Abstract
The glycoprotein hormone receptors (thyrotrophin receptor, TSHr; luteinizing hormone/chorionic gonadotrophin receptor, LH/CGr; follicle-stimulating hormone receptor, FSHr) constitute a subfamily of rhodopsin-like G protein-coupled receptors (GPCRs) with a long N-terminal extracellular extension responsible for high-affinity hormone binding. These ectodomains contain two cysteine clusters flanking nine leucine-rich repeats (LRR), a motif found in several protein families involved in protein–protein interactions. Similar to the situation described recently in CCR5, we demonstrate here that the TSHr, as it is present at the cell surface, is sulfated on tyrosines in a motif located downstream of the C-terminal cysteine cluster. Sulfation of one of the two tyrosines in the motif is mandatory for high-affinity binding of TSH and activation of the receptor. Site-directed mutagenesis experiments indicate that the motif, which is conserved in all members of the glycoprotein hormone receptor family, seems to play a similar role in the LH/CG and FSH receptors.
Keywords: glycoprotein/GPCR/hormones/sulfation/tyrosine
Introduction
The glycoprotein hormone receptors (thyrotrophin receptor, TSHr; luteinizing hormone/chorionic gonadotrophin receptor, LH/CGr; follicle-stimulating hormone receptor, FSHr) constitute a subfamily of G protein-coupled receptors (GPCRs) with a clear-cut modular structure. Their C-terminal moiety is made up of a serpentine portion with seven transmembrane segments typical of rhodopsin-like GPCRs. Their N-terminal domains, responsible for high-affinity hormone binding, are particularly long (359–414 residues) and contain nine leucine-rich repeats (LRRs; Vassart et al., 1995; Gether, 2000; Themmen and Huhtaniemi, 2000). LRRs have been found in several protein families involved in protein–protein interactions (Kobe and Deisenhofer, 1995b). Structural information at atomic resolution is available for the interaction of a prototypic LRR protein, RNase inhibitor, with its natural ligand, RNase (Kobe and Deisenhofer, 1993, 1995a). By analogy with this structure (Jiang et al., 1995; Kajava et al., 1995; Bhowmick et al., 1996), the interaction of glycoprotein hormones with their receptors is assumed to involve specific contacts with the LRR-containing segment and experimental evidence for this has been provided (Braun et al., 1991; Rapoport et al., 1998).
The sensitivity of the interactions of TSH with its receptor to ionic strength (Grossmann et al., 1998) and results from site-directed mutagenesis experiments (Szkudlinski et al., 1996; Grossmann et al., 1997, 1998) suggest that ionic bonds are important factors contributing to high-affinity hormone binding. Recently, the well documented role of tyrosine sulfation in a variety of protein–protein interactions (Huttner, 1982; Hortin et al., 1989; Leyte et al., 1991; Bundgaard et al., 1995; Somers et al., 2000; Dong et al., 2001) has been extended to some members of the chemokine or chemoattractant GPCRs. Sulfation of specific tyrosines in the N-terminal domain of CCR5 was shown to contribute to the binding of its natural ligands MIP1α and MIP1β, as well as of HIV-1 gp120 (Farzan et al., 1999). Similarly, sulfated tyrosines are involved in the formation of the docking site for human C5a receptor (Farzan et al., 2001).
In the present study, we identified a conserved motif in the ectodomain of glycoprotein hormone receptors which is subjected to sulfation of specific tyrosine residues. The motif is located downstream of the LRRs, close to the top of the first transmembrane helix. This post-translational modification is shown to be important for high-affinity hormone binding and receptor activation by TSH, LH/CG or FSH. Interestingly, sulfation of the tyrosines is completely dispensable for activation of the TSH receptor by the stimulating autoantibodies of patients with Graves’ disease.
Results
The monoclonal antibody Mab15 interferes with binding and activation of the TSHr by TSH
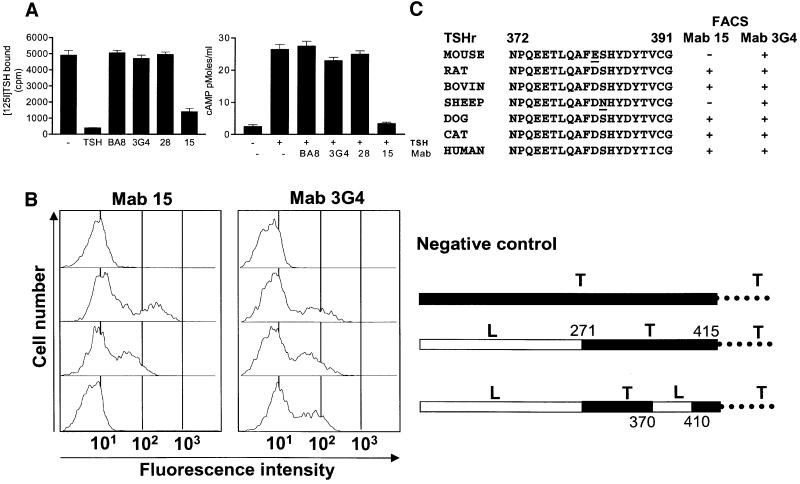
Amongst a series of monoclonal antibodies (Mabs) obtained by genetic immunization of mice with TSHr cDNA and described elsewhere (Costagliola et al., 1998a,b; Ho et al., 2001), Mab15 was shown to block both the binding of TSH and the activation of TSHr by its hormone (Figure 1A). The effect was specific, as three other Mabs against different epitopes were without effect. The epitope of Mab15 was mapped by flow immunocytometry of transfected COS-7 cells. The initial analysis of the binding of Mab15 to a series of LH/CG–TSH receptor chimeras pointed to a segment of the ectodomain between positions 370 and 410, immediately upstream of the first transmembrane helix (Figure 1B). More precise mapping was provided by testing the reactivity of Mab15 towards the TSHr of various species presenting spontaneous amino acid substitutions in the segment of interest (Figure 1C). These experiments identified two residues, D382 and S383, which are most probably part of the epitope.
Fig. 1. Mab15 interferes with binding and activation of the TSHr by TSH. (A) The left side shows inhibition of [125I]bTSH binding to intact CHO cells expressing hTSHr. Binding was measured after a 4 h incubation at room temperature with [125I]TSH (30 000 c.p.m.) and 10 µl of hybridoma supernatant. The right side shows inhibition of TSH-stimulated cAMP accumulation in intact CHO cells expressing hTSHr. (B) Mapping of the epitope of Mab15 with TSHr–LH/CGr chimeras transfected in COS cells. Constructs expressing fusion proteins containing LHr ectodomain fragments (white segment) inserted in TSHr (black segment) and the respective binding profile of Mab15 measured at the cell surface by flow cytometry are aligned on the same row. The results are expressed as histograms, with the fluorescence intensity in arbitrary fluorescence units on the x-axis (reflecting the number of receptors recognized by Mab15 or Mab3G4 at the cell surface) and the number of fluorescent cells on the y-axis (a total of 10 000 cells were analyzed). The histogram displays two populations of cells, with the second (on the right) representing the transfected cells, as detected with Mab15 or Mab3G4. (C) Mapping of the epitope of Mab15 with TSHr from various species. The results indicate that residues D382 and S383, absent in mouse and sheep TSHr, respectively (underlined), could be part of the Mab15 epitope.
