Abstract
CCAAT/enhancer binding protein alpha (C/EBPα) causes growth arrest via direct interaction with the cyclin-dependent kinases cdk2 and cdk4. In this paper, we present evidence showing that C/EBPα enhances a proteasome-dependent degradation of cdk4 during growth arrest in liver of newborn mice and in cultured cells. Overexpression of C/EBPα in several biological systems leads to a reduction of cdk4 protein levels, but not mRNA levels. Experiments with several tissue culture models reveal that C/EBPα enhances the formation of cdk4–ubiquitin conjugates and induces degradation of cdk4 through a proteasome-dependent pathway. As a result, the half-life of cdk4 is shorter and protein levels of cdk4 are reduced in cells expressing C/EBPα. Gel filtration analysis of cdk4 complexes shows that a chaperone complex cdk4–cdc37–Hsp90, which protects cdk4 from degradation, is abundant in proliferating livers that lack C/EBPα, but this complex is weak or undetectable in livers expressing C/EBPα. Our studies show that C/EBPα disrupts the cdk4–cdc37–Hsp90 complex via direct interaction with cdk4 and reduces protein levels of cdk4 by increasing proteasome-dependent degradation of cdk4.
Keywords: C/EBPα/cdk4/cell cycle/proteasome
Introduction
Tissue-specific transcription factors regulate a number of biological processes including cell growth and differentiation. The molecular mechanisms by which these transcription factors control cell proliferation are not well understood. Several recent observations present evidence that certain tissue-specific transcription factors, such as MyoD and C/EBPα, bring about growth arrest through their interaction with and inhibition of cyclin-dependent kinases (Zhang et al., 1999; Wang et al., 2001). C/EBPα belongs to a family of transcription factors that regulate a variety of biological processes (Darlington et al., 1998; Lekstrom-Himes and Xanthopoulos 1998; Poli, 1998). C/EBPα is highly expressed in fully differentiated tissues: quiescent liver and adipose tissues (Birkenmeier et al., 1989; Cao et al., 1991). Among other members of the C/EBP family, C/EBPα has been characterized as a very strong inhibitor of cell proliferation (Flodby et al., 1996; Hendricks-Taylor and Darlington, 1995; Diehl et al., 1996; Timchenko et al., 1996, 1997; Wang et al., 2001). Although C/EBPα is a transcription factor, its DNA binding activity is not required for growth arrest (Müller et al., 1999; Wang et al., 2001). A number of publications have shown that C/EBPα is capable of interacting with several proteins that are involved in the regulation of cell cycle progression. We and other investigators have previously shown that C/EBPα-mediated growth arrest is accompanied by increased expression of a cell cycle inhibitor, p21 (Timchenko et al., 1996; Cha et al., 1998), and that C/EBPα interacts with p21 and stabilizes p21 protein (Timchenko et al., 1997). However, further studies demonstrated that p21 plays a dual role in the regulation of cyclin-dependent kinases (Deng et al., 1995; Sherr and Roberts 1999), and that p21 is not crucial for C/EBPα-mediated inhibition of cell proliferation (Müller et al., 1999; Wang et al., 2001). It has also been shown that C/EBPα interacts with Rb and p107 and is able to affect E2F binding through the regulation of E2F–Rb complexes (Chen et al., 1996; Timchenko et al., 1999). Taken together, these observations suggested multiple pathways by which C/EBPα can potentially affect cell-cycle progression.
Progression through the cell cycle is driven by cyclin-dependent kinases whose activity is regulated by cyclins. Two major kinases, cdk2 and cdk4, are activated during G1, resulting in the release of Rb-dependent repression of E2F transcription and progression through S phase (Dyson 1998; Sherr and Roberts, 1999). The activity of cdk2 and cdk4 kinases is positively modulated during the cell cycle by association with cyclins E and A for cdk2, and cyclin D for cdk4. The negative regulation of cdk2 and cdk4 involves the association of cyclin-dependent kinases with specific inhibitors (CDKIs). CIP/KIP members include p21, p27 and p57, while cdk4 activity is inhibited by both CIP/KIP and ink4 inhibitors (p16, p18 and p19). Initially, p21 and p27 have been identified as strong inhibitors of cdk2 and cdk4; however, later studies demonstrated that p21 also plays a positive role in the assembly of active cdk2 and cdk4 complexes with cyclins (Sherr and Roberts, 1999). Although CIP/KIP and INK families are the major negative regulators of cdks, several recent observations present evidence that certain transcription factors also inhibit cdk activity through direct interaction. Zhang et al. have shown that a muscle-specific transcription factor, MyoD, is able to interact with cdk4 and bring about growth arrest in cultured myocytes as a result (Zhang et al., 1999). The authors also showed that transcriptional activity of MyoD is not required for the interaction with cdk4 and for inhibition of cell proliferation. This finding suggested that direct inhibition of cdks might be a common pathway for certain transcription factors to inhibit cell growth. In agreement with this suggestion, we found that C/EBPα is also capable of interacting with cdk2 and cdk4, and that this interaction is required for C/EBPα-mediated growth arrest in cultured cells (Wang et al., 2001).
Although C/EBPα activates promoters of a number of genes, data in this paper demonstrate a new function of C/EBPα: regulation of the protein expression via direct protein–protein interactions. In this paper, we determined the molecular pathway by which C/EBPα reduces protein levels of cdk4 during growth arrest in liver and in cultured cells. Using several biological models, we found that cdk4 is degraded through a proteasome-dependent pathway. Expression of C/EBPα enhances the formation of cdk4– ubiquitin conjugates and reduces the half-life of cdk4 protein. In vitro experiments suggest that C/EBPα triggers degradation of cdk4 through the disruption of a chaperone complex, cdk4–cdc37–Hsp90, which protects cdk4 from degradation.
Results
Protein levels of cdk4 are induced in livers lacking C/EBPα
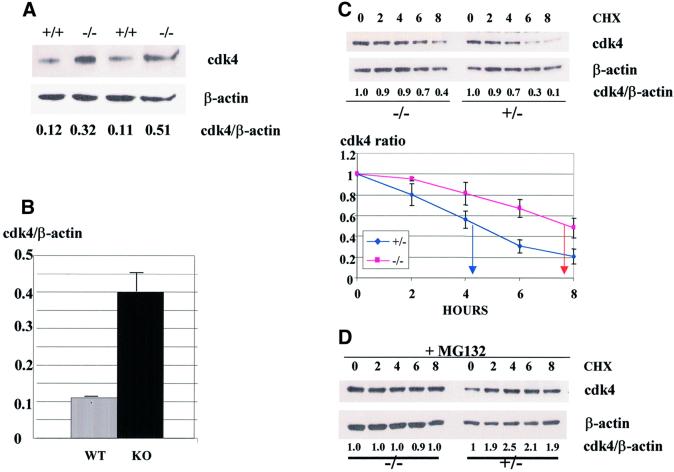
We have recently found that C/EBPα interacts with two key kinases that drive cell-cycle progression, cdk2 and cdk4, and inhibits their activities through direct interaction (Wang et al., 2001). A detailed analysis of cdk4 in livers of wild-type and C/EBPα-knockout animals indicated a complex regulation of cdk4 by C/EBPα. In addition to the direct inhibition of cdk4 activity, C/EBPα also controls protein levels of cdk4. Figure 1A shows western blotting analysis of cdk4 expression in nuclear extracts isolated from newborn livers. As can be seen, protein levels of cdk4 are elevated in livers of C/EBPα-knockout mice. Estimation of cdk4 levels as a ratio to β-actin revealed a 5- to 6-fold induction of cdk4 in nuclear extracts from C/EBPα-knockout animals. We next determined whether the difference in nuclear concentrations of cdk4 might reflect a possible difference in intracellular distribution of cdk4. Determination of cdk4 in cytoplasmic fractions of the same animals showed that, similar to nuclear extracts, protein levels of cdk4 are also induced in the cytoplasm of livers that lack C/EBPα (Figure 1A). Under the conditions of our western blot assay the variability of cdk4 levels in each genotype was within 1.6, expressed as a ratio to β-actin. On the other hand, 5- to 6-fold elevation of cdk4 protein was reproducibly observed in the nuclear extracts of all C/EBPα-knockout livers examined in these studies. To rule out a possible non-specific degradation of cdk4 during protein isolation, we performed a number of mixing experiments. Nuclear extracts from knockout livers were incubated with increasing amounts of nuclear proteins from wild-type livers, and cdk4 was determined by western blotting. No degradation of cdk4 was observed under these conditions (data not shown), indicating that the reduction of cdk4 in wild-type livers is not due to non-specific degradation, which can potentially take place during isolation of the protein. These data suggested that protein levels of cdk4 in liver are down-regulated by C/EBPα through a specific pathway, and prompted us to investigate the pathway by which C/EBPα reduces cdk4 protein levels. Since C/EBPα is a transcription factor, we first examined whether C/EBPα regulates expression of cdk4 mRNA. Northern blot analysis of cdk4 mRNA shows that levels of cdk4 mRNA do not differ significantly in wild-type and in C/EBPα-knockout livers (Figure 1B). Although there are significant (2-fold) variations in cdk4 mRNA levels between animals of the same genotype, normalization of cdk4 mRNA levels to the signals of 18S rRNA reveals that cdk4 mRNA levels are not affected by the deletion of the C/EBPα gene. Thus, these studies demonstrate that C/EBPα reduces protein levels of cdk4 in the liver of newborn mice and that C/EBPα does not affect cdk4 mRNA.
