Abstract
Background
Given the high incidence of gastrointestinal disorders in intensive rabbit production, we assessed the effects of graded levels of xylooligosaccharides (XOS) on growth performance, nutrient digestibility and intestinal health in growing rabbits.
Methods
The 35-day-old weaned rabbits (889.41 ± 0.41 g) were randomly assigned to five dietary treatments (0, 0.2, 0.3, 0.4 or 0.5 g/kg XOS) and the trial lasted for 35 d.
Results
The results revealed that linear trend responses of body weight (BW) to XOS on d 21 and 35 (P ≤ 0.05). During d 1–21, 0.2 g/kg XOS increased average daily feed intake (ADFI) while 0.5 g/kg improved feed conversion ratio (FCR) significantly (P ≤ 0.05). Weight gain rate (WGR) showed a linear trend, while FCR showed a quadratic response (P ≤ 0.05). Throughout the 35-d trial, 0.2 and 0.3 g/kg XOS enhanced ADFI, and 0.4 g/kg XOS improved FCR significantly, average daily gain (ADG) demonstrated linear dose-responsiveness, while WGR and FCR showed quadratic trends (P ≤ 0.05). Notably, 0.2 g/kg XOS elevated serum glutathione peroxidase (GSH-Px) activity and ileal secretory immunoglobulin A (sIgA) levels. Furthermore, 0.3, 0.4 and 0.5 g/kg XOS reduced jejunal malonaldehyde (MDA) content, 0.4 g/kg XOS decreased serum MDA, and 0.5 g/kg XOS elevated serum immunoglobulin M (IgM) significantly (P ≤ 0.05). 0.2, 0.4, 0.5 g/kg XOS improved the digestibility of crude fiber (CF), 0.2 and 0.4 g/kg XOS increased acid detergent fiber (ADF), and neutral detergent fiber (NDF) also increased among all treatments, although 0.5 g/kg XOS reduced cellulase activity significantly (P ≤ 0.05). Furthermore, graded levels of XOS significantly changed the relative abundance of specific bacteria, and 0.4 and 0.5 g/kg XOS enhanced the content of valeric acid significantly (P ≤ 0.05).
Conclusions
In conclusion, dietary supplementation of XOS serves as an effective nutritional strategy to optimize bacterial community in the cecum, improve fiber digestion and valeric acid production, while enhances resistance to intestinal pathogen infection and oxidative stress in rabbit production.
Supplementary Information
The online version contains supplementary material available at 10.1186/s40104-025-01268-9.
Keywords: Bacterial community, Fiber, Growing rabbit, Nutrient digestibility, Xylooligosaccharides
Introduction
Xylooligosaccharides (XOS) are oligomeric carbohydrates composed of 2–12 D-xylose units linked by β-1,4 glycosidic bonds and purified from lignocellulosic biomass [1]. These prebiotic compounds are abundantly present in agricultural byproducts, which represents a sustainable and renewable feedstock for XOS production [2, 3]. Dietary XOS supplementation demonstrates multifaceted benefits on intestinal health and growth performance of monogastric animals [4]. In broilers, XOS administration improves intestinal tissue morphology, while selectively modulating gut microbiota composition—notably elevating Bifidobacterium populations while suppressing Clostridium perfringens colonization, ultimately improving immunity and growth performance [5, 6]. Concurrently, XOS increased plasma level of immunoglobulin A (IgA), tumor necrosis factor-α (TNF-α), Immunoglobulin M (IgM) and interleukin-2 (IL-2) as well as the cecal content of butyric acid and acetic acid, while linearly reducing plasma levels of glutamic-pyruvic transaminase (GPT), cholesterol (CHO), high density lipoprotein (HDL) and very low-density lipoprotein (VLDL) linearly, which improving their intestinal health and immunity of laying hens [7, 8]. XOS supplementation demonstrates dose-dependent modulatory effects on porcine intestinal ecosystems. At lower supplementation levels (0.01% XOS), histological analysis revealed significantly increased jejunal and ileal villus height, accompanied by enhanced microbial α diversity indices and elevated short chain fatty acid (SCFA) concentrations by selective enrichment of Lactobacillus, Streptococcus, Bifidobacterium, and Turicibacter populations [9]. 0.05% XOS supplementation also showed the optimization of intestinal microbiota and increase the metabolites such as butyric acid and propionic acid [10, 11]. Notably, 0.04% XOS supplementation exhibited systemic antioxidant capacity via increase serum total antioxidant capacity (T-AOC), superoxide dismutase (SOD), and catalase activities, whereas decrease lipid peroxidation marker malondialdehyde (MDA), and significantly enhanced growth performance indices such as body weight (BW) and average daily gain (ADG) of piglets, which has the potential to prevent post-weaning intestinal dysfunction and improve production performance in piglets [12]. As a functional prebiotic, XOS demonstrate dual-action benefits for animal health and production performance: (1) protecting the gastrointestinal tract against colonization by pathogenic microbiota, (2) enhancing livestock products [13]. The unique physicochemical profile of XOS—characterized by exceptional acid tolerance (pH 2.5–8.0), high mechanical and thermal stress stability (600 MPa and 100 °C)—makes it particularly suitable for pharmaceutical formulations and functional feed development [14, 15].
Rabbits, as hindgut fermenters and obligate herbivores, have evolved specialized digestive systems adapted to process high-fiber diets consisting primarily of grass, hay and fibrous weeds. The majority of gastrointestinal disorders observed in captive rabbit populations are associated with specific nutritional imbalances, including either lower fiber intake, higher protein or carbohydrate levels in their feed [16, 17]. The gastrointestinal system of rabbits exhibits exceptional fragility. Post-weaning challenges arise from underdeveloped gastrointestinal function and intensive farming practices that utilize the prepared pellet feeds, which frequently lead to nutrition-related gastrointestinal disorders in captive rabbits [17]. Previous studies have shown that XOS can enhance the in vitro proliferation of lactic acid bacteria isolated from rabbit intestine [18]. However, adding 7.5 g/L of XOS to drinking water impairs rabbit growth and reduces nitrogen and energy retention [19].
XOS confer multiple benefits on gut health and growth performance in pigs and chickens. However, research on XOS in rabbits remains relatively limited, and its precise role in modulating intestinal digestion and production performance of rabbits remains unclear. We hypothesize that XOS supplementation may mitigate gastrointestinal complications associated with captive rabbit farming by promoting the activity of microorganisms. To test this hypothesis, we conducted a comprehensive investigation by supplementing different levels of XOS to the feed of growing rabbits. We systematically analyzed the effects of XOS on intestinal digestion and immunity. Our research provides a theoretical reference for optimizing the application of XOS in the nutritional management of rabbits.
Materials and methods
Xylooligosaccharides
XOS are provided by Yibin Yatai Biotechnology Co., Ltd. (China) with a content of 35%.
Experimental design and diets
This study performed a single-factor treatment design. A total of 150 mixed-sex New Zealand White rabbits (35 d old, body weight 889.41 ± 0.41 g) were randomly divided into 5 groups (n = 30 per group): CON (0 g/kg XOS), T1 (0.2 g/kg XOS), T2 (0.3 g/kg XOS), T3 (0.4 g/kg XOS), and T4 (0.5 g/kg XOS), all rabbits were housed in individual galvanized wire mesh cages (50 cm × 50 cm × 40 cm, length × width × height). During the experiment, all rabbits were fed twice a day with free access to feed and water. The entire experiment lasted for 35 d, and the health and death of animals was recorded daily.
According to previous studies, the experimental diet was formulated to meet the nutritional requirements of growing rabbits [20] and supplemented with 0, 0.2, 0.3, 0.4 and 0.5 g/kg XOS in the form of equivalent replacement of ball grinding bran in the basal diet [7, 11, 19]. All diets were pelleted at the feed mill of the experimental facility and stored in a dark and dry environment until the start of the trial. The basic diet composition and nutrient level are shown in Table 1.
Table 1.
