Abstract
Plasmodium sporozoites, the transmission form of the malaria parasite, successively invade salivary glands in the mosquito vector and the liver in the mammalian host. Sporozoite capacity to invade host cells is mechanistically related to their ability to glide on solid substrates, both activities depending on the transmembrane protein TRAP. Here, we show that loss-of- function mutations in two adhesive modules of the TRAP ectodomain, an integrin-like A-domain and a thrombospondin type I repeat, specifically decrease sporozoite invasion of host cells but do not affect sporozoite gliding and adhesion to cells. Irrespective of the target cell, i.e. in mosquitoes, rodents and cultured human or hamster cells, sporozoites bearing mutations in one module are less invasive, while those bearing mutations in both modules are non-invasive. In Chinese hamster ovary cells, the TRAP modules interact with distinct cell receptors during sporozoite invasion, and thus act as independently active pass keys. As these modules are also present in other members of the TRAP family of proteins in Apicomplexa, they may account for the capacity of these parasites to enter many cell types of phylogenetically distant origins.
Keywords: A-domain/cell invasion/Plasmodium/thrombospondin type I repeat/TRAP
Introduction
Apicomplexa are unicellular eukaryotes that parasitize a wide range of vertebrate and invertebrate hosts. They constitute one of the largest phyla of protozoans and contain members of major medical importance, such as Plasmodium, the causative agent of malaria, Toxoplasma and Cryptosporidium. Apicomplexa are defined by the highly polarized extracellular stages they generate (zoites), which contain a set of specialized secretory vesicles (micronemes and rhoptries). These vesicles secrete their contents at the zoite anterior tip and play a key role in attachment to and invasion of target cells. They are also involved in a unique behavior of apicomplexan zoites, called gliding motility, a substrate-dependent type of locomotion that occurs without any change in the cell shape.
Numerous lines of evidence indicate that both forward gliding on a substrate and active penetration into a cell result from backward capping of parasite surface molecules that are attached to the substrate or to host membrane receptors (reviewed in Dubremetz et al., 1998; Ménard, 2001). During gliding locomotion, zoites leave a trail of surface-associated proteins on the substrate. During cell invasion, an intimate junction forms between the zoite anterior end and the cell surface, and moves backwards as the zoite penetrates into a nascent vacuole. Although parasite actin polymers and/or polymerization are known to be central to both gliding locomotion and host cell invasion by Apicomplexa (Dobrowolski and Sibley, 1996), an uncharacterized motor is probably required for powering these processes.
Sporozoites of Plasmodium are transmitted by the mosquito vector to the mammalian host and need to invade multiple cell types during their life. They traverse the secretory cells in the mosquito salivary glands to reach the salivary duct, and once inoculated into the mammalian host, invade hepatocytes where they differentiate into the next parasite stage. Studies in Plasmodium berghei, a species that infects rodents, have shown that the sporozoite-specific transmembrane protein TRAP (thrombospondin-related anonymous protein) is essential for sporozoite gliding, cell invasion and in vivo infectivity (Sultan et al., 1997). TRAP is stored within micronemes (Rogers et al., 1992), becomes surface-exposed at the sporozoite anterior tip, particularly upon binding to host cells (Gantt et al., 2000), and is released onto the substrate during gliding locomotion (Kappe et al., 1999). Genetic alterations of the TRAP cytoplasmic tail either abrogate or modify sporozoite gliding patterns, suggesting that TRAP binds a cytoplasmic motor system (Kappe et al., 1999).
TRAP contains in its extracellular portion two long-studied adhesive modules, an A-domain of von Willebrand factor (Girma et al., 1987) and a thrombospondin type I repeat (TSR; Lawler and Hynes, 1986). A-domains are found in mammalian adhesion proteins such as integrin α-chains, numerous collagen types and matrilins. TSRs are present in very diverse proteins, including properdin, complement components, a variety of neural adhesion glycoproteins and the CS protein of Plasmodium sporozoites. In addition, combinations of A-domain(s) and TSRs are found in micronemal transmembrane proteins described in many apicomplexan genera. Independent lines of evidence suggest that these proteins have similar functions to TRAP (Carruthers and Sibley, 1999; Dessens et al., 1999; Kappe et al., 1999; Yuda et al., 1999; Templeton et al., 2000). Here, we address the contribution of the A-domain and TSR of TRAP to gliding motility and cell invasion of Plasmodium sporozoites.
Results
Rationale of mutations in the TRAP ectodomain
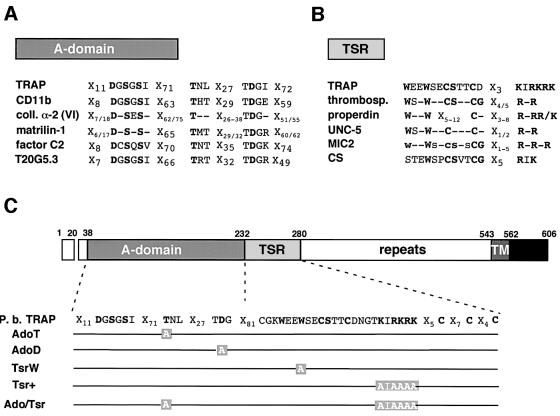
Crystallized A-domains from integrin α-chains possess a metal-binding site on the upper face of their characteristic ‘Rossman’ fold (Lee et al., 1995; Qu and Leahy, 1995; Emsley et al., 1997; Huizinga et al., 1997; Emsley et al., 2000). This site, named the metal ion-dependent adhesion site (MIDAS), is implicated in ligand binding and integrin activation (Michishita et al., 1993; Lee et al., 1995). The MIDAS contains five invariant residues scattered over three loops that coordinate a divalent cation (Mg2+ or Mn2+) an aspartate and two serines present in a DXSXS sequence, a threonine and another aspartate (Figure 1A). Substitution of either of these residues in α-chains of Mac-1 (Michishita et al., 1993), LFA-1 (Kamata et al., 1995), VLA-1 (Kern et al., 1994) and VLA-2 (Kamata and Takada, 1994) abrogates cation coordination and A-domain binding to all its known ligands, without affecting A-domain folding. Since these residues are conserved in the TRAP A-domain of all Plasmodium species (Robson et al., 1997; Templeton and Kaslow, 1997), we independently substituted the conserved threonine (Thr126) and second aspartate (Asp157) with alanine residues, creating A-domain variants AdoT and AdoD, respectively (Figure 1C). These variants were predicted to be phenotypically indistinguishable and to have lost A-domain mediated adhesive functions.
Fig. 1. Conserved residues in A-domain- and TSR-containing proteins, and TRAP variants constructed in this study. (A) Comparison of MIDAS motifs in A-domain-containing proteins (non-exhaustive list). The invariant residues of the MIDAS are highlighted in bold. TRAP, P.berghei thrombospondin-related anonymous protein; CD11b, α-subunit of the human integrin Mac-1; coll. α-2 (VI), α2-chain of human collagen VI (consensus of the three vWA-domains); matrilin-1, mouse cartilage matrix protein (consensus of the two vWA-domains); factor C2, human complement factor C2; T20G5.3, hypothetical Caenorhabditis elegans protein T20G5.3. (B) Comparison of the TSRs in TSR-containing proteins (non-exhaustive list). The central region of TSRs is shown, which includes the conserved tryptophans and the cluster of positive residues. The C-terminal conserved cysteines (between two and four) that participate in disulfide bond formation are not shown. TRAP, P.berghei thrombospondin-related anonymous protein; thrombosp., human thrombospondin (consensus of the three TSRs); properdin, human properdin (consensus of the six TSRs); UNC-5, C.elegans netrin-receptor UNC-5 (consensus of the two TSRs); MIC2, Toxoplasma gondii micronemal protein-2 (consensus of the five TSRs; lower cases = conserved in at least three TSRs); CS, P.berghei circumsporozoite protein. (C) Schematic diagram of the primary structure of P.berghei TRAP and of the mutations generated in its A-domain and TSR. In the A-domain, the conserved Thr126 and Asp157 were changed to alanines to generate mutants AdoT and AdoD, respectively. In the TSR, the conserved Trp244 was changed to alanine to generate mutant TsrW. The lysines and arginines towards the C-terminus of the motif were replaced by alanines, leading to mutant Tsr+. The double mutant Ado/Tsr is mutated both in the A-domain (Thr126→Ala) and the TSR (Lys,Arg256–261Ala). Repeats, species-specific amino-acid repeats; TM, transmembrane domain; black box, cyto plasmic domain.
