Abstract
Background
The aim of this retrospective cohort study was to evaluate the effect of day 3 embryo cell number on the clinical pregnancy and neonatal outcomes of single blastocyst transfer in frozen embryo transfer (FET) cycles.
Methods
The study included 1220 single blastocyst transfer from FET cycles conducted between January 2017 and April 2024. Patients were categorized into four groups based on day 3 embryo cell number : 110 cycles in the < 7-cell group, 743 cycles in the 7–9-cell group, 282 cycles in the 10–13-cell group, and 84 cycles in the > 13-cell group. The study compared the clinical pregnancy outcomes and neonatal outcomes among the four groups.
Results
When the maternal age was < 35 years or high-quality blastocysts were transferred, the clinical pregnancy rate (CPR) of the < 7-cell group was significantly lower than those of other three groups (all P < 0.008). Similarly, when high-quality blastocysts were transferred, the live birth rates (LBRs) of the 7–9-cell group and > 13-cell group were significantly higher than those of the < 7-cell group (all P < 0.008). In women aged < 35 years with high-quality blastocyst transfers, after adjusting for confounders, 7–9 and 10–13 groups were with significantly higher CPR (aOR 2.66, 95% CI 1.44–4.91; aOR 2.15, 95%CI 1.10–4.18 and LBR (aOR 2.50, 95%CI 1.32–4.73, aOR 1.74, 95% CI 0.88–3.46).
Conclusion
In FET cycles, a low day 3 cell number (< 7-cell) on day 3 was related to decreased CPR and LBR after blastocyst transfer, whereas a number > 9 was comparable to that of 7–9 cells.
Supplementary Information
The online version contains supplementary material available at 10.1186/s40001-025-03343-3.
Keywords: Cell number, Blastocyst, Frozen embryo transfer, Clinical pregnancy, Live birth, Neonatal
Background
In recent years, in vitro fertilization (IVF)/intracytoplasmic sperm injection (ICSI) has emerged as an effective treatment for infertility. ART has assisted the births of millions of babies, though the success rate remains moderate, due to the challenges for transfer outcomes, including embryo selection. On day 3 post-fertilization, there are significant differences in the cell number in embryos. This complicates the assessment of their developmental potential. Studies have shown that culturing embryos to the blastocyst stage can more accurately predict their implantation potential and improve synchronization between the uterus and the embryo [1]. With advancements in culture media and cryopreservation techniques, blastocyst transfer has gradually become the mainstream approach. To reduce multiple pregnancy rates and enhance maternal and fetal safety, an increasing number of reproductive centers are adopting a single blastocyst transfer strategy. This strategy has been proven to effectively reduce multiple pregnancy rates without affecting clinical pregnancy rates (CPRs) [2].
Morphological assessment methods have the advantages of simplicity and non-invasiveness [3]. Therefore, they remain the most commonly used method for selecting blastocysts in clinical practice, such as Gardner and Schoolcraft [4], the Istanbul Consensus [5] or others. However, pregnancy outcomes remain suboptimal. This highlights the need to establish a method for identifying embryos with higher implantation potential. The cell numbers on day 3 is one of the key indicators of early embryonic development. Embryos with abnormally fast or slow developmental rates may have abnormalities, leading to reduced implantation rates [5]. Recent advancements in time-lapse technology have significantly enhanced our ability to monitor embryonic development and improved the accuracy of cell number assessments [6].
The mainstream embryology theory states that the optimal blastomere number of day 3 embryos is 7–9 cells. These embryos have the highest proportion of chromosomal euploidy, and exhibit the best clinical implantation potential [5]. However, some researchers have reported different results. Their findings were not entirely consistent. For instance, Wu et al. [7] found an association between lower day 3 cell number and decreased live birth rates (LBRs) in frozen-thawed embryo transfer (FET) cycles among younger women regardless of whether high-quality or low-quality blastocysts were transferred. No significant difference was observed in women aged ≥ 35. Another study [8], however, found no significant difference in clinical pregnancy outcomes for high-quality blastocyst transfers based on cell number. Conversely, CPRs and LBRs significantly increased with higher day 3 cell numbers in the fair-quality and low-quality blastocyst subgroups. Additionally, Qiu et al. [9] revealed that in women under 35 years, higher day 3 cell numbers (> 9 cells) in vitrified-warmed day 5 single blastocyst transfer (SBT) cycles were associated with higher LBR. Furthermore, there is a lack of data on neonatal safety. Therefore, further research is needed to investigate the impact of day 3 cell number on both blastocyst clinical outcomes and neonatal outcomes.
Martha et al. [10] found that the blastocyst formation rate of day 3 embryos with ≤ 6 cells were significantly lower than those with > 6 cells, and embryos with ≥ 10 cells had a significantly greater likelihood of reaching high-quality blastocysts. Some studies also indicated that embryos with faster development rates on day 3 had similar or even significantly higher blastocyst formation rates and expansion rates compared to 8-cell embryos [11]. In addition, Pons et al. [12] further challenged the traditional view, demonstrating that blastocysts derived from faster-developing embryos were comparable in ploidy to those from 8-cell embryos. Kroener et al. also reported that a day 3 cell number > 13 was associated with a relatively low aneuploidy rate [13]. Therefore, combining the above previous studies and observed distribution within previous research [14], we divided single blastocyst transfers in FET cycles into four groups based on the day 3 cell number: the < 7-cell group, 7–9-cell group, 10–13-cell group, and > 13-cell group. This grouping method was similar to that used in previous studies [10, 14, 15]. The aim was to investigate the impact of day 3 cell number on clinical pregnancy outcomes and neonatal outcomes of vitrified-warmed single blastocyst transfer, providing evidence-based guidance for optimizing embryo selection strategies and enhancing the safety and efficacy of in vitro fertilization/intracytoplasmic sperm injection (IVF/ICSI) procedures.
Materials and methods
Study design and patients
A retrospective study was conducted at the Reproductive Medicine Center of Xingtai Meihe Reproductive and Genetic Hospital. The study included patients who underwent a frozen single blastocyst transfer between January 2017 and April 2024. Exclusion criteria comprised blastocysts derived from donor oocytes or sperm, preimplantation genetic testing (PGT), previous diagnosis of acquired or congenital uterine abnormalities (such as uterine malformations, intrauterine adhesions, endometrial polyps, and submucosal myomas) confirmed by hysterosalpingography and three-dimensional ultrasound, ovarian hypoplasia, as well as incomplete core data in the electronic medical records. The study protocol received approval from the Institutional Review Board of the hospital.
