Abstract
In pancreatic acinar cells, low, threshold concentrations of acetylcholine (ACh) or cholecystokinin (CCK) induce repetitive local cytosolic Ca2+ spikes in the apical pole, while higher concentrations elicit global signals. We have investigated the process that transforms local Ca2+ spikes to global Ca2+ transients, focusing on the interactions of multiple intracellular messengers. ACh-elicited local Ca2+ spikes were transformed into a global sustained Ca2+ response by cyclic ADP-ribose (cADPR) or nicotinic acid adenine dinucleotide phosphate (NAADP), whereas inositol 1,4,5-trisphosphate (IP3) had a much weaker effect. In contrast, the response elicited by a low CCK concentration was strongly potentiated by IP3, whereas cADPR and NAADP had little effect. Experiments with messenger mixtures revealed a local interaction between IP3 and NAADP and a stronger global potentiating interaction between cADPR and NAADP. NAADP strongly amplified the local Ca2+ release evoked by a cADPR/IP3 mixture eliciting a vigorous global Ca2+ response. Different combinations of Ca2+ releasing messengers can shape the spatio-temporal patterns of cytosolic Ca2+ signals. NAADP and cADPR are emerging as key messengers in the globalization of Ca2+ signals.
Keywords: cyclic ADP-ribose/inositol trisphosphate/local and global calcium/NAADP/pancreatic acinar cells
Introduction
Ca2+ is one of the most versatile and important intracellular messengers, as it is involved in the control of many different cellular functions (Petersen et al., 1994; Berridge et al., 1998). Hormones and neurotransmitters can generate Ca2+ signals, such as local and global cytosolic Ca2+ elevations, and these can be transient (spiking) or sustained (Petersen et al., 1994; Thomas et al., 1996; Berridge, 1997; Meldolesi and Pozzan, 1998).
The mechanisms involved in the generation of local Ca2+ signals have been extensively investigated (Petersen et al., 1994; Parker et al., 1996; Berridge, 1997; Berridge et al., 2000; Jaggar et al., 2000). Local cytosolic Ca2+ signals can be generated by opening Ca2+ channels located either in the plasma membrane or in the endoplasmic reticulum (ER) membrane (Petersen et al., 1994; Berridge, 1997). Cells possess multiple Ca2+ releasing messengers such as inositol trisphosphate (IP3), cyclic ADP-ribose (cADPR) and nicotinic acid adenine dinucleotide phosphate (NAADP) (Berridge, 1997; Guse, 1999; Petersen and Cancela, 1999; Lee, 2000; Cancela, 2001). If the Ca2+ release elicited locally by a particular stimulus is sufficiently small, a highly localized cytosolic Ca2+ spike can be generated. This is due to cytoplasmic buffers with low mobility. The mitochondria play a particularly important role with respect both to local Ca2+ buffering and local Ca2+ signal-dependent ATP generation (Pozzan et al., 1994, 2000; Rizzuto et al., 1998; Tinel et al., 1999; Rizzuto et al., 2000; Park et al., 2001a).
Several mechanisms have been proposed to explain the transformation of a local Ca2+ spike into a global Ca2+ transient. A higher concentration of a second messenger could be produced by a higher extracellular agonist concentration (Parker et al., 1996; Berridge, 1997; Ito et al., 1999) and a local Ca2+ signal could be propagated as a global Ca2+ wave via a Ca2+-induced Ca2+ release mechanism (CICR) (Parker et al., 1996; Berridge, 1997). In order to create a fully sustained Ca2+ signal, activation of Ca2+ entry must occur (Berridge, 1997; Parekh and Penner, 1997).
The pancreatic acinar cell represents an excellent system in which to investigate the mechanism of Ca2+ signal globalization. The pattern of receptor-activated cytosolic Ca2+ oscillations depends on the receptor type, the agonist concentration and the intracellular buffering of Ca2+ (Petersen et al., 1991a). The two physiologically most important agonists, acetylcholine (ACh) and cholecystokinin (CCK), can generate both local and global Ca2+ signals. At high concentrations, both agonists elicit global Ca2+ responses, but at low, just suprathreshold concentrations, ACh evokes local repetitive Ca2+ spikes, whereas CCK, in addition to such local signals also occasionally induces global and relatively long-lasting Ca2+ transients (Petersen et al., 1991a; Thorn et al., 1993).
We have previously investigated the mechanism underlying the generation of local Ca2+ spikes in the apical (secretory) pole of the cell. The local cytosolic Ca2+ spikes are due to Ca2+ release from common oscillator units composed of IP3 and ryanodine receptors. ACh activation of these common oscillator units is triggered via IP3 receptors, whereas CCK responses are triggered via a different, but convergent, pathway dependent on NAADP and cADPR receptors (Cancela and Petersen, 1998; Cancela et al., 1998, 1999, 2000; Petersen and Cancela, 1999; Cancela, 2001). However, the mechanisms involved in the generation of global Ca2+ transients and global sustained Ca2+ elevations remain unclear.
In view of the finding that physiological CCK concentrations evoke very little IP3 production (Matozaki et al., 1990) and the recent report that ACh stimulation may result in both IP3 and cADPR generation (Fukushi et al., 2001), we have investigated the functional consequences of the interaction of multiple intracellular messengers in the generation of local Ca2+ spikes and global Ca2+ transients. We have studied the process that transforms local Ca2+ spikes to global Ca2+ transients by the patch–clamp whole-cell recording technique combined with confocal Ca2+ imaging. The local Ca2+ spikes evoked by a low concentration of ACh were transformed into a global sustained Ca2+ response by cADPR or NAADP, whereas IP3 had a much weaker effect. In contrast, the CCK response was strongly potentiated by IP3, whereas cADPR and NAADP had little effect. In the absence of ACh or CCK stimulation, NAADP alone, like IP3 and cADPR, evoked cytosolic Ca2+ spiking confined to the apical pole of the cell. There were small mutually potentiating effects of cADPR and NAADP, or NAADP and IP3, whereas a cADPR/IP3 mixture was only very slightly more effective than either IP3 or cADPR alone. However, NAADP strongly amplified the local Ca2+ release evoked by a cADPR/IP3 mixture, eliciting a sustained global Ca2+ response. Our data demonstrate that different combinations of Ca2+ releasing messengers can shape the spatio-temporal pattern of Ca2+ signals. Although all the three Ca2+ releasing messengers tested could initiate local Ca2+ spikes, globalization of the signals required interactions between them.
Results
Globalization of the ACh response by cADPR and NAADP, but not IP3
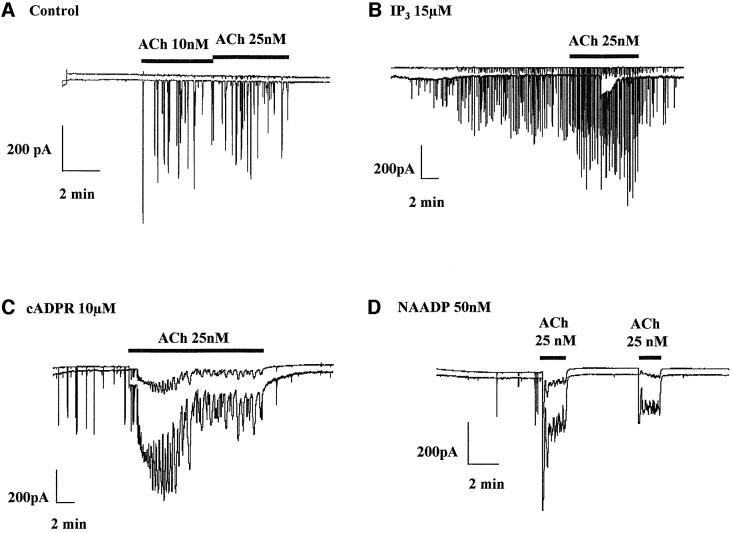
We have recently reported that CCK potentiates the Ca2+-sensitive Cl– current response to low, just suprathreshold concentrations of ACh. This potentiation is dependent on functional cADPR receptors and is blocked by a cADPR antagonist (Cancela et al., 2000). ACh at higher concentrations elicits global Ca2+ release, which could be due to increased IP3 production and/or generation of cADPR (Fukushi et al., 2001). Very high concentrations of IP3 (>100 µM) (Petersen et al., 1991b) or cADPR (100 µM) (Thorn et al., 1994) can elicit sustained cytosolic Ca2+ elevations that are global. Here we investigated whether a low cADPR concentration, which alone would elicit local Ca2+ spikes, could transform a local Ca2+ signal evoked by ACh into a global Ca2+ wave. To do so, we dialysed the cells with an intracellular solution containing 10 µM cADPR and thereafter stimulated with ACh (Figure 1). When cADPR was present in the intracellular solution, ACh evoked long-lasting Ca2+-sensitive currents (Figure 1C; n = 8), which were associated with global Ca2+ waves (n = 3; data not shown). In the absence of cADPR, ACh (as expected, see Petersen et al., 1991a; Thorn et al., 1993; Cancela et al., 2000) elicited short-lasting local Ca2+ spikes (Figure 1A; n = 3).