Composition and nutrient levels of diets (DM basis)
| Ingredients | Content, % | Nutrient | Content, %2 |
|---|---|---|---|
| Alfalfa meal | 17.50 | DE, MJ/kg | 10.20 |
| Corn | 15.00 | CP | 15.58 |
| Soybean meal | 9.20 | CF | 15.79 |
| Corn germ meal | 9.05 | NDF | 33.84 |
| Wheat bran | 15.00 | ADF | 18.96 |
| Wheat middlings | 6.00 | ADL | 5.50 |
| Soybean hull | 10.00 | ST | 16.84 |
| Rice bran | 5.00 | Ca | 0.60 |
| Rice bran and hull | 9.90 | TP | 0.52 |
| Soybean oil | 1.40 | ||
| CaCO3 | 0.50 | ||
| NaCl | 0.38 | ||
| L-Lys (≥ 98.5%) | 0.01 | ||
| DL-Met (≥ 99%) | 0.04 | ||
| L-Thr (≥ 98.5%) | 0.02 | ||
| Premix1 | 1.00 | ||
| Total | 100.00 |
1Premix provided the following per kg of the diets: VA, 6,667 IU; VD, 1,333 IU; VE, 15 IU; VK3, 1 mg; VB2, 3 mg; VB6, 0.5 mg; VB12, 10 μg; biotin, 100 μg; niacin, 35 mg; pantothenic acid, 10 mg; choline chloride, 100 mg; Fe (FeSO4·H2O), 35 mg; Cu (CuSO4·5H2O), 6 mg; Zn (ZnSO4·H2O), 35 mg; Mn (MnSO4·H2O), 8 mg; I (KI), 0.4 mg; Co (CoCl2·6H2O), 0.3 mg; Se (Na2SeO3), 0.05 mg
2Nutrient levels were calculated values
Sample collections
Feed samples were collected from each group according to the national standards of the People’s Republic of China GB/T 14699.1-2023 [21]. Fecal samples were collected from 10 animals in each group for 4 consecutive days (from d 22 to 25) and treated with 10% hydrochloric acid for nitrogen fixation, and the samples were mixed evenly and dried to constant weight, then crushed and screened 40 mesh.
On d 22 of the experiment, 6 rabbits in each group were selected for collect blood samples from the auricular vein, and the samples were then centrifuged to obtain serum. Then the rabbits (n = 6 per group) were slaughtered in an industrial slaughterhouse and the tissue of jejunal, the mucosa of ileum, the chyme of ileum and cecum were collected. All samples were stored at −80 °C for further analysis.
Growth performance
During the experiment, feed intake and body weight were recorded weekly, and growth performance were calculated according to the following formula:
Serum biochemical indices
The concentrations of total protein (TP), albumin (ALB), globulin (GLB), total cholesterol (TC), triglyceride (TG), alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), glucose (GLU) and urea in serum were determined using the fully automatic biochemistry analyzer 3100 (Hitachi, Japan), and the kits used in the analyzer were obtained from Maccura Biotechnology Co., Ltd. (Chengdu, China).
The antioxidation of serum and jejunal tissues
The activity of superoxide dismutase (SOD), glutathione peroxidase (GSH-Px) and the content of malondialdehyde (MDA) in serum and jejunal tissue were determined using commercial assay kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China), and all procedures were conducted following the provided instruction manual.
The immunity of serum and ileal mucosa
The levels of immunoglobulin A (IgA), immunoglobulin G (IgG), immunoglobulin M (IgM) in serum and the levels of secretory immunoglobulin A (sIgA), IgM and mucin 2 (MUC2) in ileum were measured using the corresponding ELISA kits (Jiangsu Meimian Industrial Co., Ltd., Yancheng, China) according to the manufacturer’s instructions.
Apparent digestibility of nutrients
The contents of gross energy (GE) in fecal and diet samples were measured following to ISO 9831-1998 [22], the contents of dry matter (DM), crude protein (CP), ether extract (EE), crude fiber (CF), neutral detergent fiber (NDF), acid detergent lignin (ADL) and acid insoluble ash (AIA) in all samples was measured according to the national standards of the People’s Republic of China GB/T 6435-2014 [23], GB/T 6432-2018 [24], GB/T 6433-2006 [25], GB/T 6434-2022 [26], GB/T 20806-2022 [27], GB/T 20805-2006 [28] and GB/T 23742-2009 [29], respectively, and acid detergent fiber (ADF) was measured by the agricultural industry standard of the People’s Republic of China NY/T 1459-2022 [30]. AIA was used as an endogenous indicator and the apparent digestibility of nutrients were calculated according to the following formula:
Apparent digestibility of nutrients (%) = [1 − (the content of AIA in the diets/the content of AIA in the fecal) × (the content of a nutrient in the fecal/the content of a nutrient in the diets)] × 100.
The activity of enzymes in the digestive tract
The enzymatic activities of amylase, lipase, and trypsin in the jejunum, as well as cellulase, hemicellulase, and pectinase in the cecum were quantified using commercial assay kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
Analysis of short chain fatty acids
The 3 g of cecal digesta were homogenized with distilled water (1:1, w/v), vortexed, and centrifuged at 12,000 × g for 10 min. 1 mL of supernatant was mixed with 0.2 mL of 25% metaphosphoric acid, incubated for 30 min, and centrifuged again under the same conditions. Subsequently, a 100 μL of the resulting supernatant was combined with 100 μL methanol, vortexed, centrifuged (12,000 × g, 10 min), and the final supernatant stored at −20 °C. Short chain fatty acid concentrations were determined using a VARIAN CP-3800 gas chromatograph (Agilent Technologies, USA).
Analysis of bacterial community in cecum
Total genomic DNA of cecum contents was extracted and verified by 1% agarose gel electrophoresis. PCR amplification was performed using forward primer (5'-ACTCCTACGGGAGGCAGCAG-3') and reverse primer (5'-GGACTACHVGGGTWTCTAAT-3') targeting the V3–V4 region of the 16S rRNA gene. Sequencing was conducted on the Illumina NovaSeq PE250 platform. Bioinformatic processing included: (1) Quality control of raw sequencing data using fastp (version 0.20.0); (2) Read assembly using FLASH (version 1.2.7); (3) OTU clustering at 97% similarity threshold and chimeric sequence removal with UPARSE (version 7.1); and (4) Taxonomic annotation using RDP classifier (version 2.2) against the SILVA 16S rRNA database (version 138) with a 70% alignment confidence threshold.
Statistical analysis
Data are expressed as mean ± standard error and analyzed using one-way ANOVA through SPSS 27.0 (SPSS Inc., Chicago, IL, USA) for statistical computation and GraphPad Prism 10.1.2 for graphical representation. Differences between CON and each treatment groups were assessed using Dunnett's multiple comparison test. Additionally, orthogonal polynomial contrast analyses (linear and quadratic) were applied to growth performance data to analyze dose-response trends across XOS supplementation levels. β-Diversity analysis was performed using Bray-Curtis distances with Adonis-based PERMANOVA. Bacterial community and physiological indices correlations were evaluated via Pearson's rank correlation coefficients. P ≤ 0.05 was denoted as different significantly. Network relationships were visualized using R 4.2.2.
