Abstract
The roX1 and roX2 genes of Drosophila produce male-specific non-coding RNAs that co-localize with the Male-Specific Lethal (MSL) protein complex. This complex mediates up-regulation of the male X chromo some by increasing histone H4 acetylation, thus contributing to the equalization of X-linked gene expression between the sexes. Both roX genes overlap two of ∼35 chromatin entry sites, DNA sequences proposed to act in cis to direct the MSL complex to the X chromosome. Although dosage compensation is essential in males, an intact roX1 gene is not required by either sex. We have generated flies lacking roX2 and find that this gene is also non-essential. However, simultaneous removal of both roX RNAs causes a striking male-specific reduction in viability accompanied by relocation of the MSL proteins and acetylated histone H4 from the X chromosome to autosomal sites and heterochromatin. Males can be rescued by roX cDNAs from autosomal transgenes, demonstrating the genetic separation of the chromatin entry and RNA-encoding functions. Therefore, the roX1 and roX2 genes produce redundant, male-specific lethal transcripts required for targeting the MSL complex.
Keywords: dosage compensation/epigenetic regulation/male-specific lethal/non-coding RNAs/roX RNA
Introduction
Organisms with divergent sex chromosomes, such as X and Y, employ various chromatin-based mechanisms of gene regulation to equalize sex chromosome expression between the two sexes (Meller, 2000). Drosophila melanogaster males up-regulate most of the genes on their X chromosome using a male-limited protein complex composed of the five male-specific lethal gene products (MSL proteins) and JIL-1 kinase (Cline and Meyer, 1996; Hilfiker et al., 1997; Jin et al., 2000). This complex binds to the X chromosome and directs acetylation of histone H4 on lysine 16 (H4Ac16), a modification associated with the elevated expression of X-linked genes (Akhtar and Becker, 2000; Smith et al., 2001). It is widely assumed that the non-coding, male-specific roX1 and roX2 RNAs play a role in dosage compensation. Both transcripts ‘paint’ the X chromosome of male, but not female Drosophila, and they colocalize with the MSL proteins in a finely banded pattern along the length of the polytenized salivary gland X chromosome (Meller et al., 1997, 2000; Franke and Baker, 1999; Gu et al., 2000). Both roX RNAs can be co-immunoprecipitated with anti-MSL antibodies, indicating that they form a stable association with these proteins (Akhtar et al., 2000; Gu et al., 2000; Meller et al., 2000). Three of the MSL proteins bind RNA or may be released from the X chromosome by RNase treatment (Richter et al., 1996; Akhtar et al., 2000). Taken together, these findings indicate that the roX RNAs participate in the MSL complex and are likely to serve a functional role in compensation. The absence of a phenotype associated with mutations in roX1 further suggested that roX1 and roX2 might be redundant. Because the roX transcripts share almost no sequence similarity, the aspect of these molecules that might confer functional redundancy is still unknown (Amrein and Axel, 1997; Franke and Baker, 1999). A prior study using large, embryonic-lethal excisions to remove roX2 suggested a requirement for the roX genes in formation of the MSL complex or in its localization to the male X chromosome, a step which precedes compensation during embryogenesis (Franke and Baker, 1999). Although this supports the previous notion that one, but not both, of these genes is essential, the roX2 deletions used for this study also removed several essential genes including the largest subunit of RNA polymerase II, RpII215, situated <10 kb distal to roX2 (Voelker, 1985). The resulting disruption of zygotic gene expression causes non-specific developmental defects that could mask the true roX– phenotype. Because dosage compensation depends on the expression of zygotic genes in response to signals from the sex determination pathway, these embryos may have a failure in this process which is unrelated to the absence of the roX RNAs. It also remained a possibility that other roX RNAs existed that were normally expressed later in development, and these had escaped detection due to the early death of the embryos used in this study.
Both roX genes are X-linked, and they overlap two of ∼35 chromatin entry sites on the X chromosome. These sites retain partial MSL complexes after the bulk of MSL binding to the X has been eliminated by mutations in the mle, msl3 or mof genes (Lyman et al., 1997; Gu et al., 1998). When moved to an autosomal location, both roX genes provide sites from which the MSL complex can spread into chromatin in cis, and the presence of these sites is believed to be the mechanism used to differentiate the X chromosome from the autosomes (Kelley et al., 1999; Meller et al., 2000). Although a non-roX chromatin entry site has not been isolated, an entry site within the roX1 gene has been studied in detail, and it lies in a few hundred base pairs of DNA internal to the transcribed region. The entry function is independent of transcription, and thus can not be a property of the roX RNA. It is instead a characteristic of the roX genomic region (Kageyama et al., 2001). As the roX1 and roX2 genes correspond to two of ∼35 X-linked chromatin entry sites, it remained an intriguing possibility that additional roX RNAs were transcribed from other entry sites. Alternatively, the roX1 and roX2 entry sites could be uniquely associated with roX transcripts. Finally, the remaining entry sites might differ qualitatively from those identified through their association with the roX genes, and it is possible that simultaneous mutation of roX1 and roX2 entry sites would prevent cis-directed dosage compensation of the male X chromosome.
To address these questions we have created flies deleted for roX2, and have used these to show that neither the roX2 chromatin entry site nor its transcript is essential. However, when combined with a roX1 mutation, a striking male-specific reduction in viability is observed. Male lethality may be rescued by expression of roX cDNAs from autosomally located transgenes, demonstrating the genetic separation of the chromatin entry function, which acts in cis, from the roX transcript, which may originate from any chromosome. Mutation of both roX genes rescues the lethality of females that inappropriately express msl2, and would otherwise be forced to up-regulate both X chromosomes. MSL protein localization to the X chromosome is profoundly disrupted in roX– males, as is H4Ac16 enrichment. These experiments reveal that the transcripts produced from roX1 and roX2 are required for targeting of the MSL complex. The male requirement for roX transcripts allows the roX genes to be classified as redundant male-specific lethals. Surprisingly, ∼5% of the roX– males emerge as developmentally delayed escapers. Their presence suggests that partial compensation can occur without any wild-type roX1 or roX2 transcripts.
