Abstract
To investigate the effects of propofol and fentanyl on the postprandial duodenal motility the intraluminal impedance technique was used. Six pigs were instrumented with a central venous catheter, a percutaneous enterogastrostomy (PEG), and an impedance catheter, which was introduced via the PEG into the duodenum through endoscopy. Over the following 3 d, duodenal motility was measured for 8-hour periods. Measurements were taken on each subject under 3 different sets of conditions: in the conscious unrestrained pig, during propofol sedation, and during sedation with propofol-fentanyl. Both, after morning feeding and during gastric nutrition via the PEG, duodenal feeding patterns and duodenal phase II of the migrating motor cycle were shortened during propofol and propofol-fentanyl sedation. In contrast, the duration of phase I was prolonged by propofol and propofol-fentanyl. In conclusion, either propofol or propofol-fentanyl sedation shortens duodenal feeding patterns, as well as phase II of the migrating motor cycle.
Résumé
Effets du propofol et du fentanyl sur la motilité duodénale du porc. La technique de l’impédance intraluminale a été utilisée pour étudier les effets du propofol et du fentanyl sur la motilité duodénale postprandiale. On a procédé chez 6 porcs à l’installation d’un cathéter veineux central, puis à une entéro-gastrostomie percutanée (EGP) suivie de l’introduction par endoscopie par la EGP d’un cathéter à impédance dans le duodénum. Dans les 3 jours suivants, la motilité intestinale a été mesurée pendant des périodes de 8 heures. Les mesures ont été effectuées sur chacun des sujets sous 3 types de conditions : animal conscient et en liberté, sous sédation au propofol et sous sédation au propofol-fentanyl. Après l’alimentation du matin ainsi qu’au cours de la nutrition par l’EGP, les modèles alimentaires duodénaux et la phase II du cycle moteur migrant du duodénum étaient raccourcis au cours des sédations au propofol et au propofol-fentanyl. Au contraire, la durée de la phase 1 a été prolongée par le propofol et le propofol-fentanyl. En conclusion, la sédation par le propofol ou par le propofol-fentanyl raccourci les modèles alimentaires duodénaux ainsi que la phase II du cycle moteur migrant.
(Traduit par Docteur André Blouin)
Introduction
Propofol is often used for sedation, as it allows for easy titration and rapid recovery, in humans and pigs (1,2). In humans, hypnotic dosages of propofol have been shown to induce a delay in gastric emptying (3,4). In vitro, propofol demonstrated a dose-dependent impairment of gastric and colonic contractile activity (5). Propofol is also frequently combined with opioids, which show further inhibitory effects on intestinal motility (6,7). In animal research, potential effects of anesthetic drugs commonly used for standard procedures should therefore be known. Hence, the present study was performed in order to accurately define the effects of propofol and fentanyl on duodenal motility in pigs.
During the last decade, the intraluminal impedance technique was used as an alternative approach for measuring intestinal motility (8–11). Recently, the impedance technique has been validated through comparison with manometry and real-time ultrasounography (12) and an inhibitory effect of ketamine on duodenal motility could be demonstrated using the impedance technique in pigs (13).
Fasting digestive tract motility is normally characterized by the periodic occurrence of the migrating motor complex (MMC), which, conventionally, is divided into 3 phases: a period of quiescence (phase I) is followed by irregular contractile activity (phase II), which, in turn, is replaced by a shorter period of regular contractions (phase III) or “activity front” of the MMC (14). The MMC is interrupted by feeding, with the initiation of continuous phase II-like activities. These phase II-like activities, also called feeding patterns, are due to crushing food into smaller particles ready to pass through the pylorus into the duodenum (15). Their duration depends on the food characteristics and they are usually terminated by a decrease in duodenal pH, an increase in osmolarity, and a rise of free fatty acids, indicating gastric emptying (16,17).
The aim of the present study was to investigate the effects of propofol and propofol-fentanyl sedation on the duration of the duodenal interdigestive phase I–III of the MMC, as assessed by the impedance technique.
Material and methods
The investigation was approved by the local Institutional Committee for Animal Ethics and followed the Canadian Council on Animal Care Guidelines on Animal Use. To avoid introducing the possibility of gender bias on intestinal motility, 6 male castrated German Landrace were used, all obtained from the same breeder and with body weights (BW) between 32 and 40 kg.
