Abstract
The protein p130 was isolated from rat brain as an inositol 1,4,5-trisphosphate-binding protein with a domain organization similar to that of phospholipase C-δ1 but lacking PLC activity. We show that p130 plays an important role in signaling by the type A receptor for γ-aminobutyric acid (GABA). Yeast twohybrid screening identified GABARAP (GABAA receptor-associated protein), which is proposed to contribute to the sorting, targeting or clustering of GABAA receptors, as a protein that interacts with p130. Furthermore, p130 competitively inhibited the binding of the γ2 subunit of the GABAA receptor to GABARAP in vitro. Electrophysiological analysis revealed that the modulation of GABA-induced Cl– current by Zn2+ or diazepam, both of which act at GABAA receptors containing γ subunits, is impaired in hippocampal neurons of p130 knockout mice. Moreover, behavioral analysis revealed that motor coordination was impaired and the intraperitoneal injection of diazepam induced markedly reduced sedative and antianxiety effects in the mutant mice. These results indicate that p130 is essential for the function of GABAA receptors, especially in response to the agents acting on a γ2 subunit.
Keywords: diazepam/GABA/knockout mice/p130
Introduction
d-myo-inositol 1,4,5-trisphosphate [Ins(1,4,5)P3], a product of receptor-induced hydrolysis of phosphatidylinositol 4,5-bisphosphate [PtdIns(4,5)P2] by phospholipase C (PLC), plays an important role as an intracellular second messenger by mobilizing Ca2+ from non-mitochondrial stores (Berridge, 1993). We previously isolated two Ins(1,4,5)P3-binding proteins with molecular masses of 130 and 85 kDa from rat brain (Kanematsu et al., 1992; Yoshida et al., 1994) with the use of an Ins(1,4,5)P3 affinity column (Hirata et al., 1985, 1990). Partial amino acid sequencing revealed that the 85 kDa molecule was PLC-δ1 (Kanematsu et al., 1992). The Ins(1,4,5)P3-binding protein with a molecular mass of 130 kDa, termed p130, was a previously unidentified molecule (Kanematsu et al., 1992; Yoshida et al., 1994). The predicted amino acid sequence of rat p130 shares 38.2% identity with that of rat PLC-δ1 (Kanematsu et al., 1996); the five identified domains of PLC-δ1 [pleckstrin homology (PH), EF-hand, putative catalytic (X and Y) and C2 domains] are all present in p130. The domain organization of p130 suggests that the protein is likely to possess a fold similar to that of PLC-δ1, a notion that is supported by the results of limited proteolysis with trypsin (Kanematsu et al., 2000). However, p130 exhibits some distinct characteristics. It is larger than the PLC-δ isozymes and it possesses unique regions both at the N-terminus, preceding the PH domain, and at the C-terminus. Moreover, the residues within the catalytic domain of PLC-δ that are critical for enzyme activity (His356 and Glu390) are not conserved in p130 (Kanematsu et al., 1996). The PH domain of p130, like that of PLC-δ1, is important for the binding of Ins(1,4,5)P3 (Takeuchi et al., 1996, 1997).
To investigate the physiological functions of p130, we previously examined the possible role of the binding of inositol compounds to the PH domain of p130 (Takeuchi et al., 1996, 1997; Hirata et al., 1998; Lemmon and Ferguson, 2000). Our results suggested that the high-affinity binding of Ins(1,4,5)P3 to the PH domain of p130 might serve to sequester Ins(1,4,5)P3 and therefore prevent its interaction with Ins(1,4,5)P3 receptors and metabolizing enzymes (Takeuchi et al., 2000).
We have now applied the yeast two-hybrid system to identify proteins that interact with p130. With the unique N-terminal region of p130 as the bait for screening a human brain cDNA library, we isolated two positive clones, one of which was shown to encode the catalytic subunit of protein phosphatase 1α (Yoshimura et al., 2001). Another clone was found to be GABARAP (GABAA receptor associated protein) that was identified as a molecule capable of binding the γ2 subunit of GABAA receptor and tubulin (Wang et al., 1999). In the present study, we studied the significance of the association of these two proteins in GABAA signaling in brain using p130–/– mice, and found that p130 is essential for the function of GABAA receptors that contain a γ2 subunit.
Results
Association between p130 and GABARAP and competition with GABAA receptor γ subunit
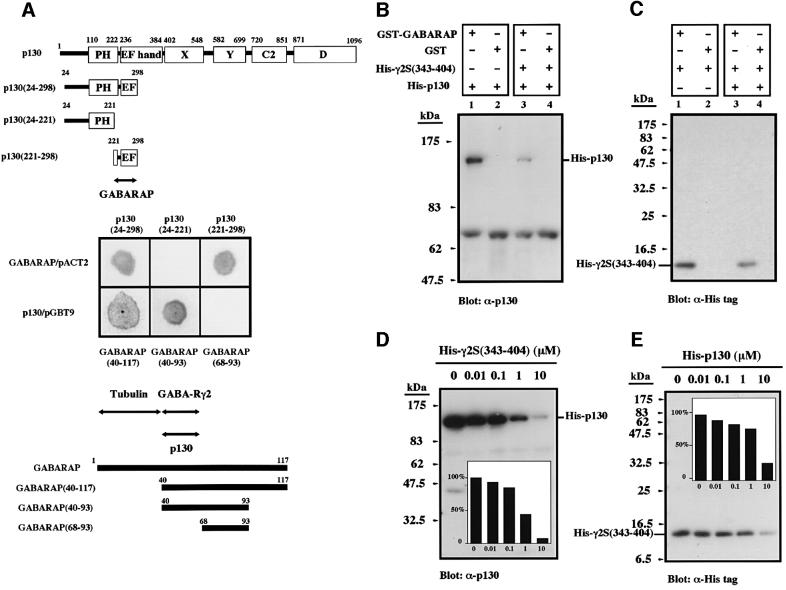
We screened a human brain cDNA library with the use of the yeast two-hybrid system. A total of 51 clones (out of 2 × 106 examined) were detected with a plasmid encoding residues 24 to 298 of rat p130 (which includes the PH domain and a portion of the EF hand motif) as the bait (Figure 1A). Ten of these clones were found to be false positives, and the remainder were divided into two groups on the basis of restriction endonuclease mapping. Sequence determination revealed that one of the clones encoded residues 40 to 117 of GABARAP; i.e. lacking the N-terminal 39 residues of the full-length protein (Wang et al., 1999). To identify the regions of these two proteins responsible for their interaction, we prepared several deletion constructs of p130 and GABARAP cDNAs in the yeast vectors pGBT9 and pACT2, respectively, and examined the association of the encoded proteins in the yeast two-hybrid assay (Figure 1A). Amino acids 24 to 298 and 221 to 298, but not residues 24 to 221, of p130 bound to GABARAP, and amino acids 40 to 93, but not residues 68 to 93, of GABARAP bound to p130. These results indicated that residues 40 to 67 of GABARAP interact with residues 221 to 298 of p130. This region of GABARAP (residues 40 to 67) has also been shown to be important for association with the intracellular loop of the γ2S subunit (Arg386 to Asp403) of the GABAA receptor (Wang et al., 1999; Wang and Olsen, 2000), suggesting that p130 might compete with the receptor γ2 subunit for binding to GABARAP.