The epitope of Mab15 is only found in mature TSHr present at the cell surface
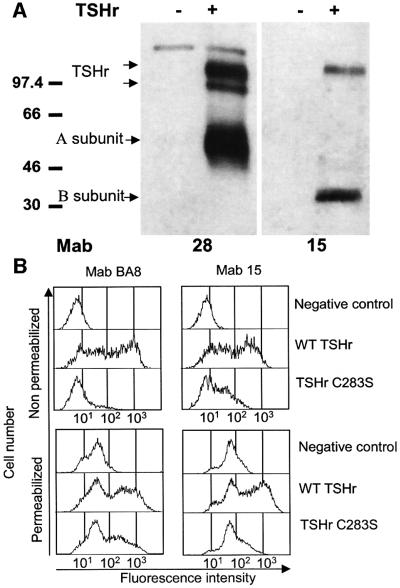
When used in western blotting experiments on extracts from transfected COS-7 cells, Mab15 only recognized the mature, fully glycosylated monomeric receptor (Figure 2A, right upper band) and the non-glycosylated B subunit resulting from the intramolecular cleavage affecting a proportion of the receptors at the cell surface (Figure 2A, right lower band; de Bernard et al., 1999; Tanaka et al., 1999; Chazenbalk et al., 2001). In contrast, Mab28, with its epitope located more upstream (see Materials and methods), recognized the mature receptor and the high mannose precursor of the intact, uncleaved receptor equally well (Figure 2A, left upper doublet). The interpretation that Mab15 recognizes an epitope present only on mature receptors present at the cell surface was confirmed by flow immunocytometry of cells transfected with a mutant TSHr receptor remaining trapped intracellularly (C283S mutant; Ho et al., 2001). While the control Mab BA8 recognizes readily the mutant in cells permeabilized with saponin, Mab15 is totally blind to the mutant (Figure 2B).
Fig. 2. The epitope of Mab15 is only found in mature TSHr present at the cell surface. (A) Solubilized COS cells transfected (+) or not (–) with wild-type TSHr were subjected to SDS–PAGE under reducing conditions, then immunoblotted with Mab28 or Mab15. Molecular mass markers are indicated (in kDa). Mab28, with its epitope located at the N-terminal extremity of the TSHr, detected three bands at ∼120, 97 and 60 kDa, corresponding to the mature holoreceptor, the immature glycosylated protein and the A subunit, respectively. Mab15 detected two bands at ∼120 and 30 kDa, corresponding to the mature holoreceptor expressed at the cell surface and the B subunit, respectively. A and B subunits are generated by the natural cleavage of TSHr occurring at the cell surface (A subunit corresponds to the N-terminal domain, from amino acid 1 to ∼305–315; B subunit corresponds to the C-terminal domain, including the serpentine portion of the receptor, from approximately amino acids 367–380 to the last amino acid at position 764; see Tanaka et al., 1999). (B) Flow cytometry analysis of intact (upper graphs) or saponin-permeabilized (lower graphs) COS cells transfected with wild-type TSHr or the C283S mutant and stained with Mab BA8 (left graphs) or Mab15 (right graphs). Flow cytometry analysis was performed as described in the text. In comparison with wild-type TSHr, the C283S mutant is poorly expressed at the cell surface, whether detected by Mab15 or Mab BA8. When permeabilized cells were used, only Mab BA8 could detect the C283S mutant accumulating inside the cells. In contrast, Mab15 was completely unable to interact with the intracellularly trapped forms of TSHr.
The epitope of Mab15 contains sulfated tyrosine residues
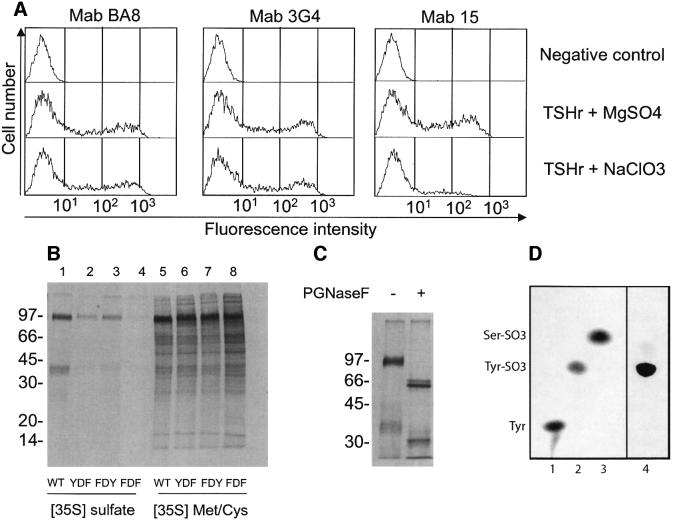
The presence of a conserved YDY tripeptide, one amino acid downstream of two residues belonging to the epitope (D382 and S383; Figure 1C), suggested that sulfation of (one of) the tyrosine(s) might be required for recognition by Mab15, which would provide an explanation for the above results. Although no precise consensus for sulfation is available, DSHYDY would fit the known requirement for acidic residues near the target tyrosines (Bundgaard et al., 1997). The first argument in favour of sulfation on tyrosine 385 and/or 387 is the decrease in immunoreactivity towards Mab15 displayed by TSHr at the surface of cells incubated with 10 mM NaClO3, a known inhibitor of tyrosine sulfation (Figure 3A). Recognition by two anti-TSHr Mabs directed against other epitopes (BA8, 3G4) is only marginally affected by such a treatment. More direct evidence for sulfation of Y385 and Y387 is provided by the decrease in [35S]sulfate incorporation in TSHr constructs harbouring substitutions at these positions. For convenience, these constructs were made on the background of a glycosylphosphatidylinositol (GPI)-anchored ectodomain of the receptor harbouring a His10 tag (Cornelis et al., 2001). Wild-type (YDY), YDF, FDY and FDF constructs were transfected in CHO cells and the cells incubated with [35S]methionine+cysteine or [35S]sulfate. Receptor constructs were affinity purified from cell extracts by nickel chromatography and the resulting material was subjected to PAGE analysis and autoradiography. As can be seen in Figure 3B, when similar amounts of [35S]methionine+cysteine-labelled receptor were loaded for each mutant, incorporation from [35S]sulfate was drastically decreased for both FDY and YDF mutants, the FDF mutant showing virtually no labelling. Incorporation of [35S]sulfate was not in N-linked carbohydrates, since it was completely resistant to treatment by N-glycosidase F (Figure 3C). The results indicate that both tyrosine residues would undergo sulfation in the wild-type TSHr.
