Abstract
Cloning by nuclear transfer (NT) has been riddled with difficulties: most clones die before birth and survivors frequently display growth abnormalities. The cross-species similarity in abnormalities observed in cloned fetuses/animals leads us to suspect the fidelity of epigenetic reprogramming of the donor genome. Here, we found that single-copy sequences, unlike satellite sequences, are demethylated in pre-implantation NT embryos. The differential demethylation pattern between genomic sequences was confirmed by analyzing single blastocysts. It suggests selective demethylation of other developmentally important genes in NT embryos. We also observed a reverse relationship between methylation levels and inner cell mass versus trophectoderm (ICM/TE) ratios, which was found to be a result of another type of differential demethylation occurring in NT blastocysts where unequal methylation was maintained between ICM and TE regions. TE-localized methylation aberrancy suggests a widespread gene dysregulation in an extra-embryonic region, thereby resulting in placental dysfunction familiar to cloned fetuses/animals. These differential demethylations among genomic sequences and between differently allocated cells produce varied overall, but specified, methylation patterns, demonstrating that epigenetic reprogramming occurs in a limited fashion in NT embryos.
Keywords: bovine/cloning/demethylation/epigenetic reprogramming/pre-implantation embryos
Introduction
Epigenetic modification of the genome ensures proper gene expression during development. DNA methylation is one of the best-studied epigenetic modifications of DNA in all organisms and is essential for the development of mammals (Li et al., 1992; Okano et al., 1999). DNA methylation is involved in a diverse range of biological processes, including tissue-specific gene expression, cell differentiation, genomic imprinting, X chromosome inactivation, regulation of chromatin structure, carcinogenesis and ageing (Reik and Surani, 1997). During early development, a dramatic reduction in methylation levels occurs in mouse (Monk et al., 1987). This process of epigenetic reprogramming in early embryos erases gamete-specific methylation patterns inherited from the parents (Monk et al., 1987; Howlett and Reik, 1991; Oswald et al., 2000). After implantation, a wave of de novo methylation takes place: most of the genomic DNA is methylated at defined developmental time points. On the other hand, housekeeping genes remain unmethylated in all cells (Bird, 1986), except for X-linked housekeeping genes, which are methylated on the inactive X chromosome in female cells and imprinted genes that show allele-specific methylation patterns (Razin and Cedar, 1994). Another demethylation–remethylation cycle of epigenetic reprogramming takes place during gametogenesis and is necessary for resetting of imprints (Chaillet et al., 1991; Stoger et al., 1993; Ariel et al., 1995; Tremblay et al., 1995; Zuccotti and Monk, 1995) or, probably, for the removal of acquired epigenetic modifications, which can be influenced by individual genetic and environmental factors (Sapienza et al., 1989; Allen et al., 1990; Reik et al., 1993).
The cloning of mammals by somatic cell nuclear transfer (NT) requires epigenetic reprogramming of the differentiated state of donor cell to a totipotent, embryonic ground state (Gurdon and Colman, 1999). It means that the donor cell must cease its own program of gene expression and assume an expression program typical of a zygotic genome. If the reprogramming process operates inefficiently, the resulting epigenetic anomaly affects embryonic development (Bestor, 1998). Poor epigenetic reprogramming in early cleavage embryos entails dysregulation of gene expression, and the accumulated action of abnormally expressed genes in cloned fetuses can disrupt their normal development (Humpherys et al., 2001). Indeed, along with the low survival rates (Wells et al., 1997; Wilmut et al., 1997; Cibelli et al., 1998; Wakayama et al., 1998), various disease phenotypes have been observed in cloned fetuses/animals, including circulatory distress, placental edema, hydrallantois, respiratory problems, immune dysfunction and kidney/brain malformation (Hill et al., 1999, 2000; Lanza et al., 2000). Even the surviving offspring show increased placental and birth weights (Wakayama et al., 1998; Hill et al., 1999; Eggan et al., 2001; Ono et al., 2001), often referred to as ‘large offspring syndrome’. It is notable that embryonic stem (ES) cell-derived mouse clones produced by either serial nuclear transfer (Amano et al., 2001) or tetraploid embryo complementation (Eggan et al., 2001) are more or less normal in fetoplacental size, unlike the large ES-derived clones produced by the conventional NT procedure.
The high-frequency and cross-species similarities in abnormalities inherent to cloned animals lead us to speculate that these various problems begin from earlier developmental stages and are caused primarily by faulty epigenetic reprogramming that should necessarily be accomplished in donor genome during pre-implantation development. We recently found, using bisulfite-sequencing technology, that various genomic repeated sequences were barely demethylated in pre-implantation-stage NT bovine embryos (Kang et al., 2001c). The methylation patterns observed in NT embryos closely resembled donor cells in the overall genomic methylation status, but were quite different from normal embryos produced in vitro or in vivo. Aberrant epigenetic reprogramming in bovine NT embryos was also observed by the analyses of metaphase chromosomes (Bourc’his et al., 2001) and interphase nuclei (Dean et al., 2001) using immunostaining technique with a 5-methylcytosine antibody. These findings in pre-implantation NT embryos led us to anticipate a widespread dysregulation of developmentally crucial genes in the resultant developing clones. A recent study using mouse ES cells as donors in cloning showed aberrant expressions of imprinted genes in cloned mice (Humpherys et al., 2001).
Here, in an effort to understand some of the parameters that give rise to abnormal development of cloned animals, we examined additional genomic sequences such as single-copy sequences, the reprogramming of which should be essential in that appropriate expression of functional genes is closely associated with normal development of NT embryos. By juxtaposing the methylation patterns of unique gene sequence to the patterns of genomic repeated sequences, we found that in the genome of NT embryos, demethylation events take place differentially according to the genomic sequences and also, more importantly, according to the locations [the inner cell mass (ICM) and the trophectoderm (TE)] in the blastocyst. The observation of TE-localized methylation aberrancy may be related to the placental dysfunction intimately observed in cloned animals. Our results indicate that the differential demethylation restricts epigenetic reprogramming events to certain genomic parts, or to certain lineage cells, of the blastocyst in pre-implantation-stage NT embryos.
Results
In order to examine the methylation status of cleavage-stage embryos, genomic DNA was isolated from each developmental stage and treated with bisulfite. Bisulfite causes deamination of unmethylated cytosines to uridine, thereby allowing discrimination between unmethylated and methylated cytosine residues through sequencing or restriction enzyme analysis. The bisulfite-treated genomic DNAs were subjected to PCR and the resulting products were digested by restriction enzyme.
