Abstract
The Disabled-2 (Dab2) gene has been proposed to act as a tumor suppressor. Cell culture studies have implicated Dab2 in signal transduction by mitogens, TGFβ and endocytosis of lipoprotein receptors. To identify in vivo functions of Dab2, targeted mutations were made in the mouse. In the absence of Dab2, embryos arrest prior to gastrulation with a phenotype reminiscent of those caused by deletion of some TGFβ signal transduction molecules involved in Nodal signaling. Dab2 is expressed in the extra-embryonic visceral endoderm but not in the epiblast. Dab2 could be conditionally deleted from the embryo without affecting normal development, showing that Dab2 is required in the visceral endoderm but dispensable in the embryo proper. Conditionally mutant Dab2–/– mice are overtly normal, but have reduced clathrin-coated pits in kidney proximal tubule cells and excrete specific plasma proteins in the urine, consistent with reduced transport by a lipoprotein receptor, megalin/gp330, in the proximal tubule. This evidence indicates that Dab2 is pleiotropic and regulates both visceral endoderm function and lipoprotein receptor trafficking in vivo.
Keywords: adaptor protein/kidney proximal tubule/megalin/Nodal/visceral endoderm
Introduction
The Disabled-2 (Dab2)/DOC2 gene is widely expressed in at least two alternative splice forms, encoding p96 and p67 proteins (Mok et al., 1994; Xu et al., 1995). Expression of Dab2 is decreased in many ovarian, mammary and prostate carcinomas, and in choriocarcinomas (Fulop et al., 1998; Mok et al., 1998; Schwahn and Medina, 1998; Tseng et al., 1998; Fazili et al., 1999). Dab2 expression inhibits proliferation of cultured cells, suggesting that its down-regulation is important for tumor initiation or progression (Tseng et al., 1998; Sheng et al., 2000). However, the cellular basis for this apparent tumor suppressor effect and the normal functions of Dab2 are unknown.
Both Dab2 and a related protein, Dab1, have features of cytoplasmic adaptor proteins, such as protein binding domains, phosphorylation sites and the absence of catalytic domains (Xu et al., 1995; Howell et al., 1997), and may thus participate in signal transduction pathways or regulate protein traffic inside cells (Pawson and Scott, 1997; Pearse et al., 2000). Indeed, Dab1 has an important signaling function during development, regulating migrations of committed but undifferentiated neurons. Genetically, Dab1 relays signals from specific lipoprotein receptors (Rice and Curran, 1999). Lipoprotein receptors are best known for their roles in importing proteins and lipids into cells, but they also have signal transduction functions (Krieger and Herz, 1994; Howell and Herz, 2001). In vitro, both Dab1 and Dab2 bind to a common sequence present in the cytoplasmic tails of lipoprotein receptors, via their phosphotyrosine binding/protein interaction (PTB/PID) domains (Trommsdorff et al., 1998; Howell et al., 1999; Margolis, 1999; Morris and Cooper, 2001). The Dab-binding sequence is also involved in trafficking lipoprotein receptors into the endocytic pathway (Chen et al., 1990). Dab2 has been found in complexes with a lipoprotein receptor, megalin (gp330/gp600), in kidney (Oleinikov et al., 2000), suggesting it could regulate megalin trafficking or signaling. In addition, the p96 splice form of Dab2 binds to the clathrin adaptor AP-2 and localizes to clathrin-coated pits, but it is not known whether p96 regulates endocytosis (Morris and Cooper, 2001).
A possible molecular mechanism for growth inhibition by Dab2 is provided by the observation that overexpression of Dab2 can interfere with mitogenic growth factor signal transduction, possibly by inhibiting Ras or MAP kinase activation (Xu et al., 1998; Tseng et al., 1999; Smith et al., 2001; Zhou and Hsieh, 2001). An alternative possible mechanism derives from studies on a TGFβ-non-responsive mutant cell line (Hocevar et al., 2001). These mutant cells express low levels of an altered form of Dab2, and expression of wild-type Dab2 restores TGFβ responsiveness. Moreover, Dab2 associates with TGFβ receptors and with their substrates, the transcription factors SMAD2 and SMAD3 (Hocevar et al., 2001). Since TGFβ inhibits proliferation of some cells, Dab2 may act as a tumor suppressor by promoting TGFβ signaling.
TGFβ-related factors relay many inductive signals during mouse embryonic development (Hogan, 1996; Zimmerman and Padgett, 2000). One such factor, Nodal, is important for establishing the anterior–posterior (A-P) axis, induction of mesoderm and definitive endoderm, and left–right asymmetry (Beddington and Robertson, 1999; Schier and Shen, 2000; Lu et al., 2001). The A-P axis is established at the egg cylinder stage, when the embryo proper is represented by a layer of embryonic ectoderm (epiblast) sheathed in a layer of extra-embryonic visceral endoderm (VE). The embryo is attached proximally to maternal tissues via more extra-embryonic cells. Nodal expression is first induced in a proximal-to-distal wave through the epiblast, and then induces the VE at the distal tip to express various genes, change shape and migrate up one side of the epiblast. This altered region of the VE is known as the anterior VE (AVE). Proteins made by the AVE include secreted inhibitors that act back on the epiblast and inhibit Nodal action. Nodal activity is thus restricted to the part of the epiblast most distant from the AVE, which is induced to form mesoderm and defines the future posterior of the embryo. The signal transduction pathways utilized by Nodal in the epiblast cells and VE cells are still being established. However, analysis of mouse mutants reveals that some TGFβ signaling components are needed for signal transduction by Nodal in the epiblast, and others for signal transduction in the VE.
We have used a targeted genetics approach to test whether Dab2 regulates signaling or transport in vivo. Our results suggest that Dab2 is needed in the VE, in part to express Nodal-induced genes, and also regulates protein transport in the kidney. Thus Dab2 is pleiotropic, with direct or indirect functions in both signal transduction and protein traffic.
Results
Targeting of the Dab2 locus
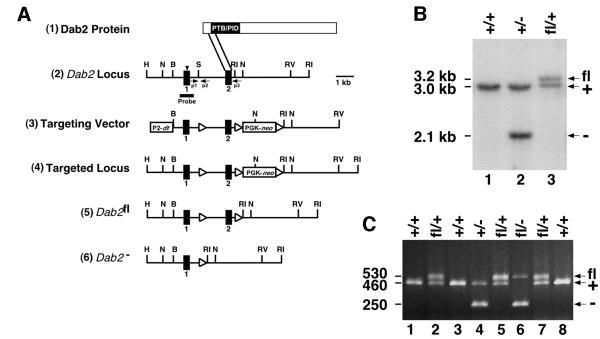
The Dab2 gene was subjected to targeted deletion in embryonic stem (ES) cells to simultaneously prepare null and conditional alleles (Figure 1A; Gu et al., 1994). A loxP site for Cre-mediated recombination (Sternberg and Hamilton, 1981) was inserted 5′ to the second coding exon, and a neomycin selection cassette flanked by loxP sites was inserted 3′ to the second exon. Cre recombinase was then transiently expressed from either of two plasmids. Expression from a strong promoter (phosphoglycerate kinase, PGK) allowed recombination between the first and third loxP sites, removing the second coding exon and the neomycin cassette and creating a null allele (Dab2–). The second coding exon encodes the beginning of the PTB domain and is found in all known Dab2 splice forms (Xu et al., 1995; Tseng et al., 1998; Cho et al., 1999; Fazili et al., 1999). If, by chance, splicing should occur from the first to third coding exon, a frameshift would occur, resulting in a truncated protein lacking most of the PTB domain. Expression from a weak promoter (cytomegalovirus, CMV) allowed recombination between the second and third loxP sites, removing the neomycin cassette but leaving the second coding exon flanked by loxP sites (floxed) (Dab2fl allele). Recombined clones were identified by PCR and verified by Southern blotting. ES cells from each type of recombination event were injected into blastocysts, and the resulting chimeric mice were mated in order to generate either Dab2+/– or Dab2fl/+ mice (Figure 1B and C).