Habituation and training
Pigs were allowed to adjust to their local housing conditions and to the investigators over a period of 9 d. The daily rhythm was maintained by making use of alternating dark and light cycles of 12 h duration each. The pigs had free access to water and were fed twice a day with a standard diet (Muskator; Muskator-Werk, Duesseldorf, Germany). Since the study protocol included enteral nutrition via a percutaneous enterogastrostomy (PEG), animals were also fed with a balanced high calorie probe food (Biosorb 1500 Multi Fibre; Pfrimmer NUTRICIA GmbH, Erlangen, Germany) for gastrointestinal adaptation to their diet. In order to adapt to laboratory conditions, the pigs were then transferred to a transportable cage. This cage enabled the pigs to turn around freely and to lie down with their legs outstretched. The animals were trained to be kept in these cages for a period of 8 h. After 4 d, the animals became accustomed to the laboratory conditions, as indicated by their behavioral signs (18,19).
Instrumentation
Instrumentation was performed on day 10. Each pig had been fasted overnight but still had free access to water. After a premedication with 4 mg/kg BW azaperone (Stresnil; Janssen-Cilag GmbH, Neuss, Germany) and 0.5 mg atropine (Atropin; B. Braun Melsungen, Melsungen, Germany), IM, 10 mg/kg BW ketamine (Ketamin; Sanofi-Cefa GmbH, Berlin, Germany) was injected, IM. Once IV access had been established, general anesthesia was subsequently induced IV with 2 to 4 mg/kg BW propofol (Propofol; Parke-Davis, Karlsruhe, Germany). Anesthesia was maintained with a continuous infusion of between 15 and 25 mg/kg BW/h propofol and with 0.5 mg/kg BW/h of (S)-ketamine ([S]-Ketanest; Parke-Davis). The pigs underwent orotracheal intubation and the lungs were mechanically ventilated. Oxygen saturation (SaO2) was maintained between 94% and 99%, and expiratory carbon dioxide partial pressure (ETCO2) was kept at 35 to 38 mmHg. Blood pressures were measured through noninvasive methods on the hind limb. Mean arterial blood pressures (MAP), heart rate (HR), SaO2, and ETCO2 were assessed at baseline and then at 15-min intervals during the surgical procedure. The animals were instrumented with a central venous catheter (CVC) inserted into the left jugular vein via venous cut down. Fluid was replaced during surgery by using a crystalloid solution at a rate of 2 mL/kg BW/h. A percutaneous enterogastrostomy (PEG, GastroPEG; B. Braun Melsungen) was performed by endoscopy. Both the CVC and the end of the PEG were tunneled, SC, towards the dorsum. Polividon-iodine solution was instilled into the SC tunnels. An impedance catheter (2 mm diameter, 100 cm length, flexible, 16 electrodes distributed over the distal 32 cm; Femu Research-Institute, University Hospital Aachen, Germany) was then introduced via the PEG into the stomach. This catheter was further introduced into the duodenum through endoscopy, with 2 to 4 proximal ring electrodes placed into the gastric antrum. Finally, the external parts of the CVC and the PEG were placed into a catheter bag, which was sutured on to the back of the pigs (20). Postoperative analgesia had already commenced under general anesthesia with the 1st dosage of nonopioid analgesics, metamizole-sodium (Novalgin; Aventis Pharma, Bad Soden, Germany), 20 mg/kg BW, IV, and carprofen (Rimadyl; Pfizer GmbH, Karlsruhe, Germany), 4 mg/kg BW, SC. The pigs were then fed a standard diet in the evening and had free access to water overnight.
Measurements
During days 11 to 13, the pigs were transported to the laboratory. On day 11, measurements were taken from conscious pigs. During morning feeding (pellets 400 g, Muskator; Muskator-Werk) and drinking, the impedance catheter was connected to the signal transducer for online measurements. Fluids were replaced with a 2 mL/kg BW/h crystalloid solution via the CVC during the investigation period. Measurements were recorded for the following 8 h. Four hours after beginning the measurements, a gastric nutrient mixture with a common substrate of 2 mL/kg BW (Biosorb; Primmer Nutrica GmbH) was infused for 30 min via the PEG. To ensure standard experimental conditions, the following parameters were monitored: Heart rate (HR), mean arterial blood pressure (MAP), respiratory rate (RR), body temperature (Temp), white blood cell count (WBC), hematocrit (HKT), and venous blood gas analysis. At the end of the measurements, the CVC and the PEG were stored in the catheter bag. Potential aberrations due to animal movement were monitored on video over the total measuring phase. To prevent postoperative pain, analgesia was maintained with IV injections of the nonopioid analgesic, metamizol, 20 mg/kg BW, via the CVC, q12h. Overnight, the animals were returned to their pens with standard housing conditions. During days 12 and 13, the feeding protocol was adhered to as on the previous day. To provide measurements during propofol sedation on day 12, sedation was induced with a bolus of propofol (2 to 4 mg/kg BW) followed by a continuous infusion of propofol (15 to 35 mg/kg BW/h) for maintenance. The dosage of propofol was guided by clinical signs to ensure appropriate depth of sedation with spontaneous breathing. The pigs received oxygen via a face-mask as required, to maintain oxygen saturation (SaO2) between 94% and 99%. Gastric nutrition was commenced as usual. Procedures and monitoring were similar to those of the previous day. On day 13, measurements commenced again during sedation, this time with propofol and fentanyl after a bolus injection of 2 to 4 mg/kg BW propofol. Sedation was maintained by using a continuous infusion of propofol at a rate of 15 to 35 mg/kg BW/h, in combination with a continuous infusion of fentanyl at a rate of 1 to 8 μg/kg BW/h. At the end of the measurements, the animals were euthanized with an overdose of barbiturate. Necropsy was subsequently performed.
