Abstract
Introduction
Acne vulgaris is chronic skin condition with considerable physical, psychological, social, and economic impacts. Adherence to Western diets with a high glycemic index and dairy content has been linked to acne. However, the associations between acne and the Mediterranean diet (MD), a non-Western diet, have not been reviewed. This review aimed to examine the associations between adherence to the MD and the development and severity of acne vulgaris.
Materials and methods
PubMed, Embase, and Web of Science databases were searched for studies examining the association between adherence to the MD and acne vulgaris. Data were extracted by two independent reviewers. The risk of bias was assessed using the Newcastle‒Ottawa Scale, and the quality of evidence was assessed using the GRADE tool. The Meta-analyses were conducted using a random-effects model to pool the odds ratio (OR) and correlation coefficient.
Results
Five case‒control studies were included, with 765 participants, 340 cases, and 425 controls. The meta-analysis found that higher adherence to the MD was not significantly associated with lower odds of acne development (OR 0.32, CI = 0.08–1.28), with high heterogeneity (Q test p = .02, I2 = 75%). The second meta-analysis of correlational studies found a significant negative correlation between adherence to the MD and acne severity (correlation coefficient= -0.29, CI= -0.55 to -0.03), with high heterogeneity (Q test p = .008, I2 = 79%). Higher consumption of vegetables was significantly associated with lower odds of acne development (OR = 0.46, 95% CI, 0.29–0.74). The quality of evidence for the associations with both acne development and severity was low.
Conclusions
This review found that higher adherence to the Mediterranean diet was significantly correlated with less severe acne, while the association with acne development was not significant. Due to the low number of studies, small sample size, and methodological limitations, more well-designed studies are needed to strengthen the evidence base.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12986-025-01033-9.
Keywords: Acne, Acne vulgaris, Mediterranean diet, Meta-analysis, Systematic review
Introduction
Acne vulgaris is a chronic skin condition of the pilosebaceous unit with a multifactorial and intricate pathophysiology that includes increased sebum production, androgenic hormonal effects, follicular hyperkeratinization, and bacterial hyperproliferation [1–3]. Acne lesions may range from mild, noninflammatory comedones to papules, pustules, nodules, and cysts and can progress into long-term complications, such as scarring and hyperpigmentation [1, 4, 5]. Furthermore, acne vulgaris is associated with psychological, social, and economic impacts, including depression, anxiety, unemployment, and productivity loss [6–9].
The historical association between acne vulgaris and diet dates back to the late nineteenth century, with multiple food items being labelled as pro-acnegenic [10]. The debate surrounding acne and diet grew polarized, as the findings of several studies conducted during the 1960 s questioned the role of chocolate and sugar as the two major culprits behind acne [11, 12]. However, the impact of diet on acne, albeit unsubstantiated, has resurged in recent decades. Particularly, adherence to diets with a high glycemic index (GI) may have a pro-acnegenic effect [13–17]. Similarly, increased dairy consumption has been linked to acne development and severity, although this has been based on observational studies and demonstrated only in certain age and sex populations that followed Western diets [18–22].
The hypothesis linking Western diets to acne might have stemmed from the negative, historical associations of high-GI food with acne. Multiple studies conducted among several indigenous populations residing in different locations, including the Arctic, Papua New Guinea, Paraguay, and South China, found no cases of acne vulgaris. However, upon exposure to the environmental factors and practices associated with industrialized societies, these populations started to accumulate cases of acne vulgaris, suggesting a potential influence of Western diets on acne pathogenesis [23–25]. This has led to dubbing acne as a “disease of Western civilization.”24 Nevertheless, the linkage between Western diets and acne is still based on evidence of low quality, as most studies were observational in nature and limited by confounding [23–25].
The Mediterranean diet (MD) is a non-Western diet with high-to-moderate intake of olive oil, white meats, seafood, fruits, vegetables, legumes, nuts, and wine, and low intake of red meats and high-GI food [26, 27]. Adherence to the MD has been suggested to be protective against acne vulgaris due to its low-GI, anti-inflammatory, and antioxidants properties. Diets rich in low-GI components help control hyperinsulinemia, which in turn modulates sebum and androgen hormones production [28–30]. Moreover, the MD contains antioxidants and anti-inflammatory nutrients, which reduce systemic inflammation and oxidative stress, both of which are implicated in the development of chronic skin diseases such as acne [31]. To the best of the authors’ knowledge, this is the first systematic review and meta-analysis to assess the association between adherence to the MD and acne outcomes.
Materials and methods
A preset protocol for this systematic review and meta-analysis was developed and registered in the PROSPERO database (CRD42024521961). This study was conducted in accordance with the Meta-analysis of Observational Studies in Epidemiology (MOOSE) guidelines (Supplementary File 1).
Search strategy and eligibility criteria
The PubMed, Embase, and Web of Science Core Collection databases were searched for studies published from the inception of each database to April 15, 2024. The search terms “acne” and “Mediterranean” were combined using the “All Fields” tool or its equivalent, in addition to the corresponding MeSh term. Such a broad search strategy is suitable for novel topics, such as the MD and acne, as it is expected to yield as many relevant articles while keeping the search time and effort efficient (Supplementary File 2). Using both All Fields and MeSh searches ensures that relevant papers indexed under the adopted terms are less likely to be missed. Alternatively, using broader, relevant terms such as “dietary pattern” and “healthy diet” would have yielded large numbers of irrelevant studies, which might have undermined the specificity of the search. Such specificity was required since this review aimed to include the studies explicitly measuring adherence to the MD as a distinct dietary pattern with validated scoring tools. Observational studies and random control trials investigating the association between adherence to the MD and acne vulgaris were eligible for inclusion. Case studies and case series were excluded. Unpublished studies were not explored. No restrictions were made on language, geographic area, year of publication, or age or gender of the target population. The search was conducted from March 20 to April 15, 2024 and was reconducted in January 2025.
Data extraction
The titles and abstracts of potentially eligible studies were initially screened, followed by reviewing full texts. More complete and recent duplicate studies were selected. The data were extracted, reviewed, and independently checked by two reviewers (S.T. and M.S.). Any disagreement between the reviewers was assessed by a third reviewer (S.Z.). The extracted data were ordered in an Excel spreadsheet. The authors of studies with missing data or analyses were contacted via email. The following information was extracted: first author’s last name, publication year, country where the research study was conducted, target population, study design, settings, and period, sample size, mean age, gender distribution, assessment of adherence to the MD and acne severity, frequencies of participants with high exposure in each group, and crude and adjusted effect estimates for the association between adherence to the MD and its items and acne development and severity.
