Abstract
Selective membrane fusion underlies subcellular compartmentation, cell growth, neurotransmission and hormone secretion. Its fundamental mechanisms are conserved among organelles, tissues and organisms. As befits a conserved process, reductionism led to its study in microorganisms. Homotypic fusion of the vacuole of Saccharomyces cerevisiae is particularly accessible to study as vacuoles are readily visualized, there is a rapid and quantitative in vitro assay of vacuole fusion, and the genetics and genomics of this organism and of vacuole fusion are highly advanced. Recent progress is reviewed in the context of general questions in the membrane fusion field.
Keywords: GTPases/phosphoinositides/SNARES
Introduction
Regulated membrane fusion has received intensive study. For example, vesicles with neurotransmitters cluster at the active zone of synapses, awaiting a voltage-triggered calcium flux to fuse and release their contents into the synaptic cleft (Jahn and Sudhof, 1999). Study of this process has benefited from excellent electrophysiology and cytology as well as extensive pharmacology, including selective toxins. Biochemical definition of proteins in synaptic complexes has been complemented by their genetic study in mice, flies, worms and yeast. In vitro synaptic fusion has been elusive, though regulated secretion in semi-intact PC12 cells has many shared features with neurons, such as requirements for ATP, calcium, chaperones and phosphoinositides (Hay and Martin, 1992; Hay et al., 1995) and toxin sensitivities (Martin and Kowalchyk, 1997). Endosome fusion has yielded novel insights, and Golgi trafficking, the first in vitro membrane fusion reaction, defined the fundamental compartment-mixing assay of fusion (Balch et al., 1984) and led to the first isolation of functional fusion catalysts. The SEC (Novick and Schekman, 1979) and vacuole protein sorting (VPS; Rothman and Stevens, 1986; Banta et al., 1988) gene selections and development of in vitro assays for endoplasmic reticulum (ER) to Golgi traffic (Baker et al., 1988) have made yeast a premier organism for studying membrane fusion.
The shared features of these trafficking systems provide the basic outline of a conserved fusion mechanism. GTPases of the Rab/Ypt family are organelle-specific ‘switches’ which cycle between their active GTP and inactive GDP form (Novick and Zerial, 1997). Cycling is regulated by GTPase activating proteins (GAPs), promoting GTP hydrolysis, and guanine nucleotide exchange factors (GEFs), promoting exchange of GTP for GDP (see Table I for a glossary). GAPs and GEFs are themselves localized and regulated in their actions. Active (GTP)Rab/Ypt proteins bind organelle-specific ‘effector’ complexes, which allow them to initiate the docking of membranes prior to fusion (Stenmark et al., 1995; Horiuchi et al., 1997; Simonsen et al., 1998; Christoforidis et al., 1999a,b). SNAREs are a second conserved family of trafficking proteins. Their helical ‘SNARE motif’ segments can assemble into a stable four-helical ‘coiled-coil’ bundle (Sutton et al., 1998). SNAREs are anchored to membranes by a C-terminal apolar domain or by acyl derivatization. SNARE complexes form in cis, between SNAREs anchored to the same membrane, or in trans, between SNAREs anchored on the apposed membranes of docked organelles. SNARE complexes are disassembled by the ATPase NSF/Sec18p and its co-chaperone α-SNAP/Sec17p. Sec1 family proteins bind to SNAREs (Misura et al., 2000); their function, though essential, is not fully understood. For all membrane fusion reactions, localized transient calcium fluxes trigger fusion by calcium activation of target proteins (Burgoyne and Morgan, 1998). Where examined, membrane fusion also requires inositol phosphatides that function as signaling molecules (Sullivan et al., 1993) or bind proteins to the membrane (Burd and Emr, 1998).
Table I. Glossary.
| Definition | |
|---|---|
| Arp2/3 complex | regulated by Bee1/Las17/WASp and triggers actin polymerization |
| Bee1/Las17p | the yeast homolog of WASp; regulates actin polymerization |
| Cdc42p | a Rho-class GTPase that regulates actin polymerization and other processes; is needed for vacuole fusion |
| Gdi1p | extracts Ypt7p and other Ypt/Rab proteins from the membrane when they are bound to GDP |
| HOPS complex | homotypic fusion and vacuole protein sorting complex (also called Class C VPS complex) includes Vps 11, 16, 18, 33, 39 and 41p. Vps33 is a Sec1p-like SNARE binding protein, Vps39p is a guanine nucleotide exchange factor for Ypt7p |
| LMA1 and 2 | low Mr activities which interact with several catalysts of vacuole fusion |
| Nyv1p | a vacuolar v-SNARE |
| Rho1p | a Rho class GTPase required for vacuole fusion. |
| SNAREs | form four helix coiled coils, in cis (on the same membrane) or in trans (on opposed, docked membranes) |
| SEC genes | encode proteins of the secretory pathway |
| Sec17p (yeast α-SNAP) | the co-chaperone of Sec18p |
| Sec18p (yeast NSF) | an ATP-driven chaperone that disassembles cis-SNARE complexes |
| V1V0 | the vacuolar ATP-driven proton pump, consisting of V1 (peripheral) domain and V0 (integral) domain |
| VAC genes | required for vacuole inheritance. Vac8p is also needed for fusion. |
| VAM genes | required for low copy number of vacuoles |
| Vam3p | the vacuolar t-SNARE |
| Vam7p | the vacuolar homolog of neuronal SNAREs SNAP23 and SNAP 25; it is a peripheral membrane protein |
| VPS genes | encode proteins needed for sorting between the Golgi, endosome and vacuole |
| VTC | vacuolar transporter chaperone, a four-membered complex (Vtc1-4) that links the stages of the vacuole fusion reaction |
| Vti1p | a v-SNARE of the vacuole and other organelles |
| Ykt6p | a v-SNARE of the vacuole and other organelles |
| Ypt/Rab GTPases | regulate trafficking as they cycle between GTP and GDP bound forms. Ypt7p is vacuolar |
Each fusion event occurs in three stages: (i) priming, the ATP-driven modification of the association status of SNAREs, Rabs and effector complexes, (ii) docking, initiated by Rab/Ypt and its effectors and leading to trans-SNARE pairing and (iii) membrane fusion, initiated by calcium flux and culminating in the fusion of membrane bilayers and mixing of lumenal contents. For different fusion reactions, priming may preceed docking, as for yeast vacuole fusion, or follow it, as for neural and PC12 cell exocytosis or trafficking from the ER to Golgi in yeast (Barlowe, 1997).
