Abstract
The process of angiogenesis has been well documented, but little is known about the biology of lymphatic endothelial cells and the molecular mechanisms controlling lymphangiogenesis. The homeobox gene Prox1 is expressed in a subpopulation of endothelial cells that, after budding from veins, gives rise to the mammalian lymphatic system. In Prox1–/– embryos, this budding becomes arrested at around embryonic day (E)11.5, resulting in embryos without lymphatic vasculature. Unlike the endothelial cells that bud off in E11.5 wild-type embryos, those of Prox1-null embryos did not co-express any lymphatic markers such as VEGFR-3, LYVE-1 or SLC. Instead, the mutant cells appeared to have a blood vascular phenotype, as determined by their expression of laminin and CD34. These results suggest that Prox1 activity is required for both maintenance of the budding of the venous endothelial cells and differentiation toward the lymphatic phenotype. On the basis of our findings, we propose that a blood vascular phenotype is the default fate of budding embryonic venous endothelial cells; upon expression of Prox1, these budding cells adopt a lymphatic vasculature phenotype.
Keywords: lymphangiogenesis/lymphatic endothelial cells/LYVE-1/Prox1/SLC/VEGFR-3
Introduction
The lymphatic system is a vascular network of thin-walled capillaries and larger vessels lined by a continuous layer of endothelial cells that drain lymph from the tissue spaces of most organs and return it to the venous system for recirculation. Although much information has been gained regarding the normal and pathological growth of the vascular system (Gale and Yancopoulos, 1999), the lack of specific lymphatic markers has made it difficult to elucidate the development of the lymphatic system. Consequently, the study of the formation of the lymphatic vasculature and its possible role in tumor metastasis has been neglected in the past, and the understanding of the precise manner by which the lymphatic system develops is still rudimentary.
Several recent reports (Banerji et al., 1999; Wigle and Oliver, 1999; Karkkainen et al., 2001; Nakano and Gunn, 2001) have described the identification of novel lymphatic markers. Furthermore, other studies have provided evidence that both VEGF-C and VEGF-D, which are ligands for the vascular endothelial growth factor receptor 3 (VEGFR-3), can enhance tumor lymphangiogenesis and lymphatic metastasis (Makinen et al., 2001; Mandriota et al., 2001; Skobe et al., 2001; Stacker et al., 2001). However, a detailed comparison of the expression patterns of these recently identified lymphatic markers during early stages of lymphatic development is not yet available.
Our previous work (Wigle and Oliver, 1999) validated Sabin’s original proposal of the venous origin of the primary lymph sacs (Sabin, 1902). Our results also indicated that the expression of Prox1 in a restricted subpopulation of endothelial cells in the embryonic veins was required to promote lymphangiogenesis and that the initial localization and subsequent migration of the lymphatic endothelial cells from the cardinal vein were polarized (the endothelial cells appear to stream together along a defined pathway). We also showed that in Prox1-null mice, budding and sprouting of lymphatic endothelial cells from the veins appear unaffected at embryonic day (E)10.5. However, both processes are arrested prematurely at around E11.5–E12.0 and, as a result of this arrest, Prox1-null mice are devoid of lymphatic vasculature (Wigle and Oliver, 1999). Ongoing studies in our laboratory had suggested that the budding and sprouting of endothelial cells from the cardinal vein which are still detectable in Prox1-null embryos at E11.5–E12.0, were no longer polarized (endothelial cells followed a random path in different directions around the cardinal vein); however, the subsequent fate of those endothelial cells was undetermined.
Is it possible that in addition to its importance in the budding and sprouting of lymphatic vessels during lymphangiogenesis, Prox1 might also play a key role in the differentiation and maturation of lymphatic endothelial cells? To address this important question, we first performed a detailed, comparative analysis of heterozygous and null Prox1 embryos between E10.5 and E14.5 using antibodies to lymphatic and blood vascular markers. Here we show that in contrast to the heterozygous Prox1 embryos, in E11.5–E12.0 mutant littermates the budding and migration of the endothelial cells from the cardinal vein are no longer polarized (follows a random path). Surprisingly, we also found that these budding endothelial cells have switched their differentiation program. In contrast to the heterozygous situation in which the budding Prox1 (β-galactosidase; β-gal)-positive cells corresponded to lymphatic endothelial cells, in Prox1-null embryos these budding β-gal-positive endothelial cells now display markers of blood vascular endothelial cells. These observations were supported by the finding that the mutant endothelial cells did not express any of the available lymphatic markers used in this study but instead they expressed blood vascular markers. Taken together, these results indicate that Prox1 activity in a restricted subpopulation of endothelial cells in the embryonic veins is required not only to promote lymphangiogenesis but also to determine a lymphatic fate.
In addition, we have also determined that Prox1, VEGFR-3, LYVE-1 and SLC are expressed similarly in lymphatic endothelial cells of normal adult and tumor tissues.
Results
Lymphatic markers during early embryonic development
To determine the mechanisms by which Prox1 regulates the budding and sprouting of lymphatic endothelial cells, we initially compared the expression of Prox1 during early murine embryonic development with that of other lymphatic markers.