Fig. 1. Protein levels of cdk4 are induced in tissues of C/EBPα-knockout mice. (A) Western analysis of cdk4 expression in liver. Nuclear extracts or cytoplasm from newborn wild-type (WT), heterozygous (H) or C/EBPα-knockout (KO) livers were loaded on a denaturing gel and probed with antibodies to cdk4. The membranes were stripped and re-probed with antibodies to β-actin to verify protein loading. C, control extract containing high levels of cdk4. Protein levels of cdk4 were calculated as their ratio to β-actin. Summaries of three to five experiments are shown below as bar graphs. (B) Northern analysis of cdk4 mRNA in livers from wild-type, heterozygous and C/EBPα-knockout littermates. Results from two animals of each genotype are shown. The membrane was re-probed with 18S rRNA probe. The intensity of signals was determined using phosphoimaging and the levels of cdk4 mRNA were calculated as a ratio to 18S rRNA. The bar graph show a summary of data with five animals per genotype. (C) Expression of cdk4 in brown fat (BF), lung and kidney tissue. Western blotting with nuclear extracts was performed as described in Materials and methods. Results with two or three animals of each genotype are shown. The table shows the induction of cdk4 protein in tissues from C/EBPα-knockout animals as ratio to the level of cdk4 in wild-type animals. Expression of C/EBPα in these tissues is shown based on published observations (Birkenmeier et al., 1989).
C/EBPα also regulates cdk4 protein levels in brown fat and lung tissue
In addition to liver, C/EBPα is also highly expressed in brown fat and lung tissues (Birkenmeier et al., 1989). To examine whether the lack of C/EBPα in these tissues affects cdk4 protein levels, cdk4 levels were examined in the brown fat, lung and kidney (C/EBPα is not expressed) of C/EBPα-knockout and wild-type littermates. Western blot analyses of nuclear extracts from three animals of each genotype are shown in Figure 1C. Normalization of cdk4 levels to the β-actin loading control shows that cdk4 levels are induced ∼4-fold in brown fat, and increased ∼3-fold in lung tissue of C/EBPα-knockout animals. In kidney, however, no induction of cdk4 protein was detected. In cytoplasmic extracts of these tissues, cdk4 levels did not significantly differ between genotypes (Figure 1C, table). This tissue-specific pattern of cdk4 induction in C/EBPα-knockout animals is in agreement with the pattern of expression of C/EBPα and with the biological role of C/EBPα in these tissues (Birkenmeier et al., 1989; Wang et al., 1995). Since the levels of cdk4 induction in tissues of C/EBPα-knockout mice correlate with the levels of C/EBPα in corresponding tissues of wild-type animals, these observations suggest that C/EBPα (either directly or indirectly) reduces protein levels of cdk4 in tissues where C/EBPα is expressed. Therefore, we investigated molecular mechanisms by which C/EBPα reduces cdk4 protein.
Half-life of cdk4 is increased in primary hepatocytes that lack C/EBPα
Newborn liver represents a mixed composition of different cell types. Therefore, we next examined whether C/EBPα-dependent regulation of cdk4 occurs in hepatocytes. To determine this, primary hepatocytes were derived from wild-type and from C/EBPα-knockout newborn livers as described in Materials and methods. The identity of the hepatocyte lineage was confirmed by measurements of hepatocyte-specific markers (see Materials and methods). Protein levels of cdk4 were determined by western blotting in these cultured cells after two to four passages. Figure 2A shows that, in agreement with data for newborn livers, cdk4 protein levels are increased in C/EBPα-knockout hepatocytes. Calculations of cdk4 levels as a ratio to β-actin showed that cdk4 is 3- to 4-fold higher in cells that lack C/EBPα (Figure 2B). Measurements of BrdU uptake in primary hepatocytes from wild-type and C/EBPα-knockout livers showed that the induction of cdk4 levels correlates with increased rate of proliferation in primary hepatocytes from C/EBPα-knockout mice (data not shown and Soriano et al., 1998). Given the observation that C/EBPα regulates protein levels of cdk4 (Figure 1), we next examined whether the half-life of cdk4 protein differs in hepatocytes expressing C/EBPα and in hepatocytes from C/EBPα-knockout livers. Protein synthesis was blocked by the addition of cyclohexamide (CHX), proteins were isolated at different time points after CHX addition, and protein levels of cdk4 were examined by western blotting. Figure 2C shows the reproducible results of these studies. In hepatocytes from heterozygous livers, cdk4 is reduced at 4, 6 and 8 h after inhibition of protein synthesis, with a half-life of ∼5 h. However, in the absence of C/EBPα, cdk4 protein is reduced after 8 h. This data demonstrates that expression of C/EBPα is required to induce cdk4 degradation and, in the absence of C/EBPα, the half-life of cdk4 is significantly longer.
Fig. 2. The half-life of cdk4 is increased in primary hepatocytes lacking C/EBPα. (A) Protein levels of cdk4 are induced in C/EBPα-knockout primary hepatocytes. Nuclear extracts from wild-type (+/+) and C/EBPα-knockout (–/–) hepatocytes were analyzed by western blotting with antibodies to cdk4. cdk4 levels were calculated as the ratio to β-actin and are shown below. (B) Induction of cdk4 levels in C/EBPα-knockout animals. The cdk4:β-actin ratio was calculated in wild-type and C/EBPα-knockout primary hepatocytes. A summary of three experiments is shown. (C) The half-life of cdk4 in wild-type and C/EBPα-knockout primary hepatocytes. The inhibitor of protein synthesis, CHX (10 µg/ml), was added to the cells. Proteins were then isolated at different time points after CHX addition (shown on the top) and analyzed by western blotting with antibodies to cdk4. The levels of cdk4 (expressed as a ratio to β-actin) are shown below. (D) The proteasome inhibitor MG132 blocks the C/EBPα-dependent degradation of cdk4. MG132 (50 µM) was added to the cells 1 h prior to CHX addition and the experiment was performed as described above.
To study further the mechanisms of C/EBPα-dependent regulation of cdk4, we determined the pathway of cdk4 degradation in primary hepatocytes. A number of cell cycle proteins are degraded via a specific proteasome pathway, therefore we tested whether this pathway also regulates protein levels of cdk4. The specific inhibitor of proteasome, MG132, was added to cells 1 h prior to CHX addition and the stability of cdk4 was examined as described above. Figure 2D shows that MG132 blocks the degradation of cdk4 in wild-type hepatocytes, and cdk4 is not reduced within the tested time period (8 h). Therefore, these studies showed that cdk4 is degraded in hepatocytes through a proteasome-dependent pathway and that C/EBPα enhances the proteasome-dependent degradation of cdk4 and shortens its half-life.