The promoter sequences of tissue-specific genes are normally demethylated in pre-implantation-stage bovine embryos produced either in vitro or by somatic cell nuclear transfer.
In order to analyze the genome of early bovine embryos produced either by in vitro fertilization (IVF) or by NT, the promoter sequences of bovine epidermal cytokeratin and mammary gland-specific β-lactoglobulin genes were chosen as targets for methylation analysis, since these promoter sequences were found previously to be heavily methylated in donor nuclei of bovine fetal fibroblasts (Kang et al., 2001a). Their hypermethylation status would be instrumental in monitoring potential changes in methylation level that can take place in donor genome of early NT embryos. In the mouse, tissue-specific gene sequences are usually heavily methylated in both oocyte and sperm, and become demethylated during early development (Razin and Shemer, 1995). If the bovine tissue-specific genes, such as the epidermal cytokeratin gene and β-lactoglobulin gene, behave similarly to the mouse versions, dynamic demethylation events could also be expected from these promoter sequences of bovine IVF embryos.
A 166 bp fragment of PCR product amplified from the promoter region of the bovine epidermal cytokeratin gene, which has seven CpG dinucleotides and four AciI recognition sequences (5′-GCGG-3′), was digested by AciI enzyme, which recognizes only the unconverted 5′-GCGG-3′ sequences. Figure 1A shows that a typical demethylation process unambiguously takes place in IVF embryos during the pre-implantation stages. The promoter sequence was initially highly methylated in sperm, matured oocyte, and one-cell fertilized egg. This hypermethylation status remained that way until, or became somewhat demethylated at, the four- to eight-cell stages, then extensively demethylated at the morula and blastocyst stages. Analysis of the promoter sequence of the mammary gland-specific β-lactoglobulin gene exhibited a similar result. A 110 bp fragment of PCR product, which carries six CpG dinucleotides and three TaqI recognition sequences (5′-TCGA-3′), was digested by TaqI (Figure 1B). This promoter region was also highly methylated in fertilized one-cell eggs, and became modified by demethylation as the embryos cleaved to later stages. The progressive pattern of demethylation closely resembled the demethylation pattern observed in mouse embryos, where genomic demethylation process occurs primarily by a replication-coupled passive mechanism (Howlett and Reik, 1991; Rougier et al., 1998; Mayer et al., 2000).
Fig. 1. Demethylation of tissue-specific gene promoter sequences in IVF (A and B) or cloned (C and D) bovine embryos. (A and C) The epidermal cytokeratin gene sequence (166 bp, four AciI recognition sites). (B and D) The β-lactoglobulin gene sequence (110 bp, three TaqI sites). The number of embryos used in each lane was ∼200 for matured oocytes, ∼100 for one-cell eggs, ∼30 of four- to eight-cell embryos, ∼10 for morulae and about six for blastocysts. Both target regions consist of unique sequences with no interrupting repeat such as transposons. Photographs, negative images. Arrows, position of intact PCR products. M, DNA size marker (bp); X, intact, undigested PCR products; O, enzyme-treated PCR products; donor, fetal bovine fibroblasts; 4/8c, four- to eight-cell embryos; mor, morulae; bl, blastocysts.
Based on these observations, we next analyzed the same promoter regions of NT embryos. The donor cells used in nuclear transplantation were bovine fetal fibroblasts, in which both promoter regions are presumed to be functionally inactive. Methylation states of the two promoter sequences are shown in Figure 1A and B (donor). In donor cells, both promoter regions were almost completely methylated. When they were transplanted into enucleated ooplasm, their initial hypermethylation was quickly modified (Figure 1C and D). In the four- to eight-cell stage embryos, the promoter sequences were already in substantially demethylated states, leaving only feint traces of digested DNA bands. The residual bands disappeared in the later cleavage stages. These results indicate that demethylation events do take place in bovine NT embryos during early developing stages, which agrees well with a recent observation of demethylation in a euchromatin DNA region of cloned bovine embryos (Bourc’his et al., 2001).
Differential demethylation patterns are shown between the epidermal cytokeratin gene promoter and the satellite I sequences in NT blastocysts
These results are in contrast to the observation of aberrant methylation of various genomic repeats in NT blastocysts; when analyzed for methylation status of various repeated sequences, bovine NT embryos closely resembled donor cells in overall genomic methylation patterns, but they were quite different from normal embryos. Our findings, therefore, indicate that the epigenetic anomaly is not a genome-wide phenomenon, and that there are sequence- or genomic region-specific differences in epigenetic modification of the donor genome in NT embryos. To verify this supposition, we examined both the satellite I sequence (Kang et al., 2001c) and the cytokeratin gene promoter sequence in single NT blastocysts and compared their methylation patterns. Analysis of individual NT blastocysts exhibited further strong evidence for differential demethylation. Cytokeratin gene sequence was undermethylated or only moderately methylated in most of the NT blastocysts, but in contrast, the satellite DNA region was substantially methylated in most of them (Figure 2A). Both target regions were normally undermethylated in individual IVF blastocysts with the exception of IVF-3 (Figure 2B). The exact methylation levels of cytokeratin promoter sequence were not determined, because of the uneven methylation among the AciI recognition sites. Heterogeneity in methylation levels of both sequences appears to be much higher among different NT embryos than among IVF embryos. A similar trend of variation in methylation level at tissue-specific gene regions was also observed in 19.5 days post-coitum (d.p.c.) cloned mice (Ohgane et al., 2001). The differential changes in methylation level between genomic sequences also appear in the genome of somatic cell system. We recently observed a similar tendency of differential methylation changes in cultured bovine fetal fibroblast cells under the ageing process (Kang et al., 2001a). In that system, various DNA sequences located in euchromatin (such as both promoter sequences of cytokeratin and β-lactoglobulin genes and other euchromatic repeats) underwent a loss of 5-methylcytosine, whereas heterochromatic DNA regions (satellites I and II, and alphoid sequences) remained unchanged in methylation levels during senescence.
Fig. 2. Differential demethylation patterns between genomic sequences in individual blastocysts derived either by NT (A) or IVF (B). Methylation patterns of the cytokeratin gene (upper panels) and the satellite sequences (lower panels) of each blastocyst are put in order by numbering along with intact, undigested PCR products below digestion panels. Judging from the rather high methylation of the cytokeratin promoter sequence observed in individual NT blastocysts in (A), the methylation level of the same genomic region shown in pooled NT blastocysts (Figure 1C) appeared to be somewhat underrated. This may be due to ‘PCR bias’ that may arise when the genomic sequence to be amplified has vastly different methylation states (Warnecke et al., 1997). In most cases, PCR bias is towards the preferential PCR amplification of unmethylated DNA. However, considering the various degree of methylation states among individual NT blastocysts in (A) (see also Figures 4B and 5B), PCR bias appears to be insignificant in the amplification of the cytokeratin sequence. Numbers are percentage digestion calculated from summed band intensity of digested fragments relative to that of the whole fragments. Arrows, position of intact PCR products. DNA size markers (bp) are shown on the left.