Results
Effects of XOS on survival rate and growth performance in growing rabbits
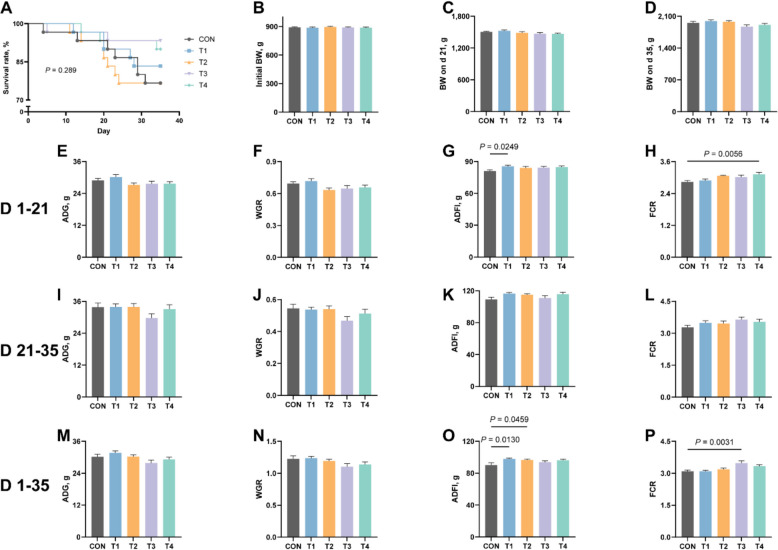
As illustrated in Fig. 1, dietary supplementation with XOS demonstrated a tendency to enhance the survival rate of experimental animals throughout the trial period, although this improvement did not significance (P > 0.05, Fig. 1A). Notably, BW on d 22 and 35 of the experimental period exhibited linear responses to XOS levels (P ≤ 0.05, Additional file 1). During the initial phase (1–21 d), significant enhancements were observed in ADFI for the T1 group (Fig. 1G) and FCR for the T4 group (P ≤ 0.05, Fig. 1H). WGR demonstrated a linear progression while FCR showed a quadratic pattern in relation to XOS levels (P ≤ 0.05, Additional file 1). Throughout the entire experimental period, XOS improved ADFI in both T1 and T2 groups, and enhanced FCR in the T3 group significantly (P ≤ 0.05, Fig. 1O and P). ADG exhibited a linear dose-response relationship to XOS levels, while both WGR and FCR displayed a quadratic trend (P ≤ 0.05, Additional file 1). However, during the later experimental phase (21–35 d), no significant treatment effects were detected for ADG, WGR, ADFI or FCR (P > 0.05). These findings collectively suggest that XOS supplementation may enhance survival rate and promote feed consumption in rabbits.
Fig. 1.
Effects of XOS on survival rate and growth performance in growing rabbits. A The survival rate of growing rabbits. B–D BW on d 0, d 21 and d 35 in growing rabbits. E–H ADG, WGR, ADFI and FCR on d 1–21 in growing rabbits. I–L ADG, WGR, ADFI and FCR on d 21–35 in growing rabbit. M–P. ADG, WGR, ADFI and FCR on d 1–35 in growing rabbits. BW, body weight; ADG, average daily gain; WGR, weight gain rate; ADFI, average daily feed intake; FCR, feed conversion ratio
Effects of XOS on serum biochemical indices in growing rabbits
As presented in Table 2, the supplementation of XOS in growing rabbit diets demonstrated no significant effects on protein such as TP, ALB, GLB or lipid metabolism parameters (TC and TG) in serum (P > 0.05). Furthermore, the enzymatic activities of ALT and ALP remained unaffected by XOS administration (P > 0.05). Compared to the CON group, AST activities also did not differ significantly according to Dunnett's multiple comparison test. Additionally, no statistically significant alterations were observed in serum GLU and Urea concentrations following XOS supplementation (P > 0.05).
Table 2.
Effects of XOS on serum biochemical indices in growing rabbits
| Items | Groups | P-value | ||||
|---|---|---|---|---|---|---|
| CON | T1 | T2 | T3 | T4 | ||
| TP, g/L | 48.64 ± 2.47 | 50.98 ± 2.40 | 50.46 ± 1.16 | 46.70 ± 2.61 | 46.14 ± 1.19 | 0.404 |
| ALB, g/L | 34.74 ± 1.84 | 35.22 ± 1.82 | 35.89 ± 0.62 | 32.42 ± 1.55 | 33.65 ± 0.81 | 0.551 |
| GLB, g/L | 12.89 ± 0.45 | 15.76 ± 1.31 | 13.81 ± 0.82 | 14.28 ± 1.65 | 12.95 ± 0.41 | 0.347 |
| TC, mmol/L | 1.70 ± 0.41 | 1.71 ± 0.12 | 1.83 ± 0.07 | 1.53 ± 0.16 | 1.55 ± 0.09 | 0.386 |
| TG, mmol/L | 1.28 ± 0.25 | 1.09 ± 0.10 | 1.05 ± 0.11 | 1.22 ± 0.16 | 0.97 ± 0.11 | 0.683 |
| ALT, U/L | 23.91 ± 3.25 | 32.10 ± 3.81 | 23.20 ± 0.57 | 29.96 ± 4.84 | 29.09 ± 3.62 | 0.404 |
| AST, U/L | 9.19 ± 2.65 | 17.59 ± 5.04 | 8.31 ± 1.45 | 8.66 ± 1.08 | 4.74 ± 0.25 | 0.048 |
| ALP, U/L | 172.40 ± 9.30 | 163.67 ± 12.37 | 197.00 ± 9.77 | 190.20 ± 28.76 | 172.67 ± 10.72 | 0.494 |
| GLU, mmol/L | 6.68 ± 0.22 | 7.07 ± 0.26 | 6.90 ± 0.10 | 6.52 ± 0.23 | 6.65 ± 0.22 | 0.439 |
| Urea, mmol/L | 3.55 ± 0.23 | 4.05 ± 0.34 | 3.45 ± 0.23 | 3.25 ± 0.25 | 3.02 ± 0.25 | 0.101 |
Multiple comparisons were analyzed using Dunnett's test
Effects of XOS on antioxidant indices in serum and jejunal tissue of growing rabbits
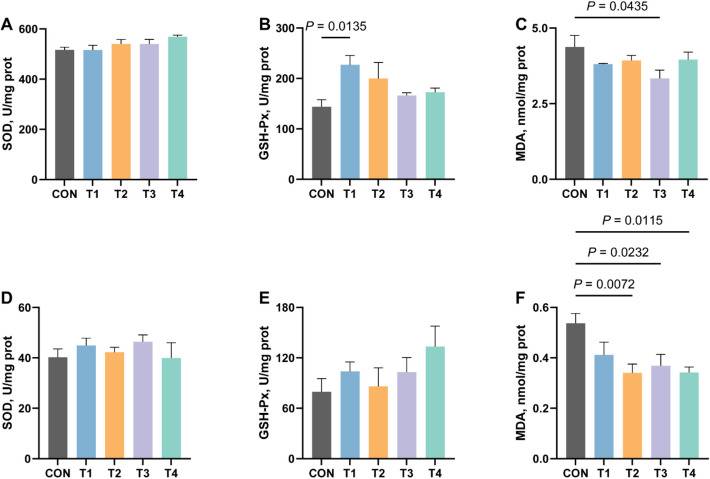
As illustrated in Fig. 2, dietary supplementation with XOS modulated antioxidant capacity in both serum and jejunal tissue of growing rabbits. Although XOS administration showed no significant alteration in SOD activity, it exhibited a significant enhancement of serum GSH-Px levels in T1 group compared to the CON group (P ≤ 0.05, Fig. 2B). The most prominent antioxidative effect of XOS was observed in its capacity to reduce the content of MDA, comparative analysis revealed that jejunal tissue MDA levels were significantly decreased in T2, T3 and T4 groups compared to the CON group, while serum MDA concentrations showed statistically significant reduction in the T3 group (P ≤ 0.05, Fig. 2C and F).
Fig. 2.
Effects of XOS on antioxidant indices in serum and jejunum of growing rabbits. A The activity of SOD in serum of growing rabbits. B The activity of GSH-Px in serum of growing rabbits. C The content of MDA in serum of growing rabbits. D The activity of SOD in jejunum of growing rabbits. E The activity of GSH-Px in jejunum of growing rabbits. F The content of MDA in jejunum of growing rabbits. SOD, superoxide dismutase; GSH-Px, glutathione peroxidase; MDA, malondialdehyde
Effects of XOS on immune indices in serum and ileum of growing rabbits
As depicted in Fig. 3, dietary supplementation with XOS elicited distinct immunomodulatory effects in growing rabbits. The T4 group demonstrated a significant increase in serum IgM levels compared to the CON group (P ≤ 0.05), while IgA and IgG concentrations remained unaffected in serum (P > 0.05). Notably, ileal analysis revealed marked enhancement of sIgA in the T1 group relative to the CON group (P ≤ 0.05). However, no statistically significant alterations were observed in ileal IgM levels or MUC2 expression among treatment groups (P > 0.05).