Results
Generation of a roX2 deletion
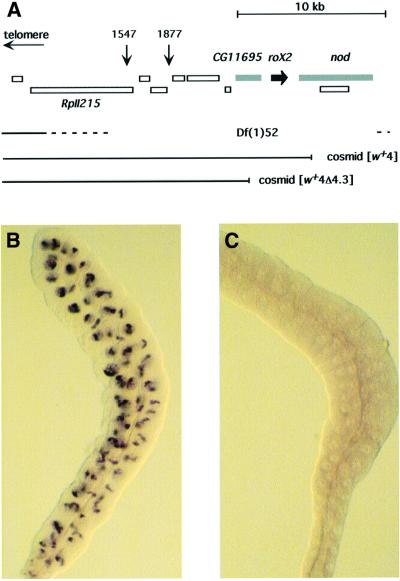
roX2 lies in a region densely packed with essential genes (Figure 1). We surveyed P-element lines with insertions cytologically mapped to the 10C region and identified two, P1547 and P1877, close to roX2. Several transcribed regions and lethal complementation groups lie between roX2 and the closest insertion, P1877. Consequently, an excision of roX2 caused by P1877 mobilization would be lethal due to disruption of other genes, regardless of the roX2– phenotype. We mobilized P1877 to generate lethal deletions, and identified several that removed roX2 (see Materials and methods). These were mapped by complementation with genomic cosmids from the region and a single deletion, Df(1)52, could be rescued by autosomal insertions of the roX2-containing cosmid [w+4] (Figure 1). Because development of Df(1)52 flies is arrested in mid embryogenesis, no larvae or adults will be obtained without restoration of a portion of the deleted region (not shown). A 4.3 kb fragment containing roX2 was removed from [w+4] to create [w+4Δ4.3], and transgenic flies carrying the deleted cosmid were generated.
Fig. 1. Deletion of roX2. (A) Identified and predicted transcripts surrounding the roX2 gene were compiled from the Drosophila genome project and Voelker et al. (1985). The insertion sites of P1877, P1547 and the proximal ends of the cosmids [w+4] and [w+4Δ4.3] are shown. The roX2 gene is indicated in black; flanking genes disrupted by Df(1)52 and not restored by [w+4Δ4.3] are denoted in gray. (B) In situ detection of roX2 in a wild-type male salivary gland. (C) In situ detection of roX2 in a Df(1)52; [w+4Δ4.3]/+ male salivary gland.
roX2 is non-essential
Females heterozygous for Df(1)52 and a balancer chromosome were mated to males carrying an autosomal insertion of the [w+4Δ4.3] cosmid, which can be followed in a w– genetic background by light yellow eye color. As anticipated, no deficiency males lacking [w+4Δ4.3] were recovered, but fertile male offspring carrying the Df(1)52 chromosome and [w+4Δ4.3] were obtained. These males were not developmentally delayed, were recovered in numbers consistent with full viability and appeared completely normal in spite of their lack of roX2. In situ hybridization to salivary glands from Df(1)52/Y; [w+4Δ4.3]/+ males detected no roX2 transcripts (Figure 1C). In addition to the deletion of roX2, these males also lack the nod gene, proximal to roX2, and the predicted zinc finger gene CG11695, distal to roX2. The nod gene product has not been reported in males and its disruption is unlikely to affect the outcome of this analysis (Zhang and Hawley, 1990). The overt normality of these males suggests that CG11695 sequences are also dispensable.
Male development is disrupted in roX double mutants
Recombinant X chromosomes carrying the mutations roX1mb710 or roX1ex6 and Df(1)52 were obtained. roX1mb710 is caused by a P-element insertion within the gene. Over 1 kb of roX1 is transcribed from the roX1mb710 chromosome, but this fragment is unstable and can not accumulate (Meller et al., 2000). roX1ex6 was created by an imprecise excision of roX1mb710 and is deleted for 1.4 kb near the 5′ end of the transcript. When using in situ hybridization conditions optimized for detection of roX1 in wild-type males, no transcripts could be detected in salivary glands from roX1ex6 males. Both roX1 alleles allow normal male development in spite of the severity of their effect on transcript accumulation. Females heterozygous for either roX1mb710 Df(1)52 or roX1ex6 Df(1)52 and a balancer chromosome were mated to yw males carrying an autosomal [w+4Δ4.3] insertion. Males inheriting the roX– chromosomes were severely affected, with only 5% survival (Table I). The eclosion of these males was delayed, most markedly for the roX1ex6 Df(1)52 chromosome, which did not appear in progeny until at least a week after adult emergence began. In spite of the low numbers of adults recovered, doubly mutant males appear healthy and fairly abundant at the end of the third larval instar. Survival of males into the third instar is characteristic of the male-specific lethal mutations, but the presence of escaper adults is not (Belote, 1983; Baker et al., 1994; Hilfiker et al., 1997).