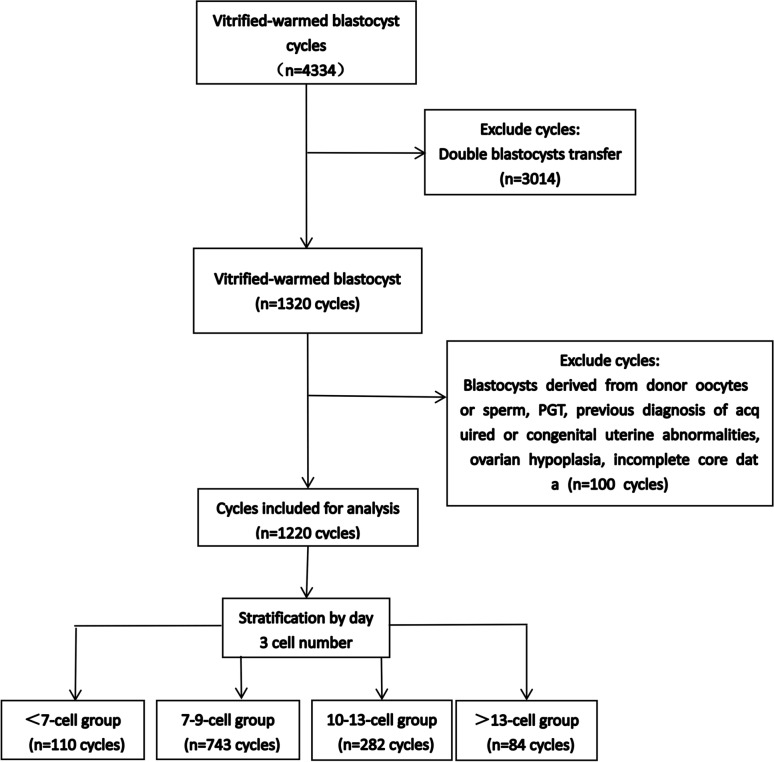
The study included 1,220 single blastocyst transfers from FET cycles. These cycles were divided into four groups based on the day 3 embryo cell number: 110 cycles in the < 7-cell group, 743 cycles in the 7–9-cell group, 282 cycles in the 10–13-cell group, and 84 cycles in the > 13-cell group (Fig. 1). Clinical pregnancy outcomes and neonatal outcomes were compared among the four groups. This study was approved by the Ethics Committee of Xingtai Meihe Reproductive and Genetic Hospital (No. 2023–15). According to the Ethics Committee of Xingtai Meihe Reproductive and Genetic Hospital, the requirement for informed consent was waived.
Fig. 1.
Scheme showing the study design
Ovarian stimulation
Ovulation promotion protocols were based on the routine protocols established by the department. Tailored ovulation induction regimens were selected based on factors such as the patient's ovarian reserve function, homogeneity of pre-basal follicles and the receptivity of the endometrium, including the long, ultra-long protocol and specific types (antagonist protocol, micro-stimulation). 6000–10,000 IU of human chorionic gonadotropin (HCG, Zhuhai Lizon Pharmaceutical) was injected when at least two leading follicles measured ≥ 18 mm. After 36–37 h, the oocytes were retrieved out by vaginal puncture under intravenous anesthesia and ultrasound guidance.
Embryo culture and assessment
All oocytes were inseminated using IVF or ICSI methods based on semen parameters. For oocytes that failed to fertilize using the IVF method, rescue ICSI (R-ICSI) would be performed in the afternoon.The embryos were then cultured individually in microdroplets containing G1-PLUS/G2-PLUS sequential culture medium (Vitrolife, Gothenburg, Sweden) in a incubator under the following conditions: 37 °C, 6% CO₂, 5% O₂, and 89% N₂. On day 3, embryos were assessed approximately 68 h after insemination. The cell number, the fragmentation, and the evenness of the blastomeres were recorded to evaluate day 3 embryos. The evenness of the blastomeres was classified into three grades: 0: The blastomeres were uniform, with size differences ≤ 10%; 1: Mildly uneven (10–49% difference); 2: Significantly uneven (≥ 50% difference). Blastocysts were scored in accordance with the Gardner blastocyst scoring system [4] on Day 5 or 6. In our laboratory, blastocysts were recorded as high-quality if they reached at least an expansion stage 3 with A or B for inner cell mass (ICM) and trophectoderm (TE), and the rest were considered low-quality. Frozen blastocysts were selected if they were at stage 3 or above and their ICM score was not C. Embryos with better grades were given higher priority for transfer, regardless of the day 3 cell number. Two highly trained embryologists graded the embryos using the same scoring standards.
Endometrial preparation
Endometrial preparation for FET was performed in artificial cycles, natural cycles, ovulation induction cycles, or down regulation + artificial cycles for women respectively. The specific protocols were as follows. Artificial cycles: During this program, oral estradiol valerate (Bayer, Leverkusen, Germany) 4–9 mg was administered daily. When the endometrial thickness was ≥ 8 mm, began intramuscular progesterone (40–100 mg, xianju, zhejiang) injection. Blastocyst transfer was performed on the sixth day of progesterone administration. Starting on the first day after transplantation, intramuscular injections of progesterone are changed to 60 mg/day, and progesterone soft capsules (Anqitan, Belgian Besins Manufacturing) at 200 mg per dose, twice daily, administered vaginally, for luteal support. Natural cycles: By detecting LH (luteinizing hormone) levels in blood/urine and performing a transvaginal ultrasound to monitor dominant follicle development, the timing of the LH surge was determined or the decision was made to use HCG (10,000 IU, Zhuhai Lizon Pharmaceutical) to trigger ovulation. Blastocyst transfer was performed 6 days after the LH surge or 7 days after HCG triggering. If a pregnancy was achieved, progesterone supplementation was continued until the first ultrasound confirming the clinical pregnancy. Down regulation + artificial cycles: Gonadotrophin releasing hormone analogue (GnRHa) 3.75 mg subcutaneous injection for 28–35 days, followed by endometrial preparation once the standard was met. The endometrial preparation protocol was the same as for the artificial cycle. Ovulation induction cycles: Oral administration of letrozole (2.5–5 mg, furui, jiangsu) for 5 days, followed by ultrasound monitoring of ovulation on day 10 of the menstrual cycle. The specific protocol is the same as for the natural cycle.