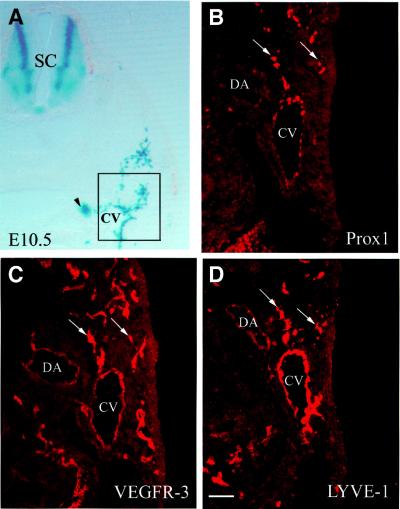
The first indication that lymphangiogenesis has begun is the specific expression of Prox1 in a restricted subpopulation of endothelial cells in the anterior cardinal vein at E9.5 (Wigle and Oliver, 1999). In wild-type embryos at E10.5, the restricted localization of Prox1 in the veins was still evident, and the first lymphatic endothelial cells had started to bud off in a polarized (not random) manner (Figure 1B). In previous studies, it has been shown that as embryonic development proceeds, expression of the VEGFR-3 gene becomes largely restricted to the lymphatic vessels, with lower levels of expression remaining in blood vessels (Kaipainen et al., 1995; Wigle and Oliver, 1999). In this study, we also detected high levels of VEGFR-3 expression in the budding Prox1-positive endothelial cells at E10.5 (Figure 1C); however, VEGFR-3 expression, although less pronounced, was still detected in the arteries and veins at that time. At E10.5, the expression pattern of Prox1 resembles that of the lymphatic endothelial hyaluronan (HA) receptor (LYVE-1) in the budding endothelial cells (Figure 1D). LYVE-1 is a member of the Link protein superfamily that recently was identified as a cell surface protein of lymphatic endothelial cells (Banerji et al., 1999; Jackson et al., 2001; Prevo et al., 2001). The only difference between the expression pattern of Prox1 and LYVE-1 at this stage was that LYVE-1 was expressed uniformly in the endothelial cells of the cardinal vein, whereas Prox1 expression in the vein was only detected in a restricted subpopulation of endothelial cells (Figure 1B).
Fig. 1. Prox1 is the earliest marker of a distinct subpopulation of endothelial cells in the cardinal vein. (A) Low power magnification of a LacZ-stained Prox1 heterozygous embryo in which the region of the cardinal vein (CV) that is examined throughout the text is highlighed by the box. The arrowhead indicates the Prox1-expressing sympathetic chain. (B–D) are adjacent sections. (B) At E10.5, Prox1 expression is detected in a subset of venous endothelial cells located near the outer margin of the cardinal vein. Initiation of budding of lymphatic endothelial cells proceeds in a polarized (following a specific path) manner (arrows). (C) VEGFR-3 expression is detected not only in the Prox-1-expressing endothelial cells (arrows) but also in the blood vascular endothelial cells. (D) The budding endothelial cells also express the lymph-specific receptor for hyaluronan (LYVE-1; arrows). However, in contrast to Prox-1, the expression of LYVE-1 in the endothelial cells lining the CV is distributed uniformly. DA, dorsal aorta; SC, spinal cord. Scale bar: 100 µm.
Interactions between LYVE-1 and the extracellular matrix glycosaminoglycan HA might regulate leukocyte migration through the lymphatic vasculature (Jackson et al., 2001). However, chemokines such as secondary lymphoid chemokine (SLC or CCL21), which are released by the lymphatic endothelium (Gunn et al., 1998, 1999; Zlotnik and Yoshie, 2000), regulate the attraction of leukocytes toward the lymphatic vessels. At E10.5, budding lymphatic endothelial cells had not yet begun to express SLC (data not shown), a finding that supports the hypothesis that these early budding endothelial cells (Prox1-, LYVE-1- and VEGFR-3-positive) are in the early stages of the lymphangiogenic pathway, prior to leukocyte intravasation.
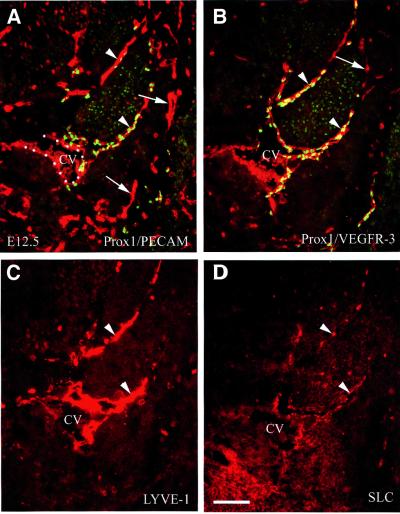
The number and distribution of lymphatic endothelial cells that had budded from the veins were greatly increased between E10.5 and E12.5. At E12.5, the number of Prox1- and LYVE-1-positive cells adjacent to the cardinal vein had clearly increased (Figure 2), but Prox1 and LYVE-1 expression was no longer detected in endothelial cells in the cardinal vein (Figure 2A and C). In the lymphatic endothelial cells, levels of VEGFR-3 remained high, whereas its expression in vascular endothelial cells had diminished substantially (Figure 2B). SLC expression was detected initially in only a subset of the budding lymphatic endothelial cells at E11.5 (Figure 6I). At E12.5, the pattern of SLC expression, while more patchy in appearance, was almost identical to that of the other lymphatic markers (Figure 2D). Maintenance of high levels of VEGFR-3 expression in the lymphatic endothelial cells, together with a reduction of its expression level in the vascular endothelial cells, as well as the beginning of expression of SLC in the Prox1- and LYVE-1-positive endothelial cells that had already budded off from the veins, is probably an indication that these cells are now specified to the lymphatic pathway.
Fig. 2. At E12.5, further development of the lymphatic vasculature is indicated by the co-expression of four lymphatic markers in the budding endothelial cells. (A) Prox1 (green) and the platelet endothelial cell adhesion molecule (PECAM-1/CD31; red) are co-expressed in budding endothelial cells (arrowheads). (B) The high level of VEGFR-3 expression (red) is maintained in lymphatic endothelial cells but to a lesser extent in blood vascular endothelial cells (arrow). The expression of LYVE-1 (C) and that of SLC (D) is restricted to the budding lymphatic endothelial cells (arrowheads). CV, cardinal vein. Scale bar: 100 µm.