Fig. 3. The epitope of Mab15 contains sulfated tyrosines. (A) Flow immunocytometry of cells treated with NaClO3. COS cells transfected with wild-type TSHr were incubated for 16 h in sulfate-free medium in which either MgSO4 or NaClO3 was added to a final concentration of 10 mM. Flow cytometry was then performed with Mabs as described in the text. Mab15, but not Mab BA8 and Mab3G4, failed to recognize TSHr expressed on cells treated with NaClO3. (B) Incorporation of [35S]sulfate in constructs with mutated tyrosines. CHO cells expressing wild-type or mutated (FDY, YDF, FDF) ECD–TSHr–10H–GPI constructs were incubated for 24 h in DMEM without cysteine and methionine, together with 500 µCi each of [35S]cysteine and [35S]methionine (lanes 5–8) or in sulfate-free medium with 1 mCi [35S]sulfate (lanes 1–4). Labelled ectodomain released with PI–PLC and purified on nickel beads were analysed by SDS–PAGE under reducing conditions and quantification of 35S-labelled material was performed by phosphoimager scanning and the image analysis program ImageQuant (Amersham Pharmacia Biotech). Molecular mass markers are indicated (in kDa). (C) Incorporation of [35S]sulfate does not occur in carbohydrates. The purified [35SO3]ECD–TSHR–10H–GPI was incubated for 16 h at 37°C with N-glycosidase F or control buffer and then analysed by SDS–PAGE under reducing conditions. (D) Sulfate is incorporated into tyrosine. The radioactive hydrolysate of [35SO3]ECD–TSHR–10H–GPI (lane 4) was run on TLC in parallel with the standards l-tyrosine (Tyr; lane 1), l-tyrosine O-sulfate (Tyr–SO3; lane 2) and l-serine O-sulfate (Ser–SO3; lane 3). The standards methionine and cysteine (not shown) co-migrated with tyrosine. The standards (lanes 1–3) and the sample (lane 4) were detected by ninhydrin (0.2%, Sigma) staining and fluorography (EN3HANCE, NEN), respectively.
Final proof that [35S]sulfate was incorporated in tyrosine sulfate of the ectodomain was provided by thin-layer electrophoresis of a Ba(OH)2 hydrolysate of the labelled GPI construct harbouring the wild-type YDY segment. The majority of the 35S-labelled material co-migrated with a sulfated tyrosine standard (Figure 3D).
Sulfation of tyrosine 385 is required for high-affinity hormone binding and receptor activation by TSH, but not by thyroid-stimulating autoantibodies
The functional role of tyrosine sulfation was explored by testing the sensitivity to TSH of COS-7 cells transfected transiently with wild-type receptor or constructs with substitutions of tyrosines in the YDY motif (YDF, YDE, FDY, EDY, EDE and FDF). All constructs were expressed at similar levels, as measured by flow immunocytometry with Mab BA8 (Figure 4A). When stimulated by 1 mIU/ml TSH, only constructs bearing a tyrosine in position 385 (wild type, YDF, YDE) responded by an increase in intracellular cAMP accumulation (Figure 4B). Substitu tion of Y385 by either glutamic acid or phenylalanine resulted in mutants completely unresponsive to TSH at this concentration. A concentration–action curve demonstrated a 10-fold increase in EC50 for these two mutants (from 0.15 to 1.5 mIU/ml; Figure 4D). The loss of affinity of the Y385 mutants was enough to completely abolish the binding of 125I-labelled bovine TSH (data not shown). In contrast with the loss of sensitivity to TSH, activation of all mutants by thyroid-stimulating autoantibodies (TSAb) was unaffected, which, besides its intrinsic interest, demonstrates that the mutant receptors are not grossly altered structurally or functionally (Figure 4B) and confirms the equal expression levels observed for each mutant by fluorescence-activated cell sorting (FACS). Evidence that the effect of substitution of Y385 was secondary to the loss of sulfation at this position is provided by the inhibitory action of incubation with NaClO3 on stimulation by bTSH of COS cells transfected with the wild-type TSHr. This treatment obliterates the epitope of Mab15 (Figure 3A) while simultaneously decreasing sensitivity to TSH but not to TSAb (Figure 4C). A concentration–effect curve demonstrated a one order of magnitude increase in EC50 for cells expressing hTSHr after treatment with NaClO3 (Figure 4E). The loss of affinity of the unsulfated hTSHr results in undetectable binding of 125I-labelled bovine TSH to cells incubated with NaClO3 (Figure 4E, inset). The importance of Y385 for stimulation of TSHr by TSH, but not by TSAb, had been noted previously; however, no relation with sulfation was provided (Kosugi et al., 1991). In the same study, residues neighbouring the tyrosines, which may contribute to sulfation (Bundgaard et al., 1997), were mutated (H384 to G and D386 to T; Kosugi et al., 1991) and the mutants generated were shown to behave as the wild-type receptor. The apparent contradiction may be solved by the results from a recent report devoted to the characterization of functional tyrosine sulfation motifs (Nicholas et al., 1999). The conclusion of this study is that secondary structure is the major determinant of sulfation, explaining that motifs deviating from the consensus [presence of acidic residues surrounding the tyrosine(s) (Bundgaard et al., 1997)] may be used efficiently.
Fig. 4. Sulfation of tyrosine 385 is required for high-affinity hormone binding and receptor activation by TSH, but not by thyroid-stimulating autoantibodies. (A) Flow cytometry of TSHr YDY mutants transfected in COS cells. Constructs (TSHr mutants EDY, FDY, YDE, YDF, EDE, FDF in pcDNA3) were transfected in COS-7 cells and flow cytometry analysis with Mab BA8 was performed as described above. (B) Responsiveness of TSHr YDY mutants to stimulation by 1 mU/ml TSH or TSAb. (C) The effect of TSH (0.3 mIU/ml) and TSAb (5%) on cAMP accumulation by COS-7 cells transfected with wild-type TSHr and treated with sulfate-free medium supplemented with MgSO4 or NaClO3. (D) Concentration–action curve of TSH on cAMP accumulation by COS-7 cells transfected with EDY and FDY mutants (EC50 of wild-type TSHr = 0.15 mIU/ml; EC50 of EDY or FDY mutants = 1.5 mIU/ml). (E) Concentration–action curve of TSH on cAMP accumulation by COS-7 cells transfected with wild-type TSHr and treated with sulfate-free medium supplemented with MgSO4 (EC50 = 0.05 mIU/ml) or NaClO3 (EC50 = 0.4 mIU/ml). Inset: [125I]TSH binding on COS-7 cells transfected with wild-type TSHr and treated with sulfate-free medium supplemented with MgSO4 or NaClO3.
Sulfation of tyrosines located at the same positions in mammalian LHr and FSHr is required for receptor activation by human LH/CG and FSH
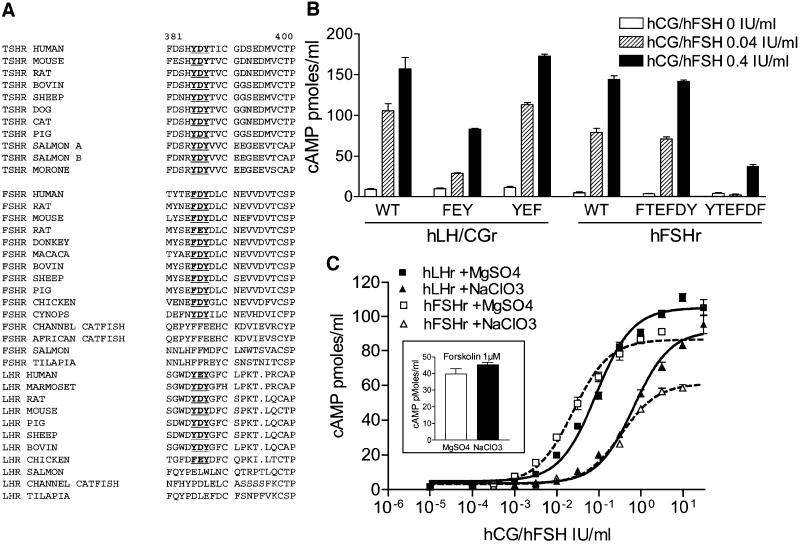
Alignment of the ectodomains of available glycoprotein hormone receptors from different mammals demonstrates complete conservation of the YDY/YEY motif between TSHr and LHr (Figure 5A). For FSHr, the motif is degenerated into FDY; however, an additional tyrosine is found three residues upstream (Figure 5A). Interestingly, in teleost fishes, whereas the YDY motif is conserved in the TSHr of all available species, it is absent in both the LHr and FSHr (Figure 5A; Kumar and Trant, 2001). Mutant human LH/CGr and FSHr (hLH/CGr and hFSHr) were constructed with phenylalanine substitution of the tyrosines in the putative sulfation motif and the functional effects were measured in transiently transfected COS-7 cells (Figure 5B). All the constructs were expressed at the cell surface at a similar level, as measured by flow immunocytometry with polyclonal antibodies specific for their respective receptor and produced by genetic immunization (data not shown). In LH/CGr (and similar to the situation in the TSHr), the FEY mutant lost sensitivity to hCG, whereas the YEF mutant behaved as the wild type. Interestingly, in the FSHr, mutation of the tyrosine residue after the conserved aspartate resulted in decreased sensitivity to FSH, while mutation of the tyrosine located more upstream was without effect (Figure 5B). As for the TSHr, incubation with NaClO3 of COS-7 cells expressing LH/CGr or FSHr decreased sensitivity to the corresponding hormone by one order of magnitude, indicating that sulfation of tyrosines of the YEY and FDY motifs is implicated, respectively (Figure 5C). Stimulation by forskolin of cAMP accumulation in COS-7 cells was completely unaffected by the NaClO3 treatment (Figure 5C, inset).