Fig. 3.
Effects of XOS on immune indices in serum and ileum of growing rabbits. A The content of IgA in serum of growing rabbits. B The content of IgG in serum of growing rabbits. C The content of IgM in serum of growing rabbits. D The content of sIgA in ileum of growing rabbits. E The content of IgM in ileum of growing rabbits. F The content of MUC2 in ileum of growing rabbits. IgA, immunoglobulin A; IgG, immunoglobulin G; IgM, immunoglobulin M; sIgA, secretory immunoglobulin A; MUC2, mucin 2
Effects of XOS on apparent digestibility of nutrient in growing rabbits
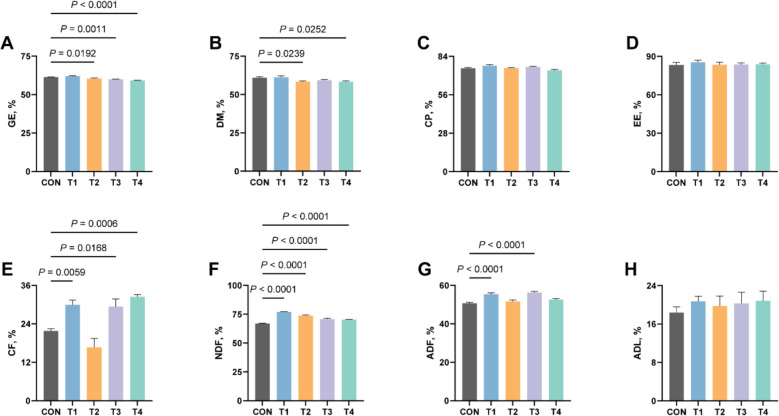
To further investigate the reasons for XOS-induced enhancement in feed intake, we evaluated its effects on nutrient digestibility in rabbits (Fig. 4). The results revealed that XOS supplementation reduced the digestibility of energy in T2, T3 and T4 groups compared to the CON group (P ≤ 0.05), while DM digestibility was reduced in T2 and T4 groups significantly (P ≤ 0.05). More importantly, XOS significantly improved fiber utilization efficiency, CF notably increased in T1, T3 and T4 groups, ADF was enhanced in T1 and T3 groups, and NDF showed significant elevation among all treatment groups (P ≤ 0.05). These findings collectively indicate that XOS supplementation substantially modulates the digestibility of energy, DM and fiber, potentially explaining its feed intake-promoting effects via improved fiber digestibility.
Fig. 4.
Effects of XOS on apparent digestibility of nutrient in growing rabbits. A The apparent digestibility of GE in growing rabbits. B The apparent digestibility of DM in growing rabbits. C The apparent digestibility of CP in growing rabbits. D The apparent digestibility of EE in growing rabbits. E The apparent digestibility of CF in growing rabbits. F The apparent digestibility of NDF in growing rabbits. G The apparent digestibility of ADF in growing rabbits. H The apparent digestibility of ADL in growing rabbits. GE, gross energy; DM, dry matter; CP, crude protein; EE, ether extract; CF, crude fiber; NDF, neutral detergent fiber; ADF, acid detergent fiber; ADL, acidic detergent lignin
Effects of XOS on the activity of digestive enzymes in growing rabbits
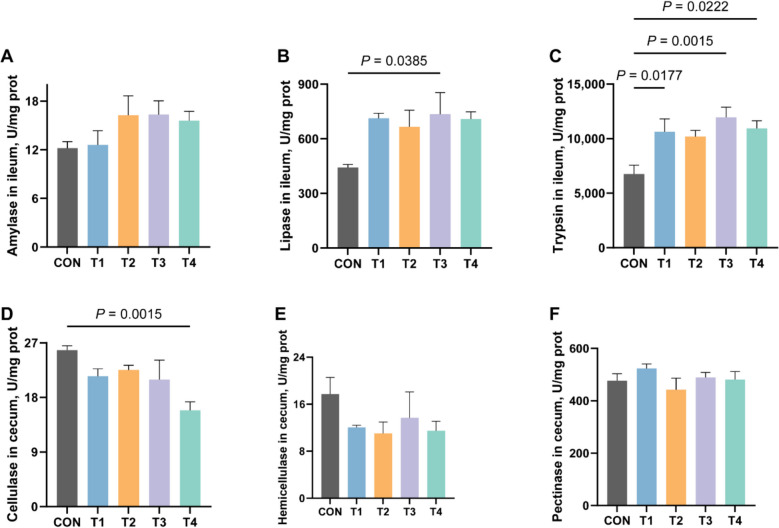
To further elucidate the mechanisms underlying the effects of XOS on nutrient digestion and absorption, we investigated its effects on digestive enzyme activities in the ileum and cecum (Fig. 5). The results demonstrated that lipase activity was significantly elevated in the T3 group compared to the CON group, while trypsin activity showed enhancement in T1, T3 and T4 groups significantly (P ≤ 0.05). However, cellulase activity in the cecum exhibited a significant reduction in the T4 group (P ≤ 0.05, Fig. 5D).
Fig. 5.
Effects of XOS on the activity of digestive enzymes in growing rabbits. A The activity of amylase in the ileum of growing rabbits. B The activity of lipase in the ileum of growing rabbits. C The activity of trypsin in the jejunum of growing rabbits. D The activity of cellulase in the caecum of growing rabbits. E The activity of hemicellulose in the caecum of growing rabbits. F The activity of pectinase in the caecum of growing rabbits
Effects of XOS on the composition and diversity of bacterial community in cecum of growing rabbits
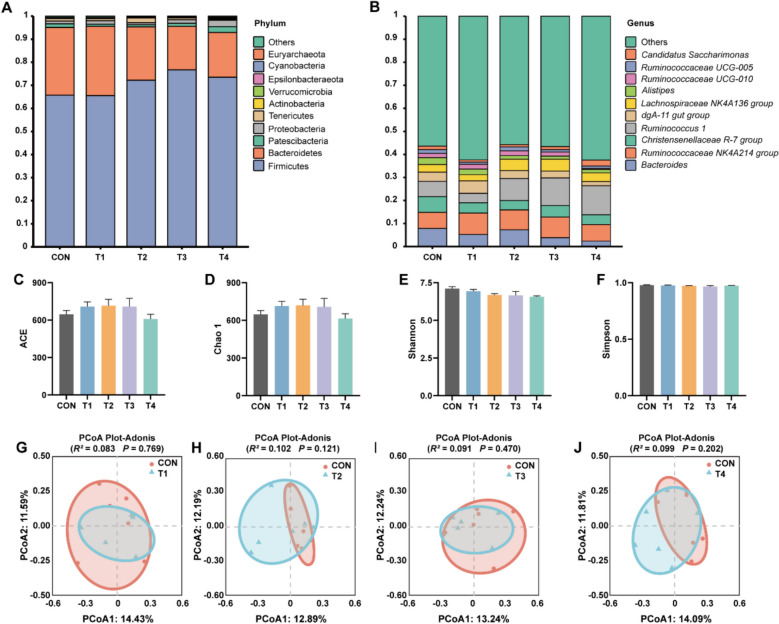
Given that rabbits primarily digest fiber through microbial activity in the cecum, a process generating SCFAs. We further analyzed bacterial community and SCFA contents in cecum (Fig. 6 and Table 3). At the phylum level, Firmicutes and Bacteroidetes predominated across all groups (CON = 95.10%, T1 = 95.52%, T2 = 95.32%, T3 = 95.53%, T4 = 92.92%), followed by Patescibacteria and Proteobacteria in CON, T1, T3 and T4 groups (CON = 2.74%, T1 = 2.52%, T3 = 2.90%, T4 = 5.28%). However, Tenericutes emerged as the secondary dominant phylum in the T2 group (2.11%). At the genus level, Bacteroides, Ruminococcaceae NK4A214 group, Christensenellaceae R-7 group and Ruminococcus 1 collectively dominated in the CON group (28.33%). The T1 group exhibited distinct dominance patterns with Ruminococcaceae NK4A214 group, dgA-11 gut group and Bacteroides constituting 19.99% of total abundance. Notably, Ruminococcus 1 and Ruminococcaceae NK4A214 group sequentially prevailed as the most abundant genera in T2, T3 and T4 groups (T2 = 18.17%, T3 = 20.99%, T4 = 19.78%), demonstrating treatment-dependent variations in genus composition (Fig. 6B). The α diversity of bacterial community analysis revealed no significant difference in ACE, Chao 1, Shannon and Simpson indices among XOS-supplemented groups compared to the CON group (P > 0.05, Fig. 6C–F). Compared to the CON group, PCoA results also demonstrated no significant difference based on Bray-Curtis distances in T1 (Adonis: R2 = 0.083, P = 0.769), T2 (Adonis: R2 = 0.102, P = 0.121), T3 (Adonis: R2 = 0.091, P = 0.470) and T4 (Adonis: R2 = 0.099, P = 0.202) groups, respectively (P > 0.05, Fig. 6G–J). These findings suggest that XOS supplementation did not broadly reshape bacterial community in cecum of growing rabbits.