Risk of bias and quality of evidence
The Newcastle‒Ottawa Scale (NOS) was used to assess the risk of bias at the study level across three domains: groups selection, groups comparability, and exposure and outcome ascertainment. The NOS assigns a score with a maximum of 9 points for each study [32]. While the original NOS manual does not specify cut-off points for categorizing final scores, this review adopted the consensus reflected in recent methodological literature and commonly used in most systematic reviews, which classifies scores of ≥ 7 points as “low risk of bias,” 2–6 points as “moderate risk of bias”, and ≤ 2 points as “high risk of bias” [33–37]. The GRADE (Grading of Recommendations, Assessment, Development, and Evaluations) tool was used to assess the quality of evidence at the outcome level based on eight factors: limitations in study design, results inconsistency, evidence indirectness, imprecision, publication bias, magnitude of effect, confounding, and dose-dependent gradient. GRADE is an “outcome-centric” tool that assesses the quality of evidence for an outcome across studies [38].
Data synthesis and analysis
The data were reported as a narrative synthesis and three meta-analyses. The main meta-analysis calculated the pooled odds ratio (OR) related to the association between adherence to the MD and the development of acne vulgaris. Similarly, the second meta-analysis calculated the pooled OR related to the associations between adherence to each item of the MD and acne development. The correlation between adherence to the MD and acne severity was synthesized with a meta-analysis of correlation measures. Studies were excluded from the meta-analyses if related data were not reported, were inappropriately reported, or were unobtainable via email. Across-study heterogeneity was assessed using Cochran’s Q and I² tests. The threshold values of I² indicating low, moderate, and high heterogeneity were 25%, 50%, and 75%, respectively. R-4.3.3 software was used to analyze the data. The pooled OR and confidence interval (CI, 95%) were calculated using the random-effects approach, which accounts for within- and across-study heterogeneity [39]. A p value less than 0.05 indicated significance for all tests except for Cochrane’s Q test (p <.10). Subgroup analyses and publication bias were not possible due to the limited number of included studies.
Results
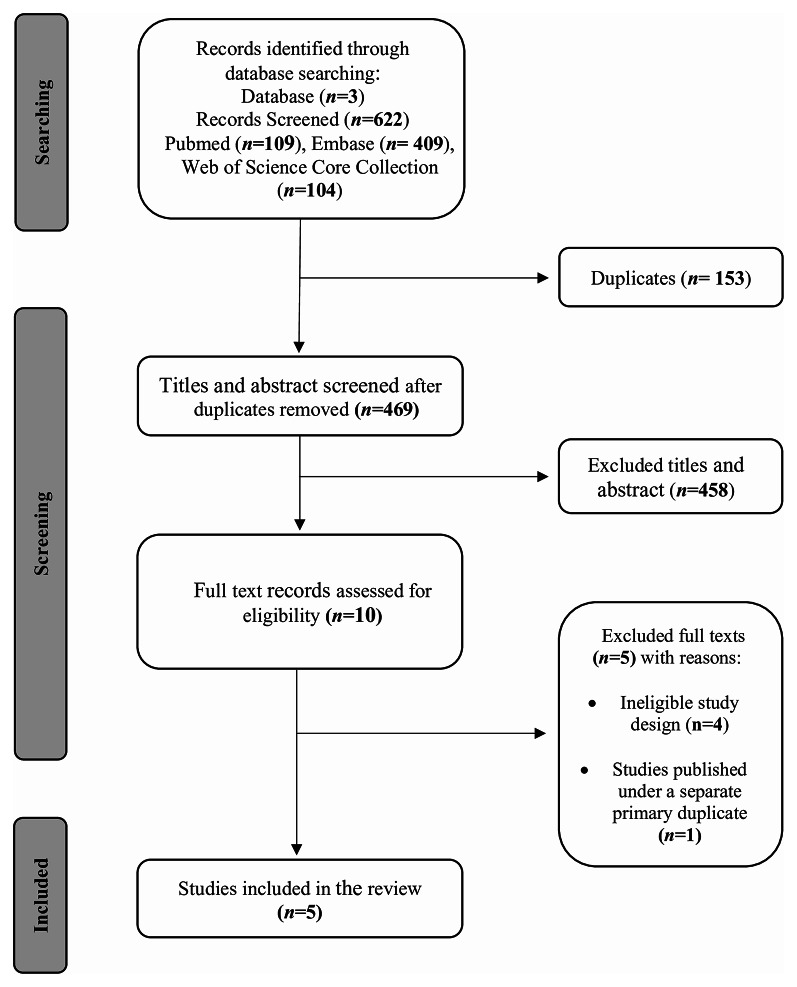
Three databases were searched and yielded a total of 622 publications, with 469 remaining after removal of duplicates. Of those, 458 publications were excluded after title and abstract screening. The full texts of the remaining 10 studies were reviewed, with five case‒control studies ultimately included in the review. Figure 1 outlines the search strategy and reasons for exclusion.
Fig. 1.
Flow diagram outlining study selection and inclusion. Flow chart outlining literature search and study selection for the systematic review and meta-analysis of the Mediterranean diet and acne vulgaris, including the reasons for exclusion
Studies characteristics
The included studies showed similarities in defined exposure, outcome, and study design but differences in target population and data analysis. All studies were conducted in Mediterranean areas, with three in Italy, [40–42] one in France, [43] and one in Palestine [44]. These were published in 2012, 2021, 2022, and 2024 [40–44]. The study durations ranged from 4 to 13 months [40, 41]. All five included studies employed a retrospective, matched, case‒control design [40–44]. The total number of participants across studies was 765, with 340 cases and 425 controls. The sample size ranged from 80 [43] to 293 [40]. The target populations were adults aged 18 to 42 years, [41]adolescents and adults aged 14 to 30 years, [42] female adolescents and adults, [43] university students aged 18 to 24 years; [44] and unreported in one study [40]. To measure exposure, four studies used the Mediterranean Diet Adherence Screener (MEDAS), [41–44] while Skroza used a validated 9-item questionnaire [40]. Four studies assessed acne severity, with three studies using the Global Acne Grading System (GAGS) [41, 42, 44] and one using the Global Evaluation Acne (GEA) scale [43]. While three studies employed a conditional regression analysis, [40, 41, 44] only two reported appropriate risk measures [40, 44] (Table 1).
Table 1.
