Abstract
Translational activation of several dormant mRNAs in vertebrate oocytes is mediated by cytoplasmic polyadenylation, a process controlled by the cytoplasmic polyadenylation element (CPE) and its binding protein CPEB. The translation of CPE-containing mRNAs does not occur en masse at any one time, but instead is temporally regulated. We show here that in Xenopus, partial destruction of CPEB controls the temporal translation of CPE-containing mRNAs. While some mRNAs, such as the one encoding Mos, are polyadenylated at prophase I, the polyadenylation of cyclin B1 mRNA requires the partial destruction of CPEB that occurs at metaphase I. CPEB destruction is mediated by a PEST box and Cdc2-catalyzed phosphorylation, and is essential for meiotic progression to metaphase II. CPEB destruction is also necessary for mitosis in the early embryo. These data indicate that a change in the CPEB:CPE ratio is necessary to activate mRNAs at metaphase I and drive the cells’ entry into metaphase II.
Keywords: CPEB/cytoplasmic polyadenylation/meiosis/Xenopus oocytes
Introduction
Vertebrate oocytes arrested at the diplotene stage of first meiotic prophase synthesize and store several mRNAs that are used later in development. Some of these maternal mRNAs, such as those encoding Mos and the cyclins, are translated when the oocytes re-enter the meiotic divisions (oocyte maturation). In Xenopus, progesterone stimulation of maturation involves several signaling events that lead to the activation of maturation-promoting factor (MPF), a heterodimer of the kinase Cdc2 and cyclin B1. MPF is stored in oocytes in an inactive form (pre-MPF), which is activated by dephosphorylation of the Cdc2 subunit. Oocytes also store monomeric Cdc2 that can be converted to active MPF by de novo synthesis of cyclin B1 (Swenson et al., 1986; Pines and Hunt, 1987). While MPF drives oocytes into metaphase I (MI), a subsequent decrease results in meiotic progression to anaphase I (AI), which is followed by a second increase and progression to metaphase II (MII). Under the control of cytostatic factor (CSF), oocytes arrest at MII even though MPF is maintained at a high level (Nebreda and Ferby, 2000).
Meiotic progression requires protein synthesis at two stages, the first of which occurs soon after oocytes are exposed to progesterone. At this time, Mos, a mitogen-activated protein kinase (MAPK) kinase kinase, is synthesized and is necessary to activate MAPK and MPF, and to transition the oocytes into MI (Sagata et al., 1989; Sheets et al., 1995). The synthesis of other proteins, such as Speedy (Ringo), a Cdc2-binding protein, may also be required for the oocyte entry into MI (Ferby et al., 1999; Lenormand et al., 1999). Protein synthesis is also required at MI and/or AI to stimulate progression to MII (Gerhart et al., 1984). Although the stockpile of cyclin B bound to Cdc2 in immature oocytes is sufficient to drive entry into MI, it (cyclin B) is degraded in MI by the anaphase-promoting complex (APC) (Peter et al., 2001; Taieb et al., 2001), but again accumulates during entry into MII (Gross et al., 2000). While cyclin B synthesis is not required for entry into MI, it is essential for progression into MII and the establishment of CSF arrest (Hochegger et al., 2001). The increased accumulation of cyclin B1 is also required for the later embryonic mitotic divisions (Minshull et al., 1989).
One feature that is common among several oocyte mRNAs, including those encoding Mos and cyclin B, is that they have short poly(A) tails when they are dormant in the arrested oocyte, and longer poly(A) tails when they are translated in the maturing oocyte. This cytoplasmic poly(A) elongation requires two 3′-untranslated region (UTR) elements, the hexanucleotide AAUAAA and the cytoplasmic polyadenylation element (CPE) (Fox et al., 1989; McGrew et al., 1989). The CPE is bound by CPEB, a zinc finger and RRM-type RNA-binding protein (Hake and Richter, 1994; Hake et al., 1998). The instigation of polyadenylation requires Aurora (also known as Eg2), which is activated soon after oocytes are exposed to progesterone (Andresson and Ruderman, 1998). Aurora phosphorylates CPEB S174 (Mendez et al., 2000a), which increases the affinity of CPEB for the cleavage and polyadenylation specificity factor (CPSF) (Mendez et al., 2000b). CPSF, in turn, binds to the AAUAAA sequence (Dickson et al., 1999), an interaction that is probably stabilized by CPEB, and recruits poly(A) polymerase to the end of the mRNA (Mendez et al., 2000b).
The CPE is not only necessary for cytoplasmic polyadenylation-induced translation in maturing oocytes, it also mediates translational repression (masking) in unstimulated oocytes (Stutz et al., 1998; de Moor and Richter, 1999; Minshall et al., 1999; Barkoff et al., 2000; Tay et al., 2000). While this translation inhibition requires CPEB (de Moor and Richter, 1999), it is not the most proximal factor effecting the repression. Instead, another protein, Maskin, is most directly responsible for masking CPE-containing mRNA translation. Maskin interacts with CPEB as well as the cap-binding protein eIF-4E (Stebbins-Boaz et al., 1999). Because Maskin binding to eIF4E would preclude the binding of eIF4G to eIF4E, translation is inhibited because eIF4G is necessary to anchor the 40S ribosomal subunit on the mRNA (Mendez and Richter, 2001). At least a partial dissociation of Maskin from eIF4E during maturation would therefore be necessary for translational activation to occur. This dissociation does indeed occur, and is mediated by cytoplasmic polyadenylation (Stebbins-Boaz et al., 1999; Q.Cao and J.D.Richter, submitted).
While CPEB, Maskin, CPSF and Aurora are involved in the general mechanism of polyadenylation-induced translation, not every CPE-containing mRNA is masked (de Moor and Richter, 1999; Barkoff et al., 2000; Nakahata et al., 2001) and not every CPE-containing mRNA is polyadenylated at the same time. Some mRNAs (Mos) are polyadenylated early during maturation while others (cyclin B1) are polyadenylated at MI and require previous Mos synthesis and Cdc2 activation (Ballantyne et al., 1997; de Moor and Richter, 1997; Mendez et al., 2001). Such events establish a hierarchical translational control during meiotic progression.
Here, we show that differential mRNA polyadenylation and meiotic progression are regulated by partial CPEB destruction. In addition to the early activating phosphorylation by Aurora, CPEB undergoes subsequent Cdc2-catalyzed phosphorylations. These later phosphorylations, particularly one at S210, target CPEB for destruction. While CPEB undergoes ubiquitylation, the phosphorylations do not appear to be involved in this process, but instead may expose a PEST box in CPEB that is also necessary for destruction (Reverte et al., 2001). While not affecting entry into meiosis, the injection of a stable CPEB mutant protein prevents the MI to MII transition. The stable CPEB also abrogates cyclin B1 mRNA polyadenylation and translation, but does not affect the earlier polyadenylation-induced translation of Mos mRNA. The stable CPEB also disrupts cyclin B1 mRNA translation and cell division in the early embryo. The differences in the 3′-UTRs of Mos and cyclin mRNAs, such as the number of CPEs that they contain, appear temporally to regulate their translation as CPEB levels change during meiotic progression.
