Abstract
The signaling mechanisms that specify, guide and coordinate cell behavior during embryonic morphogenesis are poorly understood. We report that a Xenopus homolog of the Drosophila planar cell polarity gene strabismus (stbm) participates in the regulation of convergent extension, a critical morphogenetic process required for the elongation of dorsal structures in vertebrate embryos. Overexpression of Xstbm, which is expressed broadly in early development and subsequently in the nervous system, causes severely shortened trunk structures; a similar phenotype results from inhibiting Xstbm translation using a morpholino antisense oligo. Experiments with Keller explants further demonstrate that Xstbm can regulate convergent extension in both dorsal mesoderm and neural tissue. The specification of dorsal tissues is not affected. The Xstbm phenotype resembles those obtained with several other molecules with roles in planar polarity signaling, including Dishevelled and Frizzled-7 and -8. Unlike these proteins, however, Stbm has little effect on conventional Wnt/β-catenin signaling in either frog or fly assays. Thus our results strongly support the emerging hypothesis that a vertebrate analog of the planar polarity pathway governs convergent extension movements.
Keywords: convergent extension/morphogenesis/planar cell polarity/strabismus/Xenopus
Introduction
Embryonic development involves cell determination and differentiation, but also morphogenesis; the construction of the embryo by the coordinated mechanical activities of cell populations. While the mechanisms of cell type specification are now quite well understood in many cases, relatively little is known about the control of cell behavior in development. The movements of gastrulation and neurulation, which establish the vertebrate body plan, constitute particularly dramatic episodes of morphogenesis, involving cell migration, intercalation and shape change. Although these tissue movements have been described in some detail, particularly in Xenopus, many questions remain. Specifically, the signaling events that regulate morphogenesis remain largely mysterious. How are regional patterns of cell behavior established? What are the cues that coordinate the timing and orientation of cell motility? How do extracellular signals provoke changes in cytoskeleton, adhesion and protrusive activity?
An important insight into the regulation of morphogenesis in the vertebrate embryo came with the discovery that an interfering version of the signal transduction molecule Dishevelled (Dsh) can profoundly disrupt dorsal tissue movements in Xenopus, apparently without affecting cell-type specification or differentiation (Sokol, 1996). In particular, blocking Dsh function impedes convergent extension, the many-fold narrowing and elongation of the dorsal mesoderm and posterior neural plate, resulting in a drastically shortened trunk. Convergent extension, driven by active cell intercalation, not only shapes the embryo by elongating the dorsal axial structures, but also plays important roles in gastrulation, at least in amphibians, contributing to involution of the mesoderm and closure of the blastopore (Keller et al., 2000). Although Dsh has a central role in the conventional Wnt/β-catenin signaling cascade, in Drosophila it also participates in a second, largely separate genetic network known as the planar cell polarity pathway (see below; Boutros and Mlodzik, 1999). Subsequent work in Xenopus and in the zebrafish, making use of mutants specific to one pathway or the other strongly suggests that Dsh regulates convergent extension as part of a vertebrate planar polarity pathway (Heisenberg et al., 2000; Tada and Smith, 2000; Wallingford et al., 2000).
Planar cell polarity (PCP) in Drosophila, also known as tissue polarity, describes the coordinated orientation of cells or structures within the plane of an epithelium (this is distinct from the better understood apical–basal polarity; Adler, 1992; Shulman et al., 1998; Mlodzik, 1999). The two most studied examples are the wing, in which the hairs produced by individual epithelial cells point in unison toward the distal tip, and the compound eye, whose component ommatidia acquire a precisely coordinated orientation. Genetic analyses have identified a number of genes that are required for these orderly patterns of polarization. While mutations in some planar polarity genes affect specifically wing or eye, a subset are required in both tissues as well as in other polarized developmental fields, implying that a common system for coordinating tissue polarity operates in very different contexts. In addition to Dsh, this core group of genes includes the transmembrane receptor Frizzled (Vinson and Adler, 1987), the atypical cadherin Flamingo (Usui et al., 1999), also known as Starry night (Chae et al., 1999), the small GTPase RhoA (Strutt et al., 1997), Prickle/Spiny legs (Gubb et al., 1999), the ankyrin repeat protein Diego (Feiguin et al., 2001) and the putative integral membrane protein Strabismus/Van Gogh (Taylor et al., 1998; Wolff and Rubin, 1998). Although epistasis experiments have established that Frizzled functions upstream of Dsh and RhoA (Krasnow et al., 1995; Strutt et al., 1997), it has been difficult to further define a pathway.
In Xenopus, inhibition or misexpression of either Frizzled-8 or Frizzled-7 gives a phenotype very similar to that of dominant-negative Dsh (Deardorff et al., 1998; Djiane et al., 2000; Medina et al., 2000; Sumanas et al., 2000). (That negative or positive manipulation of gene function often have indistinguishable effects seems to be a general feature of this pathway; see Discussion.) A Wnt ligand is apparently involved as well: the Xenopus Dsh phenotype is mimicked by injection of dominant-negative Wnt-11 and closely resembles the zebrafish Wnt-11 mutant silberblick (Heisenberg et al., 2000; Tada and Smith, 2000). Moreover, Wnt-5A can also disrupt dorsal morphogenetic movements (Moon et al., 1993). Thus, the signaling system suggested by these observations is often considered an alternative or ‘non-canonical’ Wnt pathway, although its relationship to the Wnt/β-catenin pathway and to a recently proposed Wnt signaling mechanism involving calcium (Kuhl et al., 2000) remains obscure. Curiously, no strong evidence yet implicates a Wnt ligand in Drosophila planar polarity.
Here we report that a Xenopus homolog of the Drosophila planar polarity gene strabismus/Van Gogh (stbm; Taylor et al., 1998; Wolff and Rubin, 1998) is involved in the regulation of convergent extension. Xstbm is expressed in the neural plate and dorsal mesoderm where convergent extension occurs, and both dorsal overexpression or reduction of expression with a morpholino antisense oligo cause trunk defects similar to those produced by Dsh and Frizzled. The effect is specific to morphogenesis: the specification and differentiation of trunk tissues is essentially normal.
Results
The effects of pathway-specific mutant forms of Dsh in Xenopus and zebrafish suggest that a vertebrate analog of the Drosophila PCP pathway participates in the regulation of convergent extension. To explore this hypothesis and to identify other molecules with roles in the same process, we tested several Drosophila planar polarity genes in the same assay: dorsal equatorial injection. We found that injection of RNA encoding Drosophila Stbm, like Dsh and Frizzled a member of the core group of genes required for proper polarity in both the Drosophila wing and eye, causes a dorsally shortened phenotype very like that seen with dominant-negative Dsh, Frizzled-7 and Frizzled-8 (see below). This observation led us to ask whether a Xenopus homolog of Stbm might have a role in dorsal morphogenesis.