Fig. 1. Targeted disruption of the Dab2 gene. (A) Dab2 targeting strategy. (1) Schematic representation of the Dab2 protein. The parallel lines indicate the region of the Dab2 protein encoded by the second coding exon and targeted for deletion. (2) Partial restriction map of the Dab2 genomic locus in which the initiation ATG is indicated by the arrowhead. (3) Targeting vector containing the diphtheria toxin gene (dt) under the control of the RNA polymerase 2 promoter (P2) and the neomycin resistance gene (neo) under the control of the PGK promoter. LoxP sites are indicated by the triangles. (4) The targeted Dab2 locus prior to Cre expression. (5) The floxed Dab2 allele (Dab2fl) and (6) the deleted Dab2 allele (Dab2–) following Cre expression. Restriction enzymes: H, HpaI; N, NcoI; B, BamHI; S, SmaI; RI, EcoRI; RV, EcoRV. (B) Southern blotting analysis of DNA prepared from a wild-type mouse (lane 1), or mice heterozygous for either the deleted (lane 2) or floxed (lane 3) allele. DNA was digested with NcoI and hybridized to the radiolabeled Dab2 probe shown in (A). The positions of the wild-type (3.0 kb, +), null (2.1 kb, –) and floxed (3.2 kb, fl) alleles are indicated. (C) PCR analysis of P8 pups born from a Dab2fl/+ × Dab2+/– mating. The positions of the wild-type (460 bp, +), null (250 bp, –) and floxed (530 bp, fl) alleles are indicated. The positions of the three PCR primers used (p1, p2 and p3) are indicated by arrows in (A).
Dab2 is required for early post-implantation development
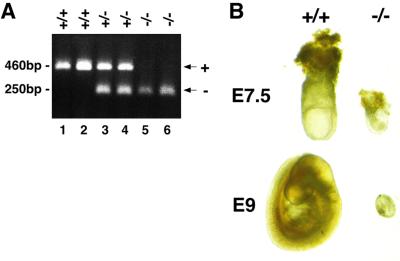
Dab2 heterozygous mice were fertile and phenotypically normal. To study the phenotype of Dab2 homozygous mice, Dab2+/– mice were intercrossed. Homozygous mutation of Dab2 is lethal in early development, as shown by the absence of Dab2–/– pups and E11.5 embryos, although at E11.5, there were a significant number of sites where dead embryos had been resorbed (Table I). Between E7.5 and E8.5, Dab2–/– embryos were found at the proper Mendelian ratio (Table I; Figure 2A), but they were significantly smaller than their littermates (Figure 2B). These embryos resembled the early egg cylinder stage, indicating that the Dab2 null embryos implant but fail to undergo gastrulation.
Table I. Genotypes of offspring from Dab2+/– × Dab2+/– matings.
| Age | +/+ | +/– | –/– | Resorbed |
|---|---|---|---|---|
| P10 | 110 (37%) | 186 (63%) | 0 (0%) | N/A |
| E11.5 | 11 (47%) | 16 (59%) | 0 (0%) | 9 |
| E8 | 8 (24%) | 17 (52%) | 8 (24%) | 0 |
Fig. 2. Analysis of Dab2+/– intercrosses. (A) PCR genotyping of E7.5 embryos resulting from Dab2+/– intercrosses. (B) Morphology of wild-type (+/+) and Dab2 null (–/–) E7.5 and E9 embryos.
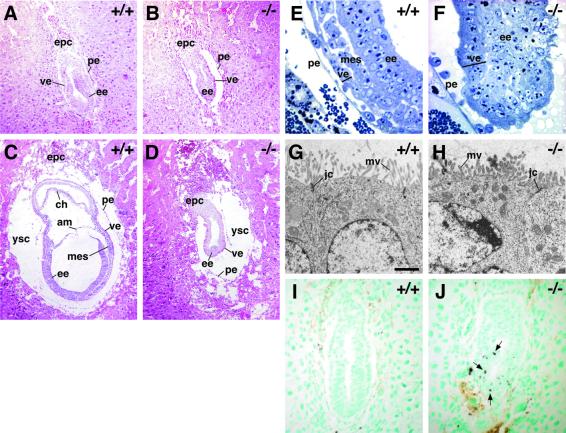
E6.5 and E7.5 mutant and wild-type embryos were examined by light and electron microscopy (Figure 3). At E6.5, wild-type embryos contain two layers of extra-embryonic (or primitive) endoderm, the outer parietal endoderm (PE) and inner VE, which surround the epiblast (EE) and the developing mesoderm. At the proximal end, trophoblast cells invade the endometrium to form the ectoplacental cone (EPC). Dab2 null embryos contain three layers that resemble the PE, VE and EE of wild- type embryos (Figure 3A and B). However, the Dab2–/– embryos were significantly smaller than their wild-type littermates, and the surrounding yolk sac cavity, between VE and PE, appeared larger. The cells of the presumed EE were jumbled, and not arranged in an epithelial sheet. The proamniotic cavity, inside the embryo, was smaller. By E7.5, the difference in size between wild-type and mutant embryos was even more obvious. At this stage in wild-type embryos, amnion, chorion and allantois had formed in the proximal part of the egg cylinder, mesoderm was invading between EE and VE, the cells of the EE were well organized, and the VE took on a thinner, more squamous morphology (Figure 3C and E). In contrast, the Dab2–/– embryos failed to grow, the amnion, chorion and allantois were absent, and the internal cells of the presumptive EE were disorganized (Figure 3D and F). Additionally, at the distal tip of Dab2–/– embryos, cells of the VE maintained their cuboidal morphology (Figure 3F). Electron microscopy (EM) of thin sections of E7.5 embryos revealed that the mutant VE was composed of well-differentiated epithelial cells with microvilli and apical junctional complexes (Figure 3G and H). This suggests that Dab2 is not needed for epithelial cell differentiation.
Fig. 3. Histology of wild-type and mutant Dab2 embryos. (A and B) Hematoxylin and eosin-stained sections of E6.5 wild-type (A) and mutant (B) embryos. (C and D) Hematoxylin and eosin-stained sections of E7.5 wild-type (C) and mutant (D) embryos. (E and F) Toluidine blue-stained thin sections from E7.5 wild-type (E) and mutant (F) embryos. (G and H) EM of E7.5 wild-type (G) and mutant (H) visceral endoderm. Bar = 2 µm. (I and J) TUNEL staining (arrows) of E6.5 wild-type (I) and mutant (J) embryos. ve, visceral endoderm; pe, parietal endoderm; ee, embryonic ectoderm; epc, ectoplacental cone; ch, chorion; am, amnion; mes, mesoderm; ysc, yolk sac cavity; jc, junctional complex; mv, microvilli.
To determine whether apoptosis is involved in the embryonic lethality, the TUNEL (TdT-mediated dUTP-biotin nick end labeling) assay was performed on E6.5 wild-type and mutant embryos (Figure 3I and J). An overall increase in TUNEL-positive brown nuclei (arrows) was observed in all of the Dab2–/– embryos analyzed as compared with wild-type embryos. This overall increase in apoptosis may contribute to the small size of Dab2–/– embryos.
Dab2 is expressed in the visceral endoderm
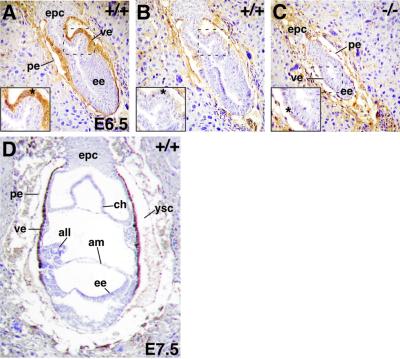
E6.5 embryos were analyzed to identify the sites of Dab2 protein expression (Figure 4). In wild-type embryos, a strong Dab2 positive signal was observed only in the cells of the VE (Figure 4A, asterisk). Staining with the secondary antibody only yielded low non-specific background staining of the PE and maternal tissues (Figure 4B). In the smaller mutant embryo, no Dab2 staining was observed in the VE (Figure 4C). In E7.5 wild-type embryos, Dab2 protein expression was still restricted to the VE (Figure 4D), as reported previously for Dab2 mRNA (Morrisey et al., 2000). Thus, Dab2 protein expression is restricted to the VE at the time when Dab2–/– embryos cease developing normally.