Table I. Simultaneous mutation of both roX genes reduces male survival.
| Female parent | Male parent | Female progeny | Male progeny |
|||
|---|---|---|---|---|---|---|
| Binsincy | roX1– Df(1)52 | roX–; [w+4Δ4.3] survival (%) | roX–; [w+4Δ4.3] delay (days) | |||
| roX1ex6 Df(1)52 | yw; [w+4Δ4.3] | 686 | 170 | 7 | 4.1 | 8 |
| Binsincy | Y + | |||||
| |
||||||
| roX1ex6 Df(1)52 | yw; [w+4Δ4.3] | 431 | 132 | 13 | 5.9 | 7 |
| Binsincy | Y [w+4Δ4.3] | |||||
| roX1mb710 Df(1)52 | yw; [w+4Δ4.3] | 1257 | 390 | 33 | 5.3 | 3 |
| Binsincy | Y [w+4Δ4.3] | |||||
Females heterozygous for a balancer chromosome and a roX– chromosome were mated to males carrying the [w+4Δ4.3] cosmid. Male survival is based on the number of females obtained from each cross.
roX RNAs are not required in females
Females heterozygous for roX1ex6 Df(1)52 and a balancer chromosome and carrying an autosomal [w+4Δ4.3] transgene were mated to roX1ex6 Df(1)52/Dp(1:Y) Bs–v+y+ males that are rescued by duplication of the roX2 region on the Y chromosome. Only half of the zygotes from this cross will inherit the [w+4Δ4.3] transgene, so roX– female survival will at best be 50% that of their sisters carrying the balancer chromosome. The roX– females were recovered at 54% the level of their sisters (Table II). Peak emergence days coincided for roX– females and their sisters (data not shown), suggesting that removal of both roX RNAs has no effect on female development.
Table II. Females are unaffected by loss of both roX RNAs.
| Female parent | Male parent | Female progeny |
|
|---|---|---|---|
| roX1ex6 Df(1)52 | roX1ex6 Df(1)52 | ||
| |
|
Binsincy |
roX1ex6 Df(1)52 |
| roX1ex6 Df(1)52; [w+4Δ4.3] | roX1ex6 Df(1)52 | 218 | 117 |
| Binsincy + | Dp(1:Y)Bs–v+y+ | ||
Females heterozygous for a balancer chromosome and a roX– chromosome, and carrying one copy of the [w+4Δ4.3] cosmid were mated to males carrying a roX– X chromosome and a rescuing duplication on the Y chromosome. Because the [w+4Δ4.3] cosmid is present in one copy in the female parent, survival of roX– daughters will at best be 50% that of their sisters carrying the balancer chromosome.
Restoration of either roX1 or roX2 in trans rescues roX– males
The roX genes have two recognized functions: they contain cis-acting DNA sequences (chromatin entry sites) from which dosage compensation spreads into surrounding chromatin; and they produce the roX transcripts. The chromatin entry site internal to the roX2 gene is removed by Df(1)52, and most of the roX1 entry site is deleted in roX1ex6 (Kageyama et al., 2001). We restored roX RNA to roX1ex6 Df(1)52; [w+4Δ4.3]/+ males to determine if the lethality associated with this X chromo some was the consequence of loss of the roX transcripts or of mutation of both roX-associated chromatin entry sites. Females homozygous for a roX1ex6 Df(1)52 chromosome and carrying one copy of the [w+4Δ4.3] transgene were mated to males homozygous for autosomal insertions of heat shock driven roX1 or roX2 cDNAs. Only half of the males will inherit the [w+4Δ4.3] cosmid that complements lethality, and consequently male survival can not exceed 50% even with full rescue of the roX– phenotype. Control crosses in which females were mated to males with no roX transgene displayed delayed emergence of male offspring (not shown) and survival of <4% (Table III). However, when roX transgenes were present, male survival increased and the eclosion time of rescued males approached that of their sisters. Hsp83-driven roX1 cDNA allows recovery of 47% of males (Table III), thus it fully rescues mutant viability. Less dramatic rescue was observed with an Hsp70-roX2 transgene, perhaps because these experiments were conducted at 25°C and the Hsp70 promoter has low activity in the absence of heat shock. Two transgenes carrying roX1 fragments driven by the Hsp83 promoter were also tested. Neither the 3′ 1 kb nor the 5′ 2.5 kb of roX1 could rescue, indicating that the intact RNA is required.
Table III. Males carrying a roX1ex6 Df(1)52 chromosome are rescued by roX cDNAs.
| Transgene | Progeny |
||
|---|---|---|---|
| Females | Males | % male survival | |
| – | 171 | 6 | 3.5 |
| Hsp83-roX1 | 266 | 125 | 47 |
| Hsp70-roX2 | 204 | 58 | 28 |
| Hsp83-roX1 3′ | 207 | 7 | 3.4 |
| Hsp83-roX1 5′ | 235 | 9 | 3.8 |
roX1ex6 Df(1)52; [w+4Δ4.3]/+ virgins were mated to males homozygous for autosomal insertions of the indicated transgenes.
Removal of both roX RNAs compromises dosage compensation
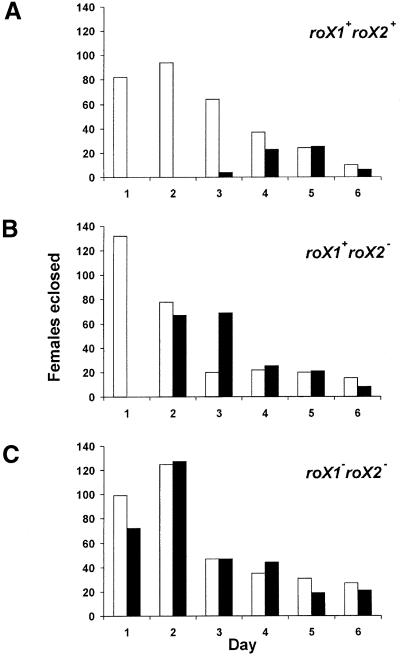
The sex-specificity of X chromosome up-regulation is achieved by tight repression of msl2 expression in females (Bashaw and Baker, 1997; Kelley et al., 1997; Gebauer et al., 1998). Inappropriate up-regulation of X chromo somes can be induced by ectopic expression of msl2 from the [w+H83M2-6I] transgene, and this is sufficient to recruit the rest of the MSL proteins to both female X chromosomes resulting in low viability, sterility and delayed development (Kelley et al., 1995). If the roX transcripts do play a vital role in dosage compensation, removal of these RNAs should block the MSL2-induced lethality of females carrying the [w+H83M2-6I] transgene. A cross introducing [w+H83M2-6I] into females with wild-type roX genes produced the anticipated reduction in viability and developmental delay of daughters inheriting this transgene (Figure 2A). We then performed a similar cross in a genetic background lacking roX1 (not shown), roX2 (Figure 2B) or mutated for both roX RNAs (Figure 2C; see Materials and methods for details). In the absence of wild-type roX RNA, female offspring carrying [w+H83M2-6I] were recovered in numbers equal to their sisters, and they display eclosion times similar to their sisters (Figure 2C). Elimination of only roX1 or roX2 resulted in an intermediate level of female lethality and developmental delay in response to the [w+H83M2-6I] transgene.