Ubiquitin–cdk4 conjugates are induced during prenatal liver development in wild-type, but not in C/EBPα-knockout animals
To investigate further the regulation of cdk4 by C/EBPα in liver, we utilized a biological model in which C/EBPα expression is induced: prenatal liver development. Because C/EBPα-knockout animals die shortly after birth, this system is the only one available for investigation of the effect of C/EBPα on cell cycle proteins in liver. The characterization of this system was described in our earlier paper (Timchenko et al., 1999). We and other investigators showed that C/EBPα expression is induced at later stages of prenatal development and that this induction causes inhibition of liver proliferation (Flodby et al., 1996; Timchenko et al., 1997, 1999).
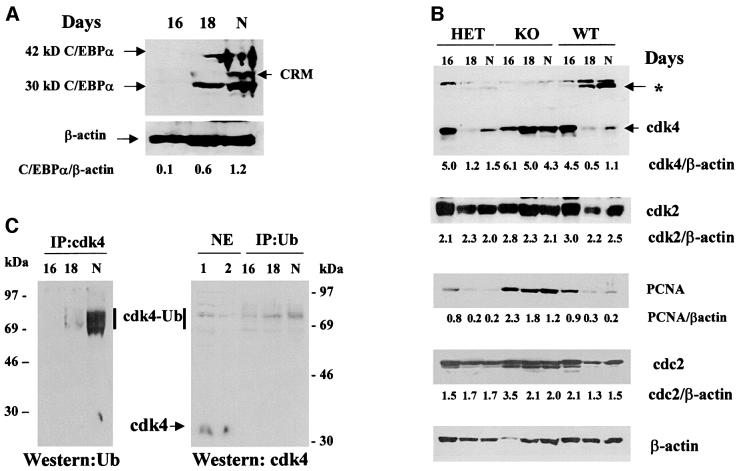
Figure 3A shows a measurement of expression of C/EBPα protein in animals used for these studies. In agreement with published data, levels of both 42 and 30 kDa isoforms of C/EBPα are increased at day 18 of gestation and in newborn livers. Calculations of C/EBPα levels (as a ratio to β-actin) (42 kDa + 30 kDa/β-actin) showed a 6- and 10-fold induction of C/EBPα at day 18 and in newborn animals, respectively. To test whether the increase in C/EBPα correlates with the reduction in cdk4 protein levels during prenatal development, nuclear extracts from 16 days and 18 days of gestation and from newborn animals (N) were analyzed by western blotting with antibodies to cdk4. Figure 3B shows the reproducible results of these studies. In the experiments presented in Figure 3B, protein extracts were isolated from three animals per genotype for days 16 and 18, and from two animals for newborn animals. At day 18 and in newborn animals, cdk4 levels are dramatically reduced in wild-type (WT) and heterozygous livers, but in C/EBPα-knockout livers levels of cdk4 remain high and do not decrease with development. Examination of the levels of two other kinases, cdk2 and cdc2, showed that although these kinases are slightly reduced during prenatal development, these alterations are minor and are not accompanied by the appearance of additional immunoreactive bands, as is observed for cdk4. To examine the proliferative status of livers during gestation, levels of an S-phase-specific protein, PCNA, were also determined by western blotting with specific antibodies. At day 16, PCNA levels were relatively high in all genotypes, while at day 18 and in newborn animals PCNA levels were significantly reduced in wild-type and heterozygous mice, but not in C/EBPα-knockout livers. These data indicate that C/EBPα- dependent reduction of cdk4 correlates with the inhibition of hepatocyte proliferation.
Fig. 3. (A) Expression of C/EBPα is increased at later stages of prenatal development. Nuclear extracts (200 µg) isolated from livers at 16, 18 and 20 (N, newborn) days of gestation were analyzed by western blotting with antibodies to C/EBPα. A pool of three livers from each genotype was used for the protein isolation. The membrane was re-probed with β-actin antibodies. Levels of C/EBPα were calculated as a ratio of C/EBPα (42 kDa + 30 kDa) to β-actin. CRM; cross reactive molecule. (B) CDK4 protein levels during prenatal development in wild-type and C/EBPα-knockout livers. Western blotting was performed with liver nuclear extracts from wild-type, heterozygous and C/EBPα-knockout mice isolated at different stages of prenatal development and from newborn mice (N). Livers from three animals per genotype were used for protein isolation. The same membrane was re-probed with antibodies to cdk4, cdk2, PCNA, cdc2 and β-actin. The levels of each protein were calculated as ratios to β-actin and are shown below each panel. Asterisk shows the position of a high molecular weight cdk4 immunoreactive protein. (C) High molecular weight cdk4 immunoreactive proteins are cdk4–ubiquitin conjugates. Ubiquitin (right) or cdk4 (left) were precipitated from nuclear extracts of wild-type animals with specific antibodies (monoclonal for ubiquitin, polyclonal for cdk4). Immunoprecipitations were analyzed by western blotting with monoclonal antibodies to cdk4 (right) or polyclonal antibodies to ubiquitin (left). Nuclear extracts from wild-type livers were run on the same gel with ubiquitin immuno precipitations and analyzed by western blotting with antibodies to cdk4. Positions of molecular weight markers are shown.
As can be seen in Figure 3B, the reduction of cdk4 during prenatal development correlates with the increase in C/EBPα at day 18 and in wild-type newborn animals, suggesting that the increase in C/EBPα causes a reduction in cdk4 levels. Since cdk4 mRNA levels are not affected by C/EBPα (Figure 1B), the failure of C/EBPα-knockout mice to reduce cdk4 before birth suggests that C/EBPα may be involved in the degradation of cdk4 protein. In these experiments, we found that the reduction of cdk4 in wild-type and heterozygous livers is accompanied by the appearance of new cdk4 immunoreactive proteins migrating to a higher molecular weight region of the gel (∼70 kDa, shown by asterisk in Figure 3B). These new bands were not detectable in C/EBPα-knockout animals at any stage of liver development. The appearance of high molecular weight immunoreactive bands is specific for cdk4, since western blotting with cdk2 or cdc2 did not detect any additional proteins interacting with antibodies to cdk2 and cdc2. Given the observation that cdk4 is degraded in hepatocytes by a proteasome-dependent pathway (Figure 2), we suggested that these new bands may be ubiquitin–cdk4 intermediate products of cdk4 degradation, and investigated this possibility. Cdk4 was immunoprecipitated from wild-type livers at different stages of prenatal development, and western analysis of immunoprecipitations was performed with monoclonal antibodies (mAbs) to ubiquitin. As can be seen in Figure 3C (left), antibodies to ubiquitin recognize the high molecular weight cdk4 immunoreactive proteins.
To verify these results, we performed immunoprecipitation with antibodies to ubiquitin and western blotting with antibodies to cdk4. To determine whether immunoprecipitated cdk4–ubiquitin conjugates corresponded to those detected by western blotting, nuclear extracts from wild-type livers were loaded on the same gel and probed with antibodies to cdk4. As can be seen, cdk4–ubiquitin conjugates detected in nuclear extracts and those immunoprecipitated with antibodies to ubiquitin migrated to the same position (Figure 3C, right). These data confirmed that the high molecular weight cdk4 proteins represent cdk4–ubiquitin. We also repeated these studies using different types of antibodies to ubiquitin and to cdk4, and reproducibly detected the interaction of high molecular weight cdk4 proteins with antibodies to both ubiquitin and cdk4. Taken together, these data show that protein levels of cdk4 are reduced in mouse liver during prenatal development, and that this reduction correlates with increased levels of cdk4–ubiquitin conjugates. Expres sion levels of cdk4 remain elevated in the liver, as does the proliferative status of the organ.
C/EBPα disrupts a chaperone cdk4–cdc37–Hsp90 complex in newborn livers via direct interaction with cdk4
Since expression of C/EBPα triggers cdk4 degradation in liver and in primary hepatocytes, we next examined putative molecular mechanisms by which C/EBPα may regulate the stability of cdk4 protein in liver. Previous studies demonstrated that cdk4 is stabilized by association with cdc37 and by the formation of a high molecular weight (450 kDa) chaperone complex, cdk4–cdc37–Hsp90 (Stepanova et al., 1996; Sherr and Roberts, 1999).