Fig. 1. Interaction of GABARAP with p130 and the γ2 subunit of the GABAA receptor. (A) The interactions of various truncation mutants of rat p130 (top) and of human GABARAP (bottom) with full-length GABARAP and p130, respectively, were examined by yeast two-hybrid analysis (center). Putative binding regions of both proteins are indicated by arrows. (B and C) Precipitation assays. His6-p130 (B) or His6-γ2S(343–404) (C) at 1 µM was incubated with GST or GST–GABARAP in the absence or presence of His6-γ2S(343–404) or His6-p130 at 1 µM, respectively. The band at 69 kDa in (B) is a non-specific signal attributable to the bovine serum albumin present in the assay mixture. (D and E) Competition assays. GST–GABARAP (1 µM) was incubated either with His6-p130 (1 µM) and the indicated concentrations of His6-γ2S(343–404) (D), or with His6-γ2S(343–404) (1 µM) and the indicated concentrations of His6-p130 (E). Insets show the percentage inhibition of His6-p130 (D) and His6-γ2S(343–404) (E) binding, as determined by densitometry.
To investigate the interactions among p130, GABA RAP, and the γ2 subunit of the GABAA receptor in vitro, we performed precipitation assays with recombinant proteins. Histidine-tagged p130 (His6-p130) and a histidine-tagged fragment of γ2S [His6-γ2S(343–404)] each bound to a glutathione S-transferase (GST) fusion protein containing full-length GABARAP but not to GST alone (Figure 1B and C). Furthermore, the presence of His6-γ2S(343–404) inhibited the binding of His6-p130 to GABARAP and vice versa, thus indicating that p130 and γ2S(343–404) compete for binding to GABARAP. The mutual inhibition of the binding of each of these two proteins to GABARAP was dose dependent (Figure 1D and E); the binding of His6-γ2S(343–404) to GABARAP, however, was more resistant to inhibition, indicating that the affinity of GABARAP for the γ2 subunit might be greater than its affinity for p130. Given that GABARAP also contains a tubulin binding site within its N-terminal 36 residues (Wang et al., 1999; Kneussel et al., 2000; Wang and Olsen, 2000) (Figure 1A), we examined the interaction between GABARAP and tubulin with crude extracts of COS-1p130 cells, a stable cell line expressing p130 (Takeuchi et al., 2000); both p130 and tubulin were precipitated from cell extracts with GST–GABARAP immobilized to glutathione–Sepharose beads (data not shown).
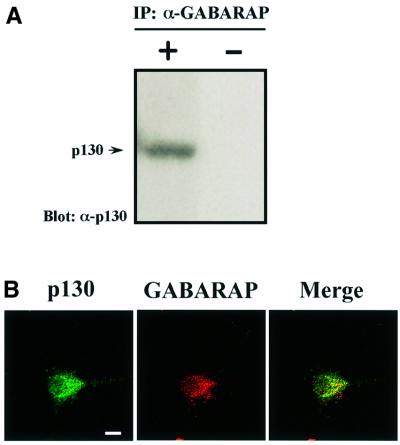
The association between p130 and GABARAP under more physiological conditions was also examined. Immunoprecipitation analysis with brain extract revealed that complex formation between p130 and GABARAP also occurs in a cellular environment (Figure 2A). The cellular localization of each of these two proteins was characterized previously (Takeuchi et al., 2000; Wang and Olsen, 2000). We now show that the majority of p130 and GABARAP co-localize as punctate structures inside cultured cortical neurons by immunofluorescence analysis, suggesting the interaction in vivo (Figure 2B). However, p130 does not appear to be enriched at inhibitory synaptic sites (data not shown). The abundance of p130 was previously shown to be highest in brain (Matsuda et al., 1998), and in situ hybridization revealed that p130 mRNA is localized in hippocampal pyramidal cells, in dentate granule cells and the pyramidal and granule cell layers of the cerebral cortex, and in the granular cell and Purkinje cell layers and cerebellar nuclei of the cerebellum (Matsuda et al., 1998). This regional distribution is similar to that of GABAA receptors (Rudolph et al., 1999). The results obtained here, together with those cited, indicated that p130 might contribute to regulation of GABAA receptor function through its association with GABARAP.
Fig. 2. Association of p130 with GABARAP in a cellular environment. (A) Immunoprecipitates (IP) prepared from mouse brain extract with antibodies to GABARAP (left lane), or in the absence of antibodies as a control (right lane), and protein G-conjugated beads were probed by immunoblot analysis with antibodies to p130. (B) Immunofluorescence localization of GABARAP (red) and p130 (green) in cultured cortical neurons using a confocal microscope. Bar: 10 µm.