Fig. 4. Embryonic expression of the Dab2 protein by immunohistochemistry. (A) Dab2 positive staining of the VE in a E6.5 wild-type embryo. (B) Background reaction with secondary antibody only. (C) Immunohistochemistry of a Dab2 null E6.5 embryo. The hatched box indicates the enlarged region. The asterisk indicates the visceral endoderm. (D) Dab2 staining of a E7.5 wild-type embryo. Note that VE is replaced by definitive endoderm at the distal tip at this stage. ve, visceral endoderm; pe, parietal endoderm; ee, embryonic ectoderm; epc, ectoplacental cone; ch, chorion; am, amnion; all, allantois; ysc, yolk sac cavity.
Functional defects in Dab2–/– visceral endoderm
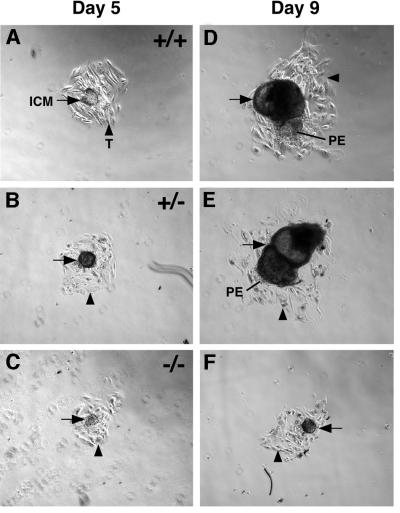
Coucouvanis and Martin (1995) showed that growth and cavitation of the inner cell mass in vitro depend on signals from the surrounding primitive endoderm. To investigate whether the primitive endoderm of Dab2 mutants can provide such signals, blastocysts were collected at E3.5 from Dab2+/– inter-crosses and cultured for 9 days (Figure 5; Table II). During the first 3–5 days in cul ture, wild-type, heterozygous and knock-out blastocysts attached to the dish and trophoblastic cells migrated out over the substrate (Figure 5A–C). Between days 5 and 9, the inner cell masses of 83% (25 out of 30) of the wild-type and heterozygous blastocysts expanded and formed large fluid-filled cavities (Figure 5D and E). In most cultures, migrating parietal endoderm cells were visible. In contrast, the inner cell masses of 93% (14 out of 15) of Dab2–/– blastocysts were significantly reduced in size or absent (Figure 5F).
Fig. 5. In vitro blastocyst outgrowths. (A) Wild-type, (B) heterozygous and (C) mutant blastocysts after 5 days in culture. The same (D) wild-type, (E) heterozygous and (F) mutant blastocysts after 9 days in culture. ICM (arrow), inner cell mass; T (arrowhead), trophectoderm; PE, parietal endoderm.
Table II. Genotype and size of inner cell mass of cultured blastocysts.
| +/+ or +/– | –/– | |
|---|---|---|
| Small | 5 | 14 |
| Large | 25 | 1 |
P > 0.9995.
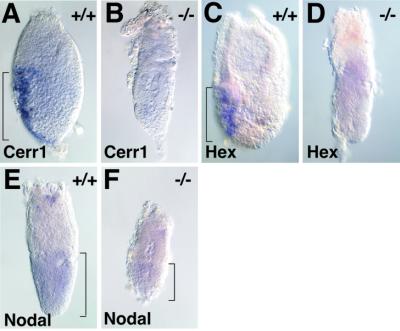
The Dab2–/– phenotype, including failure to thin the distal tip VE, elongate the extra-embryonic portion of the egg cylinder and properly organize the epiblast, resembles those of certain SMAD2 and SMAD4 mutants, in which the distal tip VE fails to differentiate into AVE in response to a Nodal signal (Nomura and Li, 1998; Sirard et al., 1998; Waldrip et al., 1998; Weinstein et al., 1998; Yang et al., 1998). Moreover, when tested, blastocysts or embryoid bodies from SMAD4 mutants failed to grow in vitro (Sirard et al., 1998; Yang et al., 1998). Therefore, we tested for the induction of the AVE markers Cerr1 (Shawlot et al., 1998) and Hex (Thomas et al., 1998). In E6.5 wild-type and heterozygous embryos, Cerr1 and Hex are expressed in the AVE (Figure 6A and C). In contrast, Cerr1 expression and Hex were not detected in Dab2 mutants (Figure 6B and D), suggesting a defect in receipt of the Nodal signal by the distal tip VE. Nodal expression was detected in the embryonic portions of the wild-type and Dab2 mutant egg cylinders (Figure 6E and F), consistent with Dab2-independent expression in the epiblast.
Fig. 6. In situ hybridization analysis. Cerr1 (A and B), Hex (C and D) and Nodal (E and F) expression in Dab2 wild-type (A, C and E) and mutant (B, D and F) E6.5 embryos. The bracket indicates a signal in the AVE in (A) and (C), and in the distal portion of the embryo in (E) and (F).
Rescue of Dab2-deficient embryos with Dab2 in extra-embryonic tissues
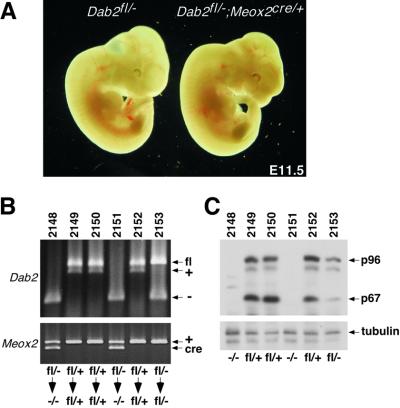
Because Dab2 expression was only detected in extra-embryonic tissues, we tested whether we could rescue the lethality of Dab2 deletion by selectively removing Dab2 from the embryo proper. Mice homozygous for the conditional Dab2fl allele (Figure 1) were mated to mice heterozygous for the Dab2 null allele and expressing Cre under the control of the Meox2 promoter (Dab2+/–; Meox2cre/+). The expression of the Cre recombinase from Meox2cre is limited to cells of the embryo proper, thus allowing for deletion of floxed alleles only in the embryo (Tallquist and Soriano, 2000). Analysis of E11.5 embryos revealed that mice whose extra-embryonic tissues were Dab2fl/–; Meox2cre/+ were normal in appearance (Figure 7A). Indeed, Dab2 conditionally null animals were born and survived, as shown by PCR genotyping of post-natal day 8 (P8) tail samples (Figure 7B). Immunoblots of P8 tail samples showed that Dab2 protein expression was essentially ablated in the conditionally null mice (Figure 7C). Both p96 and p67 were detected in the tails of mice with one (fl/–) or two (fl/+) functional copies of Dab2, with a clear effect of gene dosage on expression level. In the conditionally null (–/–) animals shown here, neither form of the Dab2 protein was detected, although, in some conditional animals, trace amounts of Dab2fl allele remained and were not analyzed further. The birth and survival of conditional null mice suggest that Dab2 is only required in extra-embryonic tissues for all steps of normal development. This is consistent with the embryonic lethality due to defective AVE induction, and further suggests that Nodal signaling in the epiblast is independent of Dab2.
Fig. 7. Rescue of Dab2-deficient mice with Meox2–Cre. (A) Morphology of E11.5 embryos with either one functional copy of Dab2 (Dab2fl/–) or conditionally null for Dab2 (Dab2fl/–; Meox2cre/+). (B) PCR analysis of P8 pups (tag number 2148–2153) born from a Dab2fl/fl × Dab2+/–; Meox2cre/+ mating. The upper panel shows the Dab2 genotype, while the lower panel shows the Meox2–Cre genotype. The bottom labels indicate the genotype before and after Cre expression. (C) Immunoblot analysis of protein lysates made from the tails of mice with the genotypes indicated in (B). Forty micrograms of total protein were resolved by 10% SDS–PAGE and immunoblotted with mouse anti-Dab2 antibodies, which recognize the N-terminus common to all known Dab2 isoforms. To verify equal loading, the blot was stripped and reprobed with mouse anti-β-tubulin antibodies.