Despite the substantial progress in understanding membrane fusion, the catalysts of each reaction stage and the connections between their actions remain unclear. To paraphrase Efraim Racker, ‘If you’re not confused by this problem, you just don’t understand it.’ This review presents recent progress in dissecting one membrane fusion event, the homotypic fusion of vacuoles from S.cerevisiae and a perspective on how we may progress from here.
Homotypic fusion of yeast vacuoles
Yeast vacuoles undergo constant fission and fusion in the cell, with a steady-state of 1–5 vacuoles in many strains. Purified vacuoles will fuse when incubated with ATP. Vacuoles are advantageous for studying membrane fusion: (i) they are readily visualized in cells and can be isolated in high purity; (ii) the genetics of trafficking to vacuoles (VPS genes; Rothman and Stevens, 1986; Banta et al., 1988; Wendland et al., 1998) and maintaining low vacuole copy number (vacuole morphology, VAM genes; Wada et al., 1992) is well-developed, with considerable overlap between the processes of heterotypic trafficking from endosome to vacuole and homotypic vacuole fusion; and (iii) vacuoles can be purified in 100 mg lots, stored frozen and used in rapid, colorimetric fusion assays. This assay has been particularly amenable to staging and biochemical dissection.
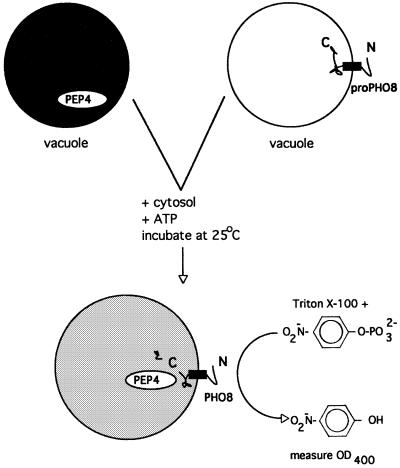
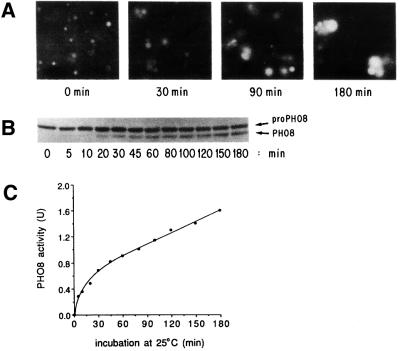
To assay fusion (Haas et al., 1994), vacuoles are isolated from two yeast strains, one with normal vacuole proteases but deleted for the PHO8 gene encoding the major phosphatase and the other deleted for vacuolar proteases and hence bearing the catalytically-inactive pro-Pho8p (Figure 1). Neither population of purified vacuoles has phosphatase activity. Upon fusion in vitro, the proteases gain access to the pro-Pho8p and convert it to the catalytically active form that can be assayed colorimetrically. Microscope observation of aliquots removed at various times from one fusion reaction shows the formation of vacuole clusters. Large, fused vacuoles form (Figure 2A) as immunoblots of the same aliquots reveals the processing of pro-Pho8p to Pho8p (Figure 2B) and a catalytic assay shows increased phosphatase activity (Figure 2C). Freshly prepared vacuoles only need ATP to fuse, though fusion needs many peripheral membrane proteins which may be lost with time or exposure to salt solutions. Biochemical and genetic studies have revealed that this is a complex and highly ordered reaction (Figure 3).
Fig. 1. Assay of homotypic vacuole fusion. Reproduced with permission from J. Cell Biol. (1994), 126, 87–97.
Fig. 2. Three assays of the kinetics of vacuole docking and fusion. All samples, withdrawn at times shown, were from one in vitro vacuole fusion reaction. Reproduced with permission from J. Cell Biol. (1994), 126, 87–97.
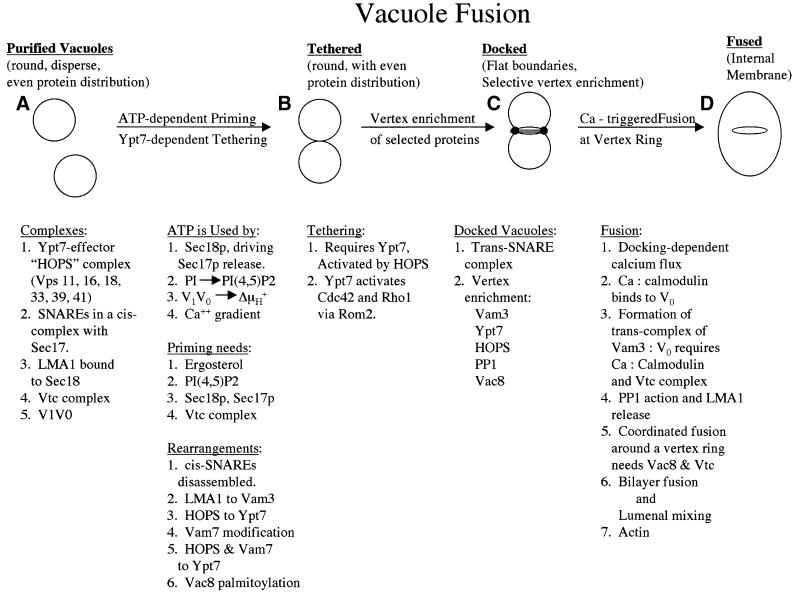
Fig. 3. Vacuole homotypic fusion. See text for details.