TSRs are ∼60 residue-long modules with two highly conserved motifs: an N-terminal WSXW tetrapeptide and a C-terminal cluster of basic residues (Figure ;1B). WSXW appears to act as a heparin binding domain (Guo et al., 1992a,b), while the stretch of basic residues in the TSR of the Plasmodium circumsporozoite (CS) protein mediates binding to sulfated glycoconjugates (Sinnis et al., 1994; Gantt et al., 1997). We generated TRAP variant TsrW, in which the distal tryptophan in the WSXW sequence, shown to be critical to the adhesive properties of the motif (Guo et al., 1992b), was substituted by alanine (Figure 1C). A second variant, Tsr+, had all positively charged residues in the 256KIRKRK261 cluster changed to alanine residues.
We also generated a TRAP double variant that combined the AdoT and Tsr+ mutations, named Ado/Tsr (Figure 1C).
Generation of clonal P.berghei parasites expressing TRAP variants
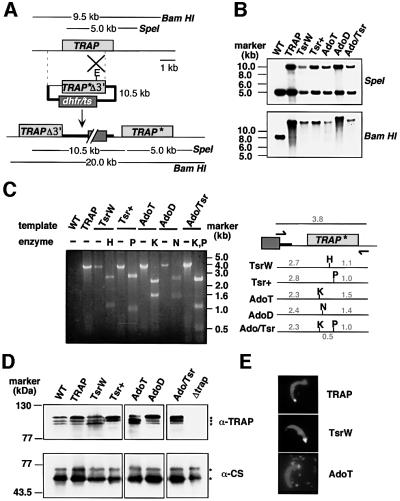
We targeted the endogenous TRAP locus in P.berghei with a series of insertion plasmids that had wild-type (WT; plasmid pTRAP) or mutated (pAdoT, pAdoD, pTsrW, pTsr+, pAdo/Tsr) TRAP targeting sequences. Homologous recombination was expected to lead to plasmid integration forming the recombinant locus depicted in Figure 2A: the first TRAP copy lacks a 3′ portion of the TRAP coding sequence and the 3′ regulatory sequences required for proper expression, whereas the second TRAP copy is a full-length gene under the control of WT regulatory sequences. Mutations were expected to be recovered in the second, full-length TRAP copy.
Fig. 2. Targeted mutagenesis at the TRAP locus. (A) Insertion strategy to generate the mutant parasite clones. The wild-type (WT) TRAP genomic locus is targeted with an EcoRI (E)-linearized plasmid containing a 3′ truncated TRAP open reading frame and the corresponding base pair substitutions (TRAP*Δ3′) as well as the mutated dhfr/ts gene for selection with pyrimethamine. Upon a single cross-over event the region of homology is duplicated and the mutation is placed in the second, full-length and expressed copy of TRAP (TRAP*). The first copy lacks the 3′ part of TRAP (TRAPΔ3′) as well as downstream regulatory sequences. The restriction fragments generated by SpeI (that cuts once in the plasmid) or BamHI (that does not cut in the plasmid) in WT TRAP and the expected recombinant locus are shown as lines with their predicted sizes. (B) Genomic Southern hybridization. A successful integration event of the targeting plasmid at the TRAP locus is verified by the presence of an additional SpeI fragment of the size of the targeting plasmid (10.5 kbp) as well as the corresponding increase in the size of the BamHI fragment. Note that the clone AdoD contains a tandem integration of two targeting plasmids, increasing the BamHI fragment from 20 to 30.5 kbp. (C) PCR analysis of the TRAP loci of WT and clonal parasite populations. The primers used for specific amplification of the last copy of TRAP, the only one to be full-length and expressed, were within the dhfr/ts resistance marker (forward primer) and the 3′ untranslated region (UTR) of TRAP (reverse primer) and are indicated by arrows. The predicted 3.8 kbp product was amplified from all parasite clones. Restriction digestion of the PCR products confirmed the presence of the corresponding mutations. Abbreviations: H, HindIII; P, PstI; K, KpnI; N, NgoMIV. Numbers indicate the size of the fragments on either side of the restriction site (in kbp). (D) Western blot analysis of sporozoite extracts. Extracts from WT or mutant, midgut sporozoites (∼100 000) were separated on a 8% SDS gel and probed with either a polyclonal serum directed against the P.berghei TRAP repeats, or a monoclonal anti-CS antibody to confirm that similar amounts of sporozoite extracts were loaded in each lane. The nature of the low molecular weight TRAP bands, as well as the reason for their changing ratios (in the WT and mutants), remains unknown. (E) Immunofluores cence stainings of salivary gland sporozoites with anti-TRAP and anti-CS antibodies. Sporozoites in mutant populations show the typical focal staining of TRAP (white) at the sporozoite apical end and the even surface distribution of CS (grey). Note also the deposit of TRAP and CS on the glass slide after gliding (shown for the AdoT parasite).
Southern blot analysis of the TRAP genomic locus of the various clones is shown in Figure 2B. In all clones, a single plasmid had integrated into TRAP, except in AdoD where two plasmids had integrated in tandem (note that only the last TRAP copy resulting from multiple integration events is full-length and correctly expressed). The presence of mutations in the expressed, full-length TRAP copy was verified after PCR amplification of the region and analysis with restriction enzymes indicative of the respective mutations (Figure 2C).
To assess TRAP production by sporozoites, recombinant erythrocytic stages were transmitted to Anopheles stephensi mosquitoes. Sporozoites were collected from mosquito midguts and sporozoite extracts were analyzed by western blot, using antibodies directed against the species-specific repeats of P.berghei TRAP (Figure 2D). Similar levels of TRAP were expressed in WT and recombinant sporozoites, except the Δtrap negative control (Sultan et al., 1997), showing that the integration strategy did not alter TRAP production and that the various substitutions did not affect protein expression or stability. To test whether mutations affected TRAP secretion onto the sporozoite surface, we stained non-permeabilized sporozoites with anti-TRAP repeat antibody (Figure 2E). A similar proportion of sporozoites (∼3%) in all clones displayed the typical surface TRAP staining pattern restricted to a sporozoite tip.
These results indicate that all recombinant P.berghei sporozoites constructed here express and secrete TRAP variants in a WT fashion. Therefore, they can be used to evaluate the contribution of TRAP adhesive modules to sporozoite gliding and host cell invasion.
Sporozoite infectivity to mosquito salivary glands depends on both TRAP modules
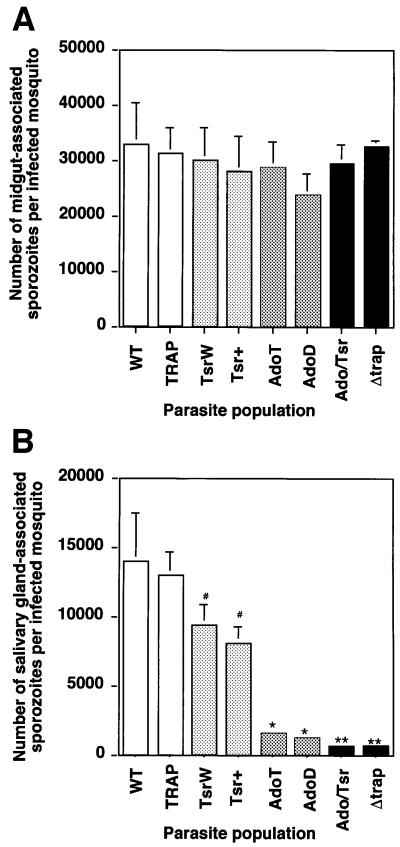
Sporozoite infectivity to mosquito salivary glands was assessed by comparing the average number of midgut and salivary gland sporozoites per infected mosquito. Throughout this paper, midgut sporozoites refer to sporozoites still contained in or already released from oocysts, while salivary gland sporozoites refer to sporozoites that are outside but attached to the glands as well as sporozoites that are inside the glands.