Measurement of gastroduodenal motility
Gastroduodenal motility was assessed by using the impedance technique. Impedance signals were stored in a personnel computer (PC). The impedance tracings were visually analyzed on the PC-screen. Locally developed software (Femu-Research Institute, University Hospital Aachen, Aachen, Germany) was used for the data acquisition, analysis, and graphic presentation. In summary, the following parameters were investigated: a) gastric motility activity, b) duodenal motor patterns, and c) duodenal bolus transport events. Gastric motility activities were def ined as wave forms, which were detected in 2 proximal channels and elevated from the baseline by more than 30%. The duodenal motor patterns were as defined Nguyen et al (21) (Figure 1). According to established manometric criteria, the interdigestive migrating motor complex (MMC) was defined as the time interval between 2 successive phase III patterns. Feeding patterns were identified as phase II-like activities, beginning during gastric food intake and ending with the termination of phase III (22). A bolus transport event (BTE) was defined as a particular impedance tracing that is related to the passage of a bolus detected over 3 measuring channels, as described by Nguyen et al (21).
Figure 1.
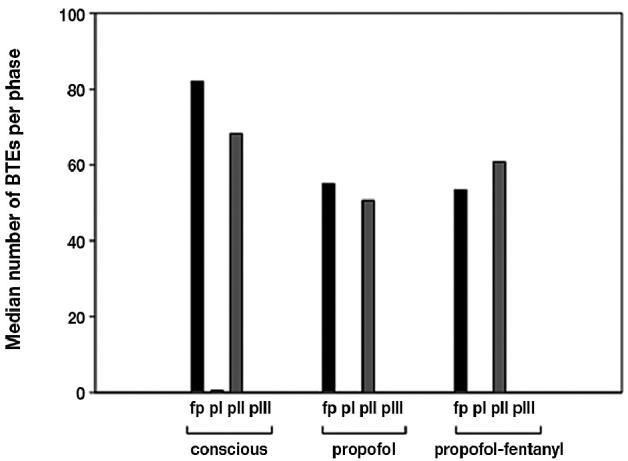
Median number of duodenal bolus transport events (BTE) during the main digestive and interdigestive phases in conscious, propofol (propofol), and propofol-fentanyl (propofol-fentanyl) sedated pigs expressed in median. Feeding patterns (fp) = feeding patterns after standard morning feeding; phase I = pI; phase II = pII; phase III = pIII.
Statistical analysis
The frequency of motility activity (motility rates/min) of each animal was analyzed by using the Kruskal-Wallis Test with a post hoc test (Bonferroni). The length of the interdigestive phase I–III of the MMC cycles was compared between the groups by using the Mann-Whitney U test. The data are expressed as median and 95% confidence interval (95% CI) or mean and standard deviation (s). Statistically significant differences were defined at P values of <0.05.
Results
Duodenal motility activity
In conscious pigs, the median duodenal feeding pattern after standard morning feeding was 174 min (90 to 213 min, 95% CI). Compared with conscious pigs, this feeding pattern was shortened during propofol and propofol-fentanyl sedation by 86% and 85%, respectively (Figure 4). The median duration of feeding pattern of 58.5 min (43.8 to 113.5 min, 95% CI) in conscious pigs given gastric nutrition via the PEG was also shortened during propofol and propofol-fentanyl sedation but by 47% and 26%, respectively (Figure 4).
Figure 4.
