Abstract
Objective
This study applies the find, organise, clarify, understand, select – plan, do, check and act (FOCUS-PDCA) programme to analyse factors affecting dental handpiece (DHP) processing quality and to optimise the workflow using this quality improvement framework.
Method
A prospective observational study was conducted at the Affiliated Ruijin Hospital of Shanghai Jiaotong University School of Medicine. The study included 2,893 DHPs processed under a routine scheme between July and December 2021 (control group) and 3,032 DHPs processed under the FOCUS-PDCA programme scheme between January and June 2022 (intervention group). The sample size was calculated using the formula for comparing two proportions, aiming to detect a significant difference in processing quality with a power of 80% and an alpha level of 0.05.
Results
The FOCUS-PDCA programme significantly improved pass rates for cleaning (chi-square [χ²] = 58.955, degree of freedom [df] = 1, P < 0.01), packaging (χ² = 27.835, df = 1, P < 0.01) and sterilisation (χ² = 89.584, df = 1, P < 0.01) compared with routine processing. Staff mastery of theoretical knowledge and operational skills was superior in the FOCUS-PDCA group (t = 16.871, df = 18, P < 0.01; t = 15.348, df = 18, P < 0.01, respectively). The implementation of the programme significantly reduced the incidence of mechanical failures, including bearing and clamping shaft issues (χ² = 5.730, df = 1, P < 0.05; χ² = 5.849, df = 1, P < 0.05, respectively). The satisfaction score for DHP processing quality was higher in the FOCUS-PDCA group, with a mean of 88.80 ± 5.88, compared with 65.13 ± 7.60 in the control group (t = 13.479, df = 58, P < 0.01).
Conclusion
Within the limitations of this study, applying the FOCUS-PDCA programme to DHP processing procedures effectively improved processing quality and reduced mechanical failures. To our knowledge, this is among the first reports applying FOCUS-PDCA to DHP processing in a hospital setting. Clinical doctors and nurses expressed high satisfaction with the quality of processing following the implementation of the improved protocols.
Keywords: FOCUS-PDCA programme, Dental handpieces, Processing quality, Mechanical failure, Dental instruments, Sterilisation, Decontamination
Introduction
Dental handpieces (DHPs) are reusable invasive medical devices that must be cleaned, decontaminated, lubricated and steam sterilised after use [1]. These devices have a complex internal design, including narrow channels that are prone to the accumulation of pathogenic microorganisms and tissue debris during operation. They are not designed for routine disassembly, making them difficult to clean, dry and sterilise. As a result, unqualified processing quality is common, affecting the daily use and turnover of the devices.
The find, organise, clarify, understand, select – plan, do, check and act (FOCUS-PDCA) programme serves as a structured approach to continuous quality improvement (CQI) in healthcare settings. It extends and enhances the plan, do, check and act (PDCA) methodology by incorporating the find, organise, clarify, understand and select (FOCUS) management cycle. Through FOCUS, followed by PDCA, this approach enables comprehensive analysis of clinical problems and the implementation of process improvements. The nine-step methodology facilitates a more thorough examination of all aspects of the programme to achieve CQI [2–4]. Since the early 20th century, this approach has been successfully applied across various healthcare domains, including central laboratory management [5], critical case management [6], management of ventilator-associated pneumonia in hospitalised patients [7] and home care [8]. Although FOCUS-PDCA has improved reprocessing of flexible endoscopes and implant trays, no prospective cohort has quantified its impact on dental handpiece pass-rates, mechanical failure or user satisfaction. This gap underpins the present study.
The primary aim of the FOCUS component is to identify potential problems, whereas the PDCA component addresses CQI. The progression from issue identification to solution implementation forms a comprehensive framework for enhancing healthcare processes.
The objective of this study was to employ the FOCUS-PDCA programme to analyse the factors affecting DHP processing quality and to implement and evaluate process improvements. To our knowledge, this is among the first reports applying FOCUS-PDCA to DHP processing in a hospital setting. The study’s novelty lies in the systematic application of this quality improvement methodology to the specific challenges of DHP processing, which have not been previously well documented in the literature.
Data and methods
General information
This research employed a prospective observational study design conducted at the Central Sterile Supply Department of the Affiliated Ruijin Hospital of Shanghai Jiaotong University School of Medicine. The study was conducted in accordance with the Declaration of Helsinki and was approved by the ethics committee of the Affiliated Ruijin Hospital of Shanghai Jiaotong University School of Medicine (approval code: RJHK2024096).