Mice conditionally null for Dab2 exhibit defects in kidney function
Dab2 conditionally null animals appear healthy and grow as rapidly as their littermates (data not shown). Moreover, female Dab2–/– mice breed and raise pups. Since Dab2 is highly expressed in a number of adult tissues, including the kidney, ovary, liver, mammary gland, intestine, uterus and heart (Fazili et al., 1999), various organs from Dab2 conditionally null mice were analyzed. Intestinal epithelium from wild-type mice expresses high levels of Dab2, yet the intestinal epithelium from Dab2 conditionally null mice appeared normal (data not shown). The kidney also appeared grossly normal, despite the absence of Dab2 protein that is normally expressed in the kidney proximal tubule (KPT) cells (Figure 8A–D).
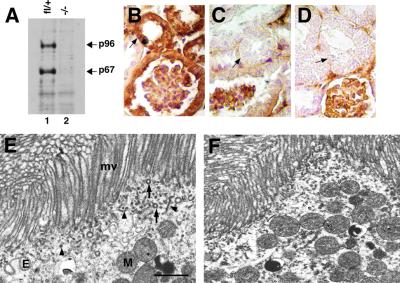
Fig. 8. Analysis of an adult kidney. (A) Immunoblot analysis of adult kidneys of the indicated genotypes. Kidney extracts (7 µg protein) were resolved on a 4–15% SDS–PAGE gradient gel and immunoblotted with mouse anti-Dab2 antibodies. (B–D) Immunohistochemistry of Dab2 localization in kidney. (B) Wild-type kidney, anti-Dab2. (C) Wild-type kidney, control antibody. (D) Conditionally null kidney, anti-Dab2. Note specific staining in the proximal tubule (arrows) and non-specific staining in the capillary knot of glomerulus. (E and F) EM of cross- sections through a KPT from a wild-type (E) and conditionally null (F) mouse. Bar = 1 µm. mv, microvilli; E, endosome; M, mitochondria; (arrow) endocytic vesicle; arrowhead, dense apical tubule.
Because Dab2 complexes with megalin (Oleinikov et al., 2000), we examined kidney function. Megalin is highly expressed in the KPT, where it is important for the reabsorption of several plasma proteins from the primary filtrate (Christensen and Birn, 2001). As a result, megalin–/– mice secrete excess quantities of vitamin D binding protein (DBP) (Nykjaer et al., 1999) and retinol binding protein (RBP) (Christensen and Willnow, 1999) in the urine, and megalin–/– KPT cells have reduced numbers of clathrin-coated pits and vesicles (Willnow et al., 1996; Nykjaer et al., 1999). EM showed that Dab2 deletion does not reduce the apical microvilli or junctional complexes between KPT cells, but the number of coated pits and endocytic vesicles near the apical membrane was significantly reduced as compared with wild type (Figure 8E and F). This suggests possible changes in transport of molecules from the apical surface of the KPT cells.
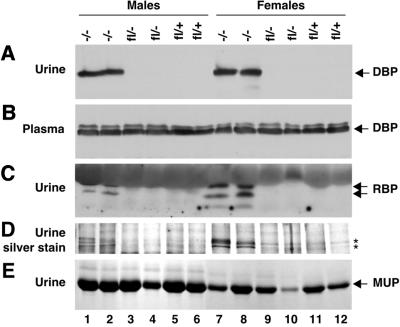
Urine was collected from Dab2fl/+, Dab2fl/– and Dab2–/– mice and analyzed for DBP, RBP and other proteins (Figure 9). While wild-type and heterozygous animals did not excrete DBP (Figure 9A, lanes 3–6 and 9–12), the urine of male and female conditionally null mice contained DBP (Figure 9A, lanes 1 and 2 and 7 and 8). Increased excretion of DBP was not a consequence of increased DBP levels in the plasma, since plasma DBP levels were unchanged (Figure 9B). Similarly, the urine of male and female conditionally null mice contained RBP (Figure 9C). Silver staining of gels also revealed increased levels of other proteins in Dab2–/– urine (Figure 9D), although total protein content and the level of the major urinary protein (MUP), a protein whose reabsorption is not dependent on megalin, were not systematically altered (Figure 9E). Interestingly, both the defect in DBP and RBP reabsorption, and the reduction in apical clathrin-coated pits, reflect the phenotypes reported for megalin-deficient mice (Christensen and Willnow, 1999; Nykjaer et al., 1999).
Fig. 9. Urine and plasma analysis of Dab2 conditionally null mice. (A) Urine samples (15 µg protein) were resolved by 12.5% SDS– PAGE and immunoblotted with rabbit anti-DBP antibodies. (B) Plasma (0.5 µl) obtained from the same mice was immunoblotted with rabbit anti-DBP antibodies. (C) Urine protein (15 µg) was resolved by 15% SDS–PAGE and immunoblotted with sheep anti-RBP antibodies. (D) Urine protein (1.5 µg) was resolved by 15% SDS–PAGE and silver stained. Asterisks indicate proteins that were increased in urine from conditionally null mice. (E) Urine protein (1.5 µg) was subjected to 15% SDS–PAGE and stained with Coommassie Blue to detect the secreted levels of the major urinary protein (MUP).
Discussion
Our genetic analysis shows that Dab2 is required for normal embryonic VE development and also facilitates a transport process in an adult epithelium. Embryos lacking Dab2 are able to implant, but they fail to gastrulate and cease developing around E6.0–6.5. The localization of Dab2 expression to the VE, the rescue of development when Dab2 is supplied in extra-embryonic tissues, the lack of induction of AVE markers, and the failure of Dab2–/– blastocysts to develop normally in vitro, all provide evidence that Dab2 is likely needed for Nodal signaling in the VE. This apparent requirement may be direct or indirect, as discussed below, and Dab2 may have additional functions in the VE that we have not detected. When Dab2 is supplied in extra-embryonic tissues, Dab2–/– embryos are efficiently rescued, implying that Dab2 is not needed for subsequent Nodal or other signaling events in the embryo proper. However, Dab2 is required for normal endocytosis in the proximal tubule cells of the kidney, as shown by the reduced number of apical coated pits and vesicles and increased excretion of DBP, RBP and certain other proteins, linking Dab2 to the megalin-dependent protein trafficking machinery.
Dab2 has an essential function in the VE
The developmental defects observed in the Dab2–/– embryos resemble some of the phenotypes described in SMAD2 and SMAD4 mutants (Nomura and Li, 1998; Sirard et al., 1998; Waldrip et al., 1998; Weinstein et al., 1998; Yang et al., 1998). These mutants fail to form a primitive streak, the proximal extra-embryonic region of the egg cylinder is truncated, and AVE markers, such as Cerr1 and Hex, are not induced in the distal VE. These phenotypes are rescued by wild-type extra-embryonic cells (Sirard et al., 1998; Waldrip et al., 1998). Thus it is likely that Dab2 is needed in the VE to respond to Nodal coming from the epiblast (Beddington and Robertson, 1999; Lu et al., 2001). However, Dab2 appears not to be needed for Nodal functions in the embryo, such as mesoderm and endoderm induction, A-P axis patterning and establishment of left–right asymmetry (Schier and Shen, 2000), since it is not detectably expressed in the epiblast at E6.5–7.5 and can be deleted without detriment. Induction of specific genes by Nodal is known to depend on different components in different cell types (Ding et al., 1998; Brennan et al., 2001). It is not clear how Dab2 functions, nor why it is dispensable in most tissues. Because Dab2 is involved in TGFβ signaling and binds to SMAD2 and TGFβ receptors (Hocevar et al., 2001), it is tempting to speculate that it acts like SARA, facilitating SMAD activation (Tsukazaki et al., 1998). However, it is also possible that Dab2 regulates expression of a cell-specific co-factor, like FAST or Cripto (Ding et al., 1998; Brennan et al., 2001), or is involved in receptor traffic or other indirect mechanisms.