Analysis of an additional number of NT blastocysts gave an interesting result: the cloned blastocysts with relatively lower methylation levels at the satellite sequence also exhibit relatively lower levels of DNA methylation at the cytokeratin gene sequence. Of eight blastocyst clones having relatively lower methylation at the satellite sequences, seven (including clones 5, 6 and 8 in Figure 2A) showed lower methylation at the cytokeratin gene promoter sequence. In addition, among the clones that carried relatively higher methylation at the cytokeratin gene sequence, six (including clones 2, 4, 9 and 11) of seven also had higher methylation at the satellite sequences; clone 3 is the exception. However, this does not agree well with the opposite case, in which the two genomic sequences behave independently without any consistent pattern: the blastocyst clones with either higher methylation at the satellite sequences or lower methylation at the cytokeratin gene promoter exhibited varied DNA methylation at the cytokeratin or the satellite sequence, respectively. These observations probably indicate that if demethylation occurs, the cytokeratin gene sequence is first affected, followed by the satellite DNA sequences, indicating a preferential demethylation of the unique sequence. The sequence, or the program, of genomic regions that become demethylated may be determined by the vulnerabilities of those methylated sequences to a potential demethylating activity that presumably exists in oocytes. Since the satellite I DNA sequences should be demethylated relatively later in the order, it could be assumed that the methylation status of the satellite sequences serves as a signal for the occurrence of a demethylation event at single-copy sequences of NT donor genome.
The methylation level has a reverse relationship with the ICM/TE ratio in NT blastocysts
In an effort to correlate the methylation levels with the structural integrity of the blastocyst, an additional parameter, the ICM/TE ratio (the proportion of ICM cells relative to trophectoderm cells), was included. The ICM/TE ratios of individual NT blastocysts were first characterized by counting the number of constituent cells using a differential stain technique (Machaty et al., 1998) (Figure 3). Following determination of the ratios, stained cells of each blastocyst were scraped up to extract their genomic DNA. Both the satellite I and cytokeratin gene promoter sequences were amplified from bisulfite-treated genomic DNA and digested with AciI. Figure 4A shows individual NT blastocysts arranged by numbering (1–19) according to their ICM/TE values. The ICM/TE values were varied (ranging between 0.01 and 16.6) among different NT embryos. The methylation status of both target sequences of each NT blastocyst was also arranged in the same order as in Figure 4A (Figure 4B). Interestingly, a potential relationship was found between the methylation levels and the ICM/TE ratios. When the NT embryos were arbitrarily subgrouped into two categories according to their ICM/TE values, i.e. a normal ICM/TE group (mean ICM/TE 0.42, range 0.3–0.6; clones 1–9) and an abnormal group (mean ICM/TE 5.84, range 1.53–16.6; clones 13–19), a significant difference in methylation levels of the satellite DNA regions was found between the groups (P <0.005, independent t-test). The NT embryos with normal ICM/TE values exhibited 51.7 ± 14.5% (mean ± SD) methylation, whereas those with higher ICM/TE values showed 23.7 ± 18.2% methylation. Although methylation levels of the cytokeratin promoter sequence in individual NT blastocysts were not represented as numerical values, a similar methylation difference appeared to exist between the groups.
Fig. 3. Photographs of differentially stained blastocysts derived by nuclear transfer. Cells in red and blue are placed in TE and ICM lineages, respectively. (A) A blastocyst with normal ICM/TE value. (B) A blastocyst having high ICM/TE value. Photographs, ×200 magnification.
Fig. 4. Methylation levels versus ICM/TE values in individual NT blastocysts. (A) ICM/TE ratios. ICM/TE values are indicated above the bars. Individual NT blastocysts are arranged (1–19) according to their ICM/TE ratios. (B) Methylation status of the satellite I sequences (upper panel) and the cytokeratin gene promoter sequence (lower panel). Numbers indicate percentage digestion calculated from summed band intensity of digested fragments relative to that of the whole fragments. The position of non-specifically amplified PCR bands is indicated by a dotted line on the lower panel. The satellite and the cytokeratin gene sequences were amplified from bisulfite-treated genomic DNA and then digested by AciI enzyme, which recognizes 5′-GCGG-3′. Methylation status of both sequences of donor fibroblasts (cell) is included. Total cell numbers of individual blastocysts are counted (cell no.).
As a control, we also analyzed blastocysts derived by IVF. IVF embryos mostly have ICM/TE ratios centered on the value of 0.6 (Figure 5A). Since IVF blastocysts were subjected to the same in vitro procedures of maturation and cultivation as the reconstructed embryos, they serve as a good reference that can exclude any potential effect produced frequently by the in vitro procedures (Humpherys et al., 2001; Young et al., 2001). When methylation levels of the satellite sequences were compared between different ICM/TE groups [a lower group (IVF1-10), average ICM/TE 0.6, versus a higher group (IVF-14 to -19), average ICM/TE 4.5], no significant difference (P >0.1) was detected in methylation levels between the lower (8.7 ± 9.1%, average ± SD) and the higher (15.0 ± 23.1%) ICM/TE group (Figure 5B). In addition, blastocysts derived by either parthenogenetic activation or IVF were analyzed as another references. Both kinds of blastocysts exhibited relatively narrow ranges of ICM/TE ratios (0.4–1.9; Figure 5C) and hypomethylation status at both genomic loci, with an exception of clone 12 (Figure 5D). Relative to IVF blastocysts, both parthenogenones and in vivo derived blastocysts appeared to have much more homogeneous methylation patterns among individuals, which leads us to recall the previous reports describing that non-physiological, in vitro culture environments may induce inappropriate epigenetic modification of genomic loci, such as imprinted regions, during early embryogenesis (Young and Fairburn, 2000; Young et al., 2001).
Fig. 5. Methylation levels versus ICM/TE values in bovine blastocysts derived either by IVF (A and B), or by parthenogenetic activation or IVF [‘parthenogenone’ or ‘in vivo’, in (C) and (D), respectively]. (A and C) ICM/TE ratios. Individual blastocysts are arranged (1–14) according to their ICM/TE ratios, which are indicated above the bars. (B and D) Methylation status of the satellite I sequences (upper panel) and the cytokeratin gene promoter sequence (lower panel). Numbers indicate percentage digestion (%). The position of non-specifically amplified PCR bands is indicated by a dotted line. Total cell numbers of individual blastocysts are indicated (cell no.).