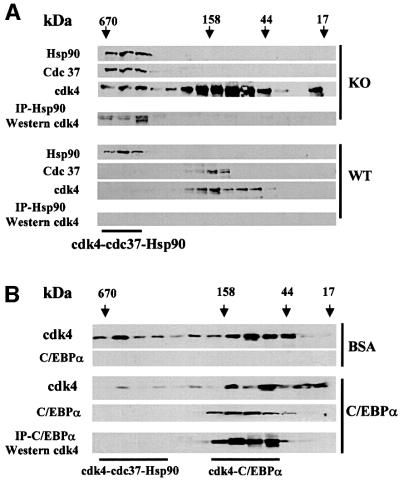
To determine whether C/EBPα affects the formation of the cdk4–cdc37–Hsp90 complex, protein extracts from livers of C/EBPα-knockout and wild-type littermates were fractionated by gel filtration (high performance liquid chromatography-based), and the presence of cdk4, cdc37 and Hsp90 in gel filtration fractions was examined by western blotting. Figure 4A shows that all three proteins are co-localized in high molecular weight fractions (400–600 kDa) of gel filtration samples from C/EBPα-knockout livers. On the other hand, no or weak signals for cdc37 and cdk4 are observed in high molecular weight fractions of samples isolated from wild-type livers expressing C/EBPα. Immunoprecipitation (Hsp90)– western (cdk4) assay shows that cdk4 is bound to Hsp90 in high molecular weight fractions of C/EBPα-knockout samples. Since cdk4 interacts directly with cdc37 (Dai et al., 1996; Stepanova et al., 1996; Lamphere et al., 1997), the presence of cdk4 in Hsp90 immunoprecipitations suggests the existence of cdk4–cdc37–Hsp90 complexes in extracts from C/EBPα-knockout livers. Thus, the comparison of wild-type and C/EBPα-knockout samples revealed that cdk4–cdc37–Hsp90 complex is abundant in C/EBPα-knockout livers, but not detectable in livers from wild-type littermates. Because C/EBPα directly interacts with cdk4 (Wang et al., 2001) (see Figure 5), we examined whether this interaction may affect the cdk4–cdc37– Hsp90 complex. Liver proteins from C/EBPα-knockout mice were preincubated with bacterially expressed, purified C/EBPα or with bovine serum albumin (BSA) as the control, and fractionated by size exclusion chromatography. Analysis of distribution of cdk4 in the gel filtration fractions shows that the incubation with C/EBPα shifts the majority of cdk4 to the lower molecular weight fractions (Figure 4B), while BSA does not affect the distribution of cdk4 within gel filtration fractions. This result suggests that C/EBPα disrupts the cdk4–cdc37–Hsp90 complex, perhaps through direct interaction (see below). In agreement with this suggestion, immunoprecipitation–western assay of gel-filtration fractions revealed that C/EBPα interacts with cdk4 and forms a complex, which is located in the lower molecular weight fractions of gel filtration. Thus, these data show that C/EBPα regulates the cdk4–cdc37–Hsp90 complex in liver and that C/EBPα is able to disrupt the complex in vitro.
Fig. 4. (A) Analysis of cdk4 complexes in livers of wild-type and C/EBPα-knockout mice. Liver proteins from C/EBPα-knockout (KO) or wild-type livers (WT) were fractionated by gel filtration and analyzed by western blotting with antibodies specific to Hsp90, cdc37 and cdk4. Three livers of each genotype were used for each protein isolation. Results presented in the figure were obtained by re-probing the same membrane. Hsp90 was immunoprecipitated from each fraction, and immunoprecipitations were examined by western blotting with antibodies to cdk4. cdc37–cdk4–Hsp90 complex is detectable in C/EBPα-knockout livers, but not in livers from wild-type littermates. (B) C/EBPα disrupts cdk4–cdc37–Hsp90 complexes. Protein extracts from C/EBPα-knockout livers were incubated with bacterially expressed, purified C/EBPα or with equal amounts of BSA (control) and separated by gel-filtration. The presence of C/EBPα and cdk4 in gel filtration fractions was examined by western blotting. C/EBPα was precipitated from gel filtration fractions and cdk4 was examined in immunoprecipitations by western blotting with monoclonal antibodies to cdk4.
Fig. 5. A growth inhibitory region of C/EBPα interacts with cdk4 and disrupts the cdk4–cdc37–Hsp90 complex. (A) Diagram showing the structure of GST–C/EBPα constructs used in these studies. The growth inhibitory region of C/EBPα (aa 140–207) is shown above. (B) GST pull-down experiment. GST–C/EBPα mutant proteins were incubated with nuclear extracts from 3T3-L1 cells, washed and analyzed by western blotting with antibodies to cdk4. The bottom part shows the Coomassie Blue stain of the same membrane. (C) A truncated C/EBPα molecule, which does not interact with cdk4, also fails to disrupt cdk4–cdc37–Hsp90 complex. Protein extracts from C/EBPα-knockout livers were incubated with GST–C/EBPα–N142 or with GST–C/EBPα–140–207 for 1 h on ice and then separated by gel filtration. Size exclusion fractions were analyzed by western blotting with antibodies to cdk4, cdc37 and Hsp90. Hsp90 was precipitated from the gel filtration fractions and the presence of cdk4 in immunoprecipitations was examined by western blotting.
A growth inhibitory region of C/EBPα is responsible for the disruption of the cdk4–cdc37–Hsp90 complex
We previously mapped a short growth inhibitory region of C/EBPα (amino acids 140–207), which interacts with cdk4 (Wang et al., 2001). Given the disruption of cdk4– cdc37–Hsp90 complex by C/EBPα, we asked whether the growth inhibitory region of C/EBPα mediates the disruption of the complex. Glutathione S-transferase (GST)– C/EBPα 140–207 and GST–C/EBPα-N142 (Figure 5A) were expressed in bacteria, purified to homogeneity and examined for the ability to disrupt the cdk4–cdc37–Hsp90 complex. Figure 5B shows the interaction of the C/EBPα mutants (used in these studies) with cdk4. In agreement with published data (Wang et al., 2001), GST–140–207 interacts with cdk4, while deletion of this region leads to failure of C/EBPα to interact with cdk4. Bacterially expressed, purified GST–140–207 (cdk interaction region) and GST–C/EBPα-N142 (truncated molecule that does not interact with cdk4) were pre-incubated with protein extracts from C/EBPα-knockout livers, and proteins were fractionated by gel filtration. Western blotting and immunoprecipitation–western assay showed that the cdk4 interacting region of C/EBPα disrupts the 450 kDa chaperone cdk4–cdc37–Hsp90 complex and shifts all three proteins to lower molecular weight fractions. On the other hand, the C/EBPα molecule that lacks this region does not affect the cdk4–cdc37–Hsp90 complex (Figure 5C). Although after the disruption of the 450 kDa chaperone complex, cdk4, cdc37 and Hsp90 partially co-localize in gel filtration fractions, the immunoprecipitation of Hsp90 and western blotting with antibodies to cdk4 indicates that cdk4 no longer interacts with cdc37–Hsp90. These data demonstrate that the growth inhibitory region of C/EBPα is sufficient to disrupt the cdk4–cdc37–Hsp90 complex. Taken together, these studies show that C/EBPα disrupts the cdk4–cdc37–Hsp90 complex via direct interaction with cdk4, and that a truncated C/EBPα protein that does not inhibit cell proliferation also fails to disrupt the complex.
cdk4 protein is degraded by the ubiquitin–proteasome pathway in cultured cells
Given the ability of C/EBPα to disrupt the cdk4–cdc37– Hsp complex in vitro and to induce degradation of cdk4 in the liver, we suggested that C/EBPα may also use this pathway for the regulation of cdk4 levels during growth arrest in culture. We therefore examined whether this pathway is operating in HT1 cells, where induction of C/EBPα causes growth arrest. Although data with newborn livers and with primary hepatocytes demonstrated that cdk4 is degraded by the proteasome, this pathway of cdk4 degradation has not been described in other cultured cells.