Results
Phosphorylation and partial destruction of CPEB at metaphase I
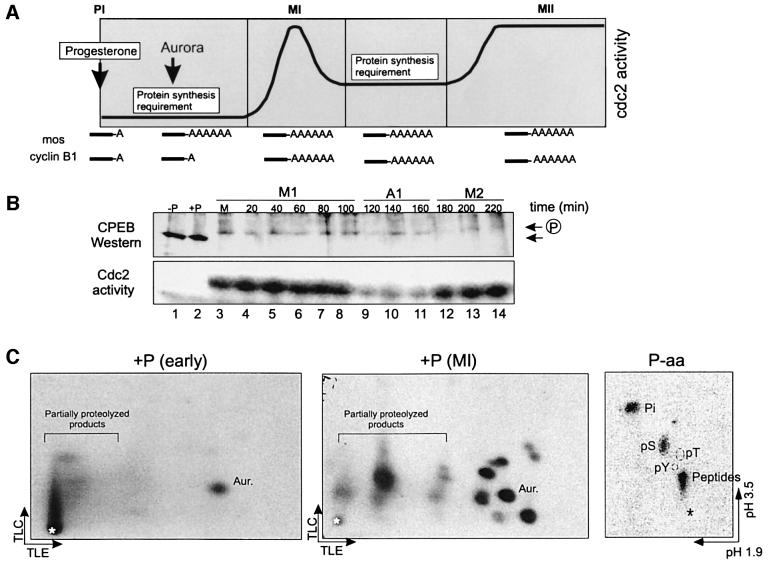
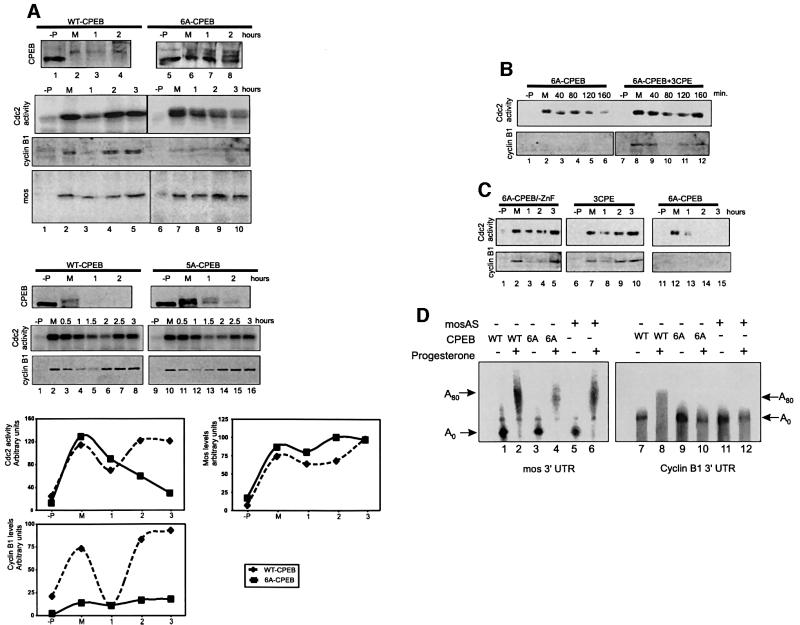
Progesterone stimulation of oocyte maturation produces two peaks of MPF activity that drive entry into MI and then into MII. One of the early signaling events is the activation of Aurora, a kinase that phosphorylates and activates CPEB, which in turn stimulates the polyadenylation and translation of Mos mRNA; Mos kinase as well as MPF are necessary for entry into MI. Cyclin B1 mRNA, which like Mos mRNA contains a CPE, remains quiescent at this time, and begins to undergo polyadenylation and translation only at MI (Figure 1A). Also taking place at MI are additional phosphorylations of CPEB, which slow its mobility in SDS–gels (Mendez et al., 2000a). This mobility shift of CPEB occurs at the beginning of MI, coincident with germinal vesicle breakdown (GVBD) and the activation of MPF (Figure 1B, lane 3). This figure also shows that coincident with the mobility shift is the partial destruction of CPEB, which varies from 70 to 90% (Hake and Richter, 1994; de Moor and Richter, 1997; Reverte et al., 2001). When CPEB phosphorylation is assessed by two-dimensional phosphopeptide mapping, it is clear that the single early phosphorylation mediated by Aurora is followed at MI by multiple phosphorylations, all of which take place on serine residues (Figure 1C).
Fig. 1. CPEB is hyperphosphorylated and degraded at metaphase I. (A) Schematic time course of meiotic progression in response to progesterone, indicating changes in Cdc2 activity, the time points at which protein synthesis is required, the time of Aurora kinase activation and the approximate times at which c-Mos and cyclin B1 mRNAs are polyadenylated. (B) Time course of CPEB hyperphosphorylation and degradation detected by western blotting (upper panel) and Cdc2 kinase activity detected by the phosphorylation of histone H1 in oocyte extracts (lower panel). Each time point corresponds to a single oocyte collected before progesterone addition (–P, lane 1), 6 h after progesterone addition but before GVBD (+P, lane 2), at GVBD (M, lane 3) or at the indicated times after GVBD (lanes 4–14). The positions of CPEB and phospho-CPEB are indicated by the arrows. The approximate times corresponding to metaphase I (M1), anaphase I (A1) and metaphase II (M2) are indicated. (C) Recombinant His-tagged CPEB was phosphorylated in vitro with extracts from oocytes stimulated with progesterone for 2 (+P, early), or 6 h (+P, MI), when maturation occurred. CPEB was then analyzed by two-dimensional phosphopeptide mapping followed by autoradiography. The arrows indicate the directions of the chromatographic (TLC) and electrophoretic (TLE) migrations. The asterisk indicates the origin. The phosphopeptide containing S174, which is phosphorylated by Aurora, is indicated (Aur.). Phosphoamino acid analysis of metabolically labeled CPEB is shown in the right panel (P-aa). The arrows indicate the directions of the electrophoretic migrations at pH 1.9 and 3.5. The positions of the phosphoserine (pS), phosphothreonine (pT) and phosphotyrosine (pY) markers, as well as free phosphate (Pi) and partially hydrolyzed products (Peptides) are indicated.
Analysis of CPEB phosphorylation
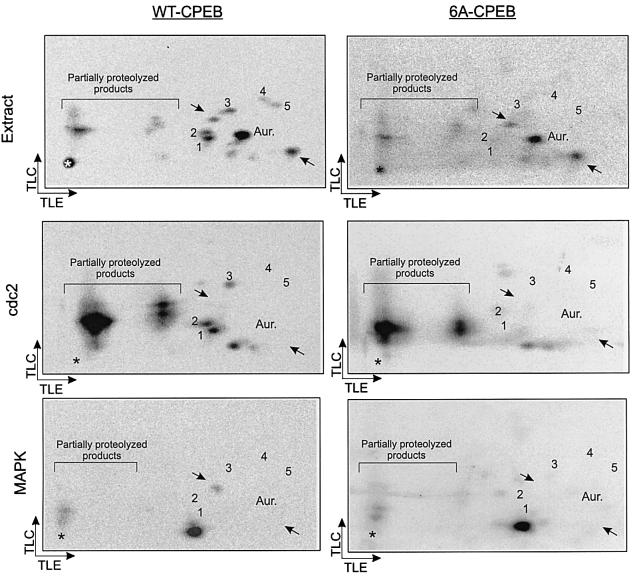
To analyze the late CPEB phosphorylations, we incuba ted Escherichia coli-expressed histidine-tagged CPEB together with [γ-32P]ATP in extracts prepared from MI stage (GVBD) oocytes. CPEB was then isolated, subjected to trypsin digestion, and the resulting phosphopeptides resolved in two dimensions and sequenced. This procedure yielded two identifiable phosphoamino acids, S138 and S248. In both cases, the serine was immediately followed by a proline, suggesting that they are targets of a proline-directed serine/threonine kinase, such as Cdc2 or MAPK, both of which are activated at MI.
To determine the remaining sites of phosphorylation, we focused on the six SP motifs in CPEB (serine residues 138, 144, 184, 210, 248 and 423). We mutated all six serine residues to alanine (6A-CPEB), and repeated the phosphorylation procedure with E.coli-expressed protein as described above. Five of the phosphopeptides that were evident with wild-type (WT) CPEB were not detected with 6A-CPEB (Figure 2, extract). Because subsequent studies demonstrated that S423 is not phosphorylated (unpublished), and because we previously identified the two-dimensional positions of the S138-, S210- and S248-containing phosphopeptides (data not shown), we have tentatively assigned serine residue numbers to each of the phosphopeptides; 1 contains S210, 2 contains S138 and 3 contains S248. Phosphopeptides 4 and 5, which were phosphorylated at a low level (Figure 1C), contain S184 and S144, although not necessarily in that order. One of the three phosphopeptides that were detected with either wild-type or 6A-CPEB substrates contains the S174 Aurora phosphorylation site (Aur.); the other two are unidentified (arrows).
