect Mag-Fura-2 fluorescence. After 10 min of intracellular dialysis, Fluo-3 fluorescence also reached a steady-state level. Mag-Fura-2 and Fluo-3 signals were separated using their distinct excitation properties (340/380 nm for Mag-Fura-2 and 488 nm for Fluo-3). Fluo-3 is weakly excited at 350–380 nm; however, the level of Fluo-3 signal did not exceed 1–2% of the signals from Mag-Fura-2 measured at the same system settings.
Calibration of Mag-Fura-2 signals
The [Ca2+]L values were calculated using the 340/380 nm ratio with the equation [Ca2+]L = K* (R – Rmin)/(Rmax – R). The Rmin, Rmax and K* values were determined using exposure of neurons to 20 µM ionomycin and four calibrating solutions with [Ca2+] <10 nM (10 mM EGTA); 100 µM; 400 µM and 10 mM; solutions were prepared as described previously (Tse et al., 1994). Values for Rmin, Rmax and K* were 0.27, 1.1 and 221 µM, respectively.
Real-time video imaging
Fluorescence images were captured using an Olympus IX70 inverted microscope (40× UV objective) equipped with a charge-coupled device (CCD)-cooled intensified camera (Pentamax Gene IV, Roper Scientific). The specimen was illuminated alternately at 340, 380 and 488 nm by a monochromator (Polychrom IV, TILL Photonics, Germany) at a cycle frequency of 3–5 Hz. Control over the experiment, image storage and off-line analysis was performed by use of MetaFluor/MetaMorph software (Universal Imaging Corporation) running on a Windows 98 workstation.
Electrophysiology and solution exchange
Electrophysiological recordings were made by using an EPC-9 amplifier run by the PC-based PULS software (both from HEKA, Germany). The pipette resistance was 3–5 MΩ. Ca2+ currents were recorded in Na+-free solution to avoid contaminating Na+ currents. Brief removal of Na+ ions did not affect either [Ca2+]i or [Ca2+]L. All solutions were applied using a fast local superfusion technique (Veselovsky et al., 1996), which ensured complete exchange of the milieu surrounding the cell within 100 ms.
Solutions and reagents
The extracellular bathing solution contained: 135 mM NaCl, 3 mM KCl, 2 mM CaCl2, 20 mM glucose, 20 mM HEPES–NaOH pH 7.4. The Ca2+-free solution contained 5 mM EGTA with no CaCl2 added. To obtain Na+-free solution, Na+ ions were substituted by TEA–Cl and 0.1 µM tetradotoxin was added. The intracellular solution contained: 122 mM CsCl2, 20 mM TEA–Cl, 3 mM Na2ATP, 10 mM HEPES–CsOH pH 7.3. For [Ca2+]i recordings, the intrapipette solution was supplemented with 0.05 mM Fluo-3 K5. All reagents were purchased from Sigma and fluorescent probes were obtained from Molecular Probes.
Acknowledgments
Acknowledgements
This research was supported by a BBSRC research grant to A.V. (ref. 34/C12751) and by a Wellcome Trust collaborative grant to N.S. and A.V. (ref. 060095).
References
- Alford S., Frenguelli,B.G., Schofield,J.G. and Collingridge,G.L. (1993) Characterization of Ca2+ signals induced in hippocampal CA1 neurones by the synaptic activation of NMDA receptors. J. Physiol. (Lond.), 469, 693–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso M.T., Barrero,M.J., Michelena,P., Carnicero,E., Cuchillo,I., Garcia,A.G., Garcia-Sancho,J., Montero,M. and Alvarez,J. (1999) Ca2+-induced Ca2+ release in chromaffin cells seen from inside the ER with targeted aequorin. J. Cell Biol., 144, 241–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M.J. (1993) Inositoltrisphosphate and calcium signalling. Nature, 361, 315–325. [DOI] [PubMed] [Google Scholar]
- Berridge M.J. (1998) Neuronal calcium signaling. Neuron, 21, 13–26. [DOI] [PubMed] [Google Scholar]
- Bezprozvanny I., Watras,J. and Ehrlich,B.E. (1991) Bell-shaped calcium-response curves of Ins(1,4,5)P3- and calcium-gated channels from endoplasmic reticulum of cerebellum. Nature, 351, 751–754. [DOI] [PubMed] [Google Scholar]
- Brorson J.R., Bleakman,D., Gibbons,S.J. and Miller,R.J. (1991) The properties of intracellular calcium stores in cultured rat cerebellar neurons. J. Neurosci., 11, 4024–4043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatton J.Y., Liu,H. and Stucki,J.W. (1995) Simultaneous measurements of Ca2+ in the intracellular stores and the cytosol of hepatocytes during hormone-induced Ca2+ oscillations. FEBS Lett., 368, 165–168. [DOI] [PubMed] [Google Scholar]
- Emptage N., Bliss,T.V.P. and Fine,A. (1999) Single synaptic events evoke NMDA receptor-mediated release of calcium from internal stores in hippocampal dendritic spines. Neuron, 22, 115–124. [DOI] [PubMed] [Google Scholar]