Fig. 2. Females are rescued from msl2 expression by removal of the roX RNAs. (A) Females display reduced viability and developmental delay if they carry the msl2-expressing transgene [w+H83M2-6I] (filled bars). Open bars indicate their sisters lacking the transgene. (B) The deletion of roX2 allows partial rescue of females carrying [w+H83M2-6I]. (C) Mutation of both roX1 and roX2 fully rescues females carrying [w+H83M2-6I]. See Materials and methods for a complete description of the genotypes used in these experiments.
Localization of the MSL proteins and H4Ac16 to the X chromosome is disrupted in roX– males
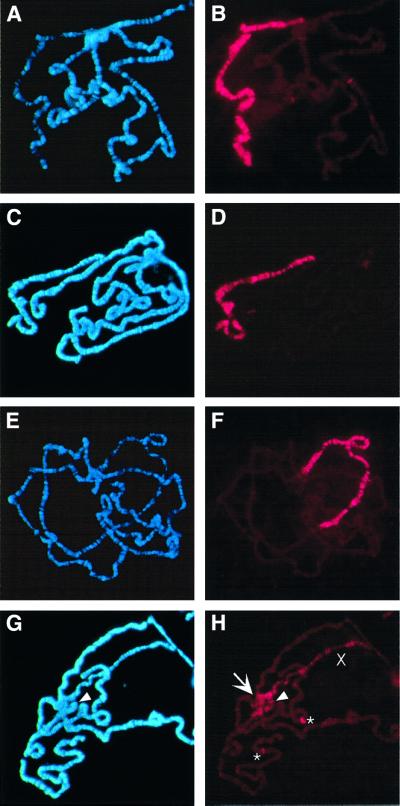
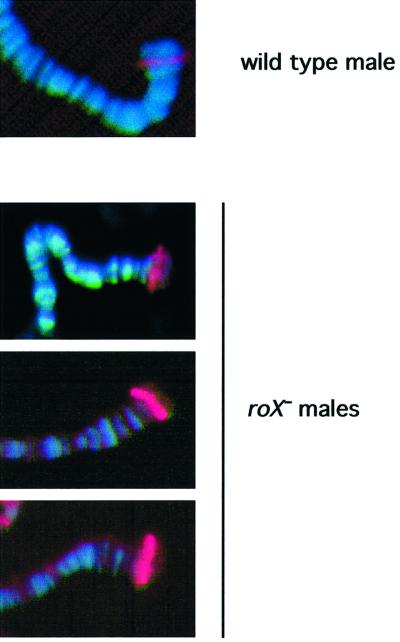
Mutation of mle, msl3 or mof results in a reduced pattern of X-chromosome binding by the remaining MSL proteins, and mutation of msl1 or msl2 leads to a complete loss of chromatin binding by the remaining members of the complex (Lyman et al., 1997; Gu et al., 1998). The removal of the roX RNAs might similarly disrupt formation of the MSL complex or affect its localization to the X chromosome. We tested this by immunolocalization of MSL1 (not shown) and MSL2 to chromosome preparations from males that were wild type, mutated for roX1 or roX2, or mutated for both roX RNAs. No differences were discernible in immunolocalization to chromosomes from wild-type males (Figure 3B) or males mutated for only one of the roX genes (Figure 3D and F). However, males lacking any wild-type roX RNA have sharply reduced overall staining of the X chromosome (Figure 3H). A number of bands retaining MSL2 do remain on the X chromosome, and this pattern is reminiscent of the reduced pattern of X-chromosome binding seen after mutations in mle, msl3 or mof. However, a few autosomal sites have strongly enhanced MSL2 binding (Figure 3H, asterisks). Notably strong staining is also seen on the fourth chromosome (arrowhead in Figure 3H), and diffuse anti-MSL2 staining appears in the heterochromatin of the chromocenter (arrow in Figure 3H). The alteration in chromatin structure associated with dosage compensation is linked to an increase in the width of the single male X chromosome to about the width of a pair of autosomes (Baker et al., 1994). Significantly, the X chromosome of roX– males appears thinner than the compensated X chromosomes from wild-type or singly mutated males.
Fig. 3. Localization of MSL2 is disrupted in males lacking both roX RNAs. (A and B) Wild-type male. (C and D) Male mutated for roX1 (roX1ex6). (E and F) Male deleted for roX2 (Df(1)52/Y; [4Δ4.3]/+). (G and H) Male mutated for both roX RNAs (roX1ex6 Df(1)52/Y; [4Δ4.3]/+). (A, C, E and G) Chromosome preparations counter stained with Hoechst 33258. (B, D, F and H) Chromosome preparations probed with antibodies against MSL2 and detected with Texas Red. In (G and H) the arrowhead points to the fourth chromosome; in (H) the arrow indicates the chromocenter and asterisks mark two strongly staining autosomal sites of MSL2 localization. ‘X’ indicates the X chromosome. Exposure times for Texas Red were between 6 and 8 s.