Fig. 2. Phosphorylation of CPEB by Cdc2 kinase and MAPK. Recombinant His-tagged CPEB variants, either WT-CPEB or a multiple point mutant in which serines 138, 144, 184, 210, 248 and 423 were replaced by alanines (6A-CPEB), were phosphorylated in vitro with extract from GVBD oocytes (Extract), purified Cdc2 kinase (cdc2) or purified MAPK (MAPK). Phosphorylated CPEB variants were then analyzed by two-dimensional phosphopeptide mapping followed by autoradiography. The arrows indicate the directions of the chromatographic (TLC) and electrophoretic (TLE) migrations. The asterisk indicates the origin. The five phosphopeptides present in WT-CPEB but not in 6A-CPEB are denoted 1–5. The phosphopeptide corresponding to S174 (Aur.), as well as two other major phosphopeptides (arrows) present both in WT- and 6A-CPEB variants are also indicated.
As mentioned above, the phosphorylation and degradation of CPEB correlate with the activation of two proline-directed serine/threonine kinases, Cdc2 and MAPK. To determine if either or both of these kinases phosphorylate CPEB, the wild-type and 6A-CPEB proteins were used as substrates for in vitro phosphorylation reactions using purified kinase. Figure 2 shows that Cdc2 generated CPEB phosphopeptides 1, 2 and 3 (S134, S210 and S248), while MAPK generated a single phosphopeptide of unknown origin that was not detected when mature oocyte extracts were used as the kinase source (compare WT-CPEB and 6A-CPEB). These data demonstrate that Cdc2, in addition to Aurora, is a likely physiological kinase that phosphorylates CPEB (but see Katsu et al., 1999). These results suggest that Cdc2 catalyzes the late phosphorylations of CPEB.
Phosphorylation-mediated CPEB destruction
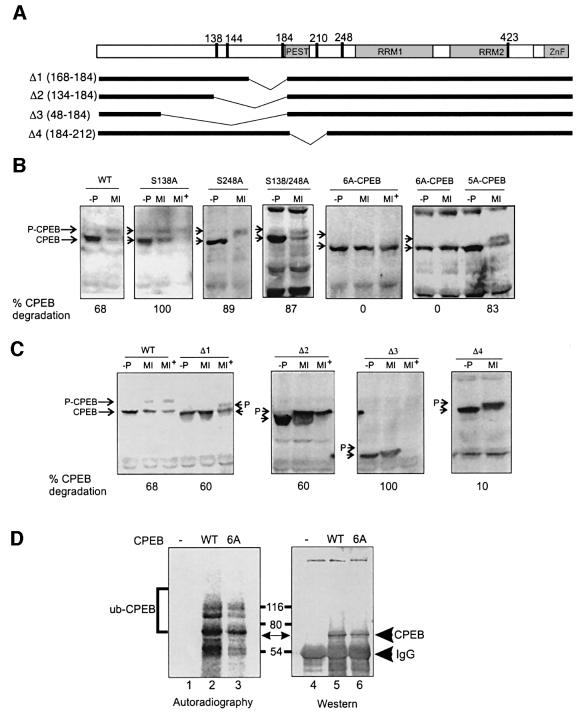
The late CPEB phosphorylations correlate with the partial destruction of the protein, which we examined by injecting oocytes with mRNAs encoding wild-type or mutant CPEB proteins fused to the myc epitope. The first series of mutations contained alanine substitutions for S138 and S248 (Figure 3A); both proteins underwent shifts in mobility and were partially destroyed in response to progesterone treatment. A double S138A S248A mutant of CPEB behaved in a similar manner (Figure 3B). However, a CPEB protein that contained alanine for serine substitutions in all six SP motifs (6A-CPEB; Figure 3A) underwent neither a shift in mobility nor destruction (Figure 3B). Thus, phosphoserines 144, 184 or 210, but not 138 and 248, appear to be involved in CPEB destruction.
Fig. 3. CPEB degradation is mediated by phosphorylation and requires the PEST box. (A) Schematic representation of CPEB showing the putative PEST box (PEST), the RNA-binding domains (RRM1 and RRM2), the zinc finger (ZnF) and the position of the six serine/proline motifs. The regions deleted in Δ1–Δ4 are indicated in the lower bars. Stage VI Xenopus oocytes were injected with mRNA coding for myc-tagged variants of CPEB. After 12 h, the oocytes were incubated in the absence (–P) or presence of progesterone and collected when the progesterone-stimulated oocytes had matured (MI) or 30 min after maturation (MI+). Extracts derived from injected oocytes, as well as from non-injected controls (not shown), were analyzed by western blotting with a myc antibody. (B) Western blot analysis of wild-type CPEB (WT), as well as point mutants in which serines 138 (S138A), 248 (S248A), 138 and 248 (S138/248A), 138, 144, 184, 210, 248 and 423 (6A-CPEB) or 138, 144, 184, 248 and 423 (5A-CPEB) were replaced by alanines. The levels of myc-CPEB were quantified and expressed as the percentage of CPEB that was degraded. (C) Western blotting of wild-type CPEB (WT), as well as the Δ1, Δ2, Δ3 and Δ4 CPEB variants. Arrows indicate the position of the Cdc2-phosphorylated (shifted) and unphosphorylated CPEB variants. The levels of myc-CPEB were quantified and expressed as the percentage of CPEB that was degraded. (D) Wild-type CPEB (WT, lane 2) and 6A-CPEB (6A, lane 3) were subjected to in vitro ubiquitylation with 32P-labeled ubiquitin, immunoprecipitated, and analyzed by SDS–PAGE, western blotting and autoradiography. A control reaction in which CPEB was not added was also carried out in parallel (lane 1). The positions of CPEB and ubiquitylated CPEB are indicated.
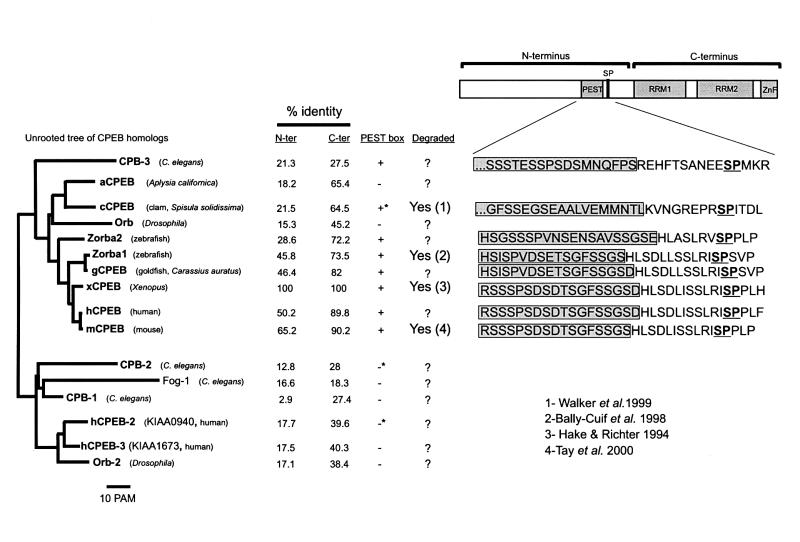
A sequence comparison between CPEB homologs of different species shows a remarkable conservation of the residue corresponding to Xenopus S210 (Figure 6). To assess whether this phospho-residue is involved in CPEB destruction, we used the 6A-CPEB template to re-introduce a serine at position 210 (5A-CPEB). In mRNA-injected oocytes, this protein displayed a slow electrophoretic migration and a significant degradation in response to progesterone (Figure 3B). This result indicates that S210 phosphorylation is sufficient to target CPEB for destruction; however, one or more other proline-directed serine phosphorylations may also be involved.
Fig. 6. Analysis of the CPEB family of RNA-binding proteins. Unrooted tree illustrating the relationship among CPEB homologs. There are two broad groups of CPEB homology, one that includes members of the ‘classical’ CPEB family, from C.elegans to human, and another that includes members that are more distantly related. The similarity of the N-terminus, which contains the PEST box and SP motifs, and the C-terminus containing the RRM RNA-binding domains and the zinc finger, is indicated as percentile identity to Xenopus CPEB. The presence of a PEST box in a position equivalent to that in Xenopus CPEB, as well as the documented degradation of CPEB homologs are also indicated: 1, Walker et al. (1999); 2, Bally-Cuif et al. (1998); 3, Hake and Richter (1994); 4, Tay et al. (2000). Note that the PEST score (logarithmic scale) of the clam CPEB homolog (cCPEB, +*) was much lower than that of the vertebrate and C.elegans CPEBs (2.49 versus an average of 13). CPB-2 and HKIAA0940 also contain PEST boxes (–*), but the positions are different from that in Xenopus CPEB, and lie within the RNA-binding C-terminus in CPB-2 and within the first 50 amino acids in HKIAA0940. Alignment of the conserved PEST boxes and SP motifs in members of the ‘classical’ CPEB group is shown in the right panel.