Unequal methylation status is maintained by the two different lineage cells of NT blastocyst
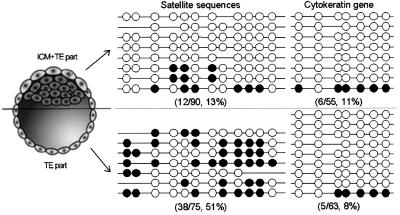
The methylation difference between the groups of NT blastocysts with different ICM/TE ratios may be due to the difference in methylation levels between ICM and TE genomes. To test this hypothesis, we further analyzed the methylation status of TE and ICM regions separately. NT blastocysts were examined under a fluorescence microscope after differential stain, and those with normal ICM/TE values were picked out and then roughly divided into two parts, a pure TE region and the remainder of the blastocyst that contains intact ICM with surrounding TE cells (Figure 6, left panel). Following genomic DNA extraction, bisulfite sequencing was performed to characterize methylation status of the 12 CpG sites of the satellite sequences (Figure 6, middle panel) (Kang et al., 2001c). There was a significant difference in the methylation status of satellite sequences between the regions (P <0.005, independent t-test); the pure TE part was 49.4 ± 20.6% (38 of 75 CpG sites) methylated, whereas the other ICM + TE part was 12.8 ± 19.1% (13 of 90) methylated. Considering that the 13% is a composite methylation value of both ICM and TE cells, ICM cells can be assumed to be much more undermethylated than they appeared. The cytokeratin gene sequence was additionally analyzed for the methylation status of the seven conserved CpG dinucleotides (Figure 6, right panel). Unlike the satellite sequences, the cytokeratin gene sequence showed nearly identical methylation profiles between the regions [11% (six of 55) in the ICM + TE part and 8% (five of 63) in the TE part]. As a control, we also examined the same sequences of IVF blastocysts, but were unable to detect differential methylation patterns between the ICM + TE and TE parts. Our results indicate that the higher methylation in NT blastocysts having normal ICM/TE ratios results from their higher proportions of TE constituents that are highly methylated, and the opposite is the case with the NT embryos with higher ICM/TE ratios. Since the methylation status of satellite sequences is preserved throughout the pre-implantation stage (see Discussion), our findings suggest that the satellite sequences are preferentially demethylated in ICM cells, whereas in the TE cells they keep their heavy methylation status, unmodified.
Fig. 6. Methylation difference between ICM + TE part and pure TE part. NT blastocysts were examined under a fluorescence microscope after differential staining, and those with seemingly normal ICM/TE values were picked out and then roughly divided into two parts, a pure TE region and the remainder of the blastocyst that contains intact ICM with surrounding TE cells (left panel). We analyzed 15 NT blastocysts in total. Methylation of each CpG was scored by sequencing PCR clones amplified from bisulfite-treated genomic DNAs of TE and ICM + TE parts. Open and closed circles indicate unmethylated and methylated CpGs, respectively. Some CpG sites are absent from some clones due to mutations in the particular copies of satellite sequences. Numbers in parentheses indicate the proportion of methylated CpG sites relative to the whole CpG sites examined.
Discussion
In summary, we observed differential demethylation events between genomic sequences and between the two distinct lineage cells. Demethylation events take place in the single-copy sequences of bovine NT embryos, in contrast to the previous observations of poor epigenetic reprogramming in various repeated sequences (Kang et al., 2001b,c). Such a differential pattern of demethylation between genomic sequences was confirmed again by the analyses of the genome of single NT blastocysts. In addition to differential demethylation between genomic sequences, another important type of differential methylation was observed between the two distinct lineage cells of the blastocyst such that unique gene sequence was demethylated in both ICM and TE lineage cells, whereas the satellite sequences remained methylated in TE cells but was demethylated in ICM cells.
Differential demethylation between unique and repeated sequences may reflect different vulnerabilities of those sequences to a demethylation-associated activity in NT embryos
We showed here that epigenetic modification takes place differentially in donor genome of NT bovine embryos. In the face of the surrounding genomic repeats that appear largely to remain methylated, the tissue-specific gene promoter sequences are rapidly demethylated. These results suggest that a potential activity, or activities, associated with demethylation is endogenously carried by the reconstituted embryos. These findings present the possibility that other single-copy genes that are important for full-term development can be also selectively demethylated in NT embryos, e.g. tissue-specific genes. If this is true and also if, as suggested previously, mammalian development is rather tolerant to epigenetic abnormalities (Humpherys et al., 2001), our findings could be the answer to the question of how viable offspring are born from unlikely NT embryos carrying abnormally methylated genomes.
It is uncertain why such a differential epigenetic modification arises between genomic sequences in early NT embryos. It is probable that the mosaic type of demethylation pattern may be related to the differences in the stabilities of DNA methylation between genomic regions. Supposing that both maintenance methylation and demethylation activities somehow co-exist in reconstructed embryos, the phenomenon of differential demethylation may be most likely explained by the fact that the single-copy sequences are more vulnerable to a demethylating activity because of their low density of CpG dinucleotides, and other genomic repeats like the satellite DNA sequences may be tightly regulated by a methylation mechanism owing to their high density of CpGs. Evidence for the different stabilities to a potential demethylating activity between genomic sequences came from the analyses of individual NT blastocysts (Figure 2A; see Results). In NT blastocysts with relatively less methylated cytokeratin gene and satellite sequences, the two different sequences appeared to be demethylated one after another; the cytokeratin gene sequence was affected first, followed by the satellite I sequences. This one-by-one demethylation pattern can arise from the different susceptibilities of these sequences to a demethylation-associated activity.
This hypothesis is based on the supposition that there is a methylation mechanism operating on genomic methylated sequences other than imprinted regions in NT pre-implantation embryos. Indeed, a maintenance methylation appears to exist. Comparison of the methylation patterns between NT blastocysts and either donor fibroblasts (Kang et al., 2001c) or NT morulae (Y.K.Kang, personal communication) manifested that their methylation patterns in the satellite sequences were very similar to, or nearly overlapped with, each other in several aspects, such overall methylation levels, relative methylation levels at individual CpG sites and methyl-CpG density of PCR strings, indicating there were no epigenetic changes during these transition periods. This type of maintenance methylation was also observed in the Bov-B LINE repeated sequences, where the donor-type methylation pattern was simply maintained during pre-implantation development of NT embryos, but not in normal embryos (Kang et al., 2001c). Although whether other genomic sequences that should be demethylated behave similarly to the satellite and the Bov-B sequences is not known, these observations indicate that at least parts of genomic regions can, by a methylation activity, completely evade the passively occurring epigenetic reprogramming process. Therefore, considering these all facts together, it is likely that the differential demethylation phenomenon comes from different vulnerabilities of those sequences to a demethylation-associated activity.