Fig. 6.
Effects of XOS on the composition and diversity of bacterial community in cecum of growing rabbits. A Bacterial community composition at the phylum level in the caecum of growing rabbits. B Bacterial community composition at the genus level in the caecum of growing rabbits. C ACE indices of α diversity in the caecum of growing rabbits. D Chao 1 indices of α diversity in the caecum of growing rabbits. E Shannon indices of α diversity in the caecum of growing rabbits. F Simpson indices of α diversity in the caecum of growing rabbits. G Comparison of β diversity between CON and T1 group. H Comparison of β diversity between CON and T2 group. I Comparison of β diversity between CON and T3 group. J Comparison of β diversity between CON and T4 group
Table 3.
Effects of XOS on short chain fatty acids in cecum of growing rabbits
| Items | Groups | P-value | ||||
|---|---|---|---|---|---|---|
| CON | T1 | T2 | T3 | T4 | ||
| Acetic acid, mg/g | 1.67 ± 0.16 | 1.28 ± 0.16 | 1.74 ± 0.31 | 1.84 ± 0.19 | 2.21 ± 0.10 | 0.058 |
| Propionic acid, mg/g | 0.19 ± 0.01 | 0.19 ± 0.05 | 0.17 ± 0.02 | 0.21 ± 0.02 | 0.23 ± 0.01 | 0.750 |
| Butyric acid, mg/g | 0.38 ± 0.07 | 0.25 ± 0.04 | 0.39 ± 0.09 | 0.36 ± 0.04 | 0.47 ± 0.02 | 0.216 |
| Valeric acid, mg/g | 0.11 ± 0.01 | 0.13 ± 0.01 | 0.13 ± 0.02 | 0.15 ± 0.01* | 0.17 ± 0.00* | 0.004 |
Multiple comparisons were analyzed using Dunnett's test, with asterisks (*) denoting significant differences between each treatment group and the CON group
Effects of XOS on short chain fatty acids in cecum of growing rabbits
As shown in Table 3, compared to the CON group, XOS supplementation did not demonstrate significant effects on the concentrations of acetic acids, propionic acids and butyric acids (P > 0.05). However, it enhanced valeric acid levels in the T3 and T4 groups significantly (P ≤ 0.05).
Correlation analysis between different bacterial communities and physiological indices of growing rabbits
To further investigate whether XOS supplementation could induce bacterial alterations, we analyzed differential bacterial composition at the genus level and identified 11 differentially genera (Fig. 7A). Specifically, compared to the CON group, the T2 group exhibited significant increases in relative abundance of Ruminococcaceae UCG-013, Anaeroplasma, Anaerovorax and Paraprevotella (P ≤ 0.05), with Coprococcus 2 demonstrating a highly significant elevation (P ≤ 0.01). In contrast, the relative abundance of Shuttleworthia was significantly reduced in the T3 group (P ≤ 0.05), Ruminococcaceae UCG-010, Butyricimonas, Ruminiclostridium 1, Family XIII AD3011 group and Faecalibaculum showed marked decreases in the T4 group (P ≤ 0.05).
Fig. 7.
Comparison of differential genus and correlation analysis with physiological indicators. A Comparison of differential genus in cecum of growing rabbits. B Correlation analysis between bacteria and physiological indicators. *, ** indicate P ≤ 0.05, P ≤ 0.01, respectively. Spearman's correlation coefficients are donated with a color gradient. Physiological indicators were correlated with each bacterium by Mantel test. Edge width corresponds to the Mantel's r statistic for the corresponding distance correlations, and edge color denotes the statistical significance
To further investigate the relationships between differential bacterial genera and host physiological indicators, Spearman correlation analysis was performed to identify potential bacterial interactions. The results revealed significant correlations between Ruminococcaceae UCG-010 and both Family XIII AD3011 group and Coprococcus 2, Family XIII AD3011 group and Faecalibaculum, as well as Paraprevotella and Coprococcus 2 (P ≤ 0.05). Notably, Faecalibaculum exhibited a highly significant correlation with Ruminococcaceae UCG-010 (P ≤ 0.01). Subsequent Mantel test analysis identified significant correlations between specific bacteria and key physiological parameters: IgM levels demonstrated significant correlations with Ruminococcaceae UCG-010 and Faecalibaculum (P ≤ 0.05), while valeric acid concentration showed a significant relationship with Shuttleworthia (P ≤ 0.05). These findings suggest potential functional linkages between differential general dynamics and critical physiological processes in the host organism.
Discussion
XOS, as a functional prebiotic additive, have been widely incorporated into food and feed industry. They not only provide nutritional benefits but also enhance animal growth performance and disease resistance [31, 32]. Previous studies indicate that dietary supplementation with 0.2 g/kg XOS significantly improved ADG of broilers [33]. In piglet trials, 0.5 g/kg XOS markedly increased BW at 56 d of age. Furthermore, graded XOS levels exhibited quadratic effects on BW at 56 d, ADG and gain to feed ratio from d 28 to 56 [34]. In another study, XOS levels showed a linear negative correlation with FCR [35]. In the present study (d 1–21), 0.2 g/kg XOS significantly enhanced ADFI, whereas 0.5 g/kg XOS improved FCR. Notably, XOS supplementation showed linear effects on WGR and quadratic effects on FCR. Over the entire trial period (d 1–35), 0.2 and 0.3 g/kg XOS increased ADFI, while 0.4 g/kg XOS increased FCR throughout the experimental phase, WGR and FCR variations displayed quadratic responses, while ADG showed linear effects, and linear relationships were also observed between XOS levels and BW on d 21 and 35. Although 0.4 and 0.5 g/kg XOS improved survival rate in growing rabbits, the differences were not statistically significant. This aligns with findings from broiler studies where XOS supplementation at 0.1 g/kg failed to induce significant changes in ADG or BW [36], suggesting that subthreshold XOS levels may be insufficient to trigger measurable improvements in BW or survival rate.
Our findings indicate that XOS administration exerted no significant effects on serum biochemical parameters, similar observations have been reported in weaned piglets [9, 35]. Notably, our study revealed that XOS supplementation demonstrated certain antioxidant effects in both jejunal tissue and serum of growing rabbits. Oxidative stress, associated with various chronic conditions including cardiovascular diseases and malignancies, can be mitigated through lifestyle modifications or antioxidant supplementation [37]. The most extensively studied lipid peroxidation products include MDA, 4-hydroxy-nonenal (HNE) and F2-isoprostane 15(S)-8-iso-prostaglandin F2α [38]. MDA, a terminal product of polyunsaturated fatty acid peroxidation, accumulates with increased free radical production [39]. Studies have demonstrated that MDA enhancing ROS generation by facilitating metmyoglobin formation and non-heme iron release, thereby promotes protein oxidation in rabbit [40]. The glutathione peroxidase family comprises four distinct mammalian selenoproteins that catalyze peroxide reduction using glutathione, providing protection against oxidative damage from dietary hydroperoxides and xenobiotic metabolism [41]. In our study, XOS supplementation at 0.2 g/kg significantly enhanced serum GSH-Px activity. Moreover, dosages of 0.3, 0.4, 0.5 g/kg XOS effectively reduced MDA content in jejunal tissue, with the 0.4 g/kg dose additionally demonstrating significant serum MDA reduction. These findings suggest that appropriate XOS supplementation enhances antioxidant capacity and confers protection against oxidative challenges from dietary components and environmental stressors.