Although isolated vacuoles just look like round bubbles (Figure 3A), appearances of simplicity are deceiving! Some of the proteins needed for fusion are present on the vacuole surface in oligomeric complexes. For example, the SNARE proteins Vam3p (t-SNARE), Vam7p (SNAP- 25 homolog), and Nyv1p, Vti1p, and Ykt6p (v-SNAREs) are present in cis-complexes along with Sec17p, yeast α-SNAP (Ungermann and Wickner, 1998; Ungermann et al., 1998a, 1999a). The hexameric HOPS (homotypic vacuole fusion and protein sorting)/Class C VPS complex (Price et al., 2000b; Sato et al., 2000; Seals et al., 2000), consisting of Vps 11, 16, 18, 33, 39 and 41 proteins, is part of a 65S complex on the vacuole surface (Nakamura et al., 1997; Price et al., 2000b); the other members of this 65S complex are unknown. The vacuole transporter chaperone (Vtc) tetrameric complex (Cohen et al., 1999; Murray and Johnson, 2000) regulates V1V0 association and is intimately involved in each stage of vacuole fusion (Muller et al., 2002; O.Muller, M.J.Bayer and A.Mayer, submitted). The initial associations of other proteins of the vacuole fusion reaction, such as Ypt7p, remain to be explored.
The initial priming stage of the reaction (Figure 3A and B) needs ATP and physiological ionic strength and temperature (Conradt et al., 1994). It is a prerequisite for productive docking (Mayer and Wickner, 1997). Sec18p hydrolyzes ATP, driving Sec17p release (Mayer et al., 1996) and the disassembly of the cis-SNARE complex (Ungermann et al., 1998a). This reaction requires both phosphatidylinositol (4,5) bisphosphate [PI(4,5)P2] (Mayer et al., 2000) and ergosterol (Kato and Wickner, 2001); since Sec18p can catalyze Sec17p release from lecithin-liposomes (Sato and Wickner, 1998), ergosterol and PI(4,5)P2 may be needed for their interactions with other proteins such as the cis-SNARE complex or might form an essential lipid phase. LMA1 (low Mr activity 1), a co-chaperone that is initially bound to Sec18p, transfers to the SNARE Vam3p, which it stabilizes (Xu et al., 1997, 1998). The HOPS complex activates Ypt7p (Wurmser et al., 2000) and remains bound as a Ypt7p effector (Seals et al., 2000). It is not known how HOPS is bound to the vacuole before it reaches Ypt7p nor do we know what triggers its transfer to Ypt7p. Vam7p also depends on Ypt7p for its continued vacuole association (Ungermann et al., 2000) and may interact directly with this GTPase, as suggested by two-hybrid analysis (Uetz et al., 2000). Both Ypt7p (Ungermann et al., 2000) and phosphatidylinositol 3-phosphate (Cheever et al., 2001) are needed to keep Vam7p on the vacuole, though the relationship between these is not known. Priming also triggers acylation of Vac8p (Veit et al., 2001), which is later required in the reaction for fusion (Y.-X.Wang et al., 2001).
Primed vacuoles associate reversibly in a ‘tethering’ reaction (Figure 3B; Ungermann et al., 1998b), followed by stable docking (Figure 3C). Tethering requires activated Ypt7p and HOPS (Price et al., 2000a,b), though other factors may be necessary. Ypt7p activation is needed for two subsequent docking steps, the activation of two Rho GTPases, Rho1p and Cdc42p (Muller et al., 2001; Eitzen et al., 2001), and the formation of trans-SNARE pairs (Ungermann et al., 1998b). Trans-pairing of SNAREs has been demonstrated for docked vacuoles (Ungermann et al., 1998b) and only involves a small percent of the vacuole SNARE proteins (Ungermann et al., 1998b; Wang et al., 2002). The composition of the trans-SNARE complex, its locale on docked vacuoles (see below), the catalysis of its formation and its subsequent actions all await further studies. Docking requires vacuole acidification (Ungermann et al., 1999b) and PI(4,5)P2 (Mayer et al., 2000; also see below), though the molecular functions of each are unclear.
Docking causes a measurable release of calcium from the vacuole (Peters and Mayer, 1998). Calcium activates calmodulin to bind to V0, the integral domain of the vacuolar H+-ATPase, triggering trans-complex formation between V0 complexes on apposed vacuoles (Peters et al., 2001). This trans-V0 complex contains the t-SNARE Vam3p but not the v-SNARE Nyv1p. Fusion is triggered by the action of protein phosphatase 1 (Conradt et al., 1994; Peters et al., 1999), though its phosphoprotein target and the opposing kinase, are unknown. Protein phosphatase 1 action triggers the release of LMA1 from the vacuoles prior to membrane fusion and contents mixing (Xu et al., 1998). The relative roles of trans-SNARE pairs and trans-V0 complexes in catalyzing the final steps of fusion are the subject of intense experimentation and debate.