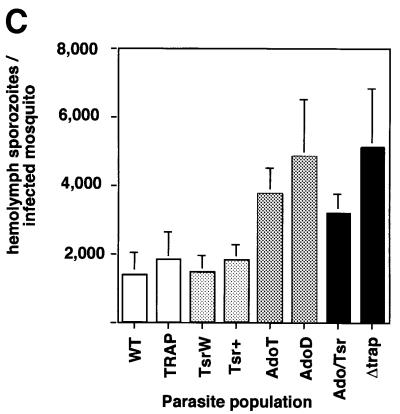
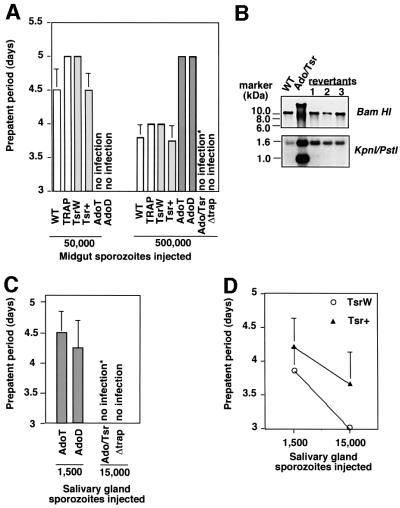
No significant difference was seen between the average numbers of midgut sporozoites in the various populations (Figure 3A). The numbers of salivary gland sporozoites are shown in Figure 3B. Comparable numbers were found in the WT and TRAP control populations (13 900 and 12 900, respectively). TsrW and Tsr+ clones produced on average 9700 and 9400 salivary gland sporozoites, respectively, which are significantly lower numbers than those of the TRAP control (P <0.05) and correspond to an ∼20% decrease in sporozoite infectivity. Greatly decreased numbers of salivary gland sporozoites were counted in the AdoT and AdoD clones (1800 and 1300, respectively), corresponding to an ∼80% decrease in infectivity. The Ado/Tsr clone produced 650 salivary gland sporozoites only, which are significantly lower numbers than those of the Ado clones (P <0.01) and correspond to an ∼95% decrease in sporozoite infectivity. Notably, a similar average number (700) of salivary gland sporozoites was counted with the Δtrap clone, which were shown to remain extracellular (Sultan et al., 1997). To confirm that the low numbers of mutant salivary gland sporozoites was not due to lack of release of sporozoite mutants from the mosquito midgut, we counted sporozoites present in the hemolymph, the fluid that bathes the mosquito cavity and carries sporozoites from the midgut to the salivary glands. As shown in Figure 3C, similar or higher numbers of hemolymph sporozoites were found in all mutant populations compared with the WT or the TRAP control. This showed that the low numbers of mutant salivary gland sporozoites are due to impairment of sporozoite invasion of salivary glands.
Fig. 3. The TRAP A-domain and TSR are important for sporozoite invasion into A.stephensi salivary glands. Represented in all graphs are the mean numbers of sporozoites (+SEM) calculated from four counts, each from an independent mosquito feeding experiment. In each population, an average of 250 mosquitoes from a minimum of four independent feeding experiments were examined. (A) Midgut sporozoites per infected mosquito at day 14 post-feeding. The variations between the various parasite populations were not statistically significant (P >0.07). (B) Salivary gland sporozoites (attached to, and within the glands) per infected mosquito at day 18 post-feeding. #, the one-tailed P values for Tsr mutants were significantly lower than the mean value for the TRAP control (TsrW: 0.024; Tsr+: 0.028); *, the mean numbers of Ado mutants were significantly lower than the mean value of the TRAP control (P <0.001); **, the mean values of the Ado/Tsr and the Δtrap mutants were significantly lower than the mean value of the Ado mutants (P <0.01). (C) Hemolymph sporozoites per infected mosquito at day 16 post-feeding. Note an increase in the numbers of hemolymph sporozoites in parasite populations that are blocked in salivary gland invasion.
Therefore, both the TRAP A-domain and TSR are involved in sporozoite infectivity to mosquito salivary glands, the A-domain playing a major role. The minor role of the TSR is shown by the decrease in infectivity of both Tsr mutants compared with the TRAP control, and of the Ado/Tsr mutant compared with both Ado mutants.
Gliding locomotion is unaffected in the TRAP mutants
Gliding locomotion was first examined in sporozoites collected from mosquito midguts and deposited on bovine serum albumin (BSA)-coated slides (Table I). In all populations, including the WT and the Ado/Tsr clone, a low proportion of sporozoites (<6%) was seen gliding and all gliders displayed a ‘weak’ pattern, defined by attachment to the substrate and gliding for less than a full circle. In contrast, Δtrap mutants did not display any locomotion. We then examined sporozoites collected from mosquito hemolymph. Again, the same phenotype was observed for all sporozoite clones and the WT: a higher proportion of sporozoites (∼20%) was seen gliding, which all displayed a ‘weak’ pattern.
Table I. Gliding motility is not affected by mutations in TRAP ectodomain.
| Parasite population | Percentage of gliding sporozoites | ||
|---|---|---|---|
| Midguta | Hemolymphb | Salivary glandsc | |
| WT | 5.5 (3.3) | n.d. | 51.3 (12.5) |
| TRAP | 3.7 (2.8) | 16.4 (4.3) | 64.8 (9.2) |
| TsrW | 2.8 (1.9) | 25.9 | 36 (12.7) |
| Tsr+ | n.d. | n.d. | 47.5 (20.5) |
| AdoT | 4.7 (4.6) | 26.7 (8.5) | 3 (1) |
| AdoD | 3 | 14 | 5 |
| Ado/Tsr | 5.8 (5.3) | 22 (8.5) | 4.3 (2.8) |
| Δtrap | 0 | 0 | 0 |
n.d., not done. Numbers in parentheses denote standard variation.
aMidgut sporozoites glide with discontinuous speed and in incomplete circles.
bHemolymph sporozoites glide similarly to midgut sporozoites.
cSalivary gland sporozoites glide with continuous and rapid speed (2–4 µm/s) and for several circles.
We next analyzed sporozoites collected from mosquito salivary glands. Unlike midgut and hemolymph sporozoites, which all originate from an extracellular location, salivary gland sporozoites are a mixture of sporozoites coming from an extracellular (the gland surface) or intracellular (inside acinar cells) location. In the WT, the vast majority of these sporozoites come from within the glands, and a large proportion (>50%) can be seen to display a ‘strong’ gliding pattern, defined by attachment to the substrate and gliding for more than a full circle (frequently several circles). Since this new gliding phenotype is most likely due, at least in part, to sporozoite internalization in the glands, the gliding penotype of a population of salivary gland sporozoites depends on its invasive capacity.
Sporozoites from the TRAP, Tsr, Ado and Ado/Tsr clones have ∼100, ∼80, ∼20 and <5% residual infectivity to the salivary glands, respectively (Figure 3B). The percentage of gliding sporozoites in each recombinant population was decreased roughly in proportion to their impaired capacity to infect salivary glands (Table I). Importantly, all gliding sporozoites in all populations, including the Ado/Tsr clone, displayed the typical ‘strong’ gliding pattern, with sporozoites occasionally gliding several circles.
Based on the indistinguishable phenotype (pattern and frequency) of midgut and hemolymph sporozoites, as well as the indistinguishable gliding pattern of salivary gland sporozoites in all populations, we conclude that loss-of-function mutations in the TRAP MIDAS and TSR have no direct consequence on sporozoite gliding. We interpret the low proportion of gliders in salivary gland mutant sporozoites as the consequence of the primary role of the domains in salivary gland invasion.
Sporozoite infectivity to the rodent liver depends on both TRAP modules
In the WT, sporozoites collected from midguts or salivary glands of mosquitoes are infective to rodents after intravenous injection. However, as for the gliding phenotype, midgut and salivary gland sporozoites have different levels of infectivity, the latter being ∼1000-fold more infective than the former. This implies that only midgut sporozoites can be used to compare infectivity with the mammalian host of sporozoite populations that have different levels of mosquito gland invasion.