Generation of p130 KO mice
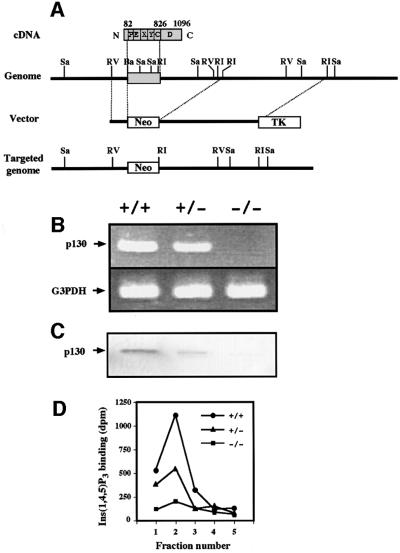
To examine the possible role of p130 interacting with GABARAP in regulation of GABAA receptor function, we generated p130 knockout mice by replacing a fragment of the protein-coding sequence (corresponding to residues 82 to 826 of rat p130) of the p130 gene in mouse embryonic stem cells with a neomycin resistance cassette by homologous recombination (Figure 3A). The success of gene targeting was confirmed by RT–PCR (Figure 3B) and immunoblot analysis (Figure 3C). Furthermore, assay of p130 function was performed by measurement of the binding of [3H]Ins(1,4,5)P3 to fractions of brain cytosol eluted from an Ins(1,4,5)P3 affinity column (Hirata et al., 1990; Kanematsu et al., 1992; Yoshida et al., 1994) (Figure 3D). Ins(1,4,5)P3 binding activity was virtually absent, and markedly reduced in the brains of p130–/– and p130+/– mice, respectively, compared with that in the brain of wild-type animals. The mice lacking p130 appeared to grow normally and were fertile.
Fig. 3. Targeted disruption of the mouse p130 gene. (A) Targeting strategy. The localization of introns and exons of the mouse p130 gene is incomplete, but the single exon that encodes at least the PH domain, EF hand, and X, Y, and C2 domains is indicated. Numbers correspond to amino acid residues of rat p130. Sa, SacI; RV, EcoRV; Ba, BamHI; RI, EcoRI; Neo, neomycin resistance gene; TK, thymidine kinase. (B) RT–PCR analysis of total RNA from the brains of p130+/+, p130+/– and p130–/– littermates with primers specific for p130 or glyceraldehyde-3-phosphate dehydrogenase (G3PDH). (C) Immunoblot analysis of cytosolic fractions of the brains of littermates with antibodies to p130 (2F9). (D) Ins(1,4,5)P3 binding assay. Brain cytosolic fractions were applied to an Ins(1,4,5)P3 affinity column, which was then washed with 0.5 M NaCl before elution with 2 M NaCl. Portions (10 µl) of eluted fractions (1 ml) were assayed for binding of [3H]Ins(1,4,5)P3, as described previously (Hirata et al., 1990; Kanematsu et al., 1992; Yoshida et al., 1994). Data are means of triplicates from an experiment that was repeated four times with similar results.
Electrophysiological analysis
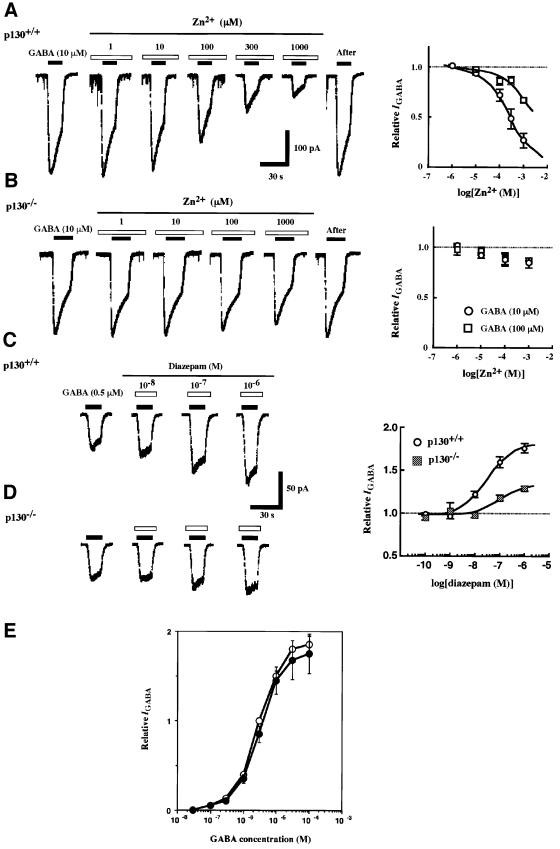
We first examined GABAA receptor-mediated Cl– current (IGABA) in nystatin-perforated patches of hippocampal CA1 cells freshly isolated from wild-type and p130 knockout mice (Figure 4). Although the current induced by 10 µM GABA was slightly smaller in cells from p130–/– mice than in those from control mice (Figure 4A and B), this difference was not statistically significant. The dose– response relationship was not changed, i.e. concentrations required for half-maximal IGABA were 2.8 and 2.9 µM for the control and mutant mice, respectively (Figure 4E). Moreover, the maximal GABA-induced currents in neurones derived from p130–/– and wild-type mice appeared to be similar (Figure 4E), suggesting no overt differences in functional cell surface-receptor number in the absence of p130.