Fig. 1. Globalization of the local ACh response by cADPR and NAADP, but not IP3. (A) Repetitive spikes of Ca2+-sensitive current were evoked by low, just suprathreshold concentrations of ACh. When IP3 at 15 µM was included in the intracellular pipette solution, the ACh-evoked response was normal (B), but when the intracellular pipette solution contained either cADPR (10 µM) (C) or NAADP (50 nM) (D) ACh elicited larger sustained responses.
In addition to cADPR, the ADP-ribosyl cyclase CD38 may form NAADP, and the NAADP receptors have also been shown to be involved in the CCK response (Cancela et al., 2000; Lee, 2000). We therefore investigated whether NAADP could also potentiate the ACh response. In this series of experiments, the cells were perfused internally with 50 nM NAADP. At this concentration, NAADP elicits short-lasting Ca2+ spikes (Cancela et al., 2000) (Figure 1D). When 25 nM ACh was applied on top of the NAADP stimulus, a sustained Ca2+-sensitive current response was observed, indicative of a global rise in the cytosolic Ca2+ concentration (Figure 1D; n = 12). In the particular cell giving rise to the trace shown in Figure 1D, the NAADP response was very weak, but the potentiation of the ACh response was remarkably strong. In all 12 cells in which NAADP elicited Ca2+ spiking, the subsequent ACh response was markedly potentiated (Figure 1D). Finally, since both the ACh- and CCK-elicited responses depend on functional IP3 receptors, we decided to investigate whether IP3 could also potentiate the ACh response. In the four cells tested with 15 µM IP3 that elicited repetitive short lasting Ca2+ spikes, additional stimulation with 25 nM ACh evoked an increase in the spiking frequency and amplitude (Figure 1B). Comparing the typical records shown in Figure 1, it can be seen that the combinations of ACh and cADPR, as well as ACh and NAADP, produced much stronger responses (Figure 1C and D) than the combination of ACh and IP3 (Figure 1B). This is remarkable, since IP3 itself was a stronger stimulus than either cADPR or NAADP.
Globalization of the CCK response by IP3, but not cADPR or NAADP
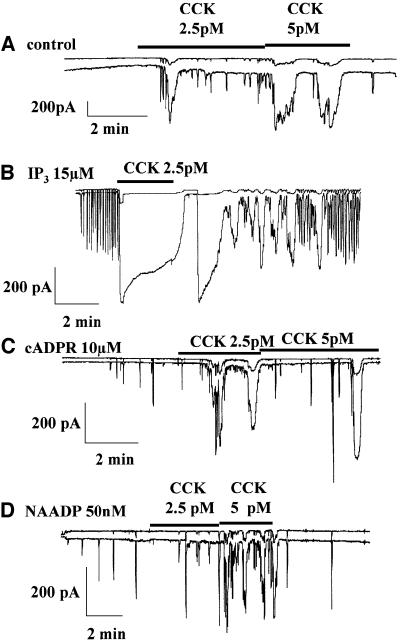
Since receptors for IP3, cADPR and NAADP have all been shown to be involved in the response to CCK (Cancela et al., 2000), we investigated whether addition of one of these messengers could alter the normal Ca2+ signal pattern elicited by a physiological CCK concentration. The cells were internally perfused with either control solution or intracellular solutions containing IP3, cADPR or NAADP, and then stimulated by 2.5 or 5 pM CCK (Figure 2). As previously documented, CCK itself elicited a mixture of short-lasting repetitive spikes and much more long-lasting transients (Figure 2A; n = 11; Petersen et al., 1991a). When CCK (2.5–5 pM) was added on top of an IP3 (15 µM) stimulus, a very large and sustained response was observed (Figure 2B; n = 7). Clearly, IP3 had markedly potentiated the CCK response. In the presence of IP3, the CCK effect was not, as usual, immediately reversible, indicating that the potentiation by IP3 was so strong that even during the period when CCK was being washed out, a substantial effect remained. During internal stimulation with cADPR (10 µM), low frequency spiking was observed (Figure 2C). When CCK (2.5 or 5 pM) was added to the external solution a fairly normal, non-potentiated, response was seen (Figure 2C; n = 9). Internal stimulation with NAADP (50 nM) also elicited Ca2+ spiking with a low frequency (Figure 2D). In this situation, CCK added on top of the internal stimulation again elicited a rather normal, non-potentiated response (Figure 2D; n = 7). These results indicate that NAADP and cADPR, in contrast to IP3, are unable to potentiate the response to CCK, although the receptors for all these messengers are involved in the generation of the normal CCK responses (Cancela et al., 2000).
Fig. 2. Globalization of the CCK response by IP3, but not cADPR or NAADP. (A) Repetitive spikes of Ca2+-sensitive current were evoked by low, just suprathreshold concentrations of CCK. (B) When IP3 (15 µM) was included in the intracellular pipette solution, the CCK-evoked response was strongly potentiated (sustained). When the intracellular pipette solution contained either cADPR (10 µM) (C) or NAADP (50 nM) (D), CCK evoked normal non-potentiated (spiking) responses.
Although low (and most likely physiologically relevant) concentrations of IP3, cADPR and NAADP each evoke local, short-lasting Ca2+ signals, the data shown in Figures 1 and 2 demonstrate that they can all be involved in global Ca2+ signal production. To understand how this can be achieved, we further characterized the functional consequences of multiple combinations of low messenger concentrations.
NAADP evokes Ca2+ spiking localized in the secretory pole
NAADP is the most potent Ca2+ releasing messenger found so far (Chini et al., 1995; Lee and Aarhus, 1995; Genazzani and Galione, 1997; Cancela et al., 1999, 2000; Lee, 2000). In pancreatic acinar cells NAADP acts as a trigger, apparently eliciting a very small primary Ca2+ release, subsequently recruiting neighbouring IP3 and ryanodine receptors, which gives rise to the cytosolic Ca2+ elevation; this is observable with currently available methodology (Cancela et al., 1999, 2000). However, the spatial localization of the Ca2+ spikes evoked by NAADP is unknown.
Figure 3 shows the result from an experiment in which the effect of NAADP (50 nM) in the internal pipette solution was assessed both by patch–clamp recording of the Ca2+-sensitive Cl– current and by simultaneous confocal imaging of the Ca2+-sensitive Fluo 4 fluorescence. As previously documented (Cancela et al., 1999, 2000), NAADP elicited repetitive short-lasting Ca2+ spikes. As shown in Figure 3, the cytosolic Ca2+ rise at the height of the spike was confined to the apical, granule-containing part of the cell (n = 7). The NAADP-elicited Ca2+ spikes are thus localized in exactly the same part of the cell as those evoked by IP3 (Thorn et al., 1993) and cADPR (Thorn et al., 1994).