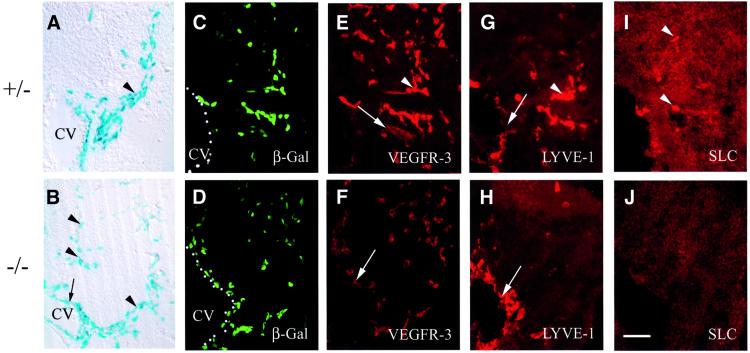
Fig. 6. At E11.5, β-gal-positive endothelial cells migrate randomly in Prox1-null embryos and do not express markers of lymphatic differentiation. (A) A LacZ-stained vibratome section showing that in Prox1 heterozygous embryos endothelial cells migrate from the cardinal vein (CV) in a polarized manner (arrowhead). (B) In contrast, in Prox1-null embryos, the β-gal-positive cells migrate in a dispersed manner (arrowheads). The heterozygous (C) and nullizygous (D) Prox1 embryos show β-gal-positive cells adjacent to the CV; however, the nullizygous embryos have fewer β-gal-positive cells. (E) Prox1 heterozygous embryos show strong expression of VEGFR-3 in lymphatic endothelial cells (arrowhead) and weaker VEGFR-3 expression in blood vascular endothelial cells (arrow). Only the weaker vascular staining (arrow) is present in the nullizygous littermate (F). In Prox1 heterozygous embryos, LYVE-1 is weakly expressed in the cardinal vein (arrow) and strongly expressed in the budding endothelial cells (arrowhead) (G). The expression of LYVE-1 in the Prox1-null embryos is restricted to the venous endothelial cells of the CV (H, arrow). The expression of SLC is detected in heterozygous embryos but only in a subset of lymphatic endothelial cells (I, arrowheads), and no SLC staining is detected in endothelial cells of Prox1-null embryos (J). Scale bar: 100 µm.
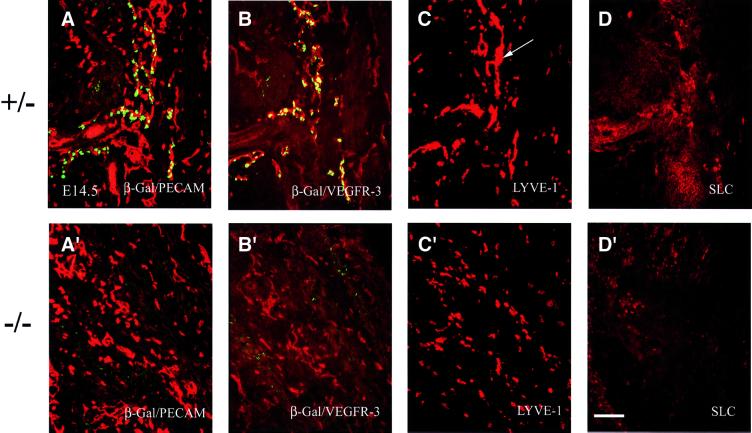
As previously shown (Wigle and Oliver, 1999) in Prox1 heterozygous embryos at E14.5, the lymphatic vasculature has spread throughout the embryo (Figure 3A). Prox1-positive endothelial cells co-expressed high levels of VEGFR-3, whereas Prox1-negative endothelial cells expressed low levels of this receptor (Figure 3B), indicating that these cells are blood vascular endothelia. The patterns of expression of LYVE-1 (Figure 3C) and SLC (Figure 3D) in adjacent sections were also similar to that of Prox1; however, LYVE-1 was also expressed in scattered non-lymphatic endothelial cells that corresponded to macro phages (data not shown; S.Banerji and D.G.Jackson, unpublished). In contrast to the well-developed lymphatic vasculature found in heterozygous E14.5 embryos, no lymphatic vasculature was present in a similar section from a Prox1 nullizygous littermate (Figure 3A′, and Wigle and Oliver, 1999). As demonstrated previously (Wigle and Oliver, 1999), the development of the blood vasculature had progressed normally, as indicated by the abundant expression of the platelet endothelial cell adhesion molecule (PECAM; Figure 3A′). The lymphatic endothelial cells that expressed high levels of VEGFR-3 were no longer present, and only cells that expressed low levels of VEGFR-3 were still detected in the blood vasculature (Figure 3B′). The capillary-like staining observed for LYVE-1 in the E14.5 heterozygous embryos was absent in Prox1-null littermates, and the only remaining staining corresponded to scattered macrophages (S.Banerji and D.G.Jackson, unpublished; Figures 3C′ and 7B). No endothelial-specific SLC expression was detected in the nullizgyous embryos at this time point (Figure 3D′).
Fig. 3. At E14.5, widespread expression of different lymphatic markers in Prox1 heterozygous embryos indicates the progression of lymphangiogenesis. Panels correspond to transverse sections taken at the level of the forelimb. (A) The lymphatic vessel network, which is indicated by the co-expression of β-gal/Prox1 (green) and CD31 (red), is interspersed with the blood vessels and capillaries, which are indicated by the expression of CD31 alone. The patterns of expression of VEGFR-3 (B), LYVE-1 (C) and SLC (D) overlap with that of Prox1/β-gal. LYVE-1 expression is detected in capillary-like structures (C, arrow) and in some scattered cells. (A′–D′) The absence of lymphatic vasculature in Prox1 nullizygous embryos is corroborated by the absence of VEGFR-3, SLC and LYVE-1 expression in endothelial cells at E14.5. (A′) CD31 expression in Prox1 null embryos indicates that the formation of the blood vascular system is normal in these mice. (B′) Low levels of VEGFR-3 expression are detected in only the blood vascular system. (C′) LYVE-1 expression is present, but only in scattered macrophages. (D′) No endothelial-specific expression of SLC is detected. Scale bar: 100 µm.