Vitrification and thawing
Blastocysts were cryopreserved on day 5 or 6 after insemination following artificial shrinkage by a laser. Vitrification and warming kits (KITAZATO, Japan) was used for both vitrification and thawing of blastocysts. Vitrification of embryos: The embryos were transferred to an equilibration solution for 10 min. Then, the embryos were exposed to the vitrification solution for 40–60 s. The embryos were loaded onto the frozen carrier with a very small volume of vitrification solution. They were then immediately immersed in liquid nitrogen. Thawing of embryos: According to the instructions of the warming kit, the frozen carrier was removed from liquid nitrogen and inserted into thawing solution (TS) for one minute and dilution solution (DS) for three minutes. It was then washed twice for five minutes each in Washing Solution 1 (WS1) and Washing Solution 2 (WS2). The criterion for blastocyst survival was re-expansion after warming. The embryos were then transferred to G2-PLUS blastocyst culture medium (Vitrolife, Gothenburg, Sweden). And the blastocyst was then transferred into the uterus two to three hours after warming.
Clinical outcomes
The primary outcome measure was clinical pregnancy rate. The secondary outcomes variables included rates of live birth, multiple pregnancies, ectopic pregnancy, and miscarriage, as well as neonatal outcomes. Neonatal outcomes included preterm birth, gestational age at delivery, proportion of males.
The CPR was calculated by dividing the number of patients with at least 1 gestational sac detected by transvaginal ultrasound (performed 28 days after embryo transfer) by the number of patients transferred. A live birth was defined as a live baby delivered after 24 weeks of pregnancy. Multiple pregnancies were defined as the presence of multiple intrauterine fetuses simultaneously. Ectopic pregnancy was diagnosed using ultrasound or laparoscopic imaging of at least one ectopic pregnancy sac. Miscarriage was defined as the loss of fetal cardiac activity within 28 weeks of confirming clinical pregnancy. Gestational age was calculated from the date of transfer by adding 19 days. Preterm birth was defined as a delivery before completing 37 weeks of gestation. Proportion of males was defined as the percentage of male infants.
Statistical analysis
All data were statistically analyzed using SPSS 22.0 for Windows (IBM, Armonk, NY, USA).
Continuous variables that did not conform to a normal distribution were expressed as the median (25th, 75th percentile), M (Q1, Q3), and were compared using the Kruskal–Wallis test. Categorical variables were expressed as frequencies and proportions and were compared using the chi-square or Fisher’s exact test, P-values < 0.05 were considered statistically significant. All data analyses used the Bonferroni correction, with a P-value of < 0.008 (0.05/6) considered statistically significant. The paper also conducted subgroup analyses based on female age, embryonic development days, and blastocyst grading to compare the CPR and LBR among the subgroups. To investigate the effect of day 3 cell number on pregnancy outcomes in < 35 years old who were transplanted with high-quality blastocysts, we performed multivariate logistic regression analyses. The female age, body mass index (BMI), duration of infertility, pattern of infertility, infertility factors, embryonic development days, pattern of insemination, evenness of blastomeres, fragmentation of day 3 embryo and expansion grade of transferred blastocysts were used as independent variables, while the CPRs or LBRs were used as dependent variables in a logistic regression analysis. A significance level of P < 0.05 was considered statistically significant.
Results
Distribution of day 3 cell number and pregnancy outcomes by cell number after blastocyst transfer
As shown in Supplementary Fig. 1, the highest number of blastocysts was observed in those with 8 cells on day 3, accounting for 38.11% (465/1220) of all blastocysts. The number of blastocysts gradually decreased as cell numbers increased and decreased progressively. When the cell number on day 3 was 4, 5, or 6 cells, the CPR and LBR after single blastocyst transfer in FET cycles were low (4-cell: 46.15%, 30.77%; 5-cell: 38.24%, 20.59%; 6-cell: 50.79%, 41.27%). When the cell number was ≥ 7, the CPR and LBR for most cell numbers tended to stabilise and showed an upward trend. As shown in Supplementary Fig. 2, when the day 3 cell number was 14 and ≥ 15, the proportion of male infants was relatively high (65.52%, 85.71%). Therefore, combining the previous studies [10–15], we divided single blastocyst transfers in FET cycles into four groups based on the day 3 cell number: the < 7-cell group, 7–9-cell group, 10–13-cell group, and > 13-cell group.
Maternal and cycle characteristics
As shown in Tables 1, 2, there were no significant differences among the four groups in terms of maternal age, BMI, duration of infertility, distribution of infertility factors, endometrial preparation method, endometrial thickness, and blastocyst expansion grades. However, significant differences were observed in the four groups regarding pattern of insemination (P = 0.001), day 3 embryo evenness (P < 0.001), day 3 embryo fragmentation (P < 0.001), transferred blastocysts grade (P < 0.001), and embryonic development days (P < 0.001). In addition, the proportion of day 5 transfers and high-quality blastocysts showed a progressive increase with an increase in the day 3 cell number (P < 0.001).
Table 1.