Fig. 5. Sulfation of homologous residues in LH/CGr and FSHr are similarly implicated in hormone recognition. (A) Alignment of glycoprotein hormone receptor sequences of various species in the YDY motif region (in bold and underlined). (B) Responsiveness of COS-7 cells expressing mutant LH/CGr (LH/CGr mutants FEY, YEF, FEF) and FSHr (FSHr mutants FTEFDY, YTEFDF, FTEFDF) to stimulation by hCG or hFSH. cAMP measurements were performed as described in the text. (C) Concentration–action curve of hCG or hFSH on cAMP accumulation in COS-7 cells transfected with wild-type LH/CGr (black lines and symbols) and FSHr (dotted lines and white symbols) and treated with sulfate-free medium supplemented with MgSO4 (squares; EC50 = 0.08 and 0.03 IU/ml for LH/CGr and FSHr, respectively) or NaClO3 (triangles; EC50 = 0.7 and 0.4 IU/ml for LH/CGr and FSHr, respectively).
Sulfation of TSHr occurs in normal thyrocytes in vivo
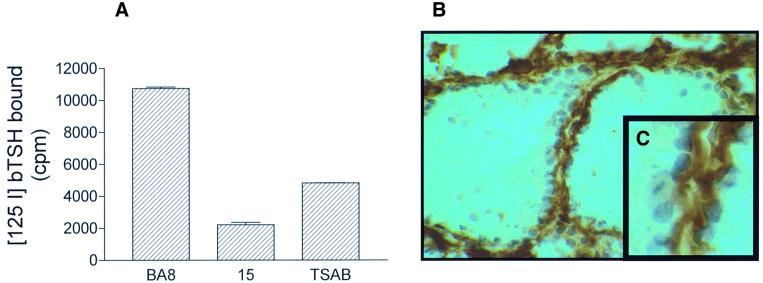
To explore whether sulfation of tyrosine 385 and 387 occurs in normal thyrocytes in vivo, we tested the ability of Mab15 (which recognizes TSHr in its sulfated form only; Figures 2 and 3A) to interact with TSHr extracted from porcine thyroid glands and with the human receptor in tissue sections. As illustrated in Figure 6A, Mab15 was shown to block the binding of 125I-labelled TSH to the porcine receptor completely. Similarly, immunohistochemistry performed on human thyroid sections with Mab5 showed positive labelling of thyroid follicles (Figure 6B). As expected, the labelling concentrated at the basolateral pole of the thyroid cells (Figure 6C). Together, these experiments demonstrate that sulfation is part of the normal post-translational modification events affecting the TSHr in vivo.
Fig. 6. Sulfation of TSHr occurs in normal thyrocytes, in vivo. (A) Interaction of Mab15 with [125I]TSH binding on porcine thyroid membrane was assayed using a commercial kit (see Materials and methods). Mab15 but not Mab BA8 inhibited [125I]TSH binding to porcine TSHr. (B) Immunoperoxidase staining of frozen thyroid section of human thyroid using Mab15. Almost all thyroid cells show a positive labelling concentrated at the basolateral pole (C).
Discussion
Sulfation of tyrosines is a late post-translational modification taking place in the trans-Golgi network and affecting a wide spectrum of membrane or secreted proteins (Huttner, 1982). In a handful of cases, a clear functional significance has been documented (Hortin et al., 1989; Leyte et al., 1991; Bundgaard et al., 1995; Dong et al., 2001), but the mechanism of how sulfation affects protein function has been addressed for GP1bα (Dong et al., 2001) and PSGL-1 only (Somers et al., 2000). Recently, sulfation of tyrosine residues of the N-terminal extension of three GPCRs belonging to the chemokine (CCR5 and CCR2B) or chemoattractant (C5A receptor) receptor family has been demonstrated (Farzan et al., 1999, 2001; Preobrazhensky et al., 2000). In CCR5 and the C5A receptor, tyrosine sulfation was required for high-affinity recognition of the receptors by their natural agonists. In addition, in CCR5, it was required for binding of gp120 of HIV-1 in the context of its co-receptor activity (Farzan et al., 1999). Glycoprotein hormones and their receptors have been used as a paradigm for the study of co-evolution of hormone–receptor couples (Moyle et al., 1994), and current information points to the LRRs in the ectodomains of the receptors as the major players in the recognition and interaction phenomena (Bhowmick et al., 1996; Rodien et al., 1998; Song et al., 2001). The present results demonstrate that sulfation of a conserved motif situated outside the LRR segment is required for efficient recognition and activation of the receptors by their cognate hormones. Mapping of functionally important contact surfaces, performed with the aid of LH/CGr–FSHr or LH/CGr–TSHr chimeras (Braun et al., 1991; Rapoport et al., 1998), failed to identify the sulfated motifs, which is compatible with the hypothesis that they must be interchangeable within the human glycoprotein hormone receptor family. Whereas this is easily understandable for the LH/CGr–TSHr couple, in which tyrosines in a homologous position seem to be implicated (the first Y of the YDY or YEY motifs, see above), it suggests that the conformation of the FSHr would be different in this region, allowing the single tyrosine of an FDY motif to fulfil the same role.
The functional importance of sulfation of the glycoprotein hormone receptors will need to be remembered when trying to produce homogeneous preparations of hormone–ectodomain complexes suitable for crystallography (Remy et al., 2001). In this context and contrary to the situation in GP1bα protein (Dong et al., 2001), the negative charge of glutamic acid does not constitute a functional substitute for sulfated tyrosine in the glycoprotein hormone receptors. On the other hand, the absence of the YDY motif in the secreted or shedded forms of the TSHr, which have been proposed to be present in the circulation (de Bernard et al., 1999; Tanaka et al., 1999), makes it unlikely that they could play a role of carrier or buffer for the hormone.