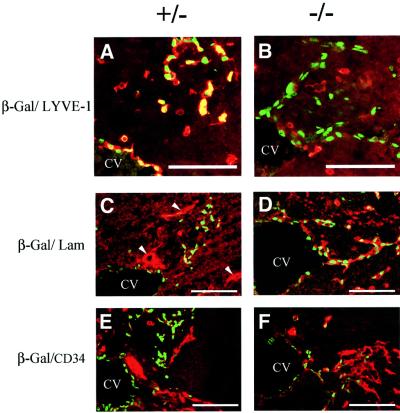
Fig. 7. At E11.5, β-gal-positive endothelial cells adopt a blood vascular phenotype in Prox1-null embryos. (A) Lymphatic endothelial cells (β-Gal, green) of heterozygous embryos are double-labeled by LYVE-1 (red). Single-labeled LYVE-1 cells correspond to macrophages. (B) In contrast, in Prox1-null embryos, budding β-gal-positive endothelial cells (green) do not express LYVE-1. (C) Lymphatic endothelial cells (β-Gal, green) of heterozygous embryos express low levels of laminin (red). Blood vessels express high levels of laminin in their continuous basement membrane (arrowheads). (D) In contrast, β-gal-positive endothelial cells (green) express high levels of laminin on their cell surfaces (red) in the Prox1-null littermates. (E) Similarly, the lymphatic endothelial cells (green) of the heterozygous embryos express very low levels of the blood vascular marker CD34 (red); however, the β-gal-positive endothelial cells of Prox1 homozygous littermates (F) express high levels of CD34. (CV, cardinal vein). Scale bar: 100 µm.
Lymphatic markers in normal adult and tumor tissues
In an effort to determine whether Prox1 might serve as a reliable marker of adult lymphatic vasculature, and whether the vasculature of different tumors express lymphatic markers in patterns that differ from those of normal adult lymphatic vasculature, we extended the comparison of the markers we used to characterize the embryonic lymphatic vasculature to two different tumor types that were surrounded by normal adult tissue.
Recent results have provided experimental evidence indicating that tumors can activate lymphangiogenesis, and that some vascular/lymphatic endothelial growth factors (VEGF-C and VEGF-D) can enhance lymphatic metastasis (Makinen et al., 2001; Mandriota et al., 2001; Skobe et al., 2001; Stacker et al., 2001). It has also been shown that angiosarcomas express markers for blood and lymphatic capillaries (Breiteneder-Geleff et al., 1999).
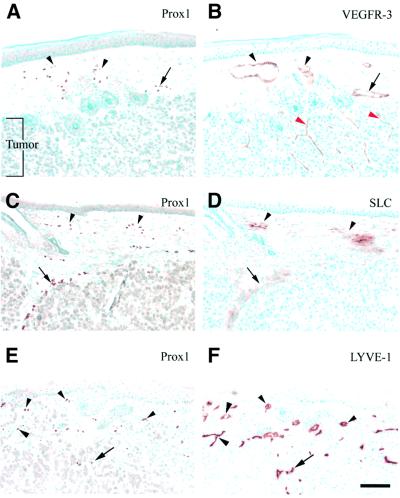
In a xenografted A431 human squamous cell carcinoma, Prox1-positive lymphatic vasculature was detected in the normal adult dermis adjacent to the tumor (Figure 4A and C) and, occasionally, within the tumor itself (Figure 4E). In adjacent sections, the Prox1-positive cells also co-expressed VEGFR-3 (Figure 4B), and weaker VEGFR-3 expression was detected in intratumoral blood vessels (Figure 4B, red arrowheads). All Prox1-positive vessels, including those proximal and those distal from the tumor, also expressed SLC (Figure 4D) and LYVE-1 (Figure 4F). Intratumoral LYVE-1 and Prox1 expression was also observed (arrows, Figure 4E and F). Similar results were also obtained in orthotopic carcinomas induced by a chemical carcinogenesis regimen (data not shown).
Fig. 4. Prox1 is a marker of the lymphatic vasculature in adult tissues. (A, C and E) Prox1 is expressed in the superficial lymphatic vessels located in normal adult tissues (arrowheads), and in the lymphatic vasculature surrounding (C) or inside (E) a squamous cell carcinoma (arrow). The co-expression of Prox1 and VEGFR-3 (B), SLC (D) and LYVE-1 (F) in adjacent sections confirmed the lymphatic-specific nature of Prox1-positive vessels. Red arrowheads in (B) indicate intratumoral VEGFR-3-positive blood vessels located inside the tumor. Scale bar: 100 µm.
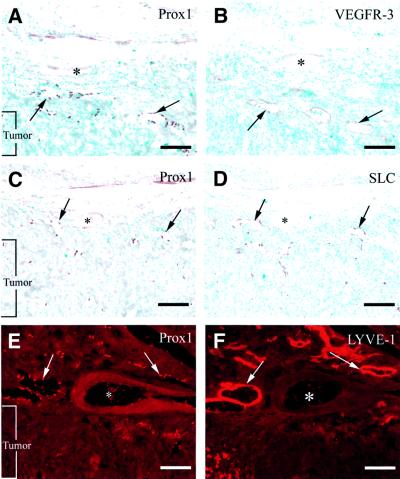
To determine whether Prox1 is a general marker of adult and tumor-associated lymphatic vasculature, we analyzed Prox1 expression in a spontaneous highly angiogenic lymphoma that developed in the leg of an Ink4d (p19ARF) mutant mouse and that was also surrounded by abundant normal tissue. Similarly to the squamous cell carcinoma, Prox1, VEGFR-3, LYVE-1 and SLC expression was detected in lymphatic vessels adjacent to the tumor (Figure 5).
Fig. 5. Prox1-positive lymphatic vessels are detected in the vicinity of a spontaneous murine lymphoma obtained from a Ink4d-null mouse. (A, C and E) Prox1-positive cells are observed in the lymphatic vessels around the tumor (arrows) but not in the blood vessels (asterisks). VEGFR-3 (B), SLC (D) and LYVE-1 (F) expression overlaps that of Prox1 in the lymphatic vessels (arrows). Scale bar: 100 µm.
