Abstract
The yeast Ppz protein phosphatases and the Hal3p inhibitory subunit are important determinants of salt tolerance, cell wall integrity and cell cycle progression. We present several lines of evidence showing that these disparate phenotypes are connected by the fact that Ppz regulates K+ transport. First, salt tolerance, cell wall integrity and cell cycle phenotypes of Ppz mutants are dependent on the Trk K+ transporters. Secondly, Ppz mutants exhibit altered activity of the Trk system, as measured by rubidium uptake. Thirdly, Ppz mutants exhibit altered intracellular K+ and pH, as expected from H+ efflux providing electrical balance during K+ uptake. Our unifying picture of Ppz phenotypes contends that activation of Trk by decreased Ppz activity results in plasma membrane depolarization (reducing uptake of toxic cations), increased intracellular K+ and turgor (compromising cell integrity), and increased intracellular pH (augmenting the expression of pH-regulated genes and facilitating α-factor recovery). In addition to providing a coherent explanation for all Ppz-dependent phenotypes, our results provide evidence for a causal relationship between intracellular cation homeostasis and a potential cell cycle checkpoint.
Keywords: cell cycle/halotolerance/pH/phosphatase/potassium
Introduction
An important feature of the physiology of living cells is the homeostasis of the major monovalent cations K+, H+ and Na+. K+ is the major intracellular cation contributing to cell turgor and ionic strength (Serrano et al., 1999). The accumulation of K+ and the extrusion of Na+ from the intracellular medium may be explained by the ability of Na+, but not K+, to displace essential Mg2+ from the catalytic sites of some enzymes such as the Hal2 phosphatase (Murguia et al., 1995; Albert et al., 2000). On the other hand, intracellular pH is tightly regulated because of its general and specific effects on proteins and biochemical reactions (Nuccitelli and Heiple, 1982). Cellular membranes contain diverse cation transport systems to ensure the right intracellular ionic composition, but the mechanisms that regulate their activity to achieve cation homeostasis are only starting to be elucidated (Serrano and Rodriguez-Navarro, 2001).
Several lines of evidence have indicated the existence of a link between cation homeostasis and the cell cycle. Specifically, previous studies have established a correlation between cytosolic alkalinization and G1 progression in yeast (Gillies et al., 1981) and animal cells (Nuccitelli and Heiple, 1982). This increased intracellular pH may be either a regulatory signal (Perona and Serrano, 1988) or merely permissive for cell proliferation (Grinstein et al., 1989). More recently, in the eukaryotic model system Saccharomyces cerevisiae, alterations in the expression of several genes encoding signal transduction proteins have been shown to affect both intracellular ion homeostasis and cell cycle, again suggesting a possible link between these two highly regulated cellular processes. Among these genes are two serine/threonine phosphatases, SIT4 and PPZ1, and a regulatory protein, Hal3p/Sis2p, all three of which are genetically and biochemically interrelated (Fernandez-Sarabia et al., 1992; Di Como et al., 1995; Ferrando et al., 1995; Posas et al., 1995; de Nadal et al., 1998; Clotet et al., 1999).
SIT4 was originally identified as a cell cycle regulator and more recently was identified in a screening searching for genes conferring lithium tolerance upon overexpression (Masuda et al., 2000). Disruption of the gene causes a slow-growth phenotype attributed to a delay in G1 cyclin expression and bud emergence (Arndt et al., 1989; Sutton et al., 1991; Fernandez-Sarabia et al., 1992). More recent reports suggest that this phosphatase may act in the TOR pathway responding to nutrient availability (Di Como and Arndt, 1996). In addition, it was reported that the SIT4 mRNA is up-regulated in response to lithium challenge and that under some conditions overexpression of the gene confers salt tolerance (Masuda et al., 2000).
The HAL3/SIS2 gene was cloned independently as both a suppressor of the growth defect of a sit4 strain and as a gene conferring halotolerance upon overexpression (Di Como et al., 1995; Ferrando et al., 1995). It has been proposed to function in a pathway parallel to that of calcineurin, modulating both intracellular cation homeostasis and G1 progression (Ferrando et al., 1995). More recent genetic and biochemical data suggest that Hal3p acts by inhibiting the protein phosphatase Ppz1p (de Nadal et al., 1998).
Ppz1p and Ppz2p are two partially redundant serine/threonine phosphatases whose modulation in expression levels affects salt tolerance, cell wall integrity and cell cycle regulation (Posas et al., 1993, 1995; Clotet et al., 1996, 1999). Disruption of the genes provides increased tolerance to toxic concentrations of lithium chloride, sensitivity to caffeine and augmented recovery after an α-factor-induced G1 block. In addition, overexpression of PPZ1 causes a pronounced slow-growth defect. As is the case with Sit4p, the cellular role of the Ppz protein phosphatases is largely undefined, although a potential role in the regulation of gene transcription and translational fidelity has been proposed (Posas et al., 1995; de Nadal et al., 2001).
In this report we demonstrate that the Ppz phosphatases and the regulatory subunit Hal3p are involved in the proper regulation of intracellular potassium concentrations and pH. Modulation of these biochemical parameters has consequences for tolerance to toxic cations, cell wall integrity and the observed cell cycle effects. Furthermore, we show that this regulation is dependent on Trk1,2p-mediated K+ transport.
Results
Disruption of PPZ1,2 mediates ENA1-4-independent sodium and lithium tolerance
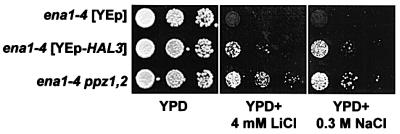
The genetic and biochemical interaction between Hal3p and the Ppz phosphatases has been thoroughly demonstrated (de Nadal et al., 1998). However, in terms of salt tolerance, the ppz1,2 Li+ phenotypes were reported to be ENA dependent, whereas HAL3 overexpression confers sodium tolerance in the absence of ENA1-4 (Ferrando et al., 1995; Posas et al., 1995). ENA1 encodes a P-type ATPase mediating Na+ and Li+ extrusion and is a major determinant of salt tolerance in S.cerevisiae (reviewed in Serrano et al., 1999). After a more detailed examination, we observed that both disruption of ppz1,2 and overexpression of HAL3 confer tolerance to lithium and sodium, independent of ENA1-4 (Figure 1). These observations expand on results reported by Ferrando et al. (1995) for HAL3, but appear to contradict those reported by Posas et al. (1995) for ppz1,2 strains. The discrepancy with the previous results may be due to the different sensitivities of the growth assays, but what is clear is that at least part of the salt tolerance conferred by reduction of Ppz phosphatase activity is independent of ENA1.
Fig. 1. ENA-independent salt tolerance phenotypes of YEp-HAL3 and ppz1,2 strains. DBY746 ena1-4::HIS3[pRS699], DBY746 ena1-4::HIS3[pRS699-HAL3] and DBY746 ena1-4::HIS3 ppz1::URA3 ppz2::TRP1 strains were grown to saturation in selective media, serially diluted in sterile water and spotted on to YPD plates containing the indicated amount of salt. Images were taken after 2–3 days of incubation at 28°C. Identical results were obtained from at least three independent colonies in two separate experiments.