Characteristics of study populations
| Study | Location | Population | Study design(matching factors) | Mean age (SD) | Settings | Number of participants | Gender (M: male, F: female) | Study period | |
|---|---|---|---|---|---|---|---|---|---|
| Cases | Controls | ||||||||
| Skroza (2012) [40] | Italy | Undefined | Case‒control (age, gender) | Total: unreported | Cases: outpatients in a dermatology department | ~ 4 months(2009) | |||
| Cases: 17 (median) | 93 | 200 |
Cases: F: 36.6% (34) M: 63.4% (59) |
||||||
| Total = 293 | |||||||||
| Controls: 16 (median) | Controls: community-based (hospital only) | Controls: F: 32.0% (64)M: 68.0% (136) | |||||||
| Barrea (2021) [41] | Italy | Adults aged 18–42 | Case‒control (age, gender, BMI) | Total:24.0 | Cases: outpatients in a dermatology department | 51 | 51 |
Total: F: 74.5% (38) M: 25.5% (13) |
~ 13 months(2019-2020) |
| Total = 102 | |||||||||
| Cases: 23.5 ± 5.9 | Controls: community-based (hospital and community) | ||||||||
| Controls: 24.5 ± 3.9 | |||||||||
| Bertolani (2021) [42] | Italy | Adolescents and adults aged14-30 years | Case‒control(age) | Total: unreported | Cases: outpatients in a dermatology department | 35 | 13 |
Cases: F: 71.4% (25) M: 28.6% (10) |
~ 12 months(2018-2019) |
| Total = 48 | |||||||||
| Cases:21.02±4.8 | Controls: unknown source | Controls: F: 53.8% (7)M: 46.2% (6) | |||||||
| Controls: 26.60 ±2.16 | |||||||||
| Ah-Thiane (2022) [43] | France | Female adolescents and adults | Case‒control(matching on age, BMI, geographic area) | Total: 19.75 ±4.3 | Cases: outpatients in a dermatology department | 40 | 40 | N/A (restricted to females) | ~ 6 months(2020) |
| Total = 80 | |||||||||
| Cases: 19.75 ±4.3 | Controls: community-based (hospital) | ||||||||
| Controls: 19.75 ± 4.3 | |||||||||
| Taha (2024) [44] | Palestine | University students |
Case‒control (age, gender, and BMI) |
Total: 20.2 | Cases: university | 121 | 121 |
Cases: F: 71.1% (86) M: 28.9% (35) |
~ 5 months(2023) |
| Total = 242 | |||||||||
| Cases:20.3 ±1.7 | Controls: community-based (university) | Controls: F: 71.1% (86)M: 28.9% (35) | |||||||
| Controls: 20.1 ±2.3 | |||||||||
Effect estimates
Association between adherence to the MD and acne development
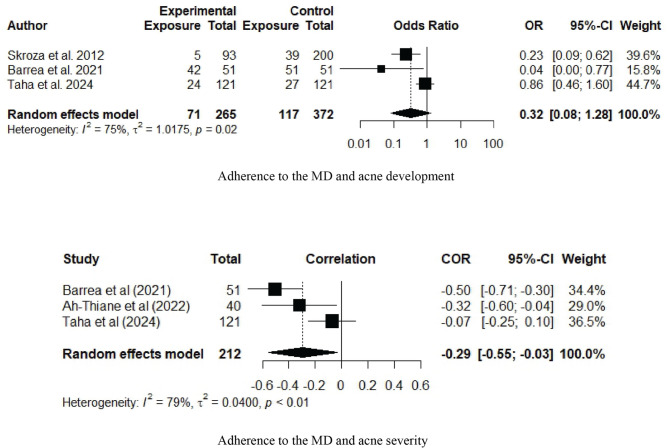
The five included studies showed that acne patients had a lower adherence to the MD than those without acne, although this difference was not statistically significant in two studies [42, 44]. The meta-analysis included three studies with a total of 637 participants, as two studies did not report a clear case‒control analysis, and no replies were received in response to the email inquiries that were sent to the authors (Table 2) [42, 43]. The meta-analysis revealed that adherence to the MD might be protective against acne development (OR 0.32, CI = 0.08–1.28), but the difference was not statistically significant (Fig. 2). The results across studies demonstrated significantly high heterogeneity (Q test p =.02, I2 = 75%, τ2 = 1.0175).
Table 2.
Outcomes of the included studies
| SStudy | Assessment of adherence to the MD | Assessment of acne severity | High adherence to the MD in the control group N (%) | High adherence to the MD in the case group N (%) | Association between adherence to the MD and acne development (i.e., differences between case and control groups) | Association between adherence to the MD and acne severity | Association between items of the MD and acne development |
|---|---|---|---|---|---|---|---|
| Skroza (2012) [40] |
80-item, 9-level food-frequency questionnaire |
Not assessed | 39/200 (19.5) | 5/39 (5.4) |
- cOR = 0.22 (CI 95%, 0.08–0.64) - aOR (full model*) = 0.30 (CI 95%, 0.10–0.85) - aOR (backward elimination model) = 0.31 (CI 95%, 0.11–0.89) |
Acne severity was not assessed | Not reported |
| Barrea (2021) [41] | MEDAS | GAGS | 37/51 (72.5) | 22/51 (43.1) |
- Compared to controls, patients with acne showed lower adherence to the MD overall (p <.001), with a higher percentage of cases in the low adherence category (17.6% vs. 0.0%, p =.005), and a lower percentage in the high adherence category (39.2% vs. 27.5%, p =.293). - No multivariate analysis was conducted. |
- Bivariate analysis: acne severity (categorical) was significantly associated (p =.007) with adherence to the MD (numerical). - Correlation analysis: r = −.504, p <.001* - Multiple regression analysis*: β =− 0.504, p <.001 |
- Olive oil as main lipid (p =.318); olive oil (p =.795); vegetables (p =.026); fruits (p =.008); red meats (p =.065); butter, cream, margarine (p =.194); soda drinks (p =.034); wine (p =.311); legumes (p =.041); fish/seafood (p <.001); commercial sweets (p =.021); nuts (p =.003); Poultry more than red meats (p =.051); use of sofrito sauce (p =.686). |
| Bertolani (2021) [42] | MEDAS | GAGS | Not reported | 27/35 (77.1) | Not reported | Adherence to the MD decreased with increasing acne severity but this was not significant (data are not reported) | Not reported |
| Ah-Thiane (2022) [43] | MEDAS | GEA | Not reported | Not reported |
N/A* *Authors conducted an erroneous correlation analysis between adherence to the MD and acne severity where control participants were included (r = −.47; p <.001), as participants without a disease cannot be assigned a “severity score” |
- Correlation analysis: r = −.32; p =.047* - Linear regression analysis*: multiple regression coefficient = − 0.17; p =.017* |
Not reported |
| Taha (2024) [44] | MEDAS | GAGS (unblinded assessor) | 27/121 (19.8) | 24/121 (22.3) |
- cOR = 0.86 (CI 95%, 0.46–1.60) - aOR (full model*) = 0.70 (CI 95%, 0.34–1.37) - aOR (backward elimination model*) = 0.88 (CI 95%, 0.47–1.62) |
- Correlation analysis: r = −.07; p =.414 - Participants with mild acne had a lower rate of high adherence to the MD than participants with moderate-to severe acne (18.7% vs. 23.3%, p =.579) |
Olive oil as main culinary lipid (p =.882); olive oil (p =.890); vegetables (p =.022*); fruits (p =.724); red meats (p =.894); butter, cream, and margarine (p =.322); soda drinks (p =.377); legumes (p =.480); fish/seafood (p =.641); commercial sweets (p =.291); nuts (p =.399); consuming poultry more than red meats (p =.193); use of sofrito sauce (p =.162) |
*Model variables:
Skroza (2012): sport activity, smoking status, familial diabetes status, familial hypertension status, familial hypercholesterolemia status, and MD score
Barrea (2021): model variables: anthropometric measurements, body composition, and adherence to the MD
AH-Thaine (2022): age, BMI, smoking, alcohol consumption, family history of acne, dairy product consumption, sugar consumption, snaking, use of cosmetics, previous local treatment, previous systemic treatment
Taha (2024): family history, milk consumption, smoking, and adherence to the MD (full model); family history and adherence to the MD (backward model)
*Abbreviations: MD: Mediterranean diet; N/A: nonapplicable; MEDAS Mediterranean Diet Adherence Screener; GAGS: Global Acne Grading System; GEA: Global Acne Evaluation
Fig. 2.