Phenotypic characterization of endothelial cells of Prox1 nullizygous embryos
After we validated Prox1, VEGFR-3, LYVE-1 and SLC as suitable markers of the embryonic and adult lymphatic vasculature, we undertook the precise phenotypic characterization of the Prox1-null embryos at E11.5. At E10.5, lymphatic endothelial cell precursors bud off from the veins in normal numbers in Prox1 nullizygous embryos. However, starting at around E11.5, fewer than normal budding Prox1 (β-gal)-positive endothelial cells were detected in Prox1 nullizygous embryos (Wigle and Oliver, 1999), and this budding was no longer polarized, but instead the endothelial cells followed a random migratory path (Figure 6A–D). As expected, the β-gal-positive endo thelial cells in the heterozygous embryos also exhibited high levels of VEGFR-3 expression (Figure 6E). Sur prisingly, only weak VEGFR-3 expression was observed in the endothelial cells of the Prox1-null littermates, indicating that those cells most probably corresponded to blood vasculature cells (Figure 6F). In heterozygous embryos at this stage, LYVE-1 is weakly expressed in the cardinal vein and strongly expressed in the budding β-gal-positive endothelial cells (Figure 6G). The expression of LYVE-1 overlapped with that of β-gal in the cardinal vein but not in the budding β-gal-positive endothelial cells of Prox1 nullizygous littermates (Figure 6H). Some heterozygous endothelial cells started to express SLC at this stage (Figure 6I); however, none of the β-gal-positive endothelial cells of the nullizygous embryos expressed detectable levels of this chemokine (Figure 6J).
Expression of blood vascular markers in endothelial cells of Prox1 homozygous embryos
Unlike the lymphatic system, the blood vessels have a distinct continuous basal membrane that contains laminin (Ezaki et al., 1990). In addition, and in contrast to lymphatic endothelial cells, blood endothelial cells express high levels of the surface glycoprotein CD34 (Paal et al., 1998; Breiteneder-Geleff et al., 1999). Therefore, we used both of these markers to help identify the β-gal-positive endothelial cells present in Prox1-null embryos at E11.5. We now confirmed by double-labeling immunohistochemistry that in contrast to the wild-type embryo in which the budding β-gal-positive endothelial cells co-expressed LYVE-1 (Figure 7A), in the mutant littermate they did not (Figure 7B). In the heterozygous embryos, β-gal-positive endothelial cells budding from the cardinal vein did not co-express laminin (Figure 7C) and only expressed very low to undetectable levels of CD34 (Figure 7E). In the mutant littermates, budding β-gal-expressing cells expressed high levels of laminin (Figure 7D) and CD34 (Figure 7F). These results indicated that in Prox1-null embryos, the budding endothelial cells which are still detected at E11.5–E12.0 have a blood vascular phenotype, instead of the wild-type lymphatic phenotype. In addition, we have determined that already at E10.5, the β-gal-positive endothelial cells budding from the cardinal vein of the mutant embryos do not co-express LYVE-1 (data not shown). However, the differences in the levels of expression of laminin and CD34 between the wild-type and the mutant littermates are not yet as obvious as at E11.5 (data not shown).
Discussion
The lack of specific markers has hampered the understanding of the mechanisms controlling the development of the lymphatic vascular system. We have shown previously that Prox1 plays a key role in lymphangiogenesis (Wigle and Oliver, 1999). We found that Prox1 activity is not required to initiate budding of endothelial cells from the cardinal vein, but rather to maintain the budding and sprouting of a restricted subpopulation of endothelial cells that give rise to the lymphatic vasculature (Wigle and Oliver, 1999). By comparing the expression of lymphatic- and blood vascular-specific markers in Prox1 heterozygous and nullizygous embryos and in normal adult tissues and tumors, we have elucidated further the role of Prox1 in the development and maintenance of the lymphatic system.
To determine the phenotypic properties of Prox1-positive cells in heterozygous and nullizygous Prox1 embryos, we investigated the expression of three other available lymphatic markers (VEGFR-3, LYVE-1 and SLC) and two blood vascular markers (laminin and CD34). Functional inactivation of VEGFR-3 in mice disrupts the development of the cardiovascular system (Dumont et al., 1998). VEGFR-3 is expressed in the endothelial cells of some fenestrated blood vessels (Partanen et al., 2000) and in angiogenic blood vessels in some tumors (Valtola et al., 1999). However, during later embryonic development, VEGFR-3 expression becomes largely restricted to the lymphatic vessels (Kaipainen et al., 1995; Wigle and Oliver, 1999). The key role that this marker plays in the lymphatic system was demonstrated by the identification of mutations in VEGFR-3 in several cases of congenital lymphedema (Karkkainen et al., 2000).
LYVE-1, a member of the Link protein superfamily, was identified recently as a lymphatic-specific receptor for the extracellular matrix glycosaminoglycan HA (Banerji et al., 1999). HA is thought to provide a hydrated environment that facilitates cell transformation and migration during development (Jackson et al., 2001). LYVE-1 may also participate in the uptake or transport of HA across the lymphatic wall (Jackson et al., 2001; Prevo et al., 2001). Immunohistochemical analyses have demonstrated LYVE-1 expression on the surface of endothelial cells of lymphatic vessels (Jackson et al., 2001; Prevo et al., 2001).
SLC (or CCL21) is released by the lymphatic endothelium (Gunn et al., 1998, 1999; Zlotnik and Yoshie, 2000). The migration of leukocytes toward cells comprising the lymphatic vasculature is regulated, at least in part, by this chemokine. SLC is expressed uniformly in adult lymphatic endothelium (Gunn et al., 1998, 1999), and is expressed in embryonic lymphatics as early as E11.5 (this work).