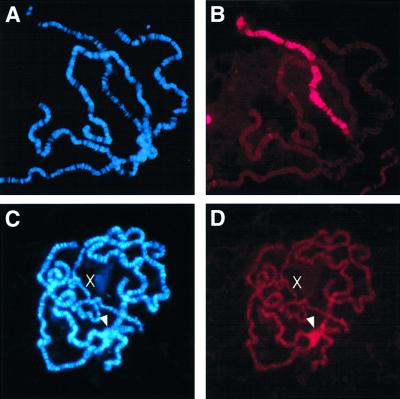
Acetylation of histone H4 on lysine 16 (H4Ac16) is linked to elevated transcription, and enrichment of H4Ac16 on the male X chromosome is believed to be central to the mechanism by which X-linked genes are up-regulated. We examined the pattern of H4Ac16 enrichment by probing chromosome preparations with an antibody specific to this acetylated isoform. Wild-type males show the anticipated pattern of H4Ac16 localization on the X chromosome (Figure 4B), but roX1– males display sharply reduced X chromosome staining and an overall increase of staining on the autosomes (Figure 4D). Similar to MSL2 localization in roX– males, there is an enrichment of H4Ac16 in the chromocenter (arrowhead in Figure 4D).
Fig. 4. Histone H4 acetylation at lysine 16 (H4Ac16) is disrupted in roX– males. (A and B) Wild-type male. (C and D) Male mutated for both roX RNAs (roX1ex6 Df(1)52/Y; [4Δ4.3]/+). (A and C) are chromosome preparations counter stained with Hoechst 33258. (B and D) are probed with an epitope-specific antibody that recognizes the H4Ac16 isoform of histone H4, detected in Texas Red. Exposure times for Texas Red is 8 s.
Autosomal sites of MSL2 binding frequently appear puffed in roX– males, and thus are presumably actively transcribed. These larvae produce poor quality chromosomes, complicating the accurate mapping of MSL2-binding sites. However, one prominent autosomal site is easily located due to its subtelomeric position at 21B. Visualization of MSL2 localization in chromosome preparations from wild-type males reveals weak signal at 21B, but in roX– males this region is often puffed and heavily labeled by anti-MSL2 antibodies (Figure 5).
Fig. 5. Autosomal MSL2 binding is enhanced in roX– males. In wild-type males (top panel) a fine band of MSL2 binding can be detected at 21B. Upon elimination of both roX RNAs (roX1ex6 Df(1)52/Y; [4Δ4.3]) this site may be puffed and heavily stained by anti-MSL2.
Discussion
The roX transcripts are redundant male-specific lethals
The X-localization of roX transcripts, their male-specificity and regulation of roX RNA accumulation by the MSL proteins all have suggested a role for these RNAs in dosage compensation. However, failure to detect a phenotype in males mutated for roX1 and the absence of precise mutations in roX2 had previously precluded formal tests of this possibility. Furthermore, slight differences between the roX RNAs, such as the ability of transcripts from roX2, but not roX1, to move to all entry sites on the X chromosome in a fly mutated for msl3, and the identification of flies and cell lines lacking roX1, but none lacking roX2, supported the notion that roX2 might be an essential gene in males (Meller et al., 2000; Smith et al., 2000). However, our mutation strategy, generation of a lethal deletion and restoration of essential gene functions with a genomic cosmid, produced healthy males lacking roX2 RNA. When the roX2 deletion was combined with a roX1 mutation, male-specific lethality was revealed. Heat shock-driven expression of either roX RNA could restore the viability of doubly mutated males, indicating that the male-specific phenotype is due to lack of roX RNA only. These experiments show that roX2 is not essential in males, and the synthetic lethality detected when both roX genes are mutated eliminates the possibility of undetected roX transcripts with similar function. The roX2 deletion removes the roX2-associated chromatin entry site at 10C, and all but 40 bp of the roX1 site is removed in roX1ex6 (Kageyama et al., 2001). Because male viability is rescued by roX RNA in trans, male lethality is consequential to loss of the roX transcripts and not to mutation of the chromatin entry sites. We can conclude that the RNA products of the roX genes are redundant male-specific lethals. The roX2 deletant that we have generated is still complex, as two genes flanking roX2 are disrupted by Df(1)52 and not restored by cosmid [w+4Δ4.3]. The rescue of male viability by roX cDNAs also demonstrates that male lethality is not an unintended consequence of other mutations associated with the Df(1)52 chromosome or the rescuing [w+4Δ4.3] transgene.
The involvement of the roX RNAs in dosage compensation can be functionally demonstrated by the rescue of females forced to express msl2. Production of the MSL2 protein triggers the formation of intact MSL complexes that bind to the X chromosome and enhance the transcription of X-linked genes. Female expression of msl2 leads to an inappropriate up-regulation of both X chromosomes resulting in a high level of lethality that can be blocked by mutations in another of the protein-coding msl genes (Kelley et al., 1995). Removal of both roX RNAs similarly rescues msl2-expressing females. Partial rescue of female lethality by elimination of only one of the roX RNAs is consistent with previous results indicating that females expressing msl2 from the [w+H83M2-6I] transgene are a sensitized genetic background in which changes in the level of components of the MSL complex may be detected (Kelley et al., 1995). These studies specifically demonstrate a block of dosage compensation when both roX RNAs are eliminated.
In contrast to the absence of escapers from mutations in the protein-coding msl genes, the doubly mutant X chromosomes used in this study did allow a low number of escaper males. This is a surprising finding in light of the disruption in MSL localization and loss of H4Ac16 enrichment on the X chromosome. It suggests that in spite of the clear importance of the roX RNAs for histone acetylation, compensation of the X chromosome is not completely compromised in the roX– males that we have generated. It is possible that the roX RNAs are peripheral to formation of the MSL complex. Residual MSL2 binding on the X chromosome of roX– males would be consistent with this. Partial complexes with reduced activity could also form in the absence of the roX RNAs, and the simultaneous enrichment at the chromocenter of MSL1, MSL2 and H4Ac16 in roX– males supports the idea that some or all of the MSL proteins may still assemble into a complex capable of acetylating histone H4.