Furthermore, our study demonstrated that dietary supplementation with 0.2 g/kg XOS significantly enhanced sIgA levels, while 0.5 g/kg XOS administration markedly increased serum IgM levels. Immunoglobulins execute their protective functions through coordinated interactions between variable and constant regions, enabling targeted neutralization and elimination of pathogenic microorganisms and toxins [42, 43]. Mechanistically, the distinctive C-terminal domain of IgA has been shown to inhibit influenza A and other sialic-acid-binding viruses [44], while simultaneously activating pathogen clearance through IgA Fc receptor (FcαRI/CD89) mediated phagocytic mechanisms [45, 46], these synergistic actions establish IgA as a critical defender against mucosal pathogen invasion [47, 48]. IgM contributes to immune homeostasis through two primary pathways: preventing autoimmune disorders via enhanced clearance of cellular debris [49, 50], and inhibiting microbial proliferation during early infection phases through viral/bacterial neutralization [51–53]. The XOS-induced elevation of sIgA and IgM observed in this investigation collectively provides robust protection against enteric toxins and pathogens.
To investigate the physiological effects of XOS in rabbit intestines, we analyzed changes in nutrient digestibility and digestive enzyme activity. The results demonstrated that dietary supplementation with 0.4 g/kg XOS significantly enhanced lipase activity, while 0.2, 0.4 and 0.5 g/kg XOS markedly increased trypsin activity. However, no improvement in the digestibility of EE or CP was observed with XOS administration. In addition, 0.5 g/kg XOS notably reduced cellulase activity without significantly affecting hemicellulase or pectinase activities, but XOS supplementation exhibited varying enhancement in the digestibility of CF, ADF and NDF paradoxically. These findings collectively suggest that XOS influences nutrient digestion and absorption, but that this effect may not be mediated through modulation of digestive enzyme activities in the intestinal tract of growing rabbits.
As herbivores, rabbits' digestion and absorption of nutrients depend not only on intestinal enzyme activity but also crucially on the functions of gut microbiota. The microbial diversity in rabbits is most abundant in the cecum and colon [54], and primarily consists of bacteria (1011/g) and yeasts (106/g) in cecum [55]. Young rabbits predominantly harbor Streptococcus and Enterobacteriaceae in their lower gastrointestinal tract, while adult rabbits are dominated by Bacteroides dominance in the small intestine, cecum and colon, with composition influenced by age, diet and antibiotic [56]. Our bacterial analysis revealed that XOS did not extensively reshape the cecal microbiota of growing rabbits, as evidenced by non-significant changes in α and β diversity. However, differential analysis demonstrated that 0.3 g/kg XOS significantly increased the relative abundance of Ruminococcaceae_UCG-013, Anaeroplasma, Anaerovorax, Paraprevotella and Coprococcus 2. Conversely, 0.4 g/kg XOS notably reduced Shuttleworthia abundance, while 0.5 g/kg XOS decreased Ruminococcaceae_UCG-010, Butyricimonas, Ruminiclostridium 1, Family XIII AD3011 group and Faecalibaculum. Notably, Ruminococcaceae_UCG-013 has been identified as a potential biomarker for obesity alleviation [57], and is associated with various fatty liver diseases in some studies [58, 59]. Paraprevotella may enhance host defense through its type IX secretion system, promoting trypsin autolysis to protect IgA from degradation and inhibiting pathogens like mouse hepatitis virus-2 [60]. Coprococcus 2 is functionally linked to carbohydrate fermentation [61, 62]. These findings suggest that XOS might influence carbohydrate metabolism, enzymatic activity, and pathogen resistance by modulating specific bacterial taxa including Coprococcus 2, Paraprevotella, Ruminococcaceae_UCG-013 and Shuttleworthia. Intriguingly, the glycolytic capacity of Shuttleworthia for producing acetate, butyrate, and lactate [63] showed a significant correlation with valeric acid levels in our study. Both 0.4 and 0.5 g/kg XOS treatments significantly increased valeric acid content, implying XOS may enhance valeric acid production by altering Shuttleworthia's metabolic activity. While our observations reveal XOS-induced bacterial differences, current data limitations permit only partial understanding of these changes, further investigations are required to elucidate their broader biological implications in gut homeostasis, disease intervention and animal husbandry applications.
Conclusion
Our findings demonstrate that XOS enhances feed consumption in rabbits, which may be attributed to improved fiber digestibility via bacterial modulation and enhanced valeric acid production. Furthermore, XOS supplementation exhibited a protective effect against oxidative stress induced by dietary challenges and potentially enhanced resistance to intestinal pathogen colonization. These combined effects highlight the potential of XOS as a multifunctional dietary supplement in rabbit farming. However, further investigations are warranted to fully elucidate the complex interactions between the microbiota and the host.
Supplementary Information
Additional file 1: Linear and quadratic analysis of XOS on growth performance in growing rabbits.
Acknowledgements
We sincerely thank Yibin Yatai Biotechnology Co., Ltd. for providing xylooligosaccharides and partial funding.
Abbreviations
- ADF
Acid detergent fiber
- ADFI
Average daily feed intake
- ADG
Average daily gain
- ADL
Acid detergent lignin
- AIA
Acid insoluble ash
- ALB
Albumin
- ALP
Alkaline phosphatase
- ALT
Alanine aminotransferase
- AST
Aspartate aminotransferase
- CF
Crude fiber
- CP
Crude protein
- DM
Dry matter
- EE
Ether extract
- FCR
Feed conversion rate
- GE
Gross energy
- GLB
Globulin
- GLU
Glucose
- GSH-Px
Glutathione peroxidase
- IgA
Immunoglobulin A
- IgG
Immunoglobulin G
- IgM
Immunoglobulin M
- MDA
Malondialdehyde
- MUC 2
Mucin 2
- NDF
Neutral detergent fiber
- SCFA
Short chain fatty acid
- sIgA
Secretory immunoglobulin A
- SOD
Superoxide dismutase
- TC
Total cholesterol
- TG
Triglyceride
- TP
Total protein
- WGR
Weight gain rate
- XOS
Xylooligosaccharides
Authors’ contributions
APM, XYP and GT: conceptualized and designed the experiments; XYP: carried out the experiment; APM and XYP: analyzed data; APM: wrote the manuscript; JNP, YBC and QYL: resources; JNP, JYC, HZ, GJ, GT: provided conceptual advice and guidance; APM and GT: revised the manuscript; GT: supervision and funding acquisition. All authors have read and approved the final manuscript.
Funding
The present study was supported by the Key Research and Development Program of Sichuan Province (2023YFN0075) and the Sichuan Rabbit Innovation Team of National Modern Agricultural Industry Technology System of China (SCCXTD-24).
Data availability
The data used to support the findings of this study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The animal use protocol in the experiment has been approved by the Sichuan Agricultural University Animal Ethical and Welfare Committee (Approval No. 20240515).
Consent for publication
Not applicable.
Competing interests
The authors declare that there are no conflicts of interest.
Footnotes
Aipeng Mao and Xiaoyan Peng contributed equally to this work.