Fig. 3. NAADP evokes Ca2+ spiking in the secretory pole. Confocal fluorescence microscopy reveals that NAADP, at 50 nM in the intracellular pipette solution, evokes repetitive Ca2+ spikes localized in the apical pole. In the picture panel below the current trace is shown (left) the transmitted light picture of the cell investigated (tip of attached patch pipette is seen), (middle) the fluorescence image between spikes, and finally (right) the fluorescence image demonstrating the position of one of the Ca2+ spikes evoked by NAADP. By comparison with the transmitted light image, it can be seen that the spike occurs in the apical granular pole of the lower right cell. Calibration of the colour coding of the cytosolic Ca2+ concentration is shown at the right-hand side.
IP3 and cADPR evoke localized Ca2+ spiking without interaction
Both IP3 and cADPR elicit local Ca2+ spikes in the secretory pole at low concentrations (<15 µM), whereas higher concentrations of IP3 or cADPR (>100 µM) elicit global Ca2+ rises (Petersen et al., 1991b, Petersen et al., 1994; Thorn et al., 1994). We investigated the effects of mixing low concentrations of IP3 and cADPR. IP3 (10–15 µM) or cADPR (10 µM) evoked repetitive local Ca2+ spikes in the granular part of the cell (Figure 4A and B; n = 4 and 5, respectively). We then perfused the cells internally with a mixture of 10 µM cADPR and 15 µM IP3, which, in all 10 cells investigated, evoked typical repetitive short-lasting Ca2+-sensitive currents corresponding to local Ca2+ elevations (Figure 4C). This indicates that there is no major cross-talk between these two messengers.
Fig. 4. A mixture of IP3 and cADPR only evokes local Ca2+ release. (A) Confocal fluorescence microscopy reveals that cADPR, at 10 µM in the intracellular pipette solution, evokes repetitive Ca2+ spikes localized in the apical pole. The repetitive Ca2+ spikes evoked by IP3 (10 µM) are localized in the apical pole (B) and the repetitive short-lasting Ca2+ spikes evoked by a mixture of cADPR (10 µM) and IP3 (15 µM) (C) are also localized in the apical pole (no mutual potentiation).
NAADP has a modest locally potentiating effect on the local Ca2+ spiking evoked by IP3
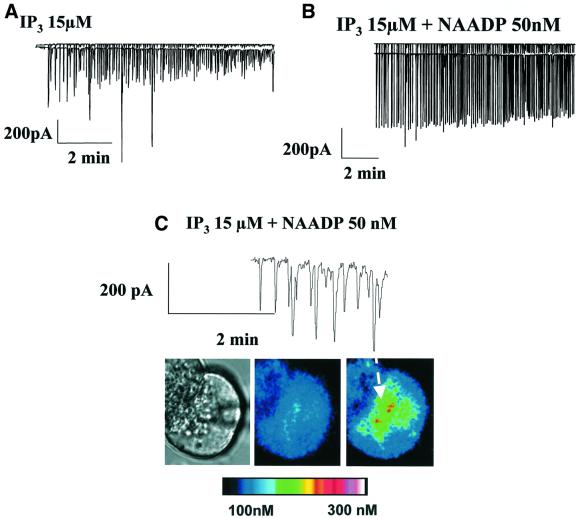
The next sets of experiments were designed to test whether a mixture of low concentrations of IP3 and NAADP could generate larger responses than IP3 or NAADP alone. Figure 5A shows a typical response to stimulation with IP3 (15 µM), consisting of repetitive short-lasting spikes (n = 9) that were associated with Ca2+ elevations confined to the apical granular pole of the cell (n = 4). In five out of six cells, a mixture of NAADP (50 nM) and IP3 (15 µM) generated relatively large, repetitive, short-lasting Ca2+-sensitive currents (Figure 5B), corresponding to local Ca2+ elevations (Figure 5C), whereas in the sixth cell, relatively large spikes on top of a small sustained elevation were observed. The main effect of adding NAADP on top of IP3 was to increase the amplitude of the local Ca2+ spikes.
Fig. 5. A mixture of NAADP and IP3 evokes locally amplified Ca2+ release. (A) IP3 (15 µM in the intracellular pipette solution) evokes repetitive spikes of Ca2+-sensitive current. The underlying Ca2+ spikes are localized in the apical pole (see Figure 4B). (B) A mixture of IP3 (15 µM) and NAADP (50 nM) evokes somewhat amplified repetitive spikes of Ca2+-sensitive current. (C) The Ca2+ spikes elicited by the mixture of IP3 and NAADP are localized in the apical pole. Calibration of the colour coding of the cytosolic Ca2+ concentration is shown at the bottom.
NAADP globalizes cADPR-elicited local Ca2+ spiking
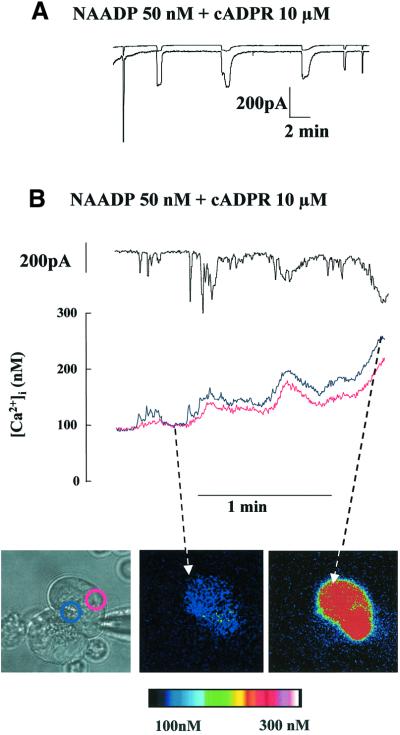
Both cADPR and NAADP receptors are involved in the Ca2+ response evoked by the hormone CCK (Cancela and Petersen, 1998; Cancela et al., 1999, 2000). The most striking feature of the response to a physiological CCK concentration (2–10 pM) is the mixture of short-lasting local Ca2+ spikes and long-lasting global Ca2+ transients (Petersen et al., 1991a). However, NAADP (Figure 3) and cADPR (Thorn et al., 1994) evoked mainly localized Ca2+ spiking in the granular part of the cell without triggering a long-lasting global Ca2+ wave. During internal perfusion with 10 µM cADPR, all the cells tested displayed short-lasting Ca2+-sensitive currents (n = 14). To investigate how CCK could generate global Ca2+ waves using these particular messengers, we performed experiments with both NAADP and cADPR in the pipette solution. Since the same enzyme, ADP-ribosyl cyclase, forms both cADPR and NAADP, this experiment might mimic a relevant physiological situation (Lee, 2000). Figure 6A shows the result of an experiment in which a mixture of NAADP and cADPR elicits repetitive, relatively long-lasting Ca2+-sensitive currents (n = 15). Figure 6B shows the result of an experiment simultaneously recording the Ca2+-sensitive current and the cytosolic Ca2+ concentration in a single pancreatic acinar cell. Initially, the mixture of NAADP and cADPR elicits repetitive local Ca2+ spiking, but gradually there is an evolution to a pattern of relatively long-lasting global Ca2+ waves (n = 6). This suggests that although both NAADP and cADPR can act separately to initiate local Ca2+ elevations in the secretory pole of the cell, they can also act together to generate the mixed pattern of local Ca2+ spikes and global Ca2+ waves seen in a typical response to a physiological level of CCK.
Fig. 6. NAADP transforms the short-lasting Ca2+ spikes evoked by cADPR into long-lasting Ca2+ spikes. (A) A mixture of NAADP (50 nM) and cADPR (10 µM) in the intracellular pipette solution evokes repetitive, long-lasting spikes of Ca2+-sensitive current with relatively long intervals between the spikes. (B) The result of an experiment simultaneously recording the Ca2+-sensitive Cl– current and the cytosolic Ca2+ concentration measured by microfluorimetry in a single pancreatic acinar cell. The short-lasting spikes are local and the long-lasting spikes are global. Calibration of the colour coding of the cytosolic Ca2+ concentration is shown at the bottom.