Baseline characteristics and cycle characteristics among the different groups
| Characteristics | < 7-cell | 7–9-cell | 10–13-cell | > 13-cell | P-value |
|---|---|---|---|---|---|
| Number of cycles | 110 | 743 | 282 | 84 | |
| Female age (years) | 31.00 (28.00,34.00) | 31.00 (2.00,34.00) | 30.00 (27.00,33.00) | 30.50 (28.00,33.00) | 0.367 |
| Female BMI (kg/m2) | 24.00 (20.80,26.70) | 23.9 (21.50,26.80) | 24.4 (21.70,26.50) | 24.60 (22.60,26.80) | 0.667 |
| Duration of infertility (years) | 3.50 (2.00,7.00) | 4.00 (2.00,6.00) | 4.00 (2.00,6.00) | 4.00 (2.75,6.00) | 0.744 |
| Pattern of infertility (%) | 0.339 | ||||
| Primary | 41.82 (46/110) | 36.07 (268/743) | 39.36 (111/282) | 30.95 (26/84) | |
| Secondary | 58.18 (64/110) | 63.93 (475/743) | 60.64 (171/282) | 69.05 (58/84) | |
| Proportion of infertility factors (%) | 0.364 | ||||
| Fallopian tube factor | 2.73 (3/110) | 4.44 (33/743) | 5.32 (15/282) | 4.76 (4/84) | |
| Ovulation disorder | 3.64 (4/110) | 4.71 (35/743) | 2.13 (6/282) | 1.19 (1/84) | |
| Endometriosis | 5.45 (6/110) | 5.38 (40/743) | 7.09 (20/282) | 7.14 (6/84) | |
| Male factor | 21.82 (24/110) | 23.28 (173/743) | 26.24 (74/282) | 22.62 (19/84) | |
| Unknown cause | 8.18 (9/110) | 6.06 (45/743) | 6.03 (17/282) | 0.00 (0/84) | |
| Others | 58.18 (64/110) | 56.12 (417/743) | 53.19 (150/282) | 64.29 (54/84) | |
| Endometrial preparation methods (%) | 0.590 | ||||
| Artificial cycles | 65.45 (72/110) | 62.58 (465/743) | 68.44 (193/282) | 71.43 (60/84) | |
| Ovulation induction cycles | 3.64 (4/110) | 2.15 (16/743) | 1.42 (4/282) | 1.19 (1/84) | |
| Natural cycles | 6.36 (7/110) | 6.19 (46/743) | 4.61 (13/282) | 3.57 (3/84) | |
| Down regulation + artificial cycles | 24.55 (27/110) | 29.07 (216/743) | 25.53 (72/282) | 23.81 (20/84) | |
| Thickness of endometrium (mm) | 9.15 (8.4,10) | 9.00 (8.30,10.00) | 9.00 (8.30,10.00) | 9.00 (8.50,9.60) | 0.330 |
| Pattern of insemination (%) | 0.001 | ||||
| IVF | 70.91 (78/110) | 79.27 (589/743) | 87.23 (246/282)ab | 88.1 (74/84)a | |
| ICSI | 26.36 (29/110) | 17.77 (132/743) | 12.41 (35/282)a | 10.71 (9/84)a | |
| R-ICSI | 2.73 (3/110) | 2.96 (22/743) | 0.35 (1/282) | 1.19 (1/84) | |
1. P value indicates differences among the four groups. P < 0.05 indicates statistically significant difference in results
2. a indicates a significant difference compared to < 7-cell group; b indicates a significant difference compared to 7–9-cell group. According to Bonferroni correction for multiple comparisons, the difference was statistically significant at P < 0.008 (0.05/6)
Table 2.
Embryo parameters among the different groups
| Characteristics | < 7-cell | 7–9-cell | 10–13-cell | > 13-cell | P-value |
|---|---|---|---|---|---|
| Number of cycles | 110 | 743 | 282 | 84 | |
| Evenness of day 3 embryo (%) | < 0.001 | ||||
| 0 | 6.36 (7/110) | 22.75 (169/743)a | 12.41 (35/282)b | 21.43 (18/84)a | |
| 1 | 85.45 (94/110) | 73.76 (548/743)a | 86.17 (243/282)b | 78.57 (66/84) | |
| 2 | 8.18 (9/110) | 3.50 (26/743) | 1.42 (4/282)a | 0.00 (0/84)a | |
| Fragmentation of day 3 embryo (%) | < 0.001 | ||||
| < 10% | 23.64 (26/110) | 40.51 (301/743)a | 52.13 (147/282)a | 50.00 (42/84)a | |
| 10–25% | 43.64 (48/110) | 53.57 (398/743) | 44.33 (125/282) | 47.62 (40/84) | |
| > 25% | 32.73 (36/110) | 5.92 (44/743)a | 3.55 (10/282)a | 2.38 (2/84)a | |
| Expansion grade (%) | 0.325 | ||||
| IV | 45.45 (50/110) | 46.57 (346/743) | 38.65 (109/282) | 39.29 (33/84) | |
| V | 50.00 (55/110) | 48.59 (361/743) | 55.32 (156/282) | 57.14 (48/84) | |
| VI | 4.55 (5/110) | 4.85 (36/743) | 6.03 (17/282) | 3.57 (3/84) | |
| Grade of transferred blastocysts (%) | < 0.001 | ||||
| High-quality | 66.36 (73/110) | 86.27 (641/743)a | 93.97 (265/282)ab | 97.62 (82/84)ab | |
| Low-quality | 33.64 (37/110) | 13.73 (102/743) | 6.03 (17/282) | 2.38 (2/84) | |
| embryonic development days (%) | < 0.001 | ||||
| Day 5 | 42.73 (47/110) | 78.33 (582/743)a | 91.49 (258/282)ab | 98.81 (83/84)ab | |
| Day 6 | 57.27 (63/110) | 21.67 (161/743) | 8.51 (24/282) | 1.19 (1/84) | |
1. P value indicates differences among the four groups. P < 0.05 indicates statistically significant difference in results
2. a indicates a significant difference compared to < 7-cell group; b indicates a significant difference compared to 7–9-cell group. According to Bonferroni correction for multiple comparisons, the difference was statistically significant at P < 0.008 (0.05/6)
Clinical outcomes and neonatal outcomes
Table 3 provides a detailed overview of pregnancy outcomes based on the day 3 embryo cell number. The CPRs of blastocysts in the 7–9-cell group (61.78%, P = 0.002), 10–13-cell group (62.41%, P = 0.004), and > 13-cell group (66.67%, P = 0.005) were significantly higher than those in the < 7-cell group (46.36%) (Fig. 2 A). The LBR in the 7–9-cell group (48.86%) was also significantly higher than in the < 7-cell group (33.64%, P = 0.003) (Fig. 2 A). However, the proportion of males in the > 13-cell group (72.09%) was significantly higher than in the < 7-cell group (42.11%, P = 0.006) (Fig. 2 A). From this data, we also could see that no significant differences were found in CPRs and LBRs among the 7–9-cell, 10–13-cell, and > 13-cell groups (Fig. 2 A). There were no significant differences among the four groups regarding the rates of ectopic pregnancy, miscarriage, monozygotic twins, preterm birth, gestational age at delivery, and birth weight.