Cloning of a Xenopus Stbm homolog
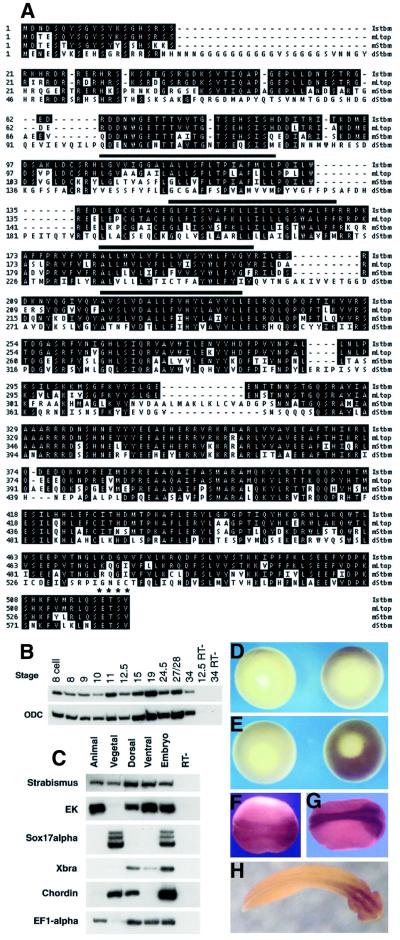
A search of public databases with the Drosophila stbm sequence turned up two incomplete, nearly identical Xenopus sequences with strong homology to the Drosophila gene. A fragment of one of the Xenopus expressed sequence tags (IMAGE clone #3301175) was used to probe a stage 11 Xenopus cDNA library, and several clones corresponding to the same gene, which we will call Xstrabismus or Xstbm, were isolated. The longest of these clones appears to contain the full open reading frame. The predicted Xstbm protein is highly homologous throughout to the product of the recently cloned mouse Ltap gene (Kibar et al., 2001) and to the human protein KAII1215 (Nagase et al., 1999), and shows extensive homology to Drosophila Stbm, especially in the C-terminal half (Figure 1A). The Xenopus cDNA reported here more closely resembles Ltap than a second, closely related mouse gene, mStbm, identified by Wolff and Rubin (1998). All five proteins contain four potential transmembrane domains and end with the putative PDZ domain binding site ETSV.
Fig. 1. Xstrabismus protein sequence and expression analysis. (A) The predicted Xstbm protein sequence is compared with those of Drosophila Stbm (dStbm; Wolff and Rubin, 1998) and the two reported mouse homologs, Ltap (Kibar et al., 2001) and mStbm (Wolff and Rubin, 1998). The Xenopus gene reported here appears to be the homolog of Ltap (90% similarity) rather than mStbm (69%). Possible transmembrane domains are indicated by black bars and the C-terminal putative PDZ domain binding site by asterisks. The Xstbm sequence has been submitted to DDBJ/EMBL/GenBank (accession number AY069979). (B and C) RT–PCR on staged whole embryos and dissected fragments of early gastrulae. Epidermal keratin (EK), Sox17α, Xbra and chordin are ectodermal, endodermal, marginal zone and dorsal markers, respectively, used to confirm dissection. Ornithine decarboxylase B (ODC) and EF1-α are ubiquitously expressed messages used as loading controls. RT–: mock reverse-transcribed sample. (D–H) In situ hybridization with Xstbm probe on early gastrula (D), midgastrula (E), midneurula (F), post-neurula (G) and tailbud stage (H) embryos. Control hybridizations with sense probes are on the left in (D) and (E). (D) and (E) are vegetal views; (F)–(H) are dorsal views, with anterior to the right.
Xstbm is expressed in the mesoderm during gastrulation and in the nervous system later
Xstbm is expressed maternally and throughout early development (Figure 1B). Analysis by dissection shows that at the start of gastrulation Xstbm RNA is present in the vegetal region, in both dorsal and ventral sectors of the marginal zone, and in the animal cap (Figure 1C). In situ hybridization confirms that during gastrula stages Xstbm is broadly expressed throughout the marginal zone and animal cap region (Figure 1D and E; data not shown). At neurula stages and afterwards, expression becomes concentrated in the neural plate and neural tube, at first along the entire anterior–posterior axis and then primarily in the anterior nervous system (Figure 1F–H). Thus Xstbm is expressed in both the mesoderm and ectoderm during gastrulation, and strongly in the neural plate at neurula stages, consistent with a role in convergent extension.
Overexpression of Xstbm blocks dorsal elongation and delays both blastopore and neural tube closure
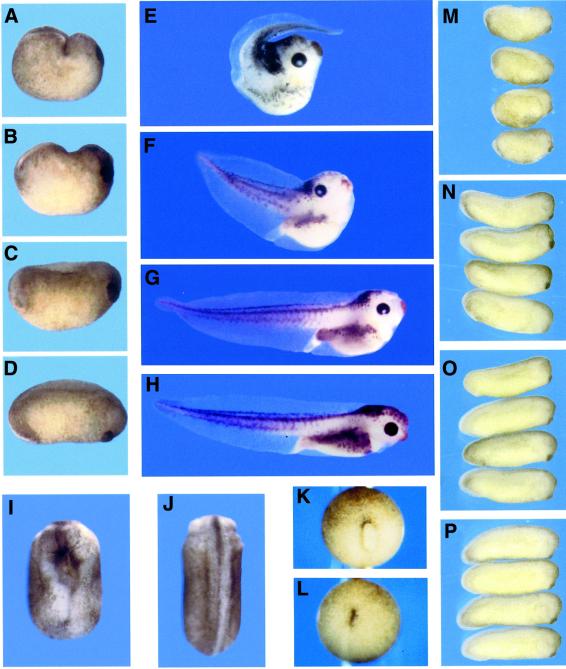
The open reading frame of Xstbm was subcloned into the expression vector pCS2 and RNA was synthesized in vitro. Injection of this message (0.5–1.0 ng) into two dorsal blastomeres at the four to eight cell stage caused severe dorsal flexure and trunk and tail truncation (Figure 2). These effects were indistinguishable from those of Drosophila Stbm. (The characterization that follows will draw on results obtained with both genes, which will be referred to collectively as Stbm.) The Stbm phenotype varies considerably, and injected embryos can be loosely classified at tadpole stages according to the severity of dorsal flexure. Some typical individuals are shown in Figure 2, while Table I records the range of results from several experiments. In the mildest form, the trunk is shortened and the head is deflected dorsally, but the tail is straight and of normal length (Figure 2G). In embryos we classify as moderately affected, the angle of the tail relative to the head is increased to as much as 90° (Figure 2F). In the severe form of the phenotype, the tail is reduced and points forward over the head (Figure 2E). In some cases the tail is split and forms poorly, probably as a consequence of incomplete closure of the blastopore (see below). We note that the degree of dorsal flexure may depend on whether the neural plate or dorsal mesoderm is most affected, as well as on the severity of the effect. It has been reported that in the case of dominant-negative Dsh, dorsal-animal injection results in greater dorsal flexure than dorsal-vegetal injection, which primarily causes shortening of the axis (Wallingford and Harland, 2001). In our hands, the principal effect of more vegetal injection of Xstbm is greater impairment of blastopore closure, although we also observe somewhat less dorsal bending (data not shown).