Strains with reduced Ppz activity are tolerant to toxic cations
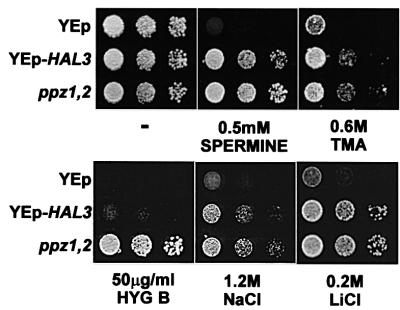
In an attempt to begin to define the ENA-independent mechanisms of PPZ/HAL3-mediated salt tolerance, we examined the tolerance phenotypes of these strains to several toxic cations. Strains harbouring these genetic alterations showed tolerance to spermine, tetramethyl ammonium and hygromycin B, in addition to LiCl and NaCl (Figure 2). This general tolerance to toxic cations has been shown to correlate with a reduction in the membrane potential, which decreases the voltage-dependent uptake of cations (Mulet et al., 1999; Goossens et al., 2000). Accordingly, we observed a decrease in methyl ammonium uptake in strains lacking PPZ1,2 (6.2 ± 0.4 as compared with 9.0 ± 0.7 nmol/mg cells, measured at 90 min). This assay is used to estimate membrane potential, which cannot be measured directly in yeast (Mulet et al., 1999)
Fig. 2. YEp-HAL3 and ppz1,2 strains are tolerant to toxic cations. W303-1A [pYPGE15], W303-1A [pYPGE15-HAL3], W303-1A and W303-1A ppz1::URA3 ppz2::TRP1 strains were grown to saturation in selective media, serially diluted in sterile water and spotted on to YPD plates containing the indicated amount of cations. Images were taken after 2–3 days of incubation at 28°C. Identical results were obtained from at least three independent colonies in three separate experiments, using various concentrations of each cation and in two different genetic backgrounds (DBY746 and W303-1A).
Cytosolic alkalinization in strains lacking PPZ1,2
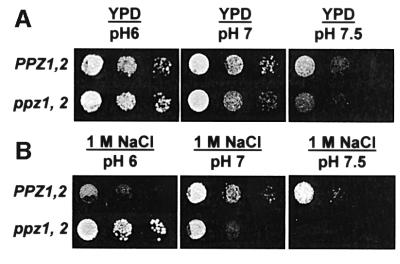
After observing that ppz1,2 mutant strains probably display changes in membrane potential, we examined whether there were also alterations in the internal pH of this strain, relative to the wild-type control. At an external pH of 6, the strain lacking PPZ1,2 had an internal pH 0.4 units higher than the wild-type control (6.80 ± 0.14 compared with 6.40 ± 0.02, respectively), as measured using 1-hydroxy-3,6,8-pyrene-trisulfonic acid (HPTS) (Pena et al., 1995). As an alternative approach to estimating intracellular pH, we also investigated the transcriptional regulation of alkaline-induced genes in these strains (Lamb et al., 2001). As demonstrated in Figure 3, ppz1,2 strains grown in rich media, buffered to pH 7, show a notably augmented induction of three alkaline-responsive genes, providing further evidence for a relative increase in the internal pH of strains lacking PPZ1,2. In addition, we studied the effect of pH on both growth and salt tolerance in these strains. We observed that strains lacking PPZ1,2 show a relative slow-growth phenotype on plates with increasing pH and that the tolerance to NaCl is markedly pH dependent: disruption of PPZ1,2 no longer confers tolerance to NaCl when tested at an external pH of 7 and this strain is inviable on plates containing 1 M NaCl at pH 7.5 (Figure 4).
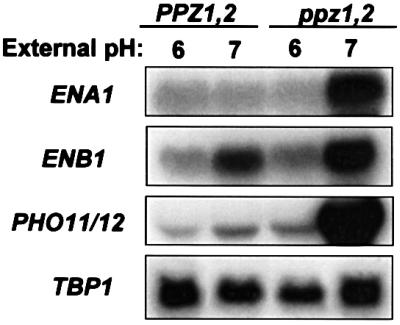
Fig. 3. Northern blot analysis of the steady-state levels of alkaline-induced transcripts. Wild-type (DBY746) and ppz1,2 (DBY746 ppz1::URA3 ppz2::TRP1) strains were grown in YPD buffered to either pH 6 or 7 with 50 mM Bis-Tris. After extraction, RNA was separated by electrophoresis and transferred to nitrocellulose. Membranes were then analysed with the radiolabelled probes indicated. TBP1 was used as an internal loading control. Identical results were obtained in two separate experiments.
Fig. 4. Growth and sodium tolerance in ppz1,2 strains is pH dependent. Wild-type (DBY746) and ppz1,2 (DBY746 ppz1::URA3 ppz2::TRP1) strains were grown to saturation in selective media, serially diluted in sterile water and spotted on to YPD plates buffered to the indicated pH, without (A) or with (B) 1 M NaCl. Images were taken after 2–3 days (pH 6.5 and 7) or 5 days (pH 7.5) of incubation at 28°C. Identical results were obtained in two separate experiments.
The in vitro activity of the Pma1p H+-pump is not altered in strains lacking PPZ1,2
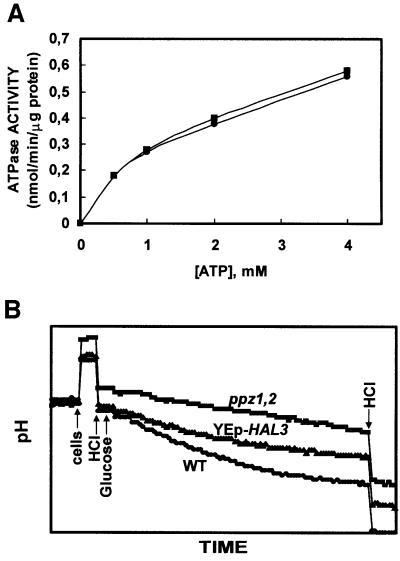
As described above, a null mutation of the Ppz phosphatase results in decreased membrane potential and increased intracellular pH. According to our current model, the plasma membrane H+-pumping ATPase (Pma1p) is the electrical generator, and the K+ transporter (Trk1,2p) is the major consumer of electrical potential (Goossens et al., 2000). The ppz1,2 mutation could decrease membrane potential by either inhibiting Pma1p or activating Trk. In the first case, intracellular pH should decrease because of less proton extrusion from the cells. In the second case, intracellular pH should increase because the enhanced K+ uptake would favour H+ efflux to keep the electrical balance. Our results are compatible with the second possibility. Accordingly, no alteration could be detected for the in vitro activity of the Pma1p enzyme in the ppz1,2 strain (Figure 5A). The in vivo activity of the proton pump can be measured by the acidification of the external medium (Portillo and Serrano, 1989). As indicated in Figure 5B, the ppz1,2 mutant and the strain overexpressing the inhibitory Hal3p subunit exhibited decreased acidification as compared with control cells. As Pma1p has a relatively acidic optimum pH (Portillo and Serrano, 1989), these results probably reflect a decrease in the in vivo activity of the enzyme due to intracellular alkalinization.
Fig. 5. Analysis of Pma1p activity in vitro and in vivo. (A) ATPase activity of plasma membranes purifed from glucose-fed cells was assayed at pH 6.5. DBY746 (squares), and DBY746 ppz1::URA3 ppz2::TRP1 (circles). Each reaction contained 2 µg of protein and the samples were incubated at 28°C for 60 min. Each data point represents the average of two independently isolated membrane preparations each assayed in duplicate. Standard deviations were <5%. (B) The strains indicated (DBY746 [pRS699], DBY746 [pRS699-HAL3] and DBY746 ppz1::URA3 ppz2::TRP1) were grown to mid-log phase, harvested, washed and resuspended in sterile water and incubated for 3 h at 28°C. Cells (30 mg) were then suspended in 10 ml of assay buffer and pH changes were recorded after the addition of 200 µmol of glucose. Cells, glucose and HCl (400 nmol for calibration) were added where indicated. Similar results were observed in two separate experiments and after overnight starvation at 4°C.