Table 3.
Clinical outcomes among the different groups
| < 7-cell | 7–9-cell | 10–13-cell | > 13-cell | P-value | |
|---|---|---|---|---|---|
| Number of cycles | 110 | 743 | 282 | 84 | |
| Clinical Pregnancy rate (%) | 46.36 (51/110) | 61.78 (459/743)a | 62.41 (176/282)a | 66.67 (56/84)a | 0.010 |
| Miscarriage rate (%) | 25.49 (13/51) | 18.95 (87/459) | 24.43 (43/176) | 25.00 (14/56) | 0.316 |
| Early miscarriage rate (%) | 15.69 (8/51) | 13.29 (61/459) | 14.77 (26/176) | 17.86 (10/56) | 0.785 |
| Late miscarriage rate (%) | 9.80 (5/51) | 5.66 (26/459) | 9.66 (17/176) | 5.36 (3/56) | 0.254 |
| Live birth rate (%) | 33.64 (37/110) | 48.86 (363/743)a | 46.45 (131/282) | 50.00 (42/84) | 0.026 |
| Ectopic pregnancy rate (%) | 0.00 (0/51) | 1.53 (7/459) | 1.14 (2/176) | 0.00 (0/56) | 0.638 |
| Monozygotic twins rate (%) | 0.00 (0/51) | 0.87 (4/459) | 1.14 (2/176) | 1.79 (1/56) | 0.800 |
| Premature delivery rate (%) | 9.8 (5/51) | 11.33 (52/459) | 6.25 (11/176) | 7.14 (4/56) | 0.241 |
| Gestational week of deliver (weeks) | 38 (38,39) | 38 (37,39) | 39 (38,39) | 39 (38,39) | 0.637 |
| Proportion of males (%) | 42.11 (16/38) | 60.54 (224/370) | 56.82 (75/132) | 72.09 (31/43)a | 0.043 |
| Birth weight [g, M (Q1, Q3)] | 3200 (3000,3600) | 3350 (3000,3600) | 3390 (3000,3750) | 3550 (3175,3875) | 0.051 |
1. P value indicates differences among the four groups. P < 0.05 indicates statistically significant difference in results
2. a indicates a significant difference compared to < 7-cell group. According to Bonferroni correction for multiple comparisons, the difference was statistically significant at P < 0.008 (0.05/6)
Fig. 2.
Clinical outcomes according to day 3 cell number after blastocyst transfer. “a” indicates a significant difference compared to < 7-cell group. According to Bonferroni correction for multiple comparisons, the difference was statistically significant at P < 0.008 (0.05/6). A All cycles, Clinical pregnancy rate, live birth rate and proportion of males: P < 0.05. B < 35 years, Clinical pregnancy rate and proportion of males: P < 0.05. C ≥ 35 years, all P > 0.05. D High-quality blastocysts, Clinical pregnancy rate and live birth rate: P < 0.05. E Low-quality blastocysts, all P > 0.05. F high-quality blastocysts in < 35 years, Clinical pregnancy rate and live birth rate: P < 0.05
Clinical and neonatal outcomes in subgroup analyses
In Fig. 2 and Supplementary Table I, a subgroup analysis is presented, evaluating the effect of age, blastocyst grade and embryonic development days on the CPRs and LBRs. When the maternal age was < 35 years or when high-quality blastocysts were transferred, the CPRs of the 7–9-cell group (62.95%, P = 0.007; 64.74%, P < 0.001), 10–13-cell group (64.37%, P = 0.003; 63.77%, P = 0.002), and > 13-cell group (67.11%, P = 0.007; 67.07%, P = 0.004) were significantly higher than those of the < 7-cell group (45.88%, 43.84%) (Fig. 2 B and D). Similarly, when high-quality blastocysts were transferred, the LBRs of the 7–9-cell group (51.33%, P < 0.001) and > 13-cell group (51.22%, P = 0.008) were significantly higher than those of the < 7-cell group (30.14%) (Fig. 2 D). No significant differences were observed among the four groups in any of the other subgroup analyses. As shown in Supplementary Table I, there was no significant difference in the impact of different cell numbers on pregnancy outcomes and the proportion of male infants on either day 5 or day 6.
To further investigate the impact of different cell numbers on pregnancy and neonatal outcomes, we conducted an analysis focusing on high-quality blastocyst transfers in women aged < 35 years. As presented in Fig. 2 F and Supplementary Table 2, The results indicated that the CPRs of blastocysts in the 7–9-cell group (65.19%, P < 0.001), 10–13-cell group (65.62%, P = 0.001), and > 13-cell group (67.57%, P = 0.004) were significantly higher than those in the < 7-cell group (42.62%). And the LBR in the 7–9-cell group (52.78%) was significantly higher than that in the < 7-cell group (32.79%, P = 0.003).
Logistic regression analysis of high-quality blastocyst transfers in woman < 35 years old
The confounders included female age, BMI, duration of infertility, pattern of infertility, infertility factors, embryonic development days, pattern of insemination, evenness of the blastomeres, fragmentation of day 3 embryo and expansion grade of transferred blastocysts. As shown in Table 4, after controlling for potential confounding factors, 7–9-cell group and 10–13-cell group were associated with higher CPR than < 7-cell group (aOR 2.66, 95% CI 1.44–4.91; aOR 2.15, 95%CI 1.10–4.18). 7–9 group was also associated with higher LBR than < 7-cell group (aOR 2.50, 95%CI 1.32–4.73).
Table 4.