Fig. 4. Impaired signaling by the γ subunit of GABAA receptors in p130–/– mice. (A and B) GABA-induced inward current (IGABA) in the presence of various concentrations of Zn2+ was measured in hippocampal CA1 cells from p130+/+ (A) and p130–/– (B) mice. The intervals of GABA (10 µM) and Zn2+ application are indicated by solid and open bars, respectively. Graphs on the right are the dose–response relations for the effect of Zn2+ on IGABA evoked by 10 or 100 µM GABA. Data are expressed relative to the current amplitude in the absence of Zn2+ and are means ± SEM of values from three to eight experiments. (C and D) Traces of IGABA induced by 0.5 µM GABA in CA1 cells from p130+/+ (C) and p130–/– (D) mice in the absence or presence of the indicated concentrations of diazepam. Graphs on the right are the dose–response relations for the effect of diazepam on IGABA induced by 0.5 µM GABA. Data are means ± SEM of values from six to nine experiments. (E) Concentration–response relationships of GABA-elicited postsynaptic currents in the wild-type (open circles) and knock-out (closed circles) hippocampal CA1 neurons. All currents were normalized to those elicited in the control neuron by 3 µM GABA. Data are means ± SEM of values from five experiments.
We then examined the effect of agents that are known to modulate the function of GABAA receptors containing the γ subunit. The effect of Zn2+ on the current evoked by 10 or 100 µM GABA was examined first. Low concentrations (∼1 µM) of Zn2+ inhibit IGABA mediated by receptors that do not contain the γ2 subunit, whereas higher concentrations of this cation are required for inhibition at receptors that do contain this subunit (Draguhn et al., 1990; Gingrich and Burkat, 1998). In neurons isolated from p130+/+ mice, Zn2+ reversibly blocked IGABA in a dose-dependent manner (Figure 4A); the concentration of Zn2+ required for half-maximal inhibition of the effect of 10 µM GABA was ∼210 µM, indicating that the receptors analyzed contained the γ2 subunit (Draguhn et al., 1990; Smart et al., 1991; Gingrich and Burkat, 1998). In contrast, Zn2+ had little effect on IGABA in the hippocampal neurons from p130–/– mice (Figure 4B) up to a concentration of 1 mM. To investigate further GABAA receptor signaling in p130–/– mice, we examined the effect of the benzodiazepine (BZ) agonist diazepam, a potent modulator of the receptors containing γ subunits (Günther et al., 1995), on IGABA in hippocampal CA1 neurons. Diazepam reversibly potentiated in a dose-dependent manner IGABA elicited by 0.5 µM GABA in neurons from p130+/+ mice (Figure 4C). In contrast, both the efficacy and potency of diazepam with regard to potentiation of IGABA were markedly reduced in neurons of p130–/– mice (Figure 4D).
These conflicting observations on Zn2+ modulation and diazepam responsiveness in the IGABA are therefore opposite to the results obtained from the study of recombinant receptors. In these systems, sensitivity to benzodiazepines is determined by the presence of a γ subunit in heteromeric αβγ receptors which are effectively antagonized by 50–100 µM Zn2+ (Smart et al., 1991), in contrast to the almost total insensitivity of IGABA in p130–/– neurons. To determine if these changes in the p130–/– mice are accompanied by changes in receptor subunit expression, we first examined the relative abundance of α/γ subunits (BZ binding sites) and α/β subunits (GABA binding sites) (Günther et al., 1995) in the GABAA receptors of p130+/+ and p130–/– mice, by measuring the binding of the BZ antagonist [3H]Ro15–1788 and the GABA antagonist [3H]SR95531 to intact hippocampal cells. Scatchard analysis of the binding data revealed no marked difference in the number of GABA binding sites or BZ binding sites between p130+/+ and p130–/– mice (data not shown). This observation is in keeping with the GABA dose–response analysis (Figure 4E) that indicates no overt loss of cell surface receptor number in the p130–/– mice. We next examined the relative abundance of receptor subunits by immunoblotting analysis with antibodies specific for α1, for β2 and β3, for γ1 and γ3, for γ2, or for δ subunits. No differences in the abundance of these subunits in both hippocampal and cortex cells were apparent between p130+/+ and p130–/– mice (data not shown). Thus, the expression of the major GABAA receptor subunits in hippocampal and cortex neurons does not appear to be affected by the lack of p130.
Given the predominantly intracellular localization of p130, this may suggest a key role for this protein in GABAA receptor assembly.
Behavioral analysis
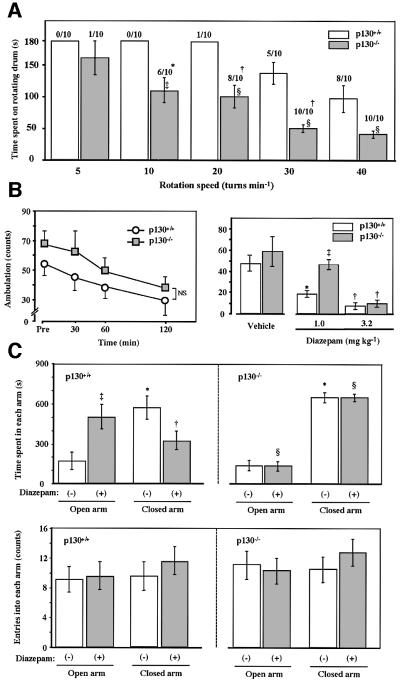
GABAA receptors are molecular substrates for the regulation of anxiety, as well as in sedation and motor coordination (Menard and Treit, 1999; Rudolph et al., 1999). We therefore compared the performance of p130–/– and wild-type mice in several behavioral tests. The rotarod test of motor coordination (Rudolph et al., 1999) revealed that such coordination was impaired in p130–/– mice, as reflected by both the incidence of falls and the time period before the first fall (Figure 5A). Hall’s open field test of motor activity (Rudolph et al., 1999) also revealed that the ambulation counts of p130–/– mice were consistently greater than those of control mice, although the difference was not statistically significant (Figure 5B). The intraperitoneal injection of diazepam at a dose of 1 mg/kg of body mass did not induce a sedative effect in the p130 knockout mice, whereas it was clearly observed with the control mice, which is consistent with the reduced responsiveness to diazepam in IGABA, but a higher dose of diazepam (3.2 mg/kg) induced sedation in mice of both genotypes (Figure 5B). Finally, we examined the effects of intraperitoneal injection of diazepam, whose anxiolytic actions are mediated by the γ subunit of GABAA receptors (Braestrup and Nielsen, 1980), on the behavior of p130+/+ and p130–/– mice in the elevated plus-maze test, which provides a measure of anxiety (Rudolph et al., 1999). No significant difference in the time spent in the open or closed arms of the maze was apparent between p130+/+ and p130–/– mice injected with vehicle. Injection of wild-type mice with diazepam (1 mg/kg) resulted in a marked increase in the time spent in the open arm, indicative of the anti-anxiety effect of this drug (Figure 5C). In contrast, the time spent by p130 knockout mice in the open or closed arms of the maze was not affected by injection of diazepam. The number of entries into the open or closed arms of the maze was not affected by diazepam administration in mice of either genotype, indicating that this dose of the drug did not impair spontaneous movements. However, injection of diazepam at a dose of 3.2 mg/kg severely reduced the spontaneous movements, similar to those seen in Hall’s open field test, in mice of either genotype (data not shown). Our electrophysiological and behavioral studies thus indicated that GABAA receptor signaling through the γ subunit is impaired in p130–/– mice.