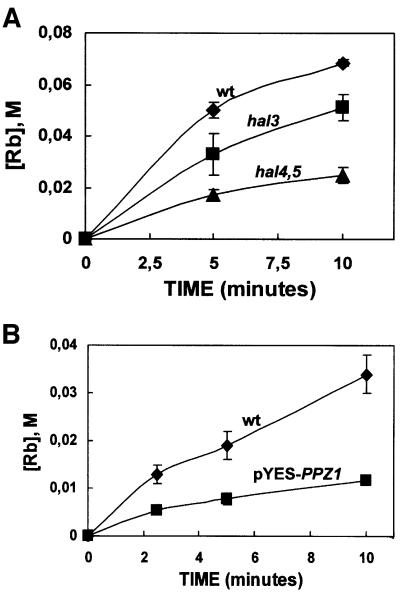
Strains with increased Ppz activity are defective in Rb+ uptake
Having discarded a role for Pma1p, we next examined whether changes in potassium transport could be detected in strains with increased Ppz activity (lacking HAL3 or overexpressing PPZ1). We chose this complementary approach based on previous experiments with the Trk1,2p-regulating protein kinases, Hal4,5p showing that, probably due to the biochemical properties of the transporters, increases in Rb+ uptake are difficult to detect. Moreover, reduced Rb+ uptake was shown to be a reliable measurement for perturbations in K+ transport (Mulet et al., 1999). Decreases in rubidium uptake were observed in strains either lacking HAL3 or overexpressing PPZ1 under the control of a galactose-inducible promoter (Figure 6). In addition, we observed the expected increase in steady state potassium levels in strains overexpressing HAL3 (96.5 ± 0.7 compared with 78.5 ± 2.1 mM in the control strain carrying the empty vector) and the expected decrease in PPZ1-overexpressing strains (70.4 ± 0.6 compared with 81.0 ± 0.8 mM under the test conditions). These data are in good agreement with results showing that strains lacking PPZ1,2 have a higher intracellular pH, very likely due to the increase in internal potassium concentrations.
Fig. 6. Rb+ uptake is modulated by the gene dosage of both HAL3 and PPZ1. (A) The strains indicated, W303-1A (diamonds); W303-1A hal3::LEU2 (squares); W303-1A hal4::LEU2, hal5::HIS3 (triangles), were grown to exponential phase in selective media, washed and incubated in the potassium starvation medium for 4 h. After further washing, the uptake of 0.2 mM Rb was determined by HPLC analysis as described in Materials and methods. (B) The experiment was performed as described in (A), except that PPZ1 expression was induced by incubation in galactose medium before the potassium starvation procedure. W303-1A [pYES2] (diamonds); W303-1A [pYES2-PPZ1] (squares). Data are the average of triplicate determinations and the error bars represent the standard deviation for each point.
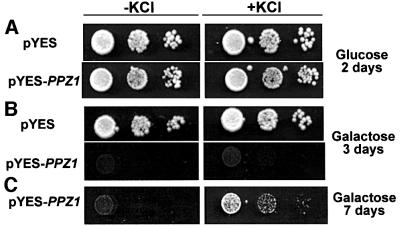
Addition of exogenous KCl partially recovers the slow-growth phenotype of PPZ1-overexpressing stains
Having observed a marked decrease in Rb+ uptake in strains conditionally overexpressing PPZ1, we hypothesized that part of the slow-growth phenotype may be attributed to sub-optimal internal K+ concentrations. As depicted in Figure 7, strains overexpressing PPZ1 clearly show an improvement in growth rate when plates are supplemented with KCl. Not surprisingly, growth is not recovered to wild-type levels, as unregulated, low affinity potassium uptake is unlikely to completely ameliorate the PPZ1-induced cell cycle block. Nevertheless, this result provides preliminary evidence indicating that the alterations observed in potassium transport are responsible not only for salt tolerance, but also for the cell cycle progression phenotypes of strains with genetically altered levels of Ppz activity.
Fig. 7. Addition of exogenous KCl partially rescues the slow-growth phenotype of a PPZ1-overexpressing strain. The strains indicated (W303-1A [pYES2] and W303-1A [pYES2-PPZ1]) were grown to saturation in selective media and then serially diluted and spotted on to plates with or without the addition of 0.1 M KCl, and using glucose (A) or galactose (B and C) as the carbon source (to induce PPZ1 expression). Images of the plates in (A) were taken after 2 days. (B) and (C) depict the same plates imaged after 3 or 7 days, respectively. Similar results were observed in two separate experiments and with addition of 0.1 or 0.05 M KCl.
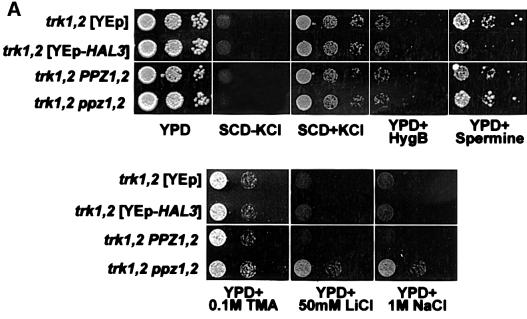
Examination of the TRK1,2 dependence of toxic cation tolerance in strains with decreased Ppz activity
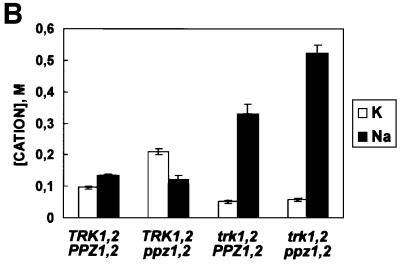
We next studied the epistatic interaction between Ppz and the high affinity K+ transporters encoded by the TRK1,2 genes with respect to tolerance to toxic cations. Figure 8A shows that both ppz1,2 and HAL3-mediated tolerance to hygromycin B, spermine and tetramethyl ammonium are completely dependent on the presence of the TRK1,2 genes (compare with Figure 2). Additionally, lithium and sodium chloride tolerance phenotypes observed upon overexpression of HAL3 are TRK1,2 dependent. How ever, the disruption of PPZ1,2 retains some tolerance to these cations even in strains lacking TRK1,2. We therefore analysed the steady state concentrations of both K+ and Na+ in strains grown in the presence of 0.8 M NaCl (Figure 8B). We observed that the K+/Na+ ratio was reversed in strains lacking PPZ1,2 as compared with the wild-type control, thus demonstrating that the absence of PPZ1,2 confers salt tolerance by accumulating K+. In addition, we show that this K+ accumulation is TRK1,2 dependent. Interestingly, the mutated strain lacking PPZ1,2 and TRK1,2, while displaying tolerance in the growth assays, accumulates more Na+ than the mutant lacking only TRK1,2. This result demonstrates that the remaining tolerance of the trk1,2 ppz1,2 strain is not due to increased sodium extrusion by ENA, but is most likely due to sequestration of excess Na+ in the vacuole. This hypothesis is based on several studies documenting a similar phenotype in yeast overexpressing the vacuolar Na+/H+ exchanger encoded by the NHX1 gene from both S.cerevisiae and Arabidopsis thaliana (Nass et al., 1997; Gaxiola et al., 1999; Darley et al., 2000).
