Abstract
The survival of motor neurons (SMN) protein complex functions in the biogenesis of spliceosomal small nuclear ribonucleoprotein particles (snRNPs) and prob ably other RNPs. All spliceosomal snRNPs have a common core of seven Sm proteins. To mediate the assembly of snRNPs, the SMN complex must be able to bring together Sm proteins with U snRNAs. We showed previously that SMN and other components of the SMN complex interact directly with several Sm proteins. Here, we show that the SMN complex also interacts specifically with U1 snRNA. The stem–loop 1 domain of U1 (SL1) is necessary and sufficient for SMN complex binding in vivo and in vitro. Substitution of three nucleotides in the SL1 loop (SL1A3) abolishes SMN interaction, and the corresponding U1 snRNA (U1A3) is impaired in U1 snRNP biogenesis. Microinjection of excess SL1 but not SL1A3 into Xenopus oocytes inhibits SMN complex binding to U1 snRNA and U1 snRNP assembly. These findings indicate that SMN complex interaction with SL1 is sequence-specific and critical for U1 snRNP biogenesis, further supporting the direct role of the SMN complex in RNP biogenesis.
Keywords: SMN/snRNP/spinal muscular atrophy/stem–loop 1/U1 snRNA
Introduction
Pre-mRNA splicing in the nucleus of eukaryotic cells is mediated by the spliceosome. The small nuclear ribonucleoprotein particles (snRNPs) U1, U2, U5 and U4/U6 are major components of the spliceosome. Each snRNP consists of one snRNA (U1, U2, U5 or U4/U6), an Sm protein core and a set of proteins that are specific to individual snRNAs (Will and Lührmann, 2001). The Sm proteins B/B′, D1, D2, D3, E, F and G are common to all spliceosomal snRNPs and are arranged into a seven-membered ring (Kambach et al., 1999; Stark et al., 2001) that assembles for each snRNA on a consensus sequence motif (PuAU4–6GPu) called the Sm site (Branlant et al., 1982; Nagai et al., 2001). This assembly process takes place in the cytoplasm shortly after the nuclear export of nascent snRNAs. After the formation of the Sm core, the 7-methyl guanosine (m7G) cap of these snRNAs is hypermethylated to become a 2,2,7-trimethyl guanosine (m3G or TMG) (Mattaj, 1986; Plessel et al., 1994). Properly assembled Sm core, cap hypermethylation and 3′ end processing of the snRNAs are required for the subsequent nuclear import of the snRNPs, where they function in splicing (Mattaj and De Robertis, 1985; Fischer and Lührmann, 1990; Hamm et al., 1990b; Fischer et al., 1993; Mattaj et al., 1993; Jarmolowski et al., 1994; Will and Lührmann, 2001).
Important and unexpected insights into the process of snRNP assembly came from studies on the function of the survival of motor neurons (SMN) protein (Liu and Dreyfuss, 1996; Fischer et al., 1997; Liu et al., 1997; Mattaj, 1998). SMN is the protein product of the spinal muscular atrophy (SMA) disease gene (Lefebvre et al., 1995). SMA is a severe neuromuscular disease characterized by degeneration of motor neurons in the spinal cord (Melki, 1997). Over 98% of SMA patients have deletions or mutations of the telomeric copy of the gene (SMN1) and produce markedly reduced levels of the SMN protein (Lefebvre et al., 1995; Burghes, 1997). SMN is part of a large multiprotein complex that also contains Gemin2 (Liu et al., 1997), the DEAD box RNA helicase Gemin3 (Charroux et al., 1999), Gemin4 (Charroux et al., 2000) and several additional as yet uncharacterized proteins. The SMN complex is present in both the nucleus and the cytoplasm of all metazoan cells, suggesting that it may have multiple functions in cells (Liu and Dreyfuss, 1996). Most lines of evidence indicate that the SMN complex functions in the assembly and metabolism of various RNPs, including snRNPs, snoRNPs, and the machineries that carry out transcription and pre-mRNA splicing (Fischer et al., 1997; Liu et al., 1997; Pellizzoni et al., 1998, 1999, 2001a,b; Buhler et al., 1999; Friesen and Dreyfuss, 2000; Meister et al., 2000, 2001; Jones et al., 2001; Mourelatos et al., 2001).
The process of snRNP assembly can be most readily studied in Xenopus oocytes, where specific reagents and intermediates can be microinjected into either the nucleus or the cytoplasm and where dissection of nuclear and cytoplasmic fractions can be readily performed. Such experiments have revealed that the SMN complex associates with spliceosomal U1, U4 and U5 snRNAs in the cytoplasm (Fischer et al., 1997; Buhler et al., 1999; Charroux et al., 2000). Antibodies against components of the SMN complex microinjected into Xenopus oocytes also inhibit the assembly of snRNPs, indicating that the SMN complex plays a crucial role in the biogenesis of snRNPs (Fischer et al., 1997; Buhler et al., 1999). In addition, overexpression of a dominant-negative SMN mutant blocks snRNP assembly in the cytoplasm of somatic cells, suggesting a general function for the SMN complex in the cytoplasmic phase of U snRNAs biogenesis (Pellizzoni et al., 1998). Recent studies have further demonstrated that the SMN complex is necessary for assembly of U1 snRNP in Xenopus egg extracts (Meister et al., 2001).
The capacity of the SMN complex to associate with and mediate the assembly of snRNPs is probably due, at least in part, to interactions between the SMN complex and snRNP proteins. Several of the components of the SMN complex interact directly with Sm proteins (Liu et al., 1997; Buhler et al., 1999; Charroux et al., 1999, 2000; Pellizzoni et al., 1999; Friesen and Dreyfuss, 2000). In particular, SMN binds avidly to RG-rich C-terminal domains that are found in the Sm proteins B, D1 and D3, whereas several SMN mutants found in SMA patients are defective in Sm protein binding (Buhler et al., 1999; Pellizzoni et al., 1999; Friesen and Dreyfuss, 2000). Importantly, SMN binds preferentially to the RG domains of D1 and D3 after arginines in specific positions are converted to symmetric dimethylarginines (sDMAs) (Friesen et al., 2001a). Thus, arginine dimethylation has a key role in the protein substrate recognition by the SMN complex, and RNP assembly is likely to be regulated by arginine methylation (Friesen et al., 2001a).
To serve in snRNP assembly, the SMN–Sm protein complex must also recruit the snRNAs. Here, we investigated the binding of U1 snRNA, an abundant and high-avidity substrate, to the SMN complex. We show that the binding is sequence-specific and is mediated by the loop of stem–loop 1 domain (SL1) of U1 snRNA. SL1 is both necessary and sufficient for the interaction with the SMN complex in vivo and in vitro. We further demonstrate that this interaction is required for U1 snRNP assembly and thus mediates the function of the SMN complex in U1 snRNP biogenesis.