We constructed several CPEB variants in which different N-terminal portions of the protein were deleted (Figure 3A). Deletion of residues 168–184 (Δ1), which contain the Aurora phosphorylation site and phosphoserine 184 site, had little effect on progesterone-mediated CPEB destruction (Figure 3C). Deletion of residues 134–184 (Δ2) or 48–184 (Δ3), which both contain phosphoserines 138 and 144 and 184, also had little effect on CPEB destruction (Figure 3C).
In addition to S210, another feature common to many members of the CPEB family is a PEST box in the N-terminal portion of the protein (Figures 2A and 6; Mendez and Richter, 2001). PEST sequences are enriched for proline (P), aspartic acid (E), serine (S) and threonine (T) residues and target proteins for destruction (Rechsteiner and Rogers, 1996). To determine whether the PEST box is involved in CPEB destruction, a CPEB variant (Δ4) that lacked this region was constructed. Following mRNA injection and progesterone stimulation, this protein was still Cdc2 phosphorylated, as indicated by its slow mobility in SDS–PAGE, but failed to undergo degradation (Figure 3C). In addition, Reverte et al. (2001) deleted a small portion of the PEST box but retained S210; this mutation resulted in a stable CPEB. These findings suggest that both the phosphorylation of S210 and the PEST box are required for CPEB degradation.
Ubiquitylation of CPEB
Reverte et al. (2001) showed that CPEB is degraded by the proteasome, and suggested that this process might involve ubiquitylation. This conjecture was based on the appearance of a slow migrating CPEB immunoreactive band when extracts were incubated in the presence of ubiquitin analogs that prevent proteasome-mediated degradation. We have taken a more direct approach to this question by subjecting recombinant CPEB to an in vitro ubiquitylation reaction. High speed supernatants of egg extracts (Salic et al., 2000), which contain active Cdc2 that phosphorylates CPEB, were incubated with [32P]ubiquitin and CPEB, which was followed by immunoprecipitation and analysis by SDS–PAGE and phosphorimaging. Figure 3D demonstrates that CPEB was indeed tagged with ubiquitin.
In a number of instances, the ubiquitylation apparatus recognizes certain proteins only when they are phosphorylated (Laney and Hochstrasser, 1999). It seemed plausible that the function of the late Cdc2-mediated CPEB phosphorylations was to act as recognition tags for ubiquitylation. Consequently, the 6A-CPEB variant was used as substrate for the ubiquitylation assay. This protein was ubiquitylated to nearly the same extent as wild-type CPEB (Figure 3D), which suggests that Cdc2-catalyzed phosphorylations regulate some downstream event, e.g. the recognition of the PEST box by the proteasome.
CPEB destruction and meiotic progression
The destruction of CPEB at MI suggests that it might be important for meiotic progression. To test this possibility, wild-type, 5A-CPEB or the Cdc2 non-phosphorylatable and non-degradable CPEB (i.e. variant 6A) proteins were injected into oocytes, which was followed by progesterone stimulation. As expected, wild-type and 5A-CPEB were phosphorylated and partially degraded at maturation while the 6A variant was neither Cdc2 phosphorylated nor destroyed (Figure 4A). The multiple 6A-CPEB immunoreactive bands most probably represent ubiquitin conjugates; these grow in intensity as maturation progresses, and CPEB is not destroyed.
Fig. 4. The stable form of CPEB blocks meiotic progression, cyclin B1 synthesis and cyclin B1 RNA polyadenylation. (A) Stage VI Xenopus oocytes were injected with WT-CPEB, 6A-CPEB or 5A-CPEB proteins and incubated in the absence (–P) or presence of progesterone. Oocytes were collected at maturation (M) or at the indicated times after maturation. Extracts derived from the injected oocytes were then analyzed for CPEB, cyclin B1 and Mos levels by western blotting, and for Cdc2 kinase activity by phosphorylation of the standard histone H1 substrate. (B) Oocytes were injected either with 6A-CPEB protein alone or 6A-CPEB protein plus 1 pmol of cyclin B1 3′-UTR containing three CPEs (6A-CPEB + 3CPE). Cdc2 activity and cyclin B1 levels were then determined as in (A). (C) Oocytes were injected either with 6A-CPEB protein alone, a 6A-CPEB variant protein in which the zinc finger was mutated (6A-CPEB/-ZnF) or the cyclin B1 3′ -UTR (3CPE). Cdc2 activity and cyclin B1 levels were then determined as in (A). (D) Oocytes were injected first either with wild-type CPEB (WT) protein, 6A-CPEB (6A) protein or Mos antisense oligodeoxynucleotides (mosAS), and then injected again either with a radioactive Mos 3′-UTR, or a radioactive cyclin B1 3′-UTR. The injected oocytes were then incubated in the absence (–) or presence (+) of progesterone. Total RNA was isolated 7 h after the progesterone was added and analyzed by electrophoresis on denaturing polyacrylamide gels followed by autoradiography. The approximate length of the poly(A) tail is indicated.
While neither wild-type CPEB, 5A-CPEB nor 6A-CPEB had any significant effect on entry into MI (GVBD as indicated by the white spot at the animal pole or the initial rise in Cdc2 activity, Figure 4A), the 6A variant inhibited progression from MI to MII. Thus, in oocytes injected with the wild-type CPEB and incubated with progesterone, Mos was synthesized prior to and at MI, and Cdc2 activity and the steady-state level of cyclin B1 peaked at MI, decreased at AI and rose again as the cells entered MII (Figure 4A). All of these events are markers for meiotic progression (Mendez and Richter, 2001). On the other hand, in oocytes injected with variant 6A, Mos was still synthesized normally and Cdc2 activity declined properly after MI, but the Cdc2 activity did not increase subsequently to drive cells into MII. Variant 6A also prevented the synthesis of cyclin B1 throughout all of meiosis (Figure 4A). The observation that the 5A-CPEB variant did not display any of the meiotic effects induced by the stable 6A-CPEB indicates that phosphorylation of S210 is sufficient not only to direct CPEB degradation (Figures 3 and 4A), but also to mediate meiotic progression.
While prophase I-arrested oocytes contain pre-MPF that is composed of Cdc2 and cyclin B, new cyclin B synthesis is not required for MI entry (Hochegger et al., 2001). On the other hand, after the degradation of cyclin B1 during MI, progression into MII does require new cyclin B1 as well as cyclin B4 and B5 synthesis, which may be functionally redundant with cyclin B1. Thus, complete ablation of all mRNAs encoding B-type cyclins does not affect MI progression, but prevents entry into MII. Interestingly, the synthesis of cyclin B4, but not of B5, is stimulated after GVBD in a pattern of accumulation similar to that of cyclin B1 (Hochegger et al., 2001), and the mRNAs encoding both proteins contain a similar arrangement of multiple CPEs. Consequently, the results in Figure 4A showing that oocytes enter MI even when injected with 6A-CPEB that prevents cyclin B1 synthesis are consistent with published reports.
We performed two sets of controls that underscore the importance of CPEB destruction for meiotic progression. First, we attempted to ‘rescue’ the 6A-CPEB variant-induced inhibition of meiotic progression and cyclin synthesis. Oocytes injected with 6A-CPEB were injected subsequently with RNA containing three repeats of the CPE (3CPE), which presumably titrates the non-degradable CPEB (de Moor and Richter, 1999). The injection of this RNA restored both Cdc2 activity and cyclin synthesis at MI and MII (Figure 4B). When injected alone, without a prior 6A-CPEB injection, this RNA had no effect on normal Cdc2 activity or cyclin B1 synthesis (Figure 4C), which rules out the possibility that the 3CPE RNA induced maturation via an independent mechanism that circumvented the requirement for CPEB degradation. Note that in the study of de Moor and Richter (1999), cyclin B1 synthesis was induced by injecting 1.5 pmol of cyclin B1 3′-UTR RNA at 10–15 h post-injection, whereas in the present work, 1 pmol of 3CPE RNA was injected and the oocytes were analyzed 5–8 h after injection. Therefore, these results not only eliminate the possibility of some deleterious bacterial contaminant of 6A-CPEB, but also reaffirm the importance of the CPEB:CPE ratio in controlling meiotic progression.