Fig. 8. Characterization of the tolerance to toxic cations mediated by YEp-HAL3 and ppz1,2 in the trk1,2 background. (A) The strains indicated (W303-1A trk1::LEU2 trk2::HIS3 [YPGE15], W303-1A trk1::LEU2 trk2::HIS3 [YPGE15-HAL3] and W303-1A trk1::LEU2 trk2::HIS3 ppz1::URA3 ppz2::TRP1) were grown to saturation in selective media supplemented with 0.2 M KCl, serially diluted in sterile water and spotted on to YPD plates containing the indicated amount of cations. Images were taken after 2–3 days (top panel) or 6 days (lower panel) of incubation at 28°C. Identical results were obtained from at least three independent colonies in three separate experiments. (B) The strains indicated were grown to OD660 = 0.5 in YPD media containing 0.8 M NaCl. Cells were washed extensively with 1.2 M sorbitol, 10 mM MgCl2, lysed by boiling and after centrifugation to remove cellular debris, internal ion concentrations were determined by HPLC analysis, as described. Similar results were observed in two separate experiments.
ppz1,2-mediated alterations in amino acid uptake are TRK1,2 dependent
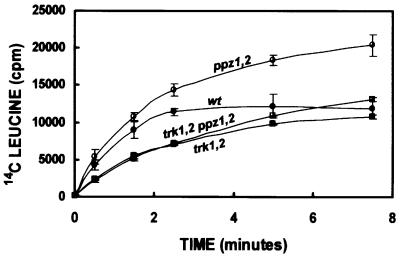
To examine the TRK1,2 dependence of ppz1,2 phenotypes in a short-term biochemical assay, we measured leucine uptake in the corresponding strains. Amino acids have been shown to be symported with protons, thus their uptake will be directly influenced by the internal pH of the cell (Vallejo and Serrano, 1989). As shown in Figure 9, strains lacking ppz1,2 display both increased initial rates and overall uptake of radiolabelled leucine, as compared with the wild-type control. This result is in good agreement with previous experiments demonstrating a relatively alkaline pH for this strain, which would create a more favourable pH gradient for amino acid symport. We observed that this phenotype also requires the presence of the TRK1,2 genes, as the trk1,2 ppz1,2 strain shows similar uptake to the strain lacking only trk1,2. We corroborated this experiment with internal pH measurements, using HTPS as described earlier. No change in internal pH was observed in trk1,2 ppz1,2 strains as compared with trk1,2.
Fig. 9. ppz1,2-mediated changes in leucine uptake depends on the presence of TRK1,2. Determination of [14C]leucine uptake was performed as described (Pascual-Ahuir et al., 2001). Data are the average of three separate experiments and error bars represent the standard deviation for each point. W303-1A (filled circles); W303-1A ppz1::URA3, ppz2::TRP1 (open circles); W303-1A trk1::LEU2 trk2::HIS3 (filled squares); W303-1A trk1::LEU2 trk2::HIS3 ppz1::URA3 ppz2::TRP1 (open squares).
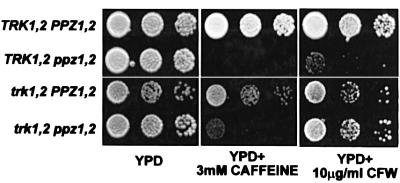
Sensitivity to both caffeine and calcofluor white of the ppz1,2 strain is partially relieved by disruption of TRK1,2
Previous studies have established a role for Ppz in the maintenance of osmotic stability. Strains lacking PPZ1,2 are sensitive to caffeine and this sensitivity can be ameliorated by the addition of sorbitol (Posas et al., 1993). In addition, disruption of PPZ1 exacerbates the lytic phenotype of the mpk1 mutant. The double mutant requires media supplemented with sorbitol for optimal growth (Lee et al., 1993). We postulated that this effect could be due to the combination of a weaker cell wall, caused by lack of MPK1, and increased turgor pressure as a result of increased Trk activity in the ppz1,2 mutant. As reported previously, disruption of PPZ1,2 causes sensitivity to very low concentrations of caffeine. This sensitivity is partially rescued by the disruption of trk1,2 in the ppz1,2 mutant (Figure 10). As the mechanism of toxicity of caffeine is not clearly defined, we corroborated these observations using another compound known to weaken the cell wall, calcofluor white. This compound is a negatively charged fluorescent dye that binds to nascent chains of chitin and glucan, thus preventing microfilament assembly and proper supramolecular organization of the cell wall (Lussier et al., 1997). Strains lacking PPZ1,2 display sensitivity to calcofluor white and, as observed with caffeine, this sensitivity is relieved by disruption of TRK1,2 in the ppz1,2 background (Figure 10B).
Fig. 10. Sensitivity of the ppz1,2 strain to both caffeine and calcofluor white is relieved by further disruption of TRK1,2. The strains indicated: W303-1A; W303-1A ppz1::URA3 ppz2::TRP1; W303-1A trk1::LEU2 trk2::HIS3; and W303-1A trk1::LEU2 trk2::HIS3 ppz1::URA3 ppz2::TRP1, were grown to saturation in selective media, serially diluted in sterile water and spotted on to YPD plates containing the indicated amount of caffeine (A) or calcofluor white (B). Images were taken after 2–3 days of incubation at 28°C. Identical results were observed in at least two separate experiments.
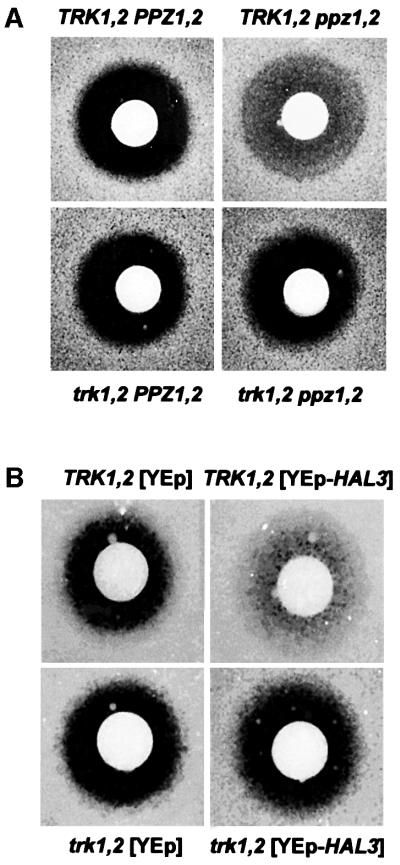
α-factor recovery
To determine whether the cell cycle effects observed in strains with decreased Ppz activity described previously were also dependent on the presence of TRK1,2, we performed α-factor recovery assays. Augmented recovery from α-factor has been shown to correlate with changes in the kinetics of transcription of G1 cyclins (Di Como et al., 1995; Clotet et al., 1999). In both cases, the ability of ppz1,2 or YEp-HAL3 strains to accelerate recovery from the G1 block imposed by α-factor required the presence of the TRK1,2 genes (Figure 11), again suggesting that changes in potassium transport are responsible, at least in part, for the phenotypes observed in strains with reduced Ppz activity.