The inclusion criteria were as follows: (1) both groups used newly purchased DHPs with confirmed structural integrity and good functionality; and (2) following clinical use, chairside pre-treatment was performed, using the water and gas system of the dental comprehensive treatment table to flush the internal waterway and gas path of the DHPs for 30 s [9].
The exclusion criterion was DHPs that had been temporarily stored in the stomatology department for more than 24 h after pre-treatment.
The sample size was calculated using a formula comparing two proportions to detect a significant difference in processing quality. Based on previous quality data indicating an approximate 94% pass rate in routine processing and an expected improvement to 98% with the FOCUS-PDCA programme, with a statistical power of 80% and an alpha level of 0.05, the minimum required sample size was calculated to be 2,800 DHPs per group.
Between July and December 2021, 2,893 DHPs processed under a routine scheme were included as the control group. Between January and June 2022, 3,032 DHPs processed under the FOCUS-PDCA programme scheme were selected as the intervention group. Consecutive sampling was used during each period, and all eligible DHPs were included in the study.
Methods
Routine processing scheme
The routine cleaning and disinfection of DHPs included manual pre-washing of water and air paths, followed by mechanical cleaning and disinfection without lubricant. The pressure used was approximately 150–200 kPa/cm², and the temperature was maintained at 90 °C for 10 min during the cleaning cycle. For oil injection and packaging, the method incorporated manual oil injection followed by drying with a pressure air gun and single packaging in paper–plastic bags. The sterilisation process utilised ordinary sterilisation baskets with flat holding, followed by pressure steam sterilisation at 134 °C for 18 min at 210–230 kPa.
FOCUS-PDCA programme processing scheme
The FOCUS-PDCA cycle served solely as a process optimisation framework; outcome quality was evaluated using independent indicators. Details of the FOCUS-PDCA programme processing are provided in Table 1 and outlined below.
Table 1.
FOCUS-PDCA program processing scheme overview
| FOCUS-PDCA program | Root cause analysis | Improvement measure |
|---|---|---|
| Find | The unqualified rate of cleaning, packaging and sterilization is higher; DHPs have long processing times, slow turnaround and frequent breakdowns | Summarize information and identify problems |
| Organize | Lack of coordination and schedule control | Set up the CQI project team, led by the head nurse |
| Clarify | Operating requirements and quality standards are unclear | Compare existing processes with industry standards and carry out assessments |
| Understand | The processing method is obsolete, the operation is arbitrary, the sense of responsibility is not strong, the training and assessment are not in place, and the management and supervision are insufficient | The reason was analyzed by using fishbone diagram |
| Select | No specific improvement plan | Add equipment and facilities, adjust processing processes, and strengthen personnel management |
| Plan | No clear implementation improvement plan | Develop an improvement plan and implement it in stages |
| Do | The old process was inefficient | Implement standardized processing procedures and strengthen training and learning |
| Check | Lack of effect evaluation | Quality control staff daily inspection, head nurse spot check, and clinical communication |
| Act | Improvement measures were not implemented consistently | Analyze the implementation process and develop standardized procedures |
Finding problems
Data analysis of DHPs processed by the disinfection supply centre between July and December 2021 revealed unqualified rates of 5.5% for cleaning, 3.18% for packaging and 6.05% for sterilisation. Clinical feedback also indicated that processing times were lengthy, turnover was slow and mechanical failures were frequent.
Organisation establishment
A CQI project team was established, with the head nurse of the central sterile supply department serving as team leader to coordinate the project and monitor progress. Team members included three quality control personnel and nursing specialists responsible for implementing the project and collecting data. The project team held monthly work improvement meetings to support smooth development and implementation.
Clarifying the status quo
The team compared current practices with industry standards, including the Hospital Disinfection Supply Centre Standard (WS310-2016), Technical Specification for Disinfection and Sterilisation of Oral Instruments (WS506-2016) and Technical Specification for Disinfection of Medical Institutions (WS/T367-2012), as well as relevant literature. An assessment of theoretical knowledge and operational skills related to DHP cleaning, disinfection, oiling, packaging and sterilisation was conducted to evaluate staff awareness of key operating steps and mastery of practical skills.