It is possible that the available roX1 mutations are not complete loss-of-function alleles. This initially seemed unlikely because mutant roX1 transcripts can not be visualized painting the X chromosome in males carrying roX1mb710 or roX1ex6 (Meller et al., 1997, 2000). However, both mutations leave the 3′ end of roX1 intact and it is possible that, in the absence of roX2, low levels of a mutated roX1 transcript could support partial X chromo some compensation. Although survival is equivalent for doubly mutated males with either roX1 allele, the delay to male emergence is most pronounced for roX1ex6 (Table I). Our detection of a subtle allelic difference between roX1ex6 and roX1mb710 implies that at least one of these mutations is not functionally null.
Function of the roX transcripts
Two lines of evidence support the idea that the MSL proteins, in the absence of the roX RNAs, can act to regulate gene expression. First, MSL1, MSL2 and H4Ac16 are similarly redistributed in roX– males, suggesting that a protein complex able to direct histone acetylation still forms in the absence of roX RNA, although its localization is disrupted. Secondly, the sites of autosomal accumulation of the MSL proteins in roX– males are often puffed, suggestive of locally high rates of transcription. Autosomal sites of MSL3 binding have been reported in wild-type males, and we have detected MSL2 at several sites, including three specifically noted as binding MSL3 (Gorman et al., 1995). The one we have focused on, 21B, displays fairly weak MSL2 staining in wild-type males but is often strongly stained and puffed in preparations from roX– males. We suggest that an MSL complex lacking roX transcripts normally functions at a few autosomal sites in wild-type males. It is plausible that these represent a handful of genes with sex-specific expression. Removal of roX RNA releases the bulk of the MSL proteins from the X chromosome, and these are consequently available at high titers for association with the autosomal sites. Puffing of these sites suggests that elevated levels of the MSL proteins can hyper-activate transcription.
Alternatively, it is possible that all targeting of the MSL proteins to chromatin requires an RNA cofactor, but only roX1 and roX2 serve to direct the MSL proteins to the X chromosome. This would evoke the presence of other roX-like RNAs that specify a more restricted set of targets, perhaps a few autosomal genes. The potential utility of a system of gene activation that can be redirected by deploying an assortment of transcripts is quite attractive; however, in this instance the restriction to a single sex would limit the range of target genes considerably.
Mislocalization of MSL2 is strikingly different in roX– males than in those lacking mle, msl3 or mof, where residual MSL2 is observed binding only to ∼35 chromatin entry sites. This points to a role for roX RNA in correct targeting of the intact complex to the X chromosome. The mechanism driving relocation of the MSL proteins to heterochromatin is at this point speculative. Two of the components of the MSL complex, MSL3 and MOF, contain variant chromodomain motifs (Lucchesi, 1996). Chromodomains are involved in targeting HP1 to heterochromatin, and localization depends on the interaction of this domain with histone H3 methylated on lysine 9, a modification found in heterochromatic regions (Bannister et al., 2001; Nakayama et al., 2001). A chromodomain found in the Polycomb protein is also involved in its localization (Platero et al., 1995). The variant chromodomains of MSL3 and MOF have been shown to bind RNA in vitro, and it is likely that the roX RNAs are their normal ligands (Akhtar et al., 2000). Removal of roX transcripts could allow these domains to engage in inappropriate protein–protein interactions that target the remaining members of the MSL complex to heterochromatin.
The chromatin entry and RNA-encoding functions of the roX genes are separable
The roX genes have two distinct functions in dosage compensation; they are the source of the roX RNA molecules, and they are chromatin entry sites that direct the MSL complex to chromatin in cis (Kelley et al., 1999). Male-lethality upon elimination of both roX RNAs rules out the existence of other equivalent RNA-encoding genes. However, there are over 30 other proposed chromatin entry sites. Because of their number it will be impractical to mutate, or possibly even to identify, all of the non-roX sites. The roX1ex6Df(1)52 chromosomes display no problems in X localization of the MSL complex if roX transcripts are supplied in trans. This indicates that all necessary cis-acting information is present, presumably supplied by remaining entry sites.
The genetic separation of the roX transcripts, which can originate from any chromosome, and the chromatin entry site, which does not require transcription and acts in cis, poses the question of why these functions are found together in the roX genes. It is possible that the proximity of entry sites to roX RNA synthesis is important for the correct assembly of the MSL complex. For example, the roX RNAs are rapidly degraded in the absence of the MSL proteins (Meller et al., 2000). If these proteins are recruited to the roX genes by the chromatin entry sites, they will be situated in the vicinity of the transcripts as they are produced, and this could be a factor in transcript stability. Rapid recruitment of the MSL proteins to the roX genes might also enhance nuclear retention of the transcripts. The roX cDNAs that were used to rescue roX– males do contain chromatin entry sites, and therefore these would have been available to fulfill any necessary organizing role in assembly of the MSL complex at the site of synthesis. It is possible that other entry sites were at one time sources of roX RNA, but because roX RNA may be supplied in trans, there is little selective pressure to retain multiple genes encoding roX transcripts. One or a few genes could supply all the needed RNA, and this perhaps represents the situation now observed in Drosophila melanogaster. However, marking the X chromosome in cis for compensation might not be efficiently assumed by a small number of X-linked loci. roX1 and roX2 could represent genes whose abundant RNA production has enabled the degradation and loss of other roX transcripts from the X chromosome.
Materials and methods
Fly stocks
Flies were raised at 25°C, 75% humidity on standard cornmeal molasses fly medium. The roX1mb710 mutation and the roX1ex6 deletion have been described previously (Meller et al., 1997; Kelley et al., 1999), as have transgenes expressing roX cDNAs under control of heat shock promoters (Meller et al., 2000).