Fig. 11. YEp-HAL3- and ppz1,2-mediated α-factor recovery depends on the presence of TRK1,2. The strains (A) W303-1A; W303-1A ppz1::URA3 ppz2::TRP1; W303-1A trk1::LEU2 trk2::HIS3; W303-1A trk1::LEU2 trk2::HIS3 ppz1::URA3 ppz2::TRP1 and (B) W303-1A [YPGE15]; W303-1A [YPGE15-HAL3]; W303-1A trk1::LEU2 trk2::HIS3 [YPGE15]; W303-1A trk1::LEU2 trk2::HIS3 [YPGE15-HAL3] were grown to an OD of 0.4 in complete media and the assay was performed as described in Materials and methods. Images of the plates were taken after 48 h and similar results were observed in four separate experiments.
Discussion
Alterations in the activity of the Ppz protein phosphatase affect salt tolerance, cell wall integrity and cell cycle progression in the yeast S.cerevisiae. In this report, we show that strains lacking PPZ1 and -2, or overexpressing the gene encoding an inhibitory subunit, Hal3p, provide salt tolerance in the absence of ENA1-4 and display phenotypes characteristic of strains with altered membrane potential. Accordingly, ppz1,2 strains have a more alkaline cytoplasmic pH, as demonstrated by direct measurements, superinduction of alkaline-responsive genes (such as ENA1), and pH-sensitive growth and salt tolerance phenotypes.
Previous reports have suggested that loss of Ppz activity confers salt tolerance by up-regulating the transcription of ENA1. Our current data demonstrate that loss of Ppz activity regulates ENA1 transcription in response to cytosolic alkalinization, not saline stress. Alkaline stress is an extremely potent and sensitive elicitor of ENA1 transcription (Garciadeblas et al., 1993). Minor increases in pH have a strongly additive effect with respect to saline induction of ENA1 transcription (L.Yenush, unpublished results). Accordingly, we have observed that relative increases in the salt induction of ENA1 in strains lacking PPZ1,2 are observed at an external pH of 7, but not of 6 (data not shown). Moreover, we observed that strains lacking PPZ1,2 respond to chronic saline stress, not by appreciably decreasing the internal concentrations of sodium, which would be expected for strains with increased ENA1 induction, but by increasing the potassium content (Figure 8B). Thus, the apparent discrepancies between our current results and previous reports can be explained as follows: decreases in Ppz activity do regulate ENA1 transcription, as reported previously, but in response to alkaline, not saline stress. This induction of the ENA1 gene will undoubtedly contribute to the strong salt tolerance phenotype of strains with decreased Ppz activity, and this increase in Ena1p activity is detectable in short-term biochemical assays. However, a TRK1,2-dependent increase in intracellular potassium is largely responsible for the adaptive response of these strains to salt challenge.
Previous studies have established an important role for alterations in membrane potential in determining salt tolerance. For example, absence of the hydrophobic polypeptide encoded by the PMP3 gene causes an increase in the membrane potential, non-specific influx of cations and the expected salt sensitivity (Navarre and Goffeau, 2000). On the other hand, a reduction in the activity of the proton ATPase, Pma1p, either by mutagenesis or by removing the activating kinase, Ptk2p, results in a decrease in membrane potential, accompanied by decreased cation uptake, thus resulting in salt tolerance (Goossens et al., 2000). A third example involves the high affinity potassium transporters encoded by the TRK1,2 genes. Disruption of TRK1,2 or their activating kinases, encoded by the HAL4 and HAL5 genes, causes a hyperpolarization of the membrane and salt sensitivity (Madrid et al., 1998; Mulet et al., 1999). Alternatively, overexpression of HAL4,5 decreases the membrane potential and confers salt tolerance (Mulet et al., 1999).
The in vitro activity of the H+-ATPase, Pma1p, is not altered in strains lacking ppz1,2. However, defects in rubidium uptake are detected in strains conditionally overexpressing PPZ1 or lacking hal3, a situation expected to be analogous to disruption of HAL4,5. Moreover, the genes encoding the K+ transporters Trk1,2p are required for ppz1,2- and HAL3-mediated tolerance to toxic concentrations of a subset of polycations and other phenotypes discussed below.
Although decreased activity of Pma1p and increased activity of Trk1,2p both result in a decrease in membrane potential by decreasing the negative charge at the inner leaflet of the plasma membrane, they have opposing effects on the internal pH of the cell. A decrease in proton efflux caused by Pma1p mutations acidifies the cytosol (Portillo and Serrano, 1989). On the other hand, an increase in Trk1,2p activity should cause an alkalinization due to proton efflux needed to maintain the electrical balance in response to increased internal potassium concentrations. Our data strongly support this hypothesis. In strains lacking PPZ1,2, we observed a relative increase in the internal pH and many ppz1,2 phenotypes are pH dependent, including growth, salt tolerance and induction of alkaline-responsive genes. All of these observations are consistent with the hypothesis that strains lacking ppz1,2 have a higher cytosolic pH and thus arrive at conditions of alkaline stress at lower external pH. In addition, we observed a TRK1,2-dependent change in amino acid uptake consistent with the expected alterations in the cytosolic pH of the respective strains.
We present two different assays that are consistent with the hypothesis that the negative effect on cell wall stability observed in strains lacking PPZ1,2 is also due to the increase in Trk activity. Using both caffeine and calcofluor white, two compounds known to compromise cell wall stability, we show that the sensitivity observed in ppz1,2 strains is relieved by additional disruption of TRK1,2. Taken together, these results indicate that the negative effect on cell wall integrity observed in strains lacking PPZ1,2 is at least partially due to the incremented turgor pressure caused by increased internal potassium concentrations.
Changes in the activity of the Ppz phosphatase have also been shown by several different experimental approaches to affect cell cycle progression (Clotet et al., 1996, 1999). In fact, HAL3, also known as SIS2, was cloned as a multicopy suppressor of the cell cycle defect observed in strains lacking the gene encoding the Sit4p protein phosphatase (Di Como et al., 1995). It was subsequently shown that this effect of Hal3p was due to its ability to inhibit Ppz1p (Clotet et al., 1999). However, the mechanism of this effect was completely unknown. Here we present data that the effects of Hal3p and Ppz1p on the cell cycle are at least partially attributable to alterations in potassium and pH homeostasis. First, we demonstrate that the PPZ1-induced cell cycle defect is partially rescued by addition of exogenous KCl. This result suggests that at least part of the defect caused by overexpression of PPZ1 is due to a lack of intracellular potassium. We also show that the effects of disrupting PPZ1,2 or overexpressing HAL3 on α-factor recovery require functional TRK1,2 genes. In our experimental system, recovery from α-factor has been shown to correlate with an acceleration in the expression of G1 cyclins, bud formation and DNA replication (Di Como et al., 1995; Clotet et al., 1999). Thus, our data indicate that high affinity potassium uptake is required for these processes.
The results presented here indicate strongly that Trk1,2p are downstream mediators of Ppz-dependent phenotypes. Whether or not Trk1,2p are direct substrates of Ppz is currently under investigation. We observe no difference in the relative amounts of TRK1,2 mRNA upon disruption of PPZ1,2, suggesting that transcriptional regulation is not involved (data not shown). The observation that overexpression of HAL3 is able to provide salt tolerance in a hal4,5 mutant demonstrates that the phenotypes caused by a decrease in Ppz activity do not require these kinases (L.Yenush, unpublished observations). Given that overexpression of HAL4,5 and decreased activity of Ppz have been shown to activate Trk1,2p, it is possible that these enzymes control these transporters directly through modulation of their phosphorylation state. Future studies will be aimed at clarifying this important point.