NAADP globalizes localized Ca2+ spiking evoked by a mixture of cADPR and IP3
The CCK response depends on functional NAADP and cADPR receptors, but there is also, as for ACh, a requirement for operational IP3 receptors. Since ACh can stimulate the ADP-ribosyl cyclase CD38 (Fukushi et al., 2001), both CCK and ACh may generate cADPR, NAADP and IP3, although the precise levels are likely to depend very much on the agonist and its concentration. However, the functional consequences of the concerted action of the three messengers have never been explored. The next sets of experiments were therefore designed to determine whether NAADP triggers a globalization of the local Ca2+ spikes evoked by a mixture of cADPR and IP3. Figure 7A and B shows that NAADP strongly potentiates the Ca2+ release evoked by the cADPR/IP3 mixture. Infusion of cells with the triple mixture NAADP (50 nM), cADPR (10 µM) and IP3 (15 µM) induced a sustained Ca2+-sensitive current, often with superimposed spikes, in six out of seven cells investigated. The sustained Ca2+-sensitive current corresponds to a sustained global Ca2+ elevation (n = 4) (Figure 7B). Comparing the responses to the mixture of NAADP and cADPR (Figure 6) with those to the triple mixture NAADP + cADPR + IP3 (Figure 7), it is apparent that whereas NAADP + cADPR typically elicited repetitive pulses of local and global Ca2+ transients, the triple mixture evoked a sustained global Ca2+ rise.
Fig. 7. NAADP transforms the local Ca2+ spikes evoked by a cADPR + IP3 mixture into a sustained global Ca2+ elevation. (A) A mixture of short-lasting spikes and sustained activation of Ca2+-sensitive current evoked by a triple mixture of NAADP (50 nM), cADPR (10 µM) and IP3 (15 µM). External application of 20 mM caffeine, used as an IP3 antagonist, abolished the sustained activation of the Ca2+-sensitive current, indicating that functional IP3 receptors are required to generate the sustained Ca2+ signal. (B) The result of an experiment simultaneously recording the Ca2+-sensitive current and the cytosolic Ca2+ concentration measured by microfluorimetry in a single pancreatic acinar cell. Confocal fluorescence microscopy reveals that the triple mixture of NAADP (50 nM), cADPR (10 µM) and IP3 (15 µM) in the intracellular pipette solution evokes a sustained global Ca2+ elevation. Calibration of the colour coding of the cytosolic Ca2+ concentration is shown at the bottom.
In order to assess the possible functional consequence of adding IP3 to a mixture of NAADP and cADPR, we measured the maximal amplitude of the Ca2+-activated Cl– current in the two sets of experiments. It has recently been demonstrated that all Ca2+-sensitive Cl– channels in pancreatic acinar cells are located in the apical (secretory) part of the plasma membrane (Park et al., 2001b). The Ca2+-dependent Cl– current is responsible for driving acinar fluid secretion, which together with the exocytotic secretion of digestive enzymes (Nemoto et al., 2001) represents the major functional consequence of the agonist-elicited cytosolic Ca2+ elevation. The mean peak amplitude of the Ca2+-dependent current response to NAADP + cADPR was (±SE) 435 ± 118 pA (n = 8), whereas the corresponding value for the triple mixture was 1283 ± 227 pA (n = 9). Thus, addition of IP3 to the mixture of NAADP + cADPR markedly enhanced the Cl– current across the apical membrane, most likely due to an enhanced amplitude of the Ca2+ concentration rise very close to the inner mouths of the Cl– channels.
Caffeine (20 mM), used as a permeant IP3 receptor antagonist, was employed to test the need for functional IP3 receptors in the concerted activity of the three messengers. Although best known as an activator of ryanodine receptors, caffeine also inhibits the opening of IP3 receptors (Wakui et al., 1990; Parker and Ivorra, 1991; Brown et al., 1992; Ehrlich et al., 1994; Petersen and Cancela, 1999). This effect is clearly not mediated by an increase in the intracellular cyclic AMP concentration (Wakui et al., 1990; Brown et al., 1992), but is likely to be a direct effect on the IP3 receptor or a closely associated protein, since it has been observed in single channel current studies of isolated IP3 receptors from cerebellum (Ehrlich et al., 1994). In the pancreatic acinar cells, caffeine (20 μM) does not release Ca2+ via ryanodine receptors and does not deplete intracellular Ca2+ stores. With IP3 present in the pipette solution there is no Ca2+ spiking when caffeine is present from the beginning of an experiment, but immediately (within seconds) after caffeine removal, Ca2+ spiking starts (see Figure 5 in Wakui et al., 1990). Furthermore, caffeine has the advantage of being extremely membrane permeant. It can therefore be applied externally and its effects are rapidly reversible (Wakui et al., 1990; Petersen and Cancela, 1999; Cancela et al., 2000). Extracellular application of 20 mM caffeine dramatically, repeatedly and reversibly reduced the Ca2+ release evoked by the triple messenger mixture (Figure 7A; n = 7). This indicates that functional IP3 receptors are required for sustained Ca2+ release.
Discussion
Our experiments demonstrate that the generation of global cytosolic Ca2+ signals depend on interaction between different Ca2+ releasing messenger pathways, which can be activated separately or in combination. We analysed the functional consequence of the concerted activity of IP3, cADPR and NAADP. Our new results demonstrate that the spatio-temporal pattern of a cytosolic Ca2+ signal can be shaped by different combinations of Ca2+ releasing messengers. One important finding from this work is that although every messenger can initiate local Ca2+ spikes, globalization of the Ca2+ signal requires interaction between these messengers. The strongest Ca2+ signals were obtained by a triple mixture containing IP3, cADPR and NAADP.
Agonist-specific Ca2+ signal patterns and messenger interactions
In pancreatic acinar cells, ACh and CCK induce specific Ca2+ signal signatures (Petersen et al., 1991a, Petersen et al., 1994). Low and physiological concentrations of both agonists elicit repetitive, short-lasting Ca2+ spikes confined to the apical granular pole (Thorn et al., 1993). These local Ca2+ spikes are sufficient to elicit exocytosis, as assessed by capacitance measurements (Maruyama et al., 1993; Maruyama and Petersen, 1994), and fluid secretion, as assessed by monitoring the Ca2+-dependent Cl– current across the apical membrane (Thorn et al., 1993; Park et al., 2001b). CCK, but not ACh, at physiological concentrations also elicits much longer-lasting global Ca2+ transients (Petersen et al., 1991a; Thorn et al., 1993). The frequency with which the global transients occur is concentration dependent. At the lower end of the physiological concentration range (1–10 pM) there are very infrequent, long, global transients, and the response essentially consists of repetitive local Ca2+ spikes, whereas at the top end long transients are seen regularly. The physiological importance of these global transients has not been clarified, but one possibility is that they are connected to the CCK-induced pancreatic growth response (Petersen et al., 1994). The results presented in Figures 1 and 2 indicate that the response to a low ACh concentration is principally triggered by IP3, since it can be markedly potentiated by cADPR and NAADP, but not by IP3, whereas the response to a physiological CCK concentration must be triggered by cADPR and NAADP, since it can be dramatically potentiated by IP3, but not by cADPR or NAADP. Clearly only complementary messengers can effectively potentiate a response. One important conclusion to be drawn from our new data is that increasing the intensity of stimulation with any one agonist is likely to produce all three messengers and consequently to generate substantial Ca2+ waves. To do so, ACh and CCK may recruit these messengers in a different sequence. These findings also provide a mechanism to explain the marked potentiation by CCK of the ACh response (Cancela et al., 2000).