Is the phenomenon of differential methylation between ICM and TE lineage cells a unique feature of NT embryos?
The phenomenon of differential demethylation appears not only between different genomic sequences but also between the different regions of the blastocysts in NT embryos. We observed in NT blastocysts that the satellite sequences remain methylated in trophectoderm cells but not in ICM cells, and that the cytokeratin gene sequence was demethylated equally in both TE and ICM regions. However, it is not known whether the unequal methylation between ICM and TE lineage cells observed in NT blastocysts is a general phenomenon that also appears in normal bovine embryos derived by fertilization. Although a similar methylation difference between ICM and TE cells was not detected in IVF embryos, this observation alone cannot exclude the possibility of differential demethylation in normal embryos. Since the satellite sequences maintain low methylation status in IVF embryos throughout pre-implantation stage (Y.K.Kang, personal communication), this makes it difficult for the two distinct lineage cells to manifest the potential ability to modify their epigenetic status differentially. We need more information on other genomic sequences that remain methylated until the blastocyst. These sequences can be very helpful for discriminating, and thus elucidating, a methylation difference between ICM and TE regions. However, such sequences appear to be rare in bovine, because the genomic sequences that have been examined until now all show hypomethylation status at the blastocyst stage (Kang et al., 2001b,c). The result with bovine IVF blastocysts is consistent with a previous observation that a low level of methylation of L1 sequences was detected uniformly across both cell lineages of the blastocyst in the mouse (Howlett and Reik, 1991). On the other hand, an early methylation study described a pattern of differential methylation in rabbit 6-day blastocysts, where high levels of methylation were found in the ICM compared with the TE (Manes and Menzel, 1981), a pattern opposite to the one in bovine NT embryos. It is presently difficult to make a decision about the generality of the phenomenon of differential methylation between the regions of the blastocyst, due to a paucity of related studies. Although the question of whether methylation status is maintained differentially between ICM and TE regions of the normal blastocyst is obscure, it has been well recognized that after implantation, the methylation level increases in fetus proper (primitive ectoderm lineage), whereas in extraembryonic tissues derived from TE or primitive endoderm lineages, methylation levels remain low throughout gastrulation as though de novo methylation has not been activated in these cells (Chapman et al., 1984; Rossant et al., 1986).
The TE-localized epigenetic anomaly may be related to placental dysfunction in fetuses derived by nuclear transfer
Whether or not the methylation difference between ICM and TE lineage cells is a unique feature of NT embryos is yet to be elucidated, but it is clear that TE lineage cells of the blastocyst clones with normal ICM/TE ratios are aberrantly methylated in the satellite sequences. TE cells do not remain as a simple epithelium after implantation, but perform several active functions such as invasion into the deciduum by synthesis of various proteases (Strickland and Richards, 1992), formation of primary trophoblastic giant cells carrying polytene chromosomes (Varmuza et al., 1988) and expression of TE-specific genes [c-fms (Regenstreif and Rossant, 1989), Mash-2 (Guillemot et al., 1994), etc.]. These various processes programmed by TE cells may be essential to establish and maintain a stable placenta and thereby to support the growth of embryo proper. The functional integrity of all these processes can be guaranteed by the proper establishment of methylation status in the corresponding cells. In this line of connection, it is likely that the TE cells of NT blastocysts stray out of the developmental orbit, since the observation of abnormal DNA methylation of the satellite sequences leads us to infer methylation aberrancy in other genomic regions, including single-copy genes with important roles in normal development of NT embryos. In fact, we have already mentioned a potential role of the satellite sequences as an indicator in determining whether demethylation takes place in NT embryos (Figure 2A; also see Results). Abnormal methylation of unique gene sequences necessarily gives rise to misregulation of gene expressions, and the cumulative action of many abnormally expressed genes may affect the post-implantation viability of NT embryos. Intuitively, placental dysfunction should be the most likely phenotype that could be explained by the epigenetic anomaly of TE lineage cells. Deficient placentation is most frequently observed in dead fetal clones of various mammalian species, and also has been recognized as a potential cause of early fetal loss (Hill et al., 1999, 2000) and neonatal mortality (Wakayama et al., 1998) in cloned animals. Although our results did not present direct evidence for this hypothesis and thus appear to be more or less suggestive, it seems natural to believe the correlation of abnormal methylation in TE cells of NT blastocysts with the placental defects observed in cloned fetuses/animals.
High occurrence of both structural and epigenetic abnormalities in NT blastocysts can considerably reduce the overall cloning efficiency
The structural and compositional abnormality is common in bovine pre-implantation embryos derived by NT (unpublished data). Bovine NT blastocysts have problems in structure in that the mean value of the ICM/TE ratio is ∼1.0. This appears largely deviated from the value (mean 0.5) of in vivo derived blastocysts, and even from the value (mean 0.7) of IVF blastocysts. The lower number of TE cells diminishes the potential for embryogenesis (Pampfer et al., 1994), and has been suggested to influence the viability of post-implantation embryos (Leppens et al., 1996). Indeed, these problems are quite familiar to the developmental defects observed in cloned fetuses/animals (Campbell et al., 1996; Schnieke et al., 1997; Wells et al., 1997; Wilmut et al., 1997). Therefore, considering the proportion (∼50%) of NT blastocysts with awry ICM/TE values, it could be anticipated that many NT embryos should be faced with poor post-implantation development. On the contrary, NT blastocysts with normal ICM/TE values may have a better chance of survival. However, it should be also considered that most of NT blastocysts with a normal ICM/TE ratio have abnormal methylation levels in their trophectoderm cells. Therefore, these findings imply that most of NT blastocysts with normal ICM/TE values have aberrancy in methylation of satellite sequences, and those with undermethylated satellite sequences have deficiencies in structural integrity. Of the 19 NT blastocysts, 10% (two of 19 individuals, clones 2 and 4, Figure 4A) seem to satisfy both epigenetic and morphological criteria. This is much less than the proportion (25–30%) of NT embryos previously suggested as the normal-appearing embryos by including the single parameter of methylation (Kang et al., 2001c).