Understanding the root cause
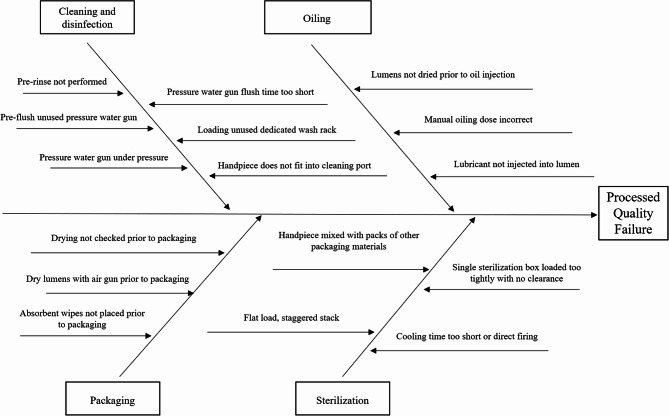
A root cause analysis of DHP processing data between July and December 2021 was conducted using fishbone diagram analysis (Fig. 1). The CQI project team identified several main reasons for the high unqualified rate of processing: (1) Outdated processing methods: Manual pre-washing had low efficiency and could not completely clean the internal cavities of DHPs. (2) Unclear processing steps: A lack of standardised procedures in oil filling and packaging led to inconsistent execution among operators. (3) Improper equipment usage: The cleaning equipment lacked adequate pressure and flow control to effectively remove the biological load.
Fig. 1.
Comprehensive fishbone diagram analysis of multiple contributing factors leading to unqualified dental handpiece (DHP) processing quality in central sterile supply department
Selection of the improvement plan
The team proposed three key areas for improvement: (1) The addition of practical equipment and facilities, such as pressure gauges, timers, automatic oilers and paper–plastic packaging sterilisation loading dividers, to enable standardised, efficient and labour-saving processing while eliminating potential risk factors. (2) The adjustment and refinement of all aspects of DHP processing, with detailed and specific operating modes and requirements for each step. (3) Strengthening personnel management through increased training frequency, improved understanding of key steps and requirements, enhanced supervision via random inspections, linking inspection results to performance evaluation and reinforcing staff responsibility through ideological education.
Planning
The team developed a staged implementation plan to improve processing procedures, including: (1) Revising DHP processing protocols, creating training materials and assessment tools and ensuring all staff understood key theoretical points and operational processes. (2) Processing DHPs according to the improved procedures, analysing results, implementing CQI measures and refining operational steps as needed.
Implementation
The CQI project team revised all aspects of the DHP processing procedures with reference to relevant standards, literature and hospital conditions, formulating standardised protocols as follows: (1) Standardised pre-washing using pressure water guns (200–250 kPa/cm²) to pulse the tube cavity for 30 s, recorded with a timer. (2) Standardised cleaning and loading using special cleaning racks with matching diameter interfaces and verification of tight connections. (3) Addition of a drying step before oil injection using pressure air guns (200–250 kPa/cm²) for 30 s, recorded with a timer [10]. (4) Improved oil injection using the M-gear of the NSK automatic oiler, followed by positioning the DHP head upside down vertically for 30 min to remove excess oil [11]. (5) Improved packaging procedures, including verification of dryness, use of low-fibre soft cloths, selection of paper–plastic bag length (minimum 15 cm), absorbent towel wrapping and placement of a chemical indicator card. (6) Standardised sterilisation loading using paper–plastic packaging sterilisation dividers with vertical placement, ≥ 2 cm spacing between packages, and divider placement in each sterilisation basket [12]. (7) Standardised cooling procedures, allowing natural cooling on sterilisation unloading carts and handling only after 30 min, verified by the timer.
Monthly training sessions were conducted to cover hospital disinfection supply centre standards, technical operation specifications, and DHP processing procedures. Quarterly operational and theoretical assessments were held to evaluate staff mastery of the improved procedures.
Checking
The effectiveness of implementation was evaluated through the following: (1) Daily quality control inspections by quality control officers, recording unqualified cleaning, packaging and sterilisation. Two independent inspectors assessed each handpiece after cleaning to ensure evaluation reliability. (2) Monthly random checks of standardised operations performed by five staff members, with results linked to individual performance evaluations. (3) Communication with clinical departments to monitor DHP failure, damage and satisfaction with processing quality.
Acting
The team analysed and summarised the execution process and improvements achieved through the FOCUS-PDCA programme, formulating standardised DHP processing flows. Any emerging issues were documented for inclusion in the next FOCUS-PDCA cycle for further improvement.