References
- 1.Santibáñez L, Henríquez C, Corro-Tejeda R, Bernal S, Armijo B, Salazar O. Xylooligosaccharides from lignocellulosic biomass: a comprehensive review. Carbohydr Polym. 2021;251:117118. 10.1016/j.carbpol.2020.117118. [DOI] [PubMed] [Google Scholar]
- 2.Palaniappan A, Antony U, Emmambux MN. Current status of xylooligosaccharides: production, characterization, health benefits and food application. Trends Food Sci Technol. 2021;111:506–19. 10.1016/j.tifs.2021.02.047. [Google Scholar]
- 3.Gruening de Mattos PB, Porto de Souza Vandenberghe L, Diestra KK, Ramos Neyra LC, Vieira S, Júnior Letti LA, et al. Recent developments in xylooligosaccharides: Sustainable production, characterization, beneficial properties and applications. Food Res Int. 2024;197(Pt1):115206. 10.1016/j.foodres.2024.115206. [DOI] [PubMed] [Google Scholar]
- 4.Baker JT, Duarte ME, Holanda DM, Kim SW. Friend or foe? Impacts of dietary xylans, xylooligosaccharides, and xylanases on intestinal health and growth performance of monogastric animals. Animals. 2021;11(3):609. 10.3390/ani11030609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rao ZY, Li Y, Yang XP, Guo YP, Zhang W, Wang ZX. Diet xylo-oligosaccharide supplementation improves growth performance, immune function, and intestinal health of broilers. Anim Nutr. 2024;17:165–76. 10.1016/j.aninu.2024.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yadav S, Singh AK, Selvaraj RK, Applegate TJ, Bhattacharya P, Shinall SB, et al. Research note: effect of dietary xylo-oligosaccharide on growth performance, intestinal histomorphology, and specific cecal bacteria in broiler chickens. Poult Sci. 2024;103(1):103189. 10.1016/j.psj.2023.103189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ding XM, Li DD, Bai SP, Wang JP, Zeng QF, Su ZW, et al. Effect of dietary xylooligosaccharides on intestinal characteristics, gut microbiota, cecal short-chain fatty acids, and plasma immune parameters of laying hens. Poult Sci. 2018;97:874–81. 10.3382/ps/pex372. [DOI] [PubMed] [Google Scholar]
- 8.Li DD, Ding XM, Zhang KY, Bai SP, Wang JP, Zeng QF, et al. Effects of dietary xylooligosaccharides on the performance, egg quality, nutrient digestibility and plasma parameters of laying hens. Anim Feed Sci Technol. 2017;225:20–6. 10.1016/j.anifeedsci.2016.12.010. [Google Scholar]
- 9.Yin J, Li FN, Kong XF, Wen CY, Guo QP, Zhang LY, et al. Dietary xylo-oligosaccharide improves intestinal functions in weaned piglets. Food Funct. 2019;10:2701–9. 10.1039/c8fo02485e. [DOI] [PubMed] [Google Scholar]
- 10.Chen YX, Xie YN, Zhong RQ, Liu L, Lin CG, Xiao L, et al. Effects of xylo-oligosaccharides on growth and gut microbiota as potential replacements for antibiotic in weaning piglets. Front Microbiol. 2021;12:641172. 10.3389/fmicb.2021.641172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tang SL, Chen YX, Deng FL, Yan XW, Zhong RQ, Meng QS, et al. Xylooligosaccharide-mediated gut microbiota enhances gut barrier and modulates gut immunity associated with alterations of biological processes in a pig model. Carbohydr Polym. 2022;294:119776. 10.1016/j.carbpol.2022.119776. [DOI] [PubMed] [Google Scholar]
- 12.Hou ZP, Wu DQ, Dai QZ. Effects of dietary xylo-oligosaccharide on growth performance, serum biochemical parameters, antioxidant function, and immunological function of nursery piglets. Rev Bras Zootecnia. 2020;49:e20190170.10.37496/rbz4920190170. [Google Scholar]
- 13.Samanta AK, Jayapal N, Jayaram C, Roy S, Kolte AP, Senani S, et al. Xylooligosaccharides as prebiotics from agricultural by-products production and applications. Bioact Carbohydr Diet Fibre. 2015;5:62–71. 10.1016/j.bcdf.2014.12.003. [Google Scholar]
- 14.Mano MCR, Neri-Numa IA, Da Silva JB, Paulino BN, Pessoa MG, Pastore GM. Oligosaccharide biotechnology: an approach of prebiotic revolution on the industry. Appl Microbiol Biotechnol. 2018;102:17–37. 10.1007/s00253-017-8564-2. [DOI] [PubMed] [Google Scholar]
- 15.Silva EK, Arruda HS, Mekala S, Pastore GM, Meireles MAA, Saldaña MDA. Xylooligosaccharides and their chemical stability under high-pressure processing combined with heat treatment. Food Hydrocolloids. 2022;124:107167. 10.1016/j.foodhyd.2021.107167. [Google Scholar]
- 16.Van Der Sluis M, Van Zeeland YRA, De Greef KH. Digestive problems in rabbit production: moving in the wrong direction? Front Vet Sci. 2024;11:1354651. 10.3389/fvets.2024.1354651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davies RR, Rees Davies JAE. Rabbit gastrointestinal physiology. Veterinary Clinics of North America: Exot Anim Pract. 2003;6:139–53. 10.1016/S1094-9194(02)00024-5. [DOI] [PubMed] [Google Scholar]
- 18.Guo H, Yang J, Gong G, Zeng L, Zhu Y, Wang Y, et al. Effect of xylo-oligosaccharide on in vitro proliferation of lactic acid bacteria from rabbits. Research Square (Preprint). 2023. 10.21203/rs.3.rs-3215079/v1. [DOI] [PubMed]
- 19.Farias-Kovac C, Simbaña F, Reyes M, Carabano R, Nicodemus N, García J. Effect of xylooligosaccharides supplementation in drinking water and feed restriction on faecal digestibility, growth traits and energy and nitrogen retention efficiency in growing rabbits. In: Proceedings of the 12th World Rabbit Congress; 2021 Nov 3–5; Nantes, France. Communication N-14. p. 1–4.
- 20.De Blas C, Mateos GG. Nutrition of the rabbit. 3rd ed. Wallingford: CAB International; 2020. [Google Scholar]
- 21.National Feed Industry Standardization Technical Committee of China. GB/T 14699.1–2023 Feed—Sampling. Beijing: Standards Press of China; 2023.
- 22.International Organization for Standardization. ISO 9831:1998 Animal Feeding Stuffs, Animal Products, and Faeces or Urine — Determination of Gross Calorific Value — Bomb Calorimeter Method. Geneva: ISO; 1998. [Google Scholar]
- 23.National Feed Industry Standardization Technical Committee of China. GB/T 6435–2014 Determination of moisture in feedstuffs. Beijing: Standards Press of China; 2014. [Google Scholar]
- 24.National Feed Industry Standardization Technical Committee of China. GB/T 6432–2018 Determination of crude protein in feeds-Kjeldahl method. Beijing: Standards Press of China; 2018. [Google Scholar]
- 25.National Feed Industry Standardization Technical Committee of China. GB/T 6433–2006 Determination of crude fat in feeds. Beijing: Standards Press of China; 2006. [Google Scholar]
- 26.National Feed Industry Standardization Technical Committee of China. GB/T 6434–2022 Determination of crude fiber content in feeds. Beijing: Standards Press of China; 2022. [Google Scholar]
- 27.National Feed Industry Standardization Technical Committee of China. GB/T 20806–2022 Determination of neutral detergent fiber (NDF) in feeds. Beijing: Standards Press of China; 2022. [Google Scholar]
- 28.National Feed Industry Standardization Technical Committee of China.GB/T 20805–2006 Determination of acid detergent lignin in feedstuffs. Beijing: Standards Press of China; 2006.
- 29.National Feed Industry Standardization Technical Committee of China. GB/T 23742–2009 Animal feeding stuffs-Determination of ash insoluble in hydrochloric acid. Beijing: Standards Press of China; 2009. [Google Scholar]
- 30.Ministry of Agriculture and Rural Affairs of the People’s Republic of China. NY/T 1459–2022 Determination of acid detergent fiber (ADF) in feeds. Beijing: Standards Press of China; 2022.