Forest plot illustrating the association between adherence to the Mediterranean diet and acne development and severity. Abbreviations: OR: odds ratio; CI: confidence interval; COR: correlation coefficient ratio; MD: Mediterranean diet. Associations are reported as odds ratios (ORs). Forest plots are presented for the ORs and 95% CIs for developing acne vulgaris in those with high adherence to the Mediterranean diet compared to those with low adherence to the Mediterranean diet (upper panel) and correlation between the severity of acne vulgaris and adherence to the Mediterranean diet (lower panel). Square size is proportional to sample sizes. Squares represent individual studies and diamonds represent pooled effect sizes
Association between adherence to the MD and acne severity
Three of the four studies that assessed acne severity were included in the meta-analysis [41, 43, 44]. Only two of these studies reported a correlation measure in their published manuscripts, [41, 43] while a correlation analysis was conducted and obtained from the authors of the third study [44]. The study by Skroza did not measure acne severity or conduct a correlation analysis, which was confirmed by an email [40] (Table 2). The meta-analysis revealed a significant negative correlation between adherence to the MD and acne severity (summary correlation coefficient= −0.29, CI= −0.55 to −0.03) (Fig. 2). The results across studies demonstrated significantly high heterogeneity (Q test p =.008, I2 = 79%, τ2 = 0.040).
Associations between adherence to the components of the MD and acne development
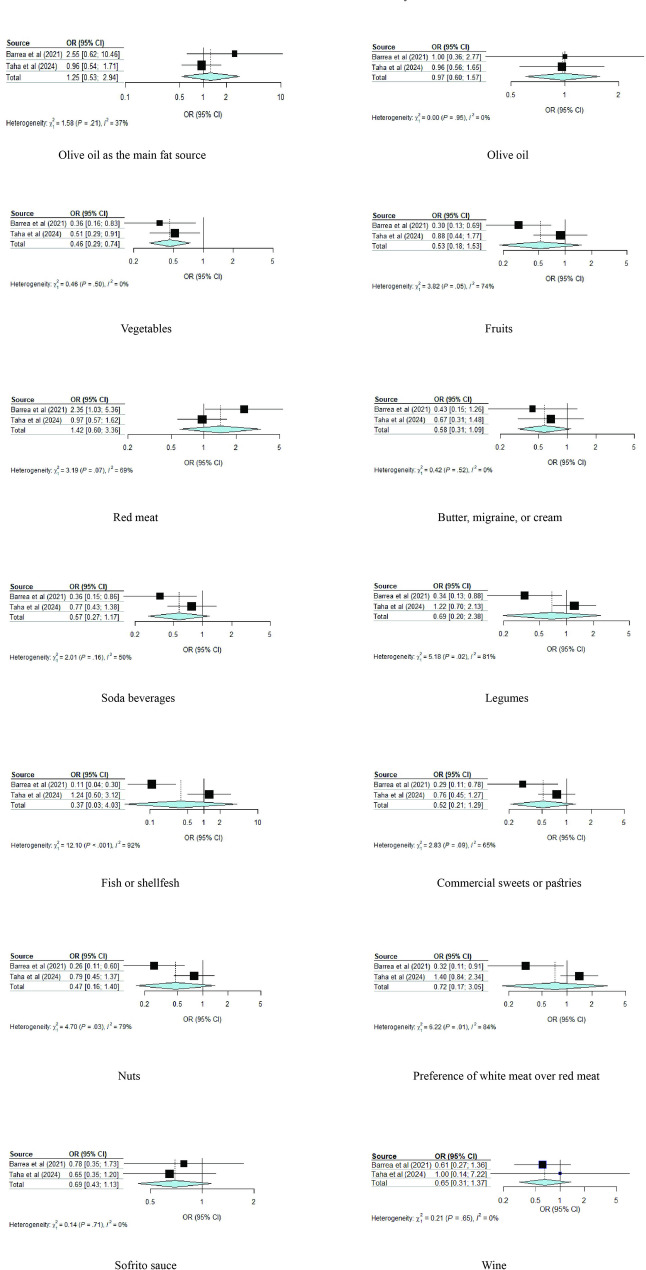
Two studies that included a total of 344 participants were included in this meta-analysis [41, 44]. All components showed negative associations with acne development, except for the use of olive oil as the main fat source and a lower consumption of red meat (Table 2). However, only a higher consumption of vegetables was significantly associated with acne development. The pooled OR of using olive oil as the main fat source was 1.25 (95%-CI, 0.53–2.94, I2 = 36.5%); consuming poultry more than red meat, 0.72 (95%-CI, 0.17–3.05, I2 = 83.9%); using sofrito sauce, 0.69 (95%-CI, 0.43–1.13, I2 = 0.0%); higher consumptions of olive oil, 0.97 (95%-CI, 0.60–1.57, I2 = 0.0%); vegetables, 0.46 (95%-CI, 0.29–0.74, I2 = 0.0%); fruits, 0.53 (95%-CI, 0.18–1.53, I2 = 73.8%); legumes, 0.69 (95%-CI, 0.20–2.38, I2 = 80.7%); fish 0.37 (95%-CI, 0.03–4.04, I2 = 91.7%); tree nuts, 0.47 (95%-CI, 0.16–1.40, I2 = 78.7%); and lower consumptions of red meat 1.41 (95%-CI, 0.60–3.36, I2 = 68.7%); butter, cream, or margarine, 0.58 (95%-CI, 0.31–1.09, I2 = 0.0%); sweet or carbonated beverages, 0.57 (95%-CI, 0.28–1.17, I2 = 50.2%); commercial sweets, 0.52 (95%-CI, 0.21–1.29, I2 = 64.6%); and wine, 0.65 (95%-CI, 0.31–1.37, I2 = 0%) (Fig. 3).
Fig. 3.