Results
The association of the SMN complex with U1 snRNA is independent of Sm core assembly
Immunoprecipitation experiments of microinjected RNAs demonstrated an association of the SMN complex with U1 and U5, and to a lesser extent U4, snRNAs (Fischer et al., 1997; Buhler et al., 1999; Charroux et al., 2000). In light of earlier studies demonstrating protein–protein interactions between components of the SMN complex and Sm proteins (Liu et al., 1997; Buhler et al., 1999; Pellizzoni et al., 1999; Charroux et al., 1999, 2000; Friesen and Dreyfuss, 2000) and the suggestion that SMN can bind RNA (Liu et al., 1996; Lorson and Androphy, 1998), we asked whether the interaction of the SMN complex with the U snRNAs is direct or occurs through the Sm proteins, which bind to the Sm site. To address this, we studied U1 snRNA interaction with the SMN complex. U1 snRNA was chosen for these experiments because it has high affinity for the SMN complex and is the most abundant and extensively characterized snRNA (Fischer et al., 1997; Charroux et al., 2000; Will and Lührmann, 2001).
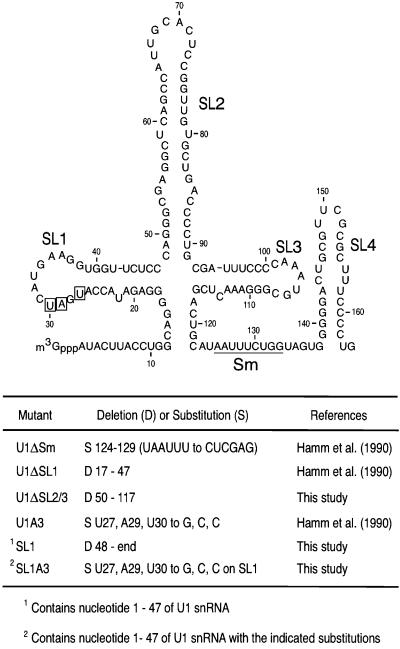
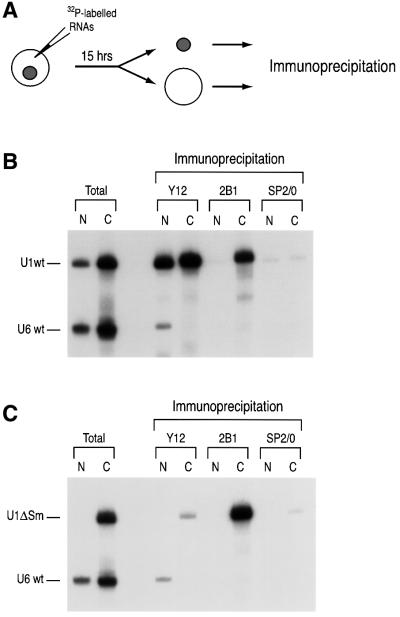
Vertebrate U1 snRNA contains four distinct stem–loop structures and the Sm site (Figure 1) (Branlant et al., 1981; Mount and Steitz, 1981). The U1ΔSm RNA, the Sm site sequence UAAUUU of which is substituted by the hexanucleotide sequence CUCGAG, does not bind Sm proteins (Hamm et al., 1987). Several RNAs including U1ΔSm and the spliceosomal U1 and U6 snRNAs were transcribed in vitro in the presence of [32P]UTP. Various mixtures of these RNAs were injected into the cytoplasm of oocytes and, after 15 h of incubation, oocytes were dissected manually into nuclear and cytoplasmic fractions, and immunoprecipitation was carried out with anti-SMN monoclonal antibody (2B1) and anti-Sm monoclonal antibody (Y12) (Figure 2A). Figure 2B and C shows that both the wild-type (wt) U1 snRNA and U1ΔSm, but not U6 snRNA, associate with the SMN complex with similar affinity. As expected, U1ΔSm was not immunoprecipitated using anti-Sm antibodies, indicating that this mutant is not assembled with the Sm proteins, and was not imported into the nucleus (Figure 2C). These data demonstrate that the interaction between the SMN complex and U1 snRNA is independent of the Sm site and therefore does not require Sm core assembly.
Fig. 1. The secondary structure of Xenopus laevis U1 snRNA and the deletion or substitution mutant U1 snRNAs used in this study. The positions of the structural elements deleted or altered in U1 snRNA are listed in the table. ΔSL1 has the entire SL1 structure deleted. ΔSL2/3 has the entire SL2 and SL3 structures deleted. The nucleotides mutated in the loop domain of SL1 of U1A3 RNA are boxed. The secondary structure is according to Branlant et al. (1981) and Mount and Steitz (1981).
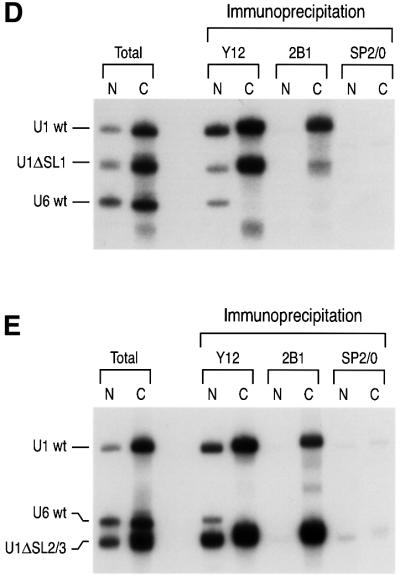
Fig. 2. Stem–loop 1 of U1 snRNA is necessary for efficient interaction of the SMN complex with U1 snRNA. (A) Experimental strategy used in this experiment. A mixture of 32P-labeled U1 and U6 snRNAs was injected into the cytoplasm of oocytes. After incubation for 15 h, the oocytes were dissected manually into nuclear (N) and cytoplasmic (C) fractions. Immunoprecipitation was then carried out from both fractions with either the anti-Sm (Y12), anti-SMN (2B1) or control non-immune (SP2/0) antibodies. (B) Microinjection and immunoprecipitation experiments of wt U1 and U6 32P-labeled snRNAs. ‘Total’ lanes correspond to 10% of the fractions used for each immunoprecipitation. Immunoprecipitation with Y12 was carried out in RSB-500 buffer (500 mM NaCl). 2B1 immunoprecipitation was performed in RSB-150 buffer. After immunoprecipitation, RNAs were purified and analyzed by electrophoresis on a 7 M urea/8% acrylamide gel and autoradiography. (C) The SMN complex binding to U1 snRNA is independent of Sm core assembly. A mixture of 32P-labeled U1ΔSm and U6 snRNAs was microinjected and analyzed for the SMN complex interaction by immunoprecipitation as described in (B). (D) SL1 domain of U1 snRNA is necessary for U1 snRNA binding of the SMN complex. A mixture of 32P-labeled U1, U6 and U1ΔSL1 snRNAs was microinjected and analyzed for the SMN complex interaction by immunoprecipitation as described in (B). (E) SL2 and SL3 domains of U1 snRNA are not necessary for the SMN complex–U1 snRNA interaction. A mixture of 32P-labeled U1, U6 and U1ΔSL2/3 snRNAs was microinjected and analyzed for the SMN complex interaction by immunoprecipitation as described in (B).