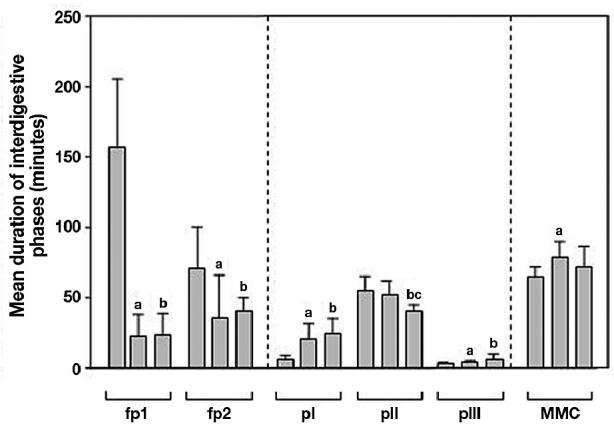
Duration (minutes) of feeding patterns after standard morning feeding (fp1), feeding patterns after gastric nutrition via PEG (fp2), phase I (pI), phase II (pII), phase III (pIII), and MMC cycle length (MMC) in conscious pigs (c) and during propofol (p) and propofol-fentanyl (pf) sedation. Data are given in mean ± s. Statistical significance of P < 0.05 is expressed as (a) for differences between c and p, (b) for differences between c and pf, and (c) for differences between p and pf.
While the conscious pigs demonstrated a median duration of 6.1 min (2.2 to 9.3 min, 95% CI) for the interdigestive phase I, in both the propofol and the propofol-fentanyl sedated pigs the duration of the interdigestive phase I was prolonged by 280% and 349%, respectively (Figure 4).
In conscious pigs, median duration of phase II was 54.9 min (38.9 to 70.1 min, 95% CI), which was comparable with that in propofol sedated pigs. However, in propofol-fentanyl sedated pigs, the duration of phase II was shortened by 24% and 17% compared with that in conscious and propofol sedated pigs, respectively (Figure 4).
In conscious pigs, the median duration of phase III was 3.4 min (2.3 to 4.1 min, 95% CI) this was extended in propofol sedated pigs by 124% and in propofol-fentanyl sedated pigs by 150% (Figure 4).
In conscious pigs, the median duration of the MMC cycle was 65.1 min (53.3 to 75.3 min, 95% CI); this was prolonged by 120% in propofol sedated pigs, but seemed to be unaffected in the propofol-fentanyl sedated pigs (Figure 4).
With regard to the BTEs, 98% of all duodenal BTEs could be found during feeding patterns and phase II, with 91% of all BTEs being of antral origin. The number of BTEs during the feeding pattern and phase II seemed to be unaffected by the use of propofol, with and without fentanyl (Figure 1). On average, every 2nd to 4th antral motility activity resulted in a duodenal BTE.
Frequency of gastric motility
During propofol sedation, the frequency of gastric motility when compared with that of conscious pigs, was decreased during the whole investigation period, except during the 6th h, Whereas with the propofol-fentanyl sedation, the frequency of gastric motility activity during the 3rd to 4th h after morning feeding was even lower that that with propofel sedation (Figure 2).
Figure 2.
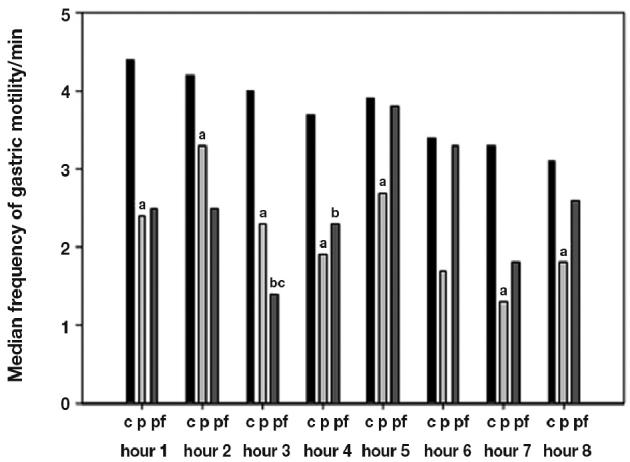
Frequency of gastric motility/min during total investigation period of 8 h in conscious (c), propofol (p), and propofol-fentanyl (pf) sedated pigs expressed in median. Statistical significance of P < 0.05 is expressed as (a) for differences between c and p, (b) for differences between c and pf, and (c) for differences between p and pf.