Fig. 5. Behavioral analysis of p130 knockout mice. (A) Rotarod test. Ten mice each of the p130+/+ and p130–/– genotypes were examined for 3 min. The number of animals that fell from the rotating drum at the indicated speeds during the period of examination is shown at the top of each column. *p <0.05, †p <0.01, versus the corresponding value for wild-type mice. Times spent on the rotating drum are presented as means ± SEM for all mice examined. ‡p <0.05, §p <0.01 versus the corresponding value for wild-type mice. (B) Hall’s open field test. Mice were placed in the ambulation chamber equipped with an infrared beam to count the number of crosses over 3 min. The left panel shows the number of crosses during 3 min beginning at the indicated time after placing the animals in the chamber. The right panel shows the number of crosses over 3 min, beginning 30 min after the intraperitoneal administration of diazepam or vehicle (0.3% carboxymethylcellulose). Data are means ± SEM of values from five mice per genotype. *p <0.05, †p <0.01, versus the corresponding value for vehicle-treated mice; ‡p <0.01 versus the corresponding value for wild-type mice. (C) Elevated plus-maze test. The test was performed 30 min after the intraperitoneal administration of diazepam (1 mg/kg) (solid bars) or vehicle (open bars). Data are means ± SEM of the time spent in the open (upper panel), and the number of entries into the closed arms (lower panel) of the maze for seven p130+/+ mice and six p130–/– mice. *p <0.01, versus the corresponding value for the open arm; †p <0.05, ‡p <0.01, versus the corresponding value for vehicle-treated mice; §p <0.01, versus the corresponding value for wild-type mice.
Discussion
This study was initiated by the finding that p130 interacts with GABARAP in a competitive manner with the γ2 subunit of GABAA receptors. To examine the significance of this interaction, we established gene-targeted mice devoid of p130. Interestingly, we found that there was no dramatic differences in the amplitude of IGABA or GABA sensitivity in hippocampal neurons between the control and mutant mice. However, these mice showed impairments of GABAA receptor modulation by both Zn2+ modulation and benzodiazepines. Both of these pharmacological markers have been shown to be controlled by the presence of γ subunits in heteromeric α/β/γ receptors, as defined by recombinant and gene targeting studies (Günther et al., 1995; Gingrich and Burkat, 1998). Behavioral performance such as measured in ambulation and anxiety models showed no apparent difference in both genotypes of mice. Only the rotarod test showed a defect in the mutant mice, which might be caused by the accumulation of small (statistically insignificant) changes in IGABA at several sites required for motor coordination.
Further analysis of the p130–/– mice revealed that there were no changes in the total number of binding sites for benzodiazepines and also that total levels of the receptor α1, β2–3, γ1–3 and δ subunits remained unaltered. Moreover, receptor density in the membranes of both strains of mice appeared to be equivalent. In vivo, most GABAA receptors are believed to be pentamers of α/β/γ subunits in a suggested ratio of 2:2:1 (Tretter et al., 1997). Benzodiazepines are believed to act at the interface of α/γ subunits, while Zn2+ acts at sites within β subunits. Therefore, one plausible mechanism behind the pharmacological changes in p130–/– mice is that this protein plays a role in the assembly of heteromeric receptors, perhaps by controlling the number and type of subunits recruited into functional pentamers. A role for p130 in receptor assembly was first indicated by the results of our yeast two-hybrid screen in which we isolated GABARAP. This protein also binds the γ2 subunit of GABAA receptors, microtubules and N-ethylmaleimide-sensitive factor (NSF) (Wang et al., 1999; Wang and Olsen, 2000; Kittler et al., 2001). GABARAP is homologous to GATE-16, which has been demonstrated to facilitate vesicular transport within the trans-Golgi network. Like GATE-16, recent high resolution studies have revealed that GABARAP is enriched on intracellular membranes, including the trans-Golgi network (Kittler et al., 2001). The predominant intracellular localization of p130 and its co-localization with GABARAP suggests that p130 may participate in GABAA receptor membrane trafficking or receptor assembly. For instance, the competitive binding of p130 and GABARAP for the receptor γ2 subunit may ensure that only mature α/β/γ receptor pentamers are transported to the cell surface. Therefore, p130 may play a central role in controlling the number and/or types of γ2 subunit that assemble into functional α/β/γ receptors, with consequent effects on receptor pharmacology. Alternatively, if GABARAP participates in the endocytic pathway as suggested for its homolog in yeast (Okazaki et al., 2000), proteolysis of incorrectly folded γ2 subunits by such organelles might be enhanced by p130. Clearly more experimental evidence is required to examine the role of p130 in GABAA receptor biosynthesis and membrane transport.