For LH/CGr and TSHr, our results provide a structural rationale to the observations of Szkundlinsky et al. (Grossmann et al., 1997) that basic residues in the α subunit of the glycoprotein hormones are important for binding to and activation of their receptors. In addition, these authors found that reversion to lysine at positions of the human α subunit where basic residues are found in non-primates leads to superagonists with a >100-fold increase in affinity and bioactivity, suggesting that interactions of the hormones with negatively charged residues have been important in the evolution of the hormone– receptor couples. In agreement with their model for TSH–TSHr interaction (Grossmann et al., 1997), we propose that the sulfated tyrosines of the YDY motif, close to the serpentine portion of the receptor, would establish a salt-sensitive electrostatic bridge with basic residues of the α subunit of the mutants or lower mammalian hormones. It is tempting to propose that this interaction constitutes an attraction common to all three glycoprotein hormones between their identical α subunits and their receptors, on top of which additional attractive and repulsive interactions, specific to the individual β subunit–ectodomain pairs, would be superimposed. In the absence of structural data on hormone–ectodomain complexes, we cannot exclude that the effect of sulfation would be indirect, affecting the structure of the ectodomain, rather than being involved in the interaction with the hormones. In the case of the TSHr, and whatever the precise structural effects of sulfation, the observation that it is completely dispensable for activation of the receptor by TSAb indicates that tyrosine sulfation is not required for the activation mechanism per se.
The presence of functionally important sulfated tyrosines in all three glycoprotein hormone receptors of mammals suggests that this post-translational modification was already present in the ancestral receptor at the origin of the gene family. Contrary to the current view that the TSHr, LHr and FSHr found in all vertebrate species would be orthologous (Kumar and Trant, 2001), the absence of a convincing sulfation motif in the FSHr and LHr homologues of fish may signify that these receptors would result from a separate duplication event involving an ancestral gene that has lost the capability to encode a sulfated receptor. Compatible with this view, the available receptor sequences from birds (chicken) and amphibians (Cynops pyrrhogaster = Salamandridae; Figure 5A) display a sulfation motif, pointing to the existence of a distinct evolutionary scenario in fish.
Finally, similar to the modulation of the bioactivity of glycoprotein hormones by differential glycosylation (Persani et al., 1998; Ferrari et al., 2001), our results suggest that tyrosine sulfation of the corresponding receptors may constitute a regulatory step allowing cells to adjust their sensitivity to hormones at a post-translational level. The present observation would justify looking at whether tyrosine sulfation plays a role in additional members of the large family of GPCRs.
Materials and methods
Reagents
Mab BA8, recognizing a conformational epitope on the TSHr extracellular domain, has been described elsewhere (Costagliola et al., 1998b). Mab3G4 (Costagliola et al., 1998a) and Mab28 (Ho et al., 2001), recognizing linear epitopes on the TSHr ectodomain (VFFEEQE, residues 354–360; and DFRVT, residues 36–45), were obtained using the same genetic immunization approach. Iodinated bTSH (bioactivity 50–60 TSH IU/mg; specific radioactivity 58 µCi/µg) was a kind gift from BRAHMS Diagnostica (Berlin, Germany). cDNAs coding for the mouse and cat TSHr were a kind gift from Peter Kopp (Northwestern University, Chicago, IL). cDNA encoding the sheep TSHr was a generous gift from Juergen Bockmann (University of Munster, Germany). Sulfated serine and sulfated tyrosine used as standards in the thin-layer chromatography (TLC) experiment were a kind gift from Hyeryun Choe (Departments of Medicine and Pediatrics, Beth Israel Hospital and Harvard Medical School, Boston, MA).
Determination of the biological activity of Mab15
Mab15 was produced by genetic immunization of NMRI mouse, using the same approach already described for Mab BA8 (Costagliola et al., 1998b).
Thyrotrophin binding inhibiting activity (TBII) was measured on a CHO cell line expressing high levels of the human thyrotrophin receptor, JP09, as described previously with minor modifications (Costagliola et al., 1998b). Briefly, 5 × 104 cells/well in 96-well plates were incubated in 0.1 ml of modified Hanks’ buffer without NaCl (isotonicity maintained with 280 mM sucrose), supplemented with 2.5% low fat milk, [125I]TSH (30 000 c.p.m.) and 10 µl of hybridoma supernatant, for 4 h at room temperature. At the end of the incubation period, the cells were rapidly rinsed with the same ice-cold buffer, solubilized with 0.2 ml of 1 N NaOH and radioactivity was measured in a gamma counter. All experiments were carried out in triplicate and results are expressed as c.p.m. bound. The ability of Mab15 to interfere with [125I]TSH binding to porcine thyroid membranes was evaluated in a commercial assay for TSHr-binding autoantibodies (TRAK assay; BRAHMS Diagnostics, Berlin, Germany). Briefly, solubilized TSHr (50 µl) was pre-incubated with 50 µl of TSAb (positive control) or with 1 µg of Mab15 or Mab BA8 (negative control) diluted in 50 µl of normal human serum. [125I]TSH was then added and incubated for 2 h at room temperature. Solubilized TSHr–[125I]TSH complexes were precipitated by polyethylene glycol. Results were expressed as [125I]TSH bound (c.p.m.).
Thyroid-stimulating (TSAb) and -blocking (TSBAb) activities were measured using a CHO cell line expressing moderate levels of the human thyrotrophin receptor, JP26 (Perret et al., 1990; Costagliola et al., 1998b). Briefly, 5 × 104 cells/well in 96-well plates were incubated in 5 mM KCl, 0.25 mM KH2PO4, 0.5 mM MgSO4, 0.4 mM Na2HPO4, 1 mM CaCl2, 0.1% glucose, 2 mM 3-isobutyl-1-methyl-xanthine (IBMX), 20 mM HEPES and 0.3% bovine serum albumin (BSA) containing 10 µl of hybridoma supernatant (total volume = 100 µl/well). After 4 h incubation at 37°C, cAMP released into the medium was measured by a radioimmunoassay (RIA) according to the method of Brooker (Brooker et al., 1979). TSAb activity was measured under the basal conditions described above and TSBAb in identical conditions but with the addition of 200 µU/ml final concentration of bovine TSH (Sigma, St Louis, MO). Duplicate samples were assayed in all experiments; results are expressed as picomoles of cAMP/ml.
Plasmid constructions
The ECD–10H–GPI construct (the TSHr ectodomain fused to a signal peptide encoding a GPI anchor and containing a His10 tag) was described previously (Cornelis et al., 2001). Constructs expressing chimeric proteins containing LHr ectodomain segments inserted in TSHr were made by inserting the appropriate PCR fragment (see Figure 1) into the TSHr pcDNA3 plasmid. cDNAs encoding LHr and FSHr were ligated into the vector pcDNA3. The receptors with tyrosine mutations were generated by the QuikChange site-directed mutagenesis method, starting with TSHr, LHr or FSHr in pBluescript SK+ and two synthetic oligonucleotide primers containing the desired mutation(s), as already described (Ho et al., 2001). After sequencing for confirmation of the mutation, a restriction fragment from each mutant obtained above was inserted in the cDNA coding for the ECD–10H–GPI construct (TSHr mutants FDY, YDF, FDF) in pEFIN3 vector (Euroscreen, Brussels, Belgium) or in full-length receptor cDNA contructs in pcDNA3 (TSHR mutants EDY, FDY, YDE, YDF, EDE, FDF; LHr mutants FEY, YEF, FEF; FSHr mutants FTEFDY, YTEFDF, FTEFDF).