Demographic data
With the exception of heart rate and the partial pressure of oxygen (pO2), which was slightly higher in propofol sedated pigs, the basic experimental conditions were comparable between all groups. The parameters monitored for equal experimental conditions are shown in Table 1. All animals were able to be included in the study. However, 2 propofol sedated pigs showed tracings involving gastric contractile activity with a lack of any duodenal motility which indicated a retrograde movement of the catheters into the stomach. In conjunction with the study protocol, these 2 pigs were sedated over the investigation period of 8 h before a necropsy was performed. Their data, obtained during the conscious state were included in the study, while the data obtained during propofol sedation, were excluded. Necropsy was subsequently performed in all the animals.
Table 1.
Demographic and hematological data of conscious, propofol (propofol), and propofol-fentanyl (propofol-fentanyl) sedated pigs presented as median values and 95% CI
| Conscious | Propofol | Propofol-fentanyl | |
|---|---|---|---|
| Body weight (kg) | 38 (36.2–39.4) | 38 (36.2–39.4) | 38 (36.2–39.4) |
| Propofol (mg/kg/h) | — | 30 (21.4–34.7) | 28 (23.7–30.8) |
| Fentanyl (μg/kg/min) | — | — | 0.05 (0.04–0.05) |
| Heart rate (/min) | 106 (99.4–113.9)a | 125 (112.8–149.9)a | 126 (109–139.8) |
| MAP (mmHg) | — | 71 (63.4–77.8) | 69 (63.9–87.4) |
| Respiratory rate (/min) | 22 (19.8–27.8) | 19 (14.0–24.7) | 23 (19.9–26.3) |
| SaO2 (%) | 97 (96.2–97.8) | 97 (95.6–98.1) | 97 (95.6–98.1) |
| EtCO2 | — | 48 (46.1–49.0) | 43 (42.7–44.4) |
| Temperature (°C) | 39.6 (39.4–40.1) | 39.4 (39.3–39.6) | 39.8 (39.5–39.9) |
| PvCO2 (mmHg) | 47.1 (39.8–51.2) | 51 (47.1–53.2) | 48.8 (46–55.2) |
| PvO2 (mmHg) | 34.9 (31.2–36.9)a | 39.8 (25.0–78.7)a | 39 (27.1–54.6) |
| pH | 7.45 (7.43–7.46) | 7.38 (7.35–7.44) | 7.4 (7.37–7.45) |
| Base excess | 7.4 (2.9–9.3) | 5.4 (3.9–8.7) | 4.9 (4.1–7.5) |
| WBC (×109/L) | 15 (12.3–27.0) | 19.7 (15.6–24.2) | 19.5 (13.5–24.3) |
| Hematocrit (L/L) | 0.29 (0.27–0.32) | 0.29 (0.27–0.31) | 0.28 (0.26–0.28) |
MAP — mean arterial blood pressure; ETCO2 — expiratory carbon dioxide partial pressures; SaO2 — oxygen saturation; PvCO2 — partial pressure of carbon dioxide; PvO2 — partial pressure of oxygen; WBC — white blood cell count
Statistical significance of P < 0.05 between 2 groups
Discussion
Using electromyography, Ruckebusch and Bueno (23) demonstrated a postprandial pattern of regular spike activities lasting for 3 h when pigs were fed q12h. Our results are comparable with those obtained by them, considering the duration of feeding patterns in conscious pigs. In humans, clinical investigations have shown that propofol and opioids both delay gastric emptying in a dose dependent manner (3,4). In contrast, gastric emptying was found to be unaffected if low dose propofol was used, demonstrating its anti-emetic properties. Therefore, propofol could not be considered as a prokinetic agent (24). Taking these aspects into account, our opinion is that the results obtained during propofol and propofol-fentanyl sedation support our hypothesis that these drugs can, at least partially, impair gastroduodenal motility through a shortened feeding pattern.
In this study, the actual time period of gastric emptying could not be measured, since investigations, like the H2-expiration-test and radiography, are difficult to perform without causing significant stress in the conscious animal. However, gastroparesis was suggested on necropsy with complete gastric food retention being found in all sedated pigs at necropsy, indicating a lack of any gastroduodenal propulsion — this being independent of the drugs used. Sedated pigs also demonstrated a reduced phase II pattern associated with a partially decreased frequency in the gastric motility cycle. This finding is of great significance, since food migration is mainly performed during phase II (25). Mushambi et al (3) also found a decrease of the transit times in the upper gastrointestinal tract during propofol sedation. Their findings therefore are supported by our results, since a shortened interdigestive phase II should be followed by a slower transit time. Furthermore, a notable reduction in the frequency of gastric motility, similar to that described in patients with gastroparesis, could be observed in pigs during propofol sedation, which might indicate a lower gastric emptying frequency (26).