The experiments with messenger mixtures revealed only a relatively minor interaction between IP3 and cADPR (Figure 4), and a modest interaction between IP3 and NAADP (Figure 5). There was a somewhat stronger potentiating interaction between cADPR and NAADP, but in order to obtain the type of global and sustained response that can result from combining CCK with IP3 (Figure 2) or ACh with cADPR or NAADP (Figure 1), it was necessary to use all three messengers together (Figure 7). Using the membrane-permeant IP3 receptor antagonist caffeine (Petersen and Cancela, 1999), we were able to demonstrate that activation of IP3 receptors is essential for maintaining a sustained response. Application of caffeine led to a fully reversible transformation from a sustained cytosolic Ca2+ elevation to repetitive baseline spiking (Figure 7).
Localization of intracellular Ca2+ release channels
IP3, cADPR and NAADP each simply evoke Ca2+ release in the apical pole. This has been well documented for IP3 and cADPR (Thorn et al., 1993, 1994). Our data (Figure 3) indicate that the short-lasting Ca2+-dependent currents evoked by NAADP are associated with Ca2+ elevations specifically in the apical pole of the cells. This result demonstrates directly that infusion of NAADP into a cell can release Ca2+ in one specific region without affecting other parts. This could be due to the exclusive presence of NAADP receptors in the apical pole or to a much higher concentration of NAADP receptors in this part of the cell than in the basal region. It could also be explained by a more diffuse presence of NAADP receptors throughout the cell, since the NAADP response is completely dependent on functional IP3 and ryanodine receptors (Cancela et al., 2000). It is known that ryanodine receptors are present throughout the acinar cell (Leite et al., 1999; Fitzsimmons et al., 2000; Straub et al., 2000), whereas IP3 receptors are preferentially localized in the apical pole (Nathanson et al., 1994; Lee et al., 1997). Most likely, cADPR elicits local Ca2+ spikes in the apical pole (Thorn et al., 1994) because of the concentration of IP3 receptors in this region, since the cADPR responses are completely dependent on functional IP3 receptors (Cancela et al., 2000). It is therefore entirely possible, and indeed likely, that the general primary localization of Ca2+ signals to the apical pole, irrespective of which Ca2+ releasing messenger is used, is principally due to the dominant presence of IP3 receptors in this part of the cell. Since all Ca2+ signal initiation depends on interaction between at least ryanodine and IP3 receptors, it must occur at sites where both these Ca2+ release channels co-exist, and the only such region is the apical pole.
The general concept concerning the organization of the major intracellular Ca2+ store, the ER, is that it forms a continuous sheet enclosing a single internal space. The continuous lumen allows Ca2+ and other small molecules to diffuse rapidly over relatively long distances (Terasaki et al., 1994; Mogami et al., 1997; Subramanian and Meyer, 1997; Park et al., 2000; Petersen et al., 2001). In the pancreatic acinar cells, the primary localization of cytosolic Ca2+ signal generation in the apical granular pole (Kasai et al., 1993; Thorn et al., 1993) is due to clustering of IP3 receptors in the most apical parts of the ER extensions into the granular area (Lee et al., 1997). Opening of these channels can mobilize Ca2+ from the whole of the ER, and particularly from the major part of this store in the basolateral part of the cell due to the ability of Ca2+ to diffuse within the lumen of the ER, from the base of the cell to its apex (Mogami et al., 1997; Park et al., 2000; Petersen et al., 2001).
Globalization of Ca2+ signals
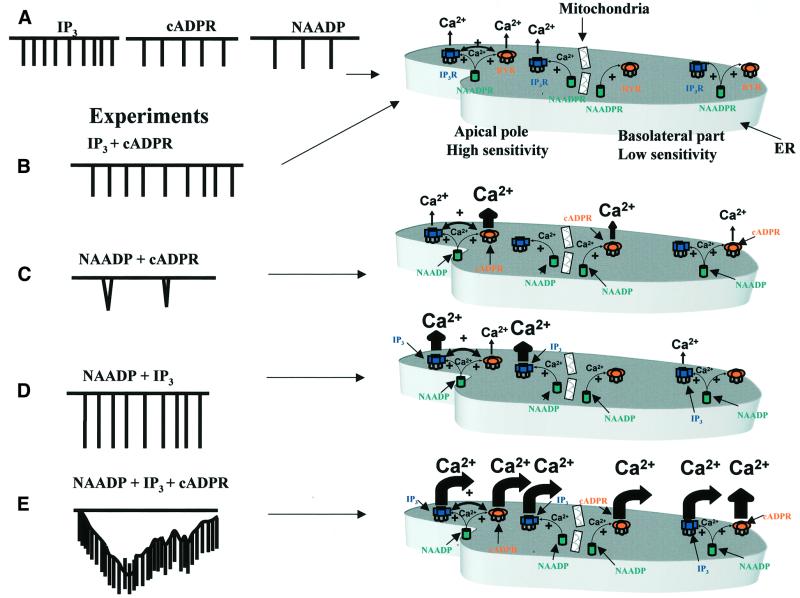
There would appear to be two separate aspects of the globalization process. Unlike the lumenally continuous ER, the cytosol is effectively compartmentalized with respect to Ca2+ diffusion by a major mitochondrial Ca2+ buffer barrier placed on the border between the apical granular pole and the rest of the cell (Tinel et al., 1999; Park et al., 2001a). This barrier undoubtedly plays a major role in mostly confining Ca2+ signals generated in the apical region to this part of the cell (Tinel et al., 1999; Park et al., 2001a). In order for a Ca2+ signal to become global, this barrier has to be overwhelmed by a substantial amount of Ca2+ released in the granular region. However, it is also known that when a Ca2+ wave progresses through the cell from the apical to the basal pole, there is a regenerative process, almost certainly due to Ca2+-induced Ca2+ release (Kasai and Augustine, 1990; Toescu et al., 1992, 1994). This means that under these circumstances Ca2+ release channels also open in the basal pole. The mechanism by which silent Ca2+ release channels in the basal pole become activated during globalization of Ca2+ signalling is not fully understood, but our data indicate that a combination of IP3, cADPR and NAADP plays an important role in this process (Figure 8).
Fig. 8. (A) Schematic model showing that every messenger on its own can initiate local Ca2+ spikes. (B) There is little interaction between IP3 and cADPR. (C) NAADP does potentiate the action of cADPR producing long-lasting global spikes at long intervals. (D) In contrast, NAADP only has a locally potentiating effect on the local IP3-evoked Ca2+ spikes. (E) When all three messengers act together a large, sustained, global Ca2+ elevation is observed. The apical pole is the most sensitive part of the cell. In the models shown to the right, the basolateral part of the cell contains poorly sensitive Ca2+ release units that cannot trigger a wave in the presence of either IP3, cADPR or NAADP alone. To generate a Ca2+ wave across the cell, a combination of potentiated Ca2+ release in the apical pole, helping to overcome the mitochondrial barrier, and sensitization of Ca2+ release channels by coincident activation of ryanodine, IP3 and NAADP receptors by their respective messengers in the basal pole is necessary.
A global Ca2+ wave is generated by the concerted activity of elementary Ca2+ release units, which act as ‘building blocks’ (Parker et al., 1996; Marchant et al., 1999; Berridge et al., 2000). The distance between these elementary units may vary between cell types, but the Ca2+ wave propagates by recruiting, in a saltatory manner, neighbouring Ca2+ release sites (Parker et al., 1996; Berridge, 1997; Boittin et al., 1998; Cannell and Soeller, 1999; Koizumi et al., 1999; Marchant et al., 1999; Berridge et al., 2000). The Ca2+ release units are recruited by several mechanisms, including Ca2+ diffusion, Ca2+-induced Ca2+ release and increase of IP3 production. All these mechanisms increase the frequency of elementary Ca2+ release events, which, once the threshold is reached, will trigger a Ca2+ wave. However, if poorly sensitive Ca2+ release units surround the most sensitive Ca2+ release units, then the Ca2+ signal remains localized and the Ca2+ wave is aborted (Parker et al., 1996; Marchant et al., 1999; Berridge et al., 2000). This is exactly what happens in our experiments with the infusion of a low concentration of either IP3, cADPR or NAADP, each of which is able to generate short-lasting Ca2+ spikes in the apical pole of the cell whithout triggering a Ca2+ wave. In this situation, the basolateral part of the cell contains poorly sensitive Ca2+ release units that cannot trigger a wave (Figure 8). A Ca2+ wave across the cell is generated by a combination of potentiated Ca2+ release in the apical pole, helping to overcome the mitochondrial barrier, and sensitization of Ca2+ release channels in the basal pole by coincident activation of ryanodine, IP3 and NAADP receptors by their respective messengers (Figure 8).