The translation elongation factor, 1Bα, has been identified as being hyperphosphorylated in a ppz1,2 mutant; however, direct dephosphorylation by Ppz could not be demonstrated (de Nadal et al., 2001). Moreover, as the pattern of phosphorylation in a ppz1,2 mutant is very similar to that of a wild-type strain (de Nadal et al., 2001), it is likely that Ppz does not have a wide range of substrates. Therefore, the hypothesis that the various phenotypes mediated by this phosphatase are attributable to the regulation of specific proteins, such as Trk1,2p, is very plausible.
Here we provide evidence that the modulation of a single physiological parameter, intracellular K+ concentrations (and the concomitant change in cytosolic pH), has implications for toxic cation tolerance, cell wall integrity and cell cycle regulation. To our knowledge, this is the first study that provides both genetic and biochemical evidence linking potassium transport and both cell wall integrity and cell cycle progression. Increases in internal potassium concentrations caused by alterations in the regulation of the Trk transporters change several physiological parameters in the cell. First, membrane potential is decreased, lowering the amount of toxic cations entering the cell non-specifically and thus providing salt tolerance. Secondly, turgor pressure is increased, exacerbating any weakening of the cell wall (such as addition of calcofluor white). Thirdly, as internal potassium increases so does the pH of the cytoplasm. Although the mechanism by which changes in Trk activity effect progression of the cell cycle have yet to be worked out, our results provide good preliminary evidence for a causal relationship between intracellular ion homeostasis and a potential cell cycle checkpoint, possibly at the level of G1 cyclin gene expression. Future experiments will determine whether, for example, either the SBF transcription factor (Swi4–Swi6) or the regulatory system that activates it (Iyer et al., 2001) are pH or K+ sensitive.
Materials and methods
Yeast strains and culture conditions
All strains of S.cerevisiae used in this work are listed in Table I. YPD contained 2% glucose, 2% peptone and 1% yeast extract. Where indica ted, YPD was supplemented with 50 mM Bis-Tris {[bis-(2-hydroxyethyl)-imino]-tris-(hydroxymethyl)-methane} (pKa 6.5, CalBiochem) and pH adjusted to 6, 7 or 7.5 with HCl. Synthetic medium (SD) contained 2% glucose, 0.7% yeast nitrogen base (Difco) without amino acids, 50 mM MES [2-(N-morpholino)ethanesulfonic acid] adjusted to pH 5.5 with Tris, and the amino acids and purine and pyrimidine bases required by the strains. The growth of yeast strains under different stress conditions was assayed by spotting serial dilutions of saturated cultures on YPD plates containing the indicated amounts of cations.
Table I. Yeast strains used in this study.
| Strain | Relevant genotype | Referencea |
|---|---|---|
| DBY746 | Matα ura3-52 leu2-3 leu2-112 his3-Δ1 trp1-289 | A.Rodriguez-Navarro |
| LY 78 | DBY746 [pRS699] | |
| LY 79 | DBY746 [pRS699-HAL3] | |
| LY 83 | DBY746 ppz1::URA3 ppz2::TRP1 | Posas et al. (1995) |
| LY 85 | DBY746 ena1-4::HIS3 | |
| LY 105 | DBY746 ena1-4::HIS3 [pRS699] | |
| LY 106 | DBY746 ena1-4::HIS3 [pRS699-HAL3] | |
| LY 102 | DBY746 ena1-4::HIS3 ppz1::URA3 ppz2::TRP1 | |
| W303-1A | Mata ade2-1 can1-100 his3-11,15 leu2-3,112 trp1-1 ura3-1 | Goosens et al. (2000) |
| LY 126 | W303-1A [pYPGE15] | |
| LY 127 | W303-1A [pYPGE15-HAL3] | |
| LY 139 | W303-1A ppz1::URA3 ppz2::TRP1 | |
| WΔ3 | W303-1A trk1::LEU2 trk2::HIS3 | Mulet et al. (1999) |
| LY 124 | W303-1A trk1::LEU2 trk2::HIS3 [pYPGE15] | |
| LY 125 | W303-1A trk1::LEU2 trk2::HIS3 [pYPGE15-HAL3] | |
| LY 140 | W303-1A trk1::LEU2 trk2::HIS3 ppz1::URA3 ppz2::TRP1 | |
| LY 134 | W303-1A [pYES2] | |
| LY 135 | W303-1A [pYES2-PPZ1] |
aUnless indicated otherwise, all strains are from this study.
Plasmids and gene disruptions
HAL3 was overexpressed by inserting the PCR-derived open reading frame into the EcoRI and XhoI sites of pYPGE15 (2 µm origin, URA3 marker, constitutive promoter from the PGK gene) (Brunelli and Pall, 1993) or the XhoI site of pRS699 (YEp352-derived vector; Hill et al., 1986), with promoter and terminator of PMA1 inserted into the HindIII site, separated by an XhoI linker for cloning. Construction of the ppz1::URA3 and ppz2::TRP1 disruption cassettes has been described previously (Posas et al., 1993; Clotet et al., 1999). The trk1,2 ppz1,2 strain was constructed by disrupting PPZ1 and PPZ2 in a strain lacking functional copies of TRK1,2 (Mulet et al., 1999). Disruptions were confirmed by genomic PCR. Disruption of ena1-4 was carried out as described (Ferrando et al., 1995). The multi-copy, galactose-inducible pYES2-PPZ1 plasmid was constructed by inserting the PCR-derived open reading frame into the BamHI and SpeI sites of the vector (Invitrogen). The HAL3 gene was disrupted as described (Ferrando et al., 1995).
Northern blot analysis
Total RNA was isolated from yeast cells that were grown to mid log-phase in buffered YPD adjusted to pH 6 or 7. Approximately 20 µg of RNA per lane were separated in formaldehyde gels and transferred to nylon membranes (Hybond-N; Amersham). Radioactively labelled probes were hybridized in PSE buffer (300 mM sodium phosphate pH 7.2, 7% SDS, 1 mM EDTA). Probes used were as follows: a 3.3 kb PCR fragment spanning the entire ENA1 gene, amplified from plasmid ML80 (a kind gift from Dr Martin Luebe), PCR fragments representing nucleotides 77–706 of TBP1, 1–1388 of PHO11 and 1–1821 of ENB1, amplified from chromosomal yeast DNA. Signal quantification was carried out using a Fujifilm BAS-1500 phosphoimager.
Assays for the Pma1 ATPase
In vitro Pma1p activity was assayed from purified plasma membranes isolated after glucose treatment, as described (Portillo and Serrano, 1988, 1989; Goossens et al., 2000). Protein concentration was measured using the Bradford reagent (Bio-Rad, Hercules, CA) and bovine globulin as the standard. Proton efflux from the cells was measured at pH 4.0 after 3 h of starvation at 28°C and re-addition of glucose (Serrano, 1980).