The VE also has transport and nutritive functions during early development (Bielinska et al., 1999). The VE of Dab2–/– embryos has a well-formed brush border, apical junctional complexes and a basement membrane, but it may be defective in transport functions, contributing to the phenotype. The VE expresses a number of proteins that are secreted basolaterally into the embryo proper (Meehan et al., 1984; Shi and Heath, 1984). The mechanism for basolateral secretion in the VE is unclear and may involve lipoprotein receptors since several of the proteins secreted by the VE are megalin ligands and megalin is highly expressed in the VE (Christensen and Birn, 2001). It is conceivable that newly synthesized megalin may associate with its ligands in the endoplasmic reticulum or Golgi, and then escort them through a basolateral secretion pathway. Thus, defective megalin trafficking could contribute to the VE defect of Dab2–/– embryos. However, it should be noted that megalin–/– embryos gastrulate successfully, suggesting that megalin is not needed when Dab2 is essential (Willnow et al., 1996), and many of the megalin ligands that are secreted by the VE are not needed until later in development (Bielinska et al., 1999).
The effects of Dab2 gene disruption resemble those observed in mutants of the GATA6 transcription factor (Morrisey et al., 1998; Koutsourakis et al., 1999). Like Dab2, GATA6 is expressed in the VE, and is needed for the development of extra-embryonic tissues in vivo and for growth and cavitation of the inner cell mass in vitro (Koutsourakis et al., 1999). Furthermore, deletion of GATA6 causes apoptosis in the epiblast (Morrisey et al., 1998). These phenotypic similarities are consistent with the observation that expression of Dab2 in the VE is dependent on GATA6 (Morrisey et al., 2000), and suggest that the death of GATA6–/– embryos may be attributed to the lack of Dab2 expression. However, in addition to Dab2, at least eight other genes depend on GATA6 for expression in ES cells (Morrisey et al., 2000), and it is possible that one or more of these other genes contributes to the GATA6–/– phenotype. Ectopic expression of Dab2 in the GATA6 null background would directly address whether Dab2 is the only GATA6-regulated gene needed for VE development. GATA6 also functions later in development, when Dab2 deletion is tolerated (Keijzer et al., 2001). Therefore, GATA6 has Dab2-independent functions.
Transport function for Dab2 in the adult kidney
Conditionally null Dab2–/– mice are outwardly normal, but they secrete DBP, RBP and certain other proteins in the urine. Two of the secreted proteins have apparent molecular weights of 63 and 53 kDa and may correspond to albumin and α-amylase, respectively, which, together with DBP and RBP, are elevated in the urine of megalin-deficient mice (Cui et al., 1996; Birn et al., 2000). Megalin is also needed to reabsorb transthyretin (thyroxine-binding protein) (Sousa et al., 2000) and transcobalamin (vitamin B12-binding protein) (Moestrup et al., 1996). The secretion of DBP and RBP, along with the altered distribution of coated pits and endocytic vesicles in KPT cells, suggest that conditionally null Dab2–/– animals have reduced megalin-mediated re-uptake. These phenotypes also resemble those caused by mutation of the RAP gene, which encodes a protein chaperone for lipoprotein receptor cell surface expression (Willnow et al., 1995; Birn et al., 2000). However, megalin-mediated transport is not completely inhibited in the Dab2–/– animals. Megalin–/– animals excrete so much DBP that it is depleted from the plasma, and shortage of vitamin D causes rickets (Nykjaer et al., 1999). Dab2–/– mice are not so severely affected. Also, most megalin–/– animals die as embryos, due to a failure in forebrain development symptomatic of starvation for cholesterol (Willnow et al., 1996). Normal brain development in conditionally null Dab2–/– mice suggests that cholesterol transport to the brain is adequate.
The molecular mechanism of Dab2 function in endocytosis likely involves direct binding to megalin (Oleinikov et al., 2000), AP-2 (Morris and Cooper, 2001), and myosin VI (Morris et al., 2002), a non-muscle myosin implicated in endocytosis (Buss et al., 2001). However, the remarkably normal development of conditionally null Dab2–/– mice suggests that Dab2 is either not a vital part of the endocytosis machinery or that it is functionally redundant with other PTB-containing adaptor proteins. Dab2 may facilitate sorting of megalin into coated pits, or alter the routing of clathrin-coated vesicles once they are internalized but before they fuse with endosomes and uncoat. The recent discoveries that a PTB protein, ARH, is required for maintaining normal levels of circulating low density lipoprotein in humans (Garcia et al., 2001), and that the PTB protein JIP-1 sorts an apolipoprotein receptor to the tips of neurites (Verhey et al., 2001), suggest that PTB proteins have different functions in the regulation of lipoprotein receptor trafficking.
Dab2 and cancer
Dab2 has many properties associated with tumor suppressor genes (Fulop et al., 1998; Mok et al., 1998; Tseng et al., 1998; Fazili et al., 1999; Sheng et al., 2000). The possibility that Dab2 is involved in Nodal signal transduction in the VE suggests that Dab2 down-regulation could contribute to the TGFβ resistance commonly found in carcinomas. Interestingly, mutations in TGFβ signaling pathway components, such as SMADs, are detected in many carcinomas, but not in the reproductive carcinomas in which Dab2 is commonly down-regulated (Massagué et al., 2000). However, to date we have failed to detect an increase in tumor incidence in our Dab2 heterozygous or conditionally null mice. It is possible that loss of Dab2 contributes to the progression of initiated tumors, in which case irradiation or carcinogen treatment may reveal an increase in tumor progression in Dab2–/– mice.
In conclusion, Dab2 is a multi-functional protein with potential roles in megalin trafficking and Nodal responses in specific cell types. These roles may be linked if, for example, Dab2 regulates traffic of Nodal receptors. Further work is needed to determine the molecular mechanisms of these in vivo functions.
Materials and methods
Construction of targeting vector
The first 915 bp of the mouse Dab2 cDNA were used as a probe to isolate a genomic clone containing at least the first two coding exons of Dab2 from a 129S4 mouse library (P.Soriano, Fred Hutchinson Cancer Research Center, Seattle, WA). The targeting vector was generated by replacing the thymidine kinase gene of pKO Scrambler NTKV-1901 (Stratagene) with the diphtheria toxin gene (Stratagene). In addition, loxP sites were added flanking the neomycin cassette to generate pKO Scrambler NTKV-DT.lp2. To generate the short arm, a loxP site was inserted into the SmaI site between the first and second coding exons, and then a 2.3 kb BglII–EcoRI fragment was cloned into the BglII/MfeI sites of NTKV-DT.lp2 (see Figure 1). The long arm was generated by cloning a 3 kb EcoRI–EcoRV fragment into the EcoRI/EcoRV sites of NTKV-DT.lp2.
Generation and genotyping of deficient and conditional embryos
AK7 ES cells were electroporated with 20 µg of NotI-linearized Dab2 targeting vector. Selection (300 µg/ml G418) was applied 24 h after electroporation and resistant clones were picked 9 days later and screened by PCR. Correctly targeted ES cells were electroporated with pPGKCrebpA (P.Soriano) or pBS185 (Sauer and Henderson, 1990), and null or floxed recombinant alleles were identified by PCR and Southern blotting. Recombined clones were injected into C57BL/6 host blastocysts and chimeric males were mated to C57BL/6 females to generate mice heterozygous for either the disrupted (Dab2–) or floxed (Dab2fl) allele. Genotyping of embryos and newborn pups was performed by PCR using three primers: a sense primer (p1, 5′-GACCACGCTGTCCTTGAACTCAG-3′) and two antisense primers (p2, 5′-CTGAAAAGAGAACACTGGAGGCTC-3′; p3, 5′-GTAAATTCTCATGGCTGTGAC TGG-3′). The resulting PCR products of 460, 530 and 250 bp correspond to the wild-type, floxed and deleted alleles, respectively.