NAADP as a key messenger in Ca2+ signal globalization
From our present work, NAADP is emerging as a key messenger in the globalization of Ca2+ signals. NAADP itself has a modest effect, since it only releases Ca2+ in the apical part of the cell; however, its unique ability to interact with ryanodine and IP3 receptor activity allows a substantial increase in the medium excitability to further activation by either cADPR or IP3. Recent work in other cell types has shown that not only pancreatic acinar cells possess several Ca2+ releasing messengers (Churchill and Galione, 2000, 2001; Cancela, 2001; Lee, 2001), suggesting that our model may be more generally valid. In systems such as ascidian oocytes, starfish oocytes, T lymphocytes and sea urchin eggs, NAADP but also cADPR and IP3 release Ca2+ from the internal stores in the same target cell (Albrieux et al., 1998; Guse et al., 1999; Berg et al., 2000; Churchill and Galione, 2000, 2001; Santella et al., 2000). These cells may have one continuous Ca2+ store or separate multiple Ca2+ stores located in different regions (Malgaroli et al., 1990; Golovina and Blaustein, 1997; Lee, 1997, 2001; Hofer et al., 1998; Churchill and Galione, 2000, 2001; Patel et al., 2001).
Our work with IP3, cADPR and NAADP has demonstrated the functional consequences of the actions of different Ca2+ releasing messengers in one target cell (Figure 8). They can be recruited individually or in combination to give an important diversity of Ca2+ signals (Figure 8). The new types of messenger interaction unravelled in our work may represent the building blocks for the more complex associations seen during stimulation with agonists. This is important, because cells in their native environment are constantly surrounded by multiple stimuli and must respond in an appropriate manner.
Materials and methods
Isolation of pancreatic acinar cells
Isolated single and double mouse pancreatic acinar cells were prepared and loaded with Fluo 4 at 60 µM in the pipette solution as described previously (Park et al., 2001a,b).
Patch–clamp recordings
Cells were investigated using the whole-cell patch–clamp configuration. From a holding potential of –30 mV, steps were made to 0 mV, the reversal potential of the two Ca2+-dependent currents through Cl– and non-selective cation channels (Thorn and Petersen, 1992). The Cl– current is by far the most important quantitatively (Park et al., 2001b). Using our solutions, the reversal potential of both the Cl– and non-selective cation currents were at 0 mV (Petersen et al., 1991a). Small deviations in ECl and Ecation and in the holding potential sometimes produce small inward or outward currents at 0 mV. At –30 mV we obtained a measure of both the Ca2+-dependent currents, which are an index of the cytosolic Ca2+ changes (Thorn et al., 1993; Tinel et al., 1999). The extracellular Na+-rich solution contained (in mM): 140 NaCl, 4.7 KCl, 1.13 MgCl2, 10 glucose, 1 CaCl2 and 10 HEPES–NaOH (pH 7.2). CCK octapeptide or ACh were added to the external solution as indicated. The internal solution contained (in mM): 140 KCl, 1.13 MgCl2, 0.05 EGTA, 2 ATP and 10 HEPES–KOH (pH 7.2). Extracellular application of CCK and ACh was performed by means of a gravity perifusion system.
Confocal imaging
Fluorescence measurements and calcium concentration calibration on Fluo 4-loaded cells (Takahashi et al., 1999; Park et al., 2001a,b) were done using a Zeiss LSM510 confocal system. The KD for Fluo 4–Ca2+ at room temperature was assumed to be 400 nM (Molecular Probes). An objective (60×) with NA 1.4 was used in all experiments. For fast scanning experiments, five frames per second final scanning speed was used. Fluo 4 was excited using a 488 nm laser light. Emitted light was collected using a BP505-550 filter. Image analysis was performed using the Zeiss confocal 510 image software as well as software developed by us. Images were divided by the first image. A linear colour scale was used in all cases.
Chemicals
NAADP, cADPR, 2,4,5-IP3, caffeine, CCK and ACh were purchased from Sigma. Fluo 4 and NAADP were from Molecular Probes.
Acknowledgments
Acknowledgements
We thank N.Burdakova for technical support. This work was supported by an MRC Programme Grant. O.H.P. is an MRC Research Professor. F.V.C. was supported by a grant from the Association pour la Recherche sur le Cancer, France (ARC).
References
- Albrieux M., Lee,H.G. and Villaz,M. (1998) Calcium signaling by cyclic ADP-ribose, NAADP and inositol trisphosphate are involved in distinct functions in ascidian oocytes. J. Biol. Chem., 273, 14566–14574. [DOI] [PubMed] [Google Scholar]
- Berg I., Potter,B.V.L., Mayr,G.W. and Guse,A.H. (2000) Nicotinic acid adenine dinucleotide phosphate (NAADP+) is an essential regulator of T-lymphocyte Ca2+ signaling. J. Cell Biol., 150, 581–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M.J. (1997) Elementary and global aspects of calcium signalling. J. Physiol., 499, 291–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M.J., Bootman,M.D. and Lipp,P. (1998) Calcium — a life and death signal. Nature, 395, 645–648. [DOI] [PubMed] [Google Scholar]
- Berridge M.J., Lipp,P. and Bootman,M.D. (2000) The versatility and universality of calcium signaling. Nature Rev. Mol. Cell. Biol., 1, 11–21. [DOI] [PubMed] [Google Scholar]
- Boittin F.X., Coussin,F., Macrez,N., Mironneau,C. and Mironneau,J. (1998) Inositol 1,4,5-trisphosphate- and ryanodine-sensitive Ca2+ release channel-dependent Ca2+ signalling in rat portal vein myocytes. Cell Calcium, 23, 303–311. [DOI] [PubMed] [Google Scholar]
- Brown G.R., Sayers,L.G., Kirk,C.J., Michell,R.H. and Michelangeli,F. (1992) The opening of the inositol 1,4,5-trisphosphate-sensitive Ca2+ channel in rat cerebellum is inhibited by caffeine. Biochem. J., 282, 309–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancela J.M. (2001) Specific Ca2+ signaling evoked by cholecystokinin and acetylcholine: the roles of NAADP, cADPR and IP3. Annu. Rev. Physiol., 63, 99–117. [DOI] [PubMed] [Google Scholar]
- Cancela J.M. and Petersen,O.H. (1998) The cyclic ADP-ribose antagonist 8-NH2-cADP-ribose blocks cholecystokinin-evoked cytosolic Ca2+ spiking in pancreatic acinar cells. Pflügers Arch., 435, 746–748. [DOI] [PubMed] [Google Scholar]
- Cancela J.M., Mogami,H., Tepikin,A.V. and Petersen,O.H. (1998) Intracellular glucose switches between cyclic ADP-ribose and inositol trisphosphate triggering of cytosolic Ca2+ spiking. Curr. Biol., 8, 865–868. [DOI] [PubMed] [Google Scholar]
- Cancela J.M., Churchill,G.C. and Galione,A. (1999) Coordination of agonist-induced Ca2+-signalling patterns by NAADP in pancreatic acinar cells. Nature, 398, 74–76. [DOI] [PubMed] [Google Scholar]