Transfection experiments
COS-7 cells were used for transient expression allowing functional assays. They were transfected by the DEAE–dextran method followed by a dimethylsulfoxide shock as described previously (Lopata et al., 1984; Govaerts et al., 2001). Two days after transfection, cells were used for cAMP determinations and flow immunocytofluorometry. Triplicate dishes were used for each assay. Each experiment was repeated at least twice. Cells transfected with the empty vector were always run as controls.
Quantification of cell surface expression of TSHr, LHr or FSHr constructs by flow immunocytometry
COS cells transiently transfected with the different cDNAs were prepared as described previously (Costagliola et al., 1998a). After detachment, they were centrifuged at 500 g for 3 min at 4°C and the supernatant discarded. They were then incubated for 30 min at room temperature in 100 µl of 0.1% phosphate-buffered saline (PBS)–BSA containing either Mab BA8, Mab3G4 or Mab15 for TSHr and LHr–TSHr chimeric constructs or polyclonal serum generated by genetic immunization of Balb/c mice (our unpublished data) for LHr and FSHr. Cells were then washed with 4 ml of 0.1% PBS–BSA and centrifuged as above. They were incubated on ice for 30 min, in the dark, with fluorescein-conjugated γ chain-specific goat anti-mouse IgG (Sigma) in the same buffer. Propidium iodide (10 µg/ml) was used for detection of damaged cells that were excluded from the analysis. Cells were washed and resuspended in 250 µl of 0.1% PBS–BSA. The immunoreactivity of permeabilized cells was determined after fixing them for 10 min on ice with 1% paraformaldehyde and treatment for 30 min at room temperature with 0.2% saponin. All subsequent steps with antibodies were performed in 0.2% saponin. The fluorescence of 10 000 cells per tube was assayed by a FACScan® flow cytofluorometer (Becton Dickinson, Erembodegem, Belgium).
Wild-type or tyrosine-mutated receptors: cAMP determination and TSH binding
Holoreceptors (TSHr, LHr, FSHr), whether wild type or tyrosine mutated, were transfected in COS cells, as described previously (Lopata et al., 1984; Govaerts et al., 2001). For cAMP determinations, culture medium was removed 48 h after transfection and replaced by Krebs–Ringer– HEPES buffer (KRH) for 30 min. Thereafter, cells were incubated for 60 min in fresh KRH supplemented with 25 µM phosphodiesterase inhibitor (Rolipram; Laboratory Logeais, Issy les Moulineaux, France) and various concentrations of bovine TSH (Sigma), hCG (Sigma) or recombinant hFSH (Organon Belge SA, Brussels, Belgium). At the end of the 1 h incubation, the medium was discarded and replaced with 0.1 M HCl. The cell extracts were dried in a vacuum concentrator, resuspended in water and diluted appropriately for cAMP measurements by RIA according to the method of Brooker (Brooker et al., 1979). Duplicate samples were assayed in all experiments; results are expressed as picomoles of cAMP/ml. Concentration–action curves were fitted with the Prism® computer program (GraphPad Software, Inc., San Diego, CA). For cAMP determination with unsulfated receptors, COS cells transfected with wild-type receptors were washed three times with PBS 24 h after transfection and incubated for 16 h in sulfate-free medium (ICN Biomedicals, Asse-Relegem, Belgium), in which either magnesium sulfate or sodium chlorate was added to a final concentration of 10 mM. TSH binding on tyrosine-mutated TSH receptor or cells treated with either magnesium sulfate or sodium chlorate was performed as described above for detection of TBII activity.
Western blot of hTSHr
Preparation of receptor. Six dishes, each containing 300 000 COS cells transfected with pcDNA3 or TSHr–pcDNA3, were treated with 5 mM EDTA and 5 mM EGTA in PBS and the cells spun down at 280 g. The cell pellet was suspended and homogenized in a Potter–Elvehjem glass homogenizer with a Teflon pestle in 1250 µl of lysis buffer [100 mM (NH4)2SO4, 20 mM Tris–HCl pH 7.5, 10% glycerol) containing protease inhibitors (Complete®; Boehringer Mannheim, Brussels, Belgium). The lysate was then centrifuged at 500 g for 10 min and the supernatant recovered for further ultracentrifugation at 30 000 g for 30 min. Lysis buffer containing 1% N-dodecyl-β-d-maltoside (200 µl; Anatrace, Maumee, OH) was added to the pellet and the suspension incubated for another 30 min at 4°C under constant rotation to allow thorough mixing. Final centrifugation was carried out at 100 000 g for 1 h and the supernatant stored at –80°C for further use. All procedures were performed at 4°C.
SDS–PAGE and immunoblotting. Laemmli sample buffer (5×) containing SDS (10 %) and β-mercaptoethanol (1 M) as a reducing agent (3 µl) was added to 10 µl of receptor protein prepared as above and denatured at 40°C for 1 h. The sample was then run on a 7% acrylamide gel and probed with Mab28 or Mab15 (culture supernatants diluted 1:50). The proteins were visualized with an anti-mouse IgG–horseradish peroxidase conjugate and the ECL Plus western blotting detection system (Amersham Pharmacia Biotech, Roosendaal, The Netherlands).
Labelling of wild-type and tyrosine-mutated ectodomain of the TSHr
Stable CHO cell lines expressing wild-type or tyrosine-mutated (FDY, YDF, FDF) ECD–10H–GPI constructs were generated as described previously (Cornelis et al., 2001). The level of expression of wild-type or mutated ectodomain expressed as a GPI anchor protein at the cell surface was measured by immunofluorimetry with Mab BA8. Twenty-four hours after seeding (5 × 106 cells/10 cm dish), cells were washed three times with PBS and incubated for 24 h in Dulbecco’s modified Eagle’s medium (DMEM) without cysteine and methionine (Sigma Aldrich, Bornem, Belgium) with 500 µCi each of [35S]cysteine and [35S]methionine (Amersham Pharmacia Biotech), or in sulfate-free medium (ICN Biomedicals) with 1 mCi of [35S]sulfate (Amersham Pharmacia Biotech). Cells were then washed three times with PBS and the labelled ectodomain, after cleavage with PI–PLC, was purified on nickel beads as described previously (Cornelis et al., 2001).
The dried pellet of purified ECD was then resuspended in 20 µl of Laemmli sample buffer containing β-mercaptoethanol as a reducing agent and denatured at 40°C for 1 h. Samples (volumes loaded were normalized to the level of expression measured by FACS) were analysed by SDS–PAGE and 35S-labelled material was quantified by phosphorimager scanning and the image analysis program ImageQuant (Molecular Dynamics, Amersham Pharmacia Biotech).
Thin-layer electrophoresis of tyrosine O-[35S]sulfate residues after digestion of ECD–TSHr
The purified [35SO3]ECD–TSHr was eluted from the nickel resin by 500 mM imidazole, 50 mM (NH4)HCO3 pH 8 and lyophilized. The sulfated amino acid residues were released from the receptor and analysed according to Huttner (1984). Briefly, the [35SO3]ECD–TSHR was solubilized in degassed 0.2 M Ba(OH)2 and hydrolysed for 20 h in a PCR block at 95°C. After neutralization by sulfuric acid, the sample was lyophilized and solubilized in N,N-dimethylformamide containing 3 µg of tyrosine-O-sulfate and 3 µg of serine-O-sulfate as standards. The sample and standards were run for 40 min at 300 V on a 0.1 mm cellulose-coated TLC plate (Merck, Leuven, Belgium) impregnated with 5% acetic acid and 0.5% pyridine. Radioactivity was detected by fluorography (EN3HANCE, NEN, Brussels, Belgium) and the standards were visualized by ninhydrin (0.2%; Sigma) staining.