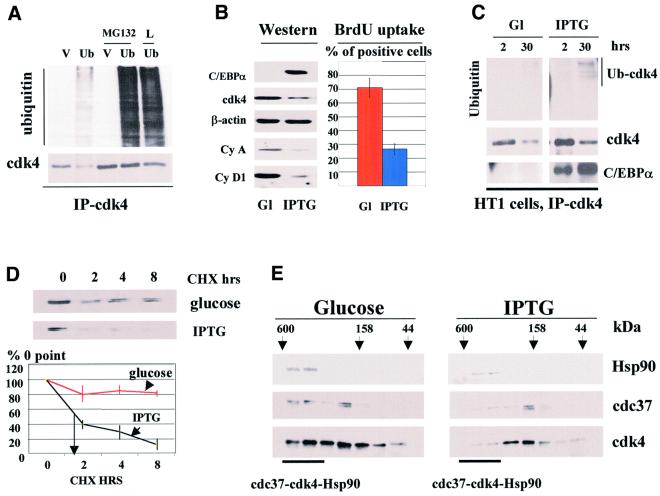
First, we tested whether degradation of cdk4 in HT1080 (parental cells for HT1 clone) and COS7 cells occurs via the proteasome pathway. The common pathway for the proteasome-dependent degradation of proteins involves formation of ubiquitin–protein conjugates followed by delivery of the ubiquitylated proteins to the catalytic 26S proteasome complex (Werma and Deshaies, 2000). Therefore, we determined whether ubiquitylation of cdk4 occurs in cultured cells. HT1080 or COS7 cells were transfected with a plamid expressing HA-tagged ubiquitin and the formation of cdk4–ubiquitin intermediates was examined. Cdk4 was precipitated from the cells and cdk4–ubiquitin conjugates were determined by western blotting with antibodies to ubiquitin. Figure 6A shows western blot analysis of cdk4 immunoprecipitations from COS7 cells transfected with empty vector or with HA-tagged ubiquitin (HA-Ub) plasmid. As can be seen, expression of HA-Ub leads to the formation of additional high molecular weight cdk4–ubiquitin conjugates (Figure 6A, lane 2). To confirm that these additional cdk4 immunoreactive bands are ubiquitin conjugates, cells were treated with several inhibitors of proteasome (lactacystine, LLnL and MG132), which block degradation of ubiquitylated proteins, but do not effect the formation of ubiquitin conjugates. These studies demonstrated a dramatic accumulation of ubiquitin–cdk4 conjugates in cells transfected with the HA-Ub plasmid (Figure 6A, lanes 4 and 5; data for lactacystine not shown). The accumulation of cdk4–ubiquitin intermediates is specific for cells transfected with HA-Ub plasmid, because cells transfected with an empty vector (control) did not show increased levels of cdk4–ubiquitin intermediates. A similar result was also observed in HT1080 cells (data not shown). These results demonstrate that in ubiquitin-transfected HT1080 and COS7 cells, cdk4 forms ubiquitin–cdk4 conjugates, suggesting that cdk4 is degraded via the ubiquitin–proteasome pathway.
Fig. 6. C/EBPα reduces cdk4–cdc37–Hsp90 complex, induces cdk4–ubiquitin conjugates and shortens the half-life of cdk4 during growth arrest in HT1 cells. (A) Expression of HA-ubiquitin leads to the formation of cdk4–ubiquitin intermediates. COS7 cells were transfected with the empty vector (V, control) or with a plasmid expressing HA-ubiquitin. The next day after transfection, cells were treated with inhibitors of the proteasome MG132 or LlnL (L) or with DMSO (control). Cdk4 was precipitated, and cdk4 immunoprecipitations were analyzed by western blotting with antibodies to the HA-tag (upper) or with antibodies to cdk4 (lower). (B) cdk4 levels are reduced in cells growth arrested by C/EBPα. HT1 cells were synchronized by serum starvation, expression of C/EBPα was induced by IPTG, and cells were plated at low density. Levels of cdk4, cyclin A, cyclin D1 and C/EBPα were determined 30 h after plating in glucose-treated (control) and IPTG-treated cells (Western). β-actin shows the re-probe of the membrane for cdk4. BrdU uptake shows percentage of glucose- and IPTG-treated cells incorporating BrdU at 18 h after plating. (C) cdk4–ubiquitin conjugates are increased in cells arrested by C/EBPα. HT1 cells were synchronized in G1. C/EBPα was induced by IPTG and cells were plated at low density. Cdk4 was precipitated from cells at 2 and 30 h, and cdk4 immunoprecipitations were analyzed by western blotting with antibodies to ubiquitin, cdk4 and C/EBPα. The result shown in the figure represents the re-probe of the same membrane with the aforementioned antibodies. The position of the cdk4–ubiquitin conjugate induced by C/EBPα is shown on the right. (D) The half-life of cdk4 is reduced in growth-arrested HT1 cells. Protein synthesis was blocked by cyclohexamide (CHX) and cdk4 levels were examined by western blotting at 0, 2, 4 and 8 h after CHX addition. The stability of cdk4 was calculated as a ratio to 0 point. A summary of three experiments is shown below. (E) cdk4–cdc37–Hsp90 complex is reduced in C/EBPα growth-arrest cells. Total proteins from control cells (glucose) and from cells arrested by C/EBPα (IPTG) were fractionated by gel filtration, and Hsp90, cdc37 and cdk4 were examined by western blotting with specific antibodies.
C/EBPα disrupts cdk4–cdc37–Hsp90 complexes, induces ubiquitin–cdk4 conjugates and shortens the half-life of cdk4 during growth arrest in HT1 cells
We next examined whether C/EBPα affects cdk4– ubiquitin intermediates during growth arrest in HT1080 cells. To do this we utilized a stable clonal cell line, HT1, which contains the C/EBPα gene under Lac-Repressor control. We have previously shown that C/EBPα causes growth arrest in these cells (Timchenko et al., 1996). Preliminary analysis of cdk4 expression in HT1 cells showed that C/EBPα has a maximum effect on cdk4 levels if C/EBPα is induced after synchronization of HT1 cells. The following studies were therefore carried out under these conditions. To synchronize HT1 cells, confluent HT1 cells were first arrested in G1 by serum starvation, C/EBPα was induced prior to trypsinization and cells were then plated at low density to initiate proliferation. Since C/EBPα inhibits proliferation of HT1 cells, we suggested that the inhibition of cell growth would correlate with the ability of C/EBPα to induce proteasome-dependent degradation of cdk4. We found that under these conditions, C/EBPα levels were relatively low after plating and reached maximum levels at 20–30 h. We also observed that the maximal reduction of cdk4 in C/EBPα-arrested cells also occurs at 30 h after plating. Figure 6B shows western analysis of cell cycle proteins 30 h after plating and induction of C/EBPα. Cdk4 levels were 2- to 3-fold lower (when expressed as a ratio to β-actin) in cells growth-arrested by C/EBPα compared with levels in dividing cells treated with glucose. Examination of two cyclins, A and D1, indicated significant reduction of these proteins in HT1 cells expressing C/EBPα. Measurement of BrdU uptake showed that 70–80% of glucose cells are synthesizing DNA, while only 25% of C/EBPα-expressing cells incorporate BrdU. These studies showed that, under the conditions of this experiment, C/EBPα causes growth arrest and cdk4 levels are reduced in C/EBPα growth-arrested cells. To examine whether C/EBPα induces proteasome-dependent degradation of cdk4, cdk4 was immunoprecipitated, and cdk4 immunoprecipitations were examined for the presence of ubiquitin–cdk4 conjugates by western blotting with antibodies to ubiquitin. The reproducible results of these studies are shown in Figure 6C. In the absence of C/EBPα, ubiquitin–cdk4 conjugates are not detectable (Figure 6C, lanes 1 and 2). Cells arrested by C/EBPα accumulate high molecular weight cdk4–ubiquitin intermediates (Figure 6C, lanes 3 and 4). The amount of cdk4–ubiquitin is proportional to the levels of C/EBPα induced by IPTG. It is interesting to note that the major cdk4–ubiquitin conjugates in HT1 cells migrate to the 70–90 kDa position, which is similar to that observed for cdk4–ubiquitin intermediates in the livers of newborn mice (Figure 3C).