The high incidence of abnormal NT embryos can substantially decrease the viability of NT embryos and thus the overall cloning efficiency. Cloning by somatic cell nuclear transfer is still a hit-and-miss procedure, and it is not clear what technical and biological factors underlie this (Solter, 2000). The characterization of more parameters that determine the developmental potentials of cloned embryos helps the current nuclear transfer technology to find its problems and to address what should be done to resolve these problems. We believe that pre-implantation-stage NT embryos are one of the most valuable materials for epigenetic reprogramming studies, and we expect that our studies with pre-implantation embryos will contribute to the understanding of the molecular basis of epigenetic reprogramming.
Materials and methods
Production of bovine embryos derived either in vitro or by NT
All the procedures for the production of bovine pre-implantation embryos, such as in vitro maturation, fertilization and cultivation were as described elsewhere (Rosenkrans et al., 1993). For NT, we removed both the first polar body and metaphase plate from the oocytes matured in vitro (Tsunoda et al., 1986). The resulting oocytes were individually fused with bovine fetal fibroblasts (Kang et al., 2001c) using one electrical pulse of 1.6 kV/Cm for 30 µs (BTX ECM2001), activated and cultured in vitro as described previously (Cibelli et al., 1998). Blastocysts were collected at 168 h after activation (NT) or fertilization (IVF). The mean number of blastomeres was ∼130 and ∼100 for IVF and NT blastocysts, respectively. Forty to 50 of four- to eight-cell stage embryos, 20–30 of morulae and 8–10 of blastocysts were obtained from either in vitro fertilized or cloned oocytes. By the procedure of in vitro maturation, we collected 200–250 oocytes at the presumptive metaphase II stage. Pronase (0.5%, Sigma) was used to remove zonae from the embryos and mature oocytes to exclude the possibility of genomic contamination, such as by residual sperm or attached cumulus cells. Parthenogenesis was induced with in vitro matured oocytes by chemical activation (Cibelli et al., 1998). Differences in methylation rates among experimental groups were analyzed by independent two-population t-test.
Genomic DNA isolation and bisulfite treatment
All procedures have been described previously (Warnecke et al., 1998). Embryos at each developmental stage were pooled into a quantity of ∼300–800 diploid genomes for each genomic DNA isolation and subsequent bisulfite treatment. Zona-free embryos were solubilized in 100 µl of lysis buffer containing 200 µg/ml of proteinase K (Rôche Molecular Biochemicals), then incubated at 55°C for 3–5 h. Genomic DNA was recovered from the lysate by ethanol precipitation in the presence of 5 µg of Escherichia coli tRNA as a carrier, then redissolved in 10 µl of distilled water. The genomic DNA was digested with BamHI enzyme in 20 µl of reaction volume for 16 h, then denatured with 0.3 N NaOH. Bisulfite modification was initiated by adding 235 µl of freshly made 5 M sodium bisulfite (pH 5, Sigma) and 13.5 µl of 10 mM hydroquinone. The reaction mixture was covered with mineral oil and incubated at 55°C for 16 h in the dark. The sample was removed from under the mineral oil and the bisulfite-treated genomic DNA was recovered using a Prep-A-Gene DNA purification kit (Bio-Rad). Desulfonation was performed by adding 0.1 vol. of 3 N NaOH and incubating at 37°C for 30 min. Following precipitation, the DNA was resuspended in 20 µl of distilled water.
PCR amplification and sequencing of the bisulfite-treated genomic DNA
Amplification of 5′ regions of the bovine epidermal cytokeratin gene and the bovine β-lactoglobulin gene was as described previously (Kang et al., 2001a). Two microliters of the bisulfite-converted genomic DNA were used as a template in each amplification reaction. PCR was repeated three times for single target regions. Satellite I DNA sequences were amplified as described previously (Kang et al., 2001c). Amplified PCR products were cloned into pGEM-T easy vector (Promega). Individual clones were sequenced using automatic sequencer (ABI PRISM 377) and complete conversions of base Cs to Ts by bisulfite treatment were confirmed.
Restriction analysis of PCR products
For AciI restriction analysis of the amplified PCR products, 0.1 vol. of pooled PCR products was reamplified under the same conditions. PCR products were purified and concentrated using a Wizard DNA purification kit (Promega). One hundred nanograms of DNA were digested with 20 U of AciI restriction enzyme (New England Biolabs) overnight at 37°C, resolved on 4% metaphore agarose gel or 5–8% polyacrylamide gel. Band intensity was calculated using a Tina20 image analyzer.
Differential staining
Differential staining of ICM and TE cells of blastocysts (day 7) was carried out using the previously described technique (Machaty et al., 1998). Briefly, the zonae pellucidae of blastocysts were removed by 0.5% pronase. After rinsing in TL-HEPES medium containing 1 mg/ml polyvinyl alcohol, zona pellucida-free embryos were exposed to a 1:5 dilution of rabbit anti-pig whole serum for 1 h. They were then rinsed three times for 5 min in TL-HEPES and placed into a 1:10 dilution of guinea pig complement containing 10 µg/ml propidium iodide and 10 µg/ml bisbenzimide for 1 h. After brief rinsing in TL-HEPES, the stained embryos were mounted on slides under cover slips and examined under UV light using an epifluorescent microscope (Olympus, Japan). Blue and red cells were counted as cells from the ICM and TE, respectively.
Acknowledgments
Acknowledgements
This work is supported by grants (NLM0050111, HSC0130134 and HSS0350134) from the Ministry of Science and Technology (MOST), Seoul, Korea.