- Cancela J.M., Gerasimenko,O.V., Gerasimenko,J.V., Tepikin,A.V. and Petersen,O.H. (2000) Two different but converging messenger pathways to intracellular Ca2+ release: the roles of NAADP, cADPR and IP3. EMBO J., 19, 2549–2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannell M.B. and Soeller,C. (1999) Mechanisms underlying calcium sparks in cardiac muscle. J. Gen. Physiol., 113, 373–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chini E.N., Beers,K.W. and Dousa,T.P. (1995) Nicotinate adenine dinucleotide phosphate (NAADP) triggers a specific calcium release system in sea urchin eggs. J. Biol. Chem., 270, 3216–3223. [DOI] [PubMed] [Google Scholar]
- Churchill G.C. and Galione,A. (2000) Spatial control of Ca2+ signaling by nicotinic acid adenine dinucleotide phosphate diffusion and gradients. J. Biol. Chem., 275, 38687–38692. [DOI] [PubMed] [Google Scholar]
- Churchill G.C. and Galione,A. (2001) NAADP induces Ca2+ oscillations via a two-pool mechanism by priming IP3 and cADPR-sensitive Ca2+ stores. EMBO J., 20, 2666–2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich B.E., Kaftan,E., Bezprozvannaya,S. and Bezprozvanny,I. (1994) The pharmacology of intracellular Ca2+ release channels. Trends Pharmacol. Sci., 15, 145–148. [DOI] [PubMed] [Google Scholar]
- Fitzsimmons T.J., Gukovsky,I., McRoberts,J.A., Rodriguez,E., Lai,F.A. and Pandol,S.J. (2000) Multiple isoforms of the ryanodine receptor are expressed in rat pancreatic acinar cells. Biochem. J., 351, 265–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushi Y. et al. (2001) Identification of cyclic ADP-ribose-dependent mechanisms in pancreatic muscarinic Ca2+ signaling using CD38 knockout mice. J. Biol. Chem., 276, 649–655. [DOI] [PubMed] [Google Scholar]
- Galione A. (1994) Cyclic ADP-ribose, the ADP-ribosyl cyclase pathway and calcium signalling. Mol. Cell. Endocrinol., 98, 125–131. [DOI] [PubMed] [Google Scholar]
- Genazzani A.A. and Galione,A. (1997) A Ca2+ release mechanism gated by the novel pyridine nucleotide, NAADP. Trends Pharmacol. Sci., 18, 108–110. [DOI] [PubMed] [Google Scholar]
- Golovina V.A. and Blaustein,M.P. (1997) Spatially and functionally distinct Ca2+ stores in sarcoplasmic and endoplasmic reticulum. Science, 275, 1643–1648. [DOI] [PubMed] [Google Scholar]
- Guse A.H. (1999) Cyclic ADP-ribose: A novel Ca2+-mobilising second messenger. Cell. Signal., 11, 309–316. [DOI] [PubMed] [Google Scholar]
- Guse A.H. et al. (1999) Regulation of calcium signalling in T lymphocytes by the second messenger cyclic ADP-ribose. Nature, 398, 70–73. [DOI] [PubMed] [Google Scholar]
- Hofer A.M., Landolfi,B., Debellis,L., Pozzan,T. and Curci,S. (1998) Free [Ca2+] dynamics measured in agonist-sensitive stores of single living intact cells: a new look at the refilling process. EMBO J., 17, 1986–1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K., Miyashita,Y. and Kasai,H. (1999) Kinetic control of multiple forms of Ca2+ spikes by inositol trisphosphate in pancreatic acinar cells. J. Cell Biol., 146, 405–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaggar J.H., Porter,V.A, Lederer,W.J. and Nelson,M.T. (2000) Calcium sparks in smooth muscle. Am. J. Physiol. Cell Physiol., 278, C235–C256. [DOI] [PubMed] [Google Scholar]
- Kasai H. and Augustine,G.J. (1990) Cytosolic Ca2+ gradients triggering unidirectional fluid secretion from exocrine pancreas. Nature, 348, 735–738. [DOI] [PubMed] [Google Scholar]
- Kasai H., Li,Y.X. and Miyashita,Y. (1993) Subcellular distribution of Ca2+ release channels underlying Ca2+ waves and oscillations in exocrine pancreas. Cell, 74, 669–677. [DOI] [PubMed] [Google Scholar]
- Koizumi S., Bootman,M.D., Bobanovic,L.K., Schell,M.J., Berridge,M.J. and Lipp,P. (1999) Characterization of elementary Ca2+ release signals in NGF-differentiated PC12 cells and hippocampal neurons. Neuron, 22, 125–137. [DOI] [PubMed] [Google Scholar]
- Lee H.C. (1997) Mechanisms of calcium signalling by cyclic ADP-ribose and NAADP. Physiol. Rev., 77, 1133–1164. [DOI] [PubMed] [Google Scholar]
- Lee H.C. (2000) NAADP: an emerging calcium signaling molecule. J. Membr. Biol., 173, 1–8. [DOI] [PubMed] [Google Scholar]
- Lee H.C. (2001) Physiological functions of cADP-ribose and NAADP as calcium messengers. Annu. Rev. Pharmacol. Toxicol., 41, 317–345. [DOI] [PubMed] [Google Scholar]
- Lee H.C. and Aarhus,R. (1995) A derivative of NADP mobilizes calcium stores insensitive to inositol trisphosphate and cyclic ADP-ribose. J. Biol. Chem., 270, 2152–2157. [DOI] [PubMed] [Google Scholar]
- Lee M.G., Xu,X., Zeng,W., Diaz,J., Wojcikiewicz,J.H., Kuo,T.H., Wuytack,F., Racymaekers,L. and Muallem,S. (1997) Polarized expression of Ca2+ channels in pancreatic and salivary gland cells. J. Biol. Chem., 272, 15765–15770. [DOI] [PubMed] [Google Scholar]
- Leite M.F., Dranoff,J.A., Gao,L. and Nathanson,M.H. (1999) Expression and subcellular localization of the ryanodine receptor in rat pancreatic acinar cells. Biochem. J., 337, 305–309. [PMC free article] [PubMed] [Google Scholar]
- Malgaroli A., Fesce,R. and Meldolesi,J. (1990) Spontaneous [Ca2+]i fluctuations in rat chromaffin cells do not require inositol 1,4,5-trisphosphate elevations but are generated by a caffeine- and ryanodine-sensitive intracellular Ca2+ store. J. Biol. Chem., 265, 3005–3008. [PubMed] [Google Scholar]
- Marchant J., Callamaras,N. and Parker,I. (1999) Initiation of IP3-mediated Ca2+ waves in Xenopus oocytes. EMBO J., 18, 5285–5299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama Y. and Petersen,O.H. (1994) Delay in granular fusion evoked by repetitive cytosolic Ca2+ spikes in mouse pancreatic acinar cells. Cell Calcium, 16, 419–430. [DOI] [PubMed] [Google Scholar]
- Maruyama Y., Inooka,G., Li,Y.X., Miyashita,Y. and Kasai,H. (1993) Agonist-induced localized Ca2+ spikes directly triggering exocytotic secretion in exocrine pancreas. EMBO J., 12, 3017–3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matozaki T., Goke,B., Tsunoda,Y., Rodriguez,M., Martinez,J. and Williams,J.A. (1990) Two functionally distinct cholecystokinin receptors show different modes of action on Ca2+ mobilization and phospholipid hydrolysis in isolated rat pancreatic acini. Studies using a new cholecystokinin analog, JMV-180. J. Biol. Chem., 265, 6247–6254. [PubMed] [Google Scholar]
- Meldolesi J. and Pozzan,T. (1998) The heterogeneity of ER Ca2+ stores has a key role in non muscle cell signaling and function. J. Cell Biol., 142, 1395–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogami H., Nakano,K., Tepikin,A.V. and Petersen,O.H. (1997) Ca2+ flow via tunnels in polarized cells: recharging of apical Ca2+ stores by focal Ca2+ entry through basal membrane patch. Cell, 88, 49–55. [DOI] [PubMed] [Google Scholar]
- Nathanson M.H., Fallon,M.B., Padfield,P.J. and Maranto,A.R. (1994) Localization of the type 3 inositol 1,4,5-trisphosphate receptor in the Ca2+ wave trigger zone of pancreatic acinar cells. J. Biol. Chem., 269, 4693–4696. [PubMed] [Google Scholar]