Recent advances
Genomics
Our knowledge of this, or other, membrane fusion pathways seems less like the coupled, sequential steps of a metabolic pathway than the fragmented understanding of a play where only some of the actors are visible on the stage, yet each speaks their lines. One path towards a more complete compilation of the actors in the fusion play was pioneered by Wada et al. (1992), who screened a collection of mutagenized yeast for those with abnormal vacuole morphology (vam phenotype), specifically those with highly fragmented vacuoles. Vacuole fragmentation suggests a defect in fusion and indeed, each of the nine genes identified in their non-saturated screen was directly involved in the fusion reaction. We screened a commercially available collection of 4828 yeast strains, each bearing a deletion in a known non-essential gene, for the vam phenotype of fragmented vacuoles. In addition to known catalysts of the vacuole fusion reaction, new genes were identified where deletion yields a vam phenotype (Seeley et al., 2002). These included open reading frames of utterly unknown function, but also genes of lipid metabolism, GTPases and effectors, cytoskeletal proteins, kinases and phosphatases, and other trafficking proteins. Though each of the nine VAM genes of Wada et al. (1992) were also VPS genes (needed for biosynthetic sorting of proteins to the vacuoles), our deletion screen only uncovered a few new VPS genes, showing that the sets of proteins catalyzing trafficking to the vacuole and vacuole fusion have only partial overlap. In this vam screen, we discovered that ergosterol is needed for vacuole priming (Kato and Wickner, 2001). We also find that the turnover of vacuolar PI(4,5)P2, by phosphatases and phospholipase C, is essential for the fusion reaction. Inhibitor studies suggest that inositol tris-phosphate or a derivative might function to activate a calcium channel, though yeast has no obvious homolog to the mammalian IP3-activated calcium transporters.
Evaluation of each new VAM gene will permit an evaluation of whether it has a direct role in fusion. Other proteins, which are essential for cell growth and which participate in vacuole fusion, may be revealed by their genetic, two-hybrid and proteomic relationships to the non-essential VAM genes as well as by biochemical fractionation of the in vitro reaction.
Vac8p
Originally found as a protein needed for vacuole inheritance (Pan and Goldfarb, 1998; Wang et al., 1998), Vac8p has been found to cluster at docking sites between vacuoles, and between vacuoles and the nucleus (Pan et al., 2000). It has recently been shown that a portion of Vac8p is palmitoylated during priming (Veit et al., 2001) and functions during membrane fusion per se (Y.-X.Wang et al., 2001). Its functional role is unknown, but its many armadillo repeats suggest homo- or hetero-oligomerization.
Rho GTPases and actin
In addition to the Ypt7p Rab-GTPase, two Rho GTPases, Rho1p and Cdc42p, are required for the docking stage of fusion (Eitzen et al., 2001; Muller et al., 2001). Rho GTPases regulate actin cytoskeleton, yet the in vitro fusion of purified vacuoles does not require cytosol. Never theless, purified vacuoles have bound G actin, which undergoes polymerization to F actin during the fusion reaction. The fusion reaction is sensitive to latrunculin B and jasplakinolide, two actin-directed drugs, and fusion is defective with vacuoles from actin mutant strains (G.Eitzen, unpublished observations). The deletion screen and two-hybrid analysis trace the relationships between Cdc42p, Cla4p, Vrp1p, PI(4,5)P2, the Arp 2/3 complex, and actin, but biochemical experiments will be needed to fully establish this pathway. The function of vacuolar actin is unknown, but might regulate protein localization on the vacuole during docking (below). While PI(4,5)P2 regulates the activity of the Bee1p–Vrp1p complex (Higgs and Pollard, 2001), it is unclear whether Rho GTPases might regulate phosphoinositide metabolism, which would connect a pathway from priming to the docking-dependent calcium flux.
The VTC complex
What factors connect the proteins at each stage of the reaction? Four VTC genes encode proteins that are found in the vacuole membrane. The VTC proteins are in direct physical association with V0 and Nyv1p and have genetic associations with V1V0 ATPase (Cohen et al., 1999; Murray and Johnson, 2000; Muller et al., 2002; O.Muller, M.J.Bayer and A.Mayer, submitted). Vtc1p and Vtc4p are needed for each aspect of priming, while Vtc3p is needed for LMA1 release and trans-V0 complex disassembly in the very terminal phases of the membrane fusion reactions (Muller et al., 2002; O.Muller, M.J.Bayer and A.Mayer, submitted).
Vam3p N-domain
Though Vam3p is homologous to other syntaxins, recent structural studies have shown that its N-terminal domain does not form a ‘closed conformation’ with its SNARE domain (Dulubova et al., 2001). Indeed, though deletion of this N-terminal domain has no obvious effect on vacuole structure in vivo or on in vitro rates of vacuole fusion, the SNARE domain of Vam3p has a crucial role in membrane fusion (Y.Wang et al., 2001).