Fig. 2. Phenotypes of Stbm- and anti-Xstbm morpholino-injected embryos. Injection of 0.5 ng of Xstbm RNA into the dorsal marginal zone at the four to eight cell stage impairs axial elongation and causes dorsal flexure. (A–C) Lateral views of post-neurula Xstbm-injected embryos show that the dorsal side is shortened (C) relative to uninjected controls (D), or buckled into a characteristic transverse fold (A and B). At tadpole stages (E–H), mild (G), moderate (F), and severe (E) phenotypes can be loosely distinguished by the degree to which the posterior axis is deflected dorsally or forward. (H) Uninjected control. Xstbm overexpression also causes neural tube closure defects [(I); compare with sibling control in (J)]. (A–H) Lateral views with dorsal up and anterior to the right; (I and J) dorsal views with anterior up. Blastopore closure is often delayed in Stbm-injected animals: an example is shown in vegetal view at stage 13 (K), when this process is complete in uninjected sibling embryos (L). (M–P) Injection of 20 ng of anti-Xstbm morpholino oligo results in embryos that are on average 30% shorter at tail-bud stages. (M) Xstbm oligo; (N) mismatch control oligo; (O) unrelated oligo; (P) uninjected.
Table I. Distribution of Stbm phenotypes.
| RNA injected | Normal | Mild | Moderate | Severe | n |
|---|---|---|---|---|---|
| dStbm, 1 ng | 5 | 24 | 24 | 66 | 119 |
| ΔPBS-Stbm, 0.5 ng | 13 | 26 | 21 | 51 | 111 |
| Xstbm, 0.5 ng | 20 | 32 | 31 | 29 | 112 |
| Xstbm, 1 ng | 7 | 6 | 22 | 66 | 101 |
| Uninjected | 239 | 6 | 0 | 2 | 247 |
Numbers (n) for each treatment shown are totals from three to six experiments. Classification of embryos is as described in the text and illustrated in Figure 2.
Stbm-injected embryos are morphologically indistinguishable from uninjected siblings through blastula and early gastrula stages: the dorsal blastopore lip appears and spreads laterally on schedule, and the cleft of Brachet forms internally between the leading edge of the migrating mesoderm and the blastocoel roof (data not shown). However, blastopore closure is often delayed in Stbm-injected embryos: by stage 13, when the blastopore has closed in controls, many Stbm-injected animals still have a small, dorso-ventrally elongated yolk plug (Figure 2K and L). By the end of neurulation the failure of dorsal elongation is evident. The dorsal side is shortened and flat or, in more severe cases, buckled into a transverse fold which brings the posterior end of the axis close to the back of the head (Figure 2A–C, compare with D). This dorsal flexure leads eventually to the misorientation of tail outgrowth and to the late phenotypes described above. Trunk and tail truncation and bending are accompanied by defects or delays in neural tube closure (Figure 2I), a central feature of the mouse Loop-tail (Lp) mutant, now identified as a stbm homolog (Kibar et al., 2001). All these features of the Stbm phenotype are also seen with Dsh (Sokol, 1996), Frizzled-7 (Djiane et al., 2000; Medina et al., 2000; Sumanas et al., 2000), and Frizzled-8 (Deardorff et al., 1998).
Reduction of Xstbm function using a morpholino antisense oligo also causes trunk shortening
Antisense ‘morpholino’ oligos have in some cases proven an effective means of specifically reducing or eliminating gene function (Heasman et al., 2000). These highly stable modified oligos act by blocking translation of targeted mRNAs. To gain further insight into Xstbm function in early development, we injected cleavage-stage embryos with a morpholino complementary to 25 bases of Xstbm, just upstream of the putative initiating ATG. Animal hemisphere injection of 20 ng of this oligo (synthesized by Gene Tools) at the two cell stage resulted in tadpoles that were 20–40% shorter at tailbud stages than embryos injected with either a mismatch oligo containing four base changes or an unrelated oligo (Figure 2M–P); this difference was significant at the P <0.001 level. Dorsal injection at the four cell stage had a similar but somewhat milder effect (data not shown). Some morpholino-injected embryos are bent dorsally, but this flexure is rarely severe and the predominant phenotype is shortening of the trunk. Thus reduction of Xstbm function, like overexpression, impairs axial elongation, supporting a role for this gene in convergent extension movements.
Stbm does not affect dorsal tissue specification
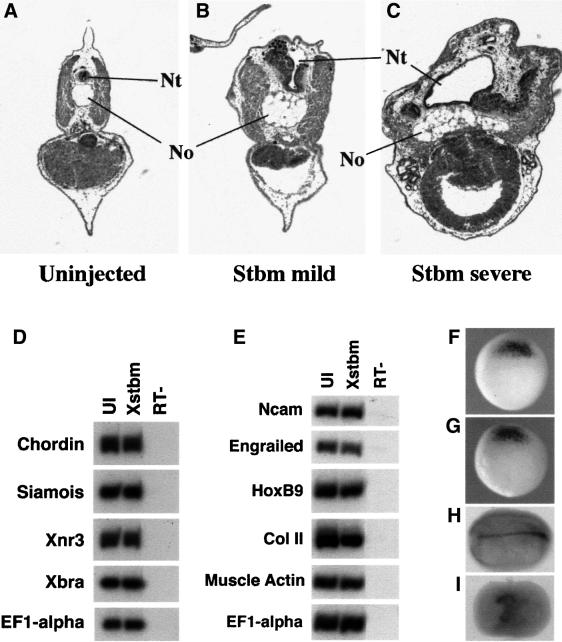
Although Stbm overexpression disturbs morphogenesis and subsequent morphology, it leaves dorsal cell type specification and differentiation relatively unperturbed. Histological analysis of Stbm-injected embryos reveals that the characteristic structures of the trunk are present, including notochord, neural tube, somites, pronephros and gut tube (Figure 3A–C). In transverse sections, however, these structures, particularly the notochord and neural tube, are wider than normal, as might be expected from the failure of convergent extension during gastrulation and neurulation. To analyze further the effect of Stbm injection on dorsal patterning, we examined the expression of dorsal markers by RT–PCR. Figure 3D shows that levels of gastrula-stage dorsal marginal zone markers, as well as the general mesodermal marker Xbra, are not altered in Stbm-injected embryos. Similarly, at later stages markers of dorsal mesoderm and neural tissue are expressed at wild-type levels (Figure 3E). Finally, in situ hybridization for chordin, a gene expressed in the gastrula organizer and in the notochord at later stages (Sasai et al., 1994), shows that while this gene is strongly expressed in Stbm-injected embryos, the expressing region fails to narrow and elongate properly during gastrulation and neurulation (Figure 3F–I).