Observation indicators
General processing indicators
(1) Qualified rates for cleaning, packaging and sterilisation were defined as follows: Cleaning qualification: following the preset cleaning process, no visible pollutants or residual debris remained inside the DHPs, and microbial test results met the requirements of WS506-2016, Technical Specifications for Disinfection and Sterilisation of Oral Instruments [13]. The assessment was conducted by two independent inspectors who had to agree on the status of the qualification. Packaging qualification: compliance with WS310-2016, Hospital Disinfection Supply Centre Part 1: Management Standards [14], including intact packaging without damage, clear labelling and protection from contamination during storage and transportation. Regular quality checks were performed by the quality control department. Sterilisation qualification: after completion of the sterilisation cycle, each batch of DHPs passed physical, chemical and biological monitoring and met the requirements of WS310-2016 and WS/T367-2012, Technical Specifications for Disinfection of Medical Institutions [15]. Qualification was determined through steriliser monitoring records and periodic bioindicator testing.
(2) Processing time: defined as the total time from receipt of the DHPs to completion of processing and packaging, including cleaning, drying, oiling, packaging and sterilisation steps. This was recorded by noting the start and end times of each step in minutes and comparing them before and after the implementation of FOCUS-PDCA.
Comparison of staff theoretical and operational mastery
Assessment was conducted during the final week of implementation using self-developed theoretical test sheets and operational rating scales for 10 staff members (eight nurses and two technical workers).
Comparison of mechanical failure incidence
Mechanical faults were categorised as bearing problems (turning needle issues, abnormal sounds, insufficient power) or clamping problems (sticking needle, inability to remove needles, improper needle installation) [16].
Comparison of clinical satisfaction
Monthly questionnaire surveys were conducted among five dental doctors or nurses, including 10 questions on processing quality, usage and turnover efficiency. The questionnaire, adapted from Chen et al. (2018) [11], demonstrated good content validity (CVI = 0.86). Responses were rated on a 10-point scale: 10 points = very satisfied, 8 points = relatively satisfied, 6 points = basically satisfied, 4 points = not very satisfied and 2 points = not satisfied.
Statistical methods
Categorical data were expressed as frequencies and percentages (%) and compared using chi-square (χ²) tests. Continuous data were expressed as mean ± standard deviation and compared using independent samples t-tests. Statistical significance was set at P < 0.05. The chi-square test was specifically applied to compare differences between groups for cleaning, packaging and sterilisation qualification rates. Independent samples t-tests were used to compare theoretical knowledge, operational skills and satisfaction scores. Effect sizes were calculated (Cramér’s V for χ² tests; Cohen’s d for t-tests) to aid interpretation. Statistical analysis was performed using SPSS 21.0 software.
Results
Comparison of dental handpiece processing quality
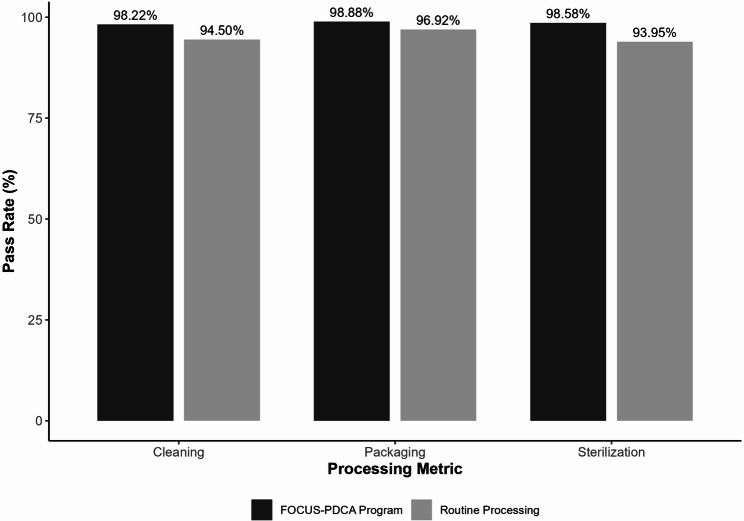
The cleaning qualified rate was 98.22% (2,978/3,032) in the FOCUS-PDCA programme group compared with 94.50% (2,734/2,893) in the routine group (χ² = 58.955, degree of freedom [df] = 1, P < 0.01). The packaging qualified rate was 98.88% (2,998/3,032) in the FOCUS-PDCA group versus 96.92% (2,804/2,893) in the routine group (χ² = 27.835, df = 1, P < 0.01). The sterilisation qualified rate was 98.58% (2,989/3,032) in the FOCUS-PDCA group compared with 93.95% (2,718/2,893) in the routine group (χ² = 89.584, df = 1, P < 0.01). The FOCUS-PDCA programme significantly improved qualification rates across all three metrics (P < 0.05) (Table 2). The corresponding Cramér’s V values ranged from 0.07 to 0.12, indicating small to moderate effects. Figure 2 illustrates the comparative pass rates for cleaning, packaging and sterilisation between the routine and FOCUS-PDCA groups, highlighting the substantial improvements achieved through the structured quality improvement approach.