We injected midgut sporozoites intravenously into rats and determined prepatent periods (PP) of infection (time until the proportion of infected erythrocytes is >0.01%). In this system (Figure 4A), one additional day in PP corresponds to a 10-fold decrease in the number of injected sporozoites (parasitemia in rats increases ∼10-fold per day). Using both WT and TRAP parasites, injection of 5 × 104 and 5 × 105 sporozoites into rats induced PP of ∼5 and ∼4 days, respectively. TsrW and Tsr+ sporozoites induced PP similar to WT sporozoites, suggesting that either the TSR is dispensable for liver infection or its role is not revealed by the test. AdoT and AdoD sporozoites had similarly decreased infectivity to rodents: injection of 5 × 104 sporozoites did not induce infection, whereas injection of 5 × 105 sporozoites induced PP of 5 days. Thus AdoT and AdoD sporozoites were ∼10-fold less infective to rodents than WT sporozoites. In contrast to single mutant sporozoites, Ado/Tsr sporozoites constantly failed to induce infection, even after injection of 5 × 105 sporozoites. In one experiment, one rat injected with Ado/Tsr sporozoites became infected with WT TRAP revertants (Figure 4B, revertant 1), which acted as internal controls linking lack of infectivity of Ado/Tsr sporozoites to the corresponding mutation.
Fig. 4. Sporozoite infectivity to the mammalian host. (A) Infectivity to rats of midgut sporozoites. Sporozoites were isolated from midguts of infected mosquitoes and intravenously injected into Sprague/Dawley rats. Shown is the average prepatent period (in days) after injection of 50 000 and 500 000 sporozoites. Ado sporozoites were less infective than TRAP sporozoites, while Ado/Tsr sporozoites were not infective. One rat injected with Ado/Tsr sporozoites became infected (marked with *); however, the resulting erythrocytic stages were revertants [see (B)]. Experiments were carried out in quadruplicate. (B) Genomic Southern hybridization of parasite red blood cell stages obtained after injection of Ado/Tsr sporozoites. Three parasite populations were obtained in rats, after injection of (i) 500 000 midgut sporozoites (one out of four experiments), (ii) 15 000 salivary gland sporozoites (one out of four experiments), and (iii) natural feeding of 100 infected mosquitoes on a rat (one out of four experiments). Parasites were revertants, i.e. arose after excision of the integration plasmid, as shown by the BamHI digestion, and had not retained the mutation in the TRAP gene, as shown by the KpnI–PstI double digestion. (C) Ado/Tsr salivary gland sporozoites are not infective. While injection of 1500 Ado (T or D) sporozoites is sufficient to cause red blood cell infection in rats, injection of 15 000 Ado/Tsr sporozoites does not infect rats. Experiments were carried out in duplicate with three replicas each. *, one rat became infected; however, the resulting erythrocytic stages were revertants [see (B), revertant 2]. (D) Tsr+ salivary gland sporozoites are less infective to rats than TsrW sporozoites after intravenous injection. Infectivity to rodents of Tsr+ and TsrW populations can be compared using salivary gland sporozoites, because these two populations have similar salivary gland infection rates. The prepatent period of infection is shown as a function of the numbers of sporozoites injected. Values are the mean PP from six experiments, each carried out in duplicate. The Tsr+ mutation leads to a consistent delay of patency, corresponding to a 5- to 10-fold decrease in infective capacity.
To gain further evidence that Ado sporozoites were infective to rats while Ado/Tsr were not, we used salivary gland sporozoites (the developmentally more advanced and potentially more infective subpopulation). Injection of 1500 AdoT or AdoD sporozoites was sufficient to induce rat infection, but injection of 10 times more Ado/Tsr salivary gland sporozoites consistently failed to induce infection (Figure 4C). One experiment using Ado/Tsr sporozoites generated erythrocytic stages, which again were revertants (Figure 4B, revertant 2). These results confirmed the lack of infectivity of Ado/Tsr sporozoites.
The reproducible difference of infectivity between Ado and Ado/Tsr mutants also showed a role of the TSR in liver infection. A second line of evidence for the involvement of the TRAP TSR in liver infection was provided by the longer PP induced by Tsr+ salivary gland sporozoites compared with TsrW sporozoites, despite their similar mosquito salivary gland infection rate (Figure 4D).
Therefore, it appears that the contributions of the TRAP binding modules to sporozoite infectivity to the mammalian host are similar to their contribution to sporozoite infectivity to the insect. Impairment of one module decreases sporozoite infectivity, the A-domain playing the major role, while impairment of both abolishes infectivity.
The A-domain and TSR are dispensable for sporozoite adhesion to cultured cells
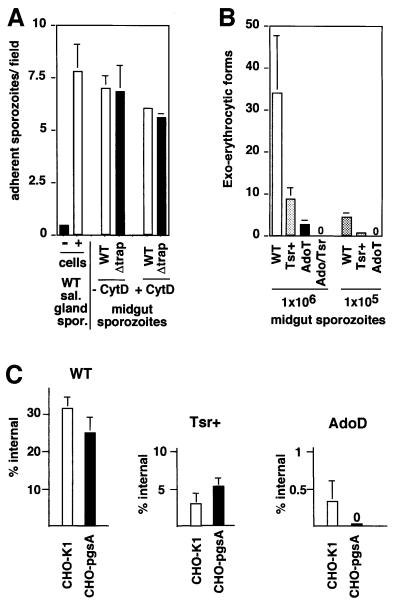
The inference that the TRAP adhesive modules were not involved in sporozoite locomotion but were important to sporozoite infectivity in each host suggested that they were specifically involved in cell invasion steps of the infectious process. To test whether the TRAP modules are required during initial sporozoite attachment to cells, we incubated midgut sporozoites with confluent HepG2 cells, the reference cell line for Plasmodium sporozoite invasion assays (Frevert et al., 1996), and labeled bound sporozoites using anti-CS repeat monoclonal antibody (Figure 5A). In the absence of cells, only background binding of WT sporozoites was observed. In contrast, WT sporozoite binding was efficient in the presence of cells, with salivary gland sporozoites being slightly more adhesive than midgut sporozoites. Next, we tested cell adhesion of midgut sporozoites of the WT, Tsr+, AdoT and Ado/Tsr clones (not shown). No significant difference was noticed in the number of adhesive sporozoites in each population. This observation led us to test whether TRAP itself was involved in sporozoite attachment to cells. For this, we compared adhesion of WT and Δtrap midgut sporozoites pre-incubated with or without the microfilament inhibitor Cytochalasin D, which precludes sporozoite internalization (Figure 5A). No significant difference was observed between the two populations. These results indicate that TRAP has no detectable role in sporozoite adhesion to cultured cells.
Fig. 5. The TRAP ectodomain is important for sporozoite invasion of, but not adhesion to, cultured cells. (A) TRAP is dispensable for sporozoite adhesion to HepG2 cells. Midgut sporozoites (50 000) were pre-incubated for 15 min in the presence or absence of 2 µM Cytochalasin D subsequently incubated for 90 min with confluent HepG2 cells, and sporozoites that remained bound to cells after washings were stained with anti-CS antibody. Adhesion is shown as the mean number of bound sporozoites in one microscopic field (400× magnification), from two sets of experiments done in quadruplicate each. (B) The TRAP A-domain and TSR are required for invasion of HepG2 cells. Midgut sporozoites (1 000 000) were added to subconfluent HepG2 cells (multiplicity of infection = 1), and EEF that develop inside cells were immunostained 48 h later. The mean numbers of EEFs from two experiments carried out at least in triplicate each are shown. (C) Cell invasion into WT CHO cells (CHO-K1) and GAG-deficient CHO cells (pgsA). Salivary gland sporozoites were incubated for 90 min with confluent CHO cells (empty bars) or pgsA derivatives (black bars) at a multiplicity of infection of 0.01, and the proportion of internalized sporozoites was assessed by differential immunostaining of extra and intracellular sporozoites (10 000 cells examined for each population). Left, GAG chains are of minor importance for WT sporozoite invasion into CHO cells. Center, TSR+ salivary gland sporozoites enter GAG-deficient cells with similar efficiency to WT cells. Right, AdoD sporozoites invade cultured cells in a GAG-dependent fashion.
The TRAP A-domain and TSR act as cell invasion ligands
We evaluated sporozoite capacity to invade HepG2 cells. Since in vivo experiments showed that the two MIDAS disruptions had, as expected, comparable impacts, and suggested that the Tsr+ mutant may be more impaired than TsrW, we focused our analysis on AdoT, Tsr+ and the Ado/Tsr mutant (AdoT/Tsr+).
Midgut sporozoites were incubated with semi-confluent cells and parasites developing intracellularly, called exo-erythrocytic forms (EEF), were immunostained (Figure 5B). WT and TRAP sporozoites generated similar numbers of EEF. Both Tsr+ and AdoT sporozoite populations had decreased invasive capacities, generating ∼3- and 10-fold fewer EEF than the WT, respectively. No EEF were detected in cells incubated with Ado/Tsr sporozoites.