SL1 of U1 snRNA is necessary for SMN complex–U1 snRNA interaction
The efficient interaction of U1ΔSm with the SMN complex suggested that other sequences of U1 snRNA mediate this interaction. To identify this sequence(s), we analyzed the binding of several deletion mutants of U1 snRNA shown in Figure 1 using the experimental procedures described above. Figure 2D shows that deletion of SL1 (U1ΔSL1) strongly impaired the association of U1 snRNA with the SMN complex. Immunoprecipitation with the Y12 antibody shows that both U1 snRNA and U1ΔSL1 were associated with the Sm proteins in the cytoplasm. However, Y12 immunoprecipitation of U1ΔSL1 from the nucleus was greatly diminished (∼4-fold compared with that of wt U1 snRNA), suggesting that deletion of SL1 strongly impairs the biogenesis of U1 snRNP. Deletion of SL2 and SL3 (U1ΔSL2/3) did not reduce the association of U1 snRNA with the SMN complex (Figure 2E). In addition, immunoprecipitation with Y12 from the nuclear and cytoplasmic fractions showed that U1ΔSL2/3 is assembled with the Sm proteins in the cytoplasm and is imported efficiently into the nucleus. Thus, unlike deletion of SL1, deletion of SL2 and SL3 does not impair the biogenesis of U1 snRNP. Similar results were obtained with individual deletions of either SL2 or SL3 (data not shown). We conclude that SL1 is necessary for efficient interaction of U1 snRNA with the SMN complex and is important for U1 snRNP biogenesis, as reflected by U1ΔSL1 accumulation in the cytoplasm.
SL1 of U1 snRNA is sufficient for SMN complex–U1 snRNA interaction
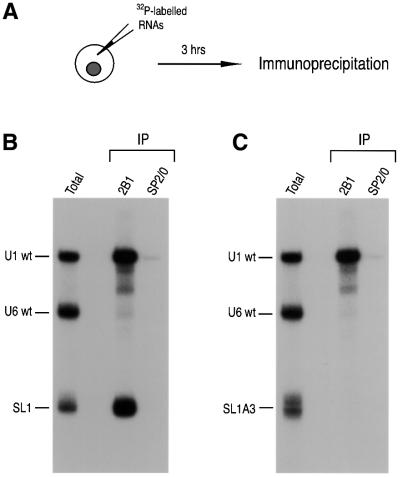
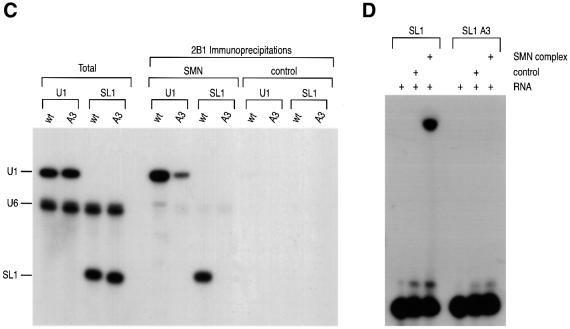
Because the experiments described above indicated that SL1 of U1 snRNA is necessary for the efficient interaction with the SMN complex, we wished to determine next whether it is also sufficient for this interaction. To do so, radiolabeled SL1 was injected into the cytoplasm of oocytes. Immunoprecipitation with 2B1 from oocyte extracts carried out after incubation for 3 h (SL1 is less stable in the oocyte than constructs containing 3′ stem loops) showed that SL1 alone interacts with the SMN complex as efficiently as wt U1 snRNA (Figure 3A and B). This indicates that SL1 is sufficient for interaction with the SMN complex.
Fig. 3. Sequence-specific interaction of the SMN complex with SL1 of U1 snRNA. (A) Experimental strategy used in this experiment. A mixture of 32P-labeled RNAs was injected into the cytoplasm of oocytes. Following a 3 h incubation, the oocytes were homogenized, and immunoprecipitated with 2B1 or SP2/0 antibodies. Immunoprecipitated RNAs were analyzed as described in Figure 2B. (B) SL1 is sufficient for U1 snRNA interaction with the SMN complex. A mixture of 32P-labeled U1, U6 snRNAs and SL1 was microinjected and analyzed as described in (A). (C) The SMN complex binding to the SL1 of U1 snRNA is sequence specific. A mixture of 32P-labeled U1, U6 snRNAs and SL1A3 was microinjected and analyzed as described in (A).
To further address the specificity of the interaction of SL1 with the SMN complex we examined the effect of mutations in SL1 on this interaction. It has been reported that mutations in SL1 can affect the binding to the U1-specific proteins U170K, U1A or U1C (Hamm et al., 1988, 1990b; Surowy et al., 1989). Several point mutants of SL1 were tested by microinjection and immunoprecipitation experiments. One previously described mutant of particular relevance is SL1A3, which contains three nucleotide changes in the loop sequence of SL1 (U27G, A29C and U30C; boxed in Figure 1) and impairs U170K binding to U1 snRNA (Hamm et al., 1990b). wt U1 and wt U6 snRNAs were injected along with SL1A3 and analyzed by immunoprecipitation with 2B1. As shown in Figure 3C, SL1A3 does not associate with the SMN complex. Although U170K interaction is dependent on Sm core assembly and is thought to take place in the nucleus (Hamm et al., 1987; Nelissen et al., 1994; Will and Lührmann, 2001), U170K binds to SL1 but not to SL1A3 and could therefore mediate the interaction with the SMN complex. To test this possibility, the point mutant A29G, which was previously shown not to interact with U170K (Surowy et al., 1989), was also studied. Immunoprecipitation with 2B1 showed that the A29G mutant associates efficiently with the SMN complex (data not shown), suggesting that the interaction between the SMN complex and SL1 is not likely to be mediated by U170K. Taken together, these results demonstrate that the interaction of the SMN complex with SL1 is sequence specific.
Purified SMN complexes interact directly with SL1 of U1 snRNA in vitro
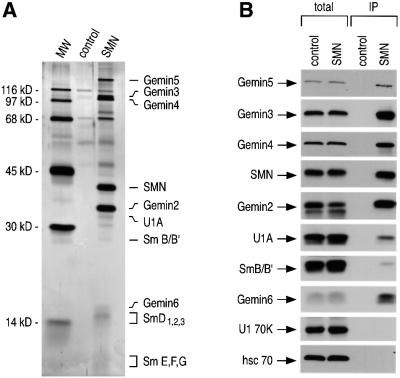
To examine the specific interaction of the SMN complex with the SL1 in a more purified and biochemically defined in vitro system, we carried out binding experiments with purified SMN complexes. Recently, we have described the purification of SMN complexes from HeLa cells under stringent conditions (500 mM NaCl) (Pellizzoni et al., 2002). These complexes are purified from cells expressing tagged SMN or Gemin2 and contain all the integral components of the complex at the native stoichiometry. Native SMN complexes were isolated by affinity chromatography and peptide elution from extracts of a stable cell line that expresses sub-stoichiometric levels of Flag-tagged Gemin2 under the control of a tetracycline-inducible promoter (see Materials and methods). Total cell extracts from the Flag-Gemin2 cells (SMN) or the parental HeLa Tet-ON cells (control) were immunoprecipitated with anti-Flag antibodies, and bound proteins were analyzed by PAGE and silver staining. These complexes isolated via Flag-Gemin2 contain all of the known components of the SMN complex, including SMN, Gemin2, Gemin3, Gemin4, Gemin5 (Gubitz et al., 2002) and Gemin6 (Pellizzoni et al., 2002), as well as smaller amounts of Sm proteins, U1A and a few as yet unidentified proteins (Figure 4A and B). Western blotting with specific antibodies showed that U170K is not present in these purified complexes (Figure 4B).