The block of proteasome activity by MG132 leads to the elevation of cdk4–ubiquitin intermediates in both C/EBPα-expressing and control cells (data not shown). In agreement with previously published data (Wang et al., 2001) re-probing the same membrane (cdk4 immunoprecipitation) with antibodies to C/EBPα showed that cdk4 interacts with C/EBPα and revealed that the accumulation of cdk4–ubiquitin conjugates is caused by the expression of C/EBPα. We next examined whether C/EBPα affects the stability of cdk4 during growth arrest. Figure 6D shows cdk4 levels after CHX-mediated block of protein synthesis in cells arrested by C/EBPα, and in control cells. In agreement with data for primary hepatocytes (Figure 2), expression of C/EBPα in HT1 cells also leads to a reduction in the half-life of cdk4. Because C/EBPα disrupts cdk4–cdc37–Hsp90 complex in vitro (Figures 4 and 5), we next examined whether this occurs during growth arrest in HT1 cells. Gel filtration analysis of total protein extracts from control (glucose) and C/EBPα-arrested HT1 cells (IPTG) is shown in Figure 6E. As can be seen, amounts of cdk4 in fractions containing cdk4–cdc37–Hsp90 complex are significantly reduced in growth-arrested cells. This observation is consistent with the ability of C/EBPα to disrupt the cdk4–cdc37–Hsp90 complex and also with the suggestion that C/EBPα enhances degradation of cdk4 via disruption of the complex. Thus, these experiments show that similar to its effect in the liver, C/EBPα induces ubiquitylation of cdk4 in cultured cells and shortens the half-life of cdk4. In both liver and HT1 cells, C/EBPα-dependent down-regulation of cdk4 correlates with a reduction in the amount of cdk4–cdc37–Hsp90 complex, which protects cdk4 from degradation.
Discussion
Although C/EBPα is a transcription factor, it interacts directly with several cell cycle associated proteins and affects their expression and activities via this interaction. The interaction of C/EBPα with p21 leads to an increase in p21 protein levels (Timchenko et al., 1996, 1997). In addition to p21, C/EBPα interacts with cyclin-dependent kinases 2 and 4, and inhibits their activities (Wang et al., 2001). Investigations into the expression and activity of cdk4 in C/EBPα-knockout mice showed that C/EBPα regulates cdk4 via multiple pathways, including direct inhibition of kinase activity and regulation of protein levels of cdk4 (Wang et al., 2001). Expression of cdk4 in mammalian cells is controlled at the transcriptional, translational and post-translational levels. The post-translational pathway for cdk4 regulation has been suggested by Stepanova et al. (1996). The authors found that a chaperone complex, cdc37–Hsp90, plays a crucial role in cdk4 regulation. One function of the chaperone complex is the proper folding and further release of cdk4 to assemble an active cdk4–cyclin D1 complex. The second role of the chaperone complex is to protect cdk4 from degradation. It has been demonstrated that in cells lacking the cdc37–Hsp90 complex, the half-life of cdk4 is shorter than in cells expressing the complex (Stepanova et al., 1996). Given the observation that C/EBPα down-regulates cdk4 protein levels in tissues of newborn mice, we examined the pathways of cdk4 degradation in liver and cultured hepatocytes, and mechanisms by which C/EBPα might trigger the degradation of cdk4 during growth arrest.
We found that in cells expressing C/EBPα, the half-life of cdk4 is significantly shorter and protein levels of cdk4 are reduced. Because several proteasome-specific inhibitors blocked the degradation of cdk4 in cells, we suggested that C/EBPα triggers a proteasome-dependent degradation of cdk4. Further analysis of pathways for degradation of cdk4 showed that, like many other cell cycle proteins, cdk4 is degraded through a proteasome-dependent pathway. We also detected accumulation of ubiquitin–cdk4 intermediates in the cultured cells expressing C/EBPα and in wild-type livers. Taken together, these data clearly indicate that C/EBPα induces degradation of cdk4 through a proteasome-dependent pathway, and suggest that ubiquitylation of cdk4 is the method of delivery of cdk4 to the catalytic cylinder of the 26S proteasome. Although ubiquitylation is the common way to induce proteasome-dependent degradation of proteins, it has been recently found that proteasome-dependent degradation of p21 occurs through direct interaction of p21 with the C8 subunit of the catalytic cylinder of 26S proteasome (Sheaf et al., 2000; Touitou et al., 2001). This pathway does not require the ubiquitylation of the p21 protein. Therefore, further studies are necessary to investigate the role of ubiquitin–cdk4 conjugates in the proteasome-dependent degradation of cdk4 in C/EBPα-expressing cells.
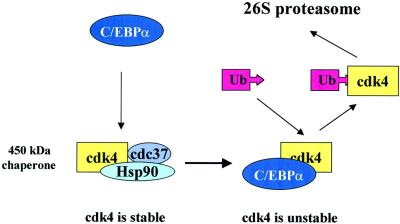
In this paper, we present data suggesting the mechanism by which C/EBPα may down-regulate cdk4 protein levels. Our data demonstrate that the interaction of C/EBPα with cdk4 leads to disruption of the cdk4–cdc37–Hsp90 complex, which has been shown to stabilize cdk4 (Stepanova et al., 1996). Gel filtration analysis indicated that the cdk4–cdc37–Hsp90 complex is abundant in livers lacking C/EBPα, and is very weak or undetectable in livers expressing C/EBPα. We also found that protein levels of Hsp90 and cdc37 do not differ in wild-type and C/EBPα-knockout livers (data not shown). This suggests that down-regulation of cdk4 in wild-type livers occurs through the disruption/prevention of formation of the complex. In agreement with this suggestion, we found that bacterially expressed purified C/EBPα disrupts the cdk4–cdc37– Hsp90 complex in vitro. The C/EBPα-mediated disruption of the complex requires the region of C/EBPα that interacts directly with cdk4. Importantly, deletion of the cdk-interacting region of C/EBPα abolishes its ability to inhibit cell proliferation (Wang et al., 2001). This suggests that the interaction of C/EBPα with cdk4 and the disruption of the cdk4–cdc37–Hsp90 complex may contribute to growth arrest. In agreement with this suggestion, C/EBPα-mediated growth arrest in HT1 cells is accompanied by a significant reduction of the complex and reduced stability of cdk4 (Figure 6). Thus, data obtained using two biological models, liver development and a stable HT1clone, showed that C/EBPα disrupts the cdc37–cdk4–Hsp90 complex during growth arrest and reduces the stability of cdk4. Based on these observations and on the ability of bacterially expressed, purified C/EBPα to disrupt the complex in vitro (Figures 4 and 5), we suggest a molecular pathway through which C/EBPα down-regulates cdk4 protein levels (Figure 7). According to this model, C/EBPα interacts with cdk4 and prevents/disrupts the cdk4–cdc37–Hsp90 complex. As a result, cdk4 becomes available for ubiquitylation and subsequent degradation by the 26S proteasome. It remains unclear whether cdk4 is ubiquitylated faster as a free protein or as a complex with C/EBPα.
Fig. 7. A hypothetical model for C/EBPα-dependent degradation of cdk4.
We have previously reported that the interaction of C/EBPα with cdk4 leads to the inhibition of cdk4 kinase activity in an in vitro kinase assay (Wang et al., 2001). The data presented in this paper demonstrate several pathways via which C/EBPα could regulate cdk4 during growth arrest. We suggest that these pathways contribute to the inhibition of cdk4 and to growth arrest. Our data demonstrate that C/EBPα-dependent reduction of cdk4 levels is observed in nuclear extracts, while in cytoplasm this effect is minor or undetectable (Figure 1). It has been also demonstrated that nuclear cdk4 functions as a regulator of cell cycle progression (Sherr and Roberts, 1999). Since C/EBPα reduces cdk4 levels in nuclei, we conclude that C/EBPα-dependent reduction of cdk4 levels may contribute to growth arrest through several putative pathways. The major pathway of cdk4-dependent regulation of the cell cycle progression is the phosphorylation of Rb family proteins. This process mainly depends on the activity of cdk4 and is regulated by type D cyclins (Sherr and Roberts, 1999). However, several recent reports suggested a second regulatory pathway by which cdk4 may affect the cell cycle. Experiments with cdk4 and p27-knockout animals showed that cdk4 titrates p27 out of the cellular pool and out of cdk2, which in turn increases cdk2 activity and contributes to cell cycle progression (Tsutsui et al., 1999; Tong and Pollard, 2001). This pathway mainly depends on the protein levels of cdk4. In this scenario, the reduction of cdk4 protein by C/EBPα would lead to an increase in the amount of free p27 capable of inhibiting cdk2 activity. Taken together, these new observations suggest that although direct inhibition of cdk2 and cdk4 is the major mechanism of C/EBPα growth arrest (Wang et al., 2001), several additional pathways can also contribute to it.