Our yeast two-hybrid analysis also revealed that p130 interacts with the catalytic subunit of protein phosphatase 1 (Yoshimura et al., 2001). Oligomeric receptor-associated ion channels in neurons are regulated by phosphorylation and dephosphorylation of various subunits (Swope et al., 1999); this is indeed the case for the GABAA receptor (Brandon et al., 2000; Moss and Smart, 2001). Receptor phosphorylation or dephosphorylation requires that the relevant protein kinase or phosphatase be targeted to the receptor. For example, Yotiao (Westphal et al., 1999) and spinophilin (neurabin-II) (Yan et al., 1999) are thought to mediate the targeting of PP1 to NMDA (N-methyl-D-aspartate)- and AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid)-sensitive glutamate receptors, respectively. It is thus possible that p130 performs an analogous function for GABAA receptors.
The human p130 gene has been identified and termed PLC-L (Kohno et al., 1995), and genes (∼70%) homologous to PLC-L have been isolated from mouse and human and termed PLC-L2 (Kikuno et al., 1999; Otsuki et al., 1999). Like rat p130 (Kanematsu et al., 1992; Yoshida et al., 1994), the native or recombinant proteins encoded by these genes do not exhibit PLC activity (Kohno et al., 1995; Kikuno et al., 1999; Otsuki et al., 1999). The presence of a similar gene in Caenorhabditis elegans suggests that this PLC-related, catalytically-inactive protein (here designated PRIP) family diverged from other PLC families early during evolution (Koyanagi et al., 1998). The PRIP family consists of at least two types of protein: type 1 is represented by p130 (Kanematsu et al., 1992; Yoshida et al., 1994; Kohno et al., 1995) and type 2 by PLC-L2 (Kikuno et al., 1999; Otsuki et al., 1999). It remains to be determined whether the type 2 proteins are also able to bind GABARAP and the catalytic subunit of protein phosphatase 1.
Finally, our studies have identified a novel role for p130 in the functional expression of GABAA receptors. The mechanism behind this remains to be determined, but as p130 is found in intracellular compartments, this strongly suggests that this protein participates in receptor assembly and transport of mature receptors to the plasma membrane.
Materials and methods
Yeast two-hybrid analysis
The XhoI–SmaI fragment (nucleotides 535–1359) of plasmid pcMT3, which contains the rat p130 cDNA (Kanematsu et al., 1996), was introduced into the yeast vector pGBT9 (SalI and blunt-ended PstI sites) to yield the bait plasmid encoding p130(24–298). Yeast cells were co-transformed with the bait plasmid and a human brain cDNA library in pACT2 (Clontech). Positive clones whose products interacted with p130 in both histidine and β-galactosidase assays were identified.
In vitro precipitation assay
The cDNAs for full-length GABARAP and the intracellular loop (residues 343–404) of the γ2S subunit of the GABAA receptor were amplified by RT–PCR with total RNA from human brain as template. The resulting GABARAP and γ2S subunit cDNAs were introduced into the BamHI–EcoRI sites of pGEX-2T (Pharmacia) and the Ecl136II–SalI sites of pETHis6-30 [pET11 (Novagen) with an added sequence for a histidine tag], respectively. The recombinant proteins were expressed in Escherichia coli and purified by affinity chromatography. Recombinant His6-p130 was prepared with a baculovirus expression system (Kanematsu et al., 2000; Takeuchi et al., 2000). Incubation of p130 or γ2S recombinant proteins with GST–GABARAP or GST alone for 1 h at 4°C was followed by the addition of glutathione–Sepharose 4B (Amersham Pharmacia Biotech.). Proteins that bound to the beads were analyzed by SDS–PAGE, followed by an immunoblot analysis with a mouse monoclonal antibody to p130 (2F9) (Yoshida et al., 1994), or antibodies to the His6 tag (Qiagen).
Immunofluorescence analysis
Cortical neurons isolated from embryonic day 18 embryo were maintained for 21 days in medium that inhibits the growth of glia. The cultured cells were fixed with 4% paraformaldehyde, permeabilized with 0.2% Triton X-100, and incubated first with 10% horse serum and then with goat antibody to GABARAP (Santa Cruz Biotech), and rabbit polyclonal antibody to p130. The cells were then exposed to Texas Red-conjugated donkey antibodies (red) to goat immunoglobulin and FITC-conjugated donkey antibodies (green) to rabbit immunoglobulin, and then viewed with a confocal microscope.
Generation of p130 knockout mice
Mouse p130 genomic DNA was isolated from a 129/Sv DNA library (Stratagene) by screening with the XhoI–SacI fragment of pcMT3 (Kanematsu et al., 1992). The targeting vector was constructed by inserting a blunt-ended EcoRV–BamHI fragment (1.1 kb) and an EcoRI–EcoRI fragment (6.3 kb) of the mouse p130 gene into the blunt-ended HindIII site and EcoRI site, respectively, of PGKNeo/PGKTK pBluescriptII (Stratagene). Four positive clones (out of a total of 288 clones analyzed) were obtained, and two of these clones (EW3 and EW17) were injected into C57/BL6 blastocysts. Chimeric males derived from each clone were crossed with C57/BL6 females. The resulting heterozygous mice were intercrossed to obtain wild-type (p130+/+), heterozygous mutant (p130+/–), and homozygous mutant (p130–/–) mice. Animal experiments were reviewed by the Committee of the Ethics on Animal Experiments in the Faculty of Medical Science, Kyushu University and carried out under the control of the Guideline for Animal Experiments in the Faculty of Medical Science, Kyushu University and The Law (No. 105) and Notification (No. 6) of the Government.
Electrophysiological analysis
Hippocampal CA1 cells were dissociated without the use of enzymes from the brains of p130+/+ and p130–/– 10- to 14-day-old mice. Electrophysiological measurements were performed with nystatin-perforated membrane patches at a holding potential of –60 mV under voltage-clamp conditions, as described previously (Rhee et al., 1999).
Behavioral analysis
The rotarod test, Hall’s open field test, and the elevated plus-maze test were performed to provide measures of motor coordination, sedation and anxiolytic activity, respectively (Rudolph et al., 1999). Mice (p130+/+ and p130–/–) were subjected to the tests at 8 to 11 weeks of age. Statistical analysis was performed using the Mann–Whitney U test.