Mutagenesis of roX2
Eight P-elements cytologically mapped to the 10C region were obtained from the Bloomington Drosophila Stock Center and tested for proximity to roX2 by inverse PCR and hybridization to overlapping cosmids spanning the roX2 region (Dalby et al., 1995). Two lethal insertions were found near roX2: P1547 disrupts RpII215 and is 9.8 kb distal to roX2; and P1877 is an insertion in CT4038, 6.8 kb distal to roX2 (Figure 1). Excisions of the roX2 region were generated by crossing dysgenic females of genotype P1877/Df(1)nod, FM7a; [w+4]; Sb P[ry+Δ2–3]/+ to X/Dp(1:Y)Bs–v+y+ males. Nineteen lethal w– chromosomes were obtained; in situ hybridization to polytene chromosomes revealed that four of these were deleted for roX2. One deletion, Df(1)52, could be rescued by the cosmid [w+4] (Figure 1). Using Southern blotting and PCR the proximal breakpoint of Df(1)52 was mapped to a series of tandem repeats at the 3′ end of nod. Failure of Df(1)52 to complement the lethality of the P1547 chromosome indicates that it is also disrupted for RpII215.
Screening a library with a roX2 probe identified cosmid [w+4], containing the 5′ end of nod and extending distally for 36 kb. Partial NotI digestion of [w+4] removed a 4.3 kb fragment containing roX2, producing [w+4Δ4.3].
Genetics
The survival of males carrying the roX– chromosome was based on the number of females emerging from each cross. Testing of the roX1ex6 Df(1)52 chromosome produced 1439 adults, and testing of roX1mb710 Df(1)52 produced 1680 adults. We discounted the possibility of escapers resulting from recombinational repair of a roX gene because: (i) we observed no evidence of recombination between the roX– chromosome and a multiply marked balancer; (ii) the developmental delay of escaping males indicates that they are severely challenged, unlike males with a single roX gene; and (iii) progeny of escapers mated to attached X females display similar male survival and developmental delay.
The survival of females carrying the [w+H83M2-6I] transgene was determined by crossing yw virgins to yw/Y; [w+H83M2-6I]/+ males (Figure 2A), ywDf(1)52; [w+4Δ4.3] virgins to ywDf(1)52/Dp(1:Y) Bs–v+y+; [w+H83M2-6I]/+ males (Figure 2B); and by crossing yw roX1ex6 Df(1)52; [w+4Δ4.3] virgins to yw roX1ex6 Df(1)52/Dp(1:Y)Bs–v+y+; [w+H83M2-6I]/+ males (Figure 2C). The [w+4Δ4.3] insertion produces a pale yellow eye color readily distinguished from the deeper eye color of the [w+H83M2-6I] transgene.
In situ hybridization and immunohistochemistry
Detection of transcripts by in situ hybridization has been described, with the exception that the current study used DIG-labeled antisense RNA probes and hybridization temperatures of 50°C (Meller et al., 1997). Probes were transcribed from a full-length roX2 cDNA (Amrein and Axel, 1997). Immunohistochemical detection of MSL2 and H4Ac16 on polytene chromosomes was done as described previously (Kelley et al., 1999). Antibody to H4Ac16 was purchased from Serotec.
Acknowledgments
Acknowledgements
We thank Dr M.Kuroda and R.Richman for gifts of antibodies, R.Kelley and X.Chu for the cosmid library, X.Deng and S.Souter for genetic and molecular analysis of roX2 deletions, and X.Chu for injections. V.H.M. would like to thank Drs J.Fuhrman and M.Kuroda for comments on the manuscript, and Drs F.R.Jackson and R.Meller for their help with instrumentation. The Bloomington Drosophila Stock Center supplied fly stocks that enabled this work to be performed. This study was supported by start-up funds provided by Tufts University and NIH grant GM58427.
References
- Akhtar A. and Becker,P.B. (2000) Activation of transcription through histone H4 acetylation by MOF, an acetyltransferase essential for dosage compensation in Drosophila. Mol. Cell, 5, 367–375. [DOI] [PubMed] [Google Scholar]
- Akhtar A., Zink,D. and Becker,P.B. (2000) Chromodomains are protein–RNA interaction modules. Nature, 407, 405–409. [DOI] [PubMed] [Google Scholar]
- Amrein H. and Axel,R. (1997) Genes expressed in neurons of adult male Drosophila. Cell, 88, 459–469. [DOI] [PubMed] [Google Scholar]
- Baker B.S., Gorman,M. and Marin,I. (1994) Dosage compensation in Drosophila. Annu. Rev. Genet., 28, 491–521. [DOI] [PubMed] [Google Scholar]
- Bannister A.J., Zegerman,P., Partridge,J.F., Miska,E.A., Thomas,J.O., Allshire,R.C. and Kouzarides,T. (2001) Selective recognition of methylated lysine 9 on histone H3 by the HP1 chromo domain. Nature, 410, 120–124. [DOI] [PubMed] [Google Scholar]
- Bashaw G.J. and Baker,B.S. (1997) The regulation of the Drosophila msl-2 gene reveals a function for Sex-lethal in translational control. Cell, 89, 789–798. [DOI] [PubMed] [Google Scholar]
- Belote J.M. (1983) Male-specific lethal mutations of Drosophila melanogaster. II. parameters of gene action during male development. Genetics, 105, 881–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline T.W. and Meyer,B.J. (1996) Vive la difference: males vs females in flies vs worms. Annu. Rev. Genet., 30, 637–702. [DOI] [PubMed] [Google Scholar]
- Dalby B., Pereira,A.J. and Goldstein,L.S. (1995) An inverse PCR screen for the detection of P element insertions in cloned genomic intervals in Drosophila melanogaster. Genetics, 139, 757–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke A. and Baker,B.S. (1999) The roX1 and roX2 RNAs are essential components of the compensasome, which mediates dosage compensation in Drosophila. Mol. Cell, 4, 117–122. [DOI] [PubMed] [Google Scholar]