Immunohistochemistry
Human thyroid glands were obtained at surgery and designed for immunohistochemistry to detect the location of TSHr using Mab15. To generate cryostat sections, the biopsies were inserted into a rat liver fragment, embedded in Tissue-Teck and rapidly frozen in isopentane cooled in liquid nitrogen. The frozen sections were subjected to immunoperoxidase staining using Mab15 culture supernatant at 1/100 (Costagliola et al., 1998b).
Acknowledgments
Acknowledgements
We thank Veronique Janssens for expert technical assistance and Hyeryun Choe for the kind gift of sulfated serine and sulfated tyrosine. We also wish to ackowledge Ho Su Chin and Michel Milinkovitch for editorial assistance and helpful discussion. This study was supported by the Belgian State Prime Minister’s Office Service for Sciences, Technology and Culture. Also supported by grants from the FRSM, FNRS, Association Recherche Biomedicale et Diagnostic and BRAHMS Diagnostics. S.C. is Chercheur Qualifié at the FNRS.
References
- Bhowmick N., Huang,J., Puett,D., Isaacs,N.W. and Lapthorn,A.J. (1996) Determination of residues important in hormone binding to the extracellular domain of the luteinizing hormone/chorionic gonadotropin receptor by site-directed mutagenesis and modeling. Mol. Endocrinol., 10, 1147–1159. [DOI] [PubMed] [Google Scholar]
- Braun T., Schofield,P.R. and Sprengel,R. (1991) Amino-terminal leucine-rich repeats in gonadotropin receptors determine hormone selectivity. EMBO J., 10, 1885–1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooker G., Harper,J.F., Terasaki,W.L. and Moylan,R.D. (1979) Radioimmunoassay of cyclic AMP and cyclic GMP. Adv. Cyclic Nucleotide Res., 10, 1–33. [PubMed] [Google Scholar]
- Bundgaard J.R., Vuust,J. and Rehfeld,J.F. (1995) Tyrosine O-sulfation promotes proteolytic processing of progastrin. EMBO J., 14, 3073–3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bundgaard J.R., Vuust,J. and Rehfeld,J.F. (1997) New consensus features for tyrosine O-sulfation determined by mutational analysis. J. Biol. Chem., 272, 21700–21705. [DOI] [PubMed] [Google Scholar]
- Chazenbalk G.D., McLachlan,S.M., Pichurin,P., Yan,X.M. and Rapoport,B. (2001) A prion-like shift between two conformational forms of a recombinant thyrotropin receptor A-subunit module: purification and stabilization using chemical chaperones of the form reactive with Graves’ autoantibodies. J. Clin. Endocrinol. Metab., 86, 1287–1293. [DOI] [PubMed] [Google Scholar]
- Cornelis S., Uttenweiler-Joseph,S., Panneels,V., Vassart,G. and Costagliola,S. (2001) Purification and characterization of a soluble bioactive amino-terminal extracellular domain of the human thyrotropin receptor. Biochemistry, 40, 9860–9869. [DOI] [PubMed] [Google Scholar]
- Costagliola S., Khoo,D. and Vassart,G. (1998a) Production of bioactive amino-terminal domain of the thyrotropin receptor via insertion in the plasma membrane by a glycosylphosphatidylinositol anchor. FEBS Lett., 436, 427–433. [DOI] [PubMed] [Google Scholar]
- Costagliola S., Rodien,P., Many,M.C., Ludgate,M. and Vassart,G. (1998b) Genetic immunization against the human thyrotropin receptor causes thyroiditis and allows production of monoclonal antibodies recognizing the native receptor. J. Immunol., 160, 1458–1465. [PubMed] [Google Scholar]
- de Bernard S., Misrahi,M., Huet,J.C., Beau,I., Desroches,A., Loosfelt,H., Pichon,C., Pernollet,J.C. and Milgrom,E. (1999) Sequential cleavage and excision of a segment of the thyrotropin receptor ectodomain. J. Biol. Chem., 274, 101–107. [DOI] [PubMed] [Google Scholar]
- Dong J., Ye,P., Schade,A.J., Gao,S., Romo,G.M., Turner,N.T., McIntire,L.V. and Lopez,J.A. (2001) Tyrosine sulfation of glycoprotein I(b)α. Role of electrostatic interactions in von Willebrand factor binding. J. Biol. Chem., 276, 16690–16694. [DOI] [PubMed] [Google Scholar]
- Farzan M., Mirzabekov,T., Kolchinsky,P., Wyatt,R., Cayabyab,M., Gerard,N.P., Gerard,C., Sodroski,J. and Choe,H. (1999) Tyrosine sulfation of the amino terminus of CCR5 facilitates HIV-1 entry. Cell, 96, 667–676. [DOI] [PubMed] [Google Scholar]
- Farzan M., Schnitzler,C.E., Vasilieva,N., Leung,D., Kuhn,J., Gerard,C., Gerard,N.P. and Choe,H. (2001) Sulfated tyrosines contribute to the formation of the C5a docking site of the human C5a anaphylatoxin receptor. J. Exp. Med., 193, 1059–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari M.C., Parini,R., Di Rocco,M.D., Radetti,G., Beck-Peccoz,P. and Persani,L. (2001) Lectin analyses of glycoprotein hormones in patients with congenital disorders of glycosylation. Eur. J. Endocrinol., 144, 409–416. [DOI] [PubMed] [Google Scholar]
- Gether U. (2000) Uncovering molecular mechanisms involved in activation of G protein-coupled receptors. Endocr. Rev., 21, 90–113. [DOI] [PubMed] [Google Scholar]
- Govaerts C., Lefort,A., Costagliola,S., Wodak,S.J., Ballesteros,J.A., Van Sande,J., Pardo,L. and Vassart,G. (2001) A conserved ASN in TM7 is a on/off switch in the activation of the TSH receptor. J. Biol. Chem., 276, 22991–22999. [DOI] [PubMed] [Google Scholar]
- Grossmann M., Weintraub,B.D. and Szkudlinski,M.W. (1997) Novel insights into the molecular mechanisms of human thyrotropin action: structural, physiological and therapeutic implications for the glycoprotein hormone family. Endocr. Rev., 18, 476–501. [DOI] [PubMed] [Google Scholar]
- Grossmann M., Leitolf,H., Weintraub,B.D. and Szkudlinski,M.W. (1998) A rational design strategy for protein hormone superagonists. Nature Biotechnol., 16, 871–875. [DOI] [PubMed] [Google Scholar]
- Ho S.C., Van Sande,J., Lefort,A., Vassart,G. and Costagliola,S. (2001) Effects of mutations involving the highly conserved S281HCC motif in the extracellular domain of the thyrotropin (TSH) receptor on TSH binding and constitutive activity. Endocrinology, 142, 2760–2767. [DOI] [PubMed] [Google Scholar]
- Hortin G.L., Farries,T.C., Graham,J.P. and Atkinson,J.P. (1989) Sulfation of tyrosine residues increases activity of the fourth component of complement. Proc. Natl Acad. Sci. USA, 86, 1338–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttner W.B. (1982) Sulphation of tyrosine residues—a widespread modification of proteins. Nature, 299, 273–276. [DOI] [PubMed] [Google Scholar]