Fig. 4. The purified native SMN complex interacts directly and specifically with the SL1 of U1 snRNA. (A) Native SMN complex purified from HeLa cells. The SMN complex (SMN) was purified as described in Materials and methods and visualized on SDS–PAGE by silver staining. Non-specific proteins purified from HeLa cells that do not express Flag-Gemin2 (control) are also shown. The known components of the SMN complex are identified. Molecular weight markers (MW) are indicated. (B) Purified native SMN complex shown in (A) was analyzed by western blotting using indicated antibodies. ‘Total’ lanes contain 5% of the input. (C) The purified SMN complex (SMN) or non-specific proteins (control) as in (A) were mixed with several 32P-labeled RNAs. The RNA–protein complexes formed were analyzed by immunoprecipitation with anti-SMN (2B1) antibody in RSB-100 containing 0.02% NP-40. Each RNA (25 000 c.p.m.) was mixed as indicated and used for the binding experiments. ‘Total’ lanes contain 25% of the input. (D) The purified SMN complex (SMN complex) or non-specific proteins (control) as in (A) were mixed with SL1 or SL1A3. The specific interaction between SL1 and the SMN complex was analyzed by in vitro gel mobility-shift assay in native PAGE.
To demonstrate that the interaction of the SMN complex with SL1 is direct, we carried out in vitro binding experiments using purified SMN complexes and in vitro transcribed snRNAs. SMN complexes purified from Flag-Gemin2 cells (SMN) or the non-specific proteins purified from HeLa Tet-ON cells (control) were incubated for 1 h with in vitro transcribed [32P]UTP-labeled U1, U1A3, SL1 or SL1A3 RNAs in the presence of U6 snRNA as an internal control. Immunoprecipitation experiments from these mixtures were carried out with anti-SMN antibodies (2B1), and bound RNAs were analyzed by denaturing PAGE and autoradiography. As shown in Figure 4C, purified SMN complexes interact efficiently with U1 snRNA in vitro. This interaction is specific because U6 snRNA is not immunoprecipitated and the interaction of the SMN complex with the U1A3 mutant is severely impaired. Furthermore, the SMN complex interacts efficiently with SL1, but not with SL1A3 mutant. The residual interaction with U1A3, but not with SL1A3, suggests the presence of additional weaker interactions of the SMN complex with regions of U1 snRNA other than SL1 (see also Figure 5A). Further analysis of the specific interaction between the SMN complex and SL1 was carried out using in vitro gel mobility-shift assay. Purified SMN complexes were mixed with [32P]UTP-labeled SL1 or SL1A3 and incubated for 1 h at 30°C. As shown in Figure 4D, purified SMN complexes specifically bind SL1 and form a stable RNA–protein complex in a native PAGE. Consistent with the microinjection experiments shown above, these experiments demonstrate that purified SMN complexes interact directly and efficiently with the stem–loop 1 domain of U1 snRNA in vitro.
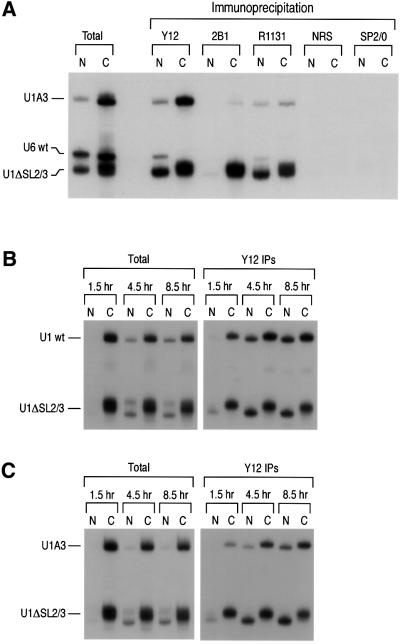
Fig. 5. U1A3 snRNA is defective in 5′ cap hypermethylation and interaction with the SMN complex. (A) A mixture of [32P]UTP-labeled U1A3, U6 and U1ΔSL2/3 snRNAs was injected into the cytoplasm of oocytes and incubated for 15 h. Nuclear and cytoplasmic fractions were used for immunoprecipitation experiments with the indicated antibodies. R1131 is a polyclonal antibody specific for trimethyl guanosine cap structure and normal rabbit serum (NRS) was used as its negative control. (B) A mixture of [32P]UTP-labeled wt U1 snRNA (U1 wt) and U1ΔSL2/3 was injected into the cytoplasm of oocytes and incubated as indicated. Nuclear and cytoplasmic fractions were used for immunoprecipitation experiments with the Y12 antibody. (C) The same experiments as described in (B) were carried out with a mixture of [32P]UTP-labeled U1A3 and U1ΔSL2/3.
U1A3 snRNA is defective in binding to the SMN complex and in cap hypermethylation in vivo
Next, we investigated the effect of mutations in SL1 on the interaction of U1 snRNA with the SMN complex and on U1 snRNP biogenesis. [32P]UTP-labeled U1A3 was injected, along with U1ΔSL2/3 and U6 RNAs, and immunoprecipitation was carried out. Consistent with the results described above, the association of U1A3 mutant with the SMN complex is almost completely abolished in the cytoplasm (Figure 5A, 2B1). Immunoprecipitation with Y12 showed that U1A3 associates with the Sm proteins in the cytoplasm of oocytes, but its nuclear import is markedly reduced (∼2-fold) compared with U1ΔSL2/3 (Figure 5A). This effect was similar to that of U1ΔSL1 (see Figure 2D). To further investigate the defect in U1A3 RNA snRNP biogenesis, we analyzed the hypermethylation of its cap structure. Proper snRNP assembly is required for TMG formation (Fischer and Lührmann, 1990; Hamm et al., 1990a). Strikingly, U1A3 from the cytoplasm as well as from the nucleus could not be efficiently immunoprecipitated with the anti-TMG-specific antibody (R1131), indicating a defect in TMG formation (Figure 5A). In contrast, hypermethylation of U1ΔSL2/3 was efficient and similar to that of U1 snRNA (Figure 5A; data not shown). Thus, both the hypermethylation of the cap structure and the association with the SMN complex are impaired in the U1A3 mutant RNA. Because the hypermethylation of the cap structure requires an assembled Sm core, it is likely that the Sm core is not properly assembled in the U1A3 mutant. To further investigate whether the impaired import of U1A3 is a reflection of a kinetic delay in the assembly of the Sm proteins, the time course of Sm protein assembly on U1A3 was monitored. [32P]UTP-labeled wt U1 or U1A3 was injected along with U1ΔSL2/3 and immunoprecipitation with Y12 was carried out at the indicated time points. As shown in Figure 5B and C, the assembly kinetics of the Sm core of U1A3 are delayed compared with those of wt U1. These data suggest that binding of the SMN complex to the SL1 domain of U1 snRNA is important for the efficient biogenesis of U1 snRNP.