For a second control, we constructed a variant of 6A-CPEB containing two point mutations in the zinc finger that abrogate mRNA binding (Hake et al., 1998). When injected into oocytes, this CPEB (6A-CPEB-ZnF) had no effect on either Cdc2 activity or cyclin B1 synthesis at either MI or MII (Figure 4C). These results show that not only does the MI to MII transition require a decreased CPEB:CPE ratio, but that this alteration is brought about by partial CPEB destruction.
Our results show that a non-phosphorylatable and non-degradable form of CPEB prevents the accumulation of cyclin B1 but not of Mos. During maturation, Mos mRNA is polyadenylated and translated before MI and Cdc2 activation, when CPEB levels are highest. However, cyclin B1 mRNA polyadenylation and translation occur when CPEB levels are low, at MI, and require both Mos synthesis and Cdc2 activation (Ballantyne et al., 1997; de Moor and Richter, 1997). Figure 4D shows that while both Mos and cyclin B1 RNAs were polyadenylated in response to progesterone (lanes 2 and 8), only Mos RNA was still polyadenylated when the Mos-coding region was ablated by the injection of an antisense oligonucleotide directed against this region (lanes 6 and 12). These data, together with those shown in Figure 4A, suggest that high CPEB levels have differential effects on Mos and cyclin RNA polyadenylation. Indeed, the injection of the non-degradable form of CPEB, 6A-CPEB, had no effect on Mos RNA polyadenylation (lane 4), but prevented cyclin B1 RNA polyadenylation (lane 10). Therefore, temporal control of specific mRNA polyadenylation during oocyte maturation seems to be dependent upon the changing levels of CPEB. Although we cannot rule out the possibility that Cdc2-mediated phosphorylation of CPEB has functions unrelated to protein destruction, this seems unlikely given that it does not affect the ability of CPEB to bind RNA (Hake et al., 1998), to associate with Maskin (Stebbins-Boaz et al., 1999) or to recruit CPSF (Mendez et al., 2000b).
CPEB destruction and embryonic cell division
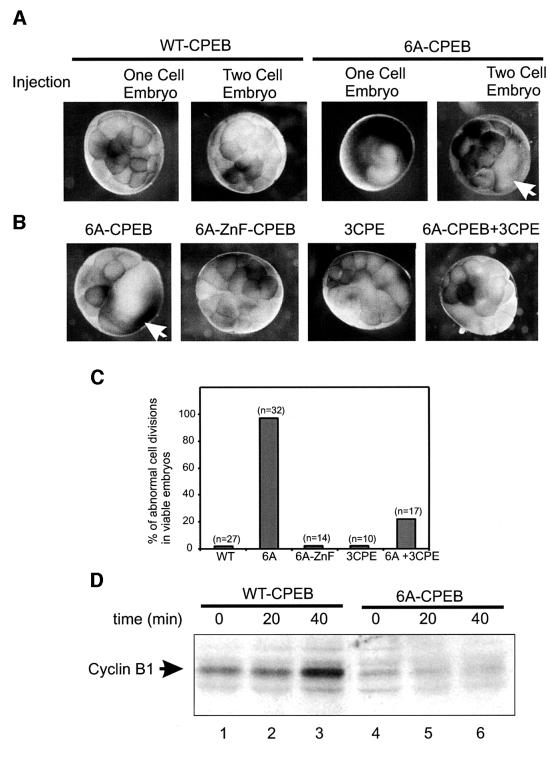
Cell division in the early embryo requires cyclin B1 synthesis in order to enter metaphase, and subsequent cyclin B1 destruction to exit metaphase (King et al., 1996). The synthesis of cyclin B1 is not constant, but instead is regulated at the level of cyclin B1 mRNA polyadenylation. Cyclin B1 mRNA is polyadenylated as cells enter meta phase, and is deadenylated as cells subsequently transition into anaphase (I.Groisman, M.Y.Jung, M.Sarkissian and J.D.Richter, submitted). Because CPEB levels in the embryo remain constantly low and are similar to those observed in the mature oocyte (Hake and Richter, 1994), we surmised that high levels of stable CPEB, equivalent to those found in the immature oocyte, might have a deleterious effect on cyclin B1 synthesis and, as a result, embryonic mitosis. When the stable 6A-CPEB variant was injected into either a fertilized egg or one cell of a two-cell embryo, cell division ceased in >90% of the embryos that remained viable (Figure 5A and C). In contrast, wild-type CPEB had no effect on cell division (Figure 5A and C). Not surprisingly, 6A-CPEB injection, but not wild-type CPEB injection, prevented the accumulation of cyclin B1 (Figure 5D). Moreover, the injection of three CPE-containing RNA rescued the 6A-CPEB phenotype by restoring normal cell division (Figure 5B and C). Figure 5 also demonstrates that CPEB-ZFn, which cannot bind RNA, had no effect on cell division. These results indicate that lowered levels of CPEB are also important to drive cyclin B1 synthesis and mitosis in the embryo.
Fig. 5. 6A-CPEB prevents embryonic cell division. (A) One-cell embryos or a single blastomere of two-cell embryos were injected with WT-CPEB or 6A-CPEB, and cultured until the control embryos (not shown) reached the 32-cell stage. (B) Single blastomeres of two-cell embryos were injected with either 6A-CPEB, 6A-ZnF-CPEB, cyclin B1 3′-UTR (3CPE) or 6A-CPEB plus cyclin B1 3′-UTR (6A-CPEB + 3CPE), and cultured until control embryos (not shown) reached the 32-cell stage. The arrows indicate the injected blastomere. (C) Score of embryos with abnormal cytokinesis depicted in (A) and (B). Numbers in parentheses indicate the number of embryos that survived the injection. (D) Single-cell embryos injected with WT-CPEB or 6A-CPEB were collected at the indicated time and analyzed for cyclin B1 levels by western blotting. Each lane corresponds to one-half embryo equivalent.
Discussion
In this study, we show that the partial destruction of CPEB at MI correlates with the appearance of seven new phosphopeptides. Mutation of these residues produces a CPEB that is not degraded after oocyte maturation. Moreover, the phosphorylation of S210, probably by Cdc2, is sufficient to induce CPEB degradation. In addition, CPEB is ubiquitylated, and requires a PEST box and the proteasome pathway for destruction (Reverte et al., 2001). Most importantly, the biological function of this partial destruction of CPEB is to activate the translation of cyclin B1 (and other such as cyclin B4) mRNA(s) at MI, and to transition the maturing oocyte from MI to MII. Finally, partial CPEB destruction is necessary for cytokinesis in the early embryo.