In the present study, we found that β-gal-positive endothelial cells that start to bud from the veins of Prox1-null embryos, but are arrested at E11.5–E12.0, do not undergo lymphatic differentiation. These cells do not express any of the lymphatic markers except VEGFR-3 (at low levels), but they do express high levels of markers such as laminin and CD34, a finding that suggests that these cells have adopted a blood vascular phenotype.
In addition to the arrest of endothelial cell budding and migration observed in Prox1-null embryos at around E11.5, the polarity (directionality) of the budding was also defective. This finding suggests that Prox1 function is required for normal maintenance of some as yet unidentified signaling mechanism that is involved in guiding the budding and migration of the lymphatic endothelial cells. This molecule may be located in the surrounding tissue on one side of the cardinal vein, and its activity is dependent on Prox1 function, in an as yet undetermined cell-autonomous or non-cell-autonomous manner.
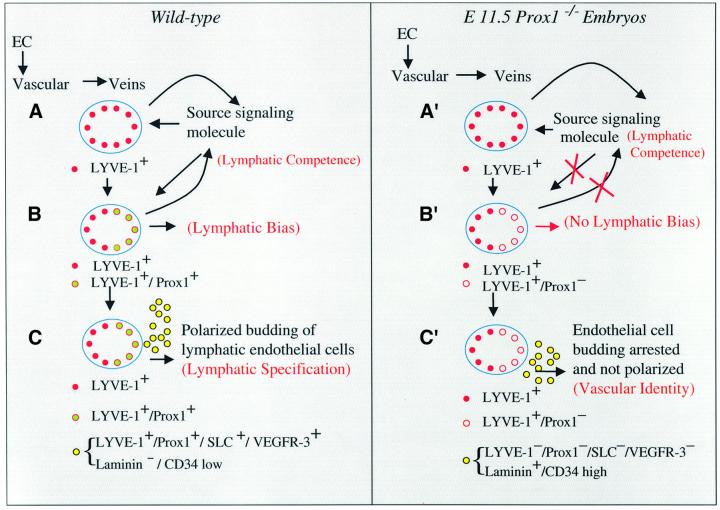
Our findings suggest that Prox1 activity is required not only to maintain budding and sprouting of a subpopulation of venous endothelial cells that will give rise to the lymphatic vasculature but also to determine the final fate of those budding endothelial cells. On the basis of our results, we have developed a working model of early lymphatic vascular development (Figure 8). After the initial formation of the vascular system, the expression of LYVE-1 and Prox1 in endothelial cells in the cardinal veins at approximately E9.5–E10.0 is probably one of the first indications that lymphangiogenesis has been initiated. All endothelial cells in the veins are probably initially bipotent and, upon the expression of at least Prox1 in a restricted subpopulation of venous endothelial cells (on only one side of the cardinal vein), those cells become committed (biased) to initiate the lymphatic differentiation program. As development proceeds, this subpopulation of LYVE-1- and Prox1- positive endothelial cells starts to bud from the veins in an initially Prox1-independent manner. However, maintenance of the budding and migration requires Prox1 activity. Normally, as the cells bud in a polarized manner, they start to express additional lymphatic endothelial markers. At this stage, SLC expression is first detected, and VEGFR-3 expression is maintained at high levels in budding lymphatic endothelial cells, but its expression becomes weaker in blood vascular endothelial cells. The expression of these four lymphatic markers may indicate that this process becomes specified irreversibly toward the lymphatic pathway. On the basis of our results, we suggest that this step is also dependent on Prox1 activity and on a feedback signaling mechanism required for the maintenance and polarized budding of these lymphatic endothelial cells. Endothelial cell budding and migration are arrested because of the lack of Prox1 function, and random budding occurs because of a failure in the feedback loop signaling mechanism. As a result, neither lymphatic bias nor lymphatic specification is accomplished. Therefore, Prox1 activity in a restricted subpopulation of endothelial cells in the embryonic veins is required not only to promote lymphangiogenesis but also to determine the lymphatic fate by the initiation of the lymphatic differentiation program of those budding venous endothelial cells. In the future, the identification of the molecules and the mechanisms involved in these developmental decisions will provide important information for our understanding of lymphangiogenesis during development and in diseases such as cancer.
Fig. 8. Proposed model for the development of the mammalian lymphatic vasculature. Upon the formation of the vascular system, LYVE-1 starts to be expressed in venous endothelial cells (EC) in the cardinal vein at approximately E9.5–E10.0. Probably at around this stage this tissue becomes competent to respond to a specific lymphatic inductive signal [(A), lymphatic competence]. Almost at the same time and following its polarized induction by some as yet unknown short-range signaling molecule, Prox1 starts to be expressed in a restricted subpopulation of endothelial cells in the cardinal vein. Initially, all endothelial cells in the veins are probably bipotent and it is upon the expression of at least Prox1 that some of those cells become biased (committed) to initiate the lymphatic differentiation program. Expression of these two markers is one of the first indications that lymphangiogenesis has been initiated [(B), lymphatic bias]. As development proceeds, this subpopulation of LYVE-1- and Prox1-positive endothelial cells starts to bud from the veins in a Prox1-independent manner. At this stage, VEGFR-3 expression still remains almost equally high in both vascular and lymphatic endothelial cells. However, maintenance of the budding and migration requires Prox1 activity and a feedback signaling mechanism. As the cells bud in a polarized manner, they start to express additional lymphatic endothelial markers. At around E11.5, SLC expression is first detected and, while the VEGFR-3 expression level remains high in budding lymphatic endothelial cells, it weakens in blood vascular endothelial cells. The expression of these four lymphatic markers may indicate that this process becomes specified irreversibly toward the lymphatic pathway [(C), lymphatic specification]. Our results suggest that this step is also dependent on Prox1 activity and on the feedback signaling mechanism required for the maintenance and polarized budding of these lymphatic endothelial cells. In Prox1-null embryos, endothelial cell budding becomes arrested at around E11.5–E12.0 due to the lack of Prox1 function, and random budding occurs because the feedback loop signaling mechanism is interrupted. As a result, the budding cells still detected in E11.5–E12.0 Prox1-null embryos do not express any of the lymphatic markers (LYVE-1, Prox1, SLC or VEGFR-3), but instead they express high levels of vascular markers (laminin and CD34). As a result, lymphatic bias, and therefore lymphatic specification, are never accomplished, and a default blood vascular identity is acquired instead.