To confirm that the Ado/Tsr sporozoites could not penetrate HepG2 cells, invasion assays were performed with salivary gland sporozoites. Examination of >5 × 104 sporozoites in five independent experiments indicated that AdoT salivary gland sporozoites invaded and developed inside cells (30 EEF), while Ado/Tsr sporozoites did not. Therefore, modifications in the TRAP adhesive modules have qualitatively comparable effects on invasion of cultured cells and in vivo infectivity to each of the sporozoite hosts.
The TSR acts via a GAG-dependent pathway and the A-domain via a GAG-independent pathway during host cell invasion
So far, only cell surface glycosaminoglycan chains (GAGs) have been reported as cell surface receptors involved in cell adhesion and/or invasion of Plasmodium sporozoites (Frevert et al., 1996). To test the contribution of cell surface GAGs during sporozoite invasion, we used WT Chinese hamster ovary (CHO)-K1 cells, which contain abundant surface GAGs, and pgsA derivatives of this cell line, which are impaired in GAG biosynthesis (Esko et al., 1985). Cells were incubated with salivary gland sporozoites, and internalized sporozoites after 90 min were counted by double immunostaining (Figure 5C). For the same reasons as stated above when assessing sporozoite gliding and infectivity to rodents, the use of salivary gland sporozoites precluded comparison of the invasive capacities between the various populations. We thus only compared the invasive capacities of a given sporozoite population in the presence or absence of cell surface GAGs.
WT sporozoites penetrated CHO cells slightly more efficiently (∼20%) than their pgsA derivatives. Although this difference was not statistically significant when applying a two-tailed test, it is in agreement with previous reports of a ∼25% decrease in infectivity of WT sporozoites in WT versus pgsA CHO cells (Frevert et al., 1996). This suggests that GAG chains have only a minor importance for sporozoite invasion into CHO cells. On the other hand, Tsr+ sporozoites penetrated both types of cells with similar efficiency. This would indicate that GAGs are only important in the presence of a functional TSR, and conversely that A-domain function does not require GAGs.
Next we tested invasion of AdoD sporozoites. We consistently saw internalized AdoD parasites in WT CHO cells in four independent experiments using 10 000 cells each. In contrast, no AdoD parasite was seen inside pgsA cells, suggesting that invasion of AdoD sporozoites requires cell surface GAGs. The residual invasive capacity of these sporozoites is mediated by the TSR, as Ado/Tsr sporozoites were never found inside WT cells.
We conclude that more than one cell surface receptor is used by the TRAP ligand system during sporozoite entry into target cells: the MIDAS-mediated role of the A-domain is mainly GAG independent, whereas TSR-mediated entry (via its C-terminal basic residues) is mainly GAG dependent.
Discussion
Prior to our work, one report (Wengelnik et al., 1999) addressed the structure–function relationship of Plas modium TRAP extracellular modules. Mutations were introduced in a P.berghei line in which the endogenous TRAP had been replaced by Plasmodium falciparum TRAP. Further PfTRAP mutagenesis in this heterologous clone led to the conclusions that the TSR, but not the A-domain, was important for sporozoite gliding, and that both modules were involved in mosquito salivary gland, but not mammalian liver infectivity. As previously discussed (Ménard and Nussenzweig, 2000), several methodological problems limit the validity of these conclusions, starting with the control sporozoites expressing WT PfTRAP which were severely impaired in all TRAP-dependent phenotypes. In contrast, based on a homologous and controlled system, our results suggest that while neither the TSR nor the A-domain is involved in sporozoite gliding motility, each contributes to sporozoite infectivity to both insect and mammalian hosts.
TRAP ectodomain: a dual ligand for entry into diverse cell types
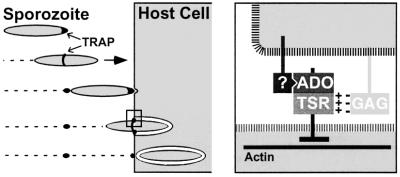
We found that all sporozoite mutant populations were impaired to similar extents in their infectivity to both target organs in vivo and in their capacity to enter into cultured cells. However, all mutants glided in a manner indistinguishable from the WT, implying that TRAP modifications did not affect protein redistribution along the sporozoite. Moreover, all mutants adhered normally to cultured cells, showing that TRAP does not act as an adhesin. Thus the steps that precede cell penetration are independent of the targeted TRAP extracellular motifs. Since cell penetration results from ‘capping’ of a tight junction, the two TRAP extracellular modules are likely to constitute the sporozoite ligands of the junction on which the sporozoite exerts force to locomote inside the cell (Figure 6).
Fig. 6. Hypothetical model for the role of the two TRAP adhesive modules during Plasmodium sporozoite invasion of host cells. Plasmodium sporozoites reach their target cells through gliding locomotion. Left part, TRAP is capped backwards during gliding motility and host cell invasion. The A-domain MIDAS and the TSR of TRAP are not required for this step. Initial host cell attachment is TRAP independent, but induces a burst of TRAP secretion at the sporozoite tip. Right, host cell invasion requires the formation of a junction between the sporozoite anterior end and the host cell. The A-domain, which binds to an as yet unidentified host cell receptor (?) in a MIDAS-dependent fashion, and the TSR, which recruits glycosaminoglycan chains (GAG) via a charged interaction, presumably act during this step of junction formation. An actin-based motor system then generates force, which is transmitted via the TRAP cytoplasmic tail and exerted on the host–parasite junction. This allows the sporozoite to locomote inside the so-called parasitophorous vacuole.
The strikingly similar impact that each TRAP modification had in all cell invasion assays also suggests that the same TRAP system directs sporozoite entry into diverse, and potentially any adherent cell type. Sporozoite invasion into mosquito salivary glands and the rodent liver, as well as penetration into human HepG2 and hamster CHO cells were all partially impaired by loss-of-function mutations in one module and abolished by mutations in both modules. The role of TSR was less important than that of the A-domain but nonetheless was significant, as shown by both the effects of Tsr mutations and the constant decrease in infectivity of Ado/Tsr compared with Ado mutants in all assays. It is difficult, however, to discriminate between additive or cooperative roles of the A-domain and the TSR during entry (which may depend on the cell type), as the impact of single modifications varied with the assay. We also showed that the two modules recruit distinct receptors on the surface of CHO cells: the TSR, but not the A-domain, mediates entry via heparan sulfate (HS). Therefore, we propose that the two TRAP modules act as a dual cell-anchoring system, with each module triggering independent pathways of cell invasion. Crucially, the finding that the double mutation constantly abolished cell entry (in vivo and in vitro) while not altering motility, raises the possibility that the two TRAP modules are the sole productive ligands for cell penetration.
What are the host cell receptors for the TRAP dual ligand?
TSRs, including the TRAP TSR (Muller et al., 1993), are adhesive modules that bind with high affinity to heparin and certain sulfated glycoconjugates (Holt et al., 1990; Cerami et al., 1992). Here, we provide evidence for the importance of TRAP TSR–HS interaction for sporozoite invasion of target cells. A similar decrease in sporozoite invasion resulted from loss-of-function mutations in the TSR of TRAP or lack of GAGs on the target cell surface. In addition, the residual invasive capacity of Ado sporozoites, which is TSR dependent, required cell surface GAGs in CHO cells. However, the nature of this TSR–HS interaction remains unclear. Cell surface-bound HS, possibly carried by syndecans, may be directly used by the sporozoite for traction and internalization. TSR–HS interaction could also modulate ligand affinity to another TRAP motif, similar to the way in which HS binding to fibronectin alters conformation of integrin-binding sites (Hynes, 1992). Finally, soluble HS shed from the plasma membrane may act as molecular bridges between the TSR and potentially any heparin-binding protein on the cell surface. Such a sulfated polysaccharides ‘recruitment strategy’, thought to be a virulence mechanism widely used by bacterial pathogens (Duensing et al., 1999), would indeed enable the parasite to adapt to variations in protein composition at the target cell surface.