Table 2.
Comparative analysis of dental handpiece processing quality metrics between control and intervention groups
| Group | Number of cases | Cleaning | Packaging | Sterilization | |||
|---|---|---|---|---|---|---|---|
| Acceptance number | Pass rate% | Acceptance number | Pass rate% | Acceptance number | Pass rate% | ||
| routine processing group | 2893 | 2734 | 94.50 | 2804 | 96.92 | 2718 | 93.95 |
| FOCUS-PDCA program processing group | 3032 | 2978 | 98.22 | 2998 | 98.88 | 2989 | 98.58 |
| χ2 value | 58.955 | 27.835 | 89.584 | ||||
| P value | < 0.01 | < 0.01 | < 0.01 | ||||
Fig. 2.
Comparison of pass rates for dental handpiece processing quality metrics between routine processing and FOCUS-PDCA program groups. All differences were statistically significant (P < 0.01)
Comparison of staff processing procedure mastery
Following implementation, theoretical knowledge scores averaged 96.10 ± 1.37 points in the FOCUS-PDCA group compared with 81.50 ± 2.36 points in the routine group (t = 16.871, df = 18, P < 0.01). Operational skills scores averaged 96.30 ± 1.34 points in the FOCUS-PDCA group versus 83.80 ± 2.20 points in the routine group (t = 15.348, df = 18, P < 0.01). Staff demonstrated significantly higher theoretical knowledge and operational skills following implementation of the FOCUS-PDCA programme (Table 3). The Cohen’s d values were 7.54 and 6.86, respectively, indicating very large effect sizes.
Table 3.
Assessment of staff competency in dental handpiece processing before and after FOCUS-PDCA implementation
| Group | Number of cases | theoretical results(points) | Operation results(points) |
|---|---|---|---|
| routine processing group | 10 | 81.50 ± 2.36 | 83.80 ± 2.20 |
| FOCUS-PDCA program processing group | 10 | 96.10 ± 1.37 | 96.30 ± 1.34 |
| T value | 16.871 | 15.348 | |
| P value | < 0.01 | < 0.01 |
Comparison of mechanical failures
The incidence of bearing failures was 0.066% (2/3,032) in the FOCUS-PDCA group versus 0.346% (10/2,893) in the routine group (χ² = 5.730, df = 1, P < 0.05). The incidence of clamping shaft failures was 0.033% (1/3,032) in the FOCUS-PDCA group versus 0.276% (8/2,893) in the routine group (χ² = 5.849, df = 1, P < 0.05). The FOCUS-PDCA programme significantly reduced both types of mechanical failures (Table 4). The Cramér’s V values were 0.032 and 0.033, respectively, indicating small but clinically meaningful effects. As shown in Fig. 3, the implementation of the FOCUS-PDCA programme resulted in substantial reductions in both bearing and clamping shaft failures, demonstrating the effectiveness of standardised processing procedures in maintaining handpiece functionality.
Table 4.
Incidence of mechanical failures in dental handpieces under different processing protocols
| Group | Number of cases | Bearing failure | clamping shaft failure | ||
|---|---|---|---|---|---|
| Occurrence number | Incidence rate% | Occurrence number | Incidence rate% | ||
| routine processing group | 2893 | 10 | 0.346 | 8 | 0.276 |
| FOCUS-PDCA program processing group | 3032 | 2 | 0.066 | 1 | 0.033 |
| χ2 value | 5.730 | 5.849 | |||
| P value | < 0.05 | < 0.05 | |||
Fig. 3.
Incidence of mechanical failures in dental handpieces under different processing protocols. All differences were statistically significant (P < 0.05)
Comparison of clinical satisfaction
The average satisfaction score was 88.80 ± 5.88 points in the FOCUS-PDCA group compared with 65.13 ± 7.60 points in the routine group (t = 13.479, df = 58, P < 0.01). Clinical satisfaction with DHP processing quality was significantly higher following implementation of the FOCUS-PDCA programme (Table 5). The Cohen’s d value was 3.52, indicating a very large effect size.
Table 5.