Materials and methods
Animals
Prox1 heterozygous and Ink4d nullizygous mice were generated as previously described (Kamijo et al., 1997; Wigle et al., 1999); these methods followed NIH-approved institutional animal care guidelines.
Immunohistochemistry
Embryos were dissected and fixed in 4% paraformaldehyde by constant shaking at 4°C for a period of 1 h to overnight. The embryos were cryoprotected in 30% sucrose dissolved in phosphate-buffered saline (PBS), embedded in tissue-freezing medium (Triangle Biomedical Sciences, Durham, NC), and cut into 10 µm sections on a cryostat. Sections for immunohistochemical analyses were treated with an Avidin/Biotin Blocking Kit (Vector Laboratories, Burlingame, CA) before the primary antisera were added.
The following primary antibodies were used in this study; rabbit anti-Prox1 (G.Oliver, unpublished), rabbit anti-mouse LYVE-1 (Prevo et al., 2001), rat anti-mouse LYVE-1 (polyclonal serum generated against mouse LYVE-1 Fc; L.A.Johnson and D.G.Jackson, unpublished); goat anti-VEGFR-3/Flt4 and goat anti-6Ckine/secondary lymphoid chemokine (R&D Systems, Minneapolis, MN), rat anti-PECAM-1/CD31, rat anti-CD34 and mouse anti-CD45 (all from Pharmingen, San Diego, CA), rat anti-laminin (Biodesign, Saco, ME) and rabbit anti-β-galactosidase (ICN Pharmaceuticals, Inc., Costa Mesa, CA). The LacZ gene was inserted in-frame into the original knock-out construct (Wigle et al., 1999) so that its product, β-gal, could be used in heterozygous and nullizygous Prox1 embryos to detect cells that express Prox1 in wild-type embryos. Primary antibodies were diluted in a solution of 20% heat-inactivated fetal bovine serum (HyClone Laboratories, Inc., Logan, UT) and 2% blocking reagent (Roche, Indianapolis, IN) in maleate buffer (100 mM maleic acid, 150 mM NaCl pH 7.5); overnight incubations were carried out in a humidified chamber at room temperature. The following Cy3-conjugated antibodies were used for fluorescence labeling: goat anti-rabbit IgG, donkey anti-goat IgG and donkey anti-rat IgG (all from Jackson ImmunoResearch Laboratiories, Inc., West Grove, PA) and Alexa 488-conjugated goat anti-rabbit IgG (Molecular Probes, Eugene, OR).
For immunohistochemical analyses, biotinylated donkey anti-rabbit IgG (Jackson ImmunoResearch Laboratories) and donkey anti-goat IgG (Santa Cruz Biotechnology, Santa Cruz, CA) were used in conjunction with the Vectastain ABC Kit (Vector Laboratories). The horseradish peroxidase-stained sections were counterstained with a 0.5% aqueous solution of methyl green (Sigma, St Louis, MO).
Tumorigenesis models
Two models of tumorigenesis were used. First, the xenograft experiment was performed as previously described (Streit et al., 1999). Briefly, human A431 squamous carcinoma cells were injected (2 × 106 cells per injection) intradermally into BALB/c (nu/nu) mice, and the mice were sacrificed 3 weeks later. Frozen tumor xenografts were cut into 6 µm sections and fixed for 30 min at 4°C in 4% paraformaldehyde.
The second tumor model was a naturally occurring T lymphoma that developed in the leg of a 6-month-old Ink4d nullizygous mouse. The tumor was dissected, fixed in 4% paraformaldehyde overnight at 4°C, cryoprotected in tissue-freezing medium and cut into 10 µm sections on a cryostat. To discriminate between a lymphoma and a myogenic tumor, sections were stained with either anti-desmin or anti-CD45 antibodies. The tumor did not stain with anti-desmin antibodies but did stain with an anti-CD45 antibody.
Acknowledgments
Acknowledgements
We thank S.Self and J.Morgan for technical assistance, and M.Roussel and C.Sherr for the Ink4d nullizygous mouse. This work was supported in part by grants GM58462 and EY12162 from the National Institutes of Health (G.O.), by a fellowship from the Medical Research Council of Canada (J.W.), by a Cancer Center Support (CORE) grant CA21765 from the National Cancer Institute, and by the American Lebanese Syrian Associated Charities (ALSAC).