Based on electromyographic measurements, the apparent migration of gastric rhythm from antrum to duodenum has been described in pigs (27). Our study suggests that nearly every 2nd to 3rd gastric motility activity was propagated through to the duodenum, independent of the use of propofol and fentanyl. In all groups, 98% of all the duodenal BTEs were detected during feeding patterns and phase II, emphasizing their important role in food migration. With 91% of all duodenal BTEs being of antral origin, regardless of the drug used, gastroduodenal coordination must be preserved. Our results are comparable with those in humans that show that during phase II, fluid transport across the pylorus could be observed in 70% of all the antral contractions detected by real-time ultrasonography (25). In that study, antral contractions were always associated with pyloric opening and a high rate (92%) of these antral contractions were propagated to the duodenum when they were associated with fluid transport.
This study focused on duodenal motility. In contrast to the duodenum, the stomach, being a large hollow organ, makes motility measurements by impedance methods and manometry more difficult to perform, so gastric motility measurements are still a challenge. Therefore, we used the frequency of gastric motility waves as a surrogate for gastric motility.
Although many different animal species can be used for intestinal research (23,26,28), we used pigs with a 32 to 40 kg body weight range, since our previous experiences had allowed us to use devices that are usually used in humans (13,18). In general, the pig is considered to be a suitable nonprimate animal model, since it adequately represents the human situation in terms of feeding patterns and its anatomy and physiology of the gastrointestinal tract (29). While in pigs being sedated by propofol, the increased PvO2 might be due to oxygen inhalation via face mask to maintain sufficient oxygen saturation, the increased heart rate could be caused by a venous dilatation during the use of propofol or by using dosages of propofol that were guided by clinical signs to ensure appropriate depth of sedation.
Although randomization would have been preferable, it would have prolonged the investigation period and increased the risk of infection. So, to avoid the use of antibiotics, with the added likelihood of influencing gastric motility (30), the investigations had to be terminated as soon as possible, and, instrumentation and measurements were performed in series, beginning with the least invasive procedure — measurements in conscious pigs — and finishing with the most disruptive procedure, propofol-fentanyl sedation.
Our results could have been influenced by the drugs used during instrumentation, even a single use of ketamine used for premedication shortened phase II of the MMC cycle, while phase I was prolonged (13). Nevertheless, the short half-life of ketamine with its analgesic effects lasting between 10 and 20 min after IM injection of 10 mg/kg BW, suggests that its potential effect on motility during the following days could be overlooked (31).
Measurements during propofol-fentanyl sedation could possibly have been influenced by the propofol used on the day before. However, observations on the pigs’ clinical conditions and eating behavior of the next morning did not suggest a potential influence of this nonrandomized design.
In addition, the porcine model used allowed for investigations of the intestinal motility in conscious and sedated animals under spontaneous ventilation. An additional study-group sedated with fentanyl alone would have been difficult to perform with the pigs probably showing signs of distress at low doses of fentanyl. With higher doses of fentanyl, ventilatory support in order to prevent hypercapnia and hypoxia might have been necessary, since hypoventilation might influence the pigs’ intestinal motility (28). Therefore, a “fentanyl only treated group” was not included.
All pigs were treated with the pyrazolone derivate metamizole and the nonsteroidal antiinflammatory drug (NSAID) carprofen for analgesia in a standardized manner. In the rat, motility of the small intestine seemed to be unaffected, gastric emptying was decreased by metamizole (32). Typical side effects of carprofen are gastrointestinal discomfort, bleeding, and ulceration. Conclusively, both drugs could, at least partially, influence motility measurements.
In order to compare 3 unpaired groups with unequal sample size the Kruskal-Wallis test with Bonferroni correction was used. Although the Kruskal-Wallis test has little power when the sample is small, the clear differences in duration of the various phases led us to the assumption that our data at least suggest significance. CVJ
Figure 3.

Duodenal impedance tracings of a conscious pig demonstrating 16 pairs of electrodes (Ch 1 = proximal channel 1, Ch 16 = distal channel 16). Top: time period of 8 h containing interdigestive phases. Middle: time period expanded. p1 = phase I, p2 = phase II, p3 = phase III.
Footnotes
This study was financially supported by START, RWTH Aachen, Germany, and B. Braun Melsungen AG, Melsungen, Germany.