Sub-organellar localization
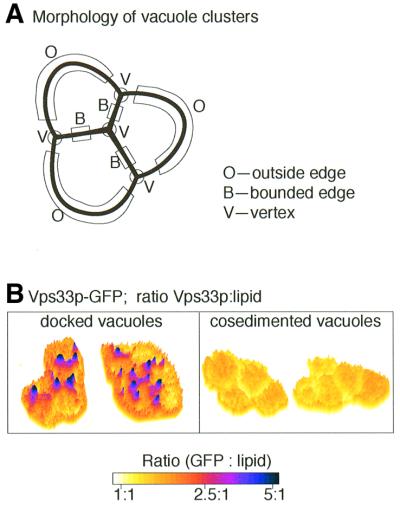
Proteins involved in the fusion process have been derivatized with green fluorescent protein (GFP), creating strains in which each GFP fusion protein is expressed at its normal level (Wang et al., 2002). This allows measurement of the relative molar abundance of each protein and its spatial distribution on docked vacuole clusters. For this purpose, the regions of vacuole membrane in a docked vacuole cluster are defined as outside edges (O), boundary membranes (B), where two vacuoles touch, and vertices (V), where boundary and outside regions end and meet (Figure 4A). Strikingly, ratiometric fluorescence microscopy, in which the ratio of a GFP-tagged protein to lipid is measured at each pixel of the image, reveals that Ypt7p and its effector HOPS complex accumulate at vertices (Figure 4B). Vac8p, the t-SNARE Vam3p and protein phosphatase 1 also accumulate at vertices. SNAREs must be released from their cis-associations for vertex enrichment to occur, showing that vertex enrichment is on the normal pathway to fusion. Since Ypt7p is almost 10 times as abundant as Vam3p, it may have a primary role in establishing these docking structures. Once docking has been established, extraction of Ypt7p leaves HOPS and Vam3p at the vertices, in accordance with biochemical findings that Ypt7p is no longer needed once docking is complete. The enrichment of Vac8p, Vam3p and protein phosphatase 1 at the vertices, three proteins involved in fusion per se, suggests that this is where fusion is initiated. Indeed, fluorescence microscopy of vacuoles in the in vitro reaction or in living cells (Wang et al., 2002) shows that fusion occurs at vertices, leaving a small fragment of membrane inside the fused vacuole. Further characterization of this vertex complex should shed light on its role in docking and fusion.
Fig. 4. Vertex enrichment of selected proteins during docking. (A) Membrane microdomains of docked vacuoles are defined as ‘outside edges’, which are not in contact with other vacuoles, ‘boundary edges’, which are opposed to other vacuoles in the cluster, and ‘vertices’, where boundary edge and outside edge membranes meet. (B) Docking-dependent enrichment of Vps33p at vertices. Left panel: purified vacuoles from cells with GFP-tagged Vps33p were stained with FM4-64, incubated in a standard fusion reaction for 30 min to allow docking and examined by ratiometric fluorescence microscopy to determine regions of membrane where tagged Vps33p is enriched. Right panel: vacuoles were not incubated under docking and fusion conditons with ATP, but rather were clustered by co-sedimentation. Reprinted with permission from Elsevier Science from L.Wang et al. (2002).
Current ‘controversies’
It remains unclear which proteins actually catalyze the last stage of the reaction, the fusion of membranes and contents mixing; there are a surfeit of candidates! Sec18p/NSF and Sec17p/α-SNAP can drive the fusion of lipsomes (Otter-Nilsson et al., 1999; Brugger et al., 2000), but act on purified vacuoles well before fusion, to separate cis-SNARE complexes (Mayer et al., 1996). Calcium can trigger the fusion of lipid bilayers directly (Wilschut et al., 1980) and indeed triggers biological membrane fusion reactions, yet its physiological effects are clearly protein-mediated. Might the proteins serve to deliver calcium to an active site where it could be a crucial and catalytic element, much as zinc functions for DNA polymerases? SNAREs can catalyze liposome fusion (Weber et al., 1998), yet their pairing in trans seems dispensible for fusion per se (Ungermann et al., 1998b). The V0 domain of the vacuolar ATPase serves as the membrane receptor for calcium/calmodulin (Peters et al., 2001), which triggers the formation of trans-complexes containing V0 and the t-SNARE Vam3p (but not the other SNAREs). V0 exhibits altered permeability when calcium/calmodulin binds and this may correspond to the expansion of a fusion pore (Peters et al., 2001).
How will the ‘real’ fusion machinery be established? Membrane fusion requires proximity (docking) and the imposition of strain on the bilayer. Though tightly regulated in the cell to prevent compartment mixing, liposome association and bilayer strain are all too readily achieved in model reactions. It will be essential to complete the task of identifying the proteins and lipids that catalyze each stage of vacuole fusion, place them in their cascades of association and order of catalytic actions, and gain mechanistic understanding of their relationships to each other.
Prospects
The stage is set for evaluating each protein of the vam deletion screen and for a systematic search for vacuole fusion catalysts that are essential for cell viability and thus were not in that screen. The connections of each subreaction will be sought: what are the initial associations of Ypt7p, HOPS and Vac8p, what are the constituents of the cis-SNARE complex, does the ATPase domain of Vps33p participate in priming, what are the roles of inositol phosphatides and ergosterol and are they enriched at vertices, what are the cascades of physical interaction during docking, what assembles proteins at vertices, how are actin filaments participating in the reaction, what is the docking-dependent calcium channel, and what are the actions of trans-SNARE pairs and trans-V0Vam3– V0Vam3 pairs? The ease of combining enzymology, genetics, genomics and cytology in the study of vacuole fusion make it a promising avenue towards finally understanding regulated membrane fusion in all its glorious complexity.
Acknowledgments
Acknowledgements
Work in my laboratory has been supported by the National Institute of General Medical Sciences and by the Human Frontiers Science Program.