An excess of SL1 inhibits U1 snRNP assembly
In order to investigate further the functional relevance of the interaction between the SMN complex and SL1 of U1 snRNA, we tested the effect of an excess of SL1 on U1 snRNP assembly. Unlabeled in vitro transcribed SL1, SL1A3 or wt U1 snRNA was microinjected into the cytoplasm of oocytes at the concentration of 300 fmol/oocyte. This represents 100-fold excess over a mixture of 32P-labeled U1, U2 and U6 snRNAs that was injected into the cytoplasm of the same oocytes 2 h later. The oocytes were then incubated for an additional 2 h, and immunoprecipitation with anti-SMN (2B1), anti-Sm (Y12) or SP2/0 antibodies was carried out. In the presence of excess amounts of unlabeled wt U1 in the cytoplasm of oocytes, the interaction of the SMN complex with radiolabeled U1 snRNA was abolished, indicating that the SMN complex was saturated by the unlabeled RNAs (compare 2B1 immunoprecipitation lanes in Figure 6A). Similarly, immunoprecipitation with Y12 showed that excess amounts of unlabeled wt U1 snRNA completed the Sm core assembly on radiolabeled U snRNAs (Figure 6A). Excess amount of unlabeled SL1 RNA in the cytoplasm of oocytes strongly reduced the binding of the SMN complex to U1 snRNA (Figure 6B). The inhibition is not complete compared with wt U1 snRNA, possibly because SL1 is less stable in vivo (data not shown). Interestingly, immunoprecipitation with Y12 showed that the assembly of U1 snRNP was inhibited by an excess of SL1, while U2 snRNP assembly was not affected severely (Figure 6B). In contrast, an excess of unlabeled SL1A3 RNA, which does not bind to the SMN complex in vivo or in vitro, did not prevent, and possibly increased, the interaction between the SMN complex and U1 snRNA. SL1A3 also did not inhibit the assembly of Sm proteins with U1 and U2 snRNAs (Figure 6B). These results are consistent with the possibility that reduction in the interaction of the SMN complex with U1 snRNA results in reduced U1 snRNP assembly. These data indicate that the interaction of the SMN complex with SL1 of U1 snRNA is important for U1 snRNP biogenesis.
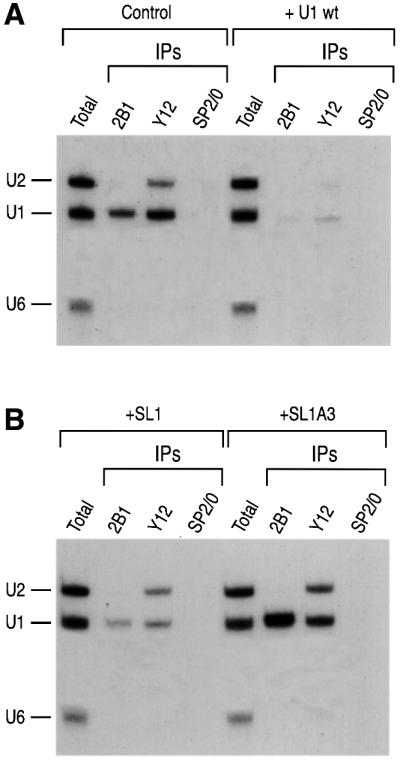
Fig. 6. An excess of SL1 inhibits SMN complex interaction with U1 snRNA and U1 snRNP assembly. Non-radioactive (cold) U1 snRNA, SL1 and SL1A3 RNAs were transcribed separately in vitro. Each RNA was injected separately into the cytoplasm of oocytes at a concentration of 300 fmol/oocyte and incubated for 2 h. The oocytes were then reinjected into the cytoplasm with a mixture of 32P-labeled U1, U2 and U6 snRNAs and incubated for an additional 2 h. The oocytes were homogenized and RNAs were immunoprecipitated with 2B1, Y12 or SP2/0 antibodies. (A) Immunoprecipitation of U snRNAs following pre-injection of water (Control) or cold U1 snRNA (+ U1 wt). (B) Immunoprecipitation of U snRNAs following pre-injection of SL1 (+SL1) or SL1A3 (+SL1A3) RNAs.
Discussion
The SMN complex plays an important role in the biogenesis of spliceosomal U snRNPs and probably of other RNPs such as snoRNPs (Fischer et al., 1997; Pellizzoni et al., 1998, 2001a,b; Buhler et al., 1999; Meister et al., 2000, 2001; Jones et al., 2001; Mourelatos et al., 2001). Immunoprecipitation experiments of microinjected RNAs into Xenopus oocytes showed that the SMN complex interacts particularly avidly and stably with U1 snRNA (Fischer et al., 1997; Buhler et al., 1999; Charroux et al., 2000) and we therefore chose to study the interaction of SMN complex with this snRNA. We show here that this interaction is sequence-specific and is mediated by the loop sequence of SL1. We conclude that to fulfill its function in the assembly of U1 snRNA, and perhaps more generally with the myriad RNPs that the SMN complex assembles or restructures, it interacts with both the protein and the RNA components in a sequence-specific manner. The interaction with the protein components is mediated by RG domains, specifically with sDMA-modified RG domains in the case of Sm proteins (Friesen and Dreyfuss, 2000; Friesen et al., 2001a). RG domains are present in most, if not all, of the substrates of the SMN complex, including SmB, SmD1, SmD3, Lsm4, fibrillarin, GAR1, RNA helicase A, hnRNP U, hnRNP Q and coilin (Liu and Dreyfuss, 1996; Friesen and Dreyfuss, 2000; Friesen et al., 2001a; Hebert et al., 2001; Jones et al., 2001; Mourelatos et al., 2001; Pellizzoni et al., 2001a,b). For the Sm proteins SmD1 and SmD3, this requires the activity of the protein arginine methyltransferase JBP1 (PRMT5) in the context of the large methylating complex, the methylosome (Friesen et al., 2001b). The binding of the SMN complex to the snRNA is, however, independent of its binding to the Sm proteins, as suggested by the direct binding to SL1, which does not contain an Sm protein binding site. It thus appears that the SMN complex recruits directly and independently the RNA target and at least some of the RNP proteins. This allows the SMN complex to function in the assembly of RNPs by first bringing together its various components into close spatial proximity. We envision the process that ensues to also involve the active participation of other components of the SMN complex, particularly the RNA helicase Gemin3.
Previous experiments have suggested that the SMN complex has the capacity to bind RNA (Liu et al., 1996; Lorson and Androphy, 1998). The significance of these interactions was not clear, however, as they were based on relatively weak interactions with ribo-homopolymers in vitro. Our results demonstrate the sequence-specific interaction of the SMN complex with SL1 of U1 snRNA and suggest that this interaction is critical for its function in the RNP assembly process.
It is not presently clear whether the binding of the SMN complex to SL1 is mediated by direct binding of SMN or via another protein in the complex. Characterization of U1 deletion mutant snRNAs by microinjection and immunoprecipitation experiments in Xenopus oocytes demonstrated that U170K binds to SL1, and U1A binds to SL2 of U1 snRNA (Hamm et al., 1987, 1990b; Query et al., 1989a,b; Scherly et al., 1989). It is not likely, however, that U170K protein is involved in the SMN complex interaction with U1 snRNA. U170K interaction with U1 snRNA requires Sm core assembly and it has been suggested to occur in the nucleus (Hamm et al., 1987; Nelissen et al., 1994). Instead, SMN complex association with U1 snRNA does not require Sm core assembly (Figure 2C) and the single point mutation of SL1 of U1 snRNA that impairs interaction with U170K (Surowy et al., 1989) does not affect the binding of the SMN complex (data not shown). In addition, in vitro binding experiments using purified native SMN complexes proved that the SMN complex interacts directly with SL1 in the absence of U170K (Figure 4C and D). No proteins other than U170K are known to bind SL1 of U1 snRNA. It is therefore likely that the interaction of the SMN complex with SL1 is mediated by direct binding of one or more of the integral core components of the SMN complex.