Acknowledgments
Acknowledgements
We thank Drs S.Hatakeyama and A.Kato (Kyushu University) for providing the yeast expression system and helping us with neuron culture, respectively. Thanks are also due to Ms Y.Yamada and K.Shimoharada for technical assistance. This work was supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Science, Sports and Culture of Japan (to T.K., H.T. and M.H.) as well as by the Kyushu University Interdisciplinary Programs in Education and Projects in Research Development (to N.A. and M.H.), The Fugaku Trust for Medicinal Research (to M.H.), The Brain Research Foundation (to M.H.), The Kowa Life Science Foundation (to T.K.), The Inamori Foundation (to T.K.) and The Uehara Memorial Foundation (to H.T.).
References
- Berridge M.J. (1993) Inositol trisphosphate and calcium signaling. Nature, 361, 315–325. [DOI] [PubMed] [Google Scholar]
- Braestrup C. and Nielsen,M. (1980) Benzodiazepine receptors. Arzneimittelforschung, 30, 852–857. [PubMed] [Google Scholar]
- Brandon N.J., Delmas,P., Kittler,J.T., McDonald,B.J., Sieghart,W., Brown,D.A., Smart,T.G. and Moss,S.J. (2000) GABAA receptor phosphorylation and functional modulation in cortical neurons by a protein kinase C-dependent pathway. J. Biol. Chem., 275, 38856–38862. [DOI] [PubMed] [Google Scholar]
- Draguhn A., Verdorn,T.A., Ewert,M., Seeburg,P.H. and Sakmann,B. (1990) Functional and molecular distinction between recombinant rat GABAA receptor subtypes by Zn2+. Neuron, 5, 781–788. [DOI] [PubMed] [Google Scholar]
- Gingrich K.J. and Burkat,P.M. (1998) Zn2+ inhibition of recombinant GABAA receptors: an allosteric, state-dependent mechanism determined by the γ-subunit. J. Physiol., 506, 609–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Günther U. et al. (1995) Benzodiazepine-insensitive mice generated by targeted disruption of the γ2 subunit gene of γ-aminobutyric acid type A receptors. Proc. Natl Acad. Sci. USA, 92, 7749–7753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata M., Sasaguri,T., Hamachi,T., Hashimoto,T., Kukita,M. and Koga,T. (1985) Irreversible inhibition of Ca2+ release in saponin-treated macrophages by the photoaffinity derivative of inositol 1,4,5-trisphosphate. Nature, 317, 723–725. [DOI] [PubMed] [Google Scholar]
- Hirata M., Watanabe,Y., Ishimatsu,T., Yanaga,F., Koga,T. and Ozaki,S. (1990) Inositol 1,4,5-trisphosphate affinity chromatography. Biochem. Biophys. Res. Commun., 168, 379–386. [DOI] [PubMed] [Google Scholar]
- Hirata M., Kanematsu,T., Takeuchi,H. and Yagisawa,H. (1998) Pleckstrin homology domain as an inositol compound binding module. Jpn. J. Pharmacol., 76, 255–263. [DOI] [PubMed] [Google Scholar]
- Kanematsu T., Takeya,H., Watanabe,Y., Ozaki,S., Yoshida,M., Koga,T., Iwanaga,S. and Hirata,M. (1992) Putative inositol 1,4,5-trisphosphate binding proteins in rat brain cytosol. J. Biol. Chem., 267, 6518–6525. [PubMed] [Google Scholar]
- Kanematsu T., Misumi,Y., Watanabe,Y., Ozaki,S., Koga,T., Iwanaga,S., Ikehara,Y. and Hirata,M. (1996) A new inositol 1,4,5-trisphosphate binding protein similar to phospholipase C-δ1. Biochem. J., 313, 319–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanematsu T., Yoshimura,K., Hidaka,K., Takeuchi,H., Katan,M. and Hirata,M. (2000) Domain organization of p130, PLC-related catalytically inactive protein, and structural basis for the lack of enzyme activity. Eur. J. Biochem., 267, 2731–2737. [DOI] [PubMed] [Google Scholar]
- Kikuno R., Nagase,T., Ishikawa,K., Hirosawa,M., Miyajima,N., Tanaka,A., Kotani,H., Nomura,N. and Ohara,O. (1999) Prediction of the coding sequences of unidentified human genes. XIV. The complete sequences of 100 new cDNA clones from brain which code for large proteins in vitro. DNA Res., 6, 197–205. [DOI] [PubMed] [Google Scholar]
- Kittler J.T., Rostaing,P., Schiavo,G., Fritschy,J.-M., Olsen,R., Triller,A. and Moss,S.J. (2001) The subcellular distribution of GABARAP and its ability to interact with NSF suggest a role for this protein in the intracellular transport of GABAA receptors. Mol. Cell. Neurosci., 18, 13–25. [DOI] [PubMed] [Google Scholar]
- Kneussel M., Haverkamp,S., Fuhrmann,J.C., Wang,H., Wässle,H., Olsen,R.W. and Betz,H. (2000) The γ-aminobutyric acid type A receptor (GABAAR)-associated protein GABARAP interacts with gephyrin but is not involved in receptor anchoring at the synapse. Proc. Natl Acad. Sci. USA, 97, 8594–8599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohno T., Otsuka,T., Takano,H., Yamamoto,T., Hamaguchi,M., Terada,M. and Yokota,J. (1995) Identification of a novel phospholipase C family gene at chromosome 2q33 that is homozygously deleted in human small cell lung carcinoma. Hum. Mol. Genet., 4, 667–674. [DOI] [PubMed] [Google Scholar]
- Koyanagi M., Ono,K., Suga,H., Iwabe,N. and Miyata,T. (1998) Phospholipase C cDNAs from sponge and hydra: antiquity of genes involved in the inositol phospholipid signaling pathway. FEBS Lett., 439, 66–70. [DOI] [PubMed] [Google Scholar]