The A-domain of TRAP has only been reported to bind heparin, but in a divalent cation-independent fashion that does not require a functional MIDAS (McCormick et al., 1999). In contrast, the major losses in infectivity of AdoT or AdoD sporozoites reported here show that most, if not all, TRAP A-domain function is MIDAS dependent. Furthermore, in CHO cells, the A-domain does not require cell surface HS for acting during sporozoite entry, as Tsr+ sporozoites have similar invasive capacities in WT and GAG– cells. A-domains that bear a MIDAS motif bind numerous ligands in a Mg2+-dependent fashion. Among integrins, the A-domain (called I-domain) of Mac-1 binds ICAM-1, iC3b, factor X and fibrinogen, while that of LFA-1 binds ICAM-1, -2, and -3, and that of VLA-2 binds fibrillar collagens and laminin. By analogy, the TRAP A-domain may be able to bind different cell surface molecules. Consequently, the sporozoite may use these TRAP A-domain partners as several possible anchors for cell invasion. Alternatively, TRAP A-domain specificity may be confined to a single cell surface receptor, which would then be ubiquitously expressed and evolutionarily conserved.
A general model of apicomplexan entry into host cells?
Plasmodium TRAP is the prototype of a family of proteins found in invasive stages of many Apicomplexa parasites, including Plasmodium ookinetes (Trottein et al., 1995), the parasite stage that traverses the mosquito midgut epithelium, human pathogens like Toxoplasma (Wan et al., 1997) and Cryptosporidium (Spano et al., 1998), and animal pathogens like Eimeria (Tomley et al., 1991) and Neospora (Lovett et al., 2000). There is now growing evidence that these proteins are functional homologs central to parasite gliding and invasive capacities. In particular, the cytoplasmic tail of Plasmodium TRAP can be functionally replaced by that of the Toxoplasma analog MIC2 (Kappe et al., 1999). The presence of at least one A-domain and TSR copy in these TRAP-related proteins in Apicomplexa, except for the A-domainless TRAPC1 of Cryptosporidium, suggests that their association may represent a basic cell anchorage system useful for invading a wide range of host cells. Restriction to the actual target cell type in vivo would be directed by a protein constitutively exposed on the parasite surface. In the case of Plasmodium sporozoites, the CS protein has been shown to bind both mosquito salivary glands (Sidjanski et al., 1997) and mammalian hepatocytes (Cerami et al., 1992; Sinnis et al., 1994) via distinct regions. Following initial adhesion, the adhesive modules of the TRAP-related protein would bind cell surface receptors, and the resulting junction would be drawn backward by connecting the cytoplasmic tail of the TRAP-related protein to an actin-based motor system (Figure 6). So far, the only known receptor of these proteins (via the TSRs) is HS, which is ubiquitous on animal adherent cells and present in lower invertebrates (Bernfield et al., 1999). Future work will determine whether the A-domain contribution to cell invasion is due to its ability to bind one evolutionarily conserved receptor, or a variety of molecules on epithelial cell surfaces.
Materials and methods
Cloning of TRAP constructs
The integration control plasmid contains the bacterial plasmid pBluescript II KS (Stratagene, La Jolla, CA), the mutant dhfr/ts gene from P.berghei, which confers resistance to the antimalarial drug pyrimethamine, and a TRAP targeting sequence that starts upstream from the TRAP promoter and ends at nucleotide 1727 of the TRAP coding sequence. The TRAP targeting sequence was cloned from genomic DNA of TRAP knockout INT parasites as described previously (Nunes et al., 1999); the 3′ deletion of TRAP was generated by digestion and religation with PstI. Base-pair substitutions were created by fusing contiguous 5′ and 3′ DNA fragments amplified by PCR from genomic TRAP. A universal forward primer (5′-GTTGTGCTTTTATTATGCATAAGTGTG-3′) for the 5′ fragment and a universal reverse primer (5′-CTGATGGCTCTTCTGGTTTTATTGG-3′) for the 3′ fragment were used in all PCRs. An additional EcoRI site was introduced at nucleotide 1470 using primers (5′-GGGAATTCATCCGTTGGTGGTACA-3′) and (5′-CGGATGAATTCCATATATAAAGGTC-3′) (the EcoRI site is underlined) to generate a unique restriction site for linearization of the integration plasmid. This site is located >700 bp downstream of the mutations to allow for maintenance of the sequence heterologies after homologous recombination (Nunes et al., 1999). For the amino acid mutations within the extracellular adhesion motifs the following reverse primers to amplify the 5′ fragment and forward primers to amplify the 3′ fragment, which introduce the mutation and a novel restriction site, were used: AdoT, 5′-GCGGGTACCATGTGGTGAATAATTATTTTGAAG-3′ and 5′-CCGGGTACCGCAAATTTAACGAGTGCATTATTGAATG-3′ (KpnI site at position 377 is underlined); AdoD, 5′-GGGGCCGGCTGTTAATTAATAACTAATTGTATTGC-3′ and 5′-GGGGCCGGCATCCCAAATAATTTAAAGAAATCTACTAC-3′) (NgoMIV site at position 476 is underlined); TsrW, 5′-CGCGAAGCTTCTTCCCATTTTCCACAAAGAGC-3′ and 5′-CGCGAAGCTTCTGAATGTTCTACTACATGTGACAATG-3′ (HindIII site at position 736 is underlined); Tsr+, 5′-CGGTGCTGCAGCTGCAATTGCTGTTCCATTTCACATGTAGTAG-3′ and 5′-CGGCTGCAGCAGTATTACATCCTAATTGTGCTGGAG-3′ (PstI site at position 784 is underlined). All PCR products were cloned into the pCRScript vector (Stratagene) and the contiguous fragments ligated and recloned into pCRScript. All fragments were sequenced and verified to differ from the WT sequence only by the desired mutations. The fragments were cloned into plasmid pTRAP (Sultan et al., 1997) by SpeI and HincII restriction digestions to replace the 837 bp WT fragment. To generate the double mutant AdoT/Tsr+, a 450 bp SpeI–AflIII fragment of pTRAPAdoT and a 700 bp AflIII–EcoRI fragment from pTRAPTsr+ were ligated with the SpeI–EcoRI-digested pTRAP.
Parasite transfection and genotypic analysis
The transfection protocol was essentially as described previously (Ménard and Janse, 1997). Southern blotting was performed with the entire TRAP open reading frame as a probe. The probe was labeled with DIG-ddUTP by random priming, and the chemiluminescence was detected using CSPD (Roche, Indianapolis, IN). For PCR analysis the second duplicate of the recombinant TRAP locus was specifically amplified using the primers OHAR4, a forward primer that hybridizes to the 3′ untranslated region (UTR) of dhfr/ts (Nunes et al., 1999) and the universal reverse primer, which hybridizes to the TRAP 3′ UTR missing in the integration plasmid pTRAP. The PCR products were digested with restriction enzymes indicative of the respective mutations. Clonal parasite populations were obtained by intravenous injection of a limited dilution of the parental population to approximately one red blood cell stage per 200 µl into 20 recipient Sprague/Dawley rats.
Mosquito infections and analysis of parasite development
Sporozoite populations were separated as described previously (Vanderberg, 1974, 1975). For collection of midgut sporozoites, the midguts of 30 infected mosquitoes were dissected on day 14 post-feeding. For collection of hemocoel sporozoites, the perfusate from 40 infected mosquitoes was pooled on day 16 post-feeding. For collection of salivary gland sporozoites, salivary glands were ground and processed on day 18 post-feeding. The number of sporozoites in the respective tissues was determined using a hemocytometer. In each experiment four separate counts were performed, and the average number of sporozoites associated with either mosquito midguts, hemocoel or salivary glands was determined from at least four independent feeding experiments. To analyze sporozoite gliding motility, sporozoites were incubated in 3% BSA-RPMI 1640 medium for 3 h prior to microscopic examination.