Histology and TUNEL assay
Embryos and tissues were fixed in 4% paraformaldehyde, paraffin embedded, sectioned (5–7 µm) and stained with hematoxylin and eosin. For thin sections and EM, samples were fixed in Karnovsky’s half-strength for 36 h, post-fixed in osmium S collidine for 8 h, and kidney samples were in-block stained with 3% uranyl acetate for 1 h. All tissues were dehydrated for 1 h each in 35, 70 and 95% ethanol, and through two changes of 100% ethanol and propylene oxide. Samples were then infiltrated with 50:50 propylene oxide:Epon 812 and placed in a vacuum oven overnight. The next day, fresh Epon 812 was added and samples were returned to the vacuum oven overnight. Tissues were embedded in fresh Epon 812 and allowed to harden in the oven for 48 h. Sections (400–600 nm) were placed on 150 mesh grids and stained for 2 h with 6% saturated uranyl acetate, then with Millonig’s lead stain for 4 min. Sections were viewed using the JEOL 100SX transmission electron microscope.
For the TUNEL assay, nuclear proteins were stripped with proteinase K (20 μg/ml) for 10 min at room temperature. The TdT reaction was accomplished as described by Gavrieli et al. (1992) except that biotin-14-dATP (Gibco-BRL) was used in place of biotinylated dUTP. Vectastain ABC and DAB kits (Vector Laboratories, Inc.) were used to visualize the biotinylated ATP. Sections were counterstained with Methyl Green.
Immunohistochemistry
Immunohistochemistry was performed with the Vectastain Elite ABC Kit (Vector Laboratories, Inc.) closely following the previously described method for Dab2 detection (Fazili et al., 1999). Briefly, 5–7 µm sections were dewaxed in Histoclear, rehydrated in serial dilutions of alcohol, and steamed for 20 min in 30 mM citrate buffer pH 4.8 for antigen retrieval. Sections were rinsed in phosphate-buffered saline (PBS) and incubated in 3% hydrogen peroxide for 5 min to block endogenous peroxidases. Sections were rinsed and blocked for 30 min in 5% normal horse serum. Slides were then incubated overnight at 4°C with a 1:200 dilution of mouse anti-Dab2 (p96) antibodies (BD Transduction Labs) diluted in 5% bovine serum albumin (BSA) in PBS. Following three 5 min washes in PBS plus 0.05% Tween-20 (PBST), sections were incubated for 30 min with horse anti-mouse biotinylated secondary antibodies diluted 1:200 in 5% horse serum. Slides were washed again in PBST and incubated for 30 min with Vectastain Elite ABC Reagent. Following the last wash of PBST, sections were incubated in DAB solution plus nickel until color change was observed (∼5 min), and reaction was stopped by rinsing in tap water. Sections were counterstained with hematoxylin.
In situ hybridization
E6.5 embryos were isolated, fixed in 4% paraformaldehyde overnight and processed for whole-mount in situ hybridization essentially as described (Hogan et al., 1994) using digoxigenin-11-UTP-labeled riboprobes. Embryos were photographed using Nomarski optics, then genotyped by PCR.
Blastocyst culture
Blastocysts were flushed at E3.5, using ES cell medium (DME, 15% FBS, 10 µM β-mercaptoethanol, 2 mM glutamine, penicillin and streptomycin) containing 20 mM HEPES pH 7.4, then cultured on gelatinized glass coverslips in ES cell medium at 37°C in 5% CO2 for 9 days. Photographs were taken on days 5 and 9 of culture. At the end of 9 days in culture, cells were scraped off and collected by mouth pipette for PCR genotyping as described above.
Urine and plasma analysis
Metabolic cages were used to collect urine from individual mice over a 24 h period. Orbital eye bleeds were performed and 200 µl of blood were collected into microfuge tubes containing 8 µl of 0.25 M EDTA to prevent clotting. Cells were removed by centrifugation. Protein concentrations were determined by Bio-Rad protein assay reagent using BSA as a standard.
Immunoblotting
Tissues were lysed on ice in lysis buffer (1% Triton X-100, 10 mM HEPES pH 7.4, 150 mM NaCl, 2 mM EDTA, 50 mM NaF, 0.2 mM Na3VO4, 1% aprotinin, 1 mM PMSF, 10 μg/ml leupeptin) followed by centrifugation at 10 000 r.p.m. for 10 min at 4°C. Samples were heated at 100°C for 5 min in 2× sample buffer, resolved by SDS–PAGE, and transferred to Immobilon P (Millipore). Filters were blocked in 25 mM Tris–HCl, 8 mM Tris-base, 150 mM NaCl, 0.05% Tween-20 and 5 mM NaF plus 2% BSA for 1 h at room temperature. Mouse anti-Dab2, rabbit anti-DBP (also known as Gc-Globulin; Dako), sheep anti-RBP (Biogenesis) and mouse anti-β-tubulin (Sigma) antibodies were applied in 1:2500, 1:1000, 1:1000 and 1:1000 dilutions, respectively, for 2 h. Goat anti-mouse IgG conjugated to horseradish peroxidase (HRP; Bio-Rad), goat anti-rabbit IgG conjugated to HRP (Bio-Rad) and protein G coupled to HRP were applied in 1:10 000, 1:10 000 and 1:2000 dilutions, respectively, for 1 h. Proteins were detected with the Renaissance chemiluminescence reagent (NEN).
Acknowledgments
Acknowledgements
We thank Nanyan Jiang and Priscilla Kronstad for excellent technical assistance, Judy Groombridge and Franque Remington for EM analysis, Richard Behringer, Jeff Wrana and Elizabeth Robertson for probes, and members of the Cooper Laboratory, Soriano Laboratory, Andre Oleinikov, Philip Howe, Jeff Wrana, Cecilia Moens and Mike Xu for helpful discussions. We are also very grateful to Philippe Soriano for providing us with the mouse genomic library, ES cells, pPGKCrebpA, helpful discussions and critical reading of this manuscript. This work was supported by grants from The Helen Hay Whitney Foundation (to S.M.M.), the National Institutes of Health, R37-CA40172 (to J.A.C.) and GM34496 (to C.O.R.), Cancer Center (CORE) Support Grant CA21765, and the American Lebanese Syrian Associated Charities.