Forest plot illustrating the association between adherence to the items of the Mediterranean diet and acne development. Forest plots are presented for the odds ratios (ORs) and 95% CIs of developing acne vulgaris in high adherence to the Mediterranean diet compared to low adherence to the Mediterranean diet for each component of the Mediterranean diet
Risk of bias
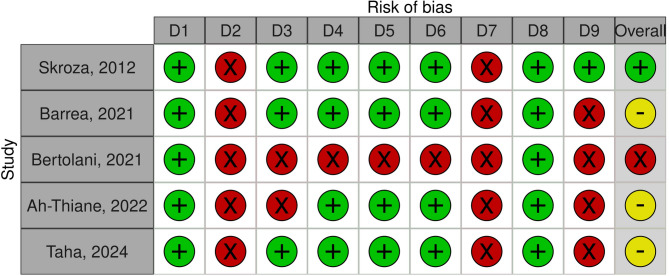
One study had a high risk of bias (NOS = 2), [42] three had a moderate risk of bias (NOS = 5–6), [41, 43, 44] and one had a low risk of bias (NOS = 7). [40] The sampling of cases was the main limitation in all studies, with either inadequate reporting of the sampling approach [40–43] or using nonrandomized techniques [44]. All studies assessed exposure using a nonblinded approach [40–44] (Fig. 4).
Fig. 4.
Traffic light plots of the domain-level judgements for each individual result using Newcastle-Ottawa Scale (NOS). Traffic light plots illustrating judgements regarding risk of bias at the study level using the Newcastle-Ottawa Scale (NOS). Domains of judgements: D1: Case definition; D2: Representativeness of the case; D3: Selection of controls; D4: Definition of controls; D5: Controlling for the most important factor; D6: Controlling for additional factors; D7: Ascertainment of exposure; D8: Same method of ascertainment; D9: Non-response rate. Legends: Å: low risk of bias ⊖ moderate risk of bias Ä: high risk of bias. Reference: McGuinness, LA, Higgins, JPT. Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Res Syn Meth. 2020; 1- 7. https://doi.org/10.1002/jrsm.1411
Quality of evidence
Acne development
The quality of evidence was weak due to risk of bias, results inconsistency, and evidence imprecision. The sampling of the control group was unreported in half of the studies, and analytical adjustment for confounding factors was suboptimal in all studies [40–44]. Second, the heterogeneity of the results (p =.02) could not be explained, as the studies had similar methodologies except for target population selection. Third, the wide confidence interval (0.08–1.28, OR = 0.32) that overlaps with no effect posed a serious limitation in evidence precision (Table 3). However, the association with a higher vegetable consumption was evaluated as having a high quality of evidence, as it was accompanied by a large magnitude of effect (OR < 0.5) without imprecision or heterogeneity of results (I2 = 0.0%) (Table 3 and Supplementary File 3).
Table 3.
Quality of evidence for the association between adherence to the Mediterranean diet and acne development and severity using the Grading of Recommendations, Assessment, Development, and Evaluations (GRADE) tool
| Outcome (number of studies) | Type of effect estimate | Limitations (risk of bias) | Inconsistency | Indirectness | Imprecision | Publication bias | Upgrading factors | Number of participants | Summary effect estimate (CI, 95%) | Overall quality of evidence | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cases | Controls | ||||||||||
|
Acne development (n=3) i.e., association between adherence to the MD and acne development |
Odds ratio | Someserious limitations | Serious inconsistency |
Noserious indirectness |
Someserious limitations | Undetected | No upgrading for large magnitude of an effect; dose‒response gradient; or effect of plausible residual confounding | 265 | 372 | OR=0.32(0.08–1.28.08.28) |
Weak ⊕⊕◯◯ |
| Total = 637 | |||||||||||
|
Acne severity (n=3) i.e., association between adherence to the MD and acne severity |
Correlation coefficient | Someserious limitations | Serious inconsistency |
Noserious indirectness |
Serious limitations | Undetected | Upgrade for dose‒response gradient | 212 | N/A | Correlation coefficient =- 0.29 (0.20–0.39.20.39) |
Weak ⊕⊕◯◯ |
| Total = 212 | |||||||||||
|
Association between adherence to the components of the MD and acne development (n=2) |
Odds ratio | Someserious limitations | Serious inconsistency |
Noserious indirectness |
Serious limitations | Undetected | No upgrading for large magnitude of an effect; dose‒response gradient; or effect of plausible residual confounding | 172 | 172 | Variable (see Figure X) |
Weak ⊕⊕◯◯ |
| Total = 344 | |||||||||||
|
Association between higher consumption of vegetables and acne development (n=2) |
Odds ratio | Someserious limitations | No serious inconsistency | No serious indirectness | No serious limitations | Undetected | Upgrade for a large magnitude of effect | 172 | 172 | OR=0.46 (0.29–0.74.29.74) | Moderate⊕⊕⊕◯ |
| Total = 344 | |||||||||||
Acne severity
The quality of evidence was evaluated as weak due to high risk of bias, results inconsistency, and evidence imprecision, but was upgraded for the inherent dose‒response gradient. First, without employing a partial correlation approach, the analyses did not sufficiently account for confounding factors. Second, correlation coefficients across studies demonstrated significant heterogeneity (p =.008, I2 = 79.3%). Moreover, serious imprecision might be concluded from the failure to meet the threshold for the optimal information size (OIS) and the wide confidence interval (Table 3 and Supplementary File 4).
Discussion
These systematic review and meta-analysis were the first to examine the associations between adherence to the MD and acne. The previous reviews that addressed the role of diet in dermatology were nonsystematic, uncoupled with a meta-analysis, or lacked a nutritional and pathological focus [45–47]. Other systematic reviews have focused on the association between diet and acne in particular but have not addressed a specific diet or conducted a meta-analysis [22, 48, 49]. This systematic review included five case‒control studies and showed a significant negative correlation between adherence to the MD and acne severity. The negative association between adherence to the MD and acne development was nonsignificant (pooled OR 0.32, CI = 0.08–1.60), with a weak quality of evidence.
The quality of evidence was poor due to methodological limitations and outcome heterogeneity. First, the methods were not appropriately in most studies, restricting judgment on the risk of bias. For example, the sampling, sourcing, and randomization of the case and control groups were underreported in most studies. This is particularly important for evaluating the quality of case‒control studies, where sampling of controls typically aims to match the characteristics and sources of the cases. These limitations may benefit from the published guidelines on the appropriate reporting of observational studies. Second, the outcomes of the included studies were heterogenous, which could not be explained by the magnitude of variability in methods across studies. Moreover, the wide confidence interval around the pooled outcome suggests an imprecise result that requires cautious interpretation despite the statistical significance.
Moreover, the nature of exposure as a dietary variable further undermines the quality of evidence, as defining and measuring diets are complex. Food chemistry, bioavailability, and biological interactions complicate the accurate assessment of exposure [50]. This is especially relevant to the MD as a diet consisting of multiple food components whose definitions and measurements are variable and intricate.