Clinical user satisfaction with dental handpiece performance following different processing protocols
| Group | Number of cases | the satisfaction score (points) |
|---|---|---|
| routine processing group | 30 | 65.13 ± 7.60 |
| FOCUS-PDCA program processing group | 30 | 88.80 ± 5.88 |
| T value | 13.479 | |
| P value | < 0.01 |
Discussion
Enhancement of quality management of dental handpiece processing via the FOCUS-PDCA programme
Dental handpieces are among the most frequently used and highly contaminated medical instruments in dentistry. As high-risk devices, they represent a major pathway for nosocomial infections in dental departments. Improper processing not only damages mechanical components, affecting performance and service life, but also increases the risk of cross-infection and medical disputes [17–20]. Taken together, our FOCUS-PDCA programme lifted the overall processing pass-rate from 94.5% to 98.6%, cut mechanical-failure incidence five-fold, and raised user-satisfaction scores by 24 points—findings that directly address this quality gap.
As a structured approach to CQI, the FOCUS-PDCA programme enables comprehensive analysis of existing problems by identifying relevant influencing factors, developing targeted improvement plans and systematically implementing them [21–24]. Previous studies have shown the programme’s effectiveness in improving disinfection quality of flexible endoscopes [2], reducing the incidence of wet packaging in foreign medical instruments [25], improving on-time surgical rates [26] and decreasing pharmacy dispensing errors [27].
In this study, application of the methodology to DHP processing revealed key weaknesses in the existing system and enabled targeted improvements in equipment, processes and personnel management. The significantly higher qualification rates for cleaning, packaging and sterilisation observed in the intervention group (P < 0.01) reflect the success of these measures. Additionally, improved theoretical knowledge and operational skills among staff indicate greater procedural compliance, which may translate into reduced reprocessing workload—a finding that warrants further confirmation. These results are consistent with prior research by Chen et al. [11], who reported similar improvements in wet pack rates and functional loss rates of DHPs through application of the PDCA cycle.
Reduction in mechanical failures and improvement of satisfaction through standardised processing
Our data showed a 5- to 8-fold reduction in failure incidence (Table 4), supporting the following mechanism: the normal operation of DHPs is closely linked to bearing performance, and mechanical failures are frequently associated with incomplete drying and improper lubrication after cleaning [13, 28].
The FOCUS-PDCA programme identified critical gaps in the processing chain and added essential steps, including pre-drying of the handpiece cavity before oil injection and switching from manual to automated oil injection with precise dosage control. These measures promoted more effective bearing lubrication and maintenance while eliminating the inconsistencies of manual processes.
Our findings offer several practical recommendations for healthcare facilities seeking to improve sterilisation protocols. First, standardised drying procedures before lubrication substantially reduce mechanical failures. Second, automated systems for critical steps such as oil injection provide more consistent results than manual methods. Third, the use of clear visual indicators (e.g. timers) improves compliance with required waiting periods. Finally, organised storage systems with appropriate spacing enhance sterilisation effectiveness.
The substantially lower incidence of mechanical failures and higher satisfaction scores among clinicians and nurses in the intervention group confirm the practical benefits of these improvements.
Limitations
Several limitations should be acknowledged when interpreting our findings. First, this study was conducted at a single medical centre, which may limit generalisability to other healthcare settings or populations. Second, although a structured approach was applied to minimise bias, time and resource constraints prevented control of all potential confounding factors.
Additionally, this study focused primarily on short-term outcomes, and further research is required to assess the long-term impact of the FOCUS-PDCA programme on DHP processing quality and mechanical performance. Finally, despite measures to maintain objectivity, observer bias cannot be completely excluded, particularly in the assessment of staff theoretical knowledge and operational skills. To reduce this potential bias, dual-reviewer assessments were used for cleaning qualification evaluations, and standardised assessment tools were applied to assess staff knowledge and skills; however, the complete elimination of subjective judgement remains challenging.
Prospects for future research
Despite these limitations, this study provides preliminary evidence supporting the use of the FOCUS-PDCA programme for DHP processing quality management. Future research could progress in several directions. First, multicentre, large-sample studies could validate these results and examine the programme’s applicability across different healthcare settings. Second, long-term follow-up studies could evaluate the sustained effects on processing quality, mechanical failure rates and healthcare costs. Third, combination approaches integrating the FOCUS-PDCA programme with other quality improvement tools may offer additional benefits. Finally, incorporating modern information technologies such as electronic health records and data analytics could provide more effective support and monitoring for programme implementation.
Conclusion
Within the limitations of this study, the FOCUS-PDCA programme effectively improved the quality of DHP processing, reduced mechanical failures and enhanced staff compliance with standardised procedures. Its systematic approach to identifying and addressing process weaknesses led to measurable improvements across multiple quality indicators. Dental clinicians and nurses reported substantially greater satisfaction with handpiece performance and availability following implementation of the revised protocols.