Rubidium uptake
Cells were inoculated in AP medium [2% glucose, 10 mM KCl, buffered with 80 mM H3PO4 (taken to pH 6.5 with Arg-base, 0.2 mg/l FeCl3, 1 mM MgSO4, 0.1 mM CaCl2), supplemented with the following vitamins and oligoelements: 20 ng/ml biotin, 500 ng/ml calcium pantotenate, 500 ng/ml nicotinic acid, 2 µg/ml thiamine chlorhydrate, 2 µg/ml pyridoxine chlorhydrate and 100 µg/ml inositol, 20 ng/ml folic acid, 200 ng/ml riboflavin and 200 ng/ml p-aminobenzoic acid, 500 ng/ml H3BO4, 50 ng/ml CuSO4, 100 ng/ml KI, 400 ng/ml MnSO4, 200 ng/ml Na2MoO4, ZnSO4] and grown overnight until an OD660 of ∼0.4, then washed twice with 20 mM MgCl2 and resuspended in AP medium without potassium. After 30 min of starvation 10 ml aliquots were taken, washed with 20 mM MgCl2 and resuspended in 0.2 ml of buffer containing 2% glucose, 50 mM succinic acid and 20 mM MgCl2. After 5 min, 5 mM RbCl was added and at the times indicated aliquots were taken, washed twice in MgCl2 and resuspended in 0.5 ml of MilliQ water. Ions were extracted by heating the cells for 10 min at 95°C and then quantified by HPLC analysis.
For measurement of the strains overexpressing PPZ1, cells were grown in AP medium containing 10 mM of potassium to an OD660 of ∼0.4, transferred to APGal media (AP media, but substituting the glucose by galactose) to induce PPZ1 expression and left overnight. At an OD660 of ∼0.6, cells were washed twice with 20 mM MgCl2 to remove all traces of potassium and resuspended in APGal without potassium. After 30 min of starvation, 10 ml aliquots were resuspended in a medium containing 2% galactose, 50 mM succinic acid pH 5.5 and 20 mM MgCl2. After 5 min incubation, 5 mM RbCl was added to the reaction and uptake was measured at the times indicated.
HPLC analysis
Sodium, potassium and rubidium ions were measured by HPLC chromatography (Waters), using an IC-PakCation M/D column and a Waters 432 conductivity detector. Elution was performed using an isocratic flux of 0.1 mM EDTA, 3 mM HNO3, prepared with MilliQ water (resistivity = 18.2 MΩ/cm) as the mobile phase. Sample analysis and preparation of standards was performed as described by the manufacturer.
Cytoplasmic pH measurements
Cytoplasmic pH was measured by monitoring the fluorescence changes of HPTS (1-hydroxypyrene-3,6,8-trisulfonic acid; Sigma), essentially as described (Pena et al., 1995). Briefly, cells were grown in 250 ml of YPD to mid-log phase, collected by centrifugation, washed twice and starved overnight at 4°C in 10 ml of sterile water. Cells were then adjusted to a concentration of 0.5 mg/ml in water. HTPS (2.85 µM) was added to 0.7 ml of cell suspension in a cuvette with a 4 mm gap. A single pulse of 1500 V, 25 µF and 200 Ω with a duration of ∼2.8 ms was applied. Cells were then washed extensively and resuspended in water to a final volume of 0.7 ml. Fluorescence was measured using an LS 50B luminescence spectrometer and FL Winlab software (Perkin Elmer) at an excitation wavelength of 460 nm and an emission wavelength of 509 nm, a slit width of 4 nm and 5 µg of cells. Measurements were taken at an external pH of 6.0 (0.1 M MES adjusted with 0.1 M triethanolamine). Maximal and minimal fluorescence were obtained by adding NH4OH and CH3COOH to final concentrations of 0.1 M.
[14C]leucine uptake
Leucine uptake experiments were performed as described (Pascual-Ahuir et al., 2001).
Pheromone response and recovery
The strains indicated were grown in YPD media to mid-log phase and equal numbers of cells were added to top agar (YPD with 0.7% agar) and spread evenly on YPD plates. Immediately after solidification, sterile cellulose discs (0.6 cm; Difco) with 14 µg of synthetic α-factor (Sigma) were placed on the nascent lawn. Images of plates were taken after a 48 h incubation at 28°C.
Acknowledgments
Acknowledgements
The authors wish to thank Dr Dolores Bernal for valuable assistance with fluorescence measurements and Drs Markus Proft and Iratxe Mendizabal for helpful discussions. L.Y. was supported by postdoctoral fellowships from the European Molecular Biology Organization and the National Science Foundation. J.M.M. was supported by a fellowship from the Conselleria de Educacio i Ciencia (Valencia). This work was supported by grants PB98-0565-C04-01 and PB98-0565-C04-02 from the Spanish Ministerio de Ciencia y Tecnología (Madrid).
References
- Albert A., Yenush,L., Gil-Mascarell,M.R., Rodriguez,P.L., Patel,S., Martinez-Ripoll,M., Blundell,T.L. and Serrano,R. (2000) X-ray structure of yeast Hal2p, a major target of lithium and sodium toxicity and identification of framework interactions determining cation sensitivity. J. Mol. Biol., 295, 927–938. [DOI] [PubMed] [Google Scholar]
- Arndt K.T., Styles,C.A. and Fink,G.R. (1989) A suppressor of a HIS4 transcriptional defect encodes a protein with homology to the catalytic subunit of protein phosphatases. Cell, 56, 527–537. [DOI] [PubMed] [Google Scholar]
- Brunelli J.P. and Pall,M.L. (1993) A series of yeast/Escherichia coli λ expression vectors designed for directional cloning of cDNAs and cre/lox-mediated plasmid excision. Yeast, 9, 1309–1318. [DOI] [PubMed] [Google Scholar]
- Clotet J., Posas,F., de Nadal,E. and Arino,J. (1996) The NH2-terminal extension of protein phosphatase PPZ1 has an essential functional role. J. Biol. Chem., 271, 26349–26355. [DOI] [PubMed] [Google Scholar]
- Clotet J., Gari,E., Aldea,M. and Arino,J. (1999) The yeast ser/thr phosphatases sit4 and ppz1 play opposite roles in regulation of the cell cycle. Mol. Cell. Biol., 19, 2408–2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darley C.P., van Wuytswinkel,O.C., van der Woude,K., Mager,W.H. and de Boer,A.H. (2000) Arabidopsis thaliana and Saccharomyces cerevisiae NHX1 genes encode amiloride sensitive electroneutral Na+/H+ exchangers. Biochem. J., 351, 241–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Nadal E., Clotet,J., Posas,F., Serrano,R., Gomez,N. and Arino,J. (1998) The yeast halotolerance determinant Hal3p is an inhibitory subunit of the Ppz1p Ser/Thr protein phosphatase. Proc. Natl Acad. Sci. USA, 95, 7357–7362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Nadal E., Fadden,R.P., Ruiz,A., Haystead,T. and Arino,J. (2001) A role for the Ppz Ser/Thr protein phosphatases in the regulation of translation elongation factor 1Bα. J. Biol. Chem., 276, 14829–14834. [DOI] [PubMed] [Google Scholar]
- Di Como C.J. and Arndt,K.T. (1996) Nutrients, via the Tor proteins, stimulate the association of Tap42 with type 2A phosphatases. Genes Dev., 10, 1904–1916. [DOI] [PubMed] [Google Scholar]
- Di Como C.J., Bose,R. and Arndt,K.T. (1995) Overexpression of SIS2, which contains an extremely acidic region, increases the expression of SWI4, CLN1 and CLN2 in sit4 mutants. Genetics, 139, 95–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Sarabia M.J., Sutton,A., Zhong,T. and Arndt,K.T. (1992) SIT4 protein phosphatase is required for the normal accumulation of SWI4, CLN1, CLN2 and HCS26 RNAs during late G1. Genes Dev., 6, 2417–2428. [DOI] [PubMed] [Google Scholar]