References
- Allen N.D., Norris,M.L. and Surani,M.A. (1990) Epigenetic control of transgene expression and imprinting by genotype-specific modifiers. Cell, 61, 853–861. [DOI] [PubMed] [Google Scholar]
- Amano T., Tani,T., Kato,Y. and Tsunoda,Y. (2001) Mouse cloned from embryonic stem (ES) cells synchronized in metaphase with nocodazole. J. Exp. Zool., 289, 139–145. [DOI] [PubMed] [Google Scholar]
- Ariel M., Robinson,E., McCarrey,J.R. and Cedar,H. (1995) Gamete-specific methylation correlates with imprinting of the murine Xist gene. Nature Genet., 9, 312–315. [DOI] [PubMed] [Google Scholar]
- Bestor T.H. (1998) Cytosine methylation and the unequal developmental potentials of the oocyte and sperm genomes. Am. J. Hum. Genet., 62, 1269–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird A.P. (1986) CpG-rich islands and the function of DNA methylation. Nature, 321, 209–213. [DOI] [PubMed] [Google Scholar]
- Bourc’his D., Le Bourhis,D., Patin,D., Niveleau,A., Comizzoli,P., Renard,J. and Viegas-Pequignot,E. (2001) Delayed and incomplete reprogramming of chromosome methylation patterns in bovine cloned embryos. Curr. Biol., 11, 1542–1546. [DOI] [PubMed] [Google Scholar]
- Campbell K.H., McWhir,J., Ritchie,W.A. and Wilmut,I. (1996) Sheep cloned by nuclear transfer from a cultured cell line. Nature, 380, 64–66. [DOI] [PubMed] [Google Scholar]
- Chaillet J.R., Vogt,T.F., Beier,D.R. and Leder,P. (1991) Parental-specific methylation of an imprinted transgene is established during gametogenesis and progressively changes during embryogenesis. Cell, 66, 77–83. [DOI] [PubMed] [Google Scholar]
- Chapman V., Forrester,L., Sanford,J., Hastie,N. and Rossant,J. (1984) Cell lineage-specific undermethylation of mouse repetitive DNA. Nature, 307, 284–286. [DOI] [PubMed] [Google Scholar]
- Cibelli J.B., Stice,S.L., Golueke,P.J., Kane,J.J., Jerry,J., Blackwell,C., Ponce de Leon,F.A. and Robl,J.M. (1998) Cloned transgenic calves produced from nonquiescent fetal fibroblasts. Science, 280, 1256–1258. [DOI] [PubMed] [Google Scholar]
- Dean W., Santos,F., Stojkovic,M., Zakhartchenko,V., Walter,J., Wolf,E. and Reik,W. (2001) Conservation of methylation reprogramming in mammalian development: aberrant reprogramming in cloned embryos. Proc. Natl Acad. Sci. USA, 98, 13734–13738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggan K., Akutsu,H., Loring,J., Jackson-Grusby,L., Klemm,M., Rideout,W.M.,III, Yanagimachi,R. and Jaenisch,R. (2001) Hybrid vigor, fetal overgrowth and viability of mice derived by nuclear cloning and tetraploid embryo complementation. Proc. Natl Acad. Sci. USA, 98, 6209–6214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillemot F., Nagy,A., Auerbach,A., Rossant,J. and Joyner,A.L. (1994) Essential role of Mash-2 in extraembryonic development. Nature, 371, 333–336. [DOI] [PubMed] [Google Scholar]
- Gurdon J.B. and Colman,A. (1999) The future of cloning. Nature, 402, 743–746. [DOI] [PubMed] [Google Scholar]
- Hill J.R. et al. (1999) Clinical and pathologic features of cloned transgenic calves and fetuses (13 case studies). Theriogenology, 51, 1451–1465. [DOI] [PubMed] [Google Scholar]
- Hill J.R., Winger,Q.A., Long,C.R., Looney,C.R., Thompson,J.A. and Westhusin,M.E. (2000) Development rates of male bovine nuclear transfer embryos derived from adult and fetal cells. Biol. Reprod., 62, 1135–1140. [DOI] [PubMed] [Google Scholar]
- Howlett S.K. and Reik,W. (1991) Methylation levels of maternal and paternal genomes during preimplantation development. Development, 113, 119–127. [DOI] [PubMed] [Google Scholar]
- Humpherys D., Eggan,K., Akutsu,H., Hochedlinger,K., Rideout, W.M.,III, Biniszkiewicz,D., Yanagimachi,R. and Jaenisch,R. (2001) Epigenetic instability in ES cells and cloned mice. Science, 293, 95–97. [DOI] [PubMed] [Google Scholar]
- Kang Y.K., Koo,D., Park,J.S., Choi,Y., Lee,K. and Han,Y. (2001a) Differential inheritance modes of DNA methylation between euchromatic and heterochromatic DNA sequences in ageing fetal bovine fibroblasts. FEBS Lett., 498, 1–5. [DOI] [PubMed] [Google Scholar]
- Kang Y.K., Koo,D., Park,J.S., Choi,Y., Lee,K. and Han,Y. (2001b) Influence of the oocyte nuclei on demethylation of donor genome in cloned bovine embryos. FEBS Lett., 499, 55–58. [DOI] [PubMed] [Google Scholar]
- Kang Y.K., Koo,D.B., Park,J.S., Choi,Y.H., Chung,A.S., Lee,K.K. and Han,Y.M. (2001c) Aberrant methylation of donor genome in cloned bovine embryos. Nature Genet., 28, 173–177. [DOI] [PubMed] [Google Scholar]
- Lanza R.P. et al. (2000) Extension of cell life-span and telomere length in animals cloned from senescent somatic cells. Science, 288, 665–669. [DOI] [PubMed] [Google Scholar]
- Leppens G., Gardner,D.K. and Sakkas,D. (1996) Co-culture of 1-cell outbred mouse embryos on bovine kidney epithelial cells: effect on development, glycolytic activity, inner cell mass:trophectoderm ratios and viability. Hum. Reprod., 11, 598–603. [DOI] [PubMed] [Google Scholar]
- Li E., Bestor,T.H. and Jaenisch,R. (1992) Targeted mutation of the DNA methyltransferase gene results in embryonic lethality. Cell, 69, 915–926. [DOI] [PubMed] [Google Scholar]
- Machaty Z., Day,B.N. and Prather,R.S. (1998) Development of early porcine embryos in vitro and in vivo. Biol. Reprod., 59, 451–455. [DOI] [PubMed] [Google Scholar]
- Manes C. and Menzel,P. (1981) Demethylation of CpG sites in DNA of early rabbit trophoblast. Nature, 293, 589–590. [DOI] [PubMed] [Google Scholar]
- Mayer W., Niveleau,A., Walter,J., Fundele,R. and Haaf,T. (2000) Demethylation of the zygotic paternal genome. Nature, 403, 501–502. [DOI] [PubMed] [Google Scholar]
- Monk M., Boubelik,M. and Lehnert,S. (1987) Temporal and regional changes in DNA methylation in the embryonic, extraembryonic and germ cell lineages during mouse embryo development. Development, 99, 371–382. [DOI] [PubMed] [Google Scholar]
- Ohgane J., Wakayama,T., Kogo,Y., Senda,S., Hattori,N., Tanaka,S., Yanagimachi,R. and Shiota,K. (2001) DNA methylation variation in cloned mice. Genesis, 30, 45–50. [DOI] [PubMed] [Google Scholar]
- Okano M., Bell,D.W., Haber,D.A. and Li,E. (1999) DNA methyltransferases Dnmt3a and Dnmt3b are essential for de novo methylation and mammalian development. Cell, 99, 247–257. [DOI] [PubMed] [Google Scholar]
- Ono Y., Shimozawa,N., Ito,M. and Kono,T. (2001) Cloned mice from fetal fibroblast cells arrested at metaphase by a serial nuclear transfer. Biol. Reprod., 64, 44–50. [DOI] [PubMed] [Google Scholar]
- Oswald J., Engemann,S., Lane,N., Mayer,W., Olek,A., Fundele,R., Dean,W., Reik,W. and Walter,J. (2000) Active demethylation of the paternal genome in the mouse zygote. Curr. Biol., 10, 475–478. [DOI] [PubMed] [Google Scholar]
- Pampfer S., Wuu,Y.D., Vanderheyden,I. and De Hertogh,R. (1994) Expression of tumor necrosis factor-α (TNF α) receptors and selective effect of TNF α on the inner cell mass in mouse blastocysts. Endocrinology, 134, 206–212. [DOI] [PubMed] [Google Scholar]
- Razin A. and Cedar,H. (1994) DNA methylation and genomic imprinting. Cell, 77, 473–476. [DOI] [PubMed] [Google Scholar]
- Razin A. and Shemer,R. (1995) DNA methylation in early development. Hum. Mol. Genet., 4, 1751–1755. [DOI] [PubMed] [Google Scholar]
- Regenstreif L.J. and Rossant,J. (1989) Expression of the c-fms proto-oncogene and of the cytokine, CSF-1, during mouse embryogenesis. Dev. Biol., 133, 284–294. [DOI] [PubMed] [Google Scholar]
- Reik W. and Surani,A. (1997) Genomic Imprinting. IRL Press, Oxford, UK.