Analysis of sporozoite infectivity
To determine the infectivity of defined numbers of sporozoites in vivo, young Sprague/Dawley rats were injected intravenously with 200 µl sporozoite suspension in RPMI 1640. The parasitemia of inoculated rodents was checked daily by a 10 min examination of a Giemsa-stained blood smear. The time point of the first detection of at least one erythrocytic stage reflects the first day of patency. To analyze the infectivity of sporozoites in cell cultures, 100 µl of sporozoite suspension was added to the cultured cells and incubated for 2 h at 37°C. Development of parasite EEF in HepG2 cells was assessed as described previously (Frevert et al., 1996). Briefly, HepG2 cells (HBA-16) were grown to subconfluency, incubated for 90 min with the parasite suspension, washed and incubated for additional 2 days in RPMI/10% fetal calf serum (FCS). The EEF were labeled with mAb 2E6, which detects Hsp70 of P.berghei, and stained with a standard peroxidase substrate assay. The average number of EEFs was counted from four wells per experimental condition. For CHO cells a two-color assay was performed as described previously (Rénia et al., 1988). Briefly, CHO cells were grown to subconfluency, incubated for 90 min with the parasite suspension and washed in CHO medium (Sigma, St Louis, MO) containing 10% FCS. To distinguish between extra- and intracellular sporozoites, extracellular sporozoites were labeled with mAb 3D11, which recognizes P.berghei CS, and goat anti-mouse-FITC (all secondary antibodies from Kirkegaard & Perry Laboratories, Gaithersburg, MD). After permeabilization of the CHO cells with 0.05% saponin for 10 min, the same mAb was used to label intracellular parasites followed by goat anti-mouse–rhodamine. The average number of intracellular parasites was counted from four wells per experimental condition. For cell adhesion assays, 100 000 midgut sporozoites were added to confluent cell layers of HepG2 or Hepa1-6 cells. The cells were incubated for 90 min at 37°C, washed four times with pre-warmed DMEM medium and fixed with 4% formaldehyde. Adherent sporozoites were stained with a combination of anti-CS 3D11 and goat anti-mouse rhodamine antibodies. For each well, 50 microscopic fields were counted in duplicate using a 400× magnification.
Western blotting and immunofluorescence
Protein samples were analyzed by SDS–PAGE and electrophoretically transferred to polyvinylidene difluoride membranes. For the detection of TRAP expression in WT and mutant P.berghei sporozoites, midgut sporozoites on day 15 post-feeding were isolated. One hundred thousand sporozoites were resuspended in 10 µl SDS sample buffer and incubated for 5 min at 70°C. The antibodies to P.berghei TRAP and CS have been described previously (Sultan et al., 1997). Bound antibody was detected with horseradish peroxidase-coupled goat anti-rabbit or anti-mouse IgG, and developed with enhanced chemiluminescence (ECL; Amersham Pharmacia, Piscataway, NJ). For immunofluorescence, salivary gland or hemocoel sporozoites were isolated and incubated in RPMI/BSA on BSA-coated slides for 30 min. The sporozoites were fixed with 2.5% paraformaldehyde (Sigma) and incubated with antibodies to TRAP and CS. Bound anti-TRAP was detected with FITC-conjugated anti-rabbit IgG and bound anti-CS with rhodamine-conjugated anti-mouse IgG.
Acknowledgments
Acknowledgements
We thank Thomas Bruderer for assistance with the generation of insertion plasmids. We are grateful to Yi Lu, Arti Jaiswal, Sandy Nosseir, Lynn Chacko, Ivette Caro and Jean Nonon for excellent technical assistance. We thank Friedrich Frischknecht, Jayne Raper and Arturo Zychlinsky for helpful discussions and reviewing this manuscript. This work was supported by the Deutsche Forschungsgemeinschaft (to K.M.), the National Institute of Health (to V.N.), and a Career Award in the Biomedical Sciences from the Burroughs Wellcome Fund (to R.M.). R.M. is a Howard Hughes Medical Institute International Research Scholar.
References
- Bernfield M., Gotte,M., Park,P.W., Reizes,O., Fitzgerald,M.L., Lincecum,J. and Zako,M. (1999) Functions of cell surface proteoglycans. Annu. Rev. Biochem., 68, 729–777. [DOI] [PubMed] [Google Scholar]
- Carruthers V.B. and Sibley,L.D. (1999) Mobilization of intracellular calcium stimulates microneme discharge in Toxoplasma gondii. Mol. Microbiol., 31, 421–428. [DOI] [PubMed] [Google Scholar]
- Cerami C., Frevert,U., Sinnis,P., Takacs,B., Clavijo,P., Santos,M.J. and Nussenzweig,V. (1992) The basolateral domain of the hepatocyte plasma membrane bears receptors for the circumsporozoite protein of Plasmodium falciparum sporozoites. Cell, 70, 1021–1033. [DOI] [PubMed] [Google Scholar]
- Dessens J.T., Beetsma,A.L., Dimopoulos,G., Wengelnik,K., Crisanti,A., Kafatos,F.C. and Sinden,R.E. (1999) CTRP is essential for mosquito infection by malaria ookinetes. EMBO J., 18, 6221–6227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrowolski J.M. and Sibley,L.D. (1996) Toxoplasma invasion of mammalian cells is powered by the actin cytoskeleton of the parasite. Cell, 84, 933–939. [DOI] [PubMed] [Google Scholar]
- Dubremetz J.F., Garcia-Réguet,N., Conseil,V. and Fourmaux,M.N. (1998) Apical organelles and host cell-invasion by Apicomplexa. Int. J. Parasitol., 28, 1007–1013. [DOI] [PubMed] [Google Scholar]
- Duensing T.D., Wing,J.S. and van Putten,J.P.M. (1999) Sulfated polysaccharide-directed recruitment of mammalian host proteins: a novel strategy in microbial pathogenesis. Infect. Immun., 67, 4463–4468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emsley J., King,S.L., Bergelson,J.M. and Liddington,R.C. (1997) Crystal structure of the I domain from integrin α2β1. J. Biol. Chem., 272, 28512–28517. [DOI] [PubMed] [Google Scholar]
- Emsley J., Knight,C.G., Farndale,R.W., Barnes,M.J. and Liddington,R.C. (2000) Structural basis of collagen recognition by integrin α2β1. Cell, 101, 47–56. [DOI] [PubMed] [Google Scholar]
- Esko J.D., Stewart,T.E. and Taylor,W.H. (1985) Animal cell mutants defective in glycosaminoglycan biosynthesis. Proc. Natl Acad. Sci. USA, 82, 3197–3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frevert U., Sinnis,P., Esko,J.D. and Nussenzweig,V. (1996) Cell surface glycosaminoglycans are not obligatory for Plasmodium berghei sporozoite invasion in vitro. Mol. Biochem. Parasitol., 76, 257–266. [DOI] [PubMed] [Google Scholar]
- Gantt S.M., Clavijo,P., Bai,X., Esko,J.D. and Sinnis,P. (1997) Cell adhesion to a motif shared by the malaria circumsporozoite protein and thrombospondin is mediated by its glycosaminoglycan-binding region and not by CSVTCG. J. Biol. Chem., 272, 19205–19213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gantt S., Persson,C., Rose,K., Birkett,A.J., Abagyan,R. and Nussenzweig,V. (2000) Antibodies against TRAP do not inhibit Plasmodium sporozoite infectivity in vivo. Infect. Immun., 68, 3667–3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girma J.P., Meyer,D., Verweij,C.L., Pannekoek,H. and Sixma,J.J. (1987) Structure–function relationship of human von Willebrand factor. Blood, 70, 605–611. [PubMed] [Google Scholar]
- Guo N.H., Krutzsch,H.C., Negre,E., Vogel,T., Blake,D.A. and Roberts,D.D. (1992a) Heparin- and sulfatide-binding peptides from the type I repeats of human thrombospondin promote melanoma cell adhesion. Proc. Natl Acad. Sci. USA, 89, 3040–3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo N.H., Krutzsch,H.C., Negre,E., Zabrenetzky,V.S. and Roberts,D.D. (1992b) Heparin-binding peptides from the type I repeats of thrombospondin. Structural requirements for heparin binding and promotion of melanoma cell adhesion and chemotaxis. J. Biol. Chem., 267, 19349–19355. [PubMed] [Google Scholar]