Logistic regression analysis of high-quality blastocyst transfers in woman < 35 years old
| Clinical pregnancy rate | Live birth rate | |||||
|---|---|---|---|---|---|---|
| aOR | 95%CI | P-value | aOR | 95%CI | P-value | |
| Day 3 embryo cell number | ||||||
| < 7-cell | 1 (Reference) | 1 (Reference) | ||||
| 7–9-cell | 2.66 | 1.44–4.91 | 0.002 | 2.50 | 1.32–4.73 | 0.005 |
| 10–13-cell | 2.15 | 1.10–4.18 | 0.025 | 1.74 | 0.88–3.46 | 0.112 |
| > 13-cell | 2.13 | 0.96–4.72 | 0.062 | 1.72 | 0.77–3.81 | 0.184 |
Discussion
In the present study, it was found that in patients < 35 years old or when high-quality blastocysts were transferred, a low day 3 cell number (< 7 cells) was associated with reduced CPR and LBR following single blastocyst transfers. No significant differences were observed between groups with > 7 cells. Therefore, blastocysts with > 9 cells on day 3 may achieve similar pregnancy outcomes to those with 7–9 cells. The same result was found in patients aged < 35 years with high-quality blastocyst transfer. In patients ≥ 35 years or when low-quality blastocysts were transferred, cell number on day 3 had no significant impact on pregnancy outcomes.
The day 3 embryo cell number is an important factor that affected embryo quality cultured in vitro. In this study, Table 2 showed that the < 7-cell group had more fragments and a lower proportion of forming day 5 blastocysts and high-quality blastocysts. These findings suggest that day 3 embryos with fewer cells might exhibit a prolonged cell cycle duration, slower developmental progression and a greater incidence of cell fragmentation. All of these factors might reduce implantation potential, leading to poor clinical outcomes [16]. The reason might be that the rate of chromosomal aneuploidy in blastocysts with < 7 cells was lower. Previous studies [12] have shown that compared to the 8-cell embryos on day 3, slower-developing day 3 embryos had a significantly reduced likelihood of forming diploid blastocysts, leading to decreased CPRs and LBRs [8]. However, in this study, the day 3 cell number in blastocysts had no significant impact on pregnancy outcomes in patients aged ≥ 35 years. This is similar to the results of Wu's research [7], which showed that a low day 3 cell number was related to decreased CPR and LBR after blastocyst transfer in young women, but had less effect on older women. Studies have shown that embryos from older women had higher rates of aneuploidy [17, 18]. Given this, older women's embryos already had a high aneuploidy baseline, which might weaken the influence of cell number on chromosomal status. While there was no definitive explanation for how maternal age affected aneuploidy rates, it was hypothesized that environmental and intrinsic factors could alter age-related chromosomal meiotic segregation [19].
Some research reports indicated that the blastocyst formation rate of faster developing embryos on day 3 was comparable to that of 8-cell embryos [11, 12]. Other studies, however, showed a statistically significant increase [10, 19]. Additionally, a retrospective study by Luna et al. [10] indicated that faster developing embryos (≥ 10 cells) were more likely to develop into high-quality 4AA or 5AA blastocysts compared to embryos with intermediate division rates. This study was consistent with the aforementioned findings: as the cell number in day 3 embryos increased, the quality of transferred blastocysts significantly improved. Furthermore, the pregnancy outcomes of high-quality blastocysts were significantly superior to those of low-quality blastocysts [20]. This may be one reason why the pregnancy rates in the 7–9 cell group, 10–13 cell group, and > 13 cell group were significantly higher than those in the < 7 cell group. Similarly, in previous studies, CPRs and LBRs increased significantly as the day 3 embryo cell number increased after blastocyst transfer in FET cycles [8]. Additionally, Qiu et al. [9] revealed that higher day 3 cell number (> 9 cells) in vitrified-warmed day 5 single blastocyst transfer (SBT) cycles were associated with higher LBR. However, in the subgroup analysis of low-quality blastocysts, there were no significant differences in pregnancy outcomes between groups. This may be due to the lower pregnancy outcomes of low-quality blastocysts, which weakened the influence of day 3 cell number on pregnancy outcomes. Furthermore, since our center did not transfer blastocysts with grade C ICM, the low-quality blastocysts in this study did not include those with grade C ICM. This may have some impact on the results to a certain extent.
Normally, embryos cultured in vitro develop into blastocysts on day 5 after fertilization, but some slower-developing embryos may develop into blastocysts on day 6 or later. In this study, embryos with more cells on day 3 had a higher proportion of developing into blastocysts on day 5, compared to embryos with fewer cells on day 3. Some studies have indicated that both high-quality and low-quality blastocysts on day 5 could lead to higher CPRs and LBRs than those on day 6 [21, 22]. This may also be one of the reasons why the < 7-cell group showed a significantly lower CPR (46.36%) and LBR (33.64%) compared 7–9 cell, 10–13-cell and > 13-cell group (CPR: 61.78%, 62.41%, 66.67%; LBR: 48.86%, 46.45%, 50.00%). However there are studies do not support this viewpoint. For instance, many studies [23, 24] found that when high-quality blastocysts were transferred, the clinical pregnancy outcomes of day 5 were similar to those of day 6.
The human sex ratio is important for maintaining gender balance. The secondary sex ratio (SSR), which is the number of male births compared to female births. In this study, the proportion of males in the > 13-cell group was significantly higher than in the < 7-cell group (P = 0.006). The reason may be that male embryos exhibit faster growth rates than female embryos [25]. Alfarawati et al. found that among the slowest growing embryos, 60% were female [26]. One possible explanation for the delayed development of female embryos is that they have a higher requirement for glucose than male embryos during the pre-implantation stage [27–29]. However, no significant differences were observed between groups in subsequent subgroup analyses, which may be related to the limited sample size. Further observation is warranted. However, in the day 6 and low-quality subgroups, the > 13-cell group did not result in any births. The main reason is that embryos with > 13-cell develop faster and are more likely to form blastocysts on day 5. At the same time, embryos that develop more rapidly are more likely to develop into high-quality blastocysts. Therefore, most embryos with > 13-cell formed blastocysts on day 5 and high-quality blastocysts. In this study, 98.81% of embryos with > 13-cell formed blastocysts on day 5, and 97.62% formed high-quality blastocysts. As a result, the day 6 group and the low-quality subgroup had too few patients, and no babies were born. In summary, in clinical practice, blastocysts with ≥ 7 cells on day 3 can be preferentially selected for transplantation.