Although the interaction of the SMN complex with SL1 of U1 snRNA could be readily detected and is therefore particularly avid, it is likely that the complex also interacts with other RNA sequences found in its many other RNP targets. For example, the SMN complex is required for the assembly of other Sm snRNPs (Fischer et al., 1997; Buhler et al., 1999) that do not contain SL1. It is thus possible that the SMN complex can interact with several different RNA sequences and/or that there is a common, as yet unidentified, RNA sequence or structural element in the many different substrates of the SMN complex. A clear definition of the RNA binding activity of the SMN complex will greatly advance the understanding of its precise molecular functions.
U1A3 was originally characterized as the most defective mutant RNA in the splicing complementation experiments in Xenopus oocytes. Microinjection of RNAs and immunoprecipitation experiments from oocyte total extracts showed that U1A3 assembles with the Sm proteins but is defective in U170K binding (Hamm et al., 1990b). Consistent with these results, our data show that U1A3 associates with the Sm proteins in the cytoplasm of oocytes, but the nuclear import of U1A3 is severely impaired compared with wt U1 (Figure 5A). The reduced hypermethylation of U1A3 in both the nuclear and cytoplasmic fractions provides an explanation for this behavior. Hypermethylation of the cap structure of U1 requires the assembly of the Sm core (Fischer and Lührmann, 1990; Hamm et al., 1990a) and it is possible that the Sm core may not be properly assembled on U1A3 snRNA. SMN complex interaction with U1A3 is also strongly impaired both in vitro and in vivo. The lack of SMN complex association with U1A3 is consistent with this possibility and with a role of SMN interaction with SL1 in U1 snRNP assembly. This possibility is further supported by kinetic and competition experiments in vivo. The assembly of the Sm core on U1A3 is delayed compared with that on wt U1, and it results in the impaired import of assembled U1A3 (Figure 5B and C). Previous studies showed that excess amount of cold RNAs can specifically sequester target proteins in oocytes (Hamm et al., 1987). This type of protein-sequestering assay was used for the delineation of sequence elements of U1 snRNA that are responsible for U1-specific protein interactions in oocyte microinjection. The titration of the SMN complex by cold SL1 RNA suggests that the interaction with the SL1 domain of U1 snRNA of the SMN complex plays a role in U1 snRNP biogenesis. Consistent with this, mutations that abolish SL1 interaction with the SMN complex do not inhibit U1 snRNP assembly.
Materials and methods
Construction of U1 snRNA mutants and in vitro transcription of RNAs
Construction of all deletion or substitution mutants of U1 snRNA DNAs was carried out by PCR according to Imai et al. (1991). In vitro transcription and 32P-labeling of RNAs was carried out as described in Kataoka et al. (2000). [32P]UTP-labeled RNAs were purified by denaturing gel electrophoresis and precipitated with ethanol. RNAs were resuspended in de-ionized distilled water.
Xenopus laevis oocyte microinjections
Injections were carried out as described in Fischer et al. (1997). Briefly, oocytes were harvested and incubated for 2 h in modified Barth’s solution containing 0.2% collagenase type II (Sigma). Defolliculated stage V and VI oocytes were collected and used the next day for microinjection. In a typical injection experiments, 20 nl of 32P-labeled RNAs (usually ∼1 × 106 c.p.m./µl for each RNA) were injected into the cytoplasm of oocytes. After incubation, oocytes were manually dissected to prepare nuclear and cytoplasmic fractions for further analysis.
Immunoprecipitation of RNA–protein complexes and analysis of RNAs
Immunoprecipitation of RNA–protein complexes was carried out as described in Kataoka et al. (2000). For a typical immunoprecipitation experiment, both nuclear and cytoplasmic fractions were homogenized in 300 µl of ice-cold RSB-150 buffer (10 mM Tris–HCl pH 7.5, 150 mM NaCl, 2.5 mM MgCl2) and the insoluble material was pelleted by centrifugation. The cleared supernatant was incubated with antibodies bound to protein A–Sepharose (Pharmacia). Immunoprecipitation was performed for 30 min at 4°C with constant shaking, and subsequently washed five times with 1 ml of ice-cold RSB-150 buffer. The immunoprecipitated RNAs were isolated by proteinase K treatment followed by phenol–chloroform extraction and ethanol precipitation. RNAs were analyzed by 7 M urea/8% PAGE and autoradiography.
Generation of a stable cell line expressing Flag-Gemin2 and affinity purification of native SMN complexes
The detailed description of the cell line that expresses Flag-tagged Gemin2 and the characterization of the purified native SMN complexes will be presented elsewhere (Pellizzoni et al., 2002). Briefly, the open reading frame of Gemin2 fused at the N-terminus to the amino acid sequence of the Flag epitope (Flag-Gemin2) was generated by PCR amplification using specific primers and was cloned into the pTRE vector (Clontech) under the control of the tetracycline-responsive element. HeLa Tet-ON cells (Clontech) that constitutively express the tetracycline transactivator were co-transfected with the pTRE plasmid encoding Flag-Gemin2, and stable clones were selected by antibiotics. Flag-Gemin2 or HeLa Tet-ON (control) cells were grown in the presence of doxycycline (5 µg/ml). Total cell extracts in RSB-100 buffer containing 0.1% NP-40 and protease inhibitors were incubated with anti-Flag beads (Sigma) for 2 h at 4°C. Supernatants were discarded and beads were extensively washed with RSB-100 containing 0.02% NP-40. Three washes were performed with 10 bed volumes of RSB-500 containing 0.02% NP-40 for 15 min at 4°C. Following three washes with RSB-100 containing 0.02% NP-40, bound proteins were eluted with 10 bed volumes of the same buffer containing 0.25–0.5 mg/ml 3X-Flag peptide (Sigma) for 1 h at 4°C.
Analysis of the purified SMN complex
Purified SMN complexes isolated from the stable cell line described above were separated by 12.5% SDS–PAGE and detected by silver staining. Western blotting of the purified SMN complex was carried out according to Pellizzoni et al. (2001b).
In vitro gel mobility-shift assay of the SMN complex
SL1 or SL1A3 (10 000 c.p.m.) was mixed with 100 ng of the purified SMN complexes and tRNA (10 µM) and reaction mixtures were incubated for 1 h at 30°C. RNA–protein mixtures were loaded onto a native gel containing 4% glycerol, 0.5× Tris–borate EDTA (TBE) buffer and 6% polyacrylamide (acrylamide/bisacrylamide ratio, 80:1). Prior to loading, a gel was pre-run for 1 h at 4°C. The gel was run for ∼2 h in 0.5× TBE buffer at 4°C with a constant current of 35 mA. The gel was exposed for autoradiography.