References
- Baker D., Hicke,L., Rexach,M., Schleyer,M. and Schekman,R. (1988) Reconstitution of SEC gene product-dependent intercompartmental protein transport. Cell, 54, 335–344. [DOI] [PubMed] [Google Scholar]
- Balch W.E., Glick,B.S. and Rothman,J.E. (1984) Sequential inter mediates in the pathway of intercompartmental transport in a cell-free system. Cell, 39, 525–536. [DOI] [PubMed] [Google Scholar]
- Banta L.M., Robinson,J.S., Klionsky,D.J. and Emr,S.D. (1988) Organelle assembly in yeast: characterization of yeast mutants defective in vacuolar biogenesis and protein sorting. J. Cell Biol., 107, 1369–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlowe C. (1997) Coupled ER to Golgi transport reconstituted with purified cytosolic proteins. J. Cell Biol., 139, 1097–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugger B., Nickel,W., Weber,T., Parlati,F., McNew,J.A., Rothman,J.E. and Sollner,T. (2000) Putative fusogenic activity of NSF is restricted to a lipid mixture whose coalescence is also triggered by other factors. EMBO J., 19, 1272–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burd C.G. and Emr,S.D. (1998) Phosphatidylinositol (3)-phosphate signaling mediated by specific binding to RING FYVE domains. Mol. Cell, 2, 157–162. [DOI] [PubMed] [Google Scholar]
- Burgoyne R.D. and Morgan,A. (1998) Calcium sensors in regulated exocytosis. Cell Calcium, 24, 367–376. [DOI] [PubMed] [Google Scholar]
- Cheever M.L., Sato,T.K., de Beer,T., Kutateladze,T.G., Emr,S.D. and Overduin,M. (2001) Phox domain interaction with PtdIns(3)P targets the Vam7 t-SNARE to vacuole membranes. Nature Cell Biol., 3, 613–618. [DOI] [PubMed] [Google Scholar]
- Christoforidis S., McBride,H.M., Burgoyne,R.D. and Zerial,M. (1999a) The Rab5 effector EEA1 is a core component of endosome docking. Nature, 397, 621–625. [DOI] [PubMed] [Google Scholar]
- Christoforidis S., Miaczynska,M., Ashman,K., Wilm,M., Zhao,L., Yip,S.-C., Waterfield,M.D., Backer,J.M. and Zerial,M. (1999b) Phosphatidylinositol-3-OH kinases are Rab5 effectors. Nature Cell Biol., 1, 249–252. [DOI] [PubMed] [Google Scholar]
- Cohen A., Perzov,N., Nelson,H. and Nelson,N. (1999) A novel family of yeast chaperons involved in the distribution of V-ATPase and other membrane proteins. J. Biol. Chem., 274, 26885–26893. [DOI] [PubMed] [Google Scholar]
- Conradt B., Haas,A. and Wickner,W. (1994) Determination of four biochemically distinct, sequential stages during vacuole inheritance in vitro. J. Cell Biol., 126, 99–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulubova I., Yamaguchi,T., Wang,Y., Sudhof,T.C. and Rizo,J. (2001) Vam3p structure reveals conserved and divergent properties of syntaxins. Nature Struct. Biol., 8, 258–264. [DOI] [PubMed] [Google Scholar]
- Eitzen G., Thorngren,N. and Wickner,W. (2001) Rho1p and Cdc42p act after Ypt7p to regulate vacuole docking and fusion. EMBO J., 20, 5650–5656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas A., Conradt,B. and Wickner,W. (1994) G-protein ligands inhibit in vitro reactions of vacuole inheritance. J. Cell Biol., 126, 87–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay J.C. and Martin,T.F.J. (1992) Resolution of regulated secretion into sequential MgATP-dependent and calcium-dependent stages mediated by distinct cytosolic components. J. Cell Biol., 119, 139–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay J.C., Fisette,P.L., Jenkins,G.H., Fukami,K., Takenawa,T., Anderson, R.A. and Martin,T.F.J. (1995) ATP-dependent inositide phosphorylation required for Ca2+-activated secretion. Nature, 374, 173–177. [DOI] [PubMed] [Google Scholar]
- Higgs H.N. and Pollard,T.D. (2001) Regulation of actin filament network formation through Arp 2/3 complex: activation by a diverse array of proteins. Annu. Rev. Biochem., 70, 649–676. [DOI] [PubMed] [Google Scholar]
- Horiuchi H. et al. (1997) A novel Rab5 GDP/GTP exchange factor complexed to rabaptin-5 links nucleotide exchange to effector recruitment and function. Cell, 90, 1149–1159. [DOI] [PubMed] [Google Scholar]
- Jahn R. and Sudhof,T.C. (1999) Membrane fusion and exocytosis. Annu. Rev. Biochem., 68, 863–911. [DOI] [PubMed] [Google Scholar]
- Kato M. and Wickner,W. (2001) Ergosterol is required for the Sec18p/ATP-dependent priming step of homotypic vacuole fusion. EMBO J., 20, 4253–4262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin T.F.J. and Kowalchyk,J.A. (1997) Docked secretory vesicles undergo Ca2+-activated exocytosis in a cell-free system. J. Biol. Chem., 272, 14447–14453. [DOI] [PubMed] [Google Scholar]
- Mayer A. and Wickner,W. (1997) Docking of yeast vacuoles is catalyzed by the Ras-like GTPase Ypt7p after symmetric priming by Sec18p (NSF). J. Cell Biol., 136, 307–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer A., Wickner,W. and Haas,A. (1996) Sec18p (NSF)-driven release of Sec17p (α-SNAP) can precede docking and fusion of yeast vacuoles. Cell, 85, 83–94. [DOI] [PubMed] [Google Scholar]