The strengths of the present study lay in the detailed division of the day 3 cell number, providing valuable insights into the neonatal outcomes after single blastocyst transfer at different cell numbers on day 3. However, the study had certain limitations. First, this was a retrospective cohort study. The distribution of cycle numbers across groups was uneven, which may introduce some bias. Second, when there were a large number of day 3 fragments, their size was similar to that of blastomeres. They were likely to be misidentified as blastomeres, leading to an incorrect classification as faster-developing embryos. Although most of these embryos could be excluded using blastocyst culture technology, some of them had the potential to form blastocysts. Therefore, the ability to distinguish between large fragments and cells needs to be further enhanced in the future. Third, this was a single-centre study. In vitro fertilization centers around the world use different culture media and environments, which may lead to inconsistent conclusions. Fourth, since our center did not use grade C ICM for transfer, the low-quality blastocysts in this study did not include those with grade C ICM. This may also lead to some degree of bias in the results. Consequently, the findings of this study require validation through future prospective, multicenter clinical research.
Conclusion
In FET cycles, < 7-cell on day 3 was related to decreased CPR and LBR after blastocyst transfer, whereas a number > 9 was comparable to that of 7–9 cells, especially when the maternal age was < 35 years or when high-quality blastocysts were transferred. Therefore, the day 3 embryo cell number may serve as an important criterion for selecting higher quality blastocysts in FET cycles. Blastocysts with ≥ 7 cells on day 3 can be preferentially selected for transplantation especially in < 35 years or when high-quality blastocysts are transferred. The findings of this study require validation through future prospective, multicenter clinical research.
Supplementary Information
Supplementary Material 1: Supplementary Figure 1. Distribution of day 3 cell number and pregnancy outcomes by cell number after blastocyst transfer. Supplementary Figure 2. Proportion of male infants by day 3 cell number after blastocyst transfer. Supplementary Table 1. Clinical and neonatal outcomes in subgroup analyses by age, blastocyst grade and embryonic development days. Supplementary Table 2. Comparison of pregnancy outcomes after high-quality blastocyst transfer in patients < 35 years.
Acknowledgements
Not applicable.
Abbreviations
- FET
Frozen embryo transfer
- IVF
In vitro fertilization
- ICSI
Intracytoplasmic sperm injection
- R-ICSI
Rescue intracytoplasmic sperm injection
- aOR
Adjusted odds ratio
- CI
Confdence interval
- CPR
Clinical pregnancy rate
- LBR
Live birth rate
- Gn
Gonadotropin
- HCG
Human chorionic gonadotropin
- BMI
Body mass index
- ICM
Inner cell mass
- TE
Trophectoderm
Author contributions
Fangfang Dai and Shusong Wang contributed to the conception and design of the study. Linlin Tao and Bo zheng contributed to data interpretation and drafted the manuscript. Guozhen Li, and Zhiwei Yang and Yasong Geng contributed to data analysis. Haoyang Dai contributed to data acquisition. The authors read and approved the final manuscript.
Funding
Not applicable.
Data availability
Data is provided within the manuscript or supplementary information files.
Declarations
Ethics approval and consent to participate
This study followed the Declaration of Helsinki and was performed in accordance with the relevant local guidelines and regulations. The study was approved by the research ethics board at Xingtai Meihe Reproductive and Genetic Hospital (approval number: 2023-15). The requirement for informed consent was waived owing to the retrospective nature of the study, and data from all patients were used anonymously. The research was conducted ethically in accordance with the World Medical Association Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
+Lin-Lin Tao and Bo Zheng are joint first authors.
References
- 1.Glujovsky D, Farquhar C. Cleavage-stage or blastocyst transfer: what are the benefits and harms. Fertil Steril. 2016;106(2):244–50. [DOI] [PubMed] [Google Scholar]
- 2.Electronic PCotASfRM, address: ASRM@asrm.org, Practice Committee of the Society for Assisted Reproductive Technology. Guidance on the limits to the number of embryos to transfer: a committee opinion. Fertil Steril. 2017;107(4):901–3. 10.1016/j.fertnstert.2017.02.107. [DOI] [PubMed] [Google Scholar]
- 3.Abeyta M, Behr B. Morphological assessment of embryo viability. Semin Reprod Med. 2014;32(2):114–26. 10.1055/s-0033-1363553. [DOI] [PubMed] [Google Scholar]
- 4.Gardner DK, Schoolcraft WB. Culture and transfer of human blastocysts. Curr Opin Obstet Gynecol. 1999;11(3):307–11. 10.1097/00001703-199906000-00013. [DOI] [PubMed] [Google Scholar]
- 5.Alpha Scientists in Reproductive Medicine and ESHRE Special Interest Group of E. The Istanbul consensus workshop on embryo assessment: proceedings of an expert meeting. Hum Reprod. 2011;26(6):1270–83. 10.1093/humrep/der037. [DOI] [PubMed] [Google Scholar]
- 6.Gallego RD, Remohí J, Meseguer M. Time-lapse imaging: the state of the art†. Biol Reprod. 2019;101(6):1146–54. 10.1093/biolre/ioz035. [DOI] [PubMed] [Google Scholar]
- 7.Wu J, Zhang J, Kuang Y, Chen Q, Wang Y. The effect of day 3 cell number on pregnancy outcomes in vitrified-thawed single blastocyst transfer cycles. Hum Reprod. 2020;35(11):2478–87. 10.1093/humrep/deaa209. [DOI] [PubMed] [Google Scholar]
- 8.Wang J, Diao Z, Fang J, et al. The influence of day 3 embryo cell number on the clinical pregnancy and live birth rates of day 5 single blastocyst transfer from frozen embryo transfer cycles. BMC Pregnanc Childbirth. 2022;22(1):980. 10.1186/s12884-022-05337-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qiu P, Ye R, Li P, Huang H, Ding L. Effect of day 3 cell number on the live birth rate of vitrified-warmed day 5 single blastocyst transfer in young women. BMC Pregnancy Childbirth. 2024;24(1):289. 10.1186/s12884-024-06468-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luna M, Copperman AB, Duke M, Ezcurra D, Sandler B, Barritt J. Human blastocyst morphological quality is significantly improved in embryos classified as fast on day 3 (>or=10 cells), bringing into question current embryological dogma. Fertil Steril. 2008;89(2):358–63. 10.1016/j.fertnstert.2007.03.030. [DOI] [PubMed] [Google Scholar]