CPEB subfamilies and PEST boxes
A sequence comparison of CPEB homologs shows that they can be placed into two subfamilies (Figure 6). One subfamily includes those ‘classical’ CPEBs, from Caenorhabditis elegans to humans, with high homology to the founding CPEB member from Xenopus. Based on the high identity within the C-terminal region that is devoted to RNA binding, we believe that all of these proteins will bind CPE-like sequences. With two exceptions, members of this family contain a PEST box followed by an SP pair that corresponds to S210–P211 in Xenopus. Moreover, four members of this CPEB subfamily are degraded in a cell cycle-dependent manner with concomitant changes in apparent molecular weight. This observation suggests a causal link between the phosphorylation and destruction of these proteins in a manner similar to those described for Xenopus CPEB. On the other hand, members of the second CPEB sub family, while also having fairly high homology with the RNA-binding region that indicates an interaction with CPE-like sequences, lack an equivalent PEST box and have no obvious residue that corresponds to S210 in Xenopus. We propose that the activities of the two CPEB subfamily members may be functionally related to the presence or absence of a PEST box, i.e. the instability of members of the first CPEB subfamily could be important for them to perform certain activities such as the translational induction of cyclin B1 mRNA by Xenopus CPEB. Conversely, the hypothesized stability of members of the second CPEB subfamily could be essential for them to conduct their duties, which might include mRNA transport or stability as well as translation. Finally, Figure 6 also presents a proposed nomenclature for human CPEB proteins
CPEB destruction and meiotic progression
CPEB phosphorylation and degradation correlate with entry into MI and the cytoplasmic polyadenylation and translation of the ‘late’ or class II mRNAs, such as cyclin B1 mRNA. The injection of a stable CPEB variant into oocytes in an amount roughly equal to that present in immature oocytes had no effect on oocyte maturation, entry in MI or Mos synthesis, but prevented progression to MII and cyclin B1 synthesis. Moreover, the polyadenylation of cyclin B1 mRNA, but not Mos mRNA, was inhibited by this stable CPEB variant. These data are consistent with the observation that new cyclin B synthesis is only required for the MI to MII transition but not for the prophase I (G2-like) to MI transition where the stockpile of pre-MPF is sufficient (Hochegger et al., 2001). The cyclin B family is composed of four functionally redundant proteins B1, B2, B4 and B5; ablation of all four member mRNAs results in inhibition of entry into MII (Hochegger et al., 2001). Of those cyclins, B2 and B5 are present in high levels in immature oocytes and the protein levels decrease after MI activation of the APC. On the other hand, immature oocytes contain low levels of cyclins B1 and B4, which only accumulate until after GVBD when CPEB is phosphorylated and partially degraded, and cyclin B1 mRNA is translated. Both cyclin B1 and B4 mRNAs contain multiple CPEs and, because the function of both encoded proteins is redundant and sufficient to mediate MI to MII transition, the overexpression of the stable CPEB variant 6A may also prevent cyclin B4 mRNA translation.
CPEB destruction and translational activation
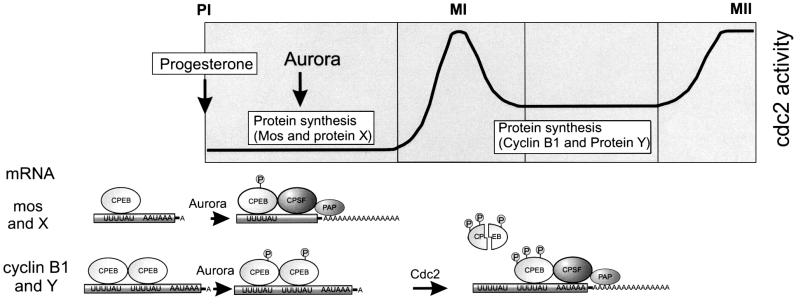
The first stage of maturation at which protein synthesis is required is entry into MI (GVBD). While Mos mRNA translation is necessary for MI (Sagata et al., 1989; Sheets et al., 1995), so too may be the translation of other CPE-containing mRNAs (Barkoff et al., 1998). These early translation events occur when CPEB levels are at their zenith, and immediately follow the activation of CPEB by Aurora (Mendez et al., 2000a). However, the late translation events (e.g. cyclin B1 mRNA) that take place at MI require the previous synthesis of Mos and the activation of Cdc2 kinase (Ballantyne et al., 1997; de Moor and Richter, 1997). We show here that an important Cdc2 activity is to trigger the partial destruction of CPEB, which in turn stimulates the translation of cyclin B1 mRNA. The seemingly paradoxical result, that a stimulatory factor such as CPEB needs to be destroyed before specific mRNA translation can ensue, may be explained if one considers the differences between the 3′-UTRs of class I and class II mRNAs. The 3′-UTRs of class I mRNAs (e.g. Mos, cyclin A1) (Ballantyne et al., 1997) all contain only a single obvious CPE, whereas the 3′-UTRs of class II mRNAs (e.g. cyclin B1) contain two or more CPEs (Figure 7). If a CPEB molecule occupies each CPE, then the class I mRNAs would be polyadenylated as soon as CPEB is activated by Aurora because CPEB would simply recruit CPSF. Class II mRNAs, however, may not be polyadenylated at this time because multiple CPEB proteins could dimerize (or multimerize), which could prevent a CPEB–CPSF interaction. In support of this notion, CPEB proteins do form dimers (unpublished). At MI, when CPEB is partially destroyed, a stochastic model would predict that only a single CPEB would remain per mRNA, even if those mRNAs contain multiple CPEs. At this time, CPEB would be free to interact with CPSF and promote polyadenylation. Other features of the mRNAs or other RNA-binding proteins may also contribute to the differential activation by the polyadenylation machinery.
Fig. 7. Proposed model for the regulation of late polyadenylation by CPEB degradation. Class I mRNAs, such as those encoding Mos and a putative protein X, are polyadenylated early after oocytes are exposed to progesterone, in response to the phosphorylation of CPEB by the Aurora kinase. Synthesis of these proteins is required for both the activation of Cdc2 kinase and for entry into metaphase I (MI), and accounts for the early requirement for protein synthesis, prior to MI. Members of the class II mRNAs, such as those encoding cyclin B1 and a putative protein Y (e.g. cyclin B4), are not, however, polyadenylated solely in response of Aurora kinase activation. We hypothesize that the failure of these mRNAs to recruit CPSF and PAP immediately and be polyadenylated at a time coincidental with Mos mRNA may reside in the different number of CPEs that they contain (cf. de Moor and Richter, 1997). That is, mos mRNA contains a single CPE, and thus a simple CPEB–CPSF interaction would occur and promote polyadenylation. Cyclin mRNA, on the other hand, contains two CPEs, which would facilitate the simultaneous binding of two CPEBs. Two CPEBs (or multiple CPEBs for RNAs that contain >2 CPEs) would bind one another to the exclusion of a CPEB–CPSF interaction, thereby preventing polyadenylation. At MI, Cdc2 catalyzes a second round of CPEB phosphorylation that leads to its partial destruction. As a result of this destruction, CPEB dimers (or multimers) would not be present, and CPSF would be recruited appropriately and stimulate the late polyadenylation of class II mRNAs. This late polyadenylation event, which could stimulate the translation of several mRNAs, would account for the protein synthesis needed to enter MII.
Saving some CPEB for the embryo
During maturation, CPEB appears to undergo a partial destruction that is spatially restricted; almost all the stable CPEB is found at the animal pole (Groisman et al., 2000). This stable CPEB is inherited by the fertilized egg and promotes the essential CPE-dependent cyclin B1 mRNA translation that occurs in the early embryo (Groisman et al., 2000). Interestingly, the embryonic polyadenylation takes place in two compartments, one that is soluble and one that is tethered to the spindles and centrosomes of the embryonic blastomeres in the animal pole (Groisman et al., 2000). Because it interacts with microtubules, CPEB seems likely to anchor the polyadenylation machinery to the mitotic apparatus. Moreover, the region of CPEB that binds the microtubules is the PEST sequence (Groisman et al., 2000), which we show here is also necessary for CPEB destruction. Because protein–protein interactions mediated by the PEST box can impair targeted degradation (Fenwick et al., 2000), we thought that the ∼30% of CPEB that remains stable at maturation does so because it is bound to microtubules via the PEST box. However, the treatment of oocytes with agents that either depolymerize or stabilize microtubules had no discernible effect on CPEB destruction, suggesting that a masking of the PEST box by microtubules is not necessary for the stability of this small but important amount of CPEB.
Materials and methods
Reagents
CPEB and myc antibodies, myc-CPEB and his-CPEB (Hake and Richter, 1994), the cyclin B1 3′-UTR (Tay et al., 2000), and the Mos 3′-UTR and Mos antisense oligonucleotide (de Moor and Richter, 1997) have been described. The Xenopus cyclin B1 antibody was a gift from J.L.Maller, University of Colorado School of Medicine. Mos antibody (Santa Cruz), ubiquitin and PKA (Sigma), and MAPK and Cdc2 (Calbiochem) were purchased from commercial sources.
In vitro CPEB phosphorylation
Extracts were prepared by homogenizing five oocytes in 100 µl of H1 kinase buffer (80 mM Na β-glycerophosphate, 20 mM EGTA, 15 mM MgCl2, 50 mM NaVaO4) with protease inhibitors (10 µg/ml each of leupeptin, pepstatin and chymostatin) and centrifuged for 5 min at 4°C. Kinase assays were performed in 30 µl of buffer containing 20 mM Tris pH 7.7, 10 mM MgCl2, 50 mM KCl, 1 mM dithiothreitol and 30 µM ATP (0.16 mCi/ml [γ-32P]ATP) with 10 µl of oocyte extract or the indicated purified kinase. Recombinant CPEB and two-dimensional phosphopeptide analysis have been described (Mendez et al., 2000a).