- 31.de Mello Capetti CC, Vacilotto MM, Dabul ANG, Sepulchro AGV, Pellegrini VOA, Polikarpov I. Recent advances in the enzymatic production and applications of xylooligosaccharides. World J Microbiol Biotechnol. 2021;37(10):169. 10.1007/s11274-021-03139-7. [DOI] [PubMed]
- 32.Chen YX, Xie YN, Ajuwon KM, Zhong RQ, Li T, Chen L, et al. Xylo-oligosaccharides, preparation and application to human and animal health: a review. Front Nutr. 2021;8:731930. 10.3389/fnut.2021.731930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yuan L, Li WL, Huo QQ, Du CH, Wang ZX, Yi BD, et al. Effects of xylo-oligosaccharide and flavomycin on the immune function of broiler chickens. PeerJ. 2018;6:e4435. 10.7717/peerj.4435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen YX, Xie YN, Zhong RQ, Han H, Liu L, Chen L, et al. Effects of graded levels of xylo-oligosaccharides on growth performance, serum parameters, intestinal morphology, and intestinal barrier function in weaned piglets. J Anim Sci. 2021;99:skab183. 10.1093/jas/skab183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pang JM, Zhou XJ, Ye H, Wu YJ, Wang ZY, Lu DD, et al. The high level of xylooligosaccharides improves growth performance in weaned piglets by increasing antioxidant activity, enhancing immune function, and modulating gut microbiota. Front Nutr. 2021;8:764556. 10.3389/fnut.2021.764556. [DOI] [PMC free article] [PubMed]
- 36.Singh AK, Mishra B, Bedford MR, Jha R. Effects of supplemental xylanase and xylooligosaccharides on production performance and gut health variables of broiler chickens. J Anim Sci Biotechnol. 2021;12:98. 10.1186/s40104-021-00617-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Willcox JK, Ash SL, Catignani GL. Antioxidants and prevention of chronic disease. Crit Rev Food Sci Nutr. 2004;44:275–95. 10.1080/10408690490468489. [DOI] [PubMed] [Google Scholar]
- 38.Tsikas D. Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: analytical and biological challenges. Anal Biochem. 2017;524:13–30. 10.1016/j.ab.2016.10.021. [DOI] [PubMed] [Google Scholar]
- 39.Gaweł S, Wardas M, Niedworok E, Wardas P. malondialdehyde (MDA) as a lipid peroxidation marker. Wiadomosci Lek Wars Pol. 1960;2004(57):453–5. [PubMed] [Google Scholar]
- 40.Wang ZM, He ZF, Emara AM, Gan X, Li HJ. Effects of malondialdehyde as a byproduct of lipid oxidation on protein oxidation in rabbit meat. Food Chem. 2019;288:405–12. 10.1016/j.foodchem.2019.02.126. [DOI] [PubMed] [Google Scholar]
- 41.Brigelius-Flohé R. Tissue-specific functions of individual glutathione peroxidases. Free Radic Biol Med. 1999;27:951–65. 10.1016/S0891-5849(99)00173-2. [DOI] [PubMed] [Google Scholar]
- 42.Janda A, Bowen A, Greenspan NS, Casadevall A. Ig constant region effects on variable region structure and function. Front Microbiol. 2016;7:22. 10.3389/fmicb.2016.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schroeder HW Jr, Cavacini L. Structure and function of immunoglobulins. J Allergy Clin Immunol. 2010;125:S41–S52. 10.1016/j.jaci.2009.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maurer MA, Meyer L, Bianchi M, Turner HL, Le NPL, Steck M, et al. Glycosylation of human IgA directly inhibits influenza a and other sialic-acid-binding viruses. Cell Rep. 2018;23:90–9. 10.1016/j.celrep.2018.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Herr AB, Ballister ER, Bjorkman PJ. Insights into IgA-mediated immune responses from the crystal structures of human FcαRI and its complex with IgA1-Fc. 2003;423(6940):614-20. 10.1038/nature01685. [DOI] [PubMed]
- 46.Breedveld A, Van Egmond M. IgA and FcαRI: pathological roles and therapeutic opportunities. Front Immunol. 2019;10:553. 10.3389/fimmu.2019.00553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.De Sousa-Pereira P, Woof JM. IgA: structure, function, and developability. Antibodies. 2019;8:57. 10.3390/antib8040057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mantis NJ, Rol N, Corthésy B. Secretory IgA’s complex roles in immunity and mucosal homeostasis in the gut. Mucosal Immunol. 2011;4:603–11. 10.1038/mi.2011.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Quartier P, Potter PK, Ehrenstein MR, Walport MJ, Botto M. Predominant role of IgM-dependent activation of the classical pathway in the clearance of dying cells by murine bone marrow-derived macrophages in vitro. Eur J Immunol. 2005;35:252–60. 10.1002/eji.200425497. [DOI] [PubMed] [Google Scholar]
- 50.Grönwall C, Vas J, Silverman GJ. Protective roles of natural IgM antibodies. Front Immunol. 2012;3:66. 10.3389/fimmu.2012.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Racine R, Winslow GM. IgM in microbial infections: taken for granted? Immunol Lett. 2009;125:79–85. 10.1016/j.imlet.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Reynolds AE, Kuraoka M, Kelsoe G. Natural IgM is produced by CD5− plasma cells that occupy a distinct survival niche in bone marrow. J Immunol. 2014;194:231–42. 10.4049/jimmunol.1401203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Boes M, Prodeus AP, Schmidt T, Carroll MC, Chen J. A critical role of natural immunoglobulin M in immediate defense against systemic bacterial infection. J Exp Med. 1998;188:2381–6. 10.1084/jem.188.12.2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cotozzolo E, Cremonesi P, Curone G, Menchetti L, Riva F, Biscarini F, et al. Characterization of bacterial microbiota composition along the gastrointestinal tract in rabbits. Animals. 2020;11(1):31. 10.3390/ani11010031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Forsythe SJ, Parker DS. Nitrogen metabolism by the microbial flora of the rabbit caecum. J Appl Bacteriol. 1985;58:363–9. 10.1111/j.1365-2672.1985.tb01475.x. [DOI] [PubMed] [Google Scholar]
- 56.Fann MK, O’Rourke D. Normal bacterial flora of the rabbit gastrointestinal tract: a clinical approach. Semin Avian Exot Pet Med. 2001;10:45–7. 10.1053/saep.2001.19794. [Google Scholar]
- 57.Feng JF, Ma HL, Huang YT, Li JC, Li WD. Ruminococcaceae_UCG-013 promotes obesity resistance in mice. Biomedicines. 2022;10:3272. 10.3390/biomedicines10123272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhai QL, Wu HY, Zheng SY, Zhong T, Du CJ, Yuan JJ, et al. Association between gut microbiota and NAFLD/NASH: a bidirectional two-sample mendelian randomization study. Front Cell Infect Microbiol. 2023;13:1294826. 10.3389/fcimb.2023.1294826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zazueta A, Valenzuela-Pérez L, Ortiz-López N, Pinto-León A, Torres V, Guiñez D, et al. Alteration of gut microbiota composition in the progression of liver damage in patients with metabolic dysfunction-associated steatotic liver disease (MASLD). Int J Mol Sci. 2024;25:4387. 10.3390/ijms25084387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li YX, Watanabe E, Kawashima Y, Plichta DR, Wang ZJ, Ujike M, et al. Identification of trypsin-degrading commensals in the large intestine. Nature. 2022;609:582–9. 10.1038/s41586-022-05181-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pidcock SE, Skvortsov T, Santos FG, Courtney SJ, Sui-Ting K, Creevey CJ, et al. Phylogenetic systematics of Butyrivibrio and Pseudobutyrivibrio genomes illustrate vast taxonomic diversity, open genomes and an abundance of carbohydrate-active enzyme family isoforms. Microb Genom. 2021;7(10):000638. 10.1099/mgen.0.000638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Alessi AM, Gray V, Farquharson FM, Flores-López A, Shaw S, Stead D, et al. β-Glucan is a major growth substrate for human gut bacteria related to Coprococcus eutactus. Environ Microbiol. 2020;22:2150–64. 10.1111/1462-2920.14977. [DOI] [PubMed] [Google Scholar]
- 63.Downes J, Munson MA, Radford DR, Spratt DA, Wade WG. Shuttleworthia satelles gen. nov., sp. nov., isolated from the human oral cavity. Int J Syst Evol Microbiol. 2002;52:1469–75. 10.1099/00207713-52-5-1469. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Linear and quadratic analysis of XOS on growth performance in growing rabbits.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author on reasonable request.