- Lemmon M.A. and Ferguson,K.M. (2000) Signal-dependent membrane targeting by pleckstrin homology (PH) domains. Biochem. J., 350, 1–18. [PMC free article] [PubMed] [Google Scholar]
- Matsuda M., Kanematsu,T., Takeuchi,H., Kukita,T. and Hirata,M. (1998) Localization of a novel inositol 1,4,5-trisphosphate binding protein, p130 in rat brain. Neurosci. Lett., 257, 97–100. [DOI] [PubMed] [Google Scholar]
- Menard J. and Treit,D. (1999) Effects of centrally administered anxiolytic compounds in animal models of anxiety. Neurosci. Biobehav. Rev., 23, 591–613. [DOI] [PubMed] [Google Scholar]
- Moss S.J. and Smart,T.G. (2001) Constructing inhibitory synapses. Nature Rev. Neurosci., 2, 240–250. [DOI] [PubMed] [Google Scholar]
- Okazaki N., Yan,J., Yuasa,S., Ueno,T., Kominami,E., Masuho,Y., Koga,H. and Muramatsu,M. (2000) Interaction of the Unc-51-like kinase and microtubule-associated protein light chain 3 related proteins in the brain. Mol. Brain Res., 85, 1–12. [DOI] [PubMed] [Google Scholar]
- Otsuki M., Fukami,K., Kohno,T., Yokota,J. and Takenawa,T. (1999) Identification and characterization of a new phospholipase C-like protein, PLC-L2. Biochem. Biophys. Res. Commun., 266, 97–103. [DOI] [PubMed] [Google Scholar]
- Rhee J.S., Ishibashi,H. and Akaike,N. (1999) Calcium channels in the GABAergic presynaptic nerve terminals projecting to meynert neurons of the rat. J. Neurochem., 72, 800–807. [DOI] [PubMed] [Google Scholar]
- Rudolph U., Crestani,F., Benke,D., Brünig,I., Benson,J.A., Fritschy,J.-M., Martin,J.R., Bluethmann,H. and Möhler,H. (1999) Benzo diazepine actions mediated by specific γ-aminobutyric acidA receptor subtypes. Nature, 401, 796–800. [DOI] [PubMed] [Google Scholar]
- Smart T.G., Moss,S.J., Xie,X. and Huganir,R.L. (1991) GABAA receptors are differentially sensitive to zinc—Dependence on subunit composition. Br. J. Pharmacol., 103, 1837–1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swope S.L., Moss,S.J., Raymond,L.A. and Huganir,R.L. (1999) Regulation of ligand-gated ion channels by protein phosphorylation. Adv. Second Messenger Phosphoprotein Res., 33, 49–78. [DOI] [PubMed] [Google Scholar]
- Takeuchi H. et al. (1996) Localization of a high affinity inositol 1,4,5-trisphosphate/inositol 1,4,5,6-tetrakisphosphate binding domain to the pleckstrin homology module of a new 130-kDa protein: characterization of the determinants of structural specificity. Biochem. J., 318, 561–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi H., Kanematsu,T., Misumi,Y., Sakane,F., Konishi,H., Kikkawa,U., Watanabe,Y., Katan,M. and Hirata,M. (1997) Distinct specificity in the binding of inositol phosphates by pleckstrin homology domains of pleckstrin, RAC-protein kinase, diacylglycerol kinase and a new 130-kDa protein. Biochim. Biophys. Acta, 1359, 275–285. [DOI] [PubMed] [Google Scholar]
- Takeuchi H., Oike,M., Paterson,H.F., Allen,V., Kanematsu,T., Ito,Y., Erneux,C., Katan,M. and Hirata,M. (2000) Inhibition of calcium signalling by p130, PLC-related catalytically inactive protein: critical role of the p130PH domain. Biochem. J., 349, 357–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tretter V., Ehya,N., Fuchs,K. and Sieghart,W. (1997) Stoichiometry and assembly of a recombinant GABAA receptor subtype. J. Neurosci., 17, 2728–2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Bedford,F.K., Brandon,N.J., Moss,S.J. and Olsen,R.W. (1999) GABAA-receptor-associated protein links GABAA receptors and the cytoskeleton. Nature, 397, 69–72. [DOI] [PubMed] [Google Scholar]
- Wang H. and Olsen,R.W. (2000) Binding of the GABAA receptor-associated protein (GABARAP) to microtubules and microfilaments suggests involvement of the cytoskeleton in GABARAP-GABAA receptor interaction. J. Neurochem., 75, 644–655. [DOI] [PubMed] [Google Scholar]
- Westphal R.S., Tavalin,S.J., Lin,J.W., Alto,N.M., Fraser,I.D.C., Langeberg,L.K., Sheng,M. and Scott,J.D. (1999) Regulation of NMDA receptors by an associated phosphatase-kinase signaling complex. Science, 285, 93–96. [DOI] [PubMed] [Google Scholar]
- Yan Z., Hsieh-Wilson,L., Feng,J., Tominaga,K., Allen,P.B., Fienberg,A.A., Nairn,A. and Greemgard,P. (1999) Protein phosphatase 1 modulation of neostriatal AMPA channels: regulation by DARPP-32 and spinophilin. Nature Neurosci., 2, 13–17. [DOI] [PubMed] [Google Scholar]
- Yoshida M., Kanematsu,T., Watanabe,Y., Koga,T., Ozaki,S., Iwanaga,S. and Hirata,M. (1994) d-myo-Inositol 1,4,5-trisphosphate binding proteins in rat brain membranes. J. Biochem. (Tokyo), 115, 973–980. [DOI] [PubMed] [Google Scholar]
- Yoshimura K. et al. (2001) Interaction of p130 with, and consequent inhibition of, the catalytic subunit of protein phosphatase 1α. J. Biol. Chem., 276, 17908–17913. [DOI] [PubMed] [Google Scholar]