Fig. 3. Histological and molecular analysis of Stbm-injected embryos. Histological sections reveal that Stbm-injected tadpoles (B and C) contain dorsal axial tissues, including notochord (No), neural tube (Nt) and somitic muscle; however, these structures are wider than in control embryos (A). Sections are transverse at the level of the trunk, with dorsal up. RT–PCR analyses at early gastrula (D) and late neurula (E) stages show that the expression of dorsal markers is not affected by injection of Xstbm RNA (0.5 ng) into the dorsal marginal zone. Xnr3, siamois and chordin are organizer markers; Xbra is a general mesodermal marker; Ncam, engrailed-2 and Hox B9 are general neural, midbrain–hindbrain boundary and spinal cord markers, respectively. Collagen type II (Col II) is expressed in the notochord and muscle actin is a marker of somitic muscle at this stage. UI: uninjected embryo. Chordin in situ hybridization shows that expression of this dorsal marker at the start of gastrulation is unaffected by Xstbm injection [(F): uninjected; (G): Xstbm]. By the end of neurulation, however, the chordin expression domain, corresponding to the notochord, is highly elongated in control embryos (H) but short and broad in injected embryos (I).
Stbm overexpression prevents elongation of activin-induced animal caps
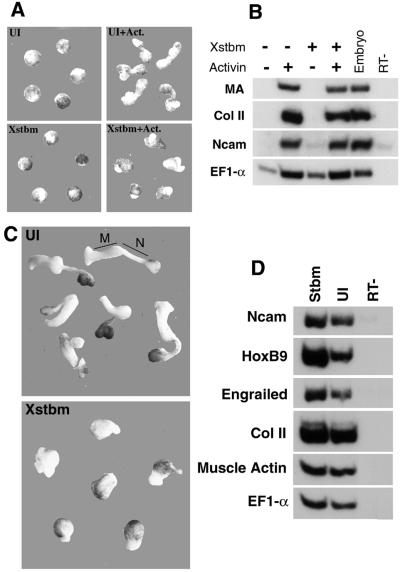
Convergent extension of the dorsal axial tissues can be studied in a simpler assay in which these movements can be separated from the complex mechanical context of the gastrulating embryo. When blastula animal cap explants are exposed to activin protein, dorsal mesoderm is induced and the explants elongate dramatically. The close correlation of cap elongation with the induction of notochord and muscle suggests that it results from convergent extension movements comparable to those performed by these cell populations in vivo (Smith and Howard, 1992). As in the intact embryo, Stbm overexpression blocks animal cap elongation in response to activin without preventing mesoderm induction: markers of notochord, somitic muscle and neural plate are well expressed in Stbm-injected, activin-induced caps (Figure 4A and B). Thus the failure of morphogenesis, both in caps and in whole embryos, is not merely a consequence of altered specification of the cell populations normally involved in this process.
Fig. 4. Stbm blocks elongation of activin-induced animal caps and Keller sandwich explants. (A) Animal cap explants cultured in activin-containing medium extend during gastrula and neurula stages; this elongation is blocked by animal hemisphere injection of 0.5 ng of Xstbm. Explants are shown at stage 18. (B) RT–PCR from the same experiment demonstrates that markers of the cell populations that normally participate in convergent extension—notochord (collagen II), somitic mesoderm (muscle actin, MA) and neuroectoderm (Ncam)—are expressed at wild-type levels in Xstbm-injected caps. UI: uninjected caps. (C) Keller sandwiches were made from embryos injected with Xstbm RNA (0.5 ng) and from control embryos at early gastrula stages (see Materials and methods). After culture until sibling embryos reach the end of neurulation, control explants are highly elongated, and mesodermal (M) and neural (N) domains can be distinguished by pigmentation and morphology. Explants from Xstbm-injected embryos extend poorly and do not become clearly divided into mesodermal and neural domains. (D) Both mesodermal and neural markers are well expressed in Stbm-injected explants. Engrailed and HoxB9 are anterior and posterior neural markers, respectively.
Both neural and mesodermal convergent extension are blocked by Stbm
During gastrulation and neurulation, both the dorsal mesoderm (prospective notochord and somites) and the prospective posterior neuroectoderm converge and extend. In normal development, the extending neural plate and the underlying dorsal mesoderm are closely apposed and, after involution has stopped, mechanically linked; thus failure of these movements in either tissue could block their realization in the other. Convergent extension in the two tissues can be analyzed separately using Keller sandwiches, which consist of explants of the early gastrula dorsal marginal zone joined together in pairs, inner face to inner face (Keller and Danilchik, 1988). In this format involution does not occur, dorsal mesoderm and prospective neural plate remain attached only at their edges, and the extension of each region can be followed independently. Explants made from control embryos converge and extend vigorously over gastrula and neurula stages, resulting in a characteristic doubly elongated shape (Figure 4C, top). Histological and molecular analysis has confirmed that one long, tapered domain consists of neural tissue, while the other contains notochord and paraxial mesoderm, as expected from the fate map (Keller and Danilchik, 1988; Doniach, 1992). In contrast, in explants made from embryos injected with stbm RNA, neither mesodermal nor ectodermal domains extend (Figure 4C, bottom). Markers of both dorsal mesoderm and neural tissue are well expressed in Stbm-injected explants, including HoxB9, a marker of spinal cord, the most vigorously extending region of the neural plate (Figure 4D). Thus, as was recently shown for dominant-negative Dsh (Wallingford and Harland, 2001), Stbm can block convergent extension in both posterior neural tissue and dorsal axial mesoderm.
Stbm has little effect on conventional Wnt signaling
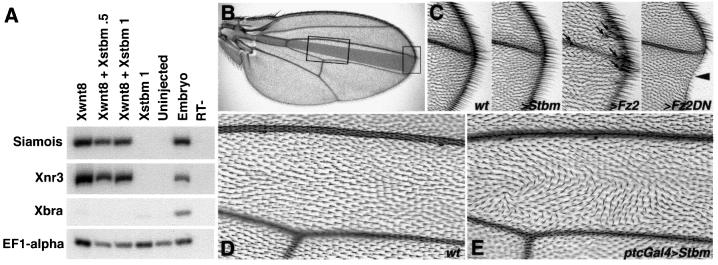
Although the stbm phenotype in Drosophila is consistent with a role in tissue polarity rather than in wingless/armadillo signaling (Taylor et al., 1998; Wolff and Rubin, 1998), the complex and poorly understood relationship between the two pathways and the possible interaction of Stbm with Dsh through the Dsh PDZ domain (see below) prompted us to examine the effect of Stbm overexpression on conventional Wnt signaling in both Xenopus and Drosophila assays. In early Xenopus development, the organizer genes siamois and Xnr3 are direct targets of the canonical Wnt pathway, acting through β-catenin and Lef/TCF transcription factors; both genes can be induced in animal cap explants by Xwnt8 and other Wnt family members (Smith et al., 1995; Brannon and Kimelman, 1996; Carnac et al., 1996; McKendry et al., 1997; Fan et al., 1998). Co-injection of stbm RNA does not prevent siamois and Xnr3 induction by Xwnt8, although in some experiments a mild reduction was observed (Figure 5A). Note also that Stbm does not activate these genes on its own and that their expression in the whole embryo is not affected by Stbm injection (Figure 3). Looking at the induction of ectopic dorsal axes by ventral injection of Xwnt8 gave similar results. In some experiments co-injection of Stbm led to a reduction in the number of second axes induced by Xwnt8; in others, no effect was seen (data not shown).