- Huttner W.B. (1984) Determination and occurence of tyrosine O-sulfate in proteins. Methods Enzymol., 107, 200–223. [DOI] [PubMed] [Google Scholar]
- Jiang X., Dreano,M., Buckler,D.R., Cheng,S., Ythier,A., Wu,H., Hendrickson,W.A. and el Tayar,N. (1995) Structural predictions for the ligand-binding region of glycoprotein hormone receptors and the nature of hormone–receptor interactions. Structure, 3, 1341–1353. [DOI] [PubMed] [Google Scholar]
- Kajava A.V., Vassart,G. and Wodak,S.J. (1995) Modeling of the three-dimensional structure of proteins with the typical leucine-rich repeats. Structure, 3, 867–877. [DOI] [PubMed] [Google Scholar]
- Kobe B. and Deisenhofer,J. (1993) Crystal structure of porcine ribonuclease inhibitor, a protein with leucine-rich repeats. Nature, 366, 751–756. [DOI] [PubMed] [Google Scholar]
- Kobe B. and Deisenhofer,J. (1995a) A structural basis of the interactions between leucine-rich repeats and protein ligands. Nature, 374, 183–186. [DOI] [PubMed] [Google Scholar]
- Kobe B. and Deisenhofer,J. (1995b) Proteins with leucine-rich repeats. Curr. Opin. Struct. Biol., 5, 409–416. [DOI] [PubMed] [Google Scholar]
- Kosugi S., Ban,T., Akamizu,T. and Kohn,L.D. (1991) Site-directed mutagenesis of a portion of the extracellular domain of the rat thyrotropin receptor important in autoimmune thyroid disease and nonhomologous with gonadotropin receptors. Relationship of functional and immunogenic domains. J. Biol. Chem., 266, 19413–19418. [PubMed] [Google Scholar]
- Kumar R.S. and Trant,J.M. (2001) Piscine glycoprotein hormone (gonadotropin and thyrotropin) receptors: a review of recent developments. Comp. Biochem. Physiol. B Biochem. Mol. Biol., 129, 347–355. [DOI] [PubMed] [Google Scholar]
- Leyte A., van Schijndel,H.B., Niehrs,C., Huttner,W.B., Verbeet,M.P., Mertens,K. and van Mourik,J.A. (1991) Sulfation of Tyr1680 of human blood coagulation factor VIII is essential for the interaction of factor VIII with von Willebrand factor. J. Biol. Chem., 266, 740–746. [PubMed] [Google Scholar]
- Lopata M.A., Cleveland,D.W. and Sollner-Webb,B. (1984) High level transient expression of a chloramphenicol acetyl transferase gene by DEAE–dextran mediated DNA transfection coupled with a dimethyl sulfoxide or glycerol shock treatment. Nucleic Acids Res., 12, 5707–5717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyle W.R., Campbell,R.K., Myers,R.V., Bernard,M.P., Han,Y. and Wang,X. (1994) Co-evolution of ligand–receptor pairs. Nature, 368, 251–255. [DOI] [PubMed] [Google Scholar]
- Nicholas H.B. Jr, Chan,S.S. and Rosenquist,G.L. (1999) Reevaluation of the determinants of tyrosine sulfation. Endocrine, 11, 285–292. [DOI] [PubMed] [Google Scholar]
- Perret J., Ludgate,M., Libert,F., Gerard,C., Dumont,J.E., Vassart,G. and Parmentier,M. (1990) Stable expression of the human TSH receptor in CHO cells and characterization of differentially expressing clones. Biochem. Biophys. Res. Commun., 171, 1044–1050. [DOI] [PubMed] [Google Scholar]
- Persani L., Borgato,S., Romoli,R., Asteria,C., Pizzocaro,A. and Beck-Peccoz,P. (1998) Changes in the degree of sialylation of carbohydrate chains modify the biological properties of circulating thyrotropin isoforms in various physiological and pathological states. J. Clin. Endocrinol. Metab., 83, 2486–2492. [DOI] [PubMed] [Google Scholar]
- Preobrazhensky A.A., Dragan,S., Kawano,T., Gavrilin,M.A., Gulina,I.V., Chakravarty,L. and Kolattukudy,P.E. (2000) Monocyte chemotactic protein-1 receptor CCR2B is a glycoprotein that has tyrosine sulfation in a conserved extracellular N-terminal region. J. Immunol., 165, 5295–5303. [DOI] [PubMed] [Google Scholar]
- Rapoport B., Chazenbalk,G.D., Jaume,J.C. and McLachlan,S.M. (1998) The thyrotropin (TSH) receptor: interaction with TSH and autoantibodies. Endocr. Rev., 19, 673–716. [DOI] [PubMed] [Google Scholar]
- Remy J.J., Nespoulous,C., Grosclaude,J., Grebert,D., Couture,L., Pajot,E. and Salesse,R. (2001) Purification and structural analysis of a soluble human chorionogonadotropin hormone–receptor complex. J. Biol. Chem., 276, 1681–1687. [DOI] [PubMed] [Google Scholar]
- Rodien P., Bremont,C., Sanson,M.L., Parma,J., Van Sande,J., Costagliola,S., Luton,J.P., Vassart,G. and Duprez,L. (1998) Familial gestational hyperthyroidism caused by a mutant thyrotropin receptor hypersensitive to human chorionic gonadotropin. N. Engl. J. Med., 339, 1823–1826. [DOI] [PubMed] [Google Scholar]
- Somers W.S., Tang,J., Shaw,G.D. and Camphausen,R.T. (2000) Insights into the molecular basis of leukocyte tethering and rolling revealed by structures of P- and E-selectin bound to SLe(X) and PSGL-1. Cell, 103, 467–479. [DOI] [PubMed] [Google Scholar]
- Song Y.S., Ji,I., Beauchamp,J., Isaacs,N.W. and Ji,T.H. (2001) Hormone interactions to Leu-rich repeats in the gonadotropin receptors. I. Analysis of Leu-rich repeats of human luteinizing hormone/chorionic gonadotropin receptor and follicle-stimulating hormone receptor. J. Biol. Chem., 276, 3426–3435. [DOI] [PubMed] [Google Scholar]
- Szkudlinski M.W., Teh,N.G., Grossmann,M., Tropea,J.E. and Weintraub,B.D. (1996) Engineering human glycoprotein hormone superactive analogues. Nature Biotechnol., 14, 1257–1263. [DOI] [PubMed] [Google Scholar]
- Tanaka K., Chazenbalk,G., McLachlan,S. and Rapoport,B. (1999) Subunit structure of thyrotropin receptors expressed on the cell surface. J. Biol. Chem., 274, 33979–33984. [DOI] [PubMed] [Google Scholar]
- Themmen A.P.N. and Huhtaniemi,I.T. (2000) Mutations of gonado tropins and gonadotropin receptors: elucidating the physiology and pathophysiology of pituitary–gonadal function. Endocr. Rev., 21, 551–583. [DOI] [PubMed] [Google Scholar]
- Vassart G., Refetoff,S. and Dumont,J.E. (1995) Thyroid disorders. In Scriver,C.R., Beaudet,A.L., Sly,W.S. and Valle,D. (eds), The Metabolic and Molecular Bases of Inherited Diseases. McGraw-Hill Inc., New York, NY, pp. 2883–2928.