- Nemoto T., Kimura,R., Ito,K., Tachikawa,A., Miyashita,Y., Iino,M. and Kasai,H. (2001) Sequential replenishment mechanism of exocytosis in pancreatic acini. Nature Cell Biol., 3, 253–258. [DOI] [PubMed] [Google Scholar]
- Parekh A. and Penner,R. (1997) Store depletion and calcium influx. Physiol. Rev., 77, 901–930. [DOI] [PubMed] [Google Scholar]
- Park M.K., Petersen,O.H. and Tepikin,A.V. (2000) The endoplasmic reticulum as one continuous Ca2+ pool: visualization of rapid Ca2+ movement and equilibration. EMBO J., 19, 5729–5739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park M.K., Ashby,M.C., Erdemli,G., Petersen,O.H. and Tepikin,A.V. (2001a) Perinuclear, perigranular and sub-plasmalemmal mitochondria have distinct functions in the regulation of cellular calcium transport. EMBO J., 20, 1863–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park M.K., Lomax,R.B., Tepikin,A.V. and Petersen,O.H. (2001b) Local uncaging of caged Ca2+ reveals distribution of Ca2+-activated Cl– channels in pancreatic acinar cells. Proc. Natl Acad. Sci. USA, 98, 10948–10953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker I. and Ivorra,I. (1991) Caffeine inhibits inositol trisphosphate-mediated liberation of intracellular calcium in Xenopus oocytes. J. Physiol., 433, 229–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker I., Choi,J. and Yao,Y. (1996) Elementary events of InsP3-induced Ca2+ liberation in Xenopus oocytes: hot spots, puffs and blips. Cell Calcium, 20, 105–121. [DOI] [PubMed] [Google Scholar]
- Patel S., Churchill,G.C. and Galione,A. (2001) Coordination of Ca2+ signalling by NAADP. Trends Biochem. Sci., 26, 482–489. [DOI] [PubMed] [Google Scholar]
- Perez-Tersic C.M., Chini,E.N., Shen,S.S., Dousa,T.P. and Clapham,D.E. (1995) Ca2+ release triggered by nicotinate adenine dinucleotide phosphate in intact sea urchin eggs. Biochem. J., 312, 955–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen C.C.H., Toescu,E.C. and Petersen,O.H. (1991a) Different patterns of receptor-activated cytoplasmic Ca2+ oscillations in single pancreatic acinar cells: dependence on receptor type, agonist concentration and intracellular Ca2+ buffering. EMBO J., 10, 527–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen C.C.H., Toescu,E.C., Potter,B.V.L. and Petersen,O.H. (1991b) Inositol trisphosphate produces different patterns of cytoplasmic Ca2+ spiking depending on its concentration. FEBS Lett., 293, 179–182. [DOI] [PubMed] [Google Scholar]
- Petersen O.H. and Cancela,J.M. (1999) New Ca2+-releasing messengers: are they important in the nervous system? Trends Neurosci., 22, 488–494. [DOI] [PubMed] [Google Scholar]
- Petersen O.H., Petersen,C.C.H. and Kasai,H. (1994) Calcium and hormone action. Annu. Rev. Physiol., 56, 297–319. [DOI] [PubMed] [Google Scholar]
- Petersen O.H., Tepikin,A.V. and Park,M.K. (2001) The endoplasmic reticulum: one continuous or several separate Ca2+ stores. Trends Neurosci., 24, 271–276. [DOI] [PubMed] [Google Scholar]
- Pozzan T., Rizzuto,R., Volpe,P. and Meldolesi,J. (1994) Molecular and cellular physiology of intracellular Ca2+ stores. Physiol. Rev., 74, 595–636. [DOI] [PubMed] [Google Scholar]
- Pozzan T., Magalhaes,P. and Rizzuto,R. (2000) The comeback of mitochondria to calcium signaling. Cell Calcium, 28, 279–283. [DOI] [PubMed] [Google Scholar]
- Rizzuto R., Pinton,P., Carrington,W., Fay,F.S., Fogarty,K.E., Lifshitz,L.S., Tuft,R.A. and Pozzan,T. (1998) Close contacts with the endoplasmic reticulum as determinants of mitochondrial Ca2+ responses. Science, 280, 1763–1766. [DOI] [PubMed] [Google Scholar]
- Rizzuto R., Bernadi,P. and Pozzan,T. (2000) Mitochondria as all-round players of the calcium game. J. Physiol., 529, 37–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santella L., Kyozuka,K., Genazzani,A.A., De Riso,L. and Carafoli,E. (2000) Nicotinic acid adenine dinucleotide phosphate-induced Ca2+ release. J. Biol. Chem., 275, 8301–8306. [DOI] [PubMed] [Google Scholar]
- Straub S.V., Giovannucci,D.R. and Yule,D.I. (2000) Calcium wave propagation in pancreatic acinar cells: functional interaction of inositol 1,4,5-trisphosphate receptors, ryanodine receptors and mitochondria. J. Gen. Physiol., 116, 547–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian K. and Meyer,T. (1997) Calcium-induced restructuring of nuclear envelope and endoplasmic reticulum calcium stores. Cell, 89, 963–971. [DOI] [PubMed] [Google Scholar]
- Takahashi A., Camacho,P., Lechleiter,J.D. and Herman,B. (1999) Measurement of intracellular calcium. Physiol. Rev., 79, 1089–1125. [DOI] [PubMed] [Google Scholar]
- Terasaki M., Traverse Slater,N., Fein,A., Schmidek,A. and Reese,T.S. (1994) Continuous network of endoplasmic reticulum in cerebellar Purkinje neurons. Proc. Natl Acad. Sci. USA, 91, 7510–7514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas A.P., Bird,G.S.J., Hajnoczky,G., Robb-Gaspers,L.D. and Putney,J.W. (1996) Spatial and temporal aspects of cellular calcium signalling. FASEB J., 10, 1505–1517. [PubMed] [Google Scholar]
- Thorn P. and Petersen,O.H. (1992) Activation of non-selective cation channels by physiological cholecystokinin concentrations in mouse pancreatic acinar cells. J. Gen. Physiol., 100, 11–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorn P., Lawrie,A.M., Smith,P.M., Gallacher,D.V. and Petersen,O.H. (1993) Local and global cytosolic Ca2+ oscillations in exocrine cells evoked by agonists and inositol trisphosphate. Cell, 74, 661–668. [DOI] [PubMed] [Google Scholar]
- Thorn P., Gerasimenko,O. and Petersen,O.H. (1994) Cyclic ADP-ribose regulation of ryanodine receptors involved in agonist evoked cytosolic Ca2+ oscillations in pancreatic acinar cells. EMBO J., 13, 2038–2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinel H., Cancela,J.M., Mogami,H., Gerasimenko,J.V., Gerasimenko, O.V., Tepikin,A.V. and Petersen,O.H. (1999) Active mitochondria surrounding the pancreatic acinar granule region prevent spreading of inositol trisphosphate-evoked local cytosolic Ca2+ signals. EMBO J., 18, 4999–5008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toescu E.C., Lawrie,A.M., Petersen,O.H. and Gallacher,D.V. (1992) Spatial and temporal distribution of agonist-evoked cytoplasmic Ca2+ signals in exocrine acinar cells analysed by digital image microscopy. EMBO J., 11, 1623–1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toescu E.C., Gallacher,D.V. and Petersen,O.H. (1994) Identical regional mechanisms of intracellular free Ca2+ concentration increase during polarized agonist-evoked Ca2+ response in pancreatic acinar cells. Biochem. J., 304, 313–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakui M., Osipchuk,Y.V. and Petersen,O.H. (1990) Receptor-activated cytoplasmic Ca2+ spiking mediated by inositol trisphosphate is due to Ca2+-induced Ca2+ release. Cell, 63, 1025–1032. [DOI] [PubMed] [Google Scholar]