Acknowledgements
No funding or sponsorship was received for this study or publication of this article.
Abbreviations
- DHP
Dental handpiece
- CQI
Continuous quality improvement
- PDCA
Plan, do, check and act
- FOCUS
Find, organise, clarify, understand and select
Authors’ contributions
(I) Conception and design: Che FL (II) Administrative support: Li JQ and Li SD (III) Provision of study materials or patients: Ji KW and Jin MZ (IV) Collection and assembly of data: Qian LM and Che FL (V) Data analysis and interpretation: Li JQ, Jin MZ and Qian LM (VI) Manuscript writing: All authors (VII) Final approval of manuscript: All authors.
Funding
This research did not receive any funding support.
Data availability
All data generated or analyzed during this study are included in this published article.
Declarations
Ethics approval and consent to participate
This study was conducted in accordance with the Declaration of Helsinki and approved by the ethics committee of Affiliated Ruijin Hospital of Shanghai Jiaotong University School of Medicine. (approval code: RJHK2024096).
Consent for publication
Not Applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Deasy EC, Scott TA, Swan JS, O’Donnell MJ, Coleman DC. Effective cleaning and decontamination of the internal air and water channels, heads and head-gears of multiple contra-angle dental handpieces using an enzymatic detergent and automated washer-disinfection in a dental hospital setting. J Hosp Infect. 2022;128:80–8. 10.1016/j.jhin.2022.07.019. [DOI] [PubMed] [Google Scholar]
- 2.Yang J, Zhu L, Yang C, Xu H. Application of FOCUS-PDCA mode in endoscopy center to improve the disinfection quality of flexible endoscopes. Chin J Nosocom Infect. 2017;27(18):4252–4. 10.11816/cn.ni2017-172447. [Google Scholar]
- 3.Chai J, Wu C, Wang W, Ying YJ, Jiang XX, Xiong XL. Continuous quality improvement in the management of hypoglycemia during Hemodialysis in patients with diabetic nephropathy. Chin J Nurs. 2015;50(02):170–4. 10.3761/j.issn.0254-1769.2015.02.010. [Google Scholar]
- 4.Ding S, Zhu L, Dong K, Zhao HB, Wang Y, Wang L, You ZY, et al. Application of FOCUS-PDCA to improve PDA scanning rate during nurse administration. Nurs Res. 2019;33(07):1262–4. 10.12102/j.issn.1009-6493.2019.07.044. [Google Scholar]
- 5.Pappou EP, Temple LK, Patil S, Smith JJ, Wei IH, et al. Quality of life and function after rectal cancer surgery with and without sphincter preservation. Front Oncol. 2022;12:944843. 10.3389/fonc.2022.944843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Niu Y, Zhang L, Sun X. Efficacy of seamless care management under FOCUS-PDCA for patients with acute cerebral infarction complicated with dysphagia and its influence on nutritional status and neurological functions. Altern Ther Health Med. 2024;30(8):251–7. [PubMed] [Google Scholar]
- 7.Oliveira L, Teixeira A, Duarte I. The appraisal of Self-Care agency Scale-Revised (ASAS-R): reliability and validity among Portuguese medical students. Int J Environ Res Public Health. 2022;19(17):10848. 10.3390/ijerph191710848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yanarda? MZ, ?zer ?. Investigating Self-Care agency and Well-Being of elderly people. Soc Work Public Health. 2021;36(4):496–508. 10.1080/19371918.2021.1915908. [DOI] [PubMed]
- 9.Shen Y, Wei J, Wu Y. Comparison of four methods to reduce the oily wet pack rate of dental mobile phones. China Med Device Inform. 2021;27:34–5. 10.3969/j.issn.1006-6586.2021.07.013. [Google Scholar]
- 10.Chen X, Zhou X, Li J, Liu FL. Study on the drying methods of Non-removable lacunar devices. Chin J Disinfection. 2015;32(1):93–4. 10.11726/j.issn.1001-7658.2015.01.0093.02. [Google Scholar]
- 11.Chen M, Tang Y, Lv G, Gu MY, Lin CM, Zhang AF. Application of PDCA cycle in reducing the wet pack rate and functional loss rate of dental handpieces. Qilu J Nurs. 2018;24(20):46–8. 10.3969/j.issn.1006-7256.2018.20.017. [Google Scholar]