Additionally, individual eating habits, including diurnal, daily, and seasonal variations in food consumption, are difficult to capture and thus may pose additional methodological challenges [51–53]. This is in addition to the numerous nutritional and nonnutritional factors that may have synergistic, additive, or confounding effects on study outcomes. Adjusting for the effects of these factors is mostly infeasible because they are difficult to identify [54]. For instance, physical activity may have an effect whose magnitude and direction cannot be precisely determined. Furthermore, recall bias, which threatens research validity, is particularly common in the case‒control study design adopted in all included studies in this review [55]. These limitations highlight the need for proposing solutions aimed at minimizing the methodological challenges inherent in defining and quantifying adherence to diets and the impact of this adherence on health outcomes. Overall, further well-designed studies are needed to substantiate the evidence base, given the methodological limitations, including the inherent challenges in nutrition research, observational design, limited sample size, and small number of included studies.
Beyond the Western-Eastern dichotomy of diets, the biological plausibility of the impact of the MD on acne can be explained by examining the MD content. This impact might be attributed to the low GI and dairy content and the high antioxidant and anti-inflammatory effects of some of the MD components. High-GI diets cause hyperinsulinemia and increase insulin-like growth factor-1 (IGF-1), which results in multiple changes that are implicated in the pathophysiology of acne, including stimulation of keratinocyte proliferation, sebum production, and lipogenesis [28–30]. Moreover, IGF-1 upregulates androgen signaling through activating 5α-reductase and androgen receptors in the skin [56]. Research has shown that the severity of acne is correlated with IGF-1 and androgen levels [57–59]. Similarly, dairy consumption causes hyperinsulinemia and induces IGF-1 secretion [60, 61].
These systematic review and meta-analyses should be interpreted in light of their limitations. First, all five studies included in this review were observational case‒control studies. Assessing whole diets, such as the MD, using an experimental study design can be difficult, resource-consuming, or even impossible. Often, rigorously designed observational studies can provide stronger evidence than poorly designed experimental studies. Nevertheless, these justifications do not eliminate the high susceptibility to bias that is inherent in observational study designs. Moreover, the other factors considered in the evaluation of the quality of evidence did not compensate for the observational study design. Second, the low number of included studies as a limitation was not offset by employing a large sample size to enhance the strength of the pooled effect estimate. The lack of association could be attributed to the small sample size of the included studies, which may differ when additional studies are conducted in the future. Similarly, neither assessment of publication bias nor subgroup analysis was possible or meaningful due to the low number of studies. Furthermore, the heterogeneity of the outcomes could not be explained. Although the target populations differed in age range, central tendency measures indicated that the participants in different studies were similar in age, possibly due to the epidemiology of acne vulgaris, where patients are predominantly adolescents and young adults. Finally, this review did not include unpublished studies, which might have limited the scope of inclusion. Despite these limitations, this was the first systematic review and meta-analysis to address the association between acne and dietary intake evaluated by adherence to a certain diet. It adhered to an a priori registered protocol to ensure that the results would be born out of rigorous methodology, which is essential for the integrity and validity of systematic reviews and meta-analyses.
Conclusions
This study found a negative correlation between adherence to the MD and acne severity with a poor quality of evidence base, but no significant associations were found with acne development. Among the MD items, only a high consumption of vegetables was significantly associated with acne development. More well-designed studies are needed, given the observational design, limited sample size, methodological drawbacks, and small number of included studies.
Supplementary Information
Acknowledgements
We express our sincere gratitude to the An-Najah National University Administration for their invaluable collaboration and generous permission granted to conduct this study.
Abbreviations
- GI
Glycemic index
- MD
The mediterranean diet
- MOOSE
Meta-analysis of observational studies in epidemiology
- GRADE
Grading of recommendations, assessment, development, and evaluations
- MEDAS
The mediterranean diet adherence screener
- GAGS
Global acne grading system
- GEA
The global evaluation acne scale
- IGF-1
Insulin-like growth factor-1
Author contributions
All authors contributed substantially to the study. ST conceptualized the study, conducted an independent literature review, retrieved and reviewed the studies, analyzed the data, and wrote the manuscript. MS conducted an independent literature review, retrieved and reviewed the studies, participated in manuscript writing, and ensured the integrity of the data. SHZ reviewed the manuscript, resolved any disagreements between the two other reviewers, and enhanced the intellectual content of the study.
Funding
No funding was received for this study.
Data availability
The data collected and analyzed for this study are available from the corresponding author upon reasonable request.
Declarations
Ethics approval
Ethical approval and informed consent were not required for this systematic review or meta-analysis.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bellew S, Thiboutot D, Del Rosso JQ. Pathogenesis of acne vulgaris: what’s new, what’s interesting and what may be clinically relevant. J Drugs Dermatol. 2011;10(6):582–5. [PubMed] [Google Scholar]