- Gebauer F., Merendino,L., Hentze,M.W. and Valcarcel,J. (1998) The Drosophila splicing regulator Sex-lethal directly inhibits translation of male-specific-lethal 2 mRNA. RNA, 4, 142–150. [PMC free article] [PubMed] [Google Scholar]
- Gorman M., Franke,A. and Baker,B.S. (1995) Molecular characterization of the male-specific lethal-3 gene and investigations of the regulation of dosage compensation in Drosophila. Development, 121, 463–475. [DOI] [PubMed] [Google Scholar]
- Gu W., Szauter,P. and Lucchesi,J.C. (1998) Targeting of MOF, a putative histone acetyl transferase, to the X chromosome of Drosophila melanogaster. Dev. Genet., 22, 56–64. [DOI] [PubMed] [Google Scholar]
- Gu W., Wei,X., Pannuti,A. and Lucchesi,J.C. (2000) Targeting the chromatin-remodeling MSL complex of Drosophila to its sites of action on the X chromosome requires both acetyl transferase and ATPase activities. EMBO J., 19, 5202–5211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilfiker A., Hilfiker-Kleiner,D., Pannuti,A. and Lucchesi,J.C. (1997) mof, a putative acetyl transferase gene related to the Tip60 and MOZ human genes and to the SAS genes of yeast, is required for dosage compensation in Drosophila. EMBO J., 16, 2054–2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y., Wang,Y., Johansen,J. and Johansen,K.M. (2000) JIL-1, a chromosomal kinase implicated in regulation of chromatin structure, associates with the male specific lethal (MSL) dosage compensation complex. J. Cell Biol., 149, 1005–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kageyama Y., Mengus,G., Gilfillan,G., Kennedy,H.G., Stuckenholz,C., Kelley,R.L., Becker,P.B. and Kuroda,M.I. (2001) Association and spreading of the Drosophila dosage compensation complex from a discrete roX1 chromatin entry site. EMBO J., 20, 2236–2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley R.L., Solovyeva,I., Lyman,L.M., Richman,R., Solovyev,V. and Kuroda,M.I. (1995) Expression of msl-2 causes assembly of dosage compensation regulators on the X chromosomes and female lethality in Drosophila. Cell, 81, 867–877. [DOI] [PubMed] [Google Scholar]
- Kelley R.L., Wang,J., Bell,L. and Kuroda,M.I. (1997) Sex lethal controls dosage compensation in Drosophila by a non-splicing mechanism. Nature, 387, 195–199. [DOI] [PubMed] [Google Scholar]
- Kelley R.L., Meller,V.H., Gordadze,P.R., Roman,G., Davis,R.L. and Kuroda,M.I. (1999) Epigenetic spreading of the Drosophila dosage compensation complex from roX RNA genes into flanking chromatin. Cell, 98, 513–522. [DOI] [PubMed] [Google Scholar]
- Lucchesi J.C. (1996) Dosage compensation in Drosophila and the ‘complex’ world of transcriptional regulation. BioEssays, 18, 541–547. [DOI] [PubMed] [Google Scholar]
- Lyman L.M., Copps,K., Rastelli,L., Kelley,R.L. and Kuroda,M.I. (1997) Drosophila male-specific lethal-2 protein: structure/function analysis and dependence on MSL-1 for chromosome association. Genetics, 147, 1743–1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meller V.H. (2000) Dosage compensation: making 1X equal 2X. Trends Cell Biol., 10, 54–59. [DOI] [PubMed] [Google Scholar]
- Meller V.H., Wu,K.H., Roman,G., Kuroda,M.I. and Davis,R.L. (1997) roX1 RNA paints the X chromosome of male Drosophila and is regulated by the dosage compensation system. Cell, 88, 445–457. [DOI] [PubMed] [Google Scholar]
- Meller V.H., Gordadze,P.R., Park,Y., Chu,X., Stuckenholz,C., Kelley,R.L. and Kuroda,M.I. (2000) Ordered assembly of roX RNAs into MSL complexes on the dosage-compensated X chromosome in Drosophila. Curr. Biol., 10, 136–143. [DOI] [PubMed] [Google Scholar]
- Nakayama J., Rice,J.C., Strahl,B.D., Allis,C.D. and Grewal,S.I. (2001) Role of histone H3 lysine 9 methylation in epigenetic control of heterochromatin assembly. Science, 292, 110–113. [DOI] [PubMed] [Google Scholar]
- Platero J.S., Hartnett,T. and Eissenberg,J.C. (1995) Functional analysis of the chromo domain of HP1. EMBO J., 14, 3977–3986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter L., Bone,J.R. and Kuroda,M.I. (1996) RNA-dependent association of the Drosophila maleless protein with the male X chromosome. Genes Cells, 1, 325–336. [DOI] [PubMed] [Google Scholar]
- Smith E.R., Pannuti,A., Gu,W., Steurnagel,A., Cook,R.G., Allis,C.D. and Lucchesi,J.C. (2000) The Drosophila MSL complex acetylates histone H4 at lysine 16, a chromatin modification linked to dosage compensation. Mol. Cell. Biol., 20, 312–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith E.R., Allis,C.D. and Lucchesi,J.C. (2001) Linking global histone acetylation to the transcription enhancement of X-chromosomal genes in Drosophila males. J. Biol. Chem., 276, 31483–31486. [DOI] [PubMed] [Google Scholar]
- Voelker R.A., Wisely,G.B., Huang,S.-M. and Gyurkovics,H. (1985) Genetic and molecular variation in the RpII215 region of Drosophila melanogaster. Mol. Gen. Genet., 201, 437–445. [Google Scholar]
- Zhang P. and Hawley,R.S. (1990) The genetic analysis of distributive segregation in Drosophila melanogaster. II. Further genetic analysis of the nod locus. Genetics, 125, 115–127. [DOI] [PMC free article] [PubMed] [Google Scholar]