- 11.MacKenna A, Crosby J, ZegersHochschild F. Embryo early cleavage, number of blastomeres and morphology at day three as factors to predict blastocyst development. J Bras Reprod Assist. 2013;17(03):158–61. 10.5935/1518-0557.20130053. [Google Scholar]
- 12.Pons MC, Carrasco B, Parriego M, et al. Deconstructing the myth of poor prognosis for fast-cleaving embryos on day 3. Is it time to change the consensus. J Assist Reprod Genet. 2019;36(11):2299–305. 10.1007/s10815-019-01574-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kroener LL, Ambartsumyan G, Pisarska MD, Briton-Jones C, Surrey M, Hill D. Increased blastomere number in cleavage-stage embryos is associated with higher aneuploidy. Fertil Steril. 2015;103(3):694–8. 10.1016/j.fertnstert.2014.12.090. [DOI] [PubMed] [Google Scholar]
- 14.Tao LL, Zheng B, Li GZ, Geng YS, Guo YY, Dai HY, et al. Effect of day 3 embryo cell number on the pregnancy and neonatal outcomes of day 4 single embryo transfer from fresh cycles. BMC Pregnancy Childbirth. 2024;24(1):75. 10.1186/s12884-024-06976-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tang L, Sun C, He Y, Hou H, Shang Y, Li L, et al. Effect of blastomere cell number on ART outcome of fresh single day 3 embryo transfer. BMC Pregnancy Childbirth. 2024;24(1):629. 10.1186/s12884-024-06825-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kong X, Yang S, Gong F, et al. The relationship between cell number, division behavior and developmental potential of cleavage stage human embryos: a time-lapse study. PLoS ONE. 2016;11(4):e0153697. 10.1371/journal.pone.0153697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mazzilli R, Cimadomo D, Vaiarelli A, et al. Effect of the male factor on the clinical outcome of intracytoplasmic sperm injection combined with preimplantation aneuploidy testing: observational longitudinal cohort study of 1,219 consecutive cycles. Fertil Steril. 2017;108(6):961-72.e3. 10.1016/j.fertnstert.2017.08.033. [DOI] [PubMed] [Google Scholar]
- 18.Ubaldi FM, Cimadomo D, Capalbo A, et al. Preimplantation genetic diagnosis for aneuploidy testing in women older than 44 years: a multicenter experience. Fertil Steril. 2017;107(5):1173–80. 10.1016/j.fertnstert.2017.03.007. [DOI] [PubMed] [Google Scholar]
- 19.Pellestor F, Andréo B, Arnal F, Humeau C, Demaille J. Maternal aging and chromosomal abnormalities: new data drawn from in vitro unfertilized human oocytes. Hum Genet. 2003;112(2):195–203. 10.1007/s00439-002-0852-x. [DOI] [PubMed] [Google Scholar]
- 20.Wang C, Shu J, Lin R, et al. Choosing the optimal blastocyst by morphology score versus developmental rate in frozen-thawed embryo transfer cycles. Hum Fertil (Camb). 2020. 10.1080/14647273.2020.1778199. [DOI] [PubMed] [Google Scholar]
- 21.Ferreux L, Bourdon M, Sallem A, et al. Live birth rate following frozen-thawed blastocyst transfer is higher with blastocysts expanded on day 5 than on day 6. Hum Reprod. 2018;33(3):390–8. 10.1093/humrep/dey004. [DOI] [PubMed] [Google Scholar]
- 22.Sciorio R, Thong KJ, Pickering SJ. Increased pregnancy outcome after day 5 versus day 6 transfers of human vitrified-warmed blastocysts. Zygote. 2019;27(5):279–84. 10.1017/S0967199419000273. [DOI] [PubMed] [Google Scholar]
- 23.Yang H, Yang Q, Dai S, et al. Comparison of differences in development potentials between frozen-thawed D5 and D6 blastocysts and their relationship with pregnancy outcomes. J Assist Reprod Genet. 2016;33(7):865–72. 10.1007/s10815-016-0712-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu TF, Chen MJ, Lee MS, et al. Comparison of clinical outcome between day 5 and day 6 single blastocyst transfers in cycles undergoing preimplantation genetic testing for aneuploidy. Taiwan J Obstet Gynecol. 2023;62(3):429–33. 10.1016/j.tjog.2023.03.005. [DOI] [PubMed] [Google Scholar]
- 25.Narvaez JL, Chang J, Boulet SL, Davies MJ, Kissin DM. Trends and correlates of the sex distribution among U.S. assisted reproductive technology births. Fertil Steril. 2019;112(2):305–14. 10.1016/j.fertnstert.2019.03.034. [DOI] [PubMed] [Google Scholar]
- 26.Alfarawati S, Fragouli E, Colls P, Stevens J, Gutierrez-Mateo C, Schoolcraft WB, et al. The relationship between blastocyst morphology, chromosomal abnormality, and embryo gender. Fertil Steril. 2011;95(2):520–4. 10.1016/j.fertnstert.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 27.Garcia-Herreros M, Aparicio IM, Rath D, Fair T, Lonergan P. Differential glycolytic and glycogenogenic transduction pathways in male and female bovine embryos produced in vitro. Reprod Fertil Dev. 2012;24(2):344–52. 10.1071/RD11080. [DOI] [PubMed] [Google Scholar]
- 28.Nasiri N, Karimian L, Hassani F, Gourabi H, Alipour H, Zolfaghari Z, et al. Total antioxidant capacity; a potential biomarker for non-invasive sex prediction in culture medium of preimplantation human embryos. Cell J (Yakhteh). 2019;21(3):253–8. 10.22074/cellj.2019.6115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gardner DK, Wale PL, Collins R, Lane M. Glucose consumption of single postcompaction human embryos is predictive of embryo sex and live birth outcome. Hum Reprod. 2011;26(8):1981–6. 10.1093/humrep/der143. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material 1: Supplementary Figure 1. Distribution of day 3 cell number and pregnancy outcomes by cell number after blastocyst transfer. Supplementary Figure 2. Proportion of male infants by day 3 cell number after blastocyst transfer. Supplementary Table 1. Clinical and neonatal outcomes in subgroup analyses by age, blastocyst grade and embryonic development days. Supplementary Table 2. Comparison of pregnancy outcomes after high-quality blastocyst transfer in patients < 35 years.
Data Availability Statement
Data is provided within the manuscript or supplementary information files.