- Mayer A., Scheglmann,D., Dove,S., Glatz,A., Wickner,W. and Haas,A. (2000) Phosphatidylinositol-(4,5)-bisphosphate regulates two steps of homotypic vacuole fusion. Mol. Biol. Cell, 11, 807–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misura K.M.S., Scheller,R.H. and Wels,W.I. (2000) Three-dimensional structure of the neuronal-Sec1-syntaxin 1a complex. Nature, 404, 355–362. [DOI] [PubMed] [Google Scholar]
- Muller O., Johnson,D.I. and Mayer,A. (2001) Cdc42p functions at the docking stage of intracellular membrane fusion. EMBO J., 20, 5657–5665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller O., Bayer,M.J., Peters,C., Andersen,J.S., Mann,M. and Mayer,A. (2002) The VTC proteins in vacuole fusion: coupling NSF activity to V0trans-complex formation. EMBO J., 21, 259–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray J.M. and Johnson,D.I. (2000) Isolation and characterization of Nrf1p, a novel negative regulator of the Cdc42p GTPase in Schizosaccharomyces pombe. Genetics, 154, 155–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura N., Hirata,A., Ohsumi,Y. and Wada,Y. (1997) Vam2/Vps41p and Vam6/Vps39p are components of a protein complex on the vacuolar membrane and involved in the vacuolar assembly in the yeast Saccharomyces cerevisiae. J. Biol. Chem., 272, 11344–11349. [DOI] [PubMed] [Google Scholar]
- Novick P. and Schekman,R. (1979) Secretion and cell-surface growth are blocked in a temperature-sensitive mutant of Saccharomyces cerevisiae. Proc. Natl Acad. Sci. USA, 76, 1858–1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick P. and Zerial,M. (1997) The diversity of Rab proteins in vesicled transport. Curr. Opin. Cell Biol., 9, 496–504. [DOI] [PubMed] [Google Scholar]
- Otter-Nilsson M., Hendriks,R., Pecheur-Huet,E.-I., Hoekstra,D. and Nilsson,T. (1999) Cytosolic ATPases, p97 and NSF, are sufficient to mediate rapid membrane fusion. EMBO J., 18, 2074–2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan X. and Goldfarb,D.S. (1998) YEB3/VAC8 encodes a myristylated armadillo protein of the Saccharomyces cerevisiae vacuolar membrane that functions in vacuole fusion and inheritance. J. Cell Sci., 111, 2137–2147. [DOI] [PubMed] [Google Scholar]
- Pan X., Roberts,P., Chen,Y., Kvam,E., Shulga,N., Huang,K., Lemmon,S. and Goldfarb,D.S. (2000) Nucleus–vacuole junctions in Saccharomyces cerevisiae are formed through the direct interaction of Vac8p with Nvj1p. Mol. Biol. Cell, 11, 2445–2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters C. and Mayer,A. (1998) Ca2+/calmodulin signals the completion of docking and triggers a late step of vacuole fusion. Nature, 396, 575–580. [DOI] [PubMed] [Google Scholar]
- Peters C., Andrews,P.D., Stark,M.J.R., Cesaro-Tadic,S., Glatz,A., Podtelejnikov,A., Mann,M. and Mayer,A. (1999) Control of the terminal step of intracellular membrane fusion by protein phosphatase 1. Science, 285, 1084–1087. [DOI] [PubMed] [Google Scholar]
- Peters C., Bayer,M.J., Buhler,S., Andersen,J.S., Mann,M. and Mayer,A. (2001) Trans-complex formation by proteolipid channels in the terminal phase of membrane fusion. Nature, 409, 581–587. [DOI] [PubMed] [Google Scholar]
- Price A., Wickner,W. and Ungermann,C. (2000a) Vacuole protein sorting (VPS) proteins needed for transport vesicle budding from the Golgi are also required for the docking step of homotypic vacuole fusion. J. Cell Biol., 148, 1223–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price A., Seals,D., Wickner,W. and Ungermann,C. (2000b) The docking stage of yeast vacuole fusion requires the transfer of proteins from a cis-SNARE complex to a Rab/Ypt protein. J. Cell Biol., 148, 1231–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman J.H. and Stevens,T.H. (1986) Protein sorting in yeast: mutants defective in vacuole biogenesis mislocalize vacuolar proteins into the late secretory pathway. Cell, 47, 1041–1051. [DOI] [PubMed] [Google Scholar]
- Sato K. and Wickner,W. (1998) Detergent solubilization, purification, and functional reconstitution of an assembled vacuole v- and t-SNARE complex. Science, 281, 700–702. [DOI] [PubMed] [Google Scholar]
- Sato T.K., Rehling,P. and Emr,S.D. (2000) Class C Vps protein complex regulates vacuolar SNARE pairing and is required for vesicle docking/fusion. Mol. Cell, 6, 661–671. [DOI] [PubMed] [Google Scholar]
- Seals D., Eitzen,G., Margolis,N., Wickner,W. and Price,A. (2000) A Ypt/Rab effector complex containing the Sec1 homolog Vps33p is required for homotypic vacuole fusion. Proc. Natl Acad. Sci. USA, 97, 9402–9407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley E.S., Kato,M., Margolis,N., Wickner,W. and Eitzen,G. (2002) Genomic analysis of homotypic vacuole fusion. Mol. Biol. Cell, 13, 782–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonsen A. et al. (1998) EEA1 links PI(3)K function to Rab5 regulation of endosome fusion. Nature, 394, 494–498. [DOI] [PubMed] [Google Scholar]