References
- Banerji S., Ni,J., Wang,S.X., Clasper,S., Su,J., Tammi,R., Jones,M. and Jackson,D.G. (1999) LYVE-1, a new homologue of the CD44 glycoprotein, is a lymph-specific receptor for hyaluronan. J. Cell Biol., 144, 789–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breiteneder-Geleff S. et al. (1999) Angiosarcomas express mixed endothelial phenotypes of blood and lymphatic capillaries: podoplanin as a specific marker for lymphatic endothelium. Am. J. Pathol., 154, 385–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont D.J., Jussila,L., Taipale,J., Lymboussaki,A., Mustonen,T., Pajusola,K., Breitman,M. and Alitalo,K. (1998) Cardiovascular failure in mouse embryos deficient in VEGF receptor-3. Science, 282, 946–949. [DOI] [PubMed] [Google Scholar]
- Ezaki T., Matsuno,K., Fujii,H., Hayashi,N., Miyakawa,K., Ohmori,J. and Kotani,M. (1990) A new approach for identification of rat lymphatic capillaries using a monoclonal antibody. Arch. Histol. Cytol., 53, 77–86. [DOI] [PubMed] [Google Scholar]
- Gale N.W. and Yancopoulos,G.D. (1999) Growth factors acting via endothelial cell-specific receptor tyrosine kinases: VEGFs, angiopoietins and ephrins in vascular development. Genes Dev., 13, 1055–1066. [DOI] [PubMed] [Google Scholar]
- Gunn M.D., Tangemann,K., Tam,C., Cyster,J.G., Rosen,S.D. and Williams,L.T. (1998) A chemokine expressed in lymphoid high endothelial venules promotes the adhesion and chemotaxis of naive T lymphocytes. Proc. Natl Acad. Sci. USA, 95, 258–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunn M.D., Kyuwa,S., Tam,C., Kakiuchi,T., Matsuzawa,A., Williams,L.T. and Nakano,H. (1999) Mice lacking expression of secondary lymphoid organ chemokine have defects in lymphocyte homing and dendritic cell localization. J. Exp. Med., 189, 451–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson D.G., Prevo,R., Clasper,S. and Banerji,S. (2001) LYVE-1, the lymphatic system and tumor lymphangiogenesis. Trends Immunol., 22, 317–321. [DOI] [PubMed] [Google Scholar]
- Kaipainen A., Korhonen,J., Mustonen,T., van Hinsbergh,V.W., Fang,G.H., Dumont,D., Breitman,M. and Alitalo,K. (1995) Expression of the fms-like tyrosine kinase 4 gene becomes restricted to lymphatic endothelium during development. Proc. Natl Acad. Sci. USA, 92, 3566–3570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamijo T., Zindy,F., Roussel,M.F., Quelle,D.E., Downing,J.R., Ashmun,R.A., Grosveld,G. and Sherr,C.J. (1997) Tumor suppression at the mouse locus mediated by the alternative reading frame product p19ARF. Cell, 91, 649–659. [DOI] [PubMed] [Google Scholar]
- Karkkainen M.J., Ferrell,R.E., Lawrence,E.C., Kimak,M.A., Levinson,K.L., McTigue,M.A., Alitalo,K. and Finegold,D.N. (2000) Missense mutations interfere with VEGFR-3 signalling in primary lymphoedema. Nature Genet., 25, 153–159. [DOI] [PubMed] [Google Scholar]
- Karkkainen M.J., Jussila,L., Ferrell,R.E., Finegold,D.N. and Alitalo,K. (2001) Molecular regulation of lymphangiogenesis and targets for tissue oedema. Trends Mol. Med., 7, 18–22. [DOI] [PubMed] [Google Scholar]
- Makinen T. et al. (2001) Inhibition of lymphangiogenesis with resulting lymphedema in transgenic mice expressing soluble VEGF receptor-3. Nature Med., 7, 199–205. [DOI] [PubMed] [Google Scholar]
- Mandriota S.J. et al. (2001) Vascular endothelial growth factor-C-mediated lymphangiogenesis promotes tumour metastasis. EMBO J., 20, 672–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano H. and Gunn,M.D. (2001) Gene duplications at the chemokine locus on mouse chromosome 4: multiple strain-specific haplotypes and the deletion of secondary lymphoid-organ chemokine and EBI-1 ligand chemokine genes in the plt mutation. J. Immunol., 166, 361–369. [DOI] [PubMed] [Google Scholar]
- Paal E., Thompson,L.D. and Heffess,C.S. (1998) A clinicopathologic and immunohistochemical study of ten pancreatic lymphangiomas and a review of the literature. Cancer, 82, 2150–2158. [DOI] [PubMed] [Google Scholar]
- Partanen T.A., Arola,J., Saaristo,A., Jussila,L., Ora,A., Miettinen,M., Stacker,S.A., Achen,M.G. and Alitalo,K. (2000) VEGF-C and VEGF-D expression in neuroendocrine cells and their receptor, VEGFR-3, in fenestrated blood vessels in human tissues. FASEB J., 14, 2087–2096. [DOI] [PubMed] [Google Scholar]
- Prevo R., Banerji,S., Ferguson,D.J., Clasper,S. and Jackson,D.G. (2001) Mouse LYVE-1 is an endocytic receptor for hyaluronan in lymphatic endothelium. J. Biol. Chem., 276, 19420–19430. [DOI] [PubMed] [Google Scholar]
- Sabin F.R. (1902) On the origin of the lymphatic system from the veins and the development of the lymph hearts and thoracic duct in the pig. Am. J. Anat., 1, 367–389. [Google Scholar]
- Skobe M. et al. (2001) Induction of tumor lymphangiogenesis by VEGF-C promotes breast cancer metastasis. Nature Med., 7, 192–198. [DOI] [PubMed] [Google Scholar]
- Stacker S.A. et al. (2001) VEGF-D promotes the metastatic spread of tumor cells via the lymphatics. Nature Med., 7, 186–191. [DOI] [PubMed] [Google Scholar]
- Streit M., Riccardi,L., Velasco,P., Brown,L.F., Hawighorst,T., Bornstein, P. and Detmar,M. (1999) Thrombospondin-2: a potent endogenous inhibitor of tumor growth and angiogenesis. Proc. Natl Acad. Sci. USA, 96, 14888–14893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valtola R. et al. (1999) VEGFR-3 and its ligand VEGF-C are associated with angiogenesis in breast cancer. Am. J. Pathol., 154, 1381–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigle J.T. and Oliver,G. (1999) Prox1 function is required for the development of the murine lymphatic system. Cell, 98, 769–778. [DOI] [PubMed] [Google Scholar]
- Wigle J.T., Chowdhury,K., Gruss,P. and Oliver,G. (1999) Prox1 function is crucial for mouse lens-fibre elongation. Nature Genet., 21, 318–322. [DOI] [PubMed] [Google Scholar]
- Zlotnik A. and Yoshie,O. (2000) Chemokines: a new classification system and their role in immunity. Immunity, 12, 121–127. [DOI] [PubMed] [Google Scholar]