- 12.Jin M, Che F, Qian L. Design and application of sterilization loading separator for Paper-plastic packaging articles. Chin J Nurs. 2018;53(05):631–2. 10.3761/j.issn.0254-1769.2018.05.025. [Google Scholar]
- 13.Standard Operating Procedure for. Disinfection and sterilization of dental devices WS 506–2016. Chin J Infect Control. 2017;16(08):784–92. [Google Scholar]
- 14.CSSD. Part 1: management specification WS 310.1–2016. Chin J Infect Control. 2017;16(09):887–92. 10.3969/j.issn.1671-9638.2017.00.023. [Google Scholar]
- 15.Yao X, Gong Y, Zhang Y, Zhou J, Wu M, Lu Q, et al. Investigation on the implementation of WS/T 367–2012 technical standard for disinfection in medical institutions. Chin J Infect Control. 2020;19(08):728–32. [Google Scholar]
- 16.Yin L, Li X, Niu L, Zhang XP. Retrospective study on organic detergent cleaning to reduce the occurrence of mechanical failure in dental high-speed turbine handpieces. J Practical Stomatology. 2021;37:164–6. 10.3969/j.issn.1001-3733.2021.02.004. [Google Scholar]
- 17.Yalamanchi P, Yu J, Chandler L, Mirza N. High-level disinfection of otorhinolaryngology clinical instruments: an evaluation of the efficacy and cost-effectiveness of instrument storage. Otolaryngol Head Neck Surg. 2018;158(1):163–6. 10.1177/0194599817738977. [DOI] [PubMed] [Google Scholar]
- 18.Huang M, Xu P, Liu C, Xiao LM. Timeliness study of idle irrigation of dental handpieces to control lubricant environmental pollution. J Nurs. 2014;21(23):55–8. 10.16460/j.issn.1008-9969.2014.23.0055.04. [Google Scholar]
- 19.Hu P, Zhang Y. Discussion on handling process of dental handpiece in disinfection supply center of hospital. China Med Equip. 2014;29(12):85–7. 10.3969/j.issn.1674-1633.2014.12.027. [Google Scholar]
- 20.Jiang X, Yu Q, Song G, Qu LL. Role of whole-process quality traceability management in dental medical device disinfection and nosocomial infection prevention and control. Chin Med Equip. 2021;18(05):155–8. 10.3969/j.issn.1672-8270.2021.05.038. [Google Scholar]
- 21.Cadnum JL, Li DF, Redmond SN, John AR, Pearlmutter B, Donskey CJ. Effectiveness of ultraviolet-C light and a high-level disinfection cabinet for decontamination of N95 respirators. Pathog Immun. 2020;5(1):52–67. 10.20411/pai.v5i1.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Armellino D, Walsh TJ, Petraitis V, Kowalski W. Assessment of focused multivector ultraviolet disinfection withshadowless delivery using 5-point multisided sampling ofpatientcare equipment without manual-chemical disinfection. Am J Infect Control. 2019;47(4):409–14. 10.1016/j.ajic.2018.09.019. [DOI] [PubMed] [Google Scholar]
- 23.Meyer J, Nippak P, Cumming A. An evaluation of cleaning practices at a teaching hospital. Am J Infect Control. 2021;49(1):40–3. 10.1016/j.ajic.2020.06.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li Q, Guo W, Liu D, Guo D, Zhou Z. Quality management practice of endoscopic devices based on FOCUS-PDCA management method. China Med Equip. 2019;34(5):140–3. 10.3969/j.issn.1674-1633.2019.05.036. [Google Scholar]
- 25.Sun H, Yang L, Xiang M, Zhao L. Reducing the incidence of sterilization wet pack of foreign medical devices using FOCUS-PDCA procedure. Nurs Res. 2018;32(14):2232–7. 10.12102/j.issn.1009-6493.2018.14.020. [Google Scholar]
- 26.Zhu X. Application of FOCUS-PDCA program in improving the punctual stroke rate of the first operation. J Nurses’ Refresh Stud. 2010;25(8):696–7. 10.3969/j.issn.1002-6975.2010.08.010. [Google Scholar]
- 27.Liu R, Bagotinib, Zhu Q. Application of FOCUS-PDCA in reducing the error rate of pharmacy dispensing. Chin J Hosp Pharm. 2020;40(24):2595–9. 10.13286/j.1001-5213.2020.24.20. 2610. [Google Scholar]
- 28.Wen Q, Li X, Ming Q, Wang YXX, Yu C, Gu Z. Comparative study on different drying methods of high-speed dental handpieces. Nurses’ Refresh J. 2022;37(14):1335–7. 10.16821/j.cnki.hsjx.2022.14.018. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.