- Stenmark H., Vitale,G., Ullrich,O. and Zerial,M. (1995) Rabaptin-5 is a direct effector of the small GTPase Rab5 in endocytic membrane fusion. Cell, 83, 423–432. [DOI] [PubMed] [Google Scholar]
- Sullivan K.M.C., Busa,W.B. and Wilson,K.L. (1993) Calcium mobilization is required for nuclear vesicle fusion in vitro: implications for membrane traffic and IP3 receptor function. Cell, 73, 1411–1422. [DOI] [PubMed] [Google Scholar]
- Sutton R.B., Fasshauer,D., Jahn,R. and Brunger,A.T. (1998) Crystal structure of a SNARE complex involved in synaptic exocytosis at 2.4Å resolution. Nature, 395, 347–353. [DOI] [PubMed] [Google Scholar]
- Uetz P. et al. (2000) A comprehensive analysis of protein–protein interactions in Saccharomyces cerevisiae. Nature, 403, 623–627. [DOI] [PubMed] [Google Scholar]
- Ungermann C. and Wickner,W. (1998) Vamp7, a vacuolar SNAP-25 homolog, is required for SNARE complex integrity and vacuole docking and fusion. EMBO J., 17, 3269–3276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungermann C., Nichols,B.J., Pelham,H.R.B. and Wickner,W. (1998a) A vacuolar v-t-SNARE complex, the predominant form in vivo and on isolated vacuoles, is disassembled and activated for docking and fusion. J. Cell Biol., 140, 61–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungermann C., Sato,K. and Wickner,W. (1998b) Defining the functions of trans-SNARE pairs. Nature, 396, 543–548. [DOI] [PubMed] [Google Scholar]
- Ungermann C., von Mollard,G.F., Jensen,O.N., Margolis,N., Stevens,T. and Wickner,W. (1999a) Three v-SNAREs and two t-SNAREs, present in a pentameric cis-SNARE complex on isolated vacuoles, are essential for homotypic fusion. J. Cell Biol., 145, 1435–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungermann C., Wickner,W. and Xu,Z. (1999b) Vacuole acidification is required for trans-SNARE pairing, LMA1 release and homotypic fusion. Proc. Natl Acad. Sci. USA, 96, 11194–11199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungermann C., Price,A. and Wickner,W. (2000) A new role for a SNARE protein as a regulator of the Ypt7 Rab-dependent stage of docking. Proc. Natl Acad. Sci. USA, 97, 8889–8891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veit M., Laage,R., Dietrich,L., Wang,L. and Ungermann,C. (2001) Vac8p release from the SNARE complex and its palmitoylation are coupled and essential for vacuole fusion. EMBO J., 20, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada Y., Ohsumi,Y. and Anraku,Y. (1992) Genes for directing vacuole morphogenesis in Saccharomyces cerevisiae. I. Isolation and characterization of two classes of vam mutants. J. Biol. Chem., 267, 18665–18670. [PubMed] [Google Scholar]
- Wang L., Seeley,E.S., Wickner,W. and Merz,A.J. (2002). Vacuole fusion at a ring of vertex docking sites leaves membrane fragments within the organelle. Cell, 108, 357–369. [DOI] [PubMed] [Google Scholar]
- Wang Y., Dulubova,I., Rizo,J. and Sudhof,T.C. (2001) Functional analysis of conserved structural elements in yeast syntaxin Vam3p. J. Biol. Chem., 276, 28598–28605. [DOI] [PubMed] [Google Scholar]
- Wang Y.-X., Catlett,N.L. and Weisman,L.S. (1998) Vac8p, a vacuolar protein with Armadillo repeats, functions in both vacuole inheritance and protein targeting from the cytoplasms to vacuole. J. Biol. Chem., 140, 1063–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y-X., Kauffman,E.J., Duex,J.E. and Weisman,L.S. (2001) Fusion of docked membranes requires the armadillo repeat protein Vac8p. J. Biol. Chem., 276, 35133–35140 [DOI] [PubMed] [Google Scholar]
- Weber T., Zemelman,B.V., McNew,J.A., Westermann,B., Gmachl,M., Parlati,F., Sollner,T.H. and Rothman,J.E. (1998) SNAREpins: minimal machinery for membrane fusion. Cell, 92, 759–772. [DOI] [PubMed] [Google Scholar]
- Wendland W., Emr,S.D. and Riezman,H. (1998) Protein traffic in the yeast endocytic and vacuolar protein sorting pathways. Curr. Opin. Cell Biol., 10, 513–522. [DOI] [PubMed] [Google Scholar]
- Wilschut J., Duzgunes,N., Fraley,R. and Papahadjopoulos,D. (1980) Kinetics of calcium ion induced fusion of phosphatidylserine vesicles followed by a new assay of mixing of aqueous vesicle contents. Biochemistry, 19, 6011–1021. [DOI] [PubMed] [Google Scholar]
- Wurmser A.E., Sato,T.K. and Emr,S.D. (2000) New component of the vacuolar class C Vps complex couples nucleotide exchange on the Ypt7 GTPase to SNARE-dependent docking/fusion. J. Cell Biol., 151, 551–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z., Mayer,A., Muller,E. and Wickner,W. (1997) A heterodimer of thioredoxin and I2B cooperates with Sec18p (NSF) to promote yeast vacuole inheritance. J. Cell Biol., 136, 299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z., Sato,K. and Wickner,W. (1998) LMA1 binds to vacuoles at Sec18p (NSF), transfers upon ATP hydrolysis to a t-SNARE (Vam3p) complex and is released during fusion. Cell, 93, 1125–1134. [DOI] [PubMed] [Google Scholar]