References
- Beddington R.S. and Robertson,E.J. (1999) Axis development and early asymmetry in mammals. Cell, 96, 195–209. [DOI] [PubMed] [Google Scholar]
- Bielinska M., Narita,N. and Wilson,D.B. (1999) Distinct roles for visceral endoderm during embryonic mouse development. Int. J. Dev. Biol., 43, 183–205. [PubMed] [Google Scholar]
- Birn H., Vorum,H., Verroust,P.J., Moestrup,S.K. and Christensen,E.I. (2000) Receptor-associated protein is important for normal processing of megalin in kidney proximal tubules. J. Am. Soc. Nephrol., 11, 191–202. [DOI] [PubMed] [Google Scholar]
- Brennan J., Lu,C.C., Norris,D.P., Rodriguez,T.A., Beddington,R.S. and Robertson,E.J. (2001) Nodal signalling in the epiblast patterns the early mouse embryo. Nature, 411, 965–969. [DOI] [PubMed] [Google Scholar]
- Buss F., Arden,S.D., Lindsay,M., Luzio,J.P. and Kendrick-Jones,J. (2001) Myosin VI isoform localized to clathrin-coated vesicles with a role in clathrin-mediated endocytosis. EMBO J., 20, 3676–3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W.J., Goldstein,J.L. and Brown,M.S. (1990) NPXY, a sequence often found in cytoplasmic tails, is required for coated pit-mediated internalization of the low density lipoprotein receptor. J. Biol. Chem., 265, 3116–3123. [PubMed] [Google Scholar]
- Cho S.Y., Cho,S.Y., Lee,S.H. and Park,S.S. (1999) Differential expression of mouse Disabled 2 gene in retinoic acid-treated F9 embryonal carcinoma cells and early mouse embryos. Mol. Cells, 9, 179–184. [PubMed] [Google Scholar]
- Christensen E.I. and Birn,H. (2001) Megalin and cubilin: synergistic endocytic receptors in renal proximal tubule. Am. J. Physiol. Renal Physiol., 280, F562–F573. [DOI] [PubMed] [Google Scholar]
- Christensen E.I. and Willnow,T.E. (1999) Essential role of megalin in renal proximal tubule for vitamin homeostasis. J. Am. Soc. Nephrol., 10, 2224–2236. [DOI] [PubMed] [Google Scholar]
- Coucouvanis E. and Martin,G.R. (1995) Signals for death and survival: a two-step mechanism for cavitation in the vertebrate embryo. Cell, 83, 279–287. [DOI] [PubMed] [Google Scholar]
- Cui S., Verroust,P.J., Moestrup,S.K. and Christensen,E.I. (1996) Megalin/gp330 mediates uptake of albumin in renal proximal tubule. Am. J. Physiol., 271, F900–F907. [DOI] [PubMed] [Google Scholar]
- Ding J., Yang,L., Yan,Y.T., Chen,A., Desai,N., Wynshaw-Boris,A. and Shen,M.M. (1998) Cripto is required for correct orientation of the anterior–posterior axis in the mouse embryo. Nature, 395, 702–707. [DOI] [PubMed] [Google Scholar]
- Fazili Z., Sun,W., Mittelstaedt,S., Cohen,C. and Xu,X.-X. (1999) Disabled-2 inactivation is an early step in ovarian tumorigenicity. Oncogene, 18, 3104–3113. [DOI] [PubMed] [Google Scholar]
- Fulop V., Colitti,C.V., Genest,D., Berkowitz,R.S., Yiu,G.K., Ng,S.-W., Szepesi,J. and Mok,S.C. (1998) DOC-2/hdab2, a candidate tumor suppressor gene involved in the development of gestational trophoblastic diseases. Oncogene, 17, 419–424. [DOI] [PubMed] [Google Scholar]
- Garcia C.K. et al. (2001) Autosomal recessive hypercholesterolemia caused by mutations in a putative LDL receptor adaptor protein. Science, 292, 1394–1398. [DOI] [PubMed] [Google Scholar]
- Gavrieli Y., Sherman,Y. and Ben-Sasson,S.A. (1992) Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J. Cell Biol., 119, 493–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu H., Marth,J.D., Orban,P.C., Mossmann,H. and Rajewsky,K. (1994) Deletion of a DNA polymerase β gene segment in T cells using cell type-specific gene targeting. Science, 265, 103–106. [DOI] [PubMed] [Google Scholar]
- Hocevar B.A., Smine,A., Xu,X.-X. and Howe,P.H. (2001) The adaptor molecule Disabled-2 links the transforming growth factor β receptors to the Smad pathway. EMBO J., 20, 2789–2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan B., Beddington,R., Costantini,F. and Lacy,E. (1994) Manipulat ing the Mouse Embryo: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Hogan B.L. (1996) Bone morphogenetic proteins in development. Curr. Opin. Genet. Dev., 6, 432–438. [DOI] [PubMed] [Google Scholar]
- Howell B.W. and Herz,J. (2001) The LDL receptor gene family: signaling functions during development. Curr. Opin. Neurobiol., 11, 74–81. [DOI] [PubMed] [Google Scholar]
- Howell B.W., Gertler,F.B. and Cooper,J.A. (1997) Mouse disabled (mDab1): a Src binding protein implicated in neuronal development. EMBO J., 16, 121–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell B.W., Lanier,L.M., Frank,R., Gertler,F.B. and Cooper,J.A. (1999) The disabled 1 phosphotyrosine-binding domain binds to the internalization signals of transmembrane glycoproteins and to phospholipids. Mol. Cell. Biol., 19, 5179–5188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keijzer R., van Tuyl,M., Meijers,C., Post,M., Tibboel,D., Grosveld,F. and Koutsourakis,M. (2001) The transcription factor GATA6 is essential for branching morphogenesis and epithelial cell differentiation during fetal pulmonary development. Development, 128, 503–511. [DOI] [PubMed] [Google Scholar]
- Koutsourakis M., Langeveld,A., Patient,R., Beddington,R. and Grosveld,F. (1999) The transcription factor GATA6 is essential for early extraembryonic development. Development, 126, 723–732. [PubMed] [Google Scholar]
- Krieger M. and Herz,J. (1994) Structures and functions of multiligand lipoprotein receptors: macrophage scavenger receptors and LDL receptor-related protein (LRP). Annu. Rev. Biochem., 63, 601–637. [DOI] [PubMed] [Google Scholar]
- Lu C.C., Brennan,J. and Robertson,E.J. (2001) From fertilization to gastrulation: axis formation in the mouse embryo. Curr. Opin. Genet. Dev., 11, 384–392. [DOI] [PubMed] [Google Scholar]
- Margolis B. (1999) The PTB domain: the name doesn’t say it all. Trends Endocrinol. Metab., 10, 262–267. [DOI] [PubMed] [Google Scholar]
- Massagué J., Blain,S.W. and Lo,R.S. (2000) TGFβ signaling in growth control, cancer and heritable disorders. Cell, 103, 295–309. [DOI] [PubMed] [Google Scholar]
- Meehan R.R., Barlow,D.P., Hill,R.E., Hogan,B.L. and Hastie,N.D. (1984) Pattern of serum protein gene expression in mouse visceral yolk sac and fetal liver. EMBO J., 3, 1881–1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moestrup S.K., Birn,H., Fischer,P.B., Petersen,C.M., Verroust,P.J., Sim,R.B., Christensen,E.I. and Nexo,E. (1996) Megalin-mediated endocytosis of transcobalamin–vitamin-B12 complexes suggests a role of the receptor in vitamin-B12 homeostasis. Proc. Natl Acad. Sci. USA, 93, 8612–8617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mok S.C., Wong,K.-K., Chan,R.K.W., Lau,C.C., Tsao,S.-W., Knapp,R.C. and Berkowitz,R.S. (1994) Molecular cloning of differentially expressed genes in human epithelial ovarian cancer. Gynecol. Oncol., 52, 247–252. [DOI] [PubMed] [Google Scholar]
- Mok S.C. et al. (1998) DOC-2, a candidate tumor suppressor gene in human epithelial ovarian cancer. Oncogene, 16, 2381–2387. [DOI] [PubMed] [Google Scholar]
- Morris S.M. and Cooper,J.A. (2001) Disabled-2 colocalizes with the LDLR in clathrin-coated pits and interacts with AP-2. Traffic, 2, 111–123. [DOI] [PubMed] [Google Scholar]
- Morris S.M., Arden,S., Roberts,R., Kendrick-Jones,J., Cooper,J.A., Luzio,J.P. and Buss,F. (2002) Myosin VI binds to and localises with Dab2, potentially linking receptor mediated endocytosis and the actin cytoskeleton. Traffic, 3, in press. [DOI] [PubMed] [Google Scholar]