Fig. 5. Stbm overexpression has little effect on canonical Wnt/β-catenin signaling in Xenopus or Drosophila. (A) The dorsal genes siamois and Xnr3 are direct targets of Wnt/β-catenin pathway activation in vivo and can be induced in animal cap explants by ectopically expressed Wnts. In the experiment shown here, induction of these genes by Xwnt8 RNA (1 pg) is not significantly reduced by co-injection of Xstbm. Neither these markers nor the mesodermal marker Xbra are induced by Xstbm alone. Animal caps were harvested at stage 10.25 for RT–PCR. (B–E) The effects of Stbm overexpression in Drosophila wings. The ptcGal4, UAS-Stbm genotype is shown in (C) and (E); compare with wild-type (D) and left panel in (C). In the overview (B), the ptcGal4 expression domain is shaded in gray and areas corresponding to the enlarged views in (C)–(E) are boxed. Note that in wild type (D) all wing cell hairs point distally (left to right), whereas in ptcGal4, UAS-Stbm (E) the hairs form swirls and wavy patterns, reflecting defects in PCP. In contrast, no effects diagnostic of changes in canonical Wg-signaling are seen within the ptcGal4 expression domain. For comparison see two right-most panels in (C), showing typical gain (>Fz2; ectopic bristles indicated by arrows) and loss of function (>Fz2DN; notches in wing margin and loss of marginal bristles; arrowhead) wing margin phenotypes. Anterior is up and distal right.
To corroborate the finding that Stbm does not significantly affect canonical Wnt signaling, we analyzed the effect of Stbm overexpression in Drosophila development using the Gal4/UAS system (Brand and Perrimon, 1993). Wing patterning is the ideal context for this analysis as the phenotypes for canonical Wg/Wnt signaling as well as planar cell polarity are well described. In the wing, tissue close to the wing margin (the source of Wg in larval stages) is most sensitive to canonical Wg signaling levels: reduction leads to notches in the wing margin while over-activation results in ectopic marginal bristles (see Figure 5C). On the other hand, either reduction (loss of function) or overexpression of proteins involved in the generation of planar cell polarity causes characteristic defects in the orientation of wing cell hairs, exemplified by wavy and swirly patches. Whereas overexpression of Stbm in either the wing or the eye imaginal disc disrupted planar polarity, leading to misoriented wing hairs (Figure 5E) or ommatidia (data not shown), we never observed defects suggestive of either loss or gain of function of canonical Wg signaling (Figure 5C). In particular, although driving Stbm expression in a stripe of cells at the anterio-posterior compartment boundary (between wing veins 3 and 4 in the adult wing, shaded in grey in Figure 5B) with ptcGal4 led to clear planar polarity phenotypes (Figure 5E) in each of over 50 wings analyzed; in no case were appreciable effects seen at the wing margin (Figure 5C). Similar observations were made with dppGal4 (expressed in the same domain as ptcGal4) and with apGal4 (expressed on the dorsal thorax and wing surface), and no Wg-signaling effects were observed in the embryo when Stbm was overexpressed there (not shown). We thus conclude that overexpression of Stbm disrupts planar polarity, but has at best a weak effect on canonical Wnt signaling.
The PDZ-domain binding site of Stbm is not required for its activity in Xenopus embryos
It has been proposed that the Stbm C-terminal sequence ETSV constitutes a PDZ domain binding site (Wolff and Rubin, 1998). This is particularly intriguing since Dsh contains a PDZ domain. To explore the role of this highly conserved motif in Xenopus convergent extension, we constructed a mutant version of the Drosophila cDNA, ΔPBS-Stbm, which lacks these residues. Injection of RNA encoding this mutant produces a phenotype indistinguishable from that of the wild-type message (Figure 6). This result suggests that the activity of overexpressed Stbm in Xenopus embryos cannot be attributed simply to the C-terminal PDZ-binding domain, although it does not prove that this domain is not required (see Discussion).
Fig. 6. A C-terminal Stbm mutant retains the ability to block dorsal extension. Dorsal injection of 0.5 ng ΔPBS-Stbm RNA gives a range of dorsally shortened phenotypes indistinguishable from that seen with wild-type stbm RNA. More severely affected individuals are shown at top; a milder example is at bottom.
Discussion
The results presented here show that a Xenopus homolog of the Drosophila planar polarity gene strabismus/Van Gogh blocks convergent extension when overexpressed in the dorsal mesoderm and prospective neural tissue, resulting in severely shortened dorsal trunk structures. Injection of a Xstbm morpholino antisense oligo results in a similar but not identical impairment of axial elongation (see below). This dramatic effect on morphogenesis is achieved without disturbing tissue specification or differentiation: notochord, muscle and neural tissue form and the expression of dorsal markers is unchanged. Xstbm is broadly expressed during gastrula stages, becoming concentrated later in the neural plate, and thus is present at the appropriate time and place to regulate convergent extension. Experiments with animal cap explants and Keller sandwiches confirm the effect on convergent extension and support the independence of dorsal morphogenesis and cell type specification. In addition, the observation that Xstbm blocks extension in Keller sandwiches argues that this gene, like Dsh (Wallingford and Harland, 2001), can regulate morphogenesis in both dorsal mesoderm and prospective neural tissue, suggesting that a common molecular pathway governs cell behavior in these disparate cell types.
The resemblance between gain and loss of function Xstbm phenotypes is consistent with results obtained with other genes involved in convergent extension in Xenopus and with PCP components in Drosophila (see Introduction). A probable explanation for this unusual feature of planar polarity genes is that proper functioning of the pathway requires a gradient of activation (Adler et al., 1997), which could be destroyed by either under- or over-stimulation. The Xstbm morpholino phenotype differs from that of overexpression, however, in that it is less severe and involves less dorsal flexure. We believe that the morpholino effect probably represents an incomplete loss of function, perhaps because Xstbm, like many Xenopus genes, exists in two closely related forms, only one of which is effectively blocked by the morpholino. In fact, the two incomplete ESTs corresponding to Xstbm in the database are essentially identical at the amino acid level but differ at approximately one in every 10–15 nucleotides. The relative lack of dorsal bending could simply reflect reduced severity. Alternatively, it might suggest that the morpholino affects Xstbm function more strongly in the mesoderm than in the neural plate, perhaps because of expression differences between the two variant forms.
The planar polarity pathway and convergent extension
The phenotype resulting from dorsal injection of stbm RNA strongly resembles those produced by interfering or wild-type forms of Dsh (Sokol, 1996), Frizzled-7 (Djiane et al., 2000; Medina et al., 2000; Sumanas et al., 2000), Frizzled-8 (Deardorff et al., 1998), casein kinase I (McKay et al., 2001) and Wnt-11 (Tada and Smith, 2000), suggesting that these genes, and perhaps also small GTPases of the Rho subfamily (Djiane et al., 2000), may act in a common pathway. Experiments with more specific Dsh mutants in Xenopus and zebrafish have been used to argue that this ‘dominant-negative Dsh’ phenotype results from disruption of a vertebrate PCP pathway (Heisenberg et al., 2000; Tada and Smith, 2000; Wallingford et al., 2000). Our results with strabismus, another gene belonging to the PCP group in Drosophila, strongly support this idea. Unlike Dsh, Frizzled and casein kinase I, which also participate in Wnt/β-catenin signaling, Stbm has so far been implicated only in the establishment of planar polarity. In our hands, overexpression of Stbm has at most a weak effect on canonical Wnt signaling in either Xenopus or Drosophila assays. Thus it appears that more than a Frizzled–Dsh interaction is conserved between the pathway controlling planar polarity in Drosophila and that regulating convergent extension in Xenopus.