Competition experiments with unlabeled RNAs
For competition experiments using unlabeled RNAs, each oocyte was pre-injected with 300 fmol of the indicated in vitro transcribed cold RNAs. After 2 h of incubation, a mixture of radiolabeled U snRNAs was injected into the cytoplasm of oocytes and incubated for an additional 2 h. The oocytes were then homogenized in ice-cold immunoprecipitation buffer (10 mM Tris–HCl pH 7.5, 500 mM NaCl, 2.5 mM MgCl2) for immunoprecipitation.
Acknowledgments
Acknowledgements
We are grateful to Dr Iain W.Mattaj for providing plasmids. We also thank Drs Joan Steitz and Reinhard Lührmann for the anti-Sm Y12 monoclonal antibody and the anti-TMG cap polyclonal antibody R1131, respectively. We are grateful to the members of our laboratory, especially Drs Severine Massenet, Amelie Gubitz and Westley Friesen, for helpful discussions and comments on this manuscript. We are also grateful to Gina Daly for secretarial assistance. This work was supported by the Association Française Contre les Myopathies (AFM) and by a grant from the National Institutes of Health. G.D. is an Investigator of the Howard Hughes Medical Institute.
References
- Branlant C., Krol,A., Ebel,J.P., Gallinaro,H., Lazar,E. and Jacob,M. (1981) The conformation of chicken, rat and human U1A RNAs in solution. Nucleic Acids Res., 9, 841–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branlant C., Krol,A., Ebel,J.P., Lazar,E., Haendler,B. and Jacob,M. (1982) U2 RNA shares a structural domain with U1, U4 and U5 RNAs. EMBO J., 1, 1259–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhler D., Raker,V., Lührmann,R. and Fischer,U. (1999) Essential role for the tudor domain of SMN in spliceosomal U snRNP assembly: implications for spinal muscular atrophy. Hum. Mol. Genet., 8, 2351–2357. [DOI] [PubMed] [Google Scholar]
- Burghes A.H. (1997) When is a deletion not a deletion? When it is converted. Am. J. Hum. Genet., 61, 9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charroux B., Pellizzoni,L., Perkinson,R.A., Shevchenko,A., Mann,M. and Dreyfuss,G. (1999) Gemin3: a novel DEAD box protein that interacts with SMN, the spinal muscular atrophy gene product and is a component of gems. J. Cell Biol., 147, 1181–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charroux B., Pellizzoni,L., Perkinson,R.A., Yong,J., Shevchenko,A., Mann,M. and Dreyfuss,G. (2000) Gemin4: a novel component of the SMN complex that is found in both gems and nucleoli. J. Cell Biol., 148, 1177–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer U. and Lührmann,R. (1990) An essential signaling role for the m3G cap in the transport of U1 snRNP to the nucleus. Science, 249, 786–790. [DOI] [PubMed] [Google Scholar]
- Fischer U., Sumpter,V., Sekine,M., Satoh,T. and Lührmann,R. (1993) Nucleo-cytoplasmic transport of U snRNPs: definition of a nuclear location signal in the Sm core domain that binds a transport receptor independently of the m3G cap. EMBO J., 12, 573–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer U., Liu,Q. and Dreyfuss,G. (1997) The SMN–SIP1 complex has an essential role in spliceosomal snRNP biogenesis. Cell, 90, 1023–1029. [DOI] [PubMed] [Google Scholar]
- Friesen W.J. and Dreyfuss,G. (2000) Specific sequences of the Sm and Sm-like (Lsm) proteins mediate their interaction with the spinal muscular atrophy disease gene product (SMN). J. Biol. Chem., 275, 26370–26375. [DOI] [PubMed] [Google Scholar]
- Friesen W.J., Massenet,S., Paushkin,S., Wyce,A. and Dreyfuss,G. (2001a) SMN, the product of the spinal muscular atrophy gene, binds preferentially to dimethylarginine-containing protein targets. Mol. Cell, 7, 1111–1117. [DOI] [PubMed] [Google Scholar]
- Friesen W.J., Paushkin,S., Wyce,A., Massenet,S., Pesiridis,G.S., Van Duyne,G., Rappsilber,J., Mann,M. and Dreyfuss,G. (2001b) The methylosome: a 20S complex containing JBP1 and pICln produces dimethylarginine-modified Sm proteins. Mol. Cell. Biol., 21, 8289–8300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubitz A.K., Mourelatos,Z., Abel,L., Rappsilber,J., Mann,M. and Dreyfuss,G. (2002) Gemin5: a novel WD repeat protein component of the SMN complex that binds Sm proteins. J. Biol. Chem., in press. [DOI] [PubMed] [Google Scholar]
- Hamm J., Kazmaier,M. and Mattaj,I.W. (1987) In vitro assembly of U1 snRNPs. EMBO J., 6, 3479–3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamm J., van Santen,V.L., Spritz,R.A. and Mattaj,I.W. (1988) Loop I of U1 small nuclear RNA is the only essential RNA sequence for binding of specific U1 small nuclear ribonucleoprotein particle proteins. Mol. Cell. Biol., 8, 4787–4791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamm J., Darzynkiewicz,E., Tahara,S.M. and Mattaj,I.W. (1990a) The trimethylguanosine cap structure of U1 snRNA is a component of a bipartite nuclear targeting signal. Cell, 62, 569–577. [DOI] [PubMed] [Google Scholar]
- Hamm J., Dathan,N.A., Scherly,D. and Mattaj,I.W. (1990b) Multiple domains of U1 snRNA, including U1 specific protein binding sites, are required for splicing. EMBO J., 9, 1237–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert M.D., Szymczyk,P.W., Shpargel,K.B. and Matera,A.G. (2001) Coilin forms the bridge between Cajal bodies and SMN, the spinal muscular atrophy protein. Genes Dev., 15, 2720–2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai Y., Matsushima,Y., Sugimura,T. and Terada,M. (1991) A simple and rapid method for generating a deletion by PCR. Nucleic Acids Res., 19, 2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarmolowski A., Boelens,W.C., Izaurralde,E. and Mattaj,I.W. (1994) Nuclear export of different classes of RNA is mediated by specific factors. J. Cell Biol., 124, 627–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones K.W., Gorzynski,K., Hales,C.M., Fischer,U., Badbanchi,F., Terns,R.M. and Terns,M.P. (2001) Direct interaction of the spinal muscular atrophy disease protein SMN with the small nucleolar RNA-associated protein fibrillarin. J. Biol. Chem., 276, 38645–38651. [DOI] [PubMed] [Google Scholar]
- Kambach C., Walke,S., Young,R., Avis,J.M., de la Fortelle,E., Raker, V.A., Lührmann,R., Li,J. and Nagai,K. (1999) Crystal structures of two Sm protein complexes and their implications for the assembly of the spliceosomal snRNPs. Cell, 96, 375–387. [DOI] [PubMed] [Google Scholar]