- Morrisey E.E., Tang,Z., Sigrist,K., Lu,M.M., Jiang,F., Ip,H.S. and Parmacek,M.S. (1998) GATA6 regulates HNF4 and is required for differentiation of visceral endoderm in the mouse embryo. Genes Dev., 12, 3579–3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrisey E.E., Musco,S., Chen,M.Y.Z., Lu,M.M., Leiden,J.M. and Parmacek,M.S. (2000) The gene encoding the mitogen-responsive phosphoprotein Dab2 is differentially regulated by GATA-6 and GATA-4 in the visceral endoderm. J. Biol. Chem., 275, 19949–19954. [DOI] [PubMed] [Google Scholar]
- Nomura M. and Li,E. (1998) Smad2 role in mesoderm formation, left–right patterning and craniofacial development. Nature, 393, 786–790. [DOI] [PubMed] [Google Scholar]
- Nykjaer A., Dragun,D., Walther,D., Vorum,H., Jacobsen,C., Herz,J., Melsen,F., Christensen,E.I. and Willnow,T.E. (1999) An endocytic pathway essential for renal uptake and activation of the steroid 25-(OH) vitamin D3. Cell, 96, 507–515. [DOI] [PubMed] [Google Scholar]
- Oleinikov A.V., Zhao,J. and Makker,S.P. (2000) Cytosolic adaptor protein Dab2 is an intracellular ligand of endocytic receptor gp600/megalin. Biochem. J., 347, 613–621. [PMC free article] [PubMed] [Google Scholar]
- Pawson T. and Scott,J.D. (1997) Signaling through scaffold, anchoring and adaptor proteins. Science, 278, 2075–2080. [DOI] [PubMed] [Google Scholar]
- Pearse B.M.F., Smith,C.J. and Owen,D.J. (2000) Clathrin coat construction in endocytosis. Curr. Opin. Struct. Biol., 10, 220–228. [DOI] [PubMed] [Google Scholar]
- Rice D.S. and Curran,T. (1999) Mutant mice with scrambled brains: understanding the signaling pathways that control cell positioning in the CNS. Genes Dev., 13, 2758–2773. [DOI] [PubMed] [Google Scholar]
- Sauer B. and Henderson,N. (1990) Targeted insertion of exogenous DNA into the eukaryotic genome by the Cre recombinase. New Biol., 2, 441–449. [PubMed] [Google Scholar]
- Schier A.F. and Shen,M.M. (2000) Nodal signalling in vertebrate development. Nature, 403, 385–389. [DOI] [PubMed] [Google Scholar]
- Schwahn D.J. and Medina,D. (1998) p96, a MAPK-related protein, is consistently downregulated during mouse mammary carcinogenesis. Oncogene, 17, 1173–1178. [DOI] [PubMed] [Google Scholar]
- Shawlot W., Deng,J.M. and Behringer,R.R. (1998) Expression of the mouse cerberus-related gene, Cerr1, suggests a role in anterior neural induction and somitogenesis. Proc. Natl Acad. Sci. USA, 95, 6198–6203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng Z., Sun,W., Smith,E., Cohen,C., Sheng,Z. and Xu,X.-X. (2000) Restoration of positioning control following Disabled-2 expression in ovarian and breast tumor cells. Oncogene, 19, 4847–4854. [DOI] [PubMed] [Google Scholar]
- Shi W.K. and Heath,J.K. (1984) Apolipoprotein expression by murine visceral yolk sac endoderm. J. Embryol. Exp. Morphol., 81, 143–152. [PubMed] [Google Scholar]
- Sirard C. et al. (1998) The tumor suppressor gene Smad4/Dpc4 is required for gastrulation and later for anterior development of the mouse embryo. Genes Dev., 12, 107–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith E.R., Capo-Chichi,C.D., He,J., Smedberg,J.L., Yang,D.H., Prowse,A.H., Godwin,A.K., Hamilton,T.C. and Xu,X.X. (2001) Disabled-2 mediates c-Fos suppression and the cell growth regulatory activity of retinoic acid in embryonic carcinoma cells. J. Biol. Chem., 276, 47303–47310. [DOI] [PubMed] [Google Scholar]
- Sousa M.M., Norden,A.G., Jacobsen,C., Willnow,T.E., Christensen,E.I., Thakker,R.V., Verroust,P.J., Moestrup,S.K. and Saraiva,M.J. (2000) Evidence for the role of megalin in renal uptake of transthyretin. J. Biol. Chem., 275, 38176–38181. [DOI] [PubMed] [Google Scholar]
- Sternberg N. and Hamilton,D. (1981) Bacteriophage P1 site-specific recombination. I. Recombination between loxP sites. J. Mol. Biol., 150, 467–486. [DOI] [PubMed] [Google Scholar]
- Tallquist M.D. and Soriano,P. (2000) Epiblast-restricted cre expression in MORE mice: a tool to distinguish embryonic vs. extra-embryonic gene function. Genesis, 26, 113–115. [DOI] [PubMed] [Google Scholar]
- Thomas P.Q., Brown,A. and Beddington,R.S. (1998) Hex: a homeobox gene revealing peri-implantation asymmetry in the mouse embryo and an early transient marker of endothelial cell precursors. Development, 125, 85–94. [DOI] [PubMed] [Google Scholar]
- Trommsdorff M., Borg,J.-P., Margolis,B. and Herz,J. (1998) Interaction of cytosolic adaptor proteins with neuronal apolipoprotein E receptors and the amyloid precursor protein. J. Biol. Chem., 273, 33556–33560. [DOI] [PubMed] [Google Scholar]
- Tseng C.P., Ely,B.D., Li,Y., Pong,R.C. and Hsieh,J.T. (1998) Regulation of rat DOC-2 gene during castration-induced rat ventral prostate degeneration and its growth inhibitory function in human prostatic carcinoma cells. Endocrinology, 139, 3542–3553. [DOI] [PubMed] [Google Scholar]
- Tseng C.P., Ely,B.D., Pong,R.C., Wang,Z., Zhou,J. and Hsieh,J.T. (1999) The role of DOC-2/DAB2 protein phosphorylation in the inhibition of AP-1 activity. J. Biol. Chem., 274, 31981–31986. [DOI] [PubMed] [Google Scholar]
- Tsukazaki T., Chiang,T.A., Davison,A.F., Attisano,L. and Wrana,J.L. (1998) SARA, a FYVE domain protein that recruits Smad2 to the TGFβ receptor. Cell, 95, 779–791. [DOI] [PubMed] [Google Scholar]
- Verhey K.J., Meyer,D., Deehan,R., Blenis,J., Schnapp,B.J., Rapoport,T.A. and Margolis,B. (2001) Cargo of kinesin identified as JIP scaffolding proteins and associated signaling molecules. J. Cell Biol., 152, 959–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldrip W.R., Bikoff,E.K., Hoodless,P.A., Wrana,J.L. and Robertson,E.J. (1998) Smad2 signaling in extraembryonic tissues determines anterior–posterior polarity of the early mouse embryo. Cell, 92, 797–808. [DOI] [PubMed] [Google Scholar]
- Weinstein M., Yang,X., Li,C., Xu,X., Gotay,J. and Deng,C.X. (1998) Failure of egg cylinder elongation and mesoderm induction in mouse embryos lacking the tumor suppressor smad2. Proc. Natl Acad. Sci. USA, 95, 9378–9383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willnow T.E., Armstrong,S.A., Hammer,R.E. and Herz,J. (1995) Functional expression of low density lipoprotein receptor-related protein is controlled by receptor-associated protein in vivo. Proc. Natl Acad. Sci. USA, 92, 4537–4541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willnow T.E., Hilpert,J., Armstrong,S.A., Rohlmann,A., Hammer,R.E., Burns,D.K. and Herz,J. (1996) Defective forebrain development in mice lacking gp330/megalin. Proc. Natl Acad. Sci. USA, 93, 8460–8464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X.-X., Yang,W., Jackowski,S. and Rock,C.O. (1995) Cloning of a novel phosphoprotein regulated by colony-stimulating factor 1 shares a domain with the Drosophila disabled gene product. J. Biol. Chem., 270, 14184–14191. [DOI] [PubMed] [Google Scholar]
- Xu X.-X., Yi,T., Tang,B. and Lambeth,J.D. (1998) Disabled-2 (Dab2) is an SH3 domain-binding partner of Grb2. Oncogene, 16, 1561–1569. [DOI] [PubMed] [Google Scholar]
- Yang X., Li,C., Xu,X. and Deng,C. (1998) The tumor suppressor SMAD4/DPC4 is essential for epiblast proliferation and mesoderm induction in mice. Proc. Natl Acad. Sci. USA, 95, 3667–3672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J. and Hsieh,J.T. (2001) The inhibitory role of DOC-2/DAB2 in growth factor receptors-mediated signal cascade: DOC-2/DAB2-mediated inhibition of Erk phosphorylation via binding to Grb2. J. Biol. Chem., 276, 27793–27798. [DOI] [PubMed] [Google Scholar]
- Zimmerman C.M. and Padgett,R.W. (2000) Transforming growth factor β signaling mediators and modulators. Gene, 249, 17–30. [DOI] [PubMed] [Google Scholar]