- Ferrando A., Kron,S.J., Rios,G., Fink,G.R. and Serrano,R. (1995) Regulation of cation transport in Saccharomyces cerevisiae by the salt tolerance gene HAL3. Mol. Cell. Biol., 15, 5470–5481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia de blas B., Rubio,F., Quintero,F.J., Banuelos,M.A., Haro,R. and Rodriguez-Navarro,A. (1993) Differential expression of two genes encoding isoforms of the ATPase involved in sodium efflux in Saccharomyces cerevisiae. Mol. Gen. Genet., 236, 363–368. [DOI] [PubMed] [Google Scholar]
- Gaxiola R.A., Rao,R., Sherman,A., Grisafi,P., Alper,S.L. and Fink,G.R. (1999) The Arabidopsis thaliana proton transporters, AtNhx1 and Avp1, can function in cation detoxification in yeast. Proc. Natl Acad. Sci. USA, 96, 1480–1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillies R.J., Ugurbil,K., den Hollander,J.A. and Shulman,R.G. (1981) 31P NMR studies of intracellular pH and phosphate metabolism during cell division cycle of Saccharomyces cerevisiae. Proc. Natl Acad. Sci. USA, 78, 2125–2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goossens A., de La,F.N., Forment,J., Serrano,R. and Portillo,F. (2000) Regulation of yeast H(+)-ATPase by protein kinases belonging to a family dedicated to activation of plasma membrane transporters. Mol. Cell. Biol., 20, 7654–7661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinstein S., Rotin,D. and Mason,M.J. (1989) Na+/H+ exchange and growth factor-induced cytosolic pH changes. Role in cellular proliferation. Biochim. Biophys. Acta, 988, 73–97. [DOI] [PubMed] [Google Scholar]
- Hill J.E., Myers,A.M., Koermer,T.-J. and Tzagoloff,A. (1986) Yeast/E.coli shuttle vectors with multiple unique restriction sites. Yeast, 2, 163–167. [DOI] [PubMed] [Google Scholar]
- Iyer V.R., Horak,C.E., Scafe C.S., Botstein,D., Snyder M. and Brown,P.O. (2001) Genomic binding sites of the yeast cell-cycle transcription factors SBF and MBF. Nature, 409, 533–538. [DOI] [PubMed] [Google Scholar]
- Lamb T.M., Xu,W., Diamond,A. and Mitchell,A.P. (2001) Alkaline response genes of Saccharomyces cerevisiae and their relationship to the RIM101 pathway. J. Biol. Chem., 276, 1850–1856. [DOI] [PubMed] [Google Scholar]
- Lee K.S., Hines,L.K. and Levin,D.E. (1993) A pair of functionally redundant yeast genes (PPZ1 and PPZ2) encoding type 1-related protein phosphatases function within the PKC1-mediated pathway. Mol. Cell. Biol., 13, 5843–5853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lussier M. et al. (1997) Large scale identification of genes involved in cell surface biosynthesis and architecture in Saccharomyces cerevisiae. Genetics, 147, 435–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madrid R., Gomez,M.J., Ramos,J. and Rodriguez-Navarro,A. (1998) Ectopic potassium uptake in trk1 trk2 mutants of Saccharomyces cerevisiae correlates with a highly hyperpolarized membrane potential. J. Biol. Chem., 273, 14838–14844. [DOI] [PubMed] [Google Scholar]
- Masuda C.A., Ramirez,J., Pena,A. and Montero-Lomeli,M. (2000) Regulation of monovalent ion homeostasis and pH by the Ser–Thr protein phosphatase SIT4 in Saccharomyces cerevisiae. J. Biol. Chem., 275, 30957–30961. [DOI] [PubMed] [Google Scholar]
- Mulet J.M., Leube,M.P., Kron,S.J., Rios,G., Fink,G.R. and Serrano,R. (1999) A novel mechanism of ion homeostasis and salt tolerance in yeast: the Hal4 and Hal5 protein kinases modulate the Trk1–Trk2 potassium transporter. Mol. Cell. Biol., 19, 3328–3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murguia J.R., Belles,J.M. and Serrano,R. (1995) A salt-sensitive 3′(2′),5′-bisphosphate nucleotidase involved in sulfate activation. Science, 267, 232–234. [DOI] [PubMed] [Google Scholar]
- Nass R., Cunningham,K.W. and Rao,R. (1997) Intracellular seques tration of sodium by a novel Na+/H+ exchanger in yeast is enhanced by mutations in the plasma membrane H+-ATPase. Insights into mechanisms of sodium tolerance. J. Biol. Chem., 272, 26145–26152. [DOI] [PubMed] [Google Scholar]
- Navarre C. and Goffeau,A. (2000) Membrane hyperpolarization and salt sensitivity induced by deletion of PMP3, a highly conserved small protein of yeast plasma membrane. EMBO J., 19, 2515–2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuccitelli R. and Heiple,J.M. (1982) Summary of the evidence and discussion concerning the involvment of pHi in the control of cellular function. In Nuccitelly,R. and Deamer,D.W. (eds), Intracellular pH. Its Measurement, Regulation and Utilization in Cellular Function. Alan R.Liss, New York, NY, pp. 567–586.
- Pascual-Ahuir A., Serrano,R. and Proft,M. (2001) The Sko1p repressor and Gcn4p activator antagonistically modulate stress-regulated transcription in Saccharomyces cerevisiae. Mol. Cell. Biol., 21, 16–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pena A., Ramirez,J., Rosas,G. and Calahorra,M. (1995) Proton pumping and the internal pH of yeast cells, measured with pyranine introduced by electroporation. J. Bacteriol., 177, 1017–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perona R. and Serrano,R. (1988) Increased pH and tumorigenicity of fibroblasts expressing a yeast proton pump. Nature, 334, 438–440. [DOI] [PubMed] [Google Scholar]
- Portillo F. and Serrano,R. (1988) Dissection of functional domains of the yeast proton-pumping ATPase by directed mutagenesis. EMBO J., 7, 1793–1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portillo F. and Serrano,R. (1989) Growth control strength and active site of yeast plasma membrane ATPase studied by site-directed mutagenesis. Eur. J. Biochem., 186, 501–507. [DOI] [PubMed] [Google Scholar]
- Posas F., Casamayor,A. and Arino,J. (1993) The PPZ protein phosphatases are involved in the maintenance of osmotic stability of yeast cells. FEBS Lett., 318, 282–286. [DOI] [PubMed] [Google Scholar]
- Posas F., Camps,M. and Arino,J. (1995) The PPZ protein phosphatases are important determinants of salt tolerance in yeast cells. J. Biol. Chem., 270, 13036–13041. [DOI] [PubMed] [Google Scholar]
- Serrano R. (1980) Effect of ATPase inhibitors on the proton pump of respiratory-deficient yeast. Eur. J. Biochem., 105, 419–424. [DOI] [PubMed] [Google Scholar]
- Serrano R. and Rodriguez-Navarro,A. (2001) Ion homeostasis during salt stress in plants. Curr. Opin. Cell Biol., 13, 399–404. [DOI] [PubMed] [Google Scholar]
- Serrano R. et al. (1999) A glimpse of the mechanisms of ion homeostasis during salt stress. J. Exp. Bot., 50, 1023–1036. [Google Scholar]
- Sutton A., Immanuel,D. and Arndt,K.T. (1991) The SIT4 protein phosphatase functions in late G1 for progression into S phase. Mol. Cell. Biol., 11, 2133–2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallejo C.G. and Serrano,R. (1989) Physiology of mutants with reduced expression of plasma membrane H+-ATPase. Yeast, 5, 307–319. [DOI] [PubMed] [Google Scholar]