CPE phosphoamino acid analysis
Oocytes were incubated overnight in buffer containing 100 µCi/ml of [32P]orthophosphate and 50 µg/ml streptomycin. Progesterone was then added and the oocytes were collected at 50% GVBD; CPEB subsequently was immunoprecipitated (Hake and Richter, 1994), resolved by SDS–PAGE, blotted onto a PVDF membrane, hydrolyzed with 6 M HCl and resolved by two-dimensional electrophoresis (Van der Geer and Hunter, 1994).
In vitro ubiquitylation
Extracts were prepared as described (Murray, 1991). Briefly, after dejellying, eggs were transferred to a centrifuge tube containing cytochalasin B (10 mg/ml), packed for 30 s at 30 g, and then crushed at 21 000 g for 5 min. The cytoplasmic layer was recovered, centrifuged twice more, supplemented with energy mix and protease inhibitors, and the high-speed supernatant (S100) obtained (Salic et al., 2000). The in vitro ubiquitylation reaction contained 30 µl of supernatant, 32P-labeled ubiquitin (21 µCi/ml) and 20 µg of the indicated CPEB variants. The reaction was incubated at 22°C for 1 h and then diluted 50-fold with immunoprecipitation buffer (0.1 M NaCl, 1 mM MgCl2, 50 mM Tris–HCl pH 8, 0.5 % NP-40, 80 mM β-glycerophosphate and 10 µg each of pepstatin, chymostatin and leupeptin) containing 50 µg/ml of RNase A, mixed with 50 µl of protein A–Sepharose and coupled with CPEB antibody by incubation for 4 h at 4°C. The mixtures were centrifuged and the pellets washed with immunoprecipitation buffer. The immunoprecipitate was eluted with SDS sample buffer and probed on a western blot for CPEB. Labeled ubiquitin was obtained by phosphorylation with PKA (Hauser et al., 1998).
H1 kinase assays, western blots, cytoplasmic polyadenylation analyses and Mos oligonucleotide injection experiments have been described previously (Hake and Richter, 1994; de Moor and Richter, 1997).
Construction of CPEB mutant proteins
Point mutations in CPEB were made with a Chamaleon mutagenesis kit (Stratagene, La Jolla, CA). The selection primers, located in the pMyc-CPEB (Hake and Richter, 1994), were: 5′-CCTCGAGGGGCGGGCCCGTACCCAATTCGCCC-3′, which changed a KpnI site to an SrfI site, or 5′-CCGTCGACGTCGAGGGGGGGGCCGGTACCC-3′, which deleted XhoI and ApaI sites. This primer was used in conjunction with the following mutation primers to create new CPEB variants: S138A, 5′-GTCTTTAGTATGCTGAACGCCCCCATGGGG-3′; S248A, 5′-GCGGCAGCAACTGTGGCGCCACTTGGC-3′; S144A, 5′-CCCCATGGGGAAGCCAGCACCCTTGGGC-3′; S423A, 5′-GCAACTTTGTGCGCG CTCCATCACAACGGC-3′; S210A, 5′-CCTAATTTCAAGTCTTCGCATCGCTCCTCCG-3′; and A210S, 5′-CCTAATTTCAAGTCTTCGAATCTCTCCTCCG-3′. To create the deletion mutants of CPEB, the myc-CPEB S184A was used as a template for PCR amplification using the following templates: Δ1 antisense 5′-GGCGCGCTCAGCAAAGGAGTAGGATACTTCTCCATGAGG-3′; Δ2 antisense 5′-GGCGCGCTAAAGACAGACTGAGCAGCTGTTGGAGTTTG-3′; Δ3 antisense 5′-GGC GCGCTGAAATCCAGAGAGTTGTCCGACATGGC-3′; Δ1–3 sense 5′-GCCGCTCTAGATGGAACAGAAGCTGATTAGC-3′; Δ4 antisense 5′-CCCACAAATACAGTCTTACTGGGATCCAGCCG-3′; and Δ4 sense 5′-CCAGCGCGCCGCTGCATTTCCTCCCACTTGG-3′. The products of Δ1–3 were digested with XbaI–BssHII and ligated into myc-CPEB S184A digested with the same enzymes. The product of Δ4 was digested with BssHII–BamHI and ligated into myc-CPEB S184A digested with the same enzymes. The His-tagged forms of the mutant CPEBs were obtained by subcloning the NheI–SacI fragment of the myc-CPEB variants into the NheI–SacI sites of pHis-CPEB (Hake and Richter, 1994).
Acknowledgments
Acknowledgements
We thank James Maller for the cyclin antibody, and Mercedes Fernandez and members of the Richter laboratory for discussions and critically reading the manuscript. R.M. was supported by a Leukemia and Lymphoma Society of America Special Fellow award and D.B. was supported in part by an Institutional NIH Postdoctoral Training Grant, and by a National Research Service Award. This work was supported by grants from the NIH to J.D.R.
References
- Andresson T. and Ruderman,J.V. (1998) The kinase Eg2 is a component of the Xenopus oocyte progesterone-activated signaling pathway. EMBO J., 17, 5627–5637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballantyne S., Daniel,D.L.,Jr and Wickens,M. (1997) A dependent pathway of cytoplasmic polyadenylation reactions linked to cell cycle control by c-mos and CDK1 activation. Mol. Biol. Cell, 8, 1633–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bally-Cuif L., Schatz,W.J. and Ho,R.K. (1998) Characterization of the zebrafish Orb/CPEB-related RNA binding protein and localization of maternal components in the zebrafish oocyte. Mech. Dev., 77, 31–47. [DOI] [PubMed] [Google Scholar]
- Barkoff A., Ballantyne,S. and Wickens,M. (1998) Meiotic maturation in Xenopus requires polyadenylation of multiple mRNAs. EMBO J., 17, 3168–3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkoff A.F., Dickson,K.S., Gray,N.K. and Wickens,N. (2000) Translational control of cyclin B1 mRNA during meiotic maturation: coordinated repression and cytoplasmic polyadenylation. Dev. Biol., 220, 97–109. [DOI] [PubMed] [Google Scholar]
- de Moor C.H. and Richter,J.D. (1997) The Mos pathway regulates cytoplasmic polyadenylation in Xenopus oocytes. Mol. Cell. Biol., 17, 6419–6426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Moor C.H. and Richter,J.D. (1999) Cytoplasmic polyadenylation elements mediate masking and unmasking of cyclin B1 mRNA. EMBO J., 18, 2294–2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson K.S., Bilger,A., Ballantyne,S. and Wickens,M.P. (1999) The cleavage and polyadenylation specificity factor in Xenopus laevis oocytes is a cytoplasmic factor involved in regulated polyadenylation. Mol. Cell. Biol., 19, 5707–5717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenwick C., Na,S.Y., Voll,R.E., Zhong,H., Im,S.Y., Lee,J.W. and Ghosh,S. (2000) A subclass of Ras proteins that regulate the degradation of IκB. Science, 287, 869–873. [DOI] [PubMed] [Google Scholar]
- Ferby I., Blazquez,M., Palmer,A., Eritja,R. and Nebreda,A.R. (1999) A novel p34cdc2-binding and activating protein that is necessary and sufficient to trigger G(2)/M progression in Xenopus oocytes. Genes Dev., 13, 2177–2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox C.A., Sheets,M.D. and Wickens,M.P. (1989) Poly(A) addition during maturation of frog oocytes: distinct nuclear and cytoplasmic activities and regulation by the sequence UUUUUAU. Genes Dev., 3, 2151–2162. [DOI] [PubMed] [Google Scholar]
- Gerhart J., Wu,M. and Kirschner,M. (1984) Cell cycle dynamics of an M-phase-specific cytoplasmic factor in Xenopus laevis oocytes and eggs. J. Cell Biol., 98, 1247–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groisman I., Huang,Y.S., Mendez,R., Cao,Q., Theurkauf,W. and Richter,J.D. (2000) CPEB, maskin and cyclin B1 mRNA at the mitotic apparatus: implications for local translational control of cell division. Cell, 103, 435–447. [DOI] [PubMed] [Google Scholar]