- Frenguelli B.G., Irving,A.J. and Collingridge,G.L. (1996) Ca2+ stores and hippocampal synaptic plasticity. Semin. Neurosci., 8, 301–309. [Google Scholar]
- Friel D.D. and Tsien,R.W. (1992) A caffeine- and ryanodine-sensitive Ca2+ store in bullfrog sympathetic neurones modulates effects of Ca2+ entry on [Ca2+]i. J. Physiol. (Lond.), 450, 217–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galione A., Lee,H.C. and Busa,W.B. (1991) Ca2+-induced Ca2+ release in sea urchin egg homogenates: modulation by cyclic ADP-ribose. Science, 253, 1143–1146. [DOI] [PubMed] [Google Scholar]
- Garaschuk O., Yaari,Y. and Konnerth,A. (1997) Release and sequestration of calcium by ryanodine-sensitive stores in rat hippocampal neurones. J. Physiol. (Lond.), 502, 13–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golovina V.A. and Blaustein,M.P. (1997) Spatially and functionally distinct Ca2+ stores in sarcoplasmic and endoplasmic reticulum. Science, 275, 1643–1648. [DOI] [PubMed] [Google Scholar]
- Golovina V.A. and Blaustein,M.P. (2000) Unloading and refilling of two classes of spatially resolved endoplasmic reticulum Ca2+ stores in astrocytes. Glia, 31, 15–28. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie,M. and Tsien,R.Y. (1985) A new generation of Ca2+ indicators with greatly improved fluorescence properties. J. Biol. Chem., 260, 3440–3450. [PubMed] [Google Scholar]
- Han S., Schiefer,A. and Isenberg,G. (1994) Ca2+ load of guinea-pig ventricular myocytes determines efficacy of brief Ca2+ currents as trigger for Ca2+ release. J. Physiol. (Lond.), 480, 411–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham G.E., Arnold,F.J. and Bading,H. (2001) Nuclear calcium signaling controls CREB-mediated gene expression triggered by synaptic activity. Nature Neurosci., 4, 261–267. [DOI] [PubMed] [Google Scholar]
- Hernandez-Cruz A., Escobar,A.L. and Jimenez,N. (1997) Ca2+-induced Ca2+ release phenomena in mammalian sympathetic neurons are critically dependent on the rate of rise of trigger Ca2+. J. Gen. Physiol., 109, 147–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer A.M. and Schulz,I. (1996) Quantification of intraluminal free [Ca] in the agonist-sensitive internal calcium store using compartmental ized fluorescent indicators: some considerations. Cell Calcium, 20, 235–242. [DOI] [PubMed] [Google Scholar]
- Hua S.Y., Nohmi,M. and Kuba,K. (1993) Characteristics of Ca2+ release induced by Ca2+ influx in cultured bullfrog sympathetic neurones. J. Physiol. (Lond.), 464, 245–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua S.Y., Tokimasa,T., Takasawa,S., Furuya,Y., Nohmi,M., Okamoto,H. and Kuba,K. (1994) Cyclic ADP-ribose modulates Ca2+ release channels for activation by physiological Ca2+ entry in bullfrog sympathetic neurons. Neuron, 12, 1073–1079. [DOI] [PubMed] [Google Scholar]
- Kano M., Garaschuk,O., Verkhratsky,A. and Konnerth,A. (1995) Ryanodine receptor-mediated intracellular calcium release in rat cerebellar Purkinje neurones. J. Physiol. (Lond.), 487, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krizaj D., Bao,J.X., Schmitz,Y., Witkovsky,P. and Copenhagen,D.R. (1999) Caffeine-sensitive calcium stores regulate synaptic transmission from retinal rod photoreceptors. J. Neurosci., 19, 7249–7261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipscombe D., Madison,D.V., Poenie,M., Reuter,H., Tsien,R.W. and Tsien,R.Y. (1988) Imaging of cytosolic Ca2+ transients arising from Ca2+ stores and Ca2+ channels in sympathetic neurons. Neuron, 1, 355–365. [DOI] [PubMed] [Google Scholar]
- Llano I., DiPolo,R. and Marty,A. (1994) Calcium-induced calcium release in cerebellar Purkinje neurones. Neuron, 12, 663–673. [DOI] [PubMed] [Google Scholar]
- Lukyanenko V., Gyorke,I. and Gyorke,S. (1996) Regulation of calcium release by calcium inside the sarcoplasmic reticulum in ventricular myocytes. Pflügers Arch., 432, 1047–1054. [DOI] [PubMed] [Google Scholar]