Nakai and Ishikawa have recently confirmed the role of the cdc37–Hsp90 chaperone in the stabilization of cell cycle proteins. The authors showed that another cell cycle kinase, cdc2, is also stabilized by it (Nakai and Ishikawa 2001). Taking this finding into account, we examined whether C/EBPα may also affect cdc2 in newborn livers. Analysis of cdc2 expression in the livers from C/EBPα-knockout and from wild-type littermates showed no significant difference in the protein levels of cdc2 (Figure 3). This observation is consistent with data showing that C/EBPα does not affect cdc2 activity in an in vitro kinase assay (Wang et al., 2001) and indicates that the effect of C/EBPα on cdk4 is specific and requires direct interaction with cdk4. Although C/EBPα is a transcription factor and activates transcription of target genes, a growing number of observations have highlighted another pathway through which C/EBPα regulates protein expression: control of protein stability. We have demonstrated previously that the major mechanism of C/EBPα-dependent regulation of a cell cycle inhibitor p21 is the stabilization of p21 protein through direct interaction with and protection of p21 from degradation (Timchenko et al., 1997). Taken together, these data show that the regulation of protein levels of cell cycle proteins by the transcription factor C/EBPα does not necessarily require its transcriptional activity. C/EBPα is able to regulate protein levels of p21 and cdk4 through direct interaction. In the case of p21, the interaction leads to the stabilization of p21 and to an increase in p21 levels, while the interaction of C/EBPα with cdk4 results in the reduction of cdk4 levels. These alterations are consistent with the growth inhibitory function of C/EBPα, and suggest multiple pathways of growth arrest mediated by C/EBPα.
Materials and methods
Materials and plasmids
Antibodies to C/EBPα (14AA), cdk4 (C-22), cdk2 (M2), cdc2 p34 (17), PCNA (PC10), ubiquitin (P1A6), cyclin A, cyclin D1, cdc37 and Hsp90 were purchased from Santa Cruz Biotechnology. Monoclonal antibodies to β-actin are from Sigma. GST–C/EBPα deletion mutants have been described previously (Wang et al., 2001). HA-Ub plasmid was kindly provided by Dr X.Wu.
Protein isolation and western analysis
Experiments were performed using total protein extracts and nuclear extracts isolated from liver or from cultured cells. The procedures for isolation of total proteins and nuclear extracts have been described previously (Timchenko et al., 1996, 1997). Western blot analysis was also carried out as described previously (Timchenko et al., 1996). Briefly, proteins were loaded on a 12% SDS gel and transferred onto membranes (PVDF or nitrocellulose; Bio-Rad, Hercules, CA). Membranes were blocked with 10% dry milk dissolved in TBS-T (20 mM Tris–HCl pH 7.5, 150 mM NaCl, 0.1% Tween-20). The membrane was probed with specific antibodies. Intensity of protein signals was determined by laser densitometry. Protein levels of C/EBPα, cdk4, cdk2, cdc2 and PCNA were calculated as the ratio to β-actin after re-probing the membrane with antibodies to β-actin. Immunoreactive proteins were detected using the enhanced chemiluminescence protocol (Amersham).
Co-immunoprecipitation (Co-IP) studies
To determine how cdk4–ubiquitin conjugates were formed in the livers of newborn mice, cdk4 was immunoprecipitated with polyclonal antibodies, washed five times with phosphate-buffered saline (PBS) and analyzed by western blotting with mAbs to ubiquitin (P1A6; Santa Cruz Biotechnology). To confirm the existence of cdk4–ubiquitin conjugates, Co-IP studies were performed using immunoprecipitation with antibodies to ubiquitin, and western blotting with antibodies to cdk4. To examine cdk4–ubiquitin intermediates in cultured cells, HT180 or COS7 cells were transfected with a plasmid expressing HA-tagged ubiquitin. Proteasome-dependent degradation was inhibited by the addition of several specific inhibitors, including lactacystine, LLnL and MG 132 (50 µM of each). Protein extracts were isolated, and ubiquitin was precipitated with antibodies to the HA-tag (Santa Cruz Biotechnology) and loaded on a denaturing gel. The membrane was analyzed by western blotting with antibodies to cdk4.
Gel filtration chromatography
Gel filtration chromatography was performed using Bio-Sil SEC 400 or Bio-Sil SEC 250 columns (Bio-Rad). 300 µl (0.6–1 mg) of proteins from wild-type or C/EBPα-knockout livers were loaded onto the column and separated in gel filtration buffer (50 mM Tris–HCl pH 7.5, 100 mM NaCl, 5 mM β-mercaptoethanol) at a flow rate of 0.3–0.5 ml/min. The molecular mass standards (Bio-Rad) were run in parallel to calibrate the column: thyroglobulin (670 kDa), γ globulin (158 kDa), ovalbumin (44 kDa), myoglobin (17 kDa). Fractions (250–300 µl) were collected, and 30–50 µl of each fraction was analyzed by western blotting with corresponding antibodies. For detection of cdk4–cdc37–Hsp90 complex, Hsp90 was immunoprecipitated from each fraction and cdk4 was determined in immunoprecipitations using western blotting with specific antibodies. To examine the effect of C/EBPα on cdc37–Hsp90 complexes, liver nuclear extracts from C/EBPα-knockout livers were incubated with C/EBPα proteins purified to homogeneity: his-C/EBPα, GST–C/EBPα–N140 or GST–140–207 prior to gel fractionation. One microgram of each protein was used for the incubation with 0.5 mg of nuclear extract. The interaction of cdk4 with C/EBPα was examined using a GST pull-down assay as described previously (Wang et al., 2001).
Measurements of BrdU uptake and ubiquitylation of cdk4 in a stable clonal line HT1
Previously, we have generated a stable clonal cell line, HT1, which contains C/EBPα under Lac-Repressor control. In these cells, induction of C/EBPα by IPTG causes growth arrest (Timchenko et al., 1996). HT1 cells were synchronized in G1 by serum starvation and high density. C/EBPα was induced by 10 mM IPTG at the time of release of serum starvation-dependent growth arrest. Control cells were released to grow by low density plating with rich media containing 10 mM glucose. Cell cycle progression in control cells and in C/EBPα-expressing cells was monitored by BrdU uptake. BrdU (1 µg/ml) was added for 1 h at different time points after plating (6, 18, 30 and 48 h), cells were fixed, and the amount of BrdU-positive cells was calculated after immunostaining with mAbs to BrdU (Sigma). In control cells, a peak of BrdU incorporation was observed at 18 h. Expression of C/EBPα, cdk4, cyclin A and cyclin D1 was examined by western blotting with specific antibodies. For detection of cdk4–ubiquitin conjugates, cdk4 was immunoprecipitated from HT1 cells and probed with antibodies to ubiquitin.
Generation of primary hepatocytes and analysis of cdk4 expression
Mouse primary hepatocytes were harvested from the livers of newborn mice. Briefly, the livers were minced and digested in a solution containing collagenase P (0.4 mg/ml), dispase (0.6 U/ml), and soybean trypsin inhibitor (0.04 mg/ml). Hepatocytes were plated on primaria dishes (Becton Dickinson, Franklin Lakes, NJ), and grown in M/M medium (3:1 minimal essential medium: Waymouth’s medium) supplemented with 10% fetal bovine serum, dexamethasone (10–6 M), epidermal growth factor (EGF; 50 ng/ml) and insulin (5 µg/ml). The identity of the hepatocyte lineage was verified by immunohistochemistry for the epithelial-specific markers E-cadherin and zonula occludens, and by analysis of the hepatocyte-specific markers C/EBPα, hepatocyte nuclear factors -1, -3 and -4, and albumin.
Acknowledgments
Acknowledgements
We thank Xiangwei Wu for the vector expressing HA-ubiquitin. The authors thank Gretchen Darlington for discussion on experimental data, and Margie Wilde for technical assistance. We also thank Alana Welm for helpful suggestions and critical review of the paper. This work is supported by National Institutes of Health grant Nos AG00765, GM55188 (to N.A.T.) and DK-54921 (to J.H.A.).