- Kataoka N., Yong,J., Kim,V.N., Velazquez,F., Perkinson,R.A., Wang,F. and Dreyfuss,G. (2000) Pre-mRNA splicing imprints mRNA in the nucleus with a novel RNA-binding protein that persists in the cytoplasm. Mol. Cell, 6, 673–682. [DOI] [PubMed] [Google Scholar]
- Lefebvre S. et al. (1995) Identification and characterization of a spinal muscular atrophy-determining gene. Cell, 80, 155–165. [DOI] [PubMed] [Google Scholar]
- Liu Q. and Dreyfuss,G. (1996) A novel nuclear structure containing the survival of motor neurons protein. EMBO J., 15, 3555–3565. [PMC free article] [PubMed] [Google Scholar]
- Liu Q., Siomi,H., Siomi,M.C., Fischer,U., Zhang,Y., Wan,L. and Dreyfuss,G. (1996) Molecular characterization of the protein products of the fragile X syndrome gene and the survival of motor neurons gene. Cold Spring Harb. Symp. Quant. Biol., 61, 689–697. [PubMed] [Google Scholar]
- Liu Q., Fischer,U., Wang,F. and Dreyfuss,G. (1997) The spinal muscular atrophy disease gene product, SMN and its associated protein SIP1 are in a complex with spliceosomal snRNP proteins. Cell, 90, 1013–1021. [DOI] [PubMed] [Google Scholar]
- Lorson C.L. and Androphy,E.J. (1998) The domain encoded by exon 2 of the survival motor neuron protein mediates nucleic acid binding. Hum. Mol. Genet., 7, 1269–1275. [DOI] [PubMed] [Google Scholar]
- Mattaj I.W. (1986) Cap trimethylation of U snRNA is cytoplasmic and dependent on U snRNP protein binding. Cell, 46, 905–911. [DOI] [PubMed] [Google Scholar]
- Mattaj I.W. (1998) Ribonucleoprotein assembly: clues from spinal muscular atrophy. Curr. Biol., 8, R93–R95. [DOI] [PubMed] [Google Scholar]
- Mattaj I.W. and De Robertis,E.M. (1985) Nuclear segregation of U2 snRNA requires binding of specific snRNP proteins. Cell, 40, 111–118. [DOI] [PubMed] [Google Scholar]
- Mattaj I.W., Boelens,W., Izaurralde,E., Jarmolowski,A. and Kambach,C. (1993) Nucleocytoplasmic transport and snRNP assembly. Mol. Biol. Rep., 18, 79–83. [DOI] [PubMed] [Google Scholar]
- Meister G., Buhler,D., Laggerbauer,B., Zobawa,M., Lottspeich,F. and Fischer,U. (2000) Characterization of a nuclear 20S complex containing the survival of motor neurons (SMN) protein and a specific subset of spliceosomal Sm proteins. Hum. Mol. Genet., 9, 1977–1986. [DOI] [PubMed] [Google Scholar]
- Meister G., Buhler,D., Pillai,R., Lottspeich,F. and Fischer,U. (2001) A multiprotein complex mediates the ATP-dependent assembly of spliceosomal U snRNPs. Nature Cell Biol., 3, 945–949. [DOI] [PubMed] [Google Scholar]
- Melki J. (1997) Spinal muscular atrophy. Curr. Opin. Neurol., 10, 381–385. [DOI] [PubMed] [Google Scholar]
- Mount S.M. and Steitz,J.A. (1981) Sequence of U1 RNA from Drosophila melanogaster: implications for U1 secondary structure and possible involvement in splicing. Nucleic Acids Res., 9, 6351–6368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mourelatos Z., Abel,L., Yong,J., Kataoka,N. and Dreyfuss,G. (2001) SMN interacts with a novel family of hnRNP and spliceosomal proteins. EMBO J., 20, 5443–5452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai K., Muto,Y., Pomeranz Krummel,D.A., Kambach,C., Ignjatovic, T., Walke,S. and Kuglstatter,A. (2001) Structure and assembly of the spliceosomal snRNPs. Novartis Medal Lecture. Biochem. Soc. Trans., 29, 15–26. [DOI] [PubMed] [Google Scholar]
- Nelissen R.L., Will,C.L., van Venrooij,W.J. and Lührmann,R. (1994) The association of the U1-specific 70K and C proteins with U1 snRNPs is mediated in part by common U snRNP proteins. EMBO J., 13, 4113–4125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellizzoni L., Kataoka,N., Charroux,B. and Dreyfuss,G. (1998) A novel function for SMN, the spinal muscular atrophy disease gene product, in pre-mRNA splicing. Cell, 95, 615–624. [DOI] [PubMed] [Google Scholar]
- Pellizzoni L., Charroux,B. and Dreyfuss,G. (1999) SMN mutants of spinal muscular atrophy patients are defective in binding to snRNP proteins. Proc. Natl Acad. Sci. USA, 96, 11167–11172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellizzoni L., Baccon,J., Charroux,B. and Dreyfuss,G. (2001a) The survival of motor neurons (SMN) protein interacts with the snoRNP proteins fibrillarin and GAR1. Curr. Biol., 11, 1079–1088. [DOI] [PubMed] [Google Scholar]
- Pellizzoni L., Charroux,B., Rappsilber,J., Mann,M. and Dreyfuss,G. (2001b) A functional interaction between the survival motor neuron complex and RNA polymerase II. J. Cell Biol., 152, 75–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellizzoni L., Baccon,J., Rappsilber,J., Mann,M. and Dreyfuss,G. (2002) Purification of native SMN complexes and identification of Gemin6 as a novel component. J. Biol. Chem., in press. [DOI] [PubMed] [Google Scholar]
- Plessel G., Fischer,U. and Lührmann,R. (1994) m3G cap hypermethylation of U1 small nuclear ribonucleoprotein (snRNP) in vitro: evidence that the U1 small nuclear RNA-(guanosine-N2)-methyltransferase is a non-snRNP cytoplasmic protein that requires a binding site on the Sm core domain. Mol. Cell. Biol., 14, 4160–4172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Query C.C., Bentley,R.C. and Keene,J.D. (1989a) A common RNA recognition motif identified within a defined U1 RNA binding domain of the 70K U1 snRNP protein. Cell, 57, 89–101. [DOI] [PubMed] [Google Scholar]
- Query C.C., Bentley,R.C. and Keene,J.D. (1989b) A specific 31-nucleotide domain of U1 RNA directly interacts with the 70K small nuclear ribonucleoprotein component. Mol. Cell. Biol., 9, 4872–4881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherly D., Boelens,W., van Venrooij,W.J., Dathan,N.A., Hamm,J. and Mattaj,I.W. (1989) Identification of the RNA binding segment of human U1 A protein and definition of its binding site on U1 snRNA. EMBO J., 8, 4163–4170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark H., Dube,P., Lührmann,R. and Kastner,B. (2001) Arrangement of RNA and proteins in the spliceosomal U1 small nuclear ribonucleoprotein particle. Nature, 409, 539–542. [DOI] [PubMed] [Google Scholar]
- Surowy C.S., van Santen,V.L., Scheib-Wixted,S.M. and Spritz,R.A. (1989) Direct, sequence-specific binding of the human U1-70K ribonucleoprotein antigen protein to loop I of U1 small nuclear RNA. Mol. Cell. Biol., 9, 4179–4186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Will C.L. and Lührmann,R. (2001) Spliceosomal UsnRNP biogenesis, structure and function. Curr. Opin. Cell Biol., 13, 290–301. [DOI] [PubMed] [Google Scholar]