Although the evidence is now compelling that PCP or non-canonical Wnt signaling is required for convergent extension, it is not at all clear what this pathway actually regulates and how convergent extension is, in turn, affected. Even basic questions are unanswered: for example, it is not known when and where PCP signaling is required. Although it is generally assumed that the pathway operates in the intercalating cell population itself, this has not been rigorously demonstrated. Perhaps the most interesting question is whether PCP signals act during vertebrate morphogenesis to polarize cells, as they do in Drosophila. Since convergent extension depends on directional cell rearrangement (mediolateral intercalation must predominate over intercalation in other orientations for net change in tissue shape to occur; Keller et al., 2000), it is tempting to suppose that PCP signals might provide this directionality by imposing or maintaining polarized cell motility. In support of this hypothesis, Wallingford et al. (2000) found that protrusive activity, which is normally largely restricted to the mediolateral ends of intercalating mesodermal cells, was randomly oriented in explants expressing dominant-negative Dsh.
The molecular function of Strabismus
The number of genes known to take part in the regulation of planar polarity in Drosophila continues to grow, and some intriguing interactions among them have emerged, in particular governing subcellular location. Yet many elements of this genetically defined network continue to defy arrangement into a simple pathway, and little is known about how most components function at the molecular level. strabismus/Van Gogh in particular has been reported to interact genetically with frizzled and prickle (Taylor et al., 1998), but no biochemical interactions with other proteins have yet been demonstrated. The primary sequence of the protein gives little hint of molecular function beyond suggesting that Stbm is an integral membrane protein. The last four amino acids, conserved in Drosophila and vertebrate homologs, meet the consensus for a PDZ domain binding site. We find that a form in which these residues are deleted is still capable of inhibiting convergent extension. This result should be interpreted with caution, however, since the over- and underexpression phenotypes may be difficult to distinguish. Thus it is formally possible that the C-terminal mutant acts as a dominant-negative, although we have not observed rescue of the ΔPBS-Stbm mutant on co-injection with wild type (data not shown). For the same reason, it cannot be determined from our results whether Xstbm plays a positive or negative role in a pathway regulating convergent extension. It may prove difficult to sort out this confusion in the absence of a downstream readout of planar polarity signaling other than the disruption of morphogenesis.
Expression of Xstbm
We report here the identification of a Xenopus Stbm homolog which we have named Xstbm. This protein shows considerable homology throughout its length to Drosophila Stbm, and is nearly identical to the product of the recently cloned mouse gene Ltap (Kibar et al., 2001) and to the human protein KIAA1215. The expression patterns of the Xenopus and mouse genes are also quite similar, although there are differences in detail. Both genes are expressed throughout the forming neural plate and early neural tube. The extent of mesodermal expression is less clear: Kibar et al. (2001) report that Ltap is not expressed in the notochord at embryonic day (E) 7.5–8.5, but is present in what appears to be paraxial mesoderm below the posterior neural plate. In contrast, Xstbm is expressed maternally and is broadly distributed at early gastrula stages, including in the marginal zone. It is worth noting that there are apparently at least two mouse Stbm homologs, since Ltap is not identical to the previously reported mouse Stbm homolog mStbm, whose pattern of expression has not been described (Wolff and Rubin, 1998). Thus, an as yet undiscovered Xenopus homolog with similar properties may be expressed elsewhere.
Stbm phenotype in the mouse
The importance of Strabismus to early vertebrate development is strongly bolstered by the recent report that a Stbm homolog (Ltap) is mutated in Lp mice (Kibar et al., 2001). The best-known feature of the Lp mutant phenotype is failure of neural tube closure, which we also observe in Stbm-injected Xenopus embryos. Moreover, there are additional intriguing similarities. Earlier analyses of Lp embryos showed that axial elongation was impaired and that the notochord was abnormally thick, and suggested that the failure of elongation may be the underlying cause of the neural tube defects (Smith and Stein, 1962). Thus the mouse phenotype and our Xenopus results are mutually supportive, and together argue that this gene functions in a pathway regulating early dorsal morphogenesis in all vertebrates.
Materials and methods
Embryological and histological methods
Eggs were fertilized in vitro and injected at cleavage stages by standard methods. Activin-containing conditioned medium from oocytes (a gift from Chenbei Chang and Ali Hemmati-Brivanlou) was used at 1:300–1:1000 dilution in animal cap experiments. Keller sandwiches were prepared as described in Keller et al. (1999). Briefly, dorsal marginal zone explants extending about 45° to each side of the midline and from the bottle cells halfway to the animal pole were cut from early gastrulae (stage 10+ to 10.25). Involuted material was stripped from the inner surface, leaving just the pre-involution dorsal mesoderm and prospective neuroectoderm. Explants were then pressed together inner surface to inner surface, allowed to heal and cultured in 0.5× modified mammalian Ringers’ (MMR). Embryo lengths, defined as longest dimension, were measured at stage 27–28 using a SPOT Insight digital camera and accompanying software. Results were analyzed using the Student’s t-test.
For histological analysis, de-membranated embryos were fixed in 4% formaldehyde in 1× phosphate-buffered saline (PBS) for 2–12 h, embedded in Paraplast, sectioned at 10 µm, and stained in hematoxylin and eosin. Whole-mount in situ hybridization (Harland, 1991) was performed according to established protocols. The chordin in situ probe was a gift from Alin Vonica and Barry Gumbiner. The Xstbm probe was transcribed with the T7 polymerase (Promega) from NotI-linearized Xstbm in pBS.
RNA extraction, RT–PCR and RNA synthesis
RNA was extracted from caps and embryos by proteinase K digestion in lysis buffer followed by DNase treatment as described previously (Wilson et al., 1997). Reverse transcription and PCR were carried out as in Darken and Wilson (2001). Capped RNA for injection was produced in vitro from linearized plasmid using the Message Machine kit from Ambion. Xstbm primers were CCAATTGCCTTCATGCTGCT (up) and GGATTCCAGTATCCGTACTC (down); other primer sequences are available on request.
Cloning of Xstbm
Two nearly identical putative Xenopus Stbm homologs were identified by BLAST search of the NCBI EST database with the Drosophila Stbm cDNA sequence (Wolff and Rubin, 1998), and the corresponding clones were obtained from Research Genetics (Huntsville, AL). One clone (IMAGE #3301175) was used to prepared a radiolabeled random-primed DNA probe, which was then used to screen a stage 11 lambdaZAP cDNA library (a gift from Peter Klein) at low stringency. Positive clones were plaque-purified, excised from the phage vector and sequenced at the ends. Several phage inserts were found to represent a single gene, apparently corresponding to the EST clone used to probe the library, which we have named Xstrabismus or Xstbm. One clone appears to contain the complete open reading frame coding for a 512 amino acid protein: an initiating methionine at a position analogous to those identified in the two mouse homologs (mStbm and Ltap) is preceded by an in-frame stop codon, and the reading frame extends to a stop codon at the same position as the mouse and Drosophila homologs.