References
- Birkenmeier E.B., Gwynn,B., Howard,S., Jerry,J., Gordon,J.I., Landschulz,W.H. and McKnight,S.L. (1989) Tissue-specific expression, developmental regulation and genetic mapping of the gene encoding CCAAT/enhancer binding protein. Genes Dev., 3, 1146–1156. [DOI] [PubMed] [Google Scholar]
- Cao Z., Umek,R.M. and McKnight,S.L. (1991) Regulated expression of three C/EBP isoforms during adipose conversion of 3T3-L1 cells. Genes Dev., 5, 1538–1552. [DOI] [PubMed] [Google Scholar]
- Cha H.H., Cram,E.J., Wang,E.C., Huang,A.J., Kasler,H.G. and Firestone,G.L. (1998) Glucocorticoids stimulate p21 gene expression by targeting multiple transcriptional elements within a steroid responsive region of the p21waf1/cip1 promoter in rat hepatoma cells. J. Biol. Chem., 273, 1998–2007. [DOI] [PubMed] [Google Scholar]
- Chen P.-L., Riley,D.J., Chen,Y. and Lee,W.-H. (1996) Retinoblastoma protein positively regulates terminal adipocyte differentiation through direct interaction with C/EBPs. Genes Dev., 10, 2794–2804. [DOI] [PubMed] [Google Scholar]
- Dai K., Kobayashi,R. and Beach,D. (1996) Physical interaction of mammalian CDC37 with cdk4. J. Biol. Chem., 271, 22030–22034. [DOI] [PubMed] [Google Scholar]
- Darlington G.J., Ross,S.E. and MacDougald,O.A. (1998). The role of C/EBP genes in adipocyte differentiation. J. Biol. Chem., 273, 30057–30060. [DOI] [PubMed] [Google Scholar]
- Deng C., Zhang,P., Harper,J.W., Elledge,S.J. and Leder,P. (1995) Mice lacking p21CIP1/WAF1 undergo normal development, but are defective in G1 checkpoint control. Cell, 82, 675–684. [DOI] [PubMed] [Google Scholar]
- Diehl A.M., Johns,D.C., Yang,S., Lin,H., Yin,M., Matelist,L.A. and Lowrence,J.H. (1996) Adenovirus-mediated transfer of CCAAT/Enhancer binding protein α identifies a dominant antiproliferative role for this isoform in hepatocytes. J. Biol. Chem., 271, 7343–7353. [DOI] [PubMed] [Google Scholar]
- Dyson N. (1998) The regulation of E2F by RB-family proteins. Genes Dev., 12, 2245–2262. [DOI] [PubMed] [Google Scholar]
- Flodby P.C., Barlow,H., Kalefjord,L., Ahrlund-Richer,L. and Xanthopolous,K.G. (1996) Increased hepatic cell proliferation and lung abnormalities in mice deficient in CCAAT/Enhancer binding protein α. J. Biol. Chem., 271, 24753–24760. [DOI] [PubMed] [Google Scholar]
- Hendricks-Taylor L.R. and Darlington,G.J. (1995) Inhibition of cell proliferation by C/EBPα occurs in many cell types, does not require the presence of p53 or Rb and is not affected by large T-antigen. Nucleic Acids Res., 23, 4726–4733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamphere L., Fiore,F., Xu,X., Bruziela,L., Sarder,C., Draetta,G.F. and Gyuris,J. (1997) Interaction between cdc37 and cdk4 in human cells. Oncogene, 15, 1999–2004. [DOI] [PubMed] [Google Scholar]
- Lekstrom-Himes J. and Xanthopoulos,K.G. (1998) Biological role of the CCAAT/Enhancer-binding protein family of transcription factors. J. Biol. Chem., 273, 28545–28548. [DOI] [PubMed] [Google Scholar]
- Müller C., Alunni-Fabbroni,M., Kowenz-Leutz,E., Mo,X., Tommasino,M. and Leutz,A. (1999) Separation of C/EBPα-mediated proliferation arrest and differentiation pathways. Proc. Natl Acad. Sci. USA, 96, 7276–7281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakai A. and Ishikawa,T. (2001) Cell cycle transition under stress conditions by vertebrate heart shock factor. EMBO J., 20, 2885–2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poli V. (1998) The role of C/EBP isoforms in the control of inflammatory and native immunity functions. J. Biol. Chem., 273, 29279–29282. [DOI] [PubMed] [Google Scholar]
- Sheaf R.G., Singer,J.D., Swanger,J., Smitherman,M., Roberts,J.M. and Clurman,B. (2000) Proteasome turnover of p21 does not require p21 ubiquitination. Mol. Cell., 5, 402–410. [DOI] [PubMed] [Google Scholar]
- Sherr C.J. and Roberts,J.M. (1999) CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev., 13, 1501–1512. [DOI] [PubMed] [Google Scholar]
- Soriano H., Kang,D.C., Finegold,M.J., Hicks,M.J., Wang,N.-D., Harrison,W. and Darlington,G.J. (1998) Lack of C/EBPα gene expression results in increased DNA synthesis and an increased frequency of immortalization of freshly isolated mice hepatocytes. Hepatology, 27, 392–401. [DOI] [PubMed] [Google Scholar]
- Stepanova L., Leng,X., Parker,S.B. and Harper,J.W. (1996) Mammalian p50cdc37 is a protein kinase-targeting subunit of Hsp 90 that binds and stabilizes cdk4. Genes Dev., 10, 1091–1502. [DOI] [PubMed] [Google Scholar]
- Timchenko N.A., Wilde,M., Nakanishi,M., Smith,J.R. and Darlington,G.J. (1996) CCAAT/enhancer-binding protein α (C/EBPα) inhibits cell proliferation through the p21 (WAF-/CIP-1/SDI-1) protein. Genes Dev., 10, 804–815. [DOI] [PubMed] [Google Scholar]
- Timchenko N.A, Harris,T.E., Wilde,M., Bilyeu,T.A., Burgess-Beusse,B.L., Finegold,M.J. and Darlington,G.J. (1997) CCAAT/enhancer binding protein α regulates p21 protein and hepatocyte proliferation in newborn mice. Mol. Cell. Biol., 17, 7353–7361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timchenko N.A., Wilde,M. and Darlington,G.J. (1999) C/EBPα regulates formation of S-phase-specific E2F-p107 complexes in livers of newborn mice. Mol. Cell. Biol., 19, 2936–2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong W. and Pollard,J. (2001) Genetic evidence for the interaction of cyclin D1 and p27 in mice. Mol. Cell. Biol., 21, 1319–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touitou R., Richardson,J., Bose,S., Nakanishi,M., Rivett,J. and Allday,M.J. (2001) A degradation signal located in the C-terminus of p21WAF1/CIP1 is binding site for the C8 a-subunit of the 20S proteasome. EMBO J., 20, 2367–2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsui T., Hesabi,B., Moons,D.S., Pandolfi,P.P., Hansel,K.S., Koff,A. and Kiyokawa,H. (1999) Targeted disruption of cdk4 delays cell cycle entry with enhanced p27 activity. Mol. Cell. Biol., 19, 7011–7019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Iakova,P., Goode,T., Welm,A.L., Roesler,W. and Timchenko,N.A. (2001) C/EBPα arrests cell proliferation through direct inhibition of cdk2 and cdk4. Mol. Cell, 8, 817–828. [DOI] [PubMed] [Google Scholar]
- Wang N.D., Finegold,M.J., Bradley,A., Ou,C.M., Abdelsayed,S.V., Wilde,M.D., Taylor,L.R., Wilson,D.R. and Darlington,G.J. (1995) Impaired energy homeostasis in C/EBPα knockout mice. Science, 269, 1108–1112. [DOI] [PubMed] [Google Scholar]
- Werma R. and Deshaies,R.J. (2000) A proteasome howdunit: the case of missing signal. Cell, 101, 341–344. [DOI] [PubMed] [Google Scholar]
- Zhang J.M., Zhao,X., Wei,Q. and Paterson,B.M. (1999) Direct inhibition of G1 cdk kinase activity by MyoD promotes myoblast cell cycle withdrawal and terminal differentiation. EMBO J., 18, 6983–6993. [DOI] [PMC free article] [PubMed] [Google Scholar]