- Holt G.D., Pangburrn,M.K. and Ginsburg,V. (1990) Properdin binds to sulfatide [Gal(3-SO4)β 1–1 Cer] and has a sequence homology with other proteins that bind sulfated glycoconjugates. J. Biol. Chem., 265, 2852–2855. [PubMed] [Google Scholar]
- Huizinga E.G., van der Plas,R.M., Kroon,J., Sixma,J.J. and Gros,P. (1997) Crystal structure of the A3 domain of human von Willebrand factor: implications for collagen binding. Structure, 5, 1147–1156. [DOI] [PubMed] [Google Scholar]
- Hynes R.O. (1992) Integrins: versatility, modulation and signaling in cell adhesion. Cell, 69, 11–25. [DOI] [PubMed] [Google Scholar]
- Kamata T. and Takada,Y. (1994) Direct binding of collagen to the I domain of integrin α2β1 (VLA-2, CD49b/CD29) in a divalent cation-independent manner. J. Biol. Chem., 269, 26006–26010. [PubMed] [Google Scholar]
- Kamata T., Wright,R. and Takada,Y. (1995) Critical threonine and aspartic acid residues within the I domains of β2 integrins for interactions with intercellular adhesion molecule 1 (ICAM-1) and C3bi. J. Biol. Chem., 270, 12531–12535. [DOI] [PubMed] [Google Scholar]
- Kappe S., Bruderer,T., Gantt,S., Fujioka,H., Nussenzweig,V. and Ménard,R. (1999) Conservation of a gliding motility and cell invasion machinery in apicomplexan parasites. J. Cell Biol., 147, 937–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern A., Briesewitz,R., Bank,I. and Marcantonio,E.E. (1994) The role of the I domain in ligand binding of the human integrin α1β1. J. Biol. Chem., 269, 22811–22816. [PubMed] [Google Scholar]
- Lawler J. and Hynes,R.O. (1986) The structure of human thrombospondin, an adhesive glycoprotein with multiple calcium-binding sites and homologies with several different proteins. J. Cell Biol., 10, 1635–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.-O., Rieu,P., Arnaout,M.A. and Liddington,R. (1995) Crystal structure of the A domain from the α subunit of integrin CR3 (CD11b/CD18). Cell, 80, 631–638. [DOI] [PubMed] [Google Scholar]
- Lovett J.L., Howe,D.K. and Sibley,L.D. (2000) Molecular characteriz ation of a thrombospondin-related anonymous protein homologue in Neospora caninum. Mol. Biochem. Parasitol., 107, 33–43. [DOI] [PubMed] [Google Scholar]
- McCormick C.J., Tuckwell,D.S., Crisanti,A., Humphries,M.J. and Hollingdale,M.R. (1999) Identification of heparin as a ligand for the A-domain of Plasmodium falciparum thrombospondin-related adhesion protein. Mol. Biochem. Parasitol., 100, 111–124. [DOI] [PubMed] [Google Scholar]
- Ménard R. (2001) Gliding motility and cell invasion by Apicomplexa: insights from the Plasmodium sporozoite. Cell. Microbiol., 3, 63–73. [DOI] [PubMed] [Google Scholar]
- Ménard R. and Janse,C. (1997) Gene targeting in malaria parasites. Methods, 13, 148–157. [DOI] [PubMed] [Google Scholar]
- Ménard R. and Nussenzweig,V. (2000) Structure–function analysis of malaria proteins by gene targeting. Parasitol. Today, 16, 222–224. [DOI] [PubMed] [Google Scholar]
- Michishita M., Videm,V. and Arnaout,M.A. (1993) A novel divalent cation-binding site in the A domain of the β2 integrin CR3 (CD11b/CD18) is essential for ligand binding. Cell, 72, 857–867. [DOI] [PubMed] [Google Scholar]
- Muller H.-M., Reckmann,I., Hollingdale,M.R., Bujard,H., Robson,K.J. and Crisanti,A. (1993) Thrombospondin-related anonymous protein (TRAP) of Plasmodium falciparum binds specifically to sulfated glycoconjugates and to HepG2 hepatoma cells suggesting a role for this molecule in sporozoite invasion of hepatocytes. EMBO J., 12, 2881–2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes A., Thathy,V., Bruderer,T., Sultan,A.A., Nussenzweig,R.S. and Ménard,R. (1999) Subtle mutagenesis by ends-in recombination in malaria parasites. Mol. Cell. Biol., 19, 2895–2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu A. and Leahy,D.J. (1995) Crystal structure of the I-domain from the CD11a/CD18 (LFA-1, αLβ2) integrin. Proc. Natl Acad. Sci. USA, 92, 10277–10281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rénia L., Miltgen,F., Charoenvit,Y., Ponnudurai,T., Verhave,J.P., Collins,W.E. and Mazier,D. (1988) Malaria sporozoite penetration. A new approach by double staining. J. Immunol. Methods, 112, 201–205. [DOI] [PubMed] [Google Scholar]
- Robson K.J., Naitza,S., Barker,G., Sinden,R.E. and Crisanti,A. (1997) Cloning and expression of the thrombospondin related adhesive protein gene of Plasmodium berghei. Mol. Biochem. Parasitol., 84, 1–12. [DOI] [PubMed] [Google Scholar]
- Rogers W.O. et al. (1992) Characterization of Plasmodium falciparum sporozoite surface protein 2. Proc. Natl Acad. Sci. USA, 89, 9176–9180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidjanski S.P., Vanderberg,J.P. and Sinnis,P. (1997) Anopheles stephensi salivary glands bear receptors for region I of the circumsporozoite protein of Plasmodium falciparum. Mol. Biochem. Parasitol., 90, 33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinnis P., Clavijo,P., Fenyo,D., Chait,B.T., Cerami,C. and Nussenzweig,V. (1994) Structural and functional properties of region II-plus of the malaria circumsporozoite protein. J. Exp. Med., 180, 297–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spano F., Putignani,L., Naitza,S., Puri,C., Wright,S. and Crisanti,A. (1998) Molecular cloning and expression analysis of a Cryptosporidium parvum gene encoding a new member of the thrombospondin family. Mol. Biochem. Parasitol., 92, 147–162. [DOI] [PubMed] [Google Scholar]
- Sultan A.A., Thathy,V., Frevert,U., Robson,K.J., Crisanti,A., Nussenzweig,V., Nussenzweig,R.S. and Ménard,R. (1997) TRAP is necessary for gliding motility and infectivity of Plasmodium sporozoites. Cell, 90, 511–522. [DOI] [PubMed] [Google Scholar]
- Templeton T.J. and Kaslow,D.C. (1997) Cloning and cross-species comparison of the thrombospondin-related anonymous protein (TRAP) gene from Plasmodium knowlesi, Plasmodium vivax and Plasmodium gallinaceum. Mol. Biochem. Parasitol., 84, 13–24. [DOI] [PubMed] [Google Scholar]
- Templeton T.J., Kaslow,D.C. and Fidock,D.A. (2000) Developmental arrest of the human malaria parasite Plasmodium falciparum within the mosquito midgut via CTRP gene disruption. Mol. Microbiol., 36, 1–9. [DOI] [PubMed] [Google Scholar]
- Tomley F.M., Clarke,L.E., Kawazoe,U., Dijkema,R. and Kok,J.J. (1991) Sequence of the gene encoding an immunodominant microneme protein of Eimeria tenella. Mol. Biochem. Parasitol., 49, 277–288. [DOI] [PubMed] [Google Scholar]
- Trottein F., Triglia,T. and Cowman,A.F. (1995) Molecular cloning of a gene from Plasmodium falciparum that codes for a protein sharing motifs found in adhesive molecules from mammals and plasmodia. Mol. Biochem. Parasitol., 74, 129–141. [DOI] [PubMed] [Google Scholar]
- Vanderberg J.P. (1974) Studies on the motility of Plasmodium sporozoites. J. Protozool., 21, 527–537. [DOI] [PubMed] [Google Scholar]
- Vanderberg J.P. (1975) Development of infectivity by the Plasmodium berghei sporozoite. J. Parasitol., 61, 43–50. [PubMed] [Google Scholar]
- Wan K.L., Carruthers,V., Sibley,L.D. and Ajioka,J.W. (1997) Molecular characterisation of an expressed sequence tag locus of Toxoplasma gondii encoding the micronemal protein MIC2. Mol. Biochem. Parasitol., 84, 203–214. [DOI] [PubMed] [Google Scholar]
- Wengelnik K., Spaccapelo,R., Naitza,S., Robson,K.J., Janse,C.J., Bistoni,F., Waters,A.P. and Crisanti,A. (1999) The A-domain and the thrombospondin-related motif of Plasmodium falciparum TRAP are implicated in the invasion process of mosquito salivary glands. EMBO J., 18, 5195–5204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuda M., Sakaida,H. and Chinzei,Y. (1999) Targeted disruption of the Plasmodium berghei CTRP gene reveals its essential role in malaria infection of the vector mosquito. J. Exp. Med., 190, 1711–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]