References
- 1.Kress JP, Pohlman AS, O’Connor MF, Hall JB. Daily interruption of sedative infusions in critically ill patients undergoing mechanical ventilation. N Engl J Med. 2000;342:1471–1477. doi: 10.1056/NEJM200005183422002. [DOI] [PubMed] [Google Scholar]
- 2.Kaiser GM, Fruhauf NR, Zhang H, et al. Intravenous infusion anesthesia with propofol-midazolam-fentanyl for experimental surgery in swine. J Invest Surg. 2003;16:353–357. doi: 10.1080/08941930390250223. [DOI] [PubMed] [Google Scholar]
- 3.Mushambi MC, Rowbotham DJ, Bailey SM. Gastric emptying after minor gynaecological surgery. The effect of anaesthetic technique. Anaesthesia. 1992;47:297–299. doi: 10.1111/j.1365-2044.1992.tb02167.x. [DOI] [PubMed] [Google Scholar]
- 4.Freye E, Sundermann S, Wilder-Smith OH. No inhibition of gastrointestinal propulsion after propofol- or propofol/ketamine-N2O/O2 anaesthesia. A comparison of gastro-caecal transit after isoflurane anaesthesia. Acta Anaesthesiol Scand. 1998;42:664–669. doi: 10.1111/j.1399-6576.1998.tb05299.x. [DOI] [PubMed] [Google Scholar]
- 5.Lee TL, Ang SB, Dambisya YM, Adaikan GP, Lau LC. The effect of propofol on human gastric and colonic muscle contractions. Anesth Analg. 1999;89:1246–1249. [PubMed] [Google Scholar]
- 6.Schiller LR. Review article: anti-diarrhoeal pharmacology and therapeutics. Aliment Pharmacol Ther. 1995;9:87–106. [PubMed] [Google Scholar]
- 7.Kurz A, Sessler DI. Opioid-induced bowel dysfunction: pathophysiology and potential new therapies. Drugs. 2003;63:649–671. doi: 10.2165/00003495-200363070-00003. [DOI] [PubMed] [Google Scholar]
- 8.Frieling T, Hermann S, Kuhlbusch R, et al. Comparison between intraluminal multiple electric impedance measurement and manometry in the human oesophagus. Neurogastroenterol Motil. 1996;8:45–50. doi: 10.1111/j.1365-2982.1996.tb00241.x. [DOI] [PubMed] [Google Scholar]
- 9.Wenzl TG, Moroder C, Trachterna M, et al. Esophageal pH monitoring and impedance measurement: a comparison of two diagnostic tests for gastroesophageal reflux. J Pediatr Gastroenterol Nutr. 2002;34:519–523. doi: 10.1097/00005176-200205000-00009. [DOI] [PubMed] [Google Scholar]
- 10.Sifrim D, Holloway R, Silny J, Tack J, Lerut A, Janssens J. Composition of the postprandial refluxate in patients with gastroesophageal reflux disease. Am J Gastroenterol. 2001;96:647–655. doi: 10.1111/j.1572-0241.2001.03598.x. [DOI] [PubMed] [Google Scholar]
- 11.Peter CS, Wiechers C, Bohnhorst B, Silny J, Poets CF. Influence of nasogastric tubes on gastroesophageal reflux in preterm infants: a multiple intraluminal impedance study. J Pediatr. 2002;141:277–279. doi: 10.1067/mpd.2002.126298. [DOI] [PubMed] [Google Scholar]
- 12.Imam H, Sanmiguel C, Larive B, Bhat Y, Soffer E. Study of intestinal flow by combined videofluoroscopy, manometry, and multiple intraluminal impedance. Am J Physiol Gastrointest Liver Physiol. 2004;286:G263–270. doi: 10.1152/ajpgi.00228.2003. [DOI] [PubMed] [Google Scholar]
- 13.Schnoor J, Unger JK, Koch B, Silny J, Rossaint R. Effects of a single dose of ketamine on duodenal motility activity. Can Vet J. 2005;46:147–152. [PMC free article] [PubMed] [Google Scholar]
- 14.Malagelada J, Camilleri M, Stanghellini V. Physiologic basis of gastrointestinal motility disorders. In: Malagelada J, ed. Manometric Diagnosis of Gastrointestinal Motility Disorders. New York: Thieme, 1986:1–11.