- Reik W., Romer,I., Barton,S.C., Surani,M.A., Howlett,S.K. and Klose,J. (1993) Adult phenotype in the mouse can be affected by epigenetic events in the early embryo. Development, 119, 933–942. [DOI] [PubMed] [Google Scholar]
- Rosenkrans C.F. Jr, Zeng,G.Q., McNamara,G.T., Schoff,P.K. and First,N.L. (1993) Development of bovine embryos in vitro as affected by energy substrates. Biol. Reprod., 49, 459–462. [DOI] [PubMed] [Google Scholar]
- Rossant J., Sanford,J.P., Chapman,V.M. and Andrews,G.K. (1986) Undermethylation of structural gene sequences in extraembryonic lineages of the mouse. Dev. Biol., 117, 567–573. [DOI] [PubMed] [Google Scholar]
- Rougier N., Bourc’his,D., Gomes,D.M., Niveleau,A., Plachot,M., Paldi,A. and Viegas-Pequignot,E. (1998) Chromosome methylation patterns during mammalian preimplantation development. Genes Dev., 12, 2108–2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapienza C., Paquette,J., Tran,T.H. and Peterson,A. (1989) Epigenetic and genetic factors affect transgene methylation imprinting. Development, 107, 165–168. [DOI] [PubMed] [Google Scholar]
- Schnieke A.E., Kind,A.J., Ritchie,W.A., Mycock,K., Scott,A.R., Ritchie,M., Wilmut,I., Colman,A. and Campbell,K.H. (1997) Human factor IX transgenic sheep produced by transfer of nuclei from transfected fetal fibroblasts. Science, 278, 2130–2133. [DOI] [PubMed] [Google Scholar]
- Solter D. (2000) Mammalian cloning: advances and limitations. Nature Rev. Genet., 1, 199–207. [DOI] [PubMed] [Google Scholar]
- Stoger R., Kubicka,P., Liu,C.G., Kafri,T., Razin,A., Cedar,H. and Barlow,D.P. (1993) Maternal-specific methylation of the imprinted mouse Igf2r locus identifies the expressed locus as carrying the imprinting signal. Cell, 73, 61–71. [DOI] [PubMed] [Google Scholar]
- Strickland S. and Richards,W.G. (1992) Invasion of the trophoblasts. Cell, 71, 355–357. [DOI] [PubMed] [Google Scholar]
- Tremblay K.D., Saam,J.R., Ingram,R.S., Tilghman,S.M. and Bartolomei,M.S. (1995) A paternal-specific methylation imprint marks the alleles of the mouse H19 gene. Nature Genet., 9, 407–413. [DOI] [PubMed] [Google Scholar]
- Tsunoda Y., Yasui,T., Nakamura,K., Uchida,T. and Sugie,T. (1986) Effect of cutting the zona pellucida on the pronuclear transplantation in the mouse. J. Exp. Zool., 240, 119–125. [DOI] [PubMed] [Google Scholar]
- Varmuza S., Prideaux,V., Kothary,R. and Rossant,J. (1988) Polytene chromosomes in mouse trophoblast giant cells. Development, 102, 127–134. [DOI] [PubMed] [Google Scholar]
- Wakayama T., Perry,A.C., Zuccotti,M., Johnson,K.R. and Yanagimachi,R. (1998) Full-term development of mice from enucleated oocytes injected with cumulus cell nuclei. Nature, 394, 369–374. [DOI] [PubMed] [Google Scholar]
- Warnecke P.M., Stirzaker,C., Melki,J.R., Millar,D.S., Paul,C.L. and Clark,S.J. (1997) Detection and measurement of PCR bias in quantitative methylation analysis of bisulphite-treated DNA. Nucleic Acids Res., 25, 4422–4426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warnecke P.M., Mann,J.R., Frommer,M. and Clark,S.J. (1998) Bisulfite sequencing in preimplantation embryos: DNA methylation profile of the upstream region of the mouse imprinted H19 gene. Genomics, 51, 182–190. [DOI] [PubMed] [Google Scholar]
- Wells D.N., Misica,P.M., Day,T.A. and Tervit,H.R. (1997) Production of cloned lambs from an established embryonic cell line: a comparison between in vivo- and in vitro-matured cytoplasts. Biol. Reprod., 57, 385–393. [DOI] [PubMed] [Google Scholar]
- Wilmut I., Schnieke,A.E., McWhir,J., Kind,A.J. and Campbell,K.H. (1997) Viable offspring derived from fetal and adult mammalian cells. Nature, 385, 810–813. [DOI] [PubMed] [Google Scholar]
- Young L.E. and Fairburn,H.R. (2000) Improving the safety of embryo technologies: possible role of genomic imprinting. Theriogenology, 53, 627–648. [DOI] [PubMed] [Google Scholar]
- Young L.E. et al. (2001) Epigenetic change in IGF2R is associated with fetal overgrowth after sheep embryo culture. Nature Genet., 27, 153–154. [DOI] [PubMed] [Google Scholar]
- Zuccotti M. and Monk,M. (1995) Methylation of the mouse Xist gene in sperm and eggs correlates with imprinted Xist expression and paternal X-inactivation. Nature Genet., 9, 316–320. [DOI] [PubMed] [Google Scholar]