Plasmids and morpholinos
Drosophila Stbm cDNA was a gift from Tanya Wolff. To create dStbm-CS2, which was used to synthesize message, Stbm-pMT14 was cut with EcoRI (blunted) and NotI, and ligated into pCS2 (105) at the StuI–NotI sites. ΔPBS-Stbm was made by PCR amplification of the Stbm coding region excluding the last four residues; this fragment was then subcloned into pCS2(105) at the XbaI and XhoI sites. Xstbm in pCS2 was made by digesting Xstbm-pBS with BglII, which cuts in the 3′ untranslated region, filling in, then digesting with EcoRI and dropping into the StuI–EcoRI sites in the pCS2 (105) polylinker. Dominant-negative Xdsh (Xdd1) in pCS2 was a gift from Sergei Sokol (Sokol, 1996); construction of Xwnt8-CS2 was as described previously (Darken and Wilson, 2001). Xstbm morpholino sequence is CGTTGGCGGATTTGGGTCCCCCCGA and the 4-base mismatch is CGTTaGCGGtTTTGGcTCCCaCCGA. All morpholinos are from Gene Tools, LLC.
Fly strains and genetics
The Gal4/UAS system was used to overexpress Stbm or Fz2 in specific regions (Brand and Perrimon, 1993). The following drivers were used: dppGal4, ptcGal4, apGal4, pnrGal4, enGal4 and sevGal4, as described in FlyBase. The UAS-Stbm flies were a kind gift from Tanya Wolff. All crosses were performed at 25 or 29°C. The wings were mounted in Hoyers medium.
Acknowledgments
Acknowledgements
We would like to thank Tanya Wolff, Sergei Sokol, Alin Vonica and Barry Gumbiner for plasmids or probes; Tanya Wolff for the gift of UAS-Stbm flies; Jun Wu for Fz2 wings shown in Figure 5C; Peter Klein for the stage 11 library; members of the Hemmati-Brivanlou laboratory for generous technical advice and activin-containing media; and Posy Bachvarova, Doris Hertzlinger and Dan Weinstein for helpful discussions and careful reading of the manuscript. This work was supported by a Basil O’Connor Starter Scholar Research Award from the March of Dimes Birth Defects Foundation (to P.A.W.) and NIH grant 1R01GM62197 (to M.M.). R.S.D. is supported by NIH MSTP grant GM07739, the Margaret and Herman Sokol Fellowship and the Harry E.Gould, Sr Graduate Student Scholarship.
Note added in proof
In an article which appeared after submission of this manuscript, another group reported the identification and functional analysis of Xenopus and zebrafish strabismus homologs [Park,M. and Moon,R.T. (2002) Nature Cell Biol., 4, 20–25].
References
- Adler P.N. (1992) The genetic control of tissue polarity in Drosophila. BioEssays, 14, 735–741. [DOI] [PubMed] [Google Scholar]
- Adler P.N., Krasnow,R.E. and Liu,J. (1997) Tissue polarity points from cells that have higher Frizzled levels towards cells that have lower Frizzled levels. Curr. Biol., 7, 940–949. [DOI] [PubMed] [Google Scholar]
- Boutros M. and Mlodzik,M. (1999) Dishevelled: at the crossroads of divergent intracellular signaling pathways. Mech. Dev., 83, 27–37. [DOI] [PubMed] [Google Scholar]
- Brand A.H. and Perrimon,N. (1993) Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development, 118, 401–415. [DOI] [PubMed] [Google Scholar]
- Brannon M. and Kimelman,D. (1996) Activation of Siamois by the Wnt pathway. Dev. Biol., 180, 344–347. [DOI] [PubMed] [Google Scholar]
- Carnac G., Kodjabachian,L., Gurdon,J.B. and Lemaire,P. (1996) The homeobox gene Siamois is a target of the Wnt dorsalisation pathway and triggers organiser activity in the absence of mesoderm. Development, 122, 3055–3065. [DOI] [PubMed] [Google Scholar]
- Chae J., Kim,M.J., Goo,J.H., Collier,S., Gubb,D., Charlton,J., Adler,P.N. and Park,W.J. (1999) The Drosophila tissue polarity gene starry night encodes a member of the protocadherin family. Development, 126, 5421–5429. [DOI] [PubMed] [Google Scholar]
- Darken R.S. and Wilson,P.A. (2001) Axis induction by wnt signaling: Target promoter responsiveness regulates competence. Dev. Biol., 234, 42–54. [DOI] [PubMed] [Google Scholar]
- Deardorff M.A., Tan,C., Conrad,L.J. and Klein,P.S. (1998) Frizzled-8 is expressed in the Spemann organizer and plays a role in early morphogenesis. Development, 125, 2687–2700. [DOI] [PubMed] [Google Scholar]
- Djiane A., Riou,J., Umbhauer,M., Boucaut,J. and Shi,D. (2000) Role of Frizzled 7 in the regulation of convergent extension movements during gastrulation in Xenopus laevis. Development, 127, 3091–3100. [DOI] [PubMed] [Google Scholar]
- Doniach T. (1992) Induction of anteroposterior neural pattern in Xenopus by planar signals. Development, Suppl., 183–193. [PubMed] [Google Scholar]
- Fan M.J., Gruning,W., Walz,G. and Sokol,S.Y. (1998) Wnt signaling and transcriptional control of Siamois in Xenopus embryos. Proc. Natl Acad. Sci. USA, 95, 5626–5631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feiguin F., Hannus,M., Mlodzik,M. and Eaton,S. (2001) The ankyrin repeat protein Diego mediates Frizzled-dependent planar polarization and formation of proximal-distal cortical domains. Dev. Cell, 1, 93–101. [DOI] [PubMed] [Google Scholar]
- Gubb D., Green,C., Huen,D., Coulson,D., Johnson,G., Tree,D., Collier,S. and Roote,J. (1999) The balance between isoforms of the prickle LIM domain protein is critical for planar polarity in Drosophila imaginal discs. Genes Dev., 13, 2315–2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harland R.M. (1991) In situ hybridization: an improved wholemount method for Xenopus embryos. In Kay,B.K. and Peng,H.B. (eds), Methods in Cell Biology. Vol. 36. Academic Press, San Diego, CA. [DOI] [PubMed]
- Heasman J., Kofron,M. and Wylie,C. (2000) β-catenin signaling activity dissected in the early Xenopus embryo: a novel antisense approach. Dev. Biol., 222, 124–134. [DOI] [PubMed] [Google Scholar]
- Heisenberg C.P., Tada,M., Rauch,G.J., Saude,L., Concha,M.L., Geisler,R., Stemple,D.L., Smith,J.C. and Wilson,S.W. (2000) Silberblick/Wnt11 mediates convergent extension movements during zebrafish gastrulation. Nature, 405, 76–81. [DOI] [PubMed] [Google Scholar]