- 2.Borzyszkowska D, Niedzielska M, Kozłowski M, et al. Evaluation of hormonal factors in acne vulgaris and the course of acne vulgaris treatment with contraceptive-based therapies in young adult women. Cells. 2022;11(24):4078. 10.3390/cells11244078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zouboulis CC. Acne and sebaceous gland function. Clin Dermatol. 2004;22(5):360–6. 10.1016/j.clindermatol.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 4.Holland DB, Jeremy AHT, Roberts SG, Seukeran DC, Layton AM, Cunliffe WJ. Inflammation in acne scarring: a comparison of the responses in lesions from patients prone and not prone to scar. Br J Dermatol. 2004;150(1):72–81. 10.1111/j.1365-2133.2004.05749.x. [DOI] [PubMed] [Google Scholar]
- 5.Jeremy AHT, Holland DB, Roberts SG, Thomson KF, Cunliffe WJ. Inflammatory events are involved in acne lesion initiation. J Invest Dermatol. 2003;121(1):20–7. 10.1046/j.1523-1747.2003.12321.x. [DOI] [PubMed] [Google Scholar]
- 6.Golchai J, Khani SH, Heidarzadeh A, Eshkevari SS, Alizade N, Eftekhari H. Comparison of anxiety and depression in patients with acne vulgaris and healthy individuals. Indian J Dermatol. 2010;55(4):352–4. 10.4103/0019-5154.74539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marron SE, Miranda-Sivelo A, Tomas-Aragones L, et al. Body dysmorphic disorder in patients with acne: a multicentre study. J Eur Acad Dermatol Venereol. 2020;34(2):370–6. 10.1111/jdv.15954. [DOI] [PubMed] [Google Scholar]
- 8.Cunliffe WJ. Acne and unemployment. Br J Dermatol. 1986;115(3):386. 10.1111/j.1365-2133.1986.tb05757.x. [DOI] [PubMed] [Google Scholar]
- 9.Dreno B, Bordet C, Seite S. Acne relapses: impact on quality of life and productivity. J Eur Acad Dermatol Venereol. 2019;33(5):937–43. 10.1111/jdv.15419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Unlisted authors. Diet in acne. Tex Med J (Austin). 1899;14(9):520–1. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9590958/. Accessed April 15, 2024. [PMC free article] [PubMed] [Google Scholar]
- 11.CORNBLEET T. Should we limit sugar in acne? Arch Dermatol. 1961;83(6):968–9. 10.1001/archderm.1961.01580120080019. [DOI] [PubMed] [Google Scholar]
- 12.Fulton JE Jr, Plewig G, Kligman AM. Effect of chocolate on acne vulgaris. JAMA. 1969;210(11):2071–4. 10.1001/jama.1969.03160370055011. [PubMed] [Google Scholar]
- 13.Burris J, Rietkerk W, Shikany JM, Woolf K. Differences in dietary glycemic load and hormones in New York City adults with no and moderate/severe acne. J Acad Nutr Diet. 2017;117(9):1375–83. 10.1016/j.jand.2017.03.024. [DOI] [PubMed] [Google Scholar]
- 14.Çerman AA, Aktaş E, Altunay İK, Arıcı JE, Tulunay A, Ozturk FY. Dietary glycemic factors, insulin resistance, and adiponectin levels in acne vulgaris. J Am Acad Dermatol. 2016;75(1):155–62. 10.1016/j.jaad.2016.02.1220. [DOI] [PubMed] [Google Scholar]
- 15.Smith RN, Mann NJ, Braue A, Mäkeläinen H, Varigos GA. The effect of a high-protein, low glycemic-load diet versus a conventional, high glycemic-load diet on biochemical parameters associated with acne vulgaris: a randomized, investigator-masked, controlled trial. J Am Acad Dermatol. 2007;57(2):247–56. 10.1016/j.jaad.2007.01.046. [DOI] [PubMed] [Google Scholar]
- 16.Smith RN, Mann NJ, Braue A, Mäkeläinen H, Varigos GA. A low-glycemic-load diet improves symptoms in acne vulgaris patients: a randomized controlled trial. Am J Clin Nutr. 2007;86(1):107–15. 10.1093/ajcn/86.1.107. [DOI] [PubMed] [Google Scholar]
- 17.Caperton C, Block S, Viera M, Keri J, Berman B. Double-blind, placebo-controlled study assessing the effect of chocolate consumption in subjects with a history of acne vulgaris. J Clin Aesthet Dermatol. 2014;7(5):19–23. [PMC free article] [PubMed] [Google Scholar]
- 18.Adebamowo CA, Spiegelman D, Berkey CS, et al. Milk consumption and acne in teenaged boys. J Am Acad Dermatol. 2008;58(5):787–93. 10.1016/j.jaad.2007.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adebamowo CA, Spiegelman D, Berkey CS, et al. Milk consumption and acne in adolescent girls. Dermatol Online J. 2006;12(4):1. [PubMed] [Google Scholar]
- 20.Adebamowo CA, Spiegelman D, Danby FW, Frazier AL, Willett WC, Holmes MD. High school dietary dairy intake and teenage acne. J Am Acad Dermatol. 2005;52(2):207–14. 10.1016/j.jaad.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 21.Penso L, Touvier M, Deschasaux M, et al. Association between adult acne and dietary behaviors: findings from the NutriNet-Santé prospective cohort study. JAMA Dermatol. 2020;156(8):854–62. 10.1001/jamadermatol.2020.1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meixiong J, Ricco C, Vasavda C, Ho BK. Diet and acne: a systematic review. JAAD International. 2022;7:95–112. 10.1016/j.jdin.2022.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steiner PE. Necropsies on Okinawans. Anatomic and pathologic observations. Arch Pathol. 1946;42(4):359–80. [PubMed] [Google Scholar]
- 24.Cordain L, Lindeberg S, Hurtado M, Hill K, Eaton SB, Brand-Miller J. Acne vulgaris: a disease of Western civilization. Arch Dermatol. 2002;138(12):1584–90. 10.1001/archderm.138.12.1584. [DOI] [PubMed] [Google Scholar]
- 25.Schaefer O. When The Eskimo Comes To Town. Nutrition Today. 1971;6(6):8. Accessed April 15, 2024. https://journals.lww.com/nutritiontodayonline/citation/1971/11000/when_the_eskimo_comes_to_town.3.aspx
- 26.Trichopoulou A, Lagiou P. Healthy traditional mediterranean diet: an expression of culture, history, and lifestyle. Nutr Rev. 1997;55(11 Pt 1):383–9. 10.1111/j.1753-4887.1997.tb01578.x. [DOI] [PubMed] [Google Scholar]
- 27.Trichopoulou A, Martínez-González MA, Tong TY, et al. Definitions and potential health benefits of the Mediterranean diet: views from experts around the world. BMC Med. 2014;12(1):112. 10.1186/1741-7015-12-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim H, Moon SY, Sohn MY, Lee WJ. Insulin-like growth factor-1 increases the expression of inflammatory biomarkers and sebum production in cultured sebocytes. Ann Dermatol. 2017;29(1):20–5. 10.5021/ad.2017.29.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Isard O, Knol AC, Ariès MF, et al. Propionibacterium acnes activates the IGF-1/IGF-1R system in the epidermis and induces keratinocyte proliferation. J Invest Dermatol. 2011;131(1):59–66. 10.1038/jid.2010.281. [DOI] [PubMed] [Google Scholar]
- 30.Haase I, Evans R, Pofahl R, Watt FM. Regulation of keratinocyte shape, migration and wound epithelialization by IGF-1- and EGF-dependent signalling pathways. J Cell Sci. 2003;116(15):3227–38. 10.1242/jcs.00610. [DOI] [PubMed] [Google Scholar]
- 31.Estruch R. Anti-inflammatory effects of the Mediterranean diet: the experience of the PREDIMED study. Proc Nutr Soc. 2010;69(3):333–40. 10.1017/S0029665110001539. [DOI] [PubMed] [Google Scholar]
- 32.Wells G, Shea B, O’Connell D et al. The Newcastle–Ottawa Scale (NOS) for Assessing the Quality of Non-Randomized Studies in Meta-Analysis.; 2000.