- Gross S.D., Schwab,M.S., Taieb,F.E., Lewellyn,A.L., Qian,Y.W. and Maller,J.L. (2000) The critical role of the MAP kinase pathway in meiosis II in Xenopus oocytes is mediated by p90(Rsk). Curr. Biol., 10, 430–438. [DOI] [PubMed] [Google Scholar]
- Hake L.E. and Richter,J.D. (1994) CPEB is a specificity factor that mediates cytoplasmic polyadenylation during Xenopus oocyte maturation. Cell, 79, 617–627. [DOI] [PubMed] [Google Scholar]
- Hake L.E., Mendez,R. and Richter,J.D. (1998) Specificity of RNA binding by CPEB: requirement for RNA recognition motifs and a novel zinc finger. Mol. Cell. Biol., 18, 685–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser H.P., Bardroff,M., Pyrowolakis,G. and Jentsch,S. (1998) A giant ubiquitin-conjugating enzyme related to IAP apoptosis inhibitors. J. Cell Biol., 141, 1415–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochegger H., Klotzbücher,A., Kirk,J., Howell,M., Le Guellec,K., Fletcher,K., Duncan,T., Sohail,M. and Hunt,T. (2001) New B-type cyclin synthesis is required between meiosis I and II during Xenopus oocyte maturation. Development, 128, 3795–3807. [DOI] [PubMed] [Google Scholar]
- Katsu Y., Minshall,N., Nagahama,Y. and Standart,N. (1999) Ca2+ is required for phosphorylation of clam p82/CPEB in vitro: implications for dual and independent roles of MAP and Cdc2 kinases. Dev. Biol., 209, 186–199. [DOI] [PubMed] [Google Scholar]
- King R.W., Deshaies,R.J., Peters,J.M. and Kirschner,M.W. (1996) How proteolysis drives the cell cycle. Science, 274, 1652–1659. [DOI] [PubMed] [Google Scholar]
- Laney J.D. and Hochstrasser,M. (1999) Substrate targeting in the ubiquitin system. Cell, 97, 427–430. [DOI] [PubMed] [Google Scholar]
- Lenormand J.L., Dellinger,R.W., Knudsen,K.E., Subramani,S. and Donoghue,D.J. (1999) Speedy: a novel cell cycle regulator of the G2/M transition. EMBO J., 18, 1869–1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrew L.L., Dworkin-Rastl,E., Dworkin,M.B. and Richter,J.D. (1989) Poly(A) elongation during Xenopus oocyte maturation is required for translational recruitment and is mediated by a short sequence element. Genes Dev., 3, 803–815. [DOI] [PubMed] [Google Scholar]
- Mendez R. and Richter,J.D. (2001) Translational control by CPEB: a means to the end. Nature Rev. Mol. Cell. Biol., 2, 521–529. [DOI] [PubMed] [Google Scholar]
- Mendez R., Hake,L.E., Andresson,T., Littlepage,L.E., Ruderman,J.V. and Richter,J.D. (2000a) Phosphorylation of CPE binding factor by Eg2 regulates translation of c-mos mRNA. Nature, 404, 302–307. [DOI] [PubMed] [Google Scholar]
- Mendez R., Murthy,K.G., Ryan,K., Manley,J.L. and Richter,J.D. (2000b) Phosphorylation of CPEB by Eg2 mediates the recruitment of CPSF into an active cytoplasmic polyadenylation complex. Mol. Cell, 6, 1253–1259. [DOI] [PubMed] [Google Scholar]
- Minshull J., Blow,J.J. and Hunt,T. (1989) Translation of cyclin mRNA is necessary for extracts of activated Xenopus eggs to enter mitosis. Cell, 56, 947–956. [DOI] [PubMed] [Google Scholar]
- Minshall, N., Walker,J., Dale,M. and Standart,N. (1999) Dual roles of p82, the clam CPEB homolog, in cytoplasmic polyadenylation and translational masking. RNA, 5, 27–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray A.W. (1991) Cell cycle extracts. Methods Cell Biol., 36, 581–606. [PubMed] [Google Scholar]
- Nakahata S., Katsu,Y., Mita,K., Inoue,K., Nagahama,Y. and Yamashita,M. (2001) Biochemical identification of Xenopus Pumilio as a sequence-specific cyclin B1 mRNA-binding protein that physically interacts with a Nanos homolog, Xcat-2 and a cytoplasmic polyadenylation element-binding protein. J. Biol. Chem., 276, 20945–20953. [DOI] [PubMed] [Google Scholar]
- Nebreda A.R. and Ferby,I. (2000) Regulation of the meiotic cell cycle in oocytes. Curr. Opin. Cell Biol., 12, 666–675. [DOI] [PubMed] [Google Scholar]
- Peter M., Castro,A., Lorca,T., Le Peuch,C., Magnaghi-Jaulin,L., Doree,M. and Labbe,J.C. (2001) The APC is dispensable for the first meiotic anaphase in Xenopus oocytes. Nature Cell Biol., 3, 83–87. [DOI] [PubMed] [Google Scholar]
- Rechsteiner M. and Rogers,S.W. (1996) PEST sequences and regulation by proteolysis. Trends Biochem. Sci., 21, 267–271. [PubMed] [Google Scholar]
- Reverte C.G., Ahearn,M.D. and Hake,L.E. (2001) CPEB degradation during Xenopus oocyte maturation requires a PEST domain and the 26S proteasome. Dev. Biol., 231, 447–458. [DOI] [PubMed] [Google Scholar]
- Sagata N., Daar,I., Oskarsson,M., Showalter,S.D. and Vande Woude,G.F. (1989) The product of the mos proto-oncogene as a candidate ‘initiator’ for oocyte maturation. Science, 245, 643–646. [DOI] [PubMed] [Google Scholar]
- Salic A., Lee,E., Mayer,L. and Kirschner,M.W. (2000) Control of β-catenin stability: reconstitution of the cytoplasmic steps of the Wnt pathway in Xenopus egg extracts. Mol. Cell, 5, 523–532. [DOI] [PubMed] [Google Scholar]
- Sheets M.D., Wu,M. and Wickens,M. (1995) Polyadenylation of c-mos mRNA as a control point in Xenopus meiotic maturation. Nature, 374, 511–516. [DOI] [PubMed] [Google Scholar]
- Stebbins-Boaz B., Cao,Q., de Moor,C.H., Mendez,R. and Richter,J.D. (1999) Maskin is a CPEB-associated factor that transiently interacts with elF-4E. Mol. Cell, 4, 1017–1027. [DOI] [PubMed] [Google Scholar]
- Stutz A., Conne,B., Huarte,J., Gubler,P., Volkel,V., Flandin,P. and Vassalli,J.D. (1998) Masking, unmasking and regulated polyadenyl ation cooperate in the translational control of a dormant mRNA in mouse oocytes. Genes Dev., 12, 2535–2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swenson K.I., Farrell,K.M. and Ruderman,J.V. (1986) The clam embryo protein cyclin A induces entry into M phase and the resumption of meiosis in Xenopus oocytes. Cell, 47, 891–870. [DOI] [PubMed] [Google Scholar]
- Taieb F.E., Gross,S.D., Lewellyn,A.L. and Maller,J.L. (2001) Activation of the anaphase-promoting complex and degradation of cyclin B is not required for progression from meiosis I to II in Xenopus oocytes. Curr. Biol., 11, 508–513. [DOI] [PubMed] [Google Scholar]
- Tay J., Hodgman,R. and Richter,J.D. (2000) The control of cyclin B1 mRNA translation during mouse oocyte maturation. Dev. Biol., 221, 1–9. [DOI] [PubMed] [Google Scholar]
- Van der Geer P. and Hunter,T. (1994) Phosphopeptide mapping and phosphoamino acid analysis by electrophoresis and chromatography on thin-layer cellulose plates. Electrophoresis, 15, 544–554. [DOI] [PubMed] [Google Scholar]
- Walker J., Minshull,N., Hake,L., Richter,J. and Standart,N. (1999) The clam 3′ UTR masking element-binding protein p82 is a member of the CPEB family. RNA, 5, 14–26. [DOI] [PMC free article] [PubMed] [Google Scholar]