- 15.Freinkel WD, Hinder RA. Recording the interdigestive myo- electrical complex: A new technique. S Afr Med J. 1980;58:238–240. [PubMed] [Google Scholar]
- 16.Phillips WT, Schwartz JG, Blumhardt R, McMahan CA. Linear gastric emptying of hyperosmolar glucose solutions. J Nucl Med. 1991;32:377–381. [PubMed] [Google Scholar]
- 17.Lin HC, Doty JE, Reedy TJ, Meyer JH. Inhibition of gastric emptying by sodium oleate depends on length of intestine exposed to nutrient. Am J Physiol. 1990;259:G1031–1036. doi: 10.1152/ajpgi.1990.259.6.G1031. [DOI] [PubMed] [Google Scholar]
- 18.Schnoor J, Bartz S, Klosterhalfen B, Kuepper W, Rossaint R, Unger JK. A long term porcine model for measurement of gastrointestinal motility. Lab Anim. 2003;37:145–154. doi: 10.1258/00236770360563796. [DOI] [PubMed] [Google Scholar]
- 19.Schnoor J, Kuepper T, Kuepper W, Rossaint R, Unger JK. Evaluation of a new long-term model for measurements of intestinal motility in awake and unrestrained pigs (Abstract) Eur J Anaesth. 2003;20:156. [Google Scholar]
- 20.Unger JK, Gerlach JC, Juhr NC, Rossaint R. Development of a special catheterbag to enable artif icial organ evaluation in conscious, unrestrained pigs: technical note. Int J Artif Organs. 2000;23:268–274. [PubMed] [Google Scholar]
- 21.Nguyen HN, Silny J, Wuller S, Marschall HU, Rau G, Matern S. Chyme transport patterns in human duodenum, determined by multiple intraluminal impedance technique. Am J Physiol. 1995;268:G700–708. doi: 10.1152/ajpgi.1995.268.4.G700. [DOI] [PubMed] [Google Scholar]
- 22.Fuchs KH, Maroske J, Tigges H, et al. Perioperative motility of the foregut. In: Herbert MK, Holzer P, Roewer N, eds. Problems of the Gastrointestinal Tract in Anesthesia, the Perioperative Period, and Intensive Care. Berlin, Springer, 1999:215–226.
- 23.Ruckenbusch Y, Bueno L. The effect of feeding on the motility of the stomach and small intestine in the pig. Br J Nutr. 1976;35:397–405. doi: 10.1079/bjn19760045. [DOI] [PubMed] [Google Scholar]
- 24.Chassard D, Lansiaux S, Duflo F, et al. Effects of subhypnotic doses of propofol on gastric emptying in volunteers. Anesthesiology. 2002;97:96–101. doi: 10.1097/00000542-200207000-00014. [DOI] [PubMed] [Google Scholar]
- 25.Savoye-Collet C, Savoye G, Smout A. Determinants of transpyloric fluid transport: a study using combined real-time ultrasound, manometry and impedance recording. Am J Physiol Gastrointest Liver Physiol. 2003;285:G1147–1152. doi: 10.1152/ajpgi.00208.2003. [DOI] [PubMed] [Google Scholar]
- 26.Nguyen HN, Silny J, Wuller S, Marschall HU, Rau G, Matern S. Abnormal postprandial duodenal chyme transport in patients with long standing insulin dependent diabetes mellitus. Gut. 1997;41:624–631. doi: 10.1136/gut.41.5.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fleckenstein P, Bueno L, Fioramonti J, Ruckebusch Y. Minute rhythm of electrical spike bursts of the small intestine in different species. Am J Physiol. 1982;242:G654–659. doi: 10.1152/ajpgi.1982.242.6.G654. [DOI] [PubMed] [Google Scholar]
- 28.Kimura A, Sato A, Sato Y, Trzebski A. Role of the central and arterial chemoreceptors in the response of gastric tone and motility to hypoxia, hypercapnia and hypocapnia in rats. J Auton Nerv Syst. 1993;45:77–85. doi: 10.1016/0165-1838(93)90363-y. [DOI] [PubMed] [Google Scholar]
- 29.Kararli TT. Comparison of the gastrointestinal anatomy, physiology, and biochemistry of humans and commonly used laboratory animals. Biopharm Drug Dispos. 1995;16:351–380. doi: 10.1002/bdd.2510160502. [DOI] [PubMed] [Google Scholar]
- 30.Bozkurt A, Deniz M, Yegen BC. Cefaclor, a cephalosporin antibiotic, delays gastric emptying rate by a CCK-A receptor-mediated mechanism in the rat. Br J Pharmacol. 2000;131:399–404. doi: 10.1038/sj.bjp.0703585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin HC. Dissociative Anesthetics. In: Thurmon JC, Tranquilli WJ, Benson J, eds. Lumb & Jones’ Veterinary Anesthesia. 3rd ed. Philadelphia: Lippincott Williams & Wilkins, 1993:241–296.
- 32.Rupp S, Schroth HJ, Hildebrandt U, Garth H, Feifel G. The effect of metamizole on gastric emptying and small intestine transit in the rat. (Article in German) Arzneimittelforschung. 1987;37:1051–1053. [PubMed] [Google Scholar]