- Marrion N.V. and Adams,P.R. (1992) Release of intracellular calcium and modulation of membrane currents by caffeine in bull-frog sympathetic neurones. J. Physiol. (Lond.), 445, 515–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama Y., Inooka,G., Li,Y.X., Miyashita,Y. and Kasai,H. (1993) Agonist-induced localized Ca2+ spikes directly triggering exocytotic secretion in exocrine pancreas. EMBO J., 12, 3017–3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogami H., Tepikin,A.V. and Petersen,O.H. (1998) Termination of cytosolic Ca2+ signals: Ca2+ reuptake into intracellular stores is regulated by the free Ca2+ concentration in the store lumen. EMBO J., 17, 435–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niggli E. (1999) Localized intracellular calcium signaling in muscle: calcium sparks and calcium quarks. Annu. Rev. Physiol., 61, 311–335. [DOI] [PubMed] [Google Scholar]
- Park M.K., Tepikin,A.V. and Petersen,O.H. (1999) The relationship between acetylcholine-evoked Ca2+-dependent current and the Ca2+ concentrations in the cytosol and the lumen of the endoplasmic reticulum in pancreatic acinar cells. Pflügers Arch., 438, 760–765. [DOI] [PubMed] [Google Scholar]
- Park M.K., Petersen,O.H. and Tepikin,A.V. (2000) The endoplasmic reticulum as one continuous Ca2+ pool: visualization of rapid Ca2+ movements and equilibration. EMBO J., 19, 5729–5739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paschen W. (2001) Dependence of vital cell function on endoplasmic reticulum calcium levels: implications for the mechanisms underlying neuronal cell injury in different pathological states. Cell Calcium, 29, 1–11 [DOI] [PubMed] [Google Scholar]
- Petersen O.H., Petersen,C.C. and Kasai,H. (1994) Calcium and hormone action. Annu. Rev. Physiol., 56, 297–319. [DOI] [PubMed] [Google Scholar]
- Petersen O.H., Tepikin,A. and Park,M.K. (2001) The endoplasmic reticulum: one continuous or several separate Ca2+ stores? Trends Neurosci., 24, 271–276. [DOI] [PubMed] [Google Scholar]
- Rose C.R. and Konnerth,A. (2001) Stores not just for storage. Intracellular calcium release and synaptic plasticity. Neuron, 31, 519–522. [DOI] [PubMed] [Google Scholar]
- Shmigol A., Verkhratsky,A. and Isenberg,G. (1995) Calcium-induced calcium release in rat sensory neurones. J. Physiol. (Lond.), 489, 627–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shmigol A., Svichar,N., Kostyuk,P. and Verkhratsky,A. (1996) Gradual caffeine-induced Ca2+ release in mouse dorsal root ganglion neurons is controlled by cytoplasmic and luminal Ca2+. Neuroscience, 73, 1061–1067. [DOI] [PubMed] [Google Scholar]
- Sitsapesan R. and Williams,A.J. (1990) Mechanisms of caffeine activation of single calcium-release channels of sheep cardiac sarcoplasmic reticulum. J. Physiol. (Lond.), 423, 425–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer S.A., Hirning,L.D. and Miller,R.J. (1988) The role of caffeine-sensitive calcium stores in the regulation of the intracellular free calcium concentration in rat sympathetic neurons in vitro. Mol. Pharmacol., 34, 664–673. [PubMed] [Google Scholar]
- Thomas D., Tovey,S.C., Collins,T.J., Bootman,M.D., Berridge,M.J. and Lipp,P. (2000) A comparison of fluorescent Ca2+ indicator properties and their use in measuring elementary and global Ca2+ signals. Cell Calcium, 28, 213–223. [DOI] [PubMed] [Google Scholar]
- Thorn P., Lawrie,A.M., Smith,P.M., Gallacher,D.V. and Petersen,O.H. (1993) Local and global cytosolic Ca2+ oscillations in exocrine cells evoked by agonists and inositol trisphosphate. Cell, 74, 661–668. [DOI] [PubMed] [Google Scholar]
- Tse F.W., Tse,A. and Hille,B. (1994) Cyclic Ca2+ changes in intracellular stores of gonadotropes during gonadotropin-releasing hormone-stimulated Ca2+ oscillations. Proc. Natl Acad. Sci. USA, 91, 9750–9754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usachev Y.M. and Thayer,S.A. (1997) All-or-none Ca2+ release from intracellular stores triggered by Ca2+ influx through voltage-gated Ca2+ channels in rat sensory neurons. J. Neurosci., 17, 7404–7414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usachev Y., Shmigol,A., Pronchuk,N., Kostyuk,P. and Verkhratsky,A. (1993) Caffeine-induced calcium release from internal stores in cultured rat sensory neurons. Neuroscience, 57, 845–859. [DOI] [PubMed] [Google Scholar]
- Verkhratsky A. and Petersen,O.H. (1998) Neuronal calcium stores. Cell Calcium, 24, 333–343. [DOI] [PubMed] [Google Scholar]
- Verkhratsky A. and Shmigol,A. (1996) Calcium-induced calcium release in neurones. Cell Calcium, 19, 1–14. [DOI] [PubMed] [Google Scholar]
- Veselovsky N.S., Engert,F. and Lux,H.D. (1996) Fast local superfusion technique. Pflügers Arch., 432, 351–354. [DOI] [PubMed] [Google Scholar]
- Zucker R.S. (1996) Exocytosis: a molecular and physiological perspective. Neuron, 17, 1049–1055. [DOI] [PubMed] [Google Scholar]