- Keller R.E. and Danilchik,M. (1988) Regional expression, pattern and timing of convergence and extension during gastrulation of Xenopus laevis. Development, 103, 193–210. [DOI] [PubMed] [Google Scholar]
- Keller R., Poznanski,A. and Elul,T. (1999) Experimental embryological methods for analysis of neural induction in the amphibian. Methods Mol. Biol., 97, 351–392. [DOI] [PubMed] [Google Scholar]
- Keller R., Davidson,L., Edlund,A., Elul,T., Ezin,M., Shook,D. and Skoglund,P. (2000) Mechanisms of convergence and extension by cell intercalation. Philos. Trans. R. Soc. Lond. B Biol. Sci., 355, 897–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kibar Z., Vogan,K.J., Groulx,N., Justice,M.J., Underhill,D.A. and Gros,P. (2001) Ltap, a mammalian homolog of Drosophila Strabismus/Van Gogh, is altered in the mouse neural tube mutant Loop-tail. Nature Genet., 28, 251–255. [DOI] [PubMed] [Google Scholar]
- Krasnow R.E., Wong,L.L. and Adler,P.N. (1995) Dishevelled is a component of the Frizzled signaling pathway in Drosophila. Development, 121, 4095–4102. [DOI] [PubMed] [Google Scholar]
- Kuhl M., Sheldahl,L.C., Park,M., Miller,J.R. and Moon,R.T. (2000) The Wnt/Ca2+ pathway: a new vertebrate Wnt signaling pathway takes shape. Trends Genet., 16, 279–283. [DOI] [PubMed] [Google Scholar]
- McKay R.M., Peters,J.M. and Graff,J.M. (2001) The casein kinase I family: roles in morphogenesis. Dev. Biol., 235, 378–387. [DOI] [PubMed] [Google Scholar]
- McKendry R., Hsu,S.C., Harland,R.M. and Grosschedl,R. (1997) LEF-1/TCF proteins mediate wnt-inducible transcription from the Xenopus nodal-related 3 promoter. Dev. Biol., 192, 420–431. [DOI] [PubMed] [Google Scholar]
- Medina A., Reintsch,W. and Steinbeisser,H. (2000) Xenopus Frizzled 7 can act in canonical and non-canonical Wnt signaling pathways: implications on early patterning and morphogenesis. Mech. Dev., 92, 227–237. [DOI] [PubMed] [Google Scholar]
- Mlodzik M. (1999) Planar polarity in the Drosophila eye: a multifaceted view of signaling specificity and cross-talk. EMBO J., 18, 6873–6879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon R.T., Campbell,R.M., Christian,J.L., McGrew,L.L., Shih,J. and Fraser,S. (1993) Xwnt-5A: a maternal Wnt that affects morphogenetic movements after overexpression in embryos of Xenopus laevis. Development, 119, 97–111. [DOI] [PubMed] [Google Scholar]
- Nagase T., Ishikawa,K., Kikuno,R., Hirosawa,M., Nomura,N. and Ohara,O. (1999) Prediction of the coding sequences of unidentified human genes. XV. The complete sequences of 100 new cDNA clones from brain which code for large proteins in vitro. DNA Res., 6, 337–345. [DOI] [PubMed] [Google Scholar]
- Sasai Y., Lu,B., Steinbeisser,H., Geissert,D., Gont,L.K. and De Robertis,E.M. (1994) Xenopus chordin: a novel dorsalizing factor activated by organizer-specific homeobox genes. Cell, 79, 779–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman J.M., Perrimon,N. and Axelrod,J.D. (1998) Frizzled signaling and the developmental control of cell polarity. Trends Genet., 14, 452–458. [DOI] [PubMed] [Google Scholar]
- Smith J.C. and Howard,J.E. (1992) Mesoderm-inducing factors and the control of gastrulation. Development, Suppl., 127–136. [PubMed] [Google Scholar]
- Smith J.L. and Stein,K.F. (1962) Axial elongation in the mouse and its retardation in homozygous looptail mice. J. Embryol. Exp. Morph., 10, 73–87. [PubMed] [Google Scholar]
- Smith W.C., McKendry,R., Ribisi,S.J. and Harland,R.M. (1995) A nodal-related gene defines a physical and functional domain within the Spemann organizer. Cell, 82, 37–46. [DOI] [PubMed] [Google Scholar]
- Sokol S.Y. (1996) Analysis of Dishevelled signalling pathways during Xenopus development. Curr. Biol., 6, 1456–1467. [DOI] [PubMed] [Google Scholar]
- Strutt D.I., Weber,U. and Mlodzik,M. (1997) The role of RhoA in tissue polarity and Frizzled signalling. Nature, 387, 292–295. [DOI] [PubMed] [Google Scholar]
- Sumanas S., Strege,P., Heasman,J. and Ekker,S.C. (2000) The putative wnt receptor Xenopus Frizzled-7 functions upstream of β-catenin in vertebrate dorsoventral mesoderm patterning. Development, 127, 1981–1990. [DOI] [PubMed] [Google Scholar]
- Tada M. and Smith,J.C. (2000) Xwnt11 is a target of Xenopus Brachyury: regulation of gastrulation movements via Dishevelled, but not through the canonical Wnt pathway. Development, 127, 2227–2238. [DOI] [PubMed] [Google Scholar]
- Taylor J., Abramova,N., Charlton,J. and Adler,P.N. (1998) Van Gogh: a new Drosophila tissue polarity gene. Genetics, 150, 199–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usui T., Shima,Y., Shimada,Y., Hirano,S., Burgess,R.W., Schwarz,T.L., Takeichi,M. and Uemura,T. (1999) Flamingo, a seven-pass transmembrane cadherin, regulates planar cell polarity under the control of Frizzled. Cell, 98, 585–595. [DOI] [PubMed] [Google Scholar]
- Vinson C.R. and Adler,P.N. (1987) Directional non-cell autonomy and the transmission of polarity information by the Frizzled gene of Drosophila. Nature, 329, 549–551. [DOI] [PubMed] [Google Scholar]
- Wallingford J.B. and Harland,R.M. (2001) Xenopus Dishevelled signaling regulates both neural and mesodermal convergent extension: parallel forces elongating the body axis. Development, 128, 2581–2592. [DOI] [PubMed] [Google Scholar]
- Wallingford J.B., Rowning,B.A., Vogeli,K.M., Rothbacher,U., Fraser,S.E. and Harland,R.M. (2000) Dishevelled controls cell polarity during Xenopus gastrulation. Nature, 405, 81–85. [DOI] [PubMed] [Google Scholar]
- Wilson P.A., Lagna,G., Suzuki,A. and Hemmati-Brivanlou,A. (1997) Concentration-dependent patterning of the Xenopus ectoderm by BMP4 and its signal transducer Smad1. Development, 124, 3177–3184. [DOI] [PubMed] [Google Scholar]
- Wolff T. and Rubin,G.M. (1998) Strabismus, a novel gene that regulates tissue polarity and cell fate decisions in Drosophila. Development, 125, 1149–1159. [DOI] [PubMed] [Google Scholar]