- 33.Lo CKL, Mertz D, Loeb M. Newcastle-ottawa scale: comparing reviewers’ to authors’ assessments. BMC Med Res Methodol. 2014;14(1):1–5. 10.1186/1471-2288-14-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guo Y, Lin J, Wu T, Zhou T, Mu Y. Risk factors for delirium among hospitalized adults with COVID-19: a systematic review and meta-analysis of cohort studies. Int J Nurs Stud. 2023;148:104602. 10.1016/j.ijnurstu.2023.104602. [DOI] [PubMed] [Google Scholar]
- 35.Mengist B, Desta M, Tura AK, Habtewold TD, Abajobir A. Maternal near miss in Ethiopia: protective role of antenatal care and disparity in socioeconomic inequities: a systematic review and meta-analysis. Int J Afr Nurs Sci. 2021;15:100332. 10.1016/j.ijans.2021.100332. [Google Scholar]
- 36.Turtabayev B, Joshibayev S, Kervan U, et al. Minimally invasive left ventricular assist device implantation: a systematic review of current evidence on clinical outcomes and surgical approaches. Med Sci. 2025;13(3):173. 10.3390/medsci13030173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carra MC, Romandini P, Romandini M. Risk of bias evaluation of Cross-Sectional studies: adaptation of the Newcastle-Ottawa scale. J Periodontal Res Published Online April. 2025;28. 10.1111/jre.13405. [DOI] [PubMed]
- 38.Schünemann H, Brożek J, Guyatt G, Oxman A, Working Group. GRADE Handbook for Grading Quality of Evidence and Strength of Recommendations. The GRADE ; 2013. Accessed June 7, 2024. https://training.cochrane.org/resource/grade-handbook
- 39.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88. 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 40.Skroza N, Tolino E, Semyonov L, et al. Mediterranean diet and familial dysmetabolism as factors influencing the development of acne. Scand J Public Health. 2012;40(5):466–74. 10.1177/1403494812454235. [DOI] [PubMed] [Google Scholar]
- 41.Barrea L, Donnarumma M, Cacciapuoti S, et al. Phase angle and mediterranean diet in patients with acne: two easy tools for assessing the clinical severity of disease. J Transl Med. 2021;19(1):171. 10.1186/s12967-021-02826-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bertolani M, Rodighiero E, Saleri R, et al. The influence of Mediterranean diet in acne pathogenesis and the correlation with insulin-like growth factor-1 serum levels: implications and results. Dermatol Rep. 2022. 10.4081/dr.2022.9143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ah-Thiane L, Nguyen JM, Khammari A, Dréno B. Lifestyle habits and impact of the Mediterranean diet on facial acne severity in French women: a case-control study. Int J Womens Dermatol. 2022;8(2):e017. 10.1097/JW9.0000000000000017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Taha S, Shakhshir M, Zyoud SH. Acne vulgaris and adherence to the mediterranean diet among university students: a case–control study. J Health Popul Nutr. 2024;43(1):41. 10.1186/s41043-024-00535-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Soliman YS, Hashim PW, Farberg AS, Goldenberg G. The role of diet in preventing photoaging and treating common skin conditions. Cutis. 2019;103(3):153–6. [PubMed] [Google Scholar]
- 46.Flores-Balderas X, Peña-Peña M, Rada KM, et al. Beneficial effects of plant-based diets on skin health and inflammatory skin diseases. Nutrients. 2023;15(13):2842. 10.3390/nu15132842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Diotallevi F, Campanati A, Martina E, et al. The role of nutrition in Immune-Mediated, inflammatory skin disease: a narrative review. Nutrients. 2022;14(3):591. 10.3390/nu14030591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dall’Oglio F, Nasca MR, Fiorentini F, Micali G. Diet and acne: review of the evidence from 2009 to 2020. Int J Dermatol. 2021;60(6):672–85. 10.1111/ijd.15390. [DOI] [PubMed] [Google Scholar]
- 49.National Guideline Alliance (UK). Dietary Interventions for the Treatment of Acne Vulgaris: Acne Vulgaris: Management: Evidence Review C, National Institute for Health and Care Excellence (NICE). 2021. Accessed July 5, 2024. http://www.ncbi.nlm.nih.gov/books/NBK573052/ [PubMed]
- 50.Willett W. Nutritional epidemiology: issues and challenges. Int J Epidemiol. 1987;16(2):312–7. 10.1093/ije/16.2.312. [DOI] [PubMed] [Google Scholar]
- 51.Beaton G, Burema J, Ritenbaugh C. Errors in the interpretation of dietary assessments. Am J Clin Nutr. 1997;65(4):S1100–7. 10.1093/ajcn/65.4.1100S. [DOI] [PubMed] [Google Scholar]
- 52.Milner J, MCGuire V, Feather TE, Little JA. Source of variance in 24-hour dietary recall data: implications for nutrition study design and interpretation. Carbohydrate sources, vitamins, and minerals. Am J Clin Nutr. 1983;37(6):986–95. 10.1093/ajcn/37.6.986. [DOI] [PubMed] [Google Scholar]
- 53.Basiotis PP, Thomas RG, Kelsay JL, Mertz W. Sources of variation in energy intake by men and women as determined from one year’s daily dietary records. Am J Clin Nutr. 1989;50(3):448–53. 10.1093/ajcn/50.3.448. [DOI] [PubMed] [Google Scholar]
- 54.Tarasuk VS, Brooker AS. Interpreting epidemiologic studies of diet-disease relationships. J Nutr. 1997;127(9):1847–52. 10.1093/jn/127.9.1847. [DOI] [PubMed] [Google Scholar]
- 55.Friedenreich CM, Slimani N, Riboli E. Measurement of past diet: review of previous and proposed methods. Epidemiol Rev. 1992;14(1):177–96. 10.1093/oxfordjournals.epirev.a036086. [DOI] [PubMed] [Google Scholar]
- 56.Melnik BC. Diet in acne: further evidence for the role of nutrient signalling in acne pathogenesis. Acta Derm Venereol. 2012;92(3):228–31. 10.2340/00015555-1358. [DOI] [PubMed] [Google Scholar]
- 57.Cappel M, Mauger D, Thiboutot D. Correlation between serum levels of insulin-like growth factor 1, dehydroepiandrosterone sulfate, and dihydrotestosterone and acne lesion counts in adult women. Arch Dermatol. 2005;141(3):333–8. 10.1001/archderm.141.3.333. [DOI] [PubMed] [Google Scholar]
- 58.Bertolani M, Rodighiero E, Saleri R, et al. The influence of Mediterranean diet in acne pathogenesis and the correlation with insulin-like growth factor-1 serum levels: implications and results. Dermatol Rep. 2022;14(1):9143. 10.4081/dr.2022.9143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Akpinar Kara Y. Evaluation of serum insulin-like growth factor-1, insulin, glucose levels in patients with adolescent and post-adolescent acne. J Cosmet Dermatol. 2022;21(3):1292–6. 10.1111/jocd.14327. [DOI] [PubMed] [Google Scholar]
- 60.Melnik BC, Schmitz G. Role of insulin, insulin-like growth factor-1, hyperglycaemic food and milk consumption in the pathogenesis of acne vulgaris. Exp Dermatol. 2009;18(10):833–41. 10.1111/j.1600-0625.2009.00924.x. [DOI] [PubMed] [Google Scholar]
- 61.Hoppe C, Mølgaard C, Dalum C, Vaag A, Michaelsen KF. Differential effects of casein versus whey on fasting plasma levels of insulin, IGF-1 and IGF-1/IGFBP-3: results from a randomized 7-day supplementation study in prepubertal boys. Eur J Clin Nutr. 2009;63(9):1076–83. 10.1038/ejcn.2009.34. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data collected and analyzed for this